Abstract
Objectives: With the increasing incidence of cancer, poor access to affordable anticancer medicines has been a serious public health problem in China. To help address this issue, we assessed the availability, price and affordability of pharmacotherapy for cancer in public hospitals in the Jiangsu Province, China. Methods: In 2012 and 2016, anticancer medicine availability and price information in the capital and five other cities was collected. A total of six cancer care hospitals, 26 tertiary general hospitals and 28 secondary general hospitals were sampled, using an adaptation of the World Health Organization/Health Action International methodology. Data was collected for the anticancer medicines in stock at the time of the surveys. Prices were expressed as inflation-adjusted median unit prices (MUPs). Medicine was affordable if the overall cost of all the prescribed anticancer medicines was less than 20% of the household’s capacity to pay. We used generalized estimating equations to estimate the significance of differences in availability from 2012 to 2016 and the Wilcoxon rank test to estimate the significance of differences in MUPs. Multivariate logistic regression was computed to measure predictors of affordability. Results: From 2012 to 2016 there was a significant decrease in the mean availability of originator brands (OBs) (from 7.79% to 5.71%, p = 0.012) and lowest-priced generics (LPGs) (36.29% to 32.67%, p = 0.009). The mean availability of anticancer medicines in secondary general hospitals was significantly lower than the cancer care, as well as in tertiary general hospitals. The MUPs of OBs (difference: −21.29%, p < 0.01) and their LPGs (−22.63%, p < 0.01) decreased significantly from 2012 to 2016. The OBs (16.67%) of all the anticancer medicines were found to be less affordable than LPGs (34.62% for urban residents and 30.77% for rural residents); their affordability varied among the different income regions. From 2012 to 2016, the proportion of LPGs with low availability and low affordability dropped from 30.77% to 19.23% in urban areas and 34.62% to 26.92% in rural areas, respectively. Generic substitution and medicine covered by basic medical insurance are factors facilitating affordability. Conclusion: There were concerning decreases in the availability of anticancer medicines in 2016 from already low availability in 2012. Anticancer medicines were more affordable for the patients in high-income regions than the patients in low-income regions. Governments should consider using their bargaining power to reduce procurement prices and abolish taxes on anticancer medicines. Policy should focus on the special health insurance plan for low-income patients with cancer. The goal of drug policy should ensure that first-line generic drugs are available for cancer patients and preferentially prescribed.
Keywords: availability, price, affordability, anticancer medicines, China
1. Introduction
Cancer is a major health problem responsible for 15% of overall mortality [1]. With the consolidation of the aging of the population, the number of new cases of cancer worldwide is projected to increase to 21.4 million in 2030 [2]. Globally, the high price of cancer therapy poses a challenge to cancer sufferers and governments alike. Despite much debate about how to reduce the cost of cancer, it continues to increase alarmingly [2]. A study by the Association of Oncology Social Work indicated that the financial burden experienced by cancer patients had negative impacts on their recovery; furthermore, 40% of participates reported that the cost of cancer therapy in the past year swallowed up all their savings [3]. Poor availability and the high cost of cancer treatments are great obstacles to access in many low- and middle-income countries (LMICs), where monthly medicine expenditure often exceeds annual income [4]. Furthermore, these high prices have begun to pose problems in high-income countries. For instance, in the UK, the average cost of cancer treatment had grown 10-fold in the 20 years since 1995 [5].
The mortality and morbidity of cancer have increased in China, which makes cancer the biggest health killer since 2010 [6]. According to the data report through the National Central Cancer Registry (NCCR) of China in 2019, on average, more than ten thousand patients were diagnosed with cancer every day, equating to seven patients being diagnosed with cancer per minute. Consequently, the demand for anticancer medicines will also intensify. Shortages of medicines are occurring at an alarming frequency worldwide [7,8,9,10], which severely affects both adult and pediatric patients with cancer [11,12]. The shortages primarily result from a lack of financial incentives for manufacturing low-cost anticancer medicines, and a weak system for the procurement and distribution of medicines [12,13]. The cost is also a limiting factor for access to anticancer medicines [14]. Statistically, the price of anticancer medicines accounts for approximately a quarter of all cancer costs, which has increased 10-fold in the last decade [15]. For patients with cancer, anticancer medicines are the key to gaining progression-free survival even if there is no overall survival [16].
In 2013, an editorial penned by a large group of experts in chronic myeloid leukemia (CML) drew public attention to the high prices of anticancer medicines [17]. The Union for International Cancer Control (UICC) launched an appeal for improving access to anticancer medicines in lower-resource settings in 2014, which aims to improve inequality in access to anticancer medicines [18]. Sixteen anticancer medicines were added to the 19th revision of the WHO Essential Medicine List in 2015, including three costly medicines [19]. In China, most cancer treatments are not covered by these schemes while the national basic medical insurance coverage is nearly universal [20,21,22]. As in any other country, the affordability of anticancer medicines is a hitherto unknown and grave challenge for the majority of Chinese patients with cancer. A study projected that the financial burden of cancer in China may continue to rise in the coming decades [6]. In 2014, a Chinese merchant named Yong Lu helped several thousand fellow patients buy a generic leukemia drug, Glivec, from India but was investigated for breaching the law. This caused public concern surrounding the accessibility of anticancer medicines to grow [23]. In an attempt to improve drug access, a series of policy initiatives have been undertaken by the Chinese health authority since 2009, with the aim of a material impact on access to anticancer medicines. For instance, the National Health Commission of the People’s Republic of China (NHC) has issued the ‘zero-markup’ drug reform policy with the aim of reducing patients’ medical burden since 2009. As one of the pilot reform provinces, Jiangsu achieved full promotion in public hospitals by the end of 2015. Although the reform attracted extreme concern, little is known about the changes in access to anticancer medicines after the policy was launched.
Price, availability, and affordability are the main criteria to measure whether patients can purchase medicine at an affordable price. A standardized method was developed by the World Health Organization (WHO) and Health Action International (HAI) and has been used in Malaysia, China, Pakistan, Thailand, Sri Lanka, Ethiopia, India, Malawi, Haiti, etc. Most surveys focused on the essential medicines for adults [24,25,26,27,28] and children [29,30,31,32]. Some others focused on medicines for cardiovascular patients [33,34], antidiabetics [35,36], and orphans [37,38]. A few studies have been conducted to evaluate the cost, availability, and affordability of anticancer medicines [39,40,41], but did not cover China. Muhammad et al. found that the availability of both originator brands (OBs) and lowest-priced generics (LPGs) was higher in private sectors (hospitals and pharmacies) than in public hospitals in Pakistan. For both OBs and LPGs, the high-income patients had higher affordability [39]. In another cross-sectional study of anticancer medicines in Pakistan, LPGs had lower availability but higher affordability. Owing to the high price of biologic medicines, oncologists were unwilling to prescribe them and most of the patients were prescribed non-biologics [40]. A survey conducted by the European Society for Medical Oncology (ESMO) demonstrated that the availability of anticancer medicines was low in LMICs, particularly in Eastern Europe. The affordability of cancer therapy with recent market access was the major influencing factor contributing to the inequity of accessibility [41].
In this study, we adopted the WHO and HAI standard methodology to assess the availability, price, and affordability of anticancer medicines for originator brands (OBs) and lowest-priced generics (LPGs) in 60 public hospitals (six tumor hospitals, 26 tertiary general hospitals, and 28 secondary general hospitals) across the capital and five other cities in the Jiangsu Province, China. The OB product had a unique originator pharmaceutical company, and the LPGs were defined as the same product sold under the generic name with the lowest unit price at each hospital at the time of data collection.
We hypothesized that the ‘zero-markup’ drug reform policy would lead to a decrease in the price of anticancer medicine and a gradual increase in the availability of anticancer medicine in health-care institutions. In particular, the imbalance in regional economic development, such as the urban–rural economic disparity was a major factor affecting the accessibility of anticancer medicines. To our knowledge, this is the first study to measure the regional disparity and temporal trends of access to anticancer medicines in China. It was expected that the findings of this study could provide a reference for decision-makers, which could help to improve the accessibility of anticancer medicines in China.
2. Materials and Methods
2.1. Study Design and Sample
The studies were undertaken in the Jiangsu Province first in 2012 and then again in 2016. The Jiangsu Province is located in the southeastern coast of China, with a population of over 80 million (in 2017) residing across 13 cities. The total gross domestic product of the province was 8586.98 billion RMB (~1271.81 billion USD) in 2017, ranking second of all provinces in mainland China [42]. Notably, cancer is currently one of the top health concerns for the residents of the Jiangsu Province [43]. Two cross-sectional studies were conducted to make comparisons between 2012 and 2016.
According to the different income levels, Jiangsu is divided into three regions, high, middle and low. In total, 60 hospitals providing oncology services were sampled using a multi-stage stratified random sampling. In the first stage, two cities were selected from each region. At the city level, 10 public sectors were selected from each sampling city and comprised 5 tertiary hospitals and 5 secondary hospitals. Taken together, the first stage of the program covered 6 cities in the Jiangsu Province, two high-income (Nanjing and Wuxi), two middle-income (Yangzhou and Nantong), and two low-income regions (Yancheng and Huaian). In each city, we first chose a local specialized tumor hospital as the main survey sector. After these specialized hospitals, additional general hospitals with a Department of Oncology (tertiary general hospitals and secondary general hospitals) were randomly selected within a 3 h drive of the main survey sector. In total, 6 tumor hospitals, 26 tertiary general hospitals, and 28 secondary general hospitals were included in both studies (Table 1). In China, most of the anticancer medicines, restricted prescription drugs, could not be dispensed in community pharmacies. Therefore, in this study, the retail pharmacy outlets were not included in the sampling frame.
Table 1.
Main characteristics of the 6 sample cities in both surveys.
| City | Nanjing (Capital) | Wuxi | Yangzhou | Nantong | Yancheng | Huaian |
|---|---|---|---|---|---|---|
| Economic level | High income | High income | Middle income | Middle income | Low income | Low income |
| Geographic position | South | South | Center | Center | North | North |
| No. of hospitals | 1 Tumor | 1 Tumor | 1 Tumor | 1 Tumor | 1 Tumor | 1 Tumor |
| 4 Tertiary general | 5 Tertiary general | 5 Tertiary general | 4 Tertiary general | 4 Tertiary general | 4 Tertiary general | |
| 5 Secondary general | 4 Secondary general | 4 Secondary general | 5 Secondary general | 5 Secondary general | 5 Secondary general |
2.2. Selection of Survey Medicines
According to the requirements of the WHO/HAI methodology, surveyed medicines were selected based on the local patients’ disease burden and needs. In this study, the anticancer medicines were identified based on the local cancer patients’ disease needs, the WHO essential medicine list, the 2012 national essential medicine list (NEML), feedback from several oncologists experts, and literature reviews. Based on the oncologists’ recommendation and local health statistics, a total of 40 medicines were surveyed in both years, selected from the commonly-used medicines for five malignancies (lung cancer, gastric cancer, esophageal cancer, liver cancer, and colorectal cancer) with a high morbidity and fatality rate in the Jiangsu Province. Of the 40 medicines surveyed, 12 were on the WHO essential medicine list and 10 were on the national essential medicine list (NEML). In total, there were 46 anticancer medicines on the WHO essential medicine list and 2012 NEML. The 18 essential medicines sampled covered nearly 40% of the anticancer medicines on the WHO essential medicine list and NEML. For individual medicine, the availability data was collected for two products: OBs and LPGs. Of the 40 medicines surveyed, 15 were for the treatment of lung cancer, 12 were for the treatment of gastric cancer, 8 were for the treatment of esophageal cancer, 7 were for the treatment of liver cancer and 9 were for the treatment of colorectal cancer. Table 2 lists all the surveyed medicines.
Table 2.
List of 40 anticancer medicines surveyed in the Jiangsu Province.
| Name | Dosage Form | Strength | Volume | WHO EML | NEML | Insurance Coverage in Jiangsu Province | Main Indication |
|---|---|---|---|---|---|---|---|
| Bleomycin | Vial | 15 IU | 1 | Yes | No | Class B | Esophageal cancer |
| Calcium folinate | Vial | 0.1 g | 1 | No | Yes | No | Gastric cancer, colorectal cancer |
| Capecitabine | TAB-CAP | 0.5 g | 12 | Yes | No | Class B | Colorectal cancer |
| Carboplatin | Bottle | 0.15 g/15 mL | 1 | Yes | No | Class A | Lung cancer |
| Carmofur | TAB-CAP | 50 mg | 24 | No | No | Class B | Gastric cancer, esophageal cancer, colorectal cancer |
| Cetuximab | Bottle | 100 mg/50 mL | 1 | No | No | No | Liver cancer, colorectal cancer |
| Cisplatin | Vial | 20 mg | 1 | No | Yes | Class A | Lung cancer, liver cancer |
| Cyclophosphamide | TAB-CAP | 50 mg | 24 | Yes | Yes | Class A | Lung cancer |
| Cytarabine | Vial | 100 mg | 1 | Yes | Yes | Class A | Lung cancer |
| Docetaxel | Bottle | 20 mg/0.5 mL | 1 | No | No | Class B | Lung cancer |
| Doxifluridine | TAB-CAP | 0.2 g | 50 | No | No | Class B | Gastric cancer, colorectal cancer |
| Doxorubicin | Bottle | 20 mg/10 mL | 1 | No | No | Class A | Lung cancer, gastric cancer, liver cancer |
| Endostar | Bottle | 15 mg/3 mL | 1 | No | No | No | Lung cancer |
| Epirubicin | Vial | 10 mg | 1 | No | No | Class B | Lung cancer, gastric cancer, liver cancer |
| Erlotinib | TAB-CAP | 150 mg | 7 | No | No | No | Lung cancer, liver cancer |
| Etoposide | Bottle | 100 mg/5 mL | 1 | Yes | Yes | Class A | Lung cancer |
| Fluorouracil | Vial | 0.1 g | 1 | No | No | Class A | Gastric cancer, esophageal cancer, colorectal cancer |
| Gefitinib | TAB-CAP | 0.25 g | 10 | No | No | Class B | Lung cancer |
| Gemcitabine | Vial | 0.2 g | 1 | Yes | No | Class B | Lung cancer |
| Ifosfamide | Vial | 1 g | 1 | Yes | No | Class B | Lung cancer |
| Imatinib | TAB-CAP | 0.1 g | 60 | Yes | No | Class B | Esophageal cancer |
| Irinotecan | Vial | 0.1 g | 1 | No | No | Class B | Lung cancer, esophageal cancer |
| Lentinan | Vial | 1 mg | 1 | No | No | No | Gastric cancer, liver cancer |
| Levomisole | TAB-CAP | 25 mg | 100 | No | No | No | Lung cancer |
| Methotrexate | Vial | 0.1 g | 1 | No | Yes | Class A | Lung cancer, gastric cancer, colorectal cancer |
| Mitomycin | Vial | 2 mg | 1 | No | Yes | Class A | Lung cancer, gastric cancer |
| Nedaplatin | Vial | 10 mg | 1 | No | No | Class B | Lung cancer, colorectal cancer |
| Ondansetron | Bottle | 8 mg/4 mL | 1 | No | No | No | Other adjuvant medicine |
| Oxaliplatin | Vial | 50 mg | 1 | Yes | Yes | Class B | Colorectal cancer |
| Paclitaxel | Bottle | 30 mg/5 mL | 1 | No | Yes | Class A | Lung cancer, colorectal cancer |
| Pemetrexed | Vial | 0.5 g | 1 | No | No | Class B | Lung cancer |
| Pingyangmycin | Vial | 8 mg | 1 | No | No | Class A | Esophageal cancer |
| Semustine | TAB-CAP | 50 mg | 5 | No | Yes | Class A | Lung cancer |
| Sunitinib | TAB-CAP | 12.5 mg | 28 | No | No | No | Liver cancer |
| Tegafur | Bottle | 500 mg/10 ml | 1 | No | No | Class B | Gastric cancer, colorectal cancer |
| Tegafur, Gimeracil and Oteracil Porassium Capsules | TAB-CAP | (Tegafur 20 mg, gimeracil 5.8 mg, Oteracil Porassium 19.6 mg) | 42 | No | No | No | Gastric cancer |
| Topotecan | TAB-CAP | 1 mg | 4 | No | No | No | Lung cancer |
| Trastuzumab | Vial | 0.44 g | 1 | Yes | No | No | Gastric cancer |
| Vindesine | Vial | 1 mg | 1 | No | No | Class B | Lung cancer |
| Vinorelbine | Bottle | 10 mg/mL | 1 | Yes | No | Class B | Lung cancer |
Note: WHO EML—World Health Organization essential medicine list; NEML—National Essential Medicine List; Yes—on the list; No—not on the list; Class A—Class A medicine list of basic medical insurance (BMI) in the Jiangsu Province; Class B—Class B medicine list of BMI.
2.3. Data Collection
A standardized data collection form was designed and made it convenient and efficient for the well-trained research assistants (RAs) to collect information. All the medicines surveyed were noted on a pre-designed sheet. The items were included in the standardized form as follows: facility information (hospital name, hospital level, survey date); medicine information (medicine in stock in the hospital on the day of data collection, yes or no); dosage, strength, medicine type (OB/LPG), retail price. After obtaining permission from the administrative office of the enrolled hospital, twelve research assistants (RAs) visited the hospitals to finish collecting data on the availability and prices of the anticancer medicines for patients. The patient prices were collected for all anticancer medicines found in the hospital on the day of the survey. As a quality control, the RAs checked the completeness and consistency of the search information at the end of each day. The RAs went back on the same day or the next day to fill in missing data.
2.4. Data Analysis
The availability of anticancer medicines was calculated as the proportion of all the survey hospitals where the medicine was found on the day of data collection (WHO/HAI 2008). We compared differences in availability of anticancer medicines surveyed between 2012 and 2016 using generalized estimating equations (GEE), and p < 0.05 was used to indicate a significant difference. Moreover, we compared the mean availability between different groups of medicines (OBs vs. LPGs; included in the WHO EML or not; included on the NEML or not), across different types of hospitals (tumor hospital, tertiary general hospital and secondary general hospital), across different income levels, and across years (2012 vs. 2016). The following criteria were used for describing the availability of anticancer medicines [28,35,37]:
Absent: 0% of hospitals—these anticancer medicines were not available in any hospital surveyed;
Very low: <30% of hospitals—these anticancer medicines were very difficult to find;
Low: 30–49% of hospitals—these anticancer medicines were somewhat difficult to find;
Fairly high: 50-80% of hospitals: these anticancer medicines were available in many hospitals surveyed;
High: >80% of hospitals—these anticancer medicines had acceptable availability.
Price was reported as the median unit price (MUP) in this study. The unit price was accessed by dividing the package price into the package size, which refers to the price per individual tablet, capsule, vial, or milliliter (for bottles) [44]. We also adjusted the 2016 unit prices to 2012 prices by deflating them by 8.5% [45]. According to the WHO/HAI methodology, the MUP of each medicine surveyed was calculated only if the medicine was available in at least four hospitals during the data collection [46]. We compared changes in the MUPs of different medicine groups between 2012 and 2016 and identified whether the MUP decrease or increase for the anticancer medicines between the years was significant using the Wilcoxon rank test.
Cancer treatment lasts for a long period of time and increases health-care costs. The longer the course, the heavier the financial burden the family may suffer. According to the WHO/HAI methodology, generally, if the cost of a course treatment of a medicine is no more than the lowest wage of one day, it is considered affordable. A study published by Rasha Khatib et al. [33] defined it as; “If a treatment cost per month less than 20% of the household capacity-to-pay then it can be considered as affordable.” And the method has been performed in two studies of anticancer medicine affordability in Pakistan [39,40]. In this study, this concept was modified and the affordability was measured for each medicine by low, middle, and high-income class of patients in urban and rural areas through this formula. In this study, on the premise that each of the therapeutic schemes contains a single medicine, the monthly expense of each medicine was denoted in this equation:
The expense of an anticancer medicine per month = Median unit price (MUP) × Defined daily dose (DDD) × 30 days.
The median unit price refers to the median procurement price per unit dose. Defined daily dose (DDD) is a unit of measure that refers to the average maintenance dosage of an anticancer medicine used for its main indication in adults on a daily basis. The DDD information was obtained from the authoritative medicine specification and clinical guidelines. If a treatment cost, per month, less than 20% of the average household monthly income, we regarded it as sufficiently affordability.
We compared the affordability of each medicine to different types of medicines (OBs vs. LPGs), residence (urban area vs. rural area), and both years. The logistic regression model was applied to estimate the independent associations of variables (survey year, medicine type, hospital type, income level, urban vs. rural residence, medicine covered by BMI or not, medicine’s inclusion in the WHO EML, NEML) with the affordability of medicines (affordable or unaffordable). We also performed a comprehensive analysis of the availability and affordability of the anticancer medicines by means of the four-quadrant diagram. The availability value was shown on the X-axis, while the affordability value of the medicines for patients in urban or rural areas was depicted on the Y-axis.
Data were entered using Microsoft Excel and analyzed using SPSS (version 22.0; SPSS, Inc., Chicago, IL, USA) and Matlab (version 2018; MathWorks, Natick, MA, USA).
2.5. Ethics Approval
Ethical approval to conduct this study was obtained from the Nanjing Medical University Ethics Committee (grant number: ethical review 201236).
2.6. Patient and Public Involvement
In this study, the patients and the public were not involved in the study design.
3. Results
3.1. Availability
The availability of the 40 anticancer medicines is presented in Table 3. In terms of individual medicines, 13 LPGs (32.50%) had fairly high (50–80%) or high availability (>80%) in 2012 and 14 (35.00%) in 2016, while the availability of OBs was unsatisfactory (<50%) both years. The highest availability of OBs and LPGs was 46.67% and 85.00% in 2012, respectively, and 38.33% and 83.33% in 2016. On average, the total availability of the anticancer medicines decreased significantly from 2012 to 2016 (5.71% in 2016 vs. 7.79% in 2012 for OBs, p = 0.012; 32.67% in 2016 vs. 36.29% in 2012 for LPGs, p = 0.009). Between 2012 and 2016, non-WHO essential OBs and LPGs showed a significant decrease in mean availability (p = 0.028 and p = 0.014, respectively), as did non-national essential OBs and LPGs (p = 0.013 and p = 0.025, respectively). We found that the mean availability of medicines included on the WHO EML was higher than the non-WHO essential medicines. Likewise, the mean availability of medicines included on the NEML was higher than the non-national essential medicines.
Table 3.
Availability of 40 anticancer medicines in the Jiangsu Province [n (%)].
| Name | OBs | LPGs | |||
|---|---|---|---|---|---|
| 2012 | 2016 | 2012 | 2016 | ||
| Bleomycin | 0 (0.00) | 0 (0.00) | 1 (1.67) | 7 (11.67) | |
| Calcium folinate | 0 (0.00) | 0 (0.00) | 18 (30.00) | 13 (21.67) | |
| Capecitabine | 0 (0.00) | 0 (0.00) | 51 (85.00) | 48 (80.00) | |
| Carboplatin | 16 (26.67) | 10 (16.67) | N/A | N/A | |
| Carmofur | 0 (0.00) | 0 (0.00) | 17 (28.33) | 11 (18.33) | |
| Cetuximab | 4 (6.67) | 1 (1.67) | N/A | N/A | |
| Cisplatin | 0 (0.00) | 0 (0.00) | 0 (0.00) | 11 (18.33) | |
| Cyclophosphamide | 0 (0.00) | 0 (0.00) | 1 (1.67) | 5 (8.33) | |
| Cytarabine | 17 (28.33) | 15 (25.00) | 23 (38.33) | 15 (25.00) | |
| Docetaxel | 22 (36.67) | 14 (23.33) | 50 (83.33) | 50 (83.33) | |
| Doxifluridine | 0 (0.00) | 0 (0.00) | 7 (11.67) | 3 (5.00) | |
| Doxorubicin | 0 (0.00) | 1 (1.67) | 7 (11.67) | 3 (5.00) | |
| Endostar | 0 (0.00) | 0 (0.00) | 15 (25.00) | 11 (18.33) | |
| Epirubicin | 0 (0.00) | 0 (0.00) | 51 (85.00) | 50 (83.33) | |
| Erlotinib | 15 (25.00) | 6 (10.00) | N/A | N/A | |
| Etoposide | 0 (0.00) | 0 (0.00) | 49 (81.67) | 45 (75.00) | |
| Fluorouracil | 0 (0.00) | 0 (0.00) | 23 (38.33) | 13 (21.67) | |
| Gefitinib | 11 (18.33) | 7 (11.67) | N/A | 0 (0.00) | |
| Gemcitabine | 28 (46.67) | 15 (25.00) | 52 (85.00) | 48 (80.00) | |
| Ifosfamide | 2 (3.33) | 2 (3.33) | 27 (45.00) | 24 (40.00) | |
| Imatinib | 7 (11.67) | 11 (18.33) | N/A | 0 (0.00) | |
| Irinotecan | 0 (0.00) | 0 (0.00) | 39 (65.00) | 38 (63.33) | |
| Lentinan | 0 (0.00) | 0 (0.00) | 44 (73.33) | 42 (70.00) | |
| Levomisole | 0 (0.00) | 0 (0.00) | 11 (18.33) | 3 (5.00) | |
| Methotrexate | 0 (0.00) | 0 (0.00) | 37 (61.67) | 36 (60.00) | |
| Mitomycin | 0 (0.00) | 0 (0.00) | 26 (43.33) | 19 (31.67) | |
| Nedaplatin | 0 (0.00) | 0 (0.00) | 46 (76.67) | 38 (63.33) | |
| Ondansetron | 1 (1.67) | 0 (0.00) | 14 (23.33) | 17 (28.33) | |
| Oxaliplatin | 20 (33.33) | 23 (38.33) | 52 (85.00) | 49 (81.67) | |
| Paclitaxel | 16 (26.67) | 12 (20.00) | 46 (76.67) | 42 (70.00) | |
| Pemetrexed | 12 (20.00) | 12 (20.00) | 19 (31.67) | 16 (26.67) | |
| Pingyangmycin | 0 (0.00) | 0 (0.00) | 22 (36.67) | 1 (1.67) | |
| Semustine | 0 (0.00) | 0 (0.00) | 12 (20.00) | 4 (6.67) | |
| Sunitinib | 1 (1.67) | 1 (1.67) | N/A | N/A | |
| Tegafur | 0 (0.00) | 0 (0.00) | 18 (30.00) | 15 (25.00) | |
| Tegafur, Gimeracil and Oteracil Porassium Capsules | 0 (0.00) | 0 (0.00) | 33 (55.00) | 36 (60.00) | |
| Topotecan | 0 (0.00) | 0 (0.00) | 1 (1.67) | 0 (0.00) | |
| Trastuzumab | 10 (16.67) | 4 (6.67) | N/A | N/A | |
| Vindesine | 0 (0.00) | 0 (0.00) | 23 (38.33) | 32 (53.33) | |
| Vinorelbine | 5 (8.33) | 3 (5.00) | 38 (63.33) | 39 (65.00) | |
| Avg | Total | 4.68 (7.79) | 3.43 (5.71) | 21.83 (36.29) | 19.60 (32.67) |
| WHO EM | 8.75 (4.72) | 6.92 (4.03) | 24.50 (43.06) | 23.33 (40.14) | |
| Non-WHO EM | 2.93 (4.62) | 1.93 (3.54) | 20.68 (34.75) | 18.00 (30.39) | |
| NEM | 5.30 (6.25) | 5.00 (5.64) | 26.40 (42.77) | 23.90 (39.95) | |
| Non-NEM | 4.47 (4.63) | 1.91 (3.55) | 20.30 (35.030 | 17.23 (30.72) | |
Note: OBs—originator brands; LPGs—lowest-priced generics; Avg—average; N/A—the medicine did not get approval from the department of drug administration in China. WHO EM, WHO essential medicine; Non-WHO EM—non-WHO essential medicine; NEM—national essential medicine; Non-NEM—non-national essential medicine.
The inter-sector comparison of the mean availability of medicines is shown in Table 4. Across hospital types, the mean availability of anticancer medicines surveyed in tumor and tertiary general hospitals was higher than in secondary general hospitals in both time periods. In tumor hospitals, the mean availability of OBs decreased (10.00% in 2016 vs. 10.42% in 2012) and that of LPGs increased (39.17% in 2016 vs. 35.42% in 2012). Compared with 2012, the availability of LPGs included in the WHO EML in 2016 increased significantly (p = 0.008) in tumor hospitals. In tertiary general hospitals, the mean availability in 2016 decreased compared with that of OBs (10.29% vs. 11.63%) and LPGs (40.29% vs. 42.31%) in 2012. In secondary general hospitals, the mean availability of OBs decreased (2.68% in 2016 vs. 3.66% in 2012) and that of LPGs increased (32.59% in 2016 vs. 31.07% in 2012).
Table 4.
Mean availability of anticancer medicines in the Jiangsu Province: inter-sector comparison.
| Tumor Hospital (%) | Tertiary General Hospital (%) | Secondary General Hospital (%) | |||||
|---|---|---|---|---|---|---|---|
| 2012 | 2016 | 2012 | 2016 | 2012 | 2016 | ||
| OBs | All | 10.42 | 10.00 | 11.63 | 10.29 | 3.66 | 2.68 |
| WHO EM | 18.06 | 19.44 | 23.08 | 19.87 | 5.95 | 6.25 | |
| Non-WHO EM | 7.14 | 5.95 | 6.73 | 6.18 | 2.68 | 1.15 | |
| NEM | 8.33 | 11.67 | 13.85 | 13.46 | 4.29 | 5.36 | |
| Non-NEM | 11.11 | 9.44 | 10.90 | 9.23 | 3.45 | 1.79 | |
| LPGs | All | 35.42 | 39.17 | 42.31 | 40.29 | 31.07 | 32.59 |
| WHO EM | 36.11 | 45.83 | 46.15 | 48.08 | 36.90 | 38.69 | |
| Non-WHO EM | 35.12 | 36.31 | 40.66 | 36.95 | 28.57 | 29.97 | |
| NEM | 38.33 | 43.33 | 49.62 | 49.23 | 40.00 | 39.64 | |
| Non-NEM | 34.44 | 37.78 | 39.87 | 37.31 | 28.10 | 30.24 | |
Note: OBs—originator brands; LPGs—lowest-priced generics; WHO EM—WHO essential medicine; Non-WHO EM—non-WHO essential medicine; NEM—national essential medicine; Non-NEM—non-national essential medicine.
As shown in Table 5, the mean availability of anticancer medicines surveyed in low-income regions obviously lagged behind that in high- and middle-income regions. The value of availability varied by regions. In low-income regions, the mean availability of OBs remained unchanged (10.13%) and LPGs showed modest improvement (36.13% in 2016 vs 34.50% in 2012). In particular, the mean availability of OBs included in the WHO EML in 2016 increased significantly compared with that in 2012 (p = 0.021). In middle-income regions, the mean availability of medicines decreased (7.63% in 2016 vs. 8.88% in 2012 for OBs; 36.63% in 2016 vs. 39.75% in 2012 for LPGs) and significantly changed at OBs (p = 0.041). In low-income regions, there was a significant decrease in the mean availability of OBs (2.38% in 2016 vs. 4.38% in 2012, p = 0.035) and an increase in that of LPGs (37.00% in 2016 vs. 34.88% in 2012), but no significant change in LPGs (p = 0.292).
Table 5.
Mean availability of anticancer medicines in the Jiangsu Province: inter-region comparison.
| High Income (%) | Middle Income (%) | Low Income (%) | |||||
|---|---|---|---|---|---|---|---|
| 2012 | 2016 | 2012 | 2016 | 2012 | 2016 | ||
| OBs | All | 10.13 | 10.13 | 8.88 | 7.63 | 4.38 | 2.38 |
| WHO EM | 20.42 | 21.25 | 15.00 | 13.33 | 8.33 | 5.83 | |
| Non-WHO EM | 5.71 | 5.36 | 6.25 | 5.18 | 2.68 | 0.89 | |
| NEM | 12.50 | 15.00 | 8.00 | 10.00 | 6.00 | 3.50 | |
| Non-NEM | 9.33 | 8.50 | 9.17 | 6.83 | 3.83 | 2.00 | |
| LPGs | All | 34.50 | 36.13 | 39.75 | 36.63 | 34.88 | 37.00 |
| WHO EM | 33.75 | 41.25 | 46.67 | 45.42 | 42.08 | 43.75 | |
| Non-WHO EM | 34.82 | 33.93 | 36.79 | 32.86 | 31.79 | 34.11 | |
| NEM | 41.50 | 45.50 | 46.50 | 42.00 | 44.00 | 45.00 | |
| Non-NEM | 32.17 | 33.00 | 37.50 | 34.83 | 31.83 | 34.33 | |
Note: OBs—originator brands; LPGs—lowest-priced generics; WHO EM—WHO essential medicine; Non-WHO EM—non-WHO essential medicine; NEM—national essential medicine; Non-NEM—non-national essential medicine.
3.2. Anticancer Medicine Prices
Of these 40 anticancer medicines surveyed, only 12 OBs and 26 LPGs were available in four or more hospitals. The adjusted unit prices of each individual medicine in 2016 decreased noticeably compared with those in 2012. The retail prices for OBs were expensive; Trastuzumab ranked the highest (25299.33 RMB in 2012 and 20129.47 RMB in 2016) and Carboplatin had the lowest price (15.39 RMB in 2012 and 11.09 RMB in 2016). For LPGs, Pemetrexed ranked the highest (5935.15 RMB in 2012 and 4722.32 RMB in 2016) and Carboplatin had the lowest retail price (15.39 RMB in 2012 and 11.09 RMB in 2016). A total of 28.95% and 21.05% of the medicines surveyed had a unit retail price (median) above 500 RMB in 2012 and 2016, respectively.
The comparison of the median unit prices in both years and the adjusted changes are shown in Table 6. For OBs, the MUP of medicines in the WHO EML was lower than those not in the WHO EML; for LPGs it was the opposite. The MUP of all medicines on the NEML was lower than that for those not on the NEML. The total MUP of OBs decreased from 549.05 in 2012 to 429.86 in 2016 (p < 0.01). The median range of price reduction was between 20.00% and 27.96%. Except for OBs on the NEML, there were significant reductions in other groups of OBs. The total MUP of the LPGs decreased from 120.79 in 2012 to 91.10 in 2016 (p < 0.01). Furthermore, all groups of LPGs experienced statistically significant declines in the median unit prices between 2012 and 2016, with the median rate of price reductions ranging from 20.43% to 28.40%.
Table 6.
Median unit prices of anticancer medicines and the median rate of price changes in 2012 and 2016.
| 2012 MUPs (n) | 2016 MUPs (n) | Median Rate of Change (%) | |
|---|---|---|---|
| OBs | |||
| All | 549.05 (12) | 429.86 (12) | −21.29 ** |
| WHO EM | 406.97 (7) | 275.30 (7) | −22.14 * |
| Non-WHO EM | 691.43 (5) | 550.13 (5) | −20.00 * |
| NEM | 313.95 (3) | 170.74 (3) | −22.14 |
| Non-NEM | 586.50 (9) | 466.65 (9) | −20.43 ** |
| LPGs | |||
| All | 120.79 (26) | 91.10 (26) | −22.63 ** |
| WHO EM | 148.35 (7) | 92.30 (7) | −28.40 * |
| Non-WHO EM | 117.97 (19) | 89.91 (19) | −21.14 ** |
| NEM | 18.69 (8) | 14.82 (8) | −20.43 * |
| Non-NEM | 173.31 (18) | 114.57 (18) | −24.29 ** |
Note: OBs—originator brands; LPGs—lowest-priced generics; WHO EM—WHO essential medicine; Non-WHO EM—non-WHO essential medicine; NEM—national essential medicine; Non-NEM—non-national essential medicine; MUP—median unit price (RMB); n—number of sectors surveyed; * Wilcoxon test p < 0.05, ** p < 0.01.
3.3. Affordability of Anticancer Medicines for Standard Treatment Regimens
In total, for 16 OBs and 26 LPGs, the affordability of standard treatment was calculated and found in more than four facilities. Table 7 presents the affordability of the anticancer medicines analyzed. The OBs (16.67% for all residents) of the anticancer medicines were found to be less affordable than LPGs (34.62% for urban residents and 30.77% for rural residents) in both years. In comparison, the least affordable standard treatment was the OB treatment of Pemetrexed for rural residents with low incomes (787.68% in 2012 and 662.36% in 2016).
Table 7.
Affordability of anticancer medicines in the urban and rural areas in different regions of the Jiangsu Province.
| Drug Name | Type | Total (%) | High Income (%) | Middle Income (%) | Low Income (%) | ||||
|---|---|---|---|---|---|---|---|---|---|
| 2012 | 2016 | 2012 | 2016 | 2012 | 2016 | 2012 | 2016 | ||
| Urban area | |||||||||
| Calcium folinate | LPGs | 5.71 | 3.45 | 4.69 | 2.74 | 6.09 | 3.49 | 7.77 | 4.51 |
| Capecitabine | LPGs | 6.80 | 3.92 | 5.59 | 3.11 | 7.26 | 3.96 | 9.26 | 5.12 |
| Carboplatin | OBs | 1.53 | 0.84 | 1.25 | 0.66 | 1.63 | 0.85 | 2.08 | 1.09 |
| Carmofur | LPGs | 14.69 | 8.46 | 12.08 | 6.72 | 15.67 | 8.55 | 19.99 | 11.06 |
| Cytarabine | OBs | 9.24 | 5.59 | 7.60 | 4.44 | 9.85 | 5.65 | 12.57 | 7.31 |
| LPGs | 2.08 | 1.44 | 1.71 | 1.14 | 2.22 | 1.45 | 2.83 | 1.88 | |
| Docetaxel | OBs | 271.49 | 131.75 | 223.27 | 104.56 | 289.64 | 133.13 | 369.55 | 172.19 |
| LPGs | 85.23 | 39.09 | 70.10 | 31.02 | 90.93 | 39.50 | 116.02 | 51.09 | |
| Doxorubicin | LPGs | 446.18 | 270.09 | 366.94 | 214.36 | 476.00 | 272.92 | 607.34 | 352.98 |
| Endostar | LPGs | 255.50 | 154.66 | 210.12 | 122.75 | 272.57 | 156.28 | 347.78 | 202.13 |
| Epirubicin | LPGs | 30.72 | 18.36 | 25.27 | 14.57 | 32.78 | 18.55 | 41.82 | 23.99 |
| Erlotinib | OBs | 278.16 | 168.38 | 228.76 | 133.64 | 296.75 | 170.14 | 378.63 | 220.06 |
| Etoposide | LPGs | 0.83 | 0.50 | 0.68 | 0.40 | 0.89 | 0.51 | 1.13 | 0.66 |
| Fluorouracil | LPGs | 20.73 | 11.54 | 17.05 | 9.16 | 22.11 | 11.66 | 28.22 | 15.09 |
| Gefitinib | OBs | 235.95 | 142.83 | 194.04 | 113.36 | 251.72 | 144.32 | 321.17 | 186.66 |
| Gemcitabine | OBs | 190.23 | 111.19 | 156.44 | 88.25 | 202.94 | 112.35 | 258.93 | 145.32 |
| LPGs | 110.73 | 60.32 | 91.06 | 47.87 | 118.13 | 60.95 | 150.72 | 78.83 | |
| Ifosfamide | LPGs | 33.62 | 15.91 | 27.65 | 12.63 | 35.87 | 16.08 | 45.76 | 20.80 |
| Imatinib | OBs | 368.27 | 222.93 | 302.86 | 176.92 | 392.88 | 225.26 | 501.28 | 291.34 |
| Irinotecan | LPGs | 89.83 | 54.38 | 73.87 | 43.15 | 95.83 | 54.94 | 122.27 | 71.06 |
| Lentinan | LPGs | 20.67 | 11.10 | 17.00 | 8.81 | 22.05 | 11.22 | 28.14 | 14.51 |
| Methotrexate | LPGs | 0.23 | 0.14 | 0.19 | 0.11 | 0.25 | 0.14 | 0.31 | 0.18 |
| Mitomycin | LPGs | 0.58 | 0.35 | 0.47 | 0.28 | 0.61 | 0.35 | 0.78 | 0.46 |
| Nedaplatin | LPGs | 42.40 | 19.76 | 34.87 | 15.68 | 45.23 | 19.96 | 57.71 | 25.82 |
| Ondansetron | LPGs | 44.90 | 27.18 | 36.92 | 21.57 | 47.90 | 27.46 | 61.11 | 35.52 |
| Oxaliplatin | OBs | 263.59 | 156.14 | 216.78 | 123.92 | 281.21 | 157.78 | 358.80 | 204.06 |
| LPGs | 40.63 | 17.68 | 33.41 | 14.03 | 43.34 | 17.87 | 55.30 | 23.11 | |
| Paclitaxel | OBs | 167.44 | 69.28 | 137.70 | 54.98 | 178.63 | 70.01 | 227.92 | 90.54 |
| LPGs | 32.65 | 16.27 | 26.85 | 12.91 | 34.83 | 16.44 | 44.44 | 21.27 | |
| Pemetrexed | OBs | 453.04 | 274.24 | 372.58 | 217.65 | 483.32 | 277.11 | 616.67 | 358.41 |
| LPGs | 196.70 | 119.07 | 161.77 | 94.50 | 209.85 | 120.32 | 267.75 | 155.61 | |
| Semustine | LPGs | 3.12 | 1.87 | 2.57 | 1.49 | 3.33 | 1.89 | 4.25 | 2.45 |
| Tegafur | LPGs | 47.90 | 22.40 | 39.39 | 17.78 | 51.10 | 22.64 | 65.20 | 29.28 |
| Tegafur, Gimeracil and Oteracil Porassium Capsules | LPGs | 1.28 | 0.77 | 1.05 | 0.61 | 1.36 | 0.78 | 1.74 | 1.01 |
| Trastuzumab | OBs | 67.60 | 40.92 | 55.59 | 32.48 | 72.12 | 41.35 | 92.02 | 53.48 |
| Vindesine | LPGs | 35.09 | 20.35 | 28.86 | 16.15 | 37.44 | 20.56 | 47.76 | 26.59 |
| Vinorelbine | OBs | 64.14 | 33.01 | 52.75 | 26.20 | 68.43 | 33.36 | 87.31 | 43.14 |
| LPGs | 39.72 | 20.99 | 32.66 | 16.66 | 42.37 | 21.21 | 54.06 | 27.43 | |
| Rural area | |||||||||
| Calcium folinate | LPGs | 10.04 | 7.85 | 7.81 | 5.02 | 9.54 | 7.37 | 10.07 | 8.47 |
| Capecitabine | LPGs | 11.98 | 8.91 | 9.31 | 5.69 | 11.38 | 8.36 | 12.01 | 9.61 |
| Carboplatin | OBs | 2.69 | 1.90 | 2.09 | 1.22 | 2.55 | 1.78 | 2.69 | 2.05 |
| Carmofur | LPGs | 25.86 | 19.23 | 20.11 | 12.30 | 24.56 | 18.06 | 25.93 | 20.75 |
| Cytarabine | OBs | 16.26 | 12.71 | 12.64 | 8.13 | 15.45 | 11.93 | 16.31 | 13.71 |
| LPGs | 3.66 | 3.27 | 2.84 | 2.09 | 3.48 | 3.07 | 3.67 | 3.53 | |
| Docetaxel | OBs | 477.95 | 299.46 | 371.66 | 191.50 | 454.04 | 281.19 | 479.28 | 323.09 |
| LPGs | 150.05 | 88.85 | 116.69 | 56.82 | 142.55 | 83.42 | 150.47 | 95.86 | |
| Doxorubicin | LPGs | 785.50 | 613.90 | 610.82 | 392.58 | 746.20 | 576.44 | 787.68 | 662.36 |
| Endostar | LPGs | 449.80 | 351.54 | 349.77 | 224.80 | 427.29 | 330.08 | 451.05 | 379.28 |
| Epirubicin | LPGs | 54.09 | 41.73 | 42.06 | 26.68 | 51.38 | 39.18 | 54.24 | 45.02 |
| Erlotinib | OBs | 489.70 | 382.72 | 380.80 | 244.74 | 465.20 | 359.37 | 491.06 | 412.93 |
| Etoposide | LPGs | 1.46 | 1.14 | 1.14 | 0.73 | 1.39 | 1.07 | 1.47 | 1.23 |
| Fluorouracil | LPGs | 36.49 | 26.24 | 28.38 | 16.78 | 34.67 | 24.64 | 36.59 | 28.31 |
| Gefitinib | OBs | 415.38 | 324.64 | 323.01 | 207.60 | 394.60 | 304.83 | 416.54 | 350.26 |
| Gemcitabine | OBs | 334.89 | 252.73 | 260.42 | 161.62 | 318.13 | 237.31 | 335.82 | 272.68 |
| LPGs | 194.94 | 137.11 | 151.59 | 87.68 | 185.19 | 128.74 | 195.48 | 147.93 | |
| Ifosfamide | LPGs | 59.19 | 36.17 | 46.03 | 23.13 | 56.23 | 33.96 | 59.35 | 39.03 |
| Imatinib | OBs | 648.33 | 506.70 | 504.15 | 324.02 | 615.89 | 475.77 | 650.13 | 546.68 |
| Irinotecan | LPGs | 158.14 | 123.59 | 122.97 | 79.03 | 150.23 | 116.05 | 158.58 | 133.35 |
| Lentinan | LPGs | 36.39 | 25.23 | 28.30 | 16.14 | 34.57 | 23.69 | 36.49 | 27.23 |
| Methotrexate | LPGs | 0.41 | 0.32 | 0.32 | 0.20 | 0.38 | 0.30 | 0.41 | 0.34 |
| Mitomycin | LPGs | 1.01 | 0.79 | 0.79 | 0.51 | 0.96 | 0.74 | 1.02 | 0.85 |
| Nedaplatin | LPGs | 74.64 | 44.90 | 58.04 | 28.71 | 70.91 | 42.16 | 74.85 | 48.45 |
| Ondansetron | LPGs | 79.04 | 61.77 | 61.46 | 39.50 | 75.08 | 58.00 | 79.26 | 66.65 |
| Oxaliplatin | OBs | 464.05 | 354.90 | 360.85 | 226.95 | 440.83 | 333.24 | 465.34 | 382.91 |
| LPGs | 71.53 | 40.19 | 55.62 | 25.70 | 67.95 | 37.74 | 71.73 | 43.36 | |
| Paclitaxel | OBs | 294.78 | 157.47 | 229.23 | 100.70 | 280.03 | 147.86 | 295.60 | 169.90 |
| LPGs | 57.48 | 36.98 | 44.70 | 23.65 | 54.61 | 34.73 | 57.64 | 39.90 | |
| Pemetrexed | OBs | 797.57 | 623.34 | 620.21 | 398.61 | 757.66 | 585.30 | 799.79 | 672.53 |
| LPGs | 346.29 | 270.64 | 269.28 | 173.07 | 328.97 | 254.13 | 347.25 | 292.00 | |
| Semustine | LPGs | 5.49 | 4.26 | 4.27 | 2.72 | 5.22 | 4.00 | 5.51 | 4.59 |
| Tegafur | LPGs | 84.33 | 50.92 | 65.58 | 32.57 | 80.11 | 47.82 | 84.57 | 54.94 |
| Tegafur, Gimeracil and Oteracil Porassium Capsules | LPGs | 2.25 | 1.76 | 1.75 | 1.12 | 2.14 | 1.65 | 2.26 | 1.90 |
| Trastuzumab | OBs | 119.01 | 93.01 | 92.54 | 59.48 | 113.05 | 87.33 | 119.34 | 100.35 |
| Vindesine | LPGs | 61.78 | 46.25 | 48.04 | 29.57 | 58.69 | 43.43 | 61.95 | 49.90 |
| Vinorelbine | OBs | 112.93 | 75.03 | 87.81 | 47.98 | 107.28 | 70.45 | 113.24 | 80.96 |
| LPGs | 69.92 | 47.71 | 54.37 | 30.51 | 66.42 | 44.80 | 70.12 | 51.47 | |
The universal health insurance system was established in China in 2008 mainly as a basic medical insurance scheme for urban employees, urban residents and rural residents. Currently, both schemes cover the same anticancer medicines divided into two categories and referred to as ‘Class A’ and ‘Class B’. Class A medicines require 0% out-of-pocket payment by patients. Class B medicines require urban employees to pay 25% and general residents to pay 30% of medicinal expenditure. The affordability of each medicine with different levels of insurance reimbursement is provided in Table 8.
Table 8.
Affordability of anticancer medicines in the urban and rural area in different regions of the Jiangsu Province after reimbursement.
| Drug Name | Type | Total (%) | High Income (%) | Middle Income (%) | Low Income (%) | ||||
|---|---|---|---|---|---|---|---|---|---|
| 2012 | 2016 | 2012 | 2016 | 2012 | 2016 | 2012 | 2016 | ||
| Urban area | |||||||||
| Calcium folinate | LPGs | 5.71 | 3.45 | 4.69 | 2.74 | 6.09 | 3.49 | 7.77 | 4.51 |
| Capecitabine | LPGs | 1.70 | 0.98 | 1.40 | 0.78 | 1.81 | 0.99 | 2.32 | 1.28 |
| Carboplatin | OBs | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| Carmofur | LPGs | 3.67 | 2.12 | 3.02 | 1.68 | 3.92 | 2.14 | 5.00 | 2.76 |
| Cytarabine | OBs | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| LPGs | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | |
| Docetaxel | OBs | 67.87 | 32.94 | 55.82 | 26.14 | 72.41 | 33.28 | 92.39 | 43.05 |
| LPGs | 21.31 | 9.77 | 17.52 | 7.76 | 22.73 | 9.87 | 29.00 | 12.77 | |
| Doxorubicin | LPGs | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| Endostar | LPGs | 255.50 | 154.66 | 210.12 | 122.75 | 272.57 | 156.28 | 347.78 | 202.13 |
| Epirubicin | LPGs | 7.68 | 4.59 | 6.32 | 3.64 | 8.19 | 4.64 | 10.45 | 6.00 |
| Erlotinib | OBs | 278.16 | 168.38 | 228.76 | 133.64 | 296.75 | 170.14 | 378.63 | 220.06 |
| Etoposide | LPGs | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| Fluorouracil | LPGs | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| Gefitinib | OBs | 58.99 | 35.71 | 48.51 | 28.34 | 62.93 | 36.08 | 80.29 | 46.67 |
| Gemcitabine | OBs | 47.56 | 27.80 | 39.11 | 22.06 | 50.74 | 28.09 | 64.73 | 36.33 |
| LPGs | 27.68 | 15.08 | 22.77 | 11.97 | 29.53 | 15.24 | 37.68 | 19.71 | |
| Ifosfamide | LPGs | 8.41 | 3.98 | 6.91 | 3.16 | 8.97 | 4.02 | 11.44 | 5.20 |
| Imatinib | OBs | 92.07 | 55.73 | 75.71 | 44.23 | 98.22 | 56.31 | 125.32 | 72.84 |
| Irinotecan | LPGs | 22.46 | 13.59 | 18.47 | 10.79 | 23.96 | 13.74 | 30.57 | 17.77 |
| Lentinan | LPGs | 20.67 | 11.10 | 17.00 | 8.81 | 22.05 | 11.22 | 28.14 | 14.51 |
| Methotrexate | LPGs | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| Mitomycin | LPGs | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| Nedaplatin | LPGs | 10.60 | 4.94 | 8.72 | 3.92 | 11.31 | 4.99 | 14.43 | 6.45 |
| Ondansetron | LPGs | 44.90 | 27.18 | 36.92 | 21.57 | 47.90 | 27.46 | 61.11 | 35.52 |
| Oxaliplatin | OBs | 65.90 | 39.04 | 54.19 | 30.98 | 70.30 | 39.44 | 89.70 | 51.02 |
| LPGs | 10.16 | 4.42 | 8.35 | 3.51 | 10.84 | 4.47 | 13.83 | 5.78 | |
| Paclitaxel | OBs | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| LPGs | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | |
| Pemetrexed | OBs | 113.26 | 68.56 | 93.14 | 54.41 | 120.83 | 69.28 | 154.17 | 89.60 |
| LPGs | 49.18 | 29.77 | 40.44 | 23.63 | 52.46 | 30.08 | 66.94 | 38.90 | |
| Semustine | LPGs | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| Tegafur | LPGs | 11.98 | 5.60 | 9.85 | 4.45 | 12.78 | 5.66 | 16.30 | 7.32 |
| Tegafur, Gimeracil and Oteracil Porassium Capsules | LPGs | 1.28 | 0.77 | 1.05 | 0.61 | 1.36 | 0.78 | 1.74 | 1.01 |
| Trastuzumab | OBs | 67.60 | 40.92 | 55.59 | 32.48 | 72.12 | 41.35 | 92.02 | 53.48 |
| Vindesine | LPGs | 8.77 | 5.09 | 7.21 | 4.04 | 9.36 | 5.14 | 11.94 | 6.65 |
| Vinorelbine | OBs | 16.04 | 8.25 | 13.19 | 6.55 | 17.11 | 8.34 | 21.83 | 10.79 |
| LPGs | 9.93 | 5.25 | 8.17 | 4.16 | 10.59 | 5.30 | 13.52 | 6.86 | |
| Rural area | |||||||||
| Calcium folinate | LPGs | 10.04 | 7.85 | 7.81 | 5.02 | 9.54 | 7.37 | 10.07 | 8.47 |
| Capecitabine | LPGs | 3.59 | 2.67 | 2.79 | 1.71 | 3.41 | 2.51 | 3.60 | 2.88 |
| Carboplatin | OBs | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| Carmofur | LPGs | 7.76 | 5.77 | 6.03 | 3.69 | 7.37 | 5.42 | 7.78 | 6.23 |
| Cytarabine | OBs | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| LPGs | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | |
| Docetaxel | OBs | 143.38 | 89.84 | 111.50 | 57.45 | 136.21 | 84.36 | 143.78 | 96.93 |
| LPGs | 45.02 | 26.65 | 35.01 | 17.04 | 42.76 | 25.03 | 45.14 | 28.76 | |
| Doxorubicin | LPGs | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| Endostar | LPGs | 449.80 | 351.54 | 349.77 | 224.80 | 427.29 | 330.08 | 451.05 | 379.28 |
| Epirubicin | LPGs | 16.23 | 12.52 | 12.62 | 8.00 | 15.41 | 11.75 | 16.27 | 13.51 |
| Erlotinib | OBs | 489.70 | 382.72 | 380.80 | 244.74 | 465.20 | 359.37 | 491.06 | 412.93 |
| Etoposide | LPGs | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| Fluorouracil | LPGs | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| Gefitinib | OBs | 124.62 | 97.39 | 96.90 | 62.28 | 118.38 | 91.45 | 124.96 | 105.08 |
| Gemcitabine | OBs | 100.47 | 75.82 | 78.13 | 48.48 | 95.44 | 71.19 | 100.75 | 81.80 |
| LPGs | 58.48 | 41.13 | 45.48 | 26.30 | 55.56 | 38.62 | 58.64 | 44.38 | |
| Ifosfamide | LPGs | 17.76 | 10.85 | 13.81 | 6.94 | 16.87 | 10.19 | 17.81 | 11.71 |
| Imatinib | OBs | 194.50 | 152.01 | 151.25 | 97.21 | 184.77 | 142.73 | 195.04 | 164.01 |
| Irinotecan | LPGs | 47.44 | 37.08 | 36.89 | 23.71 | 45.07 | 34.81 | 47.57 | 40.00 |
| Lentinan | LPGs | 36.39 | 25.23 | 28.30 | 16.14 | 34.57 | 23.69 | 36.49 | 27.23 |
| Methotrexate | LPGs | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| Mitomycin | LPGs | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| Nedaplatin | LPGs | 22.39 | 13.47 | 17.41 | 8.61 | 21.27 | 12.65 | 22.45 | 14.53 |
| Ondansetron | LPGs | 79.04 | 61.77 | 61.46 | 39.50 | 75.08 | 58.00 | 79.26 | 66.65 |
| Oxaliplatin | OBs | 139.21 | 106.47 | 108.26 | 68.09 | 132.25 | 99.97 | 139.60 | 114.87 |
| LPGs | 21.46 | 12.06 | 16.69 | 7.71 | 20.38 | 11.32 | 21.52 | 13.01 | |
| Paclitaxel | OBs | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| LPGs | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | |
| Pemetrexed | OBs | 239.27 | 187.00 | 186.06 | 119.58 | 227.30 | 175.59 | 239.94 | 201.76 |
| LPGs | 103.89 | 81.19 | 80.78 | 51.92 | 98.69 | 76.24 | 104.18 | 87.60 | |
| Semustine | LPGs | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| Tegafur | LPGs | 25.30 | 15.28 | 19.67 | 9.77 | 24.03 | 14.34 | 25.37 | 16.48 |
| Tegafur, Gimeracil and Oteracil Porassium Capsules | LPGs | 2.25 | 1.76 | 1.75 | 1.12 | 2.14 | 1.65 | 2.26 | 1.90 |
| Trastuzumab | OBs | 119.01 | 93.01 | 92.54 | 59.48 | 113.05 | 87.33 | 119.34 | 100.35 |
| Vindesine | LPGs | 18.53 | 13.87 | 14.41 | 8.87 | 17.61 | 13.03 | 18.58 | 14.97 |
| Vinorelbine | OBs | 33.88 | 22.51 | 26.34 | 14.39 | 32.18 | 21.14 | 33.97 | 24.29 |
| LPGs | 20.98 | 14.31 | 16.31 | 9.15 | 19.93 | 13.44 | 21.03 | 15.44 | |
After health insurance reimbursement, the affordability improved significantly. In both years, the proportion of affordable OBs increased to 33.33% and 25% for urban and rural residents respectively. Furthermore, in 2012, the proportion of affordable LPGs increased by 38.46% and 57.69% for urban and rural residents respectively. In 2016, the proportion of affordable LPGs increased by 23.07% and 42.31% for urban and rural residents respectively.
Notably, the affordability of each medicine in 2016 showed improvement for both urban and rural residents with each income level in the Jiangsu Province, compared with that in 2012. Anticancer medicines were more affordable for the patients in the high-income region than the patients in the low-income region. For rural residents, the affordability of each medicine for a one month treatment was worse than for urban residents. Compared with 2012, the difference in affordability in 2016 between the rural and urban residents increased. Most medicines required more than 20% of a household monthly income for a one month treatment. The unaffordability of medicines for urban residents in 2012 varied from 22.05% to 616.67%, and from 20.11% to 799.79% for rural residents; the lack of affordability of medicines for urban residents in 2016 varied from 20.56% to 358.41%, and from 20.75% to 672.53% for rural residents. On the whole, the lack of affordability was unreasonable.
3.4. Comprehensive Analysis of Medicine Availability and Affordability
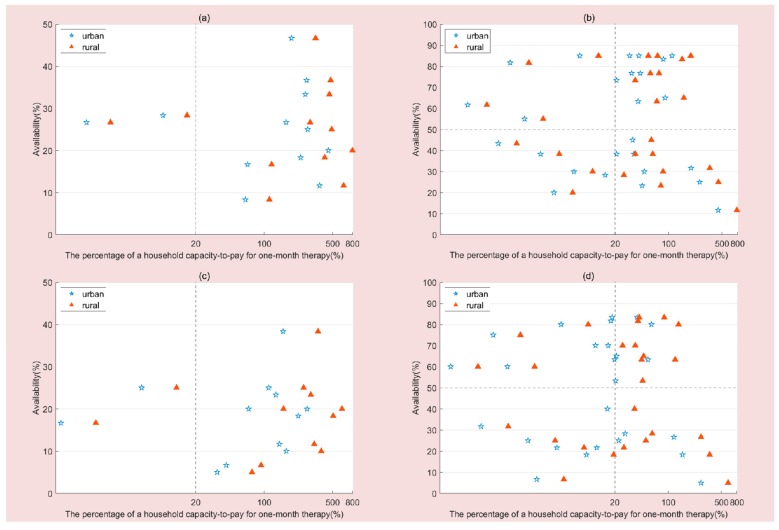
Comprehensive analysis of medicine availability and affordability for urban and rural residents is displayed in Figure 1. Figure 1a can be roughly divided into 2 quadrants. The availability of all the OBs was less than 50%. Quadrant I includes 2 (16.67%) medicines with low availability and high affordability, while quadrant II includes 10 (83.33%) medicines with low availability and low affordability. Figure 1b presents a comprehensive analysis of the availability and affordability of LPGs for urban and rural residents in 2012. There were 4 (15.38%) medicines with high availability and high affordability, 9 (34.62%) medicines with high availability and low affordability, 4 (15.38%) medicines with low availability and high affordability and 9 (34.62%) medicines with low availability and low affordability. As shown in Figure 1c,d, there is a decrease in availability but a slightly more optimistic situation in terms of affordability in 2016. Especially for LPGs, there were more anticancer medicines with high availability and high affordability (38.46% for urban residents and 15.38% for rural residents), and less with low availability and low affordability (19.23% for urban residents and 26.92% for rural residents).
Figure 1.
Comprehensive analysis of medicine availability and affordability. (a) Comprehensive analysis of OBs availability and affordability in 2012. (b) Comprehensive analysis of LPGs availability and affordability in 2012. (c) Comprehensive analysis of OBs availability and affordability in 2016. (d) Comprehensive analysis of LPGs availability and affordability in 2016.
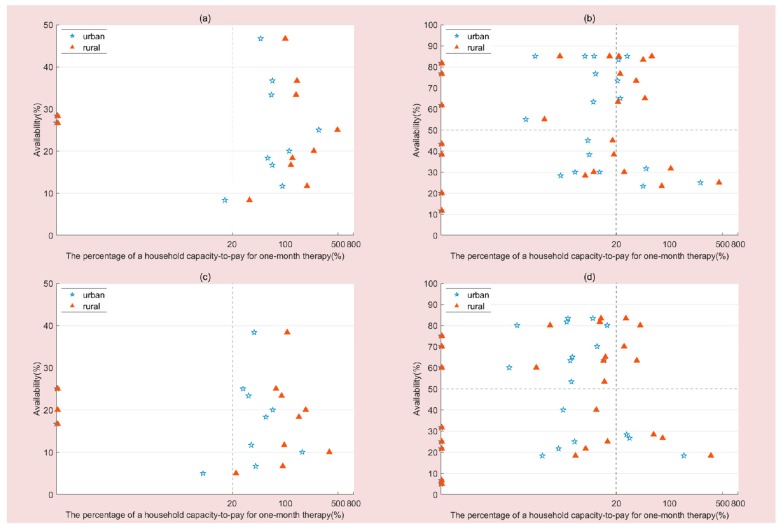
Figure 2 presents the comprehensive analysis of medicine availability and affordability following consideration of insurance reimbursement. For urban residents, the number of OBs with low availability and high affordability increased to 3 (25%) and 4 (33.33%) in 2012 and 2016, respectively. The number of LPGs with high availability and high affordability increased to 9 (34.62%) and 14 (53.85%) in 2012 and 2016, respectively. The number of LPGs with low availability and low affordability decreased to 3 (11.54%) in both years. For rural residents, the number of LPGs with high availability and high affordability increased to 9 (34.62%) and 10 (38.46%) in 2012 and 2016, respectively. The number of LPGs with low availability and low affordability decreased to 4(15.38%) and 3 (11.64%) in 2012 and 2016, respectively.
Figure 2.
Comprehensive analysis of medicine availability and affordability after insurance reimbursement. (a) Comprehensive analysis of OBs availability and affordability in 2012. (b) Comprehensive analysis of LPGs availability and affordability in 2012. (c) Comprehensive analysis of OBs availability and affordability in 2016. (d) Comprehensive analysis of LPGs availability and affordability in 2016.
3.5. Factors Associated with Affordability
The collinearity test indicated that there was no multicollinearity relationship between these variables (Variance Inflation Factor range from 1.000 to 1.314). The logistic regression model was used to estimate the independent associations of influencing factors (survey year, medicine type, hospital type, income level, urban vs. rural residence, medicine covered by BMI or not, medicine inclusion in the WHO EML or NEML) with the reporting outcome (affordable/unaffordable). However, “types of hospital” (p = 1.000) and “medicine inclusion/exclusion on the NEML” (p = 0.449) failed to enter the logistic regression equation to forecast the affordability of medicines. Table 9 indicates that the affordability of the anticancer medicines after insurance reimbursement varied significantly with survey year (p < 0.001), medicine variety (p < 0.001), urban/rural residency (p < 0.001), medicine covered by BMI (p < 0.001), and medicine inclusion/exclusion in the WHO EML (p < 0.001).
Table 9.
Factors associated with the affordability of anticancer medicines.
| Factors | B | Wald | OR (95%CI) | p |
|---|---|---|---|---|
| Survey year (reference = 2012) | ||||
| 2016 | 0.53 | 14.72 | 1.69 (1.29–2.22) | <0.001 |
| Medicine variety (reference = OBs) | ||||
| LPGs | 2.75 | 281.67 | 15.60 (11.32–21.51) | <0.001 |
| Area (reference = rural area) | ||||
| Urban area | 0.64 | 21.34 | 1.89 (1.44–2.48) | <0.001 |
| Income level (reference = low income) | ||||
| Middle income | 0.12 | 0.55 | 1.13 (0.82–1.56) | 0.457 |
| High income | 0.46 | 7.51 | 1.59 (1.14–2.20) | 0.006 |
| Covered by BMI or not (reference = not covered) | ||||
| Covered by BMI | 1.59 | 79.39 | 4.92 (3.46–6.98) | <0.001 |
| WHO essential medicine (reference = non-WHO essential medicine) | ||||
| WHO essential medicine | 1.01 | 40.84 | 2.75 (2.02–3.75) | <0.001 |
Note: Outcome (y)—affordable or not; y = 0, not affordable; y = 1, affordable; BMI, basic medical insurance in the Jiangsu Province.
Compared with 2012, patients in the Jiangsu Province in 2016 were 0.53-fold more able to afford these anticancer medicines, urban patients were 0.64-fold more than rural, and patients with high incomes were 0.46-fold more than those with low incomes. The LPG medicines were 5.46-fold more affordable than OBs. The medicines included in the WHO EML were 2.75-fold more affordable than those not included. The medicines covered by BMI were 1.59-fold more affordable than those not covered. Furthermore, the odds ratio indicated that generic substitution and medicine covered by BMI are the main contributors to affordability.
4. Discussion
In the current study, we adopted the WHO and HAI standard methodology to estimate the availability, price and affordability of anticancer medicines from the surveyed information in 6 cancer care hospitals, 26 tertiary general hospitals and 28 secondary general hospitals in the Jiangsu Province, China. As far as we know, this is the first study to assess the regional disparity and temporal variation of access to anticancer medicines in China. The Jiangsu Province is located in the eastern region of China, which is in the middle-upper level of development of China. As the first anticancer medicine survey to apply the WHO and HAI methodology to the eastern region of China, the findings of this study provide a comprehensive report on the availability, prices and affordability of anticancer medicines in East China. However, due to differences in economic development between Jiangsu and other provinces, there may be regional differences in the affordability of anticancer medicines. The main findings were as follows:
(1) On average, the overall availability of OBs (100%) and LPGs (67.50% for 2012 and 65.00% for 2016) was low (<50%) in both time periods. There was a significant decrease in the mean availability of anticancer medicines surveyed from 2012 to 2016 (from 7.79% to 5.71% for OBs, 36.29% to 32.67% for LPGs). The current study revealed that the mean availability of anticancer medicines was lower in the secondary general hospitals compared to the cancer care, as well as that in the tertiary general hospitals, which was low in low-income areas compared with middle- and high-income areas. All the hospitals surveyed had a higher mean availability of essential versus non-essential anticancer medicines.
The low availability of anticancer medicines is likely multifactorial, such as inadequate investment in research and development [38], insufficient incentive on maintaining stocks and inefficient procurement systems [47]. Our findings are contrary to the research in Pakistan that showed OBs (52.5%) were more available than LPGs (28.1%) in both public and private facilities [39]. Similar to the decreasing trend for availability in the study of essential medicines [48], we noted the decrease in anticancer medicine availability between both time periods. For public hospitals, the sales of medicines accounted for approximately 40% of their revenue [49,50]. Health service providers made higher profits from more costly pharmaceuticals since the mark-up ratio was prescribed by the government. However, the ‘zero-markup’ policy achieved full promotion in the public hospitals of the Jiangsu Province by the end of 2015, which was likely to reduce the hospitals’ revenue. Therefore, the hospitals were unable to maintain these life-saving medicines due to financial constraints. Compared to private sectors, public hospitals are more likely to face the issue of shortage or unavailability of medicines if they don’t receive timely or sufficient financial support to compensate the loss in medicine revenue, especially secondary hospitals [39].
To a large extent, the cost of medicines contributes to availability in the healthcare institutions in LMICs [51]. The regional disparity of availability is of great concern. Availability of anticancer medicines was lowest in low-income cities, which may be due to a lack of sufficient financial support. As extant literature documented, the regions with high-income levels showed a higher availability of medicines, which might be owed to the gaps in the transport system and economic level among different income regions [52]. The higher availability of essential medicines may be due to the National Essential Medicine System established by the central government of China in 2009, which aims to satisfy the public’s basic demand for health care [35,47].
The government has cut down medical expenses to satisfy public demand, while the subsidy for public hospitals remains stationary [50]. To facilitate the availability of anticancer medicines, the government should provide adequate funding to healthcare institutions and improve the equity of the allocation of health resources. In addition, there should be incentives to maintain the stocks of these lifesaving medicines in public hospitals.
(2) The inflation-adjusted median unit prices of anticancer medicines decreased significantly from 2012 to 2016, and were much higher for OBs than LPGs. This finding was consistent with a study done on the prices of the anticancer medicines in 25 provinces [53].
On the one hand, the decrease in retail price of the anticancer medicines may have been due to the ‘zero-markup’ policy [49]. Prior to year 2015, a 15% markup policy was implemented in all of the public hospitals in the Jiangsu Province. Compared with 2012, the cost of anticancer medicines with a zero-markup was much lower. On the other hand, changes to the acquisition method drove the procurement prices down. The procurement became more efficient by negotiating with wholesalers and manufacturers, since the standardized administration in the public bid for drug purchase of public hospitals was strengthened by the central government in 2014 [47]. The pharmaceutical firms could win bids only if they offered the lowest prices. The ‘zero-markup’ reform in the Jiangsu Province achieved remarkable results, attaining diffusion in the whole province and reducing the price of medicines significantly. In China, before June 2015, the price of medicine was regulated by the government. However, it was reported that price regulations had no effect on pharmaceutical price in China [54]. Drug price regulation was replaced by the market pricing policy in June 2015. In this study, we compared changes in the prices of different anticancer medicine groups between 2012 and 2016. A price increase from 2012 to 2016 was not observed in this study. Therefore, the market pricing policy could not have a negative impact on the prices of anticancer medicines.
Drug discovery and development is a complex and lengthy process. In 1994, the significant harmonization of medicine copyrights across national laws was provided by the WTO Trade-Related Aspects of Intellectual Property Rights agreement (TRIPS). The costly anticancer medicines were protected by product patent rights, which made them expensive monopolies [55]. Generally, the patent of new medicines lasts 20 years, which aims to prompt the pharmaceutical firm to recover the costs of research and development and invest again in new research [56]. In addition, high taxation contributes to the sky-high price of these OBs, which is a cruel joke for cancer sufferers [39]. Future policies are urgently needed to reduce the cost of anticancer medicines by the government in order to save lives due to their high mortality. The government should provide adequate funding to make tax-free anticancer medicines more available for cancer patients [40].
(3) Most of anticancer medicines surveyed are not affordable for patients in the absence of health insurance reimbursement. Although the affordability at three different income levels in 2016 showed improvement for both urban and rural residents compared with that in 2012, it was still unreasonable. The OBs (16.67%) of all the anticancer medicines were found to be less affordable than the LPGs (34.62% for urban residents and 30.77% for rural residents); their affordability varied among different income levels and areas. We found that both urban and rural patients with high incomes could not afford the cost of most of the anticancer medicines surveyed, let alone those with middle and low incomes. From 2012 to 2016, the proportion of LPGs with low availability and low affordability dropped from 30.77% to 19.23% in urban area and 34.62% to 26.92% in rural area, respectively. The advent of universal health insurance led to an improvement in the affordability of anticancer medicines in the Jiangsu Province. From 2012 to 2016, the proportion of LPGs with high availability and high affordability rose from 34.62% to 53.85% in urban areas and 34.62% to 38.46% in rural areas, respectively; the proportion of LPGs with low availability and low affordability dropped from 15.38% to 11.64% in rural areas, respectively. Generic substitution and medicine covered by BMI are the main factors facilitating affordability. Due to the bidding system, the pharmaceutical companies that win bids offer the prices. As a result, there is relatively little difference between the prices of the same medicine with same strength and dosage in different types of hospital. Therefore, “types of hospital” failed to enter the logistic regression equation to forecast the affordability of medicines.
Notably, the increased affordability of anticancer medicines has been observed in the Jiangsu Province. This factor might contribute to improved living standards. Meanwhile, the patient prices of anticancer medicines have dropped. However, most anticancer medicines surveyed were not affordable for patients with cancer. Unlike the medicines treating other chronic diseases, the demand for anticancer medicines is largely insensitive or inelastic to the changes in price, due to the life-threatening nature of cancer. Therefore, cancer patients have to be willing to accept and pay the cost of anticancer medicines regardless of their rising costs [57]. Meanwhile, the high cost of anticancer medicines may lead to more households being impoverished and more premature cancer deaths [58].
Policy should focus on addressing the inequity in anticancer medicine’s affordability between patients with different income levels, and between those in urban and rural areas [35]. Our study also revealed that generic substitution could lessen the cost of anticancer medicines to cancer sufferers. Governments should adjust related policies and regulations to make the approvals of generic anticancer medicines faster and improve their access to the market [59]. The preferential taxation policy and other incentive measures can be given to the non-profit generic producers in order to encourage them to manufacture low-cost high-quality anticancer medicines [51]. The government should ensure that first-line LPGs are dispensed in all the public hospitals that provide services to cancer sufferers, and that they are available for patients and preferentially prescribed [47]. Specifically, due to the implementation of the zero-markup policy, drug markups were basically eliminated. It led to a price decrease from 2012 to 2016 at the patient level. On the other hand, medical costs shifted from medicines to other medical services such as surgery and outpatient consultation. For instance, some empirical studies in the Jiangsu Province demonstrated that the total cost of medical care has increased by about 10% with the decline of drug expense after the implementation of the zero-markup policy. Meanwhile, the outpatient consultation and treatment fees have increased significantly [60]. The individual patient’s out-of-pocket expense has decreased slightly [61,62].
Our study has several limitations. First, only the availability of anticancer medicines in stock on the day of data collection was assessed at each facility, which may not accurately capture the availability of anticancer medicines in these hospitals over time. Second, the private hospitals were not considered in the two cross-sectional studies, and therefore are not reflected in the availability of anticancer medicines in all the hospitals in the Jiangsu Province. Third, the affordability may have been overestimated as other treatment costs exceeding one anticancer medicine at a time were not taken into account. The ‘zero-markup’ reform might result in lower costs of medicines but higher health service fees. Fourth, we measured affordability only at the household level. Future studies should focus on the affordability of anticancer medicines at the individual level. Finally, the new anticancer medications that were introduced between 2013 and 2016 were not included, which could lead to selection bias in the price comparison between 2012 and 2016. Due to the fact that most new drugs had higher price tags, the lack of inclusion of new drugs in 2016 could have led to biased lower prices in 2016.
5. Conclusions
This study revealed the unreasonable situation of the availability, price and affordability of anticancer medicines. The total availability of the anticancer medicines was low, as a whole, and significantly decreased from 2012 to 2016. In the Jiangsu Province, the price of anticancer medicines was controlled well after the ‘zero-markup’ reform but remained too high. Regional disparity in delivery and affordability of anticancer medicines was observed. There was more availability and affordability for the patients in high-income regions than the patients in low-income regions. In consequence, targeted policy measures should be undertaken to improve the accessibility of anticancer medicines for cancer sufferers. First, having acknowledged the existence of inequities in availability and affordability, a rise in public expenditure on cancer is indispensable. In consideration of the low availability of anticancer medicines in the Jiangsu Province, addressing the availability in hospitals that provide cancer services is a priority. Second, governments should consider using their bargaining power to reduce prices in the purchase phase and abolish taxes on anticancer medicines. Third, policy should focus on the special health insurance plan for the low-income population of cancer sufferers and lower the out-of-pocket payments for cancer therapy. Finally, the health authorities should adjust related policies to make the approvals of generic anticancer medicines faster and improve their access to the market. The goal of drug policy is to ensure that first-line generic drugs are dispensed in public sectors and are available for cancer patients and preferentially prescribed.
Acknowledgments
The authors are grateful for the support of the Jiangsu Commission of Health. The authors also appreciate the aid of all research assistants who collected data in both years.
Author Contributions
Y.Z., Y.W. and X.L. initiated the study concept; Y.Z. conducted data analysis; Y.Z. wrote the first draft of the manuscript; X.L. is the principal investigator of the project and led the data collection and contributed to the data analysis and interpretation of the data and revised the draft of the manuscript; X.S. and Y.W. participated in the data analysis and interpretation of the data; all authors read and approved the final manuscript.
Funding
This study was supported by the innovation project for the Postgraduate Research and Practice Innovation Program of the Jiangsu Province (No. KYCX19_1141); the National Natural Science Foundation of China (No. 71673147), the China Medical Board (No. 17-277), and the General Project of Philosophy and Social Science of the University of the Jiangsu Province (No. 2017SJB0277).
Conflicts of Interest
The authors declare no conflicts of interest.
References
- 1.Naghavi M., Wang H., Lozano R., Davis A., Liang X., Zhou M., Vollset S.E., Ozgoren A.A., Abdalla S., Abd-Allah F., et al. Global, regional, and national age-sex specific all-cause and cause-specific mortality for 240 causes of death, 1990–2013: A systematic analysis for the Global Burden of Disease Study 2013. Lancet. 2015;385:117–171. doi: 10.1016/S0140-6736(14)61682-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Soerjomataram I., Lortet-Tieulent J., Parkin D.M., Ferlay J., Mathers C., Forman D., Bray F. Global burden of cancer in 2008: A systematic analysis of disability-adjusted life-years in 12 world regions. Lancet. 2012;380:1840–1850. doi: 10.1016/S0140-6736(12)60919-2. [DOI] [PubMed] [Google Scholar]
- 3.Smith S.K., Nicolla J., Zafar S.Y. Bridging the gap between financial distress and available resources for patients with cancer: A qualitative study. J. Oncol. Pract. 2014;10:e368–e372. doi: 10.1200/JOP.2013.001342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Knaul F., Frenk J., Shulman L. Social Science Research Network. Harvard Global Equity Initiative; Boston, MA, USA: 2011. Closing the Cancer Divide: A Blueprint to Expand Access in Low and Middle Income Countries. [Google Scholar]
- 5.Savage P., Mahmoud S. Development and economic trends in cancer therapeutic drugs: A 5-year update 2010–2014. Br. J. Cancer. 2015;112:1037–1041. doi: 10.1038/bjc.2015.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen W., Zheng R., Baade P.D., Zhang S., Zeng H., Bray F., Jemal A., Yu X.Q., He J. Cancer statistics in China, 2015. CA Cancer J. Clin. 2016;66:115–132. doi: 10.3322/caac.21338. [DOI] [PubMed] [Google Scholar]
- 7.Gehrett B.K. A Prescription for Drug Shortages. JAMA. 2012;307:153–154. doi: 10.1001/jama.2011.2000. [DOI] [PubMed] [Google Scholar]
- 8.Gray A., Manasse H.R., Jr. Shortages of medicines: A complex global challenge. Bull. World Health Organ. 2012;90:158. doi: 10.2471/BLT.11.101303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gatesman M.L., Smith T.J. The Shortage of Essential Chemotherapy Drugs in the United States. N. Engl. J. Med. 2011;365:1653–1655. doi: 10.1056/NEJMp1109772. [DOI] [PubMed] [Google Scholar]
- 10.Kantarjian H. Chemotherapy drug shortages in the United States revisited. J. Oncol. Pract. 2014;10:329–331. doi: 10.1200/JOP.2014.001514. [DOI] [PubMed] [Google Scholar]
- 11.Havrilesky L.J., Garfield C.F., Barnett J.C., Cohn D.E. Economic impact of paclitaxel shortage in patients with newly diagnosed ovarian cancer. Gynecol. Oncol. 2012;125:631–634. doi: 10.1016/j.ygyno.2012.03.028. [DOI] [PubMed] [Google Scholar]
- 12.Becker D.J., Talwar S., Levy B.P., Thorn M., Roitman J., Blum R.H., Harrison L.B., Grossbard M.L. Impact of oncology drug shortages on patient therapy: Unplanned treatment changes. J. Oncol. Pract. 2013;9:e122–e128. doi: 10.1200/JOP.2012.000799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wagenaar B.H., Gimbel S., Hoek R., Pfeiffer J., Michel C., Manuel J.L., Cuembelo F., Quembo T., Afonso P., Gloyd S., et al. Stock-outs of essential health products in Mozambique—Longitudinal analyses from 2011 to 2013. Trop. Med. Int. Health. 2014;19:791–801. doi: 10.1111/tmi.12314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Martei Y.M., Chiyapo S., Grover S., Hanna C., Dryden-Peterson S., Pusoentsi M., Shulman L.N., Tapela N. Methodology to Forecast Volume and Cost of Cancer Drugs in Low- and Middle-Income Countries. J. Glob. Oncol. 2018;4:1–8. doi: 10.1200/JGO.17.00114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kelly R.J., Smith T.J. Delivering maximum clinical benefit at an affordable price: Engaging stakeholders in cancer care. Lancet Oncol. 2014;15:e112–e118. doi: 10.1016/S1470-2045(13)70578-3. [DOI] [PubMed] [Google Scholar]
- 16.Carrera P.M., Kantarjian H.M., Blinder V.S. The financial burden and distress of patients with cancer: Understanding and stepping-up action on the financial toxicity of cancer treatment. CA Cancer J. Clin. 2018;68:153–165. doi: 10.3322/caac.21443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Abboud C., Berman E., Cohen A., Cortes J., DeAngelo D., Deininger M., Devine S., Druker B., Fathi A., Jabbour E., et al. The price of drugs for chronic myeloid leukemia (CML) is a reflection of the unsustainable prices of cancer drugs: From the perspective of a large group of CML experts. Blood. 2013;121:4439–4442. doi: 10.1182/blood-2013-03-490003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Union for International Cancer Control (UICC) Twentieth WHO Expert Committee on the Selection and Use of Essential Medicines. World Health Organization; Geneva, Switzerland: 2015. [Google Scholar]
- 19.World Health Organization WHO Model List of Essential Medicines, 19th List. [(accessed on 3 March 2019)];2015 Available online: https://www.who.int/medicines/publications/essentialmedicines/EML_2015_FINAL_amended_NOV2015.pdf?ua=1.
- 20.Goss P.E., Strasser-Weippl K., Lee-Bychkovsky B.L., Fan L., Li J., Chavarri-Guerra Y., Liedke P.E.R., Pramesh C.S., Badovinac-Crnjevic T., Sheikine Y., et al. Challenges to effective cancer control in China, India, and Russia. Lancet Oncol. 2014;15:489–538. doi: 10.1016/S1470-2045(14)70029-4. [DOI] [PubMed] [Google Scholar]
- 21.Yip W.C.-M., Hsiao W.C., Chen W., Hu S., Ma J., Maynard A. Early appraisal of China’s huge and complex health-care reforms. Lancet. 2012;379:833–842. doi: 10.1016/S0140-6736(11)61880-1. [DOI] [PubMed] [Google Scholar]
- 22.Meng Q., Xu L. Monitoring and Evaluating Progress towards Universal Health Coverage in China. PLoS Med. 2014;11 doi: 10.1371/journal.pmed.1001694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Leukemia Patient Prosecuted for Buying Pills Overseas. [(accessed on 3 March 2019)];2015 Available online: http://www.china.org.cn/china/2014-12/22/content_34380340.htm.
- 24.Ahmad N.S., Islahudin F. Affordability of essential medicine prices in Malaysia’s private health sector. Patient Prefer. Adherence. 2018;12:1231–1237. doi: 10.2147/PPA.S151603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chahal H.S., Fort N.S., Bero L. Availability, prices and affordability of essential medicines in Haiti. J. Glob. Health. 2013;3 doi: 10.7189/jogh.03.020405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Guan X., Hu H., Man C., Shi L. A survey of availability, price and affordability of essential medicines from 2011 to 2016 in Chinese secondary and tertiary hospitals. Int. J. Equity Health. 2018;17:158. doi: 10.1186/s12939-018-0870-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Xi X., Li W., Li J., Zhu X., Fu C., Wei X., Chu S. A survey of the availability, prices and affordability of essential medicines in Jiangsu Province, China. BMC Health Serv. Res. 2015;15:345. doi: 10.1186/s12913-015-1008-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yang H., Dib H.H., Zhu M., Qi G., Zhang X. Prices, availability and affordability of essential medicines in rural areas of Hubei Province, China. Health Policy Plan. 2010;25:219–229. doi: 10.1093/heapol/czp056. [DOI] [PubMed] [Google Scholar]
- 29.Sado E., Sufa A. Availability and affordability of essential medicines for children in the Western part of Ethiopia: Implication for access. BMC Pediatr. 2016;16:40. doi: 10.1186/s12887-016-0572-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang X., Fang Y., Yang S., Jiang M., Yan K., Wu L., Lv B., Shen Q. Access to paediatric essential medicines: A survey of prices, availability, affordability and price components in Shaanxi Province, China. PLoS ONE. 2014;9:e90365. doi: 10.1371/journal.pone.0090365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sun X., Wei J., Yao Y., Chen Q., You D., Xu X., Dai J., Yao Y., Sheng J., Li X. Availability, prices and affordability of essential medicines for children: A cross-sectional survey in Jiangsu Province, China. BMJ Open. 2018;8:e023646. doi: 10.1136/bmjopen-2018-023646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gitanjali B., Manikandan S. Availability of five essential medicines for children in public health facilities in India: A snapshot survey. J. Pharmacol. Pharmacother. 2011;2:95–99. doi: 10.4103/0976-500X.81900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Khatib R., McKee M., Shannon H., Chow C., Rangarajan S., Teo K., Wei L., Mony P., Mohan V., Gupta R., et al. Availability and affordability of cardiovascular disease medicines and their effect on use in high-income, middle-income, and low-income countries: An analysis of the PURE study data. Lancet. 2016;387:61–69. doi: 10.1016/S0140-6736(15)00469-9. [DOI] [PubMed] [Google Scholar]
- 34.Vasheghani A.F., Salamzadeh J., Rasekh H.R., Najafi S., Mosadegh V. The Availability and Affordability of Cardiovascular Medicines for Secondary Prevention in Tehran Province (Iran) Iran. J. Pharm. Res. 2018;17:64–72. [PMC free article] [PubMed] [Google Scholar]
- 35.Gong S., Cai H., Ding Y., Li W., Juan X., Peng J., Jin S. The availability, price and affordability of antidiabetic drugs in Hubei province, China. Health Policy Plan. 2018;33:937–947. doi: 10.1093/heapol/czy076. [DOI] [PubMed] [Google Scholar]
- 36.Liu C., Zhang X., Liu C., Ewen M., Zhang Z., Liu G. Insulin prices, availability and affordability: A cross-sectional survey of pharmacies in Hubei Province, China. BMC Health Serv. Res. 2017;17:597. doi: 10.1186/s12913-017-2553-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gong S., Wang Y., Pan X., Zhang L., Huang R., Chen X., Hu J., Xu Y., Jin S. The availability and affordability of orphan drugs for rare diseases in China. Orphanet J. Rare Dis. 2016;11:20. doi: 10.1186/s13023-016-0392-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Guan X., Zhang J., Man C., Ni B., Shi L. How Far Have We Come? Challenges to Orphan Drug Access in China, 2011–2017. J. Pharm. Sci. 2019;108:2199–2205. doi: 10.1016/j.xphs.2019.01.012. [DOI] [PubMed] [Google Scholar]
- 39.Sarwar M.R., Iftikhar S., Saqib A. Availability of anticancer medicines in public and private sectors, and their affordability by low, middle and high-income class patients in Pakistan. BMC Cancer. 2018;18:14. doi: 10.1186/s12885-017-3980-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Saqib A., Iftikhar S., Sarwar M.R. Availability and affordability of biologic versus non-biologic anticancer medicines: A cross-sectional study in Punjab, Pakistan. BMJ Open. 2018;8:e019015. doi: 10.1136/bmjopen-2017-019015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cherny N., Sullivan R., Torode J., Saar M., Eniu A. ESMO European Consortium Study on the availability, out-of-pocket costs and accessibility of antineoplastic medicines in Europe. Ann. Oncol. 2016;27:1423–1443. doi: 10.1093/annonc/mdw213. [DOI] [PubMed] [Google Scholar]
- 42.China NBoSo . China Statistical Yearbook, 2018. China Statistics Press; Beijing, China: 2018. [Google Scholar]
- 43.Statistical Bulletin on the Development of Health and Family Planning in Jiangsu Province in 2017. [(accessed on 15 March 2019)];2017 Available online: http://wjw.jiangsu.gov.cn/module/download/downfile.jsp?classid=0&filename=0e0ca8f9770d4de88992683db9da706c.pdf. (In Chinese)
- 44.How to Use|International Medical Products Price Guide (2016) [(accessed on 15 March 2019)]; Available online: http://mshpriceguide.org/en/how-to-use/
- 45.The Rate of Inflation in China. [(accessed on 15 March 2019)];2017 Available online: http://www.pbc.gov.cn/en/3688006/index.html.
- 46.WHOCC Definition and General Considerations. [(accessed on 15 March 2019)];2018 Available online: https://www.whocc.no/ddd/definition_and_general_considera/
- 47.Fang Y., Wagner A.K., Yang S., Jiang M., Zhang F., Ross-Degnan D. Access to affordable medicines after health reform: Evidence from two cross-sectional surveys in Shaanxi Province, western China. Lancet Glob. Health. 2013;1:E227–E237. doi: 10.1016/S2214-109X(13)70072-X. [DOI] [PubMed] [Google Scholar]
- 48.Liu X., Sun X., Zhao Y., Meng Q. Financial protection of rural health insurance for patients with hypertension and diabetes: Repeated cross-sectional surveys in rural China. BMC Health Serv. Res. 2016;16:481. doi: 10.1186/s12913-016-1735-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Deng J., Tian H., Guo Y., Ma T., Sun Y., Zhang S., Yang T., Tian X. A retrospective and prospective assessment of the zero-markup drug reform in China from the perspective of policy diffusion. Int. J. Health Plan. Manag. 2018;33:e918–e929. doi: 10.1002/hpm.2562. [DOI] [PubMed] [Google Scholar]
- 50.Guan X., Qi L., Liu L. Controversy in public hospital reforms in China. Lancet Glob. Health. 2016;4:E240. doi: 10.1016/S2214-109X(16)00041-3. [DOI] [PubMed] [Google Scholar]
- 51.Kolasani B.P., Malathi D.C., Ponnaluri R.R. Variation of Cost among Anti-cancer Drugs Available in Indian Market. J. Clin. Diagn. Res. 2016;10:FC17–FC20. doi: 10.7860/JCDR/2016/22384.8918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sun J., Luo H. Evaluation on equality and efficiency of health resources allocation and health services utilization in China. Int. J. Equity Health. 2017;16:127. doi: 10.1186/s12939-017-0614-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yuan J., Zhou J., Chen Y., Liu Y., Mao Z. Analysis of Bidding Price of Anti-tumor Drugs in 25 Provinces (Region, City) China Pharm. 2016;27:4336–4339. (In Chinese) [Google Scholar]
- 54.Meng Q., Cheng G., Silver L., Sun X.J., Rehnberg C., Tomson G. The impact of China’s retail drug price control policy on hospital expenditures: A case study in two Shandong hospitals. Health Policy Plan. 2005;20:185–196. doi: 10.1093/heapol/czi018. [DOI] [PubMed] [Google Scholar]
- 55.Ruiz R., Strasser-Weippl K., Touya D., Herrero Vincent C., Hernandez-Blanquisett A., St. Louis J., Bukowski A., Goss P.E. Improving access to high-cost cancer drugs in Latin America: Much to be done. Cancer. 2017;123:1313–1323. doi: 10.1002/cncr.30549. [DOI] [PubMed] [Google Scholar]
- 56.Trade-Related Aspects of Intellectual Property Rights. [(accessed on 22 March 2019)];2019 Available online: https://www.wto.org/english/tratop_e/trips_e/trips_e.htm.
- 57.Ehni H.J. Expensive cancer drugs and just health care. Best Pract. Res. Clin. Gastroenterol. 2014;28:327–337. doi: 10.1016/j.bpg.2014.02.008. [DOI] [PubMed] [Google Scholar]
- 58.Chivukula M.V., Tisocki K. Approaches to improving access to essential cancer medicines in the WHO South-East Asia Region. WHO South East Asia J. Public Health. 2018;7:62–66. doi: 10.4103/2224-3151.239415. [DOI] [PubMed] [Google Scholar]
- 59.Learn P.A., Bach P.B. Pem and the Cost of Multicycle Maintenance. J. Thorac. Oncol. 2010;5:1111–1112. doi: 10.1097/JTO.0b013e3181e7c425. [DOI] [PubMed] [Google Scholar]
- 60.Huang W., Chen D., Zhao G. Reinforcing the Coonprehensive Pricing and Compensation. Reform of Urban Public Hospital: Case of Yangzhou. Chin. J. Health Econ. 2016;10:58–60. (In Chinese) [Google Scholar]
- 61.Cao Y., Chen J. Thoughts of Stratigies of Public Hospital Medical Pricing Reform: A Survey of Urban Public Hospitals in Jiangsu. Mod. Commer. Trade Ind. 2017;14:130–132. (In Chinese) [Google Scholar]
- 62.Hu D., Xu J., Zhang Y. Impact of removing drug markups on medical insurance fund: Practice from Jiangsu. Chin. J. Med. Insur. 2017;7:20–24. (In Chinese) [Google Scholar]