Key Points
Question
Is endovascular treatment in pediatric patients (<18 years) associated with ischemic stroke and the clinical outcome?
Findings
In this cohort study including 73 children, endovascular recanalization appeared to be safe with positive outcomes in a real-world setting (proportion of successful recanalization, 87%). The study findings suggest that neurologic outcomes of the children were mostly favorable and comparable with those noted in adult trials.
Meaning
This study appears to support the level of evidence in favor of endovascular recanalization in children with acute, large-vessel occlusion; a higher strength of recommendation may contribute to clinical outcome in children affected by arterial ischemic stroke.
Abstract
Importance
Randomized clinical trials have shown the efficacy of thrombectomy of large intracranial vessel occlusions in adults; however, any association of therapy with clinical outcomes in children is unknown.
Objective
To evaluate the use of endovascular recanalization in pediatric patients with arterial ischemic stroke.
Design, Setting, and Participants
This retrospective, multicenter cohort study, conducted from January 1, 2000, to December 31, 2018, analyzed the databases from 27 stroke centers in Europe and the United States. Included were all pediatric patients (<18 years) with ischemic stroke who underwent endovascular recanalization. Median follow-up time was 16 months.
Exposures
Endovascular recanalization.
Main Outcomes and Measures
The decrease of the Pediatric National Institutes of Health Stroke Scale (PedNIHSS) score from admission to day 7 was the primary outcome (score range: 0 [no deficit] to 34 [maximum deficit]). Secondary clinical outcomes included the modified Rankin scale (mRS) (score range: 0 [no deficit] to 6 [death]) at 6 and 24 months and rate of complications.
Results
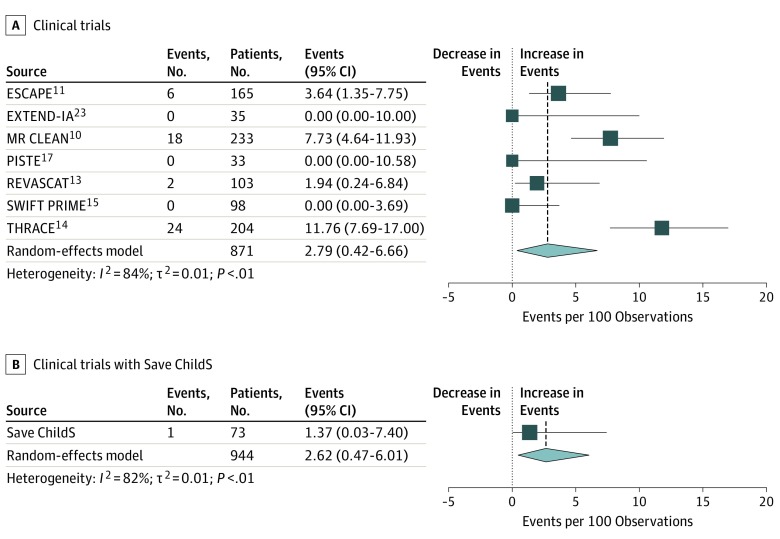
Seventy-three children from 27 participating stroke centers were included. Median age was 11.3 years (interquartile range [IQR], 7.0-15.0); 37 patients (51%) were boys, and 36 patients (49%) were girls. Sixty-three children (86%) received treatment for anterior circulation occlusion and 10 patients (14%) received treatment for posterior circulation occlusion; 16 patients (22%) received concomitant intravenous thrombolysis. Neurologic outcome improved from a median PedNIHSS score of 14.0 (IQR, 9.2-20.0) at admission to 4.0 (IQR, 2.0-7.3) at day 7. Median mRS score was 1.0 (IQR, 0-1.6) at 6 months and 1.0 (IQR, 0-1.0) at 24 months. One patient (1%) developed a postinterventional bleeding complication and 4 patients (5%) developed transient peri-interventional vasospasm. The proportion of symptomatic intracerebral hemorrhage events in the HERMES meta-analysis of trials with adults was 2.79 (95% CI, 0.42-6.66) and in Save ChildS was 1.37 (95% CI, 0.03-7.40).
Conclusions and Relevance
The results of this study suggest that the safety profile of thrombectomy in childhood stroke does not differ from the safety profile in randomized clinical trials for adults; most of the treated children had favorable neurologic outcomes. This study may support clinicians’ practice of off-label thrombectomy in childhood stroke in the absence of high-level evidence.
This cohort study examines the use of endovascular recanalization in pediatric patients with arterial ischemic stroke and clinical outcomes.
Introduction
With an estimated incidence of 2 to 8 per 100 000 children per year, childhood arterial ischemic stroke is a rare clinical event with potentially severe outcome and resulting disabilities with long-term social and financial effects.1,2 In contrast to the traditional assumption that most pediatric patients with arterial ischemic stroke have a good long-term prognosis because of neuronal plasticity, pediatric patients presenting with large-vessel occlusion in the anterior or posterior circulation or high pediatric National Institutes of Health Stroke Scale (PedNIHSS) score (score range: 0 [no deficit] to 34 [maximum deficit]) on admission are affected by high morbidity and mortality.2,3,4,5,6,7,8,9
In adults, several randomized clinical trials have shown the efficacy and safety of endovascular recanalization for large intracranial vessel occlusions with proven clinical benefit.10,11,12,13,14,15,16,17 However, the effectiveness and safety of thrombectomy in children is unknown and only small case series report feasibility and clinical outcome.18,19 To date, supportive medical management specific to the underlying etiology of AIS is considered the standard of care in pediatric patients and thrombolytic and endovascular therapy are only recommended as a last resort due to lack of level A evidence.20
The failed attempt of a prospective randomized clinical trial to assess the safety of intravenous thrombolysis in pediatric patients3 owing to a lack of recruitment underlines that randomizing pediatric patients with stroke is difficult and may never reach equipoise in the setting of evidence for a strong treatment effect in adults. Therefore, this study evaluated treatment regimens for pediatric patients with stroke with large-vessel occlusion based on real-world experience with thrombectomy in childhood stroke across 27 stroke centers in Europe and the United States.
Methods
Study Design
This retrospective, observational, multicenter cohort study, conducted from January 1, 2000, to December 31, 2018, synthesizes the analysis of radiologic databases from 27 European and US stroke centers (eTable 1 in the Supplement). Included are all pediatric patients (age <18 years) diagnosed with arterial ischemic stroke who underwent endovascular recanalization during the study period. In total, 42 tertiary stroke centers were approached; however, of those, 15 centers (36%) did not treat any pediatric patients with endovascular thrombectomy.
The study was approved by the ethics committee of the University of Muenster, Muenster, Germany, in accordance with the Declaration of Helsinki,21 with waiver of informed consent. Data are deidentified.
Characteristics and Outcome Measures
Baseline characteristics, imaging and treatment modalities, and time from symptom onset to admission were recorded. Stroke severity was assessed using the PedNIHSS on admission, after 12 to 24 hours, and after 7 days. Further clinical end points consisted of the modified Rankin scale (mRS) score (mRS 0 [no deficit] to 6 [death]) and Pediatric Stroke Outcome Measure (PSOM) (PSOM 0 [no deficit] to 10 [maximum deficit]) score at discharge, 6 months and 24 months. In most cases either the mRS or the PSOM score was recorded according to local in-hospital standards. All variables were collected from the patients’ medical records, and, if scores were not obtained at the time of treatment, also by retrospective scoring of reported neurologic examinations.
All patients underwent magnetic resonance or computed tomographic imaging before and after the intervention to rule out intracranial hemorrhage and to assess the site of vessel occlusion. The extent of early infarct was rated according to the adult Alberta Stroke Program Early CT Score (ASPECTS) or posterior circulation (PC) ASPECTS depending on vascular territory involved. ASPECTS provides segmental assessment of the vascular territory and 1 point is deducted from the initial score of 10 for every region involved (from 10 [no lesion] to 0 [maximum lesions]). Scores were determined retrospectively for all patients by the contributing center. The cause of stroke was assessed using the Childhood Arterial Ischemic Stroke Standardized Classification and Diagnostic Evaluation Classification measures.22,23
Intervention
Digital subtraction angiography was performed via the transfemoral approach. Endovascular recanalization procedures consisted of a combination of techniques using distal thrombaspiration and/or clot retrievers. Device selection as reported depended on occlusion pattern, operator preference, and availability at the time. Immediately after endovascular treatment, all patients were transferred to the pediatric intensive care unit. Twenty-four hours after treatment, or earlier in the case of clinical deterioration, a computed tomographic or magnetic resonance imaging scan was performed. Recanalization rates were classified as complete, partial, or no recanalization by the responsible neuroradiologists according to the Modified Treatment in Cerebral Infarction (mTICI) score (mTICI 0 indicates no perfusion; perfusion grade 1, antegrade reperfusion past the initial occlusion, but limited distal branch filling with little distal; reperfusion grade 2a, antegrade reperfusion of less than half of the occluded target artery previously ischemic territory; grade 2b, antegrade reperfusion of more than half of the previously occluded target artery ischemic territory; grade 2c, near-complete perfusion except for slow flow in a few distal cortical vessels or presence of small distal cortical emboli; grade 3, complete antegrade reperfusion of the previously occluded target artery ischemic territory, with absence of visualized occlusion in all distal branches).
Comparison With Trials Included in the HERMES Meta-analysis
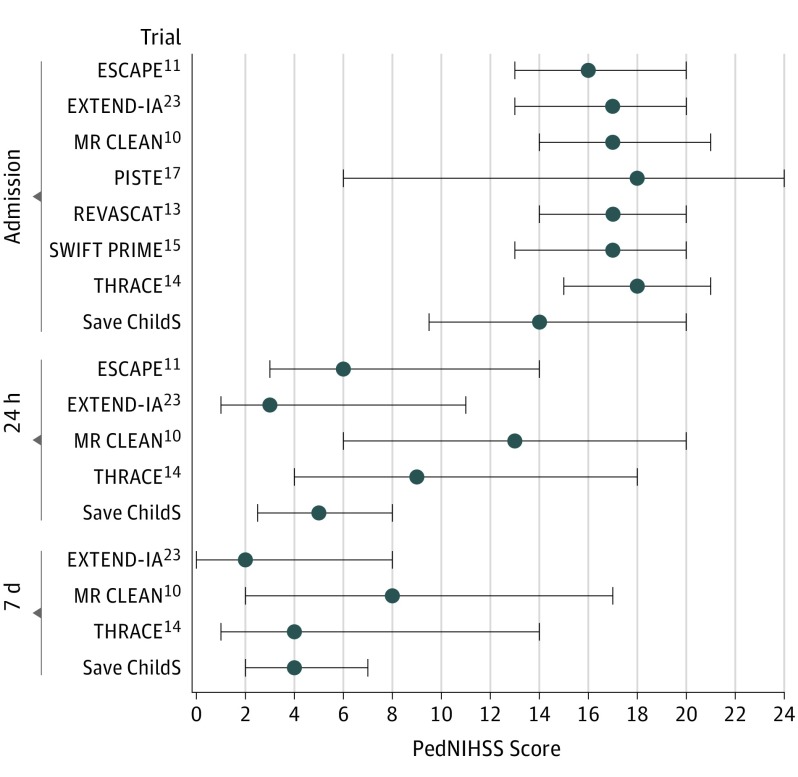
To compare clinical outcomes and safety between Save ChildS and adult trials, we extracted the following data from the intervention arms of the 7 published trials of the HERMES meta-analysis12 (ESCAPE,11 EXTEND-IA,24 MR CLEAN,10 Pragmatic Ischaemic Stroke Thrombectomy Evaluation [PISTE],17 REVASCAT,13 SWIFT PRIME,15 THRACE14): median (25th-75th percentile) NIHSS score at admission as well as 24 hours and 7 days after thrombectomy, distribution of mRS score at 90 days, and number of symptomatic intracerebral hemorrhage events during follow-up.
Statistical Analysis
All analyses were conducted on an exploratory basis given the retrospective and feasibility-driven nature of the study. Continuous variables were summarized as median (interquartile range) and binary variables as absolute and relative frequencies. Between-group comparisons were done using the Wilcoxon rank sum test for continuous variables or Fisher exact test for binary variables. Evaluation of outcomes after thrombectomy was performed descriptively only. Given the small absolute number of cases and unclear missingness patterns, we performed complete case analyses for all outcomes and report number of missing values. Incident symptomatic intracerebral hemorrhage events as the major adverse event after thrombectomy were summarized among Save ChildS and the 7 HERMES trials in a meta-analysis of proportions (function metaprop in the R package meta).25 The Freeman-Tukey Double arcsine transformation26 was used to account for 0 events in some of the studies and was evaluated if the point estimate of Save ChildS was within the 95% CI of the combined estimate of the 7 HERMES trials. Moreover, we assessed if adding Save ChildS to the meta-analysis of the HERMES trials increased the observed heterogeneity in the meta-analysis. All computations were performed with R, version 3.5.2 (R Foundation for Statistical Analysis). Findings were considered significant at P < .05.
Results
Study Population
Overall, 73 children from 27 participating centers were included; 37 patients (51%) were boys, and 36 patients (49%) were girls. Median age was 11.3 years (range, 0.7-18.0; interquartile range [IQR], 7.0-15.0), and median PedNIHSS score on admission was 14.0 (IQR, 9.2-20.0). Median time from onset to admission was 3.0 hours (IQR, 1.5-5.1) and median time from onset to recanalization was 4.0 hours (IQR, 3.0-6.9). Sixteen patients (22%) received intravenous thrombolysis before proceeding to endovascular treatment. The cause of stroke remained undetermined in 27 patients (37%). Six patients (8%) had a focal cerebral arteriopathy, 1 patient (1%) had a bilateral cerebral arteriopathy, 7 patients (10%) were classified as having aortic/cervical arteriopathy, and 32 patients (44%) were classified as having a cardioembolic cause.
Sixty-three children (86%) were treated for anterior circulation vessel occlusion and 10 patients (14%) were treated for posterior circulation occlusion. Location of the occlusions were documented as distal internal carotid artery in 21 patients (29%), M1 segment of the middle cerebral artery in 48 patients (66%), M2/M3 segment of the middle cerebral artery in 5 patients (7%), anterior cerebral artery in 1 patient (1%), posterior cerebral artery in 1 patient (1%), vertebral artery in 5 patients (7%), and basilar artery in 8 patients (11%). In addition, 1 patient (1%) developed a bilateral middle cerebral artery M1 occlusion. Baseline characteristics of the study population, including the number of missing values, are presented in the Table.
Endovascular Treatment and Clinical Outcome
Most of the thrombectomy devices used were currently available stent retrievers (60 [82%]), with 4 × 20 mm the most frequently chosen size. Aspiration catheters designed for the continuous direct aspiration first-pass technique were used as the first-line approach in 7 patients (10%). Coil retrievers (2 [3%]), braided retrievers (2 [3%]), and aspiration with teardrop separator (3 [4%]) were used infrequently. Intra-arterial thrombolysis was used as adjuvant therapy only (5 [7%]).
Angiographic outcome was good (≥mTICI 2b) in 62 of 71 patients (87%): 35 with mTICI 3, 7 with mTICI 2c, 20 with mTICI 2b) and poor (≤mTICI 2a) in 9 patients (12.6%: 2 with mTICI 0, 5 with mTICI 1, 2 with mTICI 2a). Median ASPECTS or PC ASPECTS on admission was 8.0 (IQR, 7.0-9.0) and median ASPECTS or PC ASPECTS after the intervention was 7.0 (IQR, 5.0-8.0) (eFigure in the Supplement).
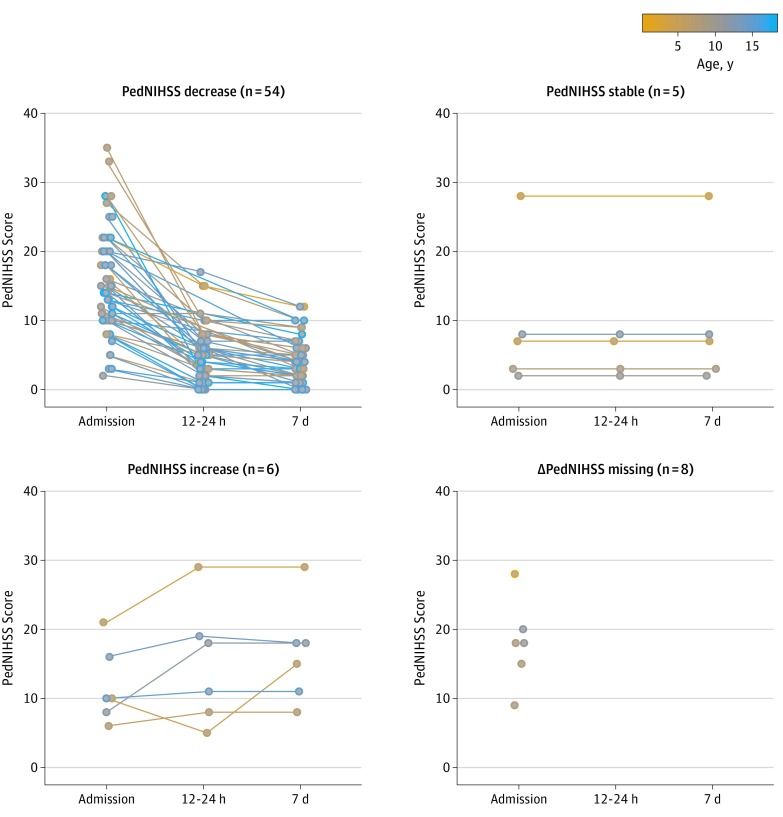
Most patients showed an improvement of neurologic deficit after thrombectomy: a median PedNIHSS score of 14.0 (IQR, 9.2-20.0) on admission improved to a median of 5.0 (IQR, 2.2-8.0) 12 to 24 hours after thrombectomy and 4.0 (IQR, 2.0-7.3) at day 7 (Table, Figure 1). A comparison of the PedNIHSS score improvement observed in Save ChildS at different times from the NIHSS values in trials included in the HERMES meta-analysis suggested that short-term neurologic improvement of our study population showed a similar pattern as observed in the adult trials (Figure 2).
Table. Patient Characteristics.
| Characteristic | No. (%) | P Value | |||
|---|---|---|---|---|---|
| All Children (N = 73) | Age, y | ||||
| 0-6 (n = 11) | 7-12 (n = 31) | 13-18 (n = 31) | |||
| Male | 37 (51) | 8 (73) | 17 (55) | 12 (39) | .14 |
| Age at stroke, median (range), y | 11.3 (7.0-15.0) | NA | NA | NA | NA |
| Onset to recanalization, median (IQR), h | 4.0 (3.0-6.9) | 6.0 (4.0-8.4) | 5.3 (3.0-10.3) | 4.0 (2.5-4.6) | .054 |
| Missing data | 10 | 3 | 5 | 2 | |
| Onset to admission, median (IQR), h | 3.0 (1.5-5.1) | 4.0 (2.0-6.9) | 4.0 (2.0-8.3) | 2.0 (1.0-3.0) | .04 |
| Missing data | 10 | 3 | 5 | 2 | |
| Uncertain onset to admission | 8 (11) | 3 (27) | 3 (10) | 2 (6) | .25 |
| Stent retriever | 60 (82) | 8 (73) | 27 (87) | 25 (81) | .50 |
| First-line ADAPT | 7 (10) | 1 (9) | 3 (10) | 3 (10) | >.99 |
| Other (coil-or braided retriever, teardrop separator) | 7 (10) | 1 (9) | 3 (10) | 3 (10) | >.99 |
| Intravenous thrombolysis | 16 (22) | 2 (18) | 3 (10) | 11 (35) | .045 |
| Intraarterial thrombolysisa | 3 (4) | 0 | 2 (6) | 1 (3) | >.99 |
| Antiplatelet | |||||
| Aspirin | 13 (18) | 0 | 10 (32) | 3 (10) | .02 |
| Clopidogrel | 10 (14) | 0 | 4 (13) | 6 (19) | .35 |
| GP2a/3b | 1 (1) | 0 | 0 | 1 (3) | >.99 |
| Heparin | |||||
| Unfractionated | 5 (7) | 2 (18) | 2 (6) | 1 (3) | .23 |
| Low molecular weight | 22 (30) | 1 (9) | 11 (35) | 10 (32) | .29 |
| Other | 4 (5) | 1 (9) | 0 | 3 (10) | .21 |
| CASCADE | |||||
| Focal cerebral arteriopathy | 6 (8) | 0 | 4 (13) | 2 (6) | .72 |
| Bilateral cerebral | 1 (1) | 0 | 0 | 1 (3) | |
| Aortic/cervical | 7 (10) | 1 (9) | 2 (6) | 4 (13) | |
| Cardioembolic | 32 (44) | 5 (45.5) | 16 (52) | 11 (35) | |
| Other | 27 (37) | 5 (4) | 9 (29) | 13 (42) | |
| CASCADE subtype | |||||
| Genetic vasculopathy | 1 (1) | 0 | 0 | 1 (3) | >.99 |
| Infectious | 4 (5) | 0 | 0 | 4 (13) | .11 |
| Hematologic/thrombotic | 2 (3) | 0 | 1 (3) | 1 (3) | >.99 |
| Inflammatory | 2 (3) | 0 | 1 (3) | 1 (3) | >.99 |
| Anterior circulation vessel occlusion | 63 (86) | 10 (91) | 24 (77) | 29 (94) | .21 |
| Distal internal carotid artery | 21 (29) | 5 (4) | 4 (13) | 12 (39) | .03 |
| M1b | 48 (66) | 8 (73) | 18 (58) | 22 (71) | .57 |
| M2b | 4 (5) | 0 | 3 (10) | 1 (3) | .51 |
| M3b | 1 (1) | 0 | 1 (3) | 0 | >.99 |
| Anterior cerebral artery | 1 (1) | 0 | 1 (3) | 0 | >.99 |
| Bilateral M1 | 1 (1) | 1 (9) | 0 | 0 | .15 |
| Posterior circulation vessel occlusion | 10 (14) | 1 (9) | 7 (23) | 2 (6) | .21 |
| Vertebral artery | 5 (7) | 1 (9) | 4 (13) | 0 | .12 |
| Basilar artery | 8 (11) | 1 (9) | 5 (16) | 2 (6) | .61 |
| Posterior cerebral artery | 1 (1) | 0 | 1 (3) | 0 | >.99 |
| PedNIHSS score on admission, median (IQR)c | 14.0 (9.2-20.0) | 16.0 (6.7-24.0) | 14.0 (9.2-19.7) | 13.0 (10.0-19.7) | .92 |
| Missing data | 2 | 2 | 0 | 0 | |
| PedNIHSS 12-24 h, median (IQR) | 5.0 (2.2-8.0) | 7.0 (4.2-13.8) | 5.0 (3.0-8.2) | 5.0 (1.9-7.0) | .34 |
| Missing data | 18 | 4 | 9 | 5 | |
| General anesthesia/deep sedation 12-24 h | 6 (8) | 2 (18) | 1 (3) | 3 (9.7) | .24 |
| PedNIHSS 7 d, median (IQR) | 4.0 (2.0-7.3) | 10.0 (4.1-22.6) | 3.5 (2.0-6.0) | 3.0 (1.0-7.0) | .06 |
| Missing data | 8 | 3 | 5 | 0 | |
| General anesthesia/deep sedation 7 d | 3 (4) | 0 | 1 (3) | 2 (6.5) | >.99 |
| ASPECTS or PC ASPECTS admission, median (IQR)d | 8.0 (7.0-9.0) | 8.0 (3.8-8.8) | 8.0 (7.0-9.0) | 8.0 (6.0-9.0) | .96 |
| Missing data | 10 | 4 | 4 | 2 | |
| ASPECTS or PC ASPECTS follow-up, median (IQR) | 7.0 (5.0-8.0) | 4.0 (1.9-8.1) | 7.0 (6.0-8.0) | 6.0 (5.0-8.0) | .10 |
| Missing data | 15 | 5 | 6 | 4 | |
| MRI | |||||
| Admission | 7 (10) | 0 | 3 (10) | 4 (13) | .67 |
| Follow-up | 8 (11) | 0 | 4 (13) | 4 (13) | .69 |
| Recanalization, mTICI e | |||||
| 0 | 2 (3) | 0 | 2 (6) | 0 | .52 |
| Missing data | 2 | 2 | 0 | 0 | |
| 1 | 5 (7) | 1 (11) | 2 (6) | 2 (6) | |
| Missing data | 2 | 2 | 0 | 0 | |
| 2a | 2 (3) | 1 (11) | 0 | 1 (3) | |
| Missing data | 2 | 2 | 0 | 0 | |
| 2b | 20 (28) | 1 (11) | 8 (26) | 11 (35) | |
| Missing data | 2 | 2 | 0 | 0 | |
| 2c | 7 (10) | 1 (11) | 2 (6) | 4 (13) | |
| Missing data | 2 | 2 | 0 | 0 | |
| 3 | 35 (49) | 5 (56) | 17 (55) | 13 (42) | |
| Missing data | 2 | 2 | 0 | 0 | |
| Complications, peri-interventional | |||||
| Spasms | 4 (5) | 0 | 2 (6) | 2 (6) | >.99 |
| Other | 1 (1) | 0 | 1 (3) | 0 | >.99 |
| Complications, postinterventional | |||||
| ICH | 1 (1) | 1 (9) | 0 | 0 | .15 |
| Malignant | 3 (4) | 0 | 0 | 3 (10) | .20 |
| Pediatric Stroke Outcome Measure score, median (IQR)f | |||||
| Discharge | 1.0 (0-2.0) | 4.0 (1.0-10.0) | 1.0 (0.5-1.0) | 0.5 (0-1.0) | .02 |
| Missing data | 21 | 5 | 12 | 4 | |
| 6 mo | 0.5 (0-1.0) | 3.0 (1.3-3.8) | 0.8 (0-1.0) | 0.5 (0-1.0) | .07 |
| Missing data | 26 | 8 | 13 | 5 | |
| 24 mo | 0.5 (0-1.0) | 3.0 (1.3-3.8) | 1.0 (0.1-1.0) | 0.3 (0-1.0) | .03 |
| Missing data | 37 | 8 | 16 | 13 | |
| Modified Rankin Scale score, median (IQR)g | |||||
| Discharge | 1.0 (0.2-2.0) | 3.5 (1.0-5.1) | 1.0 (1.0-2.0) | 1.0 (0-2.0) | .004 |
| Missing data | 2 | 1 | 1 | ||
| 6 mo | 1.0 (0-1.6) | 3.0 (1.0-3.8) | 1.0 (0-1.0) | 1.0 (0-1.3) | .03 |
| Missing data | 13 | 4 | 7 | 2 | |
| 24 mo | 1.0 (0-1.0) | 2.0 (1.0-4.2) | 1.0 (0-1.0) | 0.5 (0-1.0) | .03 |
| Missing data | 27 | 5 | 11 | 11 | |
Abbreviations: ADAPT, A Direct Aspiration First Pass Technique; ASPECTS, Alberta Stroke Program Early Computed Tomography Score; CASCADE, Childhood Arterial Ischemic Stroke Standardized Classification and Diagnostic Evaluation Classification; IQR, interquartile range; MRI, magnetic resonance imaging; mTICI, Modified Treatment in Cerebral Infarction; NA, not applicable; PC, posterior circulation; PedNIHSS, Pediatric National Institutes of Health Stroke Scale.
All antiplatelet and antithrombotic treatments were started after the intervention for stroke recurrence prevention.
Segment of the middle cerebral artery.
Possible score range: 0 (no deficit) to 34 (maximum deficit).
Provides segmental assessment of the vascular territory and 1 point is deducted from the initial score of 10 for every region involved (from 10 [no lesion] to 0 [maximum lesions]).
Possible score range: 0, no perfusion; perfusion grade 1, antegrade reperfusion past the initial occlusion but limited distal branch filling with little distal; reperfusion grade 2a, antegrade reperfusion of less than half of the occluded target artery previously ischemic territory; grade 2b, antegrade reperfusion of more than half of the previously occluded target artery ischemic territory; grade 2c, near-complete perfusion except for slow flow in a few distal cortical vessels or presence of small distal cortical emboli; grade 3, complete antegrade reperfusion of the previously occluded target artery ischemic territory, with absence of visualized occlusion in all distal branches.
Possible score range: 0 (no deficit) to 10 (maximum deficit).
Possible score range: 0 (no deficit) to 6 (death).
Figure 1. Course of Pediatric National Institutes of Health Stroke Scale (PedNIHSS) Scores in Patients of the Save ChildS Study (N = 73).
Possible score range for the PedNIHSS is 0 (no deficit) to 34 (maximum deficit).
Figure 2. Pediatric National Institutes of Health Stroke Scale (PedNIHSS) Scores (PedNIHSS) in Save ChildS at Different Times Compared With NIHSS Measured in the HERMES Meta-analysis Trials.
Possible score range for the PedNIHSS is 0 (no deficit) to 34 (maximum deficit). Error bars represent interquartile range.
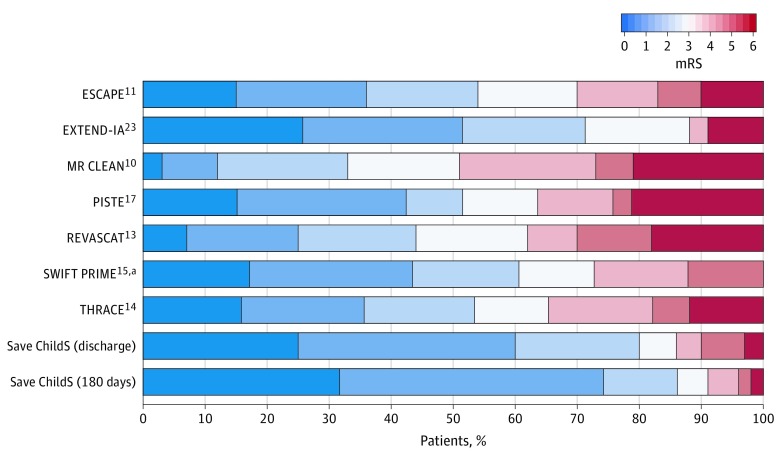
The median mRS score at discharge was 1.0 (IQR, 0.2-2.0) and further improved to 1.0 (IQR, 0-1.6) after 6 months and 1.0 (IQR, 0-1.0) after 24 months. In addition, the median PSOM score was 1.0 (IQR, 0-2.0) at discharge and 0.5 (IQR, 0-1.0) 6 and 24 months after the intervention (Table). An analysis grouped by age also suggested that the mRS score at discharge was higher in children aged 0 to 6 years (3.5; IQR, 1.0-5.1) than in the whole study cohort including all age groups 1.0 (IQR, 0.2-2.0) (Table). A comparison of mRS (at discharge and 180 days) to mRS (at 90 days) in the HERMES meta-analysis revealed an apparently lower proportion of poor outcomes in our study population (Figure 3).
Figure 3. Modified Rankin Scale (mRS) Scores in Save ChildS Measured at Discharge and 180 Days Compared With mRS Scores in the HERMES Meta-analysis Trials Measured at 90 Days.
Possible score range for the mRS is 0 (no deficit) to 6 (death).
aIn SWIFT PRIME, mRS scores of 5 and 6 were combined into 1 category mRS score greater than or equal to 5.
Safety
The patients in our study population did not appear to have periprocedural complications other than transient vasospasm, detected angiographically, which occurred in 4 patients (5%). In all cases, the vasospasms seemed to resolve after administration of nimodipine and without any clinical sequelae. In addition, 1 patient with a preexisting heart anomaly died of cardiac arrest after complete recanalization of the M1 occlusion. No vascular complications, such as arterial dissection, periprocedural thrombosis, or puncture site complications were reported. Postinterventional complications that were reported included 1 patient (1%) with symptomatic intracerebral hemorrhage and 3 patients (4%) with malignant infarction followed by decompressive hemicraniectomy.
A comparison with the adult trials included in the HERMES meta-analysis showed that the proportion of symptomatic intracerebral hemorrhage events in the HERMES trials was 2.79 (95% CI, 0.42-6.66) and in Save ChildS was 1.37 (95% CI, 0.03-7.40) (Figure 4). The heterogeneity measure I2 for symptomatic intracerebral hemorrhage across the 7 HERMES trials was 84% and decreased to 82% after adding Save ChildS data.
Figure 4. Proportion of Symptomatic Intracerebral Hemorrhage (sICH) Events in Save ChildS Compared With the Results of the HERMES Meta-analysis Trials.
A, Proportion of sICH in the 7 HERMES studies and combined estimate. B, Proportion of sICH in Save ChildS and combined estimate including 7 Hermes studies.
Discussion
Endovascular thrombectomy has emerged as the standard of care for adult patients with acute ischemic stroke due to large vessel occlusion.12 Knowledge about effectiveness and safety of mechanical recanalization in childhood stroke, however, is limited to small case series. These existing reports have been well summarized,18,19,27 but, to our knowledge, the potential to apply this technique in pediatric patients has not yet been explored systematically.
The main findings of this retrospective, multicenter cohort study are (1) endovascular thrombectomy in pediatric patients with ischemic stroke and large-vessel occlusion is feasible, as most patients underwent successful recanalization; (2) complication rates were low in children undergoing mechanical recanalization treatment (relevant intracerebral hemorrhage in 1 patient only); (3) long-term neurologic outcomes were good in most patients (median mRS score, 1 and median PSOM score, 0.5 at 6 and 24 months) and (4) short-term improvement determined by PedNIHSS score was comparable with the short-term improvement of NIHSS in adult trials (median, 14 at admission and 4 at day 7 after thrombectomy).
To our knowledge, this study is the largest available in the literature so far. The results suggest agreement with the results of 2 small, single-center case series assessing recanalization treatments in pediatric populations for acute ischemic stroke.19,28 In both previous studies, most patients were treated with intravenous thrombolysis, reducing the number of children who underwent mechanical thrombectomy to 2 patients28 and 11 patients.19 Moreover, of the 11 pediatric patients who underwent endovascular treatment in the recent study of Bigi et al,19 5 children were treated only with intraarterial thrombolysis.
One of the major concerns of neuropediatricians about the use of endovascular techniques as well as intravenous recanalization techniques in children relates to their safety. However, severe or fatal treatment-related complications appeared to be rare in our population, which is in line with previous reports.19,27,29 In our study, only 1 patient developed a symptomatic intracerebral hemorrhage after thrombectomy, which is a low incidence even compared with the recent adult trials included in the HERMES meta-analysis (Figure 4).
Furthermore, no vascular complications, such as dissections or vessel rupture, were reported during endovascular procedures. This lack of complications is particularly important because the causes of stroke are considerably different in children so that results of adult trials cannot be extrapolated to children with strokes in general. Children more often have strokes due to an underlying arteriopathy, especially of inflammatory origin, which might increase the risk of bleeding complications during endovascular procedures owing to vessel fragility.23 Even though our study included children with all types of stroke sources, only 7 patients with focal or bilateral cerebral arteriopathy were included. Thus, an a priori selection bias of thrombectomy against children with potential inflammatory vasculopathy may be inherent to a seemingly low overall hemorrhagic risk. Vascular fragility and risk of hemorrhage need to be considered and weighted carefully against a potential benefit of a recanalization treatment in this specific patient population. Underlying abnormalities are often unknown at the time of admission; therefore, the emergency decision on whether to perform thrombectomy frequently has to be made without detailed knowledge about the cause of the stroke (eTable 2 in the Supplement provides details about patients with cerebral arteriopathy).
A subanalysis of our study results grouped by patient age revealed that the PedNIHSS score, mRS score, and ASPECTS on follow-up were worse in younger patients (age 0-6 years). Admission PedNIHSS score and onset to recanalization were marginally higher, while admission ASPECTS, the rate of recanalization, and complications were not significantly different compared with those measures in patients older than 6 years. Although the statistical significance of the findings is questionable across this smaller subgroup, the data suggest that successful endovascular recanalization in younger children aged 0 to 6 years may be futile more frequently and should be performed only after careful consideration of all risks and potential benefits.
Randomized trials seeking to determine whether endovascular recanalization is safe and effective in pediatric patients would be desirable but are not likely to be conducted. Difficulties in recruitment are expected, which is underlined by the fact that a previous prospective randomized clinical trial performed to assess the safety of intravenous thrombolysis in pediatric patients was not completed.3 Furthermore, clinical equipoise of randomizing pediatric patients with stroke is unlikely against the background of a large amount of evidence for a strong treatment effect in adults. Our exploratory study currently represents an alternative attempt to gain further insights with regard to feasibility and safety of thrombectomy in pediatric patients with stroke.
Limitations
One major limitation of this study is the retrospective design involving a high number of contributing sites, which leads to missing data, especially for the long-term outcomes, and may cause selection bias. Another limitation is the lack of a control arm, that is, a comparable cohort with large-vessel occlusion and similar stroke severity not treated with endovascular thrombectomy. Based on the synthesis of evidence from small randomized clinical trials for rare diseases in pediatric populations with the evidence from adult study populations with the same disease,30 we assessed safety indirectly by a comparison with the HERMES meta-analysis in adults who underwent thrombectomy. The absence of standardized protocols of inclusion for thrombectomy in the different centers makes the study population heterogeneous. However, this heterogeneity may nonetheless provide pragmatic confidence that endovascular treatment is feasible in a not perfect clinical setting even though further improvements and standardization of protocols are desirable.
Conclusions
The findings of our study may add to the growing evidence that mechanical thrombectomy is safe in childhood stroke. This study may support clinicians’ practice of off-label thrombectomy in childhood stroke in the absence of high-level evidence.
eFigure. Course of ASPECTS in Patients of the Save ChildS Study (n = 73)
eTable 1. List of Participating Stroke Centers (n = 27)
eTable 2. Characteristics of Patients With Cerebral Arteriopathy (n = 7)
References
- 1.Mallick AA, Ganesan V, Kirkham FJ, et al. . Childhood arterial ischaemic stroke incidence, presenting features, and risk factors: a prospective population-based study. Lancet Neurol. 2014;13(1):35-43. doi: 10.1016/S1474-4422(13)70290-4 [DOI] [PubMed] [Google Scholar]
- 2.Goldenberg NA, Bernard TJ, Fullerton HJ, Gordon A, deVeber G; International Pediatric Stroke Study Group . Antithrombotic treatments, outcomes, and prognostic factors in acute childhood-onset arterial ischaemic stroke: a multicentre, observational, cohort study. Lancet Neurol. 2009;8(12):1120-1127. doi: 10.1016/S1474-4422(09)70241-8 [DOI] [PubMed] [Google Scholar]
- 3.Rivkin MJ, deVeber G, Ichord RN, et al. . Thrombolysis in pediatric stroke study. Stroke. 2015;46(3):880-885. doi: 10.1161/STROKEAHA.114.008210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Powers WJ, Rabinstein AA, Ackerson T, et al. ; American Heart Association Stroke Council . 2018 Guidelines for the early management of patients with acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2018;49(3):e46-e110. doi: 10.1161/STR.0000000000000158 [DOI] [PubMed] [Google Scholar]
- 5.Vázquez López M, de Castro de Castro P, Barredo Valderrama E, et al. . Outcome of arterial ischemic stroke in children with heart disease. Eur J Paediatr Neurol. 2017;21(5):730-737. doi: 10.1016/j.ejpn.2017.05.007 [DOI] [PubMed] [Google Scholar]
- 6.Agrawal N, Johnston SC, Wu YW, Sidney S, Fullerton HJ. Imaging data reveal a higher pediatric stroke incidence than prior US estimates. Stroke. 2009;40(11):3415-3421. doi: 10.1161/STROKEAHA.109.564633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.deVeber GA, Kirton A, Booth FA, et al. . Epidemiology and outcomes of arterial ischemic stroke in children: the Canadian Pediatric Ischemic Stroke Registry. Pediatr Neurol. 2017;69:58-70. doi: 10.1016/j.pediatrneurol.2017.01.016 [DOI] [PubMed] [Google Scholar]
- 8.deVeber GA, MacGregor D, Curtis R, Mayank S. Neurologic outcome in survivors of childhood arterial ischemic stroke and sinovenous thrombosis. J Child Neurol. 2000;15(5):316-324. doi: 10.1177/088307380001500508 [DOI] [PubMed] [Google Scholar]
- 9.Ellis MJ, Amlie-Lefond C, Orbach DB. Endovascular therapy in children with acute ischemic stroke: review and recommendations. Neurology. 2012;79(13)(suppl 1):S158-S164. doi: 10.1212/WNL.0b013e31826958bf [DOI] [PubMed] [Google Scholar]
- 10.Berkhemer OA, Fransen PS, Beumer D, et al. ; MR CLEAN Investigators . A randomized trial of intraarterial treatment for acute ischemic stroke. N Engl J Med. 2015;372(1):11-20. doi: 10.1056/NEJMoa1411587 [DOI] [PubMed] [Google Scholar]
- 11.Goyal M, Demchuk AM, Menon BK, et al. ; ESCAPE Trial Investigators . Randomized assessment of rapid endovascular treatment of ischemic stroke. N Engl J Med. 2015;372(11):1019-1030. doi: 10.1056/NEJMoa1414905 [DOI] [PubMed] [Google Scholar]
- 12.Goyal M, Menon BK, van Zwam WH, et al. ; HERMES collaborators . Endovascular thrombectomy after large-vessel ischaemic stroke: a meta-analysis of individual patient data from five randomised trials. Lancet. 2016;387(10029):1723-1731. doi: 10.1016/S0140-6736(16)00163-X [DOI] [PubMed] [Google Scholar]
- 13.Jovin TG, Chamorro A, Cobo E, et al. ; REVASCAT Trial Investigators . Thrombectomy within 8 hours after symptom onset in ischemic stroke. N Engl J Med. 2015;372(24):2296-2306. doi: 10.1056/NEJMoa1503780 [DOI] [PubMed] [Google Scholar]
- 14.Bracard S, Ducrocq X, Mas JL, et al. ; THRACE investigators . Mechanical thrombectomy after intravenous alteplase versus alteplase alone after stroke (THRACE): a randomised controlled trial. Lancet Neurol. 2016;15(11):1138-1147. doi: 10.1016/S1474-4422(16)30177-6 [DOI] [PubMed] [Google Scholar]
- 15.Saver JL, Goyal M, Bonafe A, et al. ; SWIFT PRIME Investigators . Stent-retriever thrombectomy after intravenous t-PA vs. t-PA alone in stroke. N Engl J Med. 2015;372(24):2285-2295. doi: 10.1056/NEJMoa1415061 [DOI] [PubMed] [Google Scholar]
- 16.Nogueira RG, Jadhav AP, Haussen DC, et al. ; DAWN Trial Investigators . Thrombectomy 6 to 24 hours after stroke with a mismatch between deficit and infarct. N Engl J Med. 2018;378(1):11-21. doi: 10.1056/NEJMoa1706442 [DOI] [PubMed] [Google Scholar]
- 17.Muir KW, Ford GA, Messow CM, et al. ; PISTE Investigators . Endovascular therapy for acute ischaemic stroke: the Pragmatic Ischaemic Stroke Thrombectomy Evaluation (PISTE) randomised, controlled trial. J Neurol Neurosurg Psychiatry. 2017;88(1):38-44. doi: 10.1136/jnnp-2016-314117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sporns PB, Kemmling A, Hanning U, et al. . Thrombectomy in childhood stroke. J Am Heart Assoc. 2019;8(5):e011335. doi: 10.1161/JAHA.118.011335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bigi S, Dulcey A, Gralla J, et al. . Feasibility, safety, and outcome of recanalization treatment in childhood stroke. Ann Neurol. 2018;83(6):1125-1132. doi: 10.1002/ana.25242 [DOI] [PubMed] [Google Scholar]
- 20.Goeggel Simonetti B, Cavelti A, Arnold M, et al. . Long-term outcome after arterial ischemic stroke in children and young adults. Neurology. 2015;84(19):1941-1947. doi: 10.1212/WNL.0000000000001555 [DOI] [PubMed] [Google Scholar]
- 21.World Medical Association World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA. 2013;310(20):2191-2194. doi: 10.1001/jama.2013.281053 [DOI] [PubMed] [Google Scholar]
- 22.Bernard TJ, Manco-Johnson MJ, Lo W, et al. . Towards a consensus-based classification of childhood arterial ischemic stroke. Stroke. 2012;43(2):371-377. doi: 10.1161/STROKEAHA.111.624585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Böhmer M, Niederstadt T, Heindel W, et al. . Impact of childhood arterial ischemic stroke standardized classification and diagnostic evaluation classification on further course of arteriopathy and recurrence of childhood stroke [published online December 7, 2018]. Stroke. 2018;A118023060. doi: 10.1161/STROKEAHA.118.023060 [DOI] [PubMed] [Google Scholar]
- 24.Campbell BC, Mitchell PJ, Kleinig TJ, et al. ; EXTEND-IA Investigators . Endovascular therapy for ischemic stroke with perfusion-imaging selection. N Engl J Med. 2015;372(11):1009-1018. doi: 10.1056/NEJMoa1414792 [DOI] [PubMed] [Google Scholar]
- 25.Schwarzer G. Meta: An R package for meta-analysis. R News. 2007;7(3):40-45. [Google Scholar]
- 26.Barendregt JJ, Doi SA, Lee YY, Norman RE, Vos T. Meta-analysis of prevalence. J Epidemiol Community Health. 2013;67(11):974-978. doi: 10.1136/jech-2013-203104 [DOI] [PubMed] [Google Scholar]
- 27.Satti S, Chen J, Sivapatham T, Jayaraman M, Orbach D. Mechanical thrombectomy for pediatric acute ischemic stroke: review of the literature. J Neurointerv Surg. 2017;9(8):732-737. doi: 10.1136/neurintsurg-2016-012320 [DOI] [PubMed] [Google Scholar]
- 28.Tabone L, Mediamolle N, Bellesme C, et al. . Regional pediatric acute stroke protocol: Initial experience during 3 years and 13 recanalization treatments in children. Stroke. 2017;48(8):2278-2281. doi: 10.1161/STROKEAHA.117.016591 [DOI] [PubMed] [Google Scholar]
- 29.Cappellari M, Moretto G, Grazioli A, Ricciardi GK, Bovi P, Ciceri EFM. Primary versus secondary mechanical thrombectomy for anterior circulation stroke in children: an update. J Neuroradiol. 2018;45(2):102-107. doi: 10.1016/j.neurad.2017.11.005 [DOI] [PubMed] [Google Scholar]
- 30.Weber K, Hemmings R, Koch A. How to use prior knowledge and still give new data a chance? Pharm Stat. 2018;17(4):329-341. doi: 10.1002/pst.1862 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eFigure. Course of ASPECTS in Patients of the Save ChildS Study (n = 73)
eTable 1. List of Participating Stroke Centers (n = 27)
eTable 2. Characteristics of Patients With Cerebral Arteriopathy (n = 7)