Abstract.
Significant differences in levothyroxine (LT4) dosages for congenital hypothyroidism (CH), which can be permanent (P-CH) or transient (T-CH), and their respective cutoff values have been reported. In Japanese children, however, these values are unknown, and were thus determined in this retrospective single-center study, which included 34 patients— 19 with P-CH and 15 with T-CH. The LT4 dosages of the two groups at ages 1 and 3 yr were compared, and receiver operating characteristic (ROC) analysis was performed to identify the cutoff dosages. The results showed that the LT4 dosages of the P-CH and T-CH groups differed significantly at both ages. When LT4 dosage cutoff at 1 yr of age was set at 2.4 μg/kg/d, the sensitivity and specificity were 93% and 74%, respectively, and when it was set at 1.3 μg/kg/d at 3 yr of age, they were 80% and 84%, respectively, suggesting that when LT4 dosages are ≤2.4 μg/kg/d at 1 yr and ≤1.3 μg/kg/d at 3 yr of age, T-CH should be suspected.
Keywords: congenital hypothyroidism, transient congenital hypothyroidism, permanent congenital hypothyroidism, newborn screening, levothyroxine
Introduction
Congenital hypothyroidism (CH) can be permanent (P-CH) or transient (T-CH), and based on factors such as sex, birth wt, and levels of TSH, T4, free T4 (fT4), and Tg (1,2,3,4,5,6,7,8,9,10,11,12) at the time of neonatal screening tests, these two CH types are difficult to differentiate prior to treatment initiation. However, previous studies have reported significant differences in the levothyroxine (LT4) dosages for 3-yr-old children with P-CH vs. T-CH (1, 3, 5, 7,8,9,10,11,12), and six studies have also identified LT4 dosage cutoff values for differentiating between P-CH and T-CH in 3-yr-old children (7,8,9,10,11,12). The aim of this study was to determine the presently unknown LT4 dosage cutoff values for Japanese children of ages 1 and 3 yr.
Subjects and Methods
This retrospective single-center study was conducted at the Tokyo Metropolitan Children’s Medical Center, Fuchu-city, Tokyo, Japan between 2010 and 2016. Children above the age of 3 yr with congenital hypothyroidism (CH), detected via neonatal screening of TSH and fT4 levels, were recruited. Those with Down syndrome, central hypothyroidism, delivered prematurely (< 37 wk of gestation), drug-induced hypothyroidism, thyroid dysfunction due to maternal thyroid disease, or incomplete data, were excluded from the study. The ethics review board of the Medical Center approved the study (ID: H 28b-83), and informed consent was obtained from the parents of the study participants.
A chemiluminescence enzyme immunoassay (Lumipulse® kit; Fujirebio, Tokyo, Japan) was used to measure thyroid hormone levels, which had reference ranges as follows: TSH, 0.65–6.60 μIU/mL (birth–4 mo) and 0.33–6.60 μIU/mL (4 mo–12 yr); free T3, 1.74–5.50 pg/mL (birth–11 mo) and 1.89–5.10 pg/mL (11 mo–13 yr); and fT4, 0.96–2.0 ng/dL (birth–5 yr).
After neonatal screening, all subjects received LT4 treatment for CH, which was classified as P-CH or T-CH based on their chart information. P-CH was defined as a condition requiring lifelong hormone therapy due to insufficient thyroid hormone production, and included subjects who continued to require LT4 treatment after the 3 yr of age. They were then divided into 3 subgroups as follows: A) those who required an increased LT4 dosage after LT4 initiation, B) those with high TSH levels (> 5 μIU/mL) following LT4 dosage reduction, and C) those with very high TSH peaks (> 40 μIU/mL) based on TRH stimulation tests 1 month after the withdrawal period. T-CH was defined as a condition that required transient hormone therapy during the early stages of life, and included subjects who discontinued LT4 therapy at 3 yr of age, and did not require LT4 re-administration for more than a year after LT4 discontinuation. The maximum period of observation after LT4 discontinuation was about 3 yr.
Retrospectively, the following items in the charts of the subjects were examined: current age, sex, gestational age, birth wt, body wt at ages 1 and 3 yr, TSH and fT4 levels, age at LT4 treatment initiation, initial LT4 dosage, TRH stimulation test results, and the presence of an aplastic, hypoplastic, or ectopic thyroid gland, detected using ultrasonography. Also, increase in LT4 dosage during treatment, increase in TSH level after LT4 dose reduction, and LT4 treatment discontinuation, were determined. To assess LT4 dosages at 3 yr of age, the dosages administered at this age were used for those in P-CH subgroups A and B. For the assessment of the P-CH subgroup C and the T-CH group, the final dosages administered before discontinuation were used, and LT4 had been discontinued for 1 mo prior to TRH test. At the study center, LT4 treatment was initiated or increased if TSH levels exceeded 10 and 5 μIU/mL at initial and after initial treatment, respectively.
The LT4 dosages at ages 1 and 3 yr of the P-CH and T-CH groups were compared. The chi-square test was used to compare categorical variables, and the unpaired t test or Mann-Whitney U-test was used to compare continuous variables. Receiver-operating characteristic (ROC) analysis was performed to determine the LT4 dosage cutoff at ages 1 and 3. All statistical analyses were performed using Easy R (EZR) v3.5.2, and p < 0.05 indicated statistical significance.
Results
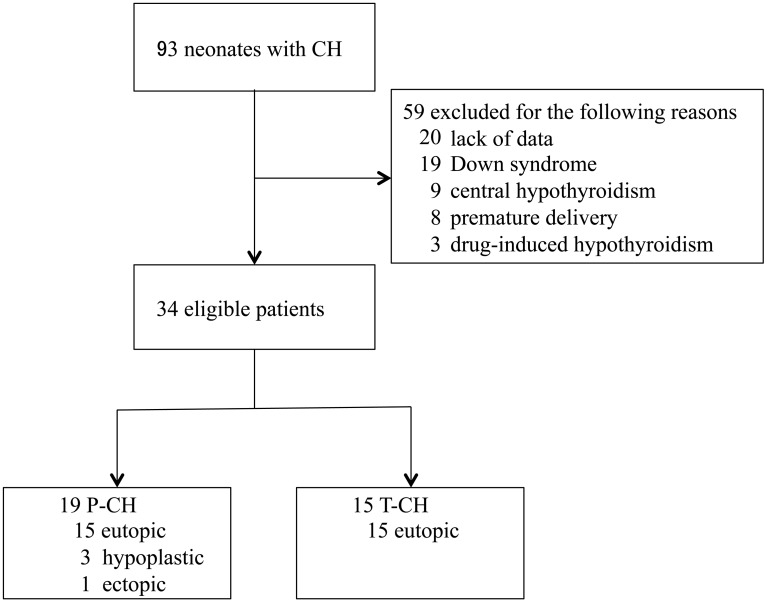
Ninety-three CH patients followed up at the study center were identified (Fig. 1), and patients with the following were excluded: Down syndrome (n = 19), central hypothyroidism (n = 9), delivered prematurely (n = 8), drug-induced hypothyroidism (n = 3), and data deficits (n = 20). The remaining 34 cases were classified as P-CH (n = 19, 56%) or T-CH (n = 15, 44%), and the P-CH group was further divided into 3 subgroups: A) increased LT4 dosage required after LT4 initiation (n = 12), B) TSH level > 5 μIU/mL following LT4 dosage reduction (n = 6), and C) TSH peaks > 40 μIU/mL detected using TRH stimulation tests after the 1 mo withdrawal period (n = 1). In P-CH subgroup C, the dose was temporarily 0 μg/kg/d at the time of evaluation in 3-yr-old. However, the peak of TSH during the TRH test was > 40 μIU/mL, and LT4 treatment had to continue after the age of 3 yr. Therefore, this subgroup C was classified as P-CH. In the 15 T-CH patients, LT4 therapy was started at first visit (after newborn screening test) and continued at the same dosage for up to 3 yr.
Fig. 1.
Flow diagram of the study selection process. CH, congenital hypothyroidism; P-CH, permanent congenital hypothyroidism; T-CH, transient congenital hypothyroidism.
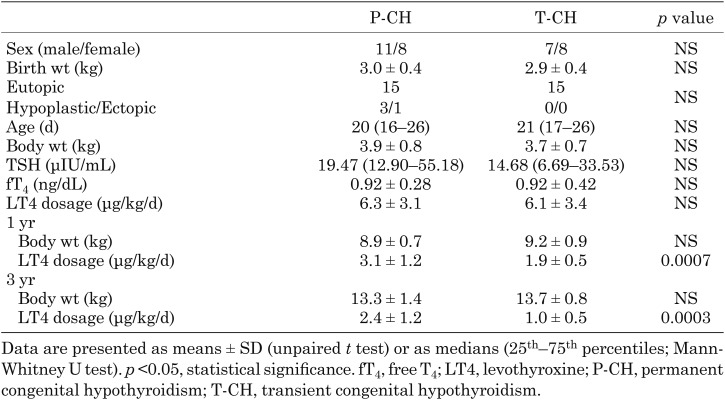
Medical record review for the 34 eligible children revealed that the number of male and female patients were 11 and 8 in the P-CH group, and 7 and 8 in the T-CH group, respectively. Their clinical characteristics shown in Table 1 reveal that there were no significant differences in sex, gestational age, birth wt, body wt, or age, TSH level or fT4 level at treatment initiation, and the presence of an aplastic, hypoplastic, or ectopic thyroid gland, between the P-CH and T-CH groups.
Table 1. Clinical characteristics of P-CH and T-CH patients.
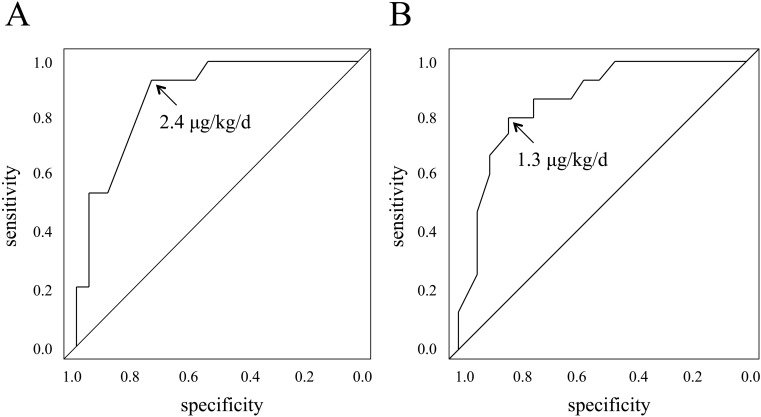
There were significant differences in the LT4 dosage between the P-CH and T-CH groups at 1 yr (3.1 ± 1.2 and 1.9 ± 0.5 μg/kg/d, respectively; p = 0.0007) and 3 yr of age (2.4 ± 1.2 and 1.0 ± 0.5 μg/kg/d, respectively; p = 0.0003). The LT4 dosage ROC curves shown in Fig. 2 revealed that when LT4 dosage cutoff at 1 yr was set at 2.4 μg/kg/d, the sensitivity and specificity were 93 and 74%, respectively, and when it was set at 1.3 μg/kg/d, at 3 yr of age, the sensitivity and specificity were 80 and 84%, respectively.
Fig. 2.
Receiver operating characteristic curves for LT4 dosages. (A) At 1 yr of age. (B) At 3 yr of age.
The P-CH group consisted of three patients with a hypoplastic thyroid gland and one with an ectopic thyroid gland. None in the T-CH group presented with an aplastic, hypoplastic, or an ectopic thyroid gland. After excluding these four patients from the P-CH group, a further analysis revealed significant differences in the LT4 dosages between the eutopic P-CH (n = 15) and eutopic T-CH (n = 15) groups at 1 (2.6 ± 0.6 and 1.9 ± 0.5 μg/kg/d, respectively; p = 0.0007) and 3 (2.0 ± 0.8 and 1.0 ± 0.5 μg/kg/d, respectively; p = 0.0007) yr. When the LT4 dosage cutoff at 1 yr was set at 2.4 μg/kg/d, the sensitivity and specificity were 93 and 67%, respectively, and when it was set at 1.3 μg/kg/d at 3 yr of age, they were 80 and 80%, respectively.
Three false negatives —namely, children administered a higher than expected LT4 dosage— were identified in the T-CH group at both 1 and 3 yr of age. All three were born between wk 37 and 40 of gestation, after an uncomplicated pregnancy and delivery, and exhibited no signs of hypothyroidism, except for jaundice. The first case involved a 12-d-old girl with a body wt of 2.8 kg (–0.5 SD) at diagnosis, and respective TSH and fT4 levels of 10.3 μIU/mL and 1.39 ng/dL, who was administered an initial LT4 dosage of 5.4 µg/kg/d. The second case involved a 17-d-old boy with a body wt of 3.7 kg (–1.0 SD) at diagnosis, and respective TSH and fT4 levels of 25.1 μIU/mL and 0.91 ng/dL, who was administered a high initial LT4 dosage of 9.4 µg/kg/d. The third case involved a 26-d-old girl with a body wt of 3.2 kg (–1.8 SD) at diagnosis, and respective TSH and fT4 levels of 24.6 μIU/mL and 1.53 ng/dL, who was administered an initial LT4 dosage of 7.8 µg/kg/d. The second case exhibited growth catch up to a wt gain of + 0.2 SD. However, the first and third cases exhibited poor wt gain after being started on LT4, with respective body wt remaining between –0.5 SD and –1.2 SD, and between –0.7 SD and –1.8 SD, respectively. Therefore, the LT4 dosages exceeded the cutoff value in these patients were aged 1 and 3 yr.
Discussion
The results of this study suggest that LT4 dosages ≤ 2.4 at 1 yr and ≤ 1.3 μg/kg/d at 3 yr of age indicate T-CH. To the best of our knowledge, this is the first report on P-CH and T-CH LT4 cutoff values in Japanese patients. Although no simple method for distinguishing T-CH from P-CH currently exists, the results of this study may eliminate the need for frequent blood tests, help predict the patient’s clinical course, and relieve parental anxiety.
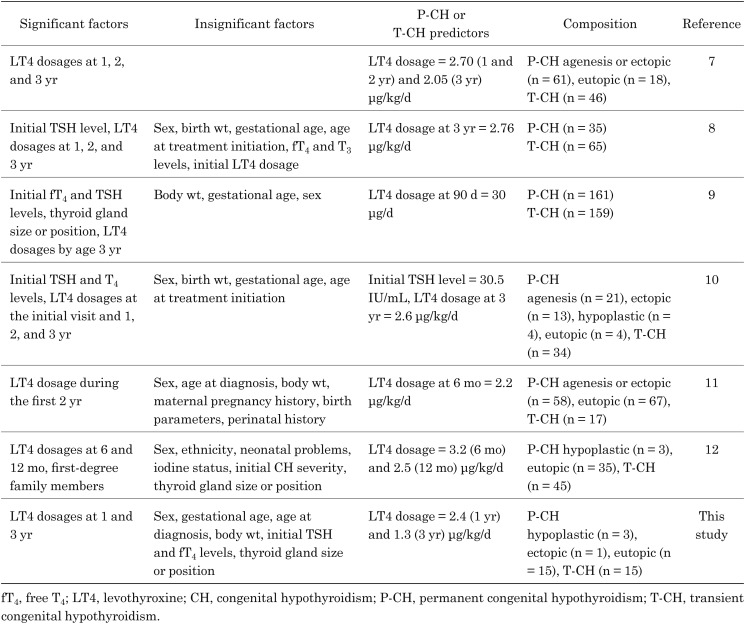
Previously identified LT4 cutoff values for differentiating between P-CH and T-CH in 3-yr-old children were 30 μg/kg/d at 90 d, 2.20–3.20 μg/kg/d at 6 mo, 2.50–2.70 μg/kg/d at 1 yr, 2.70 μg/kg/d at 2 yr, and 2.05–2.76 μg/kg/d at 3 yr in patients with eutopic thyroid glands, as shown in Table 2 (7–12). Fu et al. (9) noted that the most accurate LT4 cutoff value was 30 μg/d at 90 d.
Table 2. Summary of the studies differentiating between P-CH and T-CH.
Thyroid ultrasonography is an important diagnostic tool used to differentiate between P-CH and T-CH. Although in this study, there were no significant differences in the thyroid gland size and position between P-CH and T-CH patients, abnormal thyroid gland morphology was found to be associated with P-CH, as reported in previous studies (1, 2, 9, 10). There might have been no significant difference due to the small number of cases. Thyroid agenesis or dysgenesis patients are known to require lifelong thyroid hormone therapy. In this study, three and one patient in the P-CH group presented with a hypoplastic and an ectopic thyroid gland, respectively. Consistent with previous reports, no patient in our T-CH group presented with abnormal thyroid morphology.
This study revealed that there were no significant differences in the TSH levels at treatment initiation between the P-CH and T-CH groups. At present, using TSH levels to differentiate between P-CH and T-CH, is controversial. As reported in five previous studies, they were higher in thyroid agenesis and dysgenesis patients, than in those with eutopic thyroid glands (5, 9,10,11,12). Contrarily, in four previous studies, no significant difference in P-CH and T-CH TSH levels were reported, despite the inclusion of thyroid agenesis and dysgenesis patients in the P-CH group (1,2,3, 7). In the present study and others (8,9,10,11), LT4 dosage by the age of 3 yr was a better P-CH and T-CH predictor than TSH levels at treatment initiation.
Based on the results of this study, it is recommended that thyroid echography be initially performed to detect aplastic, hypoplastic, and ectopic thyroid glands, and LT4 treatment continued if any of these developmental abnormalities are suspected. If none is suspected, and the LT4 dosage has not been increased before the age of 3 yr, lowering the dosage to 1.3 μg/kg/d can be attempted, and if TSH levels does not increase during LT4 dosage reduction, the dosage can be discontinued after gradual tapering and TRH testing. However, if the LT4 dosage has been increased before the age of 3 yr, the LT4 dosage at age 3 yr should only be reduced after a full consideration of the case. A difficulty in the reduction process would likely suggest P-CH.
This study has at least two limitations. Firstly, only a single center was involved, and the sample size was small. Future multiple-center studies with larger sample sizes are needed. Secondly, the follow-up period after LT4 treatment discontinuation was at most 3 yr, which is insufficient for the evaluation of T-CH patients. Of the 16 T-CH patients in a study conducted by Miki et al., two (13%) relapses with slightly increased TSH levels during the follow-up were noted (13).
Conclusion
This study suggests that when LT4 dosages are ≤ 2.4 and ≤ 1.3 μg/kg/d at ages 1 and 3 yr, respectively, T-CH should be suspected.
Conflict of Interest
The authors have none to declare.
References
- 1.Eugster EA, LeMay D, Zerin JM, Pescovitz OH. Definitive diagnosis in children with congenital hypothyroidism. J Pediatr 2004;144: 643–7. doi: 10.1016/j.jpeds.2004.02.020 [DOI] [PubMed] [Google Scholar]
- 2.Rabbiosi S, Vigone MC, Cortinovis F, Zamproni I, Fugazzola L, Persani L, et al. Congenital hypothyroidism with eutopic thyroid gland: analysis of clinical and biochemical features at diagnosis and after re-evaluation. J Clin Endocrinol Metab 2013;98: 1395–402. doi: 10.1210/jc.2012-3174 [DOI] [PubMed] [Google Scholar]
- 3.Unüvar T, Demir K, Abacı A, Büyükgebiz A, Böber E. The role of initial clinical and laboratory findings in infants with hyperthyrotropinemia to predict transient or permanent hypothyroidism. J Clin Res Pediatr Endocrinol 2013;5: 170–3. doi: 10.4274/Jcrpe.931 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Korzeniewski SJ, Grigorescu V, Kleyn M, Young WI, Birbeck G, Todem D, et al. Transient hypothyroidism at 3-year follow-up among cases of congenital hypothyroidism detected by newborn screening. J Pediatr 2013;162: 177–82. doi: 10.1016/j.jpeds.2012.06.050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Skordis N, Toumba M, Savva SC, Erakleous E, Topouzi M, Vogazianos M, et al. High prevalence of congenital hypothyroidism in the Greek Cypriot population: results of the neonatal screening program 1990-2000. J Pediatr Endocrinol Metab 2005;18: 453–61. doi: 10.1515/JPEM.2005.18.5.453 [DOI] [PubMed] [Google Scholar]
- 6.Razavi Z, Mohammadi L. Permanent and transient congenital hypothyroidism in Hamadan west province of Iran. Int J Endocrinol Metab 2016;14: e38256. doi: 10.5812/ijem.38256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Messina MF, Aversa T, Salzano G, Zirilli G, Sferlazzas C, De Luca F, et al. Early discrimination between transient and permanent congenital hypothyroidism in children with eutopic gland. Horm Res Paediatr 2015;84: 159–64. doi: 10.1159/000435811 [DOI] [PubMed] [Google Scholar]
- 8.Park IS, Yoon JS, So CH, Lee HS, Hwang JS. Predictors of transient congenital hypothyroidism in children with eutopic thyroid gland. Ann Pediatr Endocrinol Metab 2017;22: 115–8. doi: 10.6065/apem.2017.22.2.115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fu C, Luo S, Li Y, Li Q, Hu X, Li M, et al. The incidence of congenital hypothyroidism (CH) in Guangxi, China and the predictors of permanent and transient CH. Endocr Connect 2017;6: 926–34. doi: 10.1530/EC-17-0289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zdraveska N, Zdravkovska M, Anastasovska V, Sukarova-Angelovska E, Kocova M. Diagnostic re-evaluation of congenital hypothyroidism in Macedonia: predictors for transient or permanent hypothyroidism. Endocr Connect 2018;7: 278–85. doi: 10.1530/EC-17-0332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Oron T, Lazar L, Ben-Yishai S, Tenenbaum A, Yackobovitch-Gavan M, Meyerovitch J, et al. Permanent vs transient congenital hypothyroidism: Assessment of predictive variables. J Clin Endocrinol Metab 2018;103: 4428–36. doi: 10.1210/jc.2018-00362 [DOI] [PubMed] [Google Scholar]
- 12.Saba C, Guilmin-Crepon S, Zénaty D, Martinerie L, Paulsen A, Simon D, et al. Early determinants of thyroid function outcomes in children with congenital hypothyroidism and a normally located thyroid gland: A regional cohort study. Thyroid 2018;28: 959–67. doi: 10.1089/thy.2018.0154 [DOI] [PubMed] [Google Scholar]
- 13.Miki K, Nose O, Miyai K, Yabuuchi H, Harada T. Transient infantile hyperthyrotrophinaemia. Arch Dis Child 1989;64: 1177–82. doi: 10.1136/adc.64.8.1177 [DOI] [PMC free article] [PubMed] [Google Scholar]