Abstract
Extranodal NK/T-cell lymphoma, nasal type (NKTL) is an aggressive type of non-Hodgkin lymphoma closely associated with Epstein-Barr virus and characterized by varying degrees of systemic inflammation. We aim to examine the prognostic significance of peripheral blood neutrophil-lymphocyte ratio (NLR) in patients with NKTL. Therefore, we conducted a retrospective review of 178 patients with biopsy-proven NKTL from the National Cancer Centre Singapore and Samsung Medical Center, South Korea. Using receiver operating curve analysis, an optimal cut-off for high NLR (>3.5) in predicting overall survival (OS) was derived. Survival analysis was performed using the Kaplan-Meier method and multivariable Cox proportional regression. In patients with high NLR, estimated 5-year OS was 25% compared to 53% in those with low NLR. In multivariable analysis, high NLR, in addition to age ≥60 years, presence of B-symptoms and stage III/IV at diagnosis, was independently correlated with worse OS (HR 2.08; 95% CI 1.36 to 3.18; p = 0.0008) and progression-free survival (HR 1.66; 95% CI 1.11 to 2.46; p = 0.0128). A new prognostic index (NABS score) derived from these factors stratified patients into low (0), low-intermediate (1), high-intermediate (2) and high (3–4) risk subgroups, which were associated with 5-year OS of 76.5%, 55.7%, 29.2% and 0% respectively. In conclusion, high NLR is an independent prognostic marker and the NABS model can be used to risk-stratify NKTL patients.
Subject terms: T-cell lymphoma, T-cell lymphoma
Introduction
Extranodal natural killer/T-cell lymphoma, nasal type (NKTL) is a rare but aggressive subtype of peripheral T-cell lymphoma (PTCL)1. The epidemiology of this entity has significant geographical variation, with a higher incidence in East Asian and Latin American populations but rare in North America and Europe2. Nonetheless, reports of NKTL in the West have been increasing steadily, possibly due to improved understanding and recognition of this disease entity3. While much remains unknown regarding the tumorigenesis of NKTL, recent studies have led to deeper insights of its clinical behavior and molecular pathobiology. NKTL classically manifests as a destructive lesion in the nasal cavity and upper aerodigestive tract, with the presence of extranasal disease characterized by more adverse clinical features and worse survival outcomes4. Most cases originate from NK cells while a minority are derived from T-cells5, and at the molecular level, we and others have shown that constitutive activation of the JAK/STAT signaling pathway plays an important role in promoting NKTL cell growth6–8.
Despite improvements in patient outcomes with the introduction of L-asparaginase-based chemotherapy regimens and modern radiotherapy in the management of NKTL, disease relapse rates remain substantial even for local disease and 5-year survival rates for advanced cases are a dismal 50%9. A recently described prognostic model (Prognostic Index of Natural Killer lymphoma, PINK) identified four risk factors—age greater than 60 years, stage III or IV disease, distant lymph node involvement and non-nasal type to be strongly associated with worse survival outcomes10. Notably, there is increasing evidence that peripheral blood indices of systemic inflammation may signify an aggressive phenotype in both solid and hematological malignancies2,11,12. Inflammation plays a pivotal role in the tumor microenvironment through the production and release of cytokines, which promotes tumorigenesis, angiogenesis, invasion and metastasis13. Mutagenic substances are also released by inflammatory cells, facilitating the development of further mutations that may provide cancer cells with a survival advantage14. In this respect, peripheral blood indices of systemic inflammation such as the neutrophil-lymphocyte ratio (NLR) has been consistently demonstrated to be prognostic in Hodgkin lymphomas15, B-cell lymphomas16, and subtypes of PTCL other than NKTL12. Therefore in this study, we aim to investigate the systemic inflammatory milieu in NKTL through the study of pre-treatment peripheral blood counts and explore its clinical prognostic relevance in these patients.
Results
Patient demographics
Table 1 shows the clinical characteristics of all 178 patients. The median age was 54.0 years (range 17.1 to 86.1 years) with 32 patients (18.0%) (over 60 years old. There was a male predominance (70.8%). One hundred and thirteen patients presented with early-stage disease (Ann-Arbor I or II), 155 had good performance status (ECOG score 0 or 1) and 74 had B symptoms at presentation. Table 2 shows the first line treatment modality patients received. One hundred and eight patients (60.7%) received chemotherapy, which included the following regimens: SMILE (n = 36), anthracycline-based (n = 20), gemcitabine-based (n = 12) and others (n = 40). Sixty patients (33.7%) received concurrent chemoradiation, 8 (4.5%) received radiation only while 2 (1.1%) received supportive care only.
Table 1.
Clinicopathological features and NLR at diagnosis.
| Characteristic (n) | Neutrophil-lymphocyte ratio at diagnosis (%) | p | |
|---|---|---|---|
| ≤3.5 | >3.5 | ||
| Total (178) | 119 (66.9) | 59 (33.1) | — |
| Sex | 0.435 | ||
| Male (126) | 82 (65.1) | 44 (34.9) | |
| Female (52) | 37 (71.2) | 15 (28.8) | |
| Age at diagnosis (years) | 0.0316 | ||
| ≥60 (32) | 18 (56.3) | 14 (43.7) | |
| <60 (146) | 101 (69.2) | 45 (30.8) | |
| Performance status (ECOG score) | 0.0025 | ||
| 0–1 (155) | 110 (71.0) | 45 (29.0) | |
| 2–4 (23) | 9 (39.1) | 14 (60.9) | |
| Ann Arbor stage | 0.255 | ||
| I-II (113) | 79 (69.9) | 34 (30.1) | |
| III-IV (65) | 40 (61.5) | 25 (38.5) | |
| Distal node involvement | 0.517 | ||
| Yes (43) | 27 (62.8) | 16 (37.2) | |
| No (135) | 92 (68.1) | 43 (31.9) | |
| B-symptoms | 0.263 | ||
| Present (74) | 46 (62.2) | 28 (37.8) | |
| Absent (104) | 73 (70.2) | 31 (29.8) | |
| LDH elevation | 0.0197 | ||
| Yes (89) | 52 (58.4) | 37 (41.6) | |
| No (88) | 66 (75.0) | 22 (25.0) | |
| EBV detection | 0.0084 | ||
| Yes (65) | 41 (63.1) | 24 (36.9) | |
| No (71) | 59 (83.1) | 12 (16.9) | |
| IPI risk group | 0.0142 | ||
| 1–2 (124) | 90 (72.6) | 34 (27.4) | |
| 3–4 (54) | 29 (53.7) | 25 (46.3) | |
| PINK score | 0.206 | ||
| 0–1 (117) | 82 (70.1) | 35 (29.9) | |
| 2–4 (61) | 37 (60.7) | 24 (39.3) | |
| Platelet-lymphocyte ratio | <0.0001 | ||
| >201 (66) | 21 (31.8) | 45 (68.2) | |
| ≤201 (112) | 98 (87.5) | 14 (12.5) | |
| Lymphocyte-monocyte ratio | <0.0001 | ||
| ≤1.8 (46) | 11 (23.9) | 35 (76.1) | |
| >1.8 (132) | 108 (81.8) | 24 (18.2) | |
Missing data: LDH (n = 1), EBV (n = 42).
Table 2.
First line treatment modality received.
| Treatment modality | n (%) | ||
|---|---|---|---|
| Overall (n = 178) | Singapore (n = 64) | Korea (n = 114) | |
| Supportive care only | 2 (1.1) | 2 (3.1) | 0 (0) |
| Radiation only | 8 (4.5) | 8 (12.5) | 0 (0) |
| Concurrent chemoradiation | 60 (33.7) | 0 (0) | 60 (52.6) |
| Chemotherapy | 108 (60.7) | 54 (84.4) | 54 (47.4) |
| • Anthracycline-based | 20 (11.2) | 15 (23.4) | 5 (4.4) |
| • Gemcitabine-based | 12 (6.7) | 12 (18.8) | 0 (0) |
| • SMILE | 36 (20.2) | 4 (6.3) | 32 (28.1) |
| • Others | 40 (22.5) | 23 (35.9) | 17 (14.9) |
Across the entire cohort, the values for NLR (median: 2.6, range: 0.7 to 24.5), platelet-lymphocyte ratio (PLR) (median: 158.4, range: 19 to 1708.9) and lymphocyte-monocyte ratio (LMR) (median: 2.8, range: 0.4 to 10.7) follow non-normal distributions (all p < 0.0001). Patients were dichotomized according to levels of NLR, PLR, LMR using optimized cut-offs to predict overall survival (OS) as derived from ROC curve analysis (>3.5, >201 and ≤1.8, respectively). The areas under the curve for NLR, PLR and LMR for OS were 0.622 (95% CI 0.546 to 0.693), 0.586 (95% CI 0.510 to 0.660), and 0.585 (95% CI 0.509 to 0.659), respectively.
Clinicopathological correlates
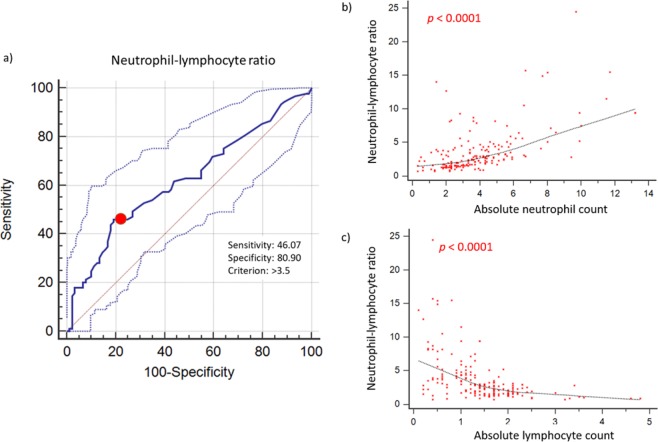
A subgroup of 59 patients (33.1%) had high NLR >3.5 at diagnosis. NLR >3.5 was significantly associated with age ≥ 60 years old (p = 0.0316), ECOG score ≥2 (p = 0.0025), elevated serum LDH (p = 0.0197), plasma Epstein-Barr virus (EBV) detection (p = 0.0084), IPI score ≥3 (p = 0.0142), PLR >201 (p < 0.0001) and LMR ≤1.8 (p < 0.0001) but not with sex, Ann-Arbor stage, distal node involvement, presence of B symptoms, and PINK score. A positive correlation was demonstrated between NLR and absolute neutrophil counts (Spearman’s rho 0.571, 95% CI 0.463 to 0.662, p < 0.0001), while a converse correlation with absolute lymphocyte counts was found (Spearman’s rho −0.591, 95% CI −0.679 to −0.486, p < 0.0001) (Fig. 1).
Figure 1.
Derivation of NLR. (a) An optimal cut-off for high NLR (>3.5) in predicting overall survival and progression-free survival was determined using receiver operating curve analyses. (b) A positive correlation was demonstrated between NLR and absolute neutrophil counts (Spearman’s rho 0.571, 95% CI 0.463 to 0.662, p <0.0001), (c) while a converse correlation with absolute lymphocyte counts was found (Spearman’s rho −0.591, 95% CI −0.679 to −0.486, p <0.0001).
Survival analyses
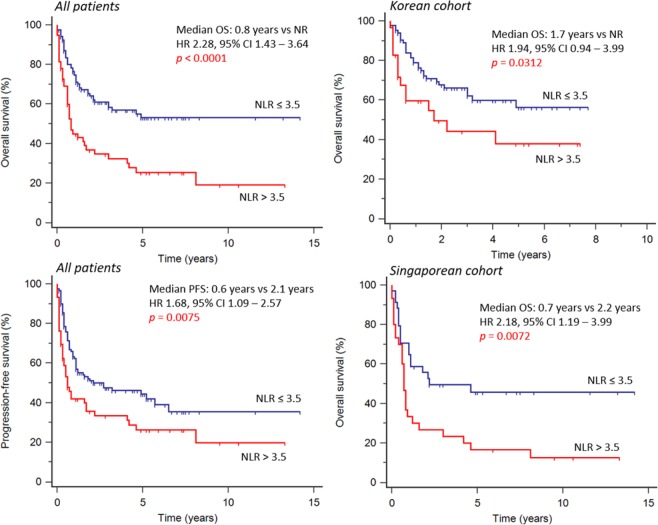
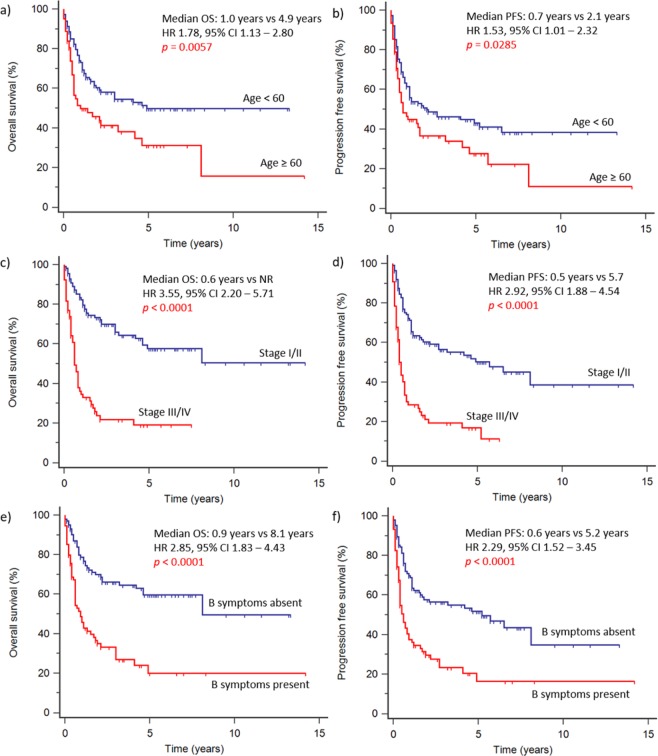
At the time of analysis, 89 patients (50%) had died. The median follow-up time was 5.2 years. In the overall cohort, NLR >3.5 was associated with worse OS (HR 2.28, 95% CI 1.43 to 3.64, p < 0.0001) (Fig. 2). Median OS was 0.8 years in patients with NLR >3.5 and 2.1 years with NLR ≤3.5. Estimated 5-year OS was 53% in patients with low NLR and 25% in those with high NLR. Under univariable analysis (Table 3), the following factors at diagnosis were statistically significant predictors for OS: NLR >3.5, age ≥60 years old, ECOG score ≥2, advanced stage (III or IV), distal node involvement, presence of B symptoms, elevated serum LDH, PLR >201, LMR ≤ 1.8, PINK score ≥ 2 and IPI score ≥ 3. These factors were also statistically significant predictors for PFS, except for PLR. Multivariate analysis (Table 4) demonstrated that high NLR was a significant independent predictor for both OS (HR 2.08, 95% CI 1.36 to 3.18, p = 0.0008) and PFS (HR 1.66, 95% CI 1.11 to 2.46, p = 0.0128). In addition, age (HR 1.70, 95% CI 1.11 to 2.62, p = 0.0156), advanced stage (HR 3.06, 95% CI 1.94 to 4.82, p <0.0001) and presence of B symptoms (HR 2.35, 95% CI 1.49 to 3.71, p = 0.0002) were also significant independent predictors of OS (Fig. 3). For PFS, advanced stage (HR 2.63, 95% CI 1.74 to 3.98, p <0.0001) and presence of B symptoms (HR 1.89, 95% CI 1.25 to 2.85, p = 0.0024) were significant independent predictors. In subgroup analysis, NLR >3.5 was a significant predictor for both poor OS (HR 2.18, 95% CI 1.19 to 3.99, p = 0.0072) and PFS (HR 1.74, 95% CI 0.98 to 3.11, p = 0.0460) in the Singaporean cohort. For the Korean cohort, high NLR was associated with poor OS (HR 1.94, 95% CI 0.94 to 3.99, p = 0.0312), but not PFS (HR 1.30, 95% CI 0.69 to 2.45, p = 0.372).
Figure 2.
Survival outcomes stratified by NLR. High NLR (>3.5) was associated with worse overall survival and progression-free survival in analysis of the entire cohort. In subgroup analysis, high NLR was consistently associated with worse overall survival both in the Singaporean and Korean cohorts.
Table 3.
Univariable survival analysis for entire cohort.
| Characteristic | Progression-free survival | Overall survival | ||
|---|---|---|---|---|
| HR (95% CI) | p | HR (95% CI) | p | |
| Sex | 0.97 | 0.8779 | 1.15 | 0.5579 |
| (male vs female) | 0.63 to 1.48 | 0.73 to 1.81 | ||
| Age at diagnosis | 1.53 | 0.0285 | 1.78 | 0.0057 |
| (≥60 vs <60 years) | 1.00 to 2.32 | 1.13 to 2.80 | ||
| ECOG score | 2.57 | 0.0001 | 3.44 | <0.0001 |
| (2–4 vs 0–1) | 1.29 to 5.11 | 1.58 to 7.49 | ||
| Stage | 2.92 | <0.0001 | 3.55 | <0.0001 |
| (III-IV vs I-II) | 1.88 to 4.54 | 2.20 to 5.71 | ||
| Distal node involved | 2.21 | 0.0001 | 2.71 | <0.0001 |
| (Yes vs No) | 1.34 to 3.64 | 1.58 to 4.66 | ||
| B-symptoms | 2.29 | <0.0001 | 2.85 | <0.0001 |
| (Present vs absent) | 1.52 to 3.45 | 1.83 to 4.43 | ||
| LDH elevation | 2.15 | 0.0001 | 2.51 | <0.0001 |
| (Yes vs No) | 1.45 to 3.17 | 1.65 to 3.83 | ||
| NLR at diagnosis | 1.68 | 0.0075 | 2.28 | <0.0001 |
| (>3.5 vs ≤3.5) | 1.09 to 2.57 | 1.43 to 3.64 | ||
| PLR at diagnosis | 1.37 | 0.1022 | 1.81 | 0.0037 |
| (>201 vs ≤201) | 0.91 to 2.05 | 1.17 to 2.81 | ||
| LMR at diagnosis | 1.55 | 0.0329 | 1.84 | 0.0047 |
| (≤1.8 vs>1.8) | 0.98 to 2.46 | 1.12 to 3.02 | ||
| PINK score | 2.95 | <0.0001 | 3.80 | <0.0001 |
| (2–4 vs 0–1) | 1.87 to 4.65 | 2.31 to 6.23 | ||
| IPI risk group | 2.85 | <0.0001 | 3.40 | <0.0001 |
| (3–4 vs 1–2) | 1.78 to 4.57 | 2.04 to 5.67 | ||
Table 4.
Multivariate survival analysis for entire cohort.
| Characteristic | Progression-free survival | Overall survival | ||
|---|---|---|---|---|
| HR (95% CI) | p | HR (95% CI) | p | |
| Age at diagnosis | — | — | 1.70 | 0.0156 |
| (≥60 vs <60 years) | 1.11 to 2.62 | |||
| Stage | 2.63 | <0.0001 | 3.06 | <0.0001 |
| (III-IV vs I-II) | 1.74 to 3.98 | 1.94 to 4.82 | ||
| B-symptoms | 1.89 | 0.0024 | 2.35 | 0.0002 |
| (Yes vs No) | 1.25 to 2.85 | 1.49 to 3.71 | ||
| NLR at diagnosis | 1.66 | 0.0128 | 2.08 | 0.0008 |
| (>3.5 vs ≤3.5) | 1.11 to 2.46 | 1.36 to 3.18 | ||
Covariates included age at diagnosis, stage, B-symptoms, distal node involvement, ECOG performance status, sex, LDH elevation, NLR, PLR and LMR.
Figure 3.
Survival outcomes stratified by age, Ann-Arbor staging and presence of B symptoms. Older age at diagnosis (≥60 years old), advanced Ann-Arbor staging (stage III/IV) and presence of B symptoms was associated with worse overall survival and progression-free survival.
A prognostic index was then derived from the 4 independent prognostic factors for OS, namely NLR, age ≥60, presence of B symptoms and advanced stage (NABS score). Patients were then further risk-stratified based on their score into low (0), low-intermediate (1), high-intermediate (2) and high (3–4) risk subgroups, which were associated with 5-year OS of 76.5%, 55.7%, 29.2% and 0%, and 2-year PFS of 64.8%, 59.5%, 37.1% and 9.4% respectively for the entire cohort (p <0.0001) (Fig. 4). The stratification into subgroups was significantly associated with both OS and PFS in both the Korean and Singaporean cohorts (p <0.0001).
Figure 4.
Overall and progression-free survival stratified by NABS score. Patients were risk-stratified based on their NABS score into low (0), low-intermediate (1), high-intermediate (2) and high (3–4) risk subgroups, which were associated with 5-year OS of 76.5%, 55.7%, 29.2% and 0%, and 2-year PFS of 64.8%, 59.5%, 37.1% and 9.4% respectively.
Gene set enrichment analysis
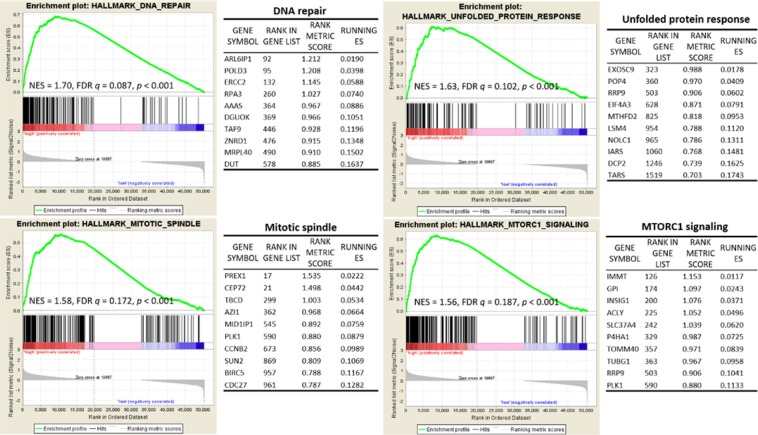
In an exploratory analysis, tumor gene expression profiles available from 8 patients were examined for differences between those with high NLR and those with low NLR (4 with NLR> 3.5 and 4 with NLR <3.5) by gene set enrichment analysis (GSEA). Using the Hallmark gene set, up-regulation of genes involved in DNA repair (NES = 1.70, FDR q = 0.087, p <0.001), genes associated with the unfolded protein response (NES = 1.63, FDR q = 0.102, p <0.001), genes important for mitotic spindle assembly (NES = 1.58, FDR q = 0.172, p <0.001) as well as those up-regulated through activation of the mTORC1 complex (NES = 1.56, FDR q = 0.187, p <0.001) were significantly enriched in cases with elevated NLR (Fig. 5).
Figure 5.
Gene set enrichment analysis (GSEA). Using the Hallmark gene set, upregulation of genes involved in DNA repair, unfolded protein response, mitotic spindle, and MTORC1 signaling were significantly enriched in cases with elevated NLR. The top 10 genes in each pathway are shown in the corresponding tables.
Discussion
Our current study demonstrates that a subset of NKTL patients possesses an elevated systemic inflammatory status as measured by peripheral blood indices. This inflammatory phenotype had adverse prognostic implications, as indicated by poor survival outcomes in these patients. Neutrophils, as part of the innate immune system, are a proxy of ongoing systemic inflammation. Increases in neutrophil counts have been shown to precede oncogenesis in some cases, while in others, malignant change stimulates the inflammatory process instead17. In other words, inflammation may facilitate survival of cancer cells, while cancer cells in turn release pro-inflammatory cytokines, augmenting each other in a perpetual spiral. The inflammatory tumor microenvironment then facilitates hallmark functions of cancer cells, such as proliferation, angiogenesis, invasion and metastasis13. Furthermore, neutrophils may contribute to functional suppression of lymphocytes18,19, facilitating tumor immune escape and promotion of metastasis20. Given these contrasting roles of neutrophils and lymphocytes in the immune regulation of cancer cells, an elevated NLR may set up an optimal immune microenvironment to promote tumor dissemination, thereby resulting in poor patient outcomes.
Previous studies in lymphomas have consistently shown that high NLR at diagnosis is a prognostic of poorer outcomes. In nodular sclerosis Hodgkin lymphoma, NLR> 6 was associated with worse OS (HR 1.54, 95% CI 1.03 to 2.29, p = 0.034) and PFS (HR 1.65, 95% CI 1.25 to 2.18, p = 0.001)15; in B-cell lymphomas, NLR ≥ 3.5 was associated with worse OS and PFS16; in subtypes of PTCL other than NKTL, NLR ≥ 4 was associated with worse OS and PFS as well12. Furthermore, lymphopenia has been associated with a worse outcome in NKTL21,22. In one study on early-stage NKTL, PLR > 185 was found to be associated with worse OS (HR 1.77, 95% CI 1.10 to 2.87, p = 0.02)23. A recent study by Zhou et al. showed that both lymphopenia and an elevated derived NLR were prognostic for worse OS and PFS24, supporting our findings.
Our own results showed that high NLR, but not PLR or LMR, was independently associated with worse PFS and OS. Taken together, it appears that the NLR may be a reliable prognostic biomarker in lymphoma. The NABS prognostic model represents a cost-effective means to easily classify patients into distinct subgroups with significantly different prognosis, which can be used to develop risk-adapted treatment approaches for patients with NKTL.
Interestingly, our results in a small subset of patients showed that NKTL associated with an elevated peripheral blood NLR and poor patient outcomes demonstrated higher expression levels of DNA repair genes. Previously, Bald et al. showed that ultraviolet radiation-induced DNA damage leads to pro-metastatic neutrophilic inflammation, stimulating perivascular invasion and metastasis in melanomas25. Though preliminary, our findings of upregulated pro-proliferative pathways including the mitotic spindle assembly as well as mTORC126 further support a more aggressive phenotype in this group of NKTL.
Our current study does have certain limitations. Firstly, as a retrospective study, information on EBV status and beta 2-microglobulin levels were not collected for all patients, limiting the ability to take into these potential confounders, as they have been found to be independent predictors of poorer outcome in patients with NKTL10,27,28. Secondly, both patient cohorts in our study received heterogenous treatments (Table 2), which may have affected their prognosis. However, the observation that NLR and NABS score was consistently prognostic in both the Singaporean and Korean patients suggests that its prognostic impact is probably independent of treatment regimen. Nonetheless, future prospective studies would be necessary to confirm our findings, especially in a larger cohort treated with non-anthracycline and L-asparaginase containing regimens, the mainstay of current treatment.
Conclusions
In patients with NKTL, peripheral blood NLR at time of diagnosis is an inexpensive clinical prognostic marker that can be added to current prognostic models to refine risk stratification. The NABS score represents a new prognostic model for risk-stratification, although further validation in a prospective study of an independent cohort is required.
Ethics approval and consent to participate
Written consent for use of biospecimens and clinical data were obtained in accordance with the Declaration of Helsinki. Tissue collection and consent protocols were performed as part of the Singapore Lymphoma Study and were under approval from the SingHealth Centralized Institution Review Board as well as the Institutional Review Board of Samsung Medical Center.
Patients and Methods
Study cohort
Retrospective review of clinical data of patients with histologically-proven NKTL seen at the National Cancer Centre Singapore (NCCS), Singapore General Hospital (SGH) and Samsung Medical Center (SMC) from 1993 to August 2016 was performed. A total of 178 patients who had available pre-treatment peripheral blood neutrophil and lymphocyte counts at the time of diagnosis were included in the final analysis29. Those with ongoing active infections, concurrent hematological disorders or receipt of corticosteroids at the time of blood draw were excluded. Clinicopathological information available included age, sex, ethnicity, presence of B symptoms at diagnosis, ECOG performance scores, LDH levels and Ann Arbor stage. National Registration Identification Cards were used to verify patient demographics. All histological parameters were reviewed by expert haematolymphoid pathologists. Patient characteristics are summarized and stratified according to NLR in Table 1.
cDNA synthesis, gene expression profiling and gene set enrichment analysis
Informed consent for use of biospecimens were obtained in accordance with the Declaration of Helsinki. Total RNA was extracted using TRIzol (Invitrogen, Carlsbad, CA, USA) and purified with RNeasy Mini Kit (Qiagen, Hilden, Germany) according to manufacturer’s instructions. The integrity of RNA was determined by electrophoresis using the 2100 Bioanalyzer (Agilent Technologies, Palo Alto, CA, USA). Total RNA (500 ng) was reverse transcribed with iScript cDNA Synthesis Kit (Bio-Rad, Hercules, CA, USA). Eight available NKTL samples were analysed by RNA-seq to estimate the transcript abundance of each protein-coding gene. mRNA sequencing was performed on Illumina HiSeq2000 (Illumina, San Diego, CA, USA) using the standard Illumina RNA-seq protocol. The reads were aligned to the human genome hg19 assembly using STAR30. Transcript abundance estimation was performed using RSEM31. For each gene, read counts are represented as “transcripts per million” (TPM) and are normalized for both sequencing depth and gene length. Gene set enrichment analysis (GSEA) was performed using the Molecular Signatures Database (MSigDB) Hallmark gene set32. The permutation type was based on phenotype, using a total of 1000 iterations. A gene set is considered to be significantly enriched if its Normalized Enrichment Score (NES) has a False Discovery Rate (FDR) q-value below 0.25 and nominal p-value <0.001.
Statistical analysis
NLR and PLR were derived by dividing the absolute neutrophil counts and absolute platelet counts by the absolute lymphocyte counts, respectively, while the LMR was derived by dividing the absolute lymphocyte counts by the absolute monocyte counts. Normality of distribution of NLR, PLR and LMR were determined using the Kolmogorov-Smirnov test.
In previous studies, empirical cutoffs for NLR had been derived using heterogeneous methods33. These studies had not demonstrated any consistent or validated cut-off values, and were highly variable (ranging from 2.1 to 5.5). Given the lack of a discriminatory method to dichotomize NLR based on biological criteria34,35, we thus selected the receiver operative characteristic (ROC) curve analysis as the most objective statistical method for our study. ROC curve analysis via the method of DeLong et al.36 was then used to derive the optimal cut-off values for each parameter as a univariable predictor of overall survival (OS). The area under the curve (AUC) was 0.622 (95% CI 0.546 to 0.693). The sensitivity and specificity were 46.1% (95% CI 35.4% –57.0%) and 80.9% (95% CI 71.2% –88.5%) using a cut-off value of 3.5. The Hosmer-Lemeshow goodness-of-fit test indicated that the model was well-calibrated (chi-square 6.0144, p = 0.6456). To address the possibility of overfitting causing optimism regarding the model’s performance, we performed internal validation using a bootstrap validation algorithm with 1000 repetitions. The bootstrap validation obtained an AUC estimate of 0.620, indicating negligible overfitting of the model.
OS, the primary survival endpoint, was determined by the interval between date of diagnosis to the date of death from any cause; while progression-free survival (PFS), the secondary survival endpoint, was determined by the interval between date of diagnosis to date of relapse or date of death from any cause, whichever comes earliest. Survival was censored at the date of the last follow-up for survivors. Comparisons of the frequencies of categorical variables were performed using Pearson’s Chi-squared tests. Correlation analysis between continuous variables were evaluated by Spearman’s rho. Kaplan-Meier analyses were conducted to identify statistically significant univariable predictors of OS, and represented by hazard ratios (HR) and 95% confidence intervals (95% CI). Multivariate Cox regression model via a stepwise procedure was employed to determine independence of significant factors identified on univariable analysis. All statistical analyses were conducted assuming a two-sided test with significance level of 0.05 unless otherwise stated, and performed using MedCalc for Windows, version 18.2.1 (MedCalc Software, Ostend, Belgium).
Acknowledgements
The authors would like to thank all patients for their participation in this study. This work was supported by the National Medical Research Council of Singapore (TCR12DEC005), Tanoto Foundation Professorship in Medical Oncology, New Century Foundation Limited, Ling Foundation, Singapore National Cancer Centre Research Fund, SHF-Foundation, as well as the SingHealth Duke-NUS Academic Medical Centre and Oncology ACP.
Author contributions
K.M.T. and J.Y.C. analyzed the data and drafted the manuscript; C.L.C. and L.T. provided pathological assessment of tissues; D.M.C., Y.L., J.W.P., T.S. processed tissue and performed sequencing experiments; J.Q.L. and B.C. performed the bioinformatic analyses; S.J.K., W.S.K., L.P.K., T.T., E.P., N.S., M.T., D.H., J.T., M.F. and S.T.L. obtained patient samples and data; C.K.O. and J.Y.C. designed the study, interpreted the results, and revised the manuscript; and all authors read and approved the final version of the manuscript.
Data availability
All data generated or analysed during this study are included in this published article. The dataset analyzed is available from the corresponding author on appropriate request.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Choon Kiat Ong, Soon Thye Lim and Jason Yongsheng Chan.
Contributor Information
Choon Kiat Ong, Email: cmrock@nccs.com.sg.
Soon Thye Lim, Email: lim.soon.thye@singhealth.com.sg.
Jason Yongsheng Chan, Email: Jason.chan.y.s@nccs.com.sg.
References
- 1.William BM, Armitage JO. International analysis of the frequency and outcomes of NK/T-cell lymphomas. Best Pract Res Clin Haematol. 2013;26:23–32. doi: 10.1016/j.beha.2013.04.003. [DOI] [PubMed] [Google Scholar]
- 2.Chan JY, Lim ST. Novel findings from the Asian Lymphoma Study Group: focus on T and NK-cell lymphomas. Int J Hematol. 2018;107:413–419. doi: 10.1007/s12185-018-2406-6. [DOI] [PubMed] [Google Scholar]
- 3.Haverkos BM, et al. Extranodal NK/T Cell Lymphoma, Nasal Type (ENKTL-NT): An Update on Epidemiology, Clinical Presentation, and Natural History in North American and European Cases. Curr Hematol Malig Rep. 2016;11:514–527. doi: 10.1007/s11899-016-0355-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Au WY, et al. Clinical differences between nasal and extranasal natural killer/T-cell lymphoma: a study of 136 cases from the International Peripheral T-Cell Lymphoma Project. Blood. 2009;113:3931–3937. doi: 10.1182/blood-2008-10-185256. [DOI] [PubMed] [Google Scholar]
- 5.Pongpruttipan T, et al. Extranodal NK/T-cell lymphoma, nasal type, includes cases of natural killer cell and alphabeta, gammadelta, and alphabeta/gammadelta T-cell origin: a comprehensive clinicopathologic and phenotypic study. Am J Surg Pathol. 2012;36:481–499. doi: 10.1097/PAS.0b013e31824433d8. [DOI] [PubMed] [Google Scholar]
- 6.Koo GC, et al. Janus kinase 3-activating mutations identified in natural killer/T-cell lymphoma. Cancer Discov. 2012;2:591–597. doi: 10.1158/2159-8290.CD-12-0028. [DOI] [PubMed] [Google Scholar]
- 7.Kucuk C, et al. Activating mutations of STAT5B and STAT3 in lymphomas derived from gammadelta-T or NK cells. Nat Commun. 2015;6:6025. doi: 10.1038/ncomms7025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Song TL, et al. Oncogenic activation of the STAT3 pathway drives PD-L1 expression in natural killer/T-cell lymphoma. Blood. 2018;132:1146–1158. doi: 10.1182/blood-2018-01-829424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kwong YL, et al. SMILE for natural killer/T-cell lymphoma: analysis of safety and efficacy from the Asia Lymphoma Study Group. Blood. 2012;120:2973–2980. doi: 10.1182/blood-2012-05-431460. [DOI] [PubMed] [Google Scholar]
- 10.Kim SJ, et al. A prognostic index for natural killer cell lymphoma after non-anthracycline-based treatment: a multicentre, retrospective analysis. Lancet Oncol. 2016;17:389–400. doi: 10.1016/S1470-2045(15)00533-1. [DOI] [PubMed] [Google Scholar]
- 11.Templeton, A. J. et al. Prognostic role of neutrophil-to-lymphocyte ratio in solid tumors: a systematic review and meta-analysis. J Natl Cancer Inst106, 10.1093/jnci/dju124 (2014). [DOI] [PubMed]
- 12.Beltran BE, et al. The neutrophil-to-lymphocyte ratio is an independent prognostic factor in patients with peripheral T-cell lymphoma, unspecified. Leukemia & Lymphoma. 2016;57:58–62. doi: 10.3109/10428194.2015.1045897. [DOI] [PubMed] [Google Scholar]
- 13.Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002;420:860–867. doi: 10.1038/nature01322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 15.Marcheselli R, et al. Neutrophil-lymphocyte ratio at diagnosis is an independent prognostic factor in patients with nodular sclerosis Hodgkin lymphoma: results of a large multicenter study involving 990 patients. Hematol Oncol. 2017;35:561–566. doi: 10.1002/hon.2359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Porrata LF, et al. Predicting survival for diffuse large B-cell lymphoma patients using baseline neutrophil/lymphocyte ratio. Am J Hematol. 2010;85:896–899. doi: 10.1002/ajh.21849. [DOI] [PubMed] [Google Scholar]
- 17.Mantovani A, Allavena P, Sica A, Balkwill F. Cancer-related inflammation. Nature. 2008;454:436–444. doi: 10.1038/nature07205. [DOI] [PubMed] [Google Scholar]
- 18.Petrie HT, Klassen LW, Kay HD. Inhibition of human cytotoxic T lymphocyte activity in vitro by autologous peripheral blood granulocytes. J Immunol. 1985;134:230–234. [PubMed] [Google Scholar]
- 19.el-Hag A, Clark RA. Immunosuppression by activated human neutrophils. Dependence on the myeloperoxidase system. J Immunol. 1987;139:2406–2413. [PubMed] [Google Scholar]
- 20.Kitamura T, Qian BZ, Pollard JW. Immune cell promotion of metastasis. Nat Rev Immunol. 2015;15:73–86. doi: 10.1038/nri3789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li N, et al. Prognostic impact of absolute lymphocyte count/absolute monocyte count ratio and prognostic score in patients with nasal-type, extranodal natural killer/T-cell lymphoma. Tumour Biol. 2017;39:1010428317705503. doi: 10.1177/1010428317705503. [DOI] [PubMed] [Google Scholar]
- 22.Huang JJ, et al. Absolute lymphocyte count is a novel prognostic indicator in extranodal natural killer/T-cell lymphoma, nasal type. Ann Oncol. 2011;22:149–155. doi: 10.1093/annonc/mdq314. [DOI] [PubMed] [Google Scholar]
- 23.Wang KF, et al. A prognostic model based on pretreatment platelet lymphocyte ratio for stage IE/IIE upper aerodigestive tract extranodal NK/T cell lymphoma, nasal type. Med Oncol. 2014;31:318. doi: 10.1007/s12032-014-0318-8. [DOI] [PubMed] [Google Scholar]
- 24.Zhou X, et al. Prognostic significance of peripheral blood absolute lymphocyte count and derived neutrophil to lymphocyte ratio in patients with newly diagnosed extranodal natural killer/T-cell lymphoma. Cancer Manag Res. 2019;11:4243–4254. doi: 10.2147/CMAR.S193397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bald T, et al. Ultraviolet-radiation-induced inflammation promotes angiotropism and metastasis in melanoma. Nature. 2014;507:109–113. doi: 10.1038/nature13111. [DOI] [PubMed] [Google Scholar]
- 26.Karmaus PWF, et al. Critical roles of mTORC1 signaling and metabolic reprogramming for M-CSF-mediated myelopoiesis. J Exp Med. 2017;214:2629–2647. doi: 10.1084/jem.20161855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yoo C, et al. Prognostic impact of beta-2 microglobulin in patients with extranodal natural killer/T cell lymphoma. Ann Hematol. 2014;93:995–1000. doi: 10.1007/s00277-014-2015-2. [DOI] [PubMed] [Google Scholar]
- 28.Kim HS, et al. Whole blood Epstein-Barr virus DNA load as a diagnostic and prognostic surrogate: extranodal natural killer/T-cell lymphoma. Leuk Lymphoma. 2009;50:757–763. doi: 10.1080/10428190902803669. [DOI] [PubMed] [Google Scholar]
- 29.Tan KM, et al. Peripheral blood neutrophil-to-lymphocyte ratio in NK/T-cell lymphoma - prognostic significance and correlation with tumor transcriptomic profiles. Annals of Oncology. 2018;29:ix87–ix93. doi: 10.1093/annonc/mdy437. [DOI] [Google Scholar]
- 30.Dobin A, et al. STAR: ultrafast universal RNA-seq aligner. Bioinformatics. 2013;29:15–21. doi: 10.1093/bioinformatics/bts635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Li B, Dewey CN. RSEM: accurate transcript quantification from RNA-Seq data with or without a reference genome. BMC Bioinformatics. 2011;12:323. doi: 10.1186/1471-2105-12-323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liberzon A, et al. The Molecular Signatures Database (MSigDB) hallmark gene set collection. Cell Syst. 2015;1:417–425. doi: 10.1016/j.cels.2015.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mazumdar M, Glassman JR. Categorizing a prognostic variable: Review of methods, code for easy implementation and applications to decision-making about cancer treatments. Statistics in Medicine. 2000;19:113–132. doi: 10.1002/(SICI)1097-0258(20000115)19:1<113::AID-SIM245>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- 34.Søreide K. Receiver-operating characteristic curve analysis in diagnostic, prognostic and predictive biomarker research. J Clin Pathol. 2009;62:1–5. doi: 10.1136/jcp.2008.061010. [DOI] [PubMed] [Google Scholar]
- 35.Mu S, et al. Prognostic role of neutrophil-to-lymphocyte ratio in diffuse large B cell lymphoma patients: an updated dose-response meta-analysis. Cancer Cell Int. 2018;18:119. doi: 10.1186/s12935-018-0609-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.DeLong ER, DeLong DM, Clarke-Pearson DL. Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics. 1988;44:837–845. doi: 10.2307/2531595. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analysed during this study are included in this published article. The dataset analyzed is available from the corresponding author on appropriate request.