Abstract
Superheated steam was used to cook barley and the volatile odor compounds and release of odorants from the steamed barley were analyzed. The main odor compounds in cooked barley were aldehydes (hexanal and (E,E)-2,4-decadienal) and acids (acetic acid and hexanoic acid). Compared to ordinary cooked barley, barley cooked by superheated steam had less odorants, and the release of odorants was reduced by almost half. Sensory evaluation revealed that this barley was preferred to ordinary cooked barley, because it had weaker smell and tasted less sour and less bitter. The steaming process steam distils and eliminates some odor compounds, while some water-soluble compounds (mainly acids) are washed away by water during steaming. Therefore, this steam cooking method, applied to barley for the first time here using a comprehensive analysis, improves the acceptability and palatability of this high-quality food rich in dietary fiber.
Electronic supplementary material
The online version of this article (10.1007/s13197-019-03907-2) contains supplementary material, which is available to authorized users.
Keywords: Superheated steam, Barley, Volatile compounds, Gas chromatography–olfactometry (GC–O), Sensory evaluation, Release of odorants
Introduction
Barley is one of the first cultivated grains in history, and to this day, remains one of the most widely consumed one on a global scale (Baik and Ullrich 2008). It is commonly found in bread as flour, malt for beer, and numerous other uses in various cuisines. Nowadays, barley is gaining popularity due to its dietary fiber content that is mainly composed of beta glucan (Izydorczyk and Dexter 2008). Its health benefits are recognized by the US Food and Drug Administration (FDA) (Baik and Ullrich 2008; Sharma and Kotari 2017).
Different processing methods can change the texture, flavor, and aroma of food. For example, extrusion processing is used to develop a new puffed barley snack and cereal with health benefits (Altan et al. 2008a, b). In Japan, pre-treated barley that can be cooked with rice is available in stores and consumed by health-conscious people. However, the consumption of barley as a staple food remains limited because of its undesirable odor (Cramer et al. 2005; Kaneko et al. 2013). Since aroma is one of the most important factors of food palatability (Dunkel et al. 2014; Shepherd 2013), removing the undesirable odor of cooked barley could make it a promising alternative to rice as a staple grain.
In our previous study (Takemitsu et al. 2016), we found that cooked rice prepared using superheated steam had a weaker smell than ordinary cooked rice, and developed a cooking machine to prepare rice on a large scale by such a method. It consisted of a continuous conveyor with a steam chamber. Hot water is introduced at regular intervals, and cooked rice comes out of the chamber ready for consumption.
In this study, the same cooking machine was applied to barley to reduce its odor. We used a waxy variety of barley (grain), which has high total dietary fiber including beta-glucan, but a relatively strong characteristic smell compared to other varieties of barley. This barley was cooked either in the steaming machine or conventionally using a rice cooker. The former cooking method resulted in reduction in some aldehydes and acid compounds relative to the latter after cooking. As a result, the steamed barley had less odor, thereby improving the acceptance of this high-quality food rich in dietary fibers.
Materials and methods
Materials
We used unpolished and milled hull-less waxy barley (Hordeum vulgare, cv BG012) provided by Hakubaku (Yamanashi, Japan).
Chemicals
The following analytical standards were used: pentanal, hexanal, 1-pentanol, 1-hexanol, nonanal, acetic acid, benzaldehyde, (E)-2-nonenal, 1-octanol, γ-hexalactone, hexanoic acid, benzyl alcohol, and 2-methoxy-4-vinylphenol from Sigma-Aldrich, Tokyo, Japan; 2-pentylfuran, 1-octen-3-ol, (E,E)-2,4-nonadienal, (E,E)-2,4-decadienal, and γ-nonalactone from Tokyo Chemical Industry, Tokyo, Japan; (E)-2-octenal, from Santa Cruz Biotechnology, Texas, USA; pentanoic acid from Wako Pure Chemical Industries, Osaka, Japan; and 3-octen-2-one, 3-methylbutyric acid, guaiacol, and β-phenethyl alcohol from Nakalai Tesque, Kyoto, Japan. Methyl tert-butyl ether (MTBE) (Nakalai Tesque) was used as an extraction solvent. Cyclohexanol (Sigma-Aldrich) was used as an internal standard.
Sensory evaluation
Sensory evaluation was conducted according to Konishi et al. (1996) with some modifications. Steamed barley was evaluated using a 7-point scale from + 3 (stronger/like extremely) to − 3 (weaker/dislike extremely), with ordinary cooked barley as the reference sample (0 on the scale; neutral) in all sessions. The testing panel consisted of 29 untrained students from Osaka Prefecture University, who objectively evaluated the aroma, texture, and taste, and gave their preference. The data were subjected to Welch’s t test analysis.
Steam distillation–extraction of unpolished barley
Raw unpolished barley (30 g) was placed in a 1 L glass vessel. Under atmospheric pressure, steam (100 °C) was blown through the barley in the vessel for 20 min, and ~ 40 mL of the distillate was collected. The distillate was extracted using 40 mL methyl tert-butyl ether (MTBE) and a separatory funnel. The upper layer of the extract was collected and dried with anhydrous Na2SO4. The solvent was removed at 52 °C using a Vigreux column (30 cm × 1.5 cm, Kiriyama Glass Works, Tokyo, Japan) and further concentrated using a stream of nitrogen gas. The concentrates were used as samples for GC–O and GC–MS analysis.
Gas chromatography–olfactometry/mass spectrometry
Steam-distilled extracts of unpolished barley were analyzed by GC–O on a chromatograph equipped with a mass spectrometer (2010 plus, Shimadzu, Kyoto, Japan) and a sniffing port (OP275, GL Sciences, Tokyo, Japan), as described previously (Takemitsu et al. 2016). A fused silica capillary column was used (DB-WAX, 60 m length, 0.25 mm i.d., 0.25 μm film thickness, Agilent J&W, Santa Clara, CA). A 2 μL sample was injected in splitless mode. The column temperature was initially maintained at 40 °C for 2 min, increased to 250 °C at a rate of 5 °C/min, and then kept constant for 20 min. The mass spectrometer was used with an ionization voltage of 70 eV (EI) and ion source temperature of 240 °C. The odorants were determined by sniffing the GC effluent (GC–O). The odor descriptions and their intensities were confirmed by three trained panelists. The odor intensities were evaluated according to the six-grade odor intensity measurement method (Ministry of the Environment 1995).
Extracts from cooked barley and raw barley, collected steam, and run-off were all analyzed by the GC–MS method described above.
Cooking methods
A superheated steam cooking machine (SRM-20, Acesystem, Osaka, Japan) was used to cook the polished barley (Takemitsu et al. 2016, 2013). The inside of the machine and the flows are shown in Fig. S1 in Electronic Supplementary Material. The dimensions of the entire machine are W 2500 × H 1650 × D 1100 (mm), with a steaming chamber of W 2100 × H 415 × D 544 (mm). The steaming chamber was preheated with steam to > 95 °C. Barley (2 kg) was soaked in tap water for 1 h, drained, and put into the preheated machine. Barley on the conveyer net belt moved at a constant speed, and was heated with steam for a total of 21 min. Saturated steam (100 °C) was used for the first half and superheated steam (125 °C) for the latter half. Hot water (80–85 °C) was added from above at regular intervals for a total of 3.75 L/kg raw barley.
An automatic electric rice cooker (SR-SU105, Panasonic, Osaka, Japan) was used to prepare ordinary cooked barley. Barley (350 g) was washed with tap water, drained, and added to the cooker with 770 mL of tap water (Takahashi and Nakazawa 1981). Then, the cooker was used in the ordinary rice cooking mode for ~ 40 min.
Extraction of volatile compounds from cooked barley
The volatile compounds from cooked barley were extracted in the same way as in our previous report (Takemitsu et al. 2016) with some modification. Cooked barley (20 g) was homogenized with 60 mL of distilled water at room temperature, and MTBE (60 mL) was added. The mixture was further homogenized and centrifuged at 1610×g for 5 min, and the upper layer was collected. This extraction was repeated three times. To remove the nonvolatile materials, solvent-assisted flavor evaporation (SAFE) was performed under reduced pressure (6.7 × 10−2 Pa) at 30 °C.
Extraction of volatile compounds from raw barley
Raw barley was ground with a Wonder Crusher WC-3 high-performance crusher (Hsiangtai Machinery Industry, Taipei, Taiwan). To extract the volatile compounds (Takemitsu et al. 2016), raw barley (5 g) was suspended in 5 mL of distilled water and extracted twice with MTBE (40 mL). The volatile compounds in the upper layer were collected by SAFE.
Water content analysis
Water content of the raw and cooked barley were measured according to oven drying methods (Lisa and Robert 2017). Briefly, the grained samples in weighing bottle (φ 50 × 30 mm) were dried by heating to 105 °C at atmospheric pressure for 48 h. Samples were weighed before and after drying to determine the amount of moisture.
Steam collection during steaming and condensation using a distillation column
Steam was collected using a distillation column attached to the outlet of the machine (Fig. S1 in ESM) during the middle 1 min of steaming. The fraction had a volume of 200 mL, and the volatiles in it were extracted twice using 100 mL of MTBE and then concentrated.
Run-off during the steaming process
Run-off was collected at the bottom of the machine during the middle 30 s of steaming; the collected volume was 840 mL. Volatiles were extracted from 400 mL of the run-off using 100 mL of MTBE, and extracts were purified by SAFE.
Isolation of the volatiles using Tenax trap
The headspace volatiles from cooked barley were collected according to our previous method (Takemitsu et al. 2016) with some modifications. Briefly, 500 g of cooked barley was put into a 5 L Smart Bag PA (GL Sciences), and the bag was placed in a thermostatic chamber (70 °C). Purified nitrogen gas was passed through the bag (100 mL/min for 30 min), and the volatiles were collected in a 60/80 mesh Tenax TA porous polymer resin (GL Sciences).
Short path thermal desorption/GC–MS analysis
The volatiles in the Tenax resin were desorbed at 280 °C for 3 min with helium gas at a flow rate of 4 mL/min, using an automated short-path thermal desorption system (OPTIC-4, ATAS GL International BV, Eindhoven, the Netherlands) (Takemitsu et al. 2016). The analytes were then cooled to − 90 °C, kept at this temperature for 3 min, then rapidly heated to 240 °C, and allowed to flow into a GC column. A GC/MS system equipped with a fused silica capillary column (30 m length, 0.25 mm i.d., 0.25 μm film thickness, Inert Cap Pure Wax, GL Sciences) was used in split mode (1:1). The column temperature was held at 40 °C for 1 min and then increased at 12.5 °C/min until reaching 240 °C. MS analysis was performed as described above.
Identification of components
The identification of volatile compounds was carried out according to Maraval et al. (2008) using the NIST 08 mass spectral library. Some compounds were confirmed by comparing with the mass spectra and retention indexes (RI) of standards. The RI was calculated using a series of n-alkanes injected under the same chromatographic conditions.
Volatile component quantification
An appropriate amount of internal standard (cyclohexanol) was added to each sample before extraction. Quantification of the individual volatile components was based on an internal standard method (Monsoor and Proctor 2004). A reference mixture consisting of a known amount of the standard volatile compounds and internal standard (cyclohexanol) was subjected to the GC–MS, and the concentration of each volatile component in the sample was calculated. Specific m/z value for each compound was used for area measurement, and m/z = 57 was used for internal standard area measurement. Significant differences between the steamed and ordinary cooked barley were analyzed by Welch’s t test.
Beta-glucan quantification
Ordinary cooked barley and steamed barley were freeze dried and ground, and the beta-glucan content was assessed using an assay kit (Megazyme, Bray, Ireland, AOAC Method 995.16, AACC Method 32-23, and ICC Standard Method No. 168).
Results and discussion
Sensory evaluation
The sensory evaluation results of cooked barley are shown in Table 1. The intensity of the aroma was significantly weaker for steamed barley than ordinary cooked barley (p < 0.01), with weaker aroma judged as more acceptable. The texture of steamed barley was harder and had more springiness, and its acceptability was divided. The steamed barley, being less bitter and less sour in taste, was significantly preferred to ordinary cooked barley (p < 0.05). Overall, the steamed barley was considered significantly more acceptable (p < 0.05).
Table 1.
Sensory evaluation of steamed barley
| Aroma | Intensity | − 0.9 ± 1.3** |
| Acceptability | 0.7 ± 1.4* | |
| Texture | Hardness | 0.5 ± 1.1* |
| Springiness | 0.7 ± 1.4* | |
| Acceptability | − 0.2 ± 1.4 | |
| Taste | Sweetness | 0.4 ± 1.4 |
| Bitterness | − 0.9 ± 1.0** | |
| Sourness | − 1.3 ± 1.2** | |
| Acceptability | 0.7 ± 1.4* | |
| Overall acceptability | 1.2 ± 1.3* |
Steamed barley was compared to ordinary cooked barley using a 7-point scale from + 3 to − 3 (+ 3 = stronger/like extremely, − 3 = weaker/dislike extremely). Ordinary cooked barley was used as the reference sample (0 on the scale) in all the sessions. Data represent the mean scores of steamed barley ± standard deviation (n = 29)
**p < 0.01, *p < 0.05 versus ordinary cooked barley (Welch’s t test)
Odor compounds in steam distillation extracts of unpolished barley
To determine the comprehensive odor compound profile of barley, unpolished barley was steam distilled. The extracts were then analyzed in duplicate with both GC–MS and GC–O (GC–O analysis relied on three trained panelists). We detected particularly strong, undesirable, and characteristic odors during the second half of the analysis. The retention index (RI) value, the associated compounds, the odor descriptions, and the odor intensity are presented in Table 2. At least 39 odor components were perceived by GC–O, of which 26 were also identified by GC–MS: twelve aldehydes, seven acids, two lactones, two alcohols, two phenols, and one ketone. Among these, our method identified nine new odor compounds from barley, namely (E,Z)-2,6-nonadienal, butanoic acid, 3-methylbutyric acid, pentanoic acid, heptanoic acid, octanoic acid, γ-hexalactone, β-phenethyl alcohol, and 2-methoxy-4-vinylphenol.
Table 2.
Odor components in steam distillation extracts from unpolished barley detected in GC–O/MS analysis
| RIa | GC–MS | No. | Identificationb | GC–O | |
|---|---|---|---|---|---|
| Compound | Barley odor descriptionc | Odor intensityd | |||
| 988 | Pentanal | [1] | A | Yoghurt | + |
| 1081 | Hexanal | [2] | A | Green | +++ |
| 1223 | (E)-2-Hexenal | [3] | B | (Green, leaf) | – |
| 1235 | 2-Pentylfuran | [4] | A | (Green) | – |
| 1256 | 1-Pentanol | [5] | A | (Grassy, fruity) | – |
| 1352 | (E)-2-Heptenal | [6] | B | Aldehyde, barley | +++ |
| 1359 | 1-Hexanol | [7] | A | (Vegetal, green) | – |
| 1400 | Nonanal | [8] | A | (Fatty, citrus, green) | – |
| 1416 | 3-Octen-2-one | [9] | A | (Nut, mushroom) | – |
| 1439 | (E)-2-Octenal | [10] | A | Aldehyde, glass | ++ |
| 1454 | 1-Octen-3-ol | [11] | A | (Mushroom) | – |
| 1459 | Acetic acid | [12] | A | Acid, grass | ++ |
| 1464 | Decanal | [13] | B | Aldehyde, rancid | + |
| 1537 | Benzaldehyde | [14] | A | (Almond) | – |
| 1547 | (E)-2-Nonenal | [15] | A | Dust | ++ |
| 1566 | 1-Octanol | [16] | A | Floral | + |
| 1570 | Unknown | [17] | Aldehyde, rancid | + | |
| 1578 | Unknown | [18] | Oriental, powder | + | |
| 1584 | (E,Z)-2,6-Nonadienal | [19] | B | Cucumber | + |
| 1585 | 3,5-Octadien-2-one | [20] | B | Fruity | + |
| 1600 | Unknown | [21] | Floral | +++ | |
| 1642 | Butanoic acid | [22] | B | Rancid, fatty, sweaty | +++++ |
| 1647 | γ-Butyrolactone | [23] | B | (Caramel, sweet) | – |
| 1656 | Phenylacetaldehyde | [24] | B | Floral | +++ |
| 1664 | Unknown | [25] | Cooked rice, aldehyde | ++ | |
| 1681 | 3-Methylbutiric acid | [26] | A | Sweaty, aldehyde | +++++ |
| 1688 | Unknown | [27] | Aldehyde, rancid | + | |
| 1712 | (E,E)-2,4-Nonadienal | [28] | A | Cooked rice | ++ |
| 1722 | γ-Hexalactone | [29] | A | Floral | + |
| 1723 | (E)-2-Undecenal | [30] | B | Cooked rice | + |
| 1749 | Pentanoic acid | [31] | A | Sour, sweaty | + |
| 1777 | (E,Z)-2,4-Decadienal | [32] | B | Rice bran, butter, rancid | ++ |
| 1826 | (E,E)-2,4-Decadienal | [33] | A | Deep-fried, cereal, burnt | +++++ |
| 1837 | Unknown | [34] | Sweet, honey | ++ | |
| 1853 | Hexanoic acid | [35] | A | Sweaty, sour | ++++ |
| 1867 | Guaiacol | [36] | A | Phenol | +++ |
| 1880 | Unknown | [37] | Cabbage | + | |
| 1891 | Benzyl alcohol | [38] | A | (Sweet, flowery) | – |
| 1919 | Unknown | [39] | Cooked rice, burnt, sour | + | |
| 1920 | Unknown | [40] | Oriental | + | |
| 1928 | Unknown | [41] | Clam, sea | + | |
| 1929 | β-Phenethyl alcohol | [42] | A | Floral | +++ |
| 1933 | Heptanoic acid | [43] | B | Sour, rancid, sweaty | + |
| 1941 | Unknown | [44] | Oriental, powder | +++ | |
| 1962 | Unknown | [45] | Seaweed | + | |
| 2055 | γ-Nonalactone | [46] | A | Sweet, milk | +++ |
| 2074 | Octanoic acid | [47] | B | Sour, aldehyde, rancid | ++++ |
| 2119 | Unknown | [48] | Barley | + | |
| 2183 | Nonanoic acid | [49] | B | (Animal, cheese, fat) | – |
| 2215 | 2-Methoxy-4-vinylphenol | [50] | A | Solvent, ink | + |
aExperimental retention indices calculated on a DB-WAX column
bReliability of the identification. A: mass spectrum and retention time identified with those of an authentic compound; B: mass spectrum agreed with mass library (NIST08) and the retention index (RI) agreed with the data in the literature (Chen et al. 2013; Maraval et al. 2008) and Flavor net database (2018)
cOdor descriptions by panelists during olfactometry. In parentheses are values from the literature and databases that are the same as b
dThe odor intensities were evaluated according to the six-grade odor intensity measurement method by three trained panelists
–, undetectable; +, barely detectable; ++, weak but recognizable; +++, easily detectable; ++++, strong; +++++, intense
Kaneko et al. (2013) analyzed volatile components obtained from polished barley by steam distillation–extraction methods, and reported that hexanal, (E)-2-nonenal, (E,E)-2,4-nonadienal, and (E,E)-2,4-decadienal exhibit high flavor dilution (FD) factors and contribute to the undesirable odor of cooked barley. These aldehydes were also found in our current study; (E,E)-2,4-decadienal had an especially strong, deep-fried, and cereal-like odor. We detected some acids that had strong unpleasant odors as well, particularly 3-methylbutyric acid (sweaty, aldehyde-like odor) and hexanoic acid (sweaty, sour odor), which contribute to the characteristic undesirable odor of barley. These acids were also detected by GC–MS from cooked rice (Takemitsu et al. 2016), but not perceived by GC–O. In general, more numerous odor compounds are detected from barley than rice.
Quantification of volatile components extracted from raw and cooked barley
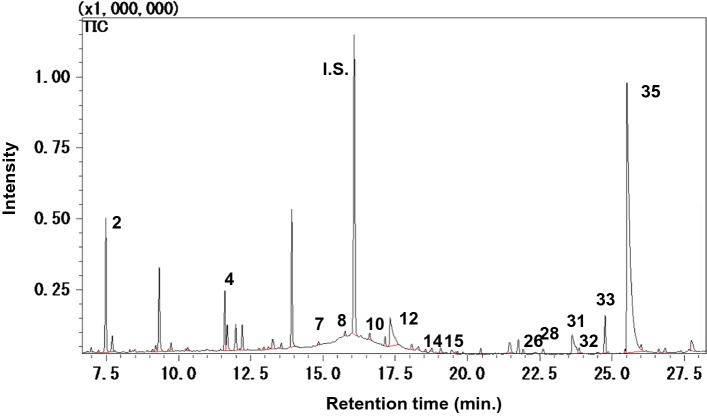
After steaming the polished barley, the odor compounds recovered in the organic extracts were analyzed by GC–MS. A typical chromatogram is shown in Fig. 1. We selected 14 compounds based on their quantity, odor threshold values, and availability of standards. The amounts of these compounds in steamed barley were compared with those in raw and ordinary cooked barley (Table 3). All the compounds, except (E)-2-octenal, benzaldehyde, and 2-pentylfuran, were reported as Key Food Odorants (KFO) (Dunkel et al. 2014).
Fig. 1.
Gas chromatogram of odor compounds in steam collected during barley steaming. For compound names designated by numbers, see Table 3. Internal standard (I.S.): cyclohexanol
Table 3.
Quantification of volatile compounds in organic aroma extracts from cooked barley
| Compound | No. | RIa | m/z b | Quantification (μg/100 g dry weight) | Ratio steamed/ordinary | Ordinary cooked ricec (μg/100 g) | Odor threshold (μg/100 g) | ||
|---|---|---|---|---|---|---|---|---|---|
| Raw barley | Ordinary cooked barley | Steamed barley | |||||||
| Hexanal | [2] | 1079 | 44 | 643 ± 63 | 112 ± 46 | 88 ± 25 | 0.8 | 14.5 | 0.91d |
| 2-Pentylfuran | [4] | 1231 | 81 | 15 ± 0.7 | 27 ± 3.5 | 26 ± 6.7 | 1.0 | 0.4 | 0.04e |
| 1-Hexanol | [7] | 1357 | 56 | 15 ± 0.9 | 4 ± 1.1 | 2 ± 0.5** | 0.4 | 10.3 | 800d |
| Nonanal | [8] | 1394 | 70 | 10 ± 1.3 | 4 ± 1.0 | 3 ± 0.7 | 0.8 | 6.6 | 0.08e |
| (E)-2-Octenal | [10] | 1430 | 70 | 10 ± 0.5 | 6 ± 1.0 | 4 ± 0.8* | 0.7 | N.D.f | 0.3e |
| Acetic acid | [12] | 1454 | 60 | 633 ± 48 | 933 ± 85 | 251 ± 48** | 0.3 | 102.4 | 20,000d |
| Benzaldehyde | [14] | 1523 | 106 | 7 ± 0.6 | 3 ± 0.4 | 2 ± 0.3* | 0.8 | 1.8 | 90d |
| (E)-2-Nonenal | [15] | 1537 | 70 | 6 ± 1.5 | 3.3 ± 0.8 | 2.7 ± 0.6 | 0.8 | N.D. | 0.007e |
| 3-Methylbutyric acid | [26] | 1685 | 60 | 19 ± 4.1 | 32 ± 3.3 | 7 ± 1.1** | 0.2 | 5.0 | 3.34d |
| (E,E)-2,4-Nonadienal | [28] | 1704 | 81 | 3.8 ± 1.0 | 4 ± 0.4 | 3 ± 0.4* | 0.8 | N.D. | – |
| Pentanoic acid | [31] | 1751 | 60 | 117 ± 31 | 127 ± 46 | 51 ± 12.7* | 0.4 | 15.0 | 300d |
| (E,Z)-2,4-Decadienalg | [32] | 1768 | 81 | Trh | 2 ± 0.3 | 1 ± 0.5 | 0.8 | N.D. | – |
| (E,E)-2,4-Decadienal | [33] | 1815 | 81 | 0.6 ± 0.4 | 26 ± 4.8 | 21 ± 4.5 | 0.8 | 0.6 | 0.006e |
| Hexanoic acid | [35] | 1852 | 60 | 261 ± 113 | 294 ± 114 | 149 ± 47* | 0.5 | 118.1 | 42d |
All the compounds except (E,Z)-2,4-decadienal were identified by mass spectrometry and retention times with those of authentic compounds. Mass spectra agreed with those in the NIST 08 mass library. Retention indexes (RI) agree with the literature (Chen et al. 2013; Zeng et al. 2009). Water contents of raw barley, ordinary cooked barley, and steamed barley were 13.1%, 65.3%, and 62.8%, respectively
The asterisk shows the significance of the difference between ordinary cooked barley and steamed barley with the Welch’s t test (**p < 0.01, *p < 0.05)
aExperimental retention indices calculated on a DB-WAX column
bm/z was used for the area measurement; m/z = 57 was used for the internal standard (cyclohexanol) area measurement
cData from Takemitsu et al. (2016)
Odor thresholds from dChen et al. (2013), eButtery et al. (1988). Quantification data are expressed as mean ± SD (n = 4–5)
fNot detected
g(E,Z)-2,4-decadienal was quantified based on the response factor of (E,E)-2,4-decadienal
hTrace level
After ordinary cooking, some odor compounds were reduced, while those of others were increased, compared with raw barley. For instance, after cooking, hexanal, nonanal, benzaldehyde, and 1-hexanol were present at less than half the initial amount. Conversely, (E,E)-2,4-decadienal dramatically increased upon ordinary cooking.
The steam-cooked barley contains fewer volatiles than those present in ordinary cooked barley, though the differences vary with each compound. The ratios of various compounds in steamed barley compared to ordinary cooked barley are displayed in Table 3. The contents of acetic acid, 3-methylbutyric acid, and 1-hexanol in steamed barley were significantly lower than those in ordinary cooked barley (p < 0.01). Remarkably, compared with raw barley, the presence of both acetic acid and 3-methylbutyric acid decreased after steaming, but increased after ordinary cooking. Both steamed and ordinary cooked barley contained nearly the same amounts of aldehydes (e.g., hexanal and (E,E)-2,4-decadienal) and 2-pentylfuran.
In our previous research on cooked rice (Takemitsu et al. 2016), we identified five major odor compounds in cooked rice: hexanal, nonanal, (E,E)-2,4-decadienal, 2-pentylfuran, and phenol. Four of the compounds (except phenol) were also found in ordinary cooked barley, in agreement with previous observations for rice (Yang et al. 2008a, b) and barley (Cramer et al. 2005; Kaneko et al. 2013). Compared to cooked rice (Takemitsu et al. 2016), the amount of (E,E)-2,4-decadienal and 2-pentylfuran was more than thirty times higher in both steamed and ordinary cooked barley. Hexanal content in steamed barley was approximately six times greater than in ordinary cooked rice. (E)-2-Octenal, (E)-2-nonenal, (E,E)-2,4-nonadienal, and (E,Z)-2,4-decadienal could be quantified from cooked barley, although they were at trace or undetectable levels in rice.
Hougen et al. (1971) analyzed various grains including barley, and concluded that the same volatile components are mainly produced, but in different characteristic amounts. Our results agree with these findings; rice and barley contain largely the same odor compounds, but barley possesses these compounds in much greater amounts.
Volatile odor components in the headspace vapor of cooked barley
Aldehydes such as hexanal, (E)-nonenal, (E,E)-2,4-nonadienal, and (E,E)-2,4-decadienal have been reported as contributing to the undesirable odor of cooked barley (Kaneko et al. 2013). To confirm the release of odorants from cooked barley, we compared the amounts of major volatile components in the headspace vapor of each cooked barley sample (Table 4). The ratio of components in steamed to ordinary cooked barley are also shown in Table 4. A ratio less than 1 indicates a compound that is relatively lower in steamed barley. We found that steamed barley contained 40% less hexanal than ordinary cooked barley, and 60–70% less other compounds (i.e., (E)-2-octenal, (E)-2-nonenal, benzaldehyde, and 2-pentylfuran). Some compounds with relatively high boiling points (e.g., (E,E)-2,4-nonadienal, (E,E)-2,4-decadienal, and some acids) were undetectable in both samples.
Table 4.
Total ion peak area of major volatile components in the headspace vapor of cooked barley
| Compound | No. | BPa | RIb | Total ion peak area (× 104) | Ratio steamed/ordinary | |
|---|---|---|---|---|---|---|
| Ordinary cooked | Steamed | |||||
| Hexanalc | [2] | 130 | 1079 | 289 ± 26 | 161 ± 7 | 0.6 |
| 2-Pentylfuran | [4] | 170 | 1228 | 840 ± 55 | 335 ± 104 | 0.4 |
| (E)-2-Octenal | [10] | 190 | 1414 | 52 ± 14 | 18 ± 2.8 | 0.3 |
| Benzaldehyde | [14] | 180 | 1496 | 47 ± 18 | 20 ± 0.9 | 0.4 |
| (E)-2-Nonenal | [15] | 189 | 1513 | 13 ± 4.2 | 4 ± 0.3 | 0.3 |
Values are expressed as mean ± SD (n = 2). All the compounds were identified as in Table 3. The headspace volatiles were collected for 30 min from 500-g samples
aBoiling points of the compounds are from Scifinder (2018) or Pub Chem (2018)
bExperimental liner retention indices calculated on an InertCap® Pure-WAX column
cThe area of hexanal was too large to measure; therefore, we measured a 100-g sample for 5 min
The difference between steamed and ordinary cooked barley was more apparent through detection of compounds in the headspace vapor than that of concentrates obtained by the extraction method. The amount of odorants released from steamed barley was less than that from ordinary cooked barley because steaming better preserves the structure of the barley grain, as has been similarly reported for steamed rice (Takemitsu et al. 2016). We had previously observed the cross-section of steamed rice grains, and found that the internal cell wall structure was preserved (Takemitsu et al. 2013). The same appears to be true for steamed barley, which leads to better retention of the odorous compounds.
Comparing the volatile compounds in collected steam and run-off
During the rice steaming process, some compounds such as aldehydes and alcohols are removed by steam distillation (Takemitsu et al. 2016). Acetic acid, which is water soluble, is leached into the run-off in large quantities. To confirm that this behavior indeed occurs during barley steaming, we collected both the steam and run-off during the middle point of steaming for analysis.
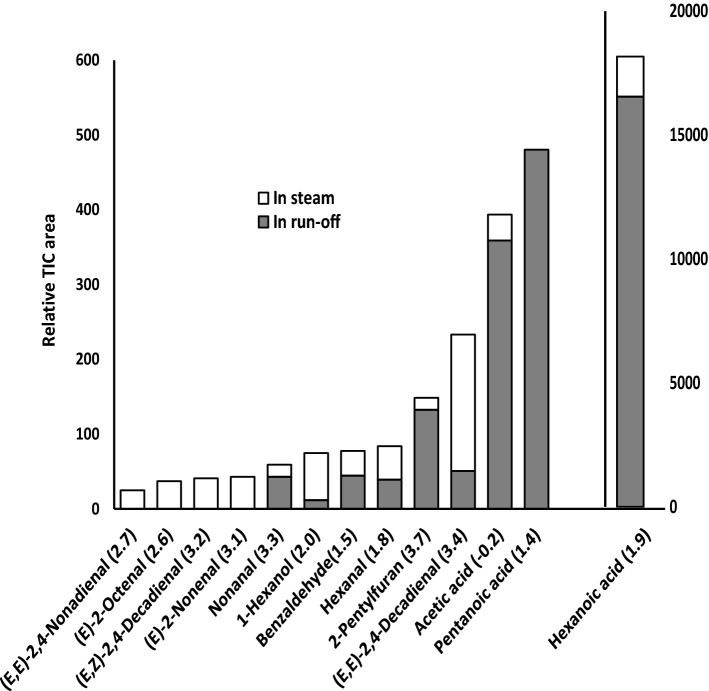
As shown in Fig. 2, hexanoic acid is the predominant component found in the run-off. Other acids (e.g., pentanoic acid and acetic acid) were also found mainly in the run-off. As a result, steamed barley contains fewer acids than ordinary cooked barley, supporting the quantification results (Table 2). On the other hand, some aldehydes and alcohols (e.g., (E)-2-octenal, (E,E)-2,4-decadienal) were detected at higher levels in the steam than in the run-off. Log p values indicate that these compounds tend to be less soluble in water and would therefore be removed mainly by steam distillation.
Fig. 2.
Relative area of odorous compounds in steam and run-off collected during the middle 1 min and 30 s of cooking, respectively. The odorous compounds in each sample were measured and converted to obtain the relative areas. In parentheses are the log p values of the compounds from Scifinder (2018) and Pub Chem (2018)
Odor compounds are known to show synergistic effects and interactions with each other. Dunkel et al. (2014) also described that a mixture of odorants does not just create a sum of the individual odors but may produce a new unique odor. Miyazawa et al. (2008) found that by adding even a subthreshold level of acetic acid to three common flavor compounds, the rated flavor intensity of each increases by a small but statistically significant degree. During the steaming process, both aldehydes and acids are eliminated, which changes the odor quality. Fewer acids present also affects the taste. While sourness is most commonly associated with acids, other taste characteristics such as bitterness, saltiness, and astringency (Da Conceicao Neta et al. 2007). In this study, sensory evaluation revealed that not only sourness, but also bitterness was weaker in steamed barley. Removal of acids using superheated steam to cook the barley is likely the reason for this.
Finally, to ensure the preservation of dietary fiber, we measured the beta-glucan content in barley. The beta-glucan contents were 1.6% in ordinary cooked barley and 1.8% in steamed barley (wet base, n = 3), and we confirmed that beta-glucan remained the same after the steaming process. This new steaming process can reduce the undesirable odor in cooked barley while preserving the dietary fiber present, making it a high-quality food with excellent nutritional value.
Conclusion
Our odor profile analysis revealed that among the odorants found in barley, the quantities of acids such as acetic acid and 3-methylbutyric acid were reduced by steam cooking. We also found that the amounts of odorants released from steamed barley were less than those released from ordinary cooked barley because steaming better preserves the structure of barley grain, and steamed barley preserved its beta-glucan content. Our steaming method improves barley’s qualities as a food and could increase the consumption of barley.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
This work was supported by Osaka Prefecture University. We thank Hakubaku Co., Ltd. for providing the barley samples. We also express our sincere thanks to the students of Osaka Prefecture University for their cooperation serving as panelists. We would like to thank Editage (www.editage.jp) for English language editing.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Altan A, McCarthy KL, Maskan M. Evaluation of snack foods from barley–tomato pomace blends by extrusion processing. J Food Eng. 2008;84:231–242. doi: 10.1016/j.jfoodeng.2007.05.014. [DOI] [Google Scholar]
- Altan A, McCarthy KL, Maskan M. Extrusion cooking of barley flour and process parameter optimization by using response surface methodology. J Sci Food Agric. 2008;88:1648–1659. doi: 10.1002/jsfa.3262. [DOI] [Google Scholar]
- Baik B-K, Ullrich SE. Barley for food: characteristics, improvement, and renewed interest. J Cereal Sci. 2008;48:233–242. doi: 10.1016/j.jcs.2008.02.002. [DOI] [Google Scholar]
- Buttery RG, Turnbaugh JG, Ling LC. Contribution of volatiles to rice aroma. J Agric Food Chem. 1988;36:1006–1009. doi: 10.1021/jf00083a025. [DOI] [Google Scholar]
- Chen S, Xu Y, Qian MC. Aroma characterization of Chinese rice wine by gas chromatography–olfactometry, chemical quantitative analysis, and aroma reconstitution. J Agric Food Chem. 2013;61:11295–11302. doi: 10.1021/jf4030536. [DOI] [PubMed] [Google Scholar]
- Cramer A-CJ, Mattinson DS, Fellman JK, Baik BK. Analysis of volatile compounds from various types of barley cultivars. J Agric Food Chem. 2005;53:7526–7531. doi: 10.1021/jf0506939. [DOI] [PubMed] [Google Scholar]
- Da Conceicao Neta ER, Johanningsmeier SD, McFeeters RF. The chemistry and physiology of sour taste: a review. J Food Sci. 2007;72:R33–R38. doi: 10.1111/j.1750-3841.2007.00282.x. [DOI] [PubMed] [Google Scholar]
- Dunkel A, Steinhaus M, Kotthoff M, Nowak B, Krautwurst D, Schieberle P, Hofmann T. Nature’s chemical signatures in human olfaction: a foodborne perspective for future biotechnology. Angew Chem Int Ed Engl. 2014;53:7124–7143. doi: 10.1002/anie.201309508. [DOI] [PubMed] [Google Scholar]
- Flavor net data base (2018) http://www.flavornet.org/flavornet.html. Accessed May 2018
- Hougen FW, Quilliam MA, Curran WA. Headspace vapors from cereal grains. J Agric Food Chem. 1971;19:182–183. doi: 10.1021/jf60173a025. [DOI] [Google Scholar]
- Izydorczyk MS, Dexter JE. Barley β-glucans and arabinoxylans: molecular structure, physicochemical properties, and uses in food products—a review. Food Res Int. 2008;41:850–868. doi: 10.1016/j.foodres.2008.04.001. [DOI] [Google Scholar]
- Kaneko S, Kodama T, Kohyama N, Watanabe H, Hayase F. Aroma components characterizing the odor of cooked barley. J Jpn Soc Food Sci Technol. 2013;60:439–442. doi: 10.3136/nskkk.60.439. [DOI] [Google Scholar]
- Konishi M, Ide K, Hatae K, Shimada A. Eating quality evaluation for cooked rice (1)-application of multiple regression on the sensory evaluation and physical measurements. J Cook Sci Jpn. 1996;29:254–263. [Google Scholar]
- Lisa JM, Robert LB. Moisture and total solids analysis. In: Nielsen SS, editor. Food analysis. 5. Basel: Springer; 2017. pp. 257–286. [Google Scholar]
- Maraval I, Mestres C, Pernin K, Ribeyre F, Boulanger R, Guichard E, Gunata Z. Odor-active compounds in cooked rice cultivars from Camargue (France) analyzed by GC–O and GC–MS. J Agric Food Chem. 2008;56:5291–5298. doi: 10.1021/jf7037373. [DOI] [PubMed] [Google Scholar]
- Ministry of the Environment, Government of Japan (1995) Offensive odor control law of Japan (English version). https://www.env.go.jp/en/laws/air/odor/index.html. Accessed 6 Mar 2019
- Miyazawa T, Gallagher M, Preti G, Wise PM. The impact of subthreshold carboxylic acids on the odor intensity of suprathreshold flavor compounds. Chemosens Percept. 2008;1:163–167. doi: 10.1007/s12078-008-9019-z. [DOI] [Google Scholar]
- Monsoor MA, Proctor A. Volatile component analysis of commercially milled head and broken rice. J Food Sci. 2004;69:C632–C636. doi: 10.1111/j.1365-2621.2004.tb09911.x. [DOI] [Google Scholar]
- Pub Chem (2018) https://pubchem.ncbi.nlm.nih.gov/. Accessed May 2018
- Scifinder (2018) https://scifinder.cas.org/scifinder/view/scifinder/scifinderExplore.jsf. Accessed May 2018
- Sharma P, Kotari S. Barley: impact of processing on physicochemical and thermal properties—review. Food Rev Int. 2017;33:359–381. doi: 10.1080/87559129.2016.1175009. [DOI] [Google Scholar]
- Shepherd GM. Neurogastronomy: how the brain creates flavor and why it matters. New York: Columbia University Press; 2013. [Google Scholar]
- Takahashi J, Nakazawa F. Cooked rice with barley (part 1) cooking conditions. J Home Econom Jpn. 1981;32:172–177. [Google Scholar]
- Takemitsu H, Hayashi Y, Sako Y, Kitamura S. Taste and freshness of cooked rice using a superheated steam rice cooking machine. J Jpn Soc Food Sci Technol. 2013;60:628–634. doi: 10.3136/nskkk.60.628. [DOI] [Google Scholar]
- Takemitsu H, Amako M, Sako Y, Shibakusa K, Kita K, Kitamura S, Inui H. Analysis of volatile odor components of superheated steam-cooked rice with a less stale flavor. Food Sci Technol Res. 2016;22:771–778. doi: 10.3136/fstr.22.771. [DOI] [Google Scholar]
- Yang DS, Lee KS, Jeong OY, Kim KJ, Kays SJ. Characterization of volatile aroma compounds in cooked black rice. J Agric Food Chem. 2008;56:235–240. doi: 10.1021/jf072360c. [DOI] [PubMed] [Google Scholar]
- Yang DS, Shewfelt RL, Lee K-S, Kays SJ. Comparison of odor-active compounds from six distinctly different rice flavor types. J Agric Food Chem. 2008;56:2780–2787. doi: 10.1021/jf072685t. [DOI] [PubMed] [Google Scholar]
- Zeng Z, Zhang H, Zhang T, Tamogami S, Chen JY. Analysis of flavor volatiles of glutinous rice during cooking by combined gas chromatography–mass spectrometry with modified headspace solid-phase microextraction method. J Food Compos Anal. 2009;22:347–353. doi: 10.1016/j.jfca.2008.11.020. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.