Abstract
Few works to date have reported the application of near-infrared spectroscopy (NIRS) for the characterization and authentication of cephalopods. This study investigated the feasibility of a portable NIRS instrument for the non-destructive freshness evaluation of fresh (F) and frozen-thawed (FT) cuttlefish (Sepia officinalis). Samples were examined by chemical, microbiological and sensorial analyses, during 13 days of conservation at 3 °C. The spectral data were collected on lateral mantle of cuttlefish, and different partial least squares discriminant analyses (PLS-DA) were applied for classification purposes. The interpretation of spectra was also investigated by applying the specific water coordinates, using aquaphotomics. Few significant differences in the wet chemistry and microbiological data were detected between F and FT during storage. The quality index method and microbiological analyses suggested similar behavior between F and FT samples until to near 9 days of shelf life. PLS-DA models with the spectral range 900–1650 nm achieved a classification precision of 0.91 between F and FT, while the performances for the prediction of storage days were less effective. The results of aquaphotomics plotted in aquagrams were suitable for the interpretation of the main physicochemical changes of cuttlefish throughout the shelf life. The water coordinates suggested a different molecular conformation of water species in the FT than F samples, with more free water molecules and a lower amount of bound species and the water solvation shell, respectively.
Electronic supplementary material
The online version of this article (10.1007/s13197-019-03957-6) contains supplementary material, which is available to authorized users.
Keywords: Near-infrared spectroscopy, Cuttlefish, Aquaphotomics, Fresh, Frozen-thawed
Introduction
In the recent decades, fish and seafood demand has greatly increased with a per capita fish consumption above 20 kg per year (Food and Agricultural Organisation, FAO 2016). Among the wide range of aquatic products, cephalopods are considered as a highly valuable group that represents 5.6% of the world seafood trade (FAO 2016). Despite being sold as fresh, dried and salted products, frozen cephalopods are the most commercialized foodstuff (Centre for the Promotion of Imports, CBI 2015). The increase in trades and the economic value of fish and seafood can involve commercial fraud while also instigating sanitary risks (Alamprese and Casiraghi 2015). Common frauds encompass the substitution of valuable species, mislabeling issues and the selling of frozen-thawed products as fresh (Fasolato et al. 2012).
Freezing is broadly applied to extend the commercial availability of seafood products, arresting microbial growth and many of the enzymatic activities. However, frozen treatments may involve qualitative decay, due to water losses, lipid oxidation, and texture and flavor modifications. Such adverse outcomes result from changes in the solutes concentration in frozen tissues that could increase enzymatic activity, and structural damage to membranes and muscle proteins, causing a decreased water holding capacity (Pavlov 2007). These alterations may also be affected by the storage duration and these phenomena could influence consumer evaluation (Cheng and Sun 2008).
In a recent investigation on protein functionality and textural properties of various cephalopods during frozen storage, Gokoglu et al. (2018) did not highlight significant modifications to the texture profiles of cuttlefish (Sepia officinalis). Nevertheless, after long conservation under frozen conditions, the sensorial evaluations may be significantly affected, with an increase in hardness, chewiness and elasticity; these modifications are more evident in cuttlefish and octopus than squid. However, the possibility for a consumer to differentiate between fresh and defrosted fish by sensorial characteristics is still debated (Pavlov 2007).
In Europe, Regulations (EU) No 1379/2013 and No 1169/2011 clarify the requirements on labelling and information to the consumer and contribute to more transparency on the market. The labels on fish, mollusks and crustaceans should show whether the product has been defrosted. For non-prepacked products, the information must be displayed on billboards or posters and, for pre-packed products, this information must accompany the commercial name. Several methods, based on different measurement parameters, such as enzymatic or chemical changes and dielectrical properties, are proposed to discriminate between fresh and defrosted fish (Uddin and Okazaki 2010). Given the perishable nature and the fact that many consumers are not able to evaluate the quality of fresh fish, the identification of frozen-thawed products is a matter of concern (Uddin and Okazaki 2010).
In the last decade, near-infrared spectroscopy (NIRS) has been suggested to recognize the fresh from frozen-thawed fish, as well as to estimate fish freshness (Fasolato et al. 2012; Kimiya et al. 2013; Ottavian et al. 2013; Uddin 2010), due to rapidness, on-site usability and accuracy of this technique. However, scarce information is currently available about the application of fast procedures, such as NIRS, on cephalopods and cuttlefish, in particular, with only a few papers that have proposed the implementation of physicochemical, sensory and microbiological analyses to evaluate the shelf life of S. officinalis (Vaz-Pires et al. 2008).
Several chemometric approaches could be suitable for the fresh/frozen-thawed authentication purposes by NIRS (Ottavian et al. 2013; Uddin 2010). The water absorbance bands could be considered as a multi-informative system that can be investigated by the multivariate spectral analysis of NIR spectra (Tsenkova 2009). The water spectrum pattern of the solvent is used as a molecular mirror of its solutes (Tsenkova et al. 2009). Since water-related H bonds are present in most natural samples, this analytical approach can be applied effectively in various fields. Moreover, this technique can provide complex structural information of samples related to the vibration behavior of molecular bonds, such as C–H, O–H and N–H (Liu et al. 2013).
This work aimed to investigate the feasibility of a portable infrared spectroscopy system to discriminate between fresh and frozen-thawed cuttlefish, to provide a rapid tool for quality assurance. Moreover, the potential impact of freshness decay on the NIRS spectral data was investigated on the classification performances of this quick and low-cost tool. NIRS was compared with microbial results and freshness evaluations, to obtain detailed information about the physicochemical changes occurring during the shelf life of this product.
Materials and methods
Sampling and experimental design
With the purpose to represent the Italian market conditions, samples of S. officinalis were caught by the trawling method in the Adriatic Sea (Italy; FAO zone 37.2.1) and from the Atlantic Ocean (France; FAO zone 27). Samples regularly fished for sale were collected from a wholesale fish plant in Chioggia (Venice), on five different times, from June to September, as five lots of five specimens each (5 × 5 = 25 samples) both Fresh (F) and FT (Frozen-thawed). The frozen samples (speed of cuttlefish freezing: 3.5 h; final temperature: − 25 °C) were stored at − 20 °C for around 5 ± 1 months. Before sampling, frozen cuttlefish were thawed overnight (16–18 h), according to the common plant procedures, at refrigerated temperature. Cuttlefish were transported under refrigerated condition (2 °C) and then stored within sterile bags until the analyses. Samples were weighed, and a specimen of the left lateral mantle (3 × 3 cm) was aseptically dissected, for the NIRS analysis. The dissected specimens and the whole cuttlefish were stored for 13 days under refrigeration temperature (3 ± 1 °C). Destructive and non-destructive analyses were performed on F and FT samples, at 0 (T0, day of sampling), 3 (T3), 6 (T6), 9 (T9) and 13 (T13) days. At each experimental time, F and FT cuttlefish were evaluated by sensory and microbiological analyses and total volatile basic nitrogen (TVB-N). The dissected specimens were examined by NIRS.
Microbiological analysis
Ten grams of cuttlefish flesh was aseptically sampled from the lateral side of the mantle. The sample was mixed in a Stomacher bag with 90 mL of peptone salt solution; samples were analyzed, according to the appropriate decimal dilutions. Total viable counts (TVC) were recorded after incubation at 30 °C for 72 h on plate count agar (Biokar Diagnostics, ZAC de Ther, Allonne, Beauvais Cedex, France) according to the ISO 4833-1. Coliforms were determined on Violet Red Bile A (Biokar Diagnostics) after incubation at 30 °C for 24 h. Enterobacteriaceae were analyzed on Violet Red Bile Glucose Agar (Biokar Diagnostics) following incubation at 37 °C for 24 h. The Coagulase-positive Staphylococcus spp. were enumerated on Baird–Parker agar (Biokar Diagnostics) with egg yolk tellurite after incubation at 37 °C for 24–48 h.
Quality index method (QIM)
Sensory evaluation was performed using a specific quality index method (QIM) scheme for freshness grading of cuttlefish (Vaz-Pires and Seixas 2006). Attributes were defined as superficial appearance, skin (dorsal and ventral side), flesh, eyes, mouth region and mucus. Vaz-pires and Seixas (2006) reported the specificity of the attributes, with the relative demerit scores. The scheme was based on eight parameters, with a range of 0–17 points, and is applied for grading of cuttlefish in the majority of commercial presentations.
Total volatile basic nitrogen (TVB-N)
The TVB-N was performed, in duplicate, as described in the regulation (EC) No 2074/2005. About 100 g of flesh was taken from at least three different points and combined by grinding. An aliquot of 10 ± 0.1 g was homogenised with 90 mL of 6% perchloric acid for 2 min. After filtering, the 50 mL extract was alkalinised with 20% NaOH in the presence of phenolphthalein. The extract was distilled. The distillate was absorbed by boric acid (3 g/100 mL) and then titrated with 0.01 mol/L HCl.
NIRS analysis
At each experimental time, the internal surface of the dissected mantle specimens was analyzed by non-destructive NIRS. The collection of NIRS spectra was achieved before the sensory rejection of samples, during the first 9 days of conservation (T0, T3, T6 and T9, for both, F and FT samples).
In this study, the portable spectrophotometer poliSPECNIR (ITPhotonics Breganze, Italy) was operated in a scanning range of 900–1650 nm at 2 nm intervals, and scans were conducted by direct contact of the sample with the probe surface. The scanning surface was a circular window, allowing an area of about 3.2 cm2 to be irradiated. Spectra were collected as an average of two repetitions, each one as an average of 10 scans. Absorbance data were stored as log(1/R), where R represented reflectance, by using poliDATA 3.0.1 software (ITPhotonics).
Statistical analyses and chemometric tools
The non-parametric combination (NPC) test was conducted using the free software NPC Test R10 (http://www.wiley.com/legacy/wileychi/pesarin/material.html). The partial and global p values were determined for the microbial count profiles (log10 CFU/g) and freshness parameters (QIM scores; TVB-N), considering five replications according to F versus FT and the storage time (T0, T3, T6, T9, T13). Permutational multivariate analysis of variance (PERMANOVA) was performed as additional multivariate analysis on the overall wet chemistry, QIM, and microbial profiles. PERMANOVA is a permutation method which highlights statistical changes in structure of groups, as well as in data from multi-factor experimental designs. A two-way PERMANOVA (Anderson 2001—Online resources 2) using fresh status (F vs. FT), storage time, and their interaction as main factors (PRIMER-E, Albany Auckland, New Zealand) was adopted. The unrestricted permutation of features was applied in a balanced design. Gower dissimilarity was applied with 999 permutations. In the case of significant effects, posteriori pair-wise comparisons were carried out. A hierarchical clustering was applied as an agglomerative approach based on the full linkage method (PRIMER-E, Albany Auckland, New Zealand). Non-metric multidimensional scaling plots (NMDS) visualized sample variability, i.e. dissimilarity between pairs of objects in a two- or three-dimensional space.
The TVC log10 CFU/g of each lot was modelled with the DMFit program, to outline the various microbial growth parameters, i.e., initial value (log10 CFU/g), lag/shoulder (h), maximum rate (log10 CFU/g/h) and final value (log10 CFU/g) (https://browser.combase.cc/membership/Login.aspx?ReturnUrl=%2f). The end of shelf life was defined as 7 log10 CFU/g (Bouletis et al. 2016). The NPC test was applied on the estimated growth parameters, to evaluate differences between F and FT samples.
A chemometric classification based on partial least square discriminant analysis (PLS-DA) model applied on NIR spectral data, were built by the use of Matlab R2017a (software V9.2.0.538062, the Mat-Works Inc., Natick, MA, USA) and PLS Toolbox (PLS Toolbox V5.8.2.1, Eigenvector Research Inc, Manson WA, USA). Different models were performed for F and FT classification and shelf life prediction by investigating the whole spectrum (900–1650 nm) and the first overtone (1300–1600 nm) of water absorbance. Models M1 (900–1650 nm) and M2 (1300–1600 nm) examined the F versus FT discrimination; M3 (900–1650 nm) and M5 (1300–1600 nm) evaluated the shelf life prediction on fresh cuttlefish and finally M4 (900–1650 nm) and M6 (1300–1600 nm) were also related to shelf life prediction of defrosted cephalopods.
For the PLS-DA performance improvement, the following pre-treatment combinations were applied: M1–M2, autoscale, standard normal variate (SNV), detrending, 2nd derivative, smoothing; M3–M4–M5, standard normal variate (SNV), detrending, 2nd derivative, smoothing; M6, mean centering, standard normal variate (SNV), detrending, 2nd, smoothing (Antonucci et al. 2011; González-Sáiz et al. 2008; Bisutti et al. 2019). The reliability of the PLS-DA models was assessed by a cross-validation method (sixfold venetian blind algorithm), to test the accuracy of the model. Each sample was assigned to a dummy dependent variable, according to physical status (F vs. FT) and the sampling time. Moreover, in the case of F versus FT classification, models were validated by using a 20% random pool of samples as a prediction set. The Matthew correlation coefficient in cross-validation (MCCcv) and in validation (MCCv) were used to evaluate the classification performances. In addition, specificity and sensitivity were calculated, to assess the performances of the calibration, together with other additional parameters (Fasolato et al. 2012; Su et al. 2017). The variable importance in projection (VIP) index was determined, to establish the relative importance of each wavelength variable within the discrimination models (Andersen and Bro 2010; Ottavian et al. 2013).
The water matrix coordinates (WAMACs) were selected by applying the procedure proposed by Bázár et al. (2016). The absorbance region corresponding to the first overtone of water was analyzed to highlight different aquaphotomes (Tsenkova et al. 2015), with respect to the shelf life of samples and the F and FT status. The selection of variables was provided by the use of loadings, regression vectors of the PLS models and by the use of VIP criterion. Different aquagrams were built on raw and pre-treated spectra (SNV), by applying the normalized absorbance values from the selected wavelength (Bázár et al. 2016).
Results and discussion
Clear discrimination between F and defrosted fish products is usually difficult, as the samples present similar physical and chemical characteristics (Uddin and Okazaki 2010). Here, several sensory and destructive analyses were performed to characterize both F and FT cuttlefish during their shelf life. As a non-destructive and fast technique, the feasibility of a portable NIRS system was evaluated, investigating the whole spectrum (900–1650 nm) and the specific range in the first overtone of water absorbance (1300–1600 nm).
Freshness characterization of samples
Sensory evaluation
In the QIM scheme, all the parameters showed an increasing linear trend and, as expected, demerit points increased during the time of preservation under refrigeration (Fig. 1). The linear regressions showed the R2 was near to 0.98 in both regressions, F and FT samples. This evaluation proved to be a good method to monitor the changes from the sensory perspective, during the shelf life of the cuttlefish (Fig. 1).
Fig. 1.
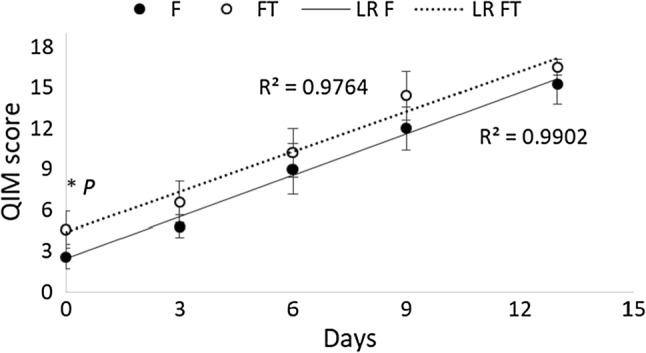
Quality index methods (QIM) scores in fresh and frozen-thawed cuttlefish. Bars represent the standard deviation (5 replicates); LR is the linear regression line; *P the non-parametric combination NPC partial test showed significant P value only at day 0 (P = 0.041, stratified by time)
Considering the more informative odor attributes (Bouletis et al. 2016; Vaz-pires and Seixas 2006), the scores of demerit scale (skin, dorsal side) gained the maximum levels from 6 to 9 days, on average, whereas, the odor of the mouth region was unacceptable only after 9–13 days. In general, it is possible to assume that the rejection of samples starts at 9 days of storage.
A significant difference between F and FT cuttlefish on QIM scores was only observed at the first evaluation. Moreover, the standard deviation suggested a large sampling variability. Many factors, such as season, caught methods, geographic area and handling practices, could affect the shelf life and QIM of seafood (Badiani et al. 2013; Nielsen and Hyldig 2004). Some discrepancies between the current results and those reported by Vaz-Pires et al. (2008) could be due to the different methods of storage. The previous scheme was developed at 2 °C, with cephalopods preserved by ice. In the present work, the refrigeration was applied at 3 °C, and the samples were kept in bags. Moreover, the origin, sampling variability and the methods used to catch the fish could justify the described inconsistencies.
Microbial evaluation of cuttlefish
The TVC, Enterobacteriaceae and coliforms were enumerated to monitor the microbiological quality of cuttlefish, as reported in Fig. 2. The initial contamination of both F and FT was substantially higher than the level reported in the literature (Vaz-Pires et al. 2008). In cuttlefish stored under different modified atmospheres, Bouletis et al. (2016) highlighted similar levels of Enterobacteriaceae and mesophilic count, with comparable trends shown in Fig. 2. A great variability of the initial microbial load was described in other studies, both in fresh and defrosted cephalopods (Bertini et al. 2004; Topic Popovic et al. 2010). This variation could explain the different microbial shelf life results reported by several authors (Bertini et al. 2004; Bouletis et al. 2016; Topic Popovic et al. 2010; Vaz-Pires et al. 2008).
Fig. 2.
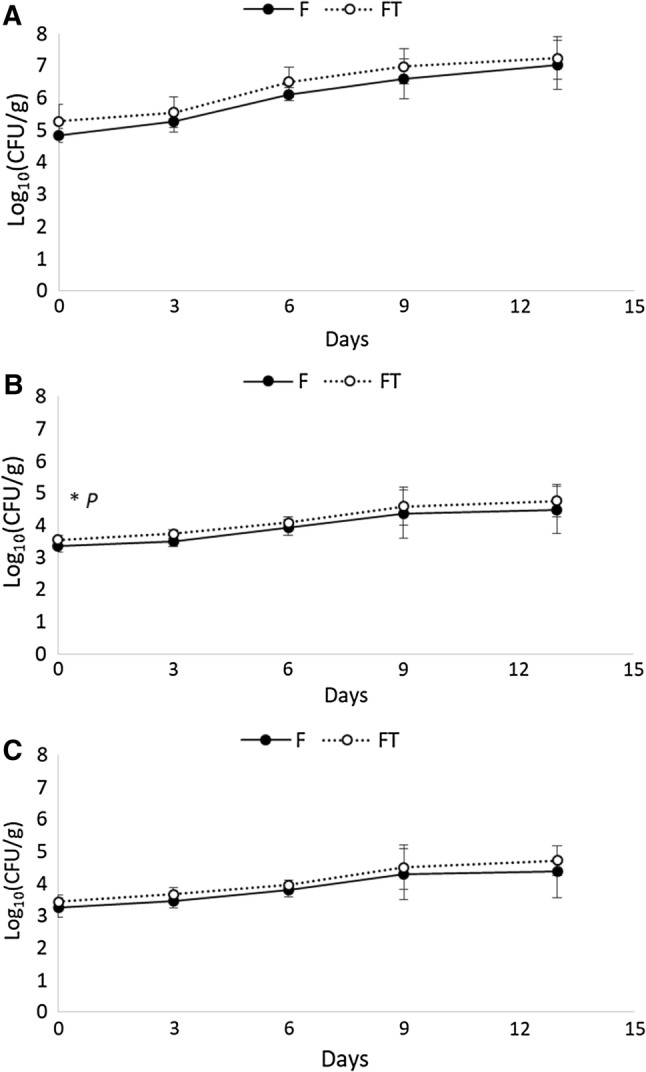
Microbiological targets evaluated in fresh and frozen-thawed cuttlefish. a total viable count; bEnterobacteriaceae; c coliforms. *P the non-parametric combination NPC partial test stratified by time showed significant P value only at day 3 for Enterobacteriaceae (P = 0.042)
No differences were observed between F and FT samples, except for Enterobacteriaceae at 3 days of conservation (Fig. 2). Staphylococcus spp. was never detected during this trial. Enterobacteriaceae and coliforms gradually increased during the shelf life. However, the differences observed were only around 1 log10 CFU/g, from the initial time (T0 = 3.4 log10 CFU/g) to the end of the shelf life (T13 = 4.5 log10 CFU/g) (Fig. 2a–c). The growth of TVC reached 7.13 log10 CFU/g, at the end of the sampling period. For fresh proteinaceous products stored under aerobic conditions (such as meat and fish), the acceptable upper limit count over 7 log10 CFU/g was suggested (Nychas et al. 2007). This threshold was also proposed in cuttlefish stored in modified atmospheres (Bouletis et al. 2016).
The DMFit regression models allowed the estimation of several growth parameters describing the behaviors of the TVC in F and FT samples (Online Resource 1). The estimated initial mesophilic load for the F and FT cuttlefish was similar (4.2 log10 CFU/g on average). Moreover, the lag phases were also comparable (around 2.9 days). Maximum growth rates were statistically different between F and FT samples (0.011 vs. 0.014 log10 CFU/g/h; P = 0.047), suggesting a faster growth of mesophilic bacteria on FT than F samples.
Similar observations were also reported for cuttlefish during storage on ice, where the spoilage occurred above the threshold limit of 7 log (Albanese et al. 2005). At these levels of TVC, the dominant microflora, mainly composed by Pseudomonas, starts to degrade amino acids and non-protein nitrogen, leading to the formation of volatile compounds involved in the perishability of the seafood. F and FT samples reached this limit between 10 and 11 days (Online Resource 1). However, the present data are far from the previous observations on cuttlefish (Vaz-Pires et al. 2008), in which the spoiled samples showed levels of contamination lower than 5 log10 CFU/g.
These data outlined a peculiar behavior of the cuttlefish collected, with a higher initial microbial load that affected the sensory traits only in the latest stage of storage. For instance, unpleasant odors and specific aspects of deterioration were unacceptable after 9 days of storage, in agreement with the TVC threshold of 7 log10 CFU/g.
Total volatile basic nitrogen (TVB-N)
Figure 3 shows the development of the TVB-N throughout storage. The trend of F and FT was comparable, even though a high variability was noted that could have affected the overall evaluation of this indicator. The chemical parameter in both groups did not excessively increase during the first 50 h of storage, but it achieved high final concentrations of 84 and 75 mg N/100 g, respectively, with a similar variability between F and FT. The TVB-N is a parameter of a late decay and is not always related to the sensory deterioration, especially when compared with other low molecular weight molecules, such as aldehydes and alcohols (Parpalani et al. 2015).
Fig. 3.
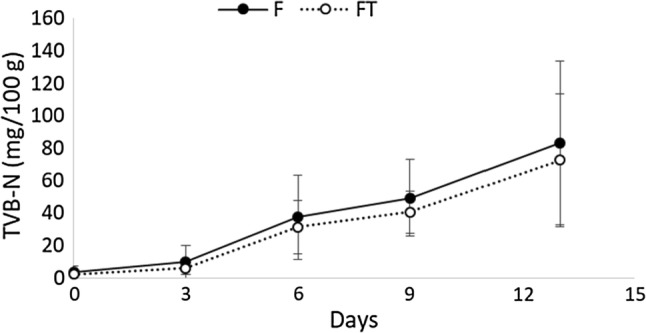
Total volatile basic nitrogen (TVB-N) values of fresh and frozen-thawed cuttlefish. Bars are the standard deviation. No significant differences were detected at each experimental time
It must be specified that the limit values of TVB-N in cephalopods is not present in any regulation. However, some authors suggested the limit of 35 mg N/100 g for cephalopods (Bouletis et al. 2016; Çağlak et al. 2014). According to this threshold, the present samples could be categorized as unsatisfactory at around 5–6 days of storage, in both F and FT samples. These data concur with Bouletis et al. (2016) while another study (Vaz-Pires et al. 2008) highlighted unacceptable levels of TVB-N only at 10 days of refrigeration. The data obtained in the current study imply that the samples had very high levels of TVB-N compared to the threshold proposed for the cuttlefish. Bouletis et al. (2016) noted a marked increase of TVB-N during the first 6 days of storage and indicated these levels were probably linked to the activity of endogenous enzymes and unrelated to the microbial growth.
PERMANOVA analysis on merged wet chemistry, sensorial, and microbial profiles, highlighted that features statistically changed as a factor of product shelf life (Online resource 2). Posteriori pair-wise comparisons showed differences between the most of the sampling times. However, cuttlefishes analyzed at 6 days (T6) were similar to those analyzed at 9 days (T9). However, the T6 specimens differed from samples at the end of the storage period (T13). The clustering analysis suggested a first segregation of cephalopods at T0 and T3 (70% of similarity), as well as a second 70% similarity cluster grouping T6 and T9, however T6 clustered uniquely together at 85% similarity (data not shown). Cuttlefishes analyzed at 0–3 days maintained their freshness descriptors, after which a loss of characteristic fresh odor and decay of freshness started at 6 days, and at 9 days off-odors and spoilage were noted.
Near-infrared spectroscopy (NIRS) characterization
Fresh (F) versus frozen-thawed (FT) classification
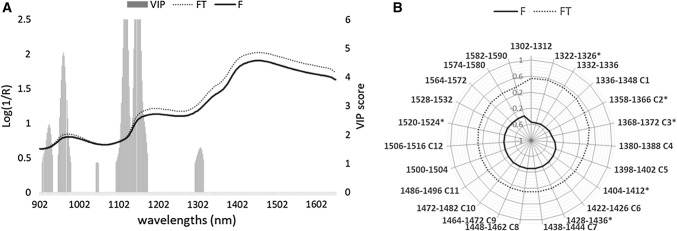
NIRS techniques are widely employed in the characterization of seafood, especially fish (Liu et al. 2013). In particular, several authors reported the application of infrared techniques for the discrimination of FT products (Fasolato et al. 2012; Ottavian et al. 2013; Uddin and Okazaki 2010). Very few data, to date, are reported for the authentication of other seafood products, such as shellfish, crustaceans or cephalopods (Qu et al. 2015). Figure 4a reports the average of the NIRS raw spectra collected from F and FT cuttlefish. The shape of the spectra showed similar behaviors. However, the absorbance values were different in several spectral ranges, such as 944–1000 and 1140–1650 nm. The VIP scores highlight the wavelengths with the most important contribution in the class discrimination after pre-treatment of the spectra. These spectral ranges selected by the VIP (908–934, 948–980, 1044–1050, 1094–1174 and 1294–1316 nm) are suitable for the classification of F and FT samples, as reported in Table 1.
Fig. 4.
a Raw near-infrared spectra of fresh and frozen-thawed cuttlefish. Variable importance in projection VIP scores (gray bars) > 1 were derived by the 2nd derivative model of partial least squares discriminant analysis. b Aquagram of average normalised absorbance values proposed for fresh (F) and frozen-thawed (F-T) cuttlefish (raw spectra). The canonical twelve Water matrix coordinates WAMACS were reported according to the code proposed by Tsenkova (2009) from C1 to C12. An asterisk highlights the characteristics water bands selected in the present study
Table 1.
Performances of the PLS-DA models (from M1 to M6) to classify fresh (F) and frozen-thawed (FT) in validation, and shelf life classes (T0, T3, T6 and T9 days) in cross-validation of cuttlefish
| Fresh versus frozen-thawed | Predicted classes | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Model/spectral range (nm) | Class | N | TPR | FPR | TNR | FNR | Err | P | F1 | MCCv | F | FT |
| M1/900–1650 | F | 20 | 0.91 | 0.09 | 0.91 | 0.09 | 0.09 | 0.91 | 0.91 | 0.80 | 18 | 2 |
| FT | 20 | 2 | 18 | |||||||||
| M2/1300–1600 | F | 20 | 0.83 | 0.17 | 0.83 | 0.17 | 0.17 | 0.83 | 0.83 | 0.60 | 16 | 4 |
| FT | 20 | 4 | 16 | |||||||||
| Shelf life | Predicted classes | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Class | Time | N | TPR | FPR | TNR | FNR | Err | P | F1 | MCCcv | T0 | T3 | T6 | T9 | |
| M3/900–1650 | F | T0 | 25 | 0.88 | 0.08 | 0.92 | 0.12 | 0.09 | 0.79 | 0.83 | 0.77 | 22 | 2 | 1 | 3 |
| T3 | 25 | 0.68 | 0.17 | 0.83 | 0.32 | 0.21 | 0.57 | 0.62 | 0.47 | 2 | 17 | 10 | 1 | ||
| T6 | 25 | 0.24 | 0.15 | 0.85 | 0.76 | 0.30 | 0.35 | 0.29 | 0.10 | 0 | 5 | 6 | 6 | ||
| T9 | 25 | 0.60 | 0.13 | 0.87 | 0.40 | 0.20 | 0.60 | 0.60 | 0.46 | 1 | 1 | 8 | 15 | ||
| T0 | T3 | T6 | T9 | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| M4/900–1650 | FT | T0 | 25 | 0.76 | 0.09 | 0.91 | 0.24 | 0.13 | 0.73 | 0.75 | 0.66 | 19 | 2 | 2 | 3 |
| T3 | 25 | 0.52 | 0.08 | 0.92 | 0.48 | 0.18 | 0.68 | 0.59 | 0.49 | 1 | 13 | 3 | 2 | ||
| T6 | 25 | 0.60 | 0.16 | 0.84 | 0.40 | 0.22 | 0.56 | 0.58 | 0.43 | 2 | 6 | 15 | 4 | ||
| T9 | 25 | 0.64 | 0.16 | 0.84 | 0.36 | 0.21 | 0.57 | 0.60 | 0.46 | 3 | 4 | 5 | 16 |
| Shelf life | Predicted classes | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Class | Time | N | TPR | FPR | TNR | FNR | Err | P | F1 | MCCcv | T0 | T3 | T6 | T9 | |
| M5/1300–1600 | F | T0 | 25 | 0.72 | 0.12 | 0.88 | 0.28 | 0.16 | 0.67 | 0.69 | 0.58 | 0 | 18 | 2 | 4 |
| T3 | 25 | 0.68 | 0.12 | 0.88 | 0.32 | 0.17 | 0.65 | 0.67 | 0.55 | 3 | 2 | 17 | 6 | ||
| T6 | 25 | 0.52 | 0.12 | 0.88 | 0.48 | 0.21 | 0.59 | 0.55 | 0.41 | 6 | 2 | 5 | 13 | ||
| T9 | 25 | 0.76 | 0.08 | 0.92 | 0.24 | 0.12 | 0.76 | 0.76 | 0.68 | 9 | 3 | 1 | 2 | ||
| T0 | T3 | T6 | T9 | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| M6/1300–1600 nm | FT | T0 | 25 | 0.48 | 0.24 | 0.76 | 0.52 | 0.31 | 0.40 | 0.44 | 0.23 | 12 | 5 | 7 | 6 |
| T3 | 25 | 0.56 | 0.12 | 0.88 | 0.44 | 0.20 | 0.61 | 0.58 | 0.45 | 3 | 14 | 3 | 3 | ||
| T6 | 25 | 0.60 | 0.23 | 0.77 | 0.40 | 0.27 | 0.47 | 0.53 | 0.35 | 8 | 6 | 15 | 3 | ||
| T9 | 25 | 0.52 | 0.03 | 0.97 | 0.48 | 0.14 | 0.87 | 0.65 | 0.60 | 2 | 0 | 0 | 13 |
Models were built according to the spectral range of 900–1650 nm, and for the reduced models (M2, M5, M6) the range of the first overtone of water absorbance at 1300–1600 nm was used
TPR true positive rate, FPR false positive rate, TNR true negative rate, FNR false negative rate, N number of spectral samples, Err misclassification error, P precision, F1 score, MCCcv and MCCv Matthews correlation coefficient in cross-validation and validation
As previously reported in fish, the region around 960–980 nm could be associated with O–H bonds, mainly related to the moisture content in the muscle tissues (Fasolato et al. 2012). The latter could be an additional factor affecting the NIRs spectral patterns in seafood products. This should be taken into account both in terms of the interpretation and the reproducibility of the spectral and aquaphotomic results. Other wavelengths have been reported for species-independent discrimination between F and FT fish (Ottavian et al. 2013). The region 908–934 nm is related to the third overtones of C–H stretching modes while the regions around 1094–1174 and 1294–1316 nm could be associated with the C–H second overtones (Barbin et al. 2013). As described in poultry and pork meat, changes in the C–H modes may be influenced by proteolysis and modification of the muscle lipids during thawing (Barbin et al. 2013; Liu and Chen 2001). Such changes may also affect the water interactions of the matrix with a different fraction of free or bound water species (Liu and Chen 2001).
Table 1 resumes the performances of NIRS classification by using spectral features. The entire spectrum (900–1650 nm) was applied for the 1st discriminant analysis (Model M1). Overall results of the validation evidenced a suitable classification for F samples (specificity, true negative rate) with high precision and F1 scores. A very low fraction of samples was incorrectly classified while the false negative rate implied a certain degree of uncorrected positive samples classified as negative. These interesting performances had a lower accuracy relative to other evaluations observed on fish muscles (Fasolato et al. 2012; Ottavian et al. 2013). However, the NIRS results suggested effective discrimination of F and FT cuttlefish not influenced by the storage period. These results highlighted that changes occurring during the shelf life do not mask the freezing effects on the muscle matrix. Even though defrosted seafood products should generally be consumed up to 24–48 h after thawing, fraudulent practices could substitute fresh products with every freshness category of defrosted seafood.
Several studies performed on fish and meat underlined that thawing affects peculiar wavelength regions related to the water (Fasolato et al. 2012; Liu and Chen 2001; Uddin and Okazaki 2010). In this context, the spectral range mainly involved in the first overtone of water absorbance (1300–1600 nm) was investigated for classification purposes, in a second PLS-DA model (M2—Table 1). The study of this region can provide useful information to discover specific dynamics of water behavior inside the samples, according to the principles of aquaphotomics (Tsenkova et al. 2009). The results of the validation indicated a loss of spectral information, as reflected by the increased false positive rate (0.17) and false negative rate values, resulting in a decreased sensibility and specificity. As described for the VIP scores (Fig. 4), the wavelengths corresponding to the first overtone of water absorbance seemed less important for the samples discrimination than the whole spectrum. Water matrix coordinates (WAMACS) were selected, as suggested by several authors (Bázár et al. 2016; Tsenkova 2009; Tsenkova et al. 2015). The specific water spectral pattern (WASP) was defined according to the loadings of the models, the regression coefficients, the VIP and the selectivity ratios, as described by Andersen and Bro (2010) and Tsenkova et al. (2015). Figure 4b presents the aquagram of the overall F and FT cuttlefish (raw spectra representation). The canonical twelve WAMACS were reported, according to the code proposed by Tsenkova (2009), from C1 to C12. Additional wavelengths were also included, as described by other authors (Bázár et al. 2016; Tsenkova et al. 2015). An asterisk highlighted the characteristic water bands selected in the present study: 1322–1326, 1358–1366 C2, 1368–1372 C3, 1404–1412, 1428–1436 and 1520–1524 nm.
The 1322–1326 and 1358–1366 nm were described as H-bonded water linked with the water solvation shell and the first overtone OH stretch (Bázár et al. 2016; Tsenkova 2009) while the 1368–1372 nm range was mainly associated with protonated water molecules (Tsenkova 2009). Absorbance values at water bands around 1404–1412 nm revealed changes also related to free water molecular species, as well as in the regions at 1428–1436 (water with H bonds—intermediate stage of water) and 1520–1524 nm (water molecule structures with one or more hydrogen bonds) (Bázár et al. 2016; Tsenkova 2009). This aquagram clearly described a different fingerprint between F and FT cuttlefish. Moreover, the shape of the aquagrams was visibly different between fresh and forzen-thawed samples in both raw and SNV representations (Fig. 4b and Online resource 4). The WASP suggested a dissimilar molecular conformation of water species in the FT than F muscle matrix, with higher free water molecules and a concomitant lower presence of bound species and a reduced amount of water solvation shell.
Shelf life prediction
The data in the second part of Table 1 predict the performances of the shelf life NIRS estimation. Two different models were built for F and FT samples (M3 and M4 models). In general, the classification in cross-validation of the storage days was quite good for F products at T0 and T3 sampling times, while for other classes was ineffective. As described for the F and FT discrimination, the application of the whole spectrum showed the better performances of classification in comparison with the reduced models (M3–M4 vs. M5–M6). Conversely, the shelf life prediction of FT cuttlefish in cross-validation seemed less effective than the prediction of the storage days for fresh products, suggesting different behaviors of these samples during storage. These data reflected the samples variability, as already noted in the microbiological and chemical analyses. As described in PERMANOVA analysis, the samples at 6 days showed similar chemical, sensory, and microbiological profiles in the cuttlefish analyzed at 9 days, this could influence the classification performances of spectral variables. Additional PLS-DA models were performed merging the freshness classes in two main groups as T0–T3 versus T6–T9 days (online resources 3). The performance of discrimination was improved (TPR: 0.74–0.83—spectral range 900–1650 nm). These PLS-DA analyses were suitable to describe a freshness cut-off, a very fresh cuttlefish and the product nearest to the spoilage phase. The spectral features have some lack for the prediction of storage period. However, the performances of classification may increase in an enlarged data set.
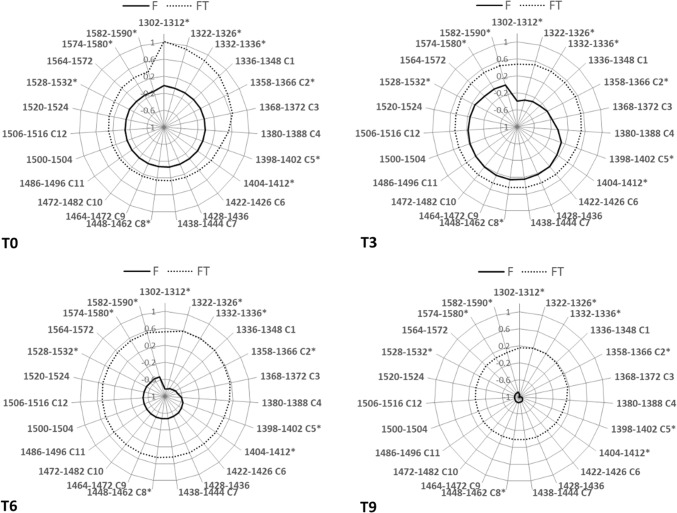
Aquagrams were also built to describe the behaviors of the WASP, according to the time of storage on raw spectra (Fig. 5). Interestingly, as shown by the graphs, the absorbance changes in the range of the first overtone of water were completely different between F and FT cuttlefish. During the first 9 days of storage, the FT samples displayed a similar pattern of WAMACS while in F samples, the aquagram shape dramatically changed throughout the shelf life (Fig. 5). After the SNV transformation, aquagrams revealed different patterns in F and FT behaviors depending on shelf life (on-line resource 5). However, once again, the F samples displayed the greatest shape changes.
Fig. 5.
Aquagram of average normalised absorbance values proposed for fresh (F) and frozen-thawed (F-T) cuttlefish at the four experimental times (T0, T3, T6, T9- raw spectra). The canonical twelve Water matrix coordinates WAMACS were reported according to the code proposed by Tsenkova (2009) from C1 to C12. An asterisk highlights the characteristics water bands selected in the present study
Nine wavelength ranges were selected: 1322–1326, 1332–1336 C2, 1358–1366, 1398–1402 C5, 1404–1412, 1448–1462 C8, 1528–1532, 1574–1580 and 1582–1590 nm. Only three regions overlapped the wavelengths selected by the F versus FT model (1322–1326, 1358–1366 and 1404–1412 nm). Three canonical WAMACs were also detected: the C2 once again described by the OH–[H2O]2 (water solvation shell; Tsenkova et al. 2009); the 1398–1402 nm C5, which was related to trapped water (C1392–1403; Kojić et al. 2014), and C8 hydrogen-bonded water molecules in the solvation shell (WS4,5 WSn:OH–(H2O)n). The remaining selected features were mainly associated with –NH groups, such as the bands around 1530 nm (N–H stretch second overtone of protein), and the wavelength region 1560–1670 nm was mainly related to –NH deformation linked to protein and peptides (Wehling 2010).
Conclusion
The discrepancy observed among chemical, sensory and microbiological analyses underlined the complexity on the freshness attribution and shelf life prediction, probably related to the variability of this wild seafood product. Although the chemical, sensory and microbiological analyses highlighted few significant differences between F and FT cuttlefish, NIRS was able to discriminate among storage treatments effectively. A PLS-DA based on the overall spectral data was satisfactory able to authenticate F products, but the performances on the shelf life delineation should be improved. The findings of the study suggested that aquaphotomics is an informative approach, capable of detecting relevant differences between F and FT cuttlefish, especially within the experimental storage times. In summary, the use of a portable NIRS instrument may help food supply chain operators and public authorities to discover frauds or inappropriate storage by a fast and non-destructive evaluation. Future studies developed on samples from various origins in an enlarged dataset are recommended to increase the performances of shelf life prediction.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
The authors would like to thank Lab-Control srl for having supported the laboratory analyses. This paper is related to the specific topics of the ECCE AQUA-MIUR Dipartimenti di Eccellenza.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Michele Sannia, Email: michele.sannia2@unibo.it.
Lorenzo Serva, Email: lorenzo.serva@unipd.it.
Stefania Balzan, Phone: +39 049 8272846, Email: stefania.balzan@unipd.it.
Severino Segato, Email: severino.segato@unipd.it.
Enrico Novelli, Email: enrico.novelli@unipd.it.
Luca Fasolato, Email: luca.fasolato@unipd.it.
References
- Alamprese C, Casiraghi E. Application of FT-NIR and FT-IR spectroscopy to fish fillet authentication. LWT Food Sci Technol. 2015;63(1):720–725. doi: 10.1016/j.lwt.2015.03.021. [DOI] [Google Scholar]
- Albanese D, Cinquanta L, Lanorte MT, Di Matteo M. Squid (Sepia officinalis) stored in active packaging: some chemical and microbiological changes. Ital J Food Sci. 2005;17(3):325–332. [Google Scholar]
- Andersen CM, Bro R. Variable selection in regression—a tutorial. J Chemom. 2010;24:728–737. doi: 10.1002/cem.1360. [DOI] [Google Scholar]
- Antonucci F, Pallottino F, Paglia G, Palma A, D’Aquino S, Menesatti P. Non-destructive estimation of mandarin maturity status through portable VIS-NIR spectrophotometer. Food Bioprocess Technol. 2011;4:809–813. doi: 10.1007/s11947-010-0414-5. [DOI] [Google Scholar]
- Badiani A, Bonaldo A, Testi S, Rotolo M, Serratore P, Giulini G, Gatta PP, et al. Good handling practices of the catch: the effect of early icing on the freshness quality of cuttlefish (Sepia officinalis L.) Food Control. 2013;32(1):327–333. doi: 10.1016/j.foodcont.2012.12.019. [DOI] [Google Scholar]
- Barbin D, Sun D, Su C. NIR hyperspectral imaging as non-destructive evaluation tool for the recognition of fresh and frozen-thawed porcine longissimus dorsi muscles. Innov Food Sci Emerg Technol. 2013;18:226–236. doi: 10.1016/j.ifset.2012.12.011. [DOI] [Google Scholar]
- Bázár G, Romvári R, Szabó A, Somogyi T, Éles V, Tsenkova R. NIR detection of honey adulteration reveals differences in water spectral pattern. Food Chem. 2016;194:873–880. doi: 10.1016/j.foodchem.2015.08.092. [DOI] [PubMed] [Google Scholar]
- Bertini S, Bresciani CM, Tiberto M, Bonardi S. Microbiological control of frozen and thawed cuttlefish (Sepia officinalis) Ital J of Food Sci. 2004;16(2):255–260. [Google Scholar]
- Bisutti V, Merlanti R, Serva L, Lucatello L, Mirisola M, Balzan S, Tenti S, Fontana F, Trevisan G, Montanucci L, Contiero B, Segato S, Capolongo F. Multivariate and machine learning approaches for honey botanical origin authentication using near infrared spectroscopy. JNIRS. 2019;27(1):65–74. doi: 10.1177/0967033518824765. [DOI] [Google Scholar]
- Bouletis AD, Arvanitoyannis IS, Hadjichristodoulou CN, Parlapani FF, Gkagtzis DC. Quality changes of cuttlefish stored under various atmosphere modifications and vacuum packaging. J Sci Food Agric. 2016;96(8):2882–2888. doi: 10.1002/jsfa.7459. [DOI] [PubMed] [Google Scholar]
- Çağlak E, Çakli Ş, Kilinc B. Comparison chemical, sensory, microbiological and textural changes of cuttlefish (Sepia officinalis) stored under different packaging. Bul J Agric Sci. 2014;20(5):1046–1053. [Google Scholar]
- CBI (2015) Product factsheet squid and cuttlefish in Europe. https://www.cbi.eu/sites/default/files/market_information/researches/product-factsheet-europe-squid-cuttlefish-2015.pdf. Accessed 19 Dec 2017
- Cheng Q, Sun DW. Factors affecting the water holding capacity of red meat products: a review of recent research advances. Crit Rev Food Sci. 2008;48(2):137–159. doi: 10.1080/10408390601177647. [DOI] [PubMed] [Google Scholar]
- Council Regulation (EU) No 1169/2011 of the European Parliament and of the Council of 25 October 2011 on the provision of food information to consumers, amending Regulations (EC) No 1924/2006 and (EC) No 1925/2006 of the European Parliament and of the Council, and repealing Commission Directive 87/250/EEC, Council Directive 90/496/EEC, Commission Directive 1999/10/EC, Directive 2000/13/EC of the European Parliament and of the Council, Commission Directives 2002/67/EC and 2008/5/EC and Commission Regulation (EC) No 608/2004
- FAO (2016) The state of world fisheries and aquaculture 2016. Contributing to food security and nutrition for all. Rome. 200 pp. http://www.fao.org/3/a-i5555e.pdf. Accessed 18 Feb 2018
- Fasolato L, Balzan S, Riovanto R, Mirisola M, Ferlito JC, Benozzo F, et al. Comparison of visible and near-infrared reflectance spectroscopy to authenticate fresh and frozen-thawed swordfish (Xiphias gladius L) J Aquat Food Prod Technol. 2012;21:493–507. doi: 10.1080/10498850.2011.615103. [DOI] [Google Scholar]
- Gokoglu N, Topuz OK, Yerlikaya P, Yatmaz HA, Ucak I. Effects of freezing and frozen storage on protein functionality and texture of some cephalopod muscles. J Aquat Food Prod T. 2018;27(2):211–218. doi: 10.1080/10498850.2017.1422168. [DOI] [Google Scholar]
- González-Sáiz JM, Estaban-Díez I, Sánchez-Gallardo C, Pizarro C. Monitoring of substrate and product concentrations in acetic fermentation processes for onion vinegar production by NIR spectroscopy: Value addition to worthless onions. Anal Bioanal Chem. 2008;391:2937–2947. doi: 10.1007/s00216-008-2186-6. [DOI] [PubMed] [Google Scholar]
- ISO 4833-1 part 1 Microbiology of the food chain—horizontal method for the enumeration of microorganisms: part 1: colony count at 30 °C by the pour plate technique
- Kimiya T, Sivertsen AH, Heia K. VIS/NIR spectroscopy for non-destructive freshness assessment of Atlantic salmon (Salmo salar L.) fillets. J Food Eng. 2013;116(3):758–764. doi: 10.1016/j.jfoodeng.2013.01.008. [DOI] [Google Scholar]
- Kojić D, Tsenkova R, Tomobe K, Yasuoka K, Yasui M. Water confined in the local field of ions. ChemPhysChem. 2014;15(18):4077–4086. doi: 10.1002/cphc.201402381. [DOI] [PubMed] [Google Scholar]
- Liu Y, Chen Y. Two-dimensional visible/near-infrared correlation spectroscopy study of thawing behavior of frozen chicken meats without exposure to air. Meat Sci. 2001;57(3):299–310. doi: 10.1016/S0309-1740(00)00106-6. [DOI] [PubMed] [Google Scholar]
- Liu D, Zeng XA, Sun DW. NIR spectroscopy and imaging techniques for evaluation of fish quality—a review. J Appl Spectrosc Rev. 2013;48(8):609–628. doi: 10.1080/05704928.2013.775579. [DOI] [Google Scholar]
- Nielsen D, Hyldig G. Influence of handling procedures and biological factors on the QIM evaluation of whole herring (Clupea harengus L.) Food Res Int. 2004;37(10):975–983. doi: 10.1016/j.foodres.2004.06.006. [DOI] [Google Scholar]
- Nychas GJE, Marshall DL, Sofos JN. Meat, poultry, and seafood. In: Doyle MP, Beuchat LR, editors. Food microbiology: fundamentals and frontiers. 3. Washington, D.C.: ASM Press; 2007. pp. 105–140. [Google Scholar]
- Ottavian M, Fasolato L, Facco P, Barolo M. Foodstuff authentication from spectral data: toward a species-independent discrimination between fresh and frozen-thawed fish samples. J Food Eng. 2013;119:765–775. doi: 10.1016/j.jfoodeng.2013.07.005. [DOI] [Google Scholar]
- Parpalani FF, Haroutounian SA, Nychas GJ, Boziaris IS. Microbiological spoilage and volatiles production of gutted European sea bass stored under air and commercial modified atmosphere package at 2 °C. Food Microbiol. 2015;50:44–53. doi: 10.1016/j.fm.2015.03.006. [DOI] [PubMed] [Google Scholar]
- Pavlov A. Changes in the meat from aquaculture species during storage at low temperature and attempts for differentiation between thawed-frozen and fresh chilled meat. A review. Bulg J Vet Med. 2007;10:67–75. [Google Scholar]
- Qu JH, Cheng JH, Sun DW, Pu H, Wang QJ, Ma J. Discrimination of shelled shrimp (Metapenaeus ensis) among fresh, frozen-thawed and cold-stored by hyperspectral imaging technique. LWT Food Sci Technol. 2015;62(1):202–209. doi: 10.1016/j.lwt.2015.01.018. [DOI] [Google Scholar]
- Regulation (EU) No 1379/2013 of the European Parliament and of the Council of 11 December 2013 on the common organization of the markets in fishery and aquaculture products, amending Council Regulations (EC) No 1184/2006 and (EC) No 1224/2009 and repealing Council Regulation (EC) No 104/2000
- Su WH, Sun DW, He JG, Zhang LB. Variation analysis in spectral indices of volatile chlorpyrifos and non-volatile imidacloprid in jujube (Ziziphus jujuba Mill.) using near-infrared hyperspectral imaging (NIR-HSI) and gas chromatograph-mass spectrometry (GC–MS) Comput Electron Agric. 2017;139:41–55. doi: 10.1016/j.compag.2017.04.017. [DOI] [Google Scholar]
- Topic Popovic N, Benussi Skukan A, Dzidara P, Coz-Rakovac R, et al. Microbiological quality of marketed fresh and frozen seafood caught off the Adriatic coast of Croatia. Vet Med. 2010;55(5):233–241. doi: 10.17221/2997-VETMED. [DOI] [Google Scholar]
- Tsenkova R. Aquaphotomics: dynamic spectroscopy of aqueous and biological systems describes peculiarities of water. J Near Infrared Spectrosc. 2009;17:303–314. doi: 10.1255/jnirs.869. [DOI] [Google Scholar]
- Tsenkova R, Meilina H, Kuroki S, Burns DH. Near infrared spectroscopy using short wavelengths and leave-one-cow-out cross-validation for quantification of somatic cells in milk. J Near Infrared Spectrosc. 2009;17:345–351. doi: 10.1255/jnirs.868. [DOI] [Google Scholar]
- Tsenkova R, Kovacs Z, Kubota Y. Aquaphotomics: near infrared spectroscopy and water states in biological systems. In: Disalvo E, editor. Membrane hydration subcellular biochemistry. Cham: Springer; 2015. [DOI] [PubMed] [Google Scholar]
- Uddin M. Differentiation of fresh and frozen-thawed fish. In: Leo M, Nollet LML, Toldrá F, editors. Handbook of seafood and seafood products analysis. Boca Raton: CRC Press; 2010. [Google Scholar]
- Uddin M, Okazaki E. Applications of vibrational spectroscopy to the analysis of fish and other aquatic food products. In: Chalmers JM, Griffiths PR, editors. Handbook of vibrational spectroscopy. Hoboken, NJ: Wiley; 2010. [Google Scholar]
- Vaz-pires P, Seixas P. Development of new quality index method (QIM) schemes for cuttlefish (Sepia officinalis) and broadtail shortfin squid (Illex coindetii) Food Control. 2006;17:942–949. doi: 10.1016/j.foodcont.2005.07.004. [DOI] [Google Scholar]
- Vaz-pires P, Seixas P, Mota M, Lapa-Guimãres J, Pickova J, Lindo A, Silva T. Sensory, microbiological, physical and chemical properties of cuttlefish (Sepia officinalis) and broadtail shortfin squid (Illex coindetii) stored in ice. LWT Food Sci Technol. 2008;41:1655–1664. doi: 10.1016/j.lwt.2007.10.003. [DOI] [Google Scholar]
- Wehling RL. Food analysis. Boston, MA: Springer; 2010. Infrared spectroscopy. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.