Abstract.
Children born small for gestational age (SGA) face an increased risk of health problems in later life, particularly persistent short stature, neurocognitive dysfunction, impaired renal and pulmonary function, decreased bone density, sensorineural hearing loss, premature adrenarche, and metabolic syndrome. Insulin resistance appears to be a key component underlying these metabolic complications. Long-term, continuous, GH treatments in short children born SGA lead to a normalization of height through childhood to adulthood. Recombinant human GH has been proven to be relatively safe. We recommend early surveillance in a growth clinic for children born SGA without catch-up growth. Obesity, insulin resistance, and the risk of metabolic syndrome increase with catch-up growth, but short stature and cognitive dysfunction increase without catch-up growth in children born SGA. A solution to this catch-up dilemma is breast feeding for a minimum of 6 to 12 mo. Because the overall prevalence of metabolic risk factors is very low, routine evaluation of metabolic parameters is not recommended for all children born SGA, but it may be useful to consider metabolic evaluations in overweight or obese children born SGA. Since children born SGA have many risk factors, long-term management from neonate to adulthood is very important.
Keywords: small for gestational age, cognition, growth, metabolic syndrome
Introduction
In Korea, 715,826 children were born in 1993, and the number of births dropped to 438,531 in 2005, similar to levels in 2013 to 2015, with only 438,420 babies born in 2015. The percentage of children with a birth weight of < 2,400 g was 2.6% in 1993 and 5.7% in 2015. According to these data from Korea’s National Statistical Office, the number of children with low birth weight has clearly been increasing (1).
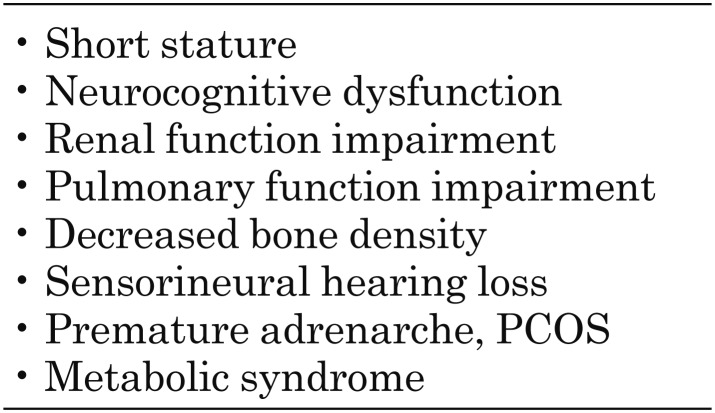
The classification of small for gestational age (SGA) has been variably defined as being at the 3rd or 10th percentile or at less than –2 standard deviation (SD) from the mean in relation to gestational age for the same sex (2). Infants born SGA are at an increased risk of persistent short stature, neurocognitive dysfunction, impaired renal and pulmonary function, decreased bone density, sensorineural hearing loss, premature adrenarche, and metabolic syndrome in later life (3) (Table 1).
Table 1. Problems associated with small for gestational age.
In this report, we reviewed mainly issues concerning neurocognitive dysfunctions, short statures, and metabolic syndromes.
Neurocognitive Dysfunction
Crude neurological handicaps, such as cerebral palsy, are extremely rare in children born SGA at term. Some studies have shown that term SGA infants are at an increased risk for mild cognitive deficits, learning difficulties, and poorer performance in childhood and adolescence (4,5,6). Studies have also demonstrated that these children have problems related to behavior and mood control in addition to psychological problems such as attention deficit hyperactive disorder (ADHD) (6). Hwang et al. reported marked behavioral problems such as delinquency, aggressiveness, anxiety, and depression as well as lower verbal Intelligence Quotient in the SGA group than in the appropriate for gestational age (AGA) group (7).
Currently, neonatologists recommend nutrient-enriched formula for preterm as well as SGA babies. However, nutrient-enriched formula has been shown to improve head growth, but not neurodevelopment in SGA infants (8), and increases the risk of rapid weight gain and eventually obesity. There is much ongoing discussion related to the issue of undernutrition vs. overnutrition. De Curtis and Rigo have discussed this problem and the various potential harmful effects in SGA children. There is controversy between the risks of rapid weight gain and reduced neurodevelopment due to undernutrition (9). In SGA babies, the quality of the food during months 6 to 12 of life appears to be very important, with breast fedding showing the best results compared to high-energy formula.
Short Stature
Accelerated growth begins between 2 wk to 3 mo after birth. Average weight and length increase from the 10th to the 25th percentile at 6 mo. Most children born premature and SGA have a slower and more prolonged catch-up growth period than term SGA children, and it can take 4 or more yr for premature children to fully catch up (10).
Birth length is the most important predictor for catch-up growth, but is not associated with gestational age, multiple births, or sex (11,12,13). Approximately 80 to 85% of children born SGA experience a rapid catch-up growth period during the first 12 months of life, while 10 to 15% do not undergo this period of catch-up growth (11, 12, 14). The mechanisms that lead to the persistence of short stature in children born SGA are not yet well understood.
Among children who are born SGA without attaining the catch-up growth by 2 yr of age, the relative risk of short stature at 18 yr of age is 5.2% for those born lighter than normal range and 7.1% for those born shorter than normal range (15).
Short children born SGA usually do not have a classic GH deficiency, but have been found to exhibit either low GH secretion or reduced sensitivity to GH (16). Approximately 50 to 60% of short children born SGA have either 24-h GH profile abnormalities or subnormal responses to arginine provocation with reduced plasma insulin-like growth factor (IGF)-I and IGF-II levels, which indicate GH insufficiency (16,17,18,19).
A wide ranges of IGF-I generation and resistance indicate that the SGA group is heterogeneous. GH stimulation testing is not required to identify candidates for GH therapy among children born SGA who fail to achieve catch-up growth (20), and GH stimulation test results do not accurately predict response to GH therapies (21). GH stimulation testing is recommended when GH deficiency (GHD) is suspected in a child born SGA. Children born SGA have abnormal bone maturation rhythms throughout childhood. In untreated, short children born SGA, bone age is typically delayed compared to chronological age. In short, prepubertal children born SGA, a spontaneous acceleration in bone maturation and a decrease in height SDS for bone age occurs from 6 to 8 yr of age. Predictions of final height based on bone age estimates have been shown to be unreliable in children born SGA who then fail to show catch-up growth (3, 22). For these reasons, bone age is not a consideration when initiating GH treatment in SGA children.
GH Treatment
Objectives of GH therapy are initiated to induce catch-up growth, to normalize height during childhood, and to achieve an adult height within the normal target range.
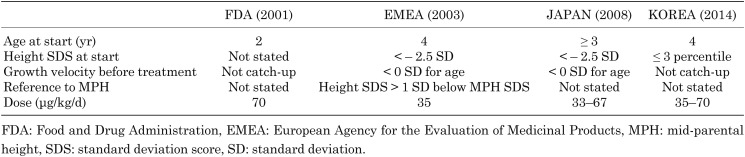
GH was approved by the Food and Drug Administration (FDA) in 2001, by the European Agency for the Evaluation of Medicinal Products (EMEA) in 2003, by Japan in 2008, and by Korea in 2014. The approved minimum age for treatment is 2 yr according to the FDA, 4 yr according to the EMEA, 3 yr according to Japan, and 4 yr according to Korea. While the FDA approved the GH dosage at 70 μg/kg/d, others have approved dosages of 33–35 μg/kg/d (Table 2).
Table 2. GH use in small for gestational age (SGA) children without catch-up growth.
The average height gain after 3 yr of GH treatment ranges from 1.2 to 2.0 SD for dosages of 35–70 μg/kg/d. The growth rate decreases over time in GH therapy (3). The annual height velocity was significantly greater among children receiving GH than among untreated children during the first and second years of treatment.
Untreated SGA children did not experience an increase in height velocity SDS (23). A multicenter, phase III clinical study in Korea reported that height velocity significantly increased from 5.36 ± 1.59 cm/yr at baseline to 10.66 ± 2.03 cm/yr at 6 mo in the treated group (24).
Van Pareren et al. (25) reported that 98% of children born SGA reached an adult height within the target height range after long-term GH treatment, and a low dose proved to be as effective as a high dose for the final height. A high dose of 100 μg/kg/d produced a dramatic and rapid increase in height SDS over 2 yr of treatment, but after discontinuation of GH treatment, height velocity decreased significantly, with a subsequent decrease in height SDS. In contrast, height SDS showed a sustained improvement in patients treated continuously with a GH dosage of 33 μg/kg/d over 6 yr (26).
Factors associated with GH responses over the first 2 to 3 yr of therapy included age and height SDS at the start of therapy, mid-parental heights, and GH dosage (21).
Ranke et al. (27) reported that the GH dose was the most important response predictor accounting for 35% of the growth response variability. Other predictors of growth response during the first year of treatment were the age at the start of treatment, weight SDS, and mid-parental height SDS.
According to the North European Small-for-Gestational-Age Study (NESGAS), IGF-I titration of GH doses in SGA children proved less effective than current dosing strategies. During the 3 yr of GH treatment, IGF-I titration resulted in physiologic IGF-I levels correlating with a wide range of GH doses and poorer GH responses. These results indicated the role of IGF-I resistance and highlighted the heterogeneity in short SGA children (28). The optimal GH dose in short children born SGA is currently under debate. To maximize the effects of GH treatments, individual GH dose adjustments are needed in short children born SGA.
GH treatments in short SGA children also had a positive effect on blood pressure and lipid metabolism in 79 SGA patients (29). Pre-treatment systolic blood pressure SDS was significantly higher and diastolic blood pressure was significantly lower in children born SGA than in healthy age-matched children. During GH therapy, both systolic and diastolic blood pressure SDS decreased significantly. After 6 yr of GH therapy, systolic blood pressure in the SGA children did not differ from that in the controls and diastolic blood pressure was even lower than that in the controls. After 4 yr of GH therapy, there were significant decreases in total cholesterol, low-density lipoprotein cholesterol, and the atherogenic index, although high-density lipoprotein cholesterol remained unchanged.
Sas et al. (30) also reported a positive effect of GH therapy on body proportions in 79 SGA children. Height, sitting height, and hand, feet, bi-acromial, and bi-iliac diameters were measured before and during GH therapy. At the start of GH therapy, mean SDS values for all measurements were significantly lower than zero, but during 6 yr of GH therapy, the mean SDS for all measurements increased significantly from baseline and closer to zero. There were significant improvements from baseline in the proportions of the hands, feet, and bi-iliac diameters in relation to height, but the bi-acromial diameters in relation to height did not change. None of the changes in body proportions differed significantly between the dosage groups.
GH treatment also had long-term beneficial effects on muscle and adipose tissue in short children born SGA. Leger et al. (31), studied long-term effects on muscle and adipose tissue in 14 short children born SGA during 3 yr of GH therapy and observed an increase in muscle tissue mass. Adipose tissue mass decreased significantly during the first year of therapy, but increased during the second and third years of therapy.
Fasting serum HbA1c or glucose levels should be monitored during GH treatment. This is especially important in children who are overweight or have a family history of type 2 diabetes (31).
IGF-I levels should not exceed 2 SDS above the mean for age and gender during treatments. If IGF-I levels are excessive (> 2 or even 3 SD), it seems reasonable to adjust the GH dose downwards until IGF-I levels are in the upper-normal range.
Recombinant human GH has been proven to be relatively safe. Treatment interruption or withdrawal as a result of adverse events is uncommon. The effect of GH on glucose metabolism in short children born SGA was generally mild and transient (32). Since insulin resistance had been reported in SGA children, fasting insulin and glycosylated hemoglobin or glucose concentrations should be monitored during and after GH treatments in these patients, particularly those with risk factors for obesity or a family history of type 2 diabetes. The French study, Sante Adulte GH Enfant, reported increased mortality rates among adults treated with recombinant GH as children, particularly in those who had received the highest dose (above 50 μg/kg/d). Specific effects in terms of death were detected because of bone tumors or cerebral hemorrhages, but not for all cancers (33).
Metabolic Syndrome
Metabolic syndrome comprises central obesity, dyslipidemia, hypertension, glucose intolerance, and insulin resistance. Insulin resistance appears to be a key component underlying these metabolic complications. Insulin resistance has been observed in babies born SGA who achieved catch-up growth at 1 yr of age (34) and until the age of 2 (35). Insulin resistance is sharply amplified by obesity and in prepubertal children, which is more evident in those with catch-up growth and rapid weight gain and BMI >17 kg/m2 (36). At 50 yr of age, the risk of insulin resistance syndrome was 10-fold higher in subjects with birth weight < 2,500 g than in subjects with birth weight ≥ 4,500 kg (37). At 22 yr of age, 2.3% of individuals born SGA were affected by metabolic syndrome compared with only 0.3% in the AGA group (33). However, Cho et al. reported that birth weight is related to current height and weight SDS in Korean adolescents, but is not related to individual components of metabolic syndrome (38). Meta-regression showed a U-shaped relationship between birth weight and later risk of type 2 diabetes (39). Birth weight and risk of coronary heart disease and stroke are inversely associated in adult women (40). Because the overall prevalence of metabolic risk factors is very low, routine evaluation of metabolic parameters is not justified in all children born SGA. This may be useful to consider, however, in overweight or obese SGA children.
In conclusion, SGA neonates require long-term management because short statures, neurocognitive dysfunctions, and metabolic syndromes persist until adulthood. Breastfeeding is recommended in SGA neonates for 6–12 mo after birth. It is important that short children born SGA who may benefit from GH treatments be referred to a pediatric endocrinologist as early as possible. To maximize the effects of GH, individual GH dose adjustments are necessary in short SGA children.
Understanding SGA heterogeneity may be the next step in further improving GH treatments.
Conflict of Interest
No potential conflict of interest relevant to this article was reported.
References
- 1.Korea Statistical Information Service [Internet]. Daejeon: Korea Statistical Information Service; [cited 2013 Jan 8]. Available from: http//www.kosis.kr.
- 2.Wollmann HA. Intrauterine growth restriction: definition and etiology. Horm Res 1998;49(Suppl 2): 1–6. doi: 10.1159/000053079 [DOI] [PubMed] [Google Scholar]
- 3.Clayton PE, Cianfarani S, Czernichow P, Johannsson G, Rapaport R, Rogol A. Management of the child born small for gestational age through to adulthood: a consensus statement of the International Societies of Pediatric Endocrinology and the Growth Hormone Research Society. J Clin Endocrinol Metab 2007;92: 804–10. doi: 10.1210/jc.2006-2017 [DOI] [PubMed] [Google Scholar]
- 4.Sommerfelt K, Andersson HW, Sonnander K, Ahlsten G, Ellertsen B, Markestad T, et al. Cognitive development of term small for gestational age children at five years of age. Arch Dis Child 2000;83: 25–30. doi: 10.1136/adc.83.1.25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hollo O, Rautava P, Korhonen T, Helenius H, Kero P, Sillanpää M. Academic achievement of small-for-gestational-age children at age 10 years. Arch Pediatr Adolesc Med 2002;156: 179–87. doi: 10.1001/archpedi.156.2.179 [DOI] [PubMed] [Google Scholar]
- 6.O’Keeffe MJ, O’Callaghan M, Williams GM, Najman JM, Bor W. Learning, cognitive, and attentional problems in adolescents born small for gestational age. Pediatrics 2003;112: 301–7. doi: 10.1542/peds.112.2.301 [DOI] [PubMed] [Google Scholar]
- 7.Yi KH, Yi YY, Hwang IT. Behavioral and intelligence outcome in 8- to 16-year-old born small for gestational age. Korean J Pediatr 2016;59: 414–20. doi: 10.3345/kjp.2016.59.10.414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Morley R, Fewtrell MS, Abbott RA, Stephenson T, MacFadyen U, Lucas A. Neurodevelopment in children born small for gestational age: a randomized trial of nutrient-enriched versus standard formula and comparison with a reference breastfed group. Pediatrics 2004;113: 515–21. doi: 10.1542/peds.113.3.515 [DOI] [PubMed] [Google Scholar]
- 9.De Curtis M, Rigo J. Extrauterine growth restriction in very-low-birthweight infants. Acta Paediatr 2004;93: 1563–8. doi: 10.1111/j.1651-2227.2004.tb00844.x [DOI] [PubMed] [Google Scholar]
- 10.Gibson AT, Carney S, Cavazzoni E, Wales JK. Neonatal and post-natal growth. Horm Res 2000;53(Suppl 1): 42–9. [DOI] [PubMed] [Google Scholar]
- 11.Karlberg J, Albertsson-Wikland K. Growth in full-term small-for-gestational-age infants: from birth to final height. Pediatr Res 1995;38: 733–9. doi: 10.1203/00006450-199511000-00017 [DOI] [PubMed] [Google Scholar]
- 12.Tuvemo T, Cnattingius S, Jonsson B. Prediction of male adult stature using anthropometric data at birth: a nationwide population-based study. Pediatr Res 1999;46: 491–5. doi: 10.1203/00006450-199911000-00001 [DOI] [PubMed] [Google Scholar]
- 13.Leger J, Levy-Marchal C, Bloch J, Pinet A, Chevenne D, Porquet D, et al. Reduced final height and indications for insulin resistance in 20 year olds born small for gestational age: regional cohort study. BMJ 1997;315: 341–7. doi: 10.1136/bmj.315.7104.341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hokken-Koelega AC, De Ridder MA, Lemmen RJ, Den Hartog H, De Muinck Keizer-Schrama SM, Drop SL. Children born small for gestational age: do they catch up? Pediatr Res 1995;38: 267–71. doi: 10.1203/00006450-199508000-00022 [DOI] [PubMed] [Google Scholar]
- 15.Albertsson-Wikland K, Karlberg J. Postnatal growth of children born small for gestational age. Acta Paediatr Suppl 1997;423: 193–5. doi: 10.1111/j.1651-2227.1997.tb18413.x [DOI] [PubMed] [Google Scholar]
- 16.de Waal WJ, Hokken-Koelega AC, Stijnen T, de Muinck Keizer-Schrama SM, Drop SL, The Dutch Working Group on Growth Hormone. Endogenous and stimulated GH secretion, urinary GH excretion, and plasma IGF-I and IGF-II levels in prepubertal children with short stature after intrauterine growth retardation. Clin Endocrinol (Oxf) 1994;41: 621–30. doi: 10.1111/j.1365-2265.1994.tb01828.x [DOI] [PubMed] [Google Scholar]
- 17.Albertsson-Wikland K. Swedish Paediatric Study Group for Growth Hormone Treatment. Growth hormone secretion and growth hormone treatment in children with intrauterine growth retardation. Acta Paediatr Scand Suppl 1989;349: 35–41, discussion 53–4. doi: 10.1111/j.1651-2227.1989.tb17166.x [DOI] [PubMed] [Google Scholar]
- 18.Stanhope R, Ackland F, Hamill G, Clayton J, Jones J, Preece MA. Physiological growth hormone secretion and response to growth hormone treatment in children with short stature and intrauterine growth retardation. Acta Paediatr Scand Suppl 1989;349: 47–52, discussion 53–4. doi: 10.1111/j.1651-2227.1989.tb17168.x [DOI] [PubMed] [Google Scholar]
- 19.Boguszewski M, Rosberg S, Albertsson-Wikland K. Spontaneous 24-hour growth hormone profiles in prepubertal small for gestational age children. J Clin Endocrinol Metab 1995;80: 2599–606. [DOI] [PubMed] [Google Scholar]
- 20.Azcona C, Albanese A, Bareille P, Stanhope R. Growth hormone treatment in growth hormone-sufficient and -insufficient children with intrauterine growth retardation/Russell-Silver syndrome. Horm Res 1998;50: 22–7. [DOI] [PubMed] [Google Scholar]
- 21.Sas T, de Waal W, Mulder P, Houdijk M, Jansen M, Reeser M, et al. Growth hormone treatment in children with short stature born small for gestational age: 5-year results of a randomized, double-blind, dose-response trial. J Clin Endocrinol Metab 1999;84: 3064–70. [DOI] [PubMed] [Google Scholar]
- 22.Job JC, Rolland A. Natural history of intrauterine growth retardation: pubertal growth and adult height. Arch Fr Pediatr 1986;43: 301–6 (in French). [PubMed] [Google Scholar]
- 23.Butenandt O, Lang G, German Study Group. Recombinant human growth hormone in short children born small for gestational age. J Pediatr Endocrinol Metab 1997;10: 275–82. [PubMed] [Google Scholar]
- 24.Lee KH, Lee BC, Ko CW, Jin DK, Yang SW, Yoo HW, et al. A single-arm, phase iii study to assess efficacy and safety after 6-month-treatment of EutropinTM Inj. (Recombinant human growth hormone) in prepubertal children with short stature due to small for gestational age. J Korean Soc Pediatr Endocrinol 2011;16: 157–64. doi: 10.6065/jkspe.2011.16.3.157 [DOI] [Google Scholar]
- 25.Van Pareren Y, Mulder P, Houdijk M, Jansen M, Reeser M, Hokken-Koelega A. Adult height after long-term, continuous growth hormone (GH) treatment in short children born small for gestational age: results of a randomized, double-blind, dose-response GH trial. J Clin Endocrinol Metab 2003;88: 3584–90. doi: 10.1210/jc.2002-021172 [DOI] [PubMed] [Google Scholar]
- 26.de Zegher F, Albertsson-Wikland K, Wollmann HA, Chatelain P, Chaussain JL, Löfström A, et al. Growth hormone treatment of short children born small for gestational age: growth responses with continuous and discontinuous regimens over 6 years. J Clin Endocrinol Metab 2000;85: 2816–21. [DOI] [PubMed] [Google Scholar]
- 27.Ranke MB, Lindberg A, Cowell CT, Wikland KA, Reiter EO, Wilton P, et al. KIGS International Board. Prediction of response to growth hormone treatment in short children born small for gestational age: analysis of data from KIGS (Pharmacia International Growth Database). J Clin Endocrinol Metab 2003;88: 125–31. doi: 10.1210/jc.2002-020867 [DOI] [PubMed] [Google Scholar]
- 28.Jensen RB, Thankamony A, O’Connell SM, Kirk J, Donaldson M, Ivarsson SA, et al. A randomised controlled trial evaluating IGF1 titration in contrast to current GH dosing strategies in children born small for gestational age: the North European Small-for-Gestational-Age Study. Eur J Endocrinol 2014;171: 509–18. doi: 10.1530/EJE-14-0419 [DOI] [PubMed] [Google Scholar]
- 29.Sas T, Mulder P, Hokken-Koelega A. Body composition, blood pressure, and lipid metabolism before and during long-term growth hormone (GH) treatment in children with short stature born small for gestational age either with or without GH deficiency. J Clin Endocrinol Metab 2000;85: 3786–92. [DOI] [PubMed] [Google Scholar]
- 30.Sas TC, Gerver WJ, Mulder PG, Cole TJ, Hokken-Koelega AC, De Waal W, et al. De Bruin RDe Waal W. Body proportions during 6 years of GH treatment in children with short stature born small for gestational age participating in a randomised, double-blind, dose-response trial. Clin Endocrinol (Oxf) 2000;53: 675–81. doi: 10.1046/j.1365-2265.2000.01155.x [DOI] [PubMed] [Google Scholar]
- 31.Leger J, Garel C, Fjellestad-Paulsen A, Hassan M, Czernichow P. Human growth hormone treatment of short-stature children born small for gestational age: effect on muscle and adipose tissue mass during a 3-year treatment period and after 1 year’s withdrawal. J Clin Endocrinol Metab 1998;83: 3512–6. [DOI] [PubMed] [Google Scholar]
- 32.Grumbach MM, Bin-Abbas BS, Kaplan SL. The growth hormone cascade: progress and long-term results of growth hormone treatment in growth hormone deficiency. Horm Res 1998;49(suppl 2): 41–57. doi: 10.1159/000053087 [DOI] [PubMed] [Google Scholar]
- 33.Carel JC, Ecosse E, Landier F, Meguellati-Hakkas D, Kaguelidou F, Rey G, et al. Long-term mortality after recombinant growth hormone treatment for isolated growth hormone deficiency or childhood short stature: preliminary report of the French SAGhE study. J Clin Endocrinol Metab 2012;97: 416–25. doi: 10.1210/jc.2011-1995 [DOI] [PubMed] [Google Scholar]
- 34.Soto N, Bazaes RA, Peña V, Salazar T, Avila A, Iñiguez G, et al. Insulin sensitivity and secretion are related to catch-up growth in small-for-gestational-age infants at age 1 year: results from a prospective cohort. J Clin Endocrinol Metab 2003;88: 3645–50. doi: 10.1210/jc.2002-030031 [DOI] [PubMed] [Google Scholar]
- 35.Lévy-Marchal C, Czernichow P. Small for gestational age and the metabolic syndrome: which mechanism is suggested by epidemiological and clinical studies? Horm Res 2006;65(Suppl 3): 123–30. [DOI] [PubMed] [Google Scholar]
- 36.Veening MA, Van Weissenbruch MM, Delemarre-Van De Waal HA. Glucose tolerance, insulin sensitivity, and insulin secretion in children born small for gestational age. J Clin Endocrinol Metab 2002;87: 4657–61. doi: 10.1210/jc.2001-011940 [DOI] [PubMed] [Google Scholar]
- 37.Barker DJ, Hales CN, Fall CH, Osmond C, Phipps K, Clark PM. Type 2 (non-insulin-dependent) diabetes mellitus, hypertension and hyperlipidaemia (syndrome X): relation to reduced fetal growth. Diabetologia 1993;36: 62–7. doi: 10.1007/BF00399095 [DOI] [PubMed] [Google Scholar]
- 38.Cho WK, Jung IA, Suh BK. Current growth status and metabolic parameters of Korean adolescents born small for gestational age: results from the Korea National Health and Nutrition Examination Surveys (KNHANES) 2010-2011. Pediatr Int 2014;56: 344–8. doi: 10.1111/ped.12278 [DOI] [PubMed] [Google Scholar]
- 39.Rich-Edwards JW, Colditz GA, Stampfer MJ, Willett WC, Gillman MW, Hennekens CH, et al. Birthweight and the risk for type 2 diabetes mellitus in adult women. Ann Intern Med 1999;130: 278–84. doi: 10.7326/0003-4819-130-4_Part_1-199902160-00005 [DOI] [PubMed] [Google Scholar]
- 40.Rich-Edwards JW, Stampfer MJ, Manson JE, Rosner B, Hankinson SE, Colditz GA, et al. Birth weight and risk of cardiovascular disease in a cohort of women followed up since 1976. BMJ 1997;315: 396–400. doi: 10.1136/bmj.315.7105.396 [DOI] [PMC free article] [PubMed] [Google Scholar]