Abstract
Meiosis is an essential cell-division process for ensuring genetic diversity across generations. Meiotic recombination ensures the accuracy of genetic interchange between homolous chromosomes and segregation of parental alleles. Programmed DNA double-strand breaks (DSBs), catalyzed by the evolutionarily conserved topoisomerase VIA (a subunit of the archaeal type II DNA topoisomerase)-like enzyme Spo11 and several other factors, is a distinctive feature of meiotic recombination initiation. The meiotic DSB formation and its regulatory mechanisms are similar among species, but certain aspects are distinct. In this review, we introduced the cumulative knowledge of the plant proteins crucial for meiotic DSB formation and technical advances in DSB detection. We also summarized the genome-wide DSB hotspot profiles for different model organisms. Moreover, we highlighted the classical views and recent advances in our knowledge of the regulatory mechanisms that ensure the fidelity of DSB formation, such as multifaceted kinase-mediated phosphorylation and the consequent high-dimensional changes in chromosome structure. We provided an overview of recent findings concerning DSB formation, distribution and regulation, all of which will help us to determine whether meiotic DSB formation is evolutionarily conserved or varies between plants and other organisms.
Keywords: meiosis, double-strand break (DSB), hotspot, homologous recombination, DSB regulation, plants, chromatin
1. Introduction
In flowering plants, reproductive cells develop in the ‘sporophytic generation’ and then differentiate into the gamete-forming ‘gametophytic generation’ [1,2]. During the reproductive process, the production of gametes requires that the genetic complement be reduced by one-half [3,4,5]. This specialized nuclear division, called meiosis, includes one round of DNA replication followed by two successive rounds of cell division, thereby ensuring the ploidy of the zygotic genome [6,7]. In the first meiotic division (meiosis I), homologous chromosomes are brought in close to pair and undergo synapsis, promoting the reciprocal exchange of parental chromosome fragments and thereby increasing the genetic diversity of the progeny [7,8,9]. Meiotic recombination is initiated by the formation of programmed DNA double-strand breaks (DSBs) catalyzed by the evolutionarily conserved type II topoisomerase–like enzyme SPO11 and several accessary proteins [9,10,11,12,13]. After resection, two SPO11 molecules remain covalently bound to each 5′ end of the nicked DNA, which is then processed by the MRX complex (Mre11-Rad50-Xrs2) with the cooperation of Sae2 to release the SPO11-bound nicked DNA oligonucleotide [14,15,16,17,18,19]. Following the action of the 5′ to 3′ exonuclease Exo1, the DNA ends are further degraded to produce 3′ single-stranded DNA tails [9,20,21,22]. The RecA family recombinases RAD51 and DMC1 bind these single-stranded DNA tails, generating DNA-protein filaments that search and invade the homologous duplex DNA [23,24,25,26,27,28,29,30,31,32,33], resulting in the formation of the recombination intermediate D-loop structure, followed by DNA repair and synthesis [34,35,36]. Consequently, the D-loop can be processed via different pathways to yield either crossovers or non-crossovers [37,38,39,40,41]. In the past decades, a growing body of knowledge related to meiotic DSB initiation has been obtained from various plant species. Although the core molecular processes that generate DSBs are largely conserved between plants and other species, the exact mechanisms involved vary among species. In this review, we introduced a generalized framework for the key players and regulatory pathways that produce DSBs and compare the conserved and non-conserved mechanisms of recombination initiation between plants and other species.
2. Conserved Spo11 and Non-Conserved Spo11 Accessary Proteins in Different Species
The programmed induction of DSBs along chromosomes is catalyzed by Spo11, which is a homolog of the A subunit of an archaeal topoisomerase (TopoVI), and this is a general mode for initiating meiotic recombination in fungi, invertebrates, mammals, and plants [4,42]. However, Spo11 does not act alone, as many other accessory proteins participate in the catalysis (Table 1). In Saccharomyces cerevisiae (S. cerevisiae), nine DSB accessory proteins have been characterized, namely Ski8, Rec114, Mei4, Mer2, Rec102, Rec104, Mre11, Rad50 and Xrs2 [4]. Ski8, a direct partner of Spo11, acts as a scaffold protein that recruits other DSB proteins to meiotic chromosomes. Rec114-Mei4-Mer2 constitutes a functional subgroup that promotes DSB formation at chromosome axes [43,44,45,46]. Rec102 and Rec104 form a subcomplex to bridge the Rec114-Mei4-Mer2 subcomplex and the Spo11–Ski8 subcomplex [44,47,48,49]. The MRX subcomplex, consisting of Mre11, Rad50 and Xrs2, is not only essential for break end resection, but is also involved in DSB formation via interaction with Mer2 [50,51,52].
Table 1.
Characterized proteins involved in meiotic double-strand breaks (DSB) formation in different organisms.
| Arabidopsis Thaliana | Oryza Sativa | Saccharomyces Cerevisiae | Schizosaccharomyces Pombe | Mus Musculus |
|---|---|---|---|---|
| DFO | ||||
| PRD1 | MEI1 | |||
| PRD2 | Mei4 | Rec24 | MEI4 | |
| PRD3 | PAIR1 | Mer2/Rec107 | Rec15 | IHO1 |
| Spo11-1,2 | Spo11-1,4 | Spo11 | Rec12 | SPO11 |
| TopoVI B | TopoVI B | TopoVI B | ||
| P31comet/BVF1 | P31comet | |||
| SDS | SDS | |||
| - | CRC1 | |||
| Rec102 | ||||
| Rec104 | ||||
| Rec6 | ||||
| Rec114 | Rec7 | REC114 | ||
| Rec10 | ||||
| Ski8/Rec103 | Rec14 | WDR61 | ||
| Mde2 | ||||
| MRE11 | Mre11 | Rad32 | ||
| RAD50 | Rad50 | Rad50 | RAD50 | |
| NBS1 | Xrs2 | Nbs1 | NBS1 |
In Schizosaccharomyces pombe (S. pombe), seven DSB accessory proteins have been identified in addition to the Spo11 ortholog Rec12 [4]. Some of these proteins have homologs in S. cerevisiae, such as Rec14 (Ski8), Rec24 (Mei4), Rec7 (Rec114) and Rec15 (Mer2), while the other three, Rec6, Rec10 and Mde2, have no clear homologs in S. cerevisiae or other organism [53,54,55,56,57,58]. The protein subcomplex is organized in both similar and dissimilar ways between S. cerevisiae and S. pombe. Rec12, Rec14 and Rec6 form the DSB catalytic core (commonly known as DSBC) in S. pombe [55]. Rec7, Rec24 and Rec15 assemble into the SFT (seven-fifteen-twenty four) subcomplex, and the interrelations among these partners are dependent on Rec10, which is the Red1 homolog in S. cerevisiae [55,56,59,60]. Although Rec10 is essential for DSB formation in S. pombe, Red1 is dispensable in S. cerevisiae [4]. Mde2 stabilizes the SFT complex via interaction with Rec15 at DSB sites. Interestingly, the Rad32-Rad50-Nbs1 complex, which corresponds to MRX in S. cerevisiae, is only required for DSB repair and not for DSB induction in S. pombe [61].
In mice, except for the conserved protein SPO11, another five proteins are indispensable for DSB formation, including REC114, MEI4, IHO1, MEI1 and TOPOVIBL [4,62,63]. The subcomplex comprising REC114, MEI4 and IHO1 is analogous to Rec114-Mei4-Mer2 in S. cerevisiae, and this subcomplex co-localizes with chromosome axes via the interaction between IHO1 and HORMAD1, which are key components of the cohesin complex [64,65,66]. MEI1 is a scaffold protein that assists with the localization of MEI4 at chromosome axes [67,68,69]. TOPOVIBL, encoding the TopoVIB subunit of TopoVI DNA topoisomerase, interacts with SPO11 to form a canonical TopoVI catalytic complex that induces DSBs [70].
The model plant Arabidopsis thaliana (Arabidopsis) has three Spo11 homologs, namely AtSpo11-1, AtSpo11-2 and AtSpo11-3. Two of them, AtSpo11-1 and AtSpo11-2, are essential for DSB formation [11,71,72,73,74], whereas AtSpo11-3 is not required for meiosis [75,76]. TopoVIB was recently identified to mediate the formation of the AtSpo11-1/AtSpo11-2 heterodimer in Arabidopsis, explaining the non-redundant nature between AtSpo11-1 and AtSpo11-2 [13,77]. In addition, AtPRD1, AtPRD2 and AtPRD3 have been demonstrated to be indispensable for DSB formation, whereas their functional mode in catalyzing DSB formation remain elusive [12,78,79]. By performing sophisticated in silico studies [4,8,78], the orthologs of AtPRD1, AtPRD2 and AtPRD3 have been suggested to be Mei1 in mice, Mei4 in yeast and Mer2 in yeast, respectively [12,80,81]. However, these proteins exhibit enormous sequence divergence with their orthologs in yeast or mice. Moreover, AtDFO encodes a plant-specific protein without any known conserved domain, and no homolog has been identified outside the plant kingdom [82].
In rice, six DSB-forming proteins have been identified so far, namely OsSPO11-1, OsSPO11-4, OsMTOPVIB, OsSDS, OsCRC1 and P31comet/OsBVF1. Although the participation of OsSPO11-1, OsSPO11-4 and OsMTOPVIB in DSB formation is conserved, as is their homologs in other species [83,84,85,86], the homologs of OsSDS and OsCRC1 in Arabidopsis, which are SDS and PCH2, respectively, are not required for DSB formation [87,88]. These findings indicate that the DSB-forming machinery has substantially diverged between monocot and dicot plants. Moreover, P31comet was first identified to be involved in the spindle assembly checkpoint in human cells [89]. However, the rice P31comet, also named OsBVF1 in an independent study, was unambiguously demonstrated to be required for DSB formation [90,91]. Taken together, these results indicate that, except for Spo11 proteins, few of the other Spo11 accessory proteins are conserved at the sequence or functional level across the eukaryotic kingdoms.
3. Defining Meiotic DSB Hotspots in Different Species
Meiotic DSBs are not randomly distributed along eukaryotic chromosomes; rather, they are concentrated within discrete regions described as DSB hotspots [92]. To generate a high-resolution physical map of the meiotic DSB landscape, several methodologies have been established over the past three decades. The early method based on gel electrophoresis was used to measure DSBs at low efficiency in yeast and mouse [93,94,95,96,97]. Recently, mapping eukaryotic DSB landscapes has been momentously intensified with advances in intricate technologies, including SPO11-oligo mapping and single-stranded DNA sequencing (SSDS). SPO11-oligo mapping is achieved by immunoprecipitating tagged-Spo11 bound with oligonucleotides, which subsequently go through end-labeling, purification and sequencing [98]. This method has been effectively applied in several yeast species, mouse and Arabidopsis (Table 2) [92,99,100,101,102]. SSDS takes advantage of an antibody specifically recognizing either one of two DNA recombinases, RAD51 or DMC1, and utilizes chromatin immunoprecipitation to enrich single-stranded DNA that has undergone single-end invasion. With the high-throughput sequencing of enriched single-stranded DNA, the genome-wide distribution of DSB hotpots was successfully obtained in maize, mouse and human [103,104,105] (Table 2).
Table 2.
Meiotic DSB hotspots identified in different species by SPO11-oligo mapping or single-stranded DNA sequencing (SSDS).
| Species | Genome Size | Chromosome No. | Number of DSBs | DSB Hotspot No. | Most Common DSB Location |
Average Width (kb) | Predominantly DSB Formation Among Transposon |
Method | Hotspot Detection | References |
|---|---|---|---|---|---|---|---|---|---|---|
| S. cerevisiae (SK1) | 12.1 Mb | 16 | ~175 | 3604–4099 | Gene promoters | 0.248–0.264 | Ty retrotransposons | SPO11-oligos | Enrichment threshold | [92,101,110,111] |
| S. cerevisiae (YPS128) | 12.1 Mb | 16 | ~175 | 4177 | Gene promoters | 0.265 | n/a | SPO11-oligos | Enrichment threshold | [112] |
| S. cerevisiae (UWOPS03-461.4) | 12.1 Mb | 16 | ~175 | 3881 | Gene promoters | 0.256 | n/a | SPO11-oligos | Enrichment threshold | [112] |
| S. pombe | 13.8 Mb | 3 | ~60 | 603 | All chromosome regions | 1.4 | n/a | Rec12-oligos | Enrichment threshold | [113] |
| M. musculus (9R×13R) | 2.8 Gb | 20 | ~250 | 9874– 15,677 |
Intergenic | ~2.000–3.400 | LTR retrotransposons SINE |
SSDS | Peak calling | [103,104] |
| M. musculus (9R) | 2.8 Gb | 20 | ~250 | 14,869 | Intergenic | ~2.000 | n/a | SSDS | Peak calling | [104] |
| M. musculus (13R) | 2.8 Gb | 20 | ~250 | 15,481 | Intergenic | ~2.000 | n/a | SSDS | Peak calling | [104] |
|
M. musculus (B6) |
2.8 Gb | 20 | ~250 | 18,313 | Intergenic | ~2.000 | n/a | SSDS | Peak calling | [104] |
|
M. musculus (B6) |
2.8 Gb | 20 | ~250 | 13,960 | Intergenic | ~0.281 | n/a | SPO11-oligos | Enrichment threshold | [100] |
| Arabidopsis thaliana | 135 Mb | 5 | ~250–300 | 5914 | Gene promoters and terminators | 0.823 | Helitron /Pogo/Tc1/Mariner DNA transposons | SPO11-1-oligos | Peak calling | [99] |
| Zea mays | 2.4 Gb | 10 | ~500 | 3126 | All chromosome regions | 1.2 | Gypsy retrotransposons | SSDS | Peak calling | [105] |
DSB hotspot designation is governed by a hierarchy of interrelated factors, including cis-regulatory elements, chromatin accessibility and high-order chromosome architecture (Table 2) [106]. Of particular prominence is the striking presence of DSB hotspots in repetitive DNA, such as transposon elements, although this aspect varies among species [10]. In maize, only ~25% of DSB hotspots have been discovered near genes, and the remaining hotspots are distributed in repetitive DNA—predominantly in Gypsy retrotransposons [105]. Although the Arabidopsis genome has fewer transposons compared with maize, a proportion of DSB hotspots were identified in Helitron/Pogo/Tc1/Mariner/MuDR DNA transposons [99]. Similarly, although most DSB hotspots tend to occur in genic regions, a significant overlap have been observed within the MULE-MuDR, TcMar-Mariner, hAT-Charlie and PiggyBac transposon families [107]. Moreover, DSB hotspots are evident in Ty retrotransposons in budding yeast. What is well established is that the meiotic recombination within or adjacent to repetitive elements is rare because this could lead to homologous recombination between non-allelic repeats, likely resulting in harmful chromosomal rearrangements and compromising genome stability [108,109]. Therefore, the paradox between the appearance of DSB hotspots and the suppression of recombination in these repetitive regions suggests that a mechanism exists to safeguard the repair of DSB in repetitive regions and prevent inadvertent crossing over.
4. Control of Meiotic DSB Formation by Protein Phosphorylation
4.1. Cyclin-Dependent Kinases (CDKs)
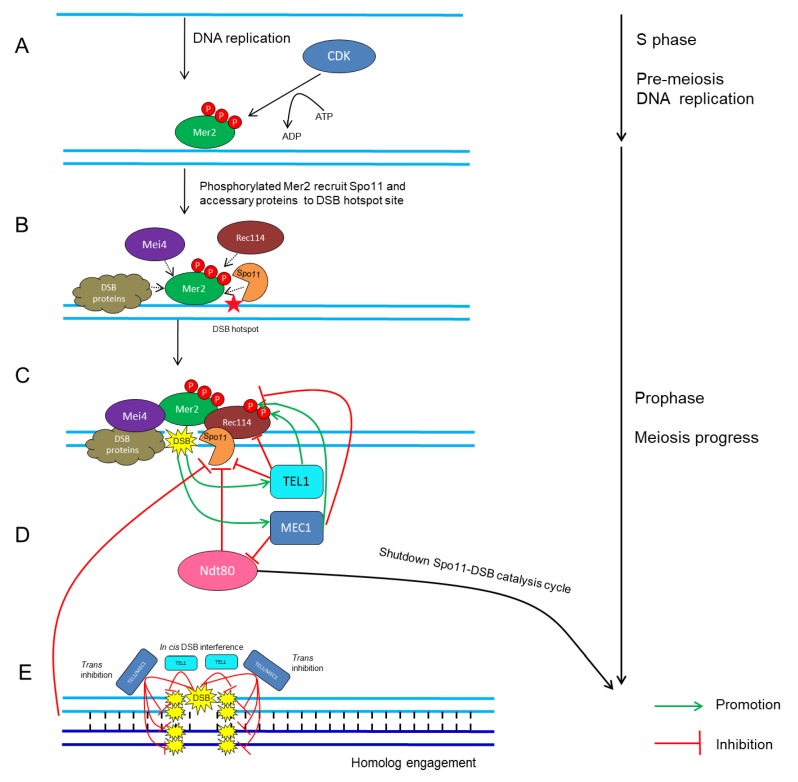
CDKs in conjunction with their cyclin partners represent an ancient molecular switch that promotes and regulates cell-cycle progression [4,114,115]. The fundamental theme of how CDKs mediate meiotic recombination initiation was mostly drawn from studies in yeast. In S. cerevisiae, the activation of Cdc28 by its two B-type cyclin partners, Clb5 and Clb6, stimulates the phosphorylation on Mer2 during pre-meiotic DNA replication (Figure 1A) [115,116,117,118,119]; subsequently, phosphorylated Mer2 recruits other Spo11-accessary proteins to initiate DSB formation (Figure 1B) [45,120,121]. Similarly, in S. pombe, the association between Cdc2 and any of the three cyclins, Crs1, Cig1 and Cig2, is crucial for DSB formation [114].
Figure 1.
Schematic network of CDK- and ATM/ATR-mediated regulatory cycles of meiotic DSB timing and number in S. cerevisiae. (A) CDK phosphorylates Mer2 during pre-meiotic DNA replication [114,115,120]. (B) Phosphorylated Mer2 recruits Rec114, Mei4, Spo11 and other DSB proteins to DSB hotspot sites [45,120,121]. (C) DSB formation catalyzed by Spo11 and accessary proteins [4,66]. (D) Recurrent DSB formation activates TEL1/MEC1-dependent positive- and negative-feedback loops, which then restrains Spo11 activity and regulates the rate and number of DSB formation [136,140,147,156]. (E) Cis DSB interference mediated by TEL1 reduces the frequency of coincident DSB formation at the region adjacent to an already-formed DSB [143,149]. Trans inhibition mediated by TEL1 and MEC1 describes the ability of a DSB formation on one chromosome to suppress DSB formation on its homolog and sister chromatid at the same or adjacent regions [141].
In Arabidopsis, there are at least five types of cell-cycle CDKs (CDKA;1, CDKB;1, CDKB1;2, CDKB2;1 and CDKB2;2) and more than 50 cyclins [122,123,124,125,126,127,128,129,130,131], of which a few have been characterized as functioning during meiosis. CDKA;1 was originally identified as the key regulator for both mitosis and meiosis progression in Arabidopsis [129,131,132], and very recently, CDKA;1 was identified as a major regulator of meiotic recombination by mediating the number and placement of crossovers of homologous chromosomes [124,131]. However, CDKA;1 seems not to regulate meiotic prophase I although the exact influence on DSB formation was not determined in that study [124]. SDS, a cyclin protein, has been shown to control the formation of crossovers, but not DSBs, which differs from the necessity of its rice homologs in DSB formation [87,127,132,133]. TAM, an A-type cyclin (CYCA1;2), is indispensable for meiosis termination at the end of the first meiotic division [134,135]. Therefore, the core CDKs or related cyclins that directly regulate DSB formation remain to be explored in plants.
4.2. Tel1/ATM and Mec1/ATR
Once DSBs are made, there are several mechanisms to maintain the proper number of DSBs within a chromosome region [95,111,136,137,138,139,140,141]. This phenomenon, termed DSB homeostasis, prevents the severe effects caused by excess or insufficient Spo11 activity [136,137,140,142,143]. In S. cerevisiae, Tel1 and the related protein kinase Mec1 act as key factors that synergistically fine-tune the number of DSBs [141,142,144]. Lack of Tel1 leads to a considerable increase in the level of Spo11-oligonucleotide complexes, which are the by-product of meiotic DSB formation, indicating that Tel1 negatively regulates DSB formation (Figure 1C) [95]. In contrast, a defect in Mec1 activity causes a dramatic reduction in DSB number via inhibition of Ndt80, which triggers the exit from pachytene and shuts down Spo11-DSB catalysis of the cell cycle [145,146]. Mec1 ensures persistent Spo11 activity and indirectly promotes DSB formation, revealing that Mec1 positively regulates DSB formation (Figure 1C) [147,148]. In addition, recent studies in S. cerevisiae have revealed a cis-regulatory machinery, termed DSB interference, that reduces the frequency of coincident DSBs at the region adjacent to the preexisting DSB [136,142,143], and this process is dependent on TEL1 over short distances of ~70–100 kb (Figure 1D) [95,149]. On the contrary, the other machinery, known as trans-inhibition, defines the ability of a DSB to occur on a single chromosome, and this suppresses DSB designation on its homolog and sister chromatid at the same locus [141]. This mechanism relies on both Tel1 and Mec1, ensuring that an interhomolog interaction surrounding a DSB will not occur twice at the allelic or two nearby chromosomal positions so that DSBs are constrained to one per pair of homologs (Figure 1E) [141]. Interestingly, homolog engagement can also restrict the number of DSBs by inhibiting Spo11 activity (Figure 1E) [101,150]. Moreover, Tel1 and/or Mec1 can phosphorylate Rec114 directly, which limits its interaction with DSB hotspots and consequently reduces DSB formation genome wide (Figure 1C) [140].
In Arabidopsis, Tel1 and Mec1 homologs exist, termed ATM and ATR, respectively. However, ATM and ATR play synergetic roles in maintenance of genomic stability in meiotic cells by processing Spo11-dependent DSBs rather than influencing DSB formation [151,152,153,154,155]. Therefore, either ATM/ATR-mediated signaling pathway is evolutionarily divergent across different species or the effects of ATM/ATR on DSB formation is too subtle to be detected by conventional methods.
5. Control of Meiosis DSB Formation in the Context of ‘Tethered Loop-Axis Complex’
Spatiotemporal control of DSB formation takes place within a specialized chromosomal structure featuring replicated sister chromosomes organized into linear looped arrays that emanate from a central proteinaceous axis [4,66,106,157,158]. However, DSBs are known to occur primarily on DNA sequences in the loops, whereas most of the Spo11 accessory proteins are located on the axis [43,44,45,66,159]. To reconcile this spatial inconsistency, different species have adapted a similar ‘tethered-loop/axis complex’ system in which DSB loci within chromatin loops can be tethered to the chromosome axis by DSB-promoting factors [66,157,160], although key players in this system are evolutionarily divergent across various species [158]. In S. cerevisiae, for example, Spp1, a PHD finger domain protein and Set1 COMPASS complex member, binds to H3K4me2/3 adjacent to DSB-prone sites on loops and interacts transiently with axis-bound Mer2, establishing a spatial linker between DSB loci and the DSB-forming machinery, thus promoting DSB formation on chromosome axes (Figure 2A) [158,161,162]. In mouse, PRDM9, the meiosis-specific histone methyltransferase, defines potential DSB sites through catalyzing trimethylation of H3K4 [163], and via its KRAB domain interacts with CXXC1, the ortholog of S. cerevisiae Spp1 [158,164], which correspondingly interacts with IHO1, a DSB-promoting protein located on chromosome axes (Figure 2B) [165,166].
Figure 2.
Different ‘tethered loop–axis complex’ models in S. cerevisiae, M. musculus and plants. (A) In S. cerevisiae, Spp1 recognizes and binds to DSB hotspots adjacent to H3K4me2/3 on loops via interaction with axis-bound Mer2 [55,161,162]. (B) In M. musculus, CXXC1 interacts with PRDM9 and IHO2, which designates DSB hotspots in loops and is located on chromosome axes, respectively; this interaction tethers the chromatin loop to the axis for DSB formation [158,163,164,165,166]. (C) In plants, the major players involved in bridging chromatin loops with axis remain uncharacterized [88,91,167,168,169].
In plants, although the formal ‘tethered-loop/axis complex’ system remains to be established, there are many indications that this may also be the case. Recently, using super-resolution microscopy, Spo11-1 and DSBs sites were found to be associated with chromosome axes in maize [167]. Additionally, several structural components of the axial elements have been demonstrated to be required for maintaining the normal number of DSB formation, such as ASY3 in Arabidopsis [168], CRC1 and P31comet in rice [88,91] and DSY2 in maize [169]. However, the direct factors bridging chromosome axis components and the DSB-forming machinery await characterization (Figure 2C).
6. Concluding Remarks
In recent years, much progress has been made toward understanding meiotic DSB initiation and distribution in plants. These advances allowed researchers to translate acquired knowledge from model species to various crops, which facilitated breeding programs [170]. However, although commonalities exist, different strategies and mechanisms for the spatiotemporal initiation and regulation of DSB formation have evolved for diverse plant species, primarily owing to differences in genome size and chromatin organization. Recent innovations in CRISPR/Cas9-mediated gene editing, as well as high-throughput sequencing have greatly accelerated functional genomics studies in many important crop species with more complex genomes, including polyploidy. Therefore, additional studies of meiotic genes that regulate the initiation of recombination in these plant species would deepen our understanding on how the designation and distinction of DSBs diverged between species from the evolutionary aspect.
Acknowledgments
We thank all the members of our laboratories for helpful discussions during the preparation of this manuscript.
Author Contributions
The manuscript was prepared by J.-L.J., T.Z., Y.-Z.W. and Y.H. All authors read and approved the final manuscript.
Funding
This work was supported by a grant from the National Natural Science Foundation of China (31671277) to Y.H.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Feng X., Zilberman D., Dickinson H. A conversation across generations: Soma-germ cell crosstalk in plants. Dev. Cell. 2013;24:215–225. doi: 10.1016/j.devcel.2013.01.014. [DOI] [PubMed] [Google Scholar]
- 2.Nakajima K. Be my baby: Patterning toward plant germ cells. Curr. Opin. Plant Biol. 2018;41:110–115. doi: 10.1016/j.pbi.2017.11.002. [DOI] [PubMed] [Google Scholar]
- 3.Saito T.T., Colaiácovo M.P. Cold Spring Harbor Symposia on Quantitative Biology. Volume 82. Cold Spring Harbor Laboratory Press; Cold Spring Harbor, NY, USA: 2018. Regulation of Crossover Frequency and Distribution during Meiotic Recombination; pp. 223–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lam I., Keeney S. Mechanism and regulation of meiotic recombination initiation. Cold Spring Harb. Perspect. Biol. 2014;7:a016634. doi: 10.1101/cshperspect.a016634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mercier R., Mezard C., Jenczewski E., Macaisne N., Grelon M. The molecular biology of meiosis in plants. Annu. Rev. Plant Biol. 2015;66:297–327. doi: 10.1146/annurev-arplant-050213-035923. [DOI] [PubMed] [Google Scholar]
- 6.Keeney S., Lange J., Mohibullah N. Self-organization of meiotic recombination initiation: General principles and molecular pathways. Annu. Rev. Genet. 2014;48:187–214. doi: 10.1146/annurev-genet-120213-092304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lambing C., Heckmann S. Tackling Plant Meiosis: From Model Research to Crop Improvement. Front. Plant Sci. 2018;9 doi: 10.3389/fpls.2018.00829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Edlinger B., Schlogelhofer P. Have a break: Determinants of meiotic DNA double strand break (DSB) formation and processing in plants. J. Exp. Bot. 2011;62:1545–1563. doi: 10.1093/jxb/erq421. [DOI] [PubMed] [Google Scholar]
- 9.Wang Y., Copenhaver G.P. Meiotic Recombination: Mixing It Up in Plants. Annu. Rev. Plant Biol. 2018 doi: 10.1146/annurev-arplant-042817-040431. [DOI] [PubMed] [Google Scholar]
- 10.Zelkowski M., Olson M.A., Wang M., Pawlowski W. Diversity and Determinants of Meiotic Recombination Landscapes. Trends Genet. TIG. 2019 doi: 10.1016/j.tig.2019.02.002. [DOI] [PubMed] [Google Scholar]
- 11.Hartung F., Wurz-Wildersinn R., Fuchs J., Schubert I., Suer S., Puchta H. The catalytically active tyrosine residues of both SPO11-1 and SPO11-2 are required for meiotic double-strand break induction in Arabidopsis. Plant Cell. 2007;19:3090–3099. doi: 10.1105/tpc.107.054817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.De Muyt A., Vezon D., Gendrot G., Gallois J.L., Stevens R., Grelon M. AtPRD1 is required for meiotic double strand break formation in Arabidopsis thaliana. EMBO J. 2007;26:4126–4137. doi: 10.1038/sj.emboj.7601815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vrielynck N., Chambon A., Vezon D., Pereira L., Chelysheva L., De Muyt A., Mezard C., Mayer C., Grelon M. A DNA topoisomerase VI-like complex initiates meiotic recombination. Science. 2016;351:939–943. doi: 10.1126/science.aad5196. [DOI] [PubMed] [Google Scholar]
- 14.Waterworth W.M., Altun C., Armstrong S.J., Roberts N., Dean P.J., Young K., Weil C.F., Bray C.M., West C.E. NBS1 is involved in DNA repair and plays a synergistic role with ATM in mediating meiotic homologous recombination in plants. Plant J. 2007;52:41–52. doi: 10.1111/j.1365-313X.2007.03220.x. [DOI] [PubMed] [Google Scholar]
- 15.Bleuyard J.Y., Gallego M.E., White C.I. Meiotic defects in the Arabidopsis rad50 mutant point to conservation of the MRX complex function in early stages of meiotic recombination. Chromosoma. 2004;113:197–203. doi: 10.1007/s00412-004-0309-1. [DOI] [PubMed] [Google Scholar]
- 16.Puizina J., Siroky J., Mokros P., Schweizer D., Riha K. Mre11 deficiency in Arabidopsis is associated with chromosomal instability in somatic cells and Spo11-dependent genome fragmentation during meiosis. Plant Cell. 2004;16:1968–1978. doi: 10.1105/tpc.104.022749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ji J., Tang D., Wang K., Wang M., Che L., Li M., Cheng Z. The role of OsCOM1 in homologous chromosome synapsis and recombination in rice meiosis. Plant J. 2012;72:18–30. doi: 10.1111/j.1365-313X.2012.05025.x. [DOI] [PubMed] [Google Scholar]
- 18.Wang Y., Jiang L., Zhang T., Jing J., He Y. ZmCom1 Is Required for Both Mitotic and Meiotic Recombination in Maize. Front. Plant Sci. 2018;9:1005. doi: 10.3389/fpls.2018.01005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Uanschou C., Siwiec T., Pedrosa-Harand A., Kerzendorfer C., Sanchez-Moran E., Novatchkova M., Akimcheva S., Woglar A., Klein F., Schlogelhofer P. A novel plant gene essential for meiosis is related to the human CtIP and the yeast COM1/SAE2 gene. EMBO J. 2007;26:5061–5070. doi: 10.1038/sj.emboj.7601913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sun H., Treco D., Szostak J.W. Extensive 3′-overhanging, single-stranded DNA associated with the meiosis-specific double-strand breaks at the ARG4 recombination initiation site. Cell. 1991;64:1155–1161. doi: 10.1016/0092-8674(91)90270-9. [DOI] [PubMed] [Google Scholar]
- 21.Zakharyevich K., Ma Y., Tang S., Hwang P.Y., Boiteux S., Hunter N. Temporally and biochemically distinct activities of Exo1 during meiosis: Double-strand break resection and resolution of double Holliday junctions. Mol. Cell. 2010;40:1001–1015. doi: 10.1016/j.molcel.2010.11.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cao L., Alani E., Kleckner N. A pathway for generation and processing of double-strand breaks during meiotic recombination in S. cerevisiae. Cell. 1990;61:1089–1101. doi: 10.1016/0092-8674(90)90072-M. [DOI] [PubMed] [Google Scholar]
- 23.Singh G., Da Ines O., Gallego M.E., White C.I. Analysis of the impact of the absence of RAD51 strand exchange activity in Arabidopsis meiosis. PLoS ONE. 2017;12:e0183006. doi: 10.1371/journal.pone.0183006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Su H., Cheng Z., Huang J., Lin J., Copenhaver G.P., Ma H., Wang Y. Arabidopsis RAD51, RAD51C and XRCC3 proteins form a complex and facilitate RAD51 localization on chromosomes for meiotic recombination. PLoS Genet. 2017;13:e1006827. doi: 10.1371/journal.pgen.1006827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bleuyard J.Y., Gallego M.E., Savigny F., White C.I. Differing requirements for the Arabidopsis Rad51 paralogs in meiosis and DNA repair. Plant J. 2005;41:533–545. doi: 10.1111/j.1365-313X.2004.02318.x. [DOI] [PubMed] [Google Scholar]
- 26.Da Ines O., Degroote F., Goubely C., Amiard S., Gallego M.E., White C.I. Meiotic recombination in Arabidopsis is catalysed by DMC1, with RAD51 playing a supporting role. PLoS Genet. 2013;9:e1003787. doi: 10.1371/journal.pgen.1003787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kurzbauer M.T., Uanschou C., Chen D., Schlogelhofer P. The recombinases DMC1 and RAD51 are functionally and spatially separated during meiosis in Arabidopsis. Plant Cell. 2012;24:2058–2070. doi: 10.1105/tpc.112.098459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li J., Harper L.C., Golubovskaya I., Wang C.R., Weber D., Meeley R.B., McElver J., Bowen B., Cande W.Z., Schnable P.S. Functional analysis of maize RAD51 in meiosis and double-strand break repair. Genetics. 2007;176:1469–1482. doi: 10.1534/genetics.106.062604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang H., Hu Q. OsDMC1 Is Not Required for Homologous Pairing in Rice Meiosis. Plant Physiol. 2016;171:230–241. doi: 10.1104/pp.16.00167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tang D., Miao C., Li Y., Wang H., Liu X., Yu H., Cheng Z. OsRAD51C is essential for double-strand break repair in rice meiosis. Front. Plant Sci. 2014;5:167. doi: 10.3389/fpls.2014.00167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Szurman-Zubrzycka M., Baran B., Stolarek-Januszkiewicz M., Kwasniewska J., Szarejko I., Gruszka D. The dmc1 Mutant Allows an Insight into the DNA Double-Strand Break Repair during Meiosis in Barley (Hordeum vulgare L.) Front. Plant Sci. 2019;10:761. doi: 10.3389/fpls.2019.00761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kobayashi W., Liu E., Ishii H., Matsunaga S., Schlogelhofer P., Kurumizaka H. Homologous pairing activities of Arabidopsis thaliana RAD51 and DMC1. J. Biochem. 2019;165:289–295. doi: 10.1093/jb/mvy105. [DOI] [PubMed] [Google Scholar]
- 33.Pradillo M., Varas J., Oliver C., Santos J.L. On the role of AtDMC1, AtRAD51 and its paralogs during Arabidopsis meiosis. Front. Plant Sci. 2014;5:23. doi: 10.3389/fpls.2014.00023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.San Filippo J., Sung P., Klein H. Mechanism of eukaryotic homologous recombination. Annu. Rev. Biochem. 2008;77:229–257. doi: 10.1146/annurev.biochem.77.061306.125255. [DOI] [PubMed] [Google Scholar]
- 35.Lambing C., Franklin F.C.H., Wang C.-J.R. Understanding and Manipulating Meiotic Recombination in Plants. Plant Physiol. 2017;173:1530–1542. doi: 10.1104/pp.16.01530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kim J., Choi K. Signaling-mediated meiotic recombination in plants. Curr. Opin. Plant Biol. 2019;51:44–50. doi: 10.1016/j.pbi.2019.04.001. [DOI] [PubMed] [Google Scholar]
- 37.Drouaud J., Khademian H., Giraut L., Zanni V., Bellalou S., Henderson I.R., Falque M., Mezard C. Contrasted patterns of crossover and non-crossover at Arabidopsis thaliana meiotic recombination hotspots. PLoS Genet. 2013;9:e1003922. doi: 10.1371/journal.pgen.1003922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dluzewska J., Szymanska M., Ziolkowski P.A. Where to Cross Over? Defining Crossover Sites in Plants. Front. Genet. 2018;9:609. doi: 10.3389/fgene.2018.00609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Muyt A., Mercier R., Mezard C., Grelon M. Meiotic recombination and crossovers in plants. Genome Dyn. 2009;5:14–25. doi: 10.1159/000166616. [DOI] [PubMed] [Google Scholar]
- 40.Mezard C., Vignard J., Drouaud J., Mercier R. The road to crossovers: Plants have their say. Trends Genet. TIG. 2007;23:91–99. doi: 10.1016/j.tig.2006.12.007. [DOI] [PubMed] [Google Scholar]
- 41.Cifuentes M., Rivard M., Pereira L., Chelysheva L., Mercier R. Haploid meiosis in Arabidopsis: Double-strand breaks are formed and repaired but without synapsis and crossovers. PLoS ONE. 2013;8:e72431. doi: 10.1371/journal.pone.0072431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Keeney S., Giroux C.N., Kleckner N. Meiosis-specific DNA double-strand breaks are catalyzed by Spo11, a member of a widely conserved protein family. Cell. 1997;88:375–384. doi: 10.1016/S0092-8674(00)81876-0. [DOI] [PubMed] [Google Scholar]
- 43.Kumar R., Bourbon H.M., de Massy B. Functional conservation of Mei4 for meiotic DNA double-strand break formation from yeasts to mice. Genes Dev. 2010;24:1266–1280. doi: 10.1101/gad.571710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Maleki S., Neale M.J., Arora C., Henderson K.A., Keeney S. Interactions between Mei4, Rec114, and other proteins required for meiotic DNA double-strand break formation in Saccharomyces cerevisiae. Chromosoma. 2007;116:471–486. doi: 10.1007/s00412-007-0111-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Li J., Hooker G.W., Roeder G.S. Saccharomyces cerevisiae Mer2, Mei4 and Rec114 Form a Complex Required for Meiotic Double-Strand Break Formation. Genetics. 2006;173:1969. doi: 10.1534/genetics.106.058768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Menees T.M., Roeder G.S. MEI4, a yeast gene required for meiotic recombination. Genetics. 1989;123:675. doi: 10.1093/genetics/123.4.675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Arora C., Kee K., Maleki S., Keeney S. Antiviral Protein Ski8 Is a Direct Partner of Spo11 in Meiotic DNA Break Formation, Independent of Its Cytoplasmic Role in RNA Metabolism. Mol. Cell. 2004;13:549–559. doi: 10.1016/S1097-2765(04)00063-2. [DOI] [PubMed] [Google Scholar]
- 48.Salem L., Walter N., Malone R. Suppressor Analysis of the Saccharomyces cerevisiae Gene REC104 Reveals a Genetic Interaction With REC102. Genetics. 1999;151:1261. doi: 10.1093/genetics/151.4.1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kee K., Protacio R.U., Arora C., Keeney S. Spatial organization and dynamics of the association of Rec102 and Rec104 with meiotic chromosomes. EMBO J. 2004;23:1815–1824. doi: 10.1038/sj.emboj.7600184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Borde V., Lin W., Novikov E., Petrini J.H., Lichten M., Nicolas A. Association of Mre11p with Double-Strand Break Sites during Yeast Meiosis. Mol. Cell. 2004;13:389–401. doi: 10.1016/S1097-2765(04)00034-6. [DOI] [PubMed] [Google Scholar]
- 51.Johzuka K., Ogawa H. Interaction of Mre11 and Rad50: Two proteins required for DNA repair and meiosis-specific double-strand break formation in Saccharomyces cerevisiae. Genetics. 1995;139:1521–1532. doi: 10.1093/genetics/139.4.1521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ogawa H., Johzuka K., Nakagawa T., Leem S.-H., Hagihara A.H. Functions of the yeast meiotic recombination genes, MRE11 and MRE2. Adv. Biophys. 1995;31:67–76. doi: 10.1016/0065-227X(95)99383-Z. [DOI] [PubMed] [Google Scholar]
- 53.Steiner S., Kohli J., Ludin K. Functional interactions among members of the meiotic initiation complex in fission yeast. Curr. Genet. 2010;56:237–249. doi: 10.1007/s00294-010-0296-0. [DOI] [PubMed] [Google Scholar]
- 54.Lin Y., Smith G.R. Transient, meiosis-induced expression of the rec6 and rec12 genes of Schizosaccharomyces pombe. Genetics. 1994;136:769–779. doi: 10.1093/genetics/136.3.769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Miyoshi T., Ito M., Kugou K., Yamada T., Yamada S., Furuichi M., Oda A., Hirota K., Masai H., Ohta K. A Central Coupler for Recombination Initiation Linking Chromosome Architecture to S Phase Checkpoint. Mol. Cell. 2012;47:722–733. doi: 10.1016/j.molcel.2012.06.023. [DOI] [PubMed] [Google Scholar]
- 56.Bonfils S., Rozalen A.E., Smith G.R., Moreno S., Martin-Castellanos C. Functional interactions of Rec24, the fission yeast ortholog of mouse Mei4, with the meiotic recombination-initiation complex. J. Cell Sci. 2011;124:1328–1338. doi: 10.1242/jcs.079194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Martin-Castellanos C., Blanco M., Rozalen A.E., Perez-Hidalgo L., Garcia A.I., Conde F., Mata J., Ellermeier C., Davis L., San-Segundo P., et al. A large-scale screen in S. pombe identifies seven novel genes required for critical meiotic events. Curr. Biol. 2005;15:2056–2062. doi: 10.1016/j.cub.2005.10.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Gregan J., Rabitsch P.K., Sakem B., Csutak O., Latypov V., Lehmann E., Kohli J., Nasmyth K. Novel genes required for meiotic chromosome segregation are identified by a high-throughput knockout screen in fission yeast. Curr. Biol. 2005;15:1663–1669. doi: 10.1016/j.cub.2005.07.059. [DOI] [PubMed] [Google Scholar]
- 59.Lorenz A., Wells J.L., Pryce D.W., Novatchkova M., Eisenhaber F., McFarlane R.J., Loidl J. S. pombe meiotic linear elements contain proteins related to synaptonemal complex components. J. Cell Sci. 2004;117:3343–3351. doi: 10.1242/jcs.01203. [DOI] [PubMed] [Google Scholar]
- 60.Lorenz A., Estreicher A., Kohli J., Loidl J. Meiotic recombination proteins localize to linear elements in Schizosaccharomyces pombe. Chromosoma. 2006;115:330–340. doi: 10.1007/s00412-006-0053-9. [DOI] [PubMed] [Google Scholar]
- 61.Young J.A., Hyppa R.W., Smith G.R. Conserved and nonconserved proteins for meiotic DNA breakage and repair in yeasts. Genetics. 2004;167:593–605. doi: 10.1534/genetics.103.023762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Robert T., Vrielynck N., Mezard C., de Massy B., Grelon M. A new light on the meiotic DSB catalytic complex. Semin. Cell Dev. Biol. 2016;54:165–176. doi: 10.1016/j.semcdb.2016.02.025. [DOI] [PubMed] [Google Scholar]
- 63.Stanzione M., Baumann M., Papanikos F., Dereli I., Lange J., Ramlal A., Tränkner D., Shibuya H., de Massy B., Watanabe Y., et al. Meiotic DNA break formation requires the unsynapsed chromosome axis-binding protein IHO1 (CCDC36) in mice. Nat. Cell Biol. 2016;18:1208–1220. doi: 10.1038/ncb3417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kumar R., Ghyselinck N., Ishiguro K., Watanabe Y., Kouznetsova A., Hoog C., Strong E., Schimenti J., Daniel K., Toth A., et al. MEI4—A central player in the regulation of meiotic DNA double-strand break formation in the mouse. J. Cell Sci. 2015;128:1800–1811. doi: 10.1242/jcs.165464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kumar R., Oliver C., Brun C., Juarez-Martinez A.B., Tarabay Y., Kadlec J., de Massy B. Mouse REC114 is essential for meiotic DNA double-strand break formation and forms a complex with MEI4. Life Sci. Alliance. 2018;1:e201800259. doi: 10.26508/lsa.201800259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Panizza S., Mendoza M.A., Berlinger M., Huang L., Nicolas A., Shirahige K., Klein F. Spo11-Accessory Proteins Link Double-Strand Break Sites to the Chromosome Axis in Early Meiotic Recombination. Cell. 2011;146:372–383. doi: 10.1016/j.cell.2011.07.003. [DOI] [PubMed] [Google Scholar]
- 67.Libby B.J., Reinholdt L.G., Schimenti J.C. Positional cloning and characterization of Mei1, a vertebrate-specific gene required for normal meiotic chromosome synapsis in mice. Proc. Natl. Acad. Sci. USA. 2003;100:15706–15711. doi: 10.1073/pnas.2432067100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Libby B.J., De La Fuente R., O’Brien M.J., Wigglesworth K., Cobb J., Inselman A., Eaker S., Handel M.A., Eppig J.J., Schimenti J.C. The mouse meiotic mutation mei1 disrupts chromosome synapsis with sexually dimorphic consequences for meiotic progression. Dev. Biol. 2002;242:174–187. doi: 10.1006/dbio.2001.0535. [DOI] [PubMed] [Google Scholar]
- 69.Kumar R., De Massy B. Initiation of meiotic recombination in mammals. Genes. 2010;1:521–549. doi: 10.3390/genes1030521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Robert T., Nore A., Brun C., Maffre C., Crimi B., Bourbon H.M., de Massy B. The TopoVIB-Like protein family is required for meiotic DNA double-strand break formation. Science. 2016;351:943–949. doi: 10.1126/science.aad5309. [DOI] [PubMed] [Google Scholar]
- 71.Stacey N.J., Kuromori T., Azumi Y., Roberts G., Breuer C., Wada T., Maxwell A., Roberts K., Sugimoto-Shirasu K. Arabidopsis SPO11-2 functions with SPO11-1 in meiotic recombination. Plant J. 2006;48:206–216. doi: 10.1111/j.1365-313X.2006.02867.x. [DOI] [PubMed] [Google Scholar]
- 72.Grelon M., Vezon D., Gendrot G., Pelletier G. AtSPO11-1 is necessary for efficient meiotic recombination in plants. EMBO J. 2001;20:589–600. doi: 10.1093/emboj/20.3.589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Shingu Y., Tokai T., Agawa Y., Toyota K., Ahamed S., Kawagishi-Kobayashi M., Komatsu A., Mikawa T., Yamamoto M.T., Wakasa K., et al. The double-stranded break-forming activity of plant SPO11s and a novel rice SPO11 revealed by a Drosophila bioassay. BMC Mol. Biol. 2012;13:1. doi: 10.1186/1471-2199-13-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Hartung F., Puchta H. Molecular characterisation of two paralogous SPO11 homologues in Arabidopsis thaliana. Nucleic Acids Res. 2000;28:1548–1554. doi: 10.1093/nar/28.7.1548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Sprink T., Hartung F. The splicing fate of plant SPO11 genes. Front. Plant Sci. 2014;5:214. doi: 10.3389/fpls.2014.00214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Hartung F., Angelis K.J., Meister A., Schubert I., Melzer M., Puchta H. An Archaebacterial Topoisomerase Homolog Not Present in Other Eukaryotes Is Indispensable for Cell Proliferation of Plants. Curr. Biol. 2002;12:1787–1791. doi: 10.1016/S0960-9822(02)01218-6. [DOI] [PubMed] [Google Scholar]
- 77.Tang Y., Yin Z., Zeng Y., Zhang Q., Chen L., He Y., Lu P., Ye D., Zhang X. MTOPVIB interacts with AtPRD1 and plays important roles in formation of meiotic DNA double-strand breaks in Arabidopsis. Sci. Rep. 2017;7:10007. doi: 10.1038/s41598-017-10270-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.De Muyt A., Pereira L., Vezon D., Chelysheva L., Gendrot G., Chambon A., Laine-Choinard S., Pelletier G., Mercier R., Nogue F., et al. A high throughput genetic screen identifies new early meiotic recombination functions in Arabidopsis thaliana. PLoS Genet. 2009;5:e1000654. doi: 10.1371/journal.pgen.1000654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Capilla-Perez L., Solier V., Portemer V., Chambon A., Hurel A., Guillebaux A., Vezon D., Cromer L., Grelon M., Mercier R. The HEM Lines: A New Library of Homozygous Arabidopsis thaliana EMS Mutants and its Potential to Detect Meiotic Phenotypes. Front. Plant Sci. 2018;9:1339. doi: 10.3389/fpls.2018.01339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Cole F., Keeney S., Jasin M. Evolutionary conservation of meiotic DSB proteins: More than just Spo11. Genes Dev. 2010;24:1201–1207. doi: 10.1101/gad.1944710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Tesse S., Bourbon H.M., Debuchy R., Budin K., Dubois E., Liangran Z., Antoine R., Piolot T., Kleckner N., Zickler D., et al. Asy2/Mer2: An evolutionarily conserved mediator of meiotic recombination, pairing, and global chromosome compaction. Genes Dev. 2017;31:1880–1893. doi: 10.1101/gad.304543.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Zhang C., Song Y., Cheng Z.H., Wang Y.X., Zhu J., Ma H., Xu L., Yang Z.N. The Arabidopsis thaliana DSB formation (AtDFO) gene is required for meiotic double-strand break formation. Plant J. 2012;72:271–281. doi: 10.1111/j.1365-313X.2012.05075.x. [DOI] [PubMed] [Google Scholar]
- 83.Xue Z., Li Y., Zhang L., Shi W., Zhang C., Feng M., Zhang F., Tang D., Yu H., Gu M., et al. OsMTOPVIB Promotes Meiotic DNA Double-Strand Break Formation in Rice. Mol. Plant. 2016;9:1535–1538. doi: 10.1016/j.molp.2016.07.005. [DOI] [PubMed] [Google Scholar]
- 84.An X.J., Deng Z.Y., Wang T. OsSpo11-4, a rice homologue of the archaeal TopVIA protein, mediates double-strand DNA cleavage and interacts with OsTopVIB. PLoS ONE. 2011;6:e20327. doi: 10.1371/journal.pone.0020327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Yu H., Wang M., Tang D., Wang K., Chen F., Gong Z., Gu M., Cheng Z. OsSPO11-1 is essential for both homologous chromosome pairing and crossover formation in rice. Chromosoma. 2010;119:625–636. doi: 10.1007/s00412-010-0284-7. [DOI] [PubMed] [Google Scholar]
- 86.Fu M., Wang C., Xue F., Higgins J., Chen M., Zhang D., Liang W. The DNA Topoisomerase VI-B Subunit OsMTOPVIB Is Essential for Meiotic Recombination Initiation in Rice. Mol. Plant. 2016;9:1539–1541. doi: 10.1016/j.molp.2016.07.006. [DOI] [PubMed] [Google Scholar]
- 87.Wu Z., Ji J., Tang D., Wang H., Shen Y., Shi W., Li Y., Tan X., Cheng Z., Luo Q. OsSDS is essential for DSB formation in rice meiosis. Front. Plant Sci. 2015;6:21. doi: 10.3389/fpls.2015.00021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Miao C., Tang D., Zhang H., Wang M., Li Y., Tang S., Yu H., Gu M., Cheng Z. Central region component1, a novel synaptonemal complex component, is essential for meiotic recombination initiation in rice. Plant Cell. 2013;25:2998–3009. doi: 10.1105/tpc.113.113175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Yang M., Li B., Tomchick D.R., Machius M., Rizo J., Yu H., Luo X. p31comet blocks Mad2 activation through structural mimicry. Cell. 2007;131:744–755. doi: 10.1016/j.cell.2007.08.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Zhou L., Han J., Chen Y., Wang Y., Liu Y.G. Bivalent Formation 1, a plant-conserved gene, encodes an OmpH/coiled-coil motif-containing protein required for meiotic recombination in rice. J. Exp. Bot. 2017;68:2163–2174. doi: 10.1093/jxb/erx077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Ji J., Tang D., Shen Y., Xue Z., Wang H., Shi W., Zhang C., Du G., Li Y., Cheng Z. P31comet, a member of the synaptonemal complex, participates in meiotic DSB formation in rice. Proc. Natl. Acad. Sci. USA. 2016;113:10577–10582. doi: 10.1073/pnas.1607334113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Pan J., Sasaki M., Kniewel R., Murakami H., Blitzblau H.G., Tischfield S.E., Zhu X., Neale M.J., Jasin M., Socci N.D., et al. A hierarchical combination of factors shapes the genome-wide topography of yeast meiotic recombination initiation. Cell. 2011;144:719–731. doi: 10.1016/j.cell.2011.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Nicolas A., Treco D., Schultes N.P., Szostak J.W. An initiation site for meiotic gene conversion in the yeast Saccharomyces cerevisiae. Nature. 1989;338:35–39. doi: 10.1038/338035a0. [DOI] [PubMed] [Google Scholar]
- 94.Baudat F., Nicolas A. Clustering of meiotic double-strand breaks on yeast chromosome III. Proc. Natl. Acad. Sci. USA. 1997;94:5213. doi: 10.1073/pnas.94.10.5213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Garcia V., Gray S., Allison R.M., Cooper T.J., Neale M.J. Tel1(ATM)-mediated interference suppresses clustered meiotic double-strand-break formation. Nature. 2015;520:114–118. doi: 10.1038/nature13993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Qin J., Richardson L.L., Jasin M., Handel M.A., Arnheim N. Mouse strains with an active H2-Ea meiotic recombination hot spot exhibit increased levels of H2-Ea-specific DNA breaks in testicular germ cells. Mol. Cell. Biol. 2004;24:1655–1666. doi: 10.1128/MCB.24.4.1655-1666.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.De Massy B., Nicolas A. The control in cis of the position and the amount of the ARG4 meiotic double-strand break of Saccharomyces cerevisiae. EMBO J. 1993;12:1459–1466. doi: 10.1002/j.1460-2075.1993.tb05789.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Choi K., Henderson I.R. Meiotic recombination hotspots—A comparative view. Plant J. 2015;83:52–61. doi: 10.1111/tpj.12870. [DOI] [PubMed] [Google Scholar]
- 99.Choi K., Zhao X., Tock A.J., Lambing C., Underwood C.J., Hardcastle T.J., Serra H., Kim J., Cho H.S., Kim J., et al. Nucleosomes and DNA methylation shape meiotic DSB frequency in Arabidopsis thaliana transposons and gene regulatory regions. Genome Res. 2018;28:532–546. doi: 10.1101/gr.225599.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Lange J., Yamada S., Tischfield S.E., Pan J., Kim S., Zhu X., Socci N.D., Jasin M., Keeney S. The Landscape of Mouse Meiotic Double-Strand Break Formation, Processing, and Repair. Cell. 2016;167:695–708. doi: 10.1016/j.cell.2016.09.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Thacker D., Mohibullah N., Zhu X., Keeney S. Homologue engagement controls meiotic DNA break number and distribution. Nature. 2014;510:241–246. doi: 10.1038/nature13120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Underwood C.J., Choi K., Lambing C., Zhao X., Serra H., Borges F., Simorowski J., Ernst E., Jacob Y., Henderson I.R., et al. Epigenetic activation of meiotic recombination near Arabidopsis thaliana centromeres via loss of H3K9me2 and non-CG DNA methylation. Genome Res. 2018;28:519–531. doi: 10.1101/gr.227116.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Smagulova F., Gregoretti I.V., Brick K., Khil P., Camerini-Otero R.D., Petukhova G.V. Genome-wide analysis reveals novel molecular features of mouse recombination hotspots. Nature. 2011;472:375–378. doi: 10.1038/nature09869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Brick K., Smagulova F., Khil P., Camerini-Otero R.D., Petukhova G.V. Genetic recombination is directed away from functional genomic elements in mice. Nature. 2012;485:642–645. doi: 10.1038/nature11089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.He Y., Wang M., Dukowic-Schulze S., Zhou A., Tiang C.L., Shilo S., Sidhu G.K., Eichten S., Bradbury P., Springer N.M., et al. Genomic features shaping the landscape of meiotic double-strand-break hotspots in maize. Proc. Natl. Acad. Sci. USA. 2017;114:12231–12236. doi: 10.1073/pnas.1713225114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Tock A.J., Henderson I.R. Hotspots for Initiation of Meiotic Recombination. Front. Genet. 2018;9:521. doi: 10.3389/fgene.2018.00521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Yamada S., Kim S., Tischfield S.E., Jasin M., Lange J., Keeney S. Genomic and chromatin features shaping meiotic double-strand break formation and repair in mice. Cell Cycle. 2017;16:1870–1884. doi: 10.1080/15384101.2017.1361065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Vader G., Blitzblau H.G., Tame M.A., Falk J.E., Curtin L., Hochwagen A. Protection of repetitive DNA borders from self-induced meiotic instability. Nature. 2011;477:115–119. doi: 10.1038/nature10331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Sasaki M., Tischfield S.E., van Overbeek M., Keeney S. Meiotic Recombination Initiation in and around Retrotransposable Elements in Saccharomyces cerevisiae. PLoS Genet. 2013;9:e1003732. doi: 10.1371/journal.pgen.1003732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Zhu X., Keeney S. High-Resolution Global Analysis of the Influences of Bas1 and Ino4 Transcription Factors on Meiotic DNA Break Distributions in Saccharomyces cerevisiae. Genetics. 2015;201:525–542. doi: 10.1534/genetics.115.178293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Mohibullah N., Keeney S. Numerical and spatial patterning of yeast meiotic DNA breaks by Tel1. Genome Res. 2017;27:278–288. doi: 10.1101/gr.213587.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Lam I., Keeney S. Nonparadoxical evolutionary stability of the recombination initiation landscape in yeast. Science. 2015;350:932–937. doi: 10.1126/science.aad0814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Fowler K.R., Sasaki M., Milman N., Keeney S., Smith G.R. Evolutionarily diverse determinants of meiotic DNA break and recombination landscapes across the genome. Genome Res. 2014;24:1650–1664. doi: 10.1101/gr.172122.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Bustamante-Jaramillo L.F., Ramos C., Alonso L., Sesmero A., Segurado M., Martin-Castellanos C. CDK contribution to DSB formation and recombination in fission yeast meiosis. PLoS Genet. 2019;15:e1007876. doi: 10.1371/journal.pgen.1007876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Wan L., Niu H., Futcher B., Zhang C., Shokat K.M., Boulton S.J., Hollingsworth N.M. Cdc28-Clb5 (CDK-S) and Cdc7-Dbf4 (DDK) collaborate to initiate meiotic recombination in yeast. Genes Dev. 2008;22:386–397. doi: 10.1101/gad.1626408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Benjamin K.R. Control of landmark events in meiosis by the CDK Cdc28 and the meiosis-specific kinase Ime2. Genes Dev. 2003;17:1524–1539. doi: 10.1101/gad.1101503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Nasmyth K. Control of the yeast cell cycle by the Cdc28 protein kinase. Curr. Opin. Cell Biol. 1993;5:166–179. doi: 10.1016/0955-0674(93)90099-C. [DOI] [PubMed] [Google Scholar]
- 118.Stuart D., Wittenberg C. CLB5 and CLB6 are required for premeiotic DNA replication and activation of the meiotic S/M checkpoint. Genes Dev. 1998;12:2698–2710. doi: 10.1101/gad.12.17.2698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Smith K.N., Penkner A., Ohta K., Klein F., Nicolas A. B-type cyclins CLB5 and CLB6 control the initiation of recombination and synaptonemal complex formation in yeast meiosis. Curr. Biol. 2001;11:88–97. doi: 10.1016/S0960-9822(01)00026-4. [DOI] [PubMed] [Google Scholar]
- 120.Sasanuma H., Hirota K., Fukuda T., Kakusho N., Kugou K., Kawasaki Y., Shibata T., Masai H., Ohta K. Cdc7-dependent phosphorylation of Mer2 facilitates initiation of yeast meiotic recombination. Genes Dev. 2008;22:398–410. doi: 10.1101/gad.1626608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Keeney S. Mechanism and control of meiotic recombination initiation. Curr. Top. Dev. Biol. 2001;52:1–53. doi: 10.1016/S0070-2153(01)52008-6. [DOI] [PubMed] [Google Scholar]
- 122.Boudolf V., Barrôco R., de Almeida Engler J., Verkest A., Beeckman T., Naudts M., Inzé D., De Veylder L. B1-Type Cyclin-Dependent Kinases Are Essential for the Formation of Stomatal Complexes in Arabidopsis thaliana. Plant Cell. 2004;16:945–955. doi: 10.1105/tpc.021774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Boniotti M.B., Gutierrez C. A cell-cycle-regulated kinase activity phosphorylates plant retinoblastoma protein and contains, in Arabidopsis, a CDKA/cyclin D complex. Plant J. 2001;28:341–350. doi: 10.1046/j.1365-313X.2001.01160.x. [DOI] [PubMed] [Google Scholar]
- 124.Wijnker E., Harashima H., Müller K., Parra-Nuñez P., de Snoo C.B., van de Belt J., Dissmeyer N., Bayer M., Pradillo M., Schnittger A. The Cdk1/Cdk2 homolog CDKA;1 controls the recombination landscape in Arabidopsis. Proc. Natl. Acad. Sci. USA. 2019;116:12534–12539. doi: 10.1073/pnas.1820753116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Corellou F., Camasses A., Ligat L., Peaucellier G., Bouget F.Y. Atypical regulation of a green lineage-specific B-type cyclin-dependent kinase. Plant Physiol. 2005;138:1627–1636. doi: 10.1104/pp.105.059626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Adachi S., Uchimiya H., Umeda M. Expression of B2-type cyclin-dependent kinase is controlled by protein degradation in Arabidopsis thaliana. Plant Cell Physiol. 2006;47:1683–1686. doi: 10.1093/pcp/pcl034. [DOI] [PubMed] [Google Scholar]
- 127.Bulankova P., Akimcheva S., Fellner N., Riha K. Identification of Arabidopsis Meiotic Cyclins Reveals Functional Diversification among Plant Cyclin Genes. PLoS Genet. 2013;9:e1003508. doi: 10.1371/journal.pgen.1003508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Potuschak T., Doerner P. Cell cycle controls: Genome-wide analysis in Arabidopsis. Curr. Opin. Plant Biol. 2001;4:501–506. doi: 10.1016/S1369-5266(00)00207-7. [DOI] [PubMed] [Google Scholar]
- 129.Nowack M.K., Harashima H., Dissmeyer N., Zhao X.A., Bouyer D., Weimer A.K., De Winter F., Yang F., Schnittger A. Genetic Framework of Cyclin-Dependent Kinase Function in Arabidopsis. Dev. Cell. 2012;22:1030–1040. doi: 10.1016/j.devcel.2012.02.015. [DOI] [PubMed] [Google Scholar]
- 130.Richard C., Granier C., Inze D., De Veylder L. Analysis of cell division parameters and cell cycle gene expression during the cultivation of Arabidopsis thaliana cell suspensions. J. Exp. Bot. 2001;52:1625–1633. doi: 10.1093/jexbot/52.361.1625. [DOI] [PubMed] [Google Scholar]
- 131.Wijnker E., Schnittger A. Control of the meiotic cell division program in plants. Plant Reprod. 2013;26:143–158. doi: 10.1007/s00497-013-0223-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Zheng T., Nibau C., Phillips D.W., Jenkins G., Armstrong S.J., Doonan J.H. CDKG1 protein kinase is essential for synapsis and male meiosis at high ambient temperature in Arabidopsis thaliana. Proc. Natl. Acad. Sci. USA. 2014;111:2182–2187. doi: 10.1073/pnas.1318460111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Azumi Y., Liu D., Zhao D., Li W., Wang G., Hu Y., Ma H. Homolog interaction during meiotic prophase I in Arabidopsis requires the SOLO DANCERS gene encoding a novel cyclin-like protein. EMBO J. 2002;21:3081–3095. doi: 10.1093/emboj/cdf285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.D’Erfurth I., Cromer L., Jolivet S., Girard C., Horlow C., Sun Y., To J.P., Berchowitz L.E., Copenhaver G.P., Mercier R. The cyclin-A CYCA1;2/TAM is required for the meiosis I to meiosis II transition and cooperates with OSD1 for the prophase to first meiotic division transition. PLoS Genet. 2010;6:e1000989. doi: 10.1371/journal.pgen.1000989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Bulankova P., Riehs-Kearnan N., Nowack M.K., Schnittger A., Riha K. Meiotic progression in Arabidopsis is governed by complex regulatory interactions between SMG7, TDM1, and the meiosis I-specific cyclin TAM. Plant Cell. 2010;22:3791–3803. doi: 10.1105/tpc.110.078378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Lukaszewicz A., Lange J., Keeney S., Jasin M. Control of meiotic double-strand-break formation by ATM: Local and global views. Cell Cycle. 2018;17:1155–1172. doi: 10.1080/15384101.2018.1464847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Cooper T.J., Wardell K., Garcia V., Neale M.J. Homeostatic regulation of meiotic DSB formation by ATM/ATR. Exp. Cell Res. 2014;329:124–131. doi: 10.1016/j.yexcr.2014.07.016. [DOI] [PubMed] [Google Scholar]
- 138.Penedos A., Johnson A.L., Strong E., Goldman A.S., Carballo J.A., Cha R.S. Essential and Checkpoint Functions of Budding Yeast ATM and ATR during Meiotic Prophase Are Facilitated by Differential Phosphorylation of a Meiotic Adaptor Protein, Hop1. PLoS ONE. 2015;10:e0134297. doi: 10.1371/journal.pone.0134297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Shiloh Y. The ATM-mediated DNA-damage response: Taking shape. Trends Biochem. Sci. 2006;31:402–410. doi: 10.1016/j.tibs.2006.05.004. [DOI] [PubMed] [Google Scholar]
- 140.Carballo J.A., Panizza S., Serrentino M.E., Johnson A.L., Geymonat M., Borde V., Klein F., Cha R.S. Budding yeast ATM/ATR control meiotic double-strand break (DSB) levels by down-regulating Rec114, an essential component of the DSB-machinery. PLoS Genet. 2013;9:e1003545. doi: 10.1371/journal.pgen.1003545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Zhang L., Kim K.P., Kleckner N.E., Storlazzi A. Meiotic double-strand breaks occur once per pair of (sister) chromatids and, via Mec1/ATR and Tel1/ATM, once per quartet of chromatids. Proc. Natl. Acad. Sci. USA. 2011;108:20036–20041. doi: 10.1073/pnas.1117937108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Lange J., Pan J., Cole F., Thelen M.P., Jasin M., Keeney S. ATM controls meiotic double-strand-break formation. Nature. 2011;479:237–240. doi: 10.1038/nature10508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Xu L., Kleckner N. Sequence non-specific double-strand breaks and interhomolog interactions prior to double-strand break formation at a meiotic recombination hot spot in yeast. EMBO J. 1995;14:5115–5128. doi: 10.1002/j.1460-2075.1995.tb00194.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Blitzblau H.G., Hochwagen A. ATR/Mec1 prevents lethal meiotic recombination initiation on partially replicated chromosomes in budding yeast. eLife. 2013;2:e00844. doi: 10.7554/eLife.00844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Xu L., Ajimura M., Padmore R., Klein C., Kleckner N. NDT80, a meiosis-specific gene required for exit from pachytene in Saccharomyces cerevisiae. Mol. Cell. Biol. 1995;15:6572. doi: 10.1128/MCB.15.12.6572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Winter E. The Sum1/Ndt80 Transcriptional Switch and Commitment to Meiosis in Saccharomyces cerevisiae. Microbiol. Mol. Biol. Rev. 2012;76:1. doi: 10.1128/MMBR.05010-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Gray S., Allison R.M., Garcia V., Goldman A.S., Neale M.J. Positive regulation of meiotic DNA double-strand break formation by activation of the DNA damage checkpoint kinase Mec1(ATR) Open Biol. 2013;3:130019. doi: 10.1098/rsob.130019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Argunhan B., Farmer S., Leung W.K., Terentyev Y., Humphryes N., Tsubouchi T., Toyoizumi H., Tsubouchi H. Direct and indirect control of the initiation of meiotic recombination by DNA damage checkpoint mechanisms in budding yeast. PLoS ONE. 2013;8:e65875. doi: 10.1371/journal.pone.0065875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Cooper T.J., Garcia V., Neale M.J. Meiotic DSB patterning: A multifaceted process. Cell Cycle. 2016;15:13–21. doi: 10.1080/15384101.2015.1093709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Kauppi L., Barchi M., Lange J., Baudat F., Jasin M., Keeney S. Numerical constraints and feedback control of double-strand breaks in mouse meiosis. Genes Dev. 2013;27:873–886. doi: 10.1101/gad.213652.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Kim J.H., Ryu T.H., Lee S.S., Lee S., Chung B.Y. Ionizing radiation manifesting DNA damage response in plants: An overview of DNA damage signaling and repair mechanisms in plants. Plant Sci. 2019;278:44–53. doi: 10.1016/j.plantsci.2018.10.013. [DOI] [PubMed] [Google Scholar]
- 152.Culligan K.M., Britt A.B. Both ATM and ATR promote the efficient and accurate processing of programmed meiotic double-strand breaks. Plant J. 2008;55:629–638. doi: 10.1111/j.1365-313X.2008.03530.x. [DOI] [PubMed] [Google Scholar]
- 153.Garcia V., Bruchet H., Camescasse D., Granier F., Bouchez D., Tissier A. AtATM is essential for meiosis and the somatic response to DNA damage in plants. Plant Cell. 2003;15:119–132. doi: 10.1105/tpc.006577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Eckardt N.A. ATM to the rescue: Repairing DNA damage. Plant Cell. 2003;15:1–3. doi: 10.1105/tpc.150110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Roitinger E., Hofer M., Kocher T., Pichler P., Novatchkova M., Yang J., Schlogelhofer P., Mechtler K. Quantitative phosphoproteomics of the ataxia telangiectasia-mutated (ATM) and ataxia telangiectasia-mutated and rad3-related (ATR) dependent DNA damage response in Arabidopsis thaliana. Mol. Cell. Proteom. MCP. 2015;14:556–571. doi: 10.1074/mcp.M114.040352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Prugar E., Burnett C., Chen X., Hollingsworth N.M. Coordination of Double Strand Break Repair and Meiotic Progression in Yeast by a Mek1-Ndt80 Negative Feedback Loop. Genetics. 2017;206:497–512. doi: 10.1534/genetics.117.199703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Blat Y., Protacio R.U., Hunter N., Kleckner N. Physical and Functional Interactions among Basic Chromosome Organizational Features Govern Early Steps of Meiotic Chiasma Formation. Cell. 2002;111:791–802. doi: 10.1016/S0092-8674(02)01167-4. [DOI] [PubMed] [Google Scholar]
- 158.Borde V., de Massy B. Programmed induction of DNA double strand breaks during meiosis: Setting up communication between DNA and the chromosome structure. Curr. Opin. Genet. Dev. 2013;23:147–155. doi: 10.1016/j.gde.2012.12.002. [DOI] [PubMed] [Google Scholar]
- 159.Keeney S. Spo11 and the Formation of DNA Double-Strand Breaks in Meiosis. Genome Dyn. Stab. 2008;2:81–123. doi: 10.1007/7050_2007_026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Kleckner N. Chiasma formation: Chromatin/axis interplay and the role(s) of the synaptonemal complex. Chromosoma. 2006;115:175. doi: 10.1007/s00412-006-0055-7. [DOI] [PubMed] [Google Scholar]
- 161.Acquaviva L., Szekvolgyi L., Dichtl B., Dichtl B.S., de La Roche Saint Andre C., Nicolas A., Geli V. The COMPASS subunit Spp1 links histone methylation to initiation of meiotic recombination. Science. 2013;339:215–218. doi: 10.1126/science.1225739. [DOI] [PubMed] [Google Scholar]
- 162.Sommermeyer V., Beneut C., Chaplais E., Serrentino M.E., Borde V. Spp1, a member of the Set1 Complex, promotes meiotic DSB formation in promoters by tethering histone H3K4 methylation sites to chromosome axes. Mol. Cell. 2013;49:43–54. doi: 10.1016/j.molcel.2012.11.008. [DOI] [PubMed] [Google Scholar]
- 163.Hayashi K., Yoshida K., Matsui Y. A histone H3 methyltransferase controls epigenetic events required for meiotic prophase. Nature. 2005;438:374–378. doi: 10.1038/nature04112. [DOI] [PubMed] [Google Scholar]
- 164.Tian H., Billings T., Petkov P.M. CXXC1 is not essential for normal DNA double-strand break formation and meiotic recombination in mouse. PLoS Genet. 2018;14:e1007657. doi: 10.1371/journal.pgen.1007657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Imai Y., Baudat F., Taillepierre M., Stanzione M., Toth A., de Massy B. The PRDM9 KRAB domain is required for meiosis and involved in protein interactions. Chromosoma. 2017;126:681–695. doi: 10.1007/s00412-017-0631-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Parvanov E.D., Tian H., Billings T., Saxl R.L., Spruce C., Aithal R., Krejci L., Paigen K., Petkov P.M. PRDM9 interactions with other proteins provide a link between recombination hotspots and the chromosomal axis in meiosis. Mol. Biol. Cell. 2017;28:488–499. doi: 10.1091/mbc.e16-09-0686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Ronceret A., Golubovskaya I., Ku J.-C., Lee D.H., Timofejeva L., Angoa A.K.G., Kao Y.-H., Kremling K., Williams-Carrier R., Meeley R., et al. The dynamic association of SPO11-1 with conformational changes of meiotic axial elements in maize. bioRxiv. 2018 doi: 10.1101/489278. [DOI] [Google Scholar]
- 168.Ferdous M., Higgins J.D., Osman K., Lambing C., Roitinger E., Mechtler K., Armstrong S.J., Perry R., Pradillo M., Cunado N., et al. Inter-homolog crossing-over and synapsis in Arabidopsis meiosis are dependent on the chromosome axis protein AtASY3. PLoS Genet. 2012;8:e1002507. doi: 10.1371/journal.pgen.1002507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Lee D.H., Kao Y.H., Ku J.C., Lin C.Y., Meeley R., Jan Y.S., Wang C.J. The Axial Element Protein DESYNAPTIC2 Mediates Meiotic Double-Strand Break Formation and Synaptonemal Complex Assembly in Maize. Plant Cell. 2015;27:2516–2529. doi: 10.1105/tpc.15.00434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Jenkins G., Phillips D., Mikhailova E.I., Timofejeva L., Jones R.N. Meiotic genes and proteins in cereals. Cytogenet. Genome Res. 2008;120:291–301. doi: 10.1159/000121078. [DOI] [PubMed] [Google Scholar]