Abstract
Persimmons are a traditional, autumnal, and healthy fruit commonly consumed in Japan and East Asia based on the saying, “a persimmon a day keeps the doctor away.” The differences in metabolites among five major Japanese persimmon cultivars were investigated using a nuclear magnetic resonance (NMR)-based metabolomics approach. By using a broadband water suppression enhanced through T1 effects (WET) method for the sensitive detection of minor metabolites, better discrimination among cultivars and more informative details regarding their metabolic differences have been achieved compared to those achieved in conventional 1H NMR sequences. Among the nonastringent cultivars analyzed, the Taishu cultivar has the highest abundance of amino acids. The Matsumotowase-Fuyu cultivar contains ethyl-β-glycosides as characteristic components, which may relate to fruit softening. Citric acid concentration is higher in Maekawa Jiro than in other nonastringent cultivars. Among the two astringent cultivars analyzed, ethanol was significantly higher in Hiratanenashi than in Yotsumizo, which indicates different reactivity during deastringency treatments. The present study proposes an efficient and relatively quantitative metabolomics approach based on broadband WET NMR spectra.
Subject terms: Metabolomics, Analytical biochemistry
Introduction
In recent years, food science has increasingly developed, transitioning from focusing on specific components to exploring the entire composition of foods and connecting differences in composition to varietal characteristics, the nutritional value of cultivars, the physical benefits of foods, etc. This improvement has been achieved by applying advanced analytical methodologies and omics tools to food science. There is a new discipline termed “Foodomics”, which is a new approach to studying food through the integration of omics technology1,2. Among omics technologies, nuclear magnetic resonance (NMR) spectroscopy is a rapid and unbiased technology that can be used for the simultaneous analysis of components in foods as an entire mixture with minimum destruction of samples. In addition, the capacity for highly reproducible results and the quantitative properties of NMR spectroscopy are important advantages3,4. NMR has increasingly become an attractive tool in metabolomics analysis and has been combined with multivariate data analysis such as principal component analysis (PCA) and partial least-squares discriminate analysis (PLS-DA)5–8. This approach has been used to identify differences among varieties of foods and provides robust support for determining the origin of a food, the quality of cultivar selection, taste evaluation, etc9–18.
Diospyros kaki, better known as the Japanese persimmon, is the most widely cultivated species of the Diospyros genus. Persimmons are recognized as an outstanding source of biologically active components that exhibit many health benefits, such as antioxidant behavior, radical scavenging activity, antihypertensive activity, and antiatherosclerosis activity19–23. Persimmon cultivars are classified broadly into two categories: astringent cultivars and nonastringent cultivars. An astringent cultivar is not suitable for human consumption at the time of harvest due to the high amount of soluble tannins in the fruit. It is suitable for human consumption after artificial removal of its astringency or once it is overripe or dried24–26, while a nonastringent cultivar is edible at the time of harvest. It is thought that the seeds in persimmons have the ability to remove astringency during the development of the fruit by turning soluble tannins into insoluble tannins27–29. Approximately 1,000 varieties are estimated to be grown in Japan. The composition of biological components varies among different cultivars30. However, despite the variety of Japanese persimmon cultivars, there are limited reports on the compositional differences among persimmon cultivars23,31. Furthermore, no report has produced a metabolic profiling characterization of different cultivars of Japanese persimmons using NMR.
In the present study, we provide detailed and comprehensive information regarding the compositional differences among five leading commercial Japanese persimmon cultivars using NMR spectroscopy combined with multivariate data analysis. The aim of this study is to provide an efficient and relatively quantitative approach to characterize the compositional differences in different persimmon cultivars. The five cultivars include three nonastringent persimmons, ‘Taishu (TS)’, ‘Matsumotowase-Fuyu (MF)’ and ‘Maekawa Jiro (MJ)’, as well as two astringent cultivars, ‘Hiratanenashi (HN)’ and ‘Yotsumizo (YM)’. Since detection of the minor components of persimmons are hindered by the main components (sugars) and are often undetectable or detected with very poor signal-to-noise (S/N) ratios in NMR analysis, the broadband water suppression enhanced through T1 effects (WET) method, which was proposed in our previous study32, was utilized to unambiguously identify minor components.
Results and Discussion
1H NMR spectra and broadband WET spectra of five japanese persimmon cultivars
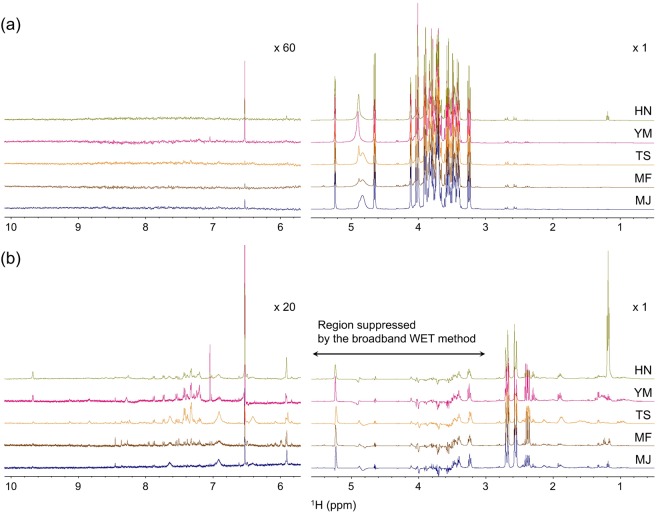
Figure 1 shows the 1H NMR spectra of juice from five different persimmon cultivars. The signal patterns of 1H NMR spectra for all five cultivars were similar. The spectral region between 3.10 and 5.40 ppm contained signals mainly from sugars (glucose, fructose and sucrose), which are the dominant components in all cultivars (Fig. 1a). As shown in the expanded spectra of the low-field region (6.0–10.0 ppm), few peaks were observed in the low-field region for all cultivars. The broadband WET method32 was successfully used as a new method for qualitatively and quantitatively observing relatively minor components in fruit juice via the simultaneous suppression of strong signals derived from major components (water and sugars) and provision of high-throughput information at a high S/N ratio for minor components in a complex mixture. As expected, new signals were detected in the low-field region with the same number of scans (128 scans) in the broadband WET spectra (Fig. 1b).
Figure 1.
Conventional 1H NMR spectra (a) and broadband WET NMR spectra (b) of five persimmon cultivars. HN, YM, TS, MF and MJ represent Hiratanenashi, Yotsumizo, Taishu, Matsumotowase-Fuyu and Maekawa Jiro, respectively.
Components were assigned according to a previous report33. Signals in the region from 0.5 ppm to 3.0 ppm were derived from protons of free amino acids including alanine (δ 1.47), 4-aminobutanoic acid (GABA) (δ 1.89, δ 2.29), aspartic acid (δ 2.66, δ 2.81), citrulline (δ 1.51, δ 1.57, δ 3.75), glutamic acid (δ 2.05, δ 2.11, δ 2.35), glutamine (δ 2.12, δ 2.43), isoleucine (δ 0.93, δ 1.24, δ 1.47, δ 1.98), leucine (δ 0.95, δ 1.71, δ 1.73), threonine (δ 1.31) and valine (δ 0.97, δ 1.02, δ 2.27) as well as organic acids including citric acid (δ 2.61, δ 2.72) and malic acid (δ 2.39, δ 2.69). The signals in the region from 6.0 ppm to 10.0 ppm were considered to be derived from acetaldehyde (δ 9.67), adenosine (δ 6.06, δ 8.31), fumaric acid (δ 6.52), trigonelline (δ 8.09, δ 8.84, δ 9.12), and uridine (δ 7.87). In this region, the broad signals were assigned to the amino protons from glutamine (δ 6.92, δ 7.62) and citrulline (δ 6.38), which were confirmed by analysis of a sample spiked with standard compounds. A portion of these assignments was also confirmed by the statistical method STOCSY34 (Supplementary Fig. S1).
Cultivar discrimination using broadband WET spectra
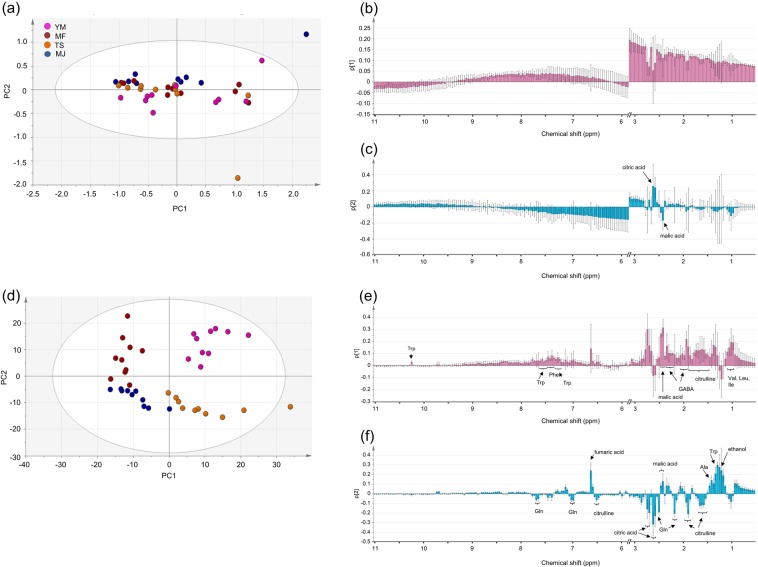
To investigate the variation in overall composition, PCA was performed on the dataset of five cultivars (10 samples of each), and the results of the multivariate data analysis for the 1H NMR and broadband WET spectra were compared. The score plots are shown in Fig. 2.
Figure 2.
PCA score plots of five cultivars derived from (a) conventional 1H NMR spectra with the exclusion of the water suppression region between 4.5 ppm and 5.2 ppm. (b) Conventional 1H NMR spectra with the regions of water and sugars (between 3.0 ppm and 5.6 ppm) excluded. (c) Broadband WET spectra with the water and sugar saturation regions within 3.0 to 5.6 ppm excluded. HN, YM, TS, MF and MJ represent Hiratanenashi, Yotsumizo, Taishu, Matsumotowase-Fuyu and Maekawa Jiro, respectively.
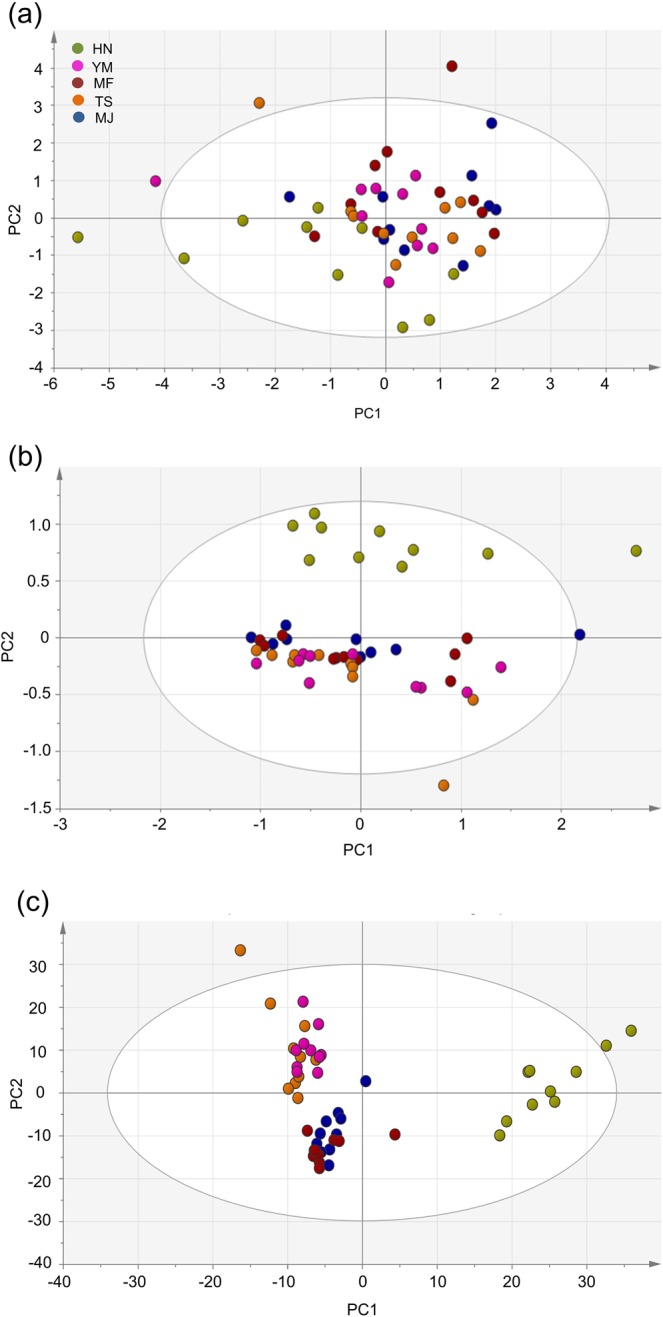
Initially, the conventional 1H NMR spectra, excluding only the water suppression region (4.5 ppm to 5.2 ppm), showed no statistically significant differences among cultivars in the score plot (Fig. 2a). Then, PCA was applied to the conventional 1H NMR spectra, except for the regions of water and sugar suppression (3.0 ppm to 5.6 ppm). The score plot showed that HN clustered far from the other four cultivars according to PC2 (Fig. 2b), indicating that this cultivar was significantly different from other cultivars and that the other four cultivars were similar in metabolite composition. This result revealed that an improvement in discrimination among cultivars was achieved by excluding the sugar region in addition to the water suppression region of 1H NMR spectra before applying PCA. Such differences among cultivars might be dictated by metabolites other than sugars because concentrations of sugars are more affected by ripeness and deastringency treatments than by cultivars. However, PC2 explained only a low percentage (17.2%) of the total variance for all five cultivars, which indicates that the discrimination result is not sufficiently informative.
PCA was then applied to the broadband WET spectra, excluding both the water and sugar saturation regions within 3.0 ppm to 5.6 ppm. The score plot (Fig. 2c) showed that the first two PCs (PC1 and PC2) explain 33.9% and 26.2% of the total variance obtained for fruit cultivars, respectively. HN was clearly separately clustered from the other 4 cultivars according to PC1, and cultivars YM and TS were separately clustered from cultivars MF and MJ according to PC2. The results showed that discrimination among cultivars was improved by using broadband WET spectra combined with the exclusion of the sugar and water regions. This improved discrimination result suggested that minor components might be responsible for cultivar discrimination because more minor components were detected in the broadband WET spectra than in the conventional 1H NMR spectra.
To further explore whether multivariate data analysis combined with broadband WET spectra can extract the features of similar cultivars more effectively than conventional 1H NMR spectra, another PCA analysis was performed using only four cultivars (YM, TS, MF and MJ) and excluding HN. The PCA score plots for conventional 1H NMR and broadband WET spectra are shown in Fig. 3, both of which exclude the regions of water and sugars (3.0 ppm to 5.6 ppm). The PCA results for the conventional 1H NMR spectra showed almost no separation among cultivars, although PC1 and PC2 explained 59.6% and 14.5% of the variance, respectively. In contrast, all four cultivars with similar metabolite compositions separately clustered on the score plot of the first two principle components derived from the broadband WET spectra, where over half of the total variance was explained by PC1 (37.2%) and PC2 (29.6%). The results showed that broadband WET spectra combined with the exclusion of the water and sugar regions exhibited good differentiation among cultivars and were very useful for investigating the differences in metabolites that are intrinsic to persimmon cultivars. The discrimination between cultivars, in PCA analysis, obtained by the use of the broadband WET NMR data could result from the minor compounds detected by the method, which contribute to the loadings for PC1 and PC2 (Fig. 3e,f). On the other hand, only citric acid and malic acid appeared in the loading plot for the analysis using the conventional NMR data (PC2, Fig. 3c).
Figure 3.
(a) PCA score plot of four cultivar samples derived from conventional 1H NMR spectra with the regions for water and sugars (between 3.0 ppm and 5.6 ppm) excluded. Loading plots for (b) PC1 and (c) PC2 of PCA analysis of conventional 1H NMR spectra (a). (d) PCA score plot of four cultivar samples derived from broadband WET spectra with the water and sugar saturation regions within 3.0 to 5.6 ppm excluded. Loading plots for (e) PC1 and (f) PC2 of PCA analysis of broadband WET spectra (d). YM, TS, MF and MJ represent Yotsumizo, Matsumotowase-Fuyu, Taishu and Maekawa Jiro, respectively.
Comparison of metabolite differences between astringent and nonastringent cultivars
OPLS-DA was used to isolate the metabolites responsible for the differences between astringent and nonastringent cultivars. The goodness-of-fit parameter R2x and the predictive ability parameter Q2 were also compared. R2x varies between 0 and 1, where 1 indicates a perfect fit between the model and the data. When Q2 is greater than 0.5, the model is considered to have good predictability, and if Q2 is greater than 0.9, then the model is considered to have excellent predictability.
Based on the results of PCA presented above, OPLS-DA was applied to the broadband WET spectra without the water and sugar saturation regions from 3.0 ppm to 5.6 ppm. For comparison, OPLS-DA was also applied to conventional 1H NMR spectra without the regions attributed to water and sugars (between 3.0 ppm and 5.6 ppm). The OPLS-DA score plots are shown in Fig. 4. In the score plot generated from the 1H NMR spectra (Fig. 4a), astringent and nonastringent cultivars were discriminated with a total variance of 63.2% (8.8% for OPLS1 and 54.4% for OPLS2). The goodness-of-fit parameters R2x and R2y were 0.940 and 0.966, respectively, and the predictive ability parameter Q2 value was 0.761.
Figure 4.
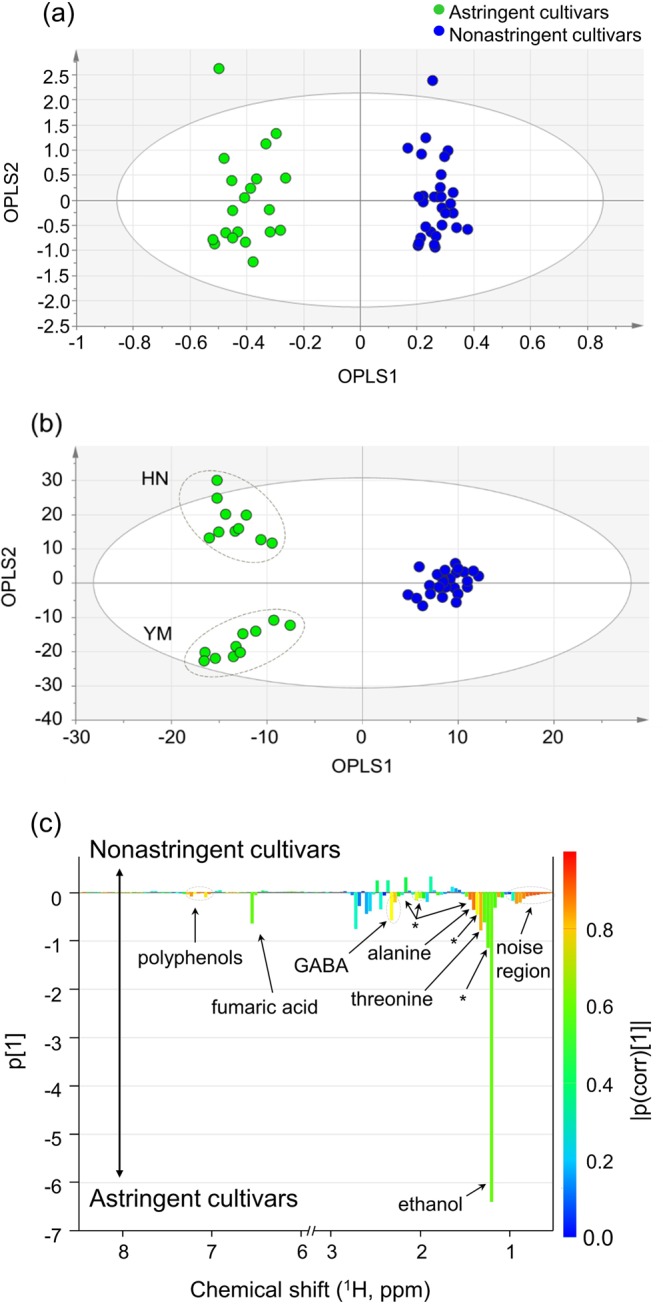
(a) OPLS-DA score plot for five cultivars derived from 1H NMR spectra with the regions for water and sugars (between 3.0 ppm and 5.6 ppm) excluded. The variances were 8.8% and 54.4% for OPLS1 and OPLS2, respectively. The goodness-of-fit parameters R2x and R2y were 0.940 and 0.966, respectively, and the Q2 value was 0.761. (b) OPLS-DA score plot and (c) OPLS-DA loading plot of five cultivars derived from broadband WET spectra with the water and sugar saturation regions within 3.0 to 5.6 ppm excluded. HN and YM represent the Hiratanenashi and Yotsumizo cultivars, respectively. Asterisks denote unknown components. The variances were 23.1% and 27.5% for OPLS1 and OPLS2, respectively. The goodness-of-fit parameters R2x and R2y were 0.870 and 0.965, respectively, and the Q2 value was 0.936.
The OPLS-DA score plot derived from broadband WET spectra (Fig. 4b) showed a total variance of 50.6% (23.1% for OPLS1 and 27.5% for OPLS2). The OPLS-DA model revealed R2x, R2y, and Q2 values of 0.870, 0.965, and 0.936, respectively, all of which indicate excellent fit and prediction abilities for this OPLS regression model. Moreover, HN and YM were separately grouped on the OPLS-DA score plot in addition to the discrimination between astringent and nonastringent cultivars.
The corresponding OPLS-DA loading plot illustrates metabolite differences among cultivars. The loading plot for OPLS1 of OPLS-DA modeled using broadband WET spectra (Fig. 4c) showed that the metabolites responsible for the differentiation between astringent and nonastringent cultivars were ethanol, fumaric acid, threonine, GABA, alanine and some unknown metabolites corresponding to several characteristic signals in astringent cultivars, such as the broad signals at 1.10–1.14 ppm, 1.26–1.30 ppm, and 2.02–2.30 ppm and the singlet signals at 7.05–7.09 ppm and 7.21–7.29 ppm that are presumed to be signals from polyphenols. A more informative characterization of metabolic differences among cultivars was achieved by comparing broadband WET spectra with the 1H NMR spectra. Threonine, GABA, and alanine can be more clearly detected (with a good S/N ratio) in the broadband WET spectra than in the conventional 1H NMR spectra, and the singlet signals at 7.05–7.09 ppm and 7.21–7.29 ppm can only be observed in broadband WET spectra.
Comparison of metabolite differences between pairs of cultivars
Since broadband WET spectra represented a suitable methodology for studying cultivar differentiation, OPLS-DA was applied to spectra of the five cultivars, and the metabolites that are mainly responsible for the separation were determined.
For the TS and MJ cultivars, the loading plot for OPLS1 (Fig. 5) showed relevant metabolites for the differentiation of the two groups. Some amino acids and organic acids were analyzed as variables of the loading plot and were considered to contribute to the differences between the TS and MJ cultivars in OPLS1. As shown in Fig. 5b, valine, leucine, isoleucine, citrulline, malic acid, aspartic acid, tryptophan, and phenylalanine were observed as typical components of TS. In contrast, ethanol, citric acid and fumaric acid were detected as biomarkers in MJ. The integral values are shown in Fig. 5c.
Figure 5.
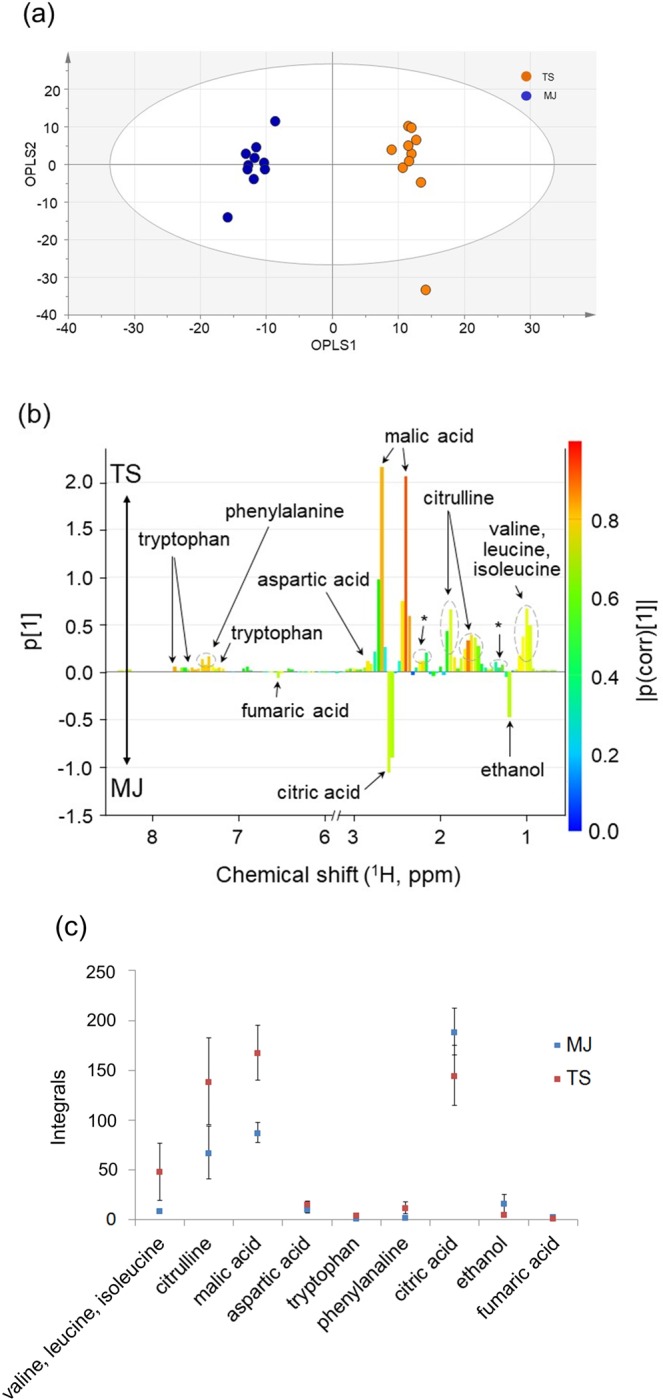
(a) OPLS-DA score plot and (b) OPLS-DA loading plot of two nonastringent cultivars (TS and MJ) derived from broadband WET spectra with the water and sugar saturation regions from 3.0 to 5.6 ppm excluded. The variances were 45.6% and 28.7% for OPLS1 and OPLS2, respectively. The goodness-of-fit parameters R2x and R2y were 0.804 and 0.982, respectively, and the Q2 value was 0.945. (c) The integral intensities of biomarkers in TS and MJ cultivars. TS and MJ represent Taishu and Maekawa Jiro, respectively. Asterisks denote unknown components. The NMR spectrum of ten persimmon fruits for each of the cultivars was measured individually (n = 10, Error bar: ±standard deviation).
Additionally, comparisons were performed between TS and MF. The total variance of the separation between the two cultivars is 0.763 (0.564 for OPLS1 and 0.199 for OPLS2). In TS, amino acids (valine, leucine, isoleucine, threonine, citrulline, GABA, glutamine, phenylalanine, and tryptophan) and malic acid were obviously higher than in MF. In addition, signals at 1.148–1.22 ppm were found to be biomarkers of the MF cultivar (Supplementary Fig. S2). These peaks were partially assigned to a series of ethyl-β-glycosides with the aid of 2D NMR spectra (1H-1H DQF-COSY, 1H-1H TOCSY, 1H-13C CT-HMBC, and 1H-1C HSQC), which are shown in Supplementary Fig. S3. Ethyl moieties were confirmed by cross-peaks in the 1H-1H DQF-COSY spectrum, which were 1.22 (t)/3.71 ppm, 1.22 (t)/3.95 ppm, 1.148 (t)/3.75 ppm, 1.154 (t)/3.75 ppm, and 1.17 (t)/3.65 ppm. The chemical shifts of –CH3 were obtained as 1.22/17.05 ppm and 1.154/17.71 ppm by the 1H-13C HSQC spectrum and overlapped with the cross-peaks at 1.148/20.36 ppm and 1.17/19.70 ppm. From the 1H-13C CT-HMBC spectrum, the cross peaks at 1.22/69.16 ppm, 1.148/82.63 ppm, 1.154/73.65 ppm, and 1.17/60.18 ppm were identified and interpreted as representing the coupling between the protons of –CH3 and the carbons of –CH2 in the ethyl moieties. Furthermore, –CH2 in the ethyl moieties was shown to be linked to a glycoside via a glycosidic bond by the cross-peaks at 4.46/69.16 ppm, 4.52/82.63 ppm, and 4.49/73.65 ppm in the 1H-13C CT-HMBC spectrum, which were considered to be derived from the anomeric protons. However, a correlation between 60.18 ppm and any saccharides was not observed in the 1H-13C HMBC spectrum. Moreover, in the 1H-1H TOCSY spectrum, the J-coupled spin systems were identified as 4.46/3.25/3.46/3.38 ppm, 4.52/3.26/3.48/3.39 ppm, and 4.49/3.31/3.48/3.38 ppm. The cross-peak at 4.46/3.25 ppm was also detected in the 1H-1H DQF-COSY spectrum, indicating that the two protons coupled with each other. The spin systems were very similar to glucose and were considered to be glycosides derived from saccharides. It was reported that the MF cultivar loses its firmness quickly after harvest, which relates to the degradation of cell wall polysaccharides by polygalacturonase (PG)35. The glycoside may originate from the degradation of cell wall polysaccharides such as polyuronide and xyloglucans during softening36. Yoshioka et al. suggested that de-esterification of polyuronides occurs with a high degree of methoxylation during the softening of apples and pears37. In addition, the sugar component ethyl-β-glucopyranoside was found in sea buckthorn fruit38 and has a very similar chemical shift to that observed in the results of this study. Therefore, we tentatively assigned those signals to ethyl-β-glycosides, possibly in the form of monosaccharides or polysaccharides.
A comparison between MF and MJ cultivars was also performed. The loading plot (Supplementary Fig. S4) for OPLS1 showed that the variables contributing to the separation were amino acids, organic acids (malic acid and citric acid), and ethyl-β-glycosides. The comparison of the integral values showed that the largest differences in integral values were those of ethyl-β-glycosides and malic acid, which were obviously abundant in the MF cultivar. In contrast, citric acid was higher in the MJ cultivar.
From the results presented above, amino acids are higher in TS than in the other two cultivars (MF and MJ), and ethyl-β-glycosides are the most abundant characteristic component in the MF cultivar. In addition, citric acid is higher in the MJ cultivar than in the other two cultivars. Variations in these metabolites may be responsible for variations in flavor, texture, ripening, and softening among cultivars. The results of the metabolite-based analyses provide meaningful data for Japanese persimmon studies and the application of this method combined with other assessments such as a quality assessment can help with the selection or modification of better quality or nutritionally important cultivars.
OPLS-DA was also performed on the two astringent cultivars for comparison (Supplementary Fig. S5). Citric acid in the HN cultivar was higher than in the YM cultivar. However, the YM cultivar included more malic acid than the HN cultivar. In the HN cultivar, ethanol was significantly higher than in the YM cultivar, and the signals at 0.86–0.98 ppm and 1.38–1.42 ppm that were unassigned (indicated with asterisks in the figure) were the marker components in the YM cultivar. Yamada et al. reported the metabolic pathway for acetaldehyde and ethanol production following CO2 and ethanol deastringency treatment, and a significant variety of treatment reactions were observed among the cultivars39. The results suggested that the two cultivars might have different enzyme activities or metabolic pathways during CTSD treatment.
Conclusions
Differences in metabolites among five major Japanese persimmon cultivars were investigated using broadband WET spectra combined with multivariate data analysis. The PCA models clearly demonstrated that cultivar discrimination of Japanese persimmons by 1H NMR spectra can be improved by removing major bulk signals (water and sugars). Improved discrimination and more informative details regarding the metabolic differences among cultivars were achieved using broadband WET spectra compared to those achieved in conventional 1H NMR sequences. The same strategy can also be applied to other fresh foods, especially those that are predominated by some major components. Some amino acids (citrulline, GABA, tryptophan, and phenylalanine), organic acids (malic acid and citric acid), and ethanol were determined to be biomarkers responsible for metabolite differences among the cultivars in this study. The results provide meaningful data for Japanese persimmon studies, and in the future, this approach combined with quality or other assessments will help in the selection or modification of better or nutritionally important cultivars.
Materials and Methods
Sample preparation
Three nonastringent (TS, MF, and MJ) and two astringent (HN and YM) persimmon cultivars were supplied by the National Agriculture and Food Research Organization (Hiroshima, Japan). TS is a new cultivar that was released in 1995, which is a mid-ripening type with very large fruits (approximately 400 g) of excellent quality (very soft with juicy flesh). Fruit can develop shallow and concentric cracks in the skin, leading to a high sugar content in the flesh beneath the cracks40,41. MF is an early-ripening cultivar selected from the ‘Fuyu’ persimmon and has a firm and crunchy flesh35. MJ is also an early-ripening cultivar and has sweet, mild, firm and flavorful flesh. HN and YM are two well-known astringent persimmon cultivars. HN is a high-quality cultivar after it is treated to remove astringency, and YM is often used to prepare dried persimmons42. Ten fruits from each of the five cultivars were collected from the same field in mid-November of same year. The two astringent persimmons were subjected to the experiments after constant temperature short duration (CTSD) treatment to remove astringency.
The fruits were ground into pulp and then centrifuged at 13,000 × g for 10 min at 4 °C. The supernatants were used as persimmon juice samples. The juices were stored at −30 °C until NMR analysis. Persimmon juice (630 μL) was mixed with 70 μL 0.1 M phosphate buffer (pH 6.5) prepared with D2O (99.9%, Shoko Co., Ltd., Tokyo, Japan). TSP-d4 solution (35 mM, Sigma-Aldrich Co. LLC., St. Louis, MO) was added to the sample as both a chemical shift and quantitative reference to a final concentration of 0.1 mM. EDTA (0.5 M, Dojindo, Kumamoto, Japan) was added to the sample to a final concentration of 1.5 mM to capture the paramagnetic ions that cause broadening of NMR signals. The mixture was then transferred into a 5-mm NMR tube.
NMR spectroscopy
NMR spectra were obtained at 20 °C on a Varian Unity INOVA-500 spectrometer (Agilent Technologies, Ltd., Santa Clara, CA) for the 1H, 1H broadband WET, 13C, 1H-13C HSQC, 1H-1H TOCSY, and 1H-1H DQF-COSY spectra, and a Varian Unity INOVA-600 spectrometer equipped with a cryogenic probe (Agilent Technologies, Ltd., Santa Clara, CA) was used for the 1H-13C CT-HMBC spectrum.
The 1H NMR spectra were recorded with a spectral width of 8,000 Hz, the number of data points was 64 k, the number of scans was 128, the acquisition time was 2.048 s, and the delay time was 5.0 s. The water signal was suppressed by a presaturation method.
The 13C NMR spectra were recorded with a spectral width of 31,422 Hz, the number of data points was 16 k, the number of scans was 52,896, the acquisition time was 1.043 s, and the delay time was 2.0 s.
The acquisition parameters of the broadband WET method27 were as follows: spectral width, 8,000 Hz; number of data points, 64 k; number of scans, 128; acquisition time, 2.048 s; and delay time, 5.0 s. The pulse length of the e-Burp pulse in the broadband WET method was 3.88 ms.
The acquisition parameters of total correlation spectroscopy (TOCSY) were as follows: spectral width, 6,700 Hz (F1) and 6,700 Hz (F2); number of data points, 512 (F1) and 2,048 (F2); number of scans, 32; acquisition time, 0.341 s; delay time, 2.0 s; field strength of MLEV-17 spin-lock pulse, 7.1 kHz; length of trim pulse, 2 ms; and mixing time, 80 ms.
For double-quantum filtered correlation spectroscopy (DQF-COSY), the spectral widths were 6,000 Hz (F1) and 6,000 Hz (F2), the number of data points were 512 (F1) and 2,048 (F2), the number of scans was 16, the acquisition time was 0.341 s, and the delay time was 2.0 s.
The acquisition parameters of the 1H–13C heteronuclear single-quantum correlation spectroscopy (HSQC) were as follows: spectral width, 6,000 Hz (1H) and 20,742 Hz (13C); number of data points, 512 (F1) and 2,048 (F2); number of scans, 256; acquisition time, 0.341 s; and delay time, 2.0 s.
The acquisition parameters of the 1H–13C constant-time heteronuclear multiple-bond correlation spectroscopy (CT-HMBC)43 were as follows: spectral width, 6,000 Hz (1H) and 28,902 Hz (13C); number of data points, 512 (F1) and 2,048 (F2); number of scans, 256; acquisition time, 0.341 s; and delay time, 2.0 s.
NMR data processing and signal assignments
All 1H NMR spectra were analyzed using FT-NMR data processing software (version 4, Sankyo publishing, Tokyo, Japan) and were manually phased, baseline corrected, and referenced to TSP at 0.00 ppm.
Signal assignments were made according to a previously described method using 2D experiments, a database, and/or spiking experiments11,12. The spin systems were confirmed by the 1H-1H DQF-COSY and 1H-1H TOCSY NMR spectra. The 1H-13C HSQC NMR spectrum was used to find correlations between protons and their neighboring carbons, and the 1H-13C CT-HMBC NMR spectrum was used to confirm the connections of quaternary carbons to protons through two- or three-bond couplings. The signals observed in 1D and 2D NMR spectra were compared with the data provided in several online databases for metabolomics, such as HMDB44, the Biological Magnetic Resonance Bank (BMRB)45, and the Madison Metabolomics Consortium Database (MMCD)46, from which several candidate components were chosen. Finally, signal assignment was accomplished by spiking authentic standard compounds (acetaldehyde, adenosine, ethanol, fumaric acid, glutamic acid, glutamine, phenylalanine, trigonelline, tryptophan, and uridine; special grade; purchased from Wako Pure Chemical Industries, Ltd., Osaka, Japan, or Nacalai Tesque Inc., Tokyo, Japan) into the juice and comparing the spectra of the resulting mixtures with those of unspiked juice.
STOCSY analysis
The 1H NMR spectral data were reduced into 0.04 ppm spectral buckets, and intensity data were normalized for each spectrum and standardized for each bucket. Then, the correlation matrix was calculated according to Cloarec et al.34. Microsoft Excel and Python with numpy and matplotlib were used for the calculation.
Multivariate data analysis
The resulting data sets were then imported into SIMCA-P version 13.0 (Umetrics, Umeå, Sweden) for further multivariate data analyses.
Prior to PCA, the bucket data were mean-centered and then scaled using Pareto scaling. Hotelling’s T2 region, shown as an ellipse in the score plots, defined the 99% confidence interval of the modeled variation. The quality of the model was described by Rx2 and Q2 values. Rx2 was defined as the proportion of variance in the data explained by the model, indicating goodness of fit. Q2 was defined as the proportion of variance in the data that was predictable by the model, indicating predictability.
OPLS-DA provided the maximum covariance between the measured data (X) and the response variable (Y). The response variables represent the intensities of the corresponding buckets assigned to separate components. The overall predictive ability of the model was assessed by cumulative Q2, which represented the fraction of the variation in Y that can be predicted by the model47.
Supplementary information
Acknowledgements
We thank Professor Akihiro Sato from the National Agriculture and Food Research Organization in Hiroshima, Japan, for kindly providing the five persimmon cultivars. This work was supported by the Japan Society for the Promotion of Science (JSPS) Grants-in-Aid for Scientific Research (S) (Grant number, 23228003) (M.T.). Some of the data shown in this study were used in the Ph.D. thesis of Ryu.
Author contributions
M.T. designed the research. S.R., F.W. and M.K. prepared the samples. S.R. and K.F. performed the experiments. S.R., T. Muramatsu and T. Miyakawa analyzed the data. S.R., T. Muramatsu, T. Miyakawa and M.T. wrote the paper. T. Muramatsu, T. Miyakawa and M.T. edited the paper.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Shoraku Ryu and Tomonari Muramatsu.
Supplementary information
is available for this paper at 10.1038/s41598-019-51489-y.
References
- 1.Trimigno A, Marincola FC, Dellarosa N, Picone G, Laghi L. Definition of food quality by NMR-based foodomics. Curr. Opin. Food Sci. 2015;4:99–104. doi: 10.1016/j.cofs.2015.06.008. [DOI] [Google Scholar]
- 2.Laghi L, Picone G, Capozzi F. Nuclear magnetic resonance for foodomics beyond food analysis. Trends Anal. Chem. 2014;59:93–102. doi: 10.1016/j.trac.2014.04.009. [DOI] [Google Scholar]
- 3.Hu F, Furihata K, Ito-Ishida M, Kaminogawa S, Tanokura M. Nondestructive observation of bovine milk by NMR spectroscopy: analysis of existing states of compounds and detection of new compounds. J. Agric. Food Chem. 2004;52:4969–4974. doi: 10.1021/jf049616o. [DOI] [PubMed] [Google Scholar]
- 4.Hu F, Furihata K, Kato Y, Tanokura M. Nondestructive quantification of organic compounds in whole milk without pretreatment by two-dimensional NMR spectroscopy. J Agric Food Chem. 2007;55:4307–4311. doi: 10.1021/jf062803x. [DOI] [PubMed] [Google Scholar]
- 5.Ryu DH, et al. Discrimination of the geographical origin of beef by 1H NMR-based metabolomics. J. Agric. Food Chem. 2010;58:10458–10466. doi: 10.1021/jf903068k. [DOI] [PubMed] [Google Scholar]
- 6.Mikros E, et al. 1H NMR-based metabonomics for the classification of greek wines according to variety, region, and vintage. Comparison with HPLC data. J. Agric. Food Chem. 2009;57:11067–11074. doi: 10.1021/jf902137e. [DOI] [PubMed] [Google Scholar]
- 7.Ohno A, Oka K, Sakuma C, Okuda H, Fukuhara K. Characterization of tea cultivated at four different altitudes using 1H NMR analysis coupled with multivariate statistics. J. Agric. Food Chem. 2011;59:5181–5187. doi: 10.1021/jf200204y. [DOI] [PubMed] [Google Scholar]
- 8.Worley B, Powers R. Multivariate analysis in metabolomics. Curr. Metabolomics. 2013;1:92–107. doi: 10.2174/2213235X11301010092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kodani Y, Miyakawa T, Komatsu T, Tanokura M. NMR-based metabolomics for simultaneously evaluating multiple determinatns of primary beef quality in Japanese Black cattle. Sci. Rep. 2017;7:1297. doi: 10.1038/s41598-017-01272-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Perez EMS, et al. HRMAS-nuclear magnetic resonance spectroscopy characterization of tomato “flavor varieties” from Almeria (Spain) Food Res. Int. 2011;44:3212–3221. doi: 10.1016/j.foodres.2011.08.012. [DOI] [Google Scholar]
- 11.Koda M, Furihata K, Wei F, Miyakawa T, Tanokura M. Metabolic discrimination of mango juice from various cultivars by band-selective NMR spectroscopy. J. Agric. Food Chem. 2012;60:1158–1166. doi: 10.1021/jf2041438. [DOI] [PubMed] [Google Scholar]
- 12.Wei F, et al. 13C NMR-based metabolomics for classification of green coffee beans according to variety and origin. J. Agric. Food Chem. 2012;60:10118–10125. doi: 10.1021/jf3033057. [DOI] [PubMed] [Google Scholar]
- 13.Beckmann M, Enot DP, Overy DP, Draper J. Representation, comparison, and interpretation of metabolome fingerprint data for total composition analysis and quality trait investigation in potato cultivars. J. Agric. Food Chem. 2007;55:3444–3451. doi: 10.1021/jf0701842. [DOI] [PubMed] [Google Scholar]
- 14.Dobson G, et al. Phytochemical diversity in tubers of potato cultivars and landraces using a GC-MS metabolomics approach. J. Agric. Food Chem. 2008;56:10280–10291. doi: 10.1021/jf801370b. [DOI] [PubMed] [Google Scholar]
- 15.Biais B, et al. 1H NMR, GC-EI-TOFMS, and data set correlation for fruit metabolomics: application to spatial metabolite analysis in melon. Anal. Chem. 2009;81:2884–2894. doi: 10.1021/ac9001996. [DOI] [PubMed] [Google Scholar]
- 16.Aprea E, et al. Metabolite profiling on apple volatile content based on solid phase microextraction and gas-chromatography time of flight mass spectrometry. J. Chromatogr. A. 2011;1218:4517–4524. doi: 10.1016/j.chroma.2011.05.019. [DOI] [PubMed] [Google Scholar]
- 17.Vermathen M, Marzorati M, Baumgartner D, Good C, Vermathen P. Investigation of different apple cultivars by high resolution magic angle spinning NMR. A feasibility study. J. Agric. Food Chem. 2011;59:12784–12793. doi: 10.1021/jf203733u. [DOI] [PubMed] [Google Scholar]
- 18.Gomez-Romero M, Segura-Carretero A, Fernandez-Gutierrez A. Metabolite profiling and quantification of phenolic compounds in methanol extracts of tomato fruit. Phytochemistry. 2010;71:1848–1864. doi: 10.1016/j.phytochem.2010.08.002. [DOI] [PubMed] [Google Scholar]
- 19.Uchida S, et al. Prolongation of life span of stroke-prone spontaneously hypertensive rats (SHRSP) ingesting persimmon tannin. Chem. Pharm. Bull. 1990;38:1049–1052. doi: 10.1248/cpb.38.1049. [DOI] [PubMed] [Google Scholar]
- 20.Chen XN, Fan JF, Yue X, Wu XR, Li LT. Radical scavenging activity and phenolic compounds in persimmon (Diospyros kaki L. cv. Mopan) J. Food Sci. 2008;73:C24–C28. doi: 10.1111/j.1750-3841.2007.00587.x. [DOI] [PubMed] [Google Scholar]
- 21.Park YS, et al. Nutraceutical value of persimmon (Diospyros kaki Thunb.) and its influence on some indices of atherosclerosis in an experiment on rats fed cholesterol-containing diet. Adv. Hortic. Sci. 2008;22:250–254. [Google Scholar]
- 22.Jang IC, et al. Antioxidant and antigenotoxic activities of different parts of persimmon (Diospyros kaki cv. Fuyu) fruit. J. Med. Plants Res. 2010;4:155–160. [Google Scholar]
- 23.Suzuki T, Someya S, Hu F, Tanokura M. Comparative study of catechin composition in five Japanese persimmons (Diospyros kaki) Food Chem. 2005;93:149–152. doi: 10.1016/j.foodchem.2004.10.017. [DOI] [Google Scholar]
- 24.Taira S, Abe K, Ooi K, Watanabe S. Influence of fruit size, defoliation, gibberellin, and growing regions on the ease of removal of astringency in Japanese persimmon. J. Jpn. Soc. Horitic. Sci. 1990;59:299–305. doi: 10.2503/jjshs.59.299. [DOI] [Google Scholar]
- 25.Taira S, Oba S, Watanabe S. Removal of astringency from ‘Hiratanenashi’ persimmon fruit with a mixture of ethanol and carbon dioxide. J. Jpn. Soc. Horitic. Sci. 1992;61:437–443. doi: 10.2503/jjshs.61.437. [DOI] [Google Scholar]
- 26.Taira S, Ooi M, Watanabe S. Volatile compounds of astringent persimmon fruits. J. Jpn. Soc. Horitic. Sci. 1996;65:177–183. doi: 10.2503/jjshs.65.177. [DOI] [Google Scholar]
- 27.Sugiura A, Tomana T. Relationships of ethanol-production by seeds of different types of Japanese persimmons and their tannin content. Hortscience. 1983;18:319–321. [Google Scholar]
- 28.Yonemori K, Matsushima J, Sugiura A. Differences in tannins of non-astringent and astringent type fruits of Japanese persimmon (Diospyros kaki Thunb.) J. Jpn. Soc. Horitic. Sci. 1983;52:135–144. doi: 10.2503/jjshs.52.135. [DOI] [Google Scholar]
- 29.Harada H, Itamura H, Taira S, Zheng GH. Natural removal of astringency in PCNA persimmon fruit CV Jiro grown in some different districts of Japan. J. Jpn. Soc. Horitic. Sci. 1990;58:807–811. doi: 10.2503/jjshs.58.807. [DOI] [Google Scholar]
- 30.Veberic R, Jurhar J, Mikulic-Petkovsek M, Stampar F, Schmitzer V. Comparative study of primary and secondary metabolites in 11 cultivars of persimmon fruit (Diospyros kaki L.) Food Chem. 2010;119:477–483. doi: 10.1016/j.foodchem.2009.06.044. [DOI] [Google Scholar]
- 31.Shiraishi M, Asakuma H. Sugar accumulation profile during the ripening process in the pollination-constant non-astringent Japanese persimmon ‘Akiou’. Hort. J. 2019;88:172–179. doi: 10.2503/hortj.UTD-023. [DOI] [Google Scholar]
- 32.Furihata K, Zhang J, Koda M, Miyakawa T, Tanokura M. Broadband WET: a novel technique for quantitative characterization of minor components in foods. Magn. Reson. Chem. 2014;52:333–338. doi: 10.1002/mrc.4066. [DOI] [PubMed] [Google Scholar]
- 33.Ryu S, et al. NMR-based analysis of the chemical composition of Japanese persimmon aqueous extracts. Magn. Reson. Chem. 2016;54:213–221. doi: 10.1002/mrc.4364. [DOI] [PubMed] [Google Scholar]
- 34.Cloarec O, et al. Statistical total correlation spectroscopy: an exploratory approach for latent biomarker identification from metabolic 1H NMR data set. Anal. Chem. 2005;77:1282–1289. doi: 10.1021/ac048630x. [DOI] [PubMed] [Google Scholar]
- 35.Niikawa T, Inari T, Ozeki T, Mitsui B. Effects of 1-methylcyclopropene on flesh firmness during storage of pollination-constant and non-astringent cultivars of Japanese persimmon (Diospyros kaki) J. Jpn. Soc. Food. Sci. Technol. 2005;52:68–73. doi: 10.3136/nskkk.52.68. [DOI] [Google Scholar]
- 36.Wakabayashi K. Changes in cell wall polysaccharides during fruit ripening. J. Plant Res. 2000;113:231–237. doi: 10.1007/PL00013932. [DOI] [Google Scholar]
- 37.Yoshioka H, Aoba K, Kashimura Y. Molecular-weight and degree of methoxylation in cell-wall polyuronide during softening in pear and apple fruit. J. Am. Soc. Hortic. Sci. 1992;117:600–606. doi: 10.21273/JASHS.117.4.600. [DOI] [Google Scholar]
- 38.Tiitinen KM, Yang BR, Haraldsson GG, Jonsdottir S, Kallio HP. Fast analysis of sugars, fruit acids, and vitamin C in sea buckthorn (Hippophae rhamnoides L.) varieties. J. Agric. Food Chem. 2006;54:2508–2513. doi: 10.1021/jf053177r. [DOI] [PubMed] [Google Scholar]
- 39.Yamada M, et al. Varietal differences in the ease of astringency removal by carbon dioxide gas and ethanol vapor treatments among Oriental astringent persimmons of Japanese and Chinese origin. Sci. Hortic. 2002;94:63–72. doi: 10.1016/S0304-4238(01)00367-3. [DOI] [Google Scholar]
- 40.Yamane H, et al. New Japanese persimmon cultivar ‘Taishuu’. Bull. Natl. Inst. Fruit Tree Sci. 2001;35:57–73. [Google Scholar]
- 41.Iwanami H, Yamada M, Sato A. A great increase of soluble solids concentration by shallow concentric skin cracks in Japanese persimmon. Sci. Hortic. 2002;94:251–256. doi: 10.1016/S0304-4238(01)00381-8. [DOI] [Google Scholar]
- 42.Matsui S, Ito S, Murata N, Banba Y. The relations of the chemical constituents to the qualitied in died persimmons. J. Jpn. Soc. Horitic. Sci. 1957;26:105–110. doi: 10.2503/jjshs.26.105. [DOI] [Google Scholar]
- 43.Furihata K, Seto H. Constant time HMBC (CT-HMBC), a new HMBC technique useful for improving separation of cross peaks. Tetrahedron Lett. 1998;39:7337–7340. doi: 10.1016/S0040-4039(98)01574-3. [DOI] [Google Scholar]
- 44.Wishart DS, et al. HMDB: Aknowledgebase for the human metabolome. Nucleic Acids Res. 2009;37:D603–D610. doi: 10.1093/nar/gkn810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ulrich EL, et al. BioMagResBank. Nucleic Acids Res. 2008;36:D402–408. doi: 10.1093/nar/gkm957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cui Q, et al. Metabolite identification via the Madison Metabolomics Consortium Database. Nat. Biotechnol. 2008;26:162–164. doi: 10.1038/nbt0208-162. [DOI] [PubMed] [Google Scholar]
- 47.Ryu, S. NMR-based analysis and characterization of minor components in Japanese persimmon (Diospyros kaki) cultivars. Ph.D. Thesis, The University of Tokyo, Tokyo, Japan (2014).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.