Abstract
Complex degenerative tears of the medial meniscus in the knee are usually treated using meniscectomy. However, this procedure increases the risk of osteoarthritis, while other treatments aimed at meniscal repair remain challenging due to the high possibility of failure. The use of synovial mesenchymal stem cells (MSCs) is an attractive additional approach for meniscal repair, as these cells have high proliferative and chondrogenic potential. In this case report, we surgically repaired a complex degenerative tear of the medial meniscus and then transplanted autologous synovial MSCs. We evaluated clinical outcomes at 2 years and assessed adverse events. We enrolled patients with clinical symptoms that included a feeling of instability in addition to pain caused by their complex degenerative tears of the medial meniscus. Two weeks after surgical repair of the torn meniscus, autologous synovial MSCs were transplanted onto the menisci of five patients. The total Lysholm knee score, the Knee Injury and Osteoarthritis Outcome Scale scores for “pain,” “daily living,” “sports activities,” and the Numerical Rating Scale were significantly increased after 2 years. Three adverse events, an increase in c-reactive protein, joint effusion, and localized warmth of the knee were recorded, although these could have been due to the meniscal repair surgery. This first-in-human study confirmed that the combination of surgical repair and synovial MSC transplantation improved the clinical symptoms in patients with a complex degenerative tear of the medial meniscus. No adverse events occurred that necessitated treatment discontinuation. These findings will serve as pilot data for a future prospective study.
Keywords: meniscus, complex degenerative tear, repair, synovium, mesenchymal stem cells, transplantation
Introduction
The meniscus, a crescent-shaped fibrocartilaginous tissue in the knee, has a role in load distribution, stability, and lubrication1. Degenerative tears of the meniscus occur frequently in middle-aged or older persons and are usually located at the posterior horn of the medial meniscus. They are generally slowly developing tears, typically involving a horizontal cleavage of the meniscus2. Symptomatic degenerative tears are a common clinical problem, and when surgery is required, the usual choice is arthroscopic partial meniscectomy rather than meniscal repair, due to the poor healing potential of the meniscus. However, meniscectomy increases the risk of osteoarthritis3.
Recent randomized studies have demonstrated no superiority of arthroscopic partial meniscectomy over non-operative treatment for degenerative tears of the meniscus4,5. The 2016 European Society of Sports Traumatology, Knee Surgery, Arthroscopy (ESSKA) meniscus consensus stated that the indications for meniscectomy should be carefully evaluated for a degenerative tear of the meniscus; however, the consensus never mentioned meniscal repair6. An instructional review by Beaufils et al. described that earlier meniscectomy in patients with degenerative tears of the meniscus may be proposed in cases with significant mechanical symptoms2. We were unable to find any comparative or large-scale clinical research studies on meniscal repairs for degenerative tears of the meniscus. Meniscal repair remains challenging, especially for complex degenerative tears, such as horizontal tears and flap tears of the meniscus.
The repair and healing of meniscal tears may require an involvement of the synovium, a thin membrane that covers the inside of the joint. Indeed, King reported approximately 80 years ago that meniscal lesions did not heal unless they communicated with the synovium and capsule7. Unfortunately, the reparative potential of synovium for meniscal lesion is limited and, therefore, spontaneous healing of the injured meniscus cannot be usually expected. To promote this process, several methods have been attempted, such as supplementation with synovial flaps8, synovial grafts9, and induction of synovium by rasping10. However, these techniques are not in common practice at present, due to the difficulty in performing the procedures or the lack of efficacious outcomes. Innovative applications using synovium are expected to be developed to preserve the meniscus instead of meniscectomy.
Mesenchymal stem cells (MSCs) isolated from synovium are attractive for meniscal repair because synovial MSCs have a high proliferative11 and chondrogenic potential12. Intraarticular injection of synovial MSCs promoted meniscus regeneration after the anterior half of the medial meniscus was resected in rats13, rabbits14, pigs15, and monkeys16. MSCs in the synovial fluid of the knee have been shown to increase in number after meniscus injury17, and the characteristics of MSCs in synovial fluid were close to synovial MSCs18, indicating a physiological role of synovial MSCs during meniscal healing in the natural course. Our preclinical study demonstrated that transplantation of synovial MSCs promoted healing after meniscal repair of extended longitudinal tears at the avascular area in pigs19. In this case report, we describe the surgical repair of a complex degenerative tear of the medial meniscus and the subsequent transplantation of autologous synovial MSCs. We evaluated the clinical outcomes after 2 years and assessed the occurrence of adverse events.
Materials and Methods
Study Design
This study was conducted in accordance with the Declaration of Helsinki and with the “Guidelines on clinical research using human stem cells” in Japan and in conformity to the “Ministerial Ordinance on Good Clinical Practice for Medical Devices” in Japan. The protocol was enrolled in a database of the National University Hospital Council of Japan (UMIN Clinical Trials Registry) and disclosed (Number UMIN000011881). The study was monitored by an independent agency (Clinical Research Support Center at the University of Tokyo, Tokyo, Japan) and approved by the Data and Safety Monitoring Board, which was also qualified by an additional agency (System Inn Nakagomi, Yamanashi, Japan) for the first 6 months. As 6 months were too short to evaluate clinical outcomes for the effectiveness of this treatment, long-term follow-up was maintained at 1 and 2 years.
Participants
The enrolled patients, who were treated between November 2013 and November 2014, had clinical symptoms that included a feeling of instability in addition to pain caused by complex degenerative meniscal tears. Preoperative magnetic resonance imaging (MRI) was performed to confirm the presence of a meniscal tear, but the eligibility of the patients was ultimately determined by arthroscopic examination just before synovium harvest. The inclusion criteria included: (1) the presence of a complex degenerative meniscal tear, including both a horizontal tear and a flap tear that normally would be treated by meniscus resection; (2) clinical symptoms of a meniscal tear (knee joint instability, limitation in range of motion, hydrarthrosis, and pain), and (3) patient age older than 20 years on the acquisition date of informed consent before the clinical study. The exclusion criteria included: (1) positive status for human immunodeficiency virus (HIV-1, HIV-2), hepatitis B virus (HBV), hepatitis C virus (HCV), or the adult T cell leukemia viruses (HTLV-1); (2) poor general condition with an active infection, active malignant tumors, or diabetes; and (3) pregnancy or breast-feeding.
Procedures
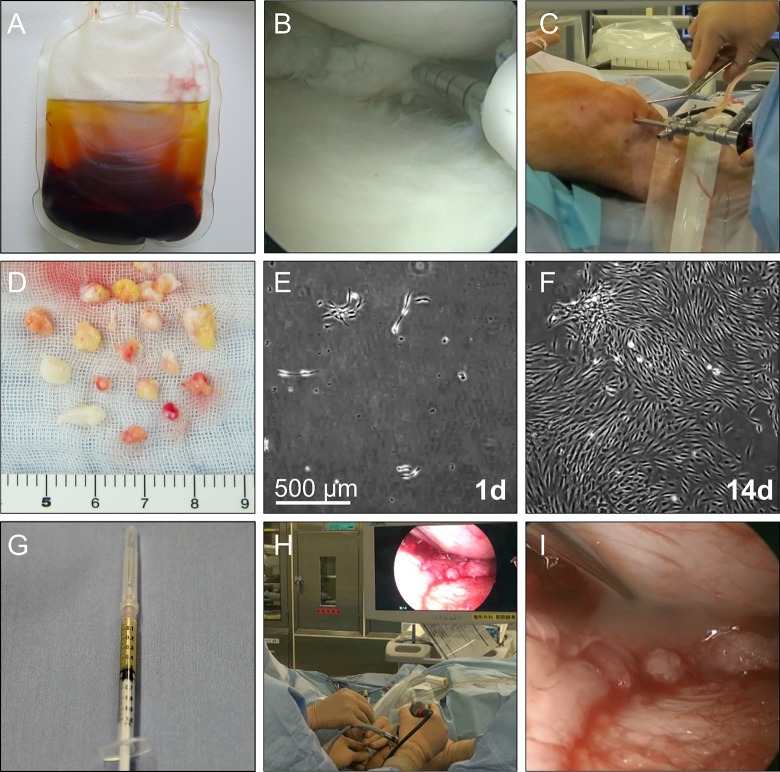
Approximately 2 weeks before synovial tissue was harvested, nearly 300 mL of whole blood was obtained using a CELLAID (JMS Co Ltd, Hiroshima, Japan), a closed-bag system containing glass beads, centrifuged for 7 min, and then serum was isolated (Fig. 1A).
Figure 1.
Procedure for transplantation of synovial MSCs onto the repaired meniscus. (A) Whole blood after centrifugation to prepare autologous human serum. (B) Arthroscopic meniscal repair. (C) Synovium harvest with a pituitary rongeur. (D) Synovium tissues as an MSC source. (E) Synovial MSCs 1 day after plating. (F) Synovial MSCs 14 days after plating. (G) Synovial MSC suspension in a syringe. (H) Arthroscopic transplantation of synovial MSCs. (I) Synovial MSC suspension placed onto the repaired meniscus.
Under lumbar spinal anesthesia, after a degenerative meniscal tear was confirmed, all-inside meniscal repairs were performed with the FasT-Fix device (Smith & Nephew Endoscopy, Andover, MA) (Fig. 1B) and inside-out meniscal repairs with the Zone-Specific Cannula System (Linvatec, Largo, FL, USA) in three cases. Then, under arthroscopic observation, the synovium with subsynovial tissue on the femur at the suprapatellar pouch was harvested with a pituitary rongeur (Fig. 1C). Approximately 20 pieces of synovial tissue, weighing nearly 0.5 g, were collected (Fig. 1D).
The cell culture was performed in a cell processing facility at the authors’ institution. The synovium was digested in a solution of 5 mg Liberase MNP-S GMP (Roche Diagnostics, Mannheim, Germany) in 5 mL Hanks’ balanced salt solution (HBSS; Thermo Fisher Scientific, Waltham, MA, USA). After 3 h, the digested cells were cultured in α-MEM (Thermo Fisher Scientific), containing 10% autologous human serum (Fig. 1E).
After the absence of contamination with bacteria, mycoplasma, virus, or endotoxin was confirmed, synovial MSCs at 14 days (Fig. 1F) were harvested with TrypLE (Thermo Fisher Scientific) at 37°C for 5 min. Thirty minutes before transplantation, primary synovial MSCs were suspended in 0.5 mL acetate Ringer’s solution (Veen-3G; Kowa, Tokyo, Japan) and drawn into a 1 mL syringe (Fig. 1G)20.
The transplantation procedure was performed at 14 days after synovial harvest. With the patient under lumber spinal anesthesia, the sutured meniscus was observed arthroscopically without irrigation fluid (Fig. 1H). An additional meniscus suture was performed in one patient with suture breakage. A suspension of synovial MSCs was placed onto the repaired meniscus through an 18-gauge needle attached to a 1 mL syringe (Fig. 1I). The patient was maintained in position for 10 min. The incisions for the portals were then closed without washing the inside of the knee.
Evaluation of Clinical Score
The Lysholm knee score was evaluated at 4 weeks, 6 weeks, 12 weeks, 24 weeks, 1 year and 2 years (Fig. 2A). The Knee Injury and Osteoarthritis Outcome Scale (KOOS), and Numerical Rating Scale (NRS) were evaluated at 4 weeks, 12 weeks, 24 week, 1 year, and 2 years. The Lysholm total score, KOOS scores for each category, and NRS score for each category ranged from 0 to 100, with 0 indicating the most severe symptoms and 100 an absence of symptoms.
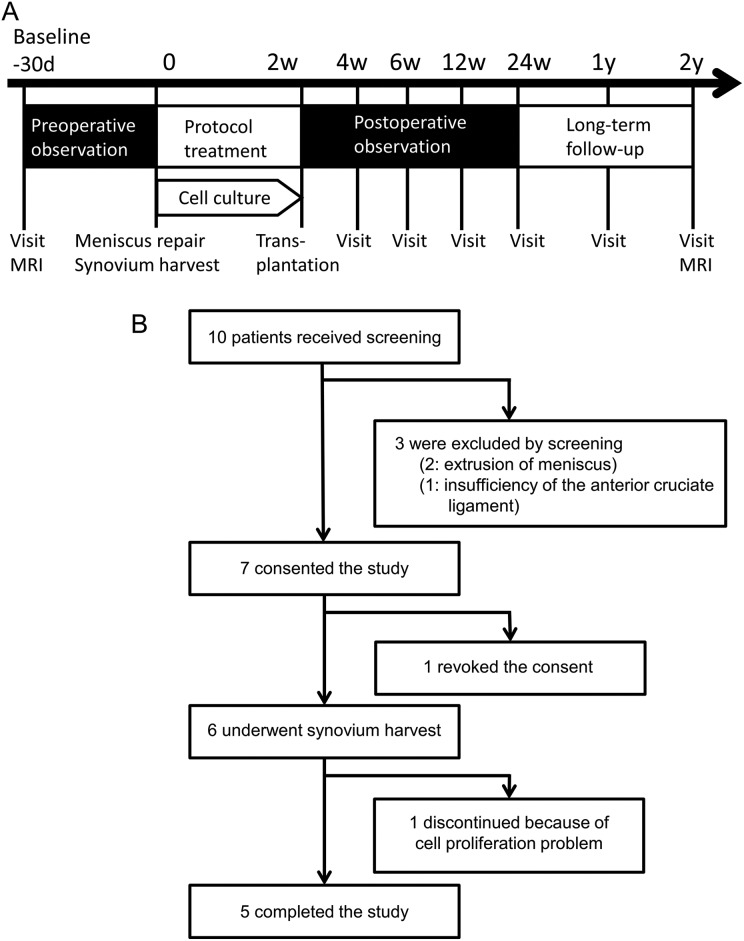
Figure 2.
Study scheme. (A) Schedule for the clinical study. (B) Enrollment of patients.
Enrollment of Patients
Of 10 patients who were candidates for enrollment, three were excluded by committee screening because of meniscus extrusion (two patients) and insufficiency of the anterior cruciate ligament (one patient), and one revoked consent. Six patients underwent an arthroscopic examination and synovial harvest; one of six was discontinued because of a cell proliferation problem, and finally five patients had a transplantation of synovial MSCs onto the repaired meniscus (Fig. 2B). There was no loss to follow-up for 2 years.
Characteristics of the Patients
Five patients received synovial MSC transplantation (Table 1). Their ages ranged from 34 to 57 years old. The meniscus symptoms developed gradually in all five patients, and the duration of the meniscus symptoms ranged from 9 months to 7 years. Three patients had previously undergone partial meniscectomy of the medial meniscus, and another patient had an extrusion of the medial meniscus in the opposite knee, and these four patients complained of knee pain in the opposite knee.
Table 1.
Characteristics of the Patients and the Number of Synovial MSC Transplanted.
| Variable | Patient 1 | Patient 2 | Patient 3 | Patient 4 | Patient 5 |
|---|---|---|---|---|---|
| Age (yr) | 34 | 57 | 45 | 50 | 55 |
| Sex | Male | Male | Male | Male | Male |
| Weight (kg) | 73 | 68 | 76 | 104 | 69 |
| Height (cm) | 172 | 167 | 177 | 177 | 174 |
| Body mass index | 24.6 | 24.5 | 24.5 | 33.3 | 22.8 |
| Index knee | Left | Right | Left | Right | Left |
| Index meniscus | Medial | Medial | Medial | Medial | Medial |
| Duration of symptoms | 7 years | 1 year | 5 years | 9 months | 9 months |
| Onset of symptoms | Gradual | Gradual | Gradual | Gradual | Gradual |
| Kellgren–Laurence grade | 1 | 1 | 0 | 0 | 1 |
| Tear location | Middle & posterior | Middle & posterior | Middle & posterior | Middle & posterior | Middle & posterior |
| Tear pattern | Flap & horizontal | Flap & horizontal | Flap, longitudinal & horizontal |
Flap & horizontal | Flap & horizontal |
| Vascularity of the torn meniscus | Avascular and vascular | Avascular | Avascular | Avascular | Avascular and vascular |
| Degenerative or traumatic | Degenerative | Degenerative | Degenerative | Degenerative | Degenerative |
| Surgical history of the opposite knee | Partial meniscectomy of MM | Partial meniscectomy of MM | Partial meniscectomy of MM | ||
| Opposite knee problem | OA, knee pain |
OA, knee pain |
MM extrusion and cyst, knee pain |
OA, knee pain |
|
| No. of cells transplanted (million) | 58 | 56 | 32 | 68 | 70 |
MM: medial meniscus, OA: osteoarthritis.
Arthroscopic Observation
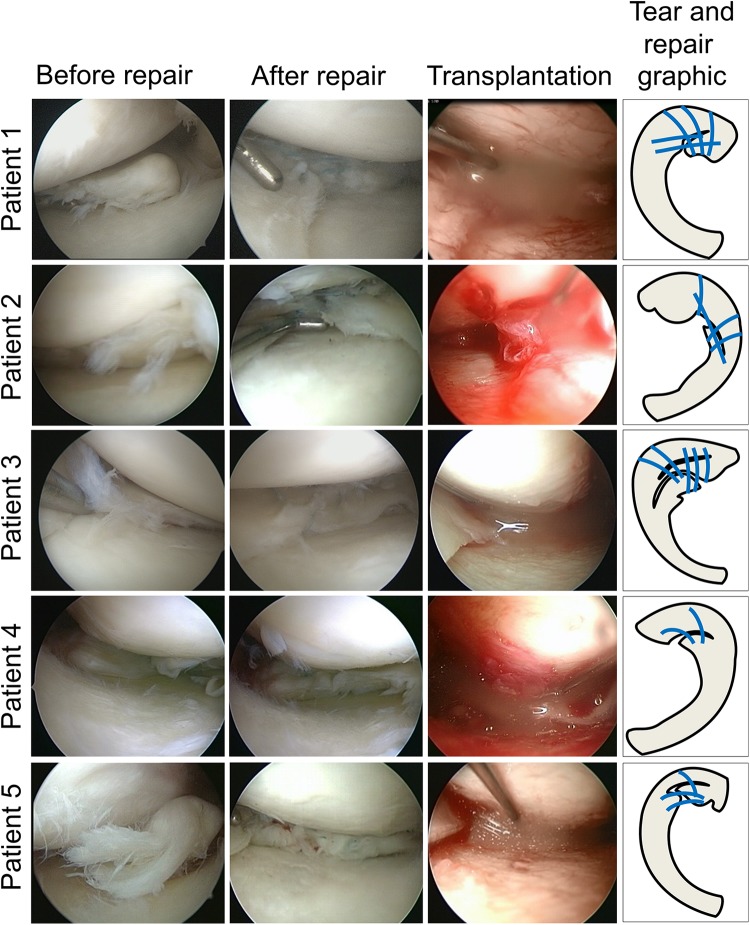
All five patients had a complex degenerative meniscal tear including horizontal tear and flap tear at the avascular area of the middle and posterior segment of the medial meniscus (Fig. 3). The torn meniscus was repaired with 2–7 threads to arrange the shape and/or to stabilize the torn meniscus. Fourteen days after the repair, synovial MSCs (32–70 million cells) were transplanted. Transplantation was completed in all five patients whose synovial MSCs were successfully prepared.
Figure 3.
Torn meniscus before and after repair and transplantation of synovial MSCs. Arthroscopic images before and after repair and during transplantation of synovial MSCs, and tear & repair with suture threads (indicated by blue lines) graphics are shown.
MRI
All MRI examinations were performed on a 3.0-T Gyroscan Intera MR unit (Philips Medical Systems, Best, Netherlands). Using the circular polarized knee coil, sagittal 3D-SE (TR: 1000 ms; TE: 35 ms; slices: 320; slice thickness: 0.36 mm) and sagittal 3D-FFE with ProSet (TR: 19 ms; TE: 7 ms; slices: 320; slice thickness: 0.36 mm) were applied. 3D meniscus images were reconstructed with Ziostation 2 (Ziosoft, Tokyo, Japan).
Assessment of Adverse Events
Adverse events were monitored continually at each visit after patients were approved for the clinical study, until 24 weeks after synovial harvest (Fig. 2A). Clinical laboratory and urine values, vital signs, and standard physical examination results were recorded and deviations from normal were noted. The knee was examined to inspect for redness, swelling, deformity, abnormal tissue presentation, and/or skin changes. X-ray and MRI were reviewed for ectopic tissue formation.
Statistical Analysis
For clinical scores (Lysholm knee score, KOOS, NRS), Student’s t-test was used to compare continuous variables (normally distributed) between values at baseline and at each visit (Fig. 2A). A p-value of 0.05 was considered to indicate statistical significance. GraphPad Prism 6 (GraphPad Software, La Jolla, CA, USA) was used for all statistical analyses.
Results
Clinical Scores
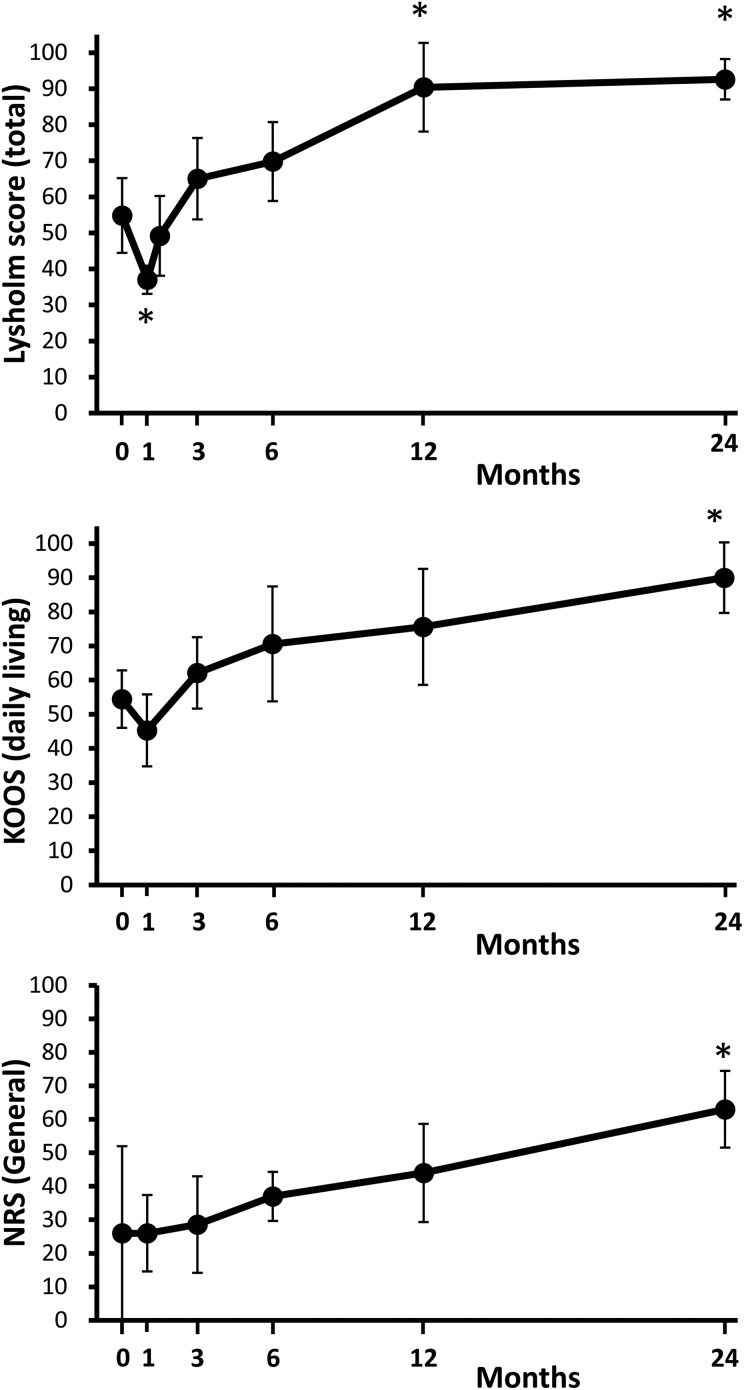
Total Lysholm knee scores decreased 4 weeks after the meniscal repair due to the invasiveness of the surgery, then increased again, and were significantly higher at 1 year than before the treatment, and maintained at 2 years (Table 2, Fig. 4). Among eight subscores, “swelling” and “pain” scores were significantly higher at 1 and 2 years than those before the treatment; furthermore, “limping” and “stairs” scores were significantly higher at 2 years. KOOS for “pain,” “daily living,” and “sports and recreational activities” increased significantly at 2 years. NRS for general and other categories continually increased and was significant at 2 years. The Tegner Activity Scale score did not decrease after the treatment in all five patients (Table 3). 3D MRI showed meniscus tears were indistinguishable at 2 years (Fig. 5).
Table 2.
Clinical Scores Before and After Meniscal Repair by Synovial MSCs.
| Pre | 4w | 6w | 12w | 24w | 1y | 2y | |
|---|---|---|---|---|---|---|---|
| Lysholm Knee Score | |||||||
| Total | 55 (44–65) | 37 (33–41)* | 49 (38–60) | 65 (54–76) | 70 (59–81) | 90 (78–103)** | 93 (87–98)** |
| Limping | 3 (3–3) | 0 (0–0) | 2 (0–3) | 2 (1–4) | 4 (3–5) | 5 (5–5) | 5 (4–5)* |
| Walker | 4 (2–5) | 2 (2–2) | 3 (2–5) | 4 (3–6) | 4 (3–6) | 5 (5–5) | 4 (3–6) |
| Swelling | 4 (1–8) | 0 (0–0) | 0 (0–0) | 4 (–1–9) | 4 (0–9) | 9 (7–11)* | 10 (10–10)* |
| Squatting | 3 (2–4) | 0 (0–0)** | 2 (1–3) | 4 (4–4) | 4 (4–4) | 5 (4–5) | 4 (3–5) |
| Blockage | 11 (8–15) | 15 (15–15) | 15 (15–15) | 12 (9–16) | 13 (11–15) | 14 (12–16) | 15 (15–15) |
| Instability | 16 (9–23) | 11 (6–16) | 13 (7–19) | 21 (15–27) | 20 (15–25) | 25 (25–25) | 25 (25–25) |
| Pain | 8 (2–14) | 7 (0–14) | 9 (2–16) | 11 (6–16) | 13 (7–19) | 20 (20–20)* | 21 (19–23)* |
| Stairs | 5 (4–7) | 2 (2–2)* | 5 (4–7) | 6 (6–6) | 7 (5–8) | 8 (6–10) | 8 (6–10)* |
| KOOS | |||||||
| Symptoms | 55 (36–74) | 40 (25–55) | 48 (31–65) | 60 (40–80) | 69 (51–86) | 83 (71–95) | |
| Pain | 48 (39–58) | 44 (31–58) | 52 (38–66) | 59 (45–74) | 67 (54–80) | 82 (72–92)* | |
| Daily living | 54 (46–63) | 45 (35–56) | 62 (52–73) | 71 (54–87) | 76 (59–93) | 90 (80–100)** | |
| Sports and recreational activities | 15 (2–28) | 7 (–2–16) | 21 (–1–43) | 33 (10–56) | 50 (23–77) | 64 (41–87)* | |
| Quality of Life | 20 (5–35) | 10 (0–20) | 18 (2–33) | 28 (11–44) | 48 (23–72) | 43 (25–60) | |
| Numerical Rating Scale (NRS) | |||||||
| General | 26 (0–52) | 26 (15–37) | 29 (14–43) | 37 (30–44) | 44 (29–59) | 63 (52–74)* | |
| Walking | 48 (28–68) | 58 (51–65) | 58 (47–69) | 70 (64–76) | 72 (61–83) | 84 (76–92)* | |
| Rest | 72 (62–82) | 82 (68–96) | 80 (66–94) | 88 (78–98) | 86 (76–96) | 94 (86–102)* | |
| Initial motion | 46 (26–66) | 62 (52–72) | 62 (51–73) | 68 (57–79) | 74 (61–87)* | 84 (74–94)* | |
| Stair | 40 (22–58) | 56 (37–75) | 54 (42–66) | 64 (51–77) | 74 (54–94)* | 78 (67–89)* | |
| Sports activity | 18 (–1–37) | 0 (0–0) | 14 (–13–41) | 14 (–13–41) | 30 (–6–66) | 34 (–7–75)* | |
| Instability | 44 (22–66) | 48 (32–64) | 42 (21–63) | 48 (31–65) | 66 (44–88) | 82 (64–100) | |
Data are shown as “mean (95% confidence interval)”. Sample number is 5 in each outcome.
**; p<0.01, *; p<0.05 with Pre (before repair by transplantation of synovial MSCs).
Figure 4.
Clinical scores before and after meniscal repair with transplantation of synovial MSCs. Lysholm knee scores, KOOS (daily living), and NRS over the 24-month follow-up period are shown. Each score ranges from 0 to 100, with lower scores indicating more severe symptoms. Data are shown as “mean (95% confidence interval).” Sample number is 5 in each outcome. *; p<0.05 with a value at time 0 (before repair with transplantation of synovial MSCs).
Table 3.
Activity Before and After Treatments.
| Patient 1 | Patient 2 | Patient 3 | Patient 4 | Patient 5 | |
|---|---|---|---|---|---|
| Tegner activity scale | |||||
| Before | 5 | 6 | 7 | 5 | 5 |
| After | 5 | 6 | 7 | 5 | 5 |
| Activity | Heavy labor | Recreational tennis | Recreational Karate | Heavy labor | Golf |
Figure 5.
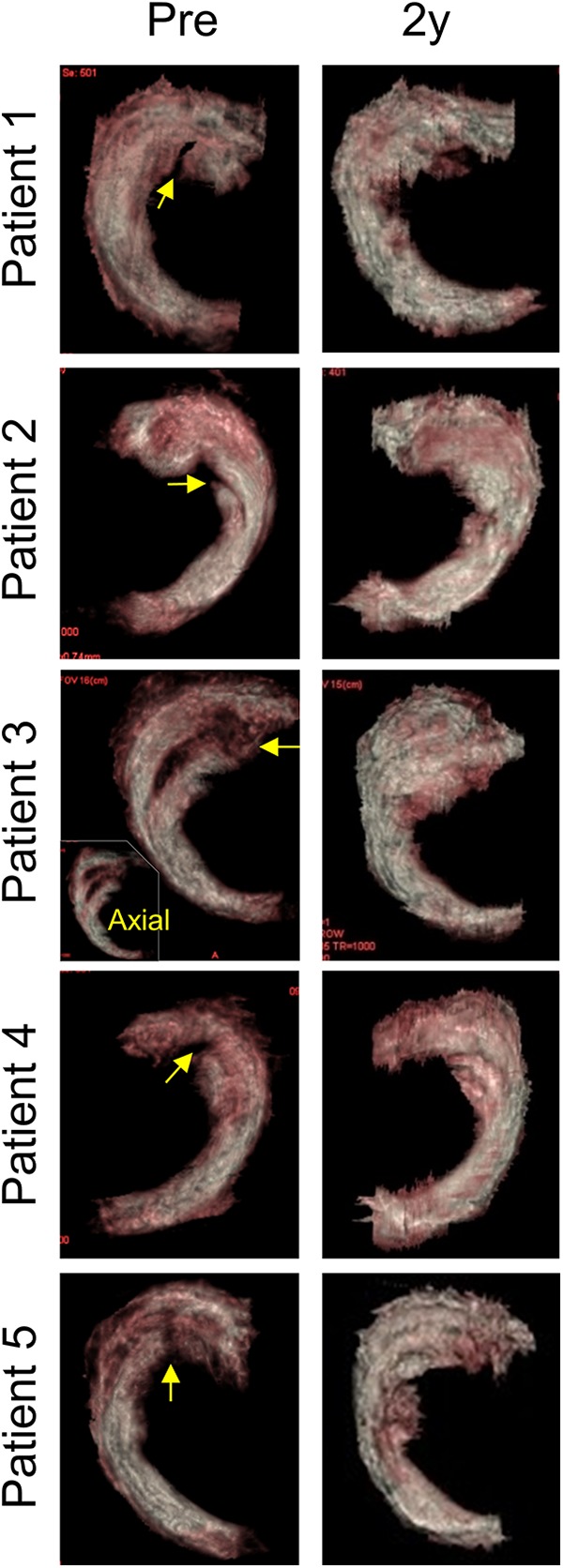
Above view 3D MR images before and after repair with transplantation of synovial MSCs. Meniscus tear is indicated with arrow. Axial view of 3D MR image is also shown in the inset.
Adverse Events
There were no major adverse events leading to study termination. A total of 39 mild adverse events were recorded among the six patients (including one patient for whom transplantation was cancelled because of a cell proliferation problem) (Table 4). The most frequently reported adverse event was knee pain after meniscal repair. There were three adverse events where a relation to the autologous synovial MSC transplantation could not be ruled out. One of them was an increase of c-reactive protein (CRP) (0.8 mg/dL) 14 days after autologous synovial MSC transplantation in patient 1, though an increase of CRP (0.2 mg/dL) was already observed 13 days after the meniscal repair and the CRP returned to a normal level 28 days after the transplantation. Two of the adverse events were effusion and localized warmth of the knee 14 days after autologous synovial MSC transplantation in patient 5, though they could have been due to an additional meniscal repair performed at the same time as MSC transplantation due to suture breakage. The localized warmth of the knee was gone at 12 weeks and the effusion at 24 weeks.
Table 4.
The Number and Rate of Adverse Events. Six patients had blood collection to prepare autologous serum, arthroscopic examination, synovial harvest, and meniscus suture. One among six was discontinued because of cell proliferation problem, and finally, five patients had transplantation of synovial MSCs on the repaired meniscus. Safety had been monitored continually since patients approved for the clinical study until 24 weeks after synovial harvest.
| Site Event | Possibly related to cell trans- plantation (n = 5) | Non-related to cell trans- plantation (n = 6) | Total (n = 6) |
|---|---|---|---|
| Lower extreme | |||
| Knee pain after meniscal repair | 0 (0%) | 6 (100%) | 6 (100%) |
| Knee pain after additional suture of meniscus | 0 (0%) | 1 (17%) | 1 (17%) |
| Knee pain at surgical wounds for scope | 0 (0%) | 4 (67%) | 4 (67%) |
| Joint effusion (affected side) | 1 (20%) | 3 (50%) | 4 (67%) |
| Joint effusion (unaffected side) | 0 (0%) | 1 (17%) | 1 (17%) |
| Localized warmth of knee | 1 (20%) | 0 (0%) | 1 (17%) |
| Retear of sutured meniscus | 0 (0%) | 1 (17%) | 1 (17%) |
| Lower leg pain | 0 (0%) | 1 (17%) | 1 (17%) |
| Lower leg numbness | 0 (0%) | 1 (17%) | 1 (17%) |
| Foot numbness | 0 (0%) | 1 (17%) | 1 (17%) |
| Contact dermatitis of thigh | 0 (0%) | 1 (17%) | 1 (17%) |
| Contact dermatitis of knee | 0 (0%) | 1 (17%) | 1 (17%) |
| Head | |||
| Headache | 0 (0%) | 1 (17%) | 1 (17%) |
| Face | |||
| Eyelid pain | 0 (0%) | 1 (17%) | 1 (17%) |
| Gingivitis | 0 (0%) | 1 (17%) | 1 (17%) |
| Upper extreme | |||
| Contact dermatitis of forearm | 0 (0%) | 1 (17%) | 1 (17%) |
| Others | |||
| Cold symptom | 0 (0%) | 2 (33%) | 2 (33%) |
| Increase of CRP | 1 (20%) | 1 (17%) | 2 (33%) |
| Increase of AST/ALT | 0 (0%) | 1 (17%) | 1 (17%) |
| Increase of LDH | 0 (0%) | 1 (17%) | 1 (17%) |
| Blood pressure increase | 0 (0%) | 1 (17%) | 1 (17%) |
| Blood pressure decrease after blood collection |
0 (0%) | 1 (17%) | 1 (17%) |
Discussion
There have been only a few clinical studies of cell therapy for meniscus injury. Whitehouse et al. inserted autologous bone marrow MSC/collagen-scaffold implants into the meniscal tear and repaired the tear in five patients (ranging from 30–38 years) with an avascular meniscal tear. Patients were followed for 2 years; three patients improved knee function scores, whereas two did not improve due to implant failures21. This method is important in that menisci with avascular meniscal tears were preserved, but we were concerned that preparation of the MSC/collagen-scaffold implant is costly and that insertion of the implant into the meniscal tear is technically demanding. Vangsness et al. injected allogeneic bone marrow MSCs or vehicle into the knee several days after more than 50% of the medial meniscus was resected in 55 patients ranging from 18–60 years. There was a significant reduction of pain in patients injected with MSCs at 2 years22. In our opinion, this study is valuable to demonstrate the safety of allogeneic bone marrow MSCs for meniscus injury; however, a meniscectomy over 50% will increase the risk of osteoarthritis progression, though meniscus volume increased by 15% in 18% patients injected with 50 million MSCs. Two other reports have been published on MSC injection without surgery for meniscus injury. Centeno et al. injected autologous bone marrow MSCs into the knee and showed an increase in meniscus cartilage volume in a 36-year-old patient complaining of knee pain23. Pak et al. injected autologous adipose tissue MSCs into knees with a stable meniscus tear and showed a reduction in knee pain in three patients24.
Previous clinical studies on the meniscus used bone marrow MSCs and adipose tissue MSCs. A comparison of the properties of the synovial MSCs used here with these other two MSC types would be interesting. No comparative studies have been made of trophic factors produced by these MSCs used for meniscus regeneration. According to our studies, the in vitro chondrogenesis potential was higher for human bone marrow MSCs and synovial MSCs than for human adipose tissue MSCs12. Bone marrow MSCs and synovial MSCs gave similar meniscus regeneration in a rat menisectomized model13. Higher yields of MSCs can be obtained from the synovium than from the bone marrow in humans11. Our previous studies showed that synovial MSCs were most useful, but trophic factors were not examined.
We isolated and prepared synovial MSCs by plating synovial nucleated cells at relatively low density, allowing cell formation, and then collecting the colonies for transplantation. This method did not require any sorting process with a specific tool. Cells prepared by this method have similar surface markers to MSCs and show multidifferentiation potential, including chondrogenesis25.
In this study, we used autologous human serum instead of fetal bovine serum (FBS), since clinical application of stem cells expanded with FBS may have some problems, including possible viral, bacterial, and prion transmission from cows26. Another potential problem with the use of FBS is an immune reaction from contamination with the highly immunogenic sialic acid derived from cows27. These problems can be overcome with the use of autologous human serum, which is recommended for the isolation and expansion of human MSCs.
For transplantation of synovial MSCs onto the repaired meniscus, a suspension of synovial MSCs (32–70 million cells/0.5 ml) was placed onto the repaired meniscus through an 18-gauge needle attached to a 1 mL syringe. This procedure was performed under arthroscopy without irrigation fluid and the patient was maintained in position for 10 min. This transplantation method was verified by a preclinical study18 using microminipigs, in which a longitudinal tear lesion was made in the avascular zone of the medial meniscus and sutured, and then a suspension of synovial MSCs labeled with green fluorescence protein (GFP) was placed onto the repaired meniscus for 10 min. After the knee was flexed and extended 100 times and washed with fluid, GFP-positive cells could be observed in the meniscus lesion and synovium around the lesion. This animal study concluded that transplantation of synovial MSCs promoted healing after meniscal repair by induction of synovium into the meniscal tear19. This method was also used clinically to repair cartilage defects20.
The most frequently reported adverse events in the present study were “knee pain after meniscal repair” and “knee pain at surgical wounds for scope”; these were recorded in all cases related. Most cases still complained of “knee pain after meniscal repair” at 24 weeks, although the pain decreased with time. “Knee pain at surgical wounds for scope” occurred at the lateral and medial portals for cell transplantation but improved in a short span of time. The third most frequently reported adverse event was “joint effusion.” Two cases with joint effusion at baseline still had joint effusion at 24 weeks. One case that had no joint effusion at baseline had a joint effusion at 12 weeks. Joint effusion at baseline could therefore be a risk factor for prolonged joint effusion.
We propose two possible effects of synovial MSCs on repaired meniscus. First, synovial MSCs attached have the potential to differentiate into meniscus cells. According to a previous study, LacZ synovial MSCs could be observed in the regenerated meniscus at 12 weeks after transplantation in a rat model14. The second possibility is that MSCs produce a wide variety of cytokines and other trophic support factors. Previous species-specific microarray analyses showed that synovial MSCs increased the gene expression of hundreds of genes, including PRG4, BMP2, and TSG-6, after transplantation into the knee in a rat osteoarthritis model28. One of the important cytokine effects caused by synovial MSCs was the induction of synovial tissue migration to the meniscus lesion or the formation of synovial tissue in the meniscus lesion in a pig model19.
Our study had the following limitations. A control group that underwent meniscal repair without MSC transplantation should have been included to clarify the effect of synovial MSCs. The effectiveness of our treatment was evaluated by clinical evaluation scores, but second-look arthroscopy should also have been performed. The ability of the repaired meniscus to maintain a function similar to a stable meniscus with age was not assessed, nor was the ability to prevent the progression of osteoarthritis.
This is the first in-human study to explore the possibility that a combination of surgical repair and synovial MSC transplantation might be an effective treatment strategy for a complex degenerative tear of the medial meniscus. No adverse events occurred that necessitated treatment discontinuation. These findings will serve as pilot data for a future prospective study.
Acknowledgments
We thank Mika Watanabe and Kimiko Takanashi for the management of our laboratory; Benjamin Larson and Ellen Roider for proofreading of the paper.
Footnotes
Ethical Approval: Ethical approval to report this case was obtained from the Medical Research Ethics Committee of the Tokyo Medical and Dental University for Human Stem Cell Clinical Research (No. 2).
Statement of Human and Animal Rights: All procedures in this study were conducted in accordance with the protocol approved by the Medical Research Ethics Committee of the Tokyo Medical and Dental University for Human Stem Cell Clinical Research (No. 2).
Statement of Informed Consent: Written informed consent was obtained from the patients for their anonymized information to be published in this article.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research and/or authorship of this article: This research was supported by the Japan Agency for Medical Research and Development (AMED) under Grant Number 19bk0104012h0002 to IS.
ORCID iD: Ichiro Sekiya  https://orcid.org/0000-0002-6331-722X
https://orcid.org/0000-0002-6331-722X
References
- 1. Fox AJ, Wanivenhaus F, Burge AJ, Warren RF, Rodeo SA. The human meniscus: a review of anatomy, function, injury, and advances in treatment. Clin Anat. 2015;28(2):269–287. [DOI] [PubMed] [Google Scholar]
- 2. Beaufils P, Becker R, Kopf S, Matthieu O, Pujol N. The knee meniscus: management of traumatic tears and degenerative lesions. EFORT Open Rev. 2017;2(5):195–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Lamplot JD, Brophy RH. The role for arthroscopic partial meniscectomy in knees with degenerative changes: a systematic review. Bone Joint J. 2016;98-B(7):934–938. [DOI] [PubMed] [Google Scholar]
- 4. Sihvonen R, Paavola M, Malmivaara A, Itälä A, Joukainen A, Nurmi H, Kalske J, Järvinen TL; Finnish Degenerative Meniscal Lesion Study (FIDELITY) Group. Arthroscopic partial meniscectomy versus sham surgery for a degenerative meniscal tear. N Engl J Med. 2013;369(26):2515–2524. [DOI] [PubMed] [Google Scholar]
- 5. Katz JN, Brophy RH, Chaisson CE, de Chaves L, Cole BJ, Dahm DL, Donnell-Fink LA, Guermazi A, Haas AK, Jones MH, Levy BA, et al. Surgery versus physical therapy for a meniscal tear and osteoarthritis. N Engl J Med. 2013;368(18):1675–1684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Beaufils P, Becker R, Kopf S, Englund M, Verdonk R, Ollivier M, Seil R. Surgical management of degenerative meniscus lesions: the 2016 ESSKA meniscus consensus. Knee Surg Sports Traumatol Arthrosc. 2017;25(2):335–346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. King D. The healing of semilunar cartilages. 1936. Clin Orthop Relat Res. 1990;(252):4–7. [PubMed] [Google Scholar]
- 8. Cisa J, Basora J, Madarnas P, Ghibely A, Navarro-Quilis A. Meniscal repair by synovial flap transfer. Healing of the avascular zone in rabbits. Acta Orthop Scand. 1995;66(1):38–40. [DOI] [PubMed] [Google Scholar]
- 9. Jitsuiki J, Ochi M, Ikuta Y. Meniscal repair enhanced by an interpositional free synovial autograft: an experimental study in rabbits. Arthroscopy. 1994;10(6):659–666. [DOI] [PubMed] [Google Scholar]
- 10. Uchio Y, Ochi M, Adachi N, Kawasaki K, Iwasa J. Results of rasping of meniscal tears with and without anterior cruciate ligament injury as evaluated by second-look arthroscopy. Arthroscopy. 2003;19(5):463–469. [DOI] [PubMed] [Google Scholar]
- 11. Nimura A, Muneta T, Koga H, Mochizuki T, Suzuki K, Makino H, Umezawa A, Sekiya I. Increased proliferation of human synovial mesenchymal stem cells with autologous human serum: comparisons with bone marrow mesenchymal stem cells and with fetal bovine serum. Arthritis Rheum. 2008;58(2):501–510. [DOI] [PubMed] [Google Scholar]
- 12. Sakaguchi Y, Sekiya I, Yagishita K, Muneta T. Comparison of human stem cells derived from various mesenchymal tissues: superiority of synovium as a cell source. Arthritis Rheum. 2005;52(8):2521–2529. [DOI] [PubMed] [Google Scholar]
- 13. Horie M, Sekiya I, Muneta T, Ichinose S, Matsumoto K, Saito H, Murakami T, Kobayashi E. Intra-articular injected synovial stem cells differentiate into meniscal cells directly and promote meniscal regeneration without mobilization to distant organs in rat massive meniscal defect. Stem Cells. 2009;27(4):878–887. [DOI] [PubMed] [Google Scholar]
- 14. Hatsushika D, Muneta T, Horie M, Koga H, Tsuji K, Sekiya I. Intraarticular injection of synovial stem cells promotes meniscal regeneration in a rabbit massive meniscal defect model. J Orthop Res. 2013;31(9):1354–1539. [DOI] [PubMed] [Google Scholar]
- 15. Hatsushika D, Muneta T, Nakamura T, Horie M, Koga H, Nakagawa Y, Tsuji K, Hishikawa S, Kobayashi E, Sekiya I. Repetitive allogeneic intraarticular injections of synovial mesenchymal stem cells promote meniscus regeneration in a porcine massive meniscus defect model. Osteoarthritis Cartilage. 2014;22(7):941–950. [DOI] [PubMed] [Google Scholar]
- 16. Kondo S, Muneta T, Nakagawa Y, Koga H, Watanabe T, Tsuji K, Sotome S, Okawa A, Kiuchi S, Ono H, Mizuno M, et al. Transplantation of autologous synovial mesenchymal stem cells promotes meniscus regeneration in aged primates. J Orthop Res. 2017;35(6):1274–1282. [DOI] [PubMed] [Google Scholar]
- 17. Matsukura Y, Muneta T, Tsuji K, Koga H, Sekiya I. Mesenchymal stem cells in synovial fluid increase after meniscus injury. Clin Orthop Relat Res. 2014;472(5):1357–1364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Sekiya I, Ojima M, Suzuki S, Yamaga M, Horie M, Koga H, Tsuji K, Miyaguchi K, Ogishima S, Tanaka H, Muneta T. Human mesenchymal stem cells in synovial fluid increase in the knee with degenerated cartilage and osteoarthritis. J Orthop Res. 2012;30(6):943–949. [DOI] [PubMed] [Google Scholar]
- 19. Nakagawa Y, Muneta T, Kondo S, Mizuno M, Takakuda K, Ichinose S, Tabuchi T, Koga H, Tsuji K, Sekiya I. Synovial mesenchymal stem cells promote healing after meniscal repair in microminipigs. Osteoarthritis Cartilage. 2015;23(6):1007–1017. [DOI] [PubMed] [Google Scholar]
- 20. Sekiya I, Muneta T, Horie M, Koga H. Arthroscopic transplantation of synovial stem cells improves clinical outcomes in knees with cartilage defects. Clin Orthop Relat Res. 2015;473(7):2316–2326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Whitehouse MR, Howells NR, Parry MC, Austin E, Kafienah W, Brady K, Goodship AE, Eldridge JD, Blom AW, Hollander AP. Repair of torn avascular meniscal cartilage using undifferentiated autologous mesenchymal stem cells: from in vitro optimization to a first-in-human study. Stem Cells Transl Med. 2017;6(4):1237–1248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Vangsness CT, Jr, Farr J, 2nd, Boyd J, Dellaero DT, Mills CR, LeRoux-Williams M. Adult human mesenchymal stem cells delivered via intra-articular injection to the knee following partial medial meniscectomy: a randomized, double-blind, controlled study. J Bone Joint Surg Am. 2014;96(2):90–98. [DOI] [PubMed] [Google Scholar]
- 23. Centeno CJ, Busse D, Kisiday J, Keohan C, Freeman M, Karli D. Regeneration of meniscus cartilage in a knee treated with percutaneously implanted autologous mesenchymal stem cells. Med Hypotheses. 2008;71(6):900–908. [DOI] [PubMed] [Google Scholar]
- 24. Pak J, Lee JH, Lee SH. Regenerative repair of damaged meniscus with autologous adipose tissue-derived stem cells. Biomed Res Int. 2014;2014:436029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Nakamura K, Tsuji K, Mizuno M, Koga H, Muneta T, Sekiya I. Initial cell plating density affects properties of human primary synovial mesenchymal stem cells. J Orthop Res. 2019;37(6):1358–1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Will RG, Ironside JW, Zeidler M, Cousens SN, Estibeiro K, Alperovitch A, Poser S, Pocchiari M, Hofman A, Smith PG. A new variant of Creutzfeldt-Jakob disease in the UK. Lancet. 1996;347(9006):921–925. [DOI] [PubMed] [Google Scholar]
- 27. Martin MJ, Muotri A, Gage F, Varki A. Human embryonic stem cells express an immunogenic nonhuman sialic acid. Nat Med. 2005;11(2):228–232. [DOI] [PubMed] [Google Scholar]
- 28. Ozeki N, Muneta T, Koga H, Nakagawa Y, Mizuno M, Tsuji K, Mabuchi Y, Akazawa C, Kobayashi E, Matsumoto K, Futamura K, et al. Not single but periodic injections of synovial mesenchymal stem cells maintain viable cells in knees and inhibit osteoarthritis progression in rats. Osteoarthritis Cartilage. 2016;24(6):1061–1070. [DOI] [PubMed] [Google Scholar]