This subgroup analysis of a randomized clinical trial compares trifluridine/tipiracil treatment with placebo for progression-free and overall survival among patients with previously treated metastatic gastric or gastroesophageal junction cancer who had or had not undergone gastrectomy.
Key Points
Question
Is trifluridine/tipiracil treatment safe and effective for the subpopulation of patients with previously treated metastatic gastric or gastroesophageal junction cancer who have undergone gastrectomy?
Findings
In this subgroup analysis of a randomized clinical trial, trifluridine/tipiracil treatment improved overall survival and progression-free survival compared with placebo among patients with previously treated metastatic gastric or gastroesophageal junction cancer and who had or had not undergone gastrectomy. No new safety concerns were reported, and hematologic toxic effects were more frequent among the subgroup who had undergone gastrectomy but were treated using dosing modifications.
Meaning
Trifluridine/tipiracil is a safe and effective treatment option for patients with pretreated metastatic gastric or gastroesophageal junction cancer regardless of previous gastrectomy.
Abstract
Importance
Trifluridine/tipiracil (FTD/TPI) treatment has shown clinical benefit in patients with pretreated metastatic gastric cancer or gastroesophageal junction cancer (mGC/GEJC). Patients who have undergone gastrectomy constitute a significant proportion of patients with mGC/GEJC.
Objective
To assess the efficacy and safety of FTD/TPI among patients with previously treated mGC/GEJC who had or had not undergone gastrectomy.
Design, Setting, and Participants
This preplanned subgroup analysis of TAGS (TAS-102 Gastric Study), a phase 3, randomized, placebo-controlled, clinical trial included patients with mGC/GEJC who had received at least 2 previous chemotherapy regimens, and was conducted at 110 academic hospitals in 17 countries in Europe, Asia, and North America, with enrollment between February 24, 2016, and January 5, 2018; the data cutoff was March 31, 2018.
Interventions
Patients were randomized 2:1 to receive oral FTD/TPI 35 mg/m2 twice daily or placebo twice daily with best supportive care on days 1 through 5 and days 8 through 12 of each 28-day treatment cycle.
Main Outcomes and Measures
The primary end point was overall survival. This subgroup analysis was conducted to examine potential trends and was not powered for statistical significance. Efficacy and safety end points were evaluated in the subgroups.
Results
Of 507 randomized patients (369 [72.8%] male; mean [SD] age, 62.5 [10.5] years), 221 (43.6%) had undergone gastrectomy (147 randomized to FTD/TPI and 74 to placebo) and 286 (56.4%) had not undergone gastrectomy (190 randomized to FTD/TPI and 96 to placebo). In the gastrectomy subgroup, the overall survival hazard ratio (HR) in the FTD/TPI group vs placebo group was 0.57 (95% CI, 0.41-0.79), and the progression-free survival HR was 0.48 (95% CI, 0.35-0.65). In the no gastrectomy subgroup, the overall survival HR in the FTD/TPI group vs placebo group was 0.80 (95% CI, 0.60-1.06), and the progression-free survival HR was 0.65 (95% CI, 0.49-0.85). Among FTD/TPI-treated patients, grade 3 or higher adverse events of any cause occurred in 122 of 145 patients (84.1%) in the gastrectomy subgroup and 145 of 190 (76.3%) in the no gastrectomy subgroup: 64 (44.1%) in the gastrectomy subgroup and 50 (26.3%) in the no gastrectomy subgroup had grade 3 or higher neutropenia, 31 (21.4%) in the gastrectomy subgroup and 33 (17.4%) in the no gastrectomy subgroup had grade 3 or higher anemia, and 21 (14.5%) in the gastrectomy subgroup and 10 (5.3%) in the no gastrectomy subgroup hD grade 3 or higher leukopenia. In the gastrectomy subgroup, 94 (64.8%) had dosing modifications because of adverse events vs 101 (53.2%) in the no gastrectomy subgroup; 15 (10.3%) in the gastrectomy group and 28 (14.7%) in the no gastrectomy group discontinued treatment because of adverse events. Treatment exposure was similar between groups.
Conclusions and Relevance
The FTD/TPI treatment was tolerable and provided efficacy benefits among patients with pretreated mGC/GEJC regardless of previous gastrectomy.
Trial Registration
ClinicalTrials.gov identifier: NCT02500043
Introduction
Survival outcomes are poor for patients with metastatic gastric cancer or gastroesophageal junction cancer (mGC/GEJC), with a median survival time of 3 to 5 months without chemotherapy.1,2 Chemotherapy extends median overall survival (OS) by nearly 7 months,2,3 but ultimately the disease becomes refractory to chemotherapy, and all patients experience disease progression after first-line treatment.4,5,6 Duration of response to second-line treatment is short,4,5,6 and there are few options for third- and later-line therapy.
Nearly half of all patients with mGC/GEJC have undergone gastrectomy.7,8,9,10,11 Patients who have undergone gastrectomy tend to be a more compromised patient subpopulation than those who have not undergone gastrectomy because the malnutrition associated with gastrectomy can delay recovery and potentially render these patients more susceptible to adverse events (AEs). In addition, many patients who have undergone gastrectomy have received adjuvant chemotherapy or radiotherapy.12,13,14 Being heavily pretreated, these patients have poorer chemotherapy tolerance.15,16 However, studies13,16,17,18,19,20,21,22,23 that have evaluated the safety and efficacy of oral chemotherapy in this subpopulation have primarily involved adjuvant therapy, and few studies7,10,15 have examined patients with metastatic disease.
Trifluridine/tipiracil (FTD/TPI), also known by the investigational drug name TAS-102, is an oral therapy comprising the thymidine analogue trifluridine and the thymidine phosphorylase inhibitor tipiracil, which inhibits trifluridine degradation.24,25 In 2015, FTD/TPI was approved for the treatment of refractory metastatic colorectal cancer at a dosage of 35 mg/m2 twice daily.26,27
In a phase 2 Japanese study (EPOC1201),28 FTD/TPI showed activity and tolerability among patients with previously treated mGC/GEJC. Although the sample sizes were small, no differences were observed in the pharmacokinetics of either FTD or TPI at the approved dose between patients who had or had not undergone gastrectomy.28
Based on these phase 2 results,28 FTD/TPI was evaluated in a global, randomized, double-blind, placebo-controlled phase 3 clinical trial (TAS-102 Gastric Study [TAGS]) of 507 patients with mGC/GEJC after disease progression following at least 2 standard chemotherapy regimens.8,29 Compared with placebo, FTD/TPI significantly improved OS, the primary end point (hazard ratio [HR], 0.69; 95% CI, 0.56-0.85; P < .001) and progression-free survival (PFS) (HR, 0.57; 95% CI, 0.47-0.70; P < .001).8 In the FTD/TPI group, grade 3 or higher AEs occurred in 80% of patients and were most frequently hematologic (neutropenia in 34% of patients and anemia in 19%); 13% discontinued treatment because of AEs. These findings supported the approval of FTD/TPI in the United States in 2019 for patients with mGC/GEJC previously treated with at least 2 lines of chemotherapy.27 We present the results of a preplanned subgroup analysis in the phase 3 TAGS trial evaluating FTD/TPI treatment in patients with mGC/GEJC who had or had not undergone gastrectomy.
Methods
Study, Design, and Participants
This study was a subgroup analysis of the TAGS trial, a global, randomized, placebo-controlled phase 3 clinical trial conducted at 110 academic hospitals in 17 countries in Europe, Asia, and North America between February 24, 2016, and January 5, 2018; the data cutoff was March 31, 2018.8 Eligible patients had mGC/GEJC, had an Eastern Cooperative Oncology Group (ECOG) performance status of 0 or 1, had received at least 2 treatment regimens for advanced disease, and were refractory to or were unable to tolerate the most recent therapy. Patients were randomized (2:1) to receive either oral FTD/TPI 35 mg/m2 twice daily plus best supportive care or placebo twice daily plus best supportive care administered on days 1 through 5 and days 8 through 12 of each 28-day treatment cycle. Treatment continued until disease progression, intolerability, patient withdrawal, or completion of the primary end point analysis, whichever occurred first. Before patient enrollment, the protocol was approved by the institutional review board or independent ethnics committee at each participating site8 (eTable 1 in Supplement 2). The study was conducted in accordance with the Declaration of Helsinki30 and Good Clinical Practice guidelines, as specified by the International Conference on Harmonization.31 All patients provided written informed consent.
The primary objective of the study was OS; secondary objectives included PFS, safety, and tolerability. Other end points were time to deterioration of ECOG performance status to 2 or more, objective response rate, and disease control rate.
Gastrectomy Subgroup Analysis
In this preplanned subgroup analysis, the efficacy and safety of FTD/TPI vs placebo were evaluated among patients who had or had not undergone gastrectomy. All patients included in the intention-to-treat assessment of the TAGS study were included in the efficacy assessments, and all patients who received 1 or more doses of study drug (as-treated population) were included in the safety assessments. Although planned, these subanalyses were not powered for statistical significance, and no formal comparisons of efficacy and safety were made between the gastrectomy and no gastrectomy subgroups.
Statistical Analysis
For the overall study, 384 events were targeted to allow the detection of an HR for death of 0.70, with 90% power at a 1-sided type I error of 0.025. Detailed statistical considerations for the phase 3 trial have been reported previously.8
Detailed end point definitions are presented in the trial protocol in Supplement 1 and the eMethods in Supplement 2. For the time-to-event end points, HRs with associated 95% CIs but not P values are presented for the subgroup analyses based on a stratified Cox proportional hazards model, along with Kaplan-Meier estimates of the medians and specific time points. In the overall study analysis, previous gastrectomy was included as a factor in a prespecified multivariate subgroup analysis for the primary end point of OS. All the subgroups used for the multivariate analysis were prespecified, but a multiplicity comparison adjustment method was not used because of the number of subgroups. Subgroup analyses were conducted to examine potential trends or estimations and not for inferential purposes. The incidence of AEs was presented by subgroup; events of interest (such as hematologic toxic events) were compared in patients treated with FTD/TPI in the 2 subgroups using estimated relative risk (RR) and associated 95% CIs. All statistical analyses were conducted using SAS statistical software, version 9.4 (SAS Institute Inc).
Results
Patient Disposition and Demographics
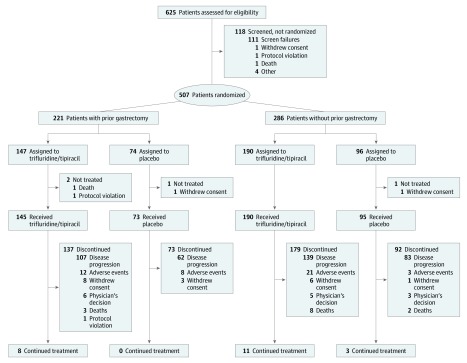
Between February 24, 2016, and January 5, 2018, 507 patients (369 [72.8%] male; mean [SD] age, 62.5 [10.5] years) were enrolled and randomized (intent-to-treat population), with 337 patients randomized to the FTD/TPI group and 170 to the placebo group. A total of 503 patients received at least 1 study drug dose (as-treated population).8
Previous gastrectomy had been performed in 147 of 337 patients (43.6%) in the FTD/TPI group and 74 of 170 patients (43.5%) in the placebo group. Of the 221 patients with previous gastrectomy, 153 (69.2%) had undergone total resection and 56 (25.3%) had undergone partial resection; resection status was unknown for 12 patients (5.4%). Patient disposition was similar between patients who had or had not undergone gastrectomy. At the data cutoff of March 31, 2018, among FTD/TPI-treated patients, 137 of 145 patients (94.5%) who had undergone gastrectomy and 179 of 190 patients (94.2%) who had not undergone gastrectomy had discontinued treatment (Figure 1). Among placebo-treated patients, 73 (100%) who had undergone gastrectomy and 92 of 95 patients (96.8%) who had not undergone gastrectomy had discontinued treatment. Disease progression was the most common reason among patients treated with FTD/TPI who discontinued (107 of 137 patients [78.1%] in the gastrectomy subgroup and 139 of 179 [77.6%] in the no gastrectomy subgroup) and among patients treated with placebo who discontinued (62 of 73 [84.9%] in the gastrectomy subgroup and 83 of 92 [90.2%] in the no gastrectomy subgroup).
Figure 1. Patient Enrollment Flowchart.
Patient baseline demographics and disease characteristics were generally similar between patients who had or had not undergone gastrectomy (eTable 2 in Supplement 2), although the proportions of some measures differed by more than 10% between the gastrectomy vs no gastrectomy subgroups. These measures that differed between the gastrectomy and no gastrectomy subgroups were: at least 3 sites of metastatic disease (94 of 221 [42.5%] vs 186 of 286 [65.0%]), previous radiotherapy (57 of 221 [25.8%] vs 40 of 286 [14.0%]), and at least 3 previous regimens (166 of 221 [75.1%] vs 151 of 286 [52.8%]). In addition, in the gastrectomy subgroup, a higher proportion of patients in the FTD/TPI group had received radiotherapy (44 of 147 patients; 29.9%) compared with the placebo group (13 of 74 patients; 17.6%), but in the no gastrectomy subgroup, the corresponding proportions of patients were similar in each treatment group (gastrectomy subgroup: 27 of 190 patients [14.2%] vs no gastrectomy subgroup: 13 of 96 [13.5%]). More patients in the gastrectomy subgroup received 1 to 2 neoadjuvant or adjuvant treatment regimens (116 of 221 [52.5%]) than those in the no gastrectomy subgroup (4 of 286 [1.4%]), but similar proportions of patients in the 2 subgroups had received at least 3 treatment regimens for metastatic cancer (gastrectomy subgroup: 113 of 221 patients [51.1%] vs no gastrectomy subgroup: 147 of 286 patients [51.4%]).
Efficacy
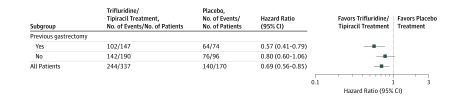
As previously reported, FTD/TPI significantly improved OS and PFS compared with placebo in the overall patient population.8 In the OS subgroup analyses, FTD/TPI was more efficacious than placebo across most prespecified subgroups, including patients who had undergone gastrectomy (Figure 2).8
Figure 2. Subgroup Analysis of Overall Survival Among Patients Who Had or Had Not Undergone Gastrectomy.
Prespecified multivariate subgroup analysis of overall survival in the TAGS (TAS-102 Gastric Study) trial based on previous gastrectomy. This figure is adapted with permission of Elsevier Science and Technology Journals, from Shitara K et al. Trifluridine/tipiracil vs placebo in patients with heavily pretreated metastatic gastric cancer (TAGS): a randomized, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol 2018;19:1437-1448; permission conveyed through Copyright Clearance Center, Inc.
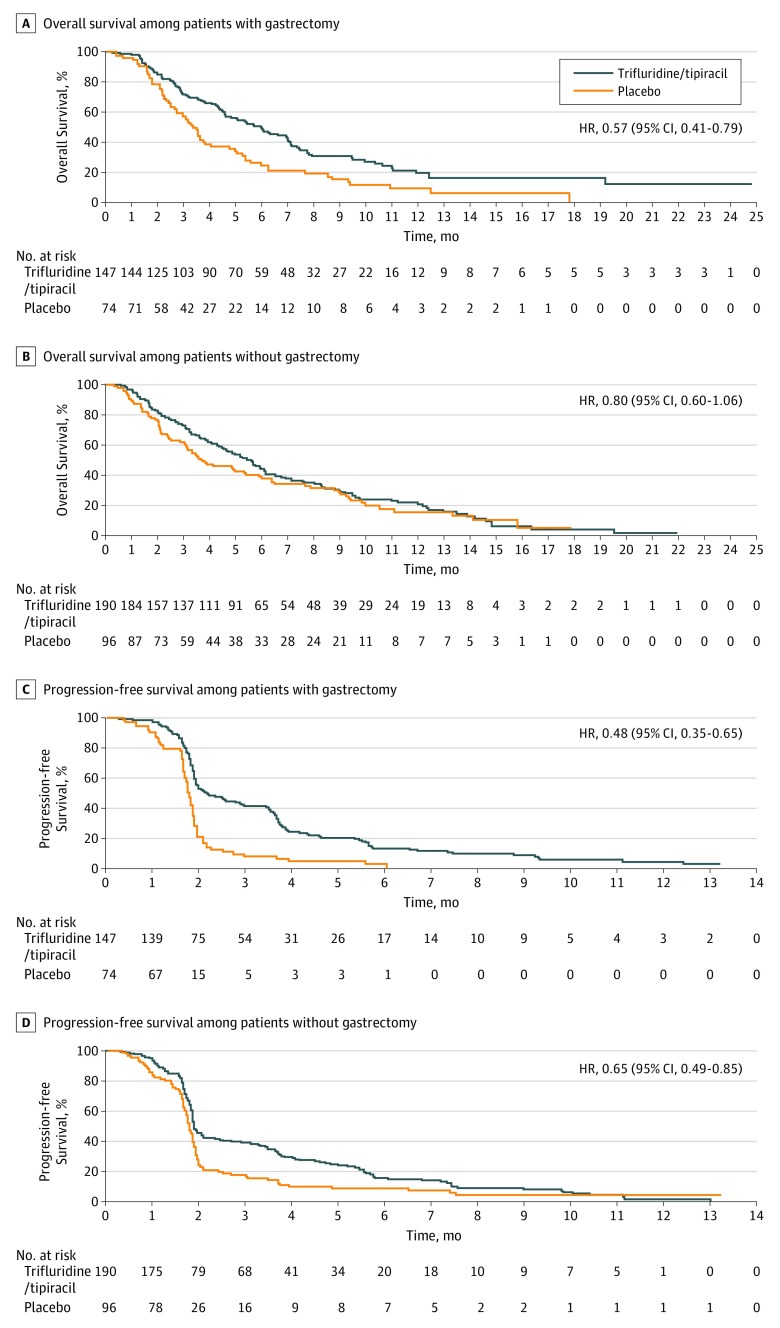
Overall survival and PFS were improved with FTD/TPI treatment in patients in both gastrectomy subgroups (Figure 3 and Table 1). Among patients who had undergone gastrectomy, in the FTD/TPI vs placebo groups, the OS HR was 0.57 (95% CI, 0.41-0.79), and the PFS HR was 0.48 (95% CI, 0.35-0.65) (Figure 3A and C). Among patients who had not undergone gastrectomy, in the FTD/TPI vs placebo groups, the OS HR was 0.80 (95% CI, 0.60-1.06), and the PFS HR was 0.65 (95% CI, 0.49-0.85) (Figure 3B and D). Additional efficacy data are provided in Table 1.
Figure 3. Overall Survival and Progression-Free Survival Among Patients Who Had or Had Not Undergone Gastrectomy.
HR indicates hazard ratio.
Table 1. Overall Survival and Progression-Free Survival Among Patients Who Had or Had Not Undergone Gastrectomya.
| End Point | Gastrectomy Subgroup | No Gastrectomy Subgroup | ||||
|---|---|---|---|---|---|---|
| Trifluridine/Tipiracil (n = 147) | Placebo (n = 74) | HR (95% CI) | Trifluridine/Tipiracil (n = 190) | Placebo (n = 96) | HR (95% CI) | |
| Overall survival, median (95% CI), mob | 6.0 (4.6-7.0) | 3.4 (2.7-3.8) | 0.57 (0.41-0.79) | 5.6 (4.6-6.2) | 3.8 (3.1-5.9) | 0.80 (0.60-1.06) |
| Rate (95% CI), % | ||||||
| 6 mo | 50 (41-58) | 24 (15-35) | NA | 44 (37-52) | 39 (30-49) | NA |
| 12 mo | 20 (12-28) | 9 (3-19) | NA | 22 (16-30) | 16 (8-26) | NA |
| Progression-free survival, median (95% CI), mob | 2.2 (1.9-3.0) | 1.8 (1.7-1.9) | 0.48 (0.35-0.65) | 1.9 (1.9-2.1) | 1.8 (1.7-1.9) | 0.65 (0.49-0.85) |
| Rate (95% CI), % | ||||||
| 4 mo | 24 (17-32) | 5 (1-12) | NA | 29 (22-36) | 10 (5-17) | NA |
| 6 mo | 13 (8-20) | 3 (1-10) | NA | 16 (10-22) | 9 (4-16) | NA |
Abbreviations: HR, hazard ratio; NA, not applicable.
Kaplan-Meier estimates in the intent-to-treat population.
95% CIs were calculated using the methods of Brookmeyer and Crowley.41
In both gastrectomy subgroups, ECOG performance status was maintained longer with FTD/TPI treatment compared with placebo (eFigure in Supplement 2). For the median time to deterioration of ECOG performance status to 2 or higher in the FTD/TPI group vs placebo group, the HR was 0.63 (95% CI, 0.46-0.87) in the gastrectomy subgroup and 0.74 (95% CI, 0.56-0.98) in the no gastrectomy subgroup.
Safety
The overall frequencies of any-cause AEs at grade 3 or higher with FTD/TPI treatment were similar among patients in the gastrectomy subgroup (84.1% [122 of 145]) and those in the no gastrectomy subgroup (76.3% [145 of 190]) (Table 2). Among patients who received FTD/TPI treatment, the incidence of hematologic AEs was higher among patients in the gastrectomy subgroup than among those in the no gastrectomy subgroup. Grade 3 or higher neutropenia was reported in 64 of 145 patients (44.1%) in the gastrectomy subgroup and 50 of 190 patients (26.3%) in the no gastrectomy subgroup, grade 3 or higher anemia in 31 (21.4%) in the gastrectomy subgroup and 33 (17.4%) in the no gastrectomy subgroup, and grade 3 or higher leukopenia in 21 (14.5%) in the gastrectomy subgroup and 10 (5.3%) in the no gastrectomy subgroup. Incidence of grade 3 or higher fatigue incidence was greater in the no gastrectomy subgroup (20 of 190 patients [10.5%]) compared with the gastrectomy subgroup (3 of 145 patients [2.1%]). Two deaths in the no gastrectomy subgroup were considered to be treatment related: 1 patient treated with FTD/TPI (0.5%) died of cardiopulmonary arrest, and 1 patient who received placebo (1.1%) died of toxic hepatitis.
Table 2. AEs Among Patients Who Had or Had Not Undergone Gastrectomya.
| Variable | Patients, No. (%) | |||||||
|---|---|---|---|---|---|---|---|---|
| Gastrectomy Subgroup | No Gastrectomy Subgroup | |||||||
| Trifluridine/Tipiracil (n = 145) | Placebo (n = 73) | Trifluridine/Tipiracil (n = 190) | Placebo (n = 95) | |||||
| Any Grade | Grade ≥3 | Any Grade | Grade ≥3 | Any Grade | Grade ≥3b | Any Grade | Grade ≥3b | |
| Any AE | 143 (98.6) | 122 (84.1) | 69 (94.5) | 44 (60.3) | 183 (96.3) | 145 (76.3) | 88 (92.6) | 53 (55.8) |
| Treatment-related AE | 128 (88.3) | 93 (64.1) | 38 (52.1) | 7 (9.6) | 143 (75.3) | 83 (43.7) | 57 (60.0) | 15 (15.8) |
| Action taken because of AE of any cause | ||||||||
| Dose modification: delay or dose | 94 (64.8) | 75 (51.7) | 15 (20.5) | 13 (17.8) | 101 (53.2) | 73 (38.4) | 22 (23.2) | 16 (16.8) |
| Treatment discontinuation | 15 (10.3) | 12 (8.3) | 12 (16.4) | 8 (11.0) | 28 (14.7) | 24 (12.6) | 16 (16.8) | 13 (13.7) |
| AEs of any cause in ≥10% of patients | ||||||||
| Hematologic | ||||||||
| Neutropeniac | 87 (60.0) | 64 (44.1) | 1 (1.4) | 0 | 89 (46.8) | 50 (26.3) | 6 (6.3) | 0 |
| Anemiad | 77 (53.1) | 31 (21.4) | 8 (11.0) | 3 (4.1) | 73 (38.4) | 33 (17.4) | 24 (25.3) | 10 (10.5) |
| Leukopeniae | 40 (27.6) | 21 (14.5) | 1 (1.4) | 0 | 38 (20.0) | 10 (5.3) | 2 (2.1) | 0 |
| Thrombocytopeniaf | 30 (20.7) | 7 (4.8) | 1 (1.4) | 0 | 30 (15.8) | 4 (2.1) | 7 (7.4) | 0 |
| Gastrointestinal | ||||||||
| Nausea | 57 (39.3) | 4 (2.8) | 26 (35.6) | 3 (4.1) | 67 (35.3) | 6 (3.2) | 27 (28.4) | 2 (2.1) |
| Diarrhea | 43 (29.7) | 5 (3.4) | 13 (17.8) | 3 (4.1) | 33 (17.4) | 4 (2.1) | 11 (11.6) | 0 |
| Vomiting | 36 (24.8) | 5 (3.4) | 12 (16.4) | 1 (1.4) | 47 (24.7) | 7 (3.7) | 22 (23.2) | 2 (2.1) |
| Abdominal pain | 26 (17.9) | 5 (3.4) | 16 (21.9) | 7 (9.6) | 29 (15.3) | 9 (4.7) | 15 (15.8) | 8 (8.4) |
| Constipation | 17 (11.7) | 1 (0.7) | 12 (16.4) | 2 (2.7) | 28 (14.7) | 3 (1.6) | 13 (13.7) | 2 (2.1) |
| Ascites | 5 (3.4) | 3 (2.1) | 4 (5.5) | 3 (4.1) | 14 (7.4) | 9 (4.7) | 12 (12.6) | 8 (8.4) |
| Upper abdominal pain | 5 (3.4) | 0 | 5 (6.8) | 0 | 17 (8.9) | 1 (0.5) | 10 (10.5) | 2 (2.1) |
| Other | ||||||||
| Decreased appetite | 50 (34.5) | 11 (7.6) | 23 (31.5) | 5 (6.8) | 65 (34.2) | 18 (9.5) | 29 (30.5) | 6 (6.3) |
| Fatigue | 36 (24.8) | 3 (2.1) | 16 (21.9) | 4 (5.5) | 53 (27.9) | 20 (10.5) | 19 (20.0) | 6 (6.3) |
| Asthenia | 30 (20.7) | 3 (2.1) | 19 (26.0) | 5 (6.8) | 35 (18.4) | 13 (6.8) | 21 (22.1) | 6 (6.3) |
| Back pain | 15 (10.3) | 1 (0.7) | 4 (5.5) | 1 (1.4) | 10 (5.3) | 1 (0.5) | 7 (7.4) | 3 (3.2) |
| Dyspnea | 12 (8.3) | 2 (1.4) | 9 (12.3) | 3 (4.1) | 12 (6.3) | 4 (2.1) | 8 (8.4) | 3 (3.2) |
| General physical health deterioration | 10 (6.9) | 10 (6.9) | 7 (9.6) | 6 (8.2) | 13 (6.8) | 12 (6.3) | 10 (10.5) | 9 (9.5) |
| Malaise | 5 (3.4) | 0 | 8 (11.0) | 1 (1.4) | 4 (2.1) | 0 | 1 (1.1) | 0 |
Abbreviation: AE, adverse event.
As-treated population.
Grade 5 AEs were reported in 1 patient (0.5%) (cardiopulmonary arrest) in the trifluridine/tipiracil group and 1 patient (1.1%) (toxic hepatitis) in the placebo group.
Neutropenia and/or decreased neutrophil count.
Anemia and/or decreased hemoglobin level.
Leukopenia and/or decreased white blood cell count.
Thrombocytopenia and/or decreased platelet count.
Among patients treated with FTD/TPI, dose modifications (delays or reductions) because of any-cause AEs occurred in 94 of 145 patients (64.8%) in the gastrectomy subgroup and 101 of 190 (53.2%) in the no gastrectomy subgroup (Table 2). The higher frequency of dose modifications in the gastrectomy subgroup was primarily because of hematologic AEs (38.6% [56 of 145] in the gastrectomy subgroup vs 28.4% [54 of 190] in the no gastrectomy subgroup). In the gastrectomy subgroup, 33 of 145 patients (22.7%) received supportive treatment for neutropenia (30 patients [20.7%] received granulocyte colony-stimulating factor) compared with 25 (13.2%) in the no gastrectomy subgroup (24 patients [12.6%] received granulocyte colony-stimulating factor) (eTable 3 in Supplement 2). The higher incidence of hematologic AEs was not associated with higher treatment discontinuation rates in the gastrectomy subgroup: 15 of 145 patients (10.3%) in the gastrectomy subgroup discontinued treatment because of AEs of any cause compared with 28 of 190 (14.7%) in the no gastrectomy subgroup. The higher treatment discontinuation rate because of AEs in the no gastrectomy subgroup was partly attributable to a higher incidence of gastrointestinal AEs associated with treatment discontinuation (10 patients [5.3%] in the no gastrectomy subgroup vs 5 [3.4%] in the gastrectomy subgroup).
Exposure to FTD/TPI was similar in the gastrectomy and no gastrectomy subgroups. Mean (SD) dose intensity was 145 (28) mg/m2 per week for patients in the gastrectomy subgroup and 151 (25) mg/m2 per week for patients in the no gastrectomy subgroup (eTable 4 in Supplement 2). Mean (SD) treatment duration was 12.7 (11.8) and 11.6 (11.2) weeks in the respective groups. Pharmacokinetic parameters for FTD/TPI were not examined in this study.
Discussion
The results of this subgroup analysis of the phase 3 TAGS study indicate that FTD/TPI was efficacious for patients with previously treated mGC/GEJC regardless of whether they had undergone gastrectomy. To our knowledge, this analysis represents the most detailed evaluation of the safety and efficacy of an oral chemotherapeutic agent for mGC/GEJC among patients who had undergone gastrectomy.
Patients who have undergone gastrectomy constitute a significant proportion (approximately 40%) of patients with mGC/GEJC who receive second- or third-line treatment.7,8,10,11 These patients tend to be more compromised nutritionally and more heavily pretreated; therefore, they may be less able to tolerate chemotherapy.13,15,16 In addition, sarcopenia is highly prevalent among patients who have undergone gastrectomy and is associated with toxic events and complications.32,33,34,35 In a phase 3 study (REGATTA),15 patients with advanced GC who had undergone gastrectomy in addition to chemotherapy did not experience survival benefit compared with patients who received chemotherapy alone. Among patients who had undergone gastrectomy, chemotherapy compliance was lower (they received fewer chemotherapy cycles) and the incidence of severe AEs was higher than in patients who had not undergone gastrectomy.15 In previous studies, gastrectomy was shown to be associated with higher incidence of AEs in patients who received adjuvant chemotherapy.16,36 In 1 report, AEs in patients receiving adjuvant chemotherapy were more frequent among patients who underwent total gastrectomy than among those who underwent subtotal gastrectomy,16 and in another report, patients who underwent gastrectomy and received the fluorouracil prodrug S-1 had higher incidences of adverse reactions than did those receiving S-1 for unresectable GC.36 It is unclear whether these increased incidences of AEs were associated with gastrectomy-induced changes in pharmacokinetic exposure. Although a number of studies21,22,23 have shown that gastrectomy does not significantly alter the absorption and pharmacokinetics of oral adjuvant chemotherapy, in at least 1 study, high fluorouracil plasma concentrations were observed in patients who had undergone gastrectomy and received adjuvant S-1.20 The pharmacokinetics and distribution of fluoropyrimidines can also be affected by the loss of lean body mass,37,38 which may be more common in patients who have undergone gastrectomy.32,39 This finding suggests that gastrectomy may be associated with higher drug exposures and, consequently, more frequent AEs.20 Together, these studies indicate that the safety of chemotherapy may need to be carefully monitored in patients who undergo gastrectomy. However, most of these data were derived from studies of adjuvant therapy, and data on chemotherapeutic agents for metastatic disease in the gastrectomy subpopulation are limited.
Among the few studies7,10,11,40 that have reported data on patients with mGC/GEJC who had undergone gastrectomy, the analyses were limited in scope, and in trials that met their primary end point, the efficacy data reported for these subgroups appeared to be conflicting. In the phase 3 ATTRACTION-2 trial,10 which evaluated nivolumab as third-line treatment in mGC, nivolumab improved OS compared with placebo in subgroups of patients who had (HR, 0.61 [95% CI, 0.47-0.80]) or had not undergone gastrectomy (HR, 0.69 [95% CI, 0.49-0.98]).10 However, in the phase 3 RAINBOW trial,11 ramucirumab plus paclitaxel in the context of second-line therapy did not improve OS compared with placebo plus paclitaxel in the gastrectomy subgroup (HR, 0.94 [95% CI, 0.70-1.26]), even though OS benefit was seen in the overall population.11 None of these studies reported safety data for the gastrectomy subpopulation.7,10,11,40
In our detailed subgroup analysis of the TAGS study, patients who had undergone partial or total gastrectomy experienced improvement in OS after receiving FTD/TPI treatment. Patients who had not undergone gastrectomy also had improved OS after receiving treatment with FTD/TPI, but this benefit was more pronounced in the gastrectomy subgroup in these analyses, the reasons for which remain unclear. In previously reported multivariate Cox regression analyses that included all prespecified factors, previous gastrectomy was not identified as a factor associated with OS after FTD/TPI treatment.8 The PFS benefits associated with FTD/TPI were seen in both the gastrectomy and no gastrectomy subgroups. In addition, FTD/TPI treatment prolonged the time to deterioration of ECOG performance status vs placebo in both the gastrectomy subgroups.
The overall safety profile of FTD/TPI was similar among patients who had or had not undergone gastrectomy, with similar frequencies of grade 3 or higher AEs in both subgroups. The main difference between the subgroups was a higher incidence of hematologic AEs (neutropenia and leukopenia) among patients who had undergone gastrectomy. These patients had received more neoadjuvant or adjuvant therapy and radiotherapy compared with those who had not undergone gastrectomy, which may provide a partial explanation for the increased myelosuppression observed. However, a preliminary analysis showed no differences in hematologic AEs between patients who received or did not receive irradiation in the 2 gastrectomy subgroups, although patient numbers were too small to draw definitive conclusions (eResults in Supplement 2). Although nutritional status was not assessed in this trial, other studies13,14 have shown that malnutrition and hypoalbuminemia associated with gastrectomy may be associated with increased incidence of hematologic AEs, including neutropenia, among patients with mGC/GEJC. Unlike the REGATTA trial,15 treatment exposure (dose intensity and treatment duration) in the TAGS trial was similar in the gastrectomy and no gastrectomy subgroups and was unlikely to account for any observed differences in safety profiles between the subgroups. However, because the pharmacokinetics of FTD/TPI were not assessed in the TAGS trial, differences in the pharmacokinetic exposure of FTD/TPI between patients who had or had not undergone gastrectomy cannot be ruled out. Hematologic AEs were associated with more frequent dose modifications among patients treated with FTD/TPI in the gastrectomy subgroup and were managed with supportive treatment; they were not associated with increased permanent treatment discontinuation rates: 10.3% of patients discontinued treatment because of AEs compared with 14.7% in the no gastrectomy subgroup. Together, these results indicate that there were no new safety concerns in patients treated with FTD/TPI who had undergone gastrectomy and that AEs were effectively managed using dose modifications and concomitant medications. To our knowledge, this was the first safety analysis of a chemotherapeutic agent used for mGC/GEJC in the subpopulation of patients who had undergone gastrectomy.
Limitations
The main limitations of this study lie in the nature of these analyses; although preplanned, they were not powered for statistical significance. This circumstance precluded a robust evaluation of the safety and efficacy of FTD/TPI in the subgroups of patients who had or had not undergone gastrectomy. The lack of pharmacokinetic exposure data in this study also limited the analysis. Although no differences in the pharmacokinetics of FTD or TPI were found between patients who had or who had not undergone gastrectomy in the phase 2 EPOC1201 study, these earlier results were derived from small numbers of patients.28
Conclusions
This subgroup analysis of the phase 3 TAGS trial showed that FTD/TPI was an effective treatment option that may improve survival outcomes and help maintain ECOG performance status among patients with previously treated mGC or mGEJC regardless of whether they had undergone gastrectomy. The benefits of FTD/TPI were especially noteworthy in the subpopulation of patients who had undergone gastrectomy, who tended to be more heavily pretreated and were less tolerant of therapy. The overall safety profile of the drug, including the incidence of severe AEs, was similar among patients who had or had not undergone gastrectomy. No new safety concerns were reported in patients who had undergone gastrectomy. Hematologic AEs associated with FTD/TPI in the gastrectomy subgroup were managed effectively with dose modifications and had no detectable association with treatment discontinuation rates. These results support the use of FTD/TPI among patients who have undergone gastrectomy, particularly in the context of the recent approval of FTD/TPI for patients with previously treated mGC/GEJC.27
Trial Protocol
eMethods. Statistical Considerations and Definitions of End Points
eTable 1. List of Institutional Review Boards/Ethics Committees in the TAGS Trial
eTable 2. Baseline Demographics and Disease Characteristics of Patients Who Had or Had Not Undergone Gastrectomy
eTable 3. Supportive Treatment for Hematologic Toxicities in Patients Who Had or Had Not Undergone Gastrectomy
eTable 4. Treatment Exposure in Patients Who Had or Had Not Undergone Gastrectomy
eFigure. Time to Deterioration of ECOG Performance Status Score to 2 or Higher in (A) Patients Who Had Undergone Gastrectomy and (B) Patients Who Had Not Undergone Gastrectomy
eResults. Hematologic Adverse Events in FTD/TPI-treated Patients Who Had or Had Not Received Prior Irradiation
Data Sharing Statement
References
- 1.Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65(2):-. doi: 10.3322/caac.21262 [DOI] [PubMed] [Google Scholar]
- 2.Van Cutsem E, Sagaert X, Topal B, Haustermans K, Prenen H. Gastric cancer. Lancet. 2016;388(10060):2654-2664. doi: 10.1016/S0140-6736(16)30354-3 [DOI] [PubMed] [Google Scholar]
- 3.Wagner AD, Syn NL, Moehler M, et al. Chemotherapy for advanced gastric cancer. Cochrane Database Syst Rev. 2017;8:CD004064. doi: 10.1002/14651858.CD004064.pub4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chrom P, Stec R, Szczylik C. Second-line treatment of advanced gastric cancer: current options and future perspectives. Anticancer Res. 2015;35(9):4575-4583. [PubMed] [Google Scholar]
- 5.Zheng Y, Zhu XQ, Ren XG. Third-line chemotherapy in advanced gastric cancer: a systematic review and meta-analysis. Medicine (Baltimore). 2017;96(24):e6884. doi: 10.1097/MD.0000000000006884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Choi IS, Choi M, Lee JH, et al. Treatment patterns and outcomes in patients with metastatic gastric cancer receiving third-line chemotherapy: a population-based outcomes study. PLoS One. 2018;13(6):e0198544. doi: 10.1371/journal.pone.0198544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shitara K, Özgüroğlu M, Bang YJ, et al. ; KEYNOTE-061 investigators . Pembrolizumab versus paclitaxel for previously treated, advanced gastric or gastro-oesophageal junction cancer (KEYNOTE-061): a randomised, open-label, controlled, phase 3 trial. Lancet. 2018;392(10142):123-133. doi: 10.1016/S0140-6736(18)31257-1 [DOI] [PubMed] [Google Scholar]
- 8.Shitara K, Doi T, Dvorkin M, et al. Trifluridine/tipiracil versus placebo in patients with heavily pretreated metastatic gastric cancer (TAGS): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2018;19(11):1437-1448. doi: 10.1016/S1470-2045(18)30739-3 [DOI] [PubMed] [Google Scholar]
- 9.Orditura M, Galizia G, Sforza V, et al. Treatment of gastric cancer. World J Gastroenterol. 2014;20(7):1635-1649. doi: 10.3748/wjg.v20.i7.1635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kang YK, Boku N, Satoh T, et al. Nivolumab in patients with advanced gastric or gastro-oesophageal junction cancer refractory to, or intolerant of, at least two previous chemotherapy regimens (ONO-4538-12, ATTRACTION-2): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2017;390(10111):2461-2471. doi: 10.1016/S0140-6736(17)31827-5 [DOI] [PubMed] [Google Scholar]
- 11.Wilke H, Muro K, Van Cutsem E, et al. ; RAINBOW Study Group . Ramucirumab plus paclitaxel versus placebo plus paclitaxel in patients with previously treated advanced gastric or gastro-oesophageal junction adenocarcinoma (RAINBOW): a double-blind, randomised phase 3 trial. Lancet Oncol. 2014;15(11):1224-1235. doi: 10.1016/S1470-2045(14)70420-6 [DOI] [PubMed] [Google Scholar]
- 12.Santarpia L, Contaldo F, Pasanisi F. Nutritional screening and early treatment of malnutrition in cancer patients. J Cachexia Sarcopenia Muscle. 2011;2(1):27-35. doi: 10.1007/s13539-011-0022-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Seo SH, Kim SE, Kang YK, et al. Association of nutritional status-related indices and chemotherapy-induced adverse events in gastric cancer patients. BMC Cancer. 2016;16(1):900. doi: 10.1186/s12885-016-2934-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li QW, Li GC, Wang YN, Long ZW, Liu XW, Zhang Z. Association of nutrition with treatment compliance and toxicities in patients undergoing chemoradiation after gastrectomy [in Chinese]. Zhonghua Wei Chang Wai Ke Za Zhi. 2013;16(6):529-533. doi: 10.3760/cma.j.issn.1671-0274.2013.06.009 [DOI] [PubMed] [Google Scholar]
- 15.Fujitani K, Yang HK, Mizusawa J, et al. ; REGATTA study investigators . Gastrectomy plus chemotherapy versus chemotherapy alone for advanced gastric cancer with a single non-curable factor (REGATTA): a phase 3, randomised controlled trial. Lancet Oncol. 2016;17(3):309-318. doi: 10.1016/S1470-2045(15)00553-7 [DOI] [PubMed] [Google Scholar]
- 16.Chou WC, Chang CL, Liu KH, et al. Total gastrectomy increases the incidence of grade III and IV toxicities in patients with gastric cancer receiving adjuvant TS-1 treatment [published correction appears in World J Surg Oncol 2013;11:310]. World J Surg Oncol. 2013;11:287. doi: 10.1186/1477-7819-11-287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sakuramoto S, Sasako M, Yamaguchi T, et al. ; ACTS-GC Group . Adjuvant chemotherapy for gastric cancer with S-1, an oral fluoropyrimidine. N Engl J Med. 2007;357(18):1810-1820. doi: 10.1056/NEJMoa072252 [DOI] [PubMed] [Google Scholar]
- 18.Milano G, Ferrero JM, François E. Comparative pharmacology of oral fluoropyrimidines: a focus on pharmacokinetics, pharmacodynamics and pharmacomodulation. Br J Cancer. 2004;91(4):613-617. doi: 10.1038/sj.bjc.6601973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Park SH, Sohn TS, Lee J, et al. Phase III trial to compare adjuvant chemotherapy with capecitabine and cisplatin versus concurrent chemoradiotherapy in gastric cancer: final report of the adjuvant chemoradiotherapy in stomach tumors trial, including survival and subset analyses. J Clin Oncol. 2015;33(28):3130-3136. doi: 10.1200/JCO.2014.58.3930 [DOI] [PubMed] [Google Scholar]
- 20.Kim WY, Nakata B, Hirakawa K. Alternative pharmacokinetics of S-1 components, 5-fluorouracil, dihydrofluorouracil and alpha-fluoro-beta-alanine after oral administration of S-1 following total gastrectomy. Cancer Sci. 2007;98(10):1604-1608. doi: 10.1111/j.1349-7006.2007.00573.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kochi M, Fujii M, Kanamori N, et al. Effect of gastrectomy on the pharmacokinetics of S-1, an oral fluoropyrimidine, in resectable gastric cancer patients. Cancer Chemother Pharmacol. 2007;60(5):693-701. doi: 10.1007/s00280-007-0415-x [DOI] [PubMed] [Google Scholar]
- 22.Lim HS, Ryu KW, Lee JH, et al. Postgastrectomy pharmacokinetic changes of S-1 in patients with localized advanced gastric cancer. J Clin Pharmacol. 2015;55(8):926-935. doi: 10.1002/jcph.499 [DOI] [PubMed] [Google Scholar]
- 23.Tsuruoka Y, Kamano T, Kitajima M, et al. Effect of gastrectomy on the pharmacokinetics of 5-fluorouracil and gimeracil after oral administration of S-1. Anticancer Drugs. 2006;17(4):393-399. doi: 10.1097/01.cad.0000203382.07114.0f [DOI] [PubMed] [Google Scholar]
- 24.Emura T, Suzuki N, Yamaguchi M, Ohshimo H, Fukushima M. A novel combination antimetabolite, TAS-102, exhibits antitumor activity in FU-resistant human cancer cells through a mechanism involving FTD incorporation in DNA. Int J Oncol. 2004;25(3):571-578. doi: 10.3892/ijo.25.3.571 [DOI] [PubMed] [Google Scholar]
- 25.Emura T, Nakagawa F, Fujioka A, et al. An optimal dosing schedule for a novel combination antimetabolite, TAS-102, based on its intracellular metabolism and its incorporation into DNA. Int J Mol Med. 2004;13(2):249-255. [PubMed] [Google Scholar]
- 26.Mayer RJ, Van Cutsem E, Falcone A, et al. ; RECOURSE Study Group . Randomized trial of TAS-102 for refractory metastatic colorectal cancer. N Engl J Med. 2015;372(20):1909-1919. doi: 10.1056/NEJMoa1414325 [DOI] [PubMed] [Google Scholar]
- 27.LONSURF (trifluridine and tipiracil) tablets, for oral use [prescribing information]. Princeton, NJ: Taiho Oncology Inc; 2019. https://www.taihooncology.com/us/prescribing-information.pdf. Accessed on April 9, 2019.
- 28.Bando H, Doi T, Muro K, et al. A multicenter phase II study of TAS-102 monotherapy in patients with pre-treated advanced gastric cancer (EPOC1201). Eur J Cancer. 2016;62:46-53. doi: 10.1016/j.ejca.2016.04.009 [DOI] [PubMed] [Google Scholar]
- 29.Arkenau H-T, Tabernero J, Shitara K, et al. . TAGS: a phase III, randomised, double-blind study of trifluridine/tipiracil (TAS-102) versus placebo in patients with refractory metastatic gastric cancer. Ann Oncol. 2018;29(suppl 8). [Google Scholar]
- 30.World Medical Association . World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA. 2013;310(20):2191-2194. doi: 10.1001/jama.2013.281053 [DOI] [PubMed] [Google Scholar]
- 31.Dixon JR Jr. The International Conference on Harmonization Good Clinical Practice guideline. Qual Assur. 1998;6(2):65-74. doi: 10.1080/105294199277860 [DOI] [PubMed] [Google Scholar]
- 32.Kugimiya N, Harada E, Oka K, et al. Loss of skeletal muscle mass after curative gastrectomy is a poor prognostic factor. Oncol Lett. 2018;16(1):1341-1347. doi: 10.3892/ol.2018.8747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yamaoka Y, Fujitani K, Tsujinaka T, Yamamoto K, Hirao M, Sekimoto M. Skeletal muscle loss after total gastrectomy, exacerbated by adjuvant chemotherapy. Gastric Cancer. 2015;18(2):382-389. doi: 10.1007/s10120-014-0365-z [DOI] [PubMed] [Google Scholar]
- 34.Zhuang C-L, Huang D-D, Pang W-Y, et al. Sarcopenia is an independent predictor of severe postoperative complications and long-term survival after radical gastrectomy for gastric cancer: analysis from a large-scale cohort. Medicine (Baltimore). 2016;95(13):e3164. doi: 10.1097/MD.0000000000003164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tan BHL, Brammer K, Randhawa N, et al. Sarcopenia is associated with toxicity in patients undergoing neo-adjuvant chemotherapy for oesophago-gastric cancer. Eur J Surg Oncol. 2015;41(3):333-338. doi: 10.1016/j.ejso.2014.11.040 [DOI] [PubMed] [Google Scholar]
- 36.Kinoshita T, Nashimoto A, Yamamura Y, et al. Feasibility study of adjuvant chemotherapy with S-1 (TS-1; tegafur, gimeracil, oteracil potassium) for gastric cancer. Gastric Cancer. 2004;7(2):104-109. doi: 10.1007/s10120-004-0278-3 [DOI] [PubMed] [Google Scholar]
- 37.Prado CM, Baracos VE, McCargar LJ, et al. Sarcopenia as a determinant of chemotherapy toxicity and time to tumor progression in metastatic breast cancer patients receiving capecitabine treatment. Clin Cancer Res. 2009;15(8):2920-2926. doi: 10.1158/1078-0432.CCR-08-2242 [DOI] [PubMed] [Google Scholar]
- 38.Prado CM, Lieffers JR, McCargar LJ, et al. Prevalence and clinical implications of sarcopenic obesity in patients with solid tumours of the respiratory and gastrointestinal tracts: a population-based study. Lancet Oncol. 2008;9(7):629-635. doi: 10.1016/S1470-2045(08)70153-0 [DOI] [PubMed] [Google Scholar]
- 39.Tegels JJ, van Vugt JL, Reisinger KW, et al. Sarcopenia is highly prevalent in patients undergoing surgery for gastric cancer but not associated with worse outcomes. J Surg Oncol. 2015;112(4):403-407. doi: 10.1002/jso.24015 [DOI] [PubMed] [Google Scholar]
- 40.Satoh T, Xu R-H, Chung HC, et al. Lapatinib plus paclitaxel versus paclitaxel alone in the second-line treatment of HER2-amplified advanced gastric cancer in Asian populations: TyTAN—a randomized, phase III study. J Clin Oncol. 2014;32(19):2039-2049. doi: 10.1200/JCO.2013.53.6136 [DOI] [PubMed] [Google Scholar]
- 41.Brookmeyer R, Crowley J. A confidence interval for the median survival time. Biometrics. 1982;38(1):29-41. doi: 10.2307/2530286 [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial Protocol
eMethods. Statistical Considerations and Definitions of End Points
eTable 1. List of Institutional Review Boards/Ethics Committees in the TAGS Trial
eTable 2. Baseline Demographics and Disease Characteristics of Patients Who Had or Had Not Undergone Gastrectomy
eTable 3. Supportive Treatment for Hematologic Toxicities in Patients Who Had or Had Not Undergone Gastrectomy
eTable 4. Treatment Exposure in Patients Who Had or Had Not Undergone Gastrectomy
eFigure. Time to Deterioration of ECOG Performance Status Score to 2 or Higher in (A) Patients Who Had Undergone Gastrectomy and (B) Patients Who Had Not Undergone Gastrectomy
eResults. Hematologic Adverse Events in FTD/TPI-treated Patients Who Had or Had Not Received Prior Irradiation
Data Sharing Statement