Abstract
Species with a broad and flexible diet may be at an advantage in a rapidly changing environment such as in today's Arctic ecosystems. Polar cod (Boreogadus saida), an abundant and ecologically important circumpolar Arctic fish, is often described as a zooplankton generalist feeder, which suggests that it may cope successfully with changes in prey composition. This description is justified based on the relatively broad diet of polar cod across sites and seasons. In this case study, we used polar cod dietary data from fall and winter and from two distinct environments, dominated either by Arctic or Atlantic water masses in Svalbard. Our results point to the importance of time and space when drawing conclusions on dietary plasticity and degree of specialization. Polar cod diet differed significantly between fall and the winter and between Arctic and Atlantic domains. Polar cod from Arctic domains displayed a strong realized population specialization on Themisto libellula in fall, and the larger dietary niche width observed in the winter was the product of realized individual specialization, with increased feeding on fish prey. Overall, we did not observe a generalized feeding behavior. If dietary niche width is to inform conservation management, we argue it must be recognized that populations from a single species may adopt seasonally contrasting degrees of dietary specialization and that these populations may differ in their vulnerability to climate‐induced changes in prey community composition.
Keywords: Arctic cod, Boreogadus saida, diet, niche width, polar cod, predator–prey interactions
By taking an alternative approach to dietary analysis, this article challenges the commonly held assumption that polar cod is a generalist predator and argues that it can display strong temporal and geographical dietary specialization.

1. INTRODUCTION
Generalist feeders display large dietary niche width comprising of high prey taxa diversity, whereas specialists display narrow dietary niche width composed of few prey taxa (Gerking, 1994). To predict the vulnerability of a species to environmental disturbances, it is essential to measure its niche width and degree of specialization. Ecological generalists will, for example, often outperform specialists in a degraded environment ultimately leading to a reduction in species diversity (Clavel, Julliard, & Devictor, 2011; Devictor, Julliard, & Jiguet, 2008). Yet, set definitions and systematic quantification measures are often missing when assessing attributes pertaining to degrees of ecological specialization (Ferry‐Graham, Bolnick, & Wainwright, 2002), which can lead to incomparable studies (Ferry‐Graham et al., 2002) and ineffective management measures (Devictor et al., 2010). Indeed, the concept of ecological specialization is flexible yet frequently treated as a rigid species attribute.
To describe dietary specialization, we can use ecological levels other than species, such as individual, population, or community (Devictor et al., 2010; Fox & Morrow, 1981). For instance, populations from a generalist species can be composed of generalist individuals displaying a high within‐individual variation in resource use or of specialist individuals displaying a high between‐individual variation in resource use (Amundsen, Gabler, & Staldvik, 1996; Bearhop, Adams, Waldron, Fuller, & MacLeod, 2004). Additionally, the degree of specialization need not be static in time and space as a species realized dietary niche width can vary seasonally and geographically (Devictor et al., 2010; Ferry‐Graham et al., 2002). Thus, fundamental specialization describes a degree of specialization that is an inherent species trait, and realized specialization describes a degree of specialization that is flexible and dependent on local conditions.
Polar cod (Boreogadus saida) is ubiquitous both in open water and below the pack‐ice (Gradinger & Bluhm, 2004; Lønne & Gulliksen, 1989) and is the most abundant species in epipelagic zones at high latitudes (Benoit, Simard, & Fortier, 2008; Fortier et al., 2015). Polar cod plays an important role in linking trophic levels and is responsible for a large transfer of energy to piscivorous organisms (Hop & Gjøsæter, 2013; Wassmann et al., 2006; Welch et al., 1992). The presence of polar cod in both the Atlantic and Arctic domains in Svalbard suggests that this species has important adaptive capacities and can tolerate a wide range of temperatures and salinity (Falk‐Petersen, Frivoll, Gulliksen, & Haug, 1986; Nahrgang et al., 2014; Renaud et al., 2012) though it was suggested that polar cod's growth and fecundity is reduced in Atlantic domains (Nahrgang et al., 2014). Based on pan‐arctic meta‐analysis, it was established that polar cod is a zooplankton generalist predator (Mueter, Nahrgang, Nelson, & Berge, 2016; Renaud et al., 2012). Polar cod have also been described as opportunist feeders (Ajiad & Gjøsæter, 1990; Majewski et al., 2016; Nakano et al., 2016) and more rarely as specialist feeders (Cui, Grebmeier, & Cooper, 2012). This variability partly stems from evaluating diet at varying spatial and temporal scales and from inconsistencies regarding the choice of a fundamental or realized approach to describing degrees of specialization. Furthermore, the difficulties and expenses associated with sampling during the polar night have greatly hampered seasonal comparisons and this study offers rare insights into seasonal variation of ecological processes in the high Arctic.
Here, we studied polar cod stomach content to evaluate and contrast the (a) seasonal and geographical variations in dietary niche width, (b) variation in ingested prey taxa between fall and winter from Atlantic and Arctic domains, and (c) degree of realized individual and population specialization in fall and winter from Arctic domain fjords and an Atlantic domain fjord using a metric of specialization developed by Amundsen et al. (1996). More generally, we aim to reveal the importance of acknowledging organizational levels (individual, population, and species) when drawing conclusions on dietary specialization.
2. MATERIALS AND METHODS
2.1. Field sampling
Polar cod were collected in three sites from the Svalbard archipelago. Billefjorden and Rijpfjorden are fjords displaying Arctic characteristics and dominated by Arctic species (Bandara et al., 2016; Nahrgang et al., 2014), whereas the sound of Smeerenburg is heavily influenced by inflow of warmer and more saline Atlantic water (Figure 1). The fish were caught using a Campelen 1800 bottom trawl with a 22 mm cod‐end mesh size aboard the R/V Helmer Hanssen. Trawls were conducted in September and October 2014, January 2015, September 2015, and January 2016, with 11 trawls in total and at depths ranging from 158 to 267 m.
Figure 1.
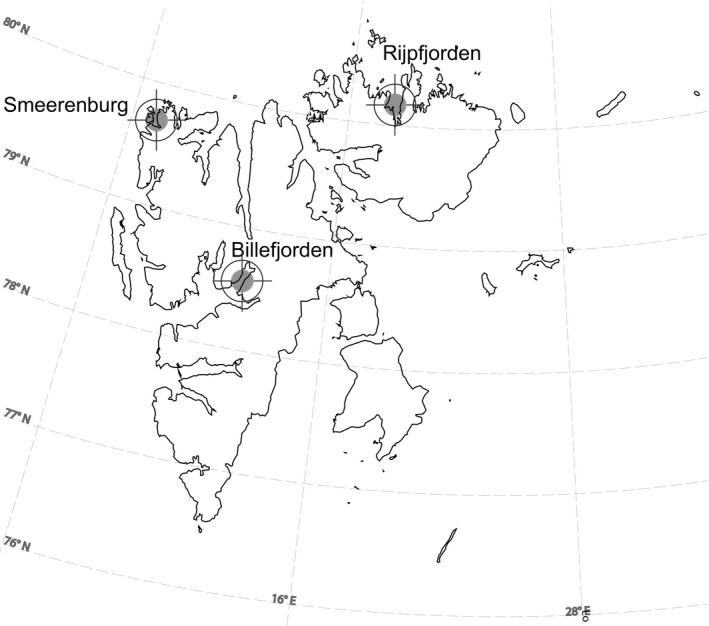
Map of Svalbard illustrating the three sampling locations; Rijpfjorden and Billefjorden being typically Arctic domain fjords and Smeerenburg being typically an Atlantic domain fjord
Polar cod were weighed, measured, and divided into three size categories, <10, 10–15, and >15 cm, based on Falk‐Petersen et al. (1986). Only individuals over 10 cm were kept for the analysis as they are likely to be mature individuals and be less limited in their diet choice due to gape size. The dissected stomachs were examined with a dissecting microscope, and prey taxa were identified to the lowest possible taxonomic level. Individuals of each prey taxa were counted and weighed. Some taxonomic levels were grouped into broader dietary categories for the analyses.
2.2. Qualitative diet analysis
In order to observe whether polar cod displayed seasonal variations in diet, we first performed a nonmetric multidimensional scaling (nMDS) analysis. Polar cod level of specialization was then directly assessed using the percent prey‐specific abundance (%Pi) versus frequency of occurrence (FO) diagram described by Amundsen et al. (1996) (Figure 2). FO and %Pi were measured using the following equations:
in which Ni is the number of stomachs with a given prey i in their stomach, N is the total number of stomachs excluding empty stomachs, Si is the total weight of prey i from all stomachs, and in which is the total prey weight of all stomachs containing prey i. Empty stomachs were excluded from the calculations along with unidentified material which would bias the results. Diagrams were generated separately for the three sites and the two seasons, and all individuals with prey in their stomach (n = 286) were retained for the analysis. Dietary niche width was calculated with the Shannon–Wiener (H′) diversity index (Spellerberg & Fedor, 2003) and Simpson's (D) diversity index (Simpson, 1949).
Figure 2.
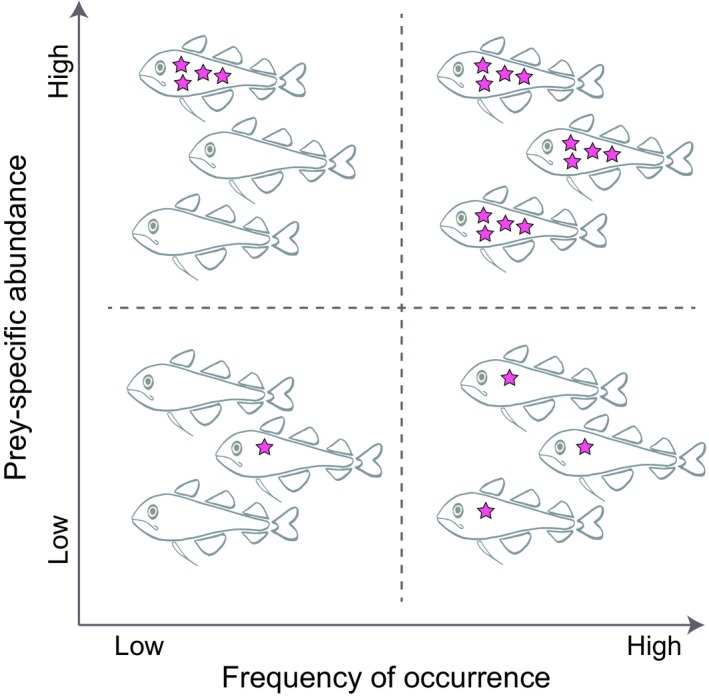
Schematic illustration adapted from the feeding strategy diagram of Amundsen et al. (1996). The original diagram that operates on a continuum was here simplified into four categories representative of four extreme circumstances at each corner of the diagram. Thus, the top left corner represents a situation with 100% prey‐specific abundance (Pi) in a single fish highlighting an extremely high between‐individual component (also called between‐phenotype component by Amundsen et al. (1996)), the top right corner represents a situation with the highest FO and 100% Pi illustrating an extremely high population specialization, the bottom left corner represents a situation with low FO and low %Pi, and the bottom right corner a situation with high FO and low %Pi illustrating a high within‐individual component (also called within‐phenotype component by Amundsen et al. (1996)) and a population generalization
2.3. Statistical diet analysis
Because of the nonindependent nature of our data, we first performed a multivariate analysis. Since our data did not meet the assumptions of a multivariate analysis of variance (MANOVA), we used a multivariate permutation analysis of variance (PERMANOVA; Anderson, 2001) to identify potential differences in diet composition between seasons and domains. The classical F test using both an analysis of variance (ANOVA) and a permutation test confirmed that our data met the assumptions of a PERMANOVA. For each sample site, prey data were normalized by dividing each measure of abundance by the total number of individual polar cod to avoid over‐representation of a prey for a given site when a higher number of polar cod from that site was analyzed. For the statistical analysis, all fish prey data (B. saida, Leptoclinus sp., Sebastes sp., and nonidentified fish) were pooled under the category Teleostei.
For statistical inference on the qualitative specialization metrics from the Amundsen et al. (1996) diagrams, we selected several preys of interest and tested their relative gravimetric abundance between sites and seasons using the Kruskal–Wallis test (α ≤ .005). The mean relative abundance of a given prey x () is a good approximation of the frequency of occurrence (FO) of that prey as it accounts for all stomachs including those that do not contain prey x (but excluding empty stomachs). Therefore, high mean relative abundance is likely to reflect high frequency of occurrence (FO) of prey x. Mean relative abundance as a proxy for FO was calculated as:
in which n is the total number of stomachs, Nx is the total amount of prey x for fish j, and N T is the total amount of prey for fish j. For consistency with the Amundsen et al. (1996) diagram, the amounts are measured in grams, but the method could also apply to numerical quantities.
The mean relative abundance of prey x in prey‐specific stomachs () is a good approximation of prey‐specific abundance (Pi) as it focuses strictly on stomachs containing prey x. Therefore, high mean relative abundance of prey x in prey‐specific stomachs is likely to reflect high prey‐specific abundance (Pi). Mean relative abundance in prey‐specific stomachs as a proxy for Pi was calculated as:
in which n p is the total number of stomachs containing prey x.
High mean relative abundance () and high mean relative abundance in prey‐specific stomachs () are analogous to general specialization, whereas low mean relative abundance () but high mean relative abundance in prey‐specific stomachs () is analogous to individual specialization. The Kruskal–Wallis analysis was conducted on the most abundant prey types (as per gravimetric measures): Themisto libellula, Teleostei, and Euphausidae. These results combined with a visual interpretation of Amundsen et al. (1996) diagrams were used to evaluate the difference in the degree of specialization between sites and seasons. All statistical analyses were performed using the R Core Team (2014) software.
3. RESULTS
3.1. Effects of season and domains on diet composition
We retained 309 stomachs for the diet analysis from a total of eleven trawl samples across two domains (Arctic and Atlantic) and seasons (Fall and Winter). The nMDS highlighted a seasonal effect in dietary composition for all populations (Figure 3). The PERMANOVA indicated that polar cod diet differed significantly between fall and winter (F 1,6 = 7.3167; p = .002), and between Arctic and Atlantic domains (F 1,6 = 2.69; p = .042); 40% of the total variance was explained by the Season factor (r 2 = .401), whereas 15% of the total variance was explained by the Domain factor (r 2 = .147). A SIMPER analysis indicated that T. libellula and Teleostei contributed to at least 70% of the observed difference between groups. Characterization of the seasonal diets follows in the analysis on degree of specialization.
Figure 3.
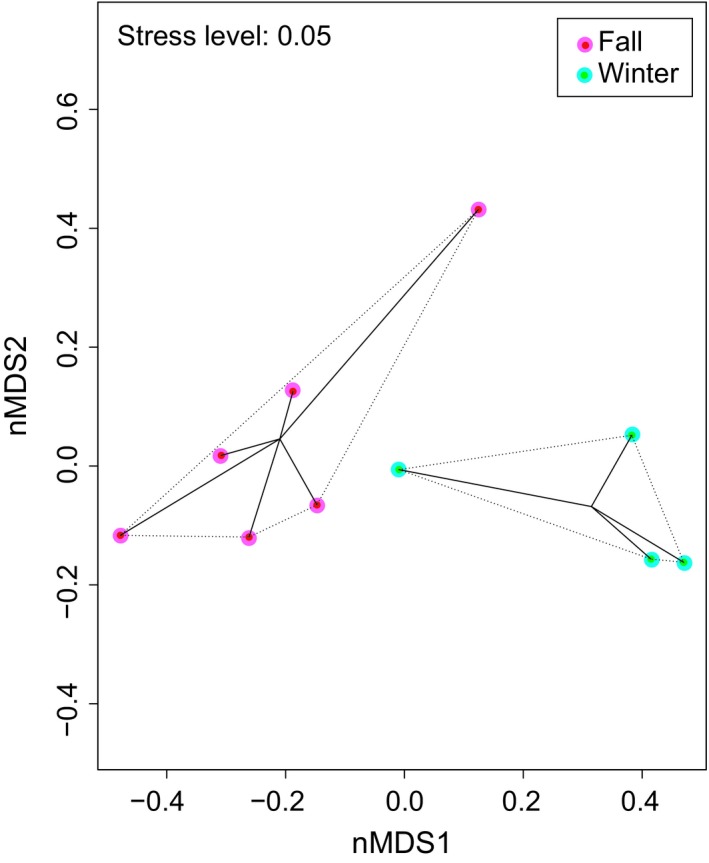
Nonmetric multidimensional scaling (nMDS) ordination of polar cod (Boreogadus saida) stomach content from Svalbard fjords during two different seasons: fall and winter
3.2. Degree of specialization
A total of 286 polar cod were included in the analysis of degree of specialization. Population specialization on T. libellula was observed for both Arctic sites (Billefjorden and Rijpfjorden) in fall. This was illustrated both by the Amundsen et al. (1996) diagrams (Figure 4) and by Figure 5a (high mean relative abundance [] and high mean relative abundance for prey‐specific stomachs []). In the Atlantic site of Smeerenburg, no such specialization was present, and Billefjorden and Rijpfjorden both exhibited a significantly different mean relative abundance of T. libellula from Smeerenburg in fall suggesting a lower frequency of occurrence of T. libellula in the Atlantic site (Figure 5a). In Smeerenburg, euphausiids prey had a relatively high frequency of occurrence and prey‐specific abundance (Figures 4 and 5b) approaching a population specialization on this taxa.
Figure 4.
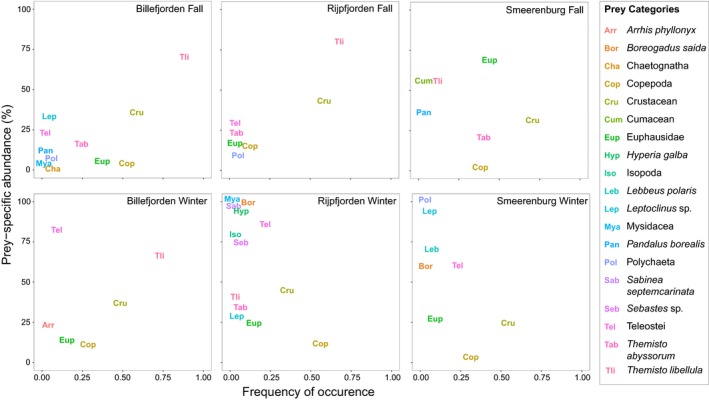
Percent prey‐specific abundance (%Pi) versus frequency of occurrence (FO) for polar cod from three Svalbard fjords during two different seasons: fall and winter
Figure 5.
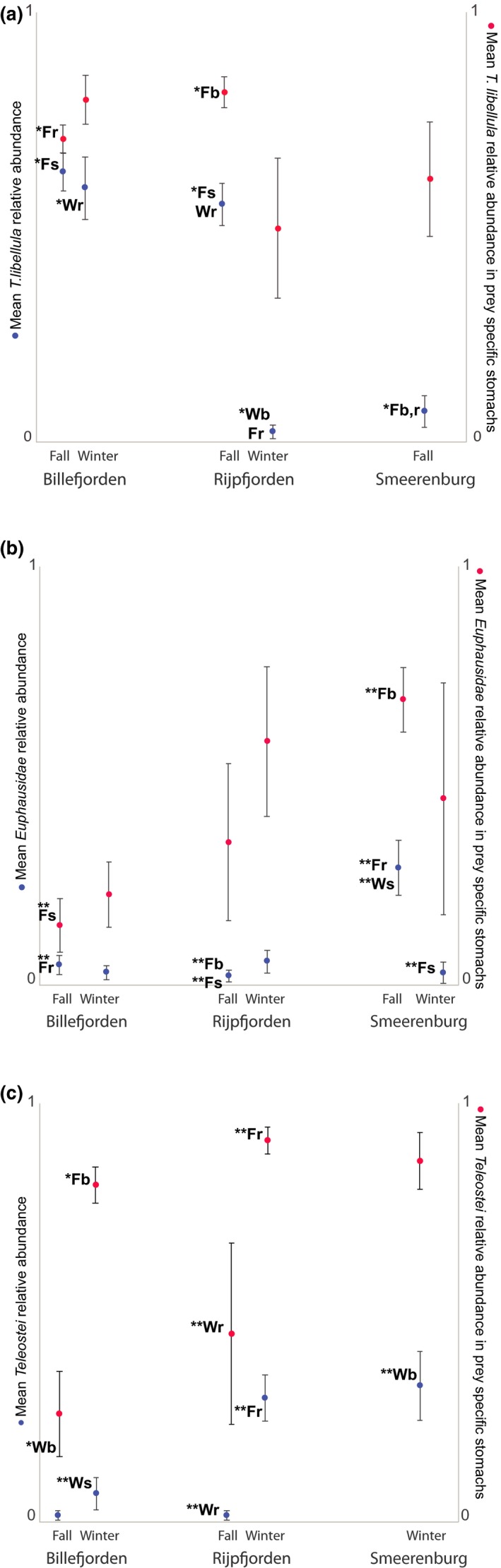
Mean value and error bars for relative (a) T. libellula, (b) Euphausidae, and (c) Teleostei abundance for polar cod from Billefjorden, Rijpfjorden, and Smeerenburg. The mean relative abundance of prey x in prey‐specific stomachs focuses strictly on stomachs containing prey x. Letters above mean value points indicate statistically significant difference from indicated site/season (F = Fall, W = Winter, s = Smeerenburg, r = Rijpfjorden, b = Billefjorden), *p < .005 and **p < .001 (Kruskal–Wallis test)
In the winter, only the Arctic fjord of Billefjorden still displayed population specialization on T. libellula, and individual specialization on fish prey was present at all sites. Though both Billefjorden and Rijpfjorden are Arctic domain fjords, the winter dietary niche width was wider in Rijpfjorden than Billefjorden (Figure 6a) with the frequency of occurrence of ingested T. libellula being much lower in Rijpfjorden. In the Atlantic site of Smeerenburg, fish prey had the highest frequency of occurrence and the highest prey‐specific abundance (Figures 4 and 5c). A shift from a realized population specialization in fall to a realized individual specialization in the winter was visible when examining each site separately (Figure 4). Overall, we did not observe a generalized feeding behavior (or high within‐individual component).
Figure 6.
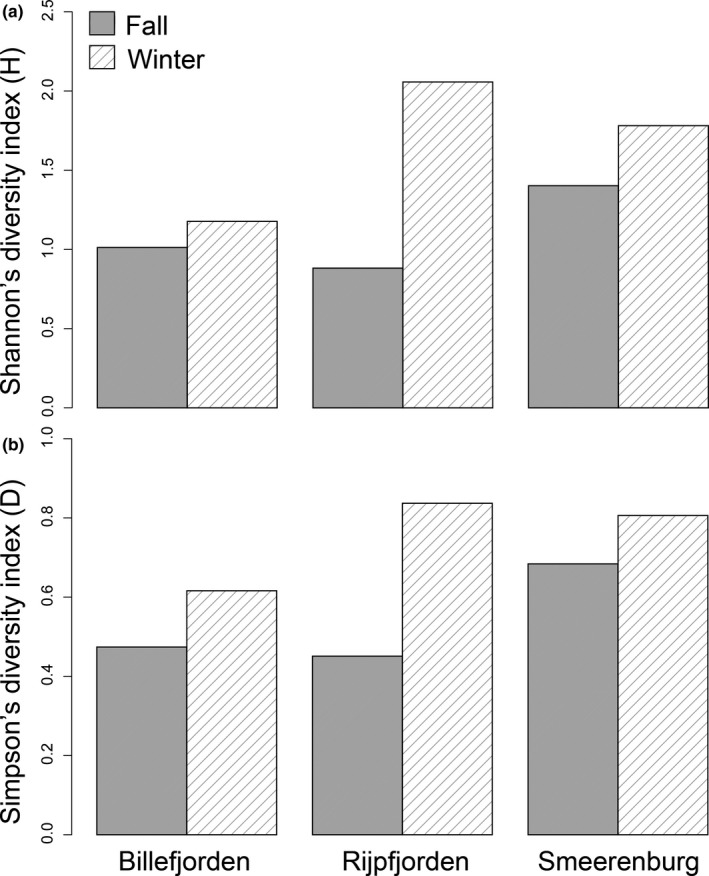
(a) Dietary niche width calculated with Shannon–Wiener (H′) and (b) Simpson's (D) diversity index separated for each site and season
3.3. Dietary niche width and prey diversity
In concordance with population specialization in Arctic sites in fall, the Shannon–Wiener index highlighted an increase in dietary niche width in the winter in each site and a greater dietary niche width in the Atlantic site in fall (Figure 6a). These trends are also displayed by the Simpson's index, which indicated a higher ingested prey diversity in the winter for all sites, and a higher ingested prey diversity in the Atlantic site in fall (Figure 6b).
4. DISCUSSION
4.1. Spatial and temporal variations in polar cod diet and degree of specialization
Polar cod is commonly depicted as an essential element of the Arctic marine food web linking primary consumers to higher trophic levels (Bradstreet et al., 1986; Melnikov & Chernova, 2013; Rice, 1995). Our study indicates that adult polar cod populations from Svalbard also act as tertiary consumers with a diet primarily composed of secondary consumer amphipod in fall and fish prey in the winter.
We noted a strong population specialization on T. libellula in Arctic domains in fall, which corresponds to prior diet observations even in the presence of other prey types such as euphausiids and copepods (Dalpadado et al., 2016). A diet primarily composed of the hyperiid amphipod T. libellula in Arctic domains in fall had previously been reported in a study comparing the diet and reproduction of polar cod between Atlantic and Arctic domains in Svalbard (Nahrgang et al., 2014). Likewise, T. libellula is frequently observed as a prey item in the North American Arctic, but important interannual variations exist (Buckley & Whitehouse, 2017). Studies from the North American Arctic report a variety of ingested amphipod species with the predominance of set families or suborders depending on the region, essentially the Hyperiidea amphipod species T. libellula and Themisto abyssorum (Majewski et al., 2016), the Senticaudata amphipod, Apherusa glacialis (Walkusz, Majewski, & Reist, 2013), and Ampeliscidae amphipods, a family of the suborder Gammaridea (Cui et al., 2012). Our study highlights the ingestion of T. abyssorum in the Atlantic site of Smeerenburg in line with evidence supporting the presence of this amphipod in Atlantic domains (Ormańczyk, Głuchowska, Olszewska, & Kwasniewski, 2017).
We observed a shift in diet from autumn to winter with fish prey becoming relatively important. Recent publications have shown how environmental factors such as temperature can affect the nutritional value of a given prey (Rosenblatt & Schmitz, 2016), which could explain predator's seasonal diet shift. The larger niche width in the winter is seemingly analogous to a generalist diet, but the diagrams indicate a strong individual specialization and between‐individual variation in resource use. Although polar cod occasionally ingest juvenile teleosts (Buckley & Whitehouse, 2017; Craig, Griffiths, Haldorson, & McElderry, 1982; Cui et al., 2012; Majewski et al., 2016; Rand et al., 2013), these results are surprising as the contribution of this prey to polar cod diet is often deemed negligible (Buckley & Whitehouse, 2017; Rand et al., 2013). Other prey species occasionally dominate polar cod diet such as mysiids (Craig et al., 1982) or euphausiids (Rand et al., 2013), and our results indicate that euphausiids ingestion seems particularly enhanced in Svalbard's Atlantic domain sites where euphausiids are dominating and where the abundance of T. libellula varies widely interannually and decreases with stronger inflow of Atlantic water (Dalpadado et al., 2016).
Altering prey availability as a result of seasonality and habitat heterogeneity, and abiotic factors such as seasonal change in light regime are suspected to explain these changes in ingested prey species compositions. These observations have led many to conclude that polar cod are opportunistic feeders (Ajiad & Gjøsæter, 1990; Bradstreet et al., 1986; Buckley & Whitehouse, 2017; Christiansen, Hop, Nilssen, & Joensen, 2012; Lowry & Frost, 1981) and zooplankton generalists (Renaud et al., 2012) and will feed on which ever prey is available and most abundant at a given moment. Our study confirms that polar cod display a generalist diet at the species level with a diverse set of invertebrates as well as fish prey, but it also highlights a strong realized specialization that varies seasonally and geographically.
4.2. The biotic and abiotic mechanisms for a realized specialization
Polar cod have the ability to forage on a wide diversity of prey yet when compared to other co‐occurring fish species, their diet often suggests a population specialization that does not appear to reflect the diversity of available prey (Cui et al., 2012; Dalpadado et al., 2016). Optimal diet theory (ODT) predicts that an increase in prey abundance and diversity should lead generalist predators to discriminate between preys and to specialize on higher value prey types (Sih & Christensen, 2001). Arctic fjords generally exhibit a diversity of potential prey such as T. libellula, T. abyssorum, euphausiids, and large calanoid copepods (Bandara et al., 2016; Cusa, 2016; Gluchowska et al., 2016; Ormańczyk et al., 2017), and the observed specialization on T. libellula could be the result of a seasonal behavioral specialization by a discriminating generalist. The predominance of T. libellula in the diet could also reflect a facility to catch this prey over other prey for a variety of reasons including handling ease, prey's color and visibility, aggregation and swimming behavior, and avoidance behavior.
If polar cod demonstrate a behavioral specialization in Arctic fjords in fall, this behavior is likely to be altered by seasonality, not just as a result of the declining abundance of a preferred prey, but also because of seasonal light regimes and the dark winter conditions encountered at high latitudes during the polar night. A decrease in light reduces the prey detection distance of visual predators (Aksnes & Utne, 1997; Langbehn & Varpe, 2017) possibly leading to a more generalized diet during the winter months. A change in foraging tactics shifting from pelagic to benthic could further explain the observed change in diet (Jørgensen & Jobling, 1990). Interestingly, the winter diet of polar cod remains quite specialized on T. libellula in Billefjorden. Polar cod's ability to adjust to a low‐light regime by changing the optical properties of its lens to a rod‐dominated retina while retaining multifocal vision (Jönsson, Varpe, Kozłowski, Berge, & Kröger, 2014) combined with more light in this lower latitude fjord (Berge et al., 2015) may permit them to continue foraging on T. libellula.
In 2017, potential fish prey species were all more abundant in summer than in winter (Geoffroy et al., 2019), yet the rather consistent individual specialization on these prey predominantly in winter suggests that other mechanisms than prey availability may be at play, such as the impaired visual ability of fish prey to escape their predators during the winter months, though polar cod might be equally impaired visually.
4.3. Limitations
One of the most important limitations of this study is the lack of prey community composition systematically sampled in concurrence with polar cod stomach samples. Methot Isaac Kidd (MIK) sampling was performed both in Rijpfjorden in fall and in winter for an adjacent project but the samples were analyzed using different methods and levels of expertise. We therefore prefer to discuss our results by referring to past studies and other manuscripts, which have investigated the community composition and abundance of zooplankton in a range of fjords in Svalbard allowing us to make well‐informed inferences (Bandara et al., 2016; Błachowiak‐Samołyk et al., 2017; Cusa, 2016; Dalpadado et al., 2016; Geoffroy et al., 2019; Kraft, Berge, Varpe, & Falk‐Petersen, 2013). Furthermore, bioenergetic measurements of the potential prey would help elucidate whether, all else being equal, there is an advantage to select for a given prey over other available prey (But see Mayzaud & Boutoute, 2015). Such information should be combined with polar cod physiological attributes and its seasonally varying energy requirements. Finally, a stable isotope analysis would nicely complete this seasonal study by bringing further insight on the seasonal and spatial component of the observed specializations. Stable isotope analysis of polar cod from Svalbard has previously been conducted (McGovern, Berge, Szymczycha, Węsławski, & Renaud, 2018; Renaud et al., 2012), but it is difficult to draw conclusions based on these analyses as they diverge from our samples both spatially and temporally.
4.4. Implications, outlook, and conclusion
Genetic variation between polar cod populations (Madsen, Nelson, Fevolden, Christiansen, & Præbel, 2016), their possible isolation, as well as a lack of understanding on migratory and reproductive patterns, calls for a narrower geographical scale description of feeding strategies. It also asks for careful consideration when implying that putative populations of polar cod may respond homogeneously to changes in prey composition. Populations that exhibit strong genetic structure could display unique population‐specific spatial and temporal specialization (Siwertsson et al., 2013). If, on the contrary, polar cod populations experience high gene flow, combining dietary data with physiological measurements might reveal essential information regarding the importance of seasonal prey selectivity and its impact on polar cod growth and fecundity. Studies have demonstrated that environmental factors can affect metabolic rate, thereby changing a predator's energetic requirements and its feeding strategy (Rowe, Figueira, Raubenheimer, Solon‐Biet, & Machovsky‐Capuska, 2018). Considering that polar cod population specialization occurs in fall, at a time period when energetic requirements are particularly high (prewinter and prereproduction, Nahrgang et al., 2014), understanding whether polar cod populations should be treated as distinct units or not seems particularly relevant.
The Arctic environment is changing rapidly, altering trophic interactions, selection pressures, and community composition (Edwards & Richardson, 2004; Hoegh‐Guldberg & Bruno, 2010; Langbehn & Varpe, 2017; Rice, 1995; Varpe, Daase, & Kristiansen, 2013; Zerba & Collins, 1992); and species response to changes induced by climate change will partly depend on their phenotypic plasticity (Munday, Warner, Monro, Pandolfi, & Marshall, 2013; Sih, Ferrari, & Harris, 2011). Studies have previously reported the selective behavior of polar cod on specific prey types (Cui et al., 2012; Hop & Gjøsæter, 2013), and we therefore suggest that polar cod specialization on T. libellula in Arctic domains in fall is a factor of both (a) an increased availability of T. libellula, and (b) a preference for T. libellula as a prey. Furthermore, we suggest that the near‐absence of T. libellula in the diet of polar cod in some sites during the winter and an increase in dietary niche width including individual specialization on fish prey likely reflects either or both (a) the low abundance of this Arctic amphipod, and (b) fish's reduced ability to rely on visual cues for prey search and predator avoidance.
CONFLICT OF INTEREST
The authors declare that they have no conflict of interest.
AUTHOR CONTRIBUTIONS
The article is based on the Master of Science project of MC. MC designed the study, collected and analyzed the data, and wrote the manuscript. JB advised on design, organized cruise time, and gave editorial advice on the manuscript. ØV advised throughout the study, participated in the design and writing, and provided support in data collection, analysis, and interpretation.
HUMAN AND ANIMAL RIGHTS
All applicable institutional and/or national guidelines for the care and use of animals were followed. Sampling and processing of polar cod was performed in compliance with the Norwegian animal welfare authorities.
ACKNOWLEDGMENTS
We are particularly thankful for Paul Renaud's feedback regarding study design and sampling. We thank Bodil Bluhm and Jan Marcin Weslwaski for valuable feedback on data interpretation. We thank Per‐Arne Amundsen for advice on the use of the Amundsen et al. (1996) diagram. We thank the Helmer Hanssen crew without whom the sampling could not have happened. We thank Janne Søreide, Daniel Vogedes, Malin Daase, Morgan Bender, Sam Eglund Newby, Maeve McGovern, Carl Ballantine, Tom Langbehn, Marvin Choquet, Olga Knyazeva, Maxime Geoffroy, and the students from the AB320 2014 and AB321 2015 for help collecting and processing samples in the field. We also thank the UiT—The Arctic University of Norway and The University Centre in Svalbard for providing the ship time.
Cusa M, Berge J, Varpe Ø. Seasonal shifts in feeding patterns: Individual and population realized specialization in a high Arctic fish. Ecol Evol. 2019;9:11112–11121. 10.1002/ece3.5615
Funding information
Funding for this study was provided by a Research Council of Norway grant to JB (project 226417), an Arctic Field Grant to MC (project 257080), and a Fulbright Arctic Initiative and a Research Council of Norway grant (project 227046) to ØV.
DATA AVAILABILITY STATEMENT
Data supporting this article are available on Dryad: https://doi.org/10.5061/dryad.6472vq6
REFERENCES
- Ajiad, A. M. , & Gjøsæter, H. (1990). Diet of polar cod, Boreogadus saida, in the Barents Sea related to fish size and geographical distribution. ICES; CM 1990/G:48. [Google Scholar]
- Aksnes, D. L. , & Utne, A. C. W. (1997). A revised model of visual range in fish. Sarsia, 82, 137–147. 10.1080/00364827.1997.10413647 [DOI] [Google Scholar]
- Amundsen, P. A. , Gabler, H. M. , & Staldvik, F. J. (1996). A new approach to graphical analysis of feeding strategy from stomach contents data—Modification of the Costello (1990) method. Journal of Fish Biology, 48(4), 607–614. 10.1111/j.1095-8649.1996.tb01455.x [DOI] [Google Scholar]
- Anderson, M. J. (2001). A new method for non‐parametric multivariate analysis of variance. Austral Ecology, 26(1), 32–46. 10.1111/j.1442-9993.2001.01070.pp.x [DOI] [Google Scholar]
- Bandara, K. , Varpe, Ø. , Søreide, J. E. , Wallenschus, J. , Berge, J. , & Eiane, K. (2016). Seasonal vertical strategies in a high‐Arctic coastal zooplankton community. Marine Ecology Progress Series, 555, 49–64. 10.3354/meps11831 [DOI] [Google Scholar]
- Bearhop, S. , Adams, C. E. , Waldron, S. , Fuller, R. A. , & MacLeod, H. (2004). Determining trophic niche width: A novel approach using stable isotope analysis. Journal of Animal Ecology, 73(5), 1007–1012. 10.1111/j.0021-8790.2004.00861.x [DOI] [Google Scholar]
- Benoit, D. , Simard, Y. , & Fortier, L. (2008). Hydroacoustic detection of large winter aggregations of Arctic cod (Boreogadus saida) at depth in ice‐covered Franklin Bay (Beaufort Sea). Journal of Geophysical Research: Oceans, 113, C06S90 10.1029/2007JC004276 [DOI] [Google Scholar]
- Berge, J. , Renaud, P. E. , Darnis, G. , Cottier, F. , Last, K. , Gabrielsen, T. M. , … Falk‐Petersen, S. (2015). In the dark: A review of ecosystem processes during the Arctic polar night. Progress in Oceanography, 139, 258–271. 10.1016/j.pocean.2015.08.005 [DOI] [Google Scholar]
- Błachowiak‐Samołyk, K. , Zwolicki, A. , Webster, C. N. , Boehnke, R. , Wichorowski, M. , Wold, A. , & Bielecka, L. (2017). Characterisation of large zooplankton sampled with two different gears during midwinter in Rijpfjorden, Svalbard. Polish Polar Research, 38(4), 459–484. 10.1515/popore-2017-0023 [DOI] [Google Scholar]
- Bradstreet, M. S. , Finley, K. J. , Sekerak, A. D. , Griffiths, W. B. , Evans, C. R. , Fabijan, M. F. , & Stallard, H. E. (1986). Aspects of the biology of Arctic cod (Boreogadus saida) and its importance in Arctic marine food chains (Report No. 1491). Canadian Technical Report of Fisheries and Aquatic Sciences. Winnipeg, Canada. [Google Scholar]
- Buckley, T. W. , & Whitehouse, G. A. (2017). Variation in the diet of Arctic Cod (Boreogadus saida) in the Pacific Arctic and Bering Sea. Environmental Biology of Fishes, 100(4), 421–442. 10.1007/s10641-016-0562-1 [DOI] [Google Scholar]
- Christiansen, J. S. , Hop, H. , Nilssen, E. M. , & Joensen, J. (2012). Trophic ecology of sympatric Arctic gadoids, Arctogadus glacialis (Peters, 1872) and Boreogadus saida (Lepechin, 1774), in NE Greenland. Polar Biology, 35(8), 1247–1257. 10.1007/s00300-012-1170-y [DOI] [Google Scholar]
- Clavel, J. , Julliard, R. , & Devictor, V. (2011). Worldwide decline of specialist species: Toward a global functional homogenization? Frontiers in Ecology and the Environment, 9(4), 222–228. 10.1890/080216 [DOI] [Google Scholar]
- Craig, P. C. , Griffiths, W. B. , Haldorson, L. , & McElderry, H. (1982). Ecological studies of Arctic cod (Boreogadus saida) in Beaufort Sea coastal waters, Alaska. Canadian Journal of Fisheries and Aquatic Sciences, 39(3), 395–406. 10.1139/f82-057 [DOI] [Google Scholar]
- Cui, X. , Grebmeier, J. M. , & Cooper, L. W. (2012). Feeding ecology of dominant groundfish in the northern Bering Sea. Polar Biology, 35(9), 1407–1419. 10.1007/s00300-012-1180-9 [DOI] [Google Scholar]
- Cusa, M. (2016). The effect of seasonality on polar cod (Boreogadus saida) dietary habits and temporal feeding strategies in Svalbard waters. Master thesis, Department of Arctic and Marine Biology, University of Tromsø, Tromsø, Norway. [Google Scholar]
- Dalpadado, P. , Hop, H. , Rønning, J. , Pavlov, V. , Sperfeld, E. , Buchholz, F. , … Wold, A. (2016). Distribution and abundance of euphausiids and pelagic amphipods in Kongsfjorden, Isfjorden and Rijpfjorden (Svalbard) and changes in their relative importance as key prey in a warming marine ecosystem. Polar Biology, 39(10), 1765–1784. 10.1007/s00300-015-1874-x [DOI] [Google Scholar]
- Devictor, V. , Clavel, J. , Julliard, R. , Lavergne, S. , Mouillot, D. , Thuiller, W. , … Mouquet, N. (2010). Defining and measuring ecological specialization. Journal of Applied Ecology, 47(1), 15–25. 10.1111/j.1365-2664.2009.01744.x [DOI] [Google Scholar]
- Devictor, V. , Julliard, R. , & Jiguet, F. (2008). Distribution of specialist and generalist species along spatial gradients of habitat disturbance and fragmentation. Oikos, 117(4), 507–514. 10.1111/j.0030-1299.2008.16215.x [DOI] [Google Scholar]
- Edwards, M. , & Richardson, A. J. (2004). Impact of climate change on marine pelagic phenology and trophic mismatch. Nature, 430, 881–884. 10.1038/nature02808 [DOI] [PubMed] [Google Scholar]
- Falk‐Petersen, I. B. , Frivoll, V. , Gulliksen, B. , & Haug, T. (1986). Occurrence and size/age relations of polar cod, Boreogadus saida (Lepechin), in Spitsbergen coastal waters. Sarsia, 71(3–4), 235–245. 10.1080/00364827.1986.10419693 [DOI] [Google Scholar]
- Ferry‐Graham, L. A. , Bolnick, D. I. , & Wainwright, P. C. (2002). Using functional morphology to examine the ecology and evolution of specialization. Integrative and Comparative Biology, 42(2), 265–277. 10.1093/icb/42.2.265 [DOI] [PubMed] [Google Scholar]
- Fortier, L. , Ferguson, S. H. , Archambault, P. , Matley, J. , Robert, D. , Darnis, G. , … Letcher, R. J. (2015). Arctic change: Impacts on marine ecosystems In Stern G. & Gaden A. (Eds.), From science to policy in the western and central Canadian Arctic. Quebec City, QC: ArcticNet Inc. [Google Scholar]
- Fox, L. A. , & Morrow, P. A. (1981). Specialization: Species property or local phenomenon? Science, 211(4485), 887–893. 10.1126/science.211.4485.887 [DOI] [PubMed] [Google Scholar]
- Geoffroy, M. , Daase, M. , Cusa, M. , Darnis, G. , Graeve, M. , Santana Hernández, N. , … Falk‐Petersen, S. (2019). Seasonality in mesopelagic sound scattering layers of the high Arctic: Variations in biomass, species assemblage, and trophic relationships. Frontiers in Marine Science. (6), Article 364, 1–18. 10.3389/fmars.2019.00364 [DOI] [Google Scholar]
- Gerking, S. D. (1994). Feeding ecology of fish, 1st ed San Diego, CA: Academic Press. [Google Scholar]
- Gluchowska, M. , Kwasniewski, S. , Prominska, A. , Olszewska, A. , Goszczko, I. , Falk‐Petersen, S. , … Weslawski, J. M. (2016). Zooplankton in Svalbard fjords on the Atlantic‐Arctic boundary. Polar Biology, 39(10), 1785–1802. 10.1007/s00300-016-1991-1 [DOI] [Google Scholar]
- Gradinger, R. R. , & Bluhm, B. A. (2004). In‐situ observations on the distribution and behavior of amphipods and Arctic cod (Boreogadus saida) under the sea ice of the High Arctic Canada Basin. Polar Biology, 27(10), 595–603. 10.1007/s00300-004-0630-4 [DOI] [Google Scholar]
- Hoegh‐Guldberg, O. , & Bruno, J. F. (2010). The impact of climate change on the world's marine ecosystems. Science, 328(5985), 1523–1528. 10.1126/science.1189930 [DOI] [PubMed] [Google Scholar]
- Hop, H. , & Gjøsæter, H. (2013). Polar cod (Boreogadus saida) and capelin (Mallotus villosus) as key species in marine food webs of the Arctic and the Barents Sea. Marine Biology Research, 9(9), 878–894. 10.1080/17451000.2013.775458 [DOI] [Google Scholar]
- Jönsson, M. , Varpe, Ø. , Kozłowski, T. , Berge, J. , & Kröger, R. H. (2014). Differences in lens optical plasticity in two gadoid fishes meeting in the Arctic. Journal of Comparative Physiology A, 200(11), 949–957. 10.1007/s00359-014-0941-z [DOI] [PubMed] [Google Scholar]
- Jørgensen, E. H. , & Jobling, M. (1990). Feeding modes in Arctic charr, Salvelinus alpinus L.: The importance of bottom feeding for the maintenance of growth. Aquaculture, 86(4), 379–385. 10.1016/0044-8486(90)90326-I [DOI] [Google Scholar]
- Kraft, A. , Berge, J. , Varpe, Ø. , & Falk‐Petersen, S. (2013). Feeding in Arctic darkness: Mid‐winter diet of the pelagic amphipods Themisto abyssorum and T. libellula . Marine Biology, 160(1), 241–248. 10.1007/s00227-012-2065-8 [DOI] [Google Scholar]
- Langbehn, T. J. , & Varpe, Ø. (2017). Sea‐ice loss boosts visual search: Fish foraging and changing pelagic interactions in polar oceans. Global Change Biology, 23(12), 5318–5330. 10.1111/gcb.13797 [DOI] [PubMed] [Google Scholar]
- Lønne, O. J. , & Gulliksen, B. (1989). Size, age and diet of polar cod, Boreogadus saida (Lepechin 1773), in ice covered waters. Polar Biology, 9(3), 187–191. 10.1007/BF00297174 [DOI] [Google Scholar]
- Lowry, L. F. , & Frost, K. J. (1981). Distribution, growth, and foods of Arctic cod (Boreogadus saida) in the Bering, Chukchi, and Beaufort seas. Canadian Field‐Naturalist, 95(2), 186–191. [Google Scholar]
- Madsen, M. L. , Nelson, R. J. , Fevolden, S. E. , Christiansen, J. S. , & Præbel, K. (2016). Population genetic analysis of Euro‐Arctic polar cod Boreogadus saida suggests fjord and oceanic structuring. Polar Biology, 39(6), 969–980. 10.1007/s00300-015-1812-y [DOI] [Google Scholar]
- Majewski, A. R. , Walkusz, W. , Lynn, B. R. , Atchison, S. , Eert, J. , & Reist, J. D. (2016). Distribution and diet of demersal Arctic Cod, Boreogadus saida, in relation to habitat characteristics in the Canadian Beaufort Sea. Polar Biology, 39(6), 1087–1098. 10.1007/s00300-015-1857-y [DOI] [Google Scholar]
- Mayzaud, P. , & Boutoute, M. (2015). Dynamics of lipid and fatty acid composition of the hyperiid amphipod Themisto: A bipolar comparison with special emphasis on seasonality. Polar Biology, 38(7), 1049–1065. 10.1007/s00300-015-1666-3 [DOI] [Google Scholar]
- McGovern, M. , Berge, J. , Szymczycha, B. , Węsławski, J. M. , & Renaud, P. E. (2018). Hyperbenthic food‐web structure in an Arctic fjord. Marine Ecology Progress Series, 603, 29–46. 10.3354/meps12713 [DOI] [Google Scholar]
- Melnikov, I. A. , & Chernova, N. V. (2013). Characteristics of under‐ice swarming of polar cod Boreogadus saida (Gadidae) in the Central Arctic Ocean. Journal of Ichthyology, 53(1), 7–15. 10.1134/S0032945213010086 [DOI] [Google Scholar]
- Mueter, F. J. , Nahrgang, J. , Nelson, R. J. , & Berge, J. (2016). The ecology of gadid fishes in the circumpolar Arctic with a special emphasis on the polar cod (Boreogadus saida). Polar Biology, 39(6), 961–967. 10.1007/s00300-016-1965-3 [DOI] [Google Scholar]
- Munday, P. L. , Warner, R. R. , Monro, K. , Pandolfi, J. M. , & Marshall, D. J. (2013). Predicting evolutionary responses to climate change in the sea. Ecology Letters, 16(12), 1488–1500. 10.1111/ele.12185 [DOI] [PubMed] [Google Scholar]
- Nahrgang, J. , Varpe, Ø. , Korshunova, E. , Murzina, S. , Hallanger, I. G. , Vieweg, I. , & Berge, J. (2014). Gender specific reproductive strategies of an Arctic key species (Boreogadus saida) and implications of climate change. PLoS ONE, 9(5), e98452 10.1371/journal.pone.0098452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakano, T. , Matsuno, K. , Nishizawa, B. , Iwahara, Y. , Mitani, Y. , Yamamoto, J. , … Watanuki, Y. (2016). Diets and body condition of polar cod (Boreogadus saida) in the northern Bering Sea and Chukchi Sea. Polar Biology, 39(6), 1081–1086. 10.1007/s00300-015-1769-x [DOI] [Google Scholar]
- Ormańczyk, M. R. , Głuchowska, M. , Olszewska, A. , & Kwasniewski, S. (2017). Zooplankton structure in high latitude fjords with contrasting oceanography (Hornsund and Kongsfjorden, Spitsbergen). Oceanologia, 59(4), 508–524. 10.1016/j.oceano.2017.06.003 [DOI] [Google Scholar]
- R Core Team (2014). R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; Retrieved from http://www.R-project.org/ [Google Scholar]
- Rand, K. M. , Whitehouse, A. , Logerwell, E. A. , Ahgeak, E. , Hibpshman, R. , & Parker‐Stetter, S. (2013). The diets of polar cod (Boreogadus saida) from August 2008 in the US Beaufort Sea. Polar Biology, 36(6), 907–912. 10.1007/s00300-013-1303-y [DOI] [Google Scholar]
- Renaud, P. E. , Berge, J. , Varpe, Ø. , Lønne, O. J. , Nahrgang, J. , Ottesen, C. , & Hallanger, I. (2012). Is the poleward expansion by Atlantic cod and haddock threatening native polar cod, Boreogadus saida? Polar Biology, 35(3), 401–412. 10.1007/s00300-011-1085-z [DOI] [Google Scholar]
- Rice, J. (1995). Food web theory, marine food webs, and what climate change may do to northern marine fish populations In Beamish R. J. (Ed.), Climate change and northern fish populations (pp. 561–568). Can Spec Publ Fish Aquat Sci. Nanaimo, BC: Research Council of Canada. [Google Scholar]
- Rosenblatt, A. E. , & Schmitz, O. J. (2016). Climate change, nutrition, and bottom‐up and top‐down food web processes. Trends in Ecology & Evolution, 31(12), 965–975. 10.1016/j.tree.2016.09.009 [DOI] [PubMed] [Google Scholar]
- Rowe, C. E. , Figueira, W. , Raubenheimer, D. , Solon‐Biet, S. M. , & Machovsky‐Capuska, G. E. (2018). Effects of temperature on macronutrient selection, metabolic and swimming performance of the Indo‐Pacific Damselfish (Abudefduf vaigiensis). Marine Biology, 165(11), 178 10.1007/s00227-018-3435-7 [DOI] [Google Scholar]
- Sih, A. , & Christensen, B. (2001). Optimal diet theory: When does it work, and when and why does it fail? Animal Behaviour, 61(2), 379–390. 10.1006/anbe.2000.1592 [DOI] [Google Scholar]
- Sih, A. , Ferrari, M. C. , & Harris, D. J. (2011). Evolution and behavioural responses to human‐induced rapid environmental change. Evolutionary Applications, 4(2), 367–387. 10.1111/j.1752-4571.2010.00166.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson, E. H. (1949). Measurement of diversity. Nature, 163(4148), 688. [Google Scholar]
- Siwertsson, A. , Knudsen, R. , Præbel, K. , Adams, C. E. , Newton, J. , & Amundsen, P. A. (2013). Discrete foraging niches promote ecological, phenotypic, and genetic divergence in sympatric whitefish (Coregonus lavaretus). Evolutionary Ecology, 27(3), 547–564. 10.1007/s10682-012-9607-x [DOI] [Google Scholar]
- Spellerberg, I. F. , & Fedor, P. J. (2003). A tribute to Claude Shannon (1916–2001) and a plea for more rigorous use of species richness, species diversity and the ‘Shannon–Wiener’ Index. Global Ecology and Biogeography, 12(3), 177–179. 10.1046/j.1466-822X.2003.00015.x [DOI] [Google Scholar]
- Varpe, Ø. , Daase, M. , & Kristiansen, T. (2015). A fish‐eye view on the new Arctic lightscape. ICES Journal of Marine Science, 72(9), 2532–2538. 10.1093/icesjms/fsv129 [DOI] [Google Scholar]
- Walkusz, W. , Majewski, A. , & Reist, J. D. (2013). Distribution and diet of the bottom dwelling Arctic cod in the Canadian Beaufort Sea. Journal of Marine Systems, 127, 65–75. 10.1016/j.jmarsys.2012.04.004 [DOI] [Google Scholar]
- Wassmann, P. , Reigstad, M. , Haug, T. , Rudels, B. , Carroll, M. L. , Hop, H. , … Pavlova, O. (2006). Food webs and carbon flux in the Barents Sea. Progress in Oceanography, 71(2–4), 232–287. 10.1016/j.pocean.2006.10.003 [DOI] [Google Scholar]
- Welch, H. E. , Bergmann, M. A. , Siferd, T. D. , Martin, K. A. , Curtis, M. F. , Crawford, R. E. , … Hop, H. (1992). Energy flow through the marine ecosystem of the Lancaster Sound region, arctic Canada. Arctic, 45(4), 343–357. 10.14430/arctic1413 [DOI] [Google Scholar]
- Zerba, K. E. , & Collins, J. P. (1992). Spatial heterogeneity and individual variation in diet of an aquatic top predator. Ecology, 73(1), 268–279. 10.2307/1938738 [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data supporting this article are available on Dryad: https://doi.org/10.5061/dryad.6472vq6


