Abstract
Chondral defects are challenging to repair because of the poor self-healing capacity of articular cartilage. The aim of this study was to compare and investigate the cartilage regeneration of stromal vascular fraction (SVF) cells and adipose-derived stem cells (ASCs) co-cultured with chondrocytes seeding on scaffolds composed of polyhydroxybutyrate (PHB)/poly-(hydroxybutyrate-co-hydroxyhexanoate) (PHBHHx). In this study, the cellular morphologies and proliferation capabilities on scaffolds were evaluated. Next, scaffolds with 1:1 co-culture of ASCs/SVF and chondrocytes were implanted into the full-thickness cartilage defects in rabbit knee for 10 weeks. Cells seeded on the scaffolds showed better adhesion, migration, and proliferation in vitro. Importantly, implantation with scaffolds with SVF and chondrocytes revealed more desirable in vivo healing outcomes. Our results illustrate a one-step surgical procedure for the regeneration of focal cartilage defects using a mixture of SVF from adipose tissue and uncultured chondrocytes.
Keywords: chondrocytes, stromal vascular fraction, co-culture, cartilage repair
Introduction
Full-thickness articular cartilage defects are among the most common injuries in orthopedics and sports medicine. They may lead to the development of osteoarthritis, resulting in serious pain and movement limitations1. As regeneration capacity of cartilage tissue is very limited, articular cartilage repair remains a challenging. There are many traditional methods such as debridement, marrow stimulation, osteochondral grafting, and autologous chondrocyte implantation can be used to treat cartilage defects2–4; however. these methods all have weaknesses, including the limitations of donor-site availability and morbidity5.
Cartilage tissue engineering provides a new option for repairing articular cartilage defect. Several studies have applied culture-expanded bone marrow-derived mesenchymal stem cells (BM-MSCs) or adipose-derived stromal cells (ASCs) for cartilage repair. Although these treatments produce good short-term results, disadvantages exist: pain and donor-site morbidity during isolation of cells and the relatively low amount of BM-MSCs in bone marrow aspirates. Patients have to undergo two surgeries—autologous cells’ collection and re-implantation after cell expansion—and in vitro cell culture would increase the risk of microbiological contamination. During the isolation of ASCs, after digestion and centrifugation, stromal vascular fraction (SVF) is resuspended and then placed in culture flasks to isolate the plastic-adherent ASC subpopulation. It is reported that SVF cells and ASC exhibit different features and properties6,7. From the perspective of clinical application, SVF cells have great advantages over ASCs, because it is possible to obtain them during the operative procedure by processing in the operating theater and putting them back into the patient without laboratory expansion, which is required for the isolation of ASCs. As adipose tissue is an abundant source of stem cells, cell numbers required for re-implantation can easily be procured.
Poly(3-hydroxybutyrate) (PHB), a member of microbial biopolyesters polyhydroxyalkanoates (PHAs) family, is the most thoroughly investigated scaffold material for its good biocompatibility to cells8. However, its high brittleness and low degradation have limited its application9. Poly (3-hydroxybutyrate-co-3-hydroxyhexanoate) (PHBHHx) is a new member of the PHAs family; it has been reported to have much better elastomeric mechanical properties than PHB and PHBV and better biocompatibility with many cells10–16. A PHB/PHBHHx blend was reported to improve mechanical properties and biocompatibility compared with PHB or PHBHHx, and chondrocytes proliferated better on the PHB/PHBHHx scaffolds than on the PHB one13,17. PHBHHx in the composite system highly favored the production of extracellular matrix of articular cartilage chondrocytes18.
In this study, a cartilage repair procedure was performed to repair articular cartilage defects in a rabbit model, integrating the in situ autologous stem cells (instead of exogenous stem cells) with PHB/PHBHHx scaffolds, which provided an appropriate physical support and residence for cell growth. This study comprised in vitro and in vivo parts. Studies in vitro detected whether PHB/PHBHHx scaffolds could support adhesion, growth, and proliferation of cells. In the in vivo study, we have compared SVF and ASC as an alternative cell source for replacing part of the chondrocytes seeded in the scaffolds, and have evaluated cartilage repair after implantation.
This study suggested that SVF is a better source than ASC for a co-implantation strategy with primary chondrocytes in cartilage repair. Our results illustrate a one-step surgical procedure for the regeneration of focal cartilage defects using a mixture of SVF from adipose tissue and uncultured chondrocytes.
Materials and Methods
Preparation of PHB/PHBHHx Scaffolds
PHB and PHBHHx (1:2 ratio by weight, provided by Tsinghua University, Beijing, PRC) were mixed with sodium chloride (NaCl) and then dissolved in chloroform (Sigma, St Louis, MO, USA) at 60°C. After agitation, the NaCl/PHA solution was cast in a Petri dish and air-dried at 20°C for 24 h. The NaCl/PHA mixtures were then removed from the Petri dish and washed several times with distilled water to remove all traces of NaCl. The PHB/PHBHHx scaffolds were frozen at –70°C, lyophilized in a freeze dryer for 24 h, and stored in a dessicator prior to use. The PHB/PHBHHx scaffolds were then cut into circular tablets (5 mm in diameter and 3 mm thickness) and exposed to gamma radiation (25 kGy) for 1 h for sterilization before cell seeding.
Cell Culture and Expansion
New Zealand white rabbits (aged 4–6 months, weighing 3.5–4.0 kg) used for this study were approved by the Institutional Animal Care and Use Committee of Zhengzhou University, China. All operations were performed according to international guidelines concerning the care and treatment of experimental animals.
Rabbit chondrocytes were isolated from hind knees cartilage by sterile dissection. Briefly, cartilage was minced and washed with PBS, then digested in Dulbecco’s Modified Eagle Medium (DMEM, Hyclone, Thermo Scientific, Waltham, MA, USA) containing 0.15% type II collagenase (Sigma-Aldrich, St. Louis, MO, USA) and 10% fetal bovine serum (FBS, Sigma-Aldrich) in a 37°C shaking water bath overnight. The digested tissue was filtered and centrifuged, then cultured in chondrocyte proliferation medium (DMEM supplemented with 10% FBS, 50 mg/mL ascorbic acid, 100 U/mL penicillin, and 100 mg/mL streptomycin) in an atmosphere of 95% air -5% CO2 at 37°C and 95% relative humidity. When the attached cells had grown to 85–90% confluence, the cells were trypsinized and passaged.
SVF of rabbit adipose tissue was isolated from the dorsal fat pads. Briefly, the dorsal fat pads were minced and washed with PBS, then digested using 0.075% type I collagenase (Sigma-Aldrich) in a 37°C shaking water bath for 60 min. After neutralizing the collagenase, the digested tissue was filtered and centrifuged, then the resulting pelleted SVF was resuspended and seeded in culture flasks with MSC proliferation medium (aɑ-MEM supplemented with 10% FBS, 100 U/mL penicillin and 100 mg/mL streptomycin). Medium was refreshed every 3 days to get rid of nonattached cells. When the attached cells had grown to 85–90% confluence, the cells were trypsinized and passaged. The cells grown on tissue culture plastic were cultured to passage 2 before experimentation.
Expanded cells are called ASCs in this article.
Cell Adhesion, Proliferation, and Morphology on Scaffolds
Rabbit ASCs were seeded at the density of 105 per mL into the flasks and on glass coverslips into the 96-well plates, which were placed in the PHB/PHBHHx scaffolds at an appropriate initial density. A control group was established in which the ASCs were seeded at the density of 105 per mL into the 96-well plates without the scaffold. For each group, three parallel holes were set up. After 2 h, 4 h, and 8 h of cell culture in the medium, 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl-tetrazolium bromide (MTT) assay (Sigma-Aldrich) was carried out per the manufacturer’s instructions to quantify the number of viable cells to determine the extent of cell adhesion on the scaffold. The absorbance at 570 nm was measured using a Thermospectronic Genesis 10 UV/Visible spectrophotometer (Thermo Electron Corp, Fitchburg, WI, USA).
The number of viable cells was quantified on days 1, 3, 5, and 7 to determine the extent of cell proliferation and the cytotoxicity of the PHB/PHBHHx scaffolds by cell counting assay kit-8 (Dojindo, Kumamoto, Japan) per the manufacturer’s instructions. The absorbance at 450 nm was measured using a Model 550 micro-plate reader (Bio-RAD, Hercules, CA, USA).
At 1, 3, 5, and 7 days, cells/scaffolds constructs were washed with PBS and fixed in 2.5% glutaraldehyde in PBS overnight at 4°C. The constructs were then stained with 1% osmium tetroxide, dehydrated in graded concentrations of alcohol, freeze-dried for 8 h, and coated with gold. The samples were examined with a scanning electron microscope (SEM, JEOL JSM-6360LV, Tokyo, Japan) at an accelerating voltage of 20 kV. The morphological structure of the PHB/PHBHHx scaffolds was also observed by SEM.
Full-thickness Articular Cartilage Defect Repair
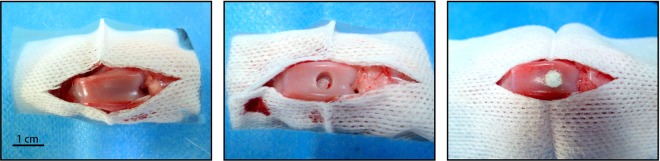
Twelve New Zealand white rabbits (24 knees) were anesthetized by auricular vein intravenous injection of 45 mg/kg body weight pentobarbital sodium. Full-thickness cartilage defects extending through the cartilage layer and penetrating the subchondral bone were created surgically at the femoropatellar groove of both hind-leg knee joints. The defects measured 4 mm in diameter and 2 mm in depth (Fig. 1).
Figure 1.
Full-thickness articular cartilage defect repair.
The knees were randomly divided into four groups: group 1 knees underwent microfracture with implanted cell-free scaffolds; group 2 knees underwent microfracture with implanted rabbit chondrocytes scaffolds; group 3 knees underwent microfracture with implanted rabbit ASC and chondrocyte (1:1) scaffolds; and group 4 knees underwent microfracture with implanted rabbit SVF and chondrocyte (1:1) scaffolds. After treatment, all rabbits were kept in cages freely with no immobilization. The rabbits were sacrificed by overdose anesthesia after 10 weeks to assess the repair process of cartilage effects.
Histology
The implantation sites were harvested and fixed in 4% paraformaldehyde for 48 h at 4°C. Samples were dehydrated through a series of graded alcohol baths and in xylene, embedded in paraffin using routine procedures. Serial sections of 5 µm were cut horizontally along the maximum diameter of the repaired sites. Histological staining was performed with hematoxylin-eosin (HE, Beyotime, Nanjing, China), alcian blue, and safranin O (Sigma-Aldrich).
International Cartilage Repair Society Scores
The International Cartilage Repair Society (ICRS) Score was used to evaluate cartilage regeneration under macroscopic outcomes19, and the ICRS Visual Histological Score was used to evaluate histological outcomes20. The samples were evaluated by 10 observers blinded to the group identities. Spearman correlation was used to test the consistence of the scores by the 10 observers.
Statistical Analysis
Stata/SE 12.1 for Windows (StataCorp LLC, College Station, TX, USA) was used for statistical analysis. The nonparametric test was used for the ICRS scores statistical analysis. The Kruskal–Wallis H test was used to compare the three groups, and the Mann–Whitney test was used between every two groups. The level of statistical significance was defined as p < 0.05.
Results
Characterization of the PHB/PHBHHx Scaffolds and Seeding of Cells
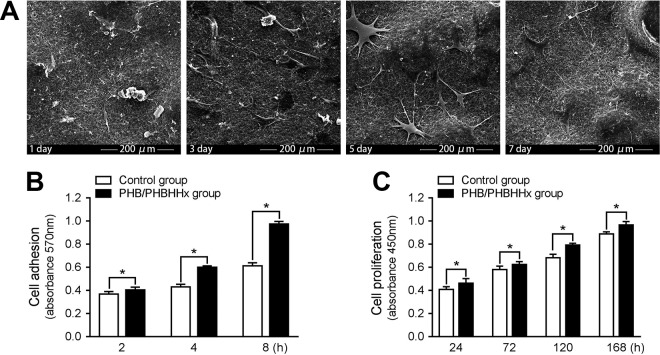
SEM micrographs showed that PHB/PHBHHx scaffolds had polygonal and interconnected pores; the magnified view of the pores was fairly uniform and had rough morphology. At 1 and 3 days after seeding in the scaffolds and culture in vitro, the cells were partially dispersed on the surface of the scaffolds, but most of the cells were infiltrated into the 3D microarchitecture. The cells rapidly migrated and proliferated, and after 5 days were grown to confluence on the surface (Fig. 2a). This indicated good biocompatibility between the cells and scaffolds.
Figure 2.
In vitro cell behavior of PHB/PHBHHx scaffolds. (a) The cell morphologies of ASCs on the PHB/PHBHHx scaffolds at different days as shown by SEM. (b) Cell adhesion of ASCs on the PHB/PHBHHx scaffolds and Petri dishes. (c) Cell proliferation of ASCs on the PHB/PHBHHx scaffolds and Petri dishes. *Significantly different from control group (p < 0.05).
In Vitro Assay of Cell Behaviors on the Scaffolds
A cell adhesion assay was indirectly performed to quantify the amount of trypsinized cells adhered onto the PHB/PHBHHx scaffolds at 2, 4, and 8 h post-seeding. The MTT showed that after 2, 4, and 8 h of culture, the cells on the scaffolds exhibited a much greater average absorbance values as compared with the control groups (p < 0.05) (Fig. 2b).
The cell proliferation rate on the scaffolds was assessed using the CCK-8 kit. The results showed that after day 1, the cells on the scaffolds had a significantly higher proliferation rate than those found on the control group (p < 0.05) (Fig. 2c).
In Vivo Evaluation of Engineered Cartilage
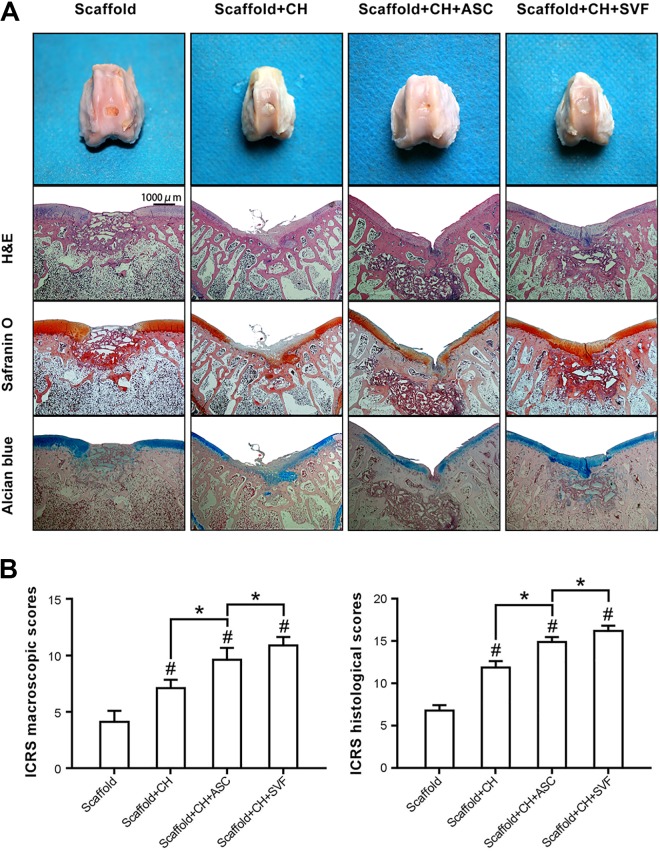
After 10 weeks of orthotopic implantation, histological staining was used for cartilage repair evaluation. In group 1, the implants formed vascularized yellow tissues, and HE staining showed that these specimens were mainly fibrous tissue and non-degraded PHB/PHBHHx scaffolds. Specimens were negative for safranin O and alcian blue staining. In group 2, positive staining for safranin O and alcian blue was observed only in some areas of a few specimens. Bits of cartilage were found in the defect. In group 3, the implants retained its similar shape and had a glistening appearance with a relatively smooth surface. HE staining further showed that the differentiated cells produced an irregular extracellular matrix, and a few sporadic lacuna-like structures were observed among the scaffolds. Specimens were positive for safranin O and alcian blue staining. In group 4, the implants exhibited a white cartilage-like appearance that resembled native cartilage. The chondrocyte-specific stainings were brighter than in group 3; the typical cartilage lacuna was also observed, and no remnants of PHB/PHBHHx existed (Fig. 3a).
Figure 3.
The evaluation of the engineered tissue after orthotopic implantation. (a) Gross view and histological staining of repaired knee articular cartilage at 10 weeks. (b) Scoring criteria using the ICRS Score to assess the integration of the scaffold into the joint. #Significantly different from control and *specified cells/scaffolds group, respectively (p < 0.05).
ICRS Cartilage Repair Assessment
ICRS macroscopic scores and ICRS histological scores by the 10 observers were consistently tested by Spearman correlation. ICRS scores after repair confirmed that SVF/chondrocytes combined with PHB/PHBHHx promoted cartilage regeneration for macroscopic and microscopic assessments, and scores for group 4 were significantly higher than those of groups 1, 2, and 3 (p < 0.05) (Fig. 3b).
Discussion
In comparison to PHB, the PHB/PHBHHx polymer blend has better surface properties which include surface free energy, surface chemical state, polarity, cell attachment, distribution, and differentiation for chondrocytes. It is useful for filaments of type II collagen to anchor and extend into deeper layers of the scaffolds21. In addition, the scaffolds could form high densities of chondrocytes and promote the synthesis of the extracellular matrix18. The 1:2 and 2:1 ratio of PHB/PHBHHx three-dimensional scaffolds were better for adhesion and proliferation of chondrocytes13. The 1:2 ratio of PHB/PHBHHx scaffolds was also good for hASC viability, chondrogenic differentiation, and glycosaminoglycan (GAG) synthesis22. It was found that the PHB/PHBHHx scaffolds could activate a redifferentiation process, which allowed chondrocytes to express and produce type II collagen18. Also the scaffolds were able to maintain the differentiated phenotype of hASCs by mimicking the extracellular matrix of cartilage, which suggested that PHB/PHBHHx has the capacity for differentiation22. The degradation rate of scaffolds affects cell growth and vitality23. It is known that the main degradation products of the PHAs family are all non-toxic24–26, and the degradation product of PHB/PHBHHx, 3-hydroxybutyrate (3HB)27, has good biocompatibility with hASCs22.
Advancement in tissue engineering for cartilage repair includes overcoming several problems about the use of the cells. ASCs are abundant, readily accessible, and easily expandable. In our previous report28, after implantation of TGF-1/electrospun 3D P3HB4HB scaffolds with 4:1 co-culture of ASCs and chondrocytes, new cartilage-like tissue was formed at the site of the defects; the results were far better than those of non-cell-seeded implantation in rabbit at up to 16 weeks. However, it was found that in comparison to ASCs, SVF cells from the same donor were more potent in facilitating chondrogenesis in co-culture with chondrocytes. SVF cells may play the part of trophic mediators in co-culture pellets with chondrocytes29. In previous studies, SVF cells showed stem cell characteristics that are very similar to expanded cells isolated from SVF, and SVF cells even appeared to be slightly better than expanded cells isolated from SVF in chondrogenic differentiation7. Even though ASC and SVF share some common features such as multilineage differentiation, ASCs have a more homogeneous composition of cell types, and resemble more mesenchymal cell lineages than SVF. SVF are composed of heterogeneous cell populations including blood-derived cells (CD45+), ASCs (CD31- CD34+ CD45- CD90+ CD105- CD146-), endothelial (progenitor) cells (CD31+ CD34+ CD45- CD90+ CD105low CD146+), pericytes (CD31- CD34- CD45- CD90+ CD105- CD146+), and other cells30; these non-mesenchymal cells enhanced the trophic effects of MSCs at least in co-culture with chondrocytes31. Therefore, SVF cells could be a promising option for cartilage regeneration.
Conclusions
In this study, our data demonstrated that PHB/PHBHHx scaffolds could support cell adhesion, growth, and proliferation, which provided a favorable environment for chondrogenic differentiation in vitro. In addition, chondrocytes form better cartilage tissue when co-implanted with SVF of adipose tissue than with ASC. Since the SVF chondrocyte combination can reduce the need for chondrocytes in cartilage regeneration, and SVF can be relatively easy and quickly isolated out of a liposuction and transplanted without further in vitro selection or expansion steps, this could provide the experimental basis for a one-step cartilage repair method in which SVF from adipose tissue and chondrocytes from the non-weight bearing joint surface are isolated, mixed, and implanted back into the patient during the same surgical procedure.
Footnotes
Ethical Approval: The study was approved by the Institutional Animal Care and Use Committee of Zhengzhou University, China.
Statement of Human and Animal Rights: All operations were performed according to international guidelines concerning the care and treatment of experimental animals.
Statement of Informed Consent: Statement of Informed Consent is not applicable for this article.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was supported by the National Natural Science Foundation of China (81500826, 81500847) and Youth Foundation of the First Affiliated Hospital of Zhengzhou University.
ORCID iD: Kai Ba  https://orcid.org/0000-0001-6533-0789
https://orcid.org/0000-0001-6533-0789
References
- 1. Dell’accio F, Vincent TL. Joint surface defects: clinical course and cellular response in spontaneous and experimental lesions. Eur Cell Mater. 2010;20:210–217. [DOI] [PubMed] [Google Scholar]
- 2. Knutsen G, Drogset JO, Engebretsen L, Grontvedt T, Isaksen V, Ludvigsen TC, Roberts S, Solheim E, Strand T, Johansen O. A randomized trial comparing autologous chondrocyte implantation with microfracture. Findings at five years. J Bone Joint Surg Am. 2007;89(10):2105–2112. [DOI] [PubMed] [Google Scholar]
- 3. Bentley G, Biant LC, Carrington RW, Akmal M, Goldberg A, Williams AM, Skinner JA, Pringle J. A prospective, randomised comparison of autologous chondrocyte implantation versus mosaicplasty for osteochondral defects in the knee. J Bone Joint Surg Br. 2003;85(2):223–230. [DOI] [PubMed] [Google Scholar]
- 4. Saris DB, Vanlauwe J, Victor J, Haspl M, Bohnsack M, Fortems Y, Vandekerckhove B, Almqvist KF, Claes T, Handelberg F, Lagae K, et al. Characterized chondrocyte implantation results in better structural repair when treating symptomatic cartilage defects of the knee in a randomized controlled trial versus microfracture. Am J Sports Med. 2008;36(2):235–246. [DOI] [PubMed] [Google Scholar]
- 5. Redler LH, Caldwell JM, Schulz BM, Levine WN. Management of articular cartilage defects of the knee. Phys Sportsmed. 2012;40(1):20–35. [DOI] [PubMed] [Google Scholar]
- 6. Varma MJ, Breuls RG, Schouten TE, Jurgens WJ, Bontkes HJ, Schuurhuis GJ, van Ham SM, van Milligen FJ. Phenotypical and functional characterization of freshly isolated adipose tissue-derived stem cells. Stem Cells Dev. 2007;16(1):91–104. [DOI] [PubMed] [Google Scholar]
- 7. Jurgens WJ, van Dijk A, Doulabi BZ, Niessen FB, Ritt MJ, van Milligen FJ, Helder MN. Freshly isolated stromal cells from the infrapatellar fat pad are suitable for a one-step surgical procedure to regenerate cartilage tissue. Cytotherapy. 2009;11(8):1052–1064. [DOI] [PubMed] [Google Scholar]
- 8. Shishatskaya EI, Volova TG. A comparative investigation of biodegradable polyhydroxyalkanoate films as matrices for in vitro cell cultures. J Mater Sci Mater Med. 2004;15(8):915–923. [DOI] [PubMed] [Google Scholar]
- 9. Qu XH, Wu Q, Chen GQ. In vitro study on hemocompatibility and cytocompatibility of poly (3-hydroxybutyrate-co-3-hydroxyhexanoate). J Biomater Sci Polym Ed. 2006;17(10):1107–1121. [Google Scholar]
- 10. Chen GQ, Wu Q. The application of polyhydroxyalkanoates as tissue engineering materials. Biomaterials. 2005;26(33):6565–6578. [DOI] [PubMed] [Google Scholar]
- 11. Qu XH, Wu Q, Liang J, Zou B, Chen GQ. Effect of 3-hydroxyhexanoate content in poly(3-hydroxybutyrate-co-3-hydroxyhexanoate) on in vitro growth and differentiation of smooth muscle cells. Biomaterials. 2006;27(15):2944–2950. [DOI] [PubMed] [Google Scholar]
- 12. Wang YW, Yang F, Wu Q, Cheng YC, Yu PH, Chen J, Chen GQ. Effect of composition of poly(3-hydroxybutyrate-co-3-hydroxyhexanoate) on growth of fibroblast and osteoblast. Biomaterials. 2005;26(7):755–761. [DOI] [PubMed] [Google Scholar]
- 13. Deng Y, Zhao K, Zhang XF, Hu P, Chen GQ. Study on the three-dimensional proliferation of rabbit articular cartilage-derived chondrocytes on polyhydroxyalkanoate scaffolds. Biomaterials. 2002;23(20):4049–4056. [DOI] [PubMed] [Google Scholar]
- 14. Wang Y, Bian YZ, Wu Q, Chen GQ. Evaluation of three-dimensional scaffolds prepared from poly(3-hydroxybutyrate-co-3-hydroxyhexanoate) for growth of allogeneic chondrocytes for cartilage repair in rabbits. Biomaterials. 2008;29(19):2858–2868. [DOI] [PubMed] [Google Scholar]
- 15. Wang YW, Wu Q, Chen GQ. Attachment, proliferation and differentiation of osteoblasts on random biopolyester poly(3-hydroxybutyrate-co-3-hydroxyhexanoate) scaffolds. Biomaterials. 2004;25(4):669–675. [DOI] [PubMed] [Google Scholar]
- 16. Yang M, Zhu S, Chen Y, Chang Z, Chen G, Gong Y, Zhao N, Zhang X. Studies on bone marrow stromal cells affinity of poly (3-hydroxybutyrate-co-3-hydroxyhexanoate). Biomaterials. 2004;25(7–8):1365–1373. [DOI] [PubMed] [Google Scholar]
- 17. Zheng Z, Deng Y, Lin XS, Zhang LX, Chen GQ. Induced production of rabbit articular cartilage-derived chondrocyte collagen II on polyhydroxyalkanoate blends. J Biomater Sci Polym Ed. 2003;14(7):615–624. [DOI] [PubMed] [Google Scholar]
- 18. Deng Y, Lin XS, Zheng Z, Deng JG, Chen JC, Ma H, Chen GQ. Poly(hydroxybutyrate-co-hydroxyhexanoate) promoted production of extracellular matrix of articular cartilage chondrocytes in vitro. Biomaterials. 2003;24(23):4273–4281. [DOI] [PubMed] [Google Scholar]
- 19. Getgood A, Henson F, Skelton C, Herrera E, Brooks R, Fortier LA, Rushton N. The augmentation of a collagen/glycosaminoglycan biphasic osteochondral scaffold with platelet-rich plasma and concentrated bone marrow aspirate for osteochondral defect repair in sheep: a pilot study. Cartilage. 2012;3(4):351–363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Mainil-Varlet P, Aigner T, Brittberg M, Bullough P, Hollander A, Hunziker E, Kandel R, Nehrer S, Pritzker K, Roberts S, Stauffer E. Histological assessment of cartilage repair: a report by the histology endpoint committee of the international cartilage repair society (ICRS). J Bone Joint Surg Am. 2003;85A(Suppl 2):45–57. [PubMed] [Google Scholar]
- 21. Zheng Z, Bei FF, Tian HL, Chen GQ. Effects of crystallization of polyhydroxyalkanoate blend on surface physicochemical properties and interactions with rabbit articular cartilage chondrocytes. Biomaterials. 2005;26(17):3537–3548. [DOI] [PubMed] [Google Scholar]
- 22. Ye C, Hu P, Ma MX, Xiang Y, Liu RG, Shang XW. PHB/PHBHHx scaffolds and human adipose-derived stem cells for cartilage tissue engineering. Biomaterials. 2009;30(26):4401–4406. [DOI] [PubMed] [Google Scholar]
- 23. Middleton JC, Tipton AJ. Synthetic biodegradable polymers as orthopedic devices. Biomaterials. 2000;21(23):2335–2346. [DOI] [PubMed] [Google Scholar]
- 24. Sun J, Dai Z, Zhao Y, Chen GQ. In vitro effect of oligo-hydroxyalkanoates on the growth of mouse fibroblast cell line L929. Biomaterials. 2007;28(27):3896–3903. [DOI] [PubMed] [Google Scholar]
- 25. Zhao Y, Zou B, Shi Z, Wu Q, Chen GQ. The effect of 3-hydroxybutyrate on the in vitro differentiation of murine osteoblast MC3T3-E1 and in vivo bone formation in ovariectomized rats. Biomaterials. 2007;28(20):3063–3073. [DOI] [PubMed] [Google Scholar]
- 26. Cheng S, Wu Q, Yang F, Xu M, Leski M, Chen GQ. Influence of DL-beta-hydroxybutyric acid on cell proliferation and calcium influx. Biomacromolecules. 2005;6(2):593–597. [DOI] [PubMed] [Google Scholar]
- 27. Cheng S, Chen GQ, Leski M, Zou B, Wang Y, Wu Q. The effect of D, L-beta-hydroxybutyric acid on cell death and proliferation in L929 cells. Biomaterials. 2006;27(20):3758–3765. [DOI] [PubMed] [Google Scholar]
- 28. Li G, Fu N, Xie J, Fu Y, Deng S, Cun X, Wei X, Peng Q, Cai X, Lin Y. Poly(3-hydroxybutyrate-co-4-hydroxybutyrate) based electrospun 3D Scaffolds for delivery of autogeneic chondrocytes and adipose-derived stem cells: evaluation of cartilage defects in rabbit. J Biomed Nanotechnol. 2015;11(1):105–116. [DOI] [PubMed] [Google Scholar]
- 29. Wu L, Prins HJ, Leijten J, Helder MN, Evseenko D, Moroni L, van Blitterswijk CA, Lin Y, Karperien M. Chondrocytes cocultured with stromal vascular fraction of adipose tissue present more intense chondrogenic characteristics than with adipose stem Ccells. Tissue Eng Part A. 2016;22(3–4):336–348. [DOI] [PubMed] [Google Scholar]
- 30. Yoshimura K, Shigeura T, Matsumoto D, Sato T, Takaki Y, Aiba-Kojima E, Sato K, Inoue K, Nagase T, Koshima I, Gonda K. Characterization of freshly isolated and cultured cells derived from the fatty and fluid portions of liposuction aspirates. J Cell Physiol. 2006;208(1):64–76. [DOI] [PubMed] [Google Scholar]
- 31. Zhang S, Ba K, Wu L, Lee S, Peault B, Petrigliano FA, McAllister DR, Adams JS, Evseenko D, Lin Y. Adventitial cells and perictyes support chondrogenesis through different mechanisms in 3-dimensional cultures with or without nanoscaffolds. J Biomed Nanotechnol. 2015;11(10):1799–1807. [DOI] [PubMed] [Google Scholar]