Abstract
Nonalcoholic fatty liver disease (NAFLD) is the most prevalent chronic liver disease worldwide. It represents a range of disorders, including simple steatosis, nonalcoholic steatohepatitis (NASH), and liver cirrhosis, and its prevalence continues to rise. In some cases, hepatocellular carcinoma (HCC) may develop. The develop;ment of non-invasive diagnostic and screening tools is needed, in order to reduce the frequency of liver biopsies. The most promising methods are those able to exclude advanced fibrosis and quantify steatosis. In this study, new perspective markers for inflammation, oxidative stress, apoptosis, and fibrogenesis; emerging scoring models for detecting hepatic steatosis and fibrosis; and new genetic, epigenetic, and multiomic studies are discussed. As isolated biochemical parameters are not specific or sensitive enough to predict the presence of NASH and fibrosis, there is a tendency to use various markers and combine them into mathematical algorithms. Several predictive models and scoring systems have been developed. Current data suggests that panels of markers (NAFLD fibrosis score, Fib-4 score, BARD score, and others) are useful diagnostic modalities to minimize the number of liver biopsies. The review unveils pathophysiological aspects related to new trends in current non-invasive biochemical, genetic, and scoring methods, and provides insight into their diagnostic accuracies and suitability in clinical practice.
Keywords: nonalcoholic fatty liver disease (NAFLD), steatosis, nonalcoholic steatohepatitis (NASH), fibrosis, biochemical diagnostic, genetic diagnostic, non-invasive scoring methods
1. Background
Nonalcoholic fatty liver disease (NAFLD) does not present as a single disease; rather, it is a spectrum of conditions, ranging from simple steatosis and nonalcoholic steatohepatitis (NASH) to liver cirrhosis, with its complications including hepatocellular carcinoma (HCC). NAFLD is a systemic condition, featuring metabolic, cardiovascular, and (hepatic/extrahepatic) cancer risks [1,2]. NAFLD is the most frequent cause of chronic liver injury in adults in developed countries [3,4]. About one-quarter of fatty liver cases develop NASH and over one-quarter of NASH patients develop severe fibrosis [1,5,6,7]. NAFLD is a precursor to type 2 diabetes (T2DM) and metabolic syndrome, and progressive liver disease develops in T2DM patients in whom the course of the disease is worsened by NAFLD [2,8,9,10]. NAFLD is highly prevalent in certain cohorts of individuals, who are potentially amenable to selective screening strategies and intensive follow-up schedules for early identification of liver-related and extrahepatic complications and for which earlier and more aggressive treatment schedules should be carried out, whenever possible [2]. Liver biopsy is an invasive diagnostic tool with little but significant hazard, and the decision of when to perform it remains to be controversial [11,12,13]. Consequently, it is necessary to search for less invasive methods for screening, distinguishing various NAFLD stages, and following their progression [14,15]. Non-invasive diagnosis is based on clinical and biochemical markers, scoring models, and algorithms of methods which have sufficient sensitivity, specificity, and reproducibility [16,17,18]. As non-invasive diagnostic methods should reduce the frequency of liver biopsies, they have to focus on two targets: Differentiation of simple steatosis from steatohepatitis and staging of fibrosis [19,20,21]. The detection of liver inflammation and fibrosis does not have only predictive value, but is important for determining the treatment threshold [22,23,24].
2. Pathophysiology of NAFLD
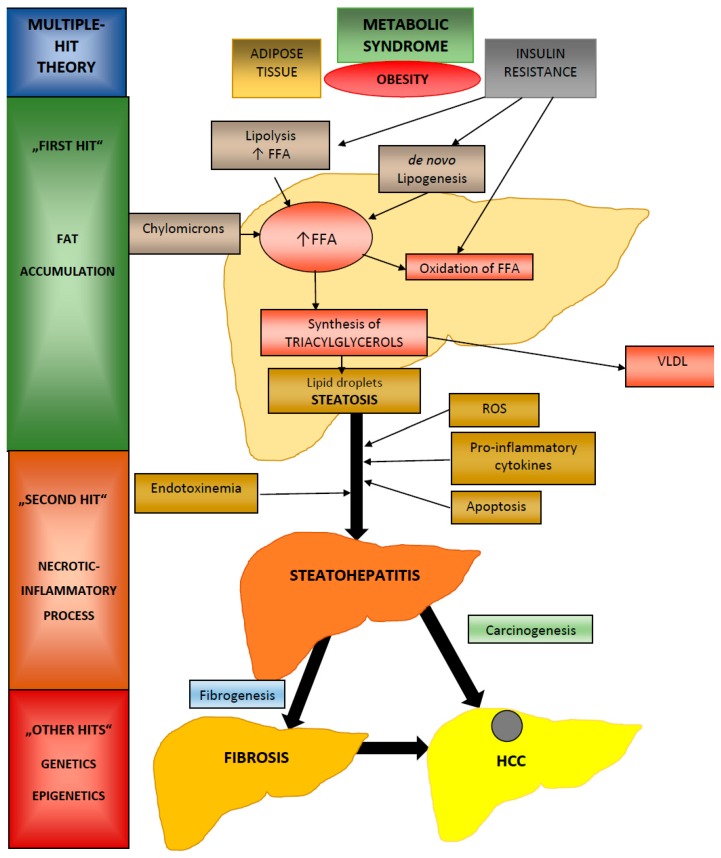
Many diagnostic markers result from pathophysiological processes in hepatocytes which are typical of fatty liver injury, such as inflammation, cell death, and oxidative stress [25,26,27,28]. The pathogenesis of NAFLD is not completely known. The “multiple hit” hypothesis considers several insults acting together on genetically predisposed subjects to induce NAFLD and provides a more accurate explanation of NAFLD pathogenesis [29] (Figure 1). Such hits include insulin resistance, hormones secreted from adipose tissue, nutritional factors, gut microbiota, and genetic and epigenetic factors [29,30,31,32]. Obesity seems to have an important position in the development of NAFLD, but NAFLD occurs even in lean patients [33,34,35]. Obesity may lead to metabolic syndrome and insulin resistance (IR). On the other hand, insulin resistance may be responsible for NAFLD in non-obese patients [30,36,37,38].
Figure 1.
Pathogenesis of nonalcoholic fatty liver disease (NAFLD). Legend: FFA—free fatty acids, VLDL—very low density lipoproteins, ROS—reactive oxygen species, HCC—hepatocellular carcinoma.
The first hit leads to fat accumulation in hepatocytes as lipid droplets in the cytoplasm and causes simple steatosis. This state is reversible, and is associated with abnormal triglyceride storage. Triglycerides are produced from free fatty acids (FFAs). The main source of FFAs are plasmatic non-esterified fatty acids (NEFAs), followed by de novo lipogenesis and dietary fats, in the form of chylomicron lipoproteins. NEFAs especially arise from the lipolysis of the adipose tissue, which is induced by IR and during fasting [3,19]. The FFAs in the liver may follow three different pathways: Beta-oxidation (mainly in the mitochondria), the export of very low density lipoproteins (VLDL) into the blood with the help of apolipoprotein B (APOB), and synthesis of triglycerides [39]. It has been proposed that excessive intra-abdominal fat can induce excessive FFA reflux into the liver through the portal circulation. Triglyceride deposition in the form of lipid droplets (liver steatosis) may represent a vulnerable condition for the second hit, which causes hepatic inflammation and necrosis and can progress to fibrosis and cirrhosis. Excessive FFA oxidation can lead to oxidative stress with free radical formation and mitochondrial dysfunction, in general leading to the state known as lipotoxicity [40]. The immune response to lipotoxicity promotes inflammatory and wound-healing processes, which can lead to fibrogenesis and NAFLD progression [29]. On one hand, inflammatory pathways are activated in the liver, propelled by Kupffer´s cells (KCs), neutrophils, and natural killer cells (NK), as well as through the production of proinflammatory cytokines (e.g. interleukin 6 (IL-6), tumor necrosis factor alpha (TNF-α), and interleukin 1 (IL-1)). On the other hand, lipotoxicity also promotes inflammatory reaction in the adipose tissue and deregulates adipocytokine production, especially through the inhibition of adiponectin and the induction of leptin [41,42,43,44,45]. The second hit also includes apoptosis and gut-derived bacterial endotoxinemia, which play important roles in the development of NAFLD [40]. The microbiota can change the whole body’s lipid metabolism, as it can shift it from oxidation to de novo production [46,47,48,49,50]. NAFLD has been associated with small intestinal bacterial overgrowth and increased permeability, which can result in endotoxinemia and the activation of Kupffer cells [46]. The necroinflammatory stage, which is typical for NASH, leads to the activation of stellate hepatic cells, which are responsible for fibrogenesis, and progenitor cells, which promote hepatocarcinogenesis [51,52,53]. For instance, insulin resistance has been considered a promotor of stellate cell proliferation and an activator of collagen 1 production [36,54,55]. The third hit includes genetic factors. Different studies have shown familial aggregation and racial varieties in the prevalence of NAFLD and have mentioned a genetic predisposition to NAFLD [56]. The most evident genetic association is with palatine-like phospholipase 3 (PNPLA3), where certain non-synonymous single nucleotide polymorphisms (SNPs) have been associated with the severity of steatosis and the presence of NASH [57] (Table 1).
Table 1.
Summary of single nucleotide polymorphisms (SNPs) related to nonalcoholic fatty liver disease (NAFLD).
| Genes incorporated in Glucose and Lipid Metabolism | |
| Apolipoprotein C III | APOC3 rs 2854116, rs 2854117 |
| Peroxisome proliferative activated receptor α, γ, peroxisome proliferator-activated receptor γ coactivator 1-α | PPAR α, rs 1800206, PPAR γ, rs1801282, PPARGC1A, rs2290602 |
| Fatty acid transport protein | FATP5, rs 56225452 |
| Adiponectin | ADIPQ, rs2241766, rs 1501299 |
| Leptin receptor | LEPR rs62589000, rs6700986 |
| Resistin | RETN rs 3745367 |
| Genes incorporated in the pathogenesis of NAFLD | |
| TNF-α, TNF-α related apoptosis inducing ligand | TNF-α rs 1800629, rs361525,TRAIL rs6763816, rs4491934 |
| Toll like receptor | TLR4 rs4986790 |
| Superoxide dismutase 2 | SOD2 rs4880 |
| Cytochrome P450 2E1 | CYP2E1 rs2031920 |
| Kruppel-like factor 6 | KLP6 rs3750816 |
| Transforming growth factor β1 | TGF-β1 rs1800471 |
| Angiotensin II, angiotensin II Type receptor | AGII rs699, AGTR1 rs3772622, rs 3772633 |
3. Clinical Evaluation of NAFLD
The majority of subjects with NAFLD are clinically asymptomatic during the pre-cirrhotic stage. Patients can complain about fatigue and uncertain discomfort in the right upper abdominal quadrant. Physical examination can reveal hepatomegaly and obesity [58]. Secondary NAFLD involves complex pathophysiological and clinical consequences that ensue when the liver becomes an ectopic site of lipid storage, owing to reasons other than its mutual association with metabolic syndrome. Disorders affecting the gonadal hormones, thyroid hormones, or growth hormones (GH) may cause secondary forms of NAFLD to develop, which exhibit pathophysiologic features and, in theory, may affect the possibility of receiving effective treatment. Some common endocrine diseases, such as polycystic ovary syndrome (PCOS), hypothyroidism, hypogonadism, and GH deficiency, may be part of a naturally occurring disease model of NAFLD in humans [27,59]. As the disease progresses, features of liver decompensation can be present (e.g., jaundice, ascites, edema, gastrointestinal bleeding, and encephalopathy) [60]. Clinical features develop with the severity of the disease; thus, clinical symptoms are not crucial for making a diagnosis of early-stage NAFLD [61,62,63]. We should check for other signs related to metabolic syndrome, such as hypertension, diabetes mellitus, and abdominal obesity. to guide us in diagnosing NAFLD [2,64,65,66,67,68]. The diagnosis of NAFLD requires evidence of hepatic steatosis; in the absence of other causes of liver fat accumulation. NAFLD is often suspected in clinical practice when an individual with features of metabolic syndrome is found to have an increase in serum aminotransferase levels. Almost 80% of patients with NAFLD, however, have no biochemical abnormalities, which has several possible explanations [36,69]. The components of the metabolic syndrome are closely associated with NAFLD. Nearly two-thirds of people with obesity and T2DM and half of patients with hyperlipidaemia and hypertension have fat identified upon liver ultrasound. The presence of multiple features of the metabolic syndrome has been associated with more severe NAFLD-related liver disease and a higher likelihood of progression to NASH and liver fibrosis [24,70,71,72]. Ultrasonography is recommended as the first-line diagnostic method in assessing steatosis [15,17], while serum biomarkers and biomarker panels are alternative tools when imaging tools are not available in larger-scale screening studies [6,18,19,24]. An increasing number of biomarker panels have been used in clinical and research applications, while most have been validated in studies with relatively small populations, or in studies with sub-optimal gold criteria [15]. Therefore, future well-designed studies are needed to develop a more effective noninvasive biomarker panel for identifying NAFLD [6,17,20,21,23]. Magnetic resonance imaging-derived proton density fat fraction (MRI-PDFF) not only presents excellent performance for diagnosing NAFLD, but also accurately detects changes in fat content during disease progression [15]. However, MRI-PDFF is costly, time-consuming, and device dependent, which makes it difficult to achieve widespread application [15]. More effective, feasible, and easily operated tools are needed for diagnosing NAFLD, especially for early steatosis [15,17,24].
4. Laboratory Evaluation of NAFLD
4.1. Routine Markers of Liver Injury and Metabolic Syndrome
The majority of subjects with NAFLD are clinically asymptomatic during the pre-cirrhotic stage, but usually we can diagnose them through abnormal liver tests; mostly through increased levels of alanine-aminotransferase (ALT), aspartate aminotransferase (AST), and gamma-glutamyl transpeptidase (GGT). Hepatic enzymes are not reliable markers, as they do not have to be elevated even in advanced NAFLD [73,74,75]. Generally, the AST to ALT ratio increases with the severity of the necroinflammatory and fibrotic changes. In most cases, the serum prothrombin time, bilirubin level, and serum albumin level are normal, except in patients with NAFLD-associated cirrhosis. The serum ferritin level is elevated in more than 20% of NAFLD patients and can be a marker for advanced disease and increased mortality [15,76]. The iron serum is associated with oxygen radicals, which contributes to necroinflammation and fibrosis, two important parameters of NAFLD. Serum iron is higher in individuals with NASH than in those with simple steatosis. Serum ferritin exhibits a moderate performance in diagnosing NASH (AUROC 0.73). Through biopsies of NAFLD patients, a scoring system which combined serum ferritin with type IV collagen 7S and fasting insulin showed the ability to predict NASH with an AUROC of 0.78–0.85 [15]. High sensitivity C-reactive protein (CRP) is a generic inflammatory marker and is also correlated with NASH [77,78]. Impaired glucose metabolism (dysglycemia and impaired glucose tolerance), insulin resistance, hyperinsulinemia, dyslipidemia (hypertriglyceridemia), and hypercholesterolemia are associated with metabolic disorders related to NAFLD [17,79,80,81,82]. If NAFLD is suspected, laboratory findings can be used but they do not refer to histological severity; however, they can predict cardiovascular morbidity and mortality [83,84,85].
4.2. Markers of Inflammation
The correlation of NAFLD with general inflammatory markers such as CRP and ferritin has been described [86,87]. A novel acute phase reactant, plasma pentraxin 3 (PTX 3), seems to be promising for distinguishing variants of NAFLD. PTX 3 is increased in NASH, but also in other inflammatory states [88]. NAFLD is also associated with subclinical systemic inflammation and corresponds well with various cytokines and adipokines. A long-recognized proinflammatory cytokine is TNF-α, which is secreted by the adipose tissue of obese individuals, as well as hepatocytes, Kupffer cells, and other cells. TNF-α is highly expressed in NASH and, in general, enhances inflammation, necrosis, apoptosis, and fibrosis [15,43]. Despite this fact, it is one of the most studied cytokines, and numerous studies have confirmed its role in the pathogenesis of NAFLD. Anti-TNF therapy hampers the development of NAFLD. Other important cytokines to mention are interleukin (IL)-6, IL-1, IL-8, and IL-18. IL-6 seems to be the most related to NASH (Table 2). In a study by Tarantino et al., an area under the receiver-operating characteristics curve (AUROC) of 0.82 was shown [41,89]. Several studies have proved the importance of interleukins in pathological pathways of NAFLD, but their precise role is not still clear [32,41,43,89]. The relationship between their levels and stages of NAFLD is controversial and, as single markers, they are not useful enough [26,43]. The development of NAFLD and metabolic syndrome are associated with chemokines produced by adipose tissue, adipokines, which are also responsible for subclinical systemic inflammation in obese individuals [26,43,90]. Adiponectin seems to be a protective cytokine [90]. It is secreted only by the visceral adipose tissue of obese subjects and has anti-inflammatory, antiproliferative, antiatherogenic, and antihyperglycemic effects [42,90]. Adiponectin is a cytokine of key importance in NAFLD, which is able to regulate liver steatosis, insulin resistance, inflammation, and fibrosis [42,43,90,91]. Adiponectin levels are low in NAFLD patients, and they are a negative predictor of NASH in adults [91]. Other notable adipocytokines are leptin, ghrelin, visfatin, resistin, and retinol-binding protein 4 (RBP4). Leptin levels correlate with total body fat, and leptin resistance may be considerable in liver fibrogenesis [92]. Ghrelin is a peptide produced mainly by the stomach, which stimulates food intake. It is correlated with insulin sensitivity, and obese individuals have lower concentrations of de-acyl ghrelin [93]. Resistin and visfatin may play roles in obesity and insulin resistance, and higher plasma levels are found in obese subjects, but their importance in the pathogenesis of NAFLD is not still clear. Most cytokines associated with liver injury in NAFLD are not sensitive and specific enough for the staging of NAFLD; thus, more research in this field is needed [24].
Table 2.
Values of AUROC for the most accurate non-invasive biochemical methods.
| Method | Field of Detection | Accuracy | Strengths | Advantages and Limitations | Reference |
|---|---|---|---|---|---|
| Biochemical Methods | |||||
| IL-6 | NASH fibrosis |
AUROC 0.83 |
95% CI: 0.67;0.98 p = 0.0024 Sensitivity 85% Specificity 86%. |
Discrimination between advanced fibrosis patients compared to mild fibrosis patients and no fibrosis patients; p < 0.001. |
[89] |
| VCAM-1 | NASH fibrosis |
AUROC 0.87 0.79 0.53 |
95% CI: 0.75;1.0 p = 0.0005 95% CI: 0.63;0.95 p = 0.0064 95% CI: 0.35;0.71 n.s. |
Distinguish between advanced fibrosis and no fibrosis. Distinguish between mild fibrosis from advanced fibrosis. Poor sensitivity for distinguish in no fibrosis compared to mild fibrosis. In children and adolescents is elevated with obesity. |
[78] |
| HA | NASH fibrosis |
AUROC 0.94 |
Cut off 25 ug/L sensitivity 90%, specificity 84% CI: 0.59–0.99. |
Discrimination between significant liver fibrosis F3 + F4 and mild to moderate, or no fibrosis (F0–F2); p < 0.001. | [94] |
| Cytokeratin 18 | NASH fibrosis | M65 AUROC 0.89 |
Cut off 750 U/L, sensitivity 80%, specificity 82%, 95% CI: 0.57–0.95. Cut-off 211 U/L, sensitivity 0.79, Specificity 0.76, 95% CI: 0.56–0.93. |
Diferentiation of patients with and without NASH. M65 p < 0.014, M30 p < 0.001. Can predict the disease severity in NASH patients. |
[94] |
| M30 AUROC 0.85 |
Cut off 750 U/L, sensitivity 80%, specificity 82%, 95% CI: 0.57–0.95. Cut-off 211 U/L, sensitivity 0.79, Specificity 0.76, 95% CI: 0.56–0.93. |
Diferentiation of patients with and without NASH. M65 p < 0.014, M30 p < 0.001. Can predict the disease severity in NASH patients. |
[15] | ||
Legend: IL-6—interleukin 6, VCAM-1—vascular cell adhesion protein 1, HA—hyaluronic acid, CI—confidence interval, AUROC—area under the receiver-operating characteristic curve.
4.3. Markers of Oxidative Stress
It is known that the increased production of reactive oxygen species (ROS) is responsible for lipid peroxidation, which leads to inflammation and fibrogenesis through stellate cell activation. The generation of ROS plays an important role in producing liver damage and initiating hepatic fibrogenesis [95,96]. Levels of thiobarbituric acid reactive substances (TBARS), nitric oxide (NO), superoxide dismutase (SOD), catalase, malonaldehyde (MDA), and vitamin E may be potential markers of disease, but it is not easy to determine their levels in serum as they are relatively volatile. Some studies have confirmed an association of markers of oxidative stress with NASH and liver fibrosis, especially TBARS, MDA, and oxidized VLDL, but results have been inconsistent [26].
4.4. Markers of Apoptosis
Cell apoptosis is related to defective permeabilization of the mitochondrial membrane and the release of proteins into the cytosol. A major intermediate filament protein in hepatocytes is cytokeratin 18 (CK 18). Several studies have proved the correlation of the CK 18 fragment level with the severity of NAFLD. Values of CK18 and its plasma fragments have shown an AUROC of 0.9 for detecting steatosis and 0.8 for detecting NASH [97,98]. CK 18 seems to be a reliable biomarker for hepatocellular injury; however, it has not yet been used in clinical practice. Another protein released during apoptosis is polypeptide-specific antigen, which could be a potential marker for fibrosis and could have clinical utility in the follow-up of obese patients with NASH [99].
4.5. Markers of Fibrogenesis
Fibrogenetic activity or extracellular matrix (ECM) turnover are typical changes in advanced-stage liver injury. Some of the most important factors in the process of extracellular matrix creation and fibrogenesis are changes in the activity of matrix metalloproteinases (MMP) and their specific inhibitors [100]. One of the most-studied matrix components HA, which is synthetized by stellate cells. Several studies have confirmed that serum hyaluronic acid is a reliable marker of hepatic fibrosis in NAFLD [94,101]. The enhanced liver fibrosis panel includes markers of fibrogenesis: Tissue inhibitor of metalloproteinase 1 (TIMP 1) and HA endaminoterminal peptide of procollagen III (P3NP). Other biomarkers for extracellular matrix turnover are transforming growth factor β, type IV collagen 7S domain, and endothelin-1 [102,103,104]. Proteomic analysis has also revealed other components of the extracellular matrix: Laminin and lumican. These markers have been proven to be predictors of fibrosis; however, they are not yet used in routine practice [89].
5. Differentiation of Steatosis, Steatohepatitis, and Fibrosis
As isolated non-invasive biochemical parameters are not specific and sensitive enough to predict the presence of NASH and fibrosis, there is a tendency to use various markers and to combine them into mathematical algorithms to avoid the use of invasive liver biopsy. Several predictive models have been developed and validated (Table 3). Non-invasive methods rely on two different approaches: A “biological” approach, based on the quantification of biomarkers in serum samples, or a “physical” approach, based on the measurement of liver stiffness, using either ultrasound or magnetic resonance-based elastography techniques. Although these approaches are complementary, they are based on different rationales. Liver stiffness corresponds to a genuine and intrinsic physical property of liver parenchyma, whereas serum biomarkers indicate several—not strictly liver-specific—clinical and serum parameters which are associated with NASH or the fibrosis state, as assessed by liver biopsy [15,17,105,106,107]. A widely used algorithm for the diagnosis of simple steatosis is the fatty liver index (FLI), which includes the body mass index (BMI), waist circumference (WC), and serum values of GGT and triglycerides. It has an AUROC of 0.834 for NAFLD [108,109,110]. Borman et al. described its limited use in obese patients with an AUROC of 0.67 [111]. A less-complicated model is the lipid accumulation product (LAP), which incorporates gender, WC, and triglyceridemia and has an AUROC for detecting steatosis of 0.79 [112]. The Hepatic Steatosis Index is another panel that includes gender, T2DM history, BMI, ALT, and AST and has an AUROC of 0.81 [113]. A novel model for predicting fatty liver disease is the ZJU index, which was validated in a Chinese population and includes BMI, fasting plasma glucose, triglycerides, and the ALT/AST ratio, which has an AUROC of 0.82 for diagnosing NAFLD and 0.896 for detecting steatosis [114]. The SteatoTest is based on the levels of α-2 macroglobulin, apolipoprotein A1, haptoglobin, total bilirubin, GGT, ALT, fasting glucose, triglycerides, cholesterol, age, gender, and BMI. This algorithm showed the worst AUROC, 0.71. In addition, it is not a cost-effective tool [115]. The AST to ALT ratio (AAR) is the simplest test for evaluating NAFLD and predicting fibrosis. In general, AAR ≥ 1 suggests the presence of advanced fibrosis or cirrhosis. McPherson et al. demonstrated that AAR can reliably exclude the presence of advanced fibrosis in patients with NAFLD using a cut-off value of 0.8, where AAR showed an AUROC of 0.83 [116]. The AST to platelet ratio index (APRI) is another elementary test which includes the AST level and platelet count and was originally used routinely for detecting chronic hepatitis C. Kruger et al. showed that APRI is an accurate bedside marker for advanced fibrosis, which can avoid the need for liver biopsy in patients with NAFLD; it showed an AUROC of 0.85 [117]. However, significantly lower AUROC values of 0.66–0.73 were confirmed in other studies [118,119]. The initials of the BARD score indicate that it incorporates BMI, AAR, and the presence of T2DM. The BARD score was validated on a cohort of Caucasian patients and was shown to have an AUROC of 0.82 and a high negative predictive value (NPV) of 97% [120]. Similar studies showed lower levels of accuracy, with AUROC values of 0.65–0.77 [116,119,121]. The NAFLD fibrosis score (NFS) is composed of six variables (age, hyperglycemia, BMI, platelet count, albumin, and AAR) and was developed in a large multicenter study of 733 patients, who were divided into two groups to estimate and validate the scoring panel. The presence of advanced fibrosis was diagnosed with high accuracy (positive predictive values of 90% and 82% in the estimation and validation groups, respectively), and liver biopsy was avoided in 75% of patients [122]. In a validation study on a Chinese population, the NPV was 91% and 79% of patients avoided liver biopsy. The highest value of AUROC (0.96) for the NAFLD fibrosis score was obtained by Demir et al. in a cohort of 267 patients [123]. The Nippon score (N score) gives the total number of risk factors and includes age, gender, history of T2DM, and hypertension. It showed an AUROC of 0.78 in a cohort of 182 Japanese patients with biopsy-confirmed NAFLD [124]. The BAAT score, which includes BMI, age, and ALT and triglyceride levels, gave an AUROC of 0.75 for moderate fibrosis and 0.92 for severe fibrosis in NAFLD patients [125]. The fibrosis-4 (FIB-4) test includes age, platelet count, and levels of ALT and AST, and was developed as a non-invasive panel to stage liver disease in subjects with HIV–hepatitis C virus (HCV) co-infection [126,127]. Several comparative studies showed that FIB-4 is the most promising diagnostic panel for distinguishing NASH from steatosis. The AUROC value for the FIB-4 index (0.87) was found to be superior to other scoring systems (NFS, APRI, AP index, AAR, BARD score, Nippon score: 0.86, 0.82, 0.81, 0.79, 0.76, and 0.71, respectively) for differentiating between advanced and mild fibrosis in 576 Japanese biopsy-proven NAFLD patients. Using the FIB-4 index, 58% of liver biopsies could be avoided [128]. The greatest AUROC value for FIB 4 (0.96), in comparison to the other panels, was obtained in a study of 165 Caucasian NAFLD patients [98]. Another study demonstrated that the FIB-4 test is the best predictive algorithm for advanced fibrosis, but it showed a smaller AUROC value, 0.80 [118]. The inferior diagnostic value of FIB 4 between noninvasive assessment systems was documented in a cohort of 228 Latin patients, with an AUROC value of 0.74 [119]. Demir et al. developed and validated the non-invasive Koeln–Essen index (NIKEI), which includes age, AAR, AST, and total bilirubin levels and showed an AUROC value of 0.97, compared with a value of 0.93 for FIB-4. The absence of severe fibrosis could be confirmed with a high level of accuracy (99–100%) [123]. The HAIR algorithm incorporates hypertension, ALT levels, and insulin resistance, and has been shown to be an independent marker for the diagnosis of NAFLD [129]. Using the HAIR model, Dixon et al. demonstrated 80% sensitivity and 89% specificity for NASH in patients after laparoscopic obesity surgery [130]. The fibrometer combines age, weight, fasting glucose, AST, ALT, ferritin levels, and platelet count. It is a test that was developed for staging fibrosis in chronic HCV (similar to other models), but has also been demonstrated to have excellent accuracy for the identification of NAFLD fibrosis, with an AUROC value of 0.94 [131]. The fibrotest (FT) is a patented formula that includes five serological markers: Haptoglobin, apolipoprotein A1, α2-macroglobulin, total bilirubin, and GGT levels. It was validated by Poynard et al. in a cohort of 761 patients with NAFLD and in morbidly obese patients with NAFLD, where it showed an AUROC value of 0.85 [115,132]. The NASH test includes gender, age, weight, height, and serum values of triglycerides, total cholesterol, ALT, AST, GGT, total bilirubin, α2-macroglobulin, haptoglobin, and apolipoprotein A1, and was shown to have an AUROC value of 0.79 for detecting NASH [133].
Table 3.
Values of AUROC for most accurate non-invasive scoring methods.
| Method | Field of Detection | Parameters Used for Calculation | Accuracy | Strengths | Advantages and Limitations | Ref. |
|---|---|---|---|---|---|---|
| Scoring Method | ||||||
|
Fatty liver index
(FLI) |
NAFLD | BMI, WC, GGT, triglycerides |
AUROC
0.83 AUROC 0.67 |
Optimal cut-off point 30 Sensitivity 79.8% Specificity 71.5% 95 % CI:0.825–0.842, p < 0.001. |
Low cutoff of 30 is used to rule out NAFLD (negative likelihood ratio 0.2). High cutoff of 60 is used (with a positive likelihood ratio of 4.3). Poorly distinguishes moderate-to severe steatosis from mild steatosis. Limited use in obese patients. |
[108] [111] |
|
Hepatic steatosis index
(HSI) |
NAFLD | Gender, Diabetes mellitus, BMI, ALT/AST ratio |
AUROC 0.81 |
Cut-off point 30 p < 0.001 Sensitivity of 93.1% Specificity of 92.4% (95 % CI: 0.81–0.824). |
At values of <30, ruled out NAFLD. At values of >36, detected NAFLD. Poorly distinguishes moderate-to severe steatosis from mild steatosis. HSI accuracy decreases in obese children. |
[113] [15] |
| SteatoTest | Steatosis | apha-2-macroglobulin, apolipoprotein A1, haptoglobin, bilirubin, GGT, ALT, glucose, triglycerides, cholesterol, age, gender, BMI |
AUROC 0.71 |
At the cut off 0.38: Sensitivity 89.7% Specificity 44.9% PPV 90.9% NPV 41.3% PPV 92.4% for the dg. of steatosis >S0 using 0.38 cut off. NPV 59.3% for the dg. of steatosis >S1 using 0.69 cut off. |
[115] | |
| NAFL Screening score | NAFLD | Age, glucose, BMI, triglycerides, ALT/AST, uric acid |
AUROC 0.87 |
At the cut-off 0.24: Sensitivity 92%; NPV 95% At the cut-off 0.44: Specificity 90%; PPV 84% |
[15] | |
|
NAFLD fibrosis score
(NFS) |
Advanced fibrosis | Age, BMI, impaired fasting glucose and/or diabetes, AST/ALT ratio, platelet count, and albumin |
AUROC 0.96 0.83 for cirrhosis 0.73 for advanced fibrosis 0.72 for significant fibrosis |
At the cutoff ≤−1.455: Sensitivity 75% Specificity 93% PPV 63%; NPV 96% |
Below the lower cutoff (≤−1.455), healthy. Above the cutoff (≥0.676), advanced fibrosis. |
[123] |
| At the cut- off ≥0.676: Sensitivity 19% Specificity 100% PPV100%; NPV 89% |
Can be used to identify those at low or high risk for advanced fibrosis or cirrhosis. | [15] | ||||
| APRI | Advanced fibrosis | AST/platelet ratio index |
AUROC 0.85 |
Optimal cut off 0.98 Sensitivity of 75% Specificity of 86% PPV 54%; NPV 93% |
[117] | |
|
AUROC 0.75 for advanced fibrosis or cirrhosis 0.70 for significant fibrosis |
Low specificity to diagnose advanced fibrosis. | [15] | ||||
| FIB-4 | Advanced fibrosis | Age, platelet count, ALT, AST |
AUROC 0.85 for cirrhosis 0.80 for advanced fibrosis 0.75 for significant fibrosis |
At the cut-off 1.3 Sensitivity 85% Specificity 65% PPV 36%; NPV 95% At the cut off 3.25 Sensitivity 26% Specificity 98% PPV 75%; NPV 85% |
Can be used to identify patients at low or high risk for advanced fibrosis or cirrhosis. | [15] |
| BARD score | Advanced fibrosis | AST, ALT, BMI and diabetes |
AUROC 0.70 for cirrhosis 0.73 for advanced fibrosis 0.64 for significant fibrosis |
Low specificity to diagnose significant fibrosis and cirrhosis. | [15] | |
|
Enhanced liver fibrosis
(ELF) |
Advanced fibrosis Mild fibrosis Fibrosis not present |
TIMP1, HA, aminoterminal peptide of pro-colagen III |
AUROC 0.90 for severe fibrosis 0.82 for moderate fibrosis 0.76 for no fibrosis |
[134] | ||
|
Hepatic steatosis index
(HIS) |
Steatosis | Gender, T2DM, BMI, ALT, AST |
AUROC 0.81 |
Sensitivity of 93.1%, at values of <30 ruled out NAFLD. Specificity of 92.4%, at values of >36 detected NAFLD. |
[113] | |
Legend:, CI—confidence interval, GGT—gamma-glutamyl transpeptidase, BMI—body mass index, WC—waist circumference, TIMP1—tissue inhibitor of matrix metalloproteinase 1, T2DM—type 2 diabetes mellitus, APRI—AST to platelet ratio index, AUROC—area under the receiver-operating characteristic curve, NPV—negative predictive value, PPV—positive predictive value.
The following tests are also known as a super combination FibroMAX (BioPredictive, Paris, France) and are used in patients at risk of chronic liver diseases: FibroTest (BioPredictive) for the quantitative assessment of fibrosis; SteatoTest (BioPredictive) for the quantitative assessment of steatosis; ActiTest (BioPredictive) for the quantitative assessment of necroinflammatory activity in chronic viral hepatitis C and B; NashTest (BioPredictive) for the categorical diagnosis of nonalcoholic steatohepatitis; and AshTest for the quantitative assessment of alcoholic steatohepatitis (also known in the USA as HCV-FibroSURE, HBV-FibroSURE, ASH-FibroSURE, and NASH-FibroSURE; LabCorp, NC, USA) [135]. Palekar et al. constructed a composite index for distinguishing steatosis from NASH by summing the risk factors of age, gender, AST level, BMI, AAR, and HA. Its accuracy was shown with an AUROC value of 0.76, obtained in a small study of 80 patients [136]. The Antwerp NAFLD significant fibrosis takes in account WC, AST, and fasting C-peptide levels, as well as ultrasound steatosis scores, and was developed by a Belgian hepatologic group. It showed an AUROC value of 0.84 in a design cohort and 0.85 in a validation cohort [137]. The enhanced liver fibrosis panel (ELF) includes tissue inhibitor of matrix metalloproteinase 1 (TIMP1), HA, and the aminoterminal peptide of procollagen III. Guha et al. validated the Original European Liver Fibrosis panel (OELF) and a simplified algorithm not containing age, the Enhanced Liver fibrosis panel (ELF), in an independent cohort of 196 patients with NAFLD. The ELF panel showed an AUROC of 0.90 for distinguishing severe fibrosis, 0.82 for moderate fibrosis, and 0.76 for no fibrosis [134]. The NAFIC score is another predictive model, designed for a Japanese population, which combines serum values of ferritin, insulin, and type IV collagen 7S, which had an AUROC of 0.79 [138].
6. Genetical Evaluation and Multi-Omics Profiles of NAFLD
NAFLD development is influenced by environmental factors, such as dietary habits and sedentary lifestyle, but requires background knowledge of genetic susceptibility [139,140,141]. Heritability also explains inter-individual and racial differences of NAFLD occurrence and progression to NASH. Indeed, a recent large study showed that the heredity level of NAFLD is 26–27% [56,142]. Genetic studies include genome-wide association studies (GWAS) and candidate gene studies. GWAS are hypothesis-free studies that identify the genetic influences on common diseases, testing associations of common variants in the human genome and polymorphic traits [139]. Candidate gene studies originate from genomic, proteomic, or other studies, and just a single nucleotide polymorphism (SNP) is selected to investigate the role of a candidate gene in a pathogenetic mechanism.
The most relevant genetic association is with palatine-like phospholipase domain, containing 3 (PNPLA3); also known as adiponutrin [57,142]. Romeo et al. demonstrated a correlation of the PNPLA3 I148M variant, non-synonymous SNP-rs 738409, with increased liver fat content [143]. However, although there was a strong link between the PNPLA3 I148M variant and NAFLD, some meta-analyses did not confirm the association of this variant with metabolic syndrome and its features [144]. Studies have also documented an interplay between PNPLA3 variant I148M and advanced fibrosis in patients with NASH, the risk of HCC development, and the presence of chronic kidney disease [145,146,147]. The frequency of the 148M allele has also been shown to vary with ethnicity [143]. Another gene found by GWAS to be a risk factor of NAFLD is the transmembrane 6 superfamily member 2 (TM6SF2), nonsynonymous variant rs58542926. Kozlitina et al. identified the association between this SNP and the hepatic triglyceride content, elevated aminotransferases, and reduced serum levels of triglycerides and LDL-cholesterol [148]. Dongiovanni et al. showed that carriers of this variant had lower values of serum lipids and more severe steatosis and fibrosis than non-carriers. In fact, they seemed to be at risk of NASH progression [149]. Other genetic variants revealed by GWAS are neurocan (NCAN-rs2228603), protein phosphatase 1, regulatory (inhibitor) subunit 3B (PPP1R3B-rs4240624), glucokinase regulator (GCKR-rs780094), and lysophospholipase-like 1 (LYPLAL1-rs12137855) [56]. Gene candidate studies have suggested a vast number of SNPs as potential genetic factors that influence the development of NAFLD and the progression of NASH [139]. However, multiple studies have found that the factor most correlated with NAFLD is the PNPLA3 gene variant I148M. The identification of these variants does not have routine utility, but has elucidated some pathogenetic mechanisms of NAFLD [32].
Epigenetic mechanisms and their influences on gene expression in the pathogenesis of NAFLD have been discussed [44,150]. Epigenetic mechanisms are heritable, reversible, and modulated by environmental stimuli; they include histone acetylation and deacetylation, DNA methylation, microRNAs, and chromatin remodeling [151,152]. MicroRNAs (miRNAs) are the most important, which regulate gene expression and protein translation. They can also be called oncogenes or tumor suppressors, because of their roles in carcinogenesis [153,154]. The most commonly expressed miRNA in the human liver is miR-122, which is involved in NAFLD and is associated with a high risk of developing HCC [154]. Epigenetics has presented potential diagnostic, prognostic, and therapeutic targets [155,156].
Multiomics profiles include genomics, proteomics, metabolomics, lipidomics, and glycomics, and these have common characteristics: They involve hypothesis-free, large-scale analyses of specific serum or tissue products during a disorder and are used, in particular, as novel biomarkers. The first proteomic profile of NAFLD was performed on a cohort of 98 obese patients, and from 12 significant protein peaks, just fibrinogen γ showed a relation to fibrosis [157]. A later study discovered 15 proteins connected to NASH and advanced fibrosis, and two diagnostic models were developed for staging NAFLD. The first model was composed of six proteins (fibrinogen β chain, retinol binding protein 4, serum amyloid P component, lumican, transgelin 2, and CD5 antigen-like), and the second involved three proteins (component C7, insulin-like growth factor acid labile subunit, and transgelin 2); the AUROC values ranged from 0.83–0.91 for different stages of NAFLD [158]. Due to multiomic studies, numerous biomarkers have been revealed to be associated with NAFLD, as they are involved in necroinflammation and fibrogenesis [159,160,161,162,163,164,165].
7. Conclusions
Numerous non-invasive methods have been developed to cover the whole spectrum of NAFLD disorders, but only some of them are reliable for differentiating among steatosis, steatohepatitis, and the degree of fibrosis and for quantifying steatosis and, thus, fulfilling the criterion of minimizing the need for liver biopsy. The most promising, widely available, and easily applicable strategies to exclude advanced liver fibrosis seem to be scoring systems (e.g., FIB-4 score, NAFLD fibrosis score, and BARD score).
As the prevalence of NAFLD has risen worldwide, and as NAFLD has been associated with increased mortality owing to cardiovascular diseases followed by cancer and liver-related causes, it is important to identify patients at high risk of NAFLD [166,167,168]. There has recently been extensive development of noninvasive methods in the NAFLD field, from serum biomarkers and imaging to omics. [169,170]. Fatty liver is a common feature of different types of liver disease. The sensitivity and specificity of ultrasonography for diagnosing fatty liver are variable. A semiquantitative ultrasound score—the ultrasonographic fatty liver indicator (US-FLI)—has been closely associated with metabolic/histological variables in patients with NAFLD. In a study by Ballestri and al. (2017), US-FLI was correlated with the steatosis percentage in each liver disease group, as well as with lobular inflammation, ballooning, portal fibrosis, grading, and staging in patients with NAFLD or HCV. US-FLI was also correlated with waist circumference, body mass index, and insulin resistance. US-FLI accurately identified the histological severity and was correlated with metabolic parameters in patients with various steatogenic liver diseases [171]. Our ability to identify NAFLD patients and to estimate steatofibrosis with various ultrasound-based techniques has undergone tremendous progress over the last few years. However, it is more difficult to capture the inflammatory component of NASH with such ultrasound-assisted techniques. Moreover, semi-quantitative, quantitative, elastographic, and contrast-enhanced ultrasound techniques are increasingly being appreciated and made available; but not all such techniques will gain success in the clinical and research areas. Therefore, further research will precisely define the roles of the most innovative ultrasonographic techniques, while reducing costs and increasing feasibility [172,173]. The diagnostic accuracy of transient hepatic elastography [174,175,176], real-time elastography [177,178], shear-wave elastography, acoustic radiation force impulse imaging (ARFI) [179,180], MR elastography [181,182,183], 1H magnetic resonance spectroscopy [184], magnetic resonance imaging-proton density fat fraction, and other imaging methods in nonalcoholic fatty liver diseases have been evaluated [169,179,185,186,187] (Table 4).
Table 4.
Values of area under the receiver-operating characteristics curve (AUROC) for the most accurate imaging methods.
| Method | Field of Detection | AUROC | Ref. |
|---|---|---|---|
| Imaging Methods | |||
| USG | Steatosis | 0.93 | [169] |
| CT | Steatosis | 0.92 | [186] |
| MRI | Steatosis | 0.99 | [186] |
| TE | Advanced fibrosis | 0.99 | [179] |
| ARFI | Advanced fibrosis | 0.97 | [179] |
Legend: USG-ultrasonography, CT-computer tomography, MRI-magnetic resonance imaging, TE-transient elastography, ARFI-acoustic radiation force impulse.
New sequential combinations of non-invasive fibrosis tests and imaging methods may provide an accurate diagnosis of advanced fibrosis [112,187,188,189,190,191,192,193,194]. Further testing and validation are needed for non-invasive diagnosis and its use in clinical practice.
Author Contributions
Conceptualization: V.K. and M.F. Writing—original draft preparation: V.K. and M.F. Writing—review and editing manuscript: V.K., J.B., and L.T. Literature analysis/search: L.T. and P.K. All team members had the option to revise the manuscript draft.
Funding
This work was supported by Scientific Grant Agency of the Ministry of Education of Slovak Republic VEGA 1/0826/18 and VEGA 1/0807/18.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Lonardo A., Nascimbeni F., Targher G., Bernardi M., Bonino F., Bugianesi E., Casini A., Gastaldelli A., Marchesini G., Marra F., et al. AISF position paper on nonalcoholic fatty liver disease (NAFLD): Updates and future directions. Dig. Liver Dis. 2017;49:471–483. doi: 10.1016/j.dld.2017.01.147. [DOI] [PubMed] [Google Scholar]
- 2.Lonardo A., Nascimbeni F., Mantovani A., Targher G. Hypertension, diabetes, atherosclerosis and NASH: Cause or consequence? J. Hepatol. 2018;68:335–352. doi: 10.1016/j.jhep.2017.09.021. [DOI] [PubMed] [Google Scholar]
- 3.Vernon G., Baranova A., Younossi Z.M. Systematic review: The epidemiology and natural history of non-alcoholic fatty liver disease and non-alcoholic steatohepatitis in adults. Aliment. Pharmacol. Ther. 2011;34:274–285. doi: 10.1111/j.1365-2036.2011.04724.x. [DOI] [PubMed] [Google Scholar]
- 4.George E.S., Roberts S.K., Nicoll A.J., Reddy A., Paris T., Itsiopoulos C., Tierney A.C. Non-alcoholic fatty liver disease patients attending two metropolitan hospitals in Melbourne, Australia: High risk status and low prevalence. Intern. Med. J. 2018;48:1369–1376. doi: 10.1111/imj.13973. [DOI] [PubMed] [Google Scholar]
- 5.Sanal M.G. Biomarkers in nonalcoholic fatty liver disease-the emperor has no clothes? World J. Gastroenterol. 2015;21:3223–3231. doi: 10.3748/wjg.v21.i11.3223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hu X.Y., Li Y., Li L.Q., Zheng Y., Lv J.H., Huang S.C., Zhang W., Liu L., Zhao L., Liu Z., et al. Risk factors and biomarkers of non-alcoholic fatty liver disease: An observational cross-sectional population survey. BMJ Open. 2018;8:e019974. doi: 10.1136/bmjopen-2017-019974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mazzoccoli G., De Cosmo S., Mazza T. The Biological Clock: A Pivotal Hub in Non-alcoholic Fatty Liver Disease Pathogenesis. Front. Physiol. 2018;9:193. doi: 10.3389/fphys.2018.00193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Oikonomou D., Georgiopoulos G., Katsi V., Kourek C., Tsioufis C., Alexopoulou A., Koutli E., Tousoulis D. Non-alcoholic fatty liver disease and hypertension: Coprevalent or correlated? Eur. J. Gastroenterol. Hepatol. 2018;30:979–985. doi: 10.1097/MEG.0000000000001191. [DOI] [PubMed] [Google Scholar]
- 9.Harada P.H., Bensenõr I.J.M., Drager L.F., Goulart A.C., Mill J.G., Lotufo P.A. Non-alcoholic fatty liver disease presence and severity are associated with aortic stiffness beyond abdominal obesity: The ELSA-Brasil. Atherosclerosis. 2019;284:59–65. doi: 10.1016/j.atherosclerosis.2019.02.005. [DOI] [PubMed] [Google Scholar]
- 10.Tellez-Plaza M., Briongos-Figuero L., Pichler G., Dominguez-Lucas A., Simal-Blanco F., Mena-Martin F.J., Bellido-Casado J., Arzua-Mouronte D., Chaves F.J., Redon J., et al. Cohort profile: The Hortega Study for the evaluation of non-traditional risk factors of cardiometabolic and other chronic diseases in a general population from Spain. BMJ Open. 2019;9:e024073. doi: 10.1136/bmjopen-2018-024073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stinton L.M., Loomba R. Recommendations for liver biopsy evaluation in non-alcoholic fatty liver disease. Minerva. Gastroenterol. Dietol. 2014;60:5–13. [PubMed] [Google Scholar]
- 12.Khurana S., Butt W., Khara H.S., Johal A.S., West S.F., Chen Z.E., Berger A.L., Diehl D.L. Bi-lobar liver biopsy via EUS enhances the assessment of disease severity in patients with non-alcoholic steatohepatitis. Hepatol. Int. 2019;13:323–329. doi: 10.1007/s12072-019-09945-4. [DOI] [PubMed] [Google Scholar]
- 13.Munsterman I.D., van Erp M., Weijers G., Bronkhorst C., de Korte C.L., Drenth J.P.H., van der Laak J.A.W.M., Tjwa E.T.T.L. A Novel Automatic Digital Algorithm that Accurately Quantifies Steatosis in NAFLD on Histopathological Whole-Slide Images. Cytom. B Clin. Cytom. 2019 doi: 10.1002/cyto.b.21790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Golabi P., Stepanova M., Pham H.T., Cable R., Rafiq N., Bush H., Gogoll TYounossi Z.M. Non-alcoholic steatofibrosis (NASF) can independently predict mortality in patients with non-alcoholic fatty liver disease (NAFLD) BMJ Open Gastroenterol. 2018;5:e000198. doi: 10.1136/bmjgast-2018-000198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhou J.H., Cai J.J., She Z.G., Li H.L. Noninvasive evaluation of nonalcoholic fatty liver disease: Current evidence and practice. World J. Gastroenterol. 2019;25:1307–1326. doi: 10.3748/wjg.v25.i11.1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang C.C., Jhu J.J. On the Application of Clustering and Classification Techniques to Analyze Metabolic Syndrome Severity Distribution Area and Critical Factors. Int. J. Environ. Res. Public Health. 2019;16:1575. doi: 10.3390/ijerph16091575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Castera L., Friedrich-Rust M., Loomba R. Noninvasive Assessment of Liver Disease in Patients with Nonalcoholic Fatty Liver Disease. Gastroenterology. 2019;156:1264–1281. doi: 10.1053/j.gastro.2018.12.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sheth H., Bagasrawala S., Shah M., Ansari R., Olithselvan ALakdawala M. The HAALT Non-invasive Scoring System for NAFLD in Obesity. Obes. Surg. 2019;29:2562–2570. doi: 10.1007/s11695-019-03880-x. [DOI] [PubMed] [Google Scholar]
- 19.Yilmaz Y., Ulukaya E. Toward a biochemical diagnosis of NASH: Insights from pathophysiology for distinguishing simple steatosis from steatohepatitis. Curr. Med. Chem. 2011;18:725–732. doi: 10.2174/092986711794480122. [DOI] [PubMed] [Google Scholar]
- 20.Boursier J., Guillaume M., Leroy V., Irlès M., Roux M., Lannes A., Foucher J., Zuberbuhler F., Delabaudière C., Barthelon J., et al. New sequential combinations of non-invasive fibrosis tests provide an accurate diagnosis of advanced fibrosis in NAFLD. J. Hepatol. 2019;71:389–396. doi: 10.1016/j.jhep.2019.04.020. [DOI] [PubMed] [Google Scholar]
- 21.Crossan C., Majumdar A., Srivastava A., Thorburn D., Rosenberg W., Pinzani M., Longworth LTsochatzis E.A. Referral pathways for patients with NAFLD based on non-invasive fibrosis tests: Diagnostic accuracy and cost analysis. Liver Int. 2019 doi: 10.1111/liv.14198. [DOI] [PubMed] [Google Scholar]
- 22.Alswat K.A., Fallatah H.I., Al-Judaibi B., Elsiesy H.A., Al-Hamoudi W.K., Qutub A.N., Alturaify NAl-Osaimi A. Position statement on the diagnosis and management of non-alcoholic fatty liver disease. Saudi Med. J. 2019;40:531–540. doi: 10.15537/smj.2019.6.23980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Önnerhag K., Hartman H., Nilsson P.M., Lindgren S. Non-invasive fibrosis scoring systems can predict future metabolic complications and overall mortality in non-alcoholic fatty liver disease (NAFLD) Scand. J. Gastroenterol. 2019;54:328–334. doi: 10.1080/00365521.2019.1583366. [DOI] [PubMed] [Google Scholar]
- 24.Jennison E., Patel J., Scorletti E., Byrne C.D. Diagnosis and management of non-alcoholic fatty liver disease. Postgrad. Med. J. 2019;95:314–322. doi: 10.1136/postgradmedj-2018-136316. [DOI] [PubMed] [Google Scholar]
- 25.Leite N.C., Villela-Nogueira C.A., Cardoso C.R., Salles G.F. Non-alcoholic fatty liver disease and diabetes: From physiopathological interplay to diagnosis and treatment. World J. Gastroenterol. 2014;20:8377–8392. doi: 10.3748/wjg.v20.i26.8377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Farrell G.C., Haczeyni FChitturi S. Pathogenesis of NASH: How Metabolic Complications of Overnutrition Favour Lipotoxicity and Pro-Inflammatory Fatty Liver Disease. Adv. Exp. Med. Biol. 2018;1061:19–44. doi: 10.1007/978-981-10-8684-7_3. [DOI] [PubMed] [Google Scholar]
- 27.Lonardo A., Mantovani A., Lugari STargher G. NAFLD in Some Common Endocrine Diseases: Prevalence, Pathophysiology, and Principles of Diagnosis and Management. Int. J. Mol. Sci. 2019;20:2841. doi: 10.3390/ijms20112841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Marchisello S., Di Pino A., Scicali R., Urbano F., Piro S., Purrello F., Rabuazzo A.M. Pathophysiological, Molecular and Therapeutic Issues of Nonalcoholic Fatty Liver Disease: An Overview Review Article. Int. J. Mol. Sci. 2019;20:1948. doi: 10.3390/ijms20081948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Buzzetti E., Pinzani M., Tsochatzis E.A. The multiple-hit pathogenesis of non-alcoholic fatty liver disease (NAFLD) Metabolism. 2016;65:1038–1048. doi: 10.1016/j.metabol.2015.12.012. [DOI] [PubMed] [Google Scholar]
- 30.Ballestri S., Nascimbeni F., Romagnoli D., Lonardo A. The independent predictors of non-alcoholic steatohepatitis and its individual histological features: Insulin resistance, serum uric acid, metabolic syndrome, alanine aminotransferase and serum total cholesterol are a clue to pathogenesis and candidate targets for treatment. Hepatol. Res. 2016;46:1074–1087. doi: 10.1111/hepr.12656. [DOI] [PubMed] [Google Scholar]
- 31.Borrelli A., Bonelli P., Tuccillo F.M., Goldfine I.D., Evans J.L., Buonaguro F.M., Mancini A. Role of gut microbiota and oxidative stress in the progression of non-alcoholic fatty liver disease to hepatocarcinoma: Current and innovative therapeutic approaches. Redox Biol. 2018;15:467–479. doi: 10.1016/j.redox.2018.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gonzalez-Jaramillo V., Portilla-Fernandez E., Glisic M., Voortman T., Ghanbari M., Bramer W., Chowdhury R., Nijsten T., Dehghan A., Franco O.H. Epigenetics and Inflammatory Markers: A Systematic Review of the Current Evidence. Int. J. Inflam. 2019;2019:6273680. doi: 10.1155/2019/6273680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kim S.S., Cho H.J., Kim H.J., Kang D.R., Berry J.R., Kim J.H., Yang M.J., Lim S.G., Kim SCheong J.Y., Cho S.W. Nonalcoholic fatty liver disease as a sentinel marker for the development of diabetes mellitus in non-obese subjects. Dig. Liver Dis. 2018;50:370–377. doi: 10.1016/j.dld.2017.12.018. [DOI] [PubMed] [Google Scholar]
- 34.Błaszczyk-Bębenek E., Piórecka B., Płonka M., Chmiel I., Jagielski P., Tuleja K., Schlegel-Zawadzka M. Risk Factors and Prevalence of Abdominal Obesity among Upper-Secondary Students. Int. J. Environ. Res. Public Health. 2019;16:1750. doi: 10.3390/ijerph16101750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.HaGani N., Moran M.R., Caspi O., Plaut P., Endevelt R., Baron-Epel O. The Relationships between Adolescents’ Obesity and the Built Environment: Are They City Dependent? Int. J. Environ. Res. Public Health. 2019;16:1579. doi: 10.3390/ijerph16091579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lonardo A., Ballestri S., Marchesini GAngulo PLoria P. Nonalcoholic fatty liver disease: A precursor of the metabolic syndrome. Dig. Liver Dis. 2015;47:181–190. doi: 10.1016/j.dld.2014.09.020. [DOI] [PubMed] [Google Scholar]
- 37.Sung K.C., Lee M.Y., Kim Y.H., Huh J.H., Kim J.Y., Wild S.H., Byrne C.D. Obesity and incidence of diabetes: Effect of absence of metabolic syndrome, insulin resistance, inflammation and fatty liver. Atherosclerosis. 2018;275:50–57. doi: 10.1016/j.atherosclerosis.2018.05.042. [DOI] [PubMed] [Google Scholar]
- 38.Golabi P., Paik J., Fukui N., Locklear C.T., de Avilla L., Younossi Z.M. Patients with Lean Nonalcoholic Fatty Liver Disease Are Metabolically Abnormal and Have a Higher Risk for Mortality. Clin. Diabetes. 2019;37:65–72. doi: 10.2337/cd18-0026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lonardo A., Loria P. Apolipoprotein synthesis in nonalcoholic steatohepatitis. Hepatology. 2002;36:514–515. doi: 10.1053/jhep.2002.34443. [DOI] [PubMed] [Google Scholar]
- 40.Lonardo A., Loria P., Argo C., Caldwell S. Perspectives on cellular dysfunction in nonalcoholic steatohepatitis: A case of ‘multiorganelle failure’? Proceedings of a virtual workshop on nonalcoholic steatohepatitis. Expert Rev. Gastroenterol. Hepatol. 2011;5:135–139. doi: 10.1586/egh.11.24. [DOI] [PubMed] [Google Scholar]
- 41.Tarantino G., Conca P., Pasanisi F., Ariello M., Mastrolia M., Arena A., Tarantino M., Scopacasa F., Vecchione R. Inflammatory markers help diagnose nonalcoholic steatohepatitis? Eur. J. Gastroenterol. Hepatol. 2009;21:504–511. doi: 10.1097/MEG.0b013e3283229b40. [DOI] [PubMed] [Google Scholar]
- 42.Moschen A.R., Wieser V., Tilg H. Adiponectin: Key player in the adipose tissue-liver crosstalk. Curr. Med. Chem. 2012;19:5467–5473. doi: 10.2174/092986712803833254. [DOI] [PubMed] [Google Scholar]
- 43.Stojsavljević S., Gomerčić Palčić M., Virović Jukić L., Smrčić Duvnjak L., Duvnjak M. Adipokines and proinflammatory cytokines, the key mediators in the pathogenesis of nonalcoholic fatty liver disease. World J. Gastroenterol. 2014;20:18070–18091. doi: 10.3748/wjg.v20.i48.18070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Stiglund N., Strand K., Cornillet M., Stål P., Thorell A., Zimmer C.L., Näslund E., Karlgren S., Nilsson H., Mellgren G., et al. Cell Phenotype and Functionality in Non-alcoholic Fatty Liver Disease. Front. Immunol. 2019;10:1255. doi: 10.3389/fimmu.2019.01255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lonardo A., Adinolfi L.E., Restivo L., Ballestri S., Romagnoli D., Baldelli E., Nascimbeni F., Loria P. Pathogenesis and significance of hepatitis C virus steatosis: An update on survival strategy of a successful pathogen. World J. Gastroenterol. 2014;20:7089–7103. doi: 10.3748/wjg.v20.i23.7089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Machado M.V., Cortez-Pinto H. Gut microbiota and nonalcoholic fatty liver disease. Ann. Hepatol. 2012;11:440–449. doi: 10.1016/S1665-2681(19)31457-7. [DOI] [PubMed] [Google Scholar]
- 47.Bertolotti M., Lonardo A., Mussi C., Baldelli E., Pellegrini E., Ballestri S., Romagnoli D., Loria P. Nonalcoholic fatty liver disease and aging: Epidemiology to management. World J. Gastroenterol. 2014;20:14185–14204. doi: 10.3748/wjg.v20.i39.14185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Magee N., Zou A., Zhang Y. Pathogenesis of Nonalcoholic Steatohepatitis: Interactions between Liver Parenchymal and Nonparenchymal Cells. BioMed Res. Int. 2016;2016:5170402. doi: 10.1155/2016/5170402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tiwari-Heckler S., Gan-Schreier H., Stremmel W., Chamulitrat W., Pathil A. Circulating Phospholipid Patterns in NAFLD Patients Associated with a Combination of Metabolic Risk Factors. Nutrients. 2018;10:649. doi: 10.3390/nu10050649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mazidi M., Katsiki N., Mikhailidis D.P., Banach M. Link between plasma trans-fatty acid and fatty liver is moderated by adiposity. Int. J. Cardiol. 2018;272:316–322. doi: 10.1016/j.ijcard.2018.07.061. [DOI] [PubMed] [Google Scholar]
- 51.Piscaglia F., Svegliati-Baroni G., Barchetti A., Pecorelli A., Marinelli S., Tiribelli C., Bellentani S. HCC-NAFLD Italian Study Group. Clinical patterns of hepatocellular carcinoma in nonalcoholic fatty liver disease: A multicenter prospective study. Hepatology. 2016;63:827–838. doi: 10.1002/hep.28368. [DOI] [PubMed] [Google Scholar]
- 52.Lonardo A., Nascimbeni F., Maurantonio M., Marrazzo A., Rinaldi L., Adinolfi L.E. Nonalcoholic fatty liver disease: Evolving paradigms. World J. Gastroenterol. 2017;23:6571–6592. doi: 10.3748/wjg.v23.i36.6571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Singhal G., Kumar G., Chan S., Fisher F.M., Ma Y., Vardeh H.G., Nasser I.A., Flier J.S., Maratos-Flier E. Deficiency of fibroblast growth factor 21 (FGF21) promotes hepatocellular carcinoma (HCC) in mice on a long term obesogenic diet. Mol. Metab. 2018;13:56–66. doi: 10.1016/j.molmet.2018.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Svegliati-Baroni G., Ridolfi F., Di Sario A., Casini A., Marucci L., Gaggiotti G., Orlandoni P., Macarri G., Perego L., Benedetti A., et al. Insulin and insulin-like growth factor-1 stimulate proliferation and type I collagen accumulation by human hepatic stellate cells: Differential effects on signal transduction pathways. Hepatology. 1999;29:1743–1751. doi: 10.1002/hep.510290632. [DOI] [PubMed] [Google Scholar]
- 55.Lonardo A., Lombardini S., Scaglioni F., Carulli L., Ricchi M., Ganazzi D., Adinolfi L.E., Ruggiero G., Carulli N., Loria P. Hepatic steatosis and insulin resistance: Does etiology make a difference? J. Hepatol. 2006;44:190–196. doi: 10.1016/j.jhep.2005.06.018. [DOI] [PubMed] [Google Scholar]
- 56.Speliotes E.K., Yerges-Armstrong L.M., Wu J., Hernaez R., Kim L.J., Palmer C.D., Gudnason V., Eiriksdottir G., Garcia M.E., Launer L.J., et al. Genome-wide association analysis identifies variants associated with nonalcoholic fatty liver disease that have distinct effects on metabolic traits. PLoS Genet. 2011;7:e1001324. doi: 10.1371/journal.pgen.1001324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Guichelaar M.M., Gawrieh S., Olivier M., Viker K., Krishnan A., Sanderson S., Malinchoc M., Watt K.D., Swain J.M., Sarr M., et al. Interactions of allelic variance of PNPLA3 with nongenetic factors in predicting nonalcoholic steatohepatitis and nonhepatic complications of severe obesity. Obesity. 2013;21:1935–1941. doi: 10.1002/oby.20327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ahmed M. Non-alcoholic fatty liver disease in 2015. World J. Hepatol. 2015;7:1450–1459. doi: 10.4254/wjh.v7.i11.1450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Targher G., Rossini M., Lonardo A. Evidence that non-alcoholic fatty liver disease and polycystic ovary syndrome are associated by necessity rather than chance: A novel hepato-ovarian axis? Endocrine. 2016;51:211–221. doi: 10.1007/s12020-015-0640-8. [DOI] [PubMed] [Google Scholar]
- 60.Sanyal A.J. Putting non-alcoholic fatty liver disease on the radar for primary care physicians: How well are we doing? BMC Med. 2018;16:148. doi: 10.1186/s12916-018-1149-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bortolotto L.A. Identifying the Impact of Metabolic Syndrome in Hypertensive Patients. Arq. Bras. Cardiol. 2018;110:522–523. doi: 10.5935/abc.20180102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lee J., Lee Y., Chung S., Cho H., Park B., Jung D. Severity of nonalcoholic fatty liver disease is associated with subclinical cerebro-cardiovascular atherosclerosis risk in Korean men. PLoS ONE. 2018;13:e0193191. doi: 10.1371/journal.pone.0193191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lonardo A., Nascimbeni F., Ballestri S., Fairweather D., Win S., Than T.A., Abdelmalek M.F., Suzuki A. Sex Differences in NAFLD: State of the Art and Identification of Research Gaps. Hepatology. 2019 doi: 10.1002/hep.30626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ballestri S., Zona S., Targher G., Romagnoli D., Baldelli E., Nascimbeni F., Roverato A., Guaraldi G., Lonardo A. Nonalcoholic fatty liver disease is associated with an almost twofold increased risk of incident type 2 diabetes and metabolic syndrome. Evidence from a systematic review and meta-analysis. J. Gastroenterol. Hepatol. 2016;31:936–944. doi: 10.1111/jgh.13264. [DOI] [PubMed] [Google Scholar]
- 65.Lin S.Y., Lin C.L., Lin C.C., Wang I.K., Hsu W.H., Kao C.H. Risk of acute coronary syndrome and peripheral arterial disease in chronic liver disease and cirrhosis: A nationwide population-based study. Atherosclerosis. 2018;270:154–159. doi: 10.1016/j.atherosclerosis.2018.01.047. [DOI] [PubMed] [Google Scholar]
- 66.Strey C.B.M., de Carli L.A., Fantinelli M., Gobbato S.S., Bassols G.F., Losekann A., Coral G.P. Impact of Diabetes Mellitus and Insulin on Nonalcoholic Fatty Liver Disease in the Morbidly Obese. Ann. Hepatol. 2018;17:585–591. doi: 10.5604/01.3001.0012.0922. [DOI] [PubMed] [Google Scholar]
- 67.Catharina A.S., Modolo R., Ritter A.M.V., Sabbatini A.R., Lopes H.F., Moreno Junior H., Faria A.P. Metabolic syndrome-Related Features in Controlled and Resistant Hypertensive Subjects. Arq. Bras. Cardiol. 2018;110:514–521. doi: 10.5935/abc.20180076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Katsiki N., Anagnostis P., Kotsa K., Goulis D.G., Mikhailidis D.P. Obesity, Metabolic Syndrome and the Risk of Microvascular Complications in Patients with Diabetes mellitus. Curr. Pharm. Des. 2019 doi: 10.2174/1381612825666190708192134. [DOI] [PubMed] [Google Scholar]
- 69.Nascimbeni F., Pais R., Bellentani S., Day C.P., Ratziu V., Loria P., Lonardo A. From NAFLD in clinical practice to answers from guidelines. J. Hepatol. 2013;59:859–871. doi: 10.1016/j.jhep.2013.05.044. [DOI] [PubMed] [Google Scholar]
- 70.Lonardo A., Targher G. NAFLD: Is There Anything New under the Sun? Int. J. Mol. Sci. 2017;18:1955. doi: 10.3390/ijms18091955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Lonardo A., Lugari S., Ballestri S., Nascimbeni F., Baldelli E., Maurantonio M. A round trip from nonalcoholic fatty liver disease to diabetes: Molecular targets to the rescue? Acta Diabetol. 2019;56:385–396. doi: 10.1007/s00592-018-1266-0. [DOI] [PubMed] [Google Scholar]
- 72.Mantovani A., Dauriz M., Byrne C.D., Lonardo A., Zoppini G., Bonora E., Targher G. Association between nonalcoholic fatty liver disease and colorectal tumours in asymptomatic adults undergoing screening colonoscopy: A systematic review and meta-analysis. Metabolism. 2018;87:1–12. doi: 10.1016/j.metabol.2018.06.004. [DOI] [PubMed] [Google Scholar]
- 73.Arora A., Sharma P. Non-invasive Diagnosis of fibrosis in non.alcoholic fatty liver disease. J. Clin. Exp. Hepatol. 2012;2:145–155. doi: 10.1016/S0973-6883(12)60103-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Schreiner A.D., Rockey D.C. Evaluation of abnormal liver tests in the adult asymptomatic patient. Curr. Opin. Gastroenterol. 2018;34:272–279. doi: 10.1097/MOG.0000000000000447. [DOI] [PubMed] [Google Scholar]
- 75.Shirin D., Peleg N., Sneh-Arbib O., Cohen-Naftaly M., Braun M., Shochat T., Issachar A., Shlomai A. The Pattern of Elevated Liver Function Tests in Nonalcoholic Fatty Liver Disease Predicts Fibrosis Stage and Metabolic-Associated Comorbidities. Dig. Dis. 2019;37:69–76. doi: 10.1159/000491428. [DOI] [PubMed] [Google Scholar]
- 76.Hagström H., Nasr P., Bottai M., Ekstedt M., Kechagias S., Hultcrantz R., Stål P. Elevated serum ferritin is associated with increased mortality in NAFLD after 16 years of follow-up. Liver Int. 2016;36:1688–1695. doi: 10.1111/liv.13144. [DOI] [PubMed] [Google Scholar]
- 77.Foroughi M., Maghsoudi Z., Khayyatzadeh S., Ghiasvand R., Askari G., Iraj B. Relationship between non-alcoholic fatty liver disease and inflammation in patients with non-alcoholic fatty liver. Adv. Biomed. Res. 2016;5:28. doi: 10.4103/2277-9175.176368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ustyol A., Aycan Ustyol E., Gurdol F., Kokali F., Bekpınar S. P-selectin, endocan, and some adhesion molecules in obese children and adolescents with non-alcoholic fatty liver disease. Scand. J. Clin. Lab. Invest. 2017;77:205–209. doi: 10.1080/00365513.2017.1292363. [DOI] [PubMed] [Google Scholar]
- 79.Dowla S., Aslibekyan S., Goss A., Fontaine K., Ashraf A.P. Dyslipidemia is associated with pediatric nonalcoholic fatty liver disease. J. Clin. Lipidol. 2018;12:981–987. doi: 10.1016/j.jacl.2018.03.089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Tabung F.K., Balasubramanian R., Liang L., Clinton S.K., Cespedes Feliciano E.M., Manson J.E., Van Horn L., Wactawski-Wende J., Clish C.B., Giovannucci E.L., et al. Identifying Metabolomic Profiles of Insulinemic Dietary Patterns. Metabolites. 2019;9:120. doi: 10.3390/metabo9060120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Chen H., Wang J., Li Z., Lam C.W.K., Xiao Y., Wu Q., Zhang W. Consumption of Sugar- Sweetened Beverages Has a Dose-Dependent Effect on the Risk of Non-Alcoholic Fatty Liver Disease: An Updated Systematic Review and Dose-Response Meta-Analysis. Int. J. Environ. Res. Public Health. 2019;16:2192. doi: 10.3390/ijerph16122192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Paquette M., Bernard S., Hegele R.A., Baass A. Chylomicronemia: Differences between familial chylomicronemia syndrome and multifactorial chylomicronemia. Atherosclerosis. 2019;283:137–142. doi: 10.1016/j.atherosclerosis.2018.12.019. [DOI] [PubMed] [Google Scholar]
- 83.Targher G. Non-alcoholic fatty liver disease and increased risk of cardiovascular disease. Atherosclerosis. 2007;191:235–240. doi: 10.1016/j.atherosclerosis.2006.08.021. [DOI] [PubMed] [Google Scholar]
- 84.Wójcik-Cichy K., Koślińska-Berkan E., Piekarska A. The influence of NAFLD on the risk of atherosclerosis and cardiovascular diseases. Clin. Exp. Hepatol. 2018;4:1–6. doi: 10.5114/ceh.2018.73155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Fujii H., Imajo K., Yoneda M., Nakahara T., Hyogo H., Takahashi H., Hara T., Tanaka S., Sumida Y., Eguchi Y., et al. Japan Study Group of Nonalcoholic Fatty Liver Disease. HOMA-IR: An independent predictor of advanced liver fibrosis in nondiabetic non-alcoholic fatty liver disease. J. Gastroenterol. Hepatol. 2019 doi: 10.1111/jgh.14595. [DOI] [PubMed] [Google Scholar]
- 86.Loria P., Lonardo A., Carulli N. Relative contribution of iron burden, HFE mutations, and insulin resistance to fibrosis in nonalcoholic fatty liver. Hepatology. 2004;39:1748. doi: 10.1002/hep.20252. [DOI] [PubMed] [Google Scholar]
- 87.Amin R.F., El Bendary A.S., Ezzat S.E., Mohamed W.S. Serum Ferritin level, microalbuminuria and non-alcoholic fatty liver disease in type 2 diabetic patients. Diabetes Metab. Syndr. 2019;13:2226–2229. doi: 10.1016/j.dsx.2019.05.030. [DOI] [PubMed] [Google Scholar]
- 88.Yoneda M., Uchiyama T., Kato S., Endo H., Fujita K., Yoneda K., Mawatari H., Iida H., Takahashi H., Kirikoshi H., et al. Plasma Pentraxin3 is a novel marker for nonalcoholic steatohepatitis (NASH) BMC Gastroenterol. 2008;8:53. doi: 10.1186/1471-230X-8-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Kar S., Paglialunga S., Jaycox S.H., Islam R., Paredes A.H. Assay validation and clinical performance of chronic inflammatory and chemokine biomarkers of NASH fibrosis. PLoS ONE. 2019;14:e0217263. doi: 10.1371/journal.pone.0217263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Nigro E., Scudiero O., Monaco M.L., Palmieri A., Mazzarella G., Costagliola C., Bianco A., Daniele A. New insight into adiponectin role in obesity and obesity-related diseases. BioMed Res. Int. 2014;2014:658913. doi: 10.1155/2014/658913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Boyraz M., Cekmez F., Karaogu A., Cinaz P., Durak M., Bideci A. Serum adiponectin, leptin, resistin and RBP4 levels in obese and metabolic syndrome children with nonalcoholic fatty liver disease. Biomark Med. 2013:737–745. doi: 10.2217/bmm.13.13. [DOI] [PubMed] [Google Scholar]
- 92.Lanthier N., Horsmans Y., leclerq I.A. The metabolic syndrome how it may influence hepatic stellate cell activation and hepatic fibrosis. Curr. Opin. Clin. Nutr. Metab. Care. 2009;12:404–411. doi: 10.1097/MCO.0b013e32832c7819. [DOI] [PubMed] [Google Scholar]
- 93.Pacifico L., Poggiogalle E., Costantino F., Anania C., Ferraro F., Chiarelli F., Chiesa C. Acylated and nonacylated ghrelin levels and their associations with insulin resistance in obese and normal weight children with metabolic synddrome. Eur. J. Endocrinol. 2009;161:861–870. doi: 10.1530/EJE-09-0375. [DOI] [PubMed] [Google Scholar]
- 94.Dvorak K., Stritesky J., Petrtyl J., Vitek L., Sroubkova R., Lenicek M., Smid V., Haluzik M., Bruha R. Use of Non-Invasive Parameters of Non-Alcoholic Steatohepatitis and Liver Fibrosis in Daily Practice–An Exploratory Case–control Study. PLoS ONE. 2014;9:e111551. doi: 10.1371/journal.pone.0111551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Sánchez-Valle V., Chávez-Tapia N.C., Uribe M., Méndez-Sánchez N. Role of oxidative stress and molecular changes in liver fibrosis: A review. Curr. Med. Chem. 2012;19:4850–4860. doi: 10.2174/092986712803341520. [DOI] [PubMed] [Google Scholar]
- 96.Cabré N., Luciano-Mateo F., Fernández-Arroyo S., Baiges-Gayà G., Hernández-Aguilera A., Fibla M., Fernández-Julià R., París M., Sabench F., Del Castillo D., et al. Laparoscopic sleeve gastrectomy reverses non-alcoholic fatty liver disease modulating oxidative stress and inflammation. Metabolism. 2019 doi: 10.1016/j.metabol.2019.07.002. [DOI] [PubMed] [Google Scholar]
- 97.Shen J., Chan H.L., Wong G.L., Chan A.W., Choi P.C., Chan H.Y., Chim A.M., Yeung D.K., Yu J., Chu W.C., et al. Assesment of non-alcoholic fatty liver disease using serum total cell death and apoptosis markers. Aliments Pharmacol. Ther. 2012;36:1057–1066. doi: 10.1111/apt.12091. [DOI] [PubMed] [Google Scholar]
- 98.Younosssi Z.M., Jarrar M., Nugent C., Randhawa M., Afendy M., Stepanova M., Rafiq N., Goodman Z., Chandhoke V., Baranova A. A novel diagnostic biomarker panel for obesity–Related nonalcoholic steatohepatitis (NASH) Obes. Surg. 2008;18:1430–1437. doi: 10.1007/s11695-008-9506-y. [DOI] [PubMed] [Google Scholar]
- 99.Tarantino G., Mazzarella C., Tarantino M., Di Minno M.N., Conca P. Could high levels of tissue polypeptide specific antigen, a marker of apoptosis detected in nonalcoholic staetohepatitis, improve after weight loss? Dis. Markers. 2009;26:55–63. doi: 10.1155/2009/292458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Markelova E.V., Romanchuk A.L., Prosekova E.V., Krasnikov V.E., Beniova S.N. Assessing the level of matrix metal proteinases 1,8,9, their tissue inhibitor type I, in cases of odontogenic phlegmons. Bratisl. Lek. Listy. 2017;118:51–55. doi: 10.4149/BLL_2017_010. [DOI] [PubMed] [Google Scholar]
- 101.Orasan O.H., Ciulei G., Cozma A., Sava M., Dumtrasci D.L. Hyaluronic acid as a biomarker of fibrosis in chronic liver diseases of different etologies. Clujul Med. 2016;89:24–31. doi: 10.15386/cjmed-554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Degertekin B., Ozenirler S., Elberg S., Akyol G. The serum endothelin-1 level in steatosis and NASH, and its relation with severity of liver fibrosis. Dig. Dis. Sci. 2007;52:2622–2628. doi: 10.1007/s10620-006-9147-8. [DOI] [PubMed] [Google Scholar]
- 103.Mahmoud A.A., Bakir A.S., Shabana S.S. Serum TGF-β, serum MMP-1, and HOMA-IR as non-invasive predictors of fibrosis in Egyptian patients with NAFLD. Saudi J. Gatroenterol. 2012;18:327–333. doi: 10.4103/1319-3767.101132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Abdelaziz R., Elbasel M., Esmat S., Essam K., Abdelaaty S. Tissue inhibitors of metalloproteinase-1 and 2 and obesity related non-alcoholic fatty liver disease. Is there a relationship. Digestion. 2015;92:130–137. doi: 10.1159/000439083. [DOI] [PubMed] [Google Scholar]
- 105.Lee Y.H., Bang H., Park Y.M., Bae J.C., Lee B.W., Kang E.S., Cha B.S., Lee H.C., Balkau B., Lee W.Y., et al. Non-laboratory-based self-assessment screening score for non-alcoholic fatty liver disease: Development, validation and comparison with other scores. PLoS ONE. 2014;9:e107584. doi: 10.1371/journal.pone.0107584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Ciećko-Michalska I., Szczepanek M., Wierzbicka-Tutka I., Zahradnik-Bilska J., Mach T. Non-invasive diagnosis of steatosis, inflammatory changes and liver fibrosis in patients with non-alcoholic fatty liver diseases. Pilot study. Arch. Med. Sci. Atheroscler. Dis. 2018;3:e179–e183. doi: 10.5114/amsad.2018.81184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Siddiqui M.S., Yamada G., Vuppalanchi R., Van Natta M., Loomba R., Guy C., Brandman D., Tonascia J., Chalasani N., Neuschwander-Tetri B., et al. NASH Clinical Research Network. Diagnostic Accuracy of Noninvasive Fibrosis Models to Detect Change in Fibrosis Stage. Clin. Gastroenterol. Hepatol. 2019;17:1877–1885. doi: 10.1016/j.cgh.2018.12.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Huang X., Xu M., Chen Y., Peng K., Huang Y., Wang P., Ding L., Lin L., Xu Y., Chen Y., et al. Validation of the fatty liver index for nonalcoholic fatty liver disease in middle aged and elderly. Medicine. 2015;94:e1682. doi: 10.1097/MD.0000000000001682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Franch-Nadal J., Caballeria L., Mata-Cases M., Mauricio D., Giraldez-García C., Mancera J., Goday A., Mundet-Tudurí X., Regidor E., PREDAPS Study Group Fatty liver index is a predictor of incident diabetes in patients with prediabetes: The PREDAPS study. PLoS ONE. 2018;13:e0198327. doi: 10.1371/journal.pone.0198327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Khang A.R., Lee H.W., Yi D., Kang Y.H., Son S.M. The fatty liver index, a simple and useful predictor of metabolic syndrome: Analysis of the Korea National Health and Nutrition Examination Survey 2010–2011. Diabetes Metab. Syndr. Obes. 2019;12:181–190. doi: 10.2147/DMSO.S189544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Borman M.A., Ladak F., Crotty P., Pollett A., Kirsch R., Pomier-Layrargues G., Beaton M., Duarte-Rojo A., Elkashab M., Myers R.P. The fatty liver index has limited utility for the detection and quantification of hepatic steatosis in obese patients. Hepatol. Int. 2013;7:592–599. doi: 10.1007/s12072-012-9401-4. [DOI] [PubMed] [Google Scholar]
- 112.Cuthbertson D.J., Weickert M.O., Lythgoe D., Sprung V.S., Dobson R., Shoajee-Moradie F., Umpleby M., Pfeiffer A.F., Thomas E.L., Bell J.D., et al. External validation of the fatty liver index and lipid accumulation product indices, using 1H-magnetic resonance spectroscopy, to identify hepatic steatosis in healthy controls and obese, insulin-resistant individuals. Eur. J. Endocrinol. 2014;171:561–569. doi: 10.1530/EJE-14-0112. [DOI] [PubMed] [Google Scholar]
- 113.Lee J.H., Kim D., Kim H.J., Lee C.H., Yang J.I., Kim W., Kim Y.J., Yoon J.H., Cho S.H., Sung M.W., et al. Hepatic steatosis index: A simple screening tool reflecting nonalcoholic fatty liver disease. Dig. Liver Dis. 2010;42:503–508. doi: 10.1016/j.dld.2009.08.002. [DOI] [PubMed] [Google Scholar]
- 114.Wang J., Xu C., Xun Y., Lu Z., Shi J., Yu C., Li Y. ZJU index: A novel model for predicting nonalcoholic fatty liver disease in a Chinese population. Sci. Rep. 2015;5:16494. doi: 10.1038/srep16494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Poynard T., Lassailly G., Diaz E., Clement K., Caïazzo R., Tordjman J., Munteanu M., Perazzo H., Demol B., Callafe R., et al. FLIP consortium. Performance of biomarkers FibroTest, ActiTest, SteatoTest, and NashTest in patients with severe obesity: Meta analysis of individual patient data. PLoS ONE. 2012;7:e30325. doi: 10.1371/journal.pone.0030325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.McPherson S., Stewart S.F., Henderson E., Burt A.D., Day C.P. Simple non-invasive fibrosis scoring systems can reliably exclude advanced fibrosis in patients with non-alcoholic fatty liver disease. Gut. 2010;59:1265–1269. doi: 10.1136/gut.2010.216077. [DOI] [PubMed] [Google Scholar]
- 117.Kruger F.C., Daniels C.R., Kidd M., Swart G., Brundyn K., van Rensburg C., Kotze M. APRI: A simple bedside marker for advanced fibrosis that can avoid liver biopsy in patients with NAFLD/NASH. S. Afr. Med. J. 2011;101:477–480. [PubMed] [Google Scholar]
- 118.Shah A.G., Lydecker A., Murray K., Tetri B.N., Contos M.J., Sanyal A.J. Nash Clinical Research Network. Comparison of noninvasive markers of fibrosis in patients with nonalcoholic fatty liver disease. Clin. Gastroenterol. Hepatol. 2009;7:1104–1112. doi: 10.1016/j.cgh.2009.05.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Pérez-Gutiérrez O.Z., Hernández-Rocha C., Candia-Balboa R.A., Arrese M.A., Benítez C., Brizuela-Alcántara D.C., Méndez-Sánchez N., Uribe M., Chávez-Tapia N.C. Validation study of systems for noninvasive diagnosis of fibrosis in nonalcoholic fatty liver disease in Latin population. Ann. Hepatol. 2013;12:416–424. doi: 10.1016/S1665-2681(19)31004-X. [DOI] [PubMed] [Google Scholar]
- 120.Raszeja-Wyszomirska J., Szymanik B., Ławniczak M., Kajor M., Chwist A., Milkiewicz P., Hartleb M. Validation of the BARD scoring system in Polish patients with nonalcoholic fatty liver disease (NAFLD) BMC Gastroenterol. 2010;10:67. doi: 10.1186/1471-230X-10-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Sun W., Cui H., Li N., Wei Y., Lai S., Yang Y., Yin X., Chen D.F. Comparison of FIB4 index, NAFLD fibrosis score and BARD score for prediction of advanced fibrosis in adult patients with non-alcoholic fatty liver disease: A meta-analysis study. Hepatol. Res. 2016;46:862–870. doi: 10.1111/hepr.12647. [DOI] [PubMed] [Google Scholar]
- 122.Angulo P., Hui J.M., Marchesini G., Bugianesi E., George J., Farrell G.C., Enders F., Saksena S., Burt A.D., Bida J.P., et al. The NAFLD fibrosis score: A noninvasive system that identifies liver fibrosis in patients with NAFLD. Hepatology. 2007;45:846–854. doi: 10.1002/hep.21496. [DOI] [PubMed] [Google Scholar]
- 123.Demir M., Lang S., Schlattjan M., Drebber U., Wedemeyer I., Nierhoff D., Kaul I., Sowa J., Canbay A., Töx U., et al. A new inexpensive and non-invasive scoring system to exclude advanced fibrosis in patients with NAFLD. PLoS ONE. 2013;8:e58360. doi: 10.1371/journal.pone.0058360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Miyaaki H., Ichikawa T., Nakao K., Yatsuhashi H., Furukawa R., Ohba K., Omagari K., Kusumoto Y., Yanagi K., Inoue O., et al. Clinicopathological study of nonalcoholic fatty liver disease in Japan: The risk factors for fibrosis. Liver Int. 2008;28:519–524. doi: 10.1111/j.1478-3231.2007.01614.x. [DOI] [PubMed] [Google Scholar]
- 125.Ratziu V., Giral P., Charlotte F., Bruckert E., Thibault V., Theodorou I., Khalil L., Turpin G., Opolon P., Poynard T. Liver fibrosis in overweight patients. Gastroenterology. 2000;118:1117–1123. doi: 10.1016/S0016-5085(00)70364-7. [DOI] [PubMed] [Google Scholar]
- 126.Sterling R.K., Lissen E., Clumeck N., Sola R., Correa M.C., Montaner J., SSulkowski M., Torriani F.J., Dieterich D.T., Thomas D.L., et al. APRICOT Clinical Investigators. Development of a simple noninvasive index to predict significant fibrosis in patients with HIV/HCV coinfection. Hepatology. 2006;43:1317–1325. doi: 10.1002/hep.21178. [DOI] [PubMed] [Google Scholar]
- 127.Nones R.B., Ivantes C.P., Pedroso M.L.A. Can FIB4 and NAFLD fibrosis scores help endocrinologists refer patients with non-alcoholic fat liver disease to a hepatologist? Arch. Endocrinol. Metab. 2017;61:276–281. doi: 10.1590/2359-3997000000233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Sumida Y., Yoneda M., Hyogo H., Itoh Y., Ono M., Fujii H., Eguchi Y., Suzuki Y., Aoki N., Kanemasa K., et al. Japan Study Group of Nonalcoholic Fatty Liver Disease (JSG-NAFLD). Validation of the FIB4 index in a Japanese nonalcoholic fatty liver disease population. BMC Gastroenterol. 2012;12:2. doi: 10.1186/1471-230X-12-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Arteaga I., Buezo I., Expósito C., Pera G., Rodríguez L., Alumà A., Auladell M.A., Torán P., Caballería L. Non-invasive markers of fibrosis in the diagnosis of non-alcoholic fatty liver disease. Gastroenterol. Hepatol. 2014;37:503–510. doi: 10.1016/j.gastrohep.2014.03.009. [DOI] [PubMed] [Google Scholar]
- 130.Dixon J.B., Bhathal P.S., O’Brien P.E. Nonalcoholic fatty liver disease: Predictors of nonalcoholic steatohepatitis and liver fibrosis in the severely obese. Gastroenterology. 2001;121:91–100. doi: 10.1053/gast.2001.25540. [DOI] [PubMed] [Google Scholar]
- 131.Calès P., Boursier J., Oberti F., Hubert I., Gallois Y., Rousselet M.C., Dib N., Moal V., Macchi L., Chevailler A., et al. FibroMeters: A family of blood tests for liver fibrosis. Gastroenterol. Clin. Biol. 2008;32:40–51. doi: 10.1016/S0399-8320(08)73992-7. [DOI] [PubMed] [Google Scholar]
- 132.Poynard T., Munteanu M., Deckmyn O., Ngo Y., Drane F., Castille J.M., Housset C., Ratziu V., Imbert-Bismut F. Validation of liver fibrosis biomarker (FibroTest) for assessing liver fibrosis progression: Proof of concept and first application in a large population. J. Hepatol. 2012;57:541–548. doi: 10.1016/j.jhep.2012.04.025. [DOI] [PubMed] [Google Scholar]
- 133.Poynard T., Ratziu V., Charlotte F., Messous D., Munteanu M., Imbert-Bismut F., Massard J., Bonyhay L., Tahiri M., Thabut D., et al. LIDO Study Group; CYTOL study group. Diagnostic value of biochemical markers (NashTest) for the prediction of non alcoholo steato hepatitis in patients with non-alcoholic fatty liver disease. BMC Gastroenterol. 2006;6:34. doi: 10.1186/1471-230X-6-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Guha I.N., Parkes J., Roderick P., Chattopadhyay D., Cross R., Harris S., Kaye P., Burt A.D., Ryder S.D., Aithal G.P., et al. Noninvasive markers of fibrosis in nonalcoholic fatty liver disease: Validating the European Liver Fibrosis Panel and exploring simple markers. Hepatology. 2008;47:455–460. doi: 10.1002/hep.21984. [DOI] [PubMed] [Google Scholar]
- 135.Morra R., Munteanu M., Imbert-Bismut F., Messous D., Ratziu V., Poynard T. FibroMAX: Towards a new universal biomarker of liver disease? Expert Rev. Mol. Diagn. 2007;7:481–490. doi: 10.1586/14737159.7.5.481. [DOI] [PubMed] [Google Scholar]
- 136.Palekar N.A., Naus R., Larson S.P., Ward J., Harrison S.A. Clinical model for distinguishing nonalcoholic steatohepatitis from simple steatosis in patients with nonalcoholic fatty liver disease. Liver Int. 2006;26:151–156. doi: 10.1111/j.1478-3231.2005.01209.x. [DOI] [PubMed] [Google Scholar]
- 137.Francque S.M., Verrijken A., Mertens I., Hubens G., Van Marck E., Pelckmans P., Michielsen P., Van Gaal L. Noninvasive assessment of nonalcoholic fatty liver disease in obese or overweight patients. Clin. Gastroenterol. Hepatol. 2012;10:1162–1168. doi: 10.1016/j.cgh.2012.06.019. [DOI] [PubMed] [Google Scholar]
- 138.Nakamura A., Yoneda M., Sumida Y., Eguchi Y., Fujii H., Hyogo H., Ono M., Suzuki Y., Kawaguchi T., Aoki N., et al. Modification of a simple clinical scoring system as a diagnostic screening tool for non-alcoholic steatohepatitis in Japanese patients with non-alcoholic fatty liver disease. J. Diabetes Investig. 2013;4:651–658. doi: 10.1111/jdi.12101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Macaluso F.S., Maida M., Petta S. Genetic background in nonalcoholic fatty liver disease: A comprehensive review. World J. Gastroenterol. 2015;21:11088–11111. doi: 10.3748/wjg.v21.i39.11088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Armstrong L.E., Guo G.L. Understanding Environmental Contaminants’ Direct Effects on Non-alcoholic Fatty Liver Disease Progression. Curr. Environ. Health Rep. 2019 doi: 10.1007/s40572-019-00231-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Wang C.W., Chuang H.Y., Liao K.W., Yu M.L., Dai C.Y., Chang W.T., Tsai C.H., Chiang H.C., Huang P.C. Urinary thiodiglycolic acid is associated with increased risk of non-alcoholic fatty liver disease in children living near a petrochemical complex. Environ. Int. 2019;131:104978. doi: 10.1016/j.envint.2019.104978. [DOI] [PubMed] [Google Scholar]
- 142.Non-alcoholic Fatty Liver Disease Study Group. Lonardo A., Bellentani S., Argo C.K., Ballestri S., Byrne C.D., Caldwell S.H., Cortez-Pinto H., Grieco A., Machado M.V., et al. Epidemiological modifiers of non-alcoholic fatty liver disease: Focus on high-risk groups. Dig. Liver Dis. 2015;47:997–1006. doi: 10.1016/j.dld.2015.08.004. [DOI] [PubMed] [Google Scholar]
- 143.Romeo S., Kozlitina J., Xing C., Pertsemlidis A., Cox D., Pennacchio L.A., Boerwinkle E., Cohen J.C., Hobbs H.H. Genetic variation in PNPLA3 confers susceptibility to nonalcoholic fatty liver disease. Nat. Genet. 2008;40:1461–1465. doi: 10.1038/ng.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Sookoian S., Pirola C.J. Meta-analysis of the influence of I148M variant of patatin-like phospholipase domain containing 3 gene (PNPLA3) on the susceptibility and histological severity of nonalcoholic fatty liver disease. Hepatology. 2011;53:1883–1894. doi: 10.1002/hep.24283. [DOI] [PubMed] [Google Scholar]
- 145.Singal A.G., Manjunath H., Yopp A.C., Beg M.S., Marrero J.A., Gopal P., Waljee A.K. The effect of PNPLA3 on fibrosis progression and development of hepatocellular carcinoma: A meta-analysis. Am. J. Gastroenterol. 2014;109:325–334. doi: 10.1038/ajg.2013.476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Liu Y.L., Patman G.L., Leathart J.B., Piguet A.C., Burt A.D., Dufour J.F., Day C.P., Daly A.K., Reeves H.L., Anstee Q.M. Carriage of the PNPLA3 rs738409 C & gt; G polymorphism confers an increased risk of non-alcoholic fatty liver disease associated hepatocellular carcinoma. J. Hepatol. 2014;61:75–81. doi: 10.1016/j.jhep.2014.02.030. [DOI] [PubMed] [Google Scholar]
- 147.Musso G., Cassader M., Gambino R. PNPLA3 rs738409 and TM6SF2 rs58542926 gene variants affect renal disease and function in nonalcoholic fatty liver disease. Hepatology. 2015;62:658–659. doi: 10.1002/hep.27643. [DOI] [PubMed] [Google Scholar]
- 148.Kozlitina J., Smagris E., Stender S., Nordestgaard B.G., Zhou H.H., Tybjærg-Hansen A., Vogt T.F., Hobbs H.H., Cohen J.C. Exomewide association study identifies a TM6SF2 variant that confers susceptibility to nonalcoholic fatty liver disease. Nat. Genet. 2014;46:352–356. doi: 10.1038/ng.2901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Dongiovanni P., Petta S., Maglio C., Fracanzani A.L., Pipitone R., Mozzi E., Motta B.M., Kaminska D., Rametta R., Grimaudo S., et al. Transmembrane 6 superfamily member 2 gene variant disentangles nonalcoholic steatohepatitis from cardiovascular disease. Hepatology. 2015;61:506–514. doi: 10.1002/hep.27490. [DOI] [PubMed] [Google Scholar]
- 150.Liang N., Damdimopoulos A., Goñi S., Huang Z., Vedin L.L., Jakobsson T., Giudici M., Ahmed O., Pedrelli M., Barilla S., et al. Hepatocyte-specific loss of GPS2 in mice reduces non-alcoholic steatohepatitis via activation of PPARα. Nat. Commun. 2019;10:1684. doi: 10.1038/s41467-019-09524-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Gallego-Durán R., Romero-Goméz M. Epigenetic mechanism in nonalcoholic fatty liver disease: An emerging field. World J. Hepatol. 2015;7:2497–2502. doi: 10.4254/wjh.v7.i24.2497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Sinton M.C., Hay D.C., Drake A.J. Metabolic control of gene transcription in non-alcoholic fatty liver disease: The role of the epigenome. Clin. Epigenet. 2019;11:104. doi: 10.1186/s13148-019-0702-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Salvoza N.C., Klinzing D.C., Gopez-Cervantes J., Baclig M.O. Association of Circulating Serum miR-34a and miR-122 with Dyslipidemia among Patients with Non-Alcoholic Fatty Liver Disease. PLoS ONE. 2016;11:e0153497. doi: 10.1371/journal.pone.0153497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Ko C.J., Li C.J., Wu M.Y., Chu P.Y. Overexpression of LGR-5 as a Predictor of Poor Outcome in Patients with Hepatocellular Carcinoma. Int. J. Environ. Res. Public Health. 2019;16:1836. doi: 10.3390/ijerph16101836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Pacifico L., Andreoli G.M., D’Avanzo M., De Mitri D., Pierimarchi P. Role of osteoprotegerin/receptor activator of nuclear factor kappa B/receptor activator of nuclear factor kappa B ligand axis in nonalcoholic fatty liver disease. World J. Gastroenterol. 2018;24:2073–2082. doi: 10.3748/wjg.v24.i19.2073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Khajehahmadi Z., Mohagheghi S., Nikeghbalian S., Geramizadeh B., Khodadadi I., Karimi J., Ghaffari M.E., Tavilani H. Downregulation of Hedgehog Ligands in Human Simple Steatosis May Protect Against Nonalcoholic Steatohepatitis: Is TAZ a Crucial Regulator? IUBMB Life. 2019 doi: 10.1002/iub.2068. [DOI] [PubMed] [Google Scholar]
- 157.Younossi Z.M., Baranova A., Ziegler K., Del Giacco L., Schlauch K., Born T.L., Elariny H., Gorreta F., VanMeter A., Younoszai A., et al. A genomic and proteomic study of the spectrum of nonalcoholic fatty liver disease. Hepatology. 2005;42:665–674. doi: 10.1002/hep.20838. [DOI] [PubMed] [Google Scholar]
- 158.Bell L.N., Theodorakis J.L., Vuppalanchi R., Saxena R., Bemis K.G., Wang M., Chalasani N. Serum proteomics and biomarker discovery across the spectrum of nonalcoholic fatty liver disease. Hepatology. 2010;51:111–120. doi: 10.1002/hep.23271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Romeo S. Notch and Nonalcoholic Fatty Liver and Fibrosis. N. Engl. J. Med. 2019;380:681–683. doi: 10.1056/NEJMcibr1815636. [DOI] [PubMed] [Google Scholar]
- 160.Romier B., Ivaldi C., Sartelet H., Heinz A., Schmelzer C.E.H., Garnotel R., Guillot A., Jonquet J., Bertin E., Guéant J.L., et al. Production of Elastin-Derived Peptides Contributes to the Development of Nonalcoholic Steatohepatitis. Diabetes. 2018;67:1604–1615. doi: 10.2337/db17-0490. [DOI] [PubMed] [Google Scholar]
- 161.Di Mauro S., Scamporrino A., Petta S., Urbano F., Filippello A., Ragusa M., Di Martino M.T., Scionti F., Grimaudo S., Pipitone R.M., et al. Serum coding and non-coding RNAs as biomarkers of NAFLD and fibrosis severity. Liver Int. 2019 doi: 10.1111/liv.14167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Kurbatova I.V., Topchieva L.V., Dudanova O.P., Shipovskaya A.A. Biochemical and molecular-genetic indicators of inflammation and apoptosis in liver cirrhosis as an outcome of the progression of non-alcoholic steatohepatitis. Ter. Arkh. 2019;91:21–27. doi: 10.26442/00403660.2019.04.000057. [DOI] [PubMed] [Google Scholar]
- 163.Mirea A.M., Toonen E.J.M., van den Munckhof I., Munsterman I.D., Tjwa E.T.T.L., Jaeger M., Oosting M., Schraa K., Rutten J.H.W., van der Graaf M., et al. Increased proteinase 3 and neutrophil elastase plasma concentrations are associated with non-alcoholic fatty liver disease (NAFLD) and type 2 diabetes. Mol. Med. 2019;25:16. doi: 10.1186/s10020-019-0084-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Barchetta I., Enhörning S., Cimini F.A., Capoccia D., Chiappetta C., Di Cristofano C., Silecchia G., Leonetti F., Melander O., Cavallo M.G. Elevated plasma copeptin levels identify the presence and severity of non-alcoholic fatty liver disease in obesity. BMC Med. 2019;17:85. doi: 10.1186/s12916-019-1319-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Asprouli E., Kalafati I.P., Sakellari A., Karavoltsos S., Vlachogiannakos J., Revenas K., Kokkinos A., Dassenakis M., Dedoussis G.V., Kalogeropoulos N. Evaluation of Plasma Trace Elements in Different Stages of Nonalcoholic Fatty Liver Disease. Biol. Trace Elem. Res. 2019;188:326–333. doi: 10.1007/s12011-018-1432-9. [DOI] [PubMed] [Google Scholar]
- 166.Lonardo A., Sookoian S., Pirola C.J., Targher G. Non-alcoholic fatty liver disease and risk of cardiovascular disease. Metabolism. 2016;65:1136–1150. doi: 10.1016/j.metabol.2015.09.017. [DOI] [PubMed] [Google Scholar]
- 167.Targher G., Byrne C.D., Lonardo A., Zoppini G., Barbui C. Non-alcoholic fatty liver disease and risk of incident cardiovascular disease: A meta-analysis. J. Hepatol. 2016;65:589–600. doi: 10.1016/j.jhep.2016.05.013. [DOI] [PubMed] [Google Scholar]
- 168.Lonardo A., Nascimbeni F., Ballestri S. NAFLD, Hepatotropic Viruses, and Cardiometabolic Risk. Hepatology. 2017;65:2122–2123. doi: 10.1002/hep.29052. [DOI] [PubMed] [Google Scholar]
- 169.Hernaez R., Lazo M., Bonekamp S., Kamel I., Brancati F.L., Guallar E., Clark J.M. Diagnostic accuracy and reliability of ultrasonography for the detection of fatty liver: A meta-analysis. Hepatology. 2011;54:1082–1090. doi: 10.1002/hep.24452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Ballestri S., Romagnoli D., Nascimbeni F., Francica G., Lonardo A. Role of ultrasound in the diagnosis and treatment of nonalcoholic fatty liver disease and its complications. Expert Rev. Gastroenterol. Hepatol. 2015;9:603–627. doi: 10.1586/17474124.2015.1007955. [DOI] [PubMed] [Google Scholar]
- 171.Ballestri S., Nascimbeni F., Baldelli E., Marrazzo A., Romagnoli D., Targher G., Lonardo A. Ultrasonographic fatty liver indicator detects mild steatosis and correlates with metabolic/histological parameters in various liver diseases. Metabolism. 2017;72:57–65. doi: 10.1016/j.metabol.2017.04.003. [DOI] [PubMed] [Google Scholar]
- 172.Ballestri S., Nascimbeni F., Lugari S., Lonardo A., Francica G. A critical appraisal of the use of ultrasound in hepatic steatosis. Expert Rev. Gastroenterol. Hepatol. 2019;13:667–681. doi: 10.1080/17474124.2019.1621164. [DOI] [PubMed] [Google Scholar]
- 173.Pu K., Wang Y., Bai S., Wei H., Zhou Y., Fan J., Qiao L. Diagnostic accuracy of controlled attenuation parameter (CAP) as a non-invasive test for steatosis in suspected non-alcoholic fatty liver disease: A systematic review and meta-analysis. BMC Gastroenterol. 2019;19:51. doi: 10.1186/s12876-019-0961-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Brener S. Transient Elastography for Assessment of Liver Fibrosis and Steatosis: An Evidence-Based Analysis. Ont. Health Technol. Assess. Ser. 2015;15:1–45. [PMC free article] [PubMed] [Google Scholar]
- 175.Cocciolillo S., Parruti G., Marzio L. CEUS and Fibroscan in non-alcoholic fatty liver disease and non-alcoholic steatohepatitis. World J. Hepatol. 2014;6:496–503. doi: 10.4254/wjh.v6.i7.496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Tovo C.V., Villela-Nogueira C.A., Leite N.C., Panke C.L., Port G.Z., Fernandes S., Buss C., Coral G.P., Cardoso A.C., Cravo C.M., et al. Transient hepatic elastography has the best performance to evaluate liver fibrosis in non-alcoholic fatty liver disease (NAFLD) Ann. Hepatol. 2019;18:445–449. doi: 10.1016/j.aohep.2018.09.003. [DOI] [PubMed] [Google Scholar]
- 177.Jeong J.Y., Kim T.Y., Sohn J.H., Kim Y., Jeong W.K., Oh Y.H., Yoo K.S. Real time shear wave elastography in chronic liver diseases: Accuracy for predicting liver fibrosis, in comparison with serum markers. World J. Gastroenterol. 2014;20:13920–13929. doi: 10.3748/wjg.v20.i38.13920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Grgurević I., Bokun T., Mustapić S., Trkulja V., Heinzl R., Banić M., Puljiz Ž., Lukšić B., Kujundžić M. Real-time two-dimensional shear wave ultrasound elastography of the liver is a reliable predictor of clinical outcomes and the presence of esophageal varices in patients with compensated liver cirrhosis. Croat Med. J. 2015;56:470–481. doi: 10.3325/cmj.2015.56.470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Yoneda M., Suzuki K., Kato S., Fujita K., Nozaki Y., Hosono K., Saito S., Nakajima A. Nonalcoholic fatty liver disease: US-based acoustic radiation force impulse elastography. Radiology. 2010;256:640–647. doi: 10.1148/radiol.10091662. [DOI] [PubMed] [Google Scholar]
- 180.Liu H., Fu J., Hong R., Liu L., Li F. Acoustic Radiation Force Impulse Elastography for the Non-Invasive Evaluation of Hepatic Fibrosis in Non-Alcoholic Fatty Liver Disease Patients: A Systematic Review & Meta-Analysis. PLoS ONE. 2015;10:e0127782. doi: 10.1371/journal.pone.0127782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Venkatesh S.K., Yin M., Ehman R.L. Magnetic resonance elastography of liver: Technique, analysis, and clinical applications. J. Magn. Reson. Imaging. 2013;37:544–555. doi: 10.1002/jmri.23731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Cui J., Ang B., Haufe W., Hernandez C., Verna E.C., Sirlin C.B., Loomba R. Comparative diagnostic accuracy of magnetic resonance elastography vs. eight clinical prediction rules for non-invasive diagnosis of advanced fibrosis in biopsy-proven non-alcoholic fatty liver disease: A prospective study. Aliments Pharmacol. Ther. 2015;41:1271–1280. doi: 10.1111/apt.13196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Heba E., Hernandez C., Haufe W., Hooker J., Andre M.P., Valasek M.A., Aryafar H., Sirlin C.B., Loomba R. Magnetic resonance elastography is superior to acoustic radiation force impulse for the Diagnosis of fibrosis in patients with biopsy-proven nonalcoholic fatty liverdisease: A prospective study. Hepatology. 2016;63:453–461. doi: 10.1002/hep.28337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Karlas T., Petroff D., Garnov N., Böhm S., Tenckhoff H., Wittekind C., Wiese M., Schiefke I., Linder N., Schaudinn A., et al. Non-invasive assessment of hepatic steatosis in patients with NAFLD using controlled attenuation parameter and 1H-MR spectroscopy. PLoS ONE. 2014;9:e91987. doi: 10.1371/journal.pone.0091987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Kang B.K., Yu E.S., Lee S.S., Lee Y., Kim N., Sirlin C.B., Cho E.Y., Yeom S.K., Byun J.H., Park S.H., et al. Hepatic fat quantification: A prospective comparison of magnetic resonance spectroscopy and analysis methods for chemical-shift gradient echo magnetic resonance imaging with histologic assessment as the reference standard. Investig. Radiol. 2012;47:368–375. doi: 10.1097/RLI.0b013e31824baff3. [DOI] [PubMed] [Google Scholar]
- 186.Lee S.S., Park S.H. Radiologic evaluation of nonalcoholic fatty liver disease. World J. Gastroenterol. 2014;20:7392–7402. doi: 10.3748/wjg.v20.i23.7392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Besutti G., Valenti L., Ligabue G., Bassi M.C., Pattacini P., Guaraldi G., Giorgi Rossi P. Accuracy of imaging methods for steatohepatitis diagnosis in non-alcoholic fatty liverdisease patients: A systematic review. Liver Int. 2019 doi: 10.1111/liv.14118. [DOI] [PubMed] [Google Scholar]
- 188.Manning P., Murphy P., Wang K., Hooker J., Wolfson T., Middleton M.S., Newton K.P., Behling C., Awai H.I., Durelle J., et al. Liver histology and diffusion-weighted MRI in children with nonalcoholic fatty liverdisease: A MAGNET study. J. Magn. Reson. Imaging. 2017;46:1149–1158. doi: 10.1002/jmri.25663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Schwimmer J.B., Behling C., Angeles J.E., Paiz M., Durelle J., Africa J., Newton K.P., Brunt E.M., Lavine J.E., Abrams S.H., et al. Magnetic resonance elastography measured shear stiffness as a biomarker of fibrosis in pediatric nonalcoholic fatty liver disease. Hepatology. 2017;66:1474–1485. doi: 10.1002/hep.29241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Guo Y., Lin H., Zhang X., Wen H., Chen S., Chen X. The influence of hepatic steatosis on the evaluation of fibrosis with non-alcoholic fatty liver disease by acoustic radiation force impulse. Conf. Proc. IEEE Eng. Med. Biol. Soc. 2017;2017:2988–2991. doi: 10.1109/EMBC.2017.8037485. [DOI] [PubMed] [Google Scholar]
- 191.Vuppalanchi R., Siddiqui M.S., Van Natta M.L., Hallinan E., Brandman D., Kowdley K., Neuschwander-Tetri B.A., Loomba R., Dasarathy S., Abdelmalek M., et al. NASH Clinical Research Network. Performance characteristics of vibration-controlled transient elastography for evaluation of nonalcoholic fatty liver disease. Hepatology. 2018;67:134–144. doi: 10.1002/hep.29489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Tovo C.V., de Mattos A.Z., Coral G.P., Branco F.S., Suwa E., de Mattos A.A. Noninvasive imaging assessment of non-alcoholic fatty liver disease: Focus onliver scintigraphy. World J. Gastroenterol. 2015;21:4432–4439. doi: 10.3748/wjg.v21.i15.4432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Lee S.J., Kim S.U. Noninvasive monitoring of hepatic steatosis: Controlled attenuation parameter and magnetic resonance imaging-proton density fat fraction in patients with nonalcoholic fatty liver disease. Expert Rev. Gastroenterol. Hepatol. 2019:523–530. doi: 10.1080/17474124.2019.1608820. [DOI] [PubMed] [Google Scholar]
- 194.Jayakumar S., Middleton M.S., Lawitz E.J., Mantry P.S., Caldwell S.H., Arnold H., Mae Diehl A., Ghalib R., Elkhashab M., Abdelmalek M.F., et al. Longitudinal correlations between MRE, MRI-PDFF, and liver histology in patients with non-alcoholic steatohepatitis: Analysis of data from a phase II trial of selonsertib. J. Hepatol. 2019;70:133–141. doi: 10.1016/j.jhep.2018.09.024. [DOI] [PubMed] [Google Scholar]