Abstract
(1) Background: As the most common eye disease diagnosed in emergency departments, conjunctivitis has caused serious health and economic burdens worldwide. However, whether air pollution may be a risk factor for conjunctivitis is still inconsistent among current evidence. (2) Methods: We searched the literature on the relationship between air pollution and conjunctivitis in multiple English databases before 18 March 2019. Meta-analysis, meta-regression, and funnel plots were used to integrate the data, identify the sources of bias, and determine the publication bias, respectively. (3) Results: A total of 2450 papers were found, 12 of which were finally included. The pooled relative risk for each 10 μg/m3 increase of air pollution on conjunctivitis was 1.0006 (95%CI: 0.9993–1.0019) for CO, 1.0287 (1.0120–1.0457) for NO2, 1.0089 (1.0030–1.0149) for O3, 1.0004 (0.9976–1.0032) for PM2.5, 1.0033 (0.9982–1.0083) for PM10, and 1.0045 (0.9908–1.0185) for SO2. In the subgroup, PM2.5 and O3 had a greater impact on conjunctivitis risk in women than in men, and people <18 years old than those ≥18 years old. Relative humidity significantly modified the risk of O3 on conjunctivitis (p = 0.023), explaining 45% of the between-study heterogeneity. (4) Conclusion: Globally, air pollution has considerable health risks for conjunctivitis. Females and the youth were more vulnerable to PM2.5, NO2, and O3. Reductions of air pollution levels are still warranted to protect the vulnerable populations.
Keywords: air pollution, conjunctivitis disease, vulnerable populations, systematic review and meta-analysis
1. Introduction
Ambient air pollution is one of the most important risk factors that affects people worldwide [1,2,3]. Numerous epidemiological investigations have revealed the short-term or long-term associations between high concentrations of air pollutants and increased health outcomes, including stroke, heart disease, lung cancer, diabetes, and chronic lung disease. Dense innervations in the ocular surface are extremely sensitive to environmental chemical substances. In addition, human eyes are only protected by a thin layer of tear film, causing them to be very susceptible to the harmful effect of air pollution [4,5,6].
Conjunctivitis disease is generally divided into two categories: infectious by pathogenic microbial factors and non-infectious by physicochemical factors. Conjunctivitis is the most common eye disease diagnosed in emergency departments and affects all ages, which has caused serious health and economic burdens around the world. For instance, in the United States, conjunctivitis accounts for almost one third of all eye-related diseases, with 4–6 million conjunctivitis visits annually and a treatment cost of nearly 800 million dollars [7]. In addition to the societal costs, conjunctivitis can directly influence the patients’ quality of life. Mild conjunctivitis can affect people’s learning and working, and severe conjunctivitis can cause irreversible damage to the eyes, such as decreased vision or even blindness [4]. Additionally, patients with conjunctivitis, particularly allergic conjunctivitis, always have coexisting symptoms, such as allergic asthma and rhinitis [8]. Therefore, identifying the environmental risk factors for conjunctivitis and then guiding the development of effective measures for reducing the incidence of conjunctivitis are important for public health in the field of ophthalmology. Recently, several studies have provided evidence that exposure to air pollution could significantly increase the risk of conjunctivitis development [9,10,11,12]. However, there is still significant controversy on which air pollutants pose the highest risk and which subpopulation of patients with conjunctivitis is particularly sensitive to air pollution. For example, Bourcier and colleagues [13] reported that NO2 is associated with a higher risk of conjunctivitis in the population than that of O3, while Larrieu et al. showed the opposite results [14]. In addition, Hong and co-authors found that the effect of O3 is greater for women with conjunctivitis than men, whereas the study of Fu et al. presented the opposite trend [5,6]. These inconsistent results indicate the necessity of quantitatively synthesizing and interpreting the current available evidence in order to provide comprehensive evidence for policymakers and protect the public’s health.
In this study, we aimed to perform a systematic review and meta-analysis to combine the global associations between air pollutants and conjunctivitis, and to identify the sensitive subgroups.
2. Materials and Methods
2.1. Data Source
We searched for articles published before 18 March 2019 in the following electronic databases: Web of Science, PubMed, Embase, and Scopus. The search terms included “conjunctivitis,” “pinkeye,” “air pollution,” “CO,” “NO2,” “SO2,” “O3,” “PM2.5,” and “PM10” (see Table A1). The reference lists of the included studies were further examined for additional studies.
2.2. Study Selection
2.2.1. Selection Criteria
The following inclusion criteria were utilized in this study:
1. Risk assessments on the relationship between air pollutants and health outcomes of conjunctivitis;
2. Studies providing quantitative effect estimates, such as the excess rate (ER), risk ratio (RR), odds ratio (OR), regression coefficient (β) or percentage and standard error (SE), and the respective 95% confidence interval (CI);
3. Literature using the following methodology: time-series, case-crossover, logistic regression, a generalized linear model (GLM), a generalized additive model (GAM), and a distributed lag model (DLM);
4. Studies that reported the link between exposures in the form of lag (day) and health outcome.
The exclusion criteria were as follows:
1. Not original research studies (e.g., commentary, communication, review, and meeting abstract);
2. Not related to outdoor air pollution (e.g., indoor, workplace, office);
3. Research related to clinical or animal experiments (e.g., drug trials, mice, and rabbits);
4. Research on conjunctivitis not caused by air pollution factors (e.g., mites and pollen);
5. The target was not conjunctivitis diseases (e.g., rhinoconjunctivitis or conjunctivitis of other organs).
2.2.2. Data Extraction
Data from all included studies that were extracted were as follows: the reference, study design, demographic data (e.g., GDP and population), average values of air pollutants, meteorological variables (e.g., temperature, relative humidity, and air pressure), and effect estimates (e.g., RR, regression coefficient, 95% confidence interval, and standard error). For articles with missing information, we contacted the corresponding authors by email to obtain the relevant data.
2.2.3. Quality Assessment
In order to distinguish between low-quality and high-quality studies, a quality assessment was performed. Due to the wide variety of study designs used in the literature, assessing the quality and their risk of bias can be difficult.
To the best of our knowledge, no validated scale has been developed to assess the quality of time-series and case-crossover studies. We selected and combined several items from the New Castle Ottawa Scale [15], the Cochrane risk of bias tool, and other tools [16], which were utilized in previous studies [17,18,19]. We created a five-point scoring system that included the following four aspects:
a. Conjunctivitis disease occurrence verification (0–1 points)
According to the International Classification of Diseases, studies on the causes of death encoded in revised version 9 (ICD-9), 10th revision, or ICPC-2 Code(s) (International Classification of Primary Care, Second Edition [20]) and official definitions of other countries are given a score of 1, but no score is given for studies that do not meet the criteria.
b. Quality of air pollutant measurements (0–1 points)
The quality of the air pollutant measurement can be judged according to the measurement frequency and the existence of missing data. If the measurement is made at least once a day and the missing data is <25%, the research score is 1; otherwise, the quality is assessed with 0 points.
c. Adjustment degree of confounders (0–3 points)
Adjustment for temperature and humidity is given 1 point. Additional adjustments, for example, seasonality, wind speed, or rainfall, acquire 2 points. If the long-term trend and days of the week are considered, 3 points are given. Zero points are given if there is no adjustment for temperature and humidity.
If the study gets full marks for all three components, the study was considered to be of a good quality. If any of the three components were zero, the study quality was considered to be low. All other studies were considered to be of a medium quality.
2.3. Data Synthesis and Statistical Analysis
The key objective of data synthesis was to unify the air pollutant concentration units, group the research population, and standardize the risk effect values. If studies used mg/m3, ppm, or ppb for the unit of measurement or unit of increment, all estimates were converted into μg/m3. Regarding population groupings, the included data were mainly divided into two groups: gender (male and female) and age group (>18 years old and <18 years old). In most studies, the risk estimates were expressed as ERs, ORs, or RRs with 95% CIs, and percent changes. The results presented as a regression coefficient and standard error were converted to RR. The summarized statistics are expressed as RRs with 95% CIs [21,22]. To pool the effect estimates, all estimates were standardized to an increment of 10 μg/m3 of air pollutant (CO, O3, SO2, NO2, PM2.5, and PM10) concentration.
The statistical analysis consisted of three steps: (1) computing the integrated estimates of each type of air pollutant using a fixed- or random-effect meta-analysis; (2) conducting a meta regression analysis based on the total population, GDP, and weather conditions; and (3) performing a sensitivity analysis. A meta-analysis was used to aggregate the risk estimates from all studies in detail. If the heterogeneity index (I2) was greater than 25%, the aggregate estimates were calculated using a random effect model; otherwise, we selected the fixed effect model [23]. The second step was to judge and test the source of heterogeneity. Heterogeneity was classified as high (I2 > 75%), medium (25 < I2 < 75%), or low (I2 < 25%) [24]. The sources of heterogeneity, such as the research design, regional GDP, geographic location (longitude and latitude, temperature, and humidity), and weather conditions, were further tested using a meta-regression analysis. Finally, we applied funnel charts and Begg’s [25] and Egger’s tests [26] to assess the potential impact of publishing bias. We conducted the sensitivity analysis by re-calculating the pooled effects by excluding each study to test whether our main findings were influenced by one study.
Statistical analysis and drawing were mainly conducted using R language software (R version 3.6.0; R Development Core Team, New Zealand, Australia).
3. Results
3.1. Search Results and Study Characteristics
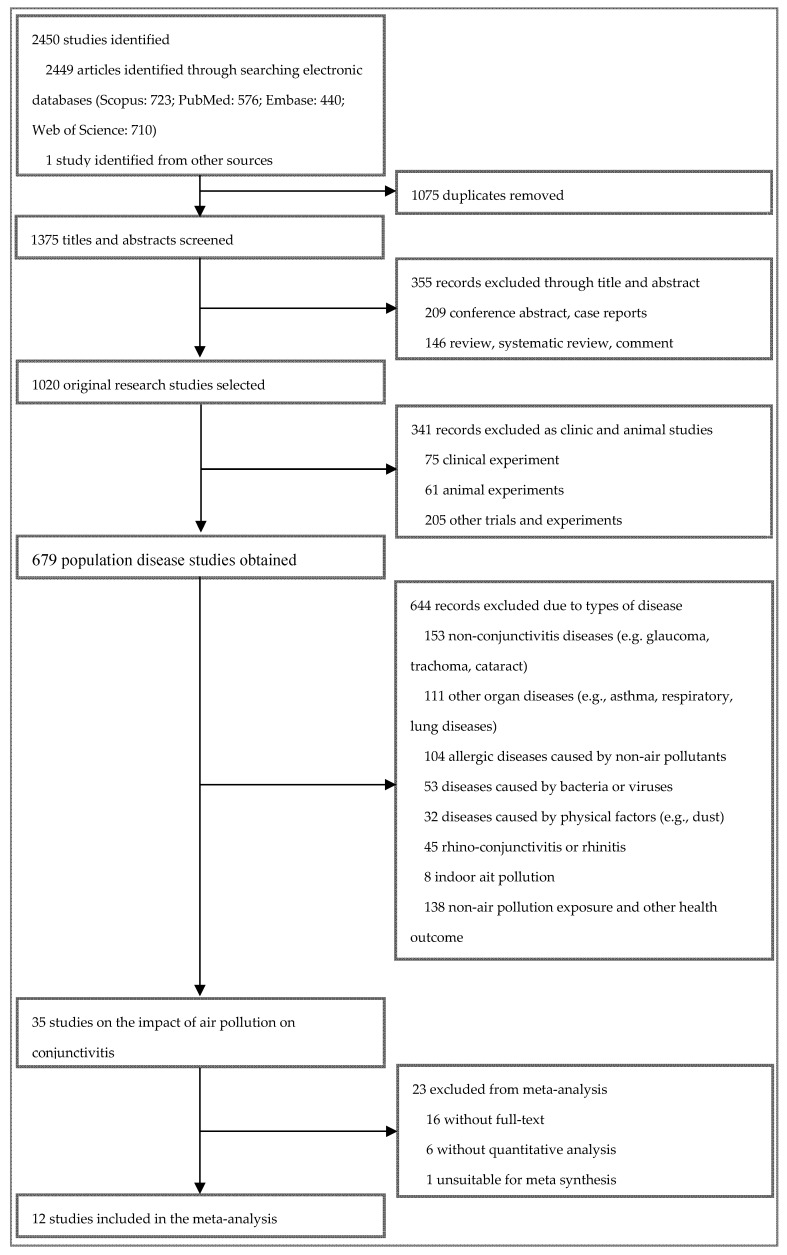
In this study, 2450 records were originally obtained from Scopus (n = 723), PubMed (n = 576), Embase (n = 440), and Web of Science (n = 710). Twelve articles from 10 regions met the inclusion criteria and were included in the meta-analysis (see Figure 1), covering 30,103,982 conjunctivitis patients. Among the 12 included studies, five were case-crossover studies [4,5,9,27,28], four were time-series studies [6,14,29,30], and three were other studies (e.g., spatial analysis and multi-level regression). Table 1 and Table A2 summarize the basic characteristics of the included studies. The number of research papers including CO, NO2, O3, PM2.5, PM10, and SO2 was two, seven, nine, four, seven, and seven, respectively.
Figure 1.
Flow chart for the study selection process.
Table 1.
The characteristics of studies included in the meta-analysis.
| Study | Location | Study Design Time-span | Study Population | Pollutant | Controlled Variables | Total Events | Lag (d/w) | Main Findings |
|---|---|---|---|---|---|---|---|---|
| Bourcier et al. (2003) [13] | Paris, France | Logistic regression 31/1/1999–31/12/1999 |
All | NO, NO2, O3, SO2, PM10 | Temperature, pressure, humidity, wind speed, day of the week |
1272 | d: 0–2 | A strong relation between NO, NO2, and conjunctivitis was observed. Atmospheric pressure, minimal humidity, and wind speed may increase the incidence of ocular surface complaints. |
| Larrieu et al. (2009) [14] | Bordeaux, France | Time series Poisson regression model 2000–2006 | All | NO2, PM10, O3 | Long-term trends, seasonality, days of the week, holidays, temperature, influenza epidemics | 179,142 | d: 0–3 | There was a much higher effect of nitrogen dioxide on visits for conjunctivitis when delayed effects were considered. Conjunctivitis was also significantly associated with PM10 and ozone levels. |
| Chang et al. (2012) [4] | Taiwan, China | Case-crossover Meta-analysis 2007–2009 |
All | CO, NO2, SO2, O3, PM10, PM2.5 | Temperature, rainfall, humidity |
26,314,960 | d: 0, 0–1 to 0–5 |
The effects on outpatient visits for nonspecific conjunctivitis were strongest for O3 and NO2. In winter, PM10 and SO2 had a more prominent impact on the risk of conjunctivitis. |
| Chiang et al. (2012) [29] | Taiwan, China (four cities) | Time series Generalized linear model 2000–2007 | All | PM10, SO2, NOx, O3 | Relative humidity, wind speed, rainfall, public holiday, calendar months and years. |
234,366 | d: 0 | There were higher risks of conjunctivitis in rural areas, but higher sensitization to air pollutants in urban cities. Children, females, and the older population were at higher risks for both types of conjunctivitis. |
| Szyszkowicz et al. (2012) [27] | Edmonton, Canada | Case-crossover Logistic regression Time-stratification 1/4/1992–31/3/2002 |
All, Sex: male, female | O3 | Long-term trends, seasonal effects, day-of-week and month-of-year effects | 7526 | d: 3–8 | For conjunctivitis, associations of these conditions with ozone exposure were observed only in females. |
| Hong et al. (2016) [6] | Shanghai, China | Time series Generalized least squares 2008–2012 | All, Sex: male, female Age: <18, 19–40, 41–60, >60 years |
SO2, NO2, PM10, PM2.5, O3 | Periodic trends | 3,211,820 | w: 1, 3 | Research revealed that higher levels of ambient NO2, O3, and temperature increased the chances of outpatient visits for allergic conjunctivitis. Meanwhile, those older than 40 years were only affected by NO2 levels. |
| Szyszkowicz et al. (2016) [28] | Ontario, Canada (nine cities) | Case-crossover Time-stratified Apr 2004–Dec 2011 |
All, Sex: male, female Age: ≤17, ≥18 years |
NO2, O3, SO2, PM2.5 | Temperature, humidity | 77,439 | d: 0–8 | There were positive associations between air pollution and ED visits for conjunctivitis, with different temporal trends and strength of association by age, sex, and season. Children and young adults were more vulnerable to conjunctivitis infections. |
| Fu et al. (2017) [5] | Hangzhou, China | Time-stratified Case-crossover Logistic regression 1/7/2014–30/6/2016 |
All, Sex: male, female Age: 0–1, 2–5, 6–18, 19–64, >65 years |
PM10, PM2.5, SO2, NO2, O3, CO | Temperature, humidity, atmospheric pressure |
9737 | d: 0, 0–1 | PM10, PM2.5, SO2, NO2, and CO were associated with the risk of conjunctivitis. SO2 was significantly associated with conjunctivitis patients between 2 and 5 years old and male. PM10 and NO2 were significantly associated with female conjunctivitis patients. |
| Jamaludin et al. (2017) [30] | Johor Bahru, Malaysian | Time series Poisson generalized linear model, negative binomial model 1/1/2012–31/12/2013 |
All | NO2, PM10, SO2 | Rainfall, temperature, humidity |
1396 | w: 14,19,20 | SO2 was the most abundant source that contributed to the eye diseases. |
| Lee et al. (2018) [11] | Daegu, Korea | Spatial analysis 1/6/2006–31/12/2014 |
All | PM10 | SO2, NO2, O3, CO | 769 | d: 0 | Incidence of conjunctivitis and keratitis varied from region to region. |
| Seo et al. (2018) [10] | Seoul, South Korea | Multi-level regression model 1/1/2011–31/12/2013 |
All | O3 | Temperature, humidity sex, age | 48,344 | d: 0 | The outpatient incidence of conjunctivitis was increased by O3. |
| Szyszkowicz et al. (2019) [9] | Edmonton, Canada | Case-crossover Time-stratified Logistic regression Apr 1992–Mar 2002 |
Sex: male, female | O3 | Temperature, humidity | 17,211 | d: 0–9 | Significant association was observed for air pollution at lag 5 day for males, and lag 1 day and lag 3 day for females. |
Note: d, day; w, week; CO, carbon monoxide; NO2, nitrogen dioxide; SO2, sulfur dioxide; O3, ozone; PM2.5, particles smaller than 2.5 μm; PM10, particles smaller than 10 μm.
3.2. Overall Analysis
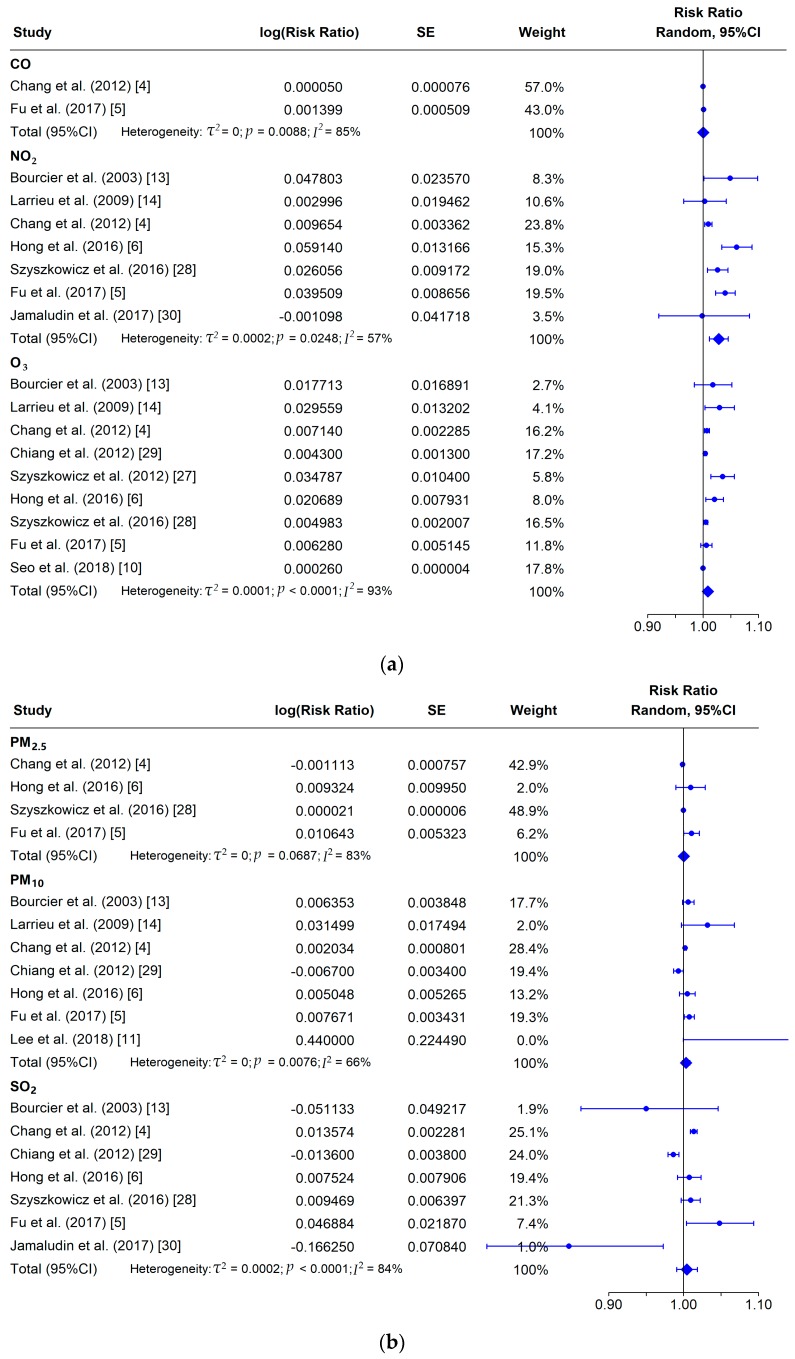
Since significant heterogeneity (I2 > 60%) was observed in the included studies, we used a random-effect meta-analysis to integrate the effect estimates of various air pollutants on conjunctivitis [31]. Figure 2 presents the pooled effect of six air pollutants on the risk of conjunctivitis among the included studies. The pooled relative risk for each 10 μg/m3 increase of air pollutants on conjunctivitis was 1.0006 (95%CI: 0.9993–1.0019) for CO, 1.0287 (95%CI: 1.0120–1.0457) for NO2, 1.0089 (95%CI: 1.0030–1.0149) for O3, 1.0004 (95%CI: 0.9976–1.0032) for PM2.5, 1.0033 (95%CI: 0.9982–1.0083) for PM10, and 1.0045 (95%CI: 0.9908–1.0185) for SO2.
Figure 2.
Forest plot of the association between conjunctivitis and exposure to air pollution: (a) CO, NO2, O3; and (b) PM2.5, PM10, SO2. Risk ratio was calculated by considering a 10 μg/m3 increase of air pollution.
3.3. Subgroup Analysis
Given the limited number of articles, we could only combine the effect estimates by subgroup for PM2.5, NO2, and O3 (Table 2). The random-effect meta-analysis was used to pool the effect risk of air pollution on conjunctivitis among subgroups as the heterogeneity was significant. Generally, the impact of air pollution was higher among females and the youth than the other groups. However, only statistically significant effects of O3 on males, with an RR value of 1.0321 (95%CI: 1.0000–1.0653), and NO2 and O3 on the youth, with corresponding RR values of 1.0472 (95%CI: 1.0249–1.0700) and 1.0357 (95%CI: 1.0156–1.0561), were found.
Table 2.
Risk analysis of air pollutants on patients with conjunctivitis, stratified by gender and age group.
| Pollutant | Groups | No. of the Studies | Heterogeneity, τ2 | Heterogeneity, p-value | Heterogeneity, I2 (%) | Summary RR (95%CI) | p-Value |
|---|---|---|---|---|---|---|---|
| PM2.5 | Male | 2 | 0.000013 | 0.2131 | 35.5 | 1.0016(0.9951–1.0081) | 0.6357 |
| Female | 2 | 0.000028 | 0.1102 | 60.8 | 1.0030(0.9943–1.0117) | 0.5050 | |
| <18year | 2 | 0.000224 | 0.0940 | 64.3 | 1.0086(0.9845–1.0332) | 0.4877 | |
| ≥18year | 2 | 0.000018 | 0.1356 | 55.1 | 1.0022(0.9952–1.0093) | 0.5324 | |
| NO2 | Male | 3 | 0.010419 | 0.0001 | 98.4 | 1.0784(0.9571–1.2151) | 0.2152 |
| Female | 3 | 0.032345 | 0.0001 | 99.6 | 1.1401(0.9233–1.4077) | 0.2231 | |
| <18year | 3 | 0.000161 | 0.2031 | 42.4 | 1.0472(1.0249–1.0700) | <0.0001 | |
| ≥18year | 3 | 0.021135 | 0.0011 | 99.5 | 1.1128(0.9371–1.3214) | 0.2228 | |
| O3 | Male | 5 | 0.000874 | 0.0083 | 88.2 | 1.0321(1.0000–1.0653) | 0.0503 |
| Female | 4 | 0.003334 | 0.0004 | 88.8 | 1.0694(0.9970–1.1471) | 0.0606 | |
| <18year | 3 | 0.000200 | 0.0160 | 72.1 | 1.0357(1.0156–1.0561) | 0.0005 | |
| ≥18year | 3 | 0.000581 | 0.0259 | 93.3 | 1.0178(0.9879–1.0487) | 0.2458 |
Note: RR—relative risk; CI—confidence interval.
3.4. Meta-Regression
In order to assess the source of the between-study heterogeneity, a meta-regression was further conducted to test the influence of city-level characteristics (e.g., GDP, longitude and latitude, average temperature, relative humidity, and duration of sunshine) on the relationship between air pollution and conjunctivitis (see Table 3). Among these factors, only the relative humidity significantly modified the risk of O3 for conjunctivitis (p = 0.023), explaining 45% of the between-study heterogeneity.
Table 3.
Meta-regression analysis of study level predictors on the association between air pollution and risk of conjunctivitis.
| Air pollutants | Covariant | IQR | Estimate | p-Value | τ2 | I2 | R 2 |
|---|---|---|---|---|---|---|---|
| NO2 | GDP | 343.07 | 0.24 (−2.69, 3.26) | 0.873 | 0.000385 | 78.586981 | 0.00 |
| Latitude | 19.21 | 0.57 (−2.25, 3.47) | 0.695 | 0.000350 | 72.085796 | 0.00 | |
| Longitude | 119.96 | 0.44 (−2.19, 3.14) | 0.745 | 0.000383 | 75.242153 | 0.00 | |
| Temperature | 4.28 | −0.43 (−2.25, 1.42) | 0.644 | 0.000497 | 85.336819 | 0.00 | |
| Humidity Duration of sunshine |
3.26 0.82 |
−2.02 (−4.35, 0.37) −2.77 (−5.60, 0.16) |
0.097 0.063 |
0.000194 0.000188 |
75.394247 63.712071 |
44.37 33.48 |
|
| O3 | GDP | 246.99 | −0.65 (−1.46, 0.17) | 0.120 | 0.000054 | 92.101803 | 0.00 |
| Latitude | 18.61 | 0.70 (−0.51, 1.91) | 0.259 | 0.000068 | 93.591351 | 0.00 | |
| Longitude | 122.10 | −0.55 (−1.37, 0.28) | 0.193 | 0.000056 | 93.062946 | 0.00 | |
| Temperature | 11.19 | −0.70 (−1.83, 0.44) | 0.227 | 0.000056 | 84.472565 | 0.00 | |
| Humidity Duration of sunshine |
4.98 0.76 |
−0.76 (−1.42, −0.10) 0.42 (−0.67, 1.52) |
0.023 0.455 |
0.000009 | 45.134099 90.739265 |
0.00 | |
| 0.000073 | 0.00 | ||||||
| PM2.5 | GDP | 238.83 | −0.40 (−1.06, 0.27) | 0.238 | 0.000018 | 66.205320 | 0.00 |
| Latitude | 7.01 | −0.07 (−0.58, 0.44) | 0.786 | 0.000047 | 63.714683 | 0.00 | |
| Longitude | 52.65 | 0.11 (−0.29, 0.51) | 0.600 | 0.000042 | 65.152911 | 0.00 | |
| Temperature | 4.28 | 0.00 (−0.55, 0.55) | 0.995 | 0.000049 | 62.464757 | 0.00 | |
| Humidity Duration of sunshine |
3.26 0.53 |
−0.17 (−1.32, 0.99) −0.52 (−1.25, 0.21) |
0.771 0.163 |
0.000030 0.000013 |
63.120757 69.255955 |
0.00 0.00 |
|
| PM10 | GDP | 266.31 | −0.71 (−2.00, 0.60) | 0.284 | 0.000033 | 69.797637 | 0.00 |
| Latitude | 12.71 | 0.51 (−0.21, 1.22) | 0.165 | 0.000021 | 59.017623 | 8.35 | |
| Longitude | 60.26 | -0.38 (−1.05, 0.30) | 0.278 | 0.000027 | 67.965036 | 0.00 | |
| Temperature | 6.13 | −0.73 (−1.77, 0.32) | 0.171 | 0.000020 | 67.213050 | 23.38 | |
| Humidity Duration of sunshine |
2.44 0.63 |
−0.32 (−0.85, 0.21) 0.04 (−1.34, 1.44) |
0.240 0.951 |
0.000020 0.000039 |
76.922096 64.984042 |
20.53 0.00 |
|
| SO2 | GDP | 230.65 | 0.20 (−2.13, 2.59) | 0.865 | 0.000314 | 89.692112 | 0.00 |
| Latitude | 15.03 | 0.99 (−1.43, 3.47) | 0.425 | 0.000319 | 89.037967 | 0.00 | |
| Longitude | 68.46 | 0.01 (−1.43, 1.47) | 0.994 | 0.000358 | 90.307176 | 0.00 | |
| Temperature | 6.58 | −0.47 (−2.26, 1.35) | 0.608 | 0.000268 | 91.617762 | 0.00 | |
| Humidity | 3.04 | −0.52 (−2.10, 1.08) | 0.523 | 0.000221 | 90.558627 | 0.00 | |
| Duration of sunshine | 0.66 | −0.71 (−4.23, 2.93) | 0.698 | 0.000380 | 89.840483 | 0.00 |
Note: CO—carbon monoxide; NO2—nitrogen dioxide; SO2—sulfur dioxide; O3—ozone; PM2.5—particles smaller than 2.5μm; PM10—particles smaller than 10μm; GDP—gross domestic product; IQR—interquartile range. The descriptive information of city-level predictors is provided in Table A2.
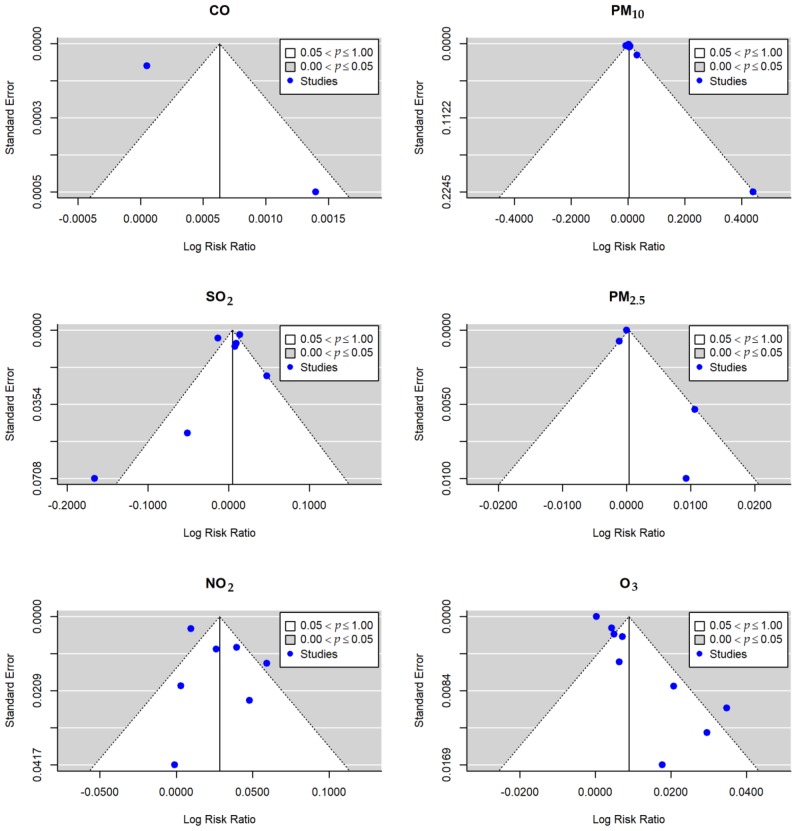
3.5. Publication Bias
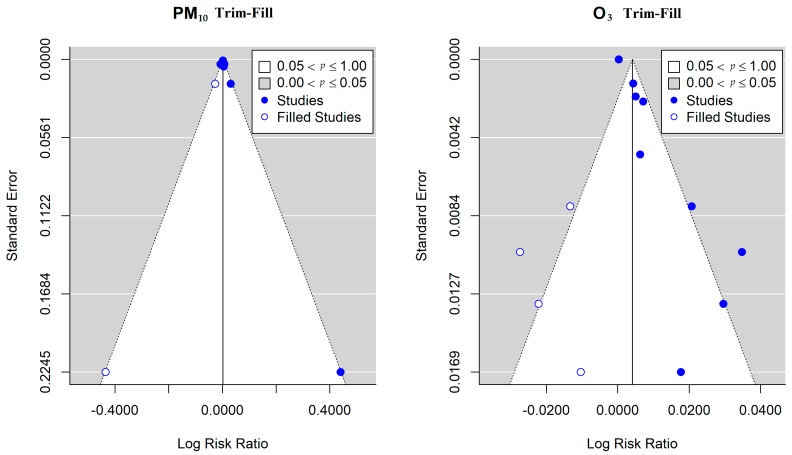
Funnel plot, Begg’s, and Egger’s tests were applied to determine whether there was publication bias. Figure 3 shows the funnel plots of the meta-analysis for the association between air pollution and the risk of conjunctivitis. The results of PM2.5, SO2, and NO2 presented a low probability of publication bias, reporting a p-value for both Begg’s test and Egger’s test of over 0.05. However, potential publication bias was detected for PM10 (Egger’s test: Z-value = 2.4238, p = 0.0154) and O3 (Egger’s test: Z-value = 5.4884, p < 0.001) (see Table 4). In addition, we performed the trim and fill method to validate the publication bias of PM10 and O3 (see Figure A1). The adjusted pooled relative risk of PM10 for total conjunctivitis was 1.0026 (95%CI: 0.9975, 1.0077) and 1.0041 (95%CI: 0.9957, 1.0126) for O3.
Figure 3.
Funnel plot showing the risk of publication bias in the meta-analysis on the risk of conjunctivitis with per 10 μg/m3 increase of air pollutants. Horizontal axis represents the log RR and vertical axis represents standard errors.
Table 4.
Begg’s test, Egger’s test, and trim-fill test on the effect of air pollutants on conjunctivitis.
| Air Pollutants | Begg’s Test | Egger’s Test | Trim-Fill-Begg’s Test | Trim-Fill-Egger’s Test | ||||
|---|---|---|---|---|---|---|---|---|
| τ | p-Value | Z-value | p-Value | τ | p-Value | Z-value | p-Value | |
| CO | 1.0000 | 1.0000 | — | — | ||||
| PM10 | 0.6190 | 0.0690 | 2.4238 | 0.0154 | 0.1715 | 0.5271 | 0.0964 | 0.9232 |
| SO2 | −0.3333 | 0.3813 | −1.6210 | 0.1050 | ||||
| PM2.5 | 0.0000 | 1.0000 | 1.8371 | 0.0662 | ||||
| NO2 | 0.0476 | 1.0000 | 0.0266 | 0.9788 | ||||
| O3 | -0.0556 | 0.9195 | 5.4884 | < 0.0001 | −0.1316 | 0.5388 | −0.0208 | 0.9834 |
Note: Egger’s test was unavailable for the CO because of the limited number of studies on the association between CO and the risk of conjunctivitis. The trim-fill test was only performed for PM10 and O3, which showed significant publication bias. CO—carbon monoxide; NO2—nitrogen dioxide; SO2—sulfur dioxide; O3—ozone; PM2.5—particles smaller than 2.5 μm; PM10—particles smaller than 10 μm.
3.6. Sensitivity Analysis
Sensitivity analyses were performed to estimate the stability of the results by recalculating the pooled effect estimates after omitting one study each time [32,33,34]. We found that the effect estimate of each 10 μg/m3 increase in the six air pollutants showed no significant change by removing one single study, suggesting that the combined results were relatively stable and reliable.
4. Discussion
To the best of our knowledge, this is the first systematic review and meta-analysis to assess the association between air pollution and conjunctivitis. Twelve studies, including 30,103,982 cases of conjunctivitis from 10 countries/regions around the world, were included. Positive associations between six common air pollutants and conjunctivitis were obtained, while statistical significance was only observed for NO2 and O3. The female subgroup and those under 18 years old were most vulnerable to the risk of conjunctivitis caused by air pollution.
4.1. Risk Analysis of Air Pollution and Conjunctivitis in the Whole Population
In the past decade, the effect of air pollution on conjunctivitis has attracted increasing interest [4,35,36]. However, the evidence so far is inconsistent (Figure 2). For instance, Fu et al. [5] revealed that the risk of NO2 and conjunctivitis in the population was significant, with an RR value of 1.0403 (95%CI: 1.0228, 1.0581), while Jamaludin et al. [30] did not find any significant effects on the risk of conjunctivitis in the population, with an RR value of 0.9989 (95%CI: 0.9205, 1.0840). For PM10, Chang et al.’s [4] study revealed that PM10 was significantly associated with the conjunctivitis risk among people, with an RR value of 1.0020 (95%CI: 1.0005, 1.0036). However, in the study of Chiang et al. [29], NO2 had no significant effect on the risk of conjunctivitis in people, with an RR value of 0.9933 (95%CI: 0.9867, 1.0000). For SO2, Fu et al.’s study [5] revealed that the risk of conjunctivitis between SO2 and the population was significant, with an RR value of 1.0480 (95%CI: 1.0040, 1.0939). In the study of Jamaludin et al. [30], SO2 had a protective effect on the conjunctivitis risk among people, with an RR value of 0.8468 (95%CI: 0.7371, 0.9730). Air pollution is gradually occupying an important position in the risk factors of conjunctivitis. Our study shows that all six air pollutants have a positive correlation with conjunctivitis. Among them, NO2 had the most significant effect, followed by O3. This may be due to differences in the physical and chemical properties between pollutants, resulting in different risk outcomes. Both NO2 and O3 are highly oxidative and irritating to the eyes [37,38,39,40]. According to the chemical properties of O3 and NO2, O3 is easily removed by a reaction, so the lifetime of NO2 is longer than that of O3 [41,42]. In addition, in terms of toxicity, the toxicity of O3 may be more complex than that of NO2 [43,44], which may have a significant potential impact on eye tissue cells. From the comprehensive analysis of the toxicity degree and lifetime of pollutants, NO2 and O3 have obvious risks for conjunctivitis in the population, among which, NO2 has the highest risk value, followed by O3.
4.2. Risk Analysis of Air Pollution and Conjunctivitis in Subgroups
According to the research analysis, PM2.5, NO2, and O3 present a higher risk for conjunctivitis in women than in men; meanwhile, PM2.5 and O3 exhibit a higher risk for conjunctivitis for people under 18 years of age than people over 18 years of age, whereas NO2 had the opposite effect. Between genders, there are three possible reasons for the greater risk of conjunctivitis in women. First, women’s physical function is generally not as good as men’s [45], so their ability to resist air pollution is relatively weak. Second, women spend more time indoors than men [46,47], and indoor air circulation is not strong, so more toxic and harmful air pollutants may more easily accumulate and then be absorbed. Third, compared with men, women prefer makeup [48], especially eye shadows, eyelashes, and contact lenses. Studies have shown that these types of eye makeup can cause discomfort to the eyes, such as dryness, pain, etc. [49,50,51,52], which may increase the risk of conjunctivitis. Therefore, in combination with the above points, the risk for females of conjunctivitis is greater than that for males. In terms of the age group, for people younger than 18 years old, the development of physical function and the defense ability is still immature and they are thus vulnerable to air pollutants. The effects of NO2 on people over 18 years of age was significantly greater than that on people under 18 years of age, which may be related to people’s living and working habits. People over the age of 18 go to work, which often involves the need to travel between cities, so there is a relatively high chance of exposure to severe air pollution scenarios [53]. Exposure to more mobile sources of pollution, such as NO2 emitted by automobiles [54], increases the risk of conjunctivitis in adults.
4.3. Source of Heterogeneity and Possible Bias
For GDP, latitude, longitude, temperature, and humidity, we observed substantial heterogeneity in the pooled effect sizes of air pollutants (NO2, O3, PM2.5, PM10, and SO2) for conjunctivitis. We found that there was a negative correlation between relative humidity and the risk of conjunctivitis for five kinds of air pollutants. There may be several explanations for this.
First, the higher the humidity in the air, the easier it is to condense and settle the solid particles in the air [55], and the easier it is to dilute the liquid or gaseous pollutants. These processes can reduce the concentration of pollutants in the air, thereby reducing the risk of conjunctivitis. Second, a high humidity will affect visibility [56], which will affect people’s travel habits; therefore, to a certain extent, it can reduce the risk of exposure to conjunctivitis. Finally, from a physiological point of view, in greater humidity, the eyes will be relatively comfortable (so it is not easy to itch the eyes, not easy to rub the eyes, etc.) and thus dry eye will not be easily caused [57]. Furthermore, it reduces the risk of conjunctivitis.
4.4. Possible Mechanisms Explaining the Relation between Conjunctivitis and Air Pollution
To date, the underlying pathophysiological mechanism of conjunctivitis caused by air pollutants is still unclear. As a human’s eyes are directly exposed to air pollution, some studies have speculated that PM2.5 [35,58,59] and PM10 [60] particles may easily cause the inadaptability of intraocular epidermal cells, leading to cell death and the inflammation of tissue cells. Second, NO2 and O3 have strong oxidative stress effects [61], which may stimulate conjunctival cell inflammation. Finally, NO2 is an acidic gas. When it enters the eyes, it easily changes the acidic and alkaline environment of the inner epidermis cells of the eyes [62], breaking the function of the eye cells and causing inflammation [63,64]. It is plausible that the association between air pollution and the risk of conjunctivitis events is a result of these important mechanistic pathways.
4.5. Limitations and Implications
Several limitations of our study should be considered. First, almost all the included references used the air pollutant data from fixed environmental monitoring stations instead of individual-level air pollutant exposures, which may have led to measurement error. Second, we included studies in the same place at different times (for example, Taiwan), which may have also had an impact on the combined value of conjunctivitis risk. Finally, few studies were available on the association between some types of air pollutants (e.g., carbon monoxide) and the risk of conjunctivitis, which led to a relatively low statistical power and limited the further stratified assessment for subgroups. Therefore, future epidemiological evidence from more countries and/or cities with a well-designed strategy is required to be able to develop more comprehensive knowledge on the effect of air pollution on the risk of conjunctivitis. Further investigations are also needed to identify the subgroups that are most vulnerable to air pollution, and the socioeconomic status should be considered. It would also be useful to explore the use of alternative exposure metrics that are more representative of individual exposure, and it would be beneficial to examine the mechanism underlying the harmful effect of air pollution on patients with conjunctivitis. Additionally, a cost-effectiveness of preventive measures for improving the air quality to reduce the incidence of conjunctivitis is also needed in future research.
5. Conclusions
This meta-analysis found that air pollution is an important factor for the risk of conjunctivitis. NO2 presented the highest impact on patients with conjunctivitis, followed by O3. For different sub-groups of patients with conjunctivitis, females and the age group under 18 years old were more sensitive to the air pollution. Notable inconsistencies in the various studies have been found for the association between air pollution and conjunctivitis, while only relative humidity significantly modified the risk of O3 for conjunctivitis, which explained 45% of the between-study heterogeneity. Our findings highlight the necessity for the reduction of air pollution levels and protection of vulnerable populations. Further research is needed to better understand the mechanisms underlying the harmful effect of air pollutants on the risk of conjunctivitis. Future well-designed epidemiological studies from more countries and/or cities are still warranted to be able to get more comprehensive knowledge and powerful evidence about the effect of air pollution on the risk of conjunctivitis and identification of the subpopulations sensitive to air pollution.
Acknowledgments
We would like to acknowledge Ms. Jing Li from Beijing Changping District Center for Disease Control and Prevention for her helpful advice on literature screening.
Abbreviations
| CO | carbon monoxide |
| NO2 | nitrogen dioxide |
| SO2 | sulfur dioxide |
| O3 | ozone |
| PM2.5 | particles smaller than 2.5 μm |
| PM10 | particles smaller than 10 μm |
| ER | excess rate |
| RR | relative risk |
| OR | odds ratio |
| β | regression coefficient |
| SE | standard error |
| CI | confidence interval |
| GLM | generalized linear model |
| GAM | generalized additive model |
| DLM | distributed lag model |
| ICD-9 | International Classification of Disease, Revision 9 |
| ICD-10 | International Classification of Disease, Revision 10 |
| ICPC-2 | Code(s) International Classification of Primary Care, Second Edition |
| GDP | gross domestic product; |
Appendix A
Table A1.
The search strategies used in the review.
| Search Field | PubMed (MeSH terms & tiab search function) |
Web of Science (TS & TI search function) |
Scopus (TITLE-ABS-KEY search function) |
Embase (ti,ab,kw search function) |
|---|---|---|---|---|
| [1] | (“conjunctivitis”[MeSH Terms] OR Conjunctivitis[Title/Abstract] OR “endophthalmitis”[MeSH Terms] OR ophthalmia[Title/Abstract] OR pinkeye[Title/Abstract] OR Pink eye[Title/Abstract]) | (TS=(“conjunctivitis” OR “endophthalmitis” OR “ophthalmia” OR “pinkeye” OR “conjunctivitis” OR “Pink eye”) OR TI=(“conjunctivitis” OR “endophthalmitis” OR “ophthalmia” OR “pinkeye” OR “conjunctivitis” OR “Pink eye”)) | TITLE-ABS-KEY(“conjunctivitis” OR “endophthalmitis” OR “ophthalmia” OR “pinkeye” OR “conjunctivitis” OR “Pink eye”) AND TITLE-ABS-KEY(“air pollution” OR “ambient air pollution” OR “outdoor air pollution” OR “atmospheric pollution”) | “conjunctivitis”:ti,ab,kw OR “endophthalmitis”:ti,ab,kw OR “ophthalmia”:ti,ab,kw OR “pinkeye”:ti,ab,kw OR “pink eye”:ti,ab,kw |
| [2] | (“air pollution”[MeSH Terms] OR air pollution[Title/Abstract] OR ambient air pollution[Title/Abstract] OR outdoor air pollution[Title/Abstract] OR atmospheric pollution[Title/Abstract]) | (TS=(“air pollution” OR “ambient air pollution” OR “outdoor air pollution” OR “atmospheric pollution”) OR TI=(“air pollution” OR “ambient air pollution” OR “outdoor air pollution” OR “atmospheric pollution”)) | TITLE-ABS-KEY(“conjunctivitis” OR “endophthalmitis” OR “ophthalmia” OR “pinkeye” OR “conjunctivitis” OR “Pink eye”) AND TITLE-ABS-KEY( “PM2.5” OR “Particulate Matter2.5” OR “particulate matter” OR “PM10” OR “Particulate Matter 10” OR “SO2” OR “Sulfur dioxide” OR “NO2” OR “Nitrogen dioxide” OR “NOx” OR “Nitrogen oxides” OR “O3” OR “ozone” OR “CO” OR “Carbon monoxide” OR “Smog” OR “black carbon”) | “air pollution”:ti,ab,kw OR “ambient air pollution”:ti,ab,kw OR “outdoor air pollution”:ti,ab,kw OR “atmospheric pollution”:ti,ab,kw |
| [3] | ( PM2.5[Title/Abstract] OR Particulate Matter2.5[Title/Abstract] OR particulate matter[MeSH Terms] OR particulate matter[Title/Abstract] OR PM10[Title/Abstract] OR Particulate Matter10[Title/Abstract] OR SO2[Title/Abstract] OR Sulfur dioxide[MeSH Terms] OR Sulfur dioxide[Title/Abstract] OR NO2[Title/Abstract] OR Nitrogen dioxide[MeSH Terms] OR Nitrogen dioxide[Title/Abstract] OR NOx[Title/Abstract] OR Nitrogen oxides[MeSH Terms] OR Nitrogen oxides[Title/Abstract] OR O3[Title/Abstract] OR ozone[MeSH Terms] OR ozone[Title/Abstract] OR CO[Title/Abstract] OR Carbon monoxide[MeSH Terms] OR Carbon monoxide[Title/Abstract] OR Smog[MeSH Terms] OR Smog[Title/Abstract] OR black carbon[MeSH Terms] OR black carbon[Title/Abstract]) | (TS=( “PM2.5” OR “Particulate Matter2.5” OR “particulate matter” OR “PM10” OR “Particulate Matter 10” OR “SO2” OR “Sulfur dioxide” OR “NO2” OR “Nitrogen dioxide” OR “NOx” OR “Nitrogen oxides” OR “O3” OR “ozone” OR “CO” OR “Carbon monoxide” OR “Smog” OR “black carbon”) OR TI=( “PM2.5” OR “Particulate Matter2.5” OR “particulate matter” OR “PM10” OR “Particulate Matter 10” OR “SO2” OR “Sulfur dioxide” OR “NO2” OR “Nitrogen dioxide” OR “NOx” OR “Nitrogen oxides” OR “O3” OR “ozone” OR “CO” OR “Carbon monoxide” OR “Smog” OR “black carbon”)) | TITLE-ABS-KEY(“conjunctivitis” OR “endophthalmitis” OR “ophthalmia” OR “pinkeye” OR “conjunctivitis” OR “Pink eye”) AND TITLE-ABS-KEY(“air pollution” OR “ambient air pollution” OR “outdoor air pollution” OR “atmospheric pollution”) AND TITLE-ABS-KEY( “PM2.5” OR “Particulate Matter2.5” OR “particulate matter” OR “PM10” OR “Particulate Matter 10” OR “SO2” OR “Sulfur dioxide” OR “NO2” OR “Nitrogen dioxide” OR “NOx” OR “Nitrogen oxides” OR “O3” OR “ozone” OR “CO” OR “Carbon monoxide” OR “Smog” OR “black carbon”) | “PM2.5”:ti,ab,kw OR “particulate matter2.5”:ti,ab,kw OR “particulate matter”:ti,ab,kw OR “PM10”:ti,ab,kw OR “particulate matter 10”:ti,ab,kw OR “SO2”:ti,ab,kw OR “sulfur dioxide”:ti,ab,kw OR “NO2”:ti,ab,kw OR “nitrogen dioxide”:ti,ab,kw OR “NOx”:ti,ab,kw OR “nitrogen oxides”:ti,ab,kw OR “O3”:ti,ab,kw OR “ozone”:ti,ab,kw OR “CO”:ti,ab,kw OR “carbon monoxide”:ti,ab,kw OR “smog”:ti,ab,kw OR “black carbon”:ti,ab,kw |
| Search strategy | ([1] AND [2]) OR ([1] AND [3]) | ([1] AND [2]) OR ([1] AND [3]) | ([1] AND [2]) OR ([1] AND [3]) | ([1] AND [2]) OR ([1] AND [3]) |
Table A2.
Supplementary information of the included literature.
| Study | Location | Population | GDP (billion dollars) | Latitude, Longitude | Temperature (°C) | Humidity (%) | Duration of Sunshine (hours) |
|---|---|---|---|---|---|---|---|
| Bourcier et al. (2003) [13] | Paris, France | 2,125,851 | 459.20 | 48.86, 2.35 | 9.31–16.90 | 54.70–89.90 | 4.54 |
| Larrieu et al. (2009) [14] | Bordeaux, France | 600,000 | 17.70 | 44.84, −0.58 | — | — | 5.57 |
| Chang et al. (2012) [4] | Taiwan, China | 23,037,031 | 392.92 | 25.03, 121.52 | 24.09 | 75.24 | 5.26 |
| Chiang et al. (2012) [29] | Taiwan, China (four cities) a | 22,689,122 | 331.01 | 25.03, 121.52 | 23.78 | 77.25 | 4.95 |
| Szyszkowicz et al. (2012) [27] | Edmonton, Canada | 626,500 | 28.80 | 53.53, -113.50 | 3.90 | 66.00 | 6.40 |
| Hong et al. (2016) [6] | Shanghai, China | 23,030,000 | 244.90 | 31.27, 121.52 | 17.20 | 69.40 | 4.88 |
| Szyszkowicz et al. (2016) [28] | Ontario, Canada (nine cities) b | 12,760,000 | 657.20 | 50.00, -85.00 | 9.09 | 72.20 | 5.64 |
| Fu et al. (2017) [5] | Hangzhou, China | 9,018,000 | 145.93 | 30.25, 120.17 | 17.90 | 74.60 | 4.69 |
| Jamaludin et al. (2017) [30] | Johor Bahru, Malaysian | 848,000 | 20.06 | 1.46, 103.76 | 25.50–27.80 | — | 5.75 |
| Lee et al. (2018) [11] | Daegu, Korea | 2,279,000 | 45.387 | 35.87, 128.60 | — | — | 6.20 |
| Seo et al. (2018) [10] | Seoul, South Korea | 10,442,426 | 280.00 | 37.53,127.02 | (7–9 month): 24.70 (1–3 month): −0.80 |
(7–9 month): 70.70 (1–3 month): 51.20 |
5.67 |
| Szyszkowicz et al. (2019) [9] | Edmonton, Canada | 626,500 | 28.8025 | 53.53, -113.50 | — | — | 6.40 |
Note: “—“, no data; “a”, Four cities included Taipei, Kaohsiung, Yunlin, and Yilan; “b”, Nine cities include Algoma, Halton, Hamilton, London, Ottawa, Peel, Toronto, Windsor, and York.
Table A3.
The quality assessment of the included literature.
| No. | Study | Conjunctivitis Disease Occurrence Verification (1 point) | Quality of Air Pollutant Measurement (1 point) | Adjustment Degree of Confounders (3 point) | Total Score (5 point) | Quality Category |
|---|---|---|---|---|---|---|
| 1 | Bourcier et al. (2003) [13] | 0 | 1 | 3 | 4 | Low quality |
| 2 | Larrieu et al. (2009) [14] | 1 | 1 | 2 | 4 | Medium quality |
| 3 | Chang et al. (2012) [4] | 1 | 1 | 2 | 4 | Medium quality |
| 4 | Chiang et al. (2012) [29] | 1 | 1 | 3 | 5 | High quality |
| 5 | Szyszkowicz et al. (2012) [27] | 1 | 1 | 2 | 4 | Medium quality |
| 6 | Hong et al. (2016) [6] | 1 | 1 | 3 | 5 | High quality |
| 7 | Szyszkowicz et al. (2016) [28] | 1 | 1 | 2 | 4 | Medium quality |
| 8 | Fu et al. (2017) [5] | 1 | 1 | 1 | 3 | Medium quality |
| 9 | Jamaludin et al. (2017) [30] | 0 | 0 | 2 | 2 | Low quality |
| 10 | Lee et al. (2018) [11] | 1 | 0 | 0 | 1 | Low quality |
| 11 | Seo et al. (2018) [10] | 1 | 0 | 1 | 2 | Low quality |
| 12 | Szyszkowicz et al. (2019) [9] | 1 | 1 | 1 | 3 | Medium quality |
Table A4.
Sensitivity meta-analysis using the leave-one-out method.
| Literature | RR(95% CI) | Z-test | p-value | Q-test | Q-p | τ2 | I2 | H2 |
|---|---|---|---|---|---|---|---|---|
| CO-3 | 1.0010(0.9990-1.0030) | 2.747 | 0.006 | 0.000 | 1.000 | 0.000000 | 0.000 | 1.000 |
| CO-8 | 1.0000(1.0000-1.0000) | 0.656 | 0.512 | 0.000 | 1.000 | 0.000000 | 0.000 | 1.000 |
| PM10-1 | 1.0030(0.9971-1.0089) | 0.873 | 0.382 | 16.265 | 0.006 | 0.000031 | 70.778 | 3.422 |
| PM10-2 | 1.0030(0.9971-1.0089) | 1.052 | 0.293 | 14.681 | 0.012 | 0.000020 | 66.979 | 3.028 |
| PM10-3 | 1.0040(0.9962-1.0119) | 1.129 | 0.259 | 17.34 | 0.004 | 0.000040 | 62.007 | 2.632 |
| PM10-4 | 1.0050(1.0011-1.0090) | 2.293 | 0.022 | 10.376 | 0.065 | 0.000009 | 40.147 | 1.671 |
| PM10-6 | 1.0030(0.9971-1.0089) | 1.006 | 0.314 | 17.192 | 0.004 | 0.000033 | 74.577 | 3.933 |
| PM10-8 | 1.0020(0.9961-1.0079) | 0.757 | 0.449 | 14.793 | 0.011 | 0.000025 | 64.491 | 2.816 |
| PM10-10 | 1.0030(0.9971-1.0089) | 1.226 | 0.220 | 13.695 | 0.018 | 0.000024 | 70.384 | 3.377 |
| SO2-1 | 1.0060(0.9923-1.0199) | 0.789 | 0.430 | 47.145 | 0.000 | 0.000193 | 86.342 | 7.322 |
| SO2-3 | 1.0010(0.9835-1.0188) | 0.155 | 0.877 | 24.433 | 0.000 | 0.000287 | 78.076 | 4.561 |
| SO2-4 | 1.0131(1.0091-1.0171) | 5.303 | 0.000 | 11.336 | 0.045 | 0.000002 | 3.03 | 1.031 |
| SO2-6 | 1.0030(0.9835-1.0229) | 0.330 | 0.742 | 48.521 | 0.000 | 0.000345 | 90.725 | 10.782 |
| SO2-7 | 1.0030(0.9835-1.0229) | 0.268 | 0.789 | 48.338 | 0.000 | 0.000348 | 90.073 | 10.073 |
| SO2-8 | 1.0020(0.9883-1.0158) | 0.224 | 0.823 | 45.144 | 0.000 | 0.000160 | 83.838 | 6.187 |
| SO2-10 | 1.0060(0.9923-1.0199) | 0.887 | 0.375 | 42.562 | 0.000 | 0.000180 | 85.515 | 6.904 |
| PM2.5-3 | 1.0050(0.9972-1.0129) | 1.051 | 0.293 | 4.856 | 0.088 | 0.000033 | 60.187 | 2.512 |
| PM2.5-6 | 1.0000(1.0000-1.0000) | 3.459 | 0.001 | 6.228 | 0.044 | 0.000000 | 0.000 | 1.000 |
| PM2.5-7 | 1.0040(0.9942-1.0139) | 0.904 | 0.366 | 5.827 | 0.054 | 0.000042 | 65.186 | 2.872 |
| PM2.5-8 | 1.0000(1.0000-1.0000) | -0.527 | 0.598 | 3.121 | 0.210 | 0.000000 | 36.356 | 1.571 |
| NO2-1 | 1.0274(1.0094-1.0457) | 2.943 | 0.003 | 23.445 | 0.000 | 0.000308 | 77.501 | 4.445 |
| NO2-2 | 1.0315(1.0134-1.0498) | 3.501 | 0.000 | 24.636 | 0.000 | 0.000293 | 76.202 | 4.202 |
| NO2-3 | 1.0356(1.0195-1.0520) | 4.463 | 0.000 | 8.329 | 0.139 | 0.000135 | 41.196 | 1.701 |
| NO2-6 | 1.0222(1.0083-1.0364) | 3.020 | 0.003 | 14.466 | 0.013 | 0.000158 | 61.183 | 2.576 |
| NO2-7 | 1.0294(1.0094-1.0498) | 2.779 | 0.005 | 24.110 | 0.000 | 0.000393 | 76.376 | 4.233 |
| NO2-8 | 1.0263(1.0064-1.0467) | 2.591 | 0.010 | 17.646 | 0.003 | 0.000341 | 72.691 | 3.662 |
| NO2-9 | 1.0294(1.0114-1.0477) | 3.392 | 0.001 | 24.980 | 0.000 | 0.000298 | 77.506 | 4.446 |
| O3-1 | 1.0090(1.0031-1.0150) | 2.783 | 0.005 | 48.211 | 0.000 | 0.000053 | 94.372 | 17.768 |
| O3-2 | 1.0070(1.0011-1.0130) | 2.802 | 0.005 | 44.353 | 0.000 | 0.000032 | 90.828 | 10.903 |
| O3-3 | 1.0101(1.0022-1.0180) | 2.622 | 0.009 | 40.214 | 0.000 | 0.000080 | 95.42 | 21.834 |
| O3-4 | 1.0111(1.0032-1.0190) | 2.793 | 0.005 | 39.621 | 0.000 | 0.000074 | 91.511 | 11.78 |
| O3-5 | 1.0050(1.0011-1.0090) | 2.982 | 0.003 | 38.258 | 0.000 | 0.000013 | 80.072 | 5.018 |
| O3-6 | 1.0070(1.0011-1.0130) | 2.648 | 0.008 | 42.643 | 0.000 | 0.000033 | 91.017 | 11.132 |
| O3-7 | 1.0111(1.0032-1.0190) | 2.745 | 0.006 | 43.741 | 0.000 | 0.000076 | 94.879 | 19.529 |
| O3-8 | 1.0101(1.0022-1.0180) | 2.689 | 0.007 | 47.910 | 0.000 | 0.000077 | 95.903 | 24.405 |
| O3-11 | 1.0111(1.0051-1.0170) | 3.218 | 0.001 | 16.862 | 0.018 | 0.000050 | 83.291 | 5.985 |
Note. The number in the column of “literature” denotes the number of the literature from Table A3 that was excluded, and effect estimates from the rest of the literature were then pooled using a meta-analysis. Q-p denotes the p-value for the Q test.
Figure A1.
Funnel plot of PM10 and O3 on conjunctivitis using Trim-fill method. The solid circles denote effect estimates from included studies and open circles denotes estimates provided by Trim-fill method.
Author Contributions
Conceptualization, J.Y. and B.W; methodology, R.C. and J.Y.; validation, R.C., C.Z., and B.L.; formal analysis, R.C. and J.Y.; data curation, R.C., H.W., and B.L.; writing—original draft preparation, R.C. and J.Y.; writing—review and editing, C.Z., F.Z., S.B., H.W., and B.W.; supervision, J.Y. and B.W.; funding acquisition, J.Y. and B.W.
Funding
This research was funded by the Fundamental Research Funds for the Central Universities (No. 11618323), the National Natural Science Foundation of China (91544215, 41373116), the National Key Research and Development Program of China (No. 2018YFC0213602), and Guangdong Provincial Science and Technology Planning Project of China (No.2017B050504002).
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Samet J.M., Gruskin S.J.T.L.R.M. Air pollution, health, and human rights. Lancet Respir. Med. 2015;3:98–100. doi: 10.1016/S2213-2600(14)70145-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sun Z., Zhu D. Exposure to outdoor air pollution and its human health outcomes: A scoping review. PLoS ONE. 2019;14:e0216550. doi: 10.1371/journal.pone.0216550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yang J., Ou C.Q., Song Y.F., Li L., Chen P.Y., Liu Q.Y. Estimating years of life lost from cardiovascular mortality related to air pollution in Guangzhou, China. Sci. Total Environ. 2016;573:1566–1572. doi: 10.1016/j.scitotenv.2016.09.014. [DOI] [PubMed] [Google Scholar]
- 4.Chang C.J., Yang H.H., Chang C.A., Tsai H.Y. Relationship between Air Pollution and Outpatient Visits for Nonspecific Conjunctivitis. Investig. Ophthalmol. Vis. Sci. 2012;53:429–433. doi: 10.1167/iovs.11-8253. [DOI] [PubMed] [Google Scholar]
- 5.Fu Q., Mo Z., Lyu D., Zhang L., Qin Z., Tang Q., Yin H., Xu P., Wu L., Lou X., et al. Air pollution and outpatient visits for conjunctivitis: A case-crossover study in Hangzhou, China. Environ. Pollut. 2017;231:1344–1350. doi: 10.1016/j.envpol.2017.08.109. [DOI] [PubMed] [Google Scholar]
- 6.Hong J., Zhong T., Li H., Xu J., Ye X., Mu Z., Lu Y., Mashaghi A., Zhou Y., Tan M., et al. Ambient air pollution, weather changes, and outpatient visits for allergic conjunctivitis: A retrospective registry study. Sci. Rep. 2016;6 doi: 10.1038/srep23858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schneider J., Scheibling C., Segall D., Sambursky R., Ohsfeldt R., Lovejoy L. Epidemiology and economic burden of conjunctivitis: A managed care perspective. J. Manag. Care Med. 2014;17:78–83. [Google Scholar]
- 8.Michailopoulos P., Almaliotis D., Georgiadou I., Papakosta D., Gougoulias K., Giouleka P., Gioulekas D., Siempis T., Karampatakis V. Allergic conjunctivitis in patients with respiratory allergic symptoms; a retrospective study in Greece. Med. Hypothesis Discov. Innov. Ophthalmol. 2017;6:3. [PMC free article] [PubMed] [Google Scholar]
- 9.Szyszkowicz M., Gordon Shaag A., Shneor E. Conjunctivitis and Exposure to Ambient Ozone. Preprints. 2019 doi: 10.20944/preprints201901.0128.v1. [DOI] [Google Scholar]
- 10.Seo J.W., Youn J.S., Park S., Joo C.K. Development of a Conjunctivitis Outpatient Rate Prediction Model Incorporating Ambient Ozone and Meteorological Factors in South Korea. Front. Pharmacol. 2018;9 doi: 10.3389/fphar.2018.01135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lee J.Y., Kim J.W., Kim E.J., Lee M.Y., Nam C.W., Chung I.S. Spatial analysis between particulate matter and emergency room visits for conjunctivitis and keratitis. Ann. Occup. Environ. Med. 2018;30 doi: 10.1186/s40557-018-0252-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nucci P., Sacchi M., Pichi F., Allegri P., Serafino M., Dello Strologo M., De Cilla S., Villani E. Pediatric Conjunctivitis and Air Pollution Exposure: A Prospective Observational Study. Semin. Ophthalmol. 2017;32:407–411. doi: 10.3109/08820538.2015.1115088. [DOI] [PubMed] [Google Scholar]
- 13.Bourcier T., Viboud C., Cohen J.C., Thomas F., Bury T., Cadiot L., Mestre O., Flahault A., Borderie V., Laroche L. Effects of air pollution and climatic conditions on the frequency of ophthalmological emergency examinations. Br. J. Ophthalmol. 2003;87:809–811. doi: 10.1136/bjo.87.7.809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Larrieu S., Lefranc A., Gault G., Chatignoux E., Couvy F., Jouves B., Filleul L. Are the Short-term Effects of Air Pollution Restricted to Cardiorespiratory Diseases? Am. J. Epidemiol. 2009;169:1201–1208. doi: 10.1093/aje/kwp032. [DOI] [PubMed] [Google Scholar]
- 15.Peterson J., Welch V., Losos M., Tugwell P. The Newcastle-Ottawa Scale (NOS) for Assessing the Quality of Nonrandomised Studies in Meta-Analyses. Ottawa Hospital Research Institute; Ottawa, ON, Canada: 2011. [Google Scholar]
- 16.Higgins J.P., Altman D.G., Gøtzsche P.C., Jüni P., Moher D., Oxman A.D., Savović J., Schulz K.F., Weeks L., Sterne J.A. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928. doi: 10.1136/bmj.d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mustafić H., Jabre P., Caussin C., Murad M.H., Escolano S., Tafflet M., Périer M.C., Marijon E., Vernerey D., Empana J.P. Main air pollutants and myocardial infarction: A systematic review and meta-analysis. JAMA. 2012;307:713–721. doi: 10.1001/jama.2012.126. [DOI] [PubMed] [Google Scholar]
- 18.Li J., Woodward A., Hou X.Y., Zhu T., Zhang J., Brown H., Yang J., Qin R., Gao J., Gu S. Modification of the effects of air pollutants on mortality by temperature: A systematic review and meta-analysis. Sci. Total Environ. 2017;575:1556–1570. doi: 10.1016/j.scitotenv.2016.10.070. [DOI] [PubMed] [Google Scholar]
- 19.Yang W.S., Wang X., Deng Q., Fan W.Y., Wang W.Y. An evidence-based appraisal of global association between air pollution and risk of stroke. Int. J. Cardiol. 2014;175:307–313. doi: 10.1016/j.ijcard.2014.05.044. [DOI] [PubMed] [Google Scholar]
- 20.World Organization of Family Doctors . ICPC-2: International Classification of Primary Care. 2nd ed. Oxford University Press; York, NY, USA: 1998. [Google Scholar]
- 21.Turner L.R., Barnett A.G., Connell D., Tonga S. Ambient temperature and cardiorespiratory morbidity: A systematic review and meta-analysis. Epidemiology. 2012;23:594–606. doi: 10.1097/EDE.0b013e3182572795. [DOI] [PubMed] [Google Scholar]
- 22.Lee W.S., Kim W.S., Lim Y.H., Hong Y.C. High Temperatures and Kidney Disease Morbidity: A Systematic Review and Meta-analysis. J. Prev. Med. Public Health. 2019;52:1. doi: 10.3961/jpmph.18.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Borenstein M., Hedges L.V., Higgins J.P., Rothstein H.R. A basic introduction to fixed-effect and random-effects models for meta-analysis. Res. Synth. Methods. 2010;1:97–111. doi: 10.1002/jrsm.12. [DOI] [PubMed] [Google Scholar]
- 24.Higgins J.P.T., Thompson S.G., Deeks J.J., Altman D.G. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Begg C.B., Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994;50:1088–1101. doi: 10.2307/2533446. [DOI] [PubMed] [Google Scholar]
- 26.Egger M., Davey Smith G., Schneider M., Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Szyszkowicz M., Porada E., Searles G., Rowe B.H. Ambient ozone and emergency department visits for skin conditions. Air Qual. Atmos. Health. 2012;5:303–309. doi: 10.1007/s11869-010-0092-5. [DOI] [Google Scholar]
- 28.Szyszkowicz M., Kousha T., Castner J. Air Pollution and Emergency Department Visits for Conjunctivitis: A Case-Crossover Study. Int. J. Occup. Med. Environ. Health. 2016;29:381–393. doi: 10.13075/ijomeh.1896.00442. [DOI] [PubMed] [Google Scholar]
- 29.Chiang C.C., Liao C.C., Chen P.C., Tsai Y.Y., Wang Y.C. Population study on chronic and acute conjunctivitis associated with ambient environment in urban and rural areas. J. Expo. Sci. Environ. Epidemiol. 2012;22:533–538. doi: 10.1038/jes.2012.37. [DOI] [PubMed] [Google Scholar]
- 30.Jamaludin A.R.B., Yusof F., Lokoman R.M., Noor Z.Z., Alias N., Aris N.M. Correlational study of air pollution-related diseases (asthma, conjunctivitis, URTI and dengue) in Johor Bahru, Malaysia. Malays. J. Fundam. Appl. Sci. 2017;13:354–361. doi: 10.11113/mjfas.v13n4-1.897. [DOI] [Google Scholar]
- 31.Higgins J.P., Green S. Cochrane Handbook for Systematic Reviews of Interventions. Volume 4 John Wiley & Sons; Hoboken, NJ, USA: 2011. [Google Scholar]
- 32.Dzhambov A.M., Dimitrova D.D. Residential road traffic noise as a risk factor for hypertension in adults: Systematic review and meta-analysis of analytic studies published in the period 2011–2017. Environ. Pollut. 2018;240:306–318. doi: 10.1016/j.envpol.2018.04.122. [DOI] [PubMed] [Google Scholar]
- 33.Sahebkar A., Cicero A.F., Simental-Mendia L.E., Aggarwal B.B., Gupta S.C. Curcumin downregulates human tumor necrosis factor-α levels: A systematic review and meta-analysis ofrandomized controlled trials. Pharmacol. Res. 2016;107:234–242. doi: 10.1016/j.phrs.2016.03.026. [DOI] [PubMed] [Google Scholar]
- 34.Sahebkar A. Are curcuminoids effective C-reactive protein-lowering agents in clinical practice? Evidence from a meta-analysis. Phytother. Res. 2014;28:633–642. doi: 10.1002/ptr.5045. [DOI] [PubMed] [Google Scholar]
- 35.Mimura T., Ichinose T., Yamagami S., Fujishima H., Kamei Y., Goto M., Takada S., Matsubara M. Airborne particulate matter (PM2.5) and the prevalence of allergic conjunctivitis in Japan. Sci. Total Environ. 2014;487:493–499. doi: 10.1016/j.scitotenv.2014.04.057. [DOI] [PubMed] [Google Scholar]
- 36.Li Z., Bian X., Yin J., Zhang X., Mu G. The Effect of Air Pollution on the Occurrence of Nonspecific Conjunctivitis. J. Ophthalmol. 2016 doi: 10.1155/2016/3628762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hamming W.J., MacPhee R.D. Relationship of nitrogen oxides in auto exhaust to eye irritation--further results of chamber studies. Atmos. Environ. 1967;1:577–584. doi: 10.1016/0004-6981(67)90025-X. [DOI] [PubMed] [Google Scholar]
- 38.Krishnan M.A., Jawahar K., Perumal V., Devaraj T., Thanarasu A., Kubendran D., Sivanesan S. Effects of ambient air pollution on respiratory and eye illness in population living in Kodungaiyur, Chennai. Atmos. Environ. 2019;203:166–171. doi: 10.1016/j.atmosenv.2019.02.013. [DOI] [Google Scholar]
- 39.Hwang S.H., Choi Y.H., Paik H.J., Wee W.R., Kim M.K., Kim D.H. Potential Importance of Ozone in the Association Between Outdoor Air Pollution and Dry Eye Disease in South KoreaOutdoor Ozone Air Pollution and Dry Eye Disease in South KoreaOutdoor Ozone Air Pollution and Dry Eye Disease in South Korea. JAMA Ophthalmol. 2016;134:503–510. doi: 10.1001/jamaophthalmol.2016.0139. [DOI] [PubMed] [Google Scholar]
- 40.Han H.K., Luk S.M., Kinsella M.T. A case report of ocular chemical injury secondary to ozone gas. Acta Ophthalmol. 2017;95:e348–e349. doi: 10.1111/aos.13171. [DOI] [PubMed] [Google Scholar]
- 41.Weschler C.J. Ozone in Indoor Environments: Concentration and Chemistry. Indoor Air. 2000;10:269–288. doi: 10.1034/j.1600-0668.2000.010004269.x. [DOI] [PubMed] [Google Scholar]
- 42.Depayras S., Kondakova T., Heipieper H.J., Feuilloley M.G., Orange N., Duclairoir-Poc C. The Hidden Face of Nitrogen Oxides Species: From Toxic Effects to Potential Cure? Emerg. Pollut. Some Strateg. Qual. Preserv. Environ. 2018:19. doi: 10.5772/intechopen.75822. [DOI] [Google Scholar]
- 43.Rietjens I.M., Poelen M.C., Hempenius R.A., Gijbels M.J., Alink G.M. Toxicity of ozone and nitrogen dioxide to alveolar macrophages: Comparative study revealing differences in their mechanism of toxic action. J. Toxicol. Environ. Health. 1986;19:555–568. doi: 10.1080/15287398609530952. [DOI] [PubMed] [Google Scholar]
- 44.Mustafa M.G., Elsayed N.M., von Dohlen F.M., Hassett C.M., Postlethwait E.M., Quinn C.L., Graham J.A., Gardner D.E. A comparison of biochemical effects of nitrogen dioxide, ozone, and their combination in mouse lung. Toxicol. Appl. Pharmacol. 1984;72:82–90. doi: 10.1016/0041-008X(84)90251-5. [DOI] [PubMed] [Google Scholar]
- 45.Miller A.E., MacDougall J.D., Tarnopolsky M.A., Sale D.G. Gender differences in strength and muscle fiber characteristics. Eur. J. Appl. Physiol. Occup. Physiol. 1993;66:254–262. doi: 10.1007/BF00235103. [DOI] [PubMed] [Google Scholar]
- 46.Brasche S., Bischof W. Daily time spent indoors in German homes – Baseline data for the assessment of indoor exposure of German occupants. Int. J. Hyg. Environ. Health. 2005;208:247–253. doi: 10.1016/j.ijheh.2005.03.003. [DOI] [PubMed] [Google Scholar]
- 47.Buonanno G., Stabile L., Morawska L. Personal exposure to ultrafine particles: The influence of time-activity patterns. Sci. Total Environ. 2014;468–469:903–907. doi: 10.1016/j.scitotenv.2013.09.016. [DOI] [PubMed] [Google Scholar]
- 48.Jones A.L., Kramer R.S.S. Facial Cosmetics and Attractiveness: Comparing the Effect Sizes of Professionally-Applied Cosmetics and Identity. PLoS ONE. 2016;11:e0164218. doi: 10.1371/journal.pone.0164218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Volpe M.G., Nazzaro M., Coppola R., Rapuano F., Aquino R.P. Determination and assessments of selected heavy metals in eye shadow cosmetics from China, Italy, and USA. Microchem. J. 2012;101:65–69. doi: 10.1016/j.microc.2011.10.008. [DOI] [Google Scholar]
- 50.Koffuor G., Anto B., Afari C., Kyei S., Gyanfosu L. Ocular discomforts following eyelash extension. J. Med. Biomed. Sci. 2012;1:55–61. [Google Scholar]
- 51.Nichols J.J., Sinnott L.T. Tear film, contact lens, and patient-related factors associated with contact lens–related dry eye. Investig. Ophthalmol. Vis. Sci. 2006;47:1319–1328. doi: 10.1167/iovs.05-1392. [DOI] [PubMed] [Google Scholar]
- 52.Velasco M.J., Bermúdez F.J., Romero J., Hita E. Variations in corneal sensitivity with hydrogel contact lenses. Acta Ophthalmol. 1994;72:53–56. doi: 10.1111/j.1755-3768.1994.tb02737.x. [DOI] [PubMed] [Google Scholar]
- 53.Kan H., Heiss G., Rose K.M., Whitsel E., Lurmann F., London S.J. Traffic exposure and lung function in adults: The Atherosclerosis Risk in Communities study. Thorax. 2007;62:873. doi: 10.1136/thx.2006.073015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bhatia S. Automobile Exhaust Emmision Control—A Review. SAE International; Pittsburgh, PA, USA: 2019. SAE Technical Paper. [Google Scholar]
- 55.Csavina J., Field J., Félix O., Corral-Avitia A.Y., Sáez A.E., Betterton E.A. Effect of wind speed and relative humidity on atmospheric dust concentrations in semi-arid climates. Sci. Total Environ. 2014;487:82–90. doi: 10.1016/j.scitotenv.2014.03.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wang X., Zhang R., Yu W. The effects of PM2. 5 concentrations and relative humidity on atmospheric visibility in Beijing. J. Geophys. Res. Atmos. 2019;124:2235–2259. doi: 10.1029/2018JD029269. [DOI] [Google Scholar]
- 57.Van Setten G., Labetoulle M., Baudouin C., Rolando M. Evidence of seasonality and effects of psychrometry in dry eye disease. Acta Ophthalmol. 2016;94:499–506. doi: 10.1111/aos.12985. [DOI] [PubMed] [Google Scholar]
- 58.Chen R., Hu B., Liu Y., Xu J., Yang G., Xu D., Chen C. Beyond PM2.5: The role of ultrafine particles on adverse health effects of air pollution. Biochim. Biophys. Acta (BBA) Gen. Subj. 2016;1860:2844–2855. doi: 10.1016/j.bbagen.2016.03.019. [DOI] [PubMed] [Google Scholar]
- 59.Gao Z.X., Song X.L., Li S.S., Lai X.R., Yang Y.L., Yang G., Li Z.J., Cui Y.H., Pan H.W. Assessment of DNA damage and cell senescence in corneal epithelial cells exposed to airborne particulate matter (PM2. 5) collected in Guangzhou, China. Investig. Ophthalmol. Vis. Sci. 2016;57:3093–3102. doi: 10.1167/iovs.15-18839. [DOI] [PubMed] [Google Scholar]
- 60.Li J., Tan G., Ding X., Wang Y., Wu A., Yang Q., Ye L., Shao Y. A mouse dry eye model induced by topical administration of the air pollutant particulate matter 10. Biomed. Pharmacother. 2017;96:524–534. doi: 10.1016/j.biopha.2017.10.032. [DOI] [PubMed] [Google Scholar]
- 61.Krishna M., Springall D., Frew A., Polak J., Holgate S. Mediators of inflammation in response to air pollution: A focus on ozone and nitrogen dioxide. J. R. Coll. Physicians Lond. 1996;30:61–66. [PMC free article] [PubMed] [Google Scholar]
- 62.Coles W.H., Jaros P.A. Dynamics of ocular surface pH. Br. J. Ophthalmol. 1984;68:549–552. doi: 10.1136/bjo.68.8.549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Callejo G., Castellanos A., Castany M., Gual A., Luna C., Acosta M.C., Gallar J., Giblin J.P., Gasull X. Acid-sensing ion channels detect moderate acidifications to induce ocular pain. Pain. 2015;156:483–495. doi: 10.1097/01.j.pain.0000460335.49525.17. [DOI] [PubMed] [Google Scholar]
- 64.Singh P., Tyagi M., Kumar Y., Gupta K., Sharma P. Ocular chemical injuries and their management. Oman J. Ophthalmol. 2013;6:83. doi: 10.4103/0974-620X.116624. [DOI] [PMC free article] [PubMed] [Google Scholar]