Abstract
If life on Earth started out in geochemical environments like hydrothermal vents, then it started out from gasses like CO2, N2 and H2. Anaerobic autotrophs still live from these gasses today, and they still inhabit the Earth's crust. In the search for connections between abiotic processes in ancient geological systems and biotic processes in biological systems, it becomes evident that chemical activation (catalysis) of these gasses and a constant source of energy are key. The H2–CO2 redox reaction provides a constant source of energy and anabolic inputs, because the equilibrium lies on the side of reduced carbon compounds. Identifying geochemical catalysts that activate these gasses en route to nitrogenous organic compounds and small autocatalytic networks will be an important step towards understanding prebiotic chemistry that operates only on the basis of chemical energy, without input from solar radiation. So, if life arose in the dark depths of hydrothermal vents, then understanding reactions and catalysts that operate under such conditions is crucial for understanding origins.
Keywords: hydrothermal vents, catalysis, activation, energy, origin of life, prebiotic chemistry
1. Introduction
When the Earth was formed 4.5 billion years ago, it was formed without life, we can safely presume. If there was any life on the freshly accreted Earth, it was destroyed at the moon forming impact, which converted the Earth into a ball of boiling magma [1]. By about 3.95 billion years ago, there was life on Earth [2]. The question of how it arose is of substantial interest. Hydrothermal vents play an important role in the question of life's origin, because they were present on the early Earth [3–7] and because they harbour continuously far-from-equilibrium conditions in an environment where H2 and CO2 interact in such a way as to generate reduced carbon compounds [8–15]. In the discussion of possible sites for life's origin, hydrothermal vents are unique by that criterion: hydrothermal vents harbour far from equilibrium conditions over geological timescales, and the approach towards equilibrium releases energy in the synthesis of reduced carbon compounds. This sets hydrothermal vents apart from all other physico-chemical settings [16]. Moreover, the release of free energy and the synthesis of reduced carbon compounds at vents are united in a common reaction sequence that operates in the laboratory without enzymes [15] and that is simultaneously the core of carbon and energy metabolism in real bacteria and archaea—acetogens and methanogens. Vents are unique among settings for the origin of metabolism (as opposed to the origin of life), because no other site for life's origin harbours chemical reactions that resemble real microbial carbon and energy metabolism.
The far-from-equilibrium conditions at alkaline hydrothermal vents entail steep redox gradients owing to a constant flux of H2-rich effluent over geological timescales [17]. The main redox reaction they harbour is the H2–CO2 system, in which the equilibrium lies far on the side of organic compounds [18], such that the reaction can proceed spontaneously as long as suitable catalysts are available and strictly reducing conditions are maintained [10,15,19,20]. In the presence of activated nitrogen species, hydrothermal vents can synthesize the building blocks of life [12,13]. Because of their abundance of chemical energy, and despite the absence of light, modern alkaline hydrothermal vents are teeming with microbial life [21,22], life that is ultimately fuelled by the reaction of H2 with CO2.
The H2–CO2 redox reaction is an attractive source of energy for the first chemical reactions en route to life, because it provides direct links between a known geochemical process (serpentinization) and known biochemical processes. These are most notably the reactions of core carbon and energy metabolism in acetogens and methanogens, anaerobic autotrophs that live from the reduction of CO2 with H2. Acetogenesis and methanogenesis represent the most primordial forms of metabolism in bacteria and archaea [23,24], rooting life's chemistry to reactions of gasses, rocks and water.
The continuity between exergonic geochemical and biochemical reactions can be seen as a virtue of hydrothermal origin theories, because it generates concrete mechanistic links between processes catalysed by minerals in the Earth's crust (exergonic CO2 reduction) [25] and processes catalysed by enzymes in the metabolism of prokaryotic lineages [26]. At hydrothermal vents, life as we know it connects to geochemistry as we know it.
2. Activation of CO2 and H2: the door to CO2 fixation
In biology, acetogens and methanogens fix CO2 via the H2-dependent reduction of CO2 to a methyl group and CO, followed by condensation of the methyl moiety and CO to a nickel bound acetyl group that is thiolytically cleaved from nickel to generate the thioester acetyl-CoA. The acetyl-CoA pathway is unique in microbial physiology, because it is carbon and energy metabolism in one. Carbon metabolism involves the H2-dependent reduction of CO2 to acetyl-CoA. Under standard physiological conditions, the synthesis of the thioester is exergonic by about –59 kJ mol−1 [27], while there is not enough energy to generate thioesters and synthesize ATP via substrate level phosphorylation [28]. Thus, for energy metabolism, acetogens that lack cytochromes and quinones couple methyl synthesis to the generation of ion gradients via electron bifurcation and ferredoxin oxidation at the membrane-bound Rnf complex [29], while methanogens that lack cytochromes generate their ion gradient by coupling the transfer of the methyl group from a nitrogen atom in methyl-tetrahydromethanopterin to a sulfur atom in coenzyme M [30].
If the acetyl-CoA pathway is the most ancient carbon fixation pathway, and various lines of evidence indicate that to be the case [14,15,23,24,27,31], there are still some dots that need to be connected. For H2 to have played a role in early chemical evolution, it required activation—it required catalysis. It is noteworthy that H2 never interacts directly with any organic oxidant (substrate) in metabolism, it always releases electrons into metabolism via a catalyst: hydrogenase. There are only three classes of hydrogenases known. All three harbour Fe atoms at their active site [32,33], all three harbour carbon metal bonds at their active site [26]. The central enzyme of the acetyl-CoA pathway, the only exergonic CO2 fixation pathway known [34,35], is bifunctional carbon monoxide dehydrogenase/acetyl-CoA synthase (CODH/ACS), which also harbours carbon metal bonds. These two activities, hydrogenase and CODH/ACS, trace to the last universal ancestor, LUCA [26]. Organisms that use the acetyl-CoA pathway employ flavin-based electron bifurcation to generate ferredoxins with a lower reducing potential than H2 [36–38]. Flavin-based electron bifurcation thus accounts for the thermodynamics of H2 oxidation, but what about the kinetics? In kinetically controlled reactions, catalysts can have an important influence on the nature of the products that accumulate—and the same is true for geochemical CO2 fixation with H2.
The H2-dependent reaction from the most oxidized form of carbon, CO2, to its most reduced form, methane (CH4), is thermodynamically favourable under reducing conditions. However, in serpentinizing, alkaline hydrothermal systems [39] the direct transfer of electrons from H2 to CO2 has a large activation energy and requires either high temperatures and high pressures [40] or, at milder conditions, chemical activation and catalysis [41,42]. The requirement for catalysis stems from kinetic barriers in the sequence of reactions from CO2 to CH4. Catalysts decrease the activation energy and thus the kinetic barrier, allowing intermediate products such as formate, acetate, methanol and pyruvate to accumulate after a short time under mild conditions [15] rather than the thermodynamically favoured end product CH4. While high temperatures, high pressures and long reaction times lead to the accumulation of CH4, the most stable product [40,43], catalysts influence the product distribution in the short term. In biology, enzymes effect such shifts from thermodynamically controlled reactions to kinetically controlled reactions [44]. In purely geological settings, however, heterogeneous catalysis can occur on mineral surfaces (figure 1)—which are not unlike the catalysts used in industry to produce hydrocarbons [15,25]. The activation of molecules on mineral surfaces is likely to have preceded the chemical activation that enzymes provide in modern organisms [46,47].
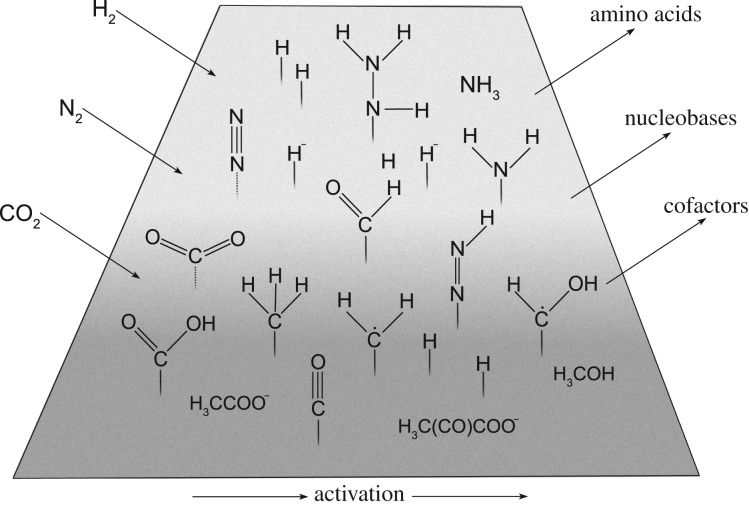
Figure 1.
Simultaneous activation of H2, CO2 and N2 on mineral surfaces leading to the formation of a variety of biologically relevant molecules, such as amino acids, nucleic acid bases and cofactors. Molecules, such as pyruvate, acetate, methanol and ammonia, are known to form on transition metal containing surfaces [15,45]. Little is known about the products obtained when the separation of N and C fixation is revoked. Heterogeneous catalysis may have been the key for early processes of protometabolism. Dashed lines indicate physisorption, non-dashed lines indicate chemisorption on the surface.
3. Adding nitrogen
In order to synthesize amino acids and nucleic acid bases, living cells have to incorporate dinitrogen (N2) into biosynthetic pathways. From a chemical point of view, N2 as a starting material is not the easiest choice in comparison to more oxidized or reduced nitrogen compounds [48]. Nevertheless, looking at early Earth's conditions, an atmosphere filled with N2 would have led to an ocean with dissolved N2 and thus—via sequestration through the Earth's crust—to a nitrogen source in serpentinizing systems [49,50]. Looking at biology, N2 fixation is considered ancient [50,51]. There is only one way for N2 to enter metabolism: via the nitrogenase complex. Nitrogenase consists of two proteins, dinitrogenase reductase, which contains an FeS-based active centre and the dinitrogenase protein, harbouring an Mo (or V, or Fe) containing Fe7S9 centre with a carbide carbon at the active site [52,53]. Mechanistically, the complex works with dinitrogenase reductase harvesting the energy of ATP hydrolysis and transferring it via conformational changes to dinitrogenase, which then binds the N2 molecule [53,54]. The following steps involve sequential hydrogenations of the nitrogen molecule. There, as for CO2 fixation, hydrogenase activity is needed to deliver electrons from H2 to N2. This hydrogenase activity is promoted by the FeS clusters of the nitrogenase complex [53,55], which is the sole entry point of N2 into metabolism. As CODH and hydrogenase, nitrogenase also traces back to LUCA [26,56].
Biology operates within constraints of temperature and pressure. Biological N2 reduction follows very different kinetics from those of the industrial process [57]. For both processes, inorganic catalysts have a central role in the reduction of N2. In industry, the reduction of N2 might resemble prebiotic FeS-based nitrogen fixation [45]. The greatest impediment to N2 reduction is its activation energy. N2 is very stable at normal atmospheric temperatures and pressures. Thus, few processes are capable of activating N2 sufficiently in order to form N-rich molecules. Industrial N2 conversion to NH3 via the Haber–Bosch process (H2-dependent) requires Fe-based catalysts such as Fe3O4, high pressure (200 bar) and temperatures exceeding 400°C [58]. The Haber–Bosch process currently consumes about 1–2% of the World's total energy production. Biological nitrogen fixation catalysed by the nitrogenase enzyme operates at ambient pressure and room temperature. Accordingly, there is immense commercial interest in the mechanism of biological N2 fixation [57].
Not unlike the stepwise use of Fe atoms found in the active sites of the nitrogenase complex, industrial N2 reduction is extremely dependent on the physico-chemical state of the catalysts. Thus, the yield of ammonia is affected as a result of several factors such as particle size, purity and subsurface dissociation of nitrogen into Fe catalysts, leading to iron nitrides such as FexN [59].
Can serpentinization reduce N2? Although there is abundant evidence for abiotic CO2 reduction in serpentinizing systems [60,61], evidence for abiotic N2 reduction is so far lacking. Laboratory simulations suggest that N2 can be reduced to ammonia (NH3) with mineral catalysts under mild hydrothermal conditions [45,62]. Incorporation of N from N2 into organic compounds under hydrothermal conditions presents a more substantial challenge for laboratory simulations. In principle, activated forms of nitrogen chemisorbed to geochemical catalysts (figure 1) might be better starting points for prebiotic synthesis of such compounds than NH3 [25], but this remains to be shown experimentally.
There are nevertheless very curious parallels between industrial hydrogenation processes and geochemical H2-dependent reactions. Serpentinization not only reduces H2O to H2 and CO2 to formate and CH4, it also generates inorganic catalysts within the Earth's crust [25]. These include magnetite, Fe3O4, which is the catalyst of choice for the industrial Haber–Bosch process (H2-dependent N2 reduction) and for Fischer–Tropsch (CO2 reduction) applications [59,63] and awaruite, Ni3Fe, which catalyses the H2-dependent reduction of CO2 to methane at high pressures and temperatures [40]. While H2 and CO2 deliver carbon and energy, for an autocatalytic network to emerge, one from which microbial metabolism could unfold, organic cofactors, bases and amino acids are required. All are nitrogenous compounds.
4. What if C, N and H are activated together?
As shown in figure 1, it is possible that mineral surfaces can activate H2, CO2 and N2 simultaneously. If so, amino acids or even bases and cofactors might be obtained via such routes. It has been reported that Fe2+ and Fe0 can catalyse reactions of 2-oxoacids with hydroxylamine to give aspartate, alanine, glycine and glutamine [64]. These should also be the first amino acids to appear in the evolution of metabolism, if metabolism evolved from a pyruvate-fed, incomplete citric acid cycle and if amino acids arose ancestrally as they do in metabolism, namely via reductive amination of the keto group in oxalacetate, pyruvate, glyoxylate and 2-oxoglutarate [9]. Pyruvate is new as a possible prebiotic compound [14]. Using hydrothermal iron minerals instead of enzymes, it is possible to synthesize pyruvate from H2 and CO2 [15]. Pyruvate now appears to be a much more readily synthesized prebiotic compound than previously assumed.
If N2 can be activated efficiently under hydrothermal conditions, nucleic acid bases might not be far away. Recent studies show that even aromatic heterocyclic compounds such as tryptophan can be formed abiotically in serpentinizing hydrothermal systems [13]. The connection of simpler amino acids like aspartate and glycine to bases is direct, they sit in the middle of the aromatic pyrimidine (aspartate and glycine) and purine (aspartate) rings. This is shown in figure 2, modified from reference [9]. In metabolism, pyrimidines are made from aspartate and carbamoyl phosphate. Carbamoyl phosphate is made from carbamate and ATP, carbamate forms spontaneously as a colourless precipitate in hot solutions containing CO2 (or carbonate) and ammonium. Four of the atoms in the pyrimidine ring come from aspartate. Purines are more complex, but the components are simple. Glycine comprises the centre of the rings, which are completed by inclusion of C1 units from formyl tetrahydrofolate [65] or from formyl phosphate (in methanogens) [66], by N from the amido group of glutamine, and, as with pyrimidines, by CO2 and N from aspartate.
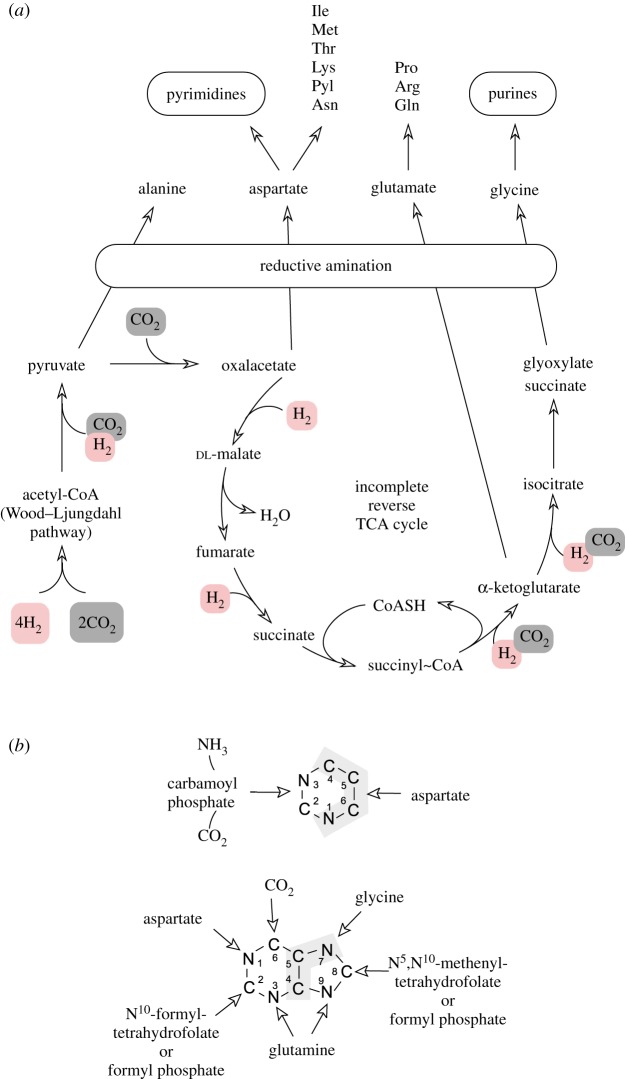
Figure 2.
A path from H2 and CO2 to nucleic acid bases (adapted from figs. 3, 4 and 6 of [9]. (a) The lower portion of the panel shows the biosynthesis of carbon backbones in microbes that use the acetyl-CoA pathway and the incomplete (horseshoe) reverse citric acid cycle. Reductive steps of CO2 fixation are indicated as H2-dependent, though reduced ferredoxin or NAD(P)H are the reductants in metabolism. The first four 2-oxoacids to arise via the route shown, and, if reductively aminated, generate Ala, Asp, Glu and Gly (upper portion of the panel). Muchowska et al. [64] showed that pyruvate, oxaloacetate, 2-oxoglutarate and glyoxylate are readily reduced to the corresponding amino acids by hydroxylamine under mild conditions in the presence of native iron. Asp is the starting point for biosynthesis of five other canonical amino acids and pyrrolysine, Glu is the starting point for synthesis of Gln, Arg and Pro. (b) Asp and Gly are central to pyrimidine and purine biosynthesis, respectively (modified from fig. 4 of [9]). The involvement of CO2 in purine and pyrimidine synthesis is noteworthy, as is the involvement of folate-bound C1 intermediates of the acetyl-CoA pathway in purine synthesis, which are replaced by the simpler intermediate formyl phosphate in methanogens. This suggests the possibility of a small prebiotic biochemical network linking CO2 reduction to nucleic acid base synthesis. (Online version in colour.)
There is a clear record of geochemical origins preserved in metabolism [26]. This record can be resurrected in the laboratory, if we find the right conditions. The four amino acids that Muchowska et al. [64] synthesize (Gly, Ala, Asp, Glu) even suggest (reveal, one might say) a connection to the evolution of the genetic code. These are the very same amino acids that are identified as ancient in different theories about the origin and evolution of the genetic code. In some theories, exactly these four (Gly, Ala, Asp, Glu) are the oldest [67]. In other theories, they are the most ancient as members of larger sets [68], while in yet other theories they rank well in order of antiquity, with Gly, Ala and Asp being the oldest, Glu coming in seventh [69]. A look at the biosynthetic families of amino acids reveals that the Asp and Glu families stand out as central.
5. Autocatalytic networks
If we assume that simultaneous activation of N2, H2 and CO2 can lead to thermodynamically stable products that include amino acids, nucleic acid bases and cofactors (that is currently a big assumption, we admit), then small chemical networks on a laboratory scale become possible. Central to various schools of thought on chemical origins are constructs called autocatalytic networks [70]. These can represent abstract mathematical constructs or they can describe interactions in real sets of molecules. As applied to molecular interactions, autocatalytic networks contain molecules that promote the synthesis of copies of themselves [71]. According to this very general definition, autocatalytic networks can provide theoretical frameworks for both the genetics first and the metabolism first approaches to prebiotic evolution. In the former, they can be sets of nucleic acids that ligate to form specific products [72], in the latter, they can be sets of metabolites that interact in such a way as to generate self-sustaining metabolic networks [24].
When describing molecular interactions, autocatalytic sets require input molecules in order to promote the synthesis of their constituent elements. This condition draws attention to a particular class of autocatalytic networks called reflexively autocatalytic food-generated networks—RAFs [73]—in which each reaction is catalysed by a molecule from within the network, and all molecules can be produced from a set of food molecules by the network itself. RAFs are particularly interesting in the context of early evolution, because they do not require a pre-existing catalyst for a reaction before it is required. The reaction can proceed uncatalysed, or rather catalysed by an unknown molecule, as long as the known catalyst is produced at some point by the network and assumes the role of catalysis in that reaction of the RAF. Moreover, when it comes to the concrete modelling of early evolution, the nature and source of the food molecules [74] that generate a given RAF or other autocatalytic set are of particular interest, because in order for the reactions in the set to take place, the overall thermodynamics of the network must be exergonic. In other words, in order for RAFs (or other autocatalytic networks) to serve as a useful model for early evolution, the set of reactants (educts) needs to release energy en route to the products (adducts), as is always the case in metabolism [18].
Of course, in cellular metabolism, the overall energetics are given by the sum of the changes in free energy for the core bioenergetic reactions [18]. For individual reactions of metabolism, the change in free energy from substrate to product is often endergonic, which is why such reactions are usually coupled to energy-releasing reactions involving exergonic electron transfer, ion gradients across the plasma membrane, or hydrolysis of high-energy bonds, such as ATP, acyl phosphates or thioesters [18,37]. Energetic coupling can also occur within RAFs, which makes them more interesting models of cellular metabolism.
It seems likely that at least a subset of the catalysts, high-energy bonds and energetic currencies that occur in modern metabolism were generally present and functional in prebiotic chemistry. Sources and transduction of modern metabolic catalysis and energy should then have analogues or homologues in geochemical settings. Regarding catalysis, there are now good indications that metals and simple organic cofactors could have promoted the emergence of cell-sized autocatalytic networks [15,64,75,76]. In physiology, the term energy metabolism generally means ATP synthesis. There are two sources of ATP in cells: chemiosmotic coupling and substrate level phosphorylation (SLP). Chemiosmotic coupling needs ion gradients as an energetic intermediate and proteins, without exception. SLP does not require ion gradients, its energy source is the Gibbs free energy of chemical reactions, and SLP reactions can take place without enzymes [77–79]. Although vents harbour natural ion gradients, ATP synthesis via chemiosmotic coupling always involves the ATPase, for which there is no known geochemical homologue or mechanistic analogue. The energy for SLP stems from the redox chemistry of carbon whereby both carbon oxidation to CO2 and H2-dependent CO2 reduction can be coupled to SLP [80]. Because the H2-dependent CO2 reducing reaction that drives SLP in acetogens (acetate synthesis) operates in the laboratory under simulated hydrothermal vent conditions with only metals and metal ions as catalysts [15], it is currently the only candidate for a primordial (geochemical) source of energy conservation (acyl phosphates via SLP) that is mechanistically linked to naturally occurring carbon redox reactions at vents.
A set of molecules that is generated by kinetically controlled reactions (the most rapidly formed products accumulate) will contain chemical energy that permits members of the set to interact further and to form an autocatalytic network that can serve as a basis for higher complexity [76]. Such a process is sketched in figure 3. The energetic input is necessarily centralized because thermodynamically stable metabolites and end products are synthesized from the core exergonic reaction, in our example the reduction of CO2 with H2 via the acetyl-CoA pathway [9,15,31].
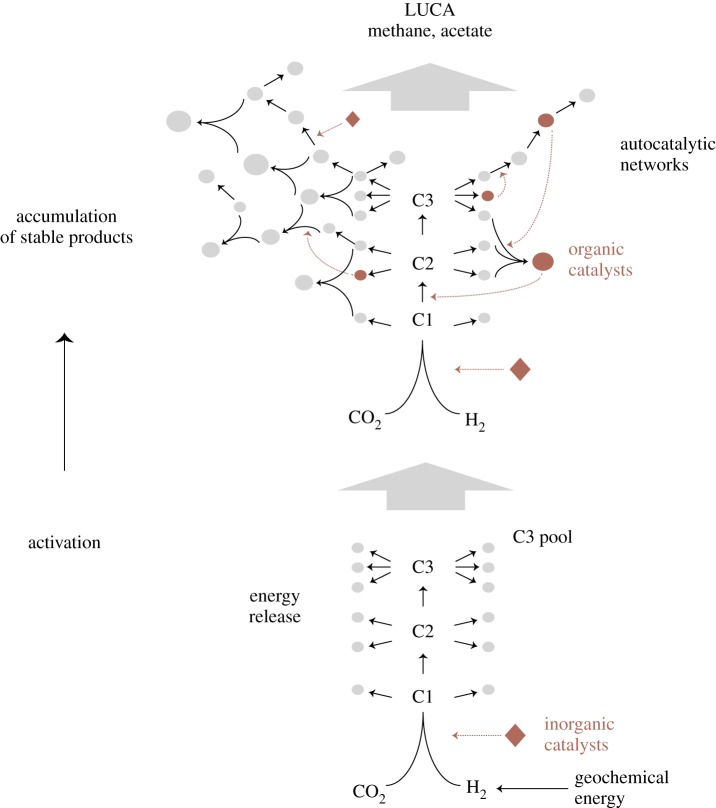
Figure 3.
Purely geochemical reactions such as CO2 fixation with H2 can give rise to autocatalytic networks and protometabolism, as long as energy is released. Kinetically controlled reactions build up a specific set of products which interact further to form an autocatalytic network that serves as a basis for higher complexity. C1, C2, C3 represent carbon compounds with 1, 2 or 3 carbon atoms such as formyl groups, acetyl groups, and pyruvate. (Online version in colour.)
6. Conclusion
Hydrothermal vents contain catalysts and chemical disequilibria that resemble life and metabolism in many ways. However, the natural chemical environment at vents does not strongly resemble metabolism in many forms of life, because metabolism is extremely diverse. Rather, it very specifically resembles the physiology of acetogens and methanogens, even down to the catalysts involved. The connections between the origin of microbial life and the chemical elements seem more tangible than ever before. Current genomic analyses indicate that the last universal common ancestor of all life, LUCA, lived from gasses: H2, CO2 and N2 [23,56]. Although our main focus is on these three gasses, it is evident that the incorporation of sulfur (S) and phosphorus (P) into early metabolism was also essential. Sulfur enters metabolism as HS– at cysteine synthesis from O-acetyl serine or O-phospho serine [81], while phosphorus enters metabolism via thioesters as acyl phosphates [9]. Under reducing conditions, H2S (HS– in alkaline vents) would be the likely sulfur source, phosphorus could enter the geochemical setting as phosphate dissolved in seawater or leached from the primordial crust, but data on phosphate under early Earth conditions is scarce [82–84]. Focusing on the enzymes that channel H2, CO2 and N2 into metabolism might uncover clues about the environment within which life arose and about the catalysts that activated these gasses at origins. The presence of carbon metal bonds in the active sites of hydrogenases, nitrogenase and carbon monoxide dehydrogenase suggest that these might be ancient relicts of the catalytic realm that led to the autocatalytic synthesis of the first organic compounds. We propose that the biology of methanogens and acetogens, anaerobic autotrophs that inhabit vents today, holds clues about the primordial catalysts that enzymes ultimately came to replace.
Acknowledgements
All authors thank the organizers of the 30/80 Meeting for the opportunity to submit to this special issue and Mike Russell for his inspirational work on the role of hydrothermal vents in the origin of life.
Data accessibility
This article has no additional data.
Authors' contributions
M.P. edited and revised the manuscript and contributed to the paragraphs on catalysis and activation of N2, CO2 and H2; J.C.X. edited and contributed to the paragraphs on autocatalysis and, together with W.F.M., drew figure 3; A.N.V. edited and contributed to paragraphs on nitrogen activation and fixation. K.K. and J.F.A. edited and advised on the content throughout the manuscript; K.K., W.F.M. and M.P. discussed and drew figure 1. W.F.M. wrote the first draft of the manuscript and sketched figure 2.
Competing interests
We declare we have no competing interests.
Funding
This work was supported by the Deutsche Forschungsgemeinschaft (grant no. MA-1426/21-1); the European Research Council (grant no. 666053); and the Volkswagen Foundation (grant no. 93046).
References
- 1.Zahnle K, Arndt N, Cockell C, Halliday A, Nisbet E, Selsis F, Sleep NH. 2007. Emergence of a habitable planet. Space Sci. Rev. 129, 35–78. ( 10.1007/s11214-007-9225-z) [DOI] [Google Scholar]
- 2.Tashiro T, Ishida A, Hori M, Igisu M, Koike M, Méjean P, Takahata N, Sano Y, Komiya T. 2017. Early trace of life from 3.95 Ga sedimentary rocks in Labrador, Canada. Nature 549, 516–518. ( 10.1038/nature24019) [DOI] [PubMed] [Google Scholar]
- 3.Baross JA, Hoffman SE. 1985. Submarine hydrothermal vents and associated gradient environments as sites for the origin and evolution of life. Orig. Life Evol. Biosph. 15, 327–345. ( 10.1007/BF01808177) [DOI] [Google Scholar]
- 4.Russell MJ, Hall AJ. 1997. The emergence of life from iron monosulphide bubbles at a submarine hydrothermal redox and pH front. J. Geol. Soc. London. 154, 377–402. (doi:10.1.1.520.7821) [DOI] [PubMed] [Google Scholar]
- 5.Nisbet E, Sleep NH. 2001. The habitat and nature of early life. Nature 409, 1083–1091. ( 10.1038/35059210) [DOI] [PubMed] [Google Scholar]
- 6.Arndt NT, Nisbet EG. 2012. Processes on the young Earth and the habitats of early life. Annu. Rev. Earth Planet. Sci. 40, 521–549. ( 10.1146/annurev-earth-042711-105316) [DOI] [Google Scholar]
- 7.Sleep NH. 2018. Geological and geochemical constraints on the origin and evolution of life. Astrobiology 18, 1199–1219. ( 10.1089/ast.2017.1778) [DOI] [PubMed] [Google Scholar]
- 8.McCollom TM, Seewald JS. 2006. Carbon isotope composition of organic compounds produced by abiotic synthesis under hydrothermal conditions. Earth Planet. Sci. Lett. 243, 74–84. ( 10.1016/j.epsl.2006.01.027) [DOI] [Google Scholar]
- 9.Martin W, Russell MJ. 2007. On the origin of biochemistry at an alkaline hydrothermal vent. Phil. Trans. R. Soc. B 362, 1887–1925. ( 10.1098/rstb.2006.1881) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McDermott JM, Seewald JS, German CR, Sylva SP. 2015. Pathways for abiotic organic synthesis at submarine hydrothermal fields. Proc. Natl Acad. Sci. USA 112, 7668–7672. ( 10.1073/pnas.1506295112) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Camprubi E, Jordan SF, Vasiliadou R, Lane N. 2017. Iron catalysis at the origin of life. IUBMB Life 69, 373–381. ( 10.1002/iub.1632) [DOI] [PubMed] [Google Scholar]
- 12.Baross JA. 2018. The rocky road to biomolecules. Nature 564, 42–43. ( 10.1038/d41586-018-07262-8) [DOI] [PubMed] [Google Scholar]
- 13.Ménez B, Pisapia C, Andreani M, Jamme F, Vanbellingen QP, Brunelle A, Richard L, Dumas P, Réfrégiers M. 2018. Abiotic synthesis of amino acids in the recesses of the oceanic lithosphere. Nature 564, 59–63. ( 10.1038/s41586-018-0684-z) [DOI] [PubMed] [Google Scholar]
- 14.Varma SJ, Muchowska KB, Chatelain P, Moran J. 2018. Native iron reduces CO2 to intermediates and endproducts of the acetyl-CoA pathway. Nat. Ecol. Evol. 2, 1019–1024. ( 10.1038/s41559-018-0542-2) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Preiner M, et al. 2019. A hydrogen dependent geochemical analogue of primordial carbon and energy metabolism. BioRxiv (doi:10.1101/682955)
- 16.Martin W, Baross J, Kelley D, Russell MJ. 2008. Hydrothermal vents and the origin of life. Nat. Rev. Microbiol. 6, 805–814. ( 10.1038/nrmicro1991) [DOI] [PubMed] [Google Scholar]
- 17.Baross JA, Martin WF. 2015. The ribofilm as a concept for life's origins. Cell 162, 13–15. ( 10.1016/j.cell.2015.06.038) [DOI] [PubMed] [Google Scholar]
- 18.Thauer RK, Jungermann K, Decker K. 1977. Energy conservation in chemotropic anaerobic bacteria. Bacteriol. Rev. 41, 100–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McCollom TM, Amend JP. 2005. A thermodynamic assessment of energy requirements for biomass synthesis by chemolithoautotrophic micro-organisms in oxic and anoxic environments. Geobiology 3, 135–144. ( 10.1111/j.1472-4669.2005.00045.x) [DOI] [Google Scholar]
- 20.Schrenk MO, Brazelton WJ, Lang SQ. 2013. Serpentinization, carbon, and deep life. Rev. Mineral. Geochemistry 75, 575–606. ( 10.2138/rmg.2013.75.18) [DOI] [Google Scholar]
- 21.Hickman-Lewis K, Cavalazzi B, Foucher F, Westall F. 2018. Most ancient evidence for life in the Barberton greenstone belt: microbial mats and biofabrics of the ∼3.47 Ga Middle Marker horizon. Precambrian Res. 312, 45–67. ( 10.1016/j.precamres.2018.04.007) [DOI] [Google Scholar]
- 22.Brazelton WJ, Schrenk MO, Kelley DS, Baross JA. 2006. Methane- and sulfur-metabolizing microbial communities dominate the Lost City Hydrothermal Field ecosystem. Appl. Environ. Microbiol. 72, 6257–6270. ( 10.1128/AEM.00574-06) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Weiss MC, Sousa FL, Mrnjavac N, Neukirchen S, Roettger M, Nelson-Sathi S, Martin WF. 2016. The physiology and habitat of the last universal common ancestor. Nat. Microbiol. 1, 16116 ( 10.1038/nmicrobiol.2016.116) [DOI] [PubMed] [Google Scholar]
- 24.Sousa FL, Martin WF. 2014. Biochemical fossils of the ancient transition from geoenergetics to bioenergetics in prokaryotic one carbon compound metabolism. Biochim. Biophys. Acta - Bioenerg. 1837, 964–981. ( 10.1016/j.bbabio.2014.02.001) [DOI] [PubMed] [Google Scholar]
- 25.Preiner M, et al. 2018. Serpentinization: connecting geochemistry, ancient metabolism and industrial hydrogenation. Life 8, 41 ( 10.3390/life8040041) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Martin WF. 2019. Carbon–metal bonds: rare and primordial in metabolism. Trends Biochem. Sci. 44, 807–818. ( 10.1016/j.tibs.2019.04.010) [DOI] [PubMed] [Google Scholar]
- 27.Fuchs G. 2011. Alternative pathways of carbon dioxide fixation: insights into the early evolution of life? Annu. Rev. Microbiol. 65, 631–658. ( 10.1146/annurev-micro-090110-102801) [DOI] [PubMed] [Google Scholar]
- 28.Martin WF. 2012. Hydrogen, metals, bifurcating electrons, and proton gradients: the early evolution of biological energy conservation. FEBS Lett. 586, 485–493. ( 10.1016/j.febslet.2011.09.031) [DOI] [PubMed] [Google Scholar]
- 29.Schuchmann K, Müller V. 2013. Direct and reversible hydrogenation of CO2 to formate by a bacterial carbon dioxide reductase. Science 342, 1382–1386. ( 10.1126/science.1244758) [DOI] [PubMed] [Google Scholar]
- 30.Thauer RK, Kaster AK, Seedorf H, Buckel W, Hedderich R. 2008. Methanogenic archaea: ecologically relevant differences in energy conservation. Nat. Rev. Microbiol. 6, 579–591. ( 10.1038/nrmicro1931) [DOI] [PubMed] [Google Scholar]
- 31.Goldford JE, Hartman H, Smith TF, Segrè D. 2017. Remnants of an ancient metabolism without phosphate. Cell 168, 1126–1134. ( 10.1016/j.cell.2017.02.001) [DOI] [PubMed] [Google Scholar]
- 32.Thauer RK, Kaster A-K, Goenrich M, Schick M, Hiromoto T, Shima S. 2010. Hydrogenases from methanogenic archaea, nickel, a novel cofactor, and H2 storage. Annu. Rev. Biochem. 79, 507–536. ( 10.1146/annurev.biochem.030508.152103) [DOI] [PubMed] [Google Scholar]
- 33.Thauer RK. 2011. Hydrogenases and the global H2 cycle. Eur. J. Inorg. Chem. 2011, 919–921. ( 10.1002/ejic.201001255) [DOI] [Google Scholar]
- 34.Berg IA. 2011. Ecological aspects of the distribution of different autotrophic CO2 fixation pathways. Appl. Environ. Microbiol. 77, 1925–1936. ( 10.1128/AEM.02473-10) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Xavier JC, Preiner M, Martin WF. 2018. Something special about CO-dependent CO2 fixation. FEBS Lett. 285, 4181–4195. ( 10.1111/febs.14664) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Buckel W, Thauer RK. 2013. Energy conservation via electron bifurcating ferredoxin reduction and proton/Na+ translocating ferredoxin oxidation. Biochim. Biophys. Acta - Bioenerg. 1827, 94–113. ( 10.1016/j.bbabio.2012.07.002) [DOI] [PubMed] [Google Scholar]
- 37.Schoelmerich MC, Müller V. 2019. Energy conservation by a hydrogenase-dependent chemiosmotic mechanism in an ancient metabolic pathway. Proc. Natl Acad. Sci. USA 116, 6329–6334. ( 10.1073/pnas.1818580116) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Peters JW, Miller AF, Jones AK, King PW, Adams MWW. 2016. Electron bifurcation. Curr. Opin. Chem. Biol. 31, 146–152. ( 10.1016/j.cbpa.2016.03.007) [DOI] [PubMed] [Google Scholar]
- 39.Klein F, Bach W. 2009. Fe-Ni-Co-O-S phase relations in peridotite–seawater interactions. J. Petrol. 50, 37–59. ( 10.1093/petrology/egn071) [DOI] [Google Scholar]
- 40.Horita J, Berndt ME. 1999. Abiogenic methane formation and isotopic fractionation under hydrothermal conditions. Sci. Rep. 285, 1055–1057. ( 10.1126/science.285.5430.1055) [DOI] [PubMed] [Google Scholar]
- 41.Maden BEH. 2000. Tetrahydrofolate and tetrahydromethanopterin compared: functionally distinct carriers in C1 metabolism. Biochem. J. 350, 609–629. ( 10.1042/0264-6021:3520935) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Appel AM, et al. 2013. Frontiers, opportunities, and challenges in biochemical and chemical catalysis of CO2 fixation. Chem. Rev. 113, 6621–6658. ( 10.1021/cr300463y) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.McCollom TM, Seewald JS. 2003. Experimental study of the hydrothermal reactivity of organic acids and acid anions: II. Acetic acid, acetate, and valeric acid. Geochim. Cosmochim. Acta 67, 3645–3664. ( 10.1016/S0016-7037(03)00135-2) [DOI] [Google Scholar]
- 44.Wolfenden R. 2011. Benchmark reaction rates, the stability of biological molecules in water, and the evolution of catalytic power in enzymes. Annu. Rev. Biochem. 80, 645–667. ( 10.1146/annurev-biochem-060409-093051) [DOI] [PubMed] [Google Scholar]
- 45.Dörr M, et al. 2003. A possible prebiotic formation of ammonia from dinitrogen on iron sulfide surfaces. Angew. Chemie Int. Ed. 42, 1540–1543. ( 10.1002/anie.200250371) [DOI] [PubMed] [Google Scholar]
- 46.Wächtershäuser G. 1988. Before enzymes and templates: theory of surface metabolism. Microbiol. Rev. 52, 452–484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Russell MJ, Hall AJ, Gize AP. 1990. Pyrite and the origin of life. Nature 344, 387 ( 10.1038/344387b0) [DOI] [Google Scholar]
- 48.Sousa FL, Thiergart T, Landan G, Nelson-Sathi S, Pereira IAC, Allen JF, Lane N, Martin WF. 2013. Early bioenergetic evolution. Phil. Trans. R. Soc. B 368, 20130088 ( 10.1098/rstb.2013.0088) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Voss M, Bange HW, Dippner JW, Middelburg JJ, Montoya JP, Ward B. 2013. The marine nitrogen cycle: recent discoveries, uncertainties and the potential relevance of climate change. Phil. Trans. R. Soc. B 368, 20130121 ( 10.1098/rstb.2013.0121) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Stüeken EE, Kipp MA, Koehler MC, Buick R. 2016. The evolution of Earth's biogeochemical nitrogen cycle. Earth-Sci. Rev. 160, 220–239. ( 10.1016/j.earscirev.2016.07.007) [DOI] [Google Scholar]
- 51.Nishizawa M, Miyazaki J, Makabe A, Koba K, Takai K. 2014. Physiological and isotopic characteristics of nitrogen fixation by hyperthermophilic methanogens: key insights into nitrogen anabolism of the microbial communities in Archean hydrothermal systems. Geochim. Cosmochim. Acta 138, 117–135. ( 10.1016/j.gca.2014.04.021) [DOI] [Google Scholar]
- 52.Hoffman BM, Lukoyanov D, Dean DR, Seefeldt LC. 2013. Nitrogenase: a draft mechanism. Acc. Chem. Res. 46, 587–595. ( 10.1021/ar300267m) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hoffman BM, Lukoyanov D, Yang Z-Y, Dean DR, Seefeldt LC. 2014. Mechanism of nitrogen fixation by nitrogenase: the next stage. Chem. Rev. 114, 4041–4062. ( 10.1021/cr400641x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Barney BM, Lee HI, Dos Santos PC, Hoffman BM, Dean DR, Seefeldt LC. 2006. Breaking the N2 triple bond: insights into the nitrogenase mechanism. Dalt. Trans. 21, 2277–2284. ( 10.1039/b517633f) [DOI] [PubMed] [Google Scholar]
- 55.Siegbahn PEM. 2019. The mechanism for nitrogenase including all steps. Phys. Chem. Chem. Phys. 21, 15 747–15 759. ( 10.1039/C9CP02073J) [DOI] [PubMed] [Google Scholar]
- 56.Weiss MC, Preiner M, Xavier JC, Zimorski V, Martin F. 2018. The last universal common ancestor between ancient Earth chemistry and the onset of genetics. PLoS Genet. 14, e1007518 ( 10.1371/journal.pgen.1007518) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Cherkasov N, Ibhadon AO, Fitzpatrick P. 2015. A review of the existing and alternative methods for greener nitrogen fixation. Chem. Eng. Process. Process Intensif. 90, 24–33. ( 10.1016/j.cep.2015.02.004) [DOI] [Google Scholar]
- 58.Leigh GJ. 2004. Haber–Bosch and other industrial processes. In Catalysts for nitrogen fixation: nitrogenases, relevant chemical models, and commercial processes (eds Smith BE, Richards RL, Newton WE), pp. 33–54. Dordrecht, The Netherlands: Kluwer Academic Publishers. [Google Scholar]
- 59.Kandemir T, Schuster ME, Senyshyn A, Behrens M, Schlögl R. 2013. The Haber–Bosch process revisited: on the real structure and stability of ‘ammonia iron’ under working conditions. Angew. Chemie - Int. Ed. 52, 12 723–12 726. ( 10.1002/anie.201305812) [DOI] [PubMed] [Google Scholar]
- 60.Etiope G, Ionescu AM. 2015. Low-temperature catalytic CO2 hydrogenation with geological quantities of ruthenium: a possible abiotic CH4 source in chromitite-rich serpentinized rocks. Geofluids 15, 438–452. ( 10.1111/gfl.1210) [DOI] [Google Scholar]
- 61.McCollom TM, Seewald JS. 2013. Serpentinites, hydrogen, and life. Elements 9, 129–134. ( 10.2113/gselements.9.2.129) [DOI] [Google Scholar]
- 62.Smirnov A, Hausner D, Laffers R, Strongin DR, Schoonen MAA. 2008. Abiotic ammonium formation in the presence of Ni–Fe metals and alloys and its implications for the Hadean nitrogen cycle. Geochem. Trans. 9, 5–20. ( 10.1186/1467-4866-9-5) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Porosoff MD, Yan B, Chen JG. 2016. Catalytic reduction of CO2 by H2 for synthesis of CO, methanol and hydrocarbons: challenges and opportunities. Energy Environ. Sci. 9, 62–73. ( 10.1039/c5ee02657a) [DOI] [Google Scholar]
- 64.Muchowska KB, Varma SJ, Moran J. 2019. Synthesis and breakdown of universal metabolic precursors promoted by iron. Nature 569, 104–107. ( 10.1038/s41586-019-1151-1) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Stryer L. 1975. Biochemistry. San Francisco, CA: Freeman. [Google Scholar]
- 66.Ownby K, Xu H, White RH. 2005. A Methanocaldococcus jannaschii archaeal signature gene encodes for a 5-formaminoimidazole-4-carboxamide-1-β-D-ribofuranosyl 5′- monophosphate synthetase: a new enzyme in purine biosynthesis. J. Biol. Chem. 280, 10 881–10 887. ( 10.1074/jbc.M413937200) [DOI] [PubMed] [Google Scholar]
- 67.Copley SD, Smith E, Morowitz HJ. 2007. The origin of the RNA world: co-evolution of genes and metabolism. Bioorg. Chem. 35, 430–443. ( 10.1016/j.bioorg.2007.08.001) [DOI] [PubMed] [Google Scholar]
- 68.Wong JTF. 2005. Coevolutlon theory of genetic code at age thirty. Bioessays 27, 416–425. ( 10.1002/bies.20208) [DOI] [PubMed] [Google Scholar]
- 69.Trifonov EN, Volkovich Z, Frenkel ZM. 2012. Multiple levels of meaning in DNA sequences, and one more. Ann. NY Acad. Sci. 1267, 35–38. ( 10.1111/j.1749-6632.2012.06589.x) [DOI] [PubMed] [Google Scholar]
- 70.Kauffman SA. 1986. Autocatalytic sets of proteins. J. Theor. Biol. 119, 1–24. ( 10.1016/S0022-5193(86)80047-9) [DOI] [PubMed] [Google Scholar]
- 71.Steel M, Hordijk W, Xavier JC. 2019. Autocatalytic networks in biology: structural theory and algorithms. J. R. Soc. Interface 16, 20180808 ( 10.1098/rsif.2018.0808) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Vaidya N, Lehman N. 2009. One RNA plays three roles to provide catalytic activity to a group I intron lacking an endogenous internal guide sequence. Nucleic Acids Res. 37, 3981–3989. ( 10.1093/nar/gkp271) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hordijk W, Steel M. 2004. Detecting autocatalytic, self-sustaining sets in chemical reaction systems. J. Theor. Biol. 227, 451–461. ( 10.1016/j.jtbi.2003.11.020) [DOI] [PubMed] [Google Scholar]
- 74.Schönheit P, Buckel W, Martin WF. 2016. On the origin of heterotrophy. Trends Microbiol. 24, 12–25. ( 10.1016/j.tim.2015.10.003) [DOI] [PubMed] [Google Scholar]
- 75.Sousa FL, Hordijk W, Steel M, Martin WF. 2015. Autocatalytic sets in E. coli metabolism. J. Syst. Chem. 6, 4 ( 10.1186/s13322-015-0009-7) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Xavier JC, Hordijk W, Kauffman SA, Steel M, Martin WF.2019. Autocatalytic chemical networks preceded proteins and RNA in evolution. BioRxiv (doi: 10.1101/693879)
- 77.Sousa FL, Preiner M, Martin WF. 2018. Native metals, electron bifurcation, and CO2 reduction in early biochemical evolution. Curr. Opin. Microbiol. 43, 77–83. ( 10.1016/j.mib.2017.12.010) [DOI] [PubMed] [Google Scholar]
- 78.Kitani A, Tsunetsugu S, Sasaki K. 1991. FeIII-ion-catalysed non-enzymatic transformation of ADP into ATP. J. Chem. Soc. Perkin Trans. 2 3, 329–331. ( 10.1039/P29910000329) [DOI] [Google Scholar]
- 79.Kitani A, Tsunetsugu S, Suzuki A, Ito S, Sasaki K. 1995. Fe(III)-ion-catalysed non-enzymatic transformation of adenosine diphosphate into adenosine triphosphate part II. Evidence of catalytic nature of Fe ions. Bioelectrochem. Bioenerg. 36, 47–51. ( 10.1016/0302-4598(94)01751-L) [DOI] [Google Scholar]
- 80.Martin WF, Thauer RK. 2017. Energy in ancient metabolism. Cell 168, 953–955. ( 10.1016/j.cell.2017.02.032) [DOI] [PubMed] [Google Scholar]
- 81.Liu Y, Beer LL, Whitman WB. 2012. Sulfur metabolism in archaea reveals novel processes. Environ. Microbiol. 14, 2632–2644. ( 10.1111/j.1462-2920.2012.02783.x) [DOI] [PubMed] [Google Scholar]
- 82.Kelley DS, Baross JA, Delaney JR. 2002. Volcanoes, fluids, and life at mid-ocean ridge spreading centers. Annu. Rev. Earth Planet. Sci. 30, 385–491. ( 10.1146/annurev.earth.30.091201.141331) [DOI] [Google Scholar]
- 83.Pasek MA. 2008. Rethinking early Earth phosphorus geochemistry. Proc. Natl Acad. Sci. USA 105, 853–858. ( 10.1073/pnas.0708205105) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Konhauser KO. 2007. Was there really an Archean phosphate crisis. Science 315, 1234 ( 10.1126/science.1136328) [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
This article has no additional data.