Abstract
Objectives:
This study aimed to explore the morphology, loadability, and releasing profiles of CalliSpheres microspheres in delivering oxaliplatin.
Methods:
Varied amount (20, 40, 60, and 80 mg oxaliplatin) and concentration (1.25, 2.5, 5.0 mg/mL oxaliplatin) of oxaliplatin were mixed with CalliSpheres microspheres with 3 sizes (50-150 μm, 100-300 μm, and 300-500 μm) to measure the loadability. Of all, 20 mg oxaliplatin-loaded CalliSpheres microspheres with 3 sizes was prepared to measure the releasing profiles, meanwhile, fetal bovine serum was added to determine the effect of serum on oxaliplatin releasing. The morphology and size distribution of CalliSpheres microspheres with 3 sizes before and after 20 mg oxaliplatin loading were detected.
Results:
Oxaliplatin amount was negatively correlated with loading efficiency with highest loadability in 20 mg oxaliplatin group (maximum 40% in 50-100 µm CalliSpheres microspheres, 52% in 100-300 µm CalliSpheres microspheres, and 52% in 300-500 µm CalliSpheres microspheres), while oxaliplatin concentration was positively associated with loading efficiency. Similar drug-releasing profiles were observed among oxaliplatin-loaded CalliSpheres microspheres with 3 sizes, and a rapid drug release was discovered in CalliSpheres microspheres with 3 sizes as well. We also found that fetal bovine serum did not affect the drug-releasing profiles of oxaliplatin-loaded CalliSpheres microspheres. In addition, CalliSpheres microspheres was modified a little to ellipse shape and less smooth after oxaliplatin loading, and it was enlarged to some extent.
Conclusion:
This study discloses drug loadability, releasing profiles, and morphology change of CalliSpheres microspheres for delivering oxaliplatin, which provides potential evidences for application of oxaliplatin-loaded drug-eluting beads in clinical practice.
Keywords: oxaliplatin, CalliSpheres microspheres, loadability, releasing profiles, morphology
Introduction
Oxaliplatin, as the third generation of platinum anticancer drugs, presents with excellent inhibitive effect on cancers featured by broad antitumor spectra, strong killing ability toward drug-resistant tumor strains and so on.1-3 Benefiting from the advantages of oxaliplatin, it has been widely applied for cancer treatment in clinical practice such as the therapy of metastatic colorectal cancer, while its neurotoxicity and cardiotoxicity due to high blood concentration still concern the physicians a lot.4-7 Therefore, it is essential to explore the methods to reduce these toxicities and increase the tumor concentration of oxaliplatin.
Along with the great improvement in biotechnology and novel materials investigation, several drug-eluting beads (DEBs) including DC beads, LC beads, HepaSphere microspheres (HSM), and CalliSpheres microspheres (CSM) have been produced to sustain high concentration of anticancer drug in target tumor and decrease the systemic toxicity, meanwhile they embolize the tumor supply vessel to make tumor necrosis, which greatly improve the efficacy and safety of transarterial chemoembolization (TACE).8-13 Among these beads, CSM is the first DEBs developed in China that illuminate good biocompatibility, suspension property, and flexibility, and it has been proved to possess satisfied loading and releasing profiles of doxorubicin.11,14,15 Considering the aforementioned features of CSM, we hypothesized that the application of CSM in loading and releasing oxaliplatin would reveal approving outcomes, while no related investigation has been disclosed. Thus, this current study aimed to explore the morphology, loadability, and releasing profiles of CSM in delivering oxaliplatin, which would provide evidence for the potential application of TACE with CSM-eluting oxaliplatin in treating cancers.
Materials and Methods
Materials Preparation
Oxaliplatin was purchased from Meilune Biological Technology Co (Dalian, China). CalliSpheres microspheres (50-150 μm, 100-300 μm, and 300-500 μm) were kindly given from Suzhou Callisyn Biomedical, Inc (Suzhou, China). Sterile water for injection was obtained from KeLun Industry Group (Sichuan, China). Methanol was purchased from Tedia Company Inc (Fairfield, Connecticut). Deionized water was obtained from a Millipore Simplicity TM system (Millipore, Billerica, MA, USA).
Preparation of Calibration Standard
The standard stock solution of 5 mg/mL oxaliplatin was prepared by dissolving oxaliplatin powder in sterile water and then stored at 4°C. The stock solution of oxaliplatin was further diluted with sterile water to prepare the calibration curves at the desired concentrations at 4, 6, 10, 15, 30, 60, 80, and 120 μg/mL.
Measurement of Oxaliplatin Loading Efficiency by CSM
Of all, 50-mg CSM with 3 sizes (50-150 μm, 100-300 μm, and 300-500 μm) was used for evaluation of oxaliplatin-loading efficiency, and 4, 8, 12, and 16 mL of 5 mg/mL oxaliplatin stock solution was prepared for detection of oxaliplatin loading efficiency of different doses (20, 40, 60, and 80 mg). CalliSpheres microspheres and oxaliplatin solution were mixed followed by agitation using Orbital Shaker (TS-1,Shanghai, China) at room temperature. Then, 20-uL upper solution was pipetted to a 1.5-mL polypropylene tube at 0, 5, 10, 15, 20, 30, 60 minutes for each sample and centrifuged at 9000g for 3 minutes at room temperature. Subsequently, 10 uL clear supernatant was obtained and mixed with 240 μL 10% methanol (vol/vol) in 1.5 mL polypropylene tubes as test sample. Finally, in order to determine oxaliplatin loading efficiency of CSM, these samples were analyzed by measuring the residual unloaded drug in the supernatant using Agilent 1260 high-performance liquid chromatography (HPLC; Agilent Technologies Co, Santa Clara, CA ,USA). In addition, 40 mg oxaliplatin with different concentrations (1.25, 2.5, and 5 mg/mL) were also prepared for evaluation of oxaliplatin loading efficiency of CSM with varied concentrations. Besides, since CSM is a marketed microsphere in China, the stirring time and stirring temperature are recommended by the manufacturer (Suzhou Callisyn Biomedical, Inc, Suzhou, China) based on their investigations and clinical use. All the experiments were conducted in triplicate.
Drug Loading Stability
For evaluating the stability of CSM, 3 replicates of 50 mg CSM at 100 to 300 µm were loaded with 20 mg oxaliplatin solution and were diluted at a ratio of 1:2, 1:4, and 1:8, respectively, while each replicate was divided into 4 groups and each group was diluted with sterile water, 0.9% saline solution, 5% glucose solution, and contrast agent ioversol injection, respectively. Later, the oxaliplatin-eluting CMS were placed at room temperature, and the concentrations of per sample were quantified at 60 minutes by the HPLC method as described before.
Measurement of Oxaliplatin-Releasing Profiles by CSM
In order to measure the oxaliplatin-releasing profiles by CSM, CSM with 3 sizes (50-150 μm, 100-300 μm, and 300-500 μm) which were loaded with 20 mg oxaliplatin were placed in 500 mL 0.9% saline solution at 37°C with a flow of 5 mL/min in the pharmacopeia flow-through apparatus 4 Sotax system (Sotax CE6, Switzerland). Then 1 mL sample was collected at 0.25, 0.5, 1, 2, 4, 8, 12 hours for each sample, and centrifuged at 10 000g for 5 minutes at room temperature. Meanwhile, after sample collection, an equal volume of 0.9% saline solution was replenished. Subsequently, the concentration of oxaliplatin in each sample was determined using Agilent 1260 HPLC (Agilent Technologies Co) and accumulating releasing rate of oxaliplatin was calculated. In addition, 100 to 300 µm CSM loaded with 20 mg oxaliplatin were incubated in 500 mL 0.9% saline solution mixed with 20% fetal bovine serum (FBS) to determine the effect of serum on oxaliplatin releasing profiles. Besides, since the releasing profiles were similar among CSM with different sizes, and 100 to 300 µm CSM was most commonly used in clinical practice, thus only 100 to 300 µm CSM group was investigated to study the effect of serum on oxaliplatin releasing profile. All the experiments were conducted in triplicate.
Morphology of CSM Before and After Oxaliplatin Loading
The morphology and size distribution of CSM with 3 sizes (50-150 μm, 100-300 μm, and 300-500 μm) before and after 20 mg oxaliplatin loading were detected by scanning electron microscopic imaging (Hitachi S 2460N, JEOL, Japan) and laser diffraction particle size analyzer (Mastersizer 3000, Malvern Panalytical Ltd, United Kingdom) respectively. All experiments were conducted in triplicate.
High-Performance Liquid Chromatography Analysis
An Agilent 1260 HPLC system (Agilent Technologies Co) consisting of a G1311B quaternary pump, a G1316A thermostated column oven, a G1329B autosampler, and a G4212B diode array detector was employed. The analyte was conducted on Eclipse Plus C18 (5 μm, 4.6 mm × 150 mm, Agilent) at 40°C. The methanol and distillation-distillation H2O were used as the mobile phase. The isocratic elution mode of 10% methanol (vol/vol) and a flow rate of 1 mL/min were employed. The temperature of autosampler was maintained at 4°C, the injection volume was 5 μL for each sample, the chromatographic run time was within 8.0 minutes, and the detection wavelength of oxaliplatin was 250 nm.
Effect of Oxaliplatin-Eluting CSM on Proliferation of Colorectal Cancer Cells
Human colorectal cancer cell lines SW620 and HT29 were purchased from Leibniz Institute DSMZ-German Collection of Microorganisms and Cell Cultures (Braunschweig, German) and were cultured in Dulbecco Modified Eagle medium (Invitrogen,Carlsbad, California, USA) supplemented with 10% FBS (Gibco, Carlsbad, California, USA), 100 U/mL penicillin (North China Pharmaceuticals Co, Ltd, China), and 100 μg/mL streptomycin (North China Pharmaceuticals Co, Ltd, China) at 37°C in a humid atmosphere of 5% CO2. A total of 200 μL medium containing 0 μM oxaliplatin solution (blank control group), 0.5 μM oxaliplatin solution (oxaliplatin group), or 0.5 μM oxaliplatin-eluting CSM (oxaliplatin-eluting CSM group) were added to treat SW620 and HT29 for 72 hours, and cell proliferation was detected by Cell Counting Kit-8 (Dojindo, Kumamoto-ken, Japan) at 0 hours, 24 hours, 48 hours, 72 hours, and 96 hours according to the instruction of manufacturer.
Statistics
Data were mainly presented as mean ± standard deviation and compared by t test or one-way ANOVA test. Data processing and analysis were performed using SPSS version 24.0 (IBM, USA). P < .05 was considered as significant.
Results
Effect of Oxaliplatin Amount on Drug-Loading Efficiency of CSM
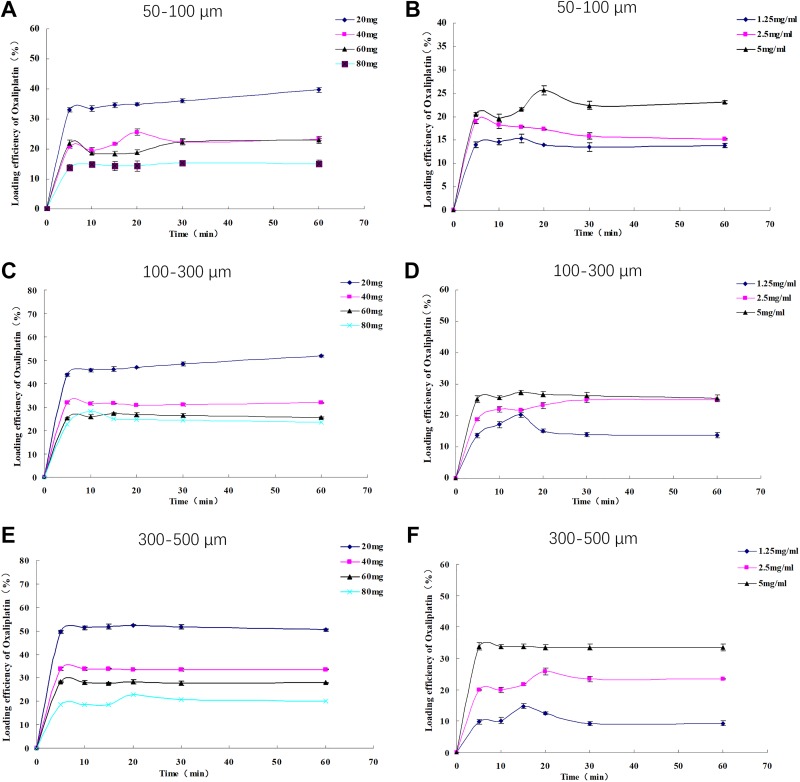
Multiple group comparison analysis revealed that oxaliplatin-loading efficiency was different among 20 mg, 40 mg, 60 mg, and 80 mg oxaliplatin groups in 50 to 100 µm CSM (Figure 1A), 100 to 300 µm CSM (Figure 1B), and 300 to 500 µm CSM (Figure 1C), and the curves disclosed that oxaliplatin amount was negatively correlated with loading efficiency with highest loadability in 20 mg oxaliplatin group (maximum 40% in 50- to 100-µm CSM, 52% in 100- to 300-µm CSM, and 52% in 300-500 µm CSM). In addition, time to maximal loading was approximately 60 minutes in 50 to 150 µm CSM and 100 to 300 µm CSM, and 20 minutes in 300 to 500 µm CSM for all doses of oxaliplatin.
Figure 1.
A-C showed the effect of different oxaliplatin amount on loading efficiency of CSM with size 50 to 150 μm, 100 to 300 μm, and 300 to 500 μm, respectively; D-F showed the effect of different oxaliplatin concentration on loading efficiency of CSM with size 50 to 150 μm, 100 to 300 μm, and 300 to 500 μm, respectively. CSM indicates CalliSpheres microspheres.
Effect of Oxaliplatin Concentration on Drug-Loading Efficiency of CSM
Multiple group comparison analysis illuminated that oxaliplatin loading efficiency was differed among 1.25 mg/mL, 2.5 mg/mL and 5 mg/mL oxaliplatin groups in 50 to 100 µm CSM (Figure 1D), 100 to 300 µm CSM (Figure 1E), and 300 to 500 µm CSM (Figure 1F), and the curves disclosed that oxaliplatin concentration was positively associated with loading efficiency with highest loadability in 5 mg/mL oxaliplatin group (maximum 26% in 50-100 µm CSM, 27% in 100-300 µm CSM, and 34% in 300-500 µm CSM). In addition, time to maximal loading was approximately 20 minutes in 50 to 150 µm CSM, 15 minutes in 100 to 300 µm CSM, and 20 minutes in 300 to 500 µm CSM for all concentrations of oxaliplatin.
Loading Stability of CSM
No difference in loading efficiency of 20 mg oxaliplatin with 100 to 300 µm CSM among different mediums or different volumes of the same medium was discovered (Supplementary Figure 1), indicating good stability of CSM.
Drug-Releasing Profiles of Oxaliplatin-Loaded CSM
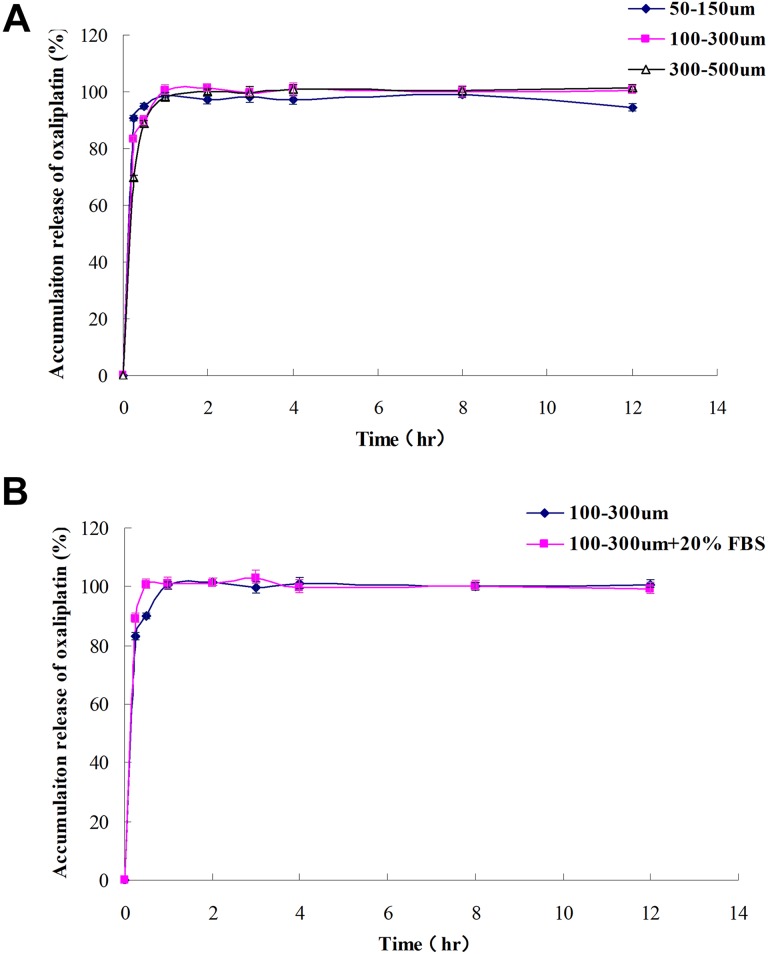
Since 20 mg oxaliplatin presented with the highest loading efficiency, 20 mg oxaliplatin-loaded CSM with 3 sizes (50-150 μm, 100-300 μm, and 300-500 μm) was prepared for drug-releasing profiles measurement. Similar drug-releasing profiles were observed among oxaliplatin-loaded CSM with 3 sizes (50-150 μm, 100-300 μm, and 300-500 μm; Figure 2A). And a rapid drug release was discovered in CSM with 3 sizes (50-150 μm, 100-300 μm, and 300-500 μm). In addition, we found that FBS did not affect the drug-releasing profiles of oxaliplatin-loaded CSM (Figure 2B).
Figure 2.
A, The releasing profile of 20 mg oxaliplatin-loaded CSM with size 50 to 150 μm, 100 to 300 μm, and 300 to 500 μm. B, The effect of FBS on releasing profiles of oxaliplatin-loaded CSM. CSM indicates CalliSphere microspheres; FBS, fetal bovine serum.
Morphology of CSM Before and After Oxaliplatin Loading
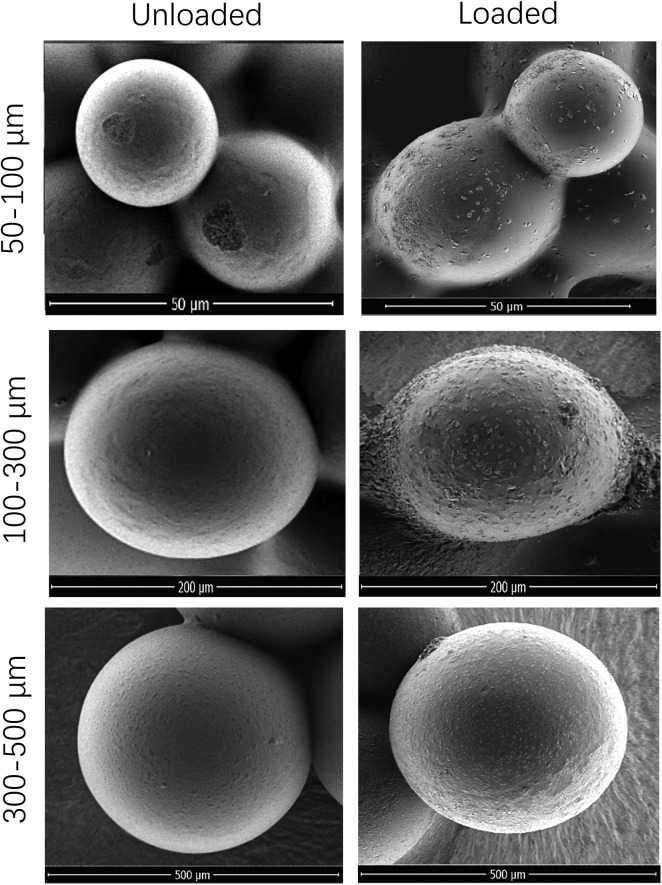
A total of 20 mg oxaliplatin-loaded CSM with 3 sizes (50-150 μm, 100-300 μm, and 300-500 μm) was prepared for morphology detection, which disclosed that the CSM was round and smooth before oxaliplatin loading, and it was modified a little to ellipse shape and less smooth after oxaliplatin loading (Figure 3). In addition, the mean particle size of CSM was enlarged by 1.7% in 50 to 150 µm CSM, 2.7% in 100 to 300 µm CSM, and 28.1% in 300 to 500 µm after 20 mg oxaliplatin loading (Table 1).
Figure 3.
Morphology of CSM with 3 sizes (50-150 μm, 100-300 μm, and 300-500 μm) before and after 20 mg oxaliplatin loading. CSM indicates CalliSphere microspheres.
Table 1.
Diameters of Unloaded and Oxaliplatin-Loaded CalliSpheres.
| Size of CalliSpheres | Mean Diameters (μm) | Increase Ratio (%) | |
|---|---|---|---|
| Unloaded | 20 mg Oxaliplatin Loaded | ||
| 50-150 μm | 119 ± 2 | 121 ± 7 | 1.7 ± 4.2 |
| 100-300 μm | 257 ± 3 | 264 ± 10 | 2.7 ± 2.7 |
| 300-500 μm | 481 ± 4 | 616 ± 25 | 28.1 ± 4.2 |
Cytotoxicity of Oxaliplatin-Eluting CSM on Colorectal Cancer Cells
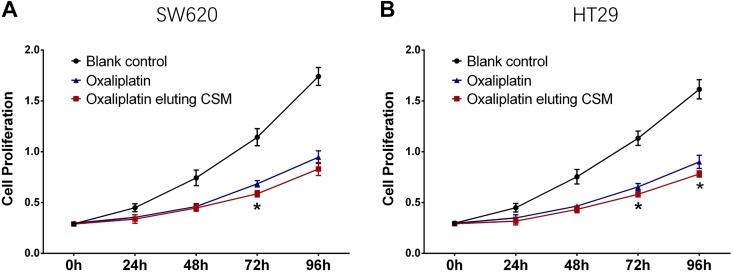
SW620 cell proliferation was similar at 0 hour, 24 hours, 48 hours, and 96 hours, while decreased at 72 hours in oxaliplatin-eluting CSM group compared to oxaliplatin group (Figure 4A); HT29 cell proliferation was similar at 0 hour, 24 hours, and 48 hours, while decreased at 72 hours and 96 hours in oxaliplatin-eluting CSM group compared to oxaliplatin group (Figure 4B). These data indicated oxaliplatin-eluting CSM increased the cytotoxicity of oxaliplatin in colorectal cancer cells to some extent.
Figure 4.
Effect of oxaliplatin-eluting CSM on colorectal cancer cells. Comparison of SW620 cell proliferation between oxaliplatin eluting CSM group and oxaliplatin group (A); comparison of HT29 cell proliferation between oxaliplatin eluting CSM group and oxaliplatin group (B). Comparison was determined by t test. CSM indicates CalliSphere microspheres.
Discussion
In this present study, we showed the oxaliplatin loading efficiency and releasing profiles of CSM with different sizes and further observed that oxaliplatin amount negatively while concentration positively correlated with loadability. Besides, oxaliplatin-eluting CSM increased the cytotoxicity of oxaliplatin in colorectal cancer cells to some extent.
In recent years, chemotherapy system with targeted and controlled release form has been widely explored to increase the drug concentration in target tumor while decrease the systemic drug-related toxicity in various cancers.8,16,17 In brief, the procedures of these methods are as follow: (1) Chemotherapy drugs are loaded by the carriers through binding (including physical adsorption, ionic bonding, and covalent bonding), encapsulation and cross-linking,9,10,16 (2) drug-loaded carriers are delivered to the tumor sites through implantation or injection.17-19 Finally, benefiting from the controlled release effect of the carriers on chemotherapy drugs, the elevated drug concentration in target tumor site and decreased drug concentration in system is realized.18,20,21 Meanwhile, novel biomaterials, which have been initiated in recent decades such as nanogel, drug-eluting stent, DEB and so on, have been developed and improved in recent years and have widely showed their potential in treating various diseases including cancers, cardiovascular diseases, analgesia applications, and so on.22-25 Among these approaches, DEB has shown its advantages in managing several cancers such as HCC via reducing systemic toxicity and increasing in-tumor chemotherapy drugs concentrations, and great efforts are still made to further improve the superiority of the materials or structures to increase the efficacy and reduce the tolerance risks.26-30 Among the types of carriers, DEB is widely accepted in clinical practice and several brands of DEB have been produced in medical application including DC beads, LC beads, HSM, and CSM, which are made of poly lactic-co-glycolic acid (PLGA), polyvinyl alcohol (PVA), or glucans.12,13,31-34
A previous study illuminates that DC beads achieve 95% doxorubicin-loading efficiency and HSM realizes 100% doxorubicin-loading efficiency, meanwhile, as to CSM, as the first DEB developed in China which mainly made of PVA, is also observed to possess similar doxorubicin loadability.11,18 However, the application of these DEBs in delivering oxaliplatin is very limited, only 3 studies (1 for noncommercial use beads and 2 for HSM) have been reported.35-37 In detail, a noncommercial use bead made of PLGA is applied to load oxaliplatin with maximum loadability 14% to 31.5%,35 and oxaliplatin-loaded HSM is introduced to treat patients with unresectable hepatic tumors which discloses encouraging outcomes, while the loading profiles are not investigated.36,37 Considering the satisfied manifestations of CSM in loading doxorubicin, we hypnotized that it would present with good loadability of oxaliplatin as well. In this present study, we observed that CSM with different sizes presented with good loading efficiency with maximum loadability 40% to 52% when loading 20 mg oxaliplatin at 5 mg/mL concentration. The possible mechanism of CSM for loading oxaliplatin might be as follows: As a PVC hydrogel modified with sulfonate groups, CSM could load oxaliplatin via multiple ways such as direct binding (including physical adsorption, ionic bonding, and covalent bonding), encapsulation or cross-linking. Besides, the loading efficiency was numerically higher in our study compared with previous report,35 which might result from that the superior binding, encapsulation, or cross-linking ability of CSM. Interesting, we firstly discovered that oxaliplatin amount negatively while concentration positively correlated with loadability, this might result from that loading burden was less when being loaded with lower amount oxaliplatin, while the contacting surface area was larger when being loaded with higher concentration oxaliplatin.
As to drug-releasing profiles, previous studies disclose that a very sustained releasing profiles are realized by DC beads, HSM, and CSM after being loaded with doxorubicin,11-13,18 while a very quick release is observed in DC beads, HSM after being loaded with irinotecan.12,13 Regarding to releasing oxaliplatin, the investigation is indeed insufficient. Only one report illustrates that a noncommercial use bead made of PLGA achieves sustained oxaliplatin release within 1200 hours.35 In addition, although another 2 reports reveal that oxaliplatin-loaded HSM presents with satisfied efficacy in treating patients with hepatic tumor, the releasing profiles of HSM for oxaliplatin is not explored.36,37 In this present study, we observed that oxaliplatin release was very quick in all sizes of CSM, this might result from that the binding or encapsulation soundness was less between CSM and oxaliplatin compared to doxorubicin. These implied that the application of oxaliplatin-loaded CSM for cancer treatment should be performed more frequently than doxorubicin-loaded CSM to sustain satisfied outcomes. Furthermore, we also observed that FBS had no effect on the releasing profiles of oxaliplatin-loaded CSM, which provides more support for the application of oxaliplatin-loaded CSM in treating cancers. Most importantly, the rapid release of oxaliplatin-eluting CSM in vitro indicates the sustained release advantage of TACE with oxaliplatin-eluting CSM is insufficient, while TACE with microspheres dose not only rely on the sustained releasing but also the targeted releasing and embolization. Such as irinotecan-eluting microspheres, although exhibit rapid release profile, is widely used to treat liver metastases of colorectal cancer.38,39
As to morphology of DEB before and after drug loading, a previous study illuminates that DC beads and HSM are both found to be less smooth and less regularly shaped after doxorubicin or irinotecan loading, and they are shrunken after doxorubicin or irinotecan loading and recovered to almost original sizes after elution.12,40 While no report has been reported on the morphology of DEB before and after oxaliplatin loading. In this study, we firstly discovered that CSM was modified a little to ellipse shape and less smooth after oxaliplatin loading, and it was enlarged to some extent. These might be explained by that binding or encapsulation soundness was less between CSM and oxaliplatin, thus the gap between them was larger than original gap between water content and CSM, thus after loading, the size was enlarged instead of shrunken.
Notably, the microspheres reduce systemic toxicities via directly releasing the chemotherapy drug to the target tumor (which reduce the concentration of chemotherapy in non-lesion tissue) instead of via the blood circulation, thus an in vivo investigation of tumor concentration and nontumor concentration with oxaliplatin-eluting CSM is meaningful, while due to lack of foundation, only in vitro investigation was performed.
In conclusion, this study discloses drug loadability, releasing profiles, and morphology change of CSM for delivering oxaliplatin, which provides potential evidences for application of oxaliplatin-loaded DEB in clinical practice.
Supplemental Material
Supplemental Material, Supplementary_Figure_1_(revised) for Morphology, Loadability, and Releasing Profiles of CalliSpheres Microspheres in Delivering Oxaliplatin: An In Vitro Study by Xiaoli Han, Qinyue Chen, Yali Sun, Limei Han and Xianyi Sha in Technology in Cancer Research & Treatment
Abbreviations
- CSM
CalliSpheres microspheres
- DEBs
drug-eluting beads
- FBS
fetal bovine serum
- HPLC
high-performance liquid chromatography
- HSM
HepaSphere microspheres
- PLGA
poly lactic-co-glycolic acid
- PVA
polyvinyl alcohol
- TACE
transarterial chemoembolization
Footnotes
Authors’ Note: Our study did not require an ethical board approval because it did not contain human or animal trials.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Xianyi Sha  https://orcid.org/0000-0002-7107-0917
https://orcid.org/0000-0002-7107-0917
Supplemental Material: Supplemental material for this article is available online.
References
- 1. Ibrahim A, Hirschfeld S, Cohen MH, Griebel DJ, Williams GA, Pazdur R. FDA drug approval summaries: oxaliplatin. Oncologist. 2004;9(1):8–12. [DOI] [PubMed] [Google Scholar]
- 2. Raymond E, Faivre S, Woynarowski JM, Chaney SG. Oxaliplatin: mechanism of action and antineoplastic activity. Semin Oncol. 1998;25(2 suppl 5):4–12. [PubMed] [Google Scholar]
- 3. Nobili S, Checcacci D, Filippelli F, et al. Bimonthly chemotherapy with oxaliplatin, irinotecan, infusional 5-fluorouracil/folinic acid in patients with metastatic colorectal cancer pretreated with irinotecan- or oxaliplatin-based chemotherapy. J Chemother. 2008;20(5):622–631. [DOI] [PubMed] [Google Scholar]
- 4. Wang LJ LZ, Zheng Z, Xiao WY. Research progress on the treatment of oxaliplatin-induced nerve toxicity. Anti-tumor Pharm. 2013;3(6):409–415. [Google Scholar]
- 5. Nagy, SG. Role of calcium and magnesium infusion in prevention of oxaliplatin neurotoxicity. A phase III trial. Chinese German J Clin Oncol. 2013;12(5):232–236. [Google Scholar]
- 6. Bullinger KL, Nardelli P, Wang Q, Rich MM, Cope TC. Oxaliplatin neurotoxicity of sensory transduction in rat proprioceptors. J Neurophysiol. 2011;106(2):704–709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hua Y, Wang W, Wang G, Zheng X, Shen Y. Preparation and evaluation of oxaliplatin long-circulating liposomes. Chinese J Modern Appl Pharm. 2018. [Google Scholar]
- 8. Xiong MH, Bao Y, Yang XZ, Zhu YH, Wang J. Delivery of antibiotics with polymeric particles. Adv Drug Deliv Rev. 2014;78:63–76. [DOI] [PubMed] [Google Scholar]
- 9. Piacentini E, Yan M, Giorno L. Development of enzyme-loaded PVA microspheres by membrane emulsification. J Membrane Sci. 2017;524:79–86. [Google Scholar]
- 10. Namur J, Citron SJ, Sellers MT, et al. Embolization of hepatocellular carcinoma with drug-eluting beads: doxorubicin tissue concentration and distribution in patient liver explants. J Hepatol. 2011;55(6):1332–1338. [DOI] [PubMed] [Google Scholar]
- 11. Zhang S, Huang C, Li Z, et al. Comparison of pharmacokinetics and drug release in tissues after transarterial chemoembolization with doxorubicin using diverse lipiodol emulsions and CalliSpheres Beads in rabbit livers. Drug Deliv. 2017;24(1):1011–1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Jordan O, Denys A, De Baere T, Boulens N, Doelker E. Comparative study of chemoembolization loadable beads: in vitro drug release and physical properties of DC bead and HepaSphere loaded with doxorubicin and irinotecan. J Vasc Interv Radiol. 2010;21(7):1084–1090. [DOI] [PubMed] [Google Scholar]
- 13. Biondi M, Fusco S, Lewis AL, Netti PA. Investigation of the mechanisms governing doxorubicin and irinotecan release from drug-eluting beads: mathematical modeling and experimental verification. J Mater Sci Mater Med. 2013;24(10):2359–2370. [DOI] [PubMed] [Google Scholar]
- 14. Liu J, Cao G, Zhang G, Zhou S, Xu W, Liu S. Interventional chemoembolization with drug-eluting microspheres of domestic CalliSpheres for the treatment of primary hepatocellular carcinomas: preliminary results in 12 patients. J Intervent Radiol. 2017;26(11):993–998. [Google Scholar]
- 15. Wu B, Zhou J, Ling G, Zhu D, Long Q. CalliSpheres drug-eluting beads versus lipiodol transarterial chemoembolization in the treatment of hepatocellular carcinoma: a short-term efficacy and safety study. World J Surg Oncol. 2018;16(1):69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Agnihotri N, Mishra R, Goda C, Arora M. Microencapsulation – a novel approach in drug delivery: a review. Indo Global J Pharm Sci. 2012;2(1):1–20. [Google Scholar]
- 17. Alagusundaram M, Chetty CMS, Umashankari K, Badarinath AV, Lavanya C, Ramkanth S. Microspheres as a novel drug delivery system—a review. Int J Chem Res. 2009;1(3):526–534. [Google Scholar]
- 18. Lewis AL, Dreher MR. Locoregional drug delivery using image-guided intra-arterial drug eluting bead therapy. J Control Release. 2012;161(2):338–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ferrer Puchol MD, la Parra C, Esteban E, et al. Comparison of doxorubicin-eluting bead transarterial chemoembolization (DEB-TACE) with conventional transarterial chemoembolization (TACE) for the treatment of hepatocellular carcinoma. Radiologia. 2011;53(3):246–253. [DOI] [PubMed] [Google Scholar]
- 20. Varela M, Real MI, Burrel M, et al. Chemoembolization of hepatocellular carcinoma with drug eluting beads: efficacy and doxorubicin pharmacokinetics. J Hepatol. 2007;46(3):474–481. [DOI] [PubMed] [Google Scholar]
- 21. Poon RT, Tso WK, Pang RW, et al. A phase I/II trial of chemoembolization for hepatocellular carcinoma using a novel intra-arterial drug-eluting bead. Clin Gastroenterol Hepatol. 2007;5(9):1100–1108. [DOI] [PubMed] [Google Scholar]
- 22. Chen J, Ding J, Xu W, et al. Receptor and microenvironment dual-recognizable nanogel for targeted chemotherapy of highly metastatic malignancy. Nano Lett. 2017;17(7):4526–4533. [DOI] [PubMed] [Google Scholar]
- 23. Li S, Zhang T, Xu W, et al. Sarcoma-targeting peptide-decorated polypeptide nanogel intracellularly delivers shikonin for upregulated osteosarcoma necroptosis and diminished pulmonary metastasis. Theranostics. 2018;8(5):1361–1375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Zhang W, Ning C, Xu W, et al. Precision-guided long-acting analgesia by Gel-immobilized bupivacaine-loaded microsphere. Theranostics. 2018;8(12):3331–3347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Zhang W, Xu W, Ning C, et al. Long-acting hydrogel/microsphere composite sequentially releases dexmedetomidine and bupivacaine for prolonged synergistic analgesia. Biomaterials. 2018;181:378–391. [DOI] [PubMed] [Google Scholar]
- 26. Li J, Xu W, Li D, et al. Locally deployable nanofiber patch for sequential drug delivery in treatment of primary and advanced orthotopic hepatomas. ACS Nano. 2018;12(7):6685–6699. [DOI] [PubMed] [Google Scholar]
- 27. Shi B, Huang K, Ding J, et al. Intracellularly swollen polypeptide nanogel assists hepatoma chemotherapy. Theranostics. 2017;7(3):703–716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Zhang Y, Zhang J, Xu W, Xiao G, Ding J, Chen X. Tumor microenvironment-labile polymer-doxorubicin conjugate thermogel combined with docetaxel for in situ synergistic chemotherapy of hepatoma. Acta Biomater. 2018;77:63–73. [DOI] [PubMed] [Google Scholar]
- 29. Wang J, Xu W, Li S, et al. Polylactide-cholesterol stereocomplex micelle encapsulating chemotherapeutic agent for improved antitumor efficacy and safety. J Biomed Nanotechnol. 2018;14(12):2102–2113. [DOI] [PubMed] [Google Scholar]
- 30. He L, Xu W, Wang X, Wang C, Ding J, Chen X. Polymer micro/nanocarrier-assisted synergistic chemohormonal therapy for prostate cancer. Biomater Sci. 2018;6(6):1433–1444. [DOI] [PubMed] [Google Scholar]
- 31. Athanasiou KA, Niederauer GG, Agrawal CM. Sterilization, toxicity, biocompatibility and clinical applications of polylactic acid/polyglycolic acid copolymers. Biomaterials. 1996;17(2):93–102. [DOI] [PubMed] [Google Scholar]
- 32. Martin R, Geller D, Espat J, et al. Safety and efficacy of trans arterial chemoembolization with drug-eluting beads in hepatocellular cancer: a systematic review. Hepato-Gastroenterol. 2012;59(113):255–260. [DOI] [PubMed] [Google Scholar]
- 33. Franca D, Medina AF, Messa LL, Souza CF, Faez R. . Chitosan spray-dried microcapsule and microsphere as fertilizer host for swellable—controlled release materials. Carbohydrate Polym. 2018;196:47–55. [DOI] [PubMed] [Google Scholar]
- 34. Inoo K, Bando H, Tabata Y. Enhanced survival and insulin secretion of insulinoma cell aggregates by incorporating gelatin hydrogel microspheres. Regenerat Ther.2018;8:29–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Lagarce F, Cruaud O, Deuschel C, Bayssas M, Griffon-Etienne G, Benoit J. Oxaliplatin loaded PLAGA microspheres: design of specific release profiles. Int J Pharm. 2002;242(1-2):243–246. [DOI] [PubMed] [Google Scholar]
- 36. Poggi G, Quaretti P, Minoia C, et al. Oxaliplatin-eluting microspheres for the treatment of intrahepatic cholangiocarcinoma: a case report. Anticancer Res. 2008;28(5B):2987–2990. [PubMed] [Google Scholar]
- 37. Poggi G, Quaretti P, Minoia C, et al. Transhepatic arterial chemoembolization with oxaliplatin-eluting microspheres (OEM-TACE) for unresectable hepatic tumors. Anticancer Res. 2008;28(6B):3835–3842. [PubMed] [Google Scholar]
- 38. Mokkarala M, Noda C, Malone C, Ramaswamy R, Akinwande O. Comparison of response and outcomes of drug-eluting bead Chemoembolization (DEB-TACE) Versus Radioembolization (TARE) for patients with colorectal cancer liver metastases. Anticancer Res. 2019;39(6):3071–3077. [DOI] [PubMed] [Google Scholar]
- 39. Fereydooni A, Letzen B, Ghani MA, et al. Irinotecan-eluting 75-150-mum embolics lobar chemoembolization in patients with colorectal cancer liver metastases: a prospective SINGLE-center Phase I Study. J vasc Interv Radiol. 2018;29(12):1646–1653. [DOI] [PubMed] [Google Scholar]
- 40. Lewis AL, Gonzalez MV, Lloyd AW, et al. DC bead: in vitro characterization of a drug-delivery device for transarterial chemoembolization. J vasc Interv Radiol. 2006;17(2 Pt 1):335–342. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Material, Supplementary_Figure_1_(revised) for Morphology, Loadability, and Releasing Profiles of CalliSpheres Microspheres in Delivering Oxaliplatin: An In Vitro Study by Xiaoli Han, Qinyue Chen, Yali Sun, Limei Han and Xianyi Sha in Technology in Cancer Research & Treatment