Key Points
Question
For adults with septic shock treated with norepinephrine, does use of selepressin, a selective vasopressin V1a receptor agonist, compared with placebo, improve patient outcome, defined as an increase in the number of days alive and free of both ventilation and vasopressor use?
Findings
In this randomized clinical trial that included 828 patients with septic shock requiring norepinephrine, treatment with selepressin compared with placebo resulted in 15.0 vs 14.4 ventilator- and vasopressor-free days within 30 days, a difference that was not statistically significant.
Meaning
Treatment with selepressin was not effective in improving ventilator- and vasopressor-free days.
Abstract
Importance
Norepinephrine, the first-line vasopressor for septic shock, is not always effective and has important catecholaminergic adverse effects. Selepressin, a selective vasopressin V1a receptor agonist, is a noncatecholaminergic vasopressor that may mitigate sepsis-induced vasodilatation, vascular leakage, and edema, with fewer adverse effects.
Objective
To test whether selepressin improves outcome in septic shock.
Design, Setting, and Participants
An adaptive phase 2b/3 randomized clinical trial comprising 2 parts that included adult patients (n = 868) with septic shock requiring more than 5 μg/min of norepinephrine. Part 1 used a Bayesian algorithm to adjust randomization probabilities to alternative selepressin dosing regimens and to trigger transition to part 2, which would compare the best-performing regimen with placebo. The trial was conducted between July 2015 and August 2017 in 63 hospitals in Belgium, Denmark, France, the Netherlands, and the United States, and follow-up was completed by May 2018.
Interventions
Random assignment to 1 of 3 dosing regimens of selepressin (starting infusion rates of 1.7, 2.5, and 3.5 ng/kg/min; n = 585) or to placebo (n = 283), all administered as continuous infusions titrated according to hemodynamic parameters.
Main Outcomes and Measures
Primary end point was ventilator- and vasopressor-free days within 30 days (deaths assigned zero days) of commencing study drug. Key secondary end points were 90-day mortality, kidney replacement therapy–free days, and ICU-free days.
Results
Among 868 randomized patients, 828 received study drug (mean age, 66.3 years; 341 [41.2%] women) and comprised the primary analysis cohort, of whom 562 received 1 of 3 selepressin regimens, 266 received placebo, and 817 (98.7%) completed the trial. The trial was stopped for futility at the end of part 1. Median study drug duration was 37.8 hours (IQR, 17.8-72.4). There were no significant differences in the primary end point (ventilator- and vasopressor-free days: 15.0 vs 14.5 in the selepressin and placebo groups; difference, 0.6 [95% CI, −1.3 to 2.4]; P = .30) or key secondary end points (90-day mortality, 40.6% vs 39.4%; difference, 1.1% [95% CI, −6.5% to 8.8%]; P = .77; kidney replacement therapy–free days: 18.5 vs 18.2; difference, 0.3 [95% CI, −2.1 to 2.6]; P = .85; ICU-free days: 12.6 vs 12.2; difference, 0.5 [95% CI, −1.2 to 2.2]; P = .41). Adverse event rates included cardiac arrhythmias (27.9% vs 25.2% of patients), cardiac ischemia (6.6% vs 5.6%), mesenteric ischemia (3.2% vs 2.6%), and peripheral ischemia (2.3% vs 2.3%).
Conclusions and Relevance
Among patients with septic shock receiving norepinephrine, administration of selepressin, compared with placebo, did not result in improvement in vasopressor- and ventilator-free days within 30 days. Further research would be needed to evaluate the potential role of selepressin for other patient-centered outcomes in septic shock.
Trial Registration
ClinicalTrials.gov Identifier: NCT02508649
This phase 2b/3 randomized clinical trial compares the effects of selepressin, a selective vasopressin V1a receptor agonist and noncatecholaminergic vasopressor, vs placebo on ventilator- and vasopressor-free days within 30 days among adult patients with septic shock receiving norepinephrine.
Introduction
Sepsis is a dysregulated host response to infection that leads to life-threatening acute organ dysfunction. Septic shock is the most severe form, characterized by vasodilatation and increased capillary permeability leading to hypotension and tissue hypoxia.1,2 Treatment includes antibiotics and resuscitation with intravenous fluids, vasopressors, and organ support. Even with prompt care, many patients develop multiple organ failure and death.1,2 The recommended first-line vasopressor is the catecholamine, norepinephrine.3 Norepinephrine is not always effective at restoring blood pressure and can cause myocardial and peripheral ischemia.4 Consequently, there is interest in alternative vasopressors, including noncatecholaminergic agents.
One such agent, vasopressin, induces vasoconstriction via V1 receptor stimulation in vascular smooth muscle. However, vasopressin has pleiotropic effects, not all beneficial in septic shock, because it also stimulates V1b and V2 receptors, resulting in increased procoagulant factors, salt and water retention, nitric oxide release, and corticosteroid stimulation.5,6 Selepressin is a selective vasopressin V1a receptor agonist,7 potentially mitigating sepsis-induced vasodilatation, vascular leakage, and tissue edema without V1b- or V2-mediated effects.8,9,10,11,12,13 These effects could be beneficial in septic shock, in which increased capillary permeability may contribute to poor outcomes via pulmonary and other vital-tissue edema, intravascular volume depletion, and impaired oxygen delivery. In a phase 2a trial in patients with septic shock, selepressin reduced norepinephrine requirements, increased the proportion of patients not receiving mechanical ventilation, and appeared to speed resolution of shock.14 These findings led to the current phase 2b/3 trial of selepressin in adult patients with vasopressor-dependent septic shock.
Methods
Trial Design and Oversight
The trial was conducted under a Special Protocol Assessment agreement with the US Food and Drug Administration and approved by relevant regulatory and ethics authorities, including institutional review boards or equivalent. The study was conducted in accordance with Good Clinical Practice guidelines, local regulations, and the ethical principles described in the Declaration of Helsinki. Written informed consent was obtained for all patients or their surrogates in accordance with local legislation.
The study was a blinded, randomized, placebo-controlled, seamless, phase 2b/3 adaptive clinical trial designed to determine the efficacy of multiple dosing regimens of selepressin and to confirm the efficacy of 1 dosing regimen in the treatment of septic shock (see study protocol and statistical analysis plan in Supplement 1). The design, published previously, had 2 parts integrated under an overarching Bayesian framework (study protocol, Supplement 1; eFigure 1 in Supplement 2), with an automatic, or “seamless,” transition from the first to the second part.15 Like a traditional phase 2b trial, part 1 tested several doses. However, prespecified response-adaptive rules adjusted the number and size of study groups, ensuring adequate power to select the best dosing strategy and transition to part 2.15 Part 2 would be triggered only if the effect size of the best-performing dosing regimen in part 1 exceeded a prespecified threshold and would consist of 1:1 fixed allocation to the optimal regimen or placebo. Unlike a traditional design, treatment effect would be estimated using all patients from both parts.
The trial was overseen by a blinded trial steering committee (TSC) and an independent, unblinded data and safety monitoring board (DSMB) (Supplement 2). The TSC oversaw trial conduct and made recommendations to the sponsor regarding all trial-related decisions. The DSMB oversaw safety and performance of the adaptive design.
Study Population
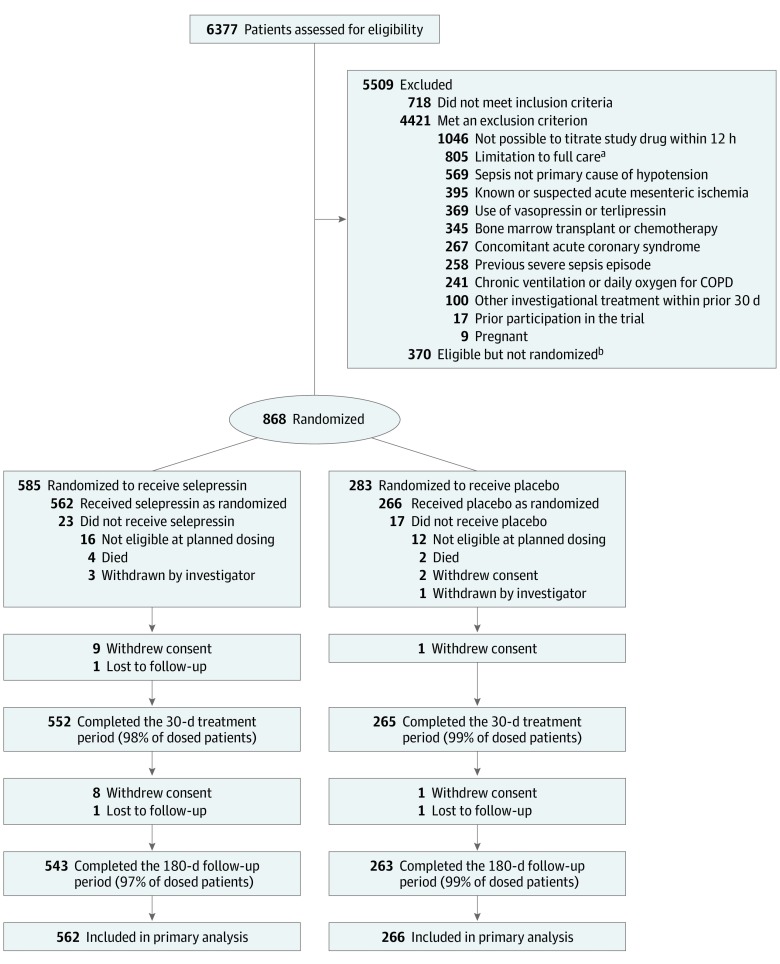
Eligible patients were 18 years or older, had proven or suspected infection, and had septic shock defined as hypotension (systolic arterial pressure <90 mm Hg or mean arterial pressure [MAP] <65 mm Hg) requiring more than 5 μg/min of norepinephrine for more than 1 hour despite more than 1 L of intravenous fluid resuscitation. Patients were excluded if they were receiving vasopressin or terlipressin, if it was not possible to start study drug within 12 hours of the onset of any use of vasopressor treatment (discounting use during surgery or other procedures if the vasopressor was completely weaned before use for sepsis-induced hypotention), or if sepsis was not the primary cause of hypotension (Figure 1) (see Supplement 2 for further entry criteria details). Two coordinating centers (United States and Belgium) reviewed data with sites before each enrollment to confirm eligibility (Supplement 2).
Figure 1. Screening, Randomization, and Follow-up of a Study of Selepressin for Septic Shock.
Patient disposition from screening through day 180 is shown. Efficacy and adverse event analyses are based on all patients who underwent randomization and received selepressin or placebo. Just before dosing, all randomized patients had to fulfill 3 predefined criteria to be eligible for receiving study drug: (1) had received a minimum of 30 mL/kg fluid in total from onset of hypotension (or less if fluid replete/overload); (2) still receiving a continuous infusion of 5 µg/min or more of norepinephrine and having done so for at least 1 hour; and (3) less than 12 hours had passed from start of vasopressor treatment for sepsis. COPD indicates chronic obstructive pulmonary disease.
aLimitations to full care included any decision to limit care, including lack of commitment to cardiopulmonary resuscitation, blood products, kidney replacement therapy, surgery to ensure source control, or mechanical ventilation if indicated.
bReasons for failure to enroll despite meeting eligibility criteria included lack of availability of study personnel or study drug and difficulty communicating with the coordinating center.
Study Interventions
Study drug was a continuous intravenous infusion of selepressin or matching placebo started within 12 hours of shock onset. Titration of study drug and other vasopressors was guided by an instruction protocol (study protocol, Supplement 1; eFigure 2, Supplement 2) based on pharmacokinetic modeling and prior clinical experience.14,15 Study drug infusion began at a rate based on patient weight. The protocol instructed the bedside clinician to maintain target MAP while weaning other vasopressors. If target MAP could not be maintained or vasopressors could not be weaned, the study drug infusion rate could be increased by up to 50%.
Study drug was weaned after all other vasopressors were discontinued and MAP was at or above target but could be restarted for sepsis-induced hypotension. The study began with 3 selepressin dosing regimen groups, defined as starting infusion rates of 1.7, 2.5, and 3.5 ng/kg/min, with the potential to add a fourth group (5 ng/kg/min). Doses were selected based on analysis of the prior feasibility study (https://clinicaltrials.gov/ct2/show/NCT01612676). Weight was assessed as per local clinical practice. Data collected on the management of shock included dose of study drug, MAP, vasopressor use, cardiovascular Sequential Organ Failure Assessment (SOFA) score16 (including study drug as a vasopressor), fluid balance, and initiation of steroids for septic shock. Weaning from mechanical ventilation was based on daily trials of spontaneous breathing, as per best practice guidelines. Other care was at the discretion of the clinical team.
Randomization
Randomization was stratified by study site, need for mechanical ventilation, baseline norepinephrine requirement, and serum creatinine level. Investigators, study personnel, and the clinical team were blinded to allocation. The first 200 patients were randomized in a fixed ratio of one-third to placebo and the remainder allocated evenly among the 3 lowest doses of the 4 possible selepressin groups (eFigure 1 in Supplement 2). Thereafter, the probability of randomization to placebo was held constant while a response-adaptive randomization algorithm determined probabilities of allocation to the selepressin groups. Probability of assignment to each of the 3 lower-dose groups was proportional to the probability that that group was most beneficial based on monthly updates of the accruing data. The fourth selepressin group (5 ng/kg/min) would only be opened if the accruing data demonstrated a 50% or greater probability that the 3.5 ng/kg/min group was superior to the 2.5 ng/kg/min group, with no safety concerns.
The study drug was prepared at the hospital by pharmacy staff not associated with the bedside clinical team. The information for treatment allocation was provided by a password-protected internet-based platform. The study drug, either placebo or selepressin, was diluted in sterile saline in identical syringe or bags blinded to treatment allocation. The label contained only the patient identification and trial name and number. Selepressin is colorless and odorless when dissolved.
End Points
The primary end point was the number of ventilator- and vasopressor-free days up to day 30, defined as days from study drug initiation to 30 days thereafter, during which the patient was alive, free of mechanical ventilation, and free of treatment with intravenous vasopressors including study drug. Any patient who died within 30 days was assigned zero days. After mechanical ventilation and vasopressors were weaned, if either were restarted before day 30 for more than 60 minutes within a 24-hour period, the intervening days were not counted as being free of ventilator or vasopressor support. Use of mechanical ventilation or vasopressors during and up to 3 hours after surgery or bedside procedure (eg, hemodialysis session) was exempt.
Vasopressor therapy was defined as any intravenous dose of norepinephrine, phenylephrine, dopamine, epinephrine, vasopressin, terlipressin, and study drug (selepressin or placebo). Mechanical ventilation was defined as invasive mechanical ventilation via endotracheal tube (including tracheostomy) or noninvasive ventilation with more than 5 cm H2O of continuous positive airway pressure and more than 5 cm H2O of pressure support when deployed to avoid intubation. Other uses of noninvasive ventilation (eg, chronic nighttime use for chronic obstructive pulmonary disease) were not counted.
There were 3 key secondary end points: 90-day all-cause mortality, 30-day kidney replacement therapy–free days, and 30-day intensive care unit (ICU)–free days. Additional prespecified end points are provided in the study protocol (Supplement 1) and Supplement 2 and included organ dysfunction (assessed by the daily SOFA score [excluding the neurologic component] while in the ICU and by need for vasopressors, mechanical ventilation, or kidney replacement therapy, analyzed as “free days,” duration, and onset of new dysfunction or failure); ICU length of stay; all-cause mortality at 30 and 180 days; daily and cumulative fluid balance and urine output while in the ICU, and health-related quality of life at 180 days using the EuroQol-5D 5-level questionnaire.17
Safety end points included incidence of adverse events with emphasis on ischemic events, hypotension, and unanticipated changes in vital signs and laboratory values. Additional prespecified end points included hospital-free days and length of stay, patient residence up to 180 days, a health economic evaluation that would be reported separately, and additional measures of the course and management of shock (MAP and vasopressor use). Several substudies of molecular and physiologic end points were planned had the study progressed further (Supplement 2).
All staff collecting outcome data were blinded to treatment allocation.
Statistical Analysis
The statistical analysis plan is detailed in the study protocol (Supplement 1). The design allowed a maximum final sample size of 1800 evaluable patients across parts 1 and 2, which provided 91% power to determine that selepressin yielded a gain of 1.5 ventilator- and vasopressor-free days compared with placebo, with a 1-tailed α error rate of .02.15 Ventilator- and vasopressor-free days is a new end point, for which a meaningful clinical difference has not been established. We chose a 1.5-day difference because that represented a 7.5% to 15% relative change assuming a control rate of between 10 and 20 ventilator- and vasopressor-free days, based on recent sepsis randomized clinical trials.18,19,20,21 We assumed smaller effects would not be considered clinically significant.
During part 1, a prespecified Bayesian inference model was used to generate monthly updated probabilities of treatment success for each selepressin group, in which probability of treatment success was the probability that a selepressin group, if tested in part 2, would be statistically superior to the placebo group by the end of the trial. If, after 200 or more patients, all selepressin groups had less than 5% probability of treatment success, the trial would stop for futility. If, with 300 or more patients, any group had more than 90% probability of treatment success, the trial transitioned to part 2. Otherwise, part 1 would continue to 800 patients at which point, if at least 1 selepressin group had more than 25% probability of treatment success, the trial would transition to part 2. Otherwise, the trial would terminate and be reported as a phase 2b trial.
All analyses included all patients who were randomized and received study drug. The primary analysis compared ventilator- and vasopressor-free days between the combined selepressin groups and placebo by the nonparametric van Elteren test,22 stratified by need for mechanical ventilation, time from onset of shock to start of study drug, and baseline norepinephrine requirement, assuming a 2-sided 5% significance level.23 Missing data during hospitalization for the primary end point were imputed using a worst-case approach. Missing data for patients withdrawn were imputed using last observed status receiving or not receiving vasopressors or mechanical ventilation carried forward to day 30. Prespecified sensitivity analyses using alternative strategies for missingness included exclusion of all patients with missing primary outcome data; use of the ratio of free to nonfree days at last observation instead of last status carried forward; and a tipping-point analysis exploring all possible combinations of “best-case” and “worst-case” scenarios (see statistical analysis plan, version 9.0, section 9.2.2, in Supplement 1). Potential heterogeneity of treatment effect was explored through prespecified subgroup analyses and analysis of treatment effect by risk of death (see statistical analysis plan in Supplement 1).
Secondary outcomes were analyzed as follows: other “free day” end points were analyzed similarly to the primary end point using the van Elteren test, continuous nonrepeated data were analyzed by negative binomial or permutation tests, continuous repeated data by repeated-measures analysis of covariance, binomial data by logistic regression, and survival-type data by Kaplan-Meier log rank. To safeguard against erroneous type I error inflation, the Hochberg procedure for adjustment on multiplicity was applied to the 3 key secondary outcomes. Because of the potential for type I error due to multiple comparisons, findings for analyses of the other secondary end points should be interpreted as exploratory.
All analyses were conducted in SAS Life Science Analytics Framework, version 4.7.1.
Results
Patients
Of 6377 screened patients, 868 were enrolled, 585 were randomly assigned to receive selepressin, and 283 to receive placebo. Forty patients did not receive study drug because of improvement in shock (n = 28), withdrawal by investigator because of safety concerns (n = 4), death (n = 6), or withdrawal of consent (n = 2). The remaining 828 comprised the analysis cohort (Figure 1). The study groups had features typical of septic shock and were well-matched at baseline (Table 1; eTable 1 in Supplement 2). Data were missing for 17 patients (2%), including 11 patients who subsequently withdrew consent or were lost to follow-up before day 30 (Figure 1) and 6 additional patients for whom the last date, but not the time, was recorded for either ventilation or vasopressor use. Overall withdrawal or loss to follow-up rates did not differ by study group (P = .97 by log-rank test). There were 17 response-adaptive randomization updates, during which the fourth selepressin group was never triggered and no study group triggered transition to part 2. Therefore, based on recommendation from the DSMB, the TSC stopped the trial for futility at the end of part 1.
Table 1. Demographic and Baseline Disease Characteristics.
| Characteristic | Selepressin (n = 562) | Placebo (n = 266) |
|---|---|---|
| Demographics | ||
| Age, mean (SD), y | 66.6 (12.8) | 65.7 (14.6) |
| Sex, No. (%) | ||
| Men | 342 (61) | 145 (55) |
| Women | 220 (39) | 121 (45) |
| Medical patients, No. (%) | 382 (68) | 204 (77) |
| Weight, mean (SD), kg | 80.2 (23.6) | 78.7 (22.2) |
| Body mass index, mean (SD)a | 27.9 (8.4) [n = 559] | 27.5 (7.2) [n = 265] |
| Severity of illness | ||
| Modified SOFA, median (IQR)b | 9 (8-11) [n = 543] | 9 (8-11) [n=258] |
| APACHE II, mean (SD)c | 25.7 (7.8) [n = 559] | 26.0 (7.7) |
| Heart rate, mean (SD), /min | 102 (23) | 101 (23) |
| Mean arterial pressure, median (IQR), mm Hg | 70 (65-76) [n = 561] | 69 (64-76) |
| Norepinephrine, median (IQR), μg/kg/min | 0.25 (0.14-0.45) [n = 561] | 0.26 (0.15-0.44) [n=265] |
| Lactate, median (IQR), mmol/L | 2.7 (1.6-4.5) [n = 526] | 2.6 (1.7-4.2) [n =255] |
| Pao2:Fio2, mean (SD), mm Hg | 221 (118) [n = 514] | 230 (141) [n = 248] |
| Hours of vasopressor therapyd | 8.2 (2.8) | 8.2 (3.0) |
| Site of infection, No. (%) | ||
| Lower respiratory tract | 222 (40) | 99 (37) |
| Intra-abdominal | 159 (28) | 63 (24) |
| Urinary tract | 82 (15) | 51 (19) |
| Skin or soft tissue | 45 (8) | 22 (8) |
| Other | 54 (10) | 31 (12) |
| Preexisting conditions, No. (%) | ||
| Cardiovascular | 332 (59) | 151 (57) |
| Cancer | 182 (32) | 70 (26) |
| Diabetes mellitus | 139 (25) | 67 (25) |
| COPD | 114 (20) | 41 (15) |
| Kidney disease | 93 (17) | 41 (15) |
| Liver cirrhosis | 43 (8) | 23 (9) |
| Charlson Comorbidity Index, mean (SD)e | 2.5 (2.0) [n = 477] | 2.5 (2.1) [n=223] |
Abbreviations: APACHE, Acute Physiologic Assessment and Chronic Health Evaluation; COPD, chronic obstructive pulmonary disease; Fio2, fraction of inspired oxygen; IQR, interquartile range; SOFA, Sequential Organ Failure Assessment.
Calculated as weight in kilograms divided by height in meters squared.
Denotes the SOFA modified to not include Glasgow Coma Scale (range, 0-16, with higher scores indicating more severe or widespread organ dysfunction). For the purpose of the trial, study drug, vasopressin, terlipressin, and phenylephrine attributed 3 points on the cardiovascular scale, and any dose of the positive inotropes milrinone and levosimendan attributed 2 points on the cardiovascular scale.
Scores range from 0 to 71, with higher scores indicating greater severity of illness. A score of approximately 26 for patients with sepsis is typically associated with a short-term mortality rate of 30% to 40%, depending on reason for admission.
Duration of vasopressor treatment administered for sepsis when study drug was initiated.
Measures the effect of coexisting conditions on mortality, with scores ranging from 0 to 29 and higher scores indicating a greater burden of illness. Age was not included in the calculation.
Efficacy End Points
The primary end point of ventilator- and vasopressor-free days did not differ significantly between the combined selepressin and placebo groups (mean, 15.0 vs 14.5 days; difference, 0.6 days [95% CI, −1.3 to 2.4]; P = .30) (Figure 2, Table 2; and eTable 2 in Supplement 2). Sensitivity analyses excluding missing or imputed data and using the alternative imputation rule were similar (P = .31 and P = .37), and the tipping-point analysis similarly showed that the results did not change significance under different assumptions about missingness. Subgroup analyses and analysis of treatment effect by deciles of risk of death showed no evidence of heterogeneity of treatment effect (eFigures 3 and 4 in Supplement 2).
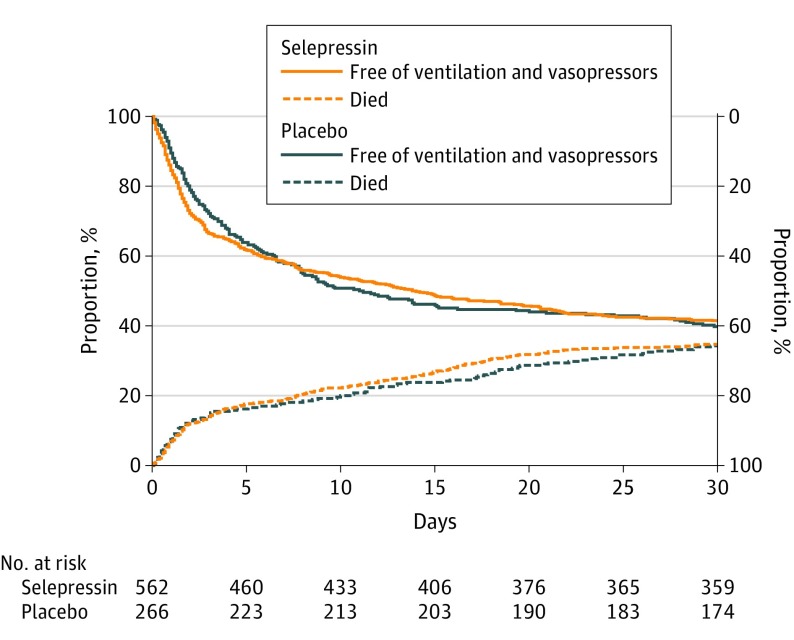
Figure 2. Days Alive and Free of Ventilation and Vasopressors.
Death and days free of ventilation and vasopressors shown as proportion of patients over time from randomization to day 30. Area above the top 2 curves corresponds to alive and free of ventilation and vasopressors; area between the top 2 curves and bottom 2 curves, alive but still receiving ventilation or vasopressors; area below bottom 2 curves, dead. The combined ventilator- and vasopressor-free days did not differ between groups (15.0 vs 14.4 days; difference, 0.6 days [95% CI, −1.3 to 2.4]; P = .30).
Table 2. Major End Pointsa.
| End Point | Unadjusted (Observed) | Adjusted (Model) | ||||||
|---|---|---|---|---|---|---|---|---|
| Selepressin | Placebo | (95% CI) | Difference (95% CI) | P Value | ||||
| No. | Mean (SD) | No. | Mean (SD) | Selepressin (n = 562) | Placebo (n = 266) | |||
| Primary End Point | ||||||||
| Ventilator- and vasopressor-free days to day 30 | 562 | 14.2 (13.1) | 266 | 14.3 (12.9) | 15.0 (13.8 to 16.2) | 14.5 (12.8 to 16.1) | 0.55 (−1.3 to 2.4) | .30 |
| Prespecified Key Secondary End Points | ||||||||
| Day 90 mortality, No. (%) | 227 (41.7) | 109 (41.1) | 40.6 | 39.4 | 1.1 [−6.5 to 8.8] | .77 | ||
| Kidney replacement therapy–free days, day 1 to day 30 | 550 | 18.0 (14.3) | 261 | 18.4 (13.9) | 18.5 (17.0 to 20.0) | 18.2 (16.1 to 20.3) | 0.29 (−2.1 to 2.6) | .85 |
| Intensive care unit–free days, day 1 to day 30 | 562 | 11.8 (11.9) | 266 | 11.7 (11.6) | 12.6 (11.5 to 13.8) | 12.2 (10.7 to 13.6) | 0.49 (−1.2 to 2.2) | .41 |
| Other Prespecified Secondary End Points | ||||||||
| Components of primary end point | ||||||||
| Days free from ventilator and vasopressors, survivors onlyb | 369 | 21.6 (10.0) | 175 | 21.8 (9.4) | 23.2 (22.2 to 24.0) | 22.2 (20.7 to 23.4) | 0.96 (−0.55 to 2.48) | NAc |
| Days free from vasopressors, survivors onlyb | 369 | 25.1 (7.2) | 175 | 25.9 (5.5) | 25.3 (24.6 to 25.9) | 25.7 (24.9 to 26.4) | −0.46 (−1.37 to 0.45) | NAc |
| Days free from ventilatorb, survivors only | 369 | 22.6 (10.1) | 175 | 22.8 (9.7) | 24.8 (23.3 to 25.9) | 23.4 (20.8 to 25.2) | 1.41 (−0.96 to 3.79) | NAc |
| Day 30 mortality, No. (%) | 193 (35.0) | 91 (34.3) | 33.6 | 32.7 | 0.9 (−6.4 to 8.2) | .81 | ||
| Day 180 mortality, No. (%) | 241 (44.4) | 116 (44.1) | 43.8 | 42.8 | 1.0 (−6.7 to 8.8) | .79 | ||
| Other free day-1 end points | ||||||||
| Vasopressor-free days to day 30 | 562 | 16.5 (13.3) | 266 | 17.0 (13.1) | 16.4 (15.1 to 17.6) | 16.8 (15.1 to 18.4) | −0.39 (−2.3 to 1.5) | .39 |
| Ventilator-free days to day 30 | 562 | 14.8 (13.5) | 266 | 15.0 (13.8) | 16.1 (14.6 to 17.5) | 15.2 (13.2 to 17.3) | 0.83 (−1.5 to 3.1) | .76 |
| Hospital-free days to day 90 | 562 | 29.8 (33.0) | 266 | 29.2 (32.6) | 30.1 (26.8 to 33.3) | 29.8 (25.4 to 34.1) | 0.32 (−4.7 to 5.3) | .67 |
| Duration of therapy, d | ||||||||
| Vasopressor to day 30 | 562 | 3.1 (4.2) | 266 | 3.4 (3.7) | 3.0 (2.7 to 3.3) | 3.3 (2.9 to 3.7) | −0.3 (−0.8 to 0.2) | .21 |
| Mechanical ventilation to day 30 | 562 | 5.9 (8.2) | 266 | 6.1 (8.1) | 6.4 (5.6 to 7.2) | 6.9 (5.7 to 8.0) | −0.48 (−1.8 to 0.8) | .67 |
| Kidney replacement therapy to day 90 | 550 | 2.0 (7.6) | 261 | 1.6 (5.5)) | 1.9 (1.3 to 2.6) | 1.6 (1.0 to 2.3) | 0.33 (−0.5 to 1.2) | .61 |
| ICU length of stay to day 30 | 562 | 9.6 (9.1) | 266 | 10.3 (9.0) | 9.6 (8.9 to 10.3) | 10.3 (9.3 to 11.4) | −0.63 (−2.1 to 0.3) | .23 |
| Hospital length of stay to day 90 | 562 | 25.4 (24.0) | 266 | 26.5 (24.1) | 25.4 (23.4 to 27.1) | 26.5 (24.0:29.6) | −1.1 (−4.9 to 2.3) | .36 |
| Quality of life at 6 mo | ||||||||
| Visual analog scaled | 259 | 69.5 (20.1) | 129 | 72.6 (20.1) | 68.9 (66.4 to 71.3) | 72.3 (68.9 to 75.7) | −3.5 (−7.7 to 0.7) | .10 |
| EQ-5D 5L index scoree | 264 | 0.75 (0.21) | 130 | 0.76 (0.22) | 0.74 (0.71 to 0.77) | 0.76 (0.72 to 0.80) | −0.02 (−0-07 to 0.03) | .48 |
Abbreviations: EQ-5D 5L, 5-level EuroQol-5D; ICU, intensive care unit; NA, not applicable.
The primary analyses for all efficacy end points are generated from statistical models as described in the study protocol in Supplement 1. There were no statistical differences in findings between adjusted and raw observed results. “Free days” reflect the time from end of last use of therapy to end of the period (30 or 90 days), with a higher number indicating a longer period free of therapy. Patients dying in the period are assigned zero free days.
“Days liberated from therapy” is similar to “free days” but without a penalty for mortality.
Subcomponents of the primary outcome are provided for illustrative purposes and are not accompanied by P values.
A patient-reported outcome with 100 representing the best thinkable health status and zero representing the worst.
Reflects the patient-reported level of physical and mental well-being.
There were also no significant differences in the 3 key secondary outcomes (90-day mortality: 40.6% vs 39.4%; difference, 1.1% [95% CI, −6.5% to 8.8%]; P = .77; kidney replacement therapy–free days: 18.5 vs 18.2; difference, 0.29 [95% CI, −2.1 to 2.6]; P = .85; ICU-free days: 12.6 vs 12.2; difference, 0.49 [95% CI, −1.2 to 2.2]; P = .41) (Table 2). There were also no significant differences in the subcomponents of the primary end point (Figure 2 and Table 2). The coagulation SOFA score was significantly higher in the selepressin group on ICU days 3 (mean, 1.73 vs 1.50; difference, 0.23 [95% CI, 0.04 to 0.41]; P = .02) and 7 (1.53 vs 1.30; difference, 0.23 [95% CI, 0.04 to 0.43]; P = .02), as was the frequency of new-onset coagulation abnormalities in the first week (62.3% vs 53.3%; odds ratio [OR], 1.45 [95% CI, 1.06 to 2.00]; P = .02), although there were no significant differences by day 30 (67.3% vs 61.1%; OR, 1.31 [95% CI, 0.95 to 1.80]; P = .10). Otherwise, organ failure, organ support, ICU and hospital length of stay, mortality, and quality-of-life end points did not differ significantly (Table 2; eFigures 5-8 and eTable 3 in Supplement 2).
Adverse Events
Adverse events are summarized in Table 3 and in eFigures 9 and 10 in Supplement 2. There were 3140 reported adverse events during the 30-day treatment period, including 275 suspected adverse drug reactions and 630 serious adverse events. Event rates were similar in both groups, including specific adverse events, such as arrhythmias, hypotension, ischemic events, and laboratory tests required for safety monitoring, including platelet count, serum creatinine level, and serum troponin level.
Table 3. Adverse Events.
| Event | No. (%) | |
|---|---|---|
| Selepressin (n = 562) | Placebo (n = 266) | |
| Adverse events of any grade | 2054 (83.8) | 1086 (88.3) |
| Adverse study drug reactions | 190 (21.0) | 85 (21.4) |
| Treatment-emergent adverse eventsa | 1029 (65.7) | 573 (73.7) |
| Adverse events of special interestb,c | 301 (42.3) | 123 (37.2) |
| Cardiac arrhythmias | 209 (27.9) | 87 (25.2) |
| Cardiac ischemia | 44 (6.6) | 17 (5.6) |
| Mesenteric ischemia | 20 (3.2) | 7 (2.6) |
| Stroke and cerebrovascular events | 14 (2.3) | 5 (1.5) |
| Peripheral ischemia | 14 (2.3) | 7 (2.3) |
| Serious adverse eventsc | 430 (51.2) | 200 (53.4) |
| Adverse events leading to discontinuation of study drugc | 44 (7.3) | 12 (4.5) |
Denote adverse events with onset during or within 12 hours of stop of study drug.
Prespecified event types monitored and collected more systematically. More details on adverse events are presented in eFigures 9 and 10 in Supplement 2.
The study was not powered to test for differences in adverse event rates.
Administration of Study Drug and Management of Shock
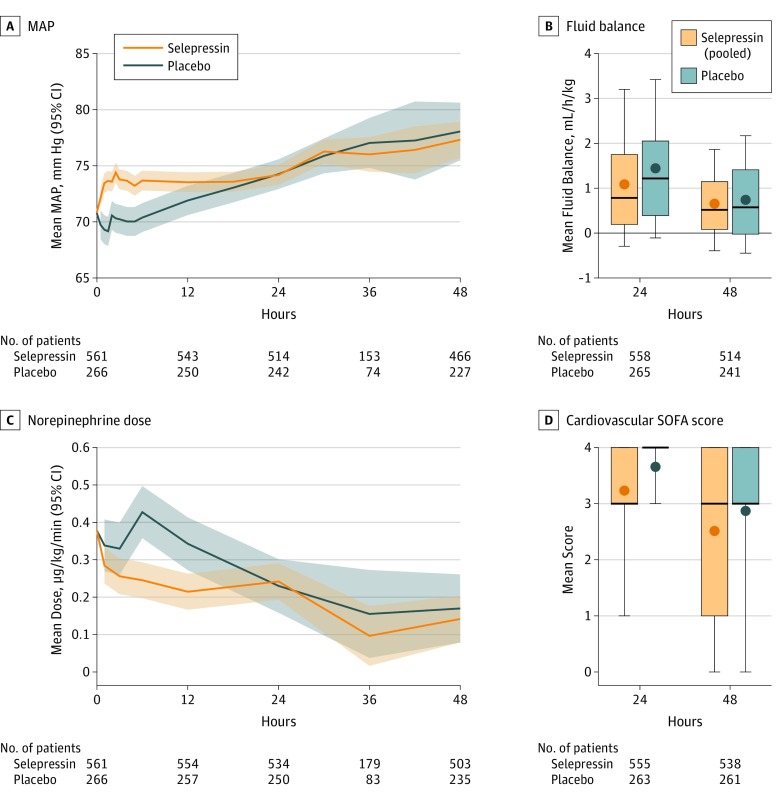
Study drug was administered for a median of 37.8 hours (interquartile range, 17.8-72.4) (eFigure 11 in Supplement 2). During infusion, there were several significant differences in the course of shock and patient care (Figure 3). Over the first 6 hours, the selepressin group had higher MAP (mean, 74 vs 70 mm Hg; difference, 3.3 mm Hg [95% CI, 1.7 to 5.0]; P < .001) (Figure 3A) and lower norepinephrine requirement (mean, 0.29 vs 0.48 μg/kg/min; absolute difference, −0.19 μg/kg/min [95% CI, −0.29 to −0.10], a 41% reduction vs 8% increase from baseline; P < .001) (Figure 3C), with norepinephrine requirements inversely correlated with the selepressin dosing regimen (eFigures 12 and 13 in Supplement 2). Over the first 2 days, the selepressin group had significantly less cardiovascular dysfunction (mean cardiovascular SOFA score, 2.5 vs 2.9; difference, −0.33 [95% CI, −0.55 to −0.11]; P = .003) (Figure 3D). Patients receiving selepressin had significantly higher urine output (100 vs 86 mL/h; difference, 14 mL/h [95% CI, 4 to 23]; P = .006) and significantly lower net fluid balance (81 vs 107 mL/h; difference, −26 mL/h [95% CI, −38 to −14]; P < .001) than those receiving placebo in the first 24 hours, but the groups were similar thereafter (Figure 3B; eFigures 14 and 15 in Supplement 2). The selepressin group was significantly less likely to receive steroids than the placebo group (13.7% vs 22.9%; OR, 0.53 [95% CI, 0.37 to 0.78]; P = .001).
Figure 3. Management of Shock During the First 48 Hours.
A, Mean (95% CI) mean arterial pressure (MAP), adjusted for baseline values. B, Mean fluid balance adjusted for body weight and for baseline values. Bottom and top margins of boxes indicate 25th and 75th quartiles, respectively; heavy lines within boxes, median values; dots within boxes, adjusted mean values; whiskers, 10th and 90th quartiles. Fluid balance was defined as intravenous fluid minus urine output, derived by assigning charted data into 24-hour intervals after commencement of study drug and analyzed by repeated-measures analysis of covariance (statistical analysis plan, version 9.0, section 9.3.4, in Supplement 1). The fluid balance differed between groups at 24 hours (81 vs 107 mL/h; difference, −26 mL/h [95% CI, −38 to −14]; P < .001) but not at 48 hours. C, Mean (95% CI) norepinephrine dose, adjusted for baseline values. D, Mean cardiovascular Sequential Organ Failure Assessment (SOFA) score. Bottom and top margins of boxes indicate 25th and 75th quartiles, respectively; heavy lines within boxes, median values; dots within boxes, adjusted mean values; whiskers, 10th and 90th quartiles. The selepressin group had significantly less cardiovascular dysfunction at 24 hours (mean cardiovascular SOFA score, 3.2 vs 3.7; difference, −0.42 [95% CI, −0.65 to −0.19]; P < .001) and at 48 hours (mean cardiovascular SOFA score, 2.5 vs 2.9; difference, −0.33 [95% CI, −0.55 to −0.11]; P = .003). All comparisons were prespecified secondary analyses.
Discussion
In this multicenter trial that compared 3 dosing regimens of selepressin, a selective vasopressin V1a receptor agonist, with placebo in the treatment of patients with septic shock, there was no evidence that selepressin offered any benefit on either the primary end point of ventilator- and vasopressor-free days at 30 days or other patient-centered outcomes, including 90-day mortality, kidney replacement therapy–free days, and ICU-free days. These findings were consistent across subgroup and secondary analyses.
Recently, the US Food and Drug Administration approved 2 noncatecholaminergic vasopressors for use in septic or vasodilatory shock: vasopressin and angiotensin II. Vasopressin has strong vasopressor activity, allowing reduction in norepinephrine use but without improvement in patient-centered outcomes, except perhaps in select subgroups.24 Angiotensin II provides short-term improvement in cardiovascular stability without significant improvement in patient-centered outcomes.25 Thus, selepressin, vasopressin, and angiotensin II all appear to have norepinephrine-sparing effects without an increase in adverse events but also without evidence that their use improves patient-centered outcomes compared with a catecholamine-based strategy. However, the present trial was designed to compare dosing regimens of selepressin with placebo, rather than with other nonadrenergic vasoactive drugs, and none of these 3 agents have been compared with each other.
To evaluate selepressin, the trial design had several novel features, including the combined organ dysfunction end point with a penalty for death, a dose-ranging strategy with response-adaptive randomization, an integrated 2-part design, pooling of treatment groups to estimate treatment effect and control type I error, a Bayesian inference model to guide interim decision-making, and pretrial simulation of operating characteristics, power, and type I error control. The lack of treatment effect limits the ability to understand the performance of all these design features. However, the trial was able to enroll patients at a rate similar to rates in prior trials, without concern expressed by sites or patients regarding the design, and the combined end point correlated closely with other patient-centered outcomes. During the trial, the monthly updates of efficacy, randomization weights, and study triggers were executed without disruption of enrollment and without corrective action by either the DSMB or any regulatory authority.
Limitations
The study has several limitations. First, patients had received norepinephrine for a median of 8 hours before enrollment, limiting the ability to determine the consequences of avoiding catecholamine-based vasopressor support earlier in septic shock. Second, although selepressin was hypothesized to reduce pulmonary edema, it was only possible to assess the clinical consequences of pulmonary edema, rather than a more detailed physiologic assessment, in this setting. Third, although the study was well-powered to assess overall effects, septic shock is a heterogenous condition, and there was limited ability to understand treatment × subgroup interactions. Fourth, because the primary analysis used a nonparametric test stratified by several patient characteristics, it was not possible to include site as a random effect. Fifth, the study was not powered to detect differences in adverse events.
Conclusions
Among patients with septic shock receiving norepinephrine, administration of selepressin, compared with placebo, did not result in improvement in vasopressor- and ventilator-free days within 30 days. Further research would be needed to evaluate the potential role of selepressin for other patient-centered outcomes in septic shock.
Study Protocol and Statistical Analysis Plan
Collaborators
Supplementary Methods
eFigures 1-15
eTables 1-3
Data Sharing Statement
Section Editor: Derek C. Angus, MD, MPH, Associate Editor, JAMA (angusdc@upmc.edu).
References
- 1.Angus DC, van der Poll T. Severe sepsis and septic shock. N Engl J Med. 2013;369(9):840-851. doi: 10.1056/NEJMra1208623 [DOI] [PubMed] [Google Scholar]
- 2.Singer M, Deutschman CS, Seymour CW, et al. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA. 2016;315(8):801-810. doi: 10.1001/jama.2016.0287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rhodes A, Evans LE, Alhazzani W, et al. Surviving Sepsis Campaign: international guidelines for management of sepsis and septic shock: 2016. Intensive Care Med. 2017;43(3):304-377. doi: 10.1007/s00134-017-4683-6 [DOI] [PubMed] [Google Scholar]
- 4.Hamzaoui O, Scheeren TWL, Teboul JL. Norepinephrine in septic shock: when and how much? Curr Opin Crit Care. 2017;23(4):342-347. doi: 10.1097/MCC.0000000000000418 [DOI] [PubMed] [Google Scholar]
- 5.Kortenoeven ML, Pedersen NB, Rosenbaek LL, Fenton RA. Vasopressin regulation of sodium transport in the distal nephron and collecting duct. Am J Physiol Renal Physiol. 2015;309(4):F280-F299. doi: 10.1152/ajprenal.00093.2015 [DOI] [PubMed] [Google Scholar]
- 6.Kaufmann JE, Oksche A, Wollheim CB, Günther G, Rosenthal W, Vischer UM. Vasopressin-induced von Willebrand factor secretion from endothelial cells involves V2 receptors and cAMP. J Clin Invest. 2000;106(1):107-116. doi: 10.1172/JCI9516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Laporte R, Kohan A, Heitzmann J, et al. Pharmacological characterization of FE 202158, a novel, potent, selective, and short-acting peptidic vasopressin V1a receptor full agonist for the treatment of vasodilatory hypotension. J Pharmacol Exp Ther. 2011;337(3):786-796. doi: 10.1124/jpet.111.178848 [DOI] [PubMed] [Google Scholar]
- 8.Maybauer MO, Maybauer DM, Enkhbaatar P, et al. The selective vasopressin type 1a receptor agonist selepressin (FE 202158) blocks vascular leak in ovine severe sepsis. Crit Care Med. 2014;42(7):e525-e533. doi: 10.1097/CCM.0000000000000300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rehberg S, Yamamoto Y, Sousse L, et al. Selective V(1a) agonism attenuates vascular dysfunction and fluid accumulation in ovine severe sepsis. Am J Physiol Heart Circ Physiol. 2012;303(10):H1245-H1254. doi: 10.1152/ajpheart.00390.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rehberg S, Ertmer C, Vincent JL, et al. Role of selective V1a receptor agonism in ovine septic shock. Crit Care Med. 2011;39(1):119-125. doi: 10.1097/CCM.0b013e3181fa3898 [DOI] [PubMed] [Google Scholar]
- 11.Su F, He X, Taccone FS, et al. Abstracts of the 42nd Critical Care Congress: January 19-23, 2013: San Juan, Puerto Rico. Crit Care Med. 2012;40:1-328. [PubMed] [Google Scholar]
- 12.He X, Su F, Taccone FS, et al. A selective V(1A) receptor agonist, selepressin, is superior to arginine vasopressin and to norepinephrine in ovine septic shock. Crit Care Med. 2016;44(1):23-31. doi: 10.1097/CCM.0000000000001380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Saad AF, Maybauer MO. The role of vasopressin and the vasopressin type V1a receptor agonist selepressin in septic shock. J Crit Care. 2017;40:41-45. doi: 10.1016/j.jcrc.2017.03.008 [DOI] [PubMed] [Google Scholar]
- 14.Russell JA, Vincent JL, Kjølbye AL, et al. Selepressin, a novel selective vasopressin V1A agonist, is an effective substitute for norepinephrine in a phase IIa randomized, placebo-controlled trial in septic shock patients. Crit Care. 2017;21(1):213. doi: 10.1186/s13054-017-1798-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lewis RJ, Angus DC, Laterre PF, et al. ; Selepressin Evaluation Programme for Sepsis-induced Shock–Adaptive Clinical Trial . Rationale and design of an adaptive phase 2b/3 clinical trial of selepressin for adults in septic shock. Ann Am Thorac Soc. 2018;15(2):250-257. doi: 10.1513/AnnalsATS.201708-669SD [DOI] [PubMed] [Google Scholar]
- 16.Vincent JL, Moreno R, Takala J, et al. ; Working Group on Sepsis-Related Problems of the European Society of Intensive Care Medicine . The SOFA (Sepsis-related Organ Failure Assessment) score to describe organ dysfunction/failure. Intensive Care Med. 1996;22(7):707-710. doi: 10.1007/BF01709751 [DOI] [PubMed] [Google Scholar]
- 17.Herdman M, Gudex C, Lloyd A, et al. Development and preliminary testing of the new five-level version of EQ-5D (EQ-5D-5L). Qual Life Res. 2011;20(10):1727-1736. doi: 10.1007/s11136-011-9903-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yealy DM, Kellum JA, Huang DT, et al. ; ProCESS Investigators . A randomized trial of protocol-based care for early septic shock. N Engl J Med. 2014;370(18):1683-1693. doi: 10.1056/NEJMoa1401602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mouncey PR, Power GS, Coats TJ. Early, goal-directed resuscitation for septic shock. N Engl J Med. 2015;373(6):577-578. [DOI] [PubMed] [Google Scholar]
- 20.Wiedemann HP, Wheeler AP, Bernard GR, et al. ; National Heart, Lung, and Blood Institute Acute Respiratory Distress Syndrome (ARDS) Clinical Trials Network . Comparison of two fluid-management strategies in acute lung injury. N Engl J Med. 2006;354(24):2564-2575. doi: 10.1056/NEJMoa062200 [DOI] [PubMed] [Google Scholar]
- 21.Annane D, Renault A, Brun-Buisson C, et al. ; CRICS-TRIGGERSEP Network . Hydrocortisone plus fludrocortisone for adults with septic shock. N Engl J Med. 2018;378(9):809-818. doi: 10.1056/NEJMoa1705716 [DOI] [PubMed] [Google Scholar]
- 22.van Elteren PH. On the combination of independent two-sample tests of Wilcoxon. Bull Int Stat Inst. 1960;37:1-13. [Google Scholar]
- 23.Cao J, Zhang S. Multiple comparison procedures. JAMA. 2014;312(5):543-544. doi: 10.1001/jama.2014.9440 [DOI] [PubMed] [Google Scholar]
- 24.Russell JA, Walley KR, Singer J, et al. ; VASST Investigators . Vasopressin versus norepinephrine infusion in patients with septic shock. N Engl J Med. 2008;358(9):877-887. doi: 10.1056/NEJMoa067373 [DOI] [PubMed] [Google Scholar]
- 25.Khanna A, English SW, Wang XS, et al. ; ATHOS-3 Investigators . Angiotensin II for the treatment of vasodilatory shock. N Engl J Med. 2017;377(5):419-430. doi: 10.1056/NEJMoa1704154 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Study Protocol and Statistical Analysis Plan
Collaborators
Supplementary Methods
eFigures 1-15
eTables 1-3
Data Sharing Statement