Abstract
Methanogens are putatively ancestral autotrophs that reduce CO2 with H2 to form biomass using a membrane-bound, proton-motive Fe(Ni)S protein called the energy-converting hydrogenase (Ech). At the origin of life, geologically sustained H+ gradients across inorganic barriers containing Fe(Ni)S minerals could theoretically have driven CO2 reduction by H2 through vectorial chemistry in a similar way to Ech. pH modulation of the redox potentials of H2, CO2 and Fe(Ni)S minerals could in principle enable an otherwise endergonic reaction. Here, we analyse whether vectorial electrochemistry can facilitate the reduction of CO2 by H2 under alkaline hydrothermal conditions using a microfluidic reactor. We present pilot data showing that steep pH gradients of approximately 5 pH units can be sustained over greater than 5 h across Fe(Ni)S barriers, with H+-flux across the barrier about two million-fold faster than OH–-flux. This high flux produces a calculated 3-pH unit-gradient (equating to 180 mV) across single approximately 25-nm Fe(Ni)S nanocrystals, which is close to that required to reduce CO2. However, the poor solubility of H2 at atmospheric pressure limits CO2 reduction by H2, explaining why organic synthesis has so far proved elusive in our reactor. Higher H2 concentration will be needed in future to facilitate CO2 reduction through prebiotic vectorial electrochemistry.
Keywords: CO2 reduction, origin of life, vectorial chemistry, energy-converting hydrogenase, alkaline hydrothermal vents, microfluidic reactor
1. Life as a guide to prebiotic chemistry
The origin of life has not been considered a question in biology until recently—prebiotic chemistry, by definition, took place before biology began. This perspective is now changing. Phylogenetics has restructured the deepest branching in the tree of life [1–6], while comparative physiology has uncovered revelatory new mechanisms of anaerobic metabolism, notably the process of flavin-based electron bifurcation [7–10]. These findings suggest that the last universal common ancestor (LUCA) was, in fact, the ancestor of bacteria and archaea [1,2,11], the eukaryotes being a derived domain [12]. The deepest branches in these groups suggest that LUCA was an obligately chemiosmotic autotroph that grew from CO2 and H2 by some form of the acetyl CoA pathway [1,13–15], extending into the formation of C2–C5 carboxylic acids, notably Krebs cycle intermediates [16–18]. We have previously argued that the metabolism of LUCA was probably similar to CO2 fixation in modern methanogens [19,20]; the notion that methanogenesis is ancient is itself a venerable idea, dating back to their discovery [21,22].
While there is no necessary link between LUCA and prebiotic chemistry, neither is there necessarily no link. Most prebiotic chemistry over decades has focused on relatively facile reactions involving reactive precursors such as cyanoacetylene activated by UV radiation [23,24]. While impressive chemistry, successfully producing informational monomers such as activated pyrimidine nucleotides [23], this ‘cyanosulfidic protometabolism' bears little resemblance to the biochemistry of known cells in terms of substrates, pathways, catalysts or energy coupling [25]. By contrast, recent work shows that strong electron donors such as native iron [26,27], or 1 V electrical potential [28], can reduce CO2 to carboxylic acids, including all five universal intermediates in life's core metabolism—acetate, pyruvate, oxaloacetate, succinate and α-ketoglutarate [26,27,29,30]. Intriguingly, glyoxylate can drive interconversions between all Krebs cycle intermediates [29]. These findings are an important proof of concept and link beautifully with the structure of metabolism, in which the hydrogenation of CO2 forms primarily C2–C5 carboxylic acids—carbon skeletons—from which are formed amino acids, fatty acids, sugars and eventually nucleotides [16–18,25,26,31]. Amino acids [29,32], fatty acids [33] and sugars [34,35] have been synthesized from carboxylic acids or their derivatives under equivalent prebiotic conditions, though nucleotides have proved more difficult so far [25]. Many of these reaction pathways were proposed to occur spontaneously in alkaline hydrothermal vents by Martin and Russell more than a decade ago [18,36] and these recent experimental findings confirm their predictions.
But there are also some issues with the use of native iron as an electron donor for CO2 fixation, or indeed any larger-scale mineral surface as a source of organic precursors. By larger scale, we mean any mineral assemblage that could not be readily inherited by daughter cells. Life requires genetic information, and prebiotic systems must obviously be capable of giving rise, through a continuous (and testable) set of steps, to the origins of the genetic code and natural selection. Genetic heredity is a specific form of growth—for RNA or DNA to be copied, one template must give rise to two, and so on, equating to growth and the formation of nucleotides. From a thermodynamic point of view, it is easier to make carboxylic acids, amino acids, fatty acids and sugars than nucleotides (which have still not been synthesized through prebiotic reactions that resemble biochemistry [25]). This means that any ‘RNA world' is necessarily dirty, contaminated with other organic molecules [37–41]. So, the question becomes: how can a growing, replicating system get better at making copies of itself, with increasingly complex prebiotic chemistry eventually giving rise to nucleotides and genetic information? This question is much easier to answer if the catalysts that drive growth (CO2 fixation) are inherited by daughter cells [42]. That, in turn, makes sense of the known process of CO2 fixation in anaerobic prokaryotes, which use inorganic mineral-like structures such as Fe(Ni)S clusters to fix CO2. These clusters are small, can self-assemble from ions in solution (through chelation) [43,44], can be oxidized and reduced in turn [45], and may be inherited directly, for example, in association with cell membranes [42]. We have shown that FeS clusters (including 4Fe–4S and 2Fe–2S clusters) can form spontaneously under alkaline conditions through interactions between Fe2+, Fe3+, S2– and amino acids such as cysteine, demonstrating a potential path to biological FeS clusters [46]. A larger-scale mineral, or even nanoparticles of native iron, could not be inherited in this way, hence do not point to a clear path from prebiotic chemistry to genetic heredity.
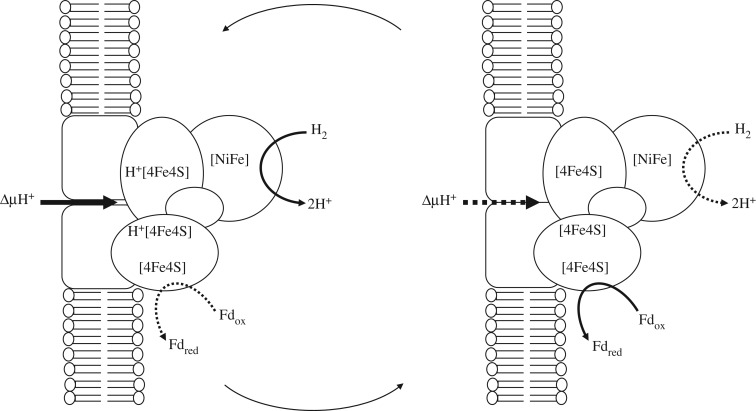
The key enzyme required to reduce CO2 to biomass in methanogens is the energy-converting hydrogenase (Ech) [45,47,48]. This is a membrane-bound, proton-motive Fe(Ni)S protein that uses H2 to reduce a low-potential ferredoxin directly [45,48,49]. The critical point here is that, even though this reduction is not favoured under standard conditions, the inward flux of protons through Ech can overcome an endergonic barrier of around 40 kJ mol−1, equating to a difference in redox potential of 200 mV, given an H2 partial pressure of 10 Pa and an oxidized-to-reduced ferredoxin ratio (Fdox/Fdred) of less than 0.01 [47]. The mechanism by which Ech reduces ferredoxin is not known, but two of the four Fe(Ni)S clusters have a pH-dependent reduction potential, becoming more reducing by –50 mV per pH unit, in rough accordance with the Nernst equation [49,50]. Given their close proximity to the transmembrane proton-pore [51], it seems plausible that these two FeS clusters could be reduced by H2 only when protonated, and could reduce ferredoxin only when deprotonated (figure 1). This putative mechanism is of major relevance to the origin of life because it implies that the most basic requirement to reduce CO2 using H2 is a dynamic proton flux across Fe(Ni)S clusters.
Figure 1.
Possible mechanism of the energy-converting hydrogenase (Ech). Ech reduces ferredoxin (Fd) with electrons from H2, despite a 200 mV difference in redox potential in methanogens [41]. (a) H+ pore is open and H+ binds to ligands associated with the two pH-modulated 4Fe–4S clusters, raising the redox potential of all four clusters through electron delocalization via quantum tunnelling and so enabling their reduction by H2. (b) H+ pore closes and H+ dissociates from ligands, lowering the redox potential of the final 4Fe–4S cluster sufficiently to reduce Fdox to Fdred.
We have previously shown that hydrothermally sustained proton gradients in alkaline vents can in principle promote the reduction of ferredoxin by H2 via Ech in the absence of any active pumping [52]. The continuous flux of protons into simple cells down a hydrothermal gradient can be maintained indefinitely, so long as their membranes are as permeable to protons as fatty-acid vesicles (which allows the escape or neutralization of protons) [52,53]. More pertinently here, prebiotic homologues of Ech with Fe(Ni)S clusters chelated by short polypeptides [43] or even amino acids [44,46] could associate with fatty-acid membranes to drive CO2 fixation in geochemical proton gradients [42]. Computer simulations show that positive feedbacks through physical interactions between dissolved ions, amino acids and fatty-acid bilayers can, in fact, promote rudimentary membrane heredity [42]. These positive feedbacks mean that the more organics that are formed, the more Fe(Ni)S clusters are chelated, and the more likely they are to associate with the membrane, driving synthesis of more organics as a ‘proto-Ech'. Such clusters are homologous with living systems [3,14], form spontaneously inside protocells [43,44], are heritable and evolvable [42]—for example, being chelated initially by amino acids [44,46], later by short non-coded polypeptide nests [54], and ultimately genetically encoded proteins [55]—can be oxidized and reduced in turn [20,47], and confer a direct selective advantage to protocells even before the emergence of genetic heredity [42].
Yet while Fe(Ni)S clusters chelated by amino acids and associated with fatty-acid membranes could in principle power protocell growth and evolution through a series of continuously connected steps, they are already quite sophisticated organic systems. How did such systems arise? Their reliance on mineral-like Fe(Ni)S clusters, hydrothermally sustained proton gradients and the abundant gases H2 and CO2, which both have pH-dependent reduction potentials, implies that a wholly inorganic form of vectorial electrochemistry could have driven CO2 fixation to generate organics at the origin of life [13,18,36,40,41,53,56–58].
2. Vectorial electrochemistry at the origin of life
If the arguments in the previous section are correct then the requirements for prebiotic CO2 fixation by H2 would be steep pH gradients across thin inorganic barriers containing Fe(Ni)S minerals, with a physical topology resembling that of cells [41,59]. This idea is not new, but exactly how geochemical proton gradients could facilitate the reduction of CO2 has been the subject of debate [53,60]. We have previously argued that the key feature is the pH-dependent reduction potentials of the reactants and catalysts — H2, CO2 and Fe(Ni)S minerals [19,20,58,61,62].
There are two cardinal factors here. First, the redox potential of H2 is not low enough to reduce CO2 at any equivalent pH, hence the difficulty of doing so in solution by scalar chemistry under prebiotic conditions. However, if H2 is dissolved in solution at a substantially higher pH than CO2, which is the case in the physically structured environment of alkaline hydrothermal vents, then in principle this reaction could proceed. Second, FeS clusters transfer single electrons rather than pairs of electrons, hence even if they are strongly reducing, they are unlikely to reduce CO2 directly [63]. One potential solution is also offered by cells: molybdenum (Mo) can receive electrons from FeS clusters and pass them on, effectively in pairs, to CO2 [41]. As in the Fe(Ni)S clusters of Ech, FeS minerals could then be reduced from Fe3+ to Fe2+ by H2 when protonated, and reduce Mo6+ to Mo4+ when deprotonated [41,64]. Mo4+ could, in turn, reduce CO2 to form organics [41]. Mo6+ is soluble in alkaline solution, hence could co-assemble with FeS minerals when oxidized by CO2 in alkaline hydrothermal conditions [41]. Mo is also notable in that its redox potential is pH-dependent [65], and displays crossed-over electron bifurcation dynamics, making it capable of driving very low-potential reductions [41,64].
The reason that pH differences across inorganic barriers could facilitate the reduction of CO2 is that the redox potential of the H2/2H+ couple falls by about –58 mV per pH unit, according to the Nernst equation, from –414 mV at pH 7 to –646 mV at pH 11 [66]. By contrast, the redox potential of the CH2O/HCOO– couple (the most endergonic step of CO2 reduction) rises from –580 mV at pH 7 to –522 mV at pH 6 [58], reflecting the likely pH of the Hadean oceans [67–70]. This means that the first steps in the reduction of CO2 by H2 are strongly endergonic if the pH is equivalent (+38 kJ mol−1) but become moderately exergonic when the pH of the reactants differs in a structured environment (–18 kJ mol−1). A difference of 5 pH units, therefore, confers a substantial free energy change of –56 kJ mol−1. Indeed, the redox potential of H2 in strongly alkaline conditions is low enough to reduce Fe2+ to Fe0, possibly enabling the formation of direct metal–carbon bonds as in some ancient metalloenzymes including [Fe–Ni] hydrogenase and radical SAM [63]. But these values assume a standard partial pressure of H2, giving a saturation value of about 0.8 mM at atmospheric pressure, as well as a very steep gradient of 5 pH units across a distance of just a few nanometres neither of which conditions might be met in abiotic compartments compared with cells.
That brings us to the crucial open question about how closely a structured inorganic environment could resemble the topology of cells. Ech is a membrane-bound Fe(Ni)S protein spanning a bilayer just 5 nm in diameter [5,44,47,48]. The FeS clusters are closely juxtaposed in space next to the transmembrane proton pore [51]. Presumably conformational changes in the transmembrane domains facilitate proton transfer (or Ech would not be able to function in reverse as a proton pump, powered by ferredoxin oxidation) [47,48]. This means that the FeS clusters should be subject to an oscillating flux of protons, with protons binding to FeS clusters or their ligands when the membrane pore is open (figure 1a) and then detaching and entering the cell when the pore is closed (figure 1b). By contrast, the inorganic barriers separating channels or pores in alkaline hydrothermal vents may be many micrometres thick [62,71–73], with nothing clearly resembling an oscillating flux of protons focused through membrane pores on a nanometre scale. It's also worth noting that the concept of pH refers to proton concentration in bulk solution and has little meaning in confined spaces. So the presence or absence of a single proton in a transmembrane channel would imply a binary switch from strong acidity to strong alkalinity, potentially much greater than 5 pH units, and therefore potentially able to drive difficult reactions with some ease [74]. This could not occur with any mixing in bulk solution, and arguably not even with unfocused transfer of protons across barriers. Given this disparity, could a broad topological equivalence hold at the nanoscale, driving CO2 fixation across inorganic barriers?
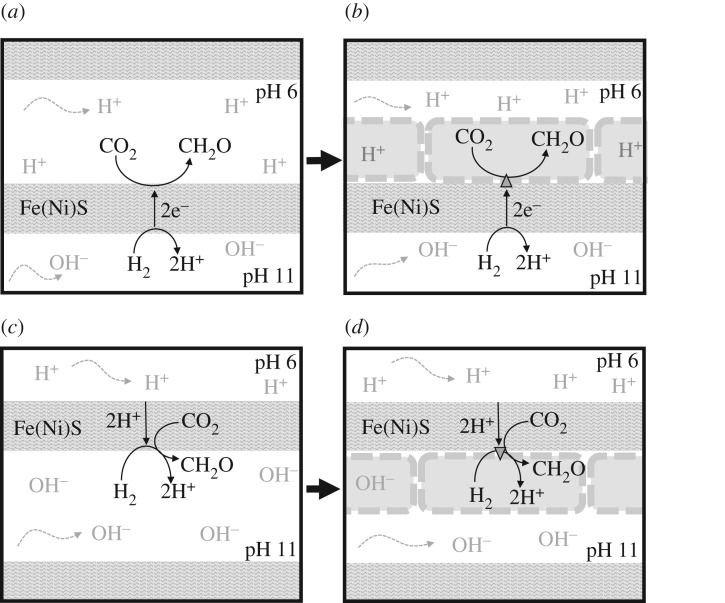
There are two possible ways in which the analogy could hold at this nano-level. First, FeS minerals such as mackinawite are semiconducting (in the longitudinal plane only) and so could theoretically transfer electrons from H2 in alkaline solution on the inside of a pore, to CO2 in acidic ocean water outside [53,62,75,76]. If so, then CO2 reduction would take place in the acidic ocean phase (figure 2a). That is not to say that organic molecules would be lost to the ocean [77]; rather, ocean waters should percolate into the labyrinth of pores inside the vent [78] and potentially even concentrate organics by thermophoresis [79,80]. But there are other issues too. Is it feasible for electrons to conduct across these relatively thick barriers, given that mackinawite only conducts in one plane [81,82]? Evidence from black-smoker vents (which are much more metal-rich, so probably better conductors) suggests that electrons can indeed conduct over several centimetres [83], but experimental work on hollow FeS vent-like structures in the laboratory suggests that they typically retain an electrical potential difference over several hours [84], implying that they do not conduct electrons readily.
Figure 2.
Topology of hypothetical vectorial chemistry across an inorganic barrier containing Fe(Ni)S nanocrystals. (a) Transfer of electrons (e–) across the whole barrier from H2 in alkaline solution (which lowers H2 redox potential) to CO2 in acidic ocean water (which raises its redox potential). (b) Topology of CO2 reduction in protocells at a later stage assuming continuity of CO2 reduction with (a). The triangle depicts Fe(Ni)S clusters in a membrane-bound proto-Ech. Note this is not topologically equivalent to CO2 reduction via Ech in modern cells. (c) Transfer of H+ across the whole barrier. CO2 reduction could hypothetically occur in a narrow region with sharp pH gradients across single 20 nm mackinawite nanocrystals close to the alkaline face. (d) Topology of CO2 reduction in protocells at a later stage assuming continuity of CO2 reduction with (c). Note that this is topologically equivalent to CO2 reduction via Ech in modern cells.
A subtler question concerns the topology, which in this case is not equivalent to that of cells. With Ech, the electrons derive from H2, which is dissolved in the relatively alkaline interior of the cell, whereas the reduction of CO2 is facilitated by an influx of protons from the more acidic exterior [47]. If electrons were transferred across a barrier, CO2 reduction would take place on the acidic exterior (figure 2a). The primary vectorial flux would then be electron flow, not proton flow. That would imply a discontinuity between prebiotic vectorial chemistry and the origins of biochemical CO2 reduction via Ech: the electrons for CO2 reduction derive from Fe2+ in the barrier outside the protocell, rather than from H2 inside the cell, and there is no primary role for a transmembrane proton gradient. A second difficulty with this hypothesis is that, at a later stage (figure 2b), protocells would eventually form on the acidic face of the barrier. However, bilayer membranes cannot self-assemble from single-chain amphiphiles (e.g. fatty acids and fatty alcohols) at even mildly acidic pH (approx. 6), so simple protocells depicted in figure 2b could only form if negatively charged head-groups are condensed onto the fatty acids, a more complex and therefore less likely scenario [85–87]. It is also possible that organics could form in the acidic phase and then accumulate by thermophoresis elsewhere in the system [79,80]. However, that too implies a discontinuity between organic synthesis and protocell formation which is at the least convoluted. We, therefore, do not favour this possibility in principle, despite having advocated it in the past [53,61,62].
The second possible way in which the analogy could hold at a nano-level would be for protons to transfer across the barrier rather than electrons. Transfer of protons rather than electrons across a barrier is more strictly analogous to the topology of the Ech, as organics would now be formed from H2 in the alkaline phase, with the flux of H+ across the barrier modulating the redox potential of H2, CO2 and Fe(Ni)S minerals on the alkaline side. For this scenario to work, however, very steep pH gradients would be required on the alkaline side of the barrier. If electrons could conduct across single nanocrystals but not across multiple crystals with scattered orientation, then these steep pH gradients would need to develop across single Fe(Ni)S nanocrystals, of approximately 20–30 nm in length. When protonated, the Fe(Ni)S nanocrystal would be reducible by H2 on its alkaline side (where the redox potential for H2/H+ couple would potentially be close to –650 mV). Conversely, when deprotonated, its redox potential would fall low enough to reduce CO2 on the acidic side of the nanocrystal (where the reduction potential for the CO2/CH2O couple could be closer to –520 mV). If that were the case, then alternating protonation and deprotonation of Fe(Ni)S nanocrystals would potentially facilitate CO2 reduction in a similar fashion to the Ech. By modulating the redox potential of Fe(Ni)S nanocrystals, H2 and CO2, sharp differences in pH alter the ΔG of the reaction, in other words, the thermodynamic driving force. That requirement, in turn, demands that H+ should transfer across the barrier much more rapidly than OH– from the alkaline side, otherwise H+ and OH– would neutralize within the barrier, dissipating any steep pH gradients and ruling out this postulated mechanism.
Whether it is really feasible for H+ to cross inorganic barriers in hydrothermal vents so much more easily than OH– is unknown. The conductance of H+ through bulk water is less than double that of OH– [88]. Unlike OH–, however, it is known that protons can conduct rapidly through intercalated nanoconfined water in layered minerals, including mackinawite, via the Grotthuss mechanism that takes place in bulk water and protein cavities [89–91]. How much faster H+ transfer might be across freshly precipitated, unmineralized barriers with a high aqueous content—likely to characterize the active regions of hydrothermal systems discussed here—remains an open question that has been debated in the literature [53] with some arguing that such steep pH gradients are not feasible in hydrothermal systems [60]. On the other hand, if the difference in mobility is substantial, then fast conductance of protons could foreshadow the mechanism of Ech in a prebiotic setting, with electron conductance taking place locally across nanocrystals rather than across the entire barrier (figure 2c). If that were the case, then the topology of protocells would be correct (figure 2d). We have previously shown that vesicles formed from single-chain amphiphiles such as fatty acids and fatty alcohols are stable at pH 11 and above [92,93], so the scheme depicted in figure 2d is feasible. The major question that we explore now is whether suitably steep H+ gradients could develop across inorganic barriers containing disordered Fe(Ni)S minerals, which could ultimately promote the reduction of CO2 by H2.
3. A microfluidic chip to simulate prebiotic vectorial electrochemistry
We have fabricated a microfluidic reactor to test these questions by simulating the flow dynamics of anoxic Hadean alkaline hydrothermal vents. The chip design is shown in figure 3a–c. Reactors were made from aluminium or polycarbonate depending on experimental requirements. All experiments were carried out in an anaerobic hood in an atmosphere of 5% H2∶95% N2. Alkaline hydrothermal fluids were simulated as a solution of sodium sulfide (NaS, 1 mM) and sodium silicate (Na6Si2O7, 10 mM). This solution was adjusted to pH 11 using NaOH as needed. H2 gas was introduced into the alkaline solution via a T-connector from a syringe containing aluminium powder and 1 M NaOH, replenished every 30 min. Hadean oceans were simulated as a solution of ferrous chloride (FeCl2, 5 mM), nickel chloride (NiCl2, 1 mM) and sodium bicarbonate (NaHCO3, 10 mM) [62,72,73]. The ocean solution was adjusted to pH 6 using HCl, this acidity reflecting likely Hadean conditions (when the atmospheric CO2 levels were at least 100-fold higher) [67–70] and favour a 50 : 50 partitioning of CO2 and . While CO2 is most likely the species reduced [94] is also possible [28]; the pH used should permit either reaction. Small bubbles of H2 could be seen in the alkaline solution, implying a concentration close to saturation at atmospheric pressure. Occasionally, small bubbles of CO2 could be observed in the acid ocean, showing partitioning from to CO2 at pH 6. These bubbles mostly progressed through the chip and rarely obstructed flow. With the exception of , which was introduced as a substrate into the acid channel only, we avoided the use of buffers as our intention was to simulate prebiotic hydrothermal environments which are not buffered.
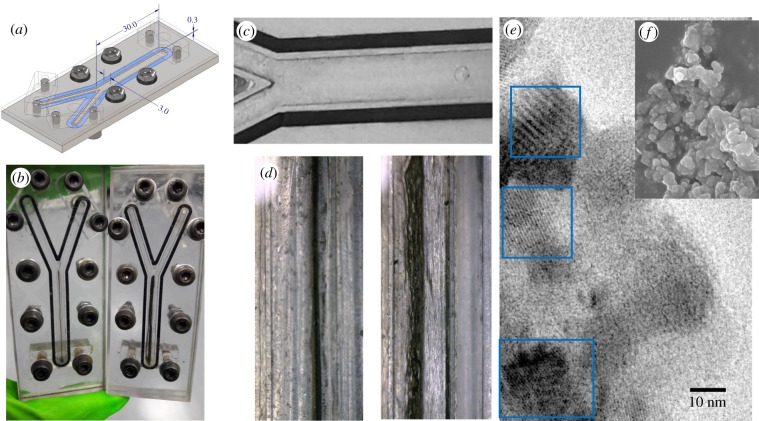
Figure 3.
Design and function of microfluidic reactor. (a) Design of reactor showing Y-shaped channel. Dimensions in millimetres. (b) Two microfluidic reactors showing fresh precipitate after 45 min (left) and 5 h (right). (c) Confluence of Y-shaped channels giving parallel laminar flow down main channel. (d) Magnification of main channel showing Fe(Ni)S precipitate after 1 h (left) and 5 h (right), showing lacy network structure. (e) Transmission electron micrograph of barrier showing lattice structure of Fe(Ni)S nanocrystals; note the disordered orientation. (f) Scanning electron micrograph showing microgeodes composed of mixed Fe minerals. (Online version in colour.)
The solutions of alkaline hydrothermal fluids and Hadean ocean were introduced through a Y-shaped channel on the chip at optimal flow rates of 50 µl min−1, giving parallel laminar flow through the main channel of the chip. The entire chip was heated to 70°C to simulate the temperature of vent fluids, which should favour the reaction between H2 and CO2 thermodynamically. A thin, stable precipitate of Fe(Ni)S minerals including mackinawite nanocrystals formed spontaneously within approximately 45 min, and thickened slowly over 5 h to fill approximately 50% of the channel (figure 3d). Mackinawite nanocrystals had been identified and characterized in earlier work [62] and were equivalent in structure here, with individual nanocrystals being 20–30 nm in length and approximately 10 nm in width, with spacing between the atomic planes of 0.3 nm, 0.5 nm and 0.5 nm for the g, h and i planes, respectively (figure 3e). High densities of these nanocrystals were observed, but with scattered orientation. This disordered orientation is unlikely to promote the transfer of electrons across the barrier, given that mackinawite is semiconducting in longitudinal plane only. That interpretation is supported by scanning electron micrographs, which show both wall structures and microgeodes composed of mixed ferrous hydroxides, carbonates, sulfides and silicates (figure 3f); there is no regular structure that would clearly promote electrical conductance. These findings are in accordance with earlier work showing the retention of an electrical potential difference over several hours [84].
Most experiments on the microfluidic chip were terminated after 5–6 h, but replacing the solutions with equivalent solutions at the same pH but lacking NaS, FeCl2 and NiCl2 (so no longer thickening the precipitate) enabled flow to be continued over 24 h without blocking the channel or causing back-pressure problems. The chip was designed with a single outflow channel, meaning that there was mixing of alkaline and acid solutions in the effluent. The rationale for mixing the effluent related to the transfer of electrical charge across the barrier, regardless of whether electrons or protons are transferred. Any charge on the barrier would oppose continued electrical flux unless there is a salt bridge (as a transfer of counter-ions across the barrier) or mixing elsewhere in the system. Mixing certainly takes place in vents, and in principle should dissipate any electrical charge. The mixing of effluent from the chip should, therefore, promote a continuous transfer of charge across the barrier in either direction, potentially facilitating CO2 reduction even if counter-ions crossed the barrier far more tardily.
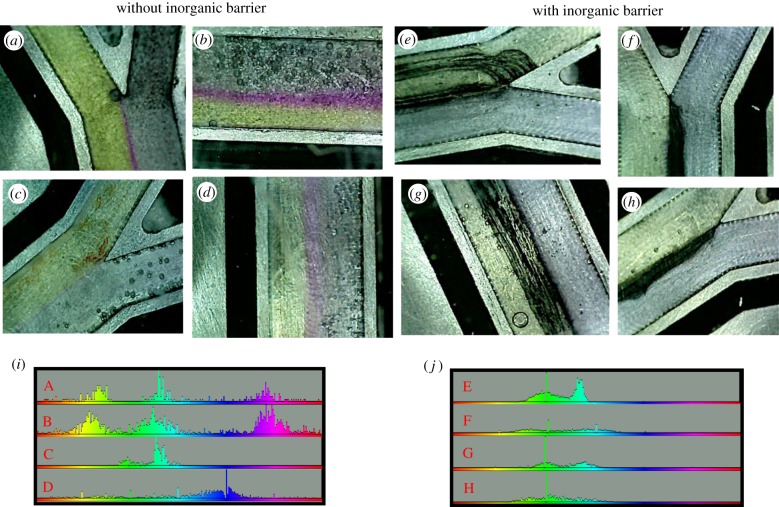
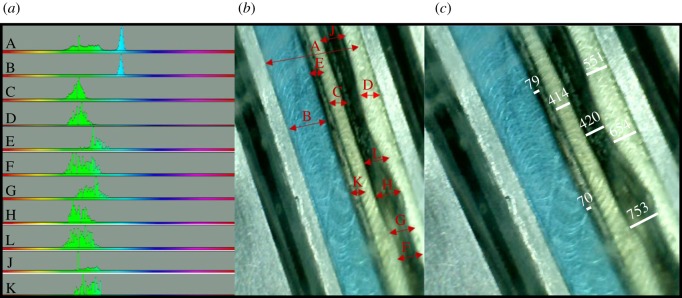
A flow rate of 50 µl min−1 can sustain laminar flow and visible pH gradients even in the absence of a barrier (using acidic and alkaline solutions lacking NaS, FeCl2 and NiCl2; figure 4a–d). This difference in pH can be maintained over 24 h in the presence of thin Fe(Ni)S barriers. With a continuous flux of solutions containing NaS, FeCl2 and NiCl2, the barrier thickened gradually over 5 h, usually (but not invariably) on the alkaline side. The Fe(Ni)S barriers tend to form lacy network structures frequently containing elongated aqueous channels within the barrier (figure 4e,g) but are occasionally dense (figure 4f,h). Inclusion of pH-sensitive dyes in the solutions (phenol red for acid ocean and bromothymol blue or universal indicator for alkaline fluids) showed that these aqueous inclusions within network barriers were almost always acidic, giving a yellow-green colour with phenol red and bromothymol blue (figure 5a). Note that at 70°C, Kw = 7.16 × 10–14, so the [H+] and [OH–] at neutral pH = 2.77 × 10–7, giving a neutral pH of 6.57. Because the alkaline fluids are pH 11 (giving an [OH–] of 2.77 × 10–3 M at 70°C) and acidic oceans are pH 6 (giving an [H+] of 2.77 × 10–6 M at 70°C), the concentration of OH– ions is 1000-fold greater than H+ ions. Despite this difference, the fact that the barrier becomes acidic in virtually all cases implies that H+ transfer across the barrier is much faster than OH– transfer (figures 4e,g and 5b). The faster transport of H+ across the barrier gives steep pH gradients, but we could only measure these directly between aqueous inclusions in the barrier and the alkaline channel, with the steepest measurable gradients being approximately 5 pH units across approximately 70 µm (figure 5c). However, this is still roughly 3500-fold greater than the approximately 20 nm length of individual Fe(Ni)S nanocrystals, across which pH gradients would arguably need to operate to facilitate CO2 reduction by vectorial chemistry under strictly prebiotic conditions. Could pH gradients be so much steeper within the precipitates?
Figure 4.
pH gradients sustained by laminar flow (50 µl min−1) on the microfluidic reactor in the absence (a–d) and presence (e–h) of freshly precipitated inorganic Fe(Ni)S barriers. pH-sensitive dyes used were phenol red (acidic channel, yellow green) and universal indicator (alkaline channel, pale blue). The colour bars (i,j) show the colour balance across the entire channel generated by ImageJ software. In the absence of a barrier (a–d), the red, dark blue, yellow and pink colours show mixing between the two flows. In the presence of barriers (e–h), there are only two colours (green and pale blue) corresponding to the acidic and alkaline channels with little if any mixing. Note that the barriers in (e) and (g) are lacy network precipitates with aqueous channels that are clearly acidic pH.
Figure 5.
Differences in pH between the acidic and alkaline channels, and aqueous channels trapped within the barrier. The dyes are phenol red (acid channel) and bromothymol blue (alkaline channel). (a) Shows the colour balance across the regions delineated in (b). There is clearly no mixing of acidic and alkaline fluids in aqueous channels within the barrier, which all retain a pH of approximately 6 (yellow-green colour). (c) Shows distances marked on the same image, with a minimal distance of 70 µm between acidic aqueous channels within the barrier and the alkaline channel. This image is representative of N = 6 equivalent runs with lacy network precipitates.
To estimate the distance across which proton gradients might in fact operate, we stopped the flow in the acidic and alkaline channels in turn to calculate the permeability of the barrier to H+ and OH–, respectively. These are approximate order of magnitude calculations. After stopping the flow in the alkaline but not the acidic channel, we measured the time taken for half of the alkaline channel to become acidic (pH 6), meaning a colour change from blue to yellow-green. The mean time for half the alkaline channel to become acidic (pH 6) was 2.7 ± 1.5 s (N = 3). The volume of the chip = 3 × 10–5 l, so half the volume of the alkaline channel alone is 7.5 × 10–6 l. The pH changed from approximately 11 to 6 in half the alkaline channel. The concentration of OH– at pH 11 = 2.77 × 10–3 M. That means the number of OH– ions neutralized in 2.66 s = 6.023 × 1023 (Avogadro's number) × 0.00277 ([OH–]) × 7.5 × 10–6 (acid volume) = 1.3 × 1016 OH– ions neutralized in 2.7 s, or 4.6 × 1015 H+ crossed the barrier in 1 s. The permeability of the barrier to protons = P = J/AC, where J = flux, A = area of precipitate and C = [H+]. Therefore, p = 4.6 × 1015/4.5 × 10–5 m2 × 2.77 × 10–6 M protons (pH 6) = 3.7 × 1025 H+/s.
For the permeability of the barrier to OH– ions, the pH changed from 6 (yellow-green) to 8 (blue) in 5.0 ± 2.0 s (N = 3). Thus, 2.77 × 10–6 M protons were neutralized in a volume of 7.5 × 10–6 l, or a total of 1.3 × 1013 H+ were neutralized in 5 s = 2.5 × 1012 OH–/s. The permeability of the barrier to OH– = 2.5 × 1012 (flux)/4.5 × 10–5 (area of barrier) × 0.00277 (concentration of OH– at pH 11) = 2.01 × 1019 OH–/s. This means the permeability of the barrier to H+ is 1.84 million-fold greater than the permeability to OH–, or approximately two million-fold more permeable to H+, allowing for the uncertainty around several of these measurements. Nonetheless, this value is clearly roughly correct given that aqueous channels within the barrier are acidic despite (i) the [OH–] is 1000-fold greater than the [H+] (pH 11 versus pH 6); (ii) the shift in pH in the alkaline channel is from pH 11 to 6 (blue to yellow-green, from a known pH of 11 to a measured pH of 6), meaning 10–3 M OH– solution is neutralized, whereas the pH shift in the acidic channel is from pH 6 to 8 (because bromothymol blue turns blue at pH 8), meaning just 10–6 M H+ solution is neutralized; and (iii) the times for H+ to transfer across the barrier were about twice those for OH–. So we are satisfied that the permeability of the Fe(Ni)S barrier to H+ is about two million-fold greater than OH–. This is consistent with the premise that H+ is transferred through intercalated, nanoconfined water in layered minerals like mackinawite (and fresh precipitates) via a Grotthuss mechanism, whereas OH– ions cannot [89–91].
The conclusion that H+ ions transfer across Fe(Ni)S barriers about two million-fold faster than OH– ions permits us to roughly constrain the likely steepness of the H+ gradient across the approximately 70 µm precipitate that was the steepest experimentally measurable gradient (5 pH units). Because [OH–] in the alkaline channel is 1000-fold greater than [H+] in the acidic channel, the difference in the rate of ions crossing the barrier should be approximately 2000-fold. That means there should be a point at which the transfer of OH– ≈ H+ at 1/2000 of the distance from the alkaline channel to the acidic inclusion, or in this case about 70/2000 = 35 nm from the alkaline channel. The balance in flux at this point should equate to pH 7, therefore, there should be a steep pH gradient over approximately 35 nm, from pH 7 to 11 in the alkaline channel, or 3 pH units across 26 nm, assuming (most simply) a linear change in concentration. If the length of a single mackinawite nanocrystal is about 20 nm, then it is feasible that prebiotic vectorial chemistry could indeed support gradients of approximately 3 pH units across single nanocrystals, potentially facilitating the reduction of CO2 as discussed above. But even if true, this value is less than the required steepness of gradient needed to reduce CO2, assuming H2 saturation at atmospheric concentration. As noted earlier, the ΔGo' for the CH2O/HCOO– couple is +38 kJ mol−1. A gradient of 5 pH units across a single mackinawite crystal would render the reduction exergonic because ΔG = –nF ΔEh, in which n is the number of electrons, F is the Faraday constant and ΔEh is the redox potential difference at 0.058 V per pH unit. In this case, ΔG = –2 × 96.5 × 0.290 V = –56 kJ mol−1, or an overall ΔG' of +38–56 = –18 kJ mol−1. But with a smaller 3-pH unit gradient across a single nanocrystal, ΔG = –2 × 96.5 × 0.174 V = –34 kJ mol−1, giving an overall ΔG' of +38–34 = +4 kJ mol−1, which thermodynamically could no longer drive the reduction of CO2, although it is on the point of doing so given the margin for uncertainty.
The reduction of CO2 by H2 depends not only on the redox potential but also on the concentration of the gases involved. A problem with a continuous-flow microfluidic chip is that H2 is poorly soluble at atmospheric pressure, saturating at 0.78 mM, which falls further at 70°C to 0.55 mM. The solubility of CO2 declines even more steeply with temperature, falling from 34 mM at 25°C to 11 mM at 70°C. However, the concentration of added to our ocean solution was 10 mM, which at pH 6 should partition to 5 mM CO2, well below saturation at 70°C. Given an initial background contamination of CH2O of approximately 20 nM [95], ΔG = ΔGo + 2.3RT log10 [CH2O]/[H2]2[CO2], then ΔG = 4 + (5.698 × log10 2 × 10–8/ 0.000552 × 0.005) = +10 kJ mol−1, so again moderately endergonic (this assumes water at unity and a requirement for 2H2 to form CH2O + H2O). Only if the pH gradients were greatly steeper than those estimated here could CO2 reduction take place. For example, if the thinnest section of the barrier was approximately 30 µm thick, giving a pH gradient of 4 pH units across approximately 15 nm, the ΔG' would be just sufficient to drive CO2 reduction. The calculations described here, therefore, explain the difficulty of reducing CO2 in a microfluidic reactor running at atmospheric pressure, and this is consistent with our failure to reproducibly detect organics in the reactor to date despite some success (S Lim, N Lane 2016, unpublished observations) [62]. Others have also struggled to synthesize organics from H2 and CO2 in microfluidic reactors [76]. However, at higher pressures the saturation of H2 increases. At Lost City, the H2 concentration is approximately 15 mM [96,97], which gives a ΔG = 4 + (5.698 × log10 2 × 10–8/0.0152 × 0.005) = –6 kJ mol−1, favouring CO2 reduction. If H2 concentration approaches 200 mM, as reported for the formation of minerals such as awaruite in hydrothermal conditions [98–100] then ΔG = –20 kJ mol−1 and CO2 reduction by H2 should proceed readily, given suitable catalysts. Other groups have also advocated the need for high-pressure to drive the reduction of CO2 by H2 [76] but to our knowledge this is the first study to constrain the dynamics of H+ gradients across Fe(Ni)S barriers in relation to CO2 reduction in a microfluidic reactor.
4. Conclusion
Phylogenetics and comparative physiology suggest that LUCA was an anaerobic autotroph that grew from the reaction between H2 and CO2 via some form of the acetyl CoA pathway [1,13–16,59] feeding into an incomplete reductive Krebs cycle [5,29,31]. However, while stronger electron donors such as Fe0 have been shown to reduce CO2 to carboxylic acids [26,27,29], the reaction between H2 and CO2 is difficult to achieve under prebiotic conditions at atmospheric pressure. Putatively ancestral autotrophs such as methanogens use a membrane-bound proton-motive Fe(Ni)S protein, the energy-converting hydrogenase, which uses the proton gradient to reduce ferredoxin and thence CO2 [7,8,48–50]. The transfer of protons across inorganic barriers containing Fe(Ni)S minerals prefigures both the magnitude and polarity of transmembrane electrochemical ion gradients in cells [41,58,59]. We have shown in a microfluidic chip that the permeability of disordered Fe(Ni)S barriers to H+ is about two million-fold greater than OH–, so steep pH gradients in the order of 3 pH units across 20–30 nm can exist across the barrier. These sharp H+ gradients could facilitate the reduction of CO2 by H2 across single mackinawite nanocrystals close to the alkaline face of the barrier. However, at atmospheric pressure, the low partial pressure of H2 means this reduction is borderline endergonic, and therefore unlikely to proceed. At partial pressures of H2 equivalent to those found in deep-sea hydrothermal systems the reaction should proceed exergonically. This warrants further experimentation in a high-pressure version of the microfluidic device presented here. The advantage of vectorial chemistry as a driving force for CO2 reduction at the origin of life is that it modulates the redox potential of H2, CO2 and Fe(Ni)S clusters, driving growth in a fashion that is topologically analogous and arguably homologous with that of cells [19,25,42,52,101]. Because membrane-associated Fe(Ni)S clusters are catalytic and self-assemble in association with fatty acid bilayers, they can in principle give rise to a form of membrane heredity that fosters growth and ultimately genetic heredity at the origin of life.
Acknowledgements
The authors thank Marco Colnaghi for valuable discussions on calculating the steepness of proton gradients, and two anonymous reviewers for their valuable comments on the manuscript. We thank Dr Brian O' Sullivan and Mr Vijay Kumar from the Department of Biochemical Engineering at UCL for their help with the design and fabrication of prototype microfluidic chips. We thank Margaret McCaul and Dermot Diamond from the Insight Centre for Data Analytics, National Centre for Sensor Research, Dublin City University, Ireland for developing these microfluidic designs. Microfluidic chips were manufactured by Parsec Precision Tooling, Dublin.
Data accessibility
Additional data on the design, fabrication and operation of the microfluidic reactor are available from the authors.
Competing interests
This work was supported by a research grant to N.L. from bgc3.
Funding
We received no funding for this study.
References
- 1.Weiss MC, Sousa FL, Mrnjavac N, Neukirchen S, Roettger M, Nelson-Sathi S, Martin WF. 2016. The physiology and habitat of the last universal common ancestor. Nat. Microbiol. 1, 1–8. ( 10.1038/nmicrobiol.2016.116) [DOI] [PubMed] [Google Scholar]
- 2.Betts HC, Puttick MN, Clark JW, Williams TA, Donoghue PCJ, Pisani D. 2018. Life's early evolution and eukaryote origin. Nat. Ecol. Evol. 2, 1556–1562. ( 10.1038/s41559-018-0644-x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nitschke W, McGlynn SE, Milner-White EJ, Russell MJ. 2013. On the antiquity of metalloenzymes and their substrates in bioenergetics. Biochim. Biophys. Acta - Bioenerg. 1827, 871–881. ( 10.1016/j.bbabio.2013.02.008) [DOI] [PubMed] [Google Scholar]
- 4.Martin WF, Weiss MC, Neukirchen S, Nelson-sathi S, Sousa FL. 2016. Physiology, phylogeny, and LUCA. Microb. Cell 3, 582–587. ( 10.15698/mic2016.12.545) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Braakman R, Smith E. 2012. The emergence and early evolution of biological carbon-fixation. PLoS Comput. Biol. 8, e1002455 ( 10.1371/journal.pcbi.1002455) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stetter KO. 2006. Hyperthermophiles in the history of life. Phil. Trans. R. Soc. B 361, 1837–1843. ( 10.1098/rstb.2006.1907) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Buckel W, Thauer RK. 2018. Flavin-based electron bifurcation, ferredoxin, flavodoxin, and anaerobic respiration with protons (Ech) or NAD+ (Rnf) as electron acceptors: a historical review. Front. Microbiol. 9, 410 ( 10.3389/fmicb.2018.00401) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Buckel W, Thauer RK. 2013. Energy conservation via electron bifurcating ferredoxin reduction and proton/Na+ translocating ferredoxin oxidation. Biochim. Biophys. Acta - Bioenerg. 1827, 94–113. ( 10.1016/j.bbabio.2012.07.002) [DOI] [PubMed] [Google Scholar]
- 9.Kaster A-K, Moll J, Parey K, Thauer RK. 2011. Coupling of ferredoxin and heterodisulfide reduction via electron bifurcation in hydrogenotrophic methanogenic archaea. Proc. Natl Acad. Sci. USA 108, 2981–2986. ( 10.1073/pnas.1016761108) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wagner T, Koch J, Ermler U, Shima S. 2017. Methanogenic heterodisulfide reductase (HdrABC-MvhAGD) uses two noncubane [4Fe–4S] clusters for reduction. Science 357, 699–703. ( 10.1126/science.1252826) [DOI] [PubMed] [Google Scholar]
- 11.Dagan T, Roettger M, Bryant D, Martin W. 2010. Genome networks root the tree of life between prokaryotic domains. Genome Biol. Evol. 2, 379–392. ( 10.1093/gbe/evq025) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Williams TA, Foster PG, Cox CJ, Embley TM. 2013. An archaeal origin of eukaryotes supports only two primary domains of life. Nature 504, 231–236. ( 10.1038/nature12779) [DOI] [PubMed] [Google Scholar]
- 13.Martin WF, Sousa FL, Lane N. 2014. Energy at life's origin. Science 344, 1092–1093. ( 10.1126/science.1251653) [DOI] [PubMed] [Google Scholar]
- 14.Russell MJ, Martin W. 2004. The rocky roots of the acetyl-CoA pathway. Trends Biochem. Sci. 29, 358–363. ( 10.1016/j.tibs.2004.05.007) [DOI] [PubMed] [Google Scholar]
- 15.Fuchs G. 2011. Alternative pathways of carbon dioxide fixation: insights into the early evolution of life? Annu. Rev. Microbiol. 65, 631–658. ( 10.1146/annurev-micro-090110-102801) [DOI] [PubMed] [Google Scholar]
- 16.Morowitz HJ, Kostelnik JD, Yang J, Cody GD. 2000. The origin of intermediary metabolism. Proc. Natl Acad. Sci. USA 97, 7704–7708. ( 10.1073/pnas.110153997) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Smith E, Morowitz HJ. 2004. Universality in intermediary metabolism. Proc. Natl Acad. Sci. USA 101, 13 168–13 173. ( 10.1073/pnas.0404922101) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Martin W, Russell MJ. 2007. On the origin of biochemistry at an alkaline hydrothermal vent. Phil. Trans. R. Soc. B 362, 1887–1925. ( 10.1098/rstb.2006.1881) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sojo V, Herschy B, Whicher A, Camprubí E, Lane N. 2016. The origin of life in alkaline hydrothermal vents. Astrobiology 16, 181–197. ( 10.1089/ast.2015.1406) [DOI] [PubMed] [Google Scholar]
- 20.Camprubi E, Jordan SF, Vasiliadou R, Lane N. 2017. Iron catalysis at the origin of life. IUBMB Life 69, 373–381. ( 10.1002/iub.1632) [DOI] [PubMed] [Google Scholar]
- 21.Woese CR. 1977. A comment on methanogenic bacteria and the primitive ecology. J. Mol. Evol. 9, 369–371. ( 10.1007/BF01796101) [DOI] [PubMed] [Google Scholar]
- 22.Decker K, Jungermann K, Thauer RK. 1970. Energy production in anaerobic organisms. Angew. Chem. Int. Ed. Engl. 9, 138–158. ( 10.1002/anie.197001381) [DOI] [PubMed] [Google Scholar]
- 23.Powner MW, Gerland B, Sutherland JD. 2009. Synthesis of activated pyrimidine ribonucleotides in prebiotically plausible conditions. Nature 459, 239–242. ( 10.1038/nature08013) [DOI] [PubMed] [Google Scholar]
- 24.Sutherland JD. 2017. Studies on the origin of life — the end of the beginning. Nat. Rev. Chem. 1, 0012 ( 10.1038/s41570-016-0012) [DOI] [Google Scholar]
- 25.Harrison S, Lane N. 2018. Life as a guide to prebiotic nucleotide synthesis. Nat. Commun. 9, 1 ( 10.1038/s41467-018-07220-y) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Varma SJ, Muchowska KB, Chatelain P, Moran J. 2018. Native iron reduces CO2 to intermediates and end-products of the acetyl-CoA pathway. Nat. Ecol. Evol. 2, 1019–1024. ( 10.1038/s41559-018-0542-2) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Muchowska KB, Varma SJ, Chevallot-Beroux E, Lethuillier-Karl L, Li G, Moran J. 2017. Metals promote sequences of the reverse Krebs cycle. Nat. Ecol. Evol. 1, 1716–1721. ( 10.1038/s41559-017-0311-7) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Roldan A, et al. 2015. Bio-inspired CO2 conversion by iron sulfide catalysts under sustainable conditions. Chem. Commun. 51, 7501–7504. ( 10.1039/c5cc02078f) [DOI] [PubMed] [Google Scholar]
- 29.Muchowska KB, Varma SJ, Moran J. 2019. Synthesis and breakdown of universal metabolic precursors promoted by iron. Nature 569, 104–107. ( 10.1038/s41586-019-1151-1) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Keller MA, Kampjut D, Harrison SA, Ralser M. 2017. Sulfate radicals enable a non-enzymatic Krebs cycle precursor. Nat. Ecol. Evol. 1, 1–9. ( 10.1038/s41559-017-0083) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Smith E, Morowitz HJ. 2016. The origin and nature of life on Earth: the emergence of the fourth geosphere . ( ) [DOI]
- 32.Huber C, Wächtershäuser G. 2003. Primordial reductive amination revisited. Tetrahedron Lett. 44, 1695–1697. ( 10.1016/S0040-4039(02)02863-0) [DOI] [Google Scholar]
- 33.McCollom TM, Ritter G, Simoneit BRT. 1999. Lipid synthesis under hydrothermal conditions by Fischer–Tropsch reactions. Orig. life Evol. Biosph. 29, 153–166. ( 10.1023/A:1006592502746) [DOI] [PubMed] [Google Scholar]
- 34.Kopetzki D, Antonietti M. 2011. Hydrothermal formose reaction. New J. Chem. 35, 1787–1794. ( 10.1039/c1nj20191c) [DOI] [Google Scholar]
- 35.Ralser M. 2018. An appeal to magic? The discovery of a non-enzymatic metabolism and its role in the origins of life. Biochem. J. 475, 2577–2592. ( 10.1042/BCJ20160866) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Martin W, Russell MJ. 2003. On the origins of cells: a hypothesis for the evolutionary transitions from abiotic geochemistry to chemoautotrophic prokaryotes, and from prokaryotes to nucleated cells. Phil. Trans. R. Soc. Lond. B 358, 59–85. ( 10.1098/rstb.2002.1183) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Amend JP, McCollom TM. 2009. Energetics of biomolecule synthesis on early earth. ACS Symp. Ser. 1025, 63–94. ( 10.1021/bk-2009-1025.ch004) [DOI] [Google Scholar]
- 38.Amend JP, LaRowe DE, McCollom TM, Shock EL. 2013. The energetics of organic synthesis inside and outside the cell. Phil. Trans. R. Soc. B 368, 20120255 ( 10.1098/rstb.2012.0255) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Amend JP, Shock EL. 1998. Energetics of amino acid synthesis in hydrothermal ecosystems. Science 281, 1659–1662. ( 10.1126/science.281.5383.1659) [DOI] [PubMed] [Google Scholar]
- 40.Russell MJ, et al. 2014. The drive to life on wet and icy worlds. Astrobiology 14, 308–343. ( 10.1089/ast.2013.1110) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nitschke W, Russell MJ. 2009. Hydrothermal focusing of chemical and chemiosmotic energy, supported by delivery of catalytic Fe, Ni, Mo/W, Co, S and Se, forced life to emerge. J. Mol. Evol. 69, 481–496. ( 10.1007/s00239-009-9289-3) [DOI] [PubMed] [Google Scholar]
- 42.West T, Sojo V, Pomiankowski A, Lane N. 2017. The origin of heredity in protocells. Phil. Trans. R. Soc. B 372, 20160419 ( 10.1098/rstb.2016.0419) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bonfio C, et al. 2017. UV-light-driven prebiotic synthesis of iron–sulfur clusters. Nat. Chem. 9, 1229–1234. ( 10.1038/nchem.2817) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bonomi F, Werth MT, Kurtz DMJ. 1985. Assembly of [FeS(SR)4]2− (n = 2, 4) in aqueous media from iron salts, thiols, and sulfur, sulfide, or thiosulfate plus rhodanese. Inorg. Chem. 2, 4331–4335. ( 10.1021/ic00219a026) [DOI] [Google Scholar]
- 45.Hedderich R, Forzi L. 2006. Energy-converting [NiFe] hydrogenases: more than just H2 activation. J. Mol. Microbiol. Biotechnol. 10, 92–104. ( 10.1159/000091557) [DOI] [PubMed] [Google Scholar]
- 46.Jordan S, Ioannou I, Vasiliadou R, Lane N. In preparation Formation of strongly reducing iron-sulfur clusters with low concentrations of single amino acids.
- 47.Thauer RK, Kaster A-K, Goenrich M, Schick M, Hiromoto T, Shima S. 2010. Hydrogenases from methanogenic archaea, nickel, a novel cofactor, and H2 storage. Annu. Rev. Biochem. 79, 507–536. ( 10.1146/annurev.biochem.030508.152103) [DOI] [PubMed] [Google Scholar]
- 48.Hedderich R. 2004. Energy-converting [NiFe] hydrogenases from archaea and extremophiles: ancestors of complex I. J. Bioenerg. Biomembr. 36, 65–75. ( 10.1023/B:JOBB.0000019599.43969.33) [DOI] [PubMed] [Google Scholar]
- 49.Kurkin S, Meuer J, Koch J, Hedderich R, Albracht SPJ. 2002. The membrane-bound [NiFe]-hydrogenase (Ech) from Methanosarcina barkeri: unusual properties of the iron–sulphur clusters. Eur. J. Biochem. 269, 6101–6111. ( 10.1046/j.1432-1033.2002.03328.x) [DOI] [PubMed] [Google Scholar]
- 50.Forzi L, Koch J, Guss AM, Radosevich CG, Metcalf WW, Hedderich R. 2005. Assignment of the [4Fe–4S] clusters of Ech hydrogenase from Methanosarcina barkeri to individual subunits via the characterization of site-directed mutants. FEBS J. 272, 4741–4753. ( 10.1111/j.1742-4658.2005.04889.x) [DOI] [PubMed] [Google Scholar]
- 51.Yu H, Wu C, Schut GJ, Haja DK, Zhao G, Peters JW, Adams MWW, Li H. 2018. Structure of an ancient respiratory system. Cell 173, 1–14. ( 10.1016/j.cell.2018.03.071) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sojo V, Pomiankowski A, Lane N. 2014. A bioenergetic basis for membrane divergence in archaea and bacteria. PLoS Biol. 12, e1001926 ( 10.1371/journal.pbio.1001926) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lane N. 2017. Proton gradients at the origin of life. Bioessays 39, 1600217 ( 10.1002/bies.201600217) [DOI] [PubMed] [Google Scholar]
- 54.Milner-White EJ, Russell MJ. 2005. Sites for phosphates and iron–sulfur thiolates in the first membranes: 3 to 6 residue anion-binding motifs (nests). Orig. Life Evol. Biosph. 35, 19–27. ( 10.1007/s11084-005-4582-7) [DOI] [PubMed] [Google Scholar]
- 55.Eck RV, Dayhoff MO. 1966. Evolution of the structure of ferredoxin based on living relics of primitive amino acid sequences. Science 152, 363–366. ( 10.1126/science.152.3720.363) [DOI] [PubMed] [Google Scholar]
- 56.Russell MJ, Hall AJ. 1997. The emergence of life from iron monosulphide bubbles at a submarine hydrothermal redox and pH front. J. Geol. Soc. Lond. 154, 377–402. ( 10.1144/gsjgs.154.3.0377) [DOI] [PubMed] [Google Scholar]
- 57.Martin W, Baross J, Kelley D, Russell MJ. 2008. Hydrothermal vents and the origin of life. Nat. Rev. Microbiol. 6, 805–814. ( 10.1038/nrmicro1991) [DOI] [PubMed] [Google Scholar]
- 58.Lane N, Martin WF. 2012. The origin of membrane bioenergetics. Cell 151, 1406–1416. ( 10.1016/j.cell.2012.11.050) [DOI] [PubMed] [Google Scholar]
- 59.Lane N, Allen JF, Martin W. 2010. How did LUCA make a living? Chemiosmosis in the origin of life. Bioessays 32, 271–280. ( 10.1002/bies.200900131) [DOI] [PubMed] [Google Scholar]
- 60.Jackson JB. 2016. Natural pH gradients in hydrothermal alkali vents were unlikely to have played a role in the origin of life. J. Mol. Evol. 83, 1–11. ( 10.1007/s00239-016-9756-6) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lane N. 2014. Bioenergetic constraints on the evolution of complex life. Cold Spring Harb. Perspect. Biol. 6, a015982 ( 10.1101/cshperspect.a015982) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Herschy B, Whicher A, Camprubi E, Watson C, Dartnell L, Ward J, Evans JRG, Lane N. 2014. An origin-of-life reactor to simulate alkaline hydrothermal vents. J. Mol. Evol. 79, 213–227. ( 10.1007/s00239-014-9658-4) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Martin WF. 2019. Carbon–metal bonds: rare and primordial in metabolism. Trends Biochem. Sci. 1577, 1–12. ( 10.1016/j.tibs.2019.04.010) [DOI] [PubMed] [Google Scholar]
- 64.Nitschke W, Russell MJ. 2011. Redox bifurcations: mechanisms and importance to life now, and at its origin. Bioessays 34, 106–109. ( 10.1002/bies.201100134) [DOI] [PubMed] [Google Scholar]
- 65.Barber MJ, Siegel LM. 1982. Oxidation–reduction potentials of molybdenum, flavin, and iron–sulfur centers in milk xanthine oxidase: variation with pH. Biochemistry 21, 1638–1647. ( 10.1021/bi00536a026) [DOI] [PubMed] [Google Scholar]
- 66.Nicholls DG, Ferguson SJ. 2002. Bioenergetics 3. London, UK: Academic Press. [Google Scholar]
- 67.Arndt NT, Nisbet EG. 2012. Processes on the young earth and the habitats of early life. Annu. Rev. Earth Planet. Sci. 40, 521–549. ( 10.1146/annurev-earth-042711-105316) [DOI] [Google Scholar]
- 68.Russell MJ, Arndt NT. 2005. Geodynamic and metabolic cycles in the Hadean. Biogeosciences 2, 97–111. ( 10.5194/bg-2-97-2005) [DOI] [Google Scholar]
- 69.Sleep NH. 2018. Geological and geochemical constraints on the origin and evolution of life. Astrobiology 18, 1199–1219. ( 10.1089/ast.2017.1778) [DOI] [PubMed] [Google Scholar]
- 70.Sleep NH. 2010. The Hadean–Archaean environment. Cold Spring Harb. Perspect. Biol. 2, a002527 ( 10.1101/cshperspect.a002527) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Russell MJ, Daniel RM, Hall AJ, Sherringham J. 1994. A hydrothermally precipitated catalytic iron sulphide membrane as a first step toward life. J. Mol. Evol. 39, 231–243. ( 10.1007/BF00160147) [DOI] [Google Scholar]
- 72.Mielke RE, Russell MJ, Wilson PR, McGlynn SE, Coleman M, Kidd R, Kanik I. 2010. Design, fabrication, and test of a hydrothermal reactor for origin-of-life experiments. Astrobiology 10, 799–810. ( 10.1089/ast.2009.0456) [DOI] [PubMed] [Google Scholar]
- 73.Barge LM, et al. 2014. Pyrophosphate synthesis in iron mineral films and membranes simulating prebiotic submarine hydrothermal precipitates. Geochim. Cosmochim. Acta 128, 1–12. ( 10.1016/j.gca.2013.12.006) [DOI] [Google Scholar]
- 74.Lane N. 2018. Hot mitochondria? PLoS Biol. 16, e2005113 ( 10.1371/journal.pbio.2005113) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Moller FM, Kriegel F, Kieß M, Sojo V, Braun D. 2017. Steep pH gradients and directed colloid transport in a microfluidic alkaline hydrothermal pore. Angew. Chem. Int. Ed. Engl. 56, 2340–2344. ( 10.1002/anie.201610781) [DOI] [PubMed] [Google Scholar]
- 76.Sojo V, Ohno A, Mcglynn SE, Yamada YMA, Nakamura R. 2019. Microfluidic reactors for carbon fixation under alkaline-hydrothermal-vent conditions. Life 9, 16 ( 10.3390/life9010016) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Wächtershäuser G. 2016. In praise of error. J. Mol. Evol. 82, 75–80. ( 10.1007/s00239-015-9727-3) [DOI] [PubMed] [Google Scholar]
- 78.Ludwig KA, Kelley DS, Butterfield DA, Nelson BK, Fru-Green G. 2006. Formation and evolution of carbonate chimneys at the Lost City Hydrothermal Field. Geochim. Cosmochim. Acta 70, 3625–3645. ( 10.1016/j.gca.2006.04.016) [DOI] [Google Scholar]
- 79.Baaske P, Weinert FM, Duhr S, Lemke KH, Russell MJ, Braun D. 2007. Extreme accumulation of nucleotides in simulated hydrothermal pore systems. Proc. Natl Acad. Sci. USA 104, 9346–9351. ( 10.1073/pnas.0609592104) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kreysing M, Keil L, Lanzmich S, Braun D. 2015. Heat flux across an open pore enables the continuous replication and selection of oligonucleotides towards increasing length. Nat. Chem. 7, 203–208. ( 10.1038/nchem.2155) [DOI] [PubMed] [Google Scholar]
- 81.Wolthers M, Charlet L, van der Linde PR, Rickard D, van der Weijden CH.. 2005. Surface chemistry of disordered mackinawite (FeS). Geochim. Cosmochim. Acta 69, 3469–3481. ( 10.1016/j.gca.2005.01.027) [DOI] [Google Scholar]
- 82.Wolthers M, van der Gaast SJ, Rickard D.. 2003. The structure of disordered mackinawite. Am. Mineral. 88, 2007–2015. ( 10.2138/am-2003-11-1245) [DOI] [Google Scholar]
- 83.Nakamura R, Takashima T, Kato S, Takai K, Yamamoto M, Hashimoto K. 2010. Electrical current generation across a black smoker chimney. Angew. Chem. Int. Ed. Engl. 49, 7692–7694. ( 10.1002/anie.201003311) [DOI] [PubMed] [Google Scholar]
- 84.Barge LM, Doloboff IJ, White LM, Stucky GD, Russell MJ, Kanik I. 2012. Characterization of iron-phosphate-silicate chemical garden structures. Langmuir 28, 3714–3721. ( 10.1021/la203727g) [DOI] [PubMed] [Google Scholar]
- 85.Maurer S. 2017. The impact of salts on single chain amphiphile membranes and implications for the location of the origin of life. Life 7, 44 ( 10.3390/life7040044) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Maurer SE, Sørensen KT, Iqbal Z, Nicholas J, Quirion K, Gioia M, Monnard P, Hanczyc MM. 2018. Vesicle self-assembly of monoalkyl amphiphiles under the effects of high ionic strength, extreme pH, and high temperature environments. Langmuir 34, 15 560–15 568. ( 10.1021/acs.langmuir.8b02830) [DOI] [PubMed] [Google Scholar]
- 87.Hargreaves WR, Deamer DW. 1978. Liposomes from ionic, single-chain amphiphiles. Biochemistry 17, 3759–3768. ( 10.1021/bi00611a014) [DOI] [PubMed] [Google Scholar]
- 88.Lee SH, Rasaiah JC. 2011. Proton transfer and mobilities of the H+ and OH– ions from studies of a dissociating model for water. J. Phys. Chem. 135, 124505 ( 10.1063/1.3632990) [DOI] [PubMed] [Google Scholar]
- 89.Russell MJ. 2018. Green rust: the simple organizing ‘seed’ of all life? Life 8, 35 ( 10.3390/life8030035) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Cukierman S. 2006. Et tu, Grotthuss! and other unfinished stories. Biochim. Biophys. Acta 1757, 876–885. ( 10.1016/j.bbabio.2005.12.001) [DOI] [PubMed] [Google Scholar]
- 91.Agmon N. 1995. The Grotthuss mechanism. Chem. Phys. Lett. 244, 456–462. ( 10.1016/0009-2614(95)00905-J) [DOI] [Google Scholar]
- 92.Jordan SF, Rammu H, Zheludev IV, Hartley AM, Maréchal A, Lane N. In press Promotion of protocell self-assembly from mixed amphiphiles at the origin of life. Nat. Ecol. Evol. [DOI] [PubMed] [Google Scholar]
- 93.Jordan SF, Nee E, Lane N. 2019. Isoprenoids enhance the stability of fatty acid membranes at the emergence of life potentially leading to an early lipid divide. Interface Focus 9, 20190067 ( 10.1098/rsfs.2019.0067) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Dzade NY, Roldan A, de Leeuw NH.. 2015. Activation and dissociation of CO2 on the (001), (011), and (111) surfaces of mackinawite (FeS): a dispersion-corrected DFT study. J. Chem. Phys. 143, 094703 ( 10.1063/1.4929470) [DOI] [PubMed] [Google Scholar]
- 95.Whicher AL.2016. Simulated alkaline hydrothermal vent environments to investigate prebiotic metabolism at the origin of life. Doctoral thesis, UCL (University College London). http://discovery.ucl.ac.uk/1515740/ .
- 96.Kelley DS, et al. 2001. An off-axis hydrothermal vent field near the Mid-Atlantic Ridge at 30° N. Nature 412, 145–149. ( 10.1038/35084000) [DOI] [PubMed] [Google Scholar]
- 97.Kelley DS, et al. 2005. A serpentinite-hosted ecosystem: the lost city hydrothermal field. Science 307, 1428–1434. ( 10.1126/science.1102556) [DOI] [PubMed] [Google Scholar]
- 98.Klein F, Bach W. 2009. Fe–Ni–Co–O–S phase relations in peridotite–seawater interactions. J. Petrol. 50, 37–59. ( 10.1093/petrology/egn071) [DOI] [Google Scholar]
- 99.Sousa FL, Preiner M, Martin WF. 2018. Native metals, electron bifurcation, and CO2 reduction in early biochemical evolution. Curr. Opin. Microbiol. 43, 77–83. ( 10.1016/j.mib.2017.12.010) [DOI] [PubMed] [Google Scholar]
- 100.Sleep NH, Meibom A, Fridriksson T, Coleman RG, Bird DK. 2004. H2-rich fluids from serpentinization: geochemical and biotic implications. Proc. Natl Acad. Sci. USA 101, 12 818–12 823. ( 10.1073/pnas.0405289101) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Whicher A, Camprubi E, Pinna S, Herschy B, Lane N. 2018. Acetyl phosphate as a primordial energy currency at the origin of life. Orig. Life Evol. Biosph. 48, 159–179. ( 10.1007/s11084-018-9555-8) [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Additional data on the design, fabrication and operation of the microfluidic reactor are available from the authors.