Key Points
Question
What is the risk of cancer incidence and cancer mortality in people with psoriasis?
Findings
This systematic review and meta-analysis included 58 unique observational studies (50 reporting on cancer incidence and 15 on cancer mortality, including 7 reporting on both). The overall risk of developing cancer was significantly elevated in people with psoriasis and for a number of site-specific cancers; the risk of cancer mortality was found to be elevated in those with severe psoriasis.
Meaning
These findings suggest that cancer is an important comorbidity in people with psoriasis, and dermatologists should be aware of this increased risk; further studies are needed to improve our knowledge about the underlying mechanisms of this increased risk.
This systematic review and meta-analysis of 58 observational studies assessed the risks of cancer incidence and mortality among people with psoriasis.
Abstract
Importance
The risk of cancer developing in people with psoriasis has raised some concern, with little clarity regarding differentiation in risk according to psoriasis severity.
Objective
To conduct a systematic review and meta-analysis of observational studies on the risk of cancer incidence and mortality in people with psoriasis.
Data Sources
Six electronic databases (MEDLINE, Embase, MEDLINE in Process, Cochrane Central Register, Web of Science, and LILACS [Literatura Latino-Americana e do Caribe em Ciências da Saúde]) were searched from inception to November 15, 2017, for eligible studies.
Study Selection
Cohort and case-control studies that provided estimates of the risk of cancer incidence or cancer mortality associated with psoriasis were included.
Data Extraction and Synthesis
Data were extracted relating to study design, study population, and risk estimates. Study-specific estimates of the relative risk (RR) were combined using a random-effects model. Heterogeneity was quantified using the I2 statistic. Data were analyzed from April 9, 2018, through February 22, 2019.
Main Outcomes and Measures
Pooled RR estimates for cancer incidence and cancer mortality for psoriasis cohorts compared with people without psoriasis.
Results
A total of 58 unique studies were included, with quality varying for the incidence and the mortality studies. Severe psoriasis (RR, 1.22; 95% CI, 1.08-1.39 [9 studies]) and all severities of psoriasis (RR, 1.18; 95% CI, 1.06-1.31 [7 studies]) were associated with an increased risk of cancer (overall), and associations were found for a range of site-specific cancers, including colon (RR, 1.18 [95% CI, 1.03-1.35]), colorectal (RR, 1.34 [95% CI, 1.06-1.70]), kidney (RR, 1.58 [95% CI, 1.11-2.24]), laryngeal (RR, 1.79 [95% CI, 1.06-3.01]), liver (RR, 1.83 [95% CI, 1.28-2.61]), lymphoma (RR, 1.40 [95% CI, 1.24-1.57]), non-Hodgkin lymphoma (RR, 1.28 [95% CI, 1.15-1.43]), keratinocyte cancers (RR, 1.71 [95% CI, 1.08-2.71]), esophageal (RR, 2.05 [95% CI, 1.04-4.07]), oral cavity (RR, 2.80 [95% CI, 1.99-3.93]), and pancreatic (RR, 1.41 [95% CI, 1.16-1.73]). Overall cancer mortality risk was higher in patients with severe psoriasis (RR, 1.22; 95% CI, 1.08-1.38 [4 studies]). Specifically, liver (RR, 1.43 [95% CI, 1.09-1.88]), esophageal (RR, 2.53 [95% CI, 1.87-3.41]), and pancreatic (RR, 1.31 [95% CI, 1.02-1.69]) cancer mortality were found to be elevated in those with severe psoriasis. The heterogeneity of estimates was often very high despite stratification. Marked attenuation of risk was found in those studies that adjusted estimates for smoking, alcohol consumption, and obesity.
Conclusions and Relevance
In this study, people with psoriasis appeared to have an increased risk of cancer incidence and cancer-related mortality involving a range of site-specific cancers. Future research examining specific lifestyle factors, treatments, and the inflammatory processes that contribute to psoriasis may help provide additional information on the underlying mechanisms for the apparent increased cancer risk.
Introduction
Psoriasis is a common, chronic inflammatory skin disease with prevalence estimates varying globally in adults from 0.91% in the United States to 8.50% in Norway.1 The condition also carries significant physical and psychosocial detriment.2 The extent of the burden imposed by psoriasis is further exacerbated through numerous comorbidities that include depression, cardiovascular disease, and psoriatic arthritis.3 In contrast to the relatively well-established nature of the associations between these comorbidities and psoriasis, the association between cancer and psoriasis is much less clear, particularly for site-specific cancers. Despite this lack of clarity, the plausibility of the association is supported through the role of inflammation in the pathogenesis of psoriasis, with chronic inflammation previously linked to increased cancer risk.4 Treatments, including the use of immunomodulatory therapies, may also increase the risk of developing cancer. Furthermore, the prevalence of known cancer risk factors such as smoking, excessive alcohol consumption, and obesity has been reported to be increased in people with psoriasis.3
An earlier systematic review5 reported an increased risk of developing several cancers in people with psoriasis. Despite the usefulness of this review, it included some cancer risk estimates that only considered psoriatic arthritis, thus limiting the generalizability of results to those people with psoriasis only. A further limitation of this study was a search strategy that resulted in the omission of several studies on multiple autoimmune conditions, which also reported separate risk estimates for psoriasis. Given these limitations, the main aim of our study was to conduct a comprehensive critical review and meta-analysis of observational studies to ascertain the risk of cancer incidence in people with psoriasis. In addition to providing the first meta-analysis, to our knowledge, of cancer mortality risk in people with psoriasis, we also aimed to understand how the risk of cancer varied according to psoriasis severity and the level of adjustment for lifestyle factors.
Methods
Search Strategy and Eligibility Criteria
Six electronic databases (MEDLINE, Embase, MEDLINE in Process and other nonindexed citations, Cochrane Central Register, Web of Science, and LILACS [Literatura Latino-Americana e do Caribe em Ciências da Saúde]) were searched from inception to November 15, 2017, for relevant studies. The details of the search strategy for MEDLINE and Embase are presented in eTable 1 in the Supplement. Reference lists of included studies were also hand searched. All included studies had to meet the following eligibility criteria: cohort or case-control study design; at least 1 study group of patients with psoriasis; and a comparison group involving patients without psoriasis or the general population. Included studies were also required to investigate cancer occurrence or cancer mortality. Case-control studies that examined cancer risk for a range of autoimmune disorders were also eligible for inclusion if data were provided separately for those individuals with psoriasis. No restrictions were applied to language, geographic region, or study period. The University of Manchester institutional review board determined that approval was not required for this study design. This study followed the Meta-analysis of Observational Studies in Epidemiology (MOOSE) reporting guidelines (eTable 2 in the Supplement).
Study Selection and Data Extraction
One author (A.M.T.) screened all titles and abstracts. Articles were sorted as definitely include, possibly include, and exclude. Articles classed as possibly include were reviewed by 4 authors (A.M.T., R.P., E.K., and D.M.A.) to determine whether the study was eligible for inclusion. Data extraction for all included studies was performed by 1 author (A.M.T.), with any issues requiring further clarification rectified through consultation with the other 3 reviewing authors. When more than 1 risk estimate was presented in a study report, the one with the greatest level of adjustment for potential confounding factors was extracted.6
Critical Appraisal Tool and Risk of Bias Assessment
To assess the risk of bias in the included cohort and case-control studies, the Newcastle-Ottawa Scale tool was used. Using the tool, each study was judged on 8 items in 3 categories, including the selection of the study groups, the comparability of the groups, and the ascertainment of the exposure of interest for case-control studies or the outcome of interest for cohort studies. Studies that received 8 or 9 of a possible 9 points were regarded as high quality, whereas studies that received 6 or 7 were regarded as fair quality, and those that received 5 or less were regarded as low quality.7
Data Analysis
Data were analyzed from April 9, 2018, through February 22, 2019. To account for between-study heterogeneity,8 a meta-analysis was performed using a Der Simonian–Laird random-effects model, with effect estimates from different studies combined through the generic inverse variance approach. For cohort studies, risk estimates were reported through risk ratios (RR), incidence rate ratios, incidence density ratios, hazard ratios, or standardized incidence/morbidity or mortality ratios. These estimates were considered relative measures of risk and were therefore pooled. Included case-control studies reported risk estimates through odds ratios. Given the differences in study design, case-control studies were examined separately from cohort studies.
We also separately analyzed study estimates restricted to people with severe psoriasis and those including people with all severities of psoriasis. Studies were categorized as involving people with severe psoriasis if their study groups involved people who had received systemic treatment for psoriasis (including methotrexate sodium, cyclosporin, acitretin, etretinate, phototherapy, or biologicals) or had been hospitalized for psoriasis.
Studies were also grouped into 2 categories according to the level of adjustment for potential confounding. Level 1 studies adjusted for age and sex, whereas level 2 studies adjusted for age, sex, and at least 1 other confounding factor (such as smoking status, alcohol consumption, or body mass index). When more than 1 study provided an estimate using the same base population, the study reporting on the most complete data was included. Estimates calculated for separate subgroups, such as mild psoriasis and severe psoriasis, within the same study were combined before meta-analysis if the pooled estimate was more relevant to the meta-analysis (eMethods in the Supplement).
Heterogeneity in the pooled analyses was evaluated through the I2 statistic8 and corresponding 95% CIs. The calculation of CIs was not possible for pooled estimates with less than 3 studies. All analyses were performed using Stata, version 15 (StataCorp LLC). Two-sided P < .05 indicated significance.
Results
Study Selection and Characteristics
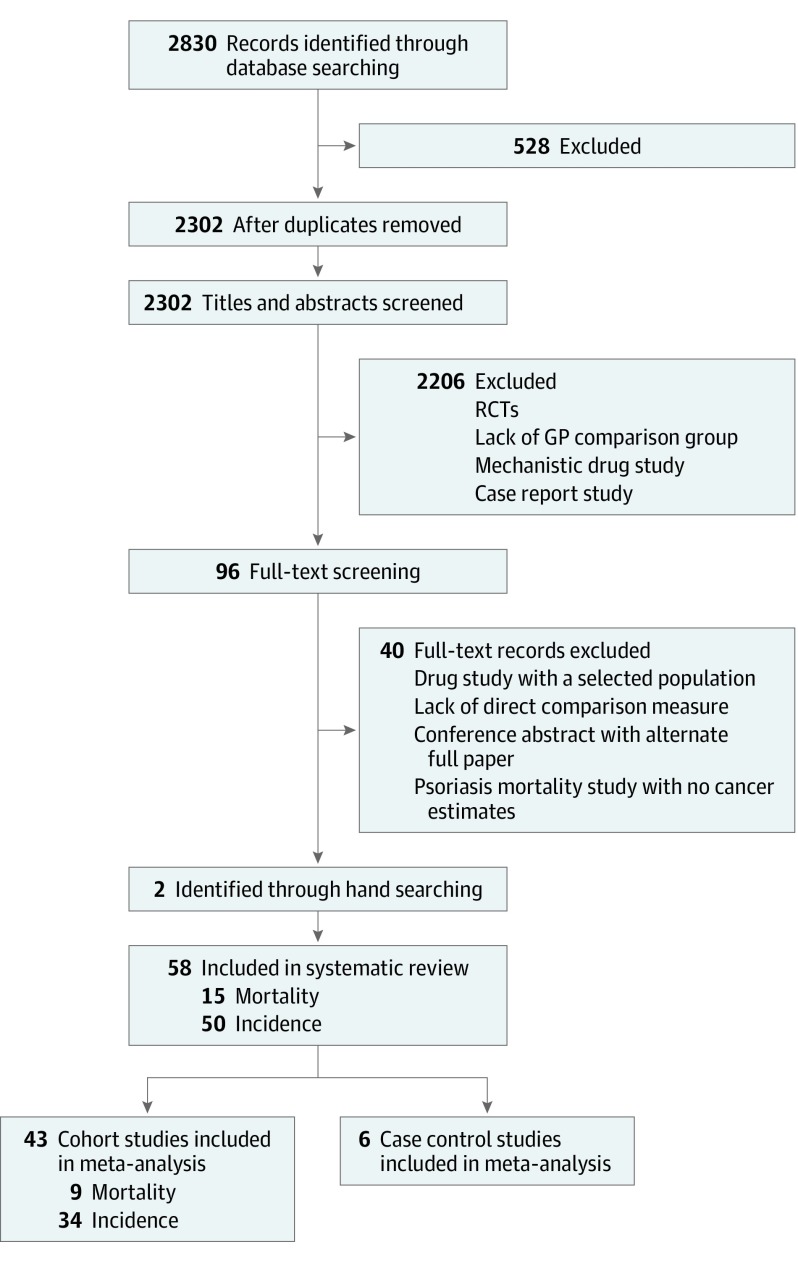
Excluding duplicates, 2302 studies were identified through database searching. Through title and abstract screening, 2206 studies were excluded for reasons noted in Figure 1. Full-text screening excluded a further 40 studies. Hand searching identified 2 additional studies, leaving the total number of included studies at 58, with 50 of those investigating the risk of cancer9,10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58 and 15 investigating cancer mortality13,15,32,33,41,48,54,59,60,61,62,63,64,65,66 (7 studies13,15,32,33,41,48,54 reported on both). Of the 50 cancer incidence studies, 28 considered only site-specific malignant neoplasms, with the remaining 22 considering all and site-specific malignant neoplasms. In contrast, of the 15 cancer mortality studies, only 1 study considered all and site-specific malignant neoplasms, with 6 studies considering only all malignant neoplasms and 8 considering only site-specific malignant neoplasms. The characteristics of the cancer incidence and cancer mortality studies are summarized in Table 1 and Table 2, respectively.
Figure 1. PRISMA Flowchart of Study Selection Process.
GP indicates general population; RCT, randomized clinical trial.
Table 1. Characteristics of Included Cancer Incidence in Psoriasis Studies.
| Source | Study Design (Outcome Measure) | Cancer Type | Population (No. of Participants) | Duration of Follow-up | Adjustment for Confounders | |
|---|---|---|---|---|---|---|
| Study | Comparison | |||||
| Alderson and Clarke,11 1983 | Cohort (SIR) | All malignant neoplasms | Patients with psoriasis in Scottish discharge records (8405) | Scottish, English, and Welsh general population (NA) | Mean: 11.5 y | Standardized: age, sex, and CT |
| Anderson et al,12 2009 | Case-control (OR) | Lymphocytic malignant neoplasms | Patients with lymphocytic cancer in Medicare database (33 721) | Cancer-free patients in Medicare database (122 531) | NA | Matched: age, sex, and CT |
| Adjusted: age, sex, CT, race, and use of health care services | ||||||
| Boffeta et al,13 2001 | Cohort (SIR) | All malignant neoplasms | Discharged patients with psoriasis from Swedish health database (9773) | Swedish general population (NA) | Mean: 11 y | Standardized: age, sex, and CT |
| Brauchli et al,14 2009 | Cohort with nested case-control (IRR) | All malignant neoplasms | Patients with psoriasis in GPRD (33 760) | Psoriasis-free patients in GPRD (36 702) | Mean: 4.6 y | Matched: age, sex, CT, GP practice, and years in GPRD |
| Adjusted: age, sex, CT, smoking status, and BMI | ||||||
| Castro et al,15 2014 | Cohort (SIR) | Hepatobiliary malignant neoplasms | Patients with psoriasis in the Swedish hospital discharge register (NR) | Swedish general population not hospitalized for any autoimmune disease (NA) | Mean: <1 y | Standardized: age, sex, CT, region, and socioeconomic status |
| Adjusted: smoking and alcoholism | ||||||
| Chen et al,16 2013 | Cohort (RR) | Keratinocyte | Patients with psoriasis in 3 population-based cohort studies (3216) | Psoriasis-free patients in 3 population-based cohort studies (185 190) | NR | NR |
| Chen et al,17 2011 | Cohort (HR) | All malignant neoplasms | Patients with psoriasis patients from NHIRD (3686) | Psoriasis-free patients from LHID (200 000) | Mean (psoriasis): 4.58 y | Adjusted: age, sex, and treatment modalities |
| Mean (control): 6.68 y | ||||||
| Chiesa et al,18 2015 | Cohort (HR) | All malignant neoplasms | Any patients with psoriasis in THIN database (198 366) | Psoriasis-free patients in THIN database (937 716) | Mean (psoriasis): 6.06-6.22 y | Matched: age and practice |
| Mean (controls): 6.39-6.57 y | Adjusted: age, sex, BMI, smoking, and alcohol consumption | |||||
| Chiou et al,19 2016 | Cohort (SIR) | All malignant neoplasms | Patients with psoriasis in NHID of Taiwan (6835) | Psoriasis-free patients in NHID of Taiwan (21 226 181) | Median: 11 y | Standardized: age |
| Cooper et al,20 1996 | Case-control (OR) | Lymphocytic malignant neoplasms | Patients with leukemia identified through cancer treatment clinical trials (624) | Random population sample attained through digit dialing (637) | NA | Matched: age, sex, race, region of residence |
| Adjusted: age, sex, race, educational level, and smoking | ||||||
| Dai et al,10 2016 | Cohort (RR) | Keratinocyte | Women with psoriasis in the NHS I and II (2645) | Women without psoriasis in the NHS I and II (155 289) | Total: 2 487 941 person-years | Adjusted: age, BMI, exercise, alcohol intake, smoking, family history of melanoma, nevi counts, susceptibility to burn, hair color, number of severe sunburns, and UV index at birth and ages 15 and 30 y |
| Egeberg et al,21 2016 | Cohort (IRR) | Skin malignant neoplasms | Patients with skin cancer from Danish Healthcare database (56 907) | Skin cancer–free patients from Danish Healthcare database (5 484 010) | Maximum: 16 y | Adjusted: age, sex, socioeconomic status, ethnic origin, and phototherapy |
| Engels et al,22 2016 | Case-control (OR) | Lymphocytic malignant neoplasms | Patients with lymphocytic cancer in the SEER-Medicare database (19 078) | Cancer-free individuals in the SEER-Medicare database (200 000) | NA | Matched: age, sex, CT, and race |
| Adjusted: age, sex, CT of case/control selection, race, and number of provider claims | ||||||
| Fallah et al,23 2014 | Cohort (SIR) | NHL | Patients with psoriasis in Swedish health care databases (131 215) | Psoriasis-free patients in Swedish health care databases (NA) | Mean (psoriasis): 7.1 y | Standardized: age, sex, CT, region, and socioeconomic status |
| Adjusted: smoking and BMI | ||||||
| Frentz and Olsen,24 1999 | Cohort (SIR) | All malignant neoplasms | Patients with psoriasis discharged from hospital (6905) | Psoriasis-free individuals from the Central Population Register (NA) | Mean: 9.3 y | Standardized: age, sex, and CT |
| Gelfand et al,25 2003 | Cohort (HR) | Lymphocytic malignant neoplasms | >65 Patients with psoriasis in GPRD (2718) | Psoriasis-free patients in the GPRD (105 203) | Median (psoriasis): 38.75 y | Adjusted: age and sex |
| Median (psoriasis free): 46 y | ||||||
| Gelfand et al,26 2006 | Cohort (HR) | Lymphocytic malignant neoplasms | Patients with psoriasis in GPRD (153 197) | Psoriasis-free patients in GPRD (765 950) | Mean (control): 5.61 y | Matched: CT and practice |
| Mean (all psoriasis): 4.54 y | Adjusted: age and sex | |||||
| Gu and Nordstrom,27 2017 | Cohort (SIR) | All malignant neoplasms | Patients aged <18 y with psoriasis in IMS Health plan database (13 174) | General population from SEER database (NA) | Mean (psoriasis): 1.5 y | Matched: age, sex, CT, region, and duration of enrollment |
| Mean (psoriasis free): 1.6 y | Standardized: age and sex | |||||
| Hannuksela et al,28 1996 | Cohort (SIR) | All malignant neoplasms | Patients with psoriasis treated with trioxsalen PUVA in Finnish hospitals (527) | Finnish general population (NA) | Mean: 10.8 y | Standardized: age and sex |
| Hannuksela-Svahn et al,29 1999 | Cohort (SIR) | All malignant neoplasms | Patients with psoriasis treated with trioxsalen PUVA in Finnish and Swedish hospitals (944) | Finnish and Swedish general population (NA) | Mean: 14.7 y | Standardized: sex |
| Hannuksela-Svahn et al,30 2000 | Cohort (SIR) | All malignant neoplasms | Patients with psoriasis in the Finnish hospital discharge records (5687) | Finnish general population (NA) | Mean: 14 y | Standardized: age, sex, and CT |
| Hannuksela-Savhn et al,31 1999 | Cohort (SIR) | All malignant neoplasms | Patients with psoriasis treated with methoxypsoralen-8 PUVA in Finnish hospital discharge records (158) | Finnish general population (NA) | Mean: 7.6 y | Standardized: age, sex, and CT |
| Hemminki et al,32 2013 | Cohort (SIR) | Brain malignant neoplasms | Patients with psoriasis in hospital discharge records (19 777) | Swedish general population (NA) | Mean: 11.9 y | Standardized: age, sex, CT, region, and socioeconomic status |
| Adjusted: obesity, smoking, alcohol, and socioeconomic features | ||||||
| Hemminki et al,33 2012 | Cohort (SIR) | Female malignant neoplasms | Patients with psoriasis in hospital discharge records (8162) | Swedish general population (NA) | Total (psoriasis): 275 971 person-years | Standardized: age, sex, CT, region, and socioeconomic status |
| Adjusted: obesity, smoking, alcohol, parity, and age at first birth | ||||||
| Ji et al,34 2009 | Cohort (SIR) | All malignant neoplasms | Patients with psoriasis previously hospitalized in Sweden (15 858) | Swedish general population (NA) | Median: 10 y | Standardized: age, sex, period, socioeconomic status, residential area, age at first birth, and parity |
| Kim et al,35 2015 | Cohort (HR) | Cervical malignant neoplasms | Female patients with psoriasis from 2 US health plans (34 665) | Female patients with hypertension from 2 US health plans (533 332) | Mean (psoriasis): 2.0 y | Adjusted: age, comorbidity score, No. of prescription drugs, alcoholism, smoking, substance abuse, sexually active status, STI presence, contraception use, HPV vaccine, diabetes, liver disease, systemic drugs, steroids, NSAIDs, and use of health care services |
| Mean (psoriasis free): 2.1 y | ||||||
| Lan et al,36 2012 | Nested case-control (HR) | All malignant neoplasms | Patients with psoriasis in the NHI (8180) | Psoriasis-free patients in the NHI (163 600) | NR | Matched: age and sex |
| Adjusted: age, sex, hypertension, dyslipidemia, and diabetes | ||||||
| Lanoy et al,37 2010 | Case-control (OR) | All malignant neoplasms | Elderly patients with cancer in the SEER-Medicare database (36 092) | Elderly cancer-free patients in the SEER-Medicare database (178 452) | NA | Adjusted: age, sex, CT, and No. of physician claims |
| Lee et al,38 2012 | Cohort (IDR) | Skin malignant neoplasms | Patients with psoriasis in the LHID (7061) | Psoriasis-free patients in the LHID (1 000 000) | Mean: 4.8 y | Standardized: age and sex |
| Li et al,39 2016 | Cohort (HR) | All malignant neoplasms | Female patients with psoriasis in the NHS (2182) | Female psoriasis-free patients in the NHS (62 808) | Total: 22 132 person-years | Adjusted: age, BMI, alcohol consumption, physical activity, physical examination, multivitamin use, smoking status, family history of cancer, contraceptive use, menopause status, history of benign breast disease, family history of breast cancer, age at first birth and parity, height, melanoma plus hair color, childhood tendency to sunburn or tan, No. of childhood sunburns, family history of melanoma, mole count, lifetime sun exposure, UV index, use of AIs, hypertension, diabetes, and parity |
| Lindelöf et al,40 1990 | Cohort (RR) | All malignant neoplasms | Patients with psoriasis in Swedish outpatient records (20 328) | Swedish general population (NA) | NR | Standardized: age and sex |
| Liu et al,41 2013 | Cohort (SIR) | Urological malignant neoplasms | Patients with psoriasis in Swedish outpatient records (19 777) | Swedish outpatients with no record of 33 selected AIs (NA) | Total (psoriasis): 275 971 person-years | Standardized: age, sex, CT, region, and socioeconomic status |
| Adjusted: obesity, smoking, and alcohol abuse | ||||||
| Margolis et al,42 2001 | Cohort (RRR) | All malignant neoplasms | Patients with psoriasis from a US claims database (16 519) | Patients with hypertension from the same claims database (234 304) | Mean (severe psoriasis): 2.36 y | Adjusted: age, sex, and region |
| Mean (less severe psoriasis): 2.27 y | ||||||
| Mean (hypertension): 2.43 y | ||||||
| McKenna et al,43 1996 | Cohort (OER) | Keratinocyte | Patients with psoriasis from the Belfast city hospital (246) | General population from North Humberside (NA) | Median: 9.5 y | Standardized: age and sex |
| Olsen et al,44 1992 | Cohort (RR) | All malignant neoplasms | Patients with psoriasis in the Danish outpatient records (6910) | Danish general population (NA) | Mean: 5.1 y | Standardized: age, sex, and CT |
| Paradisi et al,45 2017 | Cohort (RR) | Keratinocyte | Patients with psoriasis in hospital records (72 739) | Patients undergoing vascular surgery in same hospital records (25 956) | NR | Adjusted: age, sex, and phototherapy |
| Paul et al,46 2003 | Cohort (SIR) | All malignant neoplasms | Patients with psoriasis (1252) | General population from the country of the case (NA) | Median: 4.5 y | Standardized: age, sex, and country |
| Prizment et al,47 2011 | Cohort (HR) | All malignant neoplasms | Female patients with psoriasis, aged >65 y, in the IWHS (719) | Female psoriasis-free patients in the IWHS (32 191) | Mean: 11.8 y | Standardized: age |
| Adjusted: age, BMI, educational level, smoking, HT use, physical activity, and No. of live births | ||||||
| Reddy et al,48 2017 | Cohort (HR) | Melanoma and hematological cancer | Patients with psoriasis in the KPSC records (8161) | Psoriasis-free patients in the KPSC records (807 604) | NR | Matched: age and sex |
| Adjusted: race, treatments, and Charlson comorbidity index | ||||||
| Shapiro et al,49 2013 | Case-control (OR) | Skin malignant neoplasms | Patients with psoriasis in inpatient records (1079) | Patients with dermatitis in inpatient records (1079) | NA | Matched: age and sex |
| Smedby et al,50 2006 | Case-control (OR) | Lymphocytic malignant neoplasms | Patients with lymphocyctic cancer in the SCALE study (3055) | General population (3187) | NA | Matched: age, sex, and country |
| Adjusted: age, sex, country, smoking, BMI, educational level, blood transfusions, sun exposure, occupational exposure to pesticides, and family history of cancer | ||||||
| Söderberg et al,51 2006 | Case-control (OR) | Haematological malignant neoplasms | Patients with lymphocyctic cancer in the discharge registry (39 908) | General population (149 344) | NA | Matched: age, sex, and CT |
| Adjusted: age, sex, and socioeconomic status | ||||||
| Stern,52 2006 | Cohort (IRR) | Lymphoma | Patients with psoriasis in the PUVA follow-up study 1997-2005 (864) | General population using the SEER database (NA) | Total: 28 884 person-years | Standardized: age, sex, and CT |
| Adjusted: age, skin type, methotrexate exposure, and UV-B exposure | ||||||
| Stern et al,53 2002 | Cohort (RR) | Skin malignant neoplasms | All patients with psoriasis and treated with PUVA after 1989 (892) | White men in the general population using the SEER database (NA) | NR | Standardized: age |
| Stratified: treatment and period | ||||||
| Stern and Lange,54 1988 | Cohort (SMR) | All malignant neoplasms | All patients with psoriasis treated with PUVA (1380) | General population from the SEER database (NA) | Total: 13 224 person-years | Standardized: age and sex |
| Matched: age, sex, and region | ||||||
| Stern et al,9 2012 | Cohort (IRR) | Skin malignant neoplasms | Patients with psoriasis treated with PUVA from all periods (1380) | White persons in the general population (NA) | Mean (cancer): 27 y | Adjusted: age, sex, PUVA, UV-B, skin type, and methotrexate exposure |
| Mean (cancer-free): 25 y | ||||||
| Stern et al,55 1997 | Cohort (RR) | All malignant neoplasms | All patients with psoriasis treated with PUVA (1380) | White general population from SEER database (NA) | Median: 19.2 y | Standardized: age and sex |
| Sunesen et al,56 2010 | Cohort (SIR) | Skin malignant neoplasms | Patients with psoriasis from the Danish national patient registry (24 308) | General population (NA) | Mean: 8.6 y | Standardized: age, sex, and CT |
| Tseng et al,57 2013 | Case-control (OR) | All malignant neoplasms | Patients with psoriasis in hospital records (447) | Psoriasis-free patients from the same hospital records (447) | NA | Matched: age, sex, and CT |
| Zhang et al,58 2004 | Case-control (OR) | Lymphocytic malignant neoplasms | Women with lymphocytic cancer in the CTR (601) | General population of Connecticut (717) | NA | Matched: age |
| Adjusted: age, BMI, menopausal status, family history of NHL, race, educational level, tobacco use, and acohol consumption | ||||||
Abbreviations: AI, anti-inflammatory; BMI, body mass index; CT, calendar time; CTR, Connecticut Tumor Registry; GP, general practice; GPRD, General Practice Research Database; HPV, human papillomavirus; HR, hazard ratio; HT, hormonal therapy; IDR, incidence density ratio; IMS, Intercontinental Marketing Statistics; IRR, incidence rate ratio; IWHS, Iowa Women’s Health Study; KPSC, Kaiser Permanente Southern California; LHID, Longitudinal Health Insurance database; NA, not applicable; NHI, national health insurance; NHID, NHI database; NHIRD, National Health Insurance Research Database; NHL, non-Hodgkin lymphoma; NHS, Nurses’ Health Study; NMSC, nonmelanoma skin cancer; NR, not reported; NSAID, nonsteroidal AI drug; OER, occurred to expected ratio; OR, odds ratio; PUVA, psoralen–UV-A therapy; RR, risk ratio; RRR, relative RR; SCALE, Scandinavian Lymphoma Etiology Scale; SEER, Surveillance, Epidemiology, and End Results; SIR, standardized incidence ratio; SMR, standardized morbidity ratio; STI, sexually transmitted infection; THIN, The Health Improvement Network.
Table 2. Characteristics of Included Cancer Mortality in Psoriasis Studies.
| Source | Study Design (Outcome Measure) | Cancer Type | Population (No. of Participants) | Duration of Follow-up | Adjustment for Confounders | |
|---|---|---|---|---|---|---|
| Study | Comparison | |||||
| Abuabara et al,59 2010 | Cohort (HR) | All malignant neoplasms | Patients with psoriasis in primary care records (3603) | Psoriasis-free patients in primary care records (14 330) | Mean (psoriasis): 3.43 y | Adjusted: age and sex |
| Mean (psoriasis-free): 3.40 y | ||||||
| Boffeta et al,13 2001 | Cohort (SIR) | All malignant neoplasms | Discharged patients with psoriasis from Swedish health database (9773) | Swedish general population (NA) | Mean: 11 y | Standardized: age, sex, and CT |
| Castro et al,15 2014 | Cohort (SIR) | Hepatobiliary malignant neoplasms | Patients with psoriasis in the Swedish hospital discharge register (NR) | Swedish general population not hospitalized for any autoimmune disease (NA) | Mean: <1 y | Standardized: age, sex, CT, region, and socioeconomic status |
| Adjusted: smoking and alcoholism | ||||||
| Hemminki et al,32 2013 | Cohort (SIR) | Brain malignant neoplasms | Patients with psoriasis in hospital discharge records (19 777) | Swedish general population (NA) | Mean: 11.9 y | Standardized: age, sex, CT, region, and socioeconomic status |
| Adjusted: obesity, smoking, alcohol use, and socioeconomic features | ||||||
| Hemminki et al,33 2012 | Cohort (SIR) | Female malignant neoplasms | Patients with psoriasis in hospital discharge records (8162) | Swedish general population (NA) | Total (psoriasis): 275 971 person-years | Standardized: age, sex, CT, region, and socioeconomic status |
| Adjusted: obesity, smoking, alcohol use, parity, and age at first birth | ||||||
| Hemminki et al,60 2012 | Cohort (HR and SMR) | Digestive tract malignant neoplasms | Patients with psoriasis in hospital discharge records (15 592) | Swedish general population (NA) | Total (psoriasis): 222 027 person-years | Standardized: age, sex, period, region, and socioeconomic status |
| Adjusted: obesity, smoking, and alcohol use | ||||||
| Lee et al,61 2017 | Cohort (SMR) | All malignant neoplasms | Patients with psoriasis in the NHI database (80 167) | Taiwanese general population (NA) | Mean: 6.34 y | Standardized: age, sex, and CT |
| Lindqvist et al,62 2017 | Cohort (HR) | Multiple myeloma | Patients with multiple myeloma from Swedish cancer registry (8367) | General population with no hematological cancer (33 577) | NR | Matched: age, sex, and county |
| Adjusted: age, sex, and year of diagnosis | ||||||
| Liu et al,41 2013 | Cohort (SIR) | Urological malignant neoplasms | Patients with psoriasis in Swedish outpatient records (19 777) | Swedish outpatients with no record of 33 selected AIs (NA) | Total (psoriasis): 275 971 perons-years | Standardized: age, sex, CT, region, and socioeconomic status |
| Adjusted: obesity, smoking, and alcohol abuse | ||||||
| Poikolainen et al,63 1999 | Cohort (SMR) | All malignant neoplasms | Patients with psoriasis in hospital discharge records (5687) | Finnish general population (NA) | Mean: 14 y | Standardized: age, sex, and CT |
| Reddy et al,48 2017 | Cohort (HR) | Melanoma and hematological cancer | Patients with psoriasis in the KPSC records (8161) | Psoriasis-free patients in the KPSC records (807 604) | NR | Matched: age and sex |
| Adjusted: race, treatments, and Charlson comorbidity index | ||||||
| Shu et al,64 2011 | Cohort (HR) | All malignant neoplasms | Patients with psoriasis in hospital discharge records (1746) | Psoriasis-free patients in hospital discharge records (1 011 757) | Median (psoriasis): 1.83 y | Adjusted: age, sex, CT, smoking, alcohol consumption, and obesity |
| Median (psoriasis-free): 3.1 y | ||||||
| Stern and Lange,54 1988 | Cohort (SMR) | All malignant neoplasms | All patients with PUVA-treated psoriasis (1380) | General population from the SEER database (NA) | Total: 13 224 person-years | Standardized: age and sex |
| Matched: age, sex, and region | ||||||
| Stern and Huibregtse,65 2011 | Cohort (SMR and HR) | All malignant neoplasms | Patients with psoriasis from PUVA follow-up study (1376) | White US general population (NA) | Total (psoriasis): 30 817 person-years | Standardized: age, sex, and CT |
| Stratified: treatment, psoriasis severity, educational level, BMI, alcohol consumption, and smoking | ||||||
| Svedbom et al,66 2015 | Cohort (HR) | All malignant neoplasms | Patients with psoriasis in the VEGA register or Skane Health Care Register (39 074) | Swedish general population (154 775) | Mean (mild psoriasis): 4.6 y | Matched: age, sex, and region |
| Mean (severe psoriasis): 3.4 y | Adjusted: age, sex, and comorbidities | |||||
Abbreviations: AI, anti-inflammatory; BMI, body mass index; CT, calendar time; HR, hazard ratio; KPSC, Kaiser Permanente Southern California; NA, not available; NHI, national health insurance; NR, not reported; PUVA, psoralen–UV-A; SEER, Surveillance, Epidemiology, and End Results; SIR, standardized incidence ratio; SMR, standardized mortality ratio.
Study Quality
For the 50 studies examining cancer incidence, quality assessment ratings were mostly fair (29 [58%]) and the remainder graded as low (8 [16%]) and high (13 [26%]). The 15 studies of cancer mortality generally had much better quality, with most of these studies being high (8 [54%]); the remaining studies were graded as fair (5 [33%]) and low (7 [13%]). For cancer incidence and cancer mortality studies, quality was mostly lowered as a result of study participant selection. The comparability of study and comparison cohorts through matching or later adjustment also presented as an issue for a considerable number of studies. Low-quality outcome ascertainment and follow-up was the least common reason for low quality (10 studies [17%]).
Psoriasis and the Risk of Cancer Incidence
All Cancers
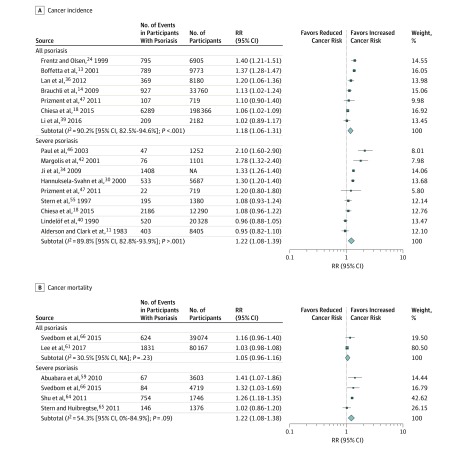
A total of 22 cohort studies11,13,14,17,18,19,24,27,28,29,30,31,34,36,39,40,42,44,46,47,54,55 reported estimates of risk of all cancer incidence in people with psoriasis. Nine severe psoriasis studies11,18,30,34,40,42,46,47,55 were suitable for inclusion in the meta-analysis (Figure 2A), producing a significantly elevated pooled RR of 1.22 (95% CI, 1.08-1.39) with high heterogeneity (I2 = 89.8% [95% CI, 82.8%-93.9%]). When taking into account psoriasis of all severities, 7 studies13,14,18,24,36,39,47 were included in the meta-analysis (Figure 2A), producing a significantly elevated pooled RR of 1.18 (95% CI, 1.06-1.31) with similarly high heterogeneity (I2 = 90.2% [95% CI, 82.5%-94.6%]). In contrast, 1 hospital-based case-control study57 also reported on the risk of all cancer in psoriasis of all severities, with a significantly reduced estimate; the corresponding odds ratio was 0.27 (95% CI, 0.16-0.44).
Figure 2. Risk of All Cancer Incidence and Mortality in Individuals With Psoriasis Stratified by Severity of Psoriasis.
NA indicates not available; RR, relative risk. Square size indicates the weight of the study; blue diamonds, the pooled RR size.
Site-Specific Cancers
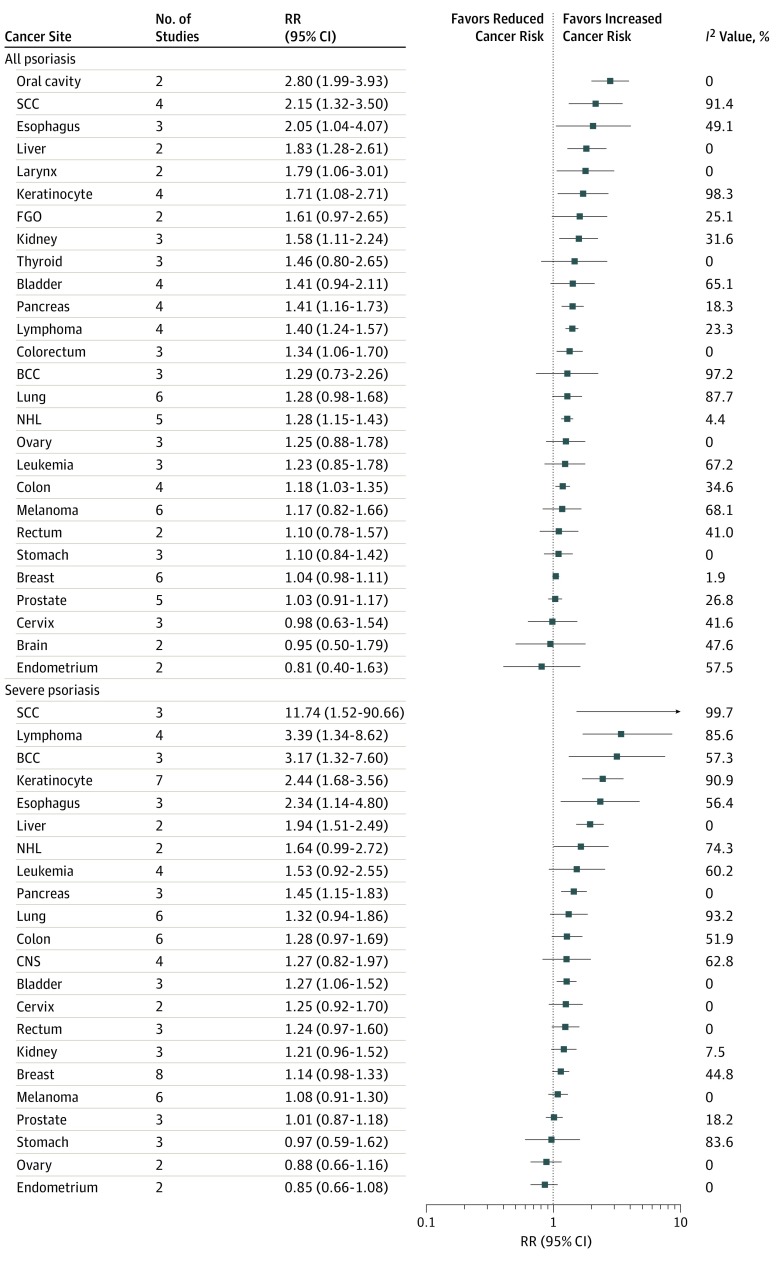
For severe psoriasis studies,10,11,15,18,21,28,30,33,34,39,40,41,42,43,46,47,52,54,55 meta-analysis was possible for a total of 22 site-specific cancers. Of these, incident risk was found to be significantly elevated in 8 site-specific cancers (Figure 3): squamous cell carcinoma (3 studies9,34,46; RR, 11.74 [95% CI, 1.52-90.66]; I2 = 99.7% [95% CI, 99.5%-99.8%]), lymphoma (4 studies18,42,46,52; RR, 3.39 [95% CI, 1.34-8.62]; I2 = 85.6% [95% CI, 64.6%-94.2%]), basal cell carcinoma (3 studies9,28,46; RR, 3.17 [95% CI, 1.32-7.60]; I2 = 57.3% [95% CI, 0%-87.8%]), keratinocyte (7 studies18,21,30,40,42,43,46; RR, 2.44 [95% CI, 1.68-3.56]; I2 = 90.9% [95% CI, 83.8%-94.9%]), esophagus (3 studies30,34,46; RR, 2.34 [95% CI, 1.14-4.80]; I2 = 56.4 [95% CI, 0%-87.5%]), liver (2 studies30,34; RR, 1.94 [95% CI, 1.51-2.49]; I2 = 0 [95% CI, not applicable [NA]), pancreas (3 studies18,30,34; RR, 1.45 [95% CI, 1.15-1.83]; I2 = 0 [95% CI, 0%-89.6%]), and bladder (3 studies11,28,41; RR, 1.27 [95% CI, 1.06-1.52]; I2 = 0 [95% CI, 0%-89.6%]). Meta-analysis was possible for 27 site-specific cancers from studies of all severities of psoriasis.10,13,14,16,17,18,23,24,26,35,38,39,44,45,47 Risk was significantly elevated in 12 site-specific cancers (Figure 3), including the oral cavity (2 studies13,24; RR, 2.80 [95% CI, 1.99-3.93]; I2 = 0 [95% CI, NA]), squamous cell carcinoma (4 studies10,13,16,24; RR, 2.15 [95% CI, 1.32-3.50]; I2 = 91.4% [95% CI, 81.2%-96.1%]), esophagus (3 studies13,14,44; RR, 2.05 [95% CI, 1.04-4.07]; I2 = 49.1% [95% CI, 0%-85.2%]), liver (2 studies13,44; RR, 1.83 [95% CI, 1.28-2.61]; I2 = 0 [95% CI, NA]), larynx (2 studies13,24; RR, 1.79 [95% CI, 1.06-3.01]; I2 = 0 [95% CI, NA]), keratinocyte (4 studies18,24,38,45; RR, 1.71 [95% CI, 1.08-2.71]; I2 = 98.3% [95% CI, 97.3%-99.0%]), kidney (3 studies13,24,39; RR, 1.58 [95% CI, 1.11-2.24]; I2 = 31.6% [95% CI, 0%-92.9%]), pancreas (4 studies13,14,18,44; RR, 1.41 [95% CI, 1.16-1.73]; I2 = 18.3% [95% CI, 0%-87.5%]), lymphoma (4 studies14,18,26,38; RR, 1.40 [95% CI, 1.24-1.57]; I2 = 23.3% [95% CI, 0%-88.3%]), colorectum (3 studies14,17,39; RR, 1.34 [95% CI, 1.06-1.70]; I2 = 0 [95% CI, 0%-89.6%]), non-Hodgkin lymphoma (5 studies13,23,24,26,39; RR, 1.28 [95% CI, 1.15-1.43]; I2 = 4.4% [95% CI, 0%-80.1%]), and colon (4 studies13,18,24,47; RR, 1.18 [95% CI, 1.03-1.35]; I2 = 34.6% [95% CI, 0%-77.1%]). Meta-analysis of a further 7 site-specific cancers was also possible for 7 case-control studies of all severities of psoriasis.12,22,37,49,50,57,58 Cancer risk was significantly elevated in T-cell lymphoma (3 studies22,50,58; RR, 2.65 [95% CI, 1.30-5.41]; I2 = 80.2% [95% CI, 37.7%-93.7%]), marginal zone lymphoma (3 studies22,50,58; RR, 1.31 [95% CI, 1.06-1.62]; I2 = 0 [95% CI, 0%-89.6%]), and B-cell lymphoma (3 studies12,50,58; RR, 1.20 [95% CI, 1.03-1.39]; I2 = 0 [95% CI, 0%-89.6%]).
Figure 3. Risk of Site-Specific Cancer Incidence by Severity of Psoriasis.
BCC indicates basal cell carcinoma; CNS, central nervous system; FGO, female genital organs; NHL, non-Hodgkin lymphoma; RR, relative risk; and SCC, squamous cell carcinoma.
Psoriasis and Risk of Cancer Mortality
All Cancers
A total of 7 cohort studies13,54,59,61,64,65,66 considered the risk of overall cancer mortality in people with psoriasis. Four studies of severe psoriasis59,64,65,66 produced a pooled RR of 1.22 (95% CI, 1.08-1.38) with considerable heterogeneity (I2 = 54.3% [95% CI, 0%-84.9%]) (Figure 2B). Only 2 studies61,66 reported mortality risk estimates from all cancers in people with psoriasis of all severities. Pooling of these 2 studies found no difference in risk of cancer mortality; the corresponding pooled RR was 1.05 (95% CI, 0.96-1.16) with slight heterogeneity (I2 = 30.5% [95% CI, NA]) (Figure 2B).
Site-Specific Cancers
Meta-analysis of cancer mortality was possible for 7 site-specific cancers from studies involving people with severe psoriasis.15,32,33,60,63,64 Of these 7 individual cancers, mortality risk was found to be significantly elevated in the following 3 sites: esophagus (2 studies60,63; RR, 2.53 [95% CI, 1.87-3.41]; I2 = 0 [95% CI, NA]), liver (2 studies63,64; RR, 1.43 [95% CI, 1.09-1.88]; I2 = 0 [95% CI, NA]), and pancreas (2 studies63,64; RR, 1.31 [95% CI, 1.02-1.69]; I2 = 0.6% [95% CI, NA]).
Subgroup Analysis
Of the 9 studies included in the meta-analysis of all cancer risk for people with severe psoriasis, 7 studies11,30,34,40,42,46,55 conducted age and sex (level 1) adjustment, with the remaining 2 studies18,47 controlling for age, sex, and other factors (level 2) adjustment. Meta-analysis of studies with level 1 adjustment produced a pooled RR of 1.25 (95% CI, 1.08-1.45; I2 = 91.9% [95% CI, 85.9%-95.4%]) (eFigure in the Supplement). Conversely, meta-analysis of studies with level 2 adjustment produced attenuated results (RR, 1.09 [95% CI. 0.97-1.22]; I2 = 0 [95% CI, NA]). Marked attenuation of all cancer incidence risk for all severities of psoriasis was also observed when comparing level 1 adjustment studies (3 studies13,24,36; RR, 1.33 [95% CI, 1.23-1.44]; I2 = 49.9% [95% CI, 0%-85.5%]) with level 2 adjustment studies (4 studies14,18,39,47; RR, 1.07 [95% CI, 1.03-1.10]; I2 = 0 [95% CI, 0%-84.7%]). Stratification of studies by the level of adjustment for 6 site-specific cancers was also undertaken for studies involving people with all severities of psoriasis. For 5 of these cancers, the pooled estimates were lower in studies involving level 2 adjustment. The risk of all cancer mortality in severe psoriasis was higher in 4 studies with level 1 adjustment,13,54,59,66 with a pooled RR of 1.29 (95% CI, 1.17-1.42; I2 = 0 [95% CI, 0%-84.7%]) compared with RR of 1.15 (95% CI, 0.94-1.41; I2 = 81.2% [95% CI, NA]) for 2 studies with level 2 adjustment64,65 (eFigure in the Supplement). Low study numbers prevented further stratification for site-specific cancer mortality.
Discussion
We found significant associations between psoriasis and the incidence of and mortality due to cancer involving a range of site-specific cancers. Cohort studies of all severities of psoriasis suggested a 1.18-fold increased risk of developing cancer compared with psoriasis-free populations. Evidence from cohort studies of severe psoriasis indicated a 1.22-fold increased risk of developing cancer when compared with populations without psoriasis, a result not significantly different from that of all psoriasis severities. With regard to cancer mortality, a 1.22-fold increased risk of dying due to cancer compared with psoriasis-free populations was observed in studies of severe psoriasis. In contrast, no significantly increased risk of cancer mortality was found in studies of all severities of psoriasis. Subgroup analysis by level of adjustment for confounders found marked attenuation of all cancer incidence and mortality risks in studies that additionally controlled for smoking, alcohol consumption, and obesity.
With regard to site-specific cancers, we observed elevated incident cancer risks for lymphoma, keratinocyte, esophageal, liver, and pancreatic cancers in studies of severe psoriasis and in those involving people with all severities of psoriasis. Similarly, esophageal, liver, and pancreatic cancers also demonstrated an increased risk when considering cancer mortality in studies of severe psoriasis.
A number of hypothesized explanations exist for the increased risk of cancer in people with psoriasis. First, psoriasis is a chronic inflammatory disease, and the link between chronic inflammation and cancer has been well reported in other conditions, such as Crohn disease and Barrett esophagus.4 This explanation may hold particular sway when considering the suggested increased risk of pancreatic cancer incidence in people with psoriasis. Indeed, previous studies have noted an association between psoriasis and pancreatitis,67 with acknowledged links between pancreatitis and pancreatic cancer. Beyond the underlying inflammatory mechanisms of psoriasis, the use of immunomodulatory agents and potentially carcinogenic therapies in psoriasis treatment has also been explored as a mechanism for increased cancer risk, particularly for cancers such as lymphoma. The use of immunomodulatory agents and increased cancer incidence has previously been suggested in other conditions, such as rheumatoid arthritis. However, a meta-analysis of the association between rheumatoid arthritis and cancer suggested a lower risk of all cancer (standardized incidence ratio, 1.05; 95% CI, 1.01-1.09) compared with that found in psoriasis.68 Of particular relevance in this regard are biological therapies, which are being increasingly used for the management of severe psoriasis. Although preliminary studies have suggested little to no increased risk of cancer incidence in patients with psoriasis receiving these therapies, further study allowing greater follow-up and increased power is required to properly examine the potential cancer risk, particularly for site-specific cancers.69 In contrast to this, the association between phototherapy in psoriasis and cancer incidence has been extensively explored, with an increased risk of squamous cell carcinoma and basal cell carcinoma being well established.9 Furthermore, the increased prevalence of known cancer risk factors in people with psoriasis, such as smoking, excessive alcohol consumption, and obesity, has also been posited as a plausible explanation for an association with cancer. The potential role of these lifestyle factors is strengthened by the attenuation of risk in those studies14,18,39,47,54,64 that adjusted estimates for lifestyle factors and the increased risk of site-specific cancers, such as esophageal cancer and liver cancer, which have been reported to be independently associated with obesity, smoking, and higher alcohol consumption.70
Despite these findings, cancer currently receives relatively little focus in guidelines for the management of psoriasis, compared with other comorbidities, such as cardiovascular disease. The National Institute for Health and Care Excellence guidelines for the assessment and management of psoriasis71 and the Canadian guidelines for psoriasis management72 do not give any consideration to cancer as a comorbidity of psoriasis. In addition, guidelines produced by the Scottish Intercollegiate Guidelines Network and the American Academy of Dermatology73 give consideration only to lymphoma. The evidence from this meta-analysis not only suggests that cancer should be given more consideration as an important comorbidity of psoriasis but also begins to present evidence that this risk could be alleviated to some extent through lifestyle behavior change. Although potential drivers of lifestyle behavior change, such as structured weight reduction and psychological counseling for alcohol use disorders, have been considered as a means to reduce the severity of psoriasis and alcohol-related mortality,74 their implementation may have the concurrent effect of attenuating some of the cancer risk. Although it has been noted that lifestyle behavior change is challenging for health care professionals to implement,75 the importance of a more holistic approach to psoriasis care involving lifestyle behavior change is reinforced through the results of this meta-analysis.
Limitations
To our knowledge, this is the only meta-analysis of cancer risk that has stratified by psoriasis severity and examined the risk of cancer mortality in people with psoriasis. The stratification provided greater insight into the factors that may be implicit in the association between psoriasis and cancer, because certain treatments are more prevalent in those with severe disease. Further value is provided by this study in the consideration of site-specific cancers.
Potential limitations also need to be acknowledged in interpreting the findings, particularly the high levels of heterogeneity between some studies. Given the observed heterogeneity, we used a random-effects model to meta-analyze studies and conducted subgroup analyses to try to explain heterogeneity. Of interest, heterogeneity was largely reduced for overall cancer risk for severe and all severities of psoriasis through the grouping of studies that adjusted risk estimates for established cancer risk factors.
With regard to adjustment for potential confounders, a possible limitation to the wider consideration of the association between psoriasis and cancer is the lens through which smoking, alcohol consumption, and obesity are considered, as confounding factors or ones that lie on the causal pathway. Because it is possible that these factors, particularly obesity, lie on the causal pathway, it may not be appropriate to consider them simply by adjustment.
Two other limitations, similarly to this study and the wider examination of the association between psoriasis and cancer, are the classification of psoriasis severity and the reporting of duration of treatment exposure. Because a large number of contemporary studies, including a considerable number in this meta-analysis, use electronic health record databases, which do not include the psoriasis area and severity index (PASI), little information is available regarding the risk of cancer according to PASI-classified psoriasis severity. Indeed, only Dai et al (2016)10 presented the risk of cancer according to PASI. Due to the lack of PASI data, studies were unable to account for psoriasis severity or, as in this study, were required to use potentially suboptimal proxies to determine severe psoriasis, such as the receiving of systemic therapies or hospitalization for psoriasis. Although this study did not focus on cancer risk according to specific treatments for psoriasis, it was notable that very few included studies reported on the length of treatment exposures, and in those that did, duration of exposure was often only provided for phototherapy. Future studies providing detailed information regarding the length of exposure to treatments, using registries such as the British Association of Dermatologists Biologic and Immunomodulators Register, are essential to understand the role of psoriasis treatments in the association between the condition and cancer.
Conclusions
People with psoriasis have an elevated risk of developing or dying of cancer, particularly for a number of site-specific cancers. To improve the understanding of the link between psoriasis and cancer, further population-based studies are needed. Where possible, considering the association according to different severities of psoriasis would be beneficial. Understanding the role of lifestyle factors in any increased cancer risk remains challenging, but studies giving greater consideration of these factors would be of benefit.
eTable 1. Database Search Strategy in MEDLINE and EMBASE
eTable 2. MOOSE Checklist for Meta-analyses of Observational Studies
eMethods. Method for Estimate Combination
eFigure. Risk of Overall Cancer Incidence and Mortality According to Severity of Psoriasis and Level of Adjustment for Confounders
eReferences.
References
- 1.Parisi R, Symmons DP, Griffiths CE, Ashcroft DM; Identification and Management of Psoriasis and Associated ComorbidiTy (IMPACT) project team . Global epidemiology of psoriasis: a systematic review of incidence and prevalence. J Invest Dermatol. 2013;133(2):377-385. doi: 10.1038/jid.2012.339 [DOI] [PubMed] [Google Scholar]
- 2.Krueger G, Koo J, Lebwohl M, Menter A, Stern RS, Rolstad T. The impact of psoriasis on quality of life: results of a 1998 National Psoriasis Foundation patient-membership survey. Arch Dermatol. 2001;137(3):280-284. doi:10-1001/pubs.Arch Dermatol.-ISSN-0003-987x-137-3-dst0024 [PubMed] [Google Scholar]
- 3.Guenther L, Gulliver W. Psoriasis comorbidities. J Cutan Med Surg. 2009;13(suppl 2):S77-S87. doi: 10.2310/7750.2009.00024 [DOI] [PubMed] [Google Scholar]
- 4.Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002;420(6917):860-867. doi: 10.1038/nature01322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pouplard C, Brenaut E, Horreau C, et al. . Risk of cancer in psoriasis: a systematic review and meta-analysis of epidemiological studies. J Eur Acad Dermatol Venereol. 2013;27(suppl 3):36-46. doi: 10.1111/jdv.12165 [DOI] [PubMed] [Google Scholar]
- 6.Fu Y, Lee CH, Chi CC. Association of psoriasis with inflammatory bowel disease: a systematic review and meta-analysis. JAMA Dermatol. 2018;154(12):1417-1423. doi: 10.1001/jamadermatol.2018.3631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ramirez JA, McIntosh AG, Strehlow R, Lawrence VA, Parekh DJ, Svatek RS. Definition, incidence, risk factors, and prevention of paralytic ileus following radical cystectomy: a systematic review. Eur Urol. 2013;64(4):588-597. doi: 10.1016/j.eururo.2012.11.051 [DOI] [PubMed] [Google Scholar]
- 8.Chapter 9: analysing data and undertaking meta-analyses. In: Deeks JJ, Higgins JPT, Altman DG; Cochrane Statistical Methods Group, eds. Cochrane Handbook for Systematic Reviews of Interventions. https://handbook-5-1.cochrane.org/chapter_9/9_analysing_data_and_undertaking_meta_analyses.htm. Published 2011. Accessed January 25, 2018. [Google Scholar]
- 9.Stern RS; PUVA Follow-Up Study . The risk of squamous cell and basal cell cancer associated with psoralen and ultraviolet A therapy: a 30-year prospective study. J Am Acad Dermatol. 2012;66(4):553-562. doi: 10.1016/j.jaad.2011.04.004 [DOI] [PubMed] [Google Scholar]
- 10.Dai H, Li WQ, Qureshi AA, Han J. Personal history of psoriasis and risk of nonmelanoma skin cancer (NMSC) among women in the United States: a population-based cohort study. J Am Acad Dermatol. 2016;75(4):731-735. doi: 10.1016/j.jaad.2016.05.021 [DOI] [PubMed] [Google Scholar]
- 11.Alderson MR, Clarke JA. Cancer incidence in patients with psoriasis. Br J Cancer. 1983;47(6):857-859. doi: 10.1038/bjc.1983.142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Anderson LA, Gadalla S, Morton LM, et al. . Population-based study of autoimmune conditions and the risk of specific lymphoid malignancies. Int J Cancer. 2009;125(2):398-405. doi: 10.1002/ijc.24287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Boffetta P, Gridley G, Lindelöf B. Cancer risk in a population-based cohort of patients hospitalized for psoriasis in Sweden. J Invest Dermatol. 2001;117(6):1531-1537. doi: 10.1046/j.0022-202x.2001.01520.x [DOI] [PubMed] [Google Scholar]
- 14.Brauchli YB, Jick SS, Miret M, Meier CR. Psoriasis and risk of incident cancer: an inception cohort study with a nested case-control analysis. J Invest Dermatol. 2009;129(11):2604-2612. doi: 10.1038/jid.2009.113 [DOI] [PubMed] [Google Scholar]
- 15.Castro FA, Liu X, Försti A, et al. . Increased risk of hepatobiliary cancers after hospitalization for autoimmune disease. Clin Gastroenterol Hepatol. 2014;12(6):1038-1045.e7. doi: 10.1016/j.cgh.2013.11.007 [DOI] [PubMed] [Google Scholar]
- 16.Chen M, Qureshi A, Li T, Han J. Personal history of psoriasis and the risk of non-melanoma skin cancers. J Invest Dermatol. 2013;133:S95. [Google Scholar]
- 17.Chen YJ, Wu CY, Chen TJ, et al. . The risk of cancer in patients with psoriasis: a population-based cohort study in Taiwan. J Am Acad Dermatol. 2011;65(1):84-91. doi: 10.1016/j.jaad.2010.04.046 [DOI] [PubMed] [Google Scholar]
- 18.Chiesa ZC, Shin DB, Takeshita J, Ogdie A, Gelfand J. The risk of cancer in patients with psoriasis: a population-based cohort study in the United Kingdom. J Invest Dermatol. 2015;135:S52. doi: 10.1001/jamadermatol.2015.4847 [DOI] [Google Scholar]
- 19.Chiou MJ, Fang YF, Kuo CF. Increased risk of cancer in patients with psoriasis: a nationwide population study. Ann Rheum Dis. 2016;75:606. doi: 10.1136/annrheumdis-2016-eular.4486 [DOI] [Google Scholar]
- 20.Cooper GS, Kamel F, Sandler DP, Davey FR, Bloomfield CD. Risk of adult acute leukemia in relation to prior immune-related conditions. Cancer Epidemiol Biomarkers Prev. 1996;5(11):867-872. [PubMed] [Google Scholar]
- 21.Egeberg A, Thyssen JP, Gislason GH, Skov L. Skin cancer in patients with psoriasis. J Eur Acad Dermatol Venereol. 2016;30(8):1349-1353. doi: 10.1111/jdv.13619 [DOI] [PubMed] [Google Scholar]
- 22.Engels EA, Parsons R, Besson C, et al. . Comprehensive evaluation of medical conditions associated with risk of non–Hodgkin lymphoma using Medicare claims (“MedWAS”). Cancer Epidemiol Biomarkers Prev. 2016;25(7):1105-1113. doi: 10.1158/1055-9965.EPI-16-0212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fallah M, Liu X, Ji J, Försti A, Sundquist K, Hemminki K. Autoimmune diseases associated with non-Hodgkin lymphoma: a nationwide cohort study. Ann Oncol. 2014;25(10):2025-2030. doi: 10.1093/annonc/mdu365 [DOI] [PubMed] [Google Scholar]
- 24.Frentz G, Olsen JH. Malignant tumours and psoriasis: a follow-up study. Br J Dermatol. 1999;140(2):237-242. doi: 10.1046/j.1365-2133.1999.02655.x [DOI] [PubMed] [Google Scholar]
- 25.Gelfand JM, Berlin J, Van Voorhees A, Margolis DJ. Lymphoma rates are low but increased in patients with psoriasis: results from a population-based cohort study in the United Kingdom. Arch Dermatol. 2003;139(11):1425-1429. doi: 10.1001/archderm.139.11.1425 [DOI] [PubMed] [Google Scholar]
- 26.Gelfand JM, Shin DB, Neimann AL, Wang X, Margolis DJ, Troxel AB. The risk of lymphoma in patients with psoriasis. J Invest Dermatol. 2006;126(10):2194-2201. doi: 10.1038/sj.jid.5700410 [DOI] [PubMed] [Google Scholar]
- 27.Gu Y, Nordstrom BL. The risk of malignancy among biologic-naïve pediatric psoriasis patients: a retrospective cohort study in a US claims database. J Am Acad Dermatol. 2017;77(2):293-301.e1. doi: 10.1016/j.jaad.2017.03.044 [DOI] [PubMed] [Google Scholar]
- 28.Hannuksela A, Pukkala E, Hannuksela M, Karvonen J. Cancer incidence among Finnish patients with psoriasis treated with trioxsalen bath PUVA. J Am Acad Dermatol. 1996;35(5 Pt 1):685-689. doi: 10.1016/S0190-9622(96)90721-5 [DOI] [PubMed] [Google Scholar]
- 29.Hannuksela-Svahn A, Sigurgeirsson B, Pukkala E, et al. . Trioxsalen bath PUVA did not increase the risk of squamous cell skin carcinoma and cutaneous malignant melanoma in a joint analysis of 944 Swedish and Finnish patients with psoriasis. Br J Dermatol. 1999;141(3):497-501. doi: 10.1046/j.1365-2133.1999.03044.x [DOI] [PubMed] [Google Scholar]
- 30.Hannuksela-Svahn A, Pukkala E, Läärä E, Poikolainen K, Karvonen J. Psoriasis, its treatment, and cancer in a cohort of Finnish patients. J Invest Dermatol. 2000;114(3):587-590. doi: 10.1046/j.1523-1747.2000.00898.x [DOI] [PubMed] [Google Scholar]
- 31.Hannuksela-Svahn A, Pukkala E, Koulu L, Jansén CT, Karvonen J. Cancer incidence among Finnish psoriasis patients treated with 8-methoxypsoralen bath PUVA. J Am Acad Dermatol. 1999;40(5 Pt 1):694-696. doi: 10.1016/S0190-9622(99)70148-9 [DOI] [PubMed] [Google Scholar]
- 32.Hemminki K, Liu X, Försti A, Ji J, Sundquist J, Sundquist K. Subsequent brain tumors in patients with autoimmune disease. Neuro Oncol. 2013;15(9):1142-1150. doi: 10.1093/neuonc/not070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hemminki K, Liu X, Ji J, Försti A, Sundquist J, Sundquist K. Effect of autoimmune diseases on risk and survival in female cancers. Gynecol Oncol. 2012;127(1):180-185. doi: 10.1016/j.ygyno.2012.07.100 [DOI] [PubMed] [Google Scholar]
- 34.Ji J, Shu X, Sundquist K, Sundquist J, Hemminki K. Cancer risk in hospitalised psoriasis patients: a follow-up study in Sweden. Br J Cancer. 2009;100(9):1499-1502. doi: 10.1038/sj.bjc.6605027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kim SC, Glynn RJ, Giovannucci E, et al. . Risk of high-grade cervical dysplasia and cervical cancer in women with systemic inflammatory diseases: a population-based cohort study. Ann Rheum Dis. 2015;74(7):1360-1367. doi: 10.1136/annrheumdis-2013-204993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lan CCE, Ko YC, Yu HS, et al. . Psoriatic patients with diabetes are prone to develop digestive organ cancers: a population-based study in Taiwan. J Dermatol Sci. 2012;68(2):82-88. doi: 10.1016/j.jdermsci.2012.08.004 [DOI] [PubMed] [Google Scholar]
- 37.Lanoy E, Engels EA. Skin cancers associated with autoimmune conditions among elderly adults. Br J Cancer. 2010;103(1):112-114. doi: 10.1038/sj.bjc.6605733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lee MS, Lin RY, Chang YT, Lai MS. The risk of developing non-melanoma skin cancer, lymphoma and melanoma in patients with psoriasis in Taiwan: a 10-year, population-based cohort study. Int J Dermatol. 2012;51(12):1454-1460. doi: 10.1111/j.1365-4632.2011.05310.x [DOI] [PubMed] [Google Scholar]
- 39.Li WQ, Han J, Cho E, et al. . Personal history of psoriasis and risk of incident cancer among women: a population-based cohort study. Br J Dermatol. 2016;174(5):1108-1111. doi: 10.1111/bjd.14301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lindelöf B, Eklund G, Lidén S, Stern RS. The prevalence of malignant tumors in patients with psoriasis. J Am Acad Dermatol. 1990;22(6, pt 1):1056-1060. doi: 10.1016/0190-9622(90)70152-8 [DOI] [PubMed] [Google Scholar]
- 41.Liu X, Ji J, Forsti A, Sundquist K, Sundquist J, Hemminki K. Autoimmune disease and subsequent urological cancer. J Urol. 2013;189(6):2262-2268. doi: 10.1016/j.juro.2012.12.014 [DOI] [PubMed] [Google Scholar]
- 42.Margolis D, Bilker W, Hennessy S, Vittorio C, Santanna J, Strom BL. The risk of malignancy associated with psoriasis. Arch Dermatol. 2001;137(6):778-783. doi:10-1001/pubs.Arch Dermatol.-ISSN-0003-987x-137-6-dea00008 [PubMed] [Google Scholar]
- 43.McKenna KE, Patterson CC, Handley J, McGinn S, Allen G. Cutaneous neoplasia following PUVA therapy for psoriasis. Br J Dermatol. 1996;134(4):639-642. doi: 10.1111/j.1365-2133.1996.tb06962.x [DOI] [PubMed] [Google Scholar]
- 44.Olsen JH, Møller H, Frentz G. Malignant tumors in patients with psoriasis. J Am Acad Dermatol. 1992;27(5, pt 1):716-722. doi: 10.1016/0190-9622(92)70244-A [DOI] [PubMed] [Google Scholar]
- 45.Paradisi A, Didona B, Tabolli S, et al. . Reduced frequency of non-melanoma skin cancer in 72 739 patients with psoriasis: a retrospective study. Eur J Dermatol. 2017;27(4):359-362. doi: 10.1684/ejd.2017.3032 [DOI] [PubMed] [Google Scholar]
- 46.Paul CF, Ho VC, McGeown C, et al. . Risk of malignancies in psoriasis patients treated with cyclosporine: a 5 y cohort study. J Invest Dermatol. 2003;120(2):211-216. doi: 10.1046/j.1523-1747.2003.12040.x [DOI] [PubMed] [Google Scholar]
- 47.Prizment AE, Alonso A, Folsom AR, et al. . Association between psoriasis and incident cancer: the Iowa’s Women’s Health Study. Cancer Causes Control. 2011;22(7):1003-1010. doi: 10.1007/s10552-011-9773-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Reddy SP, Martires K, Wu JJ. The risk of melanoma and hematologic cancers in patients with psoriasis. J Am Acad Dermatol. 2017;76(4):639-647.e2. doi: 10.1016/j.jaad.2016.09.047 [DOI] [PubMed] [Google Scholar]
- 49.Shapiro J, Pavlovski L, Cohen A, Hodak E, David M. Psoriasis and co-morbidities—new aspects. J Eur Acad Dermatol Venereol. 2013;27:39. [Google Scholar]
- 50.Smedby KE, Hjalgrim H, Askling J, et al. . Autoimmune and chronic inflammatory disorders and risk of non-Hodgkin lymphoma by subtype. J Natl Cancer Inst. 2006;98(1):51-60. doi: 10.1093/jnci/djj004 [DOI] [PubMed] [Google Scholar]
- 51.Söderberg KC, Jonsson F, Winqvist O, Hagmar L, Feychting M. Autoimmune diseases, asthma and risk of haematological malignancies: a nationwide case-control study in Sweden. Eur J Cancer. 2006;42(17):3028-3033. doi: 10.1016/j.ejca.2006.04.021 [DOI] [PubMed] [Google Scholar]
- 52.Stern RS. Lymphoma risk in psoriasis: results of the PUVA follow-up study. Arch Dermatol. 2006;142(9):1132-1135. doi: 10.1001/archderm.142.9.1132 [DOI] [PubMed] [Google Scholar]
- 53.Stern RS, Bagheri S, Nichols K; PUVA Follow Up Study . The persistent risk of genital tumors among men treated with psoralen plus ultraviolet A (PUVA) for psoriasis. J Am Acad Dermatol. 2002;47(1):33-39. doi: 10.1067/mjd.2002.124618 [DOI] [PubMed] [Google Scholar]
- 54.Stern RS, Lange R. Cardiovascular disease, cancer, and cause of death in patients with psoriasis: 10 years prospective experience in a cohort of 1380 patients. J Invest Dermatol. 1988;91(3):197-201. doi: 10.1111/1523-1747.ep12464847 [DOI] [PubMed] [Google Scholar]
- 55.Stern RS, Väkevä LH; PUVA Follow-up Study . Noncutaneous malignant tumors in the PUVA follow-up study: 1975-1996. J Invest Dermatol. 1997;108(6):897-900. doi: 10.1111/1523-1747.ep12292698 [DOI] [PubMed] [Google Scholar]
- 56.Sunesen KG, Nørgaard M, Thorlacius-Ussing O, Laurberg S. Immunosuppressive disorders and risk of anal squamous cell carcinoma: a nationwide cohort study in Denmark, 1978-2005. Int J Cancer. 2010;127(3):675-684. doi: 10.1002/ijc.25080 [DOI] [PubMed] [Google Scholar]
- 57.Tseng HW, Lin HS, Lam HC. Co-morbidities in psoriasis: a hospital-based case-control study. J Eur Acad Dermatol Venereol. 2013;27(11):1417-1425. doi: 10.1111/jdv.12028 [DOI] [PubMed] [Google Scholar]
- 58.Zhang Y, Holford TR, Leaderer B, et al. . Prior medical conditions and medication use and risk of non–Hodgkin lymphoma in Connecticut United States women. Cancer Causes Control. 2004;15(4):419-428. doi: 10.1023/B:CACO.0000027506.55846.5d [DOI] [PubMed] [Google Scholar]
- 59.Abuabara K, Azfar RS, Shin DB, Neimann AL, Troxel AB, Gelfand JM. Cause-specific mortality in patients with severe psoriasis: a population-based cohort study in the UK. Br J Dermatol. 2010;163(3):586-592. doi: 10.1111/j.1365-2133.2010.09941.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hemminki K, Liu X, Ji J, Sundquist J, Sundquist K. Effect of autoimmune diseases on mortality and survival in subsequent digestive tract cancers. Ann Oncol. 2012;23(8):2179-2184. doi: 10.1093/annonc/mdr590 [DOI] [PubMed] [Google Scholar]
- 61.Lee M-S, Yeh Y-C, Chang Y-T, Lai M-S. All-cause and cause-specific mortality in patients with psoriasis in Taiwan: a nationwide population-based study. J Invest Dermatol. 2017;137(7):1468-1473. doi: 10.1016/j.jid.2017.01.036 [DOI] [PubMed] [Google Scholar]
- 62.Lindqvist EK, Landgren O, Lund SH, et al. . History of autoimmune disease is associated with impaired survival in multiple myeloma and monoclonal gammopathy of undetermined significance: a population-based study. Ann Hematol. 2017;96(2):261-269. doi: 10.1007/s00277-016-2859-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Poikolainen K, Karvonen J, Pukkala E. Excess mortality related to alcohol and smoking among hospital-treated patients with psoriasis. Arch Dermatol. 1999;135(12):1490-1493. doi: 10.1001/archderm.135.12.1490 [DOI] [PubMed] [Google Scholar]
- 64.Shu X, Ji J, Sundquist J, Sundquist K, Hemminki K. Survival in cancer patients hospitalized for psoriasis: a population-based cohort study in Sweden. Br J Dermatol. 2011;165(1):129-136. doi: 10.1111/j.1365-2133.2011.10268.x [DOI] [PubMed] [Google Scholar]
- 65.Stern RS, Huibregtse A. Very severe psoriasis is associated with increased noncardiovascular mortality but not with increased cardiovascular risk. J Invest Dermatol. 2011;131(5):1159-1166. doi: 10.1038/jid.2010.399 [DOI] [PubMed] [Google Scholar]
- 66.Svedbom A, Dalén J, Mamolo C, et al. . Increased cause-specific mortality in patients with mild and severe psoriasis: a population-based Swedish register study. Acta Derm Venereol. 2015;95(7):809-815. doi: 10.2340/00015555-2095 [DOI] [PubMed] [Google Scholar]
- 67.Chiu H-Y, Hsieh C-F, Chiang Y-T, Huang W-F, Tsai T-F. The risk of chronic pancreatitis in patients with psoriasis: a population-based cohort study. PLoS One. 2016;11(7):e0160041. doi: 10.1371/journal.pone.0160041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Smitten AL, Simon TA, Hochberg MC, Suissa S. A meta-analysis of the incidence of malignancy in adult patients with rheumatoid arthritis. Arthritis Res Ther. 2008;10(2):R45. doi: 10.1186/ar2404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Garcia-Doval I, Descalzo MA, Mason KJ, et al. ; Psonet Network . Cumulative exposure to biological therapy and risk of cancer in patients with psoriasis: a meta-analysis of PSONET studies from Israel, Italy, Spain, the UK and Republic of Ireland. Br J Dermatol. 2018;179(4):863-871. doi: 10.1111/bjd.16715 [DOI] [PubMed] [Google Scholar]
- 70.Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65(2):87-108. doi: 10.3322/caac.21262 [DOI] [PubMed] [Google Scholar]
- 71.Samarasekera E, Sawyer L, Parnham J, Smith CH; Guideline Development Group . Assessment and management of psoriasis: summary of NICE guidance. BMJ. 2012;345:e6712. doi: 10.1136/bmj.e6712 [DOI] [PubMed] [Google Scholar]
- 72.Canadian Dermatology Association. Canadian guidelines for the management of plaque psoriasis. https://www.dermatology.ca/wp-content/uploads/2012/01/cdnpsoriasisguidelines.pdf Published 2009. Assessed January 12, 2018.
- 73.Menter A, Korman NJ, Elmets CA, et al. ; American Academy of Dermatology Work Group . Guidelines of care for the management of psoriasis and psoriatic arthritis: section 6: guidelines of care for the treatment of psoriasis and psoriatic arthritis: case-based presentations and evidence-based conclusions. J Am Acad Dermatol. 2011;65(1):137-174. doi: 10.1016/j.jaad.2010.11.055 [DOI] [PubMed] [Google Scholar]
- 74.Parisi R, Webb RT, Carr MJ, et al. . Alcohol-related mortality in patients with psoriasis: a population-based cohort study. JAMA Dermatol. 2017;153(12):1256-1262. doi: 10.1001/jamadermatol.2017.3225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Nelson PA, Keyworth C, Chisholm A, et al. ; Identification and Management of Psoriasis-Associated Co-Morbidity (IMPACT) Team . “In someone’s clinic but not in mine”—clinicians’ views of supporting lifestyle behaviour change in patients with psoriasis: a qualitative interview study. Br J Dermatol. 2014;171(5):1116-1122. doi: 10.1111/bjd.13231 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable 1. Database Search Strategy in MEDLINE and EMBASE
eTable 2. MOOSE Checklist for Meta-analyses of Observational Studies
eMethods. Method for Estimate Combination
eFigure. Risk of Overall Cancer Incidence and Mortality According to Severity of Psoriasis and Level of Adjustment for Confounders
eReferences.