Abstract
Progenitor/stem cell-based kidney regenerative strategies are a key step towards the development of novel therapeutic regimens for kidney disease treatment. However, the route of cell delivery, e.g., intravenous, intra-arterial, or intra-parenchymal, may affect the efficiency for kidney repair in different models of acute and chronic injury. Here, we describe a protocol of intra-aorta progenitor/stem cell injection in rats following either acute ischemia-reperfusion injury or acute proteinuria induced by puromycin aminonucleoside (PAN) – the experimental prototype of human minimal change disease and early stages of focal and segmental glomerulosclerosis. Vascular clips were applied across both renal pedicles for 35 min, or a single dose of PAN was injected via intra-peritoneal route, respectively. Subsequently, 2 x 106 stem cells [green fluorescent protein (GFP)-labeled c-Kit+ progenitor/stem cells or GFP-mesenchymal stem cells] or saline were injected into the suprarenal aorta, above the renal arteries, after application of a vascular clip to the abdominal aorta below the renal arteries. This approach contributed to engraftment rates of ∼10% at day 8 post ischemia-reperfusion injury, when c-Kit+ progenitor/stem cells were injected, which accelerated kidney recovery. Similar rates of engraftment were found after PAN-induced podocyte damage at day 21. With practice and gentle surgical technique, 100% of the rats could be injected successfully, and, in the week following injection, ∼ 85% of the injected rats will recover completely. Given the similarities in mammals, much of the data obtained from intra-arterial delivery of progenitor/stem cells in rodents can be tested in translational research and clinical trials with endovascular catheters in humans.
Keywords: progenitor/stem cells, acute kidney injury, ischemia-reperfusion injury, acute proteinuria, engraftment, surgical technique
Introduction
Chronic kidney disease (CKD) is a worldwide public health problem that affects millions of people of all ages, and racial and ethnic groups. CKD is incurable, requiring renal replacement therapy, that is, dialysis or, preferably, renal transplantation. However, the critical shortage of organs available for transplantation continues to severely limit this latter option1, underlying the importance of novel therapeutic regimens, such as progenitor/stem cell therapy. Mesenchymal stem cells (MSCs) have been employed therapeutically in different models of acute and chronic kidney injury. MSCs are involved in distinct mechanisms of kidney tissue repair, including not only paracrine/endocrine effects – notably immunomodulatory effects2–4 – but also low rates of tubular engraftment and differentiation5–7. In addition, the search for putative stem cells or precursors within the kidney has been the focus of extensive research. Identification of progenitor/stem cell populations in mammalian tissues is important for therapeutic applications, including the generation of new tubular, glomerular, and vascular cells for the treatment of either acute or chronic kidney injury, and also for understanding developmental processes and tissue homeostasis8. Furthermore, pursuing progenitor/stem cell-based kidney regenerative strategies is a key step towards the development of bioengineered transplantable kidneys, or kidney parts, including organoids, scaffolds, and biological devices9,10.
Of importance, efficiency of progenitor/stem cell-mediated repair is influenced by many variables, including not only the route of administration, but also the number and type of cells, number of cell injections, potency of cells, the viability of these cells, homing capacity, and severity of kidney damage11. Three major routes have been described for progenitor/stem cells delivery into the kidneys: intravenous, intra-arterial, and intra-parenchymal.
Here, we describe a step-by-step protocol for ischemia-reperfusion injury in rats followed by suprarenal aorta infusion of two different progenitor/stem cells – MSCs or kidney derived c-Kit progenitor/stem cells – immediately after the reperfusion period. Likewise, we investigated if the suprarenal route was also effective following the acute proteinuria model induced by PAN – the experimental prototype of human minimal change disease and early stages of focal and segmental glomerulosclerosis. c-Kit cells are a novel population of kidney-specific progenitor/stem cells with regenerative potential12. We describe the steps that are most critical and require caution, and a troubleshooting guide. By using the suprarenal aorta route, we were able to observe improvement in renal function and cell engraftment into damaged kidney in an acute ischemia-reperfusion model13, and in an acute proteinuria model induced by PAN14.
Materials and Methods
Cell Preparation (Timing 30–35 min)
Cell preparation is a key procedure for cell transplantation. The goal here is to work quickly and have a high rate of cell survival (viability >70%) for best results. The thawing procedure is stressful to frozen cells, and using good technique and working quickly ensures that a high proportion of the cells survive the procedure. Rat kidney c-Kit-derived progenitor/stem cells and rat MSCs were frozen in cell freezing medium containing 10% DMSO (dimethyl sulfoxide; Sigma-Aldrich, St. Louis, MO, USA) diluted in 90% fetal bovine serum (HyClone, Thermo Fisher Scientific, Logan, UT, USA) and maintained in liquid nitrogen. Cells were frozen at a concentration of 2.3–2.5 x 106 cells/ml of cell freezing solution. The thawing procedure is described in detail below.
1. Remove the cryovial containing the desired frozen cells from liquid nitrogen storage and place immediately into a 37°C water bath.
2. Thaw cells quickly (± 1 min) by gently swirling the vial in the 37°C water bath. Cells should be removed from the 37°C water bath before they are completely thawed, when there is just a small bit of ice left in the vial.
3. Before opening the cryovial, wipe the outside of the vial with 70% ethanol and transfer the cells to a biosafety cabinet. Re-suspend the cells in 5 ml of DPBS (Dulbecco’s phosphate buffered saline, calcium and magnesium free; Invitrogen, Carlsbad, CA, USA) at room temperature, and transfer them to a 15 ml centrifuge tube.
4. Mix the cells very gently, and centrifuge the cell suspension at 500 x g for 5 min. After centrifugation, check the clarity of the supernatant and that a complete pellet is visible.
5. Remove the supernatant aseptically without disturbing the pellet.
6. Add 5 ml of DPBS, mix gently, and centrifuge again at 500 x g for 5 min to remove any leftover cell freezing solution.
7. Remove the supernatant, re-suspend the pellet with 1 ml DPBS, and then pass the cell solution through the cell strainer cap tube (35 µm). Count the number of cells using a hemocytometer and check cell viability by Trypan blue exclusion.
8. Transfer the desired number of cells into a sterile, 5-ml round-bottom tube, and centrifuge again at 500 x g for 5 min.
9. Remove the supernatant and finally re-suspend the cells in a final concentration of 2 x 106 in 300 µL of saline.
10. Keep the cells on ice or 4°C until loaded into the insulin syringe. Keeping the cells on ice avoids cluster formation and maintain viability. Do not wait longer than 3 h to inject the cells to prevent a large decrease in viability.
Equipment Setup, Surgical Kit Setup, and Animal Procedures
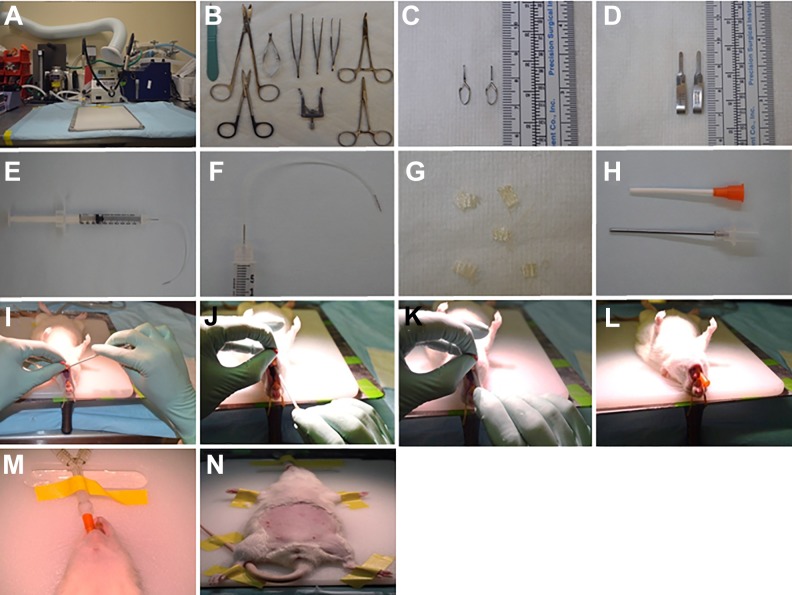
Equipment setup includes the surgical station (Fig. 1A), sterilization of the surgical kit (Fig. 1B–D), and preparation of the syringe for progenitor/stem cell or saline injection. Connect a BD ultra-fine insulin syringe (31-gauge) to sterile Tygon flexible plastic tubing (about 7 cm long). Remove a needle (31-gauge) from another BD ultra-fine insulin syringe using mosquito forceps. Insert this needle into the other side of the Tygon flexible plastic tubing (Harvard apparatus, Holliston, MA, USA). The system is now ready to be loaded with progenitor/stem cells or saline (Fig. 1E, F). In addition, cut small squares (∼ 0.5 x 0.5 cm) of Surgicel Nu-Knit absorbable hemostat (oxidized regenerated cellulose; Ethicon) for bleeding control (Fig. 1G).
Fig. 1.
Anesthesia, surgery equipment, and rat intubation. (A) Table, heating blanket, closed-circuit anesthesia system with isofluorane vaporizer, rat ventilator, and oxygen regulator. (B) Typical surgical tray setup with no. 11 scalpel, Metzenbaum scissors, micro dissection scissors, Vannas eyes scissors, Semken forceps, Graefe forceps, and Alm retractor. (C) Vessel clips used for infra-renal aorta clamping. (D) Schwartz temporary clips used for renal pedicle clamping during ischemic phase of ischemia-reperfusion injury. (E, F) Insulin syringe is connected to a Tygon flexible plastic tubing (about 7 cm long) that has already been connected to a BD ultra-fine needle (31-gauge). That syringe is used for loading stem cells or saline to be delivered into the aorta. (G) Single squares of Surgicel Nu-Knit absorbable hemostat. (H) Angiocath 14-gauge used for intubation. (I–L) Intubation procedure: rolling the tongue out of the mouth, insertion of the endotracheal tube (angiocath) directly into the illuminated space visible when the glottis is open, and placement of the endotracheal catheter to the point at which its proximal end is level with the superior incisors. (M) The animal is connected to the ventilator. Visualization of thoracic movement synchronized to the ventilator is confirmatory of endotracheal intubation. (N) The animal is taped and shaved for the surgery.
Female 2-month-old Sprague-Dawley (SD) rats (Charles River, Wilmington, MA, USA), weighing 200–250 g, were divided randomly into groups to receive kidney-derived c-Kit+ cells (n = 8), MSCs (n = 6), or saline (n = 12). The animals received standard diet and water ad libitum. For all experiments, rats were anesthetized, intubated endotracheally, and placed on mechanical ventilation (2% isoflurane and 100% oxygen). The animals were placed onto a thermostatically controlled heating mat, and body temperature was maintained at 38 ± 1°C by means of a rectal probe attached to a thermal blanket. A midline incision was performed, and nontraumatic vascular clips were applied across both renal pedicles. We clamped the renal pedicles for 35 min, and followed the rats for 8 days. Occlusion was verified visually by a change in color of the kidneys from their normal brown to dark purple. Reperfusion commenced once the artery clips were removed, and was confirmed visually by a return to normal kidney color. Subsequently, a vascular clamp was applied to the abdominal aorta below the renal arteries, and 2 x 106 cells [green fluorescent protein (GFP)-labeled c-Kit+ cells or bone-marrow-derived MSCs from GFP-SD rats, male 2-month-old SD rats, Charles River), re-suspended in a total volume of 300 µl of saline, were injected directly into the abdominal aorta above the renal arteries using a 31-gauge insulin syringe needle (BD Biosciences). The same volume of saline (300 µl) was injected into the aorta in the control group.
Blood collection was performed at different time-points: baseline (immediately prior to cell injection), and days 1, 2, 4, and 8 post ischemia-reperfusion injury. Creatinine and blood urea nitrogen (BUN) were measured at each time point (Products Vitros Chemistry, Rochester, NY, USA). Kidneys were harvested after 8 days for histological analyses.
Preparation (Pre-Anesthesia, Anesthesia, and Intubation; Timing 4–7 min)
11. Rats are kept in polycarbonate cages containing sterile bedding and access to water and rodent feed ad libitum. There is no need to fast the rats.
12. Weigh the animals to determine the amount of heparin (0.2 U/g; APP Pharmaceuticals, LLC) and buprenorphine hydrochloride (0.05–0.1 mg/kg; Webster Veterinary) to be used.
13. Anesthesia is induced in a chamber with isoflurane 3–5% for 4–6 min. Once the animal is immobilized by anesthesia, transfer it to the surgery area.
14. Place the animal, under sedation, in a supine position, and place an elastic (rubber) band over the incisors to secure the maxilla. A flexible fiber-optic light source is positioned 3–4 cm from the anterior neck for trans-illumination through the pharyngoepiglottic region. This step should be performed very quickly to avoid superficial anesthesia level and, thus, more difficult intubation.
15. Use a cotton stick to roll the tongue out of the mouth (Fig. 1I). Once the unobstructed pharyngoepiglottis is visualized, insert the endotracheal tube (angiocath; BD Becton) directly into the illuminated space visible when the glottis is open and the vocal cords are easily identified (Fig. 1 J). Placement of the catheter into the trachea is associated with a palpable step-like sliding of the catheter along the rings of the trachea (Fig. 1 K). The endotracheal catheter is then advanced into the trachea to the point at which its proximal end is level with the superior incisors (Fig. 1 L). Visualization of thoracic movement synchronized with the ventilator is confirmatory of endotracheal intubation. At this point, tape the catheter securely in place (Fig. 1 M, N).
16. Mechanical ventilation is initiated at a rate of 85–90 bpm, tidal volume ∼2.5 mL (10 ml/kg), and a mixture of oxygen and isoflurane (1.5–2%).
17. Following intubation, rats are placed on a warming blanket and maintained at 38 ± 1°C.
Ischemia-Reperfusion Injury and Progenitor/Stem Cell Injection Into Suprarenal Aorta (Timing 80–90 min)
18. Verify the adequacy of anesthesia by pinching the toe using forceps. Adequate anesthesia is characterized by a lack of response, that is, an absence of withdrawal of the extremity to pinch.
19. Use an electric trimmer to remove hair from the entire abdomen (Fig. 1 N). Remaining hair can be removed by using commercially available creams (e.g., Nair). Lubricate both eyes with eye ointment because dehydration of the eyes can cause permanent damage.
20. Disinfect the skin with iodo-alcohol to establish an aseptic field.
21. Using an insulin syringe, inject heparin, 40–50 U (0.2 U/g), via IP route.
22. Make a mid-line abdominal skin incision (about 6–7 cm long) using a No.11 stainless steel disposable scalpel. The skin incision is started just above the xiphoid process toward the lower abdomen. Then, use Semken forceps to lift the muscle and peritoneum, and cut them using Metzenbaum scissors, starting from the lower abdomen toward the xiphoid process, to expose the abdominal organs. Spare the large vessels by performing a mid-line incision in the abdominal wall following the linea alba. Be careful not to injury any underlying organs when performing the laparotomy, as this would necessitate excluding the rat from the experiment. Injury can be prevented by lifting the abdominal wall with forceps and bluntly opening the peritoneal cavity. Before enlarging the laparotomy incision, wait for air to flow into the abdomen and for any organs adhering to the anterior wall to be released.
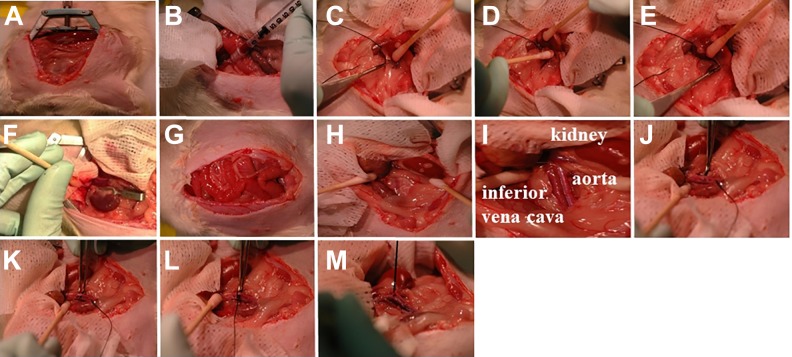
23. Expose the abdominal cavity using an abdominal retractor (Fig. 2A). Retract the intestine and cover with wet gauze. Move intestines to the left side (of the rat). Allowing the intestine to come out of the abdominal cavity can cause significant heat loss to the animal due to drying of the tissues. If drying is noted, gently moisten the tissues with warm saline.
24. Identify the portal vein and collect blood (200–300 μl) using an insulin syringe (Fig. 2B). Apply gentle compression using a cotton stick for ∼ 2 min.
25. Make sure that no bleeding arises from the portal vein. Place a square piece of the Surgicel Nu-Knit absorbable hemostat on top of the site where blood was collected, and proceed to the next step.
26. Remove the perirenal fat from the right kidney using cotton sticks and Vannas eyes scissors. Avoid large vessels by the eye. Be careful not to injury small arteries of the intra-peritoneal adipose tissue surrounding the kidneys. Be careful not to cause kidney torsion, which would necessitate excluding the rat from the experiment. Be very gentle when manipulating the kidneys.
27. Retract the intestines to the right side. Keep intestines covered with wet gauze at all times.
28. Remove the perirenal fat from the left kidney using cotton sticks and Vannas micro-dissecting (eye) scissors (Fig. 2C–E).
29. Apply the non-traumatic clip across both renal pedicles for 35 min (Schwartz temporary clip, straight, smooth; 795 g pressure, 1.7 mm jaw width, 8 mm jaw length, 1" clip length (Roboz, Gaithersburg, MD, USA; Fig. 2F). Occlusion is verified visually by a change in the color of the kidneys from brown to a dark purple. Return the intestines to the abdominal cavity and leave wet gauze covering the organs (Fig. 2G).
30. After 35 min, retract the intestines, keep them covered with wet gauze, and move them to the left side. Remove the non-traumatic clip from the right kidney, and, after moving the intestine to the right side, remove the clip from the left kidney.
31. Kidney reperfusion is confirmed visually by the normal color returning to the kidneys. Kidney reperfusion does not take long, but some areas of the kidney can take more time to become reperfused. Wait at least 10 min before moving to the next step.
32. Expose the infrarenal aorta and the inferior vena cava between the renal vessels and the iliac bifurcation using cotton sticks (Fig. 2 H, I).
33. Separate the infrarenal aorta and the inferior vena cava, ∼1.5 cm below the left renal vein, using Graefe tissue forceps (long full curve) (Fig. 2 J, K). Then, pass a silk suture (without needle) between them (Fig. 2 L, M). This step is critical because bleeding can occur from the inferior vena cava.
34. Place a damp gauze inside the cavity covering the stomach and spleen, and move these organs gently upward using cotton sticks.
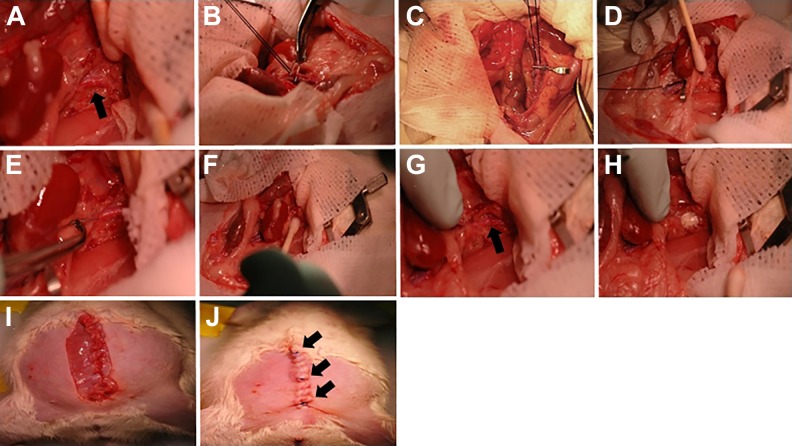
35. Identify and dissect the suprarenal aorta using cotton sticks (Fig. 3A).
36. Take the progenitor/stem cells out of the ice ∼5 min before injection in order to let them reach room temperature (± 23–25°C).
37. Using a filter pipette tip, gently mix the progenitor/stem cells by pipetting up and down ∼10 times. When mixing the progenitor/stem cells, avoid forming air bubbles.
38. Load the syringe by aspirating 300 µL of the progenitor/stem cell suspension or saline. Make sure that there are no air bubbles inside the syringe.
39. Connect the insulin syringe to the Tygon flexible plastic tubing already connected to a BD ultra-fine needle (31-gauge). Gently load the syringe with the progenitor/stem cell suspension or saline. Progenitor/stem cells or saline are now ready to be delivered.
40. Hold the silk suture between the infrarenal aorta and inferior vena cava, and, using mosquito forceps, grab the vascular clip (Vessel clips, jaw length 4 mm, width of jaw 0.75 mm, locking pressure 125 g; Harvard apparatus, Holliston, MA, USA), and proceed to clamp the infrarenal aorta (Fig. 3B, C).
41. Move the left kidney gently to the right, and keep it covered with wet gauze (Fig. 3D).
42. For intra-aorta progenitor/stem cell or saline injection, use Graefe tissue forceps, serrated slight curve, to hold the needle connected to the system including the Tygon plastic tubing and the insulin syringe. Introduce the needle into the suprarenal aorta, and deliver the progenitor/stem cells or saline very slowly (over ∼30 sec) (Fig. 3E). Immediately after placing the needle into the suprarenal aorta, the blood will travel into the tubing prior to delivering the cells. To ensure that the needle is placed correctly inside the aorta, gently aspirate until the blood enters the tubing when progenitor/stem cells or saline are being delivered (every 100 μl injected and at the end; three times in total).
43. After delivering progenitor/stem cells or saline, remove the needle and gently press a cotton stick on top of the injection site for 5–10 min (Fig. 3F). At the same time, remove the infrarenal clip and the silk suture. If no bleeding arises from the suprarenal aorta after 5–10 min (Fig. 3G), add a square piece of Surgicel Nu-Knit absorbable hemostat on top of the injection site (Fig. 3 H). After stopping the bleeding, add ∼1.0 ml of saline IP and observe if bleeding occurs. If bleeding restarts, repeat step 43.
44. Return the intestines to the abdominal cavity when no bleeding from the aorta is observed.
45. Close the peritoneum using the continuum suture technique (Fig. 3I). Perform isolated suturing of the upper, middle, and lower thirds of the peritoneum for safety. Close the skin using the same technique used for closing the peritoneum, including the safety sutures (Fig. 3 J, arrows). Inject Buprenorphine (0.05–0.1 mg/kg) via the SC route.
46. After closing the abdomen, use saline to clean the skin. Next, wipe the skin surrounding the suture with iodo-alcohol and place the animal on a warming blanket for recovery as soon as the animal exhibits signs of muscle contraction, that is, twitching, shivering, etc.
47. Rats are allowed to recover following surgery in a warm recovery chamber containing oxygen (for ∼30–60 min).
48. Rats can be transferred back to their cages when they are moving normally and do not exhibit signs of pain. It is recommended that the animals be kept in separate cages during the first 3–4 days post-operatively in order to avoid them chewing each other’s sutures, resulting in suture dehiscence. After this period, the animals can be kept in pairs for social environment purposes.
49. Add hydro gel packs to the cage to prevent post-operative dehydration. The animals can receive standard diet and water ad libitum.
Fig. 2.
Ischemia-reperfusion surgery. (A) Exposure of the abdominal cavity using the Alm retractor. (B) Blood is collected from the portal vein. (C–E) Perirenal fat is removed using cotton sticks and the Vannas micro-dissecting (eye) scissors. (F) Schwartz temporary clips are used for renal pedicle clamping. (G) During ischemia, the intestines are returned to the abdomen. Following ischemia, Schwartz temporary clips are removed and reperfusion is visually observed. (H, I) Inferior vena cava and infrarenal aorta are identified and dissected using cotton sticks. (J–M) Inferior vena cava and infrarenal aorta are separated using Graefe forceps long full curve, and a silk suture is passed between them.
Fig. 3.
Progenitor/stem cell or saline injection. (A) Suprarenal aorta is identified (arrow) and overlying tissues dissected away. (B, C) A silk suture is held between the infrarenal aorta and inferior vena cava. Using mosquito forceps, a vascular clip is applied for infrarenal aorta clamping. (D) The left kidney is moved gently to the right side and kept covered with wet gauze. (E) Progenitor/stem cells or saline are injected into suprarenal aorta using an insulin needle (31-gauge) connected to Tygon plastic tubing and an insulin syringe. (F) Suprarenal aorta is compressed gently using a cotton stick after progenitor/stem cells or saline injection. (G) Verification that no bleeding arises from the suprarenal aorta (arrow). (H) A square piece of Surgicel Nu-Knit absorbable hemostat is added on top of the segment of the suprarenal aorta where progenitor/stem cells or saline were injected. (I, J) Abdominal suturing is performed in layers. Safety suturing is also performed in the lower, middle and upper thirds to avoid dehiscence (arrows).
Kidney Perfusion Through Heart and Rat Euthanasia (Timing 12–15 min)
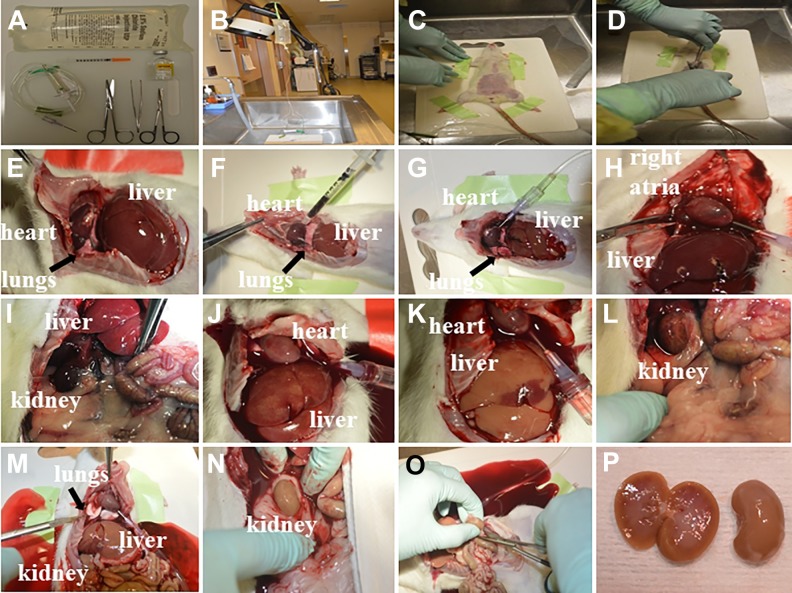
50. Dilute heparin in a saline flask (1 ml of heparin per 100 ml of saline), and mix by inverting the flask several times. Prepare the saline connected to an intravenous set. Surgical instruments include Mayo scissors, micro dissecting scissors, and Semken forceps (Fig. 4A). Cut four pieces of tape.
51. When sacrificing the rats, anesthesia is induced in a chamber with isoflurane 2–5% for 3–5 min. Once the animal is immobilized by anesthesia, inject the Euthasol solution (0.22 mL/kg) IP. The sequence of events leading to humane, painless, and rapid euthanasia following IP injection of Euthasol solution is unconsciousness with simultaneous collapse of the rat, which is induced within ∼3–5 min by pentobarbital sodium.
52. When the rat is breathing slowly, transfer the animal to the sink (Fig. 4B), check the level of anesthesia as described previously, and tape its limbs to the dissecting tray (Fig. 4C).
53. Grab the skin with forceps at the level of the diaphragm (Fig. 4D), and cut the chest to expose the heart. Cut laterally and then up, cutting through the ribs. Lift chest flap and continue cutting until the heart is easy to access (Fig. 4E). Secure the chest flap using the forceps. Cut the diaphragm. Collect the blood from the left ventricle (300–500 μl) using an insulin syringe (31-gauge) (Fig. 4F).
54. Insert a needle (18-gauge) into the left ventricle and completely open the roller clamp (Fig. 4G). Observe if the saline solution is filling the drip chamber. Next, cut the right atria using micro-dissecting scissors (Fig. 4 H).
55. Open the abdomen and verify that not only the liver is being perfused but also the kidneys (Fig. 4I–N). The lungs should change color from pink to white (Fig. 4 M).
56. When the kidneys are well perfused, remove them by cutting the renal pedicles (Fig. 4O). In the back-table (Fig. 4P), remove the perirenal fat, weigh the kidneys, and transfer the kidneys to liquid nitrogen, 10% buffered-formalin, or 4% paraformaldehyde, depending on further analyses (Fig. 5).
Fig. 4.
Kidney perfusion through the heart. (A) Typical surgical tray setup with Metzenbaum scissors, micro dissection scissors, Semken forceps, saline plastic bag, intravenous set, heparin, and insulin syringe (31-gauge). (B) Area used for euthanasia, including a sink and a support for hanging the saline plastic bag connected to the intravenous set. (C) The rat is taped onto a plastic tray. (D) Skin is grabbed with forceps at the level of diaphragm. (E) The chest is cut laterally and then up through the ribs. Chest flap is secured using forceps. (F) Blood is collected from the left ventricle using an insulin syringe (31-gauge). (G) A needle (18-gauge) is inserted into the left ventricle and the roller clamp is completely open. (H) The right atria is cut. (I–N) The abdominal window is widened and perfusion is observed in liver, kidneys, and lungs. Note the change of color of the lungs from pink to white (M, arrow). (O) Kidneys are removed after cutting the renal pedicles. (P) Representative image of the kidneys post-perfusion.
Fig. 5.
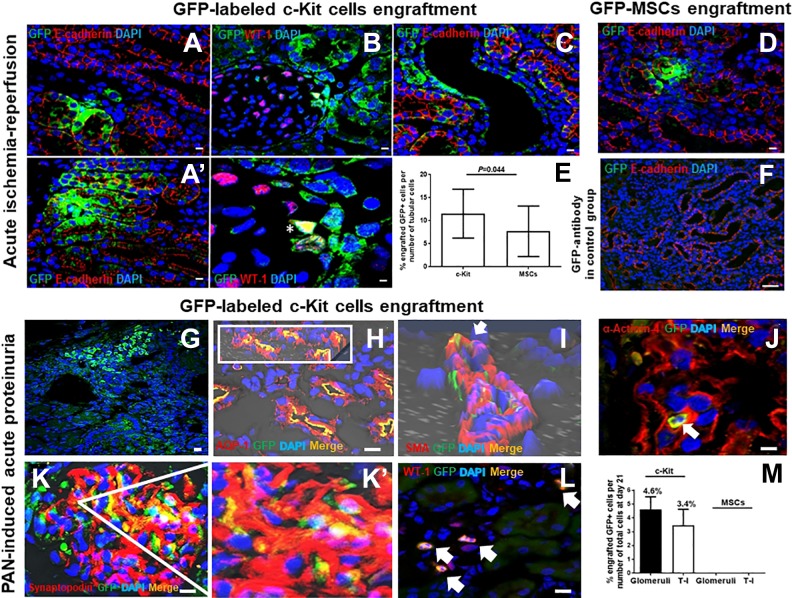
Progenitor/stem cell engraftment within the kidneys after suprarenal aorta delivery. In the acute ischemia-reperfusion model, GFP-labeled c-Kit progenitor/stem cells exhibited multi-compartment engraftment, including tubules, as shown by E-cadherin co-staining (A, A’); glomeruli in Bowman’s capsule and podocyte (B), as shown by WT-1 co-staining (*); and vascular (C). GFP-MSCs engrafted within the kidneys less frequently when compared with GFP-labeled c-Kit progenitor/stem cells (D, E). GFP antibody was used in the control group (F). Adapted from the method of Rangel and colleagues13. In the acute proteinuria model induced by puromycin aminonucleoside (PAN), GFP-labeled c-Kit progenitor/stem cells also engrafted into multi-compartments of the kidneys (G). Cells in (F) co-stained for aquaporin-1 (AQP1) (H; insert shows 3D confocal image), smooth muscle actin (SMA) (arrow; 3D confocal image) (I), α-Actinin-4 (arrow) (J), synaptopodin (K, K’), and WT-1 (arrows) (L). GFP-labeled c-Kit progenitor/stem cells engrafted into tubules and glomeruli in higher numbers when compared with GFP-MSCs (M). Adapted from the method of Rangel and colleagues14. Scale bars represent 20 µm for confocal images.
More details of reagents and equipment can be found in the supplementary file.
Immunofluorescence
c-Kit+ cells and MSCs engrafted onto adult kidneys were identified by immunohistochemical staining using anti-GFP antibody. Briefly, fragments of kidney were fixed overnight in neutral buffered formalin 10% (EMD Millipore, Burlington, MA, USA), dehydrated in alcohol, and embedded in paraffin. Sections of 4–5 µm thickness were stained with hematoxylin and eosin (H&E; Sigma-Aldrich, St. Louis, MO, USA) and Periodic Acid Schiff (PAS; Sigma-Aldrich) reagent. For immunofluorescence, renal sections were deparaffinized with xylene (EMD Millipore), and rehydrated in alcohol series and water. The sections were subsequently microwaved twice for 10 min in target retrieval solution (citrate pH 6, DakoCytomation, Carpinteria, CA, USA), and then blocked for 1 h in donkey serum (Millipore). Primary antibodies, that is, rabbit polyclonal anti-E-cadherin (Santa Cruz Biotechnology, Dallas, TX, USA) were applied overnight at 4°C. Incubations for 1 h using 568-conjugated secondary antibody (Invitrogen, Carlsbad, CA, USA) were then performed. For GFP staining, an anti-goat polyclonal anti-GFP FITC-conjugated antibody (Abcam, Cambridge, MA, USA) was applied for 1 h at 37°C. Anti-GFP antibody in saline sections was used as a control. Nuclei were labeled with 4’-6-diamidino-2-phenylindole (DAPI; Invitrogen). After that, slides were incubated with Sudan Black 0.1% (Sigma-Aldrich) for 10 min at room temperature. The slides were mounted in ProLong Gold antifade reagent (Invitrogen) for confocal analyses. In control experiments, the primary antibody was omitted. Images were obtained using a Zeiss LSM-710 confocal microscope (Analytical Imaging Core Facility, University of Miami, Miami, FL, USA).
Results
Acute Ischemia-Reperfusion Injury: Mortality
With practice and gentle surgical technique, 100% of the rats were injected successfully, and, in the week following injection, ∼ 85% of the injected rats recovered completely. Mortality (∼15%) during the following week of the ischemia-reperfusion injury was related to bleeding or acute renal failure, and was comparable among groups. Multiple attempts to inject progenitor/stem cells or saline into the suprarenal aorta were the most common cause of post-surgery bleeding. We did not observe heavy or persistent bleeding secondary to accidental trauma of perirrenal fat vessels or vena cava. In Table 1, we describe the main problems associated with bleeding, the possible reasons, and the solutions proposed to avoid or fix these adverse events. Low dose prophylactic heparin (0.2 U/g) helped avoid kidney thrombosis, and was not associated with an increased risk of bleeding.
Table 1.
Troubleshooting.
| Problem | Possible reason | Solution |
|---|---|---|
| Heavy bleeding of perirenal fat vessels | Vascular damage | Try to control blood loss by gently pressing a cotton swab on top of the focus of bleeding for 1 min. Add a squared-piece of Surgicel Nu-Knit absorbable hemostat on top of the leaky vessel. If bleeding is heavy or persistent, cautery can be used to seal the blood vessels, which helps reduce or stop bleeding. |
| Inferior vena cava bleeding | Accidental trauma | Try to control blood loss by gently pressing a cotton stick on top of the focus of bleeding for 3–5 min. Add a squared-piece of Surgicel Nu-Knit absorbable hemostat on top of the segment of the vena cava that was bleeding. Attempt dissecting an upper segment between infrarenal aorta and inferior vena cava. |
| No blood reflux when delivering stem cells or saline into suprarenal aorta | Needle is misplaced | Remove the needle and gently press a cotton stick for ∼5 min and reattempt delivering stem cells or saline. |
| Suprarenal aorta bleeding after stem cell or saline injection | Multiple injection attempts | Try to control blood loss by gently pressing a cotton stick on top of the focus of bleeding for 5–10 min. Add a squared-piece of Surgicel Nu-Knit absorbable hemostat on top of the segment of the suprarenal aorta that was bleeding. |
| Laryngo-epiglotic edema | Multiple intubation attempts | Inadequate anesthesia contributes to laryngospasm. Make sure the animal is well anesthetized when initially attempting intubation. If intubation fails twice, allow the animal recover for 5–10 min, re-anesthetize and reattempt intubation. Consider postponing the procedure for another day. |
Uremia may also contribute to bleeding in cases with multiple injection attempts. In cases with severe acute renal failure, the animals exhibited significant post-operative distress that was not alleviated by analgesics, including anorexia, failure to drink or dehydration, failure to groom, inability to move, aggressive behavior, squealing, twitching, teeth grinding, panting, labored breathing, reddish-brown nasal or ocular discharge, cold or blue extremities, and red or hot extremities. In these cases, animals were euthanized in a manner approved by the IACUC.
Progenitor/stem cell injection into aorta is not the only critical step during the procedure; endotracheal intubation is also critical. Failures occur during intubation when (a) esophageal intubation (thoracic movement is not synchronized with the ventilator or intense abdominal movement is observed); and (b) intubation is unsuccessful on the first or second attempt. Failed intubation rate was observed in ∼2% of cases. If esophageal intubation occurs, the catheter needs to be withdrawn and endotracheal intubation reattempted. If intubation is not successful on the first or second attempt, the animal will regain glottis reflexes, making subsequent intubation attempts challenging due to laryngo-epiglottic edema. Table 1 describes troubleshooting approaches.
Acute Ischemia-Reperfusion Injury: Kidney Functional and Structural Outcomes
In our study, 2–4 days following ischemia-reperfusion injury, progenitor/stem cells promoted significant renal functional recovery, as demonstrated by an improvement in creatinine and BUN levels13. Creatinine increased from ∼0.36 mg/dL at baseline to 3.15 ± 0.16 mg/dL, 2.5 ± 0.36 mg/dL, and 3.05 ± 0.55 mg/dL (mean ± SEM) in the saline, MSC, and c-Kit-treated animals, respectively, 24 h post-ischemia-reperfusion injury. However, after 48 h, creatinine started to decrease in the progenitor/stem cell-treated groups [2.11 ± 0.52 mg/dL (MSCs) and 2.45 ± 0.66 mg/dL (c-Kit)], as opposed to the saline-treated animals (4.21 ± 0.51 mg/dL). After 8 days, creatinine decreased to 0.5 ± 0.05 mg/dL, 0.65 ± 0.08 mg/dL, and 2.57 ± 0.84 mg/dL in the MSC, c-Kit, and saline treated animals, respectively (p < 0.05)13. BUN improved significantly 4 days following ischemia-reperfusion injury in the progenitor/stem-cell treated group: 61 ± 17.77 mg/dL (c-Kit) and 71.62 ± 24.18 mg/dL (MSCs), compared with 224.41 ± 46.22 mg/dL in the saline group (p < 0.01)13. Therefore, in the saline-treated group, kidney function did not return to baseline after 8 days, unlike the c-Kit- and MSC-treated groups.
Morphological analyses included the assessment of acute tubular necrosis (ATN) by semi-quantitative analysis of each individual variable (casts, brush border loss, tubular dilation, necrosis, and calcification) to augment the ATN score (maximum 7). The ATN score was ∼4 in the saline treated group, as opposed to a score of ∼3 in MSC- and c-Kit-treated groups, at the end of the study (8 days; p < 0.05), as previously documented13.
We clamped renal arteries for 35 min. However, clamping times in the literature range from 45 min to 90 min15–18. We observed higher mortality (∼40%) with clamping times ≥45 min, which was attributed to severe acute renal failure. Clamping time is not the only factor involved in the increase of creatinine and BUN after surgery; the type of clips used, the quality of the clips (old clips can loose pressure with time), and the surgical technique (renal pedicle dissection is crucial, because if the perirenal fat is not properly removed, it may compromise clip pressure) are also important. In addition, renal function recovery and tissue injury is gender-dependent, with females being more resistant than males19.
Acute Ischemia-Reperfusion Injury: Effects of Progenitor/Stem Cell Injection
After 8 days, progenitor/stem cells not only promoted higher epithelial tubular proliferation but also engrafted into kidney structures, as indicated by exposure of sections to an anti-GFP antibody (Fig. 5A–C)13. According to our previous data, on day 8 after ischemia-reperfusion injury, the number of GFP-positive c-Kit cells expressing E-cadherin was significantly higher (11.5 ± 1.1%) compared with GFP-MSCs (7.7±1.5%) (Fig. 5D–E), yet both cells were injected via the suprarenal aorta route13. These findings indicate that progenitor/stem cells have a distinct efficiency to repair kidney damage. GFP+-labeled cells were also observed within the lumen of the tubules, indicating that some cells may have been eliminated in the urine. GFP antibody was used in the control group (Fig. 5F).
Acute Proteinuria Model Induced by PAN: Outcomes
To further substantiate the finding that the suprarenal aorta route is an effective route for progenitor/stem cell delivery, we verified the therapeutic potential of either GFP-labeled c-Kit progenitor/stem cells or MSCs in a rat model of acute proteinuria induced by PAN. In all experiments, female 2-month-old SD rats weighing 200–350 g (Charles River, Wilmimgton, MA, USA) were injected with a single dose of PAN (15 mg/100 g body weight; Sigma-Aldrich, St. Louis, MO, USA) via IP route. GFP-labelled c-Kit+ cells or MSCs from GFP-SD (2 × 106 cells) or saline were injected directly into the suprarenal aorta14, as previously described for acute ischemia-reperfusion kidney injury.
Glomerular and tubule-interstitial damage was evaluated by a semi-quantitative score based on the presence of ATN (0–3), tubular casts (0–3), mesangial expansion (0–3), and interstitial inflammation (0–3) to generate a maximum overall injury score of 12, as reported previously. Yet, we did not find a difference in that score at day 21 among groups; the c-Kit treated group exhibited less damage due to ATN at day 10. Podocyte damage was documented by transmission electron microscopy (TEM) analyses, as reported in Fig. S1. Analyses of TEM and measurements of foot process effacement (FPE) indicated that foot process width (FPW) was significantly lower in the kidney-derived c-kit+ progenitor/stem cell and MSC-treated groups compared with the saline group at day 21, highlighting an important aspect of progenitor/stem cell therapy for glomerular disease14. In all groups, however, FPW was significantly higher when compared with normal kidneys, indicating that these treatments promoted partial reversal of the injury.
Therefore, we found that c-Kit+ progenitor/stem cells accelerated kidney recovery by improving FPE of podocytes. In particular, these cells engrafted in small quantity into tubules, vessels, and glomeruli, where they occasionally differentiated into podocyte-like cells (Fig. G–L). This effect was related to an up-regulation of α-Actinin-4, a protein of podocyte cytoskeleton, and mTORC2-Rictor pathway, and also to activation of autophagy14. In this model, the number of GFP-labeled c-Kit progenitor/stem cells, when these cells were injected via suprarenal aorta route, was 4.6 ± 0.91% and 3.4 ± 1.15% in the glomerular and T-I compartments, respectively (Fig. 5M). We found an average of 7.53 ± 6.6% GFP+ cells per glomeruli. From all counted glomeruli per animal (36.8 ± 1.9), 11.7 ± 9.8% (range, 2.8–22.2%) glomeruli exhibited GFP+ cell engraftment. Conversely, no engraftment was observed when MSCs were injected by suprarenal aorta route at day 21. That finding may be explained by the fact that MSCs possess mainly paracrine effects and their in vivo differentiation into renal structures is very rare20. In Figs. 5 H–L, we show immunofluorescence staining for DAPI, rabbit polyclonal anti-aquaporin-1 (AQP1) (Abcam, Cambridge, MA, USA), mouse monoclonal anti-smooth muscle actin (Sigma-Aldrich, St. Louis, MO, USA), rabbit polyclonal anti-α-Actinin-4 (Abcam, Cambridge, MA, USA), mouse monoclonal anti-synaptopodin (Progen, Heidelberg, Germany), and rabbit polyclonal anti-WT-1 (Santa Cruz, Dallas, TX, USA), respectively, applied as previously reported14. For GFP staining, rabbit or mouse polyclonal anti-GFP FITC-conjugated antibody (Abcam, Cambridge, MA, USA) was used, as described above.
Discussion
The route of progenitor/stem cell delivery, e.g., intravenous, intra-arterial, or intra-parenchymal, can affect the efficiency of kidney repair in different models of acute and chronic kidney injury. The intravenous route has been used most often, to inject not only MSCs6,21–23, but also different kidney progenitor/stem cells24–28, in several models of acute and chronic kidney injury in rodents. However, it has been documented that MSCs, bone-marrow-derived mononuclear cells, and other kidney progenitors are initially trapped inside the pulmonary microvasculature following intravenous administration29. More importantly, the number of cells, multiple intravenous injections, and cell size increase the chance of pulmonary trapping29,30. Similar observations were reported in nonhuman primates when MSCs were injected intravenously31,32.
Therefore, we tested the intra-arterial route to verify whether progenitor/stem cells would engraft into kidneys and contribute to both functional and morphological improvement. Possible intra-arterial routes for delivering progenitor/stem cells include intra-carotid2, intra-cardiac33, or intra-aorta13,34–36. When the intra-aorta route is employed, the clamps can be applied above and below the renal arteries34,35, or only below the renal arteries13,36. Bioluminescence analyses supported a distinct localization of MSCs to murine kidneys submitted to ischemia-reperfusion injury when cells were injected in the suprarenal aorta (intra-carotid), as opposed to intra-jugular vein injection, which was associated with predominant accumulation of cells in both lungs37. A recent meta-analysis including the beneficial effect of MSCs provided strong evidence in favor of arterial delivery of MSCs for kidney regeneration in small animals with both acute and chronic disease38. In larger animals (ovine), autologous MSCs delivered through renal arteries were also effective in reducing tubular injury after ischemia-reperfusion injury39.
According to the Mesenchymal Stem Cells in Solid Organ Transplantation (MISOT) study group, there is no conclusive recommendation for which route should be used in clinical trials for progenitor/stem cells administration after kidney injury40. However, there is a suggestion that intra-arterial injection of MSCs is advantageous because these cells would be delivered directly into the transplanted organs, where they can locally decrease inflammatory response. In line with these suggestions, intra-arterial infusion of allogeneic MSCs was superior to the intravenous route to treat acute kidney rejection in rodents41,42.
In acute kidney injury (AKI), suprarenal aorta injection of allogeneic MSCs to patients who have undergone on-pump cardiac surgery and were at risk of developing AKI (i.e., underlying CKD, advanced age, diabetes mellitus, congestive heart failure, chronic obstructive lung disease, and prolonged pump times) was associated with a ∼40% reduced length of hospital stay, readmission rates, and protection of renal function43. However, a phase 2, randomized, double-blind, placebo-controlled trial in 27 centers (n = 157 individuals) across North America failed to demonstrate a renoprotective effect of allogeneic human MSCs in reducing the time to recovery from AKI after cardiac surgery44. Of importance, no adverse effects were observed after intra-arterial infusion of MSC in these studies43,44.
Intravenous MSC administration was demonstrated to be safe for the treatment of impaired kidney function in glomerulonephritis secondary to systemic lupus erythromatosus, kidney transplant, and diabetic nephropathy in human subjects45–47, although longer follow-ups and multi-centric studies are necessary to confirm these findings. In other diseases, such as steroid-resistant, acute graft-versus-host disease, intravenous MSC injection was also documented to be an effective therapy48.
Although intra-parenchymal administration of progenitor/stem cells or MSCs has beneficial effects on kidney repair36,49–53, this route is less practical for clinical application, especially when the renal disease is diffuse.
Collectively, our results indicate that the protective effects of c-Kit cells were attributed not only to cell engraftment into kidney tissue, still small in quantity, and further differentiation, but also to paracrine mechanisms. MSC-treated animals exhibited lower rates of engraftment. The benefits of MSC treatment have already been well established in the literature (immunomodulatory, pro-angiogenic, anti-apoptotic, anti-fibrotic, and others)20. MSCs exhibit low capacity to differentiate in vivo into other lineages and our data is in line with these findings.
Conclusions
Suprarenal delivery of progenitor/stem cells contributed to engraftment rates of ∼10% at day 8 post ischemia-reperfusion injury and at day 21 after PAN injection, which accelerated kidney recovery. With practice and gentle surgical technique, 100% of the rats can be injected successfully, and, in the week following injection, ∼ 85% of the injected rats will recover completely.
Notably, the progenitor/stem cell injection technique reported here can be adapted easily to other preclinical studies of targeted therapies after acute ischemia-reperfusion injury and PAN-induced glomerular damage, as well as in other models of chronic kidney injury in rodents. Given the similarities in mammals, much of the data obtained with intra-arterial delivery of progenitor/stem cells in rodents can be tested in translational research and clinical trials with endovascular catheters.
Supplemental Material
Supplemental Material, Cell_Transplantation_Supplemental_File for Progenitor/Stem Cell Delivery by Suprarenal Aorta Route in Acute Kidney Injury by Érika B. Rangel, Samirah A. Gomes, Rosemeire Kanashiro-Takeuchi and Joshua M. Hare in Cell Transplantation
Footnotes
Ethical Approval: Ethical approval to perform surgical and experimental protocols were obtained from Institutional Animal Care and Use Committees (IACUC) of the University of Miami (number 10–176, Leonard M Miller School of Medicine, University of Miami, FL, USA).
Statement of Human and Animal Rights: All procedures in this study were conducted in accordance with the institutional guidelines of University of Miami, FL, USA, that are in compliance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
Statement of Informed Consent: There are no human subjects in this article and informed consent is not applicable.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research and/or authorship of this article: This work was supported by a postdoctoral research fellowship grant (1KD07-33958) from the James and Esther King Florida Biomedical Research Program to E.B.R., and National Institutes of Health RO1 grants HL107110 and AG025017 to J.M.H.
ORCID iD: Érika B Rangel  https://orcid.org/0000-0003-0982-2484
https://orcid.org/0000-0003-0982-2484
Supplemental Material: Supplemental material for this article is available online.
References
- 1. Schold JD, Segev DL. Increasing the pool of deceased donor organs for kidney transplantation. Nat Rev Nephrol. 2012;8(6):325–331. [DOI] [PubMed] [Google Scholar]
- 2. Togel F, Hu Z, Weiss K, Isaac J, Lange C, Westenfelder C. Administered mesenchymal stem cells protect against ischemic acute renal failure through differentiation-independent mechanisms. Am J Physiol Renal Physiol. 2005;289(1):F31–F42. [DOI] [PubMed] [Google Scholar]
- 3. Imberti B, Morigi M, Tomasoni S, Rota C, Corna D, Longaretti L, Rottoli D, Valsecchi F, Benigni A, Wang J, Abbate M, et al. Insulin-like growth factor-1 sustains stem cell mediated renal repair. J Am Soc Nephrol. 2007;18(11):2921–2928. [DOI] [PubMed] [Google Scholar]
- 4. Bruno S, Grange C, Deregibus MC, Calogero RA, Saviozzi S, Collino F, Morando L, Busca A, Falda M, Bussolati B, Tetta C, et al. Mesenchymal stem cell-derived microvesicles protect against acute tubular injury. J Am Soc Nephrol. 2009;20(5):1053–1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Morigi M, Imberti B, Zoja C, Corna D, Tomasoni S, Abbate M, Rottoli D, Angioletti S, Benigni A, Perico N, Alison M, et al. Mesenchymal stem cells are renotropic, helping to repair the kidney and improve function in acute renal failure. J Am Soc Nephrol. 2004;15(7):1794–1804. [DOI] [PubMed] [Google Scholar]
- 6. Herrera MB, Bussolati B, Bruno S, Fonsato V, Romanazzi GM, Camussi G. Mesenchymal stem cells contribute to the renal repair of acute tubular epithelial injury. Int J Mol Med. 2004;14(6):1035–1041. [PubMed] [Google Scholar]
- 7. Choi S, Park M, Kim J, Hwang S, Park S, Lee Y. The role of mesenchymal stem cells in the functional improvement of chronic renal failure. Stem Cells Dev. 2009;18(3):521–529. [DOI] [PubMed] [Google Scholar]
- 8. Pleniceanu O, Harari-Steinberg O, Dekel B. Concise review: kidney stem/progenitor cells: differentiate, sort out, or reprogram? Stem Cells. 2010;28(9):1649–1660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Song JJ, Guyette JP, Gilpin SE, Gonzalez G, Vacanti JP, Ott HC. Regeneration and experimental orthotopic transplantation of a bioengineered kidney. Nat Med. 2013;19(5):646–651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Becherucci F, Mazzinghi B, Allinovi M, Angelotti ML, Romagnani P. Regenerating the kidney using human pluripotent stem cells and renal progenitors. Expert Opin Biol Ther. 2018;18(7):795–806. [DOI] [PubMed] [Google Scholar]
- 11. Dimarino AM, Caplan AI, Bonfield TL. Mesenchymal stem cells in tissue repair. Front Immunol. 2013;4:201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Gomes SA, Hare JM, Rangel EB. Kidney-derived c-Kit(+) cells possess regenerative potential. Stem Cells Transl Med. 2018;7(4):317–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Rangel EB, Gomes SA, Dulce RA, Premer C, Rodrigues CO, Kanashiro-Takeuchi RM, Oskouei B, Carvalho DA, Ruiz P, Reiser J, Hare JM. C-kit(+) cells isolated from developing kidneys are a novel population of stem cells with regenerative potential. Stem Cells. 2013;31(8):1644–1656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Rangel EB, Gomes SA, Kanashiro-Takeuchi R, Saltzman RG, Wei C, Ruiz P, Reiser J, Hare JM. Kidney-derived c-kit(+) progenitor/stem cells contribute to podocyte recovery in a model of acute proteinuria. Sci Rep. 2018;8(1):14723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Okusa MD, Linden J, Macdonald T, Huang L. Selective A2A adenosine receptor activation reduces ischemia-reperfusion injury in rat kidney. Am J Physiol. 1999;277(3 Pt 2):F404–F412. [DOI] [PubMed] [Google Scholar]
- 16. Sharples EJ, Patel N, Brown P, Stewart K, Mota-Philipe H, Sheaff M, Kieswich J, Allen D, Harwood S, Raftery M, Thiemermann C, et al. Erythropoietin protects the kidney against the injury and dysfunction caused by ischemia-reperfusion. J Am Soc Nephrol. 2004;15(8):2115–2124. [DOI] [PubMed] [Google Scholar]
- 17. Molina A, Ubeda M, Escribese MM, García-Bermejo L, Sancho D, Perez de LG, Liaño F, Cabañas C, Sánchez-Madrid F, Mampaso F. Renal ischemia/reperfusion injury: functional tissue preservation by anti-activated β1 integrin therapy. J Am Soc Nephrol. 2005;16(2):374–382. [DOI] [PubMed] [Google Scholar]
- 18. De Vecchi E, Lubatti L, Beretta C, Ferrero S, Rinaldi P, Galli Kienle M, Trazzi R, Paroni R. Protection from renal ischemia-reperfusion injury by the 2-methylaminochroman U83836E. Kidney Int. 1998;54(3):857–863. [DOI] [PubMed] [Google Scholar]
- 19. Robert R, Ghazali DA, Favreau F, Mauco G, Hauet T, Goujon JM. Gender difference and sex hormone production in rodent renal ischemia reperfusion injury and repair. J Inflamm (Lond). 2011;8:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Paulini J, Higuti E, Bastos RM, Gomes SA, Rangel EB. Mesenchymal stem cells as therapeutic candidates for halting the progression of diabetic nephropathy. Stem Cells Int. 2016;2016:9521629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Villanueva S, Ewertz E, Carrión F, Tapia A, Vergara C, Céspedes C, Sáez PJ, Luz P, Irarrázabal C, Carreño JE, Figueroa F, et al. Mesenchymal stem cell injection ameliorates chronic renal failure in a rat model. Clin Sci (Lond). 2011;121(11):489–499. [DOI] [PubMed] [Google Scholar]
- 22. Villanueva S, Carreño JE, Salazar L, Vergara C, Strodthoff R, Fajre F, Céspedes C, Sáez PJ, Irarrázabal C, Bartolucci J, Figueroa F, et al. Human mesenchymal stem cells derived from adipose tissue reduce functional and tissue damage in a rat model of chronic renal failure. Clin Sci (Lond). 2013;125(4):199–210. [DOI] [PubMed] [Google Scholar]
- 23. Qian H, Yang H, Xu W, Yan Y, Chen Q, Zhu W, Cao H, Yin Q, Zhou H, Mao F, Chen Y. Bone marrow mesenchymal stem cells ameliorate rat acute renal failure by differentiation into renal tubular epithelial-like cells. Int J Mol Med. 2008;22(3):325–332. [PubMed] [Google Scholar]
- 24. Lazzeri E, Crescioli C, Ronconi E, Mazzinghi B, Sagrinati C, Netti GS, Angelotti ML, Parente E, Ballerini L, Cosmi L, Maggi L, et al. Regenerative potential of embryonic renal multipotent progenitors in acute renal failure. J Am Soc Nephrol. 2007;18(12):3128–3138. [DOI] [PubMed] [Google Scholar]
- 25. Bussolati B, Bruno S, Grange C, Buttiglieri S, Deregibus MC, Cantino D, Camussi G. Isolation of renal progenitor cells from adult human kidney. Am J Pathol. 2005;166(2):545–555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Sagrinati C, Netti GS, Mazzinghi B, Lazzeri E, Liotta F, Frosali F, Ronconi E, Meini C, Gacci M, Squecco R, Carini M, et al. Isolation and characterization of multipotent progenitor cells from the Bowman’s capsule of adult human kidneys. J Am Soc Nephrol. 2006;17(9):2443–2456. [DOI] [PubMed] [Google Scholar]
- 27. Ronconi E, Sagrinati C, Angelotti ML, Lazzeri E, Mazzinghi B, Ballerini L, Parente E, Becherucci F, Gacci M, Carini M, Maggi E, et al. Regeneration of glomerular podocytes by human renal progenitors. J Am Soc Nephrol. 2009;20(2):322–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Mazzinghi B, Ronconi E, Lazzeri E, Sagrinati C, Ballerini L, Angelotti ML, Parente E, Mancina R, Netti GS, Becherucci F, Gacci M, et al. Essential but differential role for CXCR4 and CXCR7 in the therapeutic homing of human renal progenitor cells. J Exp Med. 2008;205(2):479–490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Fischer UM, Harting MT, Jimenez F, Monzon-Posadas WO, Xue H, Savitz SI, Laine GA, Cox CS., Jr Pulmonary passage is a major obstacle for intravenous stem cell delivery: the pulmonary first-pass effect. Stem Cells Dev. 2009;18(5):683–692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Schrepfer S, Deuse T, Reichenspurner H, Fischbein MP, Robbins RC, Pelletier MP. Stem cell transplantation: the lung barrier. Transplant Proc. 2007;39(2):573–576. [DOI] [PubMed] [Google Scholar]
- 31. Feng Q, Chow PK, Frassoni F, Phua CM, Tan PK, Prasath A, Khee Hwang WY. Nonhuman primate allogeneic hematopoietic stem cell transplantation by intraosseus vs intravenous injection: engraftment, donor cell distribution, and mechanistic basis. Exp Hematol. 2008;36(11):1556–1566. [DOI] [PubMed] [Google Scholar]
- 32. Devine SM, Cobbs C, Jennings M, Bartholomew A, Hoffman R. Mesenchymal stem cells distribute to a wide range of tissues following systemic infusion into nonhuman primates. Blood. 2003;101(8):2999–3001. [DOI] [PubMed] [Google Scholar]
- 33. Sedrakyan S, Da Sacco S, Milanesi A, Shiri L, Petrosyan A, Varimezova R, Warburton D, Lemley KV, De Filippo RE, Perin L. Injection of amniotic fluid stem cells delays progression of renal fibrosis. J Am Soc Nephrol. 2012;23(4):661–673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Kunter U, Rong S, Djuric Z, Boor P, Müller-Newen G, Yu D, Floege J. Transplanted mesenchymal stem cells accelerate glomerular healing in experimental glomerulonephritis. J Am Soc Nephrol. 2006;17(8):2202–2212. [DOI] [PubMed] [Google Scholar]
- 35. Kunter U, Rong S, Boor P, Eitner F, Müller-Newen G, Djuric Z, van Roeyen CR, Konieczny A, Ostendorf T, Villa L, Milovanceva-Popovska M, et al. Mesenchymal stem cells prevent progressive experimental renal failure but maldifferentiate into glomerular adipocytes. J Am Soc Nephrol. 2007;18(6):1754–1764. [DOI] [PubMed] [Google Scholar]
- 36. Gupta S, Verfaillie C, Chmielewski D, Kren S, Eidman K, Connaire J, Heremans Y, Lund T, Blackstad M, Jiang Y, Luttun A, et al. Isolation and characterization of kidney-derived stem cells. J Am Soc Nephrol. 2006;17(11):3028–3040. [DOI] [PubMed] [Google Scholar]
- 37. Togel F, Yang Y, Zhang P, Hu Z, Westenfelder C. Bioluminescence imaging to monitor the in vivo distribution of administered mesenchymal stem cells in acute kidney injury. Am J Physiol Renal Physiol. 2008;295(1):F315–F321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Wang Y, He J, Pei X, Zhao W. Systematic review and meta-analysis of mesenchymal stem/stromal cells therapy for impaired renal function in small animal models. Nephrology (Carlton). 2013;18(3):201–208. [DOI] [PubMed] [Google Scholar]
- 39. Behr L, Hekmati M, Fromont G, Borenstein N, Noel LH, Lelievre-Pegorier M, Laborde K. Intra renal arterial injection of autologous mesenchymal stem cells in an ovine model in the postischemic kidney. Nephron Physiol. 2007;107(3):65–76. [DOI] [PubMed] [Google Scholar]
- 40. Dahlke MH, Hoogduijn M, Eggenhofer E, Popp FC, Renner P, Slowik P, Rosenauer A, Piso P, Geissler EK, Lange C, Chabannes D, et al. ; MISOT Study Group. Toward MSC in solid organ transplantation: 2008 position paper of the MISOT study group. Transplantation. 2009;88(5):614–619. [DOI] [PubMed] [Google Scholar]
- 41. De Martino M, Zonta S, Rampino T, Gregorini M, Frassoni F, Piotti G, Bedino G, Cobianchi L, Dal Canton A, Dionigi P, Alessiani M. Mesenchymal stem cells infusion prevents acute cellular rejection in rat kidney transplantation. Transplant Proc. 2010;42(4):1331–1335. [DOI] [PubMed] [Google Scholar]
- 42. Zonta S, De Martino M, Bedino G, Piotti G, Rampino T, Gregorini M, Frassoni F, Dal Canton A, Dionigi P, Alessiani M. Which is the most suitable and effective route of administration for mesenchymal stem cell-based immunomodulation therapy in experimental kidney transplantation: endovenous or arterial? Transplant Proc. 2010;42(4):1336–1340. [DOI] [PubMed] [Google Scholar]
- 43. Togel FE, Westenfelder C. Mesenchymal stem cells: a new therapeutic tool for AKI. Nat Rev Nephrol. 2010;6(3):179–183. [DOI] [PubMed] [Google Scholar]
- 44. Swaminathan M, Stafford-Smith M, Chertow GM, Warnock DG, Paragamian V, Brenner RM, Lellouche F, Fox-Robichaud A, Atta MG, Melby S, Mehta RL, et al. ; ACT-AKI investigators. Allogeneic mesenchymal stem cells for treatment of AKI after cardiac surgery. J Am Soc Nephrol. 2018;29(1):260–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. El-Ansary M, Saadi G, Abd El-Hamid SM. Mesenchymal stem cells are a rescue approach for recovery of deteriorating kidney function. Nephrology (Carlton). 2012;17(7):650–657. [DOI] [PubMed] [Google Scholar]
- 46. Kaundal U, Bagai U, Rakha A. Immunomodulatory plasticity of mesenchymal stem cells: a potential key to successful solid organ transplantation. J Transl Med. 2018;16(1):31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Packham DK, Fraser IR, Kerr PG, Segal KR. Allogeneic mesenchymal precursor cells (MPC) in diabetic nephropathy: a randomized, placebo-controlled, dose escalation study. EBioMedicine. 2016;12:263–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Le Blanc K, Frassoni F, Ball L, Locatelli F, Roelofs H, Lewis I, Lanino E, Sundberg B, Bernardo ME, Remberger M, Dini G, et al. ; Developmental Committee of the European Group for Blood and Marrow Transplantation. Mesenchymal stem cells for treatment of steroid-resistant, severe, acute graft-versus-host disease: a phase II study. Lancet. 2008;371(9624):1579–1586. [DOI] [PubMed] [Google Scholar]
- 49. Kitamura S, Yamasaki Y, Kinomura M, Sugaya T, Sugiyama H, Maeshima Y, Makino H. Establishment and characterization of renal progenitor like cells from S3 segment of nephron in rat adult kidney. FASEB J. 2005;19(13):1789–1797. [DOI] [PubMed] [Google Scholar]
- 50. Curtis LM, Chen S, Chen B, Agarwal A, Klug CA, Sanders PW. Contribution of intrarenal cells to cellular repair after acute kidney injury: subcapsular implantation technique. Am J Physiol Renal Physiol. 2008;295(1):F310–F314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Dekel B, Zangi L, Shezen E, Reich-Zeliger S, Eventov-Friedman S, Katchman H, Jacob-Hirsch J, Amariglio N, Rechavi G, Margalit R, Reisner Y. Isolation and characterization of nontubular sca-1+lin- multipotent stem/progenitor cells from adult mouse kidney. J Am Soc Nephrol. 2006;17(12):3300–3314. [DOI] [PubMed] [Google Scholar]
- 52. Alfarano C, Roubeix C, Chaaya R, Ceccaldi C, Calise D, Mias C, Cussac D, Bascands JL, Parini A. Intraparenchymal injection of bone marrow mesenchymal stem cells reduces kidney fibrosis after ischemia-reperfusion in cyclosporine-immunosuppressed rats. Cell Transplant. 2012;21(9):2009–2019. [DOI] [PubMed] [Google Scholar]
- 53. Lee SJ, Ryu MO, Seo MS, Park SB, Ahn JO, Han SM, Kang KS, Bhang DH, Youn HY. Mesenchymal stem cells contribute to improvement of renal function in a canine kidney injury model. In Vivo. 2017;31(6):1115–1124. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Material, Cell_Transplantation_Supplemental_File for Progenitor/Stem Cell Delivery by Suprarenal Aorta Route in Acute Kidney Injury by Érika B. Rangel, Samirah A. Gomes, Rosemeire Kanashiro-Takeuchi and Joshua M. Hare in Cell Transplantation