Abstract
B-1a cells are long-lived, self-renewing innate-like B cells that predominantly inhabit the peritoneal and pleural cavities. In contrast to conventional B-2 cells, B-1a cells have a receptor repertoire that is biased towards bacterial and self-antigens, promoting a rapid response to infection and clearing of apoptotic cells. Although B-1a cells are known to primarily originate from fetal tissues, the mechanisms by which they arise has been a topic of debate for many years. Here we show that in the fetal liver versus bone marrow environment, reduced IL-7R/STAT5 levels promote immunoglobulin kappa gene recombination at the early pro-B cell stage. As a result, differentiating B cells can directly generate a mature B cell receptor (BCR) and bypass the requirement for a pre-BCR and pairing with surrogate light chain. This ‘alternate pathway’ of development enables the production of B cells with self-reactive, skewed specificity receptors that are peculiar to the B-1a compartment. Together our findings connect seemingly opposing lineage and selection models of B-1a cell development and explain how these cells acquire their unique properties.
Subject terms: Central tolerance, VDJ recombination, Interleukins, B-1 cells
B-1a B cells are innate-like cells with biased reactivity to bacteria and self-antigens. Here the authors show that reduced interleukin-7 in developing fetal liver-derived pro-B cells induces premature immunoglobulin κ rearrangement, alleviating the requirement for a pre-BCR selection stage and allowing the generation of autoreactive B1-a B cells.
Introduction
B-CLL is the most common form of adult leukemia in the western world1. In the early 1980s, the T-cell antigen CD5 (Ly-1) was identified on the surface of cancerous B cells in patients with B-CLL2,3. This observation led to the search for normal CD5+ B-cell counterparts to potentially determine the cancer cell of origin. As a result of these efforts, CD5+ B cells, otherwise known as B-1a cells, were discovered in mice4,5. Further characterization of CD5+ B-1a B cells revealed that they are long-lived, self-renewing cells that predominantly reside in the pleural and peritoneal cavities where they produce natural polyreactive IgM antibodies with a biased, autoreactive repertoire. In contrast to conventional B-2 cells, B-1a cells produce antibodies with reduced junctional diversity and less somatic hypermutation6. Furthermore, Igh VH gene rearrangements favor VH12 segment usage7, generating antibodies that interact with phosphatidylcholine (PtC), a major lipid in the protective mucus layer of the gastrointestinal tract that is also present in the membranes of diverse bacteria. Thus, the B-1a receptor repertoire is biased toward bacterial and self-antigens, which is important for mounting a rapid immune response to infection and in the clearing of apoptotic cells8–10. Because B-1a cells are found in pre-immune mice, they function as an important first line of defense against bacterial pathogens. These characteristics distinguish B-1a cells from conventional B-2 cells, which have a highly diverse receptor repertoire that is important for mediating adaptive immunity.
Although B-1a cells were discovered in the early 1990s, their origin has been hotly debated since, and despite the efforts of numerous labs this remains an unresolved issue. The controversy has mainly been centered on two opposing models, the lineage model and the selection model. The lineage model proposes that a distinct B-1 progenitor cell gives rise to B-1a cells, while the selection model favors the idea that a common B-cell progenitor can acquire a B-1a or a B-2 fate depending on the type of antigen it recognizes9,11. Support for the lineage model comes from early reconstitution experiments, which reveal that fetal tissues are much more efficient at generating B-1a cells in irradiated recipient mice than adult bone marrow counterparts12. Furthermore, the first wave of B-1a cells was shown to originate in early embryos in an HSC-independent manner13–17. However, cellular barcoding experiments demonstrate that a single progenitor cell can give rise to both B-1a and B-2 cells18 challenging the notion that B-1a cells arise from a distinct lineage. Moreover, the finding that B-1a cells have a restricted and biased receptor repertoire provides support for a selection model9,19. Further support for the selection model comes from a study by Graf et al. that made use of a transgenic system to show that swapping B-2 and B-1a-specific B-cell receptors (BCRs) is sufficient to efficiently change a B-2 cell into a B-1a cell in the absence of any lineage constraints. The lineage switch is rapid, induces a proliferative burst, and cells migrate to their normal environments within the pleural and peritoneal cavities20.
Investigations have also focused on expression of specific genes that influence development. For example, the Lin28b/let7 molecular switch influences CD5+ B-1a cell fate21–23. The Lin28b and let7 miRNAs, respectively, promote and inhibit the expression of the transcription factor, Arid3a, which in turn can drive the development of CD5+ B-1a cells22,23. Nonetheless, the BCR repertoire of the resulting cells do not include PtC-specific antibodies that are characteristic of a typical B-1a cell compartment22. Thus, enforced expression of Arid3a fails to fully explain how B-1a cells develop. Another transcription factor, BHLHE41 has also been shown to be important in B-1a cell biology24. Specifically, cells deficient in this transcription factor lose B-1a cells expressing VH12/VK4 PtC-specific receptors, have impaired BCR signaling, increased proliferation, and apoptosis. BHLHE41 therefore plays an important role in B-1a maintenance by regulating self-renewal and BCR repertoire; however, it is not known whether its forced expression can drive development of these cells.
In the fetus, B-cell development takes place in the liver and moves to the bone marrow after birth. Each stage of development is marked by a particular rearrangement event that drives differentiation forward. These recombination events occur in a stage-specific manner. The first step involves the joining of the immunoglobulin heavy-chain (Igh) DH and JH gene segments within pre-pro-B cells. Rearrangement continues at the pro-B cell stage, where VH-to-DJH joining is both initiated and completed. Rearrangement of the immunoglobulin light-chain gene loci, Igk or Igl, occurs at the subsequent pre-B cell stage of development. Igh and Igk gene rearrangement is separated by a proliferative burst of large pre-B cells that allows individual cells that have successfully rearranged their heavy chain to clonally expand. At the following small pre-B cell stage, each B-cell undergoes a distinct Igk gene recombination event25. Ultimately, this results in unique heavy- and light-chain pairs that expand the antigen receptor repertoire. The successful pairing of immunoglobulin heavy chain with surrogate light chain (SLC) forms the pre-B cell receptor (pre-BCR), which is required for expansion of large pre-B cells and subsequent differentiation to the small pre-B cell stage, where Igk recombination occurs. Since SLC pairs poorly with autoreactive heavy chains, the pre-BCR provides a mechanism for negative selection of self-reactive B cells26,27.
As noted early on, B-1 cell development is quantitatively unaffected in SLC-deficient mice, and in a small fraction of B-cell progenitors in the bone marrow Igk rearrangements occur prior to rearrangements at Igh and independent of SLCs28–31. In addition, in the absence of SLC and thus pre-BCR expression, an autoreactive BCR can drive B-cell development efficiently to the stage of the immature B cell, where BCR diversification and counterselection of autoreactivity is achieved through the process of receptor editing. This has led to a model in which early B-cell development is driven by a positive signal from the pre-BCR (in the majority of progenitors) or an autoreactive BCR (in a minority of cells), with the pre-BCR having evolved as a surrogate autoreactive BCR32,33.
Downstream of pre-BCR signaling, IL-7 receptor (IL-7R) signaling is extinguished at the small pre-B cell stage. This is noteworthy because the IL-7R signaling pathway is responsible for directing the sequential ordering of recombination events, i.e., heavy-chain gene followed by light-chain gene rearrangement in classical B-cell development34,35. IL-7 is a cytokine secreted by stromal cells within the bone marrow where development takes place throughout the postnatal and adult life of the animal. In murine bone marrow, development past the pre-pro-B cell stage stringently requires IL-7R36,37. In contrast, B-cell development within the fetal liver can occur independent of IL-738,39. In terms of directing recombination, IL-7 and its downstream signaling component STAT5 have been shown to promote Igh accessibility and recombination40–42 while actively inhibiting recombination of the Igk locus34,43. Activated STAT5 enters the nucleus and forms a complex with PRC2/EZH2, which binds to the intronic enhancer of Igk (iEκ) and induces H3K27me3-mediated repression to inhibit recombination of this locus44,45. Strikingly, conditional deletion of STAT5 within pro-B cells results in a robust increase of Igk recombination at the pro-B cell stage35.
It is known that B-1a cells originate primarily from fetal tissues, however, it remains unclear what pathways drive B-1a cell development and lead to the acquisition of their unique characteristics. In addition, it has been shown that B-1a cells are efficiently generated in Il7−/− and Il7r−/− mice albeit at reduced numbers compared with wild-type38,39,46,47. Given our finding that fetal liver (FL) pro-B cells have less active cytoplasmic pSTAT5 than bone marrow (BM) pro-B cells48, we asked whether this could influence V(D)J recombination and B-cell development.
Here, we show that low levels of IL-7R/pSTAT5 signaling in the fetal liver environment promote an “alternate pathway” of B-cell development, in which increased Igk rearrangement occurs at the pro-B cell stage of development. Productive rearrangement of Igh and Igk at this stage leads directly to cell surface expression of a mature BCR, bypassing the requirement for SLC and the selection of autoreactive receptors that are characteristic of B-1a cells. Extending earlier work, we demonstrate that while SLC-independent development leads to a significant reduction in B-2 cell numbers, B-1a cells with their characteristic VH12 anti-PtC bias are still efficiently generated. Thus, reduced IL-7R/STAT5 signaling promotes an alternate pathway of development which favors the production of B-1a cells with self-reactive receptors characteristic of this B-cell subset. Together these data connect opposing lineage and selection models of B-1a cell development and explain how these cells acquire their unique properties.
Results
Igk recombination is increased in fetal liver pro-B cells
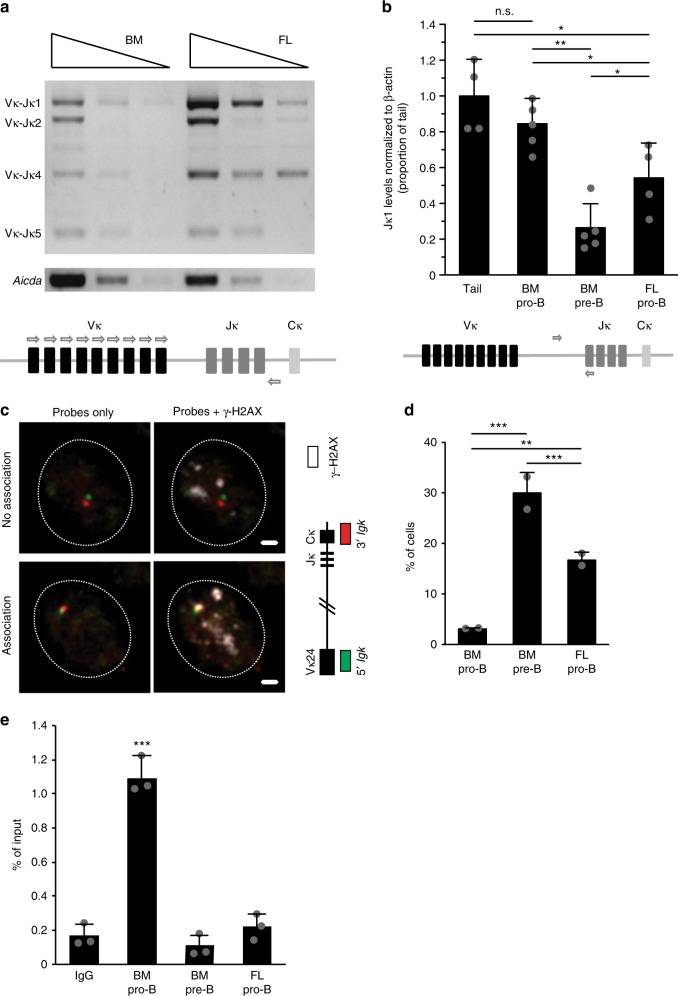
Fetal liver (FL) and bone marrow (BM)-derived progenitor cells are differentially dependent on IL-7 for development, and we have found that FL-derived pro-B cells have significantly lower levels of the downstream signaling component, phospho-STAT5 compared with their bone marrow-derived pro-B cell counterparts48. Thus, we asked whether alterations in phospho-STAT5 levels correlate with altered regulation of Igk recombination in these two anatomic locations. To explore this, we analyzed recombination from ex vivo-derived bone marrow (5–6 wk adults) and FL (E17–18) pro-B cells using semiquantitative PCR analysis (Fig. 1a). This assay uses a degenerate Vκ primer with a common reverse primer downstream of Jκ gene segments to measure recombination of a Vκ with each of the four Jκ segments49. As shown in Fig. 1a, FL pro-B cells have increased Igk recombination relative to BM pro-B cells.
Fig. 1.
Early Igk recombination is increased in the fetal liver versus bone marrow pro-B cells. a Semiquantitative PCR performed on ex vivo-derived BM pro-B (CD19+B220+IgM−CD25−CD117+) from 6-week-old mice and FL pro-B (Lin−CD19+B220+IgM−CD2−CD117+) from E17.5 mice to assess rearrangement of Vκ to each functional Jκ. Each lane represents threefold serial dilutions of input DNA. DNA levels were normalized to Aicda levels, which is located on the same chromosome as Igk. b Recombination with Jκ1 was quantified by qPCR using primers specific for the unrearranged germline sequence. DNA is quantified as a ratio between the single copy β-actin gene and the Igk germline PCR product and shown as a proportion of tail DNA. Schematics outline primer-binding sites on the Igk locus (bottom of a, b). Error bars represent the standard deviation (n = 4 biologically independent samples for tail and FL pro-B cells n = 5 biologically independent samples for BM pre-B (CD19+B220+IgM−CD117+CD25+) and BM pro-B). P-values were calculated using two-tailed T tests. c 3-D immuno-DNA FISH was performed on ex vivo sorted B cells using BAC probes specific to the distal Vκ24 (RP23–101G13) gene region and the Cκ region (RP24–387E13), shown in red and green, respectively, in conjunction with an antibody to the phosphorylated form of γ-H2AX in white. Representative images of a B cell with no γ-H2AX associated with Igk alleles (top) and with γ-H2AX associated with one Igk allele (bottom). Scale bar = 1 μm. d Percentage of B cells (BM pro-B, BM pre-B, and FL pro-B) with at least one Igk allele associated with a γ-H2AX focus. Error bars represent standard deviation (n = 2 biologically independent samples). P-values were calculated using two-tailed Fisher’s exact tests. e STAT5-ChIP-qPCR of iEκ on ex vivo sorted cells. The data are represented as a proportion of input DNA, and error bars represent the standard deviation (n = 3 biologically independent samples). For all P-values, *significant (0.05–0.01), **very significant (0.01–0.001), ***highly significant (<0.001). For b, d, and e, source data are provided as Source Data File
To address the question of altered Igk recombination between these two cell types in a more quantitative manner, we made use of a real-time PCR assay that quantifies Jκ1 rearrangement by assessing the retention of germline Igk50. In this assay, a product cannot be generated after recombination, because the sequence to which the upstream primer binds is lost or inverted during the recombination process. Quantitation of the amplified product is determined as a ratio between the single copy gene β-actin and the germline Igk band. Control tail DNA, in which no Igk rearrangement occurs, is set at 100%. Consistent with the semiquantitative experiments described above, FL pro-B cells have lower levels of Igk germline retention (61%) relative to bone marrow pro-B cells (87%) (Fig. 1b).
To determine the proportion of cells that are actively undergoing recombination at a single-cell level, we used three-dimensional (3-D) immunofluorescent in situ hybridization (FISH) assay that visualizes recombination by the association of γ-H2AX DNA repair foci with the individual alleles of antigen receptor loci51,52. In these experiments, we used two Igk BAC DNA probes that hybridize to the distal Vκ24 (RP23–101G13) gene region and the Cκ region (RP24–387E13) in combination with an antibody against the phosphorylated form of γ-H2AX (Fig. 1c). We found significantly increased association of γ-H2AX foci with the Igk locus in FL versus bone marrow-derived pro-B cells (16.7% versus 3.0%). Consistent with our previous experiments, the frequency of DNA breaks in FL pro-B cells (16.7%) is again at an intermediate level compared with BM pre-B cells (30%) where Igk recombination typically occurs (Fig. 1d). These numbers reflect the combined data from two independent experiments (Supplementary Table 1).
It is known that activated STAT5 forms a complex with PRC2 and binds to the intronic enhancer of Igk, iEκ to inhibit Igk recombination44,45,48. To determine if the increase in early Igk rearrangement could be caused by reduced STAT5-mediated repression, we analyzed enrichment of STAT5 binding on iEκ via STAT5-ChIP-qPCR. As shown in Fig. 1e, we found that FL pro-B cells have significantly less STAT5 bound to iEκ compared with BM pro-B cells, and binding levels more closely resemble those in BM pre-B cells where Igk recombination normally occurs. Taken together, these findings indicate that in the FL versus BM environment, Igk rearrangement occurs at higher levels in pro-B cells due to a decrease in STAT5-mediated repression of the Igk locus.
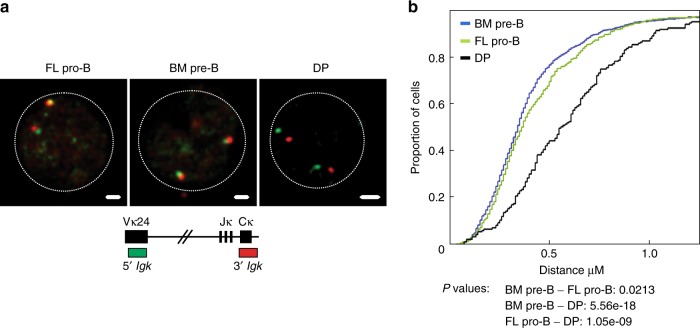
Antigen receptor loci undergo large-scale contraction through chromatin looping, which enables rearrangement between widely dispersed gene segments. Locus contraction is tightly linked both to recombination status and usage of distal gene segments49,53. To address the contraction status of the Igk locus, we measured the distance separating the distal Vκ24 and Cκ probes in interphase cells. Our analyses indicate that FL pro-B cells are significantly more contracted than wild-type (WT) double-positive (DP) T cells, a cell type in which Igk rearrangement does not occur (Fig. 2). Indeed, the level of contraction is comparable with that seen in wild-type bone marrow-derived pre-B cells, where Igk gene rearrangement is known to occur.
Fig. 2.
The Igk locus is similarly contracted in FL pro-B cells and BM pre-B cells. a Representative confocal microscopy images showing the distance separation between the probes on Igk in fetal liver pro-B cells (Lin−CD19+B220+IgM−CD2−CD117+), bone marrow pre-B cells (CD19+B220+IgM−CD117+CD25+), and double-positive T cells (CD90.2+TCRB−CD4+CD8+). b Distances separating the two ends of the locus are displayed as a cumulative frequency curve. A left shift on the curve is indicative of closer association. Bone marrow pre-B cells (blue), fetal liver pro-B cells (green), and negative control double-positive T cells (black). P-values were generated using two-sample Kolmogorov–Smirnov tests (n = 2 independent experiments)
Bypass of the pre-BCR checkpoint
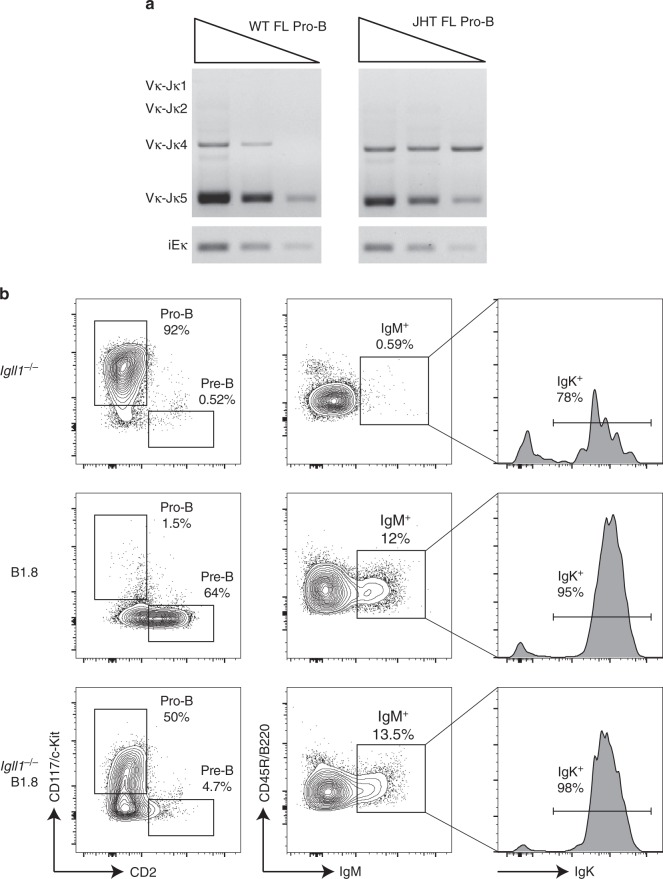
Classical bone marrow B-cell development starts with Igh recombination at the pre-pro to pro-B cell stages followed by Igk/Igl recombination at the small pre-B cell stage. However, JHT mice that lack JH segments as well as the Igh intronic enhancer, and are therefore unable to undergo Igh recombination, but can rearrange Igk/Igl independent of Igh rearrangement (Fig. 3a)29,54,55. This is also true for SLC-deficient mice, and a fraction of B cells undergoing development in the bone marrow of wild-type mice56. Given our finding that a significant number of pro-B cells rearrange Igk in the FL environment, we reasoned that productive heavy and light-chain gene rearrangement in pro-B cells could lead to expression of a mature BCR, bypassing the requirement for a pre-BCR. To address this question as a proof of principle, we examined FL B cells from mice that are deficient for a component of SLC, lambda5 (Igll1−/−), in conjunction with a transgene that has a prearranged functional heavy chain (B1.8). Igll1−/− mice display a strong developmental block at the pro-B cell stage and made very few IgM+ cells (0.59% of B cells). In contrast Igll1−/−, B1.8 and B1.8 mice produce similarly high percentages of IgM + B cells (12 and 13.5%, respectively). Importantly, B1.8 mice have a substantial pre-B cell compartment which is not observed in Igll1−/−, B1.8 mice despite a similar output of IgM+ B cells which express IgK protein (Fig. 3b). This shows that having a productive heavy- and light chain can bypass the requirement for pairing with SLC to form immature B cells that express the mature BCR. Together these data demonstrate that in the FL, expression of Igh and Igk at the pro-B cell stage can bypass the developmental block induced by surrogate light-chain deficiency by forming B cells with a mature BCR.
Fig. 3.
Early Igk rearrangement enables B cells to bypass the pre-BCR checkpoint. a Igk recombination can occur independent of Igh recombination as shown by semiquantitative PCR performed on ex vivo-derived fetal liver pro-B cells E17.5 (Lin−CD19+B220+IgM−CD2−CD117+) from wild-type and JHT mice. Each lane represents threefold serial dilutions of input DNA. DNA levels were normalized to iEκ b Representative flow-cytometry plots of fetal liver E17.5 cells from Igll1−/−, B1.8, and Igll1−/−;B1.8 littermates (from top to the bottom). Pro-B and pre-B cell gates are displayed as a percentage of CD19+IgM− cells (left), IgM+ are displayed as a percentage of CD19+ cells (middle), and IgK+ histograms are displayed as a percentage of CD19+IgM+ cells (right)
B-1a cells are efficiently generated in Igll1−/− mice
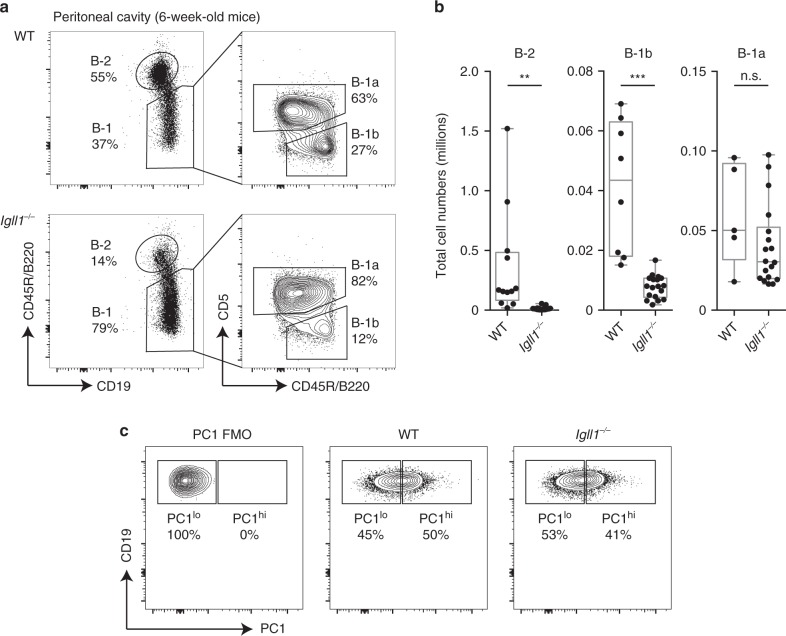
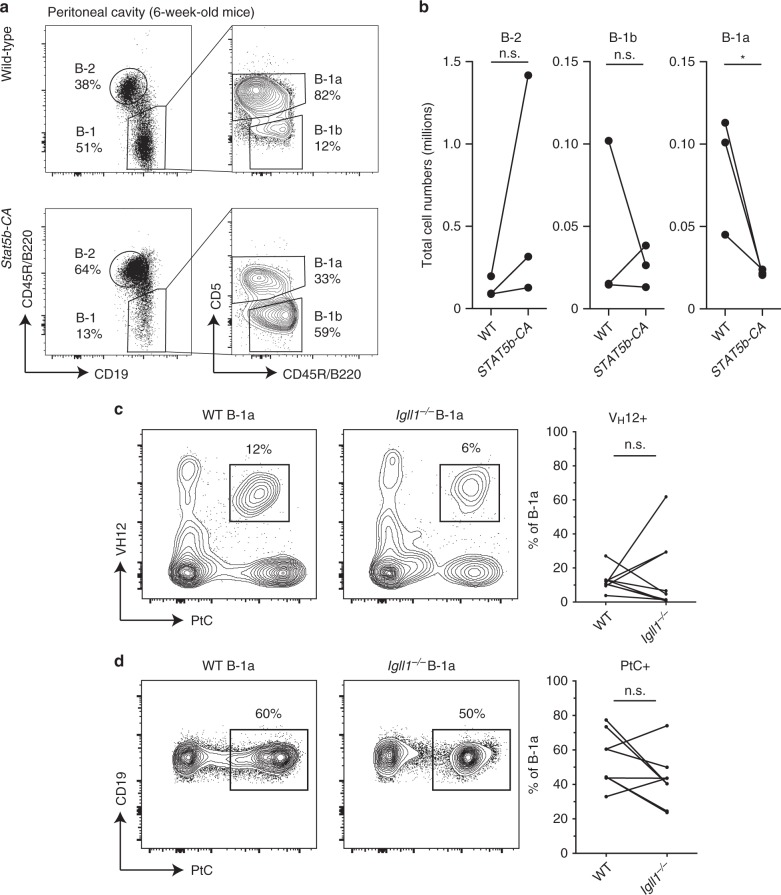
B-1a cells are primarily generated from fetal tissues, and we have shown that FL-derived B cells frequently undergo early Igk recombination allowing cells to bypass the pre-BCR selection stage. Thus, we next asked whether an absence of SLC could impact the generation of B-1a cells12,23. Here, we analyzed the B-2 as well as the B-1 compartment, which consists of both B-1a and B-1b cells. B-1b cells are distinct from B-1a cells in that they lack CD5 expression and have a memory function in protecting against bacterial infections57. As expected, peritoneal B-2 cells were significantly decreased compared with controls. Furthermore, although B-1b cells were also significantly decreased, the B-1a cell compartment remained intact. This was reflected in cell percentage, as well as total cell numbers (Fig. 4a, b). These data indicate that while B2 and B1b cell development is strongly promoted by SLC, B-1a cells do not depend on a pre-BCR checkpoint. It should be noted that B1.8 transgenic mice could not be used to analyze the B-1a cell compartment in SLC-deficient mice, because the B1.8 pre-rearranged heavy chain does not support B-1a cell development58.
Fig. 4.
B-1a cells are efficiently generated in surrogate light chain-deficient mice. a Representative flow-cytometry plots of peritoneal cavity cells from wild-type and Igll1−/− mice. B-2 and B-1 cell gates are displayed as a percentage of CD19+ cells (left). B-1a and B-1b cell gates are displayed as a percentage of B-1 cells (right). b The total cell numbers of B-2, B-1b, and B-1a cells were calculated from the peritoneal cavity of these mice. The middle line in the boxplots represents the median. The box shows first and third quartiles, and the whiskers represent the max and min values. Each dot represents cells from a different individual mouse. P-values were calculated using a two-tailed T test. Source data are provided as Source Data File. c Igll1−/− mice maintain both functionally distinct PC1lo and PC1hi subsets of B-1a cells. Representative flow-cytometry plots of peritoneal cavity cells from wild-type and Igll1−/− mice. B-2 and B-1 cell gates are displayed as a percentage of CD19+ cells (left). PC1lo and PC1hi B-1a cells are displayed as a percentage of B-1a cells (right). A PC1 FMO (fluorescence minus one) was used to define the gate
Two different subsets of B-1a cells that segregate different functions of the B-1a cell compartment have been identified by the plasma cell alloantigen 1 (PC1)59,60. PC1lo B-1a cells produce higher amounts of natural IgM, and can undergo plasma cell differentiation, while PChi B-1a cells produce less natural IgM, have PtC-reactive BCRs, and can produce IL-10. In order to determine whether or not both of these subsets of B-1a cells were represented in B-1a cells generated in SLC-deficient mice, we analyzed PC1 expression. B-1a cells from Igll1−/− mice were found to have similar proportions of PC1lo and PC1hi B-1a cells as in wild-type mice (Fig. 4c). This suggests that not only is the B-1a compartment size normal in SLC knockout mice but also the PC1lo and PC1hi subsets are maintained.
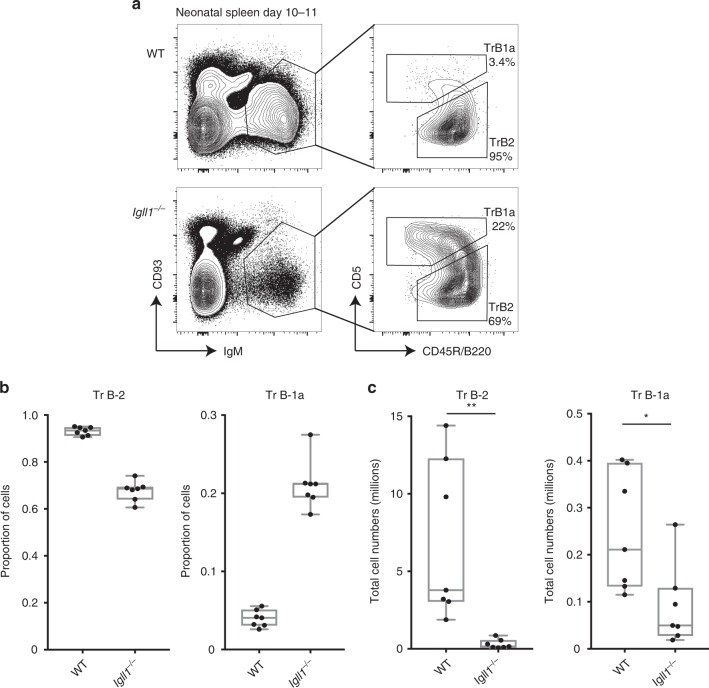
B1-a cells cannot be detected in FL, and first appear in the transitional B-cell population 1 week after birth61,62. To further analyze the impact of surrogate light-chain deficiency on the generation of B1a cells, we examined transitional B-1a cells in spleens from 10–11 days old neonatal mice. As expected, the proportion of B-2 in cells in this population was severely reduced, while the proportion of B-1a cells was found to increase. This is reflected by the finding that total numbers of transitional B-2 cells are severely depleted compared with B1a cells in Igll1−/− versus wild-type mice (Fig. 5a–c). Thus, even at this early stage of life, transitional B-1a cells from SLC-deficient mice are found at only moderately reduced levels compared with their wild-type counterparts, indicating that the presence of adult Igll1−/− B-1a cells is not a result of clonal expansion by self-renewal over time.
Fig. 5.
Transitional B-1a cells are readily detected in neonatal spleens from Igll1−/− mice. a Representative flow-cytometry plots showing transitional B-1a (CD19+CD93lo/intIgM+B220−/loCD5+) and transitional B-2 (CD19+CD93lo/intIgM+CD5−/loB220+) from wild-type and Igll1−/−. The left plot is gated on CD19+ cells from D10–11 neonatal spleen. b Graphs represent the proportion of cells that are either transitional B-1a or transitional B-2. c Graphs show the total cell numbers of transitional B-2 or transitional B-1a in millions. For both b and c, the middle line in the boxplots represents the median. The box shows first and third quartiles, and the whiskers represent the max and min values. Each dot represents cells from a different individual mouse. Source data are provided as Source Data File
B-1a cell development is inhibited in Stat5b-CA mice
Our data suggest that low phospho-STAT5 signaling induces early Igk recombination in FL pro-B cells, which promotes the efficient generation of B-1a cells in SLC-deficient mice. To further validate the role of IL-7R/STAT5 signaling in B-1a cell development, we next asked whether increased STAT5 signaling could have the opposite effect on B-1a cell development and impair the generation of this compartment. To address this question, we used mice expressing a constitutive active form of STAT5 (Stat5b-CA)63.
In these mice, we found that although B-2 and B-1b cell development were not significantly affected, all three independent littermate controlled Stat5b-CA mice exhibited a decrease in the B-1a cell compartment. This is reflected in cell proportions as well as the total cell numbers (Fig. 6a, b). Thus, STAT5 signaling inhibits B-1a cell development underscoring the importance of reduced IL-7R/STAT5 signaling in driving the development of these cells.
Fig. 6.
Constitutive phospho-STAT5 signaling selectively inhibits B-1a cell development. a Representative flow-cytometry plots of peritoneal cavity cells from wild-type and Stat5b-CA mice. B-2 (CD19+B220hi) and B-1 (CD19+B220lo) cell gates are displayed as a percentage of CD19+ cells (left). B-1a (CD19+B220loCD5+) and B-1b (CD19+B220loCD5−) cell gates are displayed as a percentage of the B-1 cells (right). b Graphs display the total cell numbers of B-2, B-1b, and B-1a cells from the peritoneal cavity of wild-type and Stat5b-CA mice. Each dot represents an individual mouse, and lines connect pairs of littermates from three different litters of mice. c, d SLC-independent B-1a cells express receptors with phosphatidylcholine (PtC)-specific VH12 gene rearrangements. Peritoneal cavity B-1a cells from wild-type and Igll1−/− mice highlight the proportion of B-1a cells that are either c VH12+, PtC+ or d PtC+. The left side shows representative flow-cytometry plots. The right side is a graphical summary of the mice from all the experiments. Each dot represents an individual mouse and lines connect pairs of littermates. P-values were calculated using a two-tailed T -test. Source data are provided as Source Data File
B-1a cells Igll1−/− mice express PtC-specific receptors
B-1a cells are known to have a strong bias for immunoglobulin rearrangements that confer specificity to PtC (VH12/VK4). In addition, the pre-BCR is known to select against B-1a-specific autoreactive heavy chains as a result of a defect in pairing of the latter with SLC26,27. Given these facts, we next asked whether B-1a cells that develop independent of SLC would promote the generation of B-1a-specific rearrangements. To address this question, we analyzed peritoneal cavity B cells for VH12 usage and specificity against PtC. As shown in Fig. 6c, B-1a cells from Igll1−/− mice express VH12 rearranged receptors. Importantly, these VH12+ B-1a cells bind PtC containing liposomes, inferring that they have the canonical VH12/VK4 gene segment bias (Fig. 6c). In addition, there is not a significant difference in the proportion of B-1a cells that have specificity against PtC (Fig. 6d). Thus, an absence of SLC supports the generation of these self-reactive B-1a cells.
Igll1−/− and wild-type B-1a cells have similar repertoires
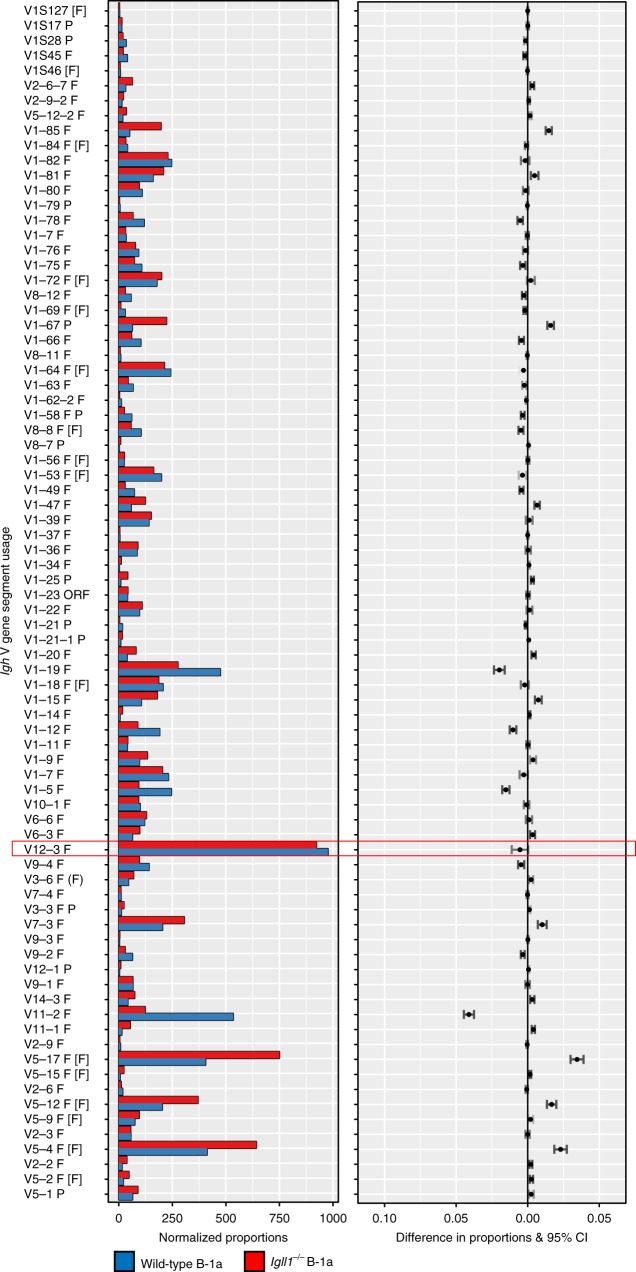
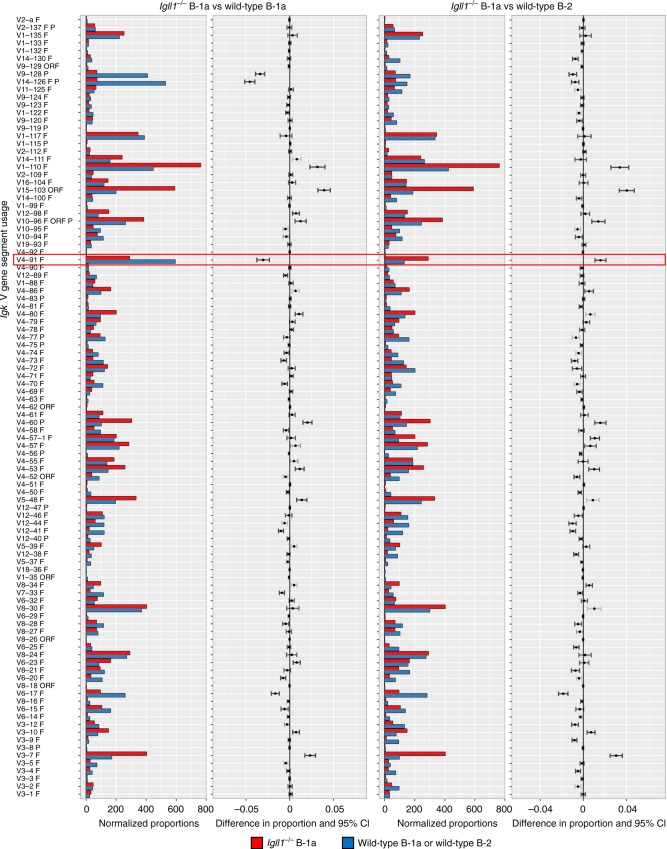
To determine how SLC deficiency affects gene segment usage, we sequenced the BCR repertoires of B-1a cells from WT and SLC-deficient mice. Briefly, peritoneal cavity B-1a cells were sorted, lysed, and the rearranged Igh products amplified by PCR. Illumina adapters and barcodes were added, and the rearrangements sequenced by next-generation sequencing. BCR repertoires were analyzed by IMGT/High-V-QUEST, and visualized using IMGT/StatClonotype64–67. These analyses revealed that B-1a cells from Igll1−/− mice have a similar overall VH usage compared with those of wild-type mice (Fig. 7). This shows that B-1a cells that develop in the absence of SLC have a wide distribution of gene segment usage, and thus do not represent a clonal expansion of a few rare progenitors. A diverse repertoire was also observed for light-chain gene segment usage (Fig. 8). As expected, VH12 (VH12-3) usage was found to be high in wild-type B-1a cells, and calculated proportions were comparable with our flow-cytometry experiments in the cells from Igll1−/− mice (Fig. 6c)7. VK4, which pairs with VH12 to confer PtC specificity, has a reduced representation in SLC-deficient B-1a cells, but it is still found at significantly higher frequency than in wild-type B-2 cells (Fig. 8). These rearrangement studies support the data from our flow-cytometry analysis and underscore the finding that B-1a cells generated in the absence of SLC maintain a VH12/VK4 gene segment bias.
Fig. 7.
Igll1−/− B-1a cells have a similar VH gene segment VH usage to wild-type controls. Representative synthesis graphs generated from IMGT/StatClonotype that compare differences in V gene segment usage between wild-type (blue) and Igll1−/− (red)-derived B-1a cells (CD19+B220loCD5+). The red box highlights VH12 (VH12-3) gene segment (n = 2 independent experiments). The graph combines a bar graph for the normalized proportions of each gene segment and the differences in proportions with significance and confidence intervals (CI)
Fig. 8.
Wild-type and Igll1−/− Igll1−/− B-1a cells have a similar VK gene segment usage to wild-type controls and maintain VK4-91 enrichment. Representative synthesis graphs generated from IMGT/StatClonotype that compare differences in Igk V gene segment usage between Igll1−/− (red) versus wild-type (blue) peritoneal cavity B-1a cells CD19+B220loCD5+ (left side) or Igll1−/− (red) peritoneal cavity B-1a cells vs wild-type (blue) peritoneal cavity B-2 cells CD19+B220+ (right side). The red box highlights VK4 (VK4-91) gene segment (n = 2 independent experiments). This graph combines a bar graph for the normalized proportions of each gene segment and the differences in proportions with significance and confidence intervals (CI)
To confirm that B-1a cells generated in SLC-deficient mice were from fetal origin, we analyzed Igh sequence for N-additions. B-1a cells are known to predominately come from fetal origins and as a consequence of low Tdt activity at this stage, they are known to have reduced N-additions compared with adult bone marrow-derived B cells. Our analysis revealed that Igll1−/− peritoneal cavity B-1a cells had similarly reduced levels of N-additions as wild-type controls (Fig. 9). The paucity of N-additions supports the idea that B-1a cells are efficiently generated in SLC-deficient mice, maintaining key B-1a cell traits like VH12 and VK4 gene segment bias and reduced levels of junctional diversity.
Fig. 9.
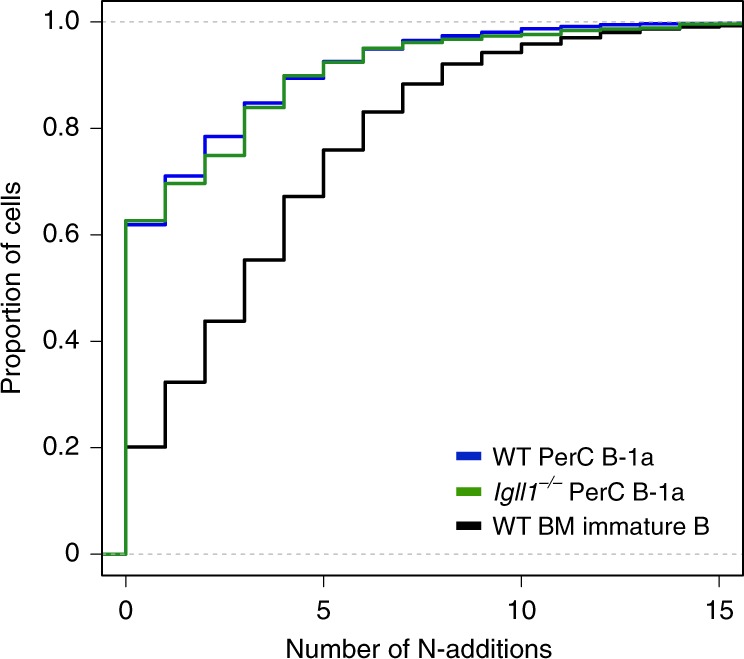
Wild-type and Igll1−/− peritoneal cavity B-1a cells have similar low levels of N-additions as compared with wild-type counterparts. Cumulative frequency graph demonstrates the proportion of cells that have a certain number of N-additions between VH and VD gene segments for wild-type peritoneal cavity B-1a cells CD19+B220loCD5+ (blue), Igll1−/− peritoneal cavity B-1a CD19+B220loCD5+ cells (green), and wild-type bone marrow immature B cells CD19 + B220 + IgMint (black) (n = 2 independent experiments)
Discussion
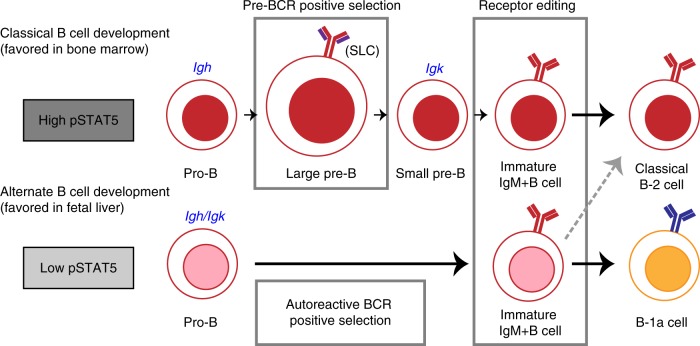
Although it was known that B-1a cells predominantly develop in the FL, the pathways driving development and the mechanisms underlying the generation of B cells with a repertoire skewed toward autoreactivity were not previously known. Indeed, this has been a subject of debate for many years. Here, we now reveal that a reduction in IL-7 signaling in the FL environment alleviates Igk repression and promotes early rearrangement of this locus in pro-B cells. Productive heavy and light-chain gene rearrangement at the pro-B cell stage can lead directly to the expression of a mature BCR, thereby bypassing the requirement for a pre-BCR checkpoint. Since PtC-specific VH gene rearrangements pair poorly with SLC26 and the pre-BCR selects against autoreactive receptors27, SLC-independent development provides an explanation for how B cells with autoreactive, PtC-specific receptors are generated. In more general terms and along Jerne’s idea that the immune system selects antibody mutants from an initial self-reactive repertoire68, B-cell progenitors can be initially positively selected by the expression of autoreactive BCRs or their evolutionary surrogate, the pre-BCR32,33. This is followed by negative selection of autoreactivity through receptor editing. The skewing of the B-1a receptor repertoire toward autoreactivity likely reflects the imprint of the initial positive selection of B-1a cells through autoreactive BCRs rather than SLC (Fig. 10).
Fig. 10.
The model of classical versus alternate B-cell development. The classical B-cell developmental pathway is favored in the bone marrow where high levels of STAT5 signaling inhibit Igk recombination at the pro-B cell stage. At the large pre-B cell stage, a productive heavy-chain pairs with SLC and pre-BCR signaling at this stage leads to proliferative expansion and positive selection. Autoreactive heavy-chains pair poorly with SLC, and will not experience positive selection in this manner. Following Igk recombination (blue) at the small pre-B cell stage, self-reactive B cells can undergo receptor editing to further select against self-reactive BCRs. The alternate B-cell developmental pathway is favored in fetal liver where low levels of STAT5 signaling in pro-B cells promotes Igk recombination at the same stage as Igh recombination (blue). This enables the generation of B cells expressing a mature BCR instead of a pre-BCR. Here, self-reactive B cells are initially positively selected by self-antigens. Although these B cells can potentially undergo receptor editing to modify their BCRs, the initial positive selection of autoreactive B cells skews the B-1a BCR repertoire toward autoreactivity
The idea that B-1 cells can develop in a SLC-independent manner is not novel. In fact, Kitamura et al. observed that in contrast to B-2 cells, B-1 cells were essentially unaffected by SLC deficiency, however, the distinct effect on the B-1a versus B-1b cell compartment was not investigated28. Our analyses demonstrate that while B-2 and B-1b cell development is severely impaired in Igll1−/− mice, B-1a cells can be generated at wild-type levels. Furthermore, SLC-independent B-1a development can occur through a mechanism that involves reduced IL-7R/STAT5 signaling and early Igk rearrangement. We found that B-1a cell development is selectively impaired in Stat5b-CA mice, providing additional support for the idea that SLC-independent B-1a cell development is favored by a decrease in IL-7 signaling. It should be noted that although Igk rearrangement can occur prior to Igh rearrangement, it is not a prerequisite for SLC-independent B-1a cell development. Indeed, a rearranged Igh that cannot associate with SLC could persist at the pro-B cell stage until subsequent productive Igk rearrangement generates a BCR independent of IGH-SLC pairing. Taken together, our findings point to a model in which B-1a cells typically develop in a SLC-independent manner. Interestingly, earlier work analyzing expression patterns of genes relevant to rearrangement in the yolk sac, para-aortic splanchnopleura, and spleen demonstrates that at E9–11, early lymphoid progenitor cells express Rag2 and VpreB, but lack expression of Igll169. The delayed expression of the SLC component, lambda5 (Igll1), points to the possibility that there is an early window in which B-cell development can occur in the absence of SLC. This stage coincides with the stage at which the earliest B-1 progenitor cells are detected (E9 in the yolk sac and para-aortic splanchnopleura)70,71. Thus, we speculate that the reason why early progenitors become B-1 cells is because they develop in a SLC-independent manner. The observation that SLC-deficient mice generate B-1a cells with autoreactive receptors supports this model27. The generation of SLC-independent autoreactive receptors does not need to occur at high frequency, because cells that acquire a B1a cell receptor undergo a rapid proliferative burst and thus even a few cells have the ability to give rise to a robust B1a cell population20. In sum, assignment of a SLC-independent pathway of B-cell development to B-1a cells provides a new perspective on B-1a development, reconciling old lineage and selection models (Fig. 10).
Methods
Mice
Igll1−/− (Jackson Laboratory 002401) and Stat5b-CA mice were maintained on a C57BL/6 background, as previously described28,36,72. B1.8 mice73 were crossed onto a Igll1−/− background. These mice were housed and cared for in accordance with IACUC guidelines and protocols approved by NYUMC (protocol #: IA15-01468) and U of Minnesota (protocol #: 1502–32347A).
Flow cytometry and antibodies
BM and FL cell populations were isolated from C57Bl/6 mice via cell sorting and analyzed by flow cytometry. B- and T-cell antibodies include: anti-CD45R/B220 (clone: RA3–6B2 cat. no. 553092), anti-CD19 (clone 1D3 cat. no. 557655), anti-IgMb (clone AF6–78 cat. no. 553520), anti-IgM (clone II/41 cat. no. 553437, 743324), anti-IgK (clone 187.1 cat. no. 562476, 562888), anti-CD117/c-Kit (clone 2B8 cat. no. 553356), anti-CD2 (clone RM2–5 cat. no. 553111), anti-CD25/IL2RA (clone PC61 cat. no. 553866), anti- CD93 (clone AA4.1 cat. no. 561990) anti-CD90.2/Thy1.2 (clone 53–2.1 cat. no. 25-0902-82), anti-CD5 (clone 53-7.3 cat. no. 553022), anti-VH12 (clone 5C5)74 anti-TCRB (clone H57–597 cat. no. 47-5961-82), anti-CD8a (clone 53-6.7 cat. no. 553031), anti-CD4 (clone RM4-5 cat. no. 553051), anti-ENPP1/PC1 (clone YE1/19.1 cat. no. 149207). All of the antibodies were obtained from BD Pharmigen, except anti-CD90.2, anti-TCRB, and anti-ENPP1/PC1 (eBioscience). Fluorescent DPOC/CHOL liposomes were acquired from FormuMax. The gating strategy was as follows: bone marrow pro-B cells B220+/CD19+/IgM−/c-kit+/CD25−/IgM−, BM pre-B cells B220+/CD19+/IgM−/c-kit−/CD25+, FL pro-B cells (E17.5) B220+/CD19+/IgM−/c-kit+/CD2−, and thymic DP cells Thy1.2+/TCRB−/CD19−/CD4+/CD8+. FL pro-B cells were sorted on CD2− instead of CD25−, because CD25 is not expressed in fetal B cells. CD2 expression is correlated with cytoplasmic μ heavy chain, and can be used to identify pre-B cells and efficiently sort pro-B cells from the FL75. From the peritoneal cavity, B-2, B-1a, and B-1b were identified as followed: B-2 cells B220hi/CD19+, B-1a cells B220lo/CD19+/CD5+, and B-1b cells B220lo/CD19+/CD5−. To obtain FL precursor cells, FLs from E15.5–17.5 were subject to lineage depletion using the following biotin-labeled antibodies: anti-CD4 (RM4–5), anti-CD8a (53-6.7), anti-TCRγδ, anti-CD49b (DX5), anti-Ly6G/Ly6C (RB6-8C5), anti-CD11c (N418), anti-CD11b (M1/70), anti-F4/80 (BM8), anti-Ter-119 (TER-119) followed by a negative selection using streptavidin magnetic rapidspheres (STEMCELL Technologies). The remaining cells were sorted as: Lin−/CD19−/IgM−/c-kit+/CD2−/B220lo. Cells were sorted using a FACSAria I (BD). The data were also collected on an LSR II (BD) and analyzed using FlowJo software.
Intracellular staining for IgK
FL E17.5 B cells were stained for surface markers (CD19, B220, IgM, IgK, CD2, and CD127), followed by permeabilization/fixation and secondary staining for IgM and IgK in accordance with the manufacturer’s instructions (BD 554714). Antibodies with different conjugated flourophores were used to distinguish between surface IgM/IgK (BD 554714 and 562476) and intracellular IgM/IgK (BD 553437 and 562888).
Semiquantitative Igk rearrangement
DNA was prepared from sorted cells by proteinase K digestion at 55 C for 4 h, 85 C for 10 min. VκJκ joints were amplified using a degenerate Vκ primer (5′-GGCTGCAGSTTCAGTGGCAGTGGRTCWGGRAC-3′), and a Jκ5 primer (5′-ATGCCACGTCAACTGATAATGAGCCCTCTCC-3′). Acidia gene was amplified as a loading control (AID F: 5′-GCCACCTTCGCAACAAGTCT-3′, AID R: 5′-CCGGGCACAGTCATAGCAC-3′). Bands were run through a 1.5% agarose gel electrophoresis and imaged using a ChemiDoc XR + (Bio-Rad). Germline retention analysis of Jκ1 was carried out by real-time quantitative PCR analysis, as previously described50.
Immuno-DNA FISH
Combined detection of γ-H2AX and Igk loci was carried out on cells adhered to poly-L lysine-coated coverslips, as previously described51. Cells were fixed with 2% paraformaldehyde/PBS for 10 min, and permeabilized for 5 min with 0.4% Triton/PBS on ice. After 30 min blocking in 2.5% BSA, 10% normal goat serum, and 0.1% Tween-20/PBS, H3S10ph staining was carried out using an antibody against phosphorylated serine-10 of H3 (Millipore) diluted at 1:400 in blocking solution for 1 h at room temperature. Cells were rinsed three times in 0.2% BSA, 0.1% Tween-20/PBS and incubated for 1 h with goat-anti-rabbit IgG Alexa 488 or 594 or 633 (Invitrogen). After three rinses in 0.1% Tween-20/PBS, cells were post fixed in 3% paraformaldehyde/PBS for 10 min, permeabilised in 0.7% Triton-X-100 in 0.1 M HCl for 15 min on ice, and incubated in 0.1 mg/ml RNase A for 30 min at 37 °C. Cells were then denatured with 1.9 M HCl for 30 min at room temperature and rinsed with cold PBS. DNA probes were denatured for 5 min at 95 °C, pre-annealed for 45 min at 37 °C, and applied to coverslips which were sealed onto slides with rubber cement and incubated overnight at 37 °C. Cells were then rinsed three times 30 min with 2x SSC at 37 °C, 2x SSC, and 1x SSC at room temperature. Cells were mounted in ProLong Gold (Invitrogen) containing DAPI to counterstain the total DNA.The Igk locus was detected using BAC DNA probes that hybridize to the distal Vκ24 gene region (RP23–101G13) and the Cκ region (RP24–387E13), in combination with an antibody against the phosphorylated form of γ-H2AX. BAC probes were directly labeled by nick translation with dUTP-A594 or dUTP-A488 (Invitrogen). FISH for locus contraction was conducted as previously published51.
Confocal microscopy and analysis
Cells were analyzed by confocal microscopy on a Leica SP5 Acousto-Optical Beam Splitter system. Optical sections separated by 0.3 μm were collected. Analysis on cells included those in which signals from both alleles could be detected, which encompassed 90–95% of the total cells imaged. Further analysis was carried out using ImageJ. Alleles were defined as colocalized with γ-H2AX if the signals overlapped. Sample sizes typically included a minimum of 100 cells, and experiments were repeated at least two or three times. Statistical significance was calculated by χ2 analysis in a pair-wise manner. For locus contraction, distances were measured between the center of mass of each BAC signal. Significant differences in distributions of empirical inter-allelic distances were determined by a nonparametric two-sample Kolmogorov–Smirnov (KS) test. To eliminate observer bias, each experiment was analyzed by at least two people.
STAT5-ChIP-qPCR
STAT5-ChIP-qPCR was conducted as previously described44. Purified DNA was then analyzed by quantitative real-time PCR. Samples were analyzed in triplicate and represent three biological replicates.
Igh and Igk repertoire analysis
Cells were sorted and lysed in 0.5 mg/mL proteinase K at 55 C for 4 h, 85 C 10 min. Lysed cells were used as a template for HotStarTaq (Qiagen) PCR amplification. Igh primers used to amplify rearrangements were adapted from VH FR3 and JH BIOMED-2 primers76,77. Additional primers were designed to capture some V genes missing from the BIOMED-2 study, which include VH12 (VH12-3). Igk primers include the degenerate V primer used in the semiquantitative PCRs with additional primers designed to capture some B-1a cell-biased gene segments, which include VK4 (VK4–91) (Supplementary Table 2). Amplified rearrangements were purified by gel extraction (Qiagen) followed by a standard end-repair reaction. Following purification steps were carried out by Ampure XP bead purification (Beckman Coulter). In order to attach Illumina-compatible adapters, samples were treated by standard dA tailing followed by adapter ligation using Quick Ligase (NEB) mixed with pre-annealed NEXTflex DNA Barcodes (Bioo Scientific). QC was carried out by tapestation, and quantified by qPCR (Kapa Biosystems). Samples were pooled and sequenced on an Illumina HiSeq 4000 (2 × 150 PE reads). Nucleotide sequences were compared with the reference equences from IMGT, the international ImMunoGeneTics information system (http://www.imgt.org) and analyzed using IMGT/HighV-QUEST67, a web portal allowing the analysis of thousands of sequences on IMGT/V-QUEST65. IMGT/StatClonotype was used to analyze statistically significant gene segment usage between samples64,66. Igh N-addition information from the region between V and D gene segments was extracted from IMGT/HighV-QUEST and graphed in R.
Reporting summary
Further information on research design is available in the Nature Research Reporting Summary linked to this article.
Supplementary information
Acknowledgements
The authors would like to thank the NYU Flow Cytometry and Cell Sorting Center as well as the NYU Genome Technology Center, both of which are supported in part by the Cancer Center Support Grant P30CA016087 at the Laura and Isaac Perlmutter Cancer Center. We would like to thank Meinrad Busslinger and Taras Kreslavsky (Research Institute of Molecular Pathology (IMP)) for suggesting the transitional B-1a cell experiment. In addition, we would like to thank the Skok lab for discussions on the study. J.A.S. is supported by NIH grants R35GM122515. J.B.W. was previously supported by the T32 CA009161 training grant (Levy) and the 2T32 AI100853-06 (Reizis) training grant. S.L.H. was supported by an American Society of Hematology (ASH) Scholar Award and by a Molecular Oncology and Immunology Training Grant NIH T32. M.A.F. is supported by NIH grants CA151845 and CA154998.
Author contributions
J.B.W., S.L.H., K.J., and J.A.S. designed the experiments. J.B.W., S.L.H., L.M.H.-H., M.M., and K.J. performed the experiments and conducted data analysis. J.B.W., J.A.S., and K.R. wrote the paper, with input from M.M., S.B.K., M.R.C., and M.A.F.
Data availability
The data that support findings of this study have been deposited in the Gene Expression Omnibus (GEO) database under the accession code GSE104111.
Competing interests
The authors declare no competing interests.
Footnotes
Peer review information Nature Communications thanks the anonymous reviewer(s) for their contribution to the peer review of this work. Peer reviewer reports are available.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary information is available for this paper at 10.1038/s41467-019-12824-z.
References
- 1.Morton LM, et al. Lymphoma incidence patterns by WHO subtype in the United States, 1992-2001. Blood. 2006;107:265–276. doi: 10.1182/blood-2005-06-2508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Royston I, Majda JA, Baird SM, Meserve BL, Griffiths JC. Human T cell antigens defined by monoclonal antibodies: the 65,000-dalton antigen of T cells (T65) is also found on chronic lymphocytic leukemia cells bearing surface immunoglobulin. J. Immunol. 1980;125:725–731. [PubMed] [Google Scholar]
- 3.Wang C-Y, Good RA, Ammirati P, Dymbort G, Evans RL. Identification of a p69,71 complex expressed on human T cells sharing determinants with B-type chronic lymphatic leukemic cells. J. Exp. Med. 1980;151:1539–1544. doi: 10.1084/jem.151.6.1539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kantor A B, Herzenberg L A. Origin of Murine B Cell Lineages. Annual Review of Immunology. 1993;11(1):501–538. doi: 10.1146/annurev.iy.11.040193.002441. [DOI] [PubMed] [Google Scholar]
- 5.Hayakawa K, Carmack CE, Shinton SA, Hardy RR. Selection of autoantibody specificities in the Ly-1 B subset. Ann. N. Y. Acad. Sci. 1992;651:346–353. doi: 10.1111/j.1749-6632.1992.tb24634.x. [DOI] [PubMed] [Google Scholar]
- 6.Kantor AB, Merrill CE, Herzenberg LA, Hilson JL. An unbiased analysis of V (H)-DJ (H) sequences from B-1a, B-1b, and conventional B cells. J. Immunol. 1997;158:1175–1186. [PubMed] [Google Scholar]
- 7.Wang H, Clarke SH. Positive selection focuses the VH12 B-cell repertoire towards a single B1 specificity with survival function. Immunol. Rev. 2004;197:51–59. doi: 10.1111/j.0105-2896.2004.0098.x. [DOI] [PubMed] [Google Scholar]
- 8.Duan B, Morel L. Role of B-1a cells in autoimmunity. Autoimmun. Rev. 2006;5:403–408. doi: 10.1016/j.autrev.2005.10.007. [DOI] [PubMed] [Google Scholar]
- 9.Berland R, Wortis HH. Origins and functions of B-1 cells with notes on the role of CD5. Annu. Rev. Immunol. 2002;20:253–300. doi: 10.1146/annurev.immunol.20.100301.064833. [DOI] [PubMed] [Google Scholar]
- 10.Baumgarth N. The double life of a B-1 cell: self-reactivity selects for protective effector functions. Nat. Rev. Immunol. 2011;11:34–46. doi: 10.1038/nri2901. [DOI] [PubMed] [Google Scholar]
- 11.Ghosn EEB, Yang Y. Hematopoietic stem cell-independent B-1a lineage. Ann. N. Y. Acad. Sci. 2015;1362:23–38. doi: 10.1111/nyas.12881. [DOI] [PubMed] [Google Scholar]
- 12.Hayakawa K, Hardy RR, Herzenberg LA, Herzenberg LA. Progenitors for Ly-1 B cells are distinct from progenitors for other B cells. J. Exp. Med. 1985;161:1554–1568. doi: 10.1084/jem.161.6.1554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kobayashi M, et al. Functional B-1 progenitor cells are present in the hematopoietic stem cell-deficient embryo and depend on Cbfβ for their development. Proc. Natl Acad. Sci. USA. 2014;111:12151–12156. doi: 10.1073/pnas.1407370111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yoshimoto M. The first wave of B lymphopoiesis develops independently of stem cells in the murine embryo. Ann. N. Y. Acad. Sci. 2015;1362:16–22. doi: 10.1111/nyas.12612. [DOI] [PubMed] [Google Scholar]
- 15.Ghosn EEB, et al. Fetal hematopoietic stem cell transplantation fails to fully regenerate the B-lymphocyte compartment. Stem Cell Rep. 2016;6:137–149. doi: 10.1016/j.stemcr.2015.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Touma M, et al. Functional role for IkBNS in T cell cytokine regulation as revealed by targeted gene disruption. J. Immunol. 2007;179:1681. doi: 10.4049/jimmunol.179.3.1681. [DOI] [PubMed] [Google Scholar]
- 17.Sawai CM, et al. Hematopoietic stem cells are the major source of multilineage hematopoiesis in adult animals. Immunity. 2016;45:597–609. doi: 10.1016/j.immuni.2016.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kristiansen TA, et al. Cellular barcoding links B-1a B cell potential to a fetal hematopoietic stem cell state at the single-cell level. Immunity. 2016;45:346–357. doi: 10.1016/j.immuni.2016.07.014. [DOI] [PubMed] [Google Scholar]
- 19.Hayakawa K, et al. Positive selection of natural autoreactive B cells. Science. 1999;285:113–116. doi: 10.1126/science.285.5424.113. [DOI] [PubMed] [Google Scholar]
- 20.Graf R, et al. BCR-dependent lineage plasticity in mature B cells. Science. 2019;363:748–753. doi: 10.1126/science.aau8475. [DOI] [PubMed] [Google Scholar]
- 21.Yuan J, Nguyen CK, Liu X, Kanellopoulou C, Muljo Sa. Lin28b reprograms adult bone. Science. 2012;335:1195–1200. doi: 10.1126/science.1216557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhou Y, et al. Lin28b promotes fetal B lymphopoiesis through the transcription factor Arid3a. J. Exp. Med. 2015;212:569–580. doi: 10.1084/jem.20141510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li Y-S, et al. A developmental switch between fetal and adult B lymphopoiesis. Ann. N. Y. Acad. Sci. 2015;1362:8–15. doi: 10.1111/nyas.12769. [DOI] [PubMed] [Google Scholar]
- 24.Kreslavsky T, et al. Essential role of the transcription factor Bhlhe41 in regulating the homeostasis and BCR repertoire of B-1a cells. Nat. Immunol. 2017;18:442–258. doi: 10.1038/ni.3694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Geier JK, Schlissel MS. Pre-BCR signals and the control of Ig gene rearrangements. Semin. Immunol. 2006;18:31–39. doi: 10.1016/j.smim.2005.11.001. [DOI] [PubMed] [Google Scholar]
- 26.Wasserman R, et al. A novel mechanism for B cell repertoire maturation based on response by B cell precursors to pre-B receptor assembly. J. Exp. Med. 1998;187:259–264. doi: 10.1084/jem.187.2.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Keenan Ra, et al. Censoring of autoreactive B cell development by the pre-B cell receptor. Science. 2008;321:696–699. doi: 10.1126/science.1157533. [DOI] [PubMed] [Google Scholar]
- 28.Kitamura D, et al. A critical role of Lambda5 protein in B cell development. Cell. 1992;69:623–631. doi: 10.1016/0092-8674(92)90293-L. [DOI] [PubMed] [Google Scholar]
- 29.Ehlich A, et al. Immunoglobulin heavy and light chain genes rearrange independently at early stages of B cell development. Cell. 1993;72:695–704. doi: 10.1016/0092-8674(93)90398-A. [DOI] [PubMed] [Google Scholar]
- 30.Kitamura D, Rajewsky K. Targeted disruption of u chain membrane exon causes loss of heavy-chain allelic exclusion. Nature. 1992;356:154–156. doi: 10.1038/356154a0. [DOI] [PubMed] [Google Scholar]
- 31.Kubagawa H, Cooper MD, Carroll AJ, Burrows PD. Light-chain gene expression before heavy-chain gene rearrangement in pre-B cells transformed by Epstein-Barr virus. Proc. Natl Acad. Sci. USA. 1989;86:2356–2360. doi: 10.1073/pnas.86.7.2356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pelanda R, et al. Receptor editing in a transgenic mouse model: site, efficiency, and role in B cell tolerance and antibody diversification. Immunity. 1997;7:765–775. doi: 10.1016/S1074-7613(00)80395-7. [DOI] [PubMed] [Google Scholar]
- 33.Eschbach C, et al. Efficient generation of B lymphocytes by recognition of self-antigens. Eur. J. Immunol. 2012;41:2397–2403. doi: 10.1002/eji.201041344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Johnson K, et al. Regulation of immunoglobulin light-chain recombination by the transcription factor IRF-4 and the attenuation of interleukin-7 signaling. Immunity. 2008;28:335–345. doi: 10.1016/j.immuni.2007.12.019. [DOI] [PubMed] [Google Scholar]
- 35.Malin S, et al. Role of STAT5 in controlling cell survival and immunoglobulin gene recombination during pro-B cell development. Nat. Immunol. 2010;11:171–179. doi: 10.1038/ni.1827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Peschon BJJ, et al. Early lymphocyte expansion is severely impaired in interleukin 7 receptor-deficient mice. J. Exp. Med. 1994;180:6–11. doi: 10.1084/jem.180.5.1955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Freeden-jeffry BU, et al. Lymphopenia in interleukin (IL)-7 gene-deleted mice identifies IL-7 as a nonredundant cytokine. J. Exp. Med. 1995;181:1519–1526. doi: 10.1084/jem.181.4.1519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Erlandsson L, et al. Impaired B-1 and B-2 B cell development and atypical splenic B cell structures in IL-7 receptor-deficient mice. Eur. J. Immunol. 2004;34:3595–3603. doi: 10.1002/eji.200425217. [DOI] [PubMed] [Google Scholar]
- 39.Hesslein DGT, Yang SY, Schatz DG. Origins of peripheral B cells in IL-7 receptor-deficient mice. Mol. Immunol. 2006;43:326–334. doi: 10.1016/j.molimm.2005.02.010. [DOI] [PubMed] [Google Scholar]
- 40.Hewitt SL, et al. Association between the Igk and Igh immunoglobulin loci mediated by the 3′ Igk enhancer induces ‘decontraction’ of the Igh locus in pre-B cells. Nat. Immunol. 2008;9:396–404. doi: 10.1038/ni1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bertolino E, et al. Regulation of interleukin 7-dependent immunoglobulin heavy-chain variable gene rearrangements by transcription factor STAT5. Nat. Immunol. 2005;6:836–843. doi: 10.1038/ni1226. [DOI] [PubMed] [Google Scholar]
- 42.Chowdhury D, Sen R. Stepwise activation of the immunoglobulin m heavy chain gene locus. EMBO J. 2001;20:6394–6403. doi: 10.1093/emboj/20.22.6394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mandal M, et al. Ras orchestrates exit from the cell cycle and light-chain recombination during early B cell development. Nat. Immunol. 2009;10:1110–1117. doi: 10.1038/ni.1785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mandal M, et al. Epigenetic repression of the Igk locus by STAT5-mediated recruitment of the histone methyltransferase Ezh2. Nat. Immunol. 2011;12:1212–1220. doi: 10.1038/ni.2136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Clark MR, Mandal M, Ochiai K, Singh H. Orchestrating B cell lymphopoiesis through interplay of IL-7 receptor and pre-B cell receptor signalling. Nat. Rev. Immunol. 2014;14:69–80. doi: 10.1038/nri3570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Carvalho TL, Mota-Santos T, Cumano A, Demengeot J, Vieira P. Arrested B lymphopoiesis and persistence of activated B cells in adult interleukin 7(-/)- mice. J. Exp. Med. 2001;194:1141–1150. doi: 10.1084/jem.194.8.1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Shriner AK, Liu H, Sun G, Guimond M, Alugupalli KR. IL-7-dependent B lymphocytes are essential for the anti-polysaccharide response and protective immunity to Streptococcus pneumoniae. J. Immunol. 2010;185:525–531. doi: 10.4049/jimmunol.0902841. [DOI] [PubMed] [Google Scholar]
- 48.Johnson K, et al. IL-7 functionally segregates the pro-B cell stage by regulating transcription of recombination mediators across cell cycle. J. Immunol. 2012;188:6084–6092. doi: 10.4049/jimmunol.1200368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Fuxa M. Pax5 induces V-to-DJ rearrangements and locus contraction of the immunoglobulin heavy-chain gene. Genes & Development. 2004;18(4):411–422. doi: 10.1101/gad.291504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhou X, Xiang Y, Garrard WT. The Ig gene enhancers, E3′ and Ed, are essential for triggering transcription. J. Immunol. 2010;185:7544–7552. doi: 10.4049/jimmunol.1002665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hewitt SL, et al. RAG-1 and ATM coordinate monoallelic recombination and nuclear positioning of immunoglobulin loci. Nat. Immunol. 2009;10:655–664. doi: 10.1038/ni.1735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chen HT, et al. Response to RAG-mediated VDJ cleavage by NBS1 and gamma-H2AX. Science. 2000;290:1962–1965. doi: 10.1126/science.290.5498.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Roldán E, et al. Locus ‘decontraction’ and centromeric recruitment contribute to allelic exclusion of the immunoglobulin heavy-chain gene. Nat. Immunol. 2005;6:31–41. doi: 10.1038/ni1150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Li Y-S, K H, Hardy RR. The regulated expression of B lineage associated genes during B cell differentiation in bone marrow and fetal liver. J. Exp. Med. 1993;178:951. doi: 10.1084/jem.178.3.951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ruetsch NR, Bosma GC, Bosma MJ. Unexpected rearrangement and expression of the immunoglobulin lambda 1 locus in scid mice. J. Exp. Med. 2000;191:1933–1943. doi: 10.1084/jem.191.11.1933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Novobrantseva BTI, et al. Rearrangement and expression of immunoglobulin light chain genes can precede heavy chain expression during normal B cell development in mice. J. Exp. Med. 1999;189:75–87. doi: 10.1084/jem.189.1.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Haas KM. B-1 lymphocytes in mice and nonhuman primates. Ann. N. Y. Acad. Sci. 2015;1362:98–109. doi: 10.1111/nyas.12760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lam KP, Rajewsky K. B cell antigen receptor specificity and surface density together determine B-1 versus B-2 cell development. J. Exp. Med. 1999;190:471–477. doi: 10.1084/jem.190.4.471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wang H, et al. Expression of plasma cell alloantigen 1 defines layered development of B-1a B-cell subsets with distinct innate-like functions. Proc. Natl Acad. Sci. USA. 2012;109:20077–20082. doi: 10.1073/pnas.1212428109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wang H, et al. New insights into heterogeneity of peritoneal B-1a cells. Ann. N. Y. Acad. Sci. 2015;1362:68–76. doi: 10.1111/nyas.12791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Pedersen GK, et al. B-1a transitional cells are phenotypically distinct and are lacking in mice deficient in IkBNS. Proc. Natl Acad. Sci. USA. 2014;111:E4119–E4126. doi: 10.1073/pnas.1415866111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Montecino-Rodriguez Encarnacion, Dorshkind Kenneth. Formation of B-1 B Cells from Neonatal B-1 Transitional Cells Exhibits NF-κB Redundancy. The Journal of Immunology. 2011;187(11):5712–5719. doi: 10.4049/jimmunol.1102416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Burchill MA, et al. Distinct effects of STAT5 activation on CD4+ and CD8+ T cell homeostasis: development of CD4+CD25+ regulatory T cells versus CD8+ memory T cells. J. Immunol. 2003;171:5853–5864. doi: 10.4049/jimmunol.171.11.5853. [DOI] [PubMed] [Google Scholar]
- 64.Aouinti S, et al. IMGT/statclonotype for pairwise evaluation and visualization of NGS IG and TR IMGT clonotype (AA) diversity or expression from IMGT/HighV-QUEST. Front. Immunol. 2016;7:1–14. doi: 10.3389/fimmu.2016.00339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Brochet X, Lefranc MP, Giudicelli V. IMGT/V-QUEST: the highly customized and integrated system for IG and TR standardized V-J and V-D-J sequence analysis. Nucleic Acids Res. 2008;36:503–508. doi: 10.1093/nar/gkn316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Aouinti S, Malouche D, Giudicelli V, Kossida S, Lefranc MP. IMGT/HighV-QUEST statistical significance of IMGT clonotype (AA) diversity per gene for standardized comparisons of next generation sequencing immunoprofiles of immunoglobulins and T cell receptors. PLoS One. 2015;10:1–22. doi: 10.1371/journal.pone.0142353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lefranc MP, et al. IMGT®, the international ImMunoGeneTics information system®. Nucleic Acids Res. 2009;37:1006–1012. doi: 10.1093/nar/gkn838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Jerne NK. The somatic generation of immune recognition. Eur. J. Immunol. 1971;1:1–9. doi: 10.1002/eji.1830010102. [DOI] [PubMed] [Google Scholar]
- 69.Andrés BDe, Gonzalo P. The first 3 days of B-cell development in the mouse embryo. Blood. 2002;100:4074–4081. doi: 10.1182/blood-2002-03-0809. [DOI] [PubMed] [Google Scholar]
- 70.Montecino-Rodriguez E, Leathers H, Dorshkind K. Identification of a B-1 B cell–specified progenitor. Nat. Immunol. 2006;7:293–301. doi: 10.1038/ni1301. [DOI] [PubMed] [Google Scholar]
- 71.Yoshimoto M, et al. Embryonic day 9 yolk sac and intra-embryonic hemogenic endothelium independently generate a B-1 and marginal zone progenitor lacking B-2 potential. Proc. Natl Acad. Sci. USA. 2011;108:1468–1473. doi: 10.1073/pnas.1015841108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Goetz CA, et al. STAT5 activation underlies IL7 receptor-dependent B cell development. J. Immunol. 2004;172:4770–4778. doi: 10.4049/jimmunol.172.8.4770. [DOI] [PubMed] [Google Scholar]
- 73.Sonoda E, et al. B cell development under the condition of allelic inclusion. Immunity. 1997;6:225–233. doi: 10.1016/S1074-7613(00)80325-8. [DOI] [PubMed] [Google Scholar]
- 74.Arnold BLW, et al. Development of B-1 cells: segregation of phosphatidyl choline-specific B cells to the B-1 population occurs after immunoglobulin gene expression. J. Exp. Med. 1994;179:1585–1595. doi: 10.1084/jem.179.5.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Sen J, Rosenberg N, Burakoff SJ. Expression and ontogeny of CD2 on murine B cells. J. Immunol. 1990;144:2925–2930. [PubMed] [Google Scholar]
- 76.van Dongen JJ, et al. Design and standardization of PCR primers and protocols for detection of clonal immunoglobulin and T-cell receptor gene recombinations in suspect lymphoproliferations: report of the BIOMED-2 concerted action BMH4-CT98-3936. Leukemia. 2003;17:2257–2317. doi: 10.1038/sj.leu.2403202. [DOI] [PubMed] [Google Scholar]
- 77.Arnaout R, et al. High-resolution description of antibody heavy-chain repertoires in humans. PLoS One. 2011;6:e22365. doi: 10.1371/journal.pone.0022365. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support findings of this study have been deposited in the Gene Expression Omnibus (GEO) database under the accession code GSE104111.