Abstract
Aims
The Digitalis Investigation Group (DIG) trial, the only large randomized trial of digoxin in heart failure, reported a neutral effect on mortality and a significant reduction in heart failure hospitalizations. Recent observational studies reported increased mortality with digoxin treatment. We present further analyses of the DIG trial displaying the inability to control bias in observational treatment comparisons despite extensive statistical adjustments.
Methods and results
Forty-four percent of the 6800 patients in the DIG trial had been treated with digoxin before randomization, and half of them were randomly withdrawn from digoxin treatment. We contrast the main randomization-based result of the DIG trial with the observational non-randomized comparison of patients pre-treated or not pre-treated with digoxin. Mortality [hazard ratio (HR) 1.22, 95% confidence interval (CI) 1.12–1.34; P < 0.001] and heart failure hospitalizations (HR 1.47, 95% CI 1.33–1.61; P < 0.001) were significantly higher in patients pre-treated with digoxin even after adjustment for baseline population differences. The higher risks for both outcomes in those who had previously received digoxin persisted even if they received placebo during the trial (HR 1.24, 95% CI 1.10–1.40; P < 0.001). This sharply contradicts the neutral effect on mortality and the significant reduction in heart failure hospitalizations observed in the randomized comparison.
Conclusion
Prescription of digoxin is an indicator of disease severity and worse prognosis, which cannot be fully accounted for by covariate adjustments in the DIG trial where patients were well-characterized. It is unlikely that weaker research approaches (observational studies of administrative data or registries) can provide more reliable estimates of the effects of cardiac glycosides.
Keywords: Cardiac glycosides , Digoxin , Mortality , Heart failure , Prescription bias , Randomization
Introduction
Numerous publications have reported conflicting results regarding the benefit of cardiac glycosides. Observational studies, analyses of registries (e.g. RIKS-HIA,1 SCAF2), and secondary analyses of randomized clinical trials done for other purposes (AFFIRM,3–5 SPORTIF III and V,6 RACE II,7 SOLVD,8 Val-HeFT,9 ROCKET-AF,10 ENGAGE AF-TIMI 48,11 and ARISTOTLE12) have reported increases, decreases, and neutral effects on mortality (sometimes based on analyses of the same data by different investigators3–5). Some of these have been published and discussed in this journal, as well.3,4,13–17 These analyses compare patients treated with those not treated with cardiac glycosides and use statistical models to adjust for differences in characteristics of the respective populations. The Digitalis Investigation Group (DIG) trial,18 the only large randomized clinical trial evaluating the efficacy of digoxin in patients with heart failure, as evidenced by a recent Cochrane Review19 reported a neutral effect on mortality and a significant reduction in heart failure hospitalizations.
A recent review argued that increases in mortality in patients treated with digoxin in observational data are most likely driven by ‘prescription bias’20: clinical deterioration leads the treating physician to prescribe an additional drug and consequently sicker patients are more likely treated than healthier patients. The conclusions of the aforementioned observational studies thus rely on the assumption that adjustment for baseline covariates appropriately addresses these prognostic differences.17
Our analysis of the DIG trial demonstrates that this assumption does not necessarily hold true: we demonstrate the existence of prescription bias after adjustment for baseline covariates in a situation where the ‘true treatment effect’ in the same data is known to be neutral for mortality and beneficial regarding heart failure hospitalizations.
Methods
The primary aim of this analysis is to assess whether adjustment in an observational analysis can lead to the same treatment effect estimate as the randomization-based analysis of the DIG trial.
The design and primary results of the DIG trial (NCT00000476) have been previously published.18,21 Interestingly, 44% of the 6800 study patients had been treated with digoxin prior to study start and were randomized to either continue digoxin or receive a placebo treatment instead (randomized withdrawal). Knowing that digoxin had no effect on overall mortality in the randomized trial,18 we compare the outcome among patients previously treated with digoxin with the outcome among those who were not treated with digoxin before the start of the study. We contrast this observational result with the neutral effect estimated in the randomization-based approach. Obviously, this observational analysis assumes that the randomized treatment added a similar effect to the average outcome in both subgroups. Therefore, we repeat the analysis (i) in patients randomized to digoxin and (ii) in patients randomized to placebo.
In both subpopulations (i) and (ii), pre-treated and not pre-treated patients were identically treated during the trial. Therefore, observed differences between patients previously treated and those newly treated with digoxin or placebo cannot be due to digoxin unless a potential withdrawal or add-on effect is present. These comparisons are ‘observational’ in that they are not based on the randomization in the trial.
We expected that—if the observational comparison was valid—the effect of digoxin should be the same irrespective of whether estimated from the randomized or the observational comparison after adjustment for population differences. The effect of digoxin is estimated with Cox proportional hazards regression in both approaches. In the observational approach (previous digoxin use vs. no previous digoxin use), the respective models are further adjusted for prognostic differences between patients pre-treated with digoxin and those not pre-treated. The following variables are included in the adjusted observational model (a P-value <0.2 in baseline comparisons was used as a criterion for inclusion into the model, see Supplementary Appendix for the respective P-values): age (years), sex, race, systolic blood pressure (mmHg), diastolic blood pressure (mmHg), body mass index (kg/m2), potassium serum level (mmol/L), ischaemic primary cause of congestive heart failure, duration of congestive heart failure (months), ejection fraction (%), chest X-ray (cardiothoracic ratio) ≤0.55, New York Heart Association (NYHA) classification III or IV, current angina, rales, elevated jugular venous pressure, peripheral oedema, dyspnoea at rest, dyspnoea at exertion, limitation of activity, S3, pulmonary congestion, previous myocardial infarction, history of hypertension, angiotensin-converting enzyme inhibitor use, hydralazine use, diuretics use, recommended digoxin dose (mg/day), and prescribed dose of study medication (mg/day). The model also includes the randomized treatment, as it may in principle alter prognosis. All analyses are performed using the SAS 9.3.22 Forest plots are created with R 3.3.123 using the forestplot package.24
Results
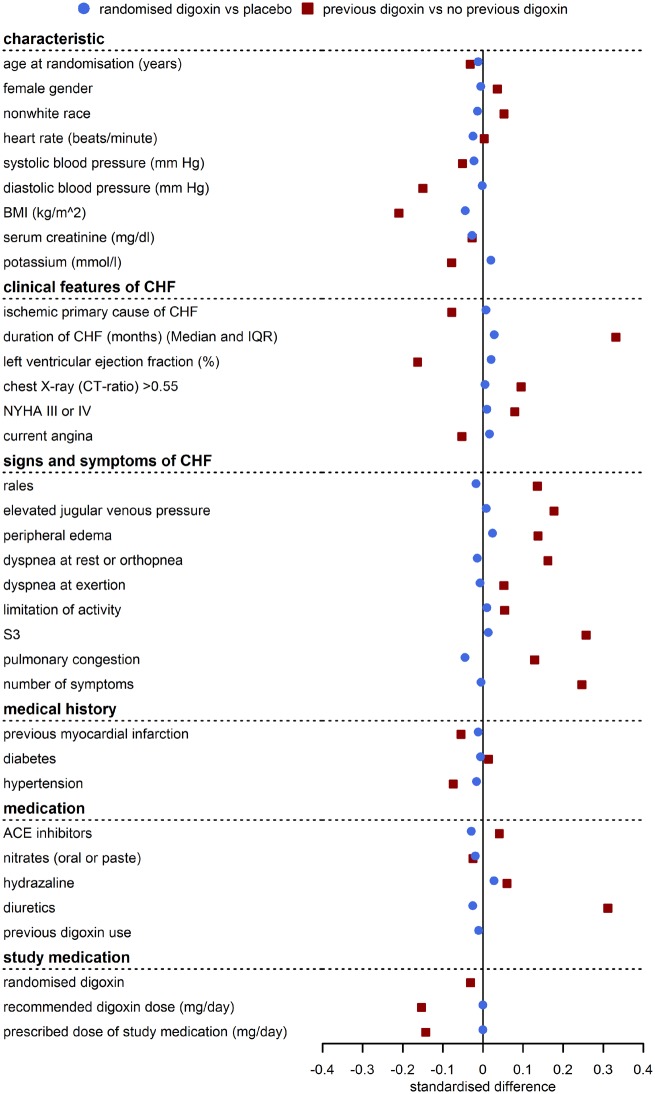
Baseline variables were well balanced between the two randomized treatment groups, but patients previously treated with digoxin systematically differed from patients not previously treated regarding many important baseline variables, especially for heart failure symptoms (e.g. rales, dyspnoea, pulmonary congestion), clinical features of heart failure (e.g. NYHA classification, ejection fraction, duration of heart failure), and use of heart failure medication (diuretics, hydralazine). Figure 1 illustrates the good balance of characteristics between those randomized to receive digoxin vs. placebo. In contrast, patients previously treated with digoxin had more frequently markers of advanced heart failure than those not previously treated with digoxin.
Figure 1.
Standardized baseline differences (difference between groups/pooled standard deviation) in the randomized comparison (blue circle) and the observational comparison (red square). Baseline characteristics are balanced between randomized treatment groups, but patients previously treated with digoxin had more advanced heart failure than previously untreated patients and standardized differences are no longer close to 0. Data from the DIG trial.
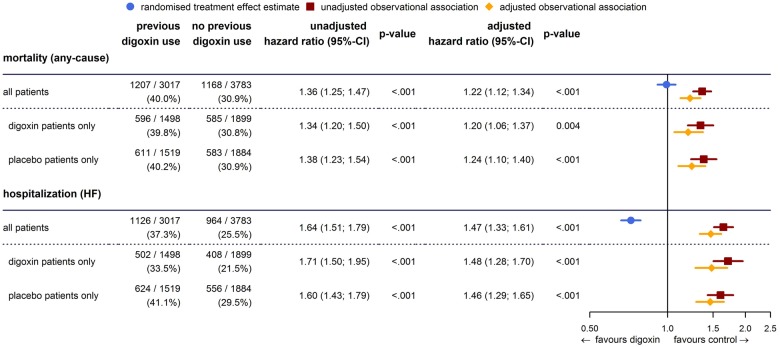
Mortality was significantly higher in patients treated with digoxin before randomization. A total of 1207 (40.0%) and 1168 (30.9%) deaths occurred in patients previously treated and those not previously treated with digoxin, respectively [hazard ratio (HR) 1.36, 95% confidence interval (CI) 1.25–1.47; P < 0.001]. The magnitude of the increase in mortality was similar in patients randomized to placebo and those randomized to digoxin [placebo: 611 (40.2%) and 583 (30.9%) deaths in pre-treated and not pre-treated patients, HR 1.38, 95% CI 1.23–1.54; P < 0.001, digoxin: 596 (39.8%) and 585 (30.8%) deaths in pre-treated and not pre-treated patients, HR 1.34, 95% CI 1.20–1.50; P < 0.001] (Figure 2).
Figure 2.
Mortality and hospitalizations for heart failure are increased in patients previously treated with digoxin, irrespective of whether treated with digoxin or placebo in the DIG trial. The randomized treatment effect estimate is included as reference.
Adjustment for baseline population differences does not explain the observed increased mortality. As we explored a variety of adjustments, differences in mortality between previously treated and not previously treated patients became smaller but persisted. For example, in all treated patients, adjustment for prognostic differences in baseline covariates reduced the HR from 1.36 to 1.22 (95% CI 1.12–1.34, P < 0.001); this reduction was of the same magnitude in the subpopulations of patients randomized to placebo or to digoxin (Figure 2).
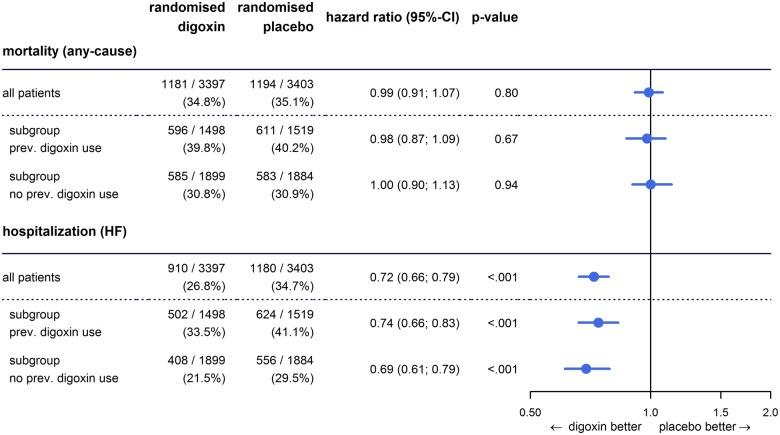
As reported previously,18 digoxin in the randomized DIG trial had no overall effect on all-cause mortality; this neutral effect is consistently observed among those in whom digoxin was withdrawn [HR for digoxin vs. placebo in previously treated patients: 0.98, 95% CI (0.87–1.09); P = 0.67] as well as among those starting de novo therapy [HR for digoxin vs. placebo in previously untreated patients: 1.00, 95% CI (0.90–1.13); P = 0.94] (Figure 3).
Figure 3.
The effect of digoxin on mortality and on hospitalizations for heart failure overall and in subgroups of pre-treated and not pre-treated patients.
Observational results were similar when time to hospitalization for heart failure was analysed: worse prognosis of patients pre-treated with digoxin led to a significant increase in the risk for heart failure hospitalizations that could not be accounted for with adjustment for population differences (adjusted HR 1.47, 95% CI 1.33–1.61; P < 0.001). Again, findings were similar irrespective of whether patients were treated with digoxin or placebo. This observational result is diametrically opposite to the results of the randomized comparison which indicated a significant reduction of hospitalizations for heart failure with digoxin (HR 0.72, 95% CI 0.66–0.79; P < 0.001) (Figure 2).
Discussion
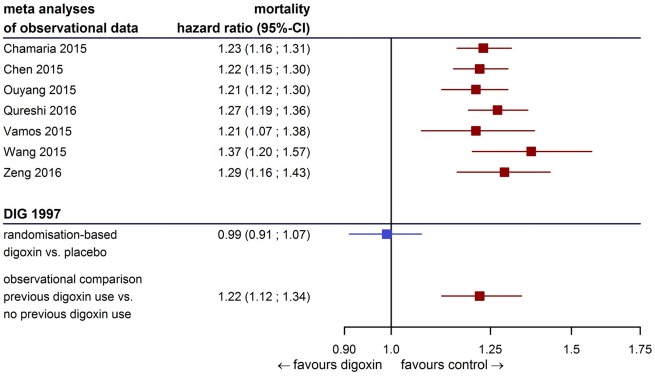
Our analysis provides evidence of prescription bias: We demonstrate that prognostic differences between patients pre-treated and not pre-treated with digoxin were so pronounced that they could not be appropriately addressed with statistical adjustment for baseline covariates. Mortality and risk for heart failure hospitalizations remained increased in those pre-treated with digoxin, even if treated with placebo in the trial. Both findings sharply contrast the results of the randomized comparison which indicated that digoxin had a neutral effect on mortality but substantially decreased heart failure hospitalizations. This implies that important prognostic variables are unmeasured. In fact, the size of prescription bias (the difference in estimate of effects between the randomized and the observational comparisons in heart failure hospitalizations) is much larger than the true effect of treatment. Given that the potential benefits of most treatments are likely to be moderate,25 biases in observational studies may far exceed these. Bias in this analysis is of the same magnitude as pooled estimates of other observational analyses provided in current meta-analyses (Figure 4). The results of this analysis cast doubts on many of the recently presented observational analyses indicating harm from digoxin treatment.
Figure 4.
Results from the DIG trial in the context of current meta-analyses. Prescription bias in the DIG trial is of the same size as pooled estimates from observational data.
In contrast to other analyses, our observational analysis is based on a randomized clinical trial that was specifically designed to assess the effects of digoxin on mortality. It is not plausible to assume that eventually more sophisticated analyses of data recorded for other purposes can produce more reliable conclusions concerning the treatment effect of digoxin. To our best knowledge, in all the aforementioned observational analyses treatment with cardiac glycosides should be interpreted as an indicator of advanced heart failure but increased mortality should not be interpreted as effect of treatment. Along the same lines a recent review argued that bias in observational analyses of treatment is not limited to digoxin in heart failure with reduced ejection fraction, but may occur in other indications, as well.26
The current ESC heart failure guidelines acknowledge the controversy about potential increases in mortality with digoxin treatment based on observational studies. Recommendations regarding digoxin treatment in heart failure are still based on the DIG trial and the respective additional analyses. This reflects that the DIG trial is still considered best evidence for treatment with cardiac glycosides. The current guideline recommendation is to consider digoxin in patients with heart failure and reduced ejection fraction in sinus rhythm if symptoms persist even after combinations of other treatments.27
Our results have wider implications beyond the case of digoxin: We present an example that proves the existence of prescription bias, which cannot be accounted for in the statistical analysis. This again challenges the role of observational studies to formally proof (or disprove) efficacy of medical treatments. In our example, not even the direction of the treatment effect was estimated correctly in the observational comparison (Take home figure). In recent times many publications are purporting to show the effects of a new therapy (usually a drug) in ‘real world’ data analyses, however, caution should be exercised when interpreting such data.
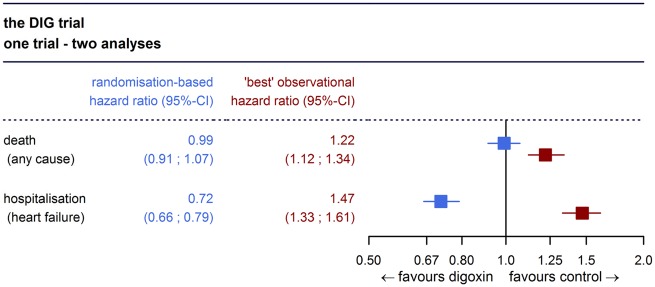
Take home figure.
The ‘best’ observational estimate of the effect of digoxin on mortality and heart failure hospitalization does not match the randomization-based estimate in the DIG trial.
Limitations
The DIG trial was conducted several years ago and both diagnosis and treatment of heart failure have changed. Well planned clinical trials document those variables that are needed to describe the patients expected to benefit from treatment and this may depend on the drug but also on the current understanding of the disease. In more recent data, information on biomarkers such as N-terminal prohormone of brain natriuretic peptide may allow better adjustment and obviously the treatment effect at the time the DIG-trial has been conducted may be different from what would be observed today. Nonetheless, data from the DIG trial is of high quality and ‘residual bias’ is substantial. We doubt that other data will overcome prescription bias.
Conclusion
Our result, derived in the context of digoxin, emphasizes the critical importance of randomized clinical trials for evaluating treatments.
Supplementary Material
Acknowledgements
This manuscript was prepared using DIG Research Materials obtained from the NHLBI. S.Y. was involved in the conduct of the DIG trial. He was supported by the Marion Burke Research Chair of the Heart and Stroke Foundation of Canada.
Funding
This work has been partly funded by the FP7-HEALTH-2013-INNOVATION-1 project: Advances in Small Trials Design for Regulatory Innovation and Excellence (ASTERIX), Grant Agreement No. 603160.
Conflict of interest: L.A.D., K.W., U.B., J.B., and A.K. are involved in the conduct of the DIGIT-HF trial28 (Eudra-CT: 2013-005326-38) funded by the Federal Ministry of Education and Research (BMBF), Germany.
See page 3342 for the editorial comment on this article (doi: 10.1093/eurheartj/ehz527)
References
- 1. Hallberg P, Lindbäck J, Lindahl B, Stenestrand U, Melhus H.. Digoxin and mortality in atrial fibrillation: a prospective cohort study. Eur J Clin Pharmacol 2007;63:959–971. [DOI] [PubMed] [Google Scholar]
- 2. Friberg L, Hammar N, Rosenqvist M.. Digoxin in atrial fibrillation: report from the Stockholm Cohort study of Atrial Fibrillation (SCAF). Heart 2010;96:275–280. [DOI] [PubMed] [Google Scholar]
- 3. Whitbeck MG, Charnigo RJ, Khairy P, Ziada K, Bailey AL, Zegarra MM, Shah J, Morales G, MacAulay T, Sorrell VL, Campbell CL, Gurley J, Anaya P, Nasr H, Bai R, Di BL, Booth DC, Jondeau G, Natale A, Roy D, Smyth S, Moliterno DJ, Elayi CS.. Increased mortality among patients taking digoxin—analysis from the AFFIRM study. Eur Heart J 2013;34:1481–1488. [DOI] [PubMed] [Google Scholar]
- 4. Gheorghiade M, Fonarow GC, van Veldhuisen DJ, Cleland JG, Butler J, Epstein AE, Patel K, Aban IB, Aronow WS, Anker SD, Ahmed A.. Lack of evidence of increased mortality among patients with atrial fibrillation taking digoxin: findings from post hoc propensity-matched analysis of the AFFIRM trial. Eur Heart J 2013;34:1489–1497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Patel NJ, Hoosien M, Deshmukh A, Badheka AO, Grover PM, Shah N, Singh V, Mehta K, Chothani A, Savani GT, Arora S, Bhalara V, Patel N, Khalpada D, Rathod A, Vazzana TJ, Lafferty J, Viles-Gonzalez JF, Mitrani RD.. Digoxin significantly improves all-cause mortality in atrial fibrillation patients with severely reduced left ventricular systolic function. Int J Cardiol 2013;169:e84–e86. [DOI] [PubMed] [Google Scholar]
- 6. Gjesdal K, Feyzi J, Olsson SB.. Digitalis: a dangerous drug in atrial fibrillation? An analysis of the SPORTIF III and V data. Heart 2008;94:191–196. [DOI] [PubMed] [Google Scholar]
- 7. Mulder BA, Van Veldhuisen DJ, Crijns HJ, Tijssen JG, Hillege HL, Alings M, Rienstra M, Van den Berg MP, Van Gelder IC.. Digoxin in patients with permanent atrial fibrillation: data from the RACE II study. Heart Rhythm 2014;11:1543–1550. [DOI] [PubMed] [Google Scholar]
- 8. Domanski M, Fleg J, Bristow M, Knox S.. The effect of gender on outcome in digitalis-treated heart failure patients. J Card Fail 2005;11:83–86. [DOI] [PubMed] [Google Scholar]
- 9. Butler J, Anand IS, Kuskowski MA, Rector T, Carson P, Cohn JN.. Digoxin use and heart failure outcomes: results from the valsartan heart failure trial (Val-HeFT). Congest Heart Fail 2010;16:191–195. [DOI] [PubMed] [Google Scholar]
- 10. Washam JB, Stevens SR, Lokhnygina Y, Halperin JL, Breithardt G, Singer DE, Mahaffey KW, Hankey GJ, Berkowitz SD, Nessel CC, Fox KAA, Califf RM, Piccini JP, Patel MR.. Digoxin use in patients with atrial fibrillation and adverse cardiovascular outcomes: a retrospective analysis of the Rivaroxaban Once Daily Oral Direct Factor Xa Inhibition Compared with Vitamin K Antagonism for Prevention of Stroke and Embolism Trial in Atrial Fibrillation (ROCKET AF). Lancet 2015;385:2363–2370. [DOI] [PubMed] [Google Scholar]
- 11. Eisen A, Ruff CT, Braunwald E, Hamershock RA, Lewis BS, Hassager C, Chao TF, Jy Le H, Mercuri M, Rutman H, Antman EM, Giugliano RP.. Digoxin use and subsequent clinical outcomes in patients with atrial fibrillation with or without heart failure in the ENGAGE AF-TIMI 48 trial. J Am Heart Assoc 2017;6:e006035. doi: 10.1161/JAHA.117.006035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Lopes RD, Rordorf R, De Ferrari GM, Leonardi S, Thomas L, Wojdyla DM, Ridefelt P, Lawrence JH, De Caterina R, Vinereanu D, Hanna M, Flaker G, Al-Khatib SM, Hohnloser SH, Alexander JH, Granger CB, Wallentin L.. Digoxin and mortality in patients with atrial fibrillation. J Am Coll Cardiol 2018;71:1063–1074. [DOI] [PubMed] [Google Scholar]
- 13. Køber L, Torp-Pedersen C, Gadsbøll N, Hildebrandt P, Høilund-Carlsen PF.. Is digoxin an independent risk factor for long-term mortality after acute myocardial infarction? Eur Heart J 1994;15:382–388. [DOI] [PubMed] [Google Scholar]
- 14. Mølstad P, Abdelnoor M.. Digitoxin-associated mortality in acute myocardial infarction. Eur Heart J 1991;12:65–69. [DOI] [PubMed] [Google Scholar]
- 15. Vamos M, Erath JW, Hohnloser SH.. Digoxin-associated mortality: a systematic review and meta-analysis of the literature. Eur Heart J 2015;36:1831–1838. [DOI] [PubMed] [Google Scholar]
- 16. Erath JW, Vamos M, Hohnloser SH.. Effects of digitalis on mortality in a large cohort of implantable cardioverter defibrillator recipients: results of a long-term follow-up study in 1020 patients. Eur Heart J Cardiovasc Pharmacother 2016;2:168–174. [DOI] [PubMed] [Google Scholar]
- 17. Bavendiek U, Aguirre Davila L, Koch A, Bauersachs J. . Assumption versus evidence: the case of digoxin in atrial fibrillation and heart failure. Eur Heart J 2017;38:2095–2099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.The Digitalis Investigation Group. The effect of digoxin on mortality and morbidity in patients with heart failure. N Engl J Med 1997;336:525–533. [DOI] [PubMed] [Google Scholar]
- 19. Hood JW, Dans AL, Guyatt GH, Jaeschke R, McMurray JJ.. Digitalis for treatment of heart failure in patients in sinus rhythm. Cochrane Database Syst Rev 2014;4:CD002901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ziff OJ, Lane DA, Samra M, Griffith M, Kirchhof P, Lip GYH, Steeds RP, Townend J, Kotecha D.. Safety and efficacy of digoxin: systematic review and meta-analysis of observational and controlled trial data. BMJ 2015;351:h4451.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.The Digitalis Investigation Group. Rationale, design, implementation, and baseline characteristics of patients in the DIG trial. Control Clin Trials 1996;17:77–97. [DOI] [PubMed] [Google Scholar]
- 22.SAS Institute Inc. The SAS System for Windows, Version 9.3. Cary, NC: SAS Institute Inc; 2010. [Google Scholar]
- 23.R Core Team. R: A Language and Environment for Statistical Computing. Vienna, Austria: R Foundation for Statistical Computing; 2016. [Google Scholar]
- 24. Gordon M, Lumley T. forestplot: Advanced Forest Plot Using ‘grid’ Graphics. R package version 1.7: 2017. https://CRAN.R-project.org/package=forestplot.
- 25. Yusuf S, Collins R, Peto R.. Why do we need some large, simple randomized trials? Stat Med 1984;3:409–422. [DOI] [PubMed] [Google Scholar]
- 26. Rush CJ, Campbell RT, Jhund PS, Petrie MC, McMurray JJV.. Association is not causation: treatment effects cannot be estimated from observational data in heart failure. Eur Heart J 2018;39:3417–3438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JGF, Coats AJS, Falk V, González-Juanatey JR, Harjola VP, Jankowska EA, Jessup M, Linde C, Nihoyannopoulos P, Parissis JT, Pieske B, Riley JP, Rosano GMC, Ruilope LM, Ruschitzka F, Rutten FH, van der Meer P.. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J 2016;37:2129–2200.27206819 [Google Scholar]
- 28. Bavendiek U, Berliner D, Aguirre Dávila L, Schwab J, Maier L, Philipp SA, Rieth A, Westenfeld R, Piorkowski C, Weber K, Hänselmann A, Oldhafer M, Schallhorn S, von der LH, Schröder C, Veltmann C, Störk S, Böhm M, Koch A, Bauersachs J; on behalf of the DIGIT-HF Investigators. Rationale and design of the DIGIT-HF trial (DIGitoxin to Improve ouTcomes in patients with advanced chronic Heart Failure): a randomized, double-blind, placebo-controlled study. Eur J Heart Fail 2019;21:676–684. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.