Abstract
Recent research has fueled a debate concerning the role of nicotine in the emergence of schizophrenia. The three main hypotheses are: (a) the self-medication effect, (b) the causal relationship hypothesis, or (c) the shared diathesis hypothesis. To explore this role, the study of nicotine consumption during the initial prodromal phase of schizophrenia offers important opportunities. In the present work, 10 relevant studies are reviewed, out of 727 retrieved citations, in order to address questions regarding the prevalence of smoking in the prodromal period, the time of smoking initiation, existing patterns of tobacco use in relation with the escalation of prodromal symptoms into first psychotic episode, and potential differences in symptomatology between smokers and nonsmokers. Even though there was considerable heterogeneity among studies, relevant findings are discussed. Prevalence of nicotine use during the prodromal period was reported to be 16.6–46%. Tobacco use was found to be taken up most often before or during the prodromal period of schizophrenia. Even though a protective role of smoking has been reported by one study, other studies report an increased risk for psychosis, with hazard ratios 2.77 (95% CI: 2.34–3.43) and 2.21 (95% CI: 1.11–4.42) for female and male heavy smokers (11–20 and >20 cigarettes/day), respectively. In a different study, the risk of onset was associated with the progressive use of cannabis and tobacco prior to onset, particularly with rapid escalation to the highest levels of use. Also, nicotine use in ultra high risk (UHR) for developing psychosis subjects is associated with elevated cognitive performance, namely better processing speed, visual learning, and spatial working memory. As a conclusion, it appears that evidence accumulates supporting a possible etiologic role of smoking, in the emergence of schizophrenia along with diverse effects on patients’ symptomatology, already demonstrable at the prodromal phase. Future research employing better-defined criteria should further explore the patterns of use and effects of nicotine during the schizophrenia prodrome.
Keywords: tobacco, smoking, psychosis, ultra high risk, conversion, clinical high risk
Introduction
The nicotine consumption rate is high in the mentally ill and seems to remain high over the last years, in contrast to the decline of smoking prevalence observed in the general population.1–3 A recent study has found that prevalence of smoking in adults with any mental disorder was 36.1%, compared to 21.4% in individuals without any mental disorder,4 but rose significantly (even four to five times higher than the healthy population) in severe or psychotic disorders such as schizophrenia, bipolar disorder, schizoaffective disorder, Posttraumatic Stress Disorder, and substance use disorder.5–7 Particularly in schizophrenia, well over 60% of the patients have been found to be smokers according to previous and more recent studies,6,8 reporting, for example, a current smoking prevalence of 64% in patients with schizophrenia versus 19% in mentally healthy controls in routine clinical settings.8 A study that included a large multi-ethnic sample of schizophrenic patients showed that 72% of them smoke daily, versus 29% of the controls.6 Moreover, individuals with schizophrenia consume more tobacco and extract higher amounts of nicotine from their cigarettes, as compared to smokers in the general population.9
Three main hypotheses have been employed as an explanatory basis for this comorbidity, which need not be mutually exclusive: (a) the self-medication hypothesis, which considers that patients use tobacco to gain relief from symptoms of the disease or from treatment-related side effects, (b) the shared diathesis hypothesis, where there is an assumed shared genetic basis between smoking and schizophrenia, and (c) the third hypothesis that includes a causal relationship between the two, according to which tobacco is viewed as a putative causal risk factor for the illness.10 Concerning the self-medication hypothesis, the anxiolytic effect of nicotine is well known, and recent data provide evidence that the inhibition of β2* nicotinic acetylcholinergic receptors (nAChRs) may relieve anxiety.11–13 Tobacco smoking has been reported to normalize prepulse inhibition (PPI) in NMDA receptor antagonist models of psychosis.14,15 In addition, nAchR dysfunction is associated with sensory processing deficits in schizophrenia, like a p50 response or prepulse inhibition of the startle response, and evidence also suggests that the α7 nAchR function contributes to the positive effect of nicotine in cognitive deficits. The α7 and β2 nAchR subunits are involved in the cognitive, affective, and negative symptoms of the illness, which show less response to antipsychotic treatment,9,16 and can be alleviated by nicotine consumption.9 Besides affecting symptoms of the illness, smoking has been reported to decrease extrapyramidal side effects either directly or by reducing antipsychotic drug levels.17–19
On the other hand, recent findings from GWAS studies have come to suggest a more complex role for tobacco use than that of solely self-medicating symptoms or adverse effects, such as a condition with some common genetic basis, or possibly one that stands for a causal factor somewhere in the pathophysiological process underlying the illness.10,20 The link between altered brain function and dimensions of schizophrenic symptoms, namely positive (hallucinations, delusions, and thought impairments), negative (diminished emotional expression, loss of motivation, and psychosocial impairments),21 or cognitive (disorganized thought, attention, and working memory deficits), has been approached by the neurotransmitter hypotheses, including dopamine and glutamate neurotransmission,22,23 alterations in the serotonergic system,24 and dysfunction of the GABAergic neurons.25 The role of the cholinergic system, that includes nicotinic and muscarinic neurotransmission, presents considerable interest. It has been reported that schizophrenic patients have reduced alpha-4 and alpha-7 nicotinic receptor brain expression in various cerebral areas.26 The fact that the cholinergic system interacts with the glutamatergic, GABAergic, and the other projection systems strengthens the hypothesis that it may play a key role in the pathophysiology of schizophrenia,27 with implications for the role of tobacco use, which interferes with these receptors.
The relation between cigarette smoking and the initial prodromal period of schizophrenia is less studied. The schizophrenia prodrome is defined as the period preceding the onset of active psychosis, characterized by the presence of heterogeneous and non-specific symptoms and types of behavior, that mark a departure from the premorbid, baseline state, and the beginning of the illness, when frank psychosis has not manifested yet.28,29 Its duration varies from some weeks to several years.30 The prodromal period can importantly be an opportunity for early intervention31 and also can offer insights into the pathophysiology of the disease.32 For the purpose of identification and intervention, the Ultra High Risk (UHR)33,34 and the Basic Symptom (BS) concept criteria35 are endorsed, which are complementary to each other and are used in recently released guidelines for early detection.31 Still, constellations of non-specific symptoms that have been described include impairment in concentration, depressive mood, sleep disturbance, anxiety, irritability/anger, suspiciousness, quarreling, aggressiveness, hyperacousia, restlessness, suicidal ideas, mood swings, preoccupation, somatic/cenesthetic symptoms, lack of appetite, and compulsive behavior.29 During the prodromal period of schizophrenia, core psychotic symptoms are present in attenuated form, as thought, speech and perception disorders,36 and comorbid diagnoses like major depression and cannabis abuse are frequently met.37 At the biological level, the prodromal period corresponds to gray matter thinning in the prefrontal cortex, parahippocampal gyrus, superior temporal gyrus, which is steeper in at-risk individuals who eventually convert to psychosis compared with non-converters. This process possibly involves inflammation, microglial activation, and deficits in NMDA-dependent synaptic plasticity.32 White matter loss and disruption of myelination may also take place, completing the conceptualization of schizophrenia as a brain dysconnectivity disorder.38 It is possible that smoking could interfere with increasing dopamine synthesis and release in the striatum, as a downstream effect of NMDA hypofunction, developing during the prodromal phase.32 Smoking has been shown to inhibit expression of genes related to myelin synthesis and maintenance in mice,39 and heavy smokers have been shown to have abnormal integrity of white matter in anterior corpus callosum;40 the impact of smoking on myelin integrity, neuroplasticity, and subsequent brain dysconnectivity in susceptible, at-risk individuals remains to be explored. Still, data about nicotine consumption patterns and effects during the prodromal phase of schizophrenia appear to be insufficient.
According to Kelly and McCreadie, the onset of smoking occurs before the onset of schizophrenia, defined as the first contact with services, by 11 years on average.41 Beratis et al found that in 86% of the patients smoking initiation preceded the disease onset and defined the time at which the first prodromal symptoms of the illness appeared.42 Sandyk and Kay found that patients with schizophrenia who consumed tobacco had a significantly earlier age of onset of the illness,43 but others have not confirmed this difference.42 Initiation of cigarette smoking may coincide with the prodromal period of the disorder and the prevalence of smoking in future schizophrenia patients was higher, according to Weiser et al.44 More recent data have shown that daily tobacco use is associated with decreased risk45,46 or increased risk of developing psychosis,47 as well as earlier onset of psychosis compared to nonsmokers.48 Nicotine dependence is related with more severe symptoms and poorer outcome in the schizophrenic population.9
In short, the complexity of the relationship between tobacco consumption and schizophrenia appears to be worthy of investigation, but the reasons why people with psychosis are keener to smoke than the general population remain unclear.48,49 The association between smoking and the prodromal period seems even less studied, even though the investigation of this relation could shed some light on pathophysiologic mechanisms of the disease. In view of this, a review of the existing literature addressing this issue appears to be warranted. This present study attempts to address some of the questions regarding this matter and can be summarized as follows:
When does the initiation of smoking occur, in relation to the onset of prodromal symptoms?
What is the prevalence of tobacco smoking in the prodromal period?
Are there patterns of tobacco use somehow related to the escalation of prodromal symptoms leading to active psychosis, and does smoking have any influence on transition rates or age of onset for psychosis?
Are there any differences between smokers and nonsmokers regarding clinical symptoms, cognition or neurobiological correlates?
Methods
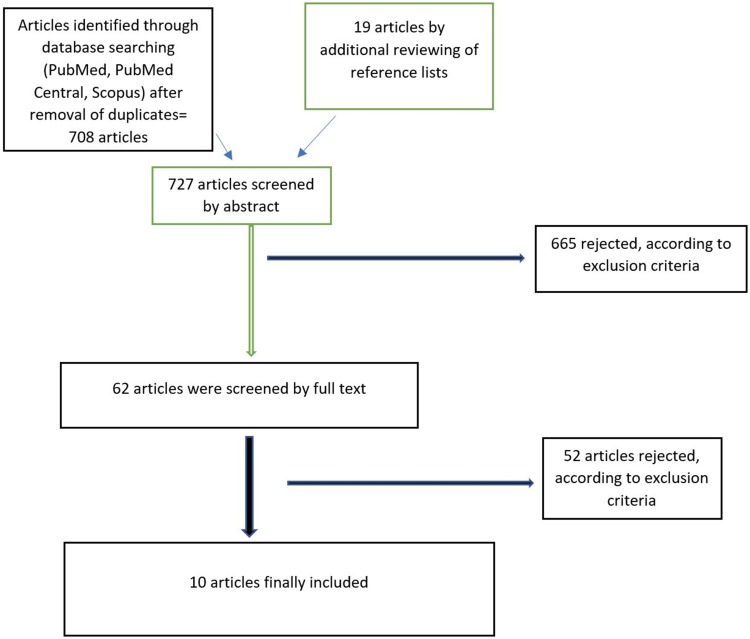
The electronic databases PubMed, PubMed Central, and Scopus were used to search for original studies relevant to nicotine use during the initial prodromal period of schizophrenia (last date accessed was the 31st of March 2018). We used the search terms “schizophrenia” or “psychosis” AND “prodromal” AND “nicotine” OR “smoking” OR “tobacco”. We also searched for additional articles in the reference lists of the retrieved publications. A flow diagram of the screening process can be seen in Figure 1.
Figure 1.
Flow diagram of the screening procedure.
The selection process was carried out by two investigators separately, AG and MS. In case there was disagreement on whether to include or exclude a study, a decision was made after discussion. Inclusion criteria that had to be met were: (a) the use of ICD or DSM criteria for the diagnosis of schizophrenia, excluding studies where recruited subjects were prospectively diagnosed with prodromal symptoms, and therefore had not yet converted to a diagnosable psychotic disorder, (b) reports of any data regarding the initial prodromal period of schizophrenia and nicotine consumption, irrespective of definition criteria for the prodrome or for smoking status, (c) the discrimination between nicotine use and use of other substances, and (d) peer-reviewed English language papers. Exclusion criteria were (a) not referring to schizophrenia, focusing on organic or substance-induced psychoses, (b) not distinguishing or controlling for the use of other substances, (c) not referring to the prodromal period, and (d) language other than English or not peer-reviewed papers, for example, conference abstracts. Data collected were the design and setting of the studies, sample sizes, definitions of the prodromal status, definition of smoking status, criteria for smoking heaviness or dependence, and main results of the study relevant to the prodrome of the disease and smoking. Gathered data were used for the narrative/qualitative synthesis of the results.
Results
Ten full-text articles have been reviewed, in which a report on the probable relationship between smoking and prodromal period was noted. Study characteristics, sample sizes, and main findings of reviewed articles regarding smoking and the prodromal period of psychosis are demonstrated in Table 1. There was considerable heterogeneity of studies, regarding sample sizes, design (longitudinal or cross-sectional) criteria employed for definition of prodromal period, and methods used to assess nicotine use.
Table 1.
Study characteristics, sample sizes, and main findings of the reviewed articles regarding smoking and the prodromal period of psychosis
| Study | Design | Setting | Sample size | Definition of diagnosis | Definition of the prodromal status | Definition of psychosis or illness onset | Assessment of tobacco use | Substance use other than Tobacco | Main findings relevant to the prodrome |
|---|---|---|---|---|---|---|---|---|---|
| Riala et al (2005)50 | Longitudinal cohort study | Northern Finland 1966 birth cohort | 11,017; finally included 10,934 (5589 men, 5345 women) | DSM-III-R | Unspecified | Age of first psychotic symptoms as recorded in case notes for psychoses; age of first admission to a mental hospital for disorders with no psychotic symptoms | Questionnaire mailed to cohort members; lifetime smoking/currently smoking/number of cigarettes smoked daily/age of onset of regular smoking; dichotomous variable, smokers/nonsmokers | Screening for (hospital-treated) substance use disorders by DSM-III-R criteria; Assessment of alcohol use by questionnaire; logistic regression model for alcohol use | Initiation of smoking precedes onset of schizophrenia by 2.3 (SD=6.60) years; there was not any statistically significant difference in the age of onset of the illness between smokers and nonsmokers |
| Diaz et al (2008)52 | Cross-sectional, Case control | Patients with Severe Mental Illness (SMI) from Central Kentucky, collected during a pharmacogenetics investigation | 805 [258 patients with schizophrenia (57% males), 166 with a mood disorder (42% males), 381 healthy controls (24% males) | DSM-IV | Unspecified; Presumably one or two years based on literature | Age at first starting psychiatric medication | Recording of smoking history (current daily smoking/ever daily smoking); Heaviness of Smoking Index (HSI) for nicotine dependence | Data on possible comorbid substance use not reported | Higher hazard of smoking initiation in patients than in controls, not explained by prodromal or other symptoms, 5 years before psychosis onset |
| Zammit et al (2003)46 | Longitudinal cohort study | Swedish Conscription cohort | 50,087 males | ICD-8 | Unspecified; Presumably up to 5 years before psychosis onset | Age at first psychiatric inpatient admission | Clinical interview; nonsmokers, light smokers (1–10 cigarettes per day), medium (11–20 cigarettes per day) and heavy smokers (>20 cigarettes per day) | Cannabis use was included as a confounder in Cox regression model | Hazard ratio of 0.8 for psychosis in medium and heavy smokers, possibly a protective effect |
| Kendler et al (2015)46 | Longitudinal cohort study | Swedish conscription cohort (men) and Swedish birth register (women) | 1,647,728 (233,879 men, 1,413,849 women) | ICD-8 and ICD-10 | A buffer period of 1, 3 and 5 years was used, in order to test the influence of a possible evolving prodrome on the smoking-schizophrenia association | First hospital discharge with diagnosis of schizophrenia or non-affective psychosis | Interview during first midwife assessment for women (week 12 of pregnancy): not smoking, light smoking (1–9 cigarettes per day), heavy smoking (≥10 cigarettes per day). Also at week 30–32 from 1990, and before pregnancy from 1998. Evaluation for army conscription for men: not smoking, light smoking (1–10 cigarettes per day), heavy smoking (≥11 cigarettes per day) | Drug abuse added as a potential confounder to Cox regression model | Dose–response relationship between smoking and future occurrence of schizophrenia, with hazard ratios 1.92 (95% CI::1.59–2.33) and 2.77 (95% CI: 2.34–3.43) for female light and heavy smokers, respectively. In the male sample: 1.62 (95% CI: 1.00–2.62) and 2.21(95% CI: 1.11–4.42), respectively. Risk did not decline as the time to schizophrenia onset increased, suggesting that the association is not substantially attributed to the presence of a prodrome. |
| Cadenhead (2011)53 | Case control, longitudinal study | The Cognitive Assessment and Risk Evaluation (CARE) program, University of California San Diego | 249 [75 Early Psychosis (76.0% males), 89 At Risk for psychosis patients (60.7% males) and 85 nonpsychiatric control subjects (45.9% males)] | DSM-IV or the Schedule for Affective Disorders and Schizophrenia for School-Age Children | Structured Interview for Prodromal Symptoms (SIPS) | Development of psychosis per the SCID/KSADS criteria | Clinical Interview. Dichotomous variable for current tobacco use: Yes/No | ANOVA was repeated with tobacco and cannabis use as between subjects factors; subjects with current substance misuse excluded | Reduced Prepulse Inhibition (PPI) in early psychosis patients who smoke compared to nonsmokers; increased PPI in at risk subjects who smoke compared to nonsmokers |
| Gupta and Mittal (2014)54 | Cross-sectional, case control study | Adolescent Development and Preventive Treatment (ADAPT) program | 67 [35 Ultra High Risk (UHR) (63% males) and 32 Healthy Control, (HC) subjects (41% males) | DSM-IV | Attenuated Positive Symptom (APS) or Genetic Risk and Deterioration (GRD) prodromal syndromes | Not applicable | Alcohol/Drug Use Scale (AUS/DUS) | Substance dependence disorder was exclusion criterion | UHR subjects smoked more frequently than HC subjects, 46% vs 22%; Elevated cognitive performance in UHR smokers vs UHR nonsmokers |
| Zhang et al (2012)51 | Cross-sectional case control study | Inpatients in the Beijing Hui-Long-Guan Psychiatric Hospital | 1336 (776 male patients with schizophrenia, 560 male healthy controls) | DSM-IV | Not specified | Not specified- presumably conferred by the recorded illness course duration | Patient, Questionnaire, Fagerstrom Test for Nicotine Dependence (FTND) |
Participants reported no dependence on any substance other than tobacco | Mean age of smoking initiation 7 years (SD=10.2) before psychosis onset, during the early prodromal or premorbid period. Positive correlation between age at starting smoking and age at onset of illness (P=0.005) |
| Buchy et al (2014)55 | Longitudinal study/cross-sectional as for the substance use measures | Multi-site NIMH study “Enhancing the Prospective Prediction of Psychosis”(PREDICT), | 170 (96 men, 74 women) at Clinical High Risk (CHR) of psychosis | Not applicable | SIPS, SOPS (Scale of Prodromal Symptoms) | Conversion assessed by SIPS criteria | Alcohol and Drug use Scale (AUS/DUS) | Cox regression for cannabis, tobacco, alcohol | No association between tobacco use at baseline and conversion to psychosis |
| Compton et al (2009)57 | Retrospective cross-sectional study | Two public-sector inpatient psychiatric units in an urban socially disadvantaged community | 109 first episode patients (83 males, 26 females) | DSM-IV | Symptom Onset in Schizophrenia inventory and complementary items from the semistructured Course of Onset and Relapse Schedule/Topography of Psychotic Episode interview | Symptom Onset in Schizophrenia inventory and complementary items from the semistructured Course of Onset and Relapse Schedule/Topography of Psychotic Episode interview | Inquiry about use —ever, weekly, and daily—for cannabis, alcohol, and tobacco, and recording of pattern of use, and corresponding age at which each level of frequency of use began | Cox regression for cannabis, tobacco, alcohol | Cox regression analysis with participants classified according to maximum frequency of use prior to onset (none, ever, weekly, or daily) indicated no significant effects of cannabis or tobacco use on risk of onset of psychotic symptoms. Significant effects of progression to both daily cannabis use and daily tobacco use on risk of onset of psychosis were observed, while progression of alcohol use had no significant effect |
| Kristensen and Cadenhead (2007)56 | Prospective longitudinal study | Health services, public schools and colleges in the community of San Diego and were seen in the Cognitive Assessment and Risk Evaluation (CARE) Program | 48 (26 males, 22 females) | DSM-IV | SIPS | SIPS | Clinical interview on a monthly basis | Nicotine use could not be dissected from cannabis use, since 7 out of 8 subjects who smoked also used cannabis | Nicotine use (amount smoked per day ranged from 0.125 to 1.5 packs per day) was found to be significantly associated with later conversion to psychosis (Fisher’s Exact Test P=0.005). |
Initiation of smoking in relation with the initial prodrome
Age of smoking initiation was studied by Riala et al who examined a 1966 birth cohort study comprising 11,017 individuals in Northern Finland.50 It was found that age of initiation of smoking preceded the illness onset in patients with schizophrenia by 2.3 (SD=6.6) years, which was significantly shorter than in patients with other mental disorders. The time difference between initiation of smoking and onset of the disease was even shorter, 0.9 (SD=5.8) years, in individuals who were not treated in a hospital for comorbid substance use disorder, comprising 77% of the group with schizophrenia. Based on the close temporal relationship, the authors concluded that smoking initiation may occur as a result of shared genetic risk between smoking and premorbid developmental deviance of schizophrenia. They also stated that this could be an indicator of the prodromal period, where patients are more prone to smoke to alleviate prodromal symptoms of the illness, in line with the self-medication hypothesis.50 In a study conducted in China, it was found that 73% of the schizophrenia patients had started smoking before the disease onset by a mean of 7.6 (SD=10.2) years. According to the authors, smoking could have been associated with prodromal symptoms, although they argue that 7 years seem to be a long period to be characterized as prodromal phase of the illness51 and thus schizophrenic patients may have started tobacco use during the premorbid period. In a different study,52 the risk of daily smoking initiation was found to be significantly higher in schizophrenia patients than in healthy controls preceding the initiation of psychiatric medication 5 years (assuming that this is a time when prodromal symptoms have not yet occurred). The authors conclude that the difference in the hazard of smoking initiation cannot be explained by either the illness, its treatment, or the prodromal period, and therefore a genetic component of this vulnerability in patients with schizophrenia must be considered.52 The age at onset of psychosis was not found to be significantly different between smokers and nonsmokers in two studies,50,51 but a positive correlation between age of smoking initiation and the age at onset of illness was reported (r = 0.12, P = 0.005).51
Prevalence of smoking in the prodromal period and risk factors for smoking initiation
Prevalence of nicotine use during the prodromal period varies among studies (16.6–46%). In the study conducted by Cadenhead, prevalence of current smokers was found to be 18% in at-risk individuals (N=89) and 32% in early psychosis patients (N=75) (converted to psychosis-and therefore no longer prodromal within the past 2 years) versus 14.1% in the control nonpsychiatric group (N=85).53 Another estimate is provided by Gupta and Mittal,54 in a smaller sample, where UHR patients (N=35) reported nicotine use at a rate of 46% versus the 22% of the control group (N=32, P≤0.05).54 In the study of Buchy et al, the rate of tobacco use in a sample of Clinical High Risk (CHR) individuals (N=170) was 33.3%, with 2.8% corresponding to dependence or abuse.55 Prevalence of nicotine use was 16.6% in the CHR sample of Kristensen and Cadenhead (2007).56 Riala et al examined a number of known risk factors for smoking in the general population, namely male sex, low primary family socioeconomic status, poor school performance, single-parent family, alcohol use, parental smoking, to see if these could account for the elevated rates of smoking in patients with schizophrenia. Only the father’s smoking at home was found to elevate the risk of smoking in the group of schizophrenia, giving an odds ratio of 3.5 (95% CI: 1.9–11.3, P=0.035). Data for maternal smoking were not available.50 Diaz et al have found that patients with schizophrenia have a higher hazard of becoming smokers 5 years before the psychosis onset, and presumably before the prodromal onset; the cumulative hazard for smoking initiation remains high after the age of 20 and at least for 10 years more, compared to healthy controls. This difference could not be demonstrated between mood disorder patients and controls.52 The authors discuss that a shared genetic vulnerability should be part of any explanatory hypothesis of this association, pointing out that people prone to schizophrenia are also prone to smoking initiation, not only in adolescence but also later (until at least their 30 years of age).52
Predictive risk for schizophrenia and transition to psychosis
Zammit et al report findings on a cohort study of Swedish conscripts, comprising 50,087 men aged 18–20.46 The cohort study was followed up for a period of 27 years regarding the development of schizophrenia, based on the Swedish National Register of Inpatient Care. The authors found that after adjusting for a number of confounding factors, including IQ, cannabis use, place of upbringing, and personality factors related to social integration (including number of close friends, relationships with women and sensitivity of the subject), there was no significant association between smoking at age 18 and developing of schizophrenia within 5 years of follow-up. They continued that this time frame corresponds to a possible prodromal period, where some of the subjects might have already started to smoke to relieve prodromal dysphoric symptoms. For medium (11 to 20 cigarettes/day) and heavy smokers (>20 cigarettes/day), there was actually a significantly lower risk for developing the disease after 5 or more years from conscription, with an adjusted hazard ratio of 0.8 (95% CI=0.7–0.9, P=0.003) This result leads the authors to assume the possibility of a protective role of smoking, that is also found to be specific to schizophrenia and not to other psychoses.46 Kendler et al in 2015 conducted larger Swedish cohort study, comprising 1,413,849 women from the birth registry (mean age of 27.3 and followed up for a mean of 18.5 years), as well as 233,879 men from the conscript registry (mean age of 18.5 and followed up for a mean period of 7.9 years). They reported that according to their findings, smoking prospectively predicts risk for schizophrenia.47 In this work, a clear dose–response relationship was demonstrated between smoking and the future occurrence of schizophrenia. Hazard ratios after adjustment for possible confounders (age, family and community, level socioeconomic status and drug abuse) were 1.92 (95% CI: 1.59–2.33) and 2.77 (95% CI: 2.34–3.43) for female light (1–10 cigarettes/day) and heavy smokers (11–20 and >20 cigarettes/day), respectively, and in the male sample 1.62 (95% CI: 1.00–2.62) and 2.21 (95% CI: 1.11–4.42), respectively. Nicotine dependence was indirectly estimated by the continuation of smoking during the third trimester of pregnancy and by higher rates of future chronic obstructive pulmonary disease (COPD). Furthermore, the risk did not decline as the time to schizophrenia onset increased, and therefore the authors assumed that the association is not substantially attributed to the presence of a prodrome. Looking at the hazard ratios calculated for relative pairs discordant for smoking, they found them to be 3.87 (95% CI=2.86–5.25) in cousins, 4.75 (95% CI=2.27–9.91) in half-sisters, 2.27 (95% CI=1.59–3.23) in full sisters, and 30% and 69% higher risk for non-affective psychosis in the light smoking and heavy smoking co-twin, respectively. It is suggested that a shared familial/genetic background only partly accounts for the comorbidity of schizophrenia and smoking.47 Not much evidence exists concerning conversion to psychosis specifically. No association was found between tobacco use (or cannabis) at baseline and transition to active phase in the study by Buchy et al, yet the authors note minimal rates of abuse or dependence in this sample, only 1.4% for each of these severity categories.55 In another study, nicotine use (amount smoked per day ranged from 0.125 to 1.5 packs per day) was found to be significantly associated with later conversion to psychosis (Fisher’s Exact Test P=0.005), but the 4 subjects out of 6 who converted smoked nicotine as well as cannabis.56 Compton et al examined a group of 109 socially disadvantaged first-episode patients, comprising patients with schizophrenia (56.9%), schizophreniform disorder (20.2%), schizoaffective disorder (7.3%), psychotic disorder not otherwise specified (11.0%), brief psychotic disorder (3.7%), and delusional disorder (0.9%), who were retrospectively assessed for prior and current alcohol, cannabis and tobacco use, and ages at onset of prodromal and psychotic symptoms. It was shown that the risk of onset was associated with progression of cannabis and tobacco use prior to onset, particularly with rapid progression to the highest levels of use of these substances.57
Effects of smoking relevant to the prodromal period
Cadenhead conducted a study in 2011 on startle reactivity and prepulse inhibition (PPI) as markers in prodromal and early psychosis. She proposed an interaction between early nicotine exposure and risk for psychosis. She suggested that neurobiological changes in the early course of illness are perhaps alterable by substances including nicotine as well as cannabis and antipsychotic medication. Smoking was associated with larger PPI in both the control and the at-risk (AR) group, defined by SIPS criteria. However, a reduction in PPI was observed in patients with early psychosis (within 2 years from onset of active psychosis) that smoked. It is argued that the observed PPI reduction in these patients may represent an interaction between early nicotine exposure and risk for psychosis with malevolent effects, whereas the larger PPI in the AR group could relate to compensatory processes close to, but before, the onset of active psychosis.53 In a similar line, supportive of the self-medication hypothesis, nicotine use in UHR for developing psychosis subjects is associated with elevated cognitive performance, as reported in the study by Gupta and Mittal, in 2014. In this study, smoking was associated with better processing speed, visual learning and spatial working memory among the UHR group.54
Discussion
The relationship between tobacco consumption and schizophrenia has gained research attention in recent years, but perhaps not to the extent at which other substances, ie, cannabis, have been studied.48 This is mirrored by the number of the studies that were found, as there is a relative lack of studies addressing the relationship between schizophrenia and nicotine, and even less articles that focus on the importance of smoking during the prodromal period of psychosis. There were some methodological issues during the search for relevant papers that should be pointed out. First, the definitions of the prodromal period and age of onset of schizophrenia vary between studies. In many cases, the prodrome is only supposed to exist for a set time period before psychosis onset.46,49,50 Also, the age of onset of psychosis is frequently calculated as either the age of first hospitalization, age at starting medication,46,47,50,52 was not specified,51 or included criteria for conversion.53,55–57 It is well known that psychosis onset can be well before first hospitalization, and Durations of Untreated Psychoses (DUPs) range between 9.3 and 147.2 weeks, according to a recent meta-analysis.58 Another shortcoming is that nicotine use is not separated by the use of other substances,56 most importantly cannabis, for which quite more literature exists.59 Not all studies use a measure or a scale for nicotine dependence,51,52,55,60,61 but rather report number of cigarettes per day,46,47,50,56,57 or treat this variable dichotomously,53 or use indirect measures for dependence.47 Also, patterns of use and longitudinal assessments of smoking habits are examined in only two studies.56,57 Some studies are based on rather small samples.54,56 Still, smoking habits in patients with schizophrenia during or even before the prodromal period present particular interest, deserve more research attention, and call for a multifaceted approach.
Prevalence of smoking in this population is high and significantly higher than healthy controls and being in this group increases risk for smoking initiation.47,52,53 This is in accordance with a recent review focusing on cardiometabolic risk factors in UHR patients; prevalence of smoking in UHR subjects was 33% (95% CI=0.24–0.42; I2=82.4%; total pooled subjects n=629), with an odds ratio of 2.3 (P=0.05; 95% CI=1.48–3.48; total pooled subjects n=938), compared to healthy controls.62 Serious concerns are raised for the physical health of this population, given the detrimental effects of smoking on respiratory and cardiovascular function, and negative influence on life expectancy. Vigorous programs for smoking cessation should try to address this need, even more because smoking cessation rates in patients with schizophrenia lag behind those of the healthy population.63,64 From a different point of view, it is this proneness of schizophrenic people to smoking that stresses the complex interplay between the illness and nicotine use and could shed light on a possible mediating or etiologic role of nicotine in the evolving disease, complementing the self-medication aspects of tobacco use.
In some of the reviewed articles, the study of the prodrome has served to rule out the self-medication hypothesis, or better disentangle the exact significance and role of smoking in the evolution of the disease.46,47,52 The reviewed studies suggest that tobacco use is taken up most often at the premorbid or the prodromal period of schizophrenia and cannot therefore be attributed to symptoms of active psychosis,50–52 possibly pointing to a shared vulnerability between the two. Accordingly, a recent meta-analysis found that the time of smoking initiation preceded the illness onset by 5.3 years on average, with prevalence of smoking at first presentation for treatment being 58.9%,65 which is even higher than during the prodromal phase, indicating maybe an escalation of use and also implicated in the study of Compton et al.57
On the other hand, evidence accumulates regarding a possible etiologic role of smoking in the pathogenesis of schizophrenia. In the articles reviewed, Zammit et al46 argue for a protective role of nicotine for medium and heavy smokers, in contrast to Kendler et al,47 who have found increased risk for the illness associated with smoking in a dose-response mode. The protective role of nicotine is a rather surprising result, but it is possible that it could be attributed to bias. This can be explained by the fact that the authors have, amongst other confounders controlled for poor social integration, stating that this is a risk factor for smoking. However, poor social integration, corresponding to sensitivity and lack of close friends or relationships, could also well be part of a premorbid personality related to schizophrenia,66 or could be a manifestation of prodromal symptoms of the illness, such as social isolation. By treating this as a confounder, a possibly significant proportion of subjects already at grave risk for schizophrenia is excluded from analysis, leading to biased results. Distribution of ages at onset for the two sexes should also be considered. Males get ill mostly around 15–25 years old, with onset of illness beyond 35 years of age being rarer for them, whereas for women two similar peaks exist, before and after 35.29 By treating this as a confounder, a possibly significant proportion of subjects already at grave risk for schizophrenia is excluded from analysis, leading to biased results. Distribution of ages at onset for the two sexes should also be considered. Males get ill mostly around 15–25 years old, with onset of illness beyond 35 years of age being rarer for them, whereas for women two similar peaks exist, before and after 35.67,68 We think that the period of observation of Kendler et al for the male and female cohort study is more fitted to the particularities of the age of onset in schizophrenia in the two sexes.
Data regarding the course of nicotine consumption during prodromal period through transition are lacking; in the study of Compton et al,57 an escalation to highest levels of use is implicated in subjects who convert to psychosis. Buchy et al failed to find an association,55 but assessment of use was only cross-sectional at baseline, with abuse or dependence being considerably low or minimal, and these may have led to a negative result. In a recent meta-analysis, risk ratio for new psychotic disorder was 2.18, a modest effect, and people who smoke in the first episode of psychosis have earlier ages of onset of psychosis.48 In the studies included in this review that focuses on the prodromal period, this difference is not found, and the age at onset of schizophrenia is not lower in patients who have started smoking before onset, compared to nonsmokers. Also, the age at onset of smoking does not differ between schizophrenia patients and controls.
The role of several neurochemical circuitries in the pathophysiology of schizophrenia has been extensively studied, including the dopaminergic pathway, the glutamate dysfunction, the serotonergic and GABAergic system, and the cholinergic system, the latter of which is probably the most relevant regarding the physiological effects of nicotine on the brain and its possible connection with schizophrenia.69 Stimulation of nicotine acetylcholine receptors in the brain dopamine receptor system may have positive effects in sensory processing deficits in schizophrenic patients,70 and this also seems true for the prodromal period, according to the reviewed studies.53,54 Particularly interesting is the increased PPI in at-risk individuals that smoke, compared to at-risk nonsmokers, implicating a positive influence of nicotine on compensatory processes before the transition to psychosis, a finding that is reversed after the establishment of active psychotic symptoms. Smoking could relieve patients from negative or cognitive symptoms by increasing the mesocortical DA activity, although DA neurotransmission is already hyperactive in subcortical regions, ie, the nigrostriatal pathway, which is related to the positive symptoms.71 Smoking could also be used by a patient as an anxiolytic agent, due to the inhibition of nicotinic Ach receptors (nAchRs).11 Anxious distress and depressive symptoms are very common in patients at risk for psychosis, particularly in early stages, and in patients with prevalent paranoid symptoms.72 Nicotine, even in healthy subjects, alters the bioavailability of neuroregulatory molecules such as catecholamines, monoamines, hypophyseal hormones, and cortisol, providing immediate improvement in affect or performance in response to environmental demands.20 In the present review, no articles examining differences in symptoms other than cognitive in at-risk individuals were identified, between smokers and nonsmokers. Nevertheless, the above could be supportive of the self-medication hypothesis in this population, also described as a prevalent hypothesis about the link between smoking and schizophrenia in many articles.71
On the other hand, a most convincing insight on the biological link between schizophrenia and smoking comes from latest genetic studies that strongly suggest that nicotine consumption and schizophrenia share some genetic liability.73,74 Recently, a multi-stage schizophrenia genome-wide association study (GWAS) was performed by Schizophrenia Working Group of the Psychiatric Genomics Consortium and 108 schizophrenia-associated genetic loci were identified. The presence of an associated variant in the CHRNA5-A3-B4 gene cluster might reflect a dose–response relationship between the heaviness of smoking and the schizophrenia risk,75 while a variant within the CHRNA5-A3-B4 gene cluster was strongly associated with the heaviness of smoking.76 A genetic variation in CHRNA7 gene has been associated with higher risk for nicotine dependence in schizophrenic patients, and there are multiple nAChR genes that have been proposed to play a role for the increased susceptibility to smoking in these patients.77,78 These could mean that there is shared genetic architecture between schizophrenia and smoking, but it could also indicate a causal association between smoking and schizophrenia.79 In the Chen et al study, the results support the self-medication hypothesis, as well as a causal link between smoking and schizophrenia, and evidence of the shared vulnerability hypothesis is provided, with shared genes involved in neuroplasticity processes and cognitive functions.80 Furthermore, some researchers have employed the method of mendelian randomization, as a means to overcome confounding in observational studies, by using genetic variants that increase risk for a condition, as a proxy measure of this condition.81 The study of Gage et al investigated causality in associations between smoking initiation and schizophrenia and was not supportive of a causal effect of schizophrenia risk on becoming a smoker, but there was some evidence of a causal effect of being a smoker, and maybe of the heaviness of smoking, on the risk of schizophrenia.79 The study of Wooton et al, which used a similar approach but captured smoking duration heaviness and cessation suggested a causal effect of smoking status on schizophrenia and depression as well, but there was also evidence for causation in the opposite direction; genetic risk for schizophrenia and depression was found to increase lifetime smoking.82
On the neurotransmitter level, effects of nicotine on the dopamine system may have similarities to psychosis, as it may cause changes in the dopaminergic system, by inducing a supersensitivity of the D2-receptors.48,83,84 This might be better understood by the dopamine–acetylcholine balance hypothesis.85 Maladaptive changes in dopaminergic signaling may be produced by high nicotine concentrations through the nAchR function alteration,9 contributing to glutaminergic signaling modulation, and thus influencing cognition and behavior.86 In conclusion, smoking can be seen as a condition that schizophrenia-prone subjects are prone to take up, and also a condition that itself has some role in the etiology of the illness.
In view of the above, could smoking during the prodromal period deteriorate the course of the disease? Findings from different studies are inconclusive on the matter. Even though there appears to be evidence of a strong association between nicotine abuse and relapses in general49,87,88 and a greater risk on hospitalization,89 other studies report no clear association between the two.90 Only one of the reviewed studies provided evidence of escalation of tobacco use as psychosis got imminent; still, because the data about nicotine consumption patterns during the prodromal phase are yet insufficient, clearly more relevant studies are needed.
Limitations of the present study are the considerable heterogeneity of the articles as well as that some studies rely on small samples. The heterogeneity included several methodological differences, such as varying sample sizes and designs, definitions of prodromal period, age of onset of schizophrenia, and assessment of nicotine use. We should also note that the use of only unspecific databases (and not more or specific ones), the limited search to English language only, and not using truncations and controlled vocabulary could have influenced the search results.
Conclusion
The reviewed articles imply a link between smoking onset and the prodromal period (or the premorbid period) of schizophrenia, explained by association and the close temporal relationship of the initiation of smoking and the onset of psychosis, the high prevalence of nicotine use in patients at the prodromal phase, the possible escalation of use, a higher hazard of developing schizophrenia in smokers in a dose-dependent mode, and the amelioration of cognitive measures in prodromal individuals who smoke. This complex interplay corresponds to three explanatory hypotheses not necessarily mutually exclusive: the shared vulnerability, the causal role, and the self-medication effect. Further research is needed to clarify whether nicotine consumption has a direct effect on the altered genetic substrate and if cigarette smoking should be examined as an environmental risk factor for developing schizophrenia. Moreover, it is very important to identify, in future studies, the role of nicotine or smoking patterns during the early, prodromal stages of the illness. The investigation of a possible link between smoking patterns and specific schizophrenic symptoms, like negative/positive symptoms or cognitive deficits would be a great challenge for the future, along with a clearer understanding of the role of the cholinergic system’s role in the pathophysiology of psychosis and possibly a consideration for public health.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Szatkowski L, McNeill A. Diverging trends in smoking behaviors according to mental health status. Nicotine Tob Res. 2015. doi: 10.1093/ntr/ntu173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mendelsohn CP, Kirby DP, Castle DJ. Smoking and mental illness. An update for psychiatrists. Australas Psychiatry. 2015. doi: 10.1177/1039856214562076 [DOI] [PubMed] [Google Scholar]
- 3.Lê Cook B, Wayne GF, Kafali EN, Liu Z, Shu C, Flores M. Trends in smoking among adults with mental illness and association between mental health treatment and smoking cessation. JAMA. 2014. doi: 10.1001/jama.2013.284985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Centers for Disease Control and Prevention (CDC). Vital signs: current cigarette smoking among adults aged ≥18 years with mental illness - United States, 2009–2011. MMWR Morb Mortal Wkly Rep 2013;62(5):81–87. [PMC free article] [PubMed] [Google Scholar]
- 5.Prochaska JJ, Das S, Young-Wolff KC. Smoking, Mental Illness, and Public Health. Annu Rev Public Health 2017;38(1):165–185. doi: 10.1146/annurev-publhealth-031816-044618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hartz SM, Pato CN, Medeiros H, et al. Comorbidity of severe psychotic disorders with measures of substance use. JAMA Psychiatry. 2014. doi: 10.1001/jamapsychiatry.2013.3726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dickerson F, Schroeder J, Katsafanas E, et al. Cigarette smoking by patients with serious mental illness, 1999–2016: an increasing disparity. Psychiatr Serv. 2017. doi: 10.1176/appi.ps.201700118 [DOI] [PubMed] [Google Scholar]
- 8.Dickerson F, Stallings CR, Origoni AE, et al. Cigarette smoking among persons with schizophrenia or bipolar disorder in routine clinical settings, 1999–2011. Psychiatr Serv. 2013. doi: 10.1176/appi.ps.201200143 [DOI] [PubMed] [Google Scholar]
- 9.Parikh V, Kutlu MG, Gould TJ. nAChR dysfunction as a common substrate for schizophrenia and comorbid nicotine addiction: current trends and perspectives. Schizophr Res. 2016;171(1–3):1–15. doi: 10.1016/j.schres.2016.01.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gage SH, Munafò MR. Rethinking the association between smoking and schizophrenia. Lancet Psychiatry. 2015;2(2):118–119. doi: 10.1016/s2215-0366(14)00057-1 [DOI] [PubMed] [Google Scholar]
- 11.Anderson SM, Brunzell DH. Anxiolytic-like and anxiogenic-like effects of nicotine are regulated via diverse action at β2-nicotinic acetylcholine receptors. Br J Pharmacol. 2015. doi: 10.1111/bph.13090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Picciotto MR, Lewis AS, van Schalkwyk GI, Mineur YS. Mood and anxiety regulation by nicotinic acetylcholine receptors: a potential pathway to modulate aggression and related behavioral states. Neuropharmacology. 2015;96:235–243. doi: 10.1016/j.neuropharm.2014.12.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kutlu MG, Parikh V, Gould TJ. Nicotine addiction and psychiatric disorders. Int Rev Neurobiol. 2015;124:171–208. doi: 10.1016/bs.irn.2015.08.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Domino EF, Mirzoyan D, Tsukada H. N-methyl-d-aspartate antagonists as drug models of schizophrenia: a surprising link to tobacco smoking. Prog Neuropsychopharmacol Biol Psychiatry. 2004;28(5):801–811. doi: 10.1016/j.pnpbp.2004.05.024 [DOI] [PubMed] [Google Scholar]
- 15.Levin ED, Petro A, Caldwell DP. Nicotine and clozapine actions on pre-pulse inhibition deficits caused by N-methyl-d-aspartate (NMDA) glutamatergic receptor blockade. Prog Neuropsychopharmacol Biol Psychiatry. 2005;29(4):581–586. doi: 10.1016/j.pnpbp.2005.01.012 [DOI] [PubMed] [Google Scholar]
- 16.Tamminga CA, Buchanan RW, Gold JM. The role of negative symptoms and cognitive dysfunction in schizophrenia outcome. Int Clin Psychopharmacol. 1998. doi: 10.1097/00004850-199803003-00004 [DOI] [PubMed] [Google Scholar]
- 17.Sandyk R. Cigarette smoking: effects on cognitive functions and drug-induced parkinsonism in chronic schizophrenia. Int J Neurosci. 1993;70(3–4):193–197. doi: 10.3109/00207459309000574 [DOI] [PubMed] [Google Scholar]
- 18.Decina P, Caracci G, Sandik R, Berman W, Mukherjee S, Scapicchio P. Cigarette smoking and neuroleptic-induced parkinsonism. Biol Psychiatry. 1990;28(6):502–508. doi: 10.1016/0006-3223(90)90483-i [DOI] [PubMed] [Google Scholar]
- 19.Desai HD, Seabolt J, Jann MW. Smoking in patients receiving psychotrophic medications: a pharmacokinetic perspective. CNS Drugs. 2001. doi: 10.2165/00023210-200115060-00005 [DOI] [PubMed] [Google Scholar]
- 20.Pomerleau OF, Rosecrans J. Neuroregulatory effects of nicotine. Psychoneuroendocrinology. 1989;14(6):407–423. doi: 10.1016/0306-4530(89)90040-1 [DOI] [PubMed] [Google Scholar]
- 21.Azaiez C, Millier A, Lançon C, et al. Health related quality of life in patients having schizophrenia negative symptoms – a systematic review. J Mark Access Heal Policy. 2018;6(1):1517573. doi: 10.1080/20016689.2018.1517573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sakurai H, Bies RR, Stroup ST, et al. Dopamine D2 receptor occupancy and cognition in schizophrenia: analysis of the CATIE data. Schizophr Bull. 2012;39(3):564–574. doi: 10.1093/schbul/sbr189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hu W, MacDonald ML, Elswick DE, Sweet RA. The glutamate hypothesis of schizophrenia: evidence from human brain tissue studies. Ann NY Acad Sci. 2014;1338(1):38–57. doi: 10.1111/nyas.12547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Selvaraj S, Arnone D, Cappai A, Howes O. Alterations in the serotonin system in schizophrenia: a systematic review and meta-analysis of postmortem and molecular imaging studies. Neurosci Biobehav Rev. 2014;45:233–245. doi: 10.1016/j.neubiorev.2014.06.005 [DOI] [PubMed] [Google Scholar]
- 25.Steeds H, Carhart-Harris RL, Stone JM. Drug models of schizophrenia. Ther Adv Psychopharmacol. 2014;5(1):43–58. doi: 10.1177/2045125314557797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ripoll N, Bronnec M, Bourin M. Nicotinic receptors and schizophrenia. Curr Med Res Opin. 2004;20(7):1057–1074. doi: 10.1185/030079904125004060 [DOI] [PubMed] [Google Scholar]
- 27.Scarr E, Gibbons AS, Neo J, Udawela M, Dean B. Cholinergic connectivity: it’s implications for psychiatric disorders. Front Cell Neurosci. 2013;7. doi: 10.3389/fncel.2013.00055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ruhrmann S, Schultze-Lutter F, Klosterkötter J. Early detection and intervention in the initial prodromal phase of schizophrenia. Pharmacopsychiatry. 2003;36(S03):162–167. doi: 10.1055/s-2003-45125 [DOI] [PubMed] [Google Scholar]
- 29.Gourzis P, Katrivanou A, Beratis S. Symptomatology of the initial prodomal phase in schizophrenia. Schizophr Bull. 2002. doi: 10.1093/oxfordjournals.schbul.a006950 [DOI] [PubMed] [Google Scholar]
- 30.Löffler W, Häfner H. [Long prodromal phase in schizophrenia. By recognizing it, the prognosis of the patient can be significantly improved]. MMW Fortschr Med 2000;142(10):26–29. German. [PubMed] [Google Scholar]
- 31.Schmidt SJ, Schultze-Lutter F, Schimmelmann BG, et al. EPA guidance on the early intervention in clinical high risk states of psychoses. Eur Psychiatry. 2015. doi: 10.1016/j.eurpsy.2015.01.013 [DOI] [PubMed] [Google Scholar]
- 32.Cannon TD. How schizophrenia develops: cognitive and brain mechanisms underlying onset of psychosis. Trends Cogn Sci. 2015. doi: 10.1016/j.tics.2015.09.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Miller TJ, McGlashan TH, Rosen JL, et al. Prodromal assessment with the structured interview for prodromal syndromes and the scale of prodromal symptoms: predictive validity, interrater reliability, and training to reliability. Schizophr Bull. 2003;29(4):703–715. doi: 10.1093/oxfordjournals.schbul.a007040 [DOI] [PubMed] [Google Scholar]
- 34.McGorry PD, Nelson B, Phillips LJ, et al. Randomized controlled trial of interventions for young people at ultra-high risk of psychosis: twelve-month outcome. J Clin Psychiatry. 2013. doi: 10.4088/JCP.12m07785 [DOI] [PubMed] [Google Scholar]
- 35.Schultze-Lutter F. Subjective symptoms of schizophrenia in research and the clinic: the basic symptom concept. Schizophr Bull. 2009. doi: 10.1093/schbul/sbn139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Klosterkötter J, Schultze-Lutter F. Diagnosing schizophrenia in the initial prodromal phase. Schizophr Res. 2000;41(1):10–11. doi: 10.1016/s0920-9964(00)90330-x [DOI] [PubMed] [Google Scholar]
- 37.Rosen J, Miller T, Dandrea J, Mcglashan T, Woods S. Comorbid diagnoses in patients meeting criteria for the schizophrenia prodrome. Schizophr Res. 2006;85(1–3):124–131. doi: 10.1016/j.schres.2006.03.034 [DOI] [PubMed] [Google Scholar]
- 38.Kalkman HO, Feuerbach D. Modulatory effects of α7 nAChRs on the immune system and its relevance for CNS disorders. Cell Mol Life Sci. 2016. doi: 10.1007/s00018-016-2175-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yu R, Deochand C, Krotow A, et al. Tobacco smoke-induced brain white matter myelin dysfunction: potential co-factor role of smoking in neurodegeneration. J Alzheimer’s Dis. 2015. doi: 10.3233/JAD-150751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lin F, Wu G, Zhu L, Lei H. Heavy smokers show abnormal microstructural integrity in the anterior corpus callosum: a diffusion tensor imaging study with tract-based spatial statistics. Drug Alcohol Depend. 2013. doi: 10.1016/j.drugalcdep.2012.09.013 [DOI] [PubMed] [Google Scholar]
- 41.Kelly C, McCreadie RG. Smoking habits, current symptoms, and premorbid characteristics of schizophrenic patients in Nithsdale, Scotland. Am J Psychiatry 1999;156(11):1751–1757. [DOI] [PubMed] [Google Scholar]
- 42.Beratis S, Katrivanou A, Gourzis P. Factors affecting smoking in schizophrenia. Compr Psychiatry. 2001;42(5):393–402. doi: 10.1053/comp.2001.26273 [DOI] [PubMed] [Google Scholar]
- 43.Sandyk R, Kay SR. Brief communication: tobacco addiction as a marker of age at onset of schizophrenia. Int J Neurosci. 1991;57(3–4):259–262. doi: 10.3109/00207459109150699 [DOI] [PubMed] [Google Scholar]
- 44.Weiser M, Reichenberg A, Grotto I, et al. Higher rates of cigarette smoking in male adolescents before the onset of schizophrenia: a historical-prospective cohort study. Am J Psychiatry. 2004;161(7):1219–1223. doi: 10.1176/appi.ajp.161.7.1219 [DOI] [PubMed] [Google Scholar]
- 45.Üneri Ö, Tural Ü, Çakin Memik N. Smoking and schizophrenia: where is the biological connection? Turk Psikiyatr Derg. 2006;17(1):1–10. [PubMed] [Google Scholar]
- 46.Zammit S, Allebeck P, Dalman C, Lundberg I, Hemmingsson T, Lewis G. Investigating the association between cigarette smoking and schizophrenia in a cohort study. Am J Psychiatry. 2003;160(12):2216–2221. doi: 10.1176/appi.ajp.160.12.2216 [DOI] [PubMed] [Google Scholar]
- 47.Kendler KS, Lönn SL, Sundquist J, Sundquist K. Smoking and schizophrenia in population cohorts of Swedish women and men: a prospective co-relative control study. Am J Psychiatry. 2015;172(11):1092–1100. doi: 10.1176/appi.ajp.2015.15010126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gurillo P, Jauhar S, Murray RM, MacCabe JH. Does tobacco use cause psychosis? Systematic review and meta-analysis. Lancet Psychiatry. 2015;2(8):718–725. doi: 10.1016/s2215-0366(15)00152-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.de Leon J, Diaz FJ. A meta-analysis of worldwide studies demonstrates an association between schizophrenia and tobacco smoking behaviors. Schizophr Res. 2005;76(2–3):135–157. doi: 10.1016/j.schres.2005.02.010 [DOI] [PubMed] [Google Scholar]
- 50.Riala K, Hakko H, Isohanni M, Pouta A, Räsänen P. Is initiation of smoking associated with the prodromal phase of schizophrenia? J Psychiatry Neurosci 2005;30(1):26–32. [PMC free article] [PubMed] [Google Scholar]
- 51.Zhang XY, Liang J, Chen DC, et al. Cigarette smoking in male patients with chronic schizophrenia in a Chinese population: prevalence and relationship to clinical phenotypes. PLoS One. 2012;7(2):e30937. doi: 10.1371/journal.pone.0030937 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Diaz FJ, Velásquez DM, Susce MT, de Leon J. The association between schizophrenia and smoking: unexplained by either the illness or the prodromal period. Schizophr Res. 2008;104(1–3):214–219. doi: 10.1016/j.schres.2008.06.004 [DOI] [PubMed] [Google Scholar]
- 53.Cadenhead KS. Startle reactivity and prepulse inhibition in prodromal and early psychosis: effects of age, antipsychotics, tobacco and cannabis in a vulnerable population. Psychiatry Res. 2011;188(2):208–216. doi: 10.1016/j.psychres.2011.04.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gupta T, Mittal VA. Nicotine usage is associated with elevated processing speed, spatial working memory, and visual learning performance in youth at ultrahigh-risk for psychosis. Psychiatry Res. 2014;220(1–2):687–690. doi: 10.1016/j.psychres.2014.07.085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Buchy L, Perkins D, Woods SW, Liu L, Addington J. Impact of substance use on conversion to psychosis in youth at clinical high risk of psychosis. Schizophr Res. 2014. doi: 10.1016/j.schres.2014.04.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kristensen K, Cadenhead KS. Cannabis abuse and risk for psychosis in a prodromal sample. Psychiatry Res. 2007. doi: 10.1016/j.psychres.2006.10.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Compton MT, Kelley ME, Ramsay CE, et al. Association of pre-onset cannabis, alcohol, and tobacco use with age at onset of prodrome and age at onset of psychosis in first-episode patients. Am J Psychiatry. 2009. doi: 10.1176/appi.ajp.2009.09030311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bora E, Yalincetin B, Akdede BB, Alptekin K. Duration of untreated psychosis and neurocognition in first-episode psychosis: a meta-analysis. Schizophr Res. 2018. doi: 10.1016/j.schres.2017.06.021 [DOI] [PubMed] [Google Scholar]
- 59.Large M, Sharma S, Compton MT, Slade T, Nielssen O. Cannabis use and earlier onset of psychosis: a systematic meta-analysis. Arch Gen Psychiatry. 2011. doi: 10.1001/archgenpsychiatry.2011.5 [DOI] [PubMed] [Google Scholar]
- 60.Stewart DW, Vinci C, Adams CE, Cohen AS, Copeland AL. Smoking topography and outcome expectancies among individuals with schizotypy. Psychiatry Res. 2013;205(3):205–212. doi: 10.1016/j.psychres.2012.11.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kulhara P, Gupta S. What is schizophrenia: a neurodevelopmental or neurodegenerative disorder or a combination of both? A critical analysis. Indian J Psychiatry. 2010;52(1):21. doi: 10.4103/0019-5545.58891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Carney R, Cotter J, Bradshaw T, Firth J, Yung AR. Cardiometabolic risk factors in young people at ultra-high risk for psychosis: a systematic review and meta-analysis. Schizophr Res. 2016;170(2–3):290–300. doi: 10.1016/j.schres.2016.01.010 [DOI] [PubMed] [Google Scholar]
- 63.Roberts E, Eden Evins A, Mcneill A, Robson D. Efficacy and tolerability of pharmacotherapy for smoking cessation in adults with serious mental illness: a systematic review and network meta-analysis. Addiction. 2016. doi: 10.1111/add.13236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Tidey JW, Miller ME. Smoking cessation and reduction in people with chronic mental illness. BMJ. 2015;h4065. doi: 10.1136/bmj.h4065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Myles N, Newall HD, Curtis J, Nielssen O, Shiers D, Large M. Tobacco use before, at, and after first-episode psychosis. J Clin Psychiatry. 2012;73(04):468–475. doi: 10.4088/jcp.11r07222 [DOI] [PubMed] [Google Scholar]
- 66.Skokou M, Gourzis P. Demographic features and premorbid personality disorder traits in relation to age of onset and sex in paranoid schizophrenia. Psychiatry Res. 2014. doi: 10.1016/j.psychres.2014.01.018 [DOI] [PubMed] [Google Scholar]
- 67.Beratis S, Gabriel J, Hoidas S. Age at onset in subtypes of schizophrenic disorders. Schizophr Bull. 1994. doi: 10.1093/schbul/20.2.287 [DOI] [PubMed] [Google Scholar]
- 68.Skokou M, Katrivanou A, Andriopoulos I, Gourzis P. Active and prodromal phase symptomatology of young-onset and late-onset paranoid schizophrenia. Rev Psiquiatr y Salud Ment. 2012. doi: 10.1016/j.rpsmen.2012.03.002 [DOI] [PubMed] [Google Scholar]
- 69.Terry Alvin J. Role of the central cholinergic system in the therapeutics of schizophrenia. Curr Neuropharmacol. 2008;6(3):286–292. doi: 10.2174/157015908785777247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Heilbronner U, Samara M, Leucht S, Falkai P, Schulze TG. The longitudinal course of schizophrenia across the lifespan. Harv Rev Psychiatry. 2016;24(2):118–128. doi: 10.1097/hrp.0000000000000092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Manzella F. Smoking in schizophrenic patients: a critique of the self-medication hypothesis. World J Psychiatry. 2015;5(1):35. doi: 10.5498/wjp.v5.i1.35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Fusar-Poli P, Nelson B, Valmaggia L, Yung AR, McGuire PK. Comorbid depressive and anxiety disorders in 509 individuals with an at-risk mental state: impact on psychopathology and transition to psychosis. Schizophr Bull. 2014. doi: 10.1093/schbul/sbs136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ripke S, Neale BM, Corvin A, et al. Biological insights from 108 schizophrenia-associated genetic loci. Nature. 2014. doi: 10.1038/nature13595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Gage SH, Munafò MR. Smoking as a causal risk factor for schizophrenia. Lancet Psychiatry. 2015;2(9):778–779. doi: 10.1016/s2215-0366(15)00333-8 [DOI] [PubMed] [Google Scholar]
- 75.Flint J, Munafò M. Genesis of a complex disease. Nature. 2014;511(7510):412–413. doi: 10.1038/nature13645 [DOI] [PubMed] [Google Scholar]
- 76.Furberg H, Kim Y, Dackor J, et al. Genome-wide meta-analyses identify multiple loci associated with smoking behavior. Nat Genet. 2010;42(5):441–447. doi: 10.1038/ng.571 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.De Luca V, Wong AHC, Muller DJ, Wong GWH, Tyndale RF, Kennedy JL. Evidence of association between smoking and α7 nicotinic receptor subunit gene in schizophrenia patients. Neuropsychopharmacology. 2004;29(8):1522–1526. doi: 10.1038/sj.npp.1300466 [DOI] [PubMed] [Google Scholar]
- 78.Saccone NL, Schwantes-An T-H, Wang JC, et al. Multiple cholinergic nicotinic receptor genes affect nicotine dependence risk in African and European Americans. Genes Brain Behav. 2010;9(7):741–750. doi: 10.1111/j.1601-183x.2010.00608.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Gage SH, Jones HJ, Taylor AE, Burgess S, Zammit S, Munafò MR. Investigating causality in associations between smoking initiation and schizophrenia using Mendelian randomization. Sci Rep. 2017;7(1):40653. doi: 10.1038/srep40653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Chen J, Bacanu SA, Yu H, et al. Genetic relationship between schizophrenia and nicotine dependence. Sci Rep. 2016. doi: 10.1038/srep25671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Davies NM, Holmes MV, Davey Smith G. Reading Mendelian randomisation studies: a guide, glossary, and checklist for clinicians. BMJ. 2018. doi: 10.1136/bmj.k601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Wootton RE, Richmond RC, Stuijfzand BG, et al. Causal effects of lifetime smoking on risk for depression and schizophrenia: evidence from a Mendelian randomisation study. bioRxiv. 2018. doi: 10.1101/381301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Howes OD, Kapur S. The dopamine hypothesis of schizophrenia: version III–the final common pathway. Schizophr Bull. 2009;35(3):549–562. doi: 10.1093/schbul/sbp006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Novak G, Seeman P, Foll BL. Exposure to nicotine produces an increase in dopamine D2HighReceptors: a possible mechanism for dopamine hypersensitivity. Int J Neurosci. 2010;120(11):691–697. doi: 10.3109/00207454.2010.513462 [DOI] [PubMed] [Google Scholar]
- 85.Aosaki T, Miura M, Suzuki T, Nishimura K, Masuda M. Acetylcholine-dopamine balance hypothesis in the striatum: an update. Geriatr Gerontol Int. 2010;10:S148–S157. doi: 10.1111/j.1447-0594.2010.00588.x [DOI] [PubMed] [Google Scholar]
- 86.Higley MJ, Picciotto MR. Neuromodulation by acetylcholine: examples from schizophrenia and depression. Curr Opin Neurobiol. 2014;29:88–95. doi: 10.1016/j.conb.2014.06.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Margolese HC, Carlos Negrete J, Tempier R, Gill K. A 12-month prospective follow-up study of patients with schizophrenia-spectrum disorders and substance abuse: changes in psychiatric symptoms and substance use. Schizophr Res. 2006. doi: 10.1016/j.schres.2005.11.019 [DOI] [PubMed] [Google Scholar]
- 88.de Leon J. Psychopharmacology: atypical antipsychotic dosing: the effect of smoking and caffeine. Psychiatr Serv. 2004. doi: 10.1176/appi.ps.55.5.491 [DOI] [PubMed] [Google Scholar]
- 89.Sullivan G, Wells KB, Morgenstern H, Leake B. Identifying modifiable risk factors for rehospitalization: a case-control study of seriously mentally ill persons in Mississippi. Am J Psychiatry. 1995. doi: 10.1176/ajp.152.12.1749 [DOI] [PubMed] [Google Scholar]
- 90.Warner R, Taylor D, Wright J, et al. Substance use among the mentally ILL: prevalence, reasons for use, and effects on illness. Am J Orthopsychiatry. 1994. doi: 10.1037/h0079489 [DOI] [PubMed] [Google Scholar]