This editorial refers to ‘Prevention of liver cancer cachexia-induced cardiac wasting and heart failure’, by J. Springer et al. doi:10.1093/eurheartj/eht302. The paper was inadvertently published in Volume 35, Issue 14 without this editorial.
In his Principles and Practices of Medicine , written over 100 years ago, William Osler described cachexia in stomach cancer as ‘progressive emaciation’ and ‘loss of strength disproportionate to loss of weight’. 1 Today, cachexia is further defined as a multifactorial syndrome characterized by an ongoing loss of skeletal muscle mass that cannot be reversed by conventional nutritional support, leading to progressive functional impairment. 2 Although the prevalence and severity vary across cancer types, cachexia occurs in the majority of patients with advanced oncological disease and is responsible for ~ 20% of cancer deaths. 3 , 4 The negative impact on functional capacity, quality of life, cancer treatment response, and prognosis in the oncology setting emphasizes the clinical importance of cachexia. Despite reductions in overall lean mass, most previous work has focused on the loss of skeletal muscle. Few studies have assessed effects of cachexia on non-skeletal muscle types, such as the myocardium. Also, whereas pre-clinical models have been developed to elucidate the underlying effects of cancer cachexia on skeletal muscle, there remains a paucity of human data to inform the development of effective therapies. 5
Although there is clear evidence that cancer therapy can negatively impact cardiac muscle function, 6 it is less clear if tumour growth alone is sufficient to cause either cardiac atrophy (similar to skeletal muscle wasting) or cardiac dysfunction. Springer and colleagues now add to a growing body of literature suggesting that tumours can lead specifically to atrophy and dysfunction of cardiac tissue. 7 Furthermore, they show that these effects are mitigated by treatment with either bisoprolol (a ß1 selective beta-blocker) or spirinolactone (an aldosterone antagonist).
The authors use a rat hepatoma model, which promotes acute global cachexia, to evaluate local effects on the heart. The loss of body weight is reflected in individual organs including the heart, characterized by a decrease in cardiac mass and contractility, and is associated with death over 2 weeks. Interestingly, treatment with bisoprolol and spironolactone—but not with the angiotensin-converting enzyme inhibitor imidapril—attenuated body weight reduction, loss of fat and skeletal muscle mass, as well as tumour-induced cardiac atrophy and dysfunction. The authors provide data implicating cardiac necrosis, apoptosis, and autophagy in the pathogenesis of cachectic hearts, with bisoprolol and spironolactone having a preferential effect on markers of apoptosis but not on markers associated with autophagy. Further, the authors present evidence of dysregulation of multiple anabolic and catabolic pathways contributing to cardiac atrophy and dysfunction in the hearts of tumour-bearing rats, with bisoprolol and spironolactone differentially modulating these pathways. Finally, the authors perform correlative studies in humans, using autopsy samples of patients with non-small cell lung cancer and colorectal cancer with or without cachexia. In these studies, there was evidence of reduced left ventricular thickness among patients with cachexia. Interestingly, all cancer patients studied demonstrated evidence of adverse cardiac remodelling, including fibrosis, independent of co-existing cachexia.
The authors are to be lauded for a well-conducted study with detailed cardiac investigations performed in both rat and human models of cancer cachexia. As ever, good studies raise additional interesting questions. First, acute atrophy affects all organs examined, including all skeletal muscle groups, white and brown adipose tissue, and the liver; this observation of such multiorgan atrophy raises the possibility that dysfunction of these other organs and not the tumour itself contributes to cardiac dysfunction. Second, it remains uncertain whether bisoprolol or spironolactone can actually reverse rather than merely attenuate cardiac atrophy in this model, similar to the reversal of cancer-induced skeletal muscle and cardiac atrophy achieved via pharmacological manipulation of the ubiquitin–proteosome pathway in tumour-bearing mice. 8 Third, data on whether these agents had any effect on tumour growth in this rat model, which in turn may attenuate cardiac atrophy, are not presented; beta-blockers have been reported to reduce breast cancer proliferation, 9 , 10 and so direct tumour effects of cardioprotective agents cannot be ruled out. Finally whereas the many signalling pathways studied and their varying responses to bisoprolol and spironolactone treatment are intriguing, further studies are needed to assess the precise signalling pathways that directly cause cardiac atrophy with in vitro and in vivo functional validation.
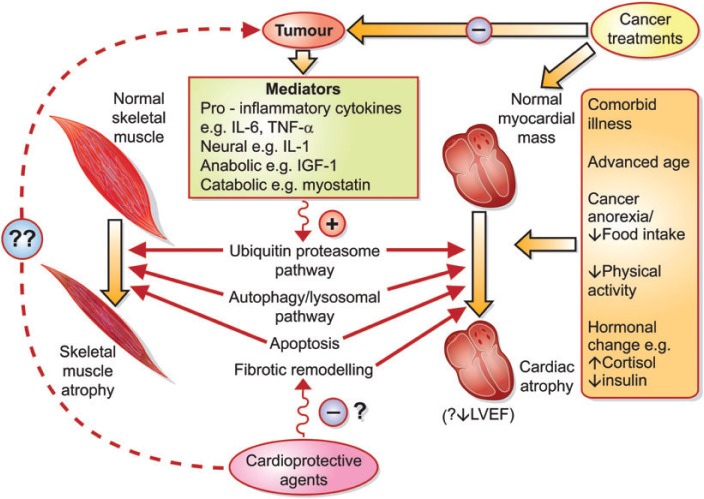
Mechanisms underlying skeletal muscle atrophy associated with cancer cachexia have previously received more attention, and this previous work may offer insights into the mechanisms contributing to cardiac atrophy (see Figure 1 ). Alterations involving a multitude of neural, proinflammatory, anabolic, and catabolic mediators have been implicated in murine models of cancer-induced skeletal muscle atrophy. 5 Increased protein degradation, resulting from activation of catabolic ubiquitin–proteosome pathway or autophagy/lysosomal pathways, interacts with suppressed protein synthesis to produce skeletal muscle atrophy in these models. The extent to which the distinct yet overlapping phenotypic states of cachexia-induced skeletal muscle and cardiac atrophy are due to separate or common pathways requires clarification. Mechanistic overlap with models of cardiac atrophy from non-cancer aetiologies such as prolonged bedrest or mechanical unloading with a ventricular assist device should also be considered. 11 Because the research to date has largely focused on skeletal muscle atrophy, the investigative focus by Springer and colleagues on cardiac atrophy, and its attenuation, is particularly welcome.
Figure 1.
Proposed model illustrating the multifactorial aetiology of cardiac and skeletal muscle atrophy in patients with cancer cachexia. The interplaying pathways leading to cardiac and skeletal muscle atrophy outlined in this figure are non-exhaustive and remain incompletely delineated. IGF-1, insulin-like growth factor-1; IL, interleukin; LVEF, left ventricular ejection fraction; TNF-a, tumour necrosis factor-a.
The translational value of animal models of cancer cachexia requires consideration. The acute onset and rapid progression of cachexia in the rat hepatoma model used by Springer et al . is not necessarily analogous to the chronic wasting observed in humans with cancer cachexia. 5 Furthermore, these models are unable to reflect the clinical complexity of oncology patients, who often present with significant age-related concomitant co-morbidities and prior, as well as concurrent, treatment with cytotoxic therapies. The inability to evaluate fully the role of such complexities in the development of cardiac atrophy and dysfunction in cancer states using animal models limits meaningful extrapolation of pre-clinical findings. Springer et al . present human data limited to cross-sectional blood levels of aldosterone, cortisol, renin, and brain natriuretic peptide among cancer patients compared with controls, as well as post-mortem cardiac examination and histological data, without description of potentially confounding pre-morbid cancer therapies. 7 In the absence of more detailed supportive human data, it is possible that Springer et al . have elegantly described the mechanisms of inevitable cardiac demise that are part of the terminal cancer process, or effects of anticancer therapy, which can be attenuated by certain medications while offering little or no symptomatic gain for patients. Findings from small clinical trials of treatments aimed at attenuating skeletal muscle loss in cancer cachexia have been mixed, and there are currently no treatments specifically approved for cancer cachexia. Therapies directed against proinflammatory cytokines implicated in muscle atrophy have achieved little or no success in the clinical setting; results of treatments with low dose insulin therapy or androgen therapy have been similarly disappointing. 12–15 Accordingly, studies specifically investigating therapies for ameliorating cancer-induced cardiac atrophy and cardiac function in humans are lacking. There also remains the need to determine whether the biochemical disarray reported by Springer et al . exists in cardiac atrophy associated with human cancers. Ultimately, clinical trials are also needed to assess the symptomatic and prognostic impact of attempts to treat cancer-induced cardiac atrophy with medications such as aldosterone antagonists or beta-blockers. Ideally, sufficient mechanistic overlap underlying skeletal muscle and cardiac atrophy exists such that a common effective therapy is plausible.
Although necessary, conducting well-designed clinical trials to identify therapies for the growing population of cancer patients ‘at risk’ for cardiac cachexia will prove challenging. Quantifying the contribution of cardiac atrophy to patients' overall burden of symptoms and clinical signs in advanced cancer is difficult, especially when multiple potential culprits are present. Different pathways dominate pathogenesis at different stages of cachexia-induced skeletal muscle atrophy; for example, the ubiquitin–proteosome pathway has been implicated in later stages of the disease process compared with lysosomal pathways. 16 Thus, the timing of treatments targeting different mechanistic pathways may need to be considered. Furthermore, clinical research involving patients in the terminal stages of cancer is ethically sensitive. Although endpoints such as echocardiographic measures of cardiac function and cardiac biomarker trends will be informative, primary outcomes should focus on symptomatic relief and quality of life measures for this patient cohort. Survival gains will need to be carefully adjusted for quality of life during any additional life years.
Overall, the interesting findings of Springer et al . underscore the expanding role for the subspecialty of cardio-oncology, which has to date focused on prevention or management of cancer treatment-related cardiomyopathy. This recent work by Springer et al . has highlighted the need also to consider cardiac effects of cancer itself and identifies avenues for translational research with potentially wider clinical impact. As a young field, cardio-oncology continues to evolve.
Funding
The Irish Board for Training in Cardiovascular Medicine [a bursary to J.G.], the National Cancer Institute (NCI) [grants to L.W.J.], National Institutes of Health (NIH) [Career Development Award, Watkins Discovery Award Program and Cardiovascular Leadership Council Investigator Award (both by Brigham and Women's Hospital) to J.M.].
Conflict of interest: none declared.
The opinions expressed in this article are not necessarily those of the Editors of the European Heart Journal or of the European Society of Cardiology.
References
- 1.Osler W. The Principles and Practice of Medicine: Cancer of the Stomach. New York: D. Appleton & Co; 1901. [Google Scholar]
- 2.Fearon K, Strasser F, Anker SD, Bosaeus I, Bruera E, Fainsinger RL, Jatoi A, Loprinzi C, MacDonald N, Mantovani G, Davis M, Muscaritoli M, Ottery F, Radbruch L, Ravasco P, Walsh D, Wilcock A, Kaasa S, Baracos VE. Definition and classification of cancer cachexia: an international consensus. Lancet Oncol. 2011;12:489–495. doi: 10.1016/S1470-2045(10)70218-7. [DOI] [PubMed] [Google Scholar]
- 3.Fearon KC. Cancer cachexia: developing multimodal therapy for a multidimensional problem. Eur J Cancer. 2008;44:1124–1132. doi: 10.1016/j.ejca.2008.02.033. [DOI] [PubMed] [Google Scholar]
- 4.Bossola M, Pacelli F, Tortorelli A, Doglietto GB. Cancer cachexia: it's time for more clinical trials. Ann Surg Oncol. 2007;14:276–285. doi: 10.1245/s10434-006-9179-5. [DOI] [PubMed] [Google Scholar]
- 5.Johns N, Stephens NA, Fearon KC. Muscle wasting in cancer. Int J Biochem Cell Biol. 2013;45:2215–2229. doi: 10.1016/j.biocel.2013.05.032. [DOI] [PubMed] [Google Scholar]
- 6.Ky B, Vejpongsa P, Yeh ET, Force T, Moslehi JJ. Emerging paradigms in cardiomyopathies associated with cancer therapies. Circ Res. 2013;113:754–764. doi: 10.1161/CIRCRESAHA.113.300218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Springer J, Tschirner A, Haghikia A, von Haehling S, Lal H, Grzesiak A, Kaschina E, Palus S, Potsch M, von Websky K, Hocher B, Latouche C, Jaisser F, Morawietz L, Coats AJ, Beadle J, Argiles JM, Thum T, Foldes G, Doehner W, Hilfiker-Kleiner D, Force T, Anker SD. Prevention of liver cancer cachexia-induced cardiac wasting and heart failure. Eur Heart J doi: 10.1093/eurheartj/eht302. . Published online ahead of print 29 August 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhou X, Wang JL, Lu J, Song Y, Kwak KS, Jiao Q, Rosenfeld R, Chen Q, Boone T, Simonet WS, Lacey DL, Goldberg AL, Han HQ. Reversal of cancer cachexia and muscle wasting by ActRIIB antagonism leads to prolonged survival. Cell. 2010;142:531–543. doi: 10.1016/j.cell.2010.07.011. [DOI] [PubMed] [Google Scholar]
- 9.Powe DG, Voss MJ, Zanker KS, Habashy HO, Green AR, Ellis IO, Entschladen F. Beta-blocker drug therapy reduces secondary cancer formation in breast cancer and improves cancer specific survival. Oncotarget. 2010;1:628–638. doi: 10.18632/oncotarget.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cakir Y, Plummer HK, 3rd, Tithof PK, Schuller HM. Beta-adrenergic and arachidonic acid-mediated growth regulation of human breast cancer cell lines. Int J Oncol. 2002;21:153–157. [PubMed] [Google Scholar]
- 11.Hill JA, Olson EN. Cardiac plasticity. N Engl J Med. 2008;358:1370–1380. doi: 10.1056/NEJMra072139. [DOI] [PubMed] [Google Scholar]
- 12.Prado CM, Bekaii-Saab T, Doyle LA, Shrestha S, Ghosh S, Baracos VE, Sawyer MB. Skeletal muscle anabolism is a side effect of therapy with the MEK inhibitor: selumetinib in patients with cholangiocarcinoma. Br J Cancer. 2012;106:1583–1586. doi: 10.1038/bjc.2012.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jatoi A, Ritter HL, Dueck A, Nguyen PL, Nikcevich DA, Luyun RF, Mattar BI, Loprinzi CL. A placebo-controlled, double-blind trial of infliximab for cancer-associated weight loss in elderly and/or poor performance non-small cell lung cancer patients (N01C9) Lung Cancer. 2010;68:234–239. doi: 10.1016/j.lungcan.2009.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lundholm K, Korner U, Gunnebo L, Sixt-Ammilon P, Fouladiun M, Daneryd P, Bosaeus I. Insulin treatment in cancer cachexia: effects on survival, metabolism, and physical functioning. Clin Cancer Res. 2007;13:2699–2706. doi: 10.1158/1078-0432.CCR-06-2720. [DOI] [PubMed] [Google Scholar]
- 15.Loprinzi CL, Kugler JW, Sloan JA, Mailliard JA, Krook JE, Wilwerding MB, Rowland KM, Jr, Camoriano JK, Novotny PJ, Christensen BJ. Randomized comparison of megestrol acetate versus dexamethasone versus fluoxymesterone for the treatment of cancer anorexia/cachexia. J Clin Oncol. 1999;17:3299–3306. doi: 10.1200/JCO.1999.17.10.3299. [DOI] [PubMed] [Google Scholar]
- 16.Jagoe RT, Redfern CP, Roberts RG, Gibson GJ, Goodship TH. Skeletal muscle mRNA levels for cathepsin B, but not components of the ubiquitin-proteasome pathway, are increased in patients with lung cancer referred for thoracotomy. Clin Sci (Lond) 2002;102:353–361. [PubMed] [Google Scholar]