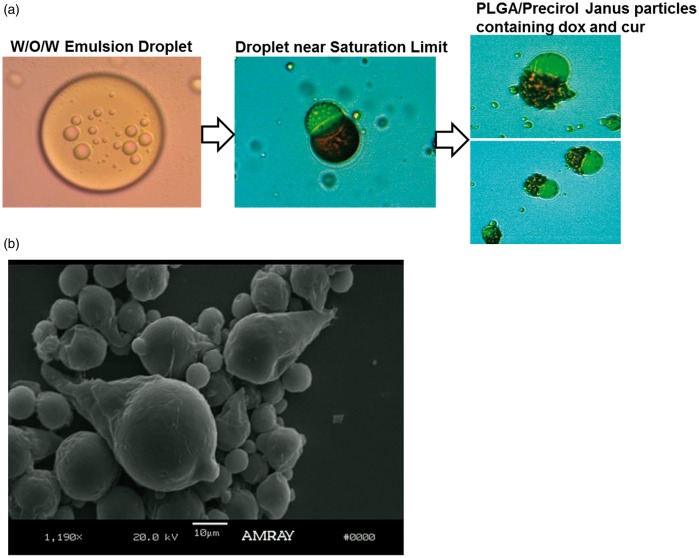
Short abstract
Bicompartmental Janus particles have many advantages in drug delivery, including co-delivery of two compounds with varying solubilities, differential release kinetics, and two surfaces available for targeting ligands. We present a novel strategy using the double emulsion method for the coencapsulation and staggered release of a hydrophobic and hydrophilic drug from anisotropic PLGA/PCL Janus particles, as well as a UV detection method to measure the release of two different compounds from Janus particles. Curcumin and quercetin were chosen as the model hydrophobic compounds for drug loading studies, while acetaminophen (APAP) and naproxen were chosen as the model hydrophilic–hydrophobic drug pair for encapsulation methods and drug loading. Also, a similar double emulsion method was also applied for PLGA/Preicrol® Janus particles containing Doxorubicin and Curcumin. Hydrophobic drugs were encapsulated by the single O/W emulsion technique. Hydrophilic compounds required special modifications due to their poor oil solubility and tendency to escape to the outer aqueous phase during the emulsification and solvent evaporation steps. In total, three different strategies for incorporating hydrophilic drugs were employed: (1) O/W emulsion with partially water miscible solvent, (2) O/W emulsion with co-solvent (i.e. acetone, methanol, ethanol), or (3) W/O/W double emulsion. The encapsulation efficiencies and drug loading percentages were measured using UV/Vis spectroscopy and compared for the different synthesis methods. It was found that the double emulsion method resulted in the highest encapsulation efficiency and drug loading of the hydrophilic drug.
Keywords: Janus particles, drug delivery, emulsion-solvent evaporation, double emulsion
Impact statement
Current drug delivery strategies fail to provide codelivery of two drugs with strongly dissimilar aqueous solubility, segregation of potentially reactive drug compounds, and sequenced drug release all simultaneously. Janus particles offer a platform for the co-encapsulation and staggered release of drugs with widely disparate solubility as well as independent release kinetics. Although emulsions were previously used for the delivery of hydrophilic compounds, none of the previous works studied dual encapsulation of disparate solubility drugs in biodegradable polymeric Janus particles, offering a platform for the coencapsulation and staggered release of drugs with disparate solubility and independent release kinetics. This work is the first to simultaneously encapsulate two disparate solubility drugs into biodegradable polymeric Janus particles. We present novel strategies for simultaneous inclusion of hydrophilic and hydrophobic drugs into Janus particles and measurement of their encapsulation efficiency. These studies will help to propel Janus particles from a concept in labs to practical clinical applications.
Introduction
The desire for drug delivery systems with tunable properties and multiple functionalities has spawned a new generation of particulate carriers, including Janus particles.1–7 Current approaches to nanoparticle-based combination therapy include administering a cocktail of single-drug nanoparticles, coencapsulating multiple drugs into a single nanoparticle core,8,9 conjugating one drug to the particle surface while encapsulating the other inside of the core,2 and covalently conjugating multiple drugs to the same polymer backbone.10 While innovative, these strategies fail to provide co-delivery of two drugs with strongly dissimilar aqueous solubility (i.e. hydrophobic and hydrophilic), segregation of potentially reactive drug compounds, and sequenced drug release all simultaneously. Janus particles offer a platform for the co-encapsulation and staggered release of drugs with widely disparate solubility as well as independent release kinetics.7,11 Staggered release profiles are especially desirable in treating certain diseases that require exposure to one active agent at a specific rate, followed by exposure to another active agent at a different rate. Combination therapy is especially useful in cancer treatment, where co-administration of multiple drugs targeting different pathways has been shown to reverse multidrug resistance, increase therapeutic efficacy, and reduce side effects.2,12,13 Co-delivery of different chemicals through various dual drug devices has been previously reported.14–17 Recent works have presented the synthesis of empty Janus particles using microfluidic devices18,19 and single emulsion polymerization.20 Dual drug encapsulation with Janus particles was recently reported using graphene oxide with a thermoresponsive method for drug delivery.21
Microparticles offer various significant advantages as drug delivery systems22–24 including: (i) protection of the encapsulated active agent against (e.g. enzymatic) degradation, (ii) the possibility to accurately control the release rate of the incorporated drug23over periods of hours to months, (iii) ease of administration (compared to alternative parenteral controlled release dosage forms, such as macro-sized implants), and (iv) the possibility of creating a desired, pre-programmed drug release profile to match the therapeutic needs of the patient.25 Another advantage is that microparticles injected into a variety of tissues tend to stay where they are placed. For example, 60 mm polymeric particles composed of a slowly degrading polymer injected at the sciatic nerve were still found in quantity at the site of injection more than eight weeks later.26 The difference between micro- and nanoparticles is also well documented in the abdominal cavity. Microparticles 5, 25, 60, and 250 mm in diameter injected into the peritoneum of mice remained there for at least two weeks. In contrast, an equal mass of nanoparticles of the same material showed almost complete clearance from the peritoneum in the same time frame.23,27 The spleens of those mice were enlarged and discolored and on light microscopy revealed numerous macrophages with a foamy appearance due to the accumulation of a large amount of polymeric material.
We have previously demonstrated the synthesis and characterization of biphasic poly (lactic-co-glycolic acid) (PLGA)/polycaprolactone (PCL) Janus particles from O/W emulsions.28,29 In this work, we present novel drug loading strategies for the simultaneous inclusion of hydrophilic and hydrophobic drugs in Janus particles and measuring their encapsulation efficiencies. Although single and double emulsions were previously used in preparing polymer nanoparticles for the delivery of hydrophilic compounds,30–32 only a few of the previous published work have focused on dual encapsulation of disparate solubility drugs in biocompatible Janus particles.7 However, this work used fluidic nanoprecipitation with co-jets and not emulsion solvent evaporation. A more recent paper done by Fan et al.33 used emulsion solvent evaporation techniques but their work was focused on charged an non-charged drugs, they did not encapsulate disparate solubility drugs. To the best of our knowledge, this work is the first to simultaneously encapsulate two disparate solubility drugs into biocompatible Janus particles using an emulsion solvent evaporation. We measure their encapsulation efficiencies using three novel synthetic routes and formulations for the inclusion of hydrophilic compounds into Janus particles. In addition, we have developed a spectroscopic method for the simultaneous measurement of two drug compounds in Janus particles. It is the authors’ hope that the studies performed here will help to propel Janus particles from a concept in academic labs to a practical clinical application.
Curcumin (CUR) and quercetin (QCT) were chosen as the model hydrophobic drug compounds, while acetaminophen (APAP) and naproxen (NPX) were chosen as the model hydrophilic–hydrophobic drug pair. Also, similar double emulsion method was also applied for doxorubicin hydrochloride (DOX) and CUR with PLGA and Precirol as a Polymer-Lipid pair. CUR and QCT are bioflavonoids with anti-inflammatory, anti-proliferative, and immunosuppressive properties used to treat a host of diseases, including multi-drug-resistant cancer,34 Alzheimer disease,35 and arthritis.36,37 APAP and NPX are often used in combination due to their additive effects in pain management and treatment of rheumatoid arthritis.38,39
The organization of this paper is as follows: The next section describes the materials and methods used in the preparation and characterization of dual-loaded Janus particles, then contains the results from the studies, followed by conclusions in the last section.
Materials and methods
Materials
Biocompatible and biodegradable40 poly(lactide-co-glycolide) (PLGA, lactide:glycolide = 65:35, M.W. = 40,000–75,000), PCL (M.W. = 42,500–65,000), poly(ethylene glycol) (PEG, M.N. = 400), CUR, QCT, NPX, APAP, DOX, dichloromethane (DCM), methanol, acetone, and tetrahydrofuran (THF) were purchased from Sigma Aldrich (St. Louis, MO, USA). Span 80, Tween 80 were obtained from Fisher Scientific (Waltham, MA, USA). Polyvinyl alcohol (PVA, 98 mol% hydrolyzed, M.W. = 9000–10,000) was obtained from Polysciences (Warrington, PA, USA). All materials used in this study are of analytical grade.
Methods
In this work, hydrophobic compounds were encapsulated into biocompatible and biodegradable Janus particles by including them in the oil phase prior to emulsification. To incorporate the hydrophilic compound, we compared three different methods:
(i) Single oil-in-water (O/W) emulsion containing a partially water-miscible solvent, (ii) O/W emulsion using a co-solvent (O/W-S), and (iii) water-in-oil-in-water (W/O/W) double emulsion. The O/W single emulsion method is not suitable for microencapsulation of water-soluble compounds due to rapid partitioning into the outer aqueous phase. The double emulsion method requires two surfactants: one for the inner aqueous phase and one for the outer aqueous phase. The hydrophilic drug is dissolved in the inner aqueous phase, which is emulsified into a polymer solution in organic solvent containing the hydrophobic drug to form the primary emulsion. The primary emulsion is then added to the outer aqueous phase containing surfactant and homogenized to produce the double emulsion. The solvent is allowed to evaporate, leaving an aqueous suspension of particles. The same factors that influence particle formation from single emulsions discussed in the previous chapter also apply to double emulsions. Additionally, there are more variables related to the internal W/O emulsion that need to be taken into consideration, such as W/O emulsifier type and concentration and internal water phase volume and composition.41 However, these factors have been widely studied elsewhere and thus are not discussed in this work.42–46
Single O/W emulsion method (O/W)
Preparation of PLGA/PCL Janus particles by the single emulsion method for hydrophobic drugs
Hydrophobic compounds (CUR and QCT) were encapsulated into PLGA/PCL Janus particles via the single O/W emulsion-solvent evaporation method. Single emulsions are suitable for the encapsulation of hydrophobic compounds because in this method, the hydrophobic compounds are dissolved in the oil phase along with the polymers. Hydrophobic compounds such as the ones used here are readily soluble in most solvents that can comprise the oil phase.
The oil phase was created by dissolving 2.5% w/v of each PLGA (lactide:glycolide = 65:35, M.W. = 40,000–75,000) and PCL ( M.W. = 42,500–65,000) in 4 mL of DCM. After that 5% QCT and 2.5% CUR are dissolved in oil phase. Separately, a 10 mL solution of 1% w/v PVA in deionized water was prepared. The oil phase was added to the water phase and emulsified. The O/W emulsion was further homogenized using either an Ultra Turrax T-25 rotor-stator, probe-tip sonicator, or Avestin Emulsiflex C-3 piston-gap high pressure homogenizer (Avestin Inc., Ottawa, Canada), depending on the desired particle size. Post-homogenization, the O/W emulsion was magnetically stirred and kept at 40°C in an open beaker to allow for solvent evaporation. Upon complete solvent removal, the size distribution was done by a Beckman Coulter’s Laser Diffraction module, and later particles were harvested by centrifugation at 20,000 r/min for 30 min. The supernatant was discarded, and the remaining powder bed was washed with deionized water. Particles were stored in a vacuum desiccator for further analysis. A schematic of the Janus particle formation process is shown in Figure 1.
Figure 1.
Overview of the modified emulsification solvent evaporation method for producing biodegradable Janus particles. Step 1: Polymer solution is added to an aqueous solution that contains the stabilizer. Step 2: The two-phase system is homogenized to form an oil-in-water emulsion. Step 3: The solvent evaporates or diffuses out of the saturated oil droplets, leading to co-precipitation of the two polymer species into Janus particles. (A color version of this figure is available in the online journal.)
Preparation of PLGA/PCL Janus particles for hydrophilic and hydrophobic drugs pair
Janus particles containing the hydrophilic (APAP) and hydrophobic compounds (NPX) were also synthesized using a single O/W emulsion-solvent evaporation method, but with some modifications to accommodate the loading of the hydrophilic APAP. Due to the fact that APAP is poorly soluble in the chlorinated hydrocarbon solvents that are typically used for O/W emulsions (i.e. DCM and chloroform), two different strategies were employed:
Single O/W emulsion containing a partially water-miscible solvent, where ethyl acetate was used as the solvent and
Single O/W emulsion using a co-solvent (O/W-S), where a mixture of DCM and Methanol was used as the solvent.
The solubility of APAP in each of these solvents is provided in Table 1.
Table 1.
Solubility of APAP in selected solvents.
| Solvent | APAP solubility (g/kg) |
|---|---|
| Water | 17.39 |
| Ethyl acetate | 10.73 |
| Acetone | 111.65 |
| Methanol | 371.61 |
| Dichloromethane (DCM) | 0.32 |
Note: Data adapted from Parhi et al.17
APAP: acetaminophen.
Single O/W emulsion containing a partially water-miscible solvent
The oil phase was created by dissolving 2.5% w/v of each PLGA (lactide:glycolide = 65:35, M.W. = 40,000–75,000) and PCL (M.W. = 42,500–65,000) in 4 mL of ethyl acetate. After that 2.5% APAP and 2.5% NPX are dissolved in oil phase. Separately, a 10 mL solution of 1% w/v PVA in deionized water was prepared. The oil phase was added to the water phase and emulsified. The O/W emulsion was further homogenized using either an Ultra Turrax T-25 rotor-stator for 5 min at 12,000 r/min. Post-homogenization, the O/W emulsion was magnetically stirred and kept in an open beaker to allow for solvent evaporation. Upon complete solvent removal, particles were harvested by centrifugation at 20,000 r/min for 30 min. The supernatant was discarded, and the remaining powder bed was washed with deionized water. Particles were stored in a vacuum desiccator for further analysis.
Single oil-in-water O/W emulsion using a co-solvent (O/W-S)
The oil phase was comprised of 5% w/v 50:50 PLGA/PCL, 2.5% w/w APAP, and 2.5% w/w NPX. For the O/W-S method, methanol was added at various methanol-to-DCM ratios from 1:1 to 1:4. The water phase was comprised of 1% w/v PVA solution. Typically, 4 mL of oil was added to 10 mL water and emulsified using an Ultra Turrax T-25 rotor-stator for 5 min at 12,000 r/min. The resultant O/W emulsion was magnetically stirred until complete solvent evaporation. Upon complete solvent removal, same procedure was followed for harvesting particles as used in previous methods.
W/O/W double emulsion method
Preparation of PLGA/PCL Janus particles by a novel double emulsion method for a hydrophilic and hydrophobic drug pair
Double W/O/W emulsions are commonly used to encapsulate hydrophilic compounds into particles. As with the single emulsion method, particles are formed from a single O/W emulsion template. However, in the double emulsion method, hydrophilic compounds are entrapped inside of W/O emulsion droplets which reside in the core of the particles. This is necessary for compounds that are insoluble in the solvent used as the oil phase.
To create PLGA/PCL Janus particles with a dual encapsulation of a hydrophilic/hydrophobic pair, we created a novel double emulsion method by modifying the double emulsion approach. The inner aqueous phase consisted of 20% w/v APAP dissolved in 75:25 PEG 400/water. The primary water in oil emulsion indicated by W1/O was formed by adding 500 µL of the PEG 400/water solution to the oil phase, which consisted of 0.25 g PLGA, 0.25 g PCL, 0.025 g NPX, and 0.2 g Span 80/Tween 80 (HLB 6) dissolved in 5 mL DCM. The W/O emulsion was homogenized using an Ultra Turrax T-25 rotor-stator for 5 min at 16,000 r/min. Finally, the W/O emulsion was added to the outer aqueous phase (12.5 mL 1% PVA w/v solution with 10% w/v NaCl) and emulsified at 6000 r/min for 2 min. The resultant W/O/W emulsion was magnetically stirred in an open beaker to allow solvent evaporation to proceed. A schematic showing the steps of the W/O/W technique is shown in Figure 2.
Figure 2.
Overview of the double emulsion solvent evaporation method used to encapsulate hydrophilic compounds inside Janus particles. First, the internal aqueous phase containing the hydrophilic compound to be encapsulated and the hydrophilic surfactant of the hydrophilic/hydrophobic surfactant blend is added to the oil phase containing the polymers, hydrophobic compound to be encapsulated, and the hydrophobic surfactant. The two solutions are emulsified to form the primary W/O emulsion, which is added to the external water phase containing surfactant and emulsified. The result is O/W emulsion droplets containing W/O emulsion droplets, or a W/O/W double emulsion. During solvent evaporation, Janus particles containing the hydrophilic compound inside W/O emulsion droplets and the hydrophobic compound are formed. (A color version of this figure is available in the online journal.)
Measuring encapsulation efficiency using UV/vis
Absorbance studies
Ultraviolet–visible spectroscopy (UV/Vis) was used to detect the concentration of drug in solution. The concentration of the drug can be calculated from standard absorbance curves of known concentrations of drug on the basis of Beer–Lambert Law, which states that there is the linear relationship between absorbance and concentration.47,48 Beer’s Law states the total absorbance is the sum of individual absorbances only if there is no interaction between absorbing species. The absorbance of each drug was obtained by deconvoluting the two spectra using the standard plots as reference. The calibration curves were constructed by preparing standards of various concentrations and plotting their absorbance at pre-selected wavelengths as a function of concentration. Once the Beer’s law equation is obtained from the standards, it can be used to quantify the amount of CUR, QCT, NPX, and APAP in solution. The following six wavelengths were selected for calibration and analysis of CUR and QCT by UV/Vis spectroscopy based on large adsorption difference ΔA needed to separate the two signals: 243, 273, 291, 429, 475, and 500 nm. QCT has peaks at 243 and 273 nm, while CUR has a sharp peak at 429 nm. CUR has a minimum at 291 nm, and QCT has close to zero absorbance at 475 and 500 nm. Similarly, APAP has a peak at 243 nm and NPX has a peak at 230 nm. Standard curves of CUR, QCT, APAP, and NPX containing absorbance values at the six selected wavelengths were constructed from 50:50 methanol/water solutions of known concentration prior to analysis. Methanol was chosen for inclusion in the buffer solution due to its solvation power for CUR, QCT, and NPX. All calibration curves for CUR, QCT, APAP, and NPX are contained in the Supplementary Appendix.
Encapsulation efficiency studies
Following centrifugation, the supernatant was analyzed for drug content. The amount of each drug present in the samples was calculated by deconvoluting the CUR and QCT spectra using the Excel solver function. The same procedure was followed for determining NPX and APAP content, except different wavelengths were selected for analysis (230, 243, 252, 272, 318, and 331 nm). The encapsulation efficiency (E.E.) was calculated using the following equation:
| (1) |
The drug loading was calculated using the following equation
| (2) |
The EE of Janus particles containing CUR and QCT was also measured by dissolving an accurately weighed amount of particles in THF (calibration curves are contained in the Supplementary Appendix). The equation used to directly measure EE is given below:
| (3) |
Direct measurements can be used for CUR and QCT because these two compounds are in the visible range and have a much higher absorption than the polymers, so their signals are not affected by the presence of the polymers. However, NPX and APAP must be measured indirectly because the PCL and PLGA signals interfere with those of NPX and APAP. In this case, the drug content of the particles is determined by measuring the free drug content released into the dissolution media and subtracting that from the initial drug loading.
Drug release profiles
In vitro release studies of PLGA/PCL Janus particles containing APAP and NPX were performed using Slid-a-lyzer mini dialysis devices with a 20,000 molecular weight cut off to mimic drug release in the gastric tract. The devices were loaded with 500 μL of the formulation and the receptor medium contained 14 mL of the phosphate buffer saline solution (PBS) at pH 7.4. The devices were kept in a water bath at 37 °C with constant shaking. The release study was run over a 48-h period with sampling at 2 h, 4 h, 6 h, 21 h, 22 h, 23 h, 24 h, 29 h and 48 h. At each time point, 3 mL of the receptor media were collected from each tube and an equal amount of fresh PBS was added back to the tube to maintain a constant volume. The samples collected at each time point were quantified using UV-Vis Spectroscopy and concentrations and drug percent profiles were plotted against time.
Results and discussion
PLGA/PCL Janus particles containing CUR and QCT
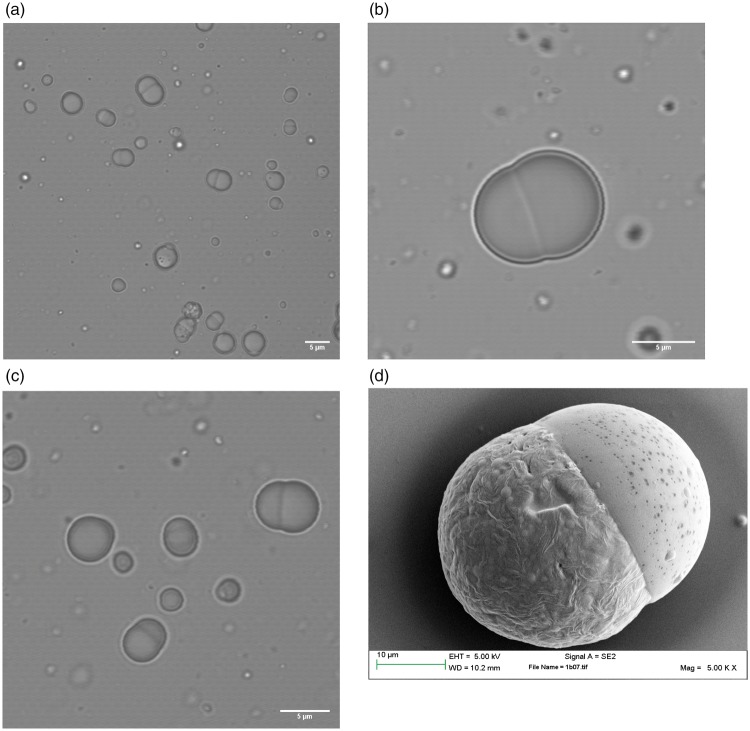
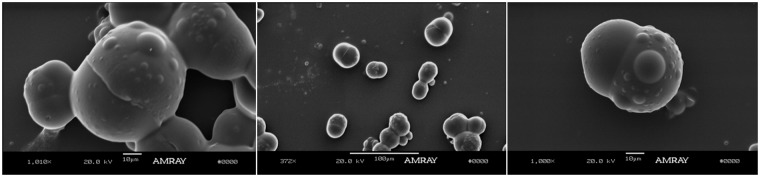
PLGA/PCL Janus particles were formed from emulsions using two different homogenization methods: manual shear and rotor-stator homogenizer. In our previous work, we also used a high pressure homogenization (high energy), which results in the smallest particles, followed by the rotor–stator mixer and manual shaking.29 In this work, manual shaking (low energy) was used as the primary emulsification method when it was necessary to produce particles large enough to visualize via optical microscopy. Manual shaking results in a broad particle size distribution with particles that can range from approximately 0.5 µm, all the way up to tens of microns. On the other hand, Janus particles produced using the rotor–stator homogenizer (moderate energy) show a more uniform size distribution. CUR and QCT were produced using a single emulsion O/W method with DCM solvent as explained in the methods sections for encapsulation of two hydrophobic drugs. The inclusion of CUR and QCT did not affect the usual dumbbell Janus particle morphology obtained in the absence of drug.29,49 SEM and optical microscope images of Janus particles containing CUR and QCT are shown in Figure 3.
Figure 3.
(a), (b) and (c) are optical microscope and (d) SEM images of PLGA/PCL Janus particles containing CUR and QCT. These different snapshots show that the inclusion of CUR and QCT does not alter the typical dumbbell structure of the Janus particles found in the absence of drug.49 The particles vary in size because they were made by manual shaking, which gives the broadest PSD. The SEM picture was taken from a different batch with a slightly larger mean size.
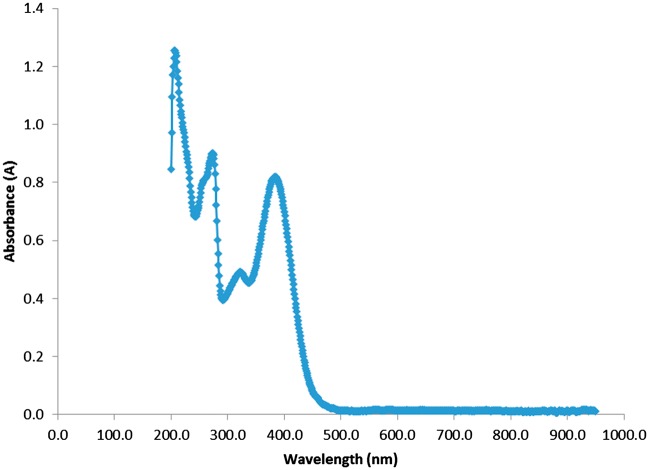
CUR and QCT were loaded at concentrations of 2.5% w/w and 5% w/w, respectively, to facilitate simultaneous UV/Vis spectroscopy measurement of both compounds in order to prevent the CUR spectrum from completely blocking out the signal of QCT. Figure 4 shows the UV spectrum generated with a mixture of both drugs, CUR and QCT, at different concentrations: CUR (2.5%) and QCT (5%), with different peaks at 243 nm, 273 nm and 429 nm. The overall spectra shown in Figure 4 include the two drugs, CUR and QCT showing different peaks at 243 nm, 273 nm, and 429 nm. However, from this curve alone it is impossible to elucidate which peak corresponds to each of the two drugs. In order to determine which peak corresponds to each drug, we have deconvoluted the overall spectra using the Solver function in Excel, which readily gives the deconvolution of the two curves. This is done by subtracting the spectrum of each individual drug from the overall spectra of the two drugs. It should be noted that calibration curves are needed to obtain the concentration of each individual drug in the mixture. Calibration curves were prepared for six different wavelengths selected based on the largest difference between each signal as well as peaks for both drugs. CUR has a peak at approximately 429 nm at a concentration of 2.5%, while QCT has peaks at 243 nm and 273 nm at 5.0 percent concentration. The concentration mismatch of 2.5% was used in order to prevent the CUR signal from completely overtaking that of QCT. The peak at 429 nm in Figure 4 corresponds to CUR’s main peak, while the peaks at approximately 243 nm, 273 nm, and 380 nm belong to QCT.
Figure 4.
UV spectrum of the mixture CUR and QCT in 50:50 methanol/water at a concentration of 0.00625 mg/mL. We used concentrations of 2.5% w/w for CUR and 5% w/w for QCT to facilitate simultaneous visualization of both compounds. The peak at 429 nm corresponds to Curcumin’s main peak, while the peaks at approximately 243 nm, 273 nm, and 380 nm belong to QCT. (A color version of this figure is available in the online journal.)
PLGA/PCL Janus particles containing APAP and NPX using the O/W emulsion method with a partially water-miscible co-solvent
Ethyl acetate was used as the partially water-miscible solvent for co-encapsulation of APAP and NPX. Although the solubility of APAP in ethyl acetate is quite low, it is higher than the solubility in DCM (10.73 g/kg vs. 0.32 g/kg). Optical images of PLGA/PCL Janus particles containing APAP and NPX are shown in Figure 5. These Janus particles appear to have holes on the surface as result of the slow evaporation rate and long residence time of the solvent ethyl acetate. UV-Vis spectra for APAP-NPX in EA are shown in Figure 6. Corresponding absorbance values for APAP and NPX were measured from the UV-Vis curve at 243 nm and 230 nm as it was showing different peaks at these wavelengths and individual concentrations of these compounds in the Janus particles were calculated from the calibration curves.
Figure 6.
Example of UV-Vis spectrum of APAP and NPX in PLGA/PCL Janus Particles for a 50:50 methanol/water mixture at a drug concentration of 8%. A small peak at 230 nm and a larger peak at 243 nm are visible, corresponding to the APAP and NPX UV spectra. (A color version of this figure is available in the online journal.)
Figure 5.
(a) and (b) are optical microscopy images of PLGA/PCL Janus particles containing APAP and NPX, demonstrating that the addition of the active pharmaceutical ingredient does not change the typical dumbbell Janus particle morphology. Some random holes (which are not uniform from particle to particle, (compare (a) and (b)), appear on the surface as a result of the slow evaporation rate and long residence time of the solvent ethyl acetate. (A color version of this figure is available in the online journal.)
PLGA/PCL Janus particles containing APAP and NPX using the O/W emulsion method with a partially water-miscible co-solvent
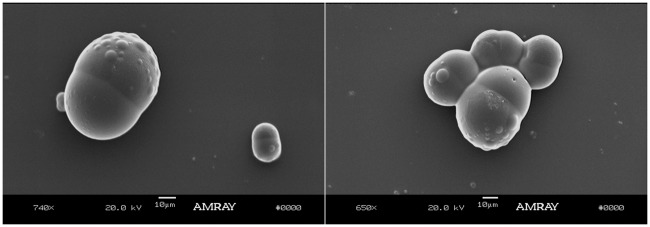
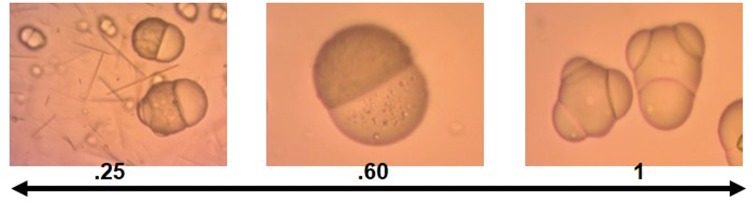
Janus particles containing APAP and NPX were prepared using the co-solvent method using methanol as the co-solvent. The resulting Janus particles are shown in Figure 7. This figure shows two SEM snapshots of Janus particles with APAP and NPX, both showing some protrusions. Protrusions in Janus particles were observed before50 but their origin is still not completely understood. We hypothesize that these protrusions occur during the solvent evaporation stage as the droplets shrink and then expand causing first tiny protrusions due to interfacial instability and finally yielding particles with a rough surface and more significant protrusions. These particles were prepared by the O/W-S method using methanol at a methanol-to-DCM ratio of 0.6. This was determined to be the optimal methanol-to-DCM volume ratio because the typical dumbbell Janus morphology was obtained and there is no drug precipitation due to poor encapsulation, which occurs when there is not enough methanol to solubilize the APAP. It is worth noticing that APAP is highly soluble in methanol, but methanol is completely miscible with water, and therefore the APAP/methanol solution will tend to leach out to water phase during solvent evaporation if the methanol is not used in the right amount. Therefore, the ratio methanol-DCM is critical to obtain the right morphology. The importance of the co-solvent to solvent ratios is shown in Figure 8. Various co-solvent-to-solvent ratios were tested in the development of the formulation. It was found that using too much co-solvent (methanol) disrupts phase separation, leading to triphasic particles (Figure 8(c)). Conversely, not having enough co-solvent causes the APAP to not be encapsulated into the particles as it prefers to remain in the water phase, resulting in the formation of free drug needles (Figure 8(a)). Figure 8(b) shows particles with the optimal ratio 0.6, which give the expected dumbbell morphology.
Figure 7.
SEM images of PLGA/PCL Janus particles containing APAP and NPX prepared by the O/W-S method using methanol at a methanol-to-DCM ratio of 0.6. This was determined to be the optimal methanol-to-DCM volume ratio because the expected dumbbell Janus morphology was obtained and because there was no drug precipitation due to poor encapsulation, which occurs when there is not enough methanol to solubilize the APAP. The first snapshot depicts also a much smaller Janus particle. This is due to the fact that these particles are made using handshake mixing which gives a much broader size distribution.
Figure 8.
PLGA/PCL Janus particles containing APAP and NPX prepared by the O/W-S method at the indicated in increasing order of methanol-DCM ratio. (a) At the lowest methanol/DCM ratio of 0.25, there is not enough methanol, and the APAP is not encapsulated into the particles as it prefers to remain in the water phase and form needles as shown. (b) The optimal methanol-DCM ratio was found to be 0.6 as shown in Figure 8b. (c) At a ratio of 1, there is excess of methanol resulting in disruption of phase separation, leading to particles with multiple compartments. (A color version of this figure is available in the online journal.)
Acetone was also tested as a co-solvent. These particles had noticeably more surface protrusions than when methanol was used as a co-solvent. Examples are shown in Figure 9, which depicts PLGA/PCL Janus particles containing APAP and NPX prepared by the O/W-S method using acetone. Similarly to the case of O/W-S method using methanol, we believe that PCL surface protrusions are present as result of the rate of evaporation of the solvent with respect to the drug it encapsulates. Similar protrusions have already been observed in the literature50 with a seeded polymerization method; however, this work does not mention the reason for the origin of the protrusions. We believe that the rate of evaporation of acetone is more strongly affected by the polymer PCL and the drug it encapsulates (NPX) than methanol and for that reason protrusions appear to be larger. More work needs to be done to elucidate the reason of the origin of the protrusions.
Figure 9.
PLGA/PCL Janus particles containing APAP and NPX prepared by the O/W-S method using acetone. We hypothesize that PCL surface protrusions are present as result of the rate of evaporation of the solvent with respect to the drug it encapsulates (NPX). Figure 9(b) also shows clear protrusions at the bottom right but the larger scale (100 µm) does not allow to completely see them in all of them.
PLGA/PCL Janus particles containing APAP and NPX using the W/O/W emulsion method
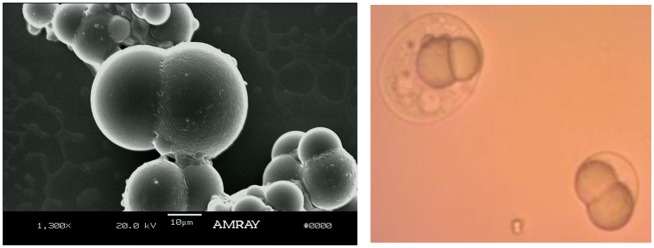
The double emulsion method was used to encapsulate APAP in inner water droplets within Janus particles containing NPX. Using a 75:25 v/v mixture of PEG 400/water as the inner aqueous phase instead of water greatly increased the amount of APAP that could be incorporated into the particles. The solubility of APAP in a 75:25 blend of PEG 400/water is 220 mg/mL, compared to only approximately 12 mg/mL in water. It is important to minimize the volume of W1 because smaller internal water phase volume has been shown to reduce porosity and burst release.51,52 Thus, a W1/O/W2 ratio of 1/10/30 was used. PLGA/PCL Janus particles containing APAP and NPX prepared by the double emulsion method are shown in Figure 10. These particles exhibit an oblong shape compared to the standard PLGA/PCL biphasic dumbbell Janus particles normally obtained from single emulsions.
Figure 10.
PLGA/PCL Janus particles containing APAP and NPX prepared by the double emulsion method. (A color version of this figure is available in the online journal.)
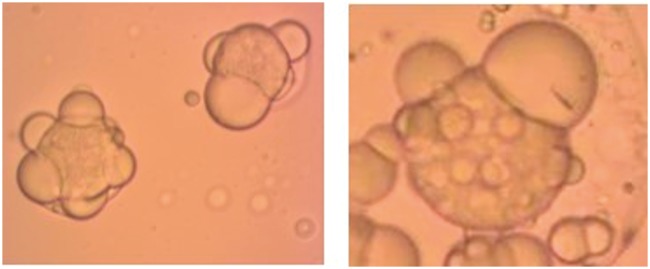
The complexity of the W/O/W emulsion process renders the formulation and process variables much more important and less flexible than a standard O/W emulsion process, and there are some commonly encountered issues associated with this emulsion process. For example, it was found that the primary W/O droplets need to be significantly smaller in diameter than the outer O/W emulsion droplets to prevent coalescence and rupture of the O/W droplets. We hypothesize that surface protrusions occur when the W/O droplets are too large relative to the O/W droplet/particle encapsulating them. Therefore, it is important to use the high pressure homogeneizer to make the W/O droplets smaller (Figure 11).
Figure 11.
Commonly encountered issues with Janus particles formed by the double W/O/W emulsion method. (a) Disrupted phase separation due to changes in thermodynamic conditions (i.e. evaporation time) and (b) protrusion of inner water droplets due to large inner W/O droplets. (A color version of this figure is available in the online journal.)
The O/W emulsion was formed by either manual shear or a lower speed setting on the rotor stator. Additionally, NaCl was added to the external aqueous phase in order to balance the osmotic pressure gradient, leading to greater emulsion stability.52,53 This allows the W/O emulsion droplets to remain small and prevents destabilization of the W/O/W emulsion. The surfactants used to produce a W/O emulsion are hydrophobic, so we hypothesize that the W/O emulsion droplets predominantly reside in the more hydrophobic PCL compartment (or Precirol®, in the case of polymer/lipid particles).
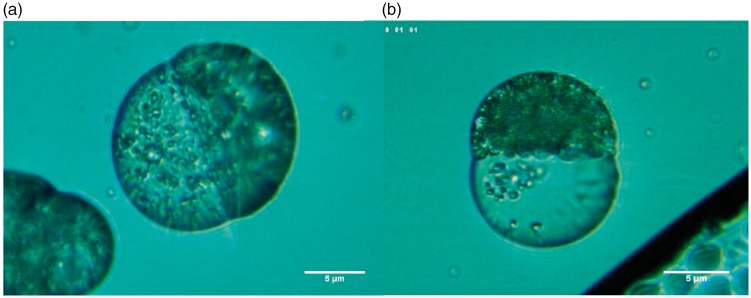
Double W/O/W emulsions were also used to simultaneously encapsulate the hydrophilic and hydrophobic compounds DOX and CUR, respectively, in PLGA/Precirol® Janus particles. In the double emulsion process, the water phase containing DOX hydrochloride is first added to the oil phase containing CUR and the polymer/lipid and homogenized to form the W/O emulsion. The W/O emulsion is subsequently added to the external water phase and homogenized, forming a W/O/W double emulsion, wherein the W/O emulsion droplets are contained inside larger O/W droplets. Initially, the double emulsion droplets are spherical. As solvent evaporation progresses, the PLGA and Precirol® phase separates into two separate compartments. The W/O emulsion droplets containing DOX hydrochloride are hydrophilic but it is surrounded by surfactants which provide a very hydrophobic coating to it, favoring the localization into the more hydrophobic Precirol® phase, while the CUR resides in the PLGA phase. This is due to the very hydrophobic nature of the surface of the W/O emulsion droplets in which DOX is encapsulated. DOX is surrounded by surfactants (Tween 80 and Span80 with a hydrophilic lipophilic balance index of approximately 6) which confer a very hydrophobic coating on its surface, thus making it more favorable to prefer the more hydrophobic compartment Precirol.
Figure 12 shows the transformation of W/O/W emulsion droplets into biphasic PLGA/Precirol® particles via solvent evaporation. Three different steps in the formation of these particles are shown in this figure. In step 1, we observe the double emulsion W/O/W consisting of W/O emulsion droplets containing DOX inside an O/W emulsion droplet containing CUR. In step 2, we see the initial phase separation of the polymer and lipid phases during solvent evaporation. The location of the drug inside each compartment can be clearly seen in these images. Those drugs were purposely chosen for this study not only because they are a good combination for cancer treatment but also because their natural color allows for a good visualization of their location inside the Janus particle without the need of the use of any additional dyes. Doxorubicin is naturally red and CUR is usually bright yellow. It can be clearly observed that the red DOX is in the Precirol compartment and the yellow CUR is in the PLGA compartment. We believe that the reason why red DOX was entrapped in the Precirol compartment is double fold: (i) DOX.HCL was formulated into W/O emulsion droplets which are stabilized by hydrophobic surfactants (a surfactant mixture with low HLB) rendering the surface hydrophobic and therefore more likely to reside in the more hydrophobic compartment which in this case is the Precirol compartment, also DOX.HCL droplets are prepared separately from the CUR in the polymer solution. So DOX.HCL resides inside the emulsion droplets and CUR is free. (ii) Also DOX.HCL is by itself hydrophilic so it would not mix with CUR, so it will end up in a place of its own. For this reason, it is noteworthy that random deposition of the two drugs did not occur, indicating that also, both the interactions CUR-CUR and DOX-DOX are both more attractive that DOX-CUR.
Figure 12.
Optical microscope pictures depicting PLGA-Precirol Janus particles in the presence DOX and CUR. Compartmentalization of the drug particles is demonstrated visually in the optical microscope pictures through localization of the red DOX to the Precirol® compartment and bright yellow CUR in the PLGA compartment, as it can be clearly observed in the optical microscope pictures in Figure 12(a). (a) PLGA/Precirol Janus particle formation from W/O/W emulsions. Step 1: W/O/W emulsion consisting of W/O emulsion droplets containing DOX inside an O/W emulsion droplet containing CUR. Step 2: Initial phase separation of the polymer and lipid phases during solvent evaporation. The red color of the drug DOX can be clearly seen in the Precirol compartment (conical part of the ice cream cone), and the yellow color of CUR can be seen in the PLGA phase. Step 3: Formation of PLGA/Precirol particles upon complete solvent evaporation (b) Fully formed PLGA/Precirol particles in the absence of any drugs. (A color version of this figure is available in the online journal.)
Step 3 shows the formation of PLGA/Precirol particles upon complete solvent evaporation.
Finally, Figure 12(b) shows a fully formed PLGA/Precirol particle in the absence of any drugs which clearly shows the typical ice cream cone morphology. When the drugs are uploaded inside the ice cream cone particles, the cone morphology changes and the lipid Precirol compartment (conical part of the ice cream cone) becomes more rounded due to the presence of the drug inside the compartment.
Particle size distribution for single and double emulsions
Particle size distribution (PSD) was done by laser diffraction, with a Beckman Coulter apparatus. A representative sample of the PSD of four sets with single and double emulsions obtained with different solvents is shown in Figure 13: CUR-QCT with DCM, APAP/NPX with ethyl acetate, APAP/NPX with DCM and Methanol, and APAP-NPX with double emulsion. The solvent plays a critical role in crystal growth and morphology. Ultimately, solvent selection is dictated by the solubility of the drug. Most poorly water-soluble drugs exhibit high solubility in at least one partially water-miscible solvent. Ethyl acetate was chosen because it has the largest solvent-to-water diffusion coefficients. This corresponds to faster dissolution of emulsion droplets and rapid drug precipitation, theoretically resulting in smaller particles. This can be seen in Figure 13, which shows that Janus with APAP-NPX made with a single emulsion process with ethyl acetate gives the smallest PSD. APAP-NPX with methanol DCM shows an almost unimodal PSD with a small peak from 10 to 20 µm, which comes from the formation of needle particles, not Janus. These particles were made with a ratio solvent – co-solvent of 0.5, yielding the aforementioned needles similar to the ones shown in Figure 8, that appear at a ratio smaller than 0.6. Lastly, we observe that the double emulsion technique shows a broad pic between 0.1 and 1 µm and a narrow distribution around 5 µm.
Figure 13.
Particle size distribution for single and double emulsions. (A color version of this figure is available in the online journal.)
Encapsulation efficiency of Janus particles
The EE of Janus particles loaded with 2.5% w/w CUR and 5% w/w QCT was calculated both directly (particles dissolved in THF) and indirectly (subtracting free drug in supernatant) with similar results (see Table 2). As expected, both CUR and QCT were loaded into Janus particles and pure PCL particles with high encapsulation efficiency.
Table 2.
Encapsulation efficiencies percentages of CUR and QCT in Janus and PCL particles prepared by the O/W emulsion technique.
|
EE (%) particles dissolved in THF (Absorbance data) |
EE (%) supernatant |
|||
|---|---|---|---|---|
| CUR | QCT | CUR | QCT | |
| Janus particles | 93.11 ± 0.86 | 92.03 ± 4.19 | 93.10 ± 1.95 | 89.55 ± 5.69 |
| PCL particles | 93.38 ± 0.55 | 86.90 ± 1.05 | 92.99 ± 0.21 | 83.97 ± 0.87 |
CUR; curcumin; QCT: quercetin; THF: tetrahydrofuran.
As mentioned previously, the encapsulation efficiency of the hydrophilic and the hydrophobic drugs, APAP and NPX, can only be measured indirectly from the supernatant due to interference from the polymers’ spectra. The EE and DL of Janus particles containing APAP and NPX synthesized via the ethyl acetate-in-water single emulsion method, the O/W emulsion method using DCM as the solvent and methanol as a co-solvent, and the W/O/W emulsion method are contained in Table 3.
Table 3.
Encapsulation efficiencies and drug loading percents of APAP and NPX in Janus particles synthesized via single and double emulsions.
|
APAP |
NPX |
|||
|---|---|---|---|---|
| Synthesis method | EE (%) | DL (%) | EE (%) | DL (%) |
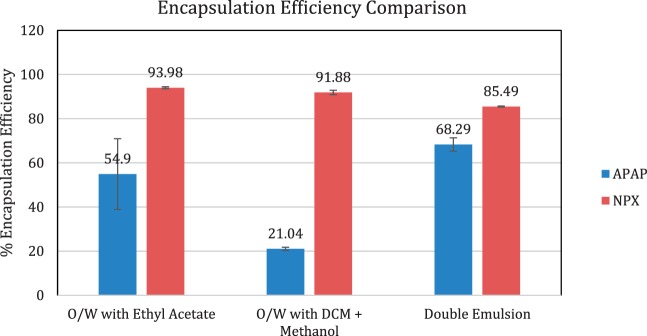
| O/W with ethyl acetate | 54.90 ± 16.01 | 4.26 ± 0.49 | 93.98 ± 0.45 | 7.22 ± 0.83 |
| O/W with DCM + methanol | 21.04 ± 0.72 | 1.69 ± 0.28 | 91.88 ± 1.00 | 7.36 ± 0.86 |
| Double emulsion | 68.29 ± 3.04 | 15.93 ± 4.39 | 85.49 ± 0.20 | 9.14 ± 2.50 |
NPX: naproxen; DCM: dichloromethane; APAP: acetaminophen.
Table 3 shows that the EE of NPX, a hydrophobic drug, was very high with all the emulsion techniques, ranging from 85 to 94%. This translated to total drug loadings ranging from 7.22% for the single O/W emulsion with ethyl acetate to 9.14% for the double emulsion. The loading of NPX was comparable for both single emulsion techniques: 7.22% when ethyl acetate was used as the oil phase and 7.36% when a mixture of DCM and methanol was used. Figure 14 shows a bar graph comparing the encapsulation efficiency of the three synthesis methods. The double emulsion method resulted in the highest EE of 68.29% for the hydrophilic drug APAP, while the single emulsion methods gave EE’s of 21.04% using DCM + Methanol as the oil phase and 54.90% using ethyl acetate as the oil phase. The drug loading for APAP was considerably lower at 4.26% and 1.69% for the O/W-EA and O/W-DCM emulsions respectively, and 15.93% in the double emulsion batch. Such a high drug loading was achieved by the double emulsion method due to the high solubility of APAP in the PEG 400/water internal water phase.
Double emulsions are frequently used for the entrapment of hydrophilic compounds. A very high concentration of APAP is possible using the W/O/W emulsion technique with PEG 400/water as the inner water phase despite the small volume of W1. For example, even with an inner water phase only 1/10th of the volume of the oil phase that contains NPX, there is a higher content of APAP than NPX (15.93% w/w total formulation vs. 9.14%). The O/W-S method using methanol resulted in the lowest EE despite APAP’s high solubility in methanol. This is due to the fact that methanol is completely miscible with water, causing most of the APAP dissolved in methanol to escape to the water phase during evaporation since APAP is soluble in water and practically insoluble in DCM. Using ethyl acetate as the solvent resulted in a moderate EE of APAP. All three methods resulted in relatively high EE of NPX, which is expected for the encapsulation of hydrophobic compounds using O/W emulsion-based techniques.
Drug release profiles
The formulations of the three different methods proposed in this work (1. Single O/W emulsion containing a partially water-miscible solvent (ethyl acetate), 2. O/W emulsion using a co-solvent (methanol) (O/W-S), and 3. W/O/W double emulsion) for the synthesis of Janus particles encapsulating APAP and NPX were subject to drug release studies and the release curves of each drug were then plotted in Figure 15. Figure 15 shows the percent drug released obtained with UV-Vis spectroscopy in 48 hours.
Figure 15.
Percent drug release for Naproxen and APAP for the three different synthesis methods. (A color version of this figure is available in the online journal.)
For the double emulsion method, after 24 h, 50.8% of the encapsulated APAP (blue curve in Figure 15) is released, whereas 81.01% of NPX is released at the same time (grey curve). There is a sustained release up to 97% at 48 h for NPX. The release of APAP goes up to 68.04% at 48 h and the positive slope indicates that it will continue to be released after 48 h.
The encapsulation efficiency for APAP for the DCM-Methanol method is approximately 21.04% as indicated in Table 3, and that is also reflected in the low cumulative drug release of encapsulated APAP in Figure 15 for this method, whereas for NPX the encapsulation is high (91.88%), showing a much higher cumulative drug release after 48 h.
It is worth noticing that the double emulsion shows a higher percent drug release than other two methods for both APAP and NPX. This is consistent with the encapsulation efficiency and drug loading of NPX and APAP in the Janus particles, which also contains higher concentrations of the drug inside the Janus particles.
The amount of encapsulated hydrophilic drug is higher for the double emulsion method as indicated in Table 3, and this is consistent with the amount released for the hydrophilic drug APAP, which is the highest for this method, followed by the single emulsion with EA and then by the single emulsion with DCM-methanol. This can be seen in the light blue, yellow, and green curves in Figure 15. It is interesting to notice that APAP has a delayed release for all the methods; in particular, it can be seen that for the single emulsion method with ethyl acetate it shows up to 1.7% release at 22 h and it reaches 35.25% at 24 h, for the single emulsion with DCM-methanol it takes up to 48 h to release 40% of the drug, and lastly, for the double emulsion method only 21% of the drug is released at 21 h. Our results confirm that Janus particles can be used for staggered dual release of drugs.
Effect of solvent
Drug solubility is typically the first consideration in solvent selection. Most poorly water-soluble drugs exhibit high solubility in at least one partially water-miscible solvent suitable for emulsion-precipitation. Since one of the novel features of this paper is the encapsulation of the hydrophilic drug along with the hydrophobic drug, the solvent selection was done based on the solubility of the hydrophilic drug in the solvent (see Table 1).
Due to the fact that APAP is poorly soluble in DCM, we chose two different strategies to increase the solubility of APAP with the solvent:
Single O/W emulsion containing a partially water-miscible solvent, where ethyl acetate was used as the solvent and
O/W emulsion using a co-solvent (O/W-S), where a mixture of DCM and Methanol was used as the solvent (methanol has high solubility with APAP).
Solvent transport phenomenon has been shown to greatly affect the size of polymer particles prepared by the emulsion–diffusion method.54,55 In particular, the solvent plays an important role in crystal growth, size, and morphology.56 Due to the amount work that would be required to address the effect of solvent in the absence of drugs, and then in the presence of drugs and surfactants, we are unable to include these points in this manuscript and will leave that for future studies. However, we have calculated the value of the solvent exchange ratios based on the work done by Choi et al.54 referred to the two solvents we used in our studies, DCM, ethyl acetate and the co-solvent, methanol (Table 4) and tried to observe our trends in comparison with their published work.
Table 4.
Calculated mutual diffusion coefficients and exchange ratios (R) of partially water-miscible solvents in water at 20 °C.
| Solvent | DAB (cm2/s) | DBA (cm2/s) | R |
|---|---|---|---|
| n-butyl lactate | 8.910 × 10−6 | 4.954 × 10−6 | 1.798 |
| Ethyl acetate | 1.105 × 10−5 | 3.460 × 10−5 | 0.319 |
| Methyl ethyl ketone | 1.147 × 10−5 | 3.530 × 10−5 | 0.325 |
| Dichloromethane | 1.17 × 10−5 | 3.57 × 10−5 | 0.3277 |
Choi et al.54 have reported the effect of the solvent in PLGA particles using solvent diffusivity and solvent exchange ratios based on the diffusivity of the solvent in water. Ethyl acetate and MEK have the largest solvent-to-water diffusion coefficients. This corresponds to faster dissolution of emulsion droplets and rapid drug precipitation, theoretically resulting in smaller particles. However, diffusion between two phases is bidirectional, counter-diffusion of water into emulsion droplets must also be taken into consideration. The solvent exchange ratio R, a parameter that has been previously used to quantify solvent diffusion from emulsion droplets and counter-diffusion of water into emulsion droplets, was calculated as follows54(2)
| (4) |
N-butyl lactate has the highest exchange ratio among the solvents used, leading to the largest particle size. In a separate study, the diameter of PLGA nanoparticles prepared using emulsion-diffusion was found to increase exponentially with the increase of the solvent exchange ratio.54 Rapid solvent exchange is implicated in the formation of local supersaturation regions, leading to aggregation.
Our results for PLGA compartments were similar to those reported in Choi et al.54 The larger the solvent exchange ratio R, the larger the particle size for the same level of shear. The small R values are consistent with the smaller size of the PLGA particles and the smaller encapsulation efficiency of APAP in the PLGA compartment as can be seen in Figure 14.
Figure 14.
Comparison of the encapsulation efficiencies for the three different synthesis methods. (A color version of this figure is available in the online journal.)
Diffusion coefficient values for water in ethyl acetate and DCM/methanol were obtained from the literature.57,58 The solvent exchange ratio R of ethyl acetate in water is R = 0.319 and the R value for DCM in water is R = 0.322. The value of DCM is slightly larger suggesting that the particle compartments with DCM will tend to be larger.
Conclusions
This paper demonstrates three different methods for incorporating hydrophilic and hydrophobic compounds, as well as a UV detection method for measuring the encapsulation efficiency and drug release of the different compounds from PLGA/PCL Janus particles. PLGA/PCL Janus particles were loaded with two hydrophobic compounds, CUR and QCT, via the standard single O/W emulsion technique and one hydrophilic compound APAP. Due to the poor solubility of the hydrophilic drug APAP in organic solvents, a co-solvent, partially water-miscible solvent, or double W/O/W emulsion was proposed to encapsulate the hydrophilic compound. Some key findings in the formulation and process optimization of the Janus encapsulated particles are: (i) the addition of NaCl to the external aqueous phase balances osmotic pressure gradient leading to greater emulsion stability; (ii) smaller internal water phase volume reduces porosity and, (iii) the W/O droplet diameter has to be much smaller than the W/O/W droplets to prevent O/W emulsion droplet rupture.
All three methods encapsulate hydrophobic compounds with high encapsulation efficiency. The double emulsion method yields the highest encapsulation efficiency of hydrophilic compounds. The optimal method will ultimately depend on the individual API(s) to be encapsulated and its solubility in the various water-miscible or partially water-miscible solvents. From the case studies presented in this work, our data suggest that the double emulsion method is the most advantageous in terms of encapsulation efficiency, compartmentalization, tunable drug release kinetics and easiness to scale up for large batches.
The Janus particles exhibit similar or greater encapsulation efficiency of CUR and QCT relative to the PCL only particles. Subtracting the drug content in the supernatant from the initial amount of drug loaded was validated as an accurate method by which to measure encapsulation efficiency in Janus particles. This is necessary in some cases where the polymeric matrix is insoluble in the solvent being used for UV analysis or if it is desired to measure drug release at discrete time points.
The hydrophobic drug NPX was encapsulated into the particles at a high encapsulation efficiency regardless of the synthesis method owing to its high oil solubility. Unlike hydrophobic drugs which are readily encapsulated by a single O/W emulsion, encapsulating hydrophilic compounds requires more complex approaches. For hydrophilic drugs, the double emulsion (W/O/W) method yields the highest encapsulation efficiency followed in decreasing order of efficiency by the single O/W ethyl acetate method and last, by the methanol-DCM method with the least encapsulation efficiency. This is consistent with measurements of the percent drug released for the three methods, which show the same trends: the double emulsion shows the highest percent drug released, followed by the single emulsion with ethyl acetate and in last place the single emulsion with methanol-DCM. The delayed released observed for the hydrophilic drug for the three methods confirms that Janus particles can be used for staggered dual release of drugs.
It is worth mentioning that double emulsions are inherently more complex than single emulsions with the addition of another phase, thus there are more variables that need to be taken into consideration. For example, if inner W/O droplets are too large, this can result in surface protrusions. In single emulsion O/W-S system, it is observed that low API solubility due to lack of solvent power can result in the formation of free drug needles due to the partitioning of the drug to the aqueous phase where it is more soluble.
Although microfluidics and electrohydrodynamic co-jetting produce monodisperse, bicompartmental Janus particles, these techniques suffer from low yield and material restrictions. The synthesis methods presented in this work are one-pot techniques amenable to scale up with little processing equipment and are not subject to material constraints. Janus particles have the potential to meet the ever-growing demand for multifaceted drug delivery systems capable of targeting and treating complex diseases.
Supplemental Material
Supplemental material, EBM876554 Supplemental Material1 for Dual drug-loaded biodegradable Janus particles for simultaneous co-delivery of hydrophobic and hydrophilic compounds by Jennifer S Winkler, Mayur Barai and Maria S Tomassone in Experimental Biology and Medicine
Supplemental Material
Supplemental material, EBM876554 Supplemental Material2 for Dual drug-loaded biodegradable Janus particles for simultaneous co-delivery of hydrophobic and hydrophilic compounds by Jennifer S Winkler, Mayur Barai and Maria S Tomassone in Experimental Biology and Medicine
Supplemental Material
Supplemental material, EBM876554 Supplemental Material3 for Dual drug-loaded biodegradable Janus particles for simultaneous co-delivery of hydrophobic and hydrophilic compounds by Jennifer S Winkler, Mayur Barai and Maria S Tomassone in Experimental Biology and Medicine
Authors’ contributions
All authors participated in the design, interpretation of the studies and analysis of the data and review of the manuscript.
DECLARATION OF CONFLICTING INTERESTS
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
FUNDING
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by grant # NSF CMMI 1235301.
References
- 1.Du JZ, O'Reilly RK. Anisotropic particles with patchy, multicompartment and Janus architectures: preparation and application. Chem Soc Rev 2011; 40:2402–16 [DOI] [PubMed] [Google Scholar]
- 2.Huang HY, Kuo WT, Chou MJ, Huang YY. Co-Delivery of anti-Vascular endothelial growth factor sirna and doxorubicin by multifunctional polymeric micelle for tumor growth suppression. J Biomed Mater Res 2011; 97A:330–8 [DOI] [PubMed] [Google Scholar]
- 3.Lee KJ, Yoon J, Lahann J. Recent advances with anisotropic particles. Curr Opin Colloid Interf Sci 2011; 16:195–202 [Google Scholar]
- 4.Liu B, Zhang W, Zhang DW, Yang XL. Facile method for large scale synthesis of magnetic inorganic-organic hybrid anisotropic Janus particles. J Colloid Interface Sci 2012; 385:34–40 [DOI] [PubMed] [Google Scholar]
- 5.Song XR, Cai Z, Zheng Y, He G, Cui FY, Gong DQ, Hou SX, Xiong SJ, Lei XJ, Wei YQ. Reversion of multidrug resistance by Co-Encapsulation of vincristine and verapamil in PLGA nanoparticles'. Eur J Pharm Sci 2009; 37:300–5 [DOI] [PubMed] [Google Scholar]
- 6.Song XR, Zhao Y, Hou SX, Xu FY, Zhao R, He JY, Cai Z, Li YB, Chen QH. Dual agents loaded plga nanoparticles: systematic study of particle size and drug entrapment efficiency. Eur J Pharm Biopharm 2008; 69:445–53 [DOI] [PubMed] [Google Scholar]
- 7.Xie H, She ZG, Wang S, Sharma G, Smith JW. One-Step fabrication of polymeric Janus nanoparticles for drug delivery. Langmuir 2012; 28:4459–63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Song XR, Cai Z, Zheng Y, He G, Cui FY, Gong DQ, Hou SX, Xiong SJ, Lei XJ, Wei YQ. Reversion of multidrug resistance by co-encapsulation of vincristine and verapamil in PLGA nanoparticles. Eur J Pharm Sci 2009; 37:300–5 [DOI] [PubMed] [Google Scholar]
- 9.Song X, Zhao Y, Hou S, Xu F, Zhao R, He J, Cai Z, Li Y, Chen Q. Dual agents loaded PLGA nanoparticles: systematic study of particle size and drug entrapment efficiency. Eur J Pharm Biopharm 2008; 69:445–53 [DOI] [PubMed] [Google Scholar]
- 10.Lee JH, Nan A. Combination drug delivery approaches in metastatic breast cancer. J Drug Deliv 2012; 2012:17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hwang S, Lahann J. Differentially degradable Janus particles for controlled release applications. Macromol Rapid Commun 2012; 33:1178–83 [DOI] [PubMed] [Google Scholar]
- 12.Hu C-M, Zhang L. Nanoparticle-based combination therapy toward overcoming drug resistance in cancer. Biochem Pharmacolog 2012; 83:1104–11 [DOI] [PubMed] [Google Scholar]
- 13.Hu C-MJ, Aryal S, Zhang L. Nanoparticle-assisted combination therapies for effective cancer treatment. Ther Deliv 2010; 1:323–4 [DOI] [PubMed] [Google Scholar]
- 14.David KI, Jaidev LR, Sethuraman S, Krishnan UM. Dual drug loaded chitosan nanoparticles – sugar-coated arsenal against pancreatic cancer. Colloids Surf B 2015; 135:689–98 [DOI] [PubMed] [Google Scholar]
- 15.Kundu A, Nandi S, Das P, Nandi AK. Fluorescent graphene oxide via polymer grafting: an efficient nanocarrier for both hydrophilic and hydrophobic drugs. ACS Appl Mater Interfaces 2015; 7:3512–23 [DOI] [PubMed] [Google Scholar]
- 16.Lockhart JN, Stevens DM, Beezer DB, Kravitz A, Harth E. Dual drug delivery of tamoxifen and quercetin: regulated metabolism for anticancer treatment with nanosponges. J Control Release 2015; 220:751–7 [DOI] [PubMed] [Google Scholar]
- 17.Parhi P, Mohanty C, Sahoo SK. Nanotechnology-based combinational drug delivery: an emerging approach for cancer therapy. Drug Discov Today 2012; 17:1044–52 [DOI] [PubMed] [Google Scholar]
- 18.Khan IU, Serra CA, Anton N, Li X, Akasov R, Messaddeq N, Kraus I, Vandamme TF. Microfluidic conceived drug loaded Janus particles in side-by-Side capillaries device. Int J Pharm 2014; 473:239–49 [DOI] [PubMed] [Google Scholar]
- 19.Li WX, Dong H, Tang GN, Ma T, Cao XD. Controllable microfluidic fabrication of Janus and microcapsule particles for drug delivery applications. RSC Adv 2015; 5:23181–8 [Google Scholar]
- 20.Zhu AD, Guo MY. Single emulsion microfluidic production of Janus and core-shell particles via off-chip polymerization. Chin J Polym Sci 2016; 34:367–77 [Google Scholar]
- 21.Khoee S, Karimi MR. Dual-drug loaded Janus graphene oxide-based thermoresponsive nanoparticles for targeted therapy. Polymer 2018; 142:80–98 [Google Scholar]
- 22.Khlibsuwan R, Siepmann F, Siepmann J, Pongjanyakul T. Chitosan-clay nanocomposite microparticles for controlled drug delivery: effects of the MAS content and TPP crosslinking. J Drug Deliv Sci Technol 2017; 40:1–10 [Google Scholar]
- 23.Kohane DS. Microparticles and nanoparticles for drug delivery. Biotechnol Bioeng 2007; 96:203–9 [DOI] [PubMed] [Google Scholar]
- 24.Ni R, Zhao J, Liu QY, Liang ZL, Muenster U, Mao SR. Nanocrystals embedded in chitosan-based respirable swellable microparticles as dry powder for sustained pulmonary drug delivery. Eur J Pharm Sci 2017; 99:137–46 [DOI] [PubMed] [Google Scholar]
- 25.Siepmann J, Siepmann F. Microparticles used as drug delivery systems. In: Richtering W (ed) Smart colloidal materials Berlin: Springer-Verlag, 2006, p. 15
- 26.Kohane DS, Lipp M, Kinney RC, Anthony DC, Louis DN, Lotan N, Langer R. Biocompatibility of lipid-protein-sugar particles containing bupivacaine in the epineurium. J Biomed Mater Res 2002; 59:450–9 [DOI] [PubMed] [Google Scholar]
- 27.Kohane DS, Tse JY, Yeo Y, Padera R, Shubina M, Langer R. Biodegradable polymeric microspheres and nanospheres for drug delivery in the peritoneum. J Biomed Mater Res 2006; 77A:351–61 [DOI] [PubMed] [Google Scholar]
- 28.Garbuzenko OB, Winkler J, Tomassone MS, Minko T. Biodegradable Janus nanoparticles for local pulmonary delivery of hydrophilic and hydrophobic molecules to the lungs. Langmuir 2014; 30:12941–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Romanski FS, Winkler JS, Riccobene RC, Tomassone MS. Production and characterization of anisotropic particles from biodegradable materials. Langmuir 2012; 28:3756–65 [DOI] [PubMed] [Google Scholar]
- 30.Rosca ID, Watari F, Uo M. Microparticle formation and its mechanism in single and double emulsion solvent evaporation. J Control Release 2004; 99:271–80 [DOI] [PubMed] [Google Scholar]
- 31.Wang H, Agarwal P, Zhao S, Xu RX, Yu J, Lu X, He X. Hyaluronic acid-decorated dual responsive nanoparticles of pluronic F127, PLGA, and chitosan for targeted co-delivery of doxorubicin and irinotecan to eliminate cancer stem-like cells. Biomaterials 2015; 72:74–89 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhai WZ, Wang B, Wang YS, He YF, Song PF, Wang RM. An efficient strategy for preparation of polymeric Janus particles with controllable morphologies and emulsifiabilities. Colloids Surf A 2016; 503:94–100 [Google Scholar]
- 33.Fan YL, Tan CH, Lui Y, Zudhistira D, Loo SCJ. Mechanistic formation of drug-encapsulated Janus particles through emulsion solvent evaporation. Rsc Adv 2018; 8:16032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhang JY, Lin MT, Zhou MJ, Yi T, Tang YN, Tang SL, Yang ZJ, Zhao ZZ, Chen HB, Cancer MGC. Combinational treatment of curcumin and quercetin against gastric 803 cells in vitro. Molecules 2015; 20:11524–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tang M, Taghibiglou C. The mechanisms of action of curcumin in Alzheimer's disease. J Alzheimers Dis. 2017; 58(4): 1003–1016 [DOI] [PubMed] [Google Scholar]
- 36.Jackson JK, Higo T, Hunter WL, Burt HM. The antioxidants curcumin and quercetin inhibit inflammatory processes associated with arthritis. Inflamm Res 2006; 55:168–75 [DOI] [PubMed] [Google Scholar]
- 37.Moorthi C, Kathiresan K. Curcumin–piperine/curcumin–quercetin/curcumin–silibinin dual drug-loaded nanoparticulate combination therapy: a novel approach to target and treat multidrug-resistant cancers. J Med Hypoth Ideas 2013; 7:15–20 [Google Scholar]
- 38.Prior MJ, Cooper KM, May LG, Bowen DL. Efficacy and safety of acetaminophen and naproxen in the treatment of tension-type headache. A randomized, double-blind, placebo-controlled trial. Cephalgia 2002; 22:740–8 [DOI] [PubMed] [Google Scholar]
- 39.Seideman P. Additive effect of combined naproxen and paracetamol in rheumatoid arthritis. Br J Rheumatol 1993; 32:1077–82 [DOI] [PubMed] [Google Scholar]
- 40.Hiep NT, Chan Khon H, Hai ND, Byong-Taek L, Toi VV, Hung LT. Biocompatibility of PCL/PLGA-BCP porous scaffold for bone tissue engineering applications. J Biomater Sci 2017; 28:864–78 [DOI] [PubMed] [Google Scholar]
- 41.Ayoub M, Ahmed N, Kalaji N, Charcosset C, Magdy A, Fessi H, Elaissari A. Study of the effect of formulation parameters/variables to control the nanoencapsulation of hydrophilic drug via double emulsion technique. J Biomed Nanotechnol 2011; 7:255–62 [DOI] [PubMed] [Google Scholar]
- 42.Astete CE, Kumar C, Sabliov CM. Size control of poly(D,L-Lactide-Co-Glycolide) and poly(D,L-Lactide-Co-Glycolide)-magnetite nanoparticles synthesized by emulsion evaporation technique. Colloids Surf A 2007; 299:209–16 [Google Scholar]
- 43.Jeffery H, Davis SS, Ohagan DT. The preparation and characterization of poly(Lactide-Co-Glycolide) microparticles.1. Oil-in-water emulsion solvent evaporation. Int J Pharm 1991; 77:169–75 [DOI] [PubMed] [Google Scholar]
- 44.Li M, Rouaud O, Poncelet D. Microencapsulation by solvent evaporation: state of the art for process engineering approaches. Int J Pharm 2008; 363:26–39 [DOI] [PubMed] [Google Scholar]
- 45.Odonnell PB, McGinity JW. Preparation of microspheres by the solvent evaporation technique. Adv Drug Deliv Rev 1997; 28:25–42 [DOI] [PubMed] [Google Scholar]
- 46.Song CX, Labhasetwar V, Murphy H, Qu X, Humphrey WR, Shebuski RJ, Levy RJ. Formulation and characterization of biodegradable nanoparticles for intravascular local drug delivery. J Controll Release 1997; 43:197–212 [Google Scholar]
- 47.Calloway DJ. Beer–lambert law. J Chem Educ 1997; 74:744 [Google Scholar]
- 48.Fuwa K, Valle BL. The physical basis of analytical atomic absorption spectrometry. The pertinence of the Beer–Lambert law. Anal Chem 1963; 35:942–6 [Google Scholar]
- 49.Mock EB, De Bruyn H, Hawkett BS, Gilbert RG, Zukoski CF. Synthesis of anisotropic nanoparticles by seeded emulsion polymerization. Langmuir 2006; 22:4037–43 [DOI] [PubMed] [Google Scholar]
- 50.Li Y, Chen SS, Demirci S, Qin SY, Xu ZH, Olson E, Liu F, Palm D, Yong X, Jiang S. Morphology evolution of Janus dumbbell nanoparticles in seeded emulsion polymerization. J Colloid Interf Sci 2019; 543:34–42 [DOI] [PubMed] [Google Scholar]
- 51.Cui F, Cun D, Tao A, Yang M, Shi K, Zhao M, Guan Y. Preparation and characterization of melittin-loaded poly (dl-lactic acid) or poly (dl-lactic-co-glycolic acid) microspheres made by the double emulsion method. J Control Release 2005; 107:310–9 [DOI] [PubMed] [Google Scholar]
- 52.Mahboubian A, Hashemein SK, Moghadam S, Atyabi F, Dinarvand R. Preparation and in-vitro evaluation of controlled release PLGA microparticles containing triptoreline. Iran J Pharm Res 2010; 9:369–78 [PMC free article] [PubMed] [Google Scholar]
- 53.Pistel KF, Kissel T. Effects of salt addition on the microencapsulation of proteins using W/O/W double emulsion technique. J Microencapsul 2000; 17:467–83 [DOI] [PubMed] [Google Scholar]
- 54.Choi S-W, Kwon HY, Kim WS, Kim JH. Thermodynamic parameters on poly(D,L-Lactide-Co-Glycolide) particle size in emulsification-diffusion process. Colloids Surf A 2002; 201:283–9 [Google Scholar]
- 55.KC, Song HS, Lee IY, Choung KI, Cho Y, Ahn EJ. Choi The effect of type of organic phase solvents on the particle size of poly(D,L-Lactide-Co-Glycolide) nanoparticles. Colloids Surf A 2006; 276:162–7 [Google Scholar]
- 56.Li T, Li B, Tomassone MS. Surface characterization of aspirin crystal planes using molecular dynamics simulations. Chem Eng Sci 2006; 61:5159–69 [Google Scholar]
- 57.Lees FP, Sarram P. Diffusion coefficient of water in some organic liquids. J Chem Eng Data 1971; 16:41–4 [Google Scholar]
- 58.Rausch MH, Lehmann J, Leipertz A, Froba AP. Mutual diffusion in binary mixtures of ionic liquids and molecular liquids by dynamic light scattering (DLS). Phys Chem Chem Phys 2011; 13:9525–33 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, EBM876554 Supplemental Material1 for Dual drug-loaded biodegradable Janus particles for simultaneous co-delivery of hydrophobic and hydrophilic compounds by Jennifer S Winkler, Mayur Barai and Maria S Tomassone in Experimental Biology and Medicine
Supplemental material, EBM876554 Supplemental Material2 for Dual drug-loaded biodegradable Janus particles for simultaneous co-delivery of hydrophobic and hydrophilic compounds by Jennifer S Winkler, Mayur Barai and Maria S Tomassone in Experimental Biology and Medicine
Supplemental material, EBM876554 Supplemental Material3 for Dual drug-loaded biodegradable Janus particles for simultaneous co-delivery of hydrophobic and hydrophilic compounds by Jennifer S Winkler, Mayur Barai and Maria S Tomassone in Experimental Biology and Medicine