Abstract
Seven different autologous chondrocyte implantation (ACI) grafts were used consecutively over a period of 18 years for the treatment of cartilage lesions in the knees. The aim was to evaluate this entire ACI patient series for graft-related or unrelated serious adverse events (SAE), graft failures, and to reveal potential risk factors for these incidents. The study group comprised 151 operated patients: classical periosteum-ACI (n = 45); ACI-seeded fibrin-collagen patch, fixed by either periosteum (n = 59), collagen membrane (n = 15), or fibrin glue (n = 6); ACI seeded alginate-agarose hydrogel (n = 14); and biomimetic collagen-hydroxyapatite scaffold injected with the ACI suspension (n = 12). The covariates analyzed as possible predicting factors were: age, gender, BMI, lesion depth, lesion size, lesion location, previous surgeries, and concomitant procedures. The Kaplan–Meier method for estimating survival curves, and Cox’s proportional hazards model to test for covariates, were used in the statistical analysis. The patients in this series, follow-up 10.1 (2.1–18.3) years, encountered 11% of graft-related SAE (risk factors: previous cartilage surgery, age over 40 years, BMI over 25 kg/m2, and meniscus surgery) and 10% of graft unrelated SAE (risk factors: meniscus surgery and osteotomy). None of these factors was a risk for definitive graft failure. The 10-year graft survival rate was 86%. Females had 2.8 times higher incidence of graft failures than males. There was a tendency toward higher graft failures after a previous cartilage surgery. Different ACI graft types offered safe and durable cartilage repair. Female gender, age over 40 years, increased weight, previous cartilage surgery, and meniscus loss showed increased risk for revision surgery or graft failures.
Keywords: knee, cartilage, lesion, repair, autologous chondrocyte implantation, long-term results
Introduction
Cartilage lesions are encountered in 6 out of 10 knee arthroscopies1–3. In addition to causing joint symptoms4, they also represent a risk factor for early degenerative joint disease with associated major source of functional disability and economic cost4. Several cartilage repair techniques have evolved over the past 30 years, but the optimal treatment protocol has still not been defined5. Autologous chondrocyte implantation (ACI) is a two-stage cartilage repair procedure based on ex-vivo chondrocyte cultivation from a small cartilage biopsy; the first clinical case series was presented in 1994, showing a very positive outcome6. According to the original surgical technique, nowadays known as the ‘periosteal-ACI’, the suspension of autologous chondrocytes was retained in the debrided cartilage lesion via a sutured and sealed autologous periosteum cover6. The original authors also confirmed long-term durability of the clinical outcome7. A demanding surgical technique, occasional periosteum hypertrophy, and difficulties in the treatment of deep, large, or uncontained lesions, led to the evolution of collagen membranes to replace periosteal cover in second-generation ACI, and three-dimensional chondral and osteochondral scaffolds that were seeded by chondrocytes in third-generation ACI8. The first patients at our institution were treated with periosteum-ACI in 1996, and our short- and long-term outcome concurred with the outcomes of the original authors9. During the intervening years, many upgrades in cell cultivation, surgical techniques, and biomaterial have become available. Over a period of 18 years, seven different ACI implant types have been used: periosteum-ACI; fibrin-collagen patch seeded by ACI and fixed by either periosteum, collagen membrane, or fibrin glue; ACI seeded alginate-agarose hydrogel; and biomimetic collagen-hydroxyapatite scaffold injected with ACI suspension. All ACI patients were followed prospectively in a registry.
The aim of the current study is to evaluate our entire ACI patient cohort graft in light of serious adverse events and graft failures, and to define the risk factors for their occurrence.
Materials and Methods
The study protocol was approved by the Slovenian National Medical Ethics Committee (#01/06/96). This prospective case series comprises 151 patients, who were treated with different types of ACI grafts due to chondral or osteochondral lesions in the knee between the years 1996 and 2013. All operations were performed by the two senior co-authors (DM and MD). There were 115 male and 36 female patients with a mean age at operation of 31.5 ± 8.7 years and an average BMI of 25.4 ± 3.5 kg/m2. A total of 99 patients suffered from chondral lesions, and another 52 from osteochondral lesions; the average total lesion size was 4.5 ± 2.3 cm2. The majority of lesions were located on medial femoral condyles (110), followed by lateral femoral condyle (25), patellofemoral area (8), and multiple lesions (8); 62 (41%) patients experienced at least one previous cartilage surgery: 47 had lesion debridement or loose body removal, while 15 had an active cartilage repair via microfractures, osteochondral autografting, or osteochondral fragment refixation. The majority (78%) of patients had intact menisci at the time of surgery, 19% had a partial resection of medial or lateral menisci, and 3% had both menisci partially resected. A total of 50 patients had ACL reconstruction done either before or simultaneously with the ACI surgery. A concomitant high tibial osteotomy was conducted in 13 patients.
The ACI procedure followed a standardized protocol (ChondroArt, Educell, Slovenia), described in detail elsewhere9, arthroscopic cartilage biopsy, chondrocyte isolation and ex vivo cultivation in autologous serum, and graft implantation 3–4 weeks later. Alternatively, the isolated chondrocytes were stored by deep freezing for delayed surgical procedure, when necessary. The ACI graft implantation was performed by mini-open or classical arthrotomy, depending on lesion characteristics and concomitant procedures. The ACI grafts have evolved over the years, different ACI types were used at different time points, and, therefore, every patient had received the ACI type that was available at the time of treatment. A total of 45 patients were operated on by classical periosteum-ACI; 80 patients received a fibrin-collagen patch (Tachocomb, Nycomed Pharma, Linz, Austria) seeded by autologous chondrocytes during cultivation and fixed either by periosteum (n = 59), collagen membrane by (Chondrogide, Geistlich Pharma AG, Wolhusen, Switzerland) (n = 15), or fibrin glue (n = 6); 14 patients were treated with alginate-agarose hydrogel (Gel4Cell, TBF, Lyon, France) seeded with autologous chondrocytes during cultivation; 12 patients were treated with a three-layered collagen-hydroxyapatite biomimetic scaffold (Maioregen, Finceramica, Italy) injected with autologous chondrocyte suspension directly upon implantation.
The current study included the entire patient series with a focus on serious adverse effects (SAE – defined as a revision surgery or hospitalization related to the index knee surgery), and graft failures (defined as revision cartilage repair or arthroplasty, or low clinical outcome together with radiological or arthroscopic evidence for graft failure). The SAEs were further delineated as graft-related (such as chronic synovitis, graft hypertrophy, arthrofibrosis, infection, loose body, minor graft failure, and others) or graft-unrelated (such as novel meniscal tear or ligament lesion, fractures around the knee, and others). Parameters analyzed as possible risk factors for SAE or graft failure were: age, gender, BMI, lesion depth (full thickness chondral vs. osteochondral), lesion size, lesion location, graft type, previous repair surgeries, and concomitant procedures (ACL reconstruction, osteotomy, or meniscectomy). Ten patients (7%) were lost from follow-up evaluation. The Kaplan–Meier method for estimating the survival curves, and Cox’s proportional hazards model to test for covariates were used. Statistical analysis was performed using IBM SPSS statistics Version 23.
Results
Graft-Related SAE
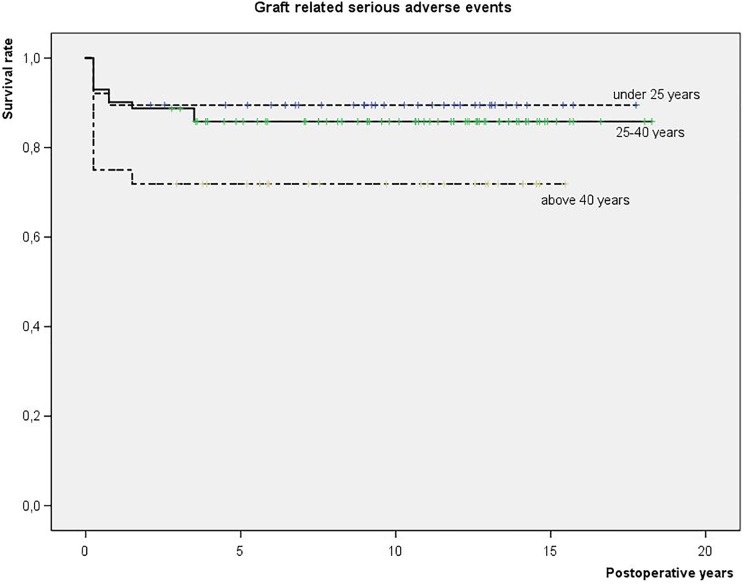
Among 141 patients available for complete follow-up, 16 graft-related SAEs were encountered (11%): 5 graft infections, 4 chronic synovitis, 4 minor (partial) graft delaminations, 2 arthrofibrosis, and 1 graft hypertrophy. All these SAE occurred within the first 2 years post-operatively. A higher incidence of graft-related SAE was encountered after previous cartilage surgeries, in patients over 40 years of age (Fig 1), and BMI over 25 kg/m2 (last two were borderline significant). There were fewer graft-related SAEs in the medial femoral condyles than in the other knee locations. A partial meniscus loss at the time of the ACI increased the likelihood for later graft-related SAE.
Fig 1.
Graft-related serious adverse events (SAE) in a prospective cohort of patients treated with different ACI grafts for cartilage lesions in the knee (N = 151): survival analysis in relation to patients’ age at the index surgery: <25, 25–40, and >40 years.
A higher incidence of SAE was encountered when ACI was combined with the alginate-agarose hydrogel or with a three-layered collagen-hydroxyapatite biometric scaffold. Gender, lesion depth, lesion size, ACL surgery, and an osteotomy had no influence on the graft related SAE (Table 1).
Table 1.
Possible Risk Factors for Graft-Related SAE, Graft-Unrelated SAE and Graft Failures.
| Graft Related SAE |
Graft unrelated SAE |
Graft failure |
|
|---|---|---|---|
| Age | p = 0.095 | p = 0.857 | p = 0.458 |
| Sex | p = 0.078 | p = 0.288 | p = 0.019* |
| BMI | p = 0.095 | p = 0.726 | p = 0.942 |
| Lesion depth | p = 0.278 | p = 0.682 | p = 0.090 |
| Lesion size | p = 0.239 | p = 0.446 | p = 0.495 |
| Lesion location | p = 0.121 | p = 0.365 | p = 0.539 |
| Previous repair | p = 0.007* | p = 0.653 | p = 0.061 |
| ACL reconstruction | p = 0.325 | p = 0.450 | p = 0.954 |
| Meniscus surgery | p = 0.002* | p = 0.035* | p = 0.540 |
| Osteotomy | p = 0.081 | p = 0.019* | p = 0.883 |
| Graft type | p = 0.002* | p = 0.089 | p = 0.793 |
*Statistically significant results as determined by Kaplan-Meyer survival analysis.
ACL, anterior cruciate ligament; SAE, Serious adverse events.
All patients with SAE underwent revision arthroscopy with synovectomy, arthrolysis, graft trimming, or loose body removal. The five patients with infections additionally received 6 weeks of corresponding antibiotic treatment. These SAEs did not represent a risk factor for later graft failure.
Graft Unrelated SAE
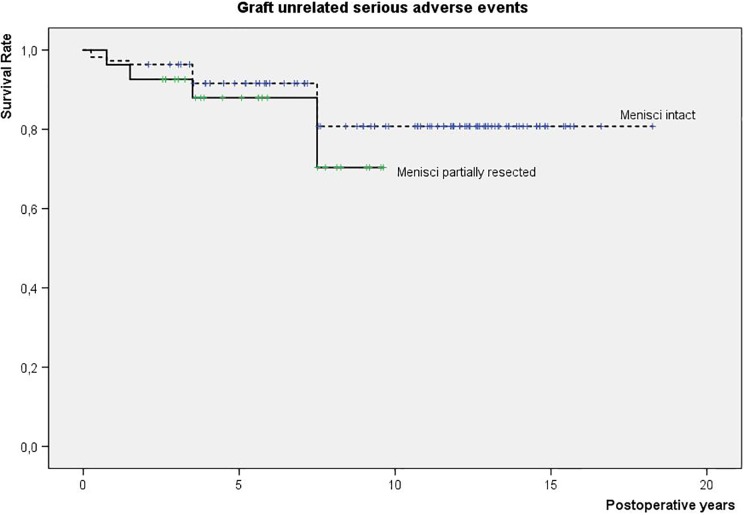
There were 15 graft-unrelated SAE encountered among 141 patients who completed the entire follow-up (10%): 8 repetitive meniscus tears, 5 ACL lesions, and 2 fractures around the knee. The majority of graft-unrelated SAE tended to occur between 1 and 7 years after the index procedure. A partial meniscus loss (Fig 2) or an osteotomy at the time of an ACI surgery seems to increase the risk of graft-unrelated SAE. Other parameters evaluated showed no significant influence (Table 1). After surgical interventions (additional meniscus resections in 13 cases, ACL reconstructions in 3 cases, and fracture osteosynthesis in 2 cases), these patients did not experience increased graft failure rates.
Fig 2.
Graft-unrelated SAE in a prospective cohort of patients treated with different ACI grafts for cartilage lesions in the knee (N = 151): survival analysis in relation to meniscus status at the index surgery: intact versus partially resected.
Graft Failures
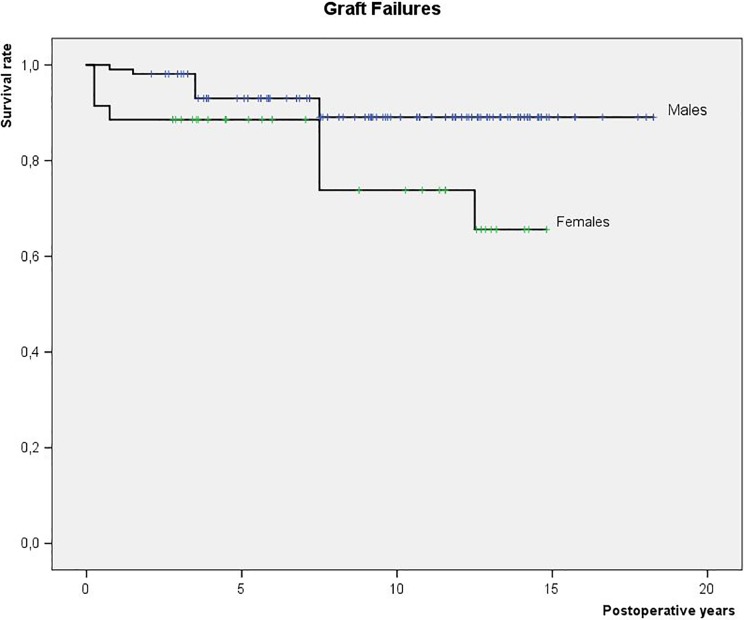
There were 19 (13%) graft failures in total among 141 patients available for the entire follow-up. The graft survival rates were as follows: 97% at 1 year, 96% at 2 years, 92% at 5 years, and 87% at 10 years. The graft failures were comparable between different ACI graft types, albeit not taking into the account different post-operative observation periods of these grafts. The females bear a 2.8 times higher risk of graft failure than males (Fig 3). A tendency was noted, albeit not statistically confirmed, that previous cartilage repair increases graft failures. The other evaluated parameters were not correlated with the ACI graft failures (Table 1).
Fig 3.
Graft failures in a prospective cohort of patients treated with different ACI grafts for cartilage lesions in the knee (N = 151): survival analysis in relation to gender.
Discussion
The primary goal of our study was to evaluate the frequency of SAE and graft failures in a prospective series of patients operated on with different types of ACI grafts. Over an average follow-up of 10 years, we encountered 13% graft failures, 11% of ACI graft-related SAE (all occurred within 2 years post-operatively), and 10% of graft-unrelated SAE (occurring 1–7 years post-operatively). The secondary goal was to identify risk factors for these events, which were: graft-related SAE: previous cartilage surgery, age over 40 years, BMI over 25kg/m2, meniscus surgery, and combining ACI with alginate-agarose or collagen-hydroxyapatite scaffold; graft unrelated SAE: meniscus surgery and osteotomy; and graft failures: female gender and previous cartilage surgery. It has also been established that adequately treated SAE did not pose a risk for subsequent graft failure.
The ACI procedure has been in use for nearly three decades6, and several clinical trials have confirmed positive clinical (70–92% good/excellent), radiologic, and histologic outcomes (hyaline-like tissue was reproduced)10,11. The longest available clinical outcome of the original authors, with a mean follow-up of 12.8 years, showed that 92% patients were satisfied and would undergo ACI again10. In spite of its safety and reliability, periosteum-ACI has been upgraded over the years to make the surgical procedure faster and less invasive (periosteum harvesting and meticulous water-tight periosteum suturing, problems with deep osteochondral lesions or uncontained lesions, wider knee exposures) and offer also biological improvement (biomaterials offer chondrocyte stability, fixation, and stimulate their chondrogenic phenotype). The replacement of periosteum with collagen membrane showed a similar clinical outcome, but with less complication in terms of graft hypertrophy12. A matrix-assisted ACI was the next step in the evolution of this procedure13. Either the chondrocytes are seeded into the biomaterial during the process of cultivation, or, alternatively, they can be added onto the scaffold during implantation14. Reports of ACI-treated patients show significantly improved scores compared with the pre-operative values, and failure rates up to 20% during short and mid-term follow-up14–16.
The medical evidence comparing different ACI procedures is scarce. Goyal et al. examined studies comparing periosteum-ACI to membrane-ACI or MACI17, and found that newer generations of ACI performed better, but the evidence was weak. The data gathered in our study mostly concur with the results above, but only a few studies on ACI offer a follow-up longer than 10 years10–12. Third generation ACI (alginate-agarose hydrogel and three-layer C-HA biomimetic scaffold) was found to have significantly higher risk for the SAE in our series. The synovitis and arthrofibrosis in C-HA biomimetic grafts were probably related to the method of scaffold seeding with autologous chondrocytes. Initially, cell suspension was delivered directly onto a dry C-HA scaffold, but, in the next stage, the C-HA scaffold was first impregnated with cell culture media and then seeded with chondrocytes. Both methods of chondrocyte seeding caused a significant HA crystal release with consequent synovitis and arthrofibrosis. When the method of seeding was upgraded to allow blood impregnation of C-HA scaffold first, and only then applying the chondrocyte suspension, the HA-crystal-related problems subsided18. The alginate-agarose hydrogel grafts revealed the highest infection rate (three out of five infections in the cohort occurred in the hydrogel subgroup). There was no evidence of cell sample contamination during the preparation process. The incidence of infections was higher compared with other studies using the same graft type: Clave et al. indicate an incidence of 1 in 30 patients19, whereas Neyret et al. found no infection among 17 patients20. During revision surgery, we found unstable or dislocated hydrogel grafts. We speculate that the mechanical micro-instability of the agarose graft leads to particle release and foreign body reaction with synovitis. Chronic synovitis represents grounds for later infection, whereas SAE, when addressed correspondingly, did not influence graft failure in the long run. It is also worth pointing out that none of the graft-related SAE occurred later than the 2nd year post-operatively. The early periods is therefore the most vulnerable for SAE, and patients should exercise caution before returning to full activities. On the other hand, the graft-unrelated SAE occurred later, i.e. already in a period of full knee activities.
Graft failure was nearly three times more common in women. These data are rather unique, and we could find no other study with similar results. However, the unequal number of female (36) and male (115) patients in our study could have influenced this result. Saris et al. searched for ideal candidates for ACI surgery outcomes21. They realized that the ACI appeared to result in a positive benefit/risk ratio when used in an unselected heterogeneous population, irrespective of the follow-up period, lesion size, and type of lesion treatment. However, a recent systematic review of the long-term ACI follow-up by Pareek et al. demonstrated that increased patient age, and a lesion size greater than 4.5 cm2 were risk factors for a higher reoperation and failure rate15. These two parameters had no influence on graft failures in our case series.
ACI cultivation for applications at our institution has been conducted in a local GMP facility since 1996, while our tertiary orthopedic institution functions as donor (for harvesting) and recipient (for implantation) center. The strength of the study presented here is to show the entire patient series from a single center, each operated on by two surgeons only. This enables a very high standardization of surgical procedures, which would otherwise be the main issue for failures22. Moreover, analysis of the entire patient series allowed us to compare seven different graft types for their safety. The limitations of our study are: different follow-up times of subgroups of ACI grafts, as they were used in a consecutive manner, and many other covariates that could bias the results. With a statistical model of survival analysis, we were able to test the most important covariates, and establish significant risk factors. We are well aware that we are not presenting the data of a randomized comparative trial, but of a real clinical situation to show all treated patients. Due to the non-homogeneity of the ACI grafts and influencing covariates, we were not able to perform a study on clinical efficacy, but focused instead on the safety of ACI surgeries.
Conclusions
This patient series with an average follow-up of over 10 years, encountered 11% of graft-related SAE (risk factors: previous cartilage surgery, age over 40 years, BMI over 25 kg/m2, and meniscus surgery) and 10% of graft-unrelated SAE (risk factors: meniscus surgery and osteotomy). None of these risk factors represented a risk for definitive graft failure when the SAE were treated accordingly. The 10-year graft survival rate was 86%. Females had 2.8 times higher incidence of graft failures, and there was also a tendency toward higher graft failures after previous cartilage surgery.
Footnotes
Ethical Approval: The study protocol was approved by the Slovenian National Medical Ethics Committee (# 01/06/96).
Statement of Human and Animal Rights: All procedures in this study were conducted in accordance with the study protocol approved by the Slovenian National Medical Ethics Committee (# 01/06/96).
Statement of Informed Consent: Written informed consent was obtained from the patients for their anonymized information to be published in this article.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: David Martinčič  https://orcid.org/0000-0002-7712-543X
https://orcid.org/0000-0002-7712-543X
References
- 1. Aroen A, Loken S, Heir S, Alvik E, Ekeland A, Granlund OG, Engebretsen L. Articular cartilage lesions in 993 consecutive knee arthroscopies. Am J Sports Med. 2004;32(1):211–215. [DOI] [PubMed] [Google Scholar]
- 2. Curl WW, Krome J, Gordon ES, Rushing J, Smith BP, Poehling GG. Cartilage injuries: a review of 31,516 knee arthroscopies. Arthroscopy. 1997;13(4):456–460. [DOI] [PubMed] [Google Scholar]
- 3. Hjelle K, Solheim E, Strand T, Muri R, Brittberg M. Articular cartilage defects in 1,000 knee arthroscopies. Arthroscopy. 2002;18(7):730–734. [DOI] [PubMed] [Google Scholar]
- 4. Alford JW, Cole BJ. Cartilage restoration, part 1: basic science, historical perspective, patient evaluation, and treatment options. Am J Sports Med. 2005;33(2):295–306. [DOI] [PubMed] [Google Scholar]
- 5. Heir S, Nerhus TK, Røtterud JH, Løken S, Ekeland A, Engebretsen L, Arøen A. Focal cartilage defects in the knee impair quality of life as much as severe osteoarthritis: a comparison of knee injury and osteoarthritis outcome score in 4 patient categories scheduled for knee surgery. Am J Sports Med. 2010;38(2):231–237. [DOI] [PubMed] [Google Scholar]
- 6. Brittberg M, Lindahl A, Nilsson A, Ohlsson C, Isaksson O, Peterson L. Treatment of deep cartilage defects in the knee with autologous chondrocyte transplantation. N Engl J Med. 1994;331(14):889–895. [DOI] [PubMed] [Google Scholar]
- 7. Brittberg M. Autologous chondrocyte implantation-technique and long-term follow-up. Injury. 2008;39(suppl 1):40–49. [DOI] [PubMed] [Google Scholar]
- 8. Mithoefer K, Peterson L, Saris DB, Mandelbaum BR. Evolution and current role of autologous chondrocyte implantation for treatment of articular cartilage defects in the football (Soccer) player. Cartilage. 2012;3(suppl 1):31–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Martinčič D, Radosavljevič D, Drobnič M. Ten-year clinical and radiographic outcomes after autologous chondrocyte implantation of femoral condyles. Knee Surg Sports Traumatol Arthrosc. 2014;22(6):1277–1283. [DOI] [PubMed] [Google Scholar]
- 10. Peterson L, Vasiliadis HS, Brittberg M, Lindahl A. Autologous chondrocyte implantation: a long-term follow-up. Am J Sports Med. 2010;38(6):1117–1124. [DOI] [PubMed] [Google Scholar]
- 11. Biant LC, Bentley G, Vijayan S, Skinner JA, Carrington RW. Long-term results of autologous chondrocyte implantation in the knee for chronic chondral and osteochondral defects. Am J Sports Med. 2014;42(9):2178–2183. [DOI] [PubMed] [Google Scholar]
- 12. Niemeyer P, Salzmann G, Feucht M, Pestka J, Porichis S, Ogon P, Südkamp N, Schmal H. First-generation versus second-generation autologous chondrocyte implantation for treatment of cartilage defects of the knee: a matched-pair analysis on long-term clinical outcome. Int Orthop. 2014;38(10):2065–2070. [DOI] [PubMed] [Google Scholar]
- 13. Kon E, Filardo G, Di Martino A, Marcacci M. ACI and MACI. J Knee Surg. 2012;25(1):17–22. [DOI] [PubMed] [Google Scholar]
- 14. Gille J, Behrens P, Schulz AP, Oheim R, Kienast B. Matrix-associated autologous chondrocyte implantation: a clinical follow-up at 15 years. Cartilage. 2016;7(4):309–315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Pareek A, Carey JL, Reardon PJ, Peterson L, Stuart MJ, Krych AJ. Long-term outcomes after autologous chondrocyte implantation: a systematic review at mean follow-up of 11.4 years. Cartilage. 2016;7(4):298–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Carey-Smith R, Ebert JR, Davies H, Garrett S, Wood DJ, Janes GC. Arthroscopic matrix-induced autologous chondrocyte implantation (MACI): a simple surgical technique. Tech Knee Surg. 2010;9:170–175. [Google Scholar]
- 17. Goyal D, Goyal A, Keyhani S, Lee EH, Hui JH. Evidence-based status of second- and third-generation autologous chondrocyte implantation over first generation: a systematic review of level I and II studies. Arthroscopy. 2013;29(11):1872–1878. [DOI] [PubMed] [Google Scholar]
- 18. Drobnic M, Martincic D, Strazar K, Radosavljevic D. Autologous chondrocytes implanted on Maioregen membrane for the treatment of chronic osteochondral lesions in the knee. Paper presented at: Abstract in ICRS - 12th World Congress Chicago. International Cartilage Repair Society, 2015. Zurich, Switzerland: International Cartilage Repair Society. [Google Scholar]
- 19. Clavé A, Potel JF, Servien E, Neyret P, Dubrana F, Stindel E. Third-generation autologous chondrocyte implantation versus mosaicplasty for knee cartilage injury: 2-year randomized trial. J Orthop Res. 2016;34(4):658–665. [DOI] [PubMed] [Google Scholar]
- 20. Selmi TA, Verdonk P, Chambat P, Dubrana F, Potel JF, Barnouin L, Neyret P. Autologous chondrocyte implantation in a novel alginate-agarose hydrogel: outcome at two years. J Bone Joint Surg Br. 2008;90(5):597–604. [DOI] [PubMed] [Google Scholar]
- 21. Vanlauwe J, Huylebroek J, Van Der Bauwhede J, Saris D, Veeckman G, Bobic V, Victor J, Almqvist KF, Verdonk P, Fortems Y, Van Lommel N, et al. Clinical outcomes of characterized chondrocyte implantation. Cartilage. 2012;3(2):173–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Filardo G, Drobnic M, Perdisa F, Kon E, Hribernik M, Marcacci M. Fibrin glue improves osteochondral scaffold fixation: study on the human cadaveric knee exposed to continuous passive motion. Osteoarthritis Cartilage. 2014;22(4):557–565. [DOI] [PubMed] [Google Scholar]