Key Points
Question
What is the association between bidirectional income change and cardiovascular disease?
Findings
In this cohort study of middle-aged, community-dwelling adults, a more than 50% income drop was significantly associated with higher risk of incident cardiovascular disease, while a more than 50% income rise was significantly associated with lower risk of incident cardiovascular disease, over a 17-year follow-up.
Meaning
A negative income change is associated with higher risk of incident cardiovascular disease, while a positive income change is associated with lower risk of incident cardiovascular disease.
Abstract
Importance
Higher income is associated with lower incident cardiovascular disease (CVD). However, there is limited research on the association between changes in income and incident CVD.
Objective
To examine the association between change in household income and subsequent risk of CVD.
Design, Setting, and Participants
The Atherosclerosis Risk In Communities (ARIC) study is an ongoing, prospective cohort of 15 792 community-dwelling men and women, of mostly black or white race, from 4 centers in the United States (Jackson, Mississippi; Washington County, Maryland; suburbs of Minneapolis, Minnesota; and Forsyth County, North Carolina), beginning in 1987. For our analysis, participants were followed up until December 31, 2016.
Exposures
Participants were categorized based on whether their household income dropped by more than 50% (income drop), remained unchanged/changed less than 50% (income unchanged), or increased by more than 50% (income rise) over a mean (SD) period of approximately 6 (0.3) years between ARIC visit 1 (1987-1989) and visit 3 (1993-1995).
Main Outcomes and Measures
Our primary outcome was incidence of CVD after ARIC visit 3, including myocardial infarction (MI), fatal coronary heart disease, heart failure (HF), or stroke during a mean (SD) of 17 (7) years. Analyses were adjusted for sociodemographic variables, health behaviors, and CVD biomarkers.
Results
Of the 8989 included participants (mean [SD] age at enrollment was 53 [6] years, 1820 participants were black [20%], and 3835 participants were men [43%]), 900 participants (10%) experienced an income drop, 6284 participants (70%) had incomes that remained relatively unchanged, and 1805 participants (20%) experienced an income rise. After full adjustment, those with an income drop experienced significantly higher risk of incident CVD compared with those whose incomes remained relatively unchanged (hazard ratio, 1.17; 95% CI, 1.03-1.32). Those with an income rise experienced significantly lower risk of incident CVD compared with those whose incomes remained relatively unchanged (hazard ratio, 0.86; 95% CI, 0.77-0.96).
Conclusions and Relevance
Income drop over 6 years was associated with higher risk of subsequent incident CVD over 17 years, while income rise over 6 years was associated with lower risk of subsequent incident CVD over 17 years. Health professionals should have greater awareness of the influence of income change on the health of their patients.
This cohort study examines the association between change in household income and subsequent risk of cardiovascular disease.
Introduction
Socioeconomic status (SES) is conceptualized as a fundamental cause of disease1; that is, no matter what type of health threat, higher-status individuals enjoy greater access to resources (such as knowledge, power, and access to powerful social connections) that they can deploy to protect their health.1 Income, along with education level and occupational prestige, is considered to be a core component of SES.2 Low income (including poverty) is associated with higher risk of myocardial infarction (MI),3 heart failure (HF),4 and stroke.5 Multiple mechanisms have been proposed to explain this association, including impeding cognitive function,6 inability to purchase access to health care, worse nutritional quality, worse neighborhoods, and higher psychosocial stress.2,7 Low SES is also associated with depression,8 which is in turn associated with cardiovascular disease (CVD) risk.9
However, it does not necessarily cost money to engage in some cardioprotective behaviors. In principle, even low-income individuals have the time to engage in regular physical activity (although it might be argued that low-income individuals who are forced to work multiple jobs might be squeezed with respect to their time budget). In addition, money can be just as easily spent to procure goods that harm health (ie, cigarettes) as improve it (ie, fitness club membership). For example, low-income individuals may spend proportionally more of their budget on obtaining cigarettes,10 although they may be less likely to spend income on alcohol.11 Despite these considerations, existing studies have shown that health behaviors, such as smoking behavior, diet, and exercise, are associated with income.12,13,14
In short, although low income is robustly associated with risk of CVD, causality remains debated. It is possible that low income is simply a marker of other characteristics that also predict worse health maintenance behaviors. For more robust causal inference, we need to observe whether changes in incomes are associated with CVD risk.
To our knowledge, the literature on income changes and risk of CVD remains limited. Elfassy et al15 examined 3937 participants of the Coronary Artery Risk Development in Young Adults (CARDIA) study (aged 23-35 years at baseline) and found that high levels of income volatility (defined as an intraindividual standard deviation of the percent income change of 52%-252%) or 2 income drops (defined as ≥25% of their previously measured income) over 15 years were associated with greater risk of CVD compared with those with low volatility and no income drops, respectively.15 In this study, we sought to determine the association between income drop or income rise and incident CVD. We hypothesized that an income drop would be associated with higher risk of CVD, while an income rise would be associated with lower risk of CVD.
Methods
Study Design
The Atherosclerosis Risk in Communities (ARIC) study is a prospective, ongoing cohort of community-dwelling men and women in 4 US geographic regions: Jackson, Mississippi; Washington County, Maryland; suburbs of Minneapolis, Minnesota; and Forsyth County, North Carolina.16 At ARIC visit 1 between 1987 and 1989, the study enrolled 15 792 participants aged 45 to 64 years. The Jackson field center only enrolled African American participants. The first 4 ARIC visits were conducted approximately 3 years apart and included an assessment of risk factors, health behaviors, and comorbidities. The institutional review board at each center approved the study, and all participants provided written informed consent at each visit.
Inclusion/Exclusion Criteria
We included all participants who attended ARIC visit 1 and excluded all participants who did not attend ARIC visit 3 (n = 2905). We excluded participants with prevalent MI, stroke, or HF at visit 1 (n = 990). Prevalent MI before visit 1 was determined from a 12-lead electrocardiogram or a previous physician diagnosis. Prevalent stroke before visit 1 was determined by self-report of a previous physician diagnosis. Prevalent HF was determined either by self-report or manifest HF by Gothenburg criteria.17,18 To minimize possibility of reverse causation (ie, incident disease resulting in income loss), we also excluded participants who developed incident MI/fatal coronary heart disease (CHD), stroke, or HF between visit 1 and visit 3 (n = 361).
Next, we excluded participants self-reported as retired at visit 3 (n = 1612). We also excluded participants not black or white or black at all centers from Minneapolis and Washington county (n = 69) owing to small numbers. Lastly, to account for missing values, we excluded study participants who were missing the total number of people in the household or if this number was 0 at visit 1 (n = 10), and we excluded participants with missing information for income at visit 1 or visit 3 (n = 856). The final analytical study cohort included 8989 participants (Figure 1).
Figure 1. CONSORT Diagram.
ARIC indicates the Atherosclerosis Risk in Communities; HF, heart failure; MI, myocardial infarction.
Measurement of Income Change Categories
Participants reported their total combined family income at visit 1 for the prior 12 months using the following categories: less than $5000, $5000 to $7999, $8000 to $11 999, $12 000 to $15 999, $16 000 to $24 999, $25 000 to $34 999, $35 000 to $49 999, and more than $50 000. There was no income data recorded at ARIC visit 2. Income brackets at visit 3 were determined for each individual from self-report using the same categories as at visit 1 with the addition of the following 3 categories: $50 000 to $74 999, $75 000 to $99 999, and more than $100 000. Because these income brackets were not included in visit 1, we combined them into a single highest income bracket of more than $50 000. We used the midpoint of each income bracket as each participant’s income at visit 1 and visit 3, as has been used in previous studies.15,19 If the participant was in the highest bracket at each visit, we multiplied the value of the highest income category by 1.5 following the Pareto distribution.20 Thus, the highest income bracket at both visits was interpreted as $75 000.
We analyzed income change between visits 1 and 3 both as a continuous variable using a restricted cubic spline curve of a Cox regression and in categories as (1) income unchanged (participants whose incomes at visit 3 remained within 50% of income at visit 1); (2) income drop (participants whose incomes fell by greater than 50% from visit 1 to visit 3); and (3) income rise (participants whose incomes increased by greater than 50% from visits 1 to 3). Sensitivity analyses were conducted using a 25% change in income.
Measurement of Covariates
The covariates used for adjustment in this study were sociodemographic variables (age, sex, race/ethnicity center, education level, occupation, number in household, insurance status, and income), health behaviors (smoking status, drinking status, sport index during leisure, and body mass index [BMI]), and biomarkers (total cholesterol, high-density lipoprotein cholesterol, lipid-lowering medications, systolic blood pressure, antihypertensive medications, diabetes, and serum creatinine), all at baseline (visit 1).
Trained staff measured covariates using standardized protocols, which were similar at visits 1 and 3. Participants answered questions regarding their age, sex, race/ethnicity, income, educational attainment, employment status, household number, health insurance status, smoking status, alcohol use, diabetes status, diabetes medication use, antihypertensive medication use in the past 2 weeks, and lipid-lowering medication use within the past 2 weeks. Study staff measured the following: (1) weight in scrub suit and standing height; BMI, calculated as weight in kilograms divided by height in meters squared; (2) fasting plasma total cholesterol in milligrams per deciliter determined by enzymatic methods (to convert to millimoles per liter, multiply by 0.0259)21; (3) sitting blood pressure by 3 measurements from a random-zero sphygmomanometer after a 5-minute rest, using the average of the last 2 measurements; and (4) serum glucose in milligrams per deciliter using a hexokinase/glucose-6-phosphate dehydrogenase method22 (prevalent type 2 diabetes was determined based on either fasting glucose level of at least 126 mg/dL [to convert to millimoles per liter, multiply by 0.0555], random glucose level of at least 200 mg/dL, self-report of physician diagnosis of diabetes, or diabetes medication use); and (5) serum creatinine level determined by the modified kinetic Jaffe method.23 Participants answered questions regarding exercise behavior using the validated Baecke questionnaire at ARIC visits 1 and 3. The sports index during leisure was calculated on a scale of 1 to 5, with higher numbers demonstrating greater physical activity levels.24,25
Measurement of Primary Outcome and Secondary Outcomes
The primary outcome was defined as incident CVD, comprising definite or probable MI, fatal CHD, incident HF, or definite or probable stroke. All participants were followed up from visit 3 to the CVD event, death, loss to follow-up, or until censoring on December 31, 2016. Secondary outcomes included (1) definite or probable MI or fatal CHD; (2) incident HF; and (3) definite or probable stroke. Cardiovascular disease events were determined after contacting participants annually (semiannually since 2012), recording hospitalizations, surveying discharge lists from hospitals, and obtaining information from the National Death Index and state death registries.18,26,27 Myocardial infarction was ascertained by trained abstractors using information regarding presence of chest pain of cardiac origin, biomarkers, and electrocardiographic findings and classified as definite or probable MI.27,28 Stroke was ascertained by trained abstractors and classified by physician reviewers on the basis of symptoms and evidence of procedures as definite or probable ischemic or hemorrhagic stroke.29 Heart failure was identified as hospitalizations with an International Classification of Diseases, Ninth Revision discharge code of 428.0 to 428.9, or International Statistical Classification of Diseases and Related Health Problems, Tenth Revision discharge code I50.18
Data Analysis
Data analyses were conducted using Stata, version 14 SE (StataCorp). Descriptive statistics were used to analyze demographic variables by each of the income change categories. We used global χ2 tests to compare categorical variables, analysis of variance to compare continuous, normal variables, and Kruskal-Wallis test for continuous, nonnormal data. A restricted cubic spline curve of a Cox regression was used to graph hazard ratios (HRs) of incident CVD vs relative income change as a continuous variable. The primary outcome and each secondary outcome were then analyzed using Cox proportional hazards multivariable modeling using income change as a categorical variable, adjusting for all the Table 1 covariates previously described in the measurement of covariates section, including baseline income, with the group whose incomes remained relatively unchanged as the reference group. Interaction analyses were conducted with respect to race/ethnicity and sex. Participants were followed up after visit 3 for a mean of 17 years, and a Kaplan-Meier curve was used to illustrate cumulative incidence of CVD. In all cases, a 2-sided P value less than .05 was deemed statistically significant.
Table 1. Sociodemographic Variables, Health Behaviors, and Biomarkers of Individuals Prior to Income Change (Visit 1) and After Income Change (Visit 3) by Income Change Category.
| Variable | No. (%) | P Value | ||
|---|---|---|---|---|
| Income Drop >50% | Income Unchanged or Changed ≤50% | Income Rise >50% | ||
| No. | 900 | 6284 | 1805 | NA |
| Sociodemographic | ||||
| Age, mean (SD), y | ||||
| Visit 1 | 55.3 (6) | 53.5 (6) | 52.5 (6) | <.001 |
| Visit 3 | 61.2 (6) | 59.4 (6) | 58.5 (6) | <.001 |
| Male | 333 (37) | 2713 (43) | 789 (44) | <.001 |
| Race/ethnicity, center | <.001 | |||
| White, Forsyth, North Carolina | 181 (20) | 1622 (26) | 369 (20) | |
| Black, Forsyth, North Carolina | 25 (3) | 125 (2) | 60 (3) | |
| Black, Jackson, Mississippi | 259 (29) | 887 (14) | 464 (26) | |
| White, Minneapolis, Minnesota | 197 (22) | 1907 (30) | 444 (25) | |
| White, Washington, Maryland | 238 (26) | 1743 (28) | 468 (26) | |
| Education | <.001 | |||
| Grade school or 0 y education | 129 (14) | 286 (5) | 124 (7) | |
| High school, no degree | 153 (17) | 681 (11) | 232 (13) | |
| High school graduate | 311 (35) | 2155 (34) | 613 (34) | |
| Vocational school | 82 (9) | 550 (9) | 156 (9) | |
| College | 184 (20) | 1846 (29) | 495 (27) | |
| Graduate school or professional school | 41 (5) | 758 (12) | 184 (10) | |
| Visit 1 occupation | <.001 | |||
| Homemaking, not working outside home | 144 (16) | 823 (13) | 188 (11) | |
| Employed, either full or part time | 590 (66) | 4368 (70) | 1311 (73) | |
| Employed, but away from job | 7 (1) | 66 (1) | 16 (1) | |
| Unemployed, looking for work | 13 (1) | 66 (1) | 27 (2) | |
| Unemployed, not looking for work | 11 (1) | 57 (1) | 27 (2) | |
| Retired and not working | 103 (12) | 659 (11) | 176 (10) | |
| Retired but working for pay | 31 (3) | 234 (4) | 54 (3) | |
| Visit 1 No. in household, mean (SD) | 2.8 (1.4) | 2.8 (1.2) | 2.9 (1.3) | <.001 |
| Visit 3 No. in household, mean (SD) | 2.3 (1.3) | 2.4 (1.0) | 2.5 (1.1) | |
| Insurance status | ||||
| Visit 1 | ||||
| Insured | 784 (87) | 5933 (95) | 1596 (88) | <.001 |
| Uninsured | 111 (12) | 346 (6) | 207 (12) | |
| Unknown | 2 (0) | 1 (0) | 2 (0) | |
| Visit 3 | ||||
| Insured | 789 (88) | 5993 (95) | 1688 (94) | <.001 |
| Uninsured | 111 (12) | 286 (5) | 117 (7) | |
| Unknown | 0 | 3 (0) | 0 | |
| Visit 1 income, $ | ||||
| Mean (SD) | 40 516 (23 491) | 43 897 (24 808) | 26 099 (15 808) | <.001 |
| Median (IQR) | 43 000 (20 500-75 000) | 30 000 (20 500-75 000) | 30 000 (10 000-43 000) | <.001 |
| Visit 3 Income, $ | ||||
| Mean (SD) | 14 655 (9060) | 43 057 (23 330) | 53 347 (26 397) | <.001 |
| Median (IQR) | 14 000 (20 500-75 000) | 43 000 (20 500-75 000) | 75 000 (20 500-75 000) | <.001 |
| Health behaviors | ||||
| Visit 1 smoking status | ||||
| Current | 241 (27) | 1314 (21) | 407 (23) | <.001 |
| Former | 267 (30) | 2040 (33) | 599 (33) | |
| Never | 392 (44) | 2926 (47) | 797 (44) | |
| Unknown | 0 | 1 (0) | 2 (0) | |
| Visit 3 smoking status | ||||
| Current | 196 (22) | 1020 (16) | 327 (18) | <.001 |
| Former | 322 (36) | 2544 (41) | 732 (41) | |
| Never | 382 (42) | 2717 (43) | 745 (41) | |
| Unknown | 0 | 2 (0) | 0 | |
| Visit 1 drinking status | ||||
| Current | 471 (53) | 3873 (62) | 986 (54) | <.001 |
| Former | 171 (19) | 964 (15) | 333 (19) | |
| Never | 255 (28) | 1423 (23) | 482 (27) | |
| Unknown | 0 | 5 (0) | 0 | |
| Visit 3 drinking status | ||||
| Current | 403 (45) | 3519 (56) | 929 (52) | <.001 |
| Former | 227 (25) | 1295 (21) | 430 (24) | |
| Never | 270 (30) | 1468 (23) | 446 (25) | |
| Unknown | 0 | 2 (0) | 0 | |
| Sport index during leisure | ||||
| Visit 1 | 2.38 (0.7) | 2.51 (0.8) | 2.44 (0.8) | <.001 |
| Visit 3 | 2.46 (0.8) | 2.54 (0.8) | 2.50 (0.8) | .007 |
| BMI, mean (SD) | ||||
| Visit 1 | 27.8 (5) | 27.3 (5) | 27.4 (5) | .01 |
| Visit 3 | 28.5 (6) | 28.3 (5) | 28.5 (6) | .19 |
| Biomarkers | ||||
| Total cholesterol, mean (SD), mg/dL | ||||
| Visit 1 | 215 (44) | 214 (40) | 213 (41) | .41 |
| Visit 3 | 209 (39) | 207 (37) | 208 (38) | .33 |
| High-density lipoprotein (SD), mg/dL | ||||
| Visit 1 | 54 (17) | 52 (17) | 53 (17) | .07 |
| Visit 3 | 54 (18) | 53 (18) | 53 (19) | .49 |
| Lipid-lowering medications | ||||
| Visit 1 | 29 (3) | 162 (3) | 37 (2) | .17 |
| Visit 3 | 78 (9) | 549 (9) | 126 (7) | .06 |
| Systolic blood pressure, mean (SD) | ||||
| Visit 1 | 121 (17) | 119 (17) | 119 (18) | <.001 |
| Visit 3 | 126 (19) | 123 (18) | 124 (19) | <.001 |
| Antihypertensive medications | ||||
| Visit 1 | 251 (28) | 1448 (23) | 404 (22) | .003 |
| Visit 3 | 336 (37) | 1978 (32) | 574 (32) | .002 |
| Diabetes | ||||
| Visit 1 | 97 (11) | 508 (8) | 158 (9) | .02 |
| Visit 3 | 138 (15) | 836 (13) | 242 (14) | .24 |
| Serum creatinine, mean (SD), mg/dL | ||||
| Visit 1 | 1.09 (0.2) | 1.09 (0.3) | 1.09 (0.2) | .97 |
| Visit 2a | 1.13 (0.3) | 1.14 (0.3) | 1.13 (0.2) | .58 |
Abbreviations: ARIC, the Atherosclerosis Risk in Communities; BMI, body mass index (calculated as weight in kilograms divided by height in meters squared); IQR, interquartile range; NA, not applicable.
SI conversion factors: To convert cholesterol levels to millimoles per liter, multiply by 0.0259; creatinine to micromoles per liter, multiply by 88.4.
This was at ARIC visit 2 because serum creatinine was not recorded at ARIC visit 3.
Results
Of the 15 792 ARIC cohort participants, 8989 fulfilled the inclusion criteria and were included in the analyses (mean [SD] age at baseline 53 [5.8] years; 3835 men [43%], 1820 African American participants [20%]). eTable 1 in the Supplement shows the characteristics of participants excluded owing to missing values. For the included participants, in the approximately 6-year period between ARIC visit 1 to visit 3, 900 participants (10%) experienced an income drop, with mean household incomes falling from $40 516 to $14 655. A total of 6284 participants (70%) had incomes that remained relatively unchanged, with mean household incomes remaining similar from $43 897 to $43 057. A total of 1805 participants (20%) experienced an income rise, with mean household incomes rising from $26 099 to $53 347.
Table 1 summarizes sociodemographic characteristics, health behaviors, and biomarkers of study participants before (visit 1) and after income change (visit 3). Before income change (visit 1), compared with the group whose incomes remained unchanged, individuals in the group who experienced an income drop were older, less likely to be male, and less likely to graduate high school. They were also less likely to have health insurance, more likely to smoke, less likely to drink alcohol, less likely to engage in physical activity, more likely to have higher BMIs, more likely to have higher systolic blood pressure, and more likely to have diabetes. At the same time, the participants experiencing an income rise were, by comparison with those whose income levels were unchanged, younger, had lower baseline incomes, were less likely to have health insurance, more likely to smoke, and less likely to drink alcohol. Income gain resulted in a greater proportion of participants with health insurance. In both the groups with income drop and income rise, there were lower proportions of smoking and drinking after income change compared with before income change. There was a significantly lower increase in BMI in the income drop group compared with the income unchanged and income rise groups (eTable 2 in the Supplement).
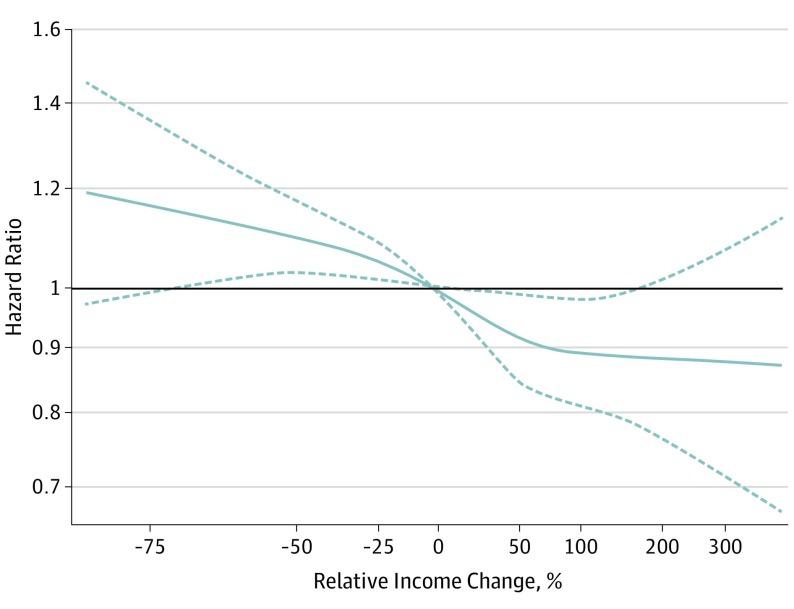
We used a Cox regression spline model to graph HRs by relative income change as a continuous variable for each ARIC participant, adjusting for all covariates at visit 1 (Figure 2). We found that a relative change in income of 50% coincided with the beginning of plateauing of the HR for incident CVD in the direction of a positive income change. In addition, the rate of change of the HR for incident CVD seemed to be decreased starting at a 50% in the direction of a negative income change. This suggested that an income change of around 50% may coincide with clinically meaningful differences in incident CVD. Using a 25% income change as our exposure variable did not result in any significant differences in CVD risk (eTable 3 in the Supplement).
Figure 2. Restricted Cubic Splines of Cox Regression Showing Hazard Ratios of Primary Outcome (Incident Cardiovascular Disease) by Relative Income Change, Adjusted for Sociodemographic Variables, Health Behaviors, and Cardiovascular Disease Biomarkers.
Dashed lines are 95% confidence intervals.
Thus, our unadjusted Kaplan-Meier results using categories of income change (income drop >50%, income changed ≤50%, and income rise >50%) suggest a gradient of risk of the primary outcome of incident CVD (Figure 3). All subsequent models in Table 2 used income change as categories and were adjusted for covariates ascertained at baseline prior to income change (visit 1). For the primary outcome, compared with the reference group whose incomes were unchanged, the group that experienced income drop had significantly higher crude HRs of incident CVD (HR, 1.46; 95% CI, 1.30-1.65). Following adjustment for sociodemographic variables, health behaviors, and biomarkers, the HR was attenuated to 1.17 (95% CI, 1.03-1.32). In addition, compared with the group whose incomes were unchanged, the group that experienced income rise had significantly lower HRs of incident CVD in the model adjusted for sociodemographic variables, health behaviors, and biomarkers (HR, 0.86; 95% CI, 0.77-0.96).
Figure 3. Unadjusted Kaplan-Meier Curve .
Kaplan-Meier curves are of time to primary outcome of incident cardiovascular disease (definite or probable myocardial infarction, fatal coronary heart disease, incident heart failure, or definite or probable stroke) by income change category.
Table 2. Association of Income Change Categories of Income Drop (n = 900), Income Unchanged (n = 6284), and Income Rise (n = 1805) With Incident Cardiovascular Disease in Participants Free of Cardiovascular Disease and Following Income Change (ARIC Visit 3).
| Income Change Category | No. of Events | Unadjusted Model | Model 1a | Model 2b | Model 3c | ||||
|---|---|---|---|---|---|---|---|---|---|
| Hazard Ratio (95% CI) | P Value | Hazard Ratio (95% CI) | P Value | Hazard Ratio (95% CI) | P Value | Hazard Ratio (95% CI) | P Value | ||
| Primary outcome of definite or probable MI, fatal coronary heart disease, incident heart failure, or definite or probable stroke | |||||||||
| Income drop | 327 | 1.46 (1.30-1.65) | <.001 | 1.20 (1.07-1.36) | .003 | 1.16 (1.02-1.31) | .02 | 1.17 (1.03-1.32) | .01 |
| Income unchanged | 1733 | 1 [Reference] | NA | 1 [Reference] | NA | 1 [Reference] | NA | 1 [Reference] | NA |
| Income rise | 457 | 0.91 (0.82-1.01) | .08 | 0.83 (0.75-0.93) | .001 | 0.85 (0.76-0.95) | .004 | 0.86 (0.77-0.96) | .007 |
| Secondary outcome of definite or probable MI or fatal coronary heart disease | |||||||||
| Income drop | 141 | 1.44 (1.20-1.72) | <.001 | 1.30 (1.08-1.57) | .005 | 1.26 (1.04-1.52) | .02 | 1.29 (1.07-1.56) | .008 |
| Income unchanged | 732 | 1 [Reference] | NA | 1 [Reference] | NA | 1 [Reference] | NA | 1 [Reference] | NA |
| Income rise | 196 | 0.93 (0.79-1.08) | .34 | 0.86 (0.73-1.02) | .08 | 0.87 (0.74-1.03) | .12 | 0.88 (0.75-1.04) | .15 |
| Secondary outcome of incident heart failure | |||||||||
| Income drop | 212 | 1.46 (1.26-1.69) | <.001 | 1.15 (0.99-1.34) | .08 | 1.08 (0.92-1.25) | .35 | 1.09 (0.93-1.27) | .28 |
| Income unchanged | 1100 | 1 [Reference] | NA | 1 [Reference] | NA | 1 [Reference] | NA | 1 [Reference] | NA |
| Income rise | 287 | 0.90 (0.79-1.02) | .11 | 0.81 (0.70-0.92) | .002 | 0.84 (0.73-0.96) | .01 | 0.83 (0.73-0.96) | .01 |
| Secondary outcome of definite or probable stroke | |||||||||
| Income drop | 97 | 1.69 (1.36-2.11) | <.001 | 1.37 (1.09-1.72) | .008 | 1.33 (1.06-1.68) | .02 | 1.33 (1.05-1.68) | .02 |
| Income unchanged | 434 | 1 [Reference] | NA | 1 [Reference] | NA | 1 [Reference] | NA | 1 [Reference] | NA |
| Income rise | 127 | 1.01 (0.83-1.24) | .89 | 0.87 (0.71-1.07) | .19 | 0.89 (0.72-1.09) | .26 | 0.93 (0.75-1.14) | .48 |
Abbreviations: ARIC, the Atherosclerosis Risk in Communities; NA, not applicable.
Model 1: adjusted for age, sex, race/ethnicity and center, household number, employment status, education level, health insurance status, and income prior to income change (ARIC visit 1).
Model 2: model 1 and additionally adjusted for smoking status, alcohol intake, sport index during leisure, and body mass index (calculated as weight in kilograms divided by height in meters squared) prior to income change (ARIC visit 1).
Model 3: model 2 and total cholesterol, high-density lipoprotein cholesterol, lipid-lowering medications, systolic blood pressure, antihypertensive medications, diabetes status, and serum creatinine levels prior to income change (ARIC visit 1).
Survival analyses were conducted for the secondary outcomes of definite or probable MI or fatal CHD, HF, and definite or probable stroke. In fully adjusted analysis, we observed that the group that experienced an income drop had significantly higher HRs of MI/fatal CHD (HR, 1.28; 95% CI, 1.06-1.55) and definite or probable stroke (HR, 1.32; 95% CI, 1.05-1.67) when compared with the group whose incomes were unchanged. In addition, after full adjustment, the group that experienced income rise had a significantly lower HR of incident HF (HR, 0.83; 95% CI, 0.72-0.96) compared with the group whose incomes were unchanged.
Lastly, we observed significant interactions for income drop by race/ethnicity (HR, 0.74; 95% CI, 0.56-0.97; P for interaction = .03) and for income rise by sex (HR, 1.38; 95% CI, 1.12-1.71; P for interaction = .003). After full adjustment, white participants had higher risk of incident CVD from an income drop compared with black participants (HR for white race, 1.28; 95% CI, 1.11-1.48 vs HR for black race, 1.01; 95% CI, 0.79-1.29). Women developed lower risk of incident CVD from an income rise compared with men (HR for women, 0.74; 95% CI, 0.63-0.87 vs HR for men, 1.01; 95% CI, 0.86-1.18) (eTable 4 in the Supplement).
Discussion
In a large sample of middle-aged, community-dwelling adults, we found that compared with the participants whose incomes were relatively unchanged over a 6-year period, participants who experienced an income drop had a 17%, significantly higher risk of incident CVD over 17 years of follow-up. These findings were mainly driven by higher risk of MI/fatal CHD and stroke. Compared with the participants whose incomes were relatively unchanged over 6 years, the group that experienced an income rise had a 14% significantly lower risk of incident CVD over 17 years, mainly driven by lower risk of HF.
There are several plausible mechanisms that can explain the observed association between income changes and risk of CVD. An unanticipated drop in income may induce changes in health behaviors. For example, income loss has been associated with a shift toward consumption of less healthy foods, eg, foods that are more energy dense and yield more calories for a given price.30,31 Income drops may also result in loss of health insurance coverage,32 although we did not observe that in this study population. Large drops in income may also lead to financial stress and worry, which are in turn linked to stress-coping behaviors such as increased intensity of cigarette smoking and alcohol use.12,33,34 Lastly, lower incomes may be associated with depression,8 which increases the risk of coronary artery disease.9 Conversely, a boost in income could increase the psychological motivation to invest for the future, including the adoption of health maintenance behaviors. We attempted to elucidate some of these mechanisms by adjusting for health insurance status and health behaviors after income change (visit 3) (eTable 5 in the Supplement). However, our HRs did not change significantly from the original model; hence, there must be other factors, beyond health behaviors and health insurance coverage, that account for these associations.
Limitations
To our knowledge, this is the largest study that examined the association between an income drop and incident CVD and the only study to address the associations between income rise and incident CVD. Some limitations of our study should be mentioned. First, there was no standard definition of what constitutes a significant income change. For example, Elfassy et al15 characterized an “income drop” as an at least 25% income reduction and reported a significant result after 2 income drops, while Pool et al35 characterized an at least 75% decrease in wealth as a drop. Our definition of a 50% change fell in between these 2 definitions. Similar to the study by Elfassy et al,15 our sensitivity analysis noted that a single 25% change in income may not be large enough to significantly affect CVD risk. Additionally, income is dynamic, and there may have been additional income drops and/or income rises after the exposure was defined at visit 3, resulting in misclassification bias. However, because those who experienced an income drop had higher incomes at baseline, while those who experienced an income rise had lower incomes at baseline, the income misclassification is likely to be nondifferential (ie, bias toward the null). A second limitation is that studies on changes in income are subject to reverse causation, in that participants who became sick may have been more likely to fall into poverty. To minimize this possibility, we excluded participants with prevalent CVD, we excluded participants who developed incident CVD between visit 1 and visit 3, and we adjusted for baseline health behaviors and biomarkers. We also conducted a sensitivity analysis excluding those with prevalent cancer or chronic lung disease at baseline, and our results were robust. However, we cannot completely account for the fact that deterioration in health, apart from the diseases we accounted for, may have occurred simultaneously with the income drop or income rise. A third limitation is that we only observed income drop and income rise over a relatively short span of time (6 years), and we do not know whether these income changes were part of a longer, fluctuating pattern over time. Fourth, it is possible that CVD effects may not be owing to the income drop but rather owing to spending many years at the lower or higher income level. We conducted a sensitivity analysis truncating follow-up at 10 years, and our confidence intervals were similar but wider and were not statistically significant for the income rise group after full adjustment (eTable 6 in the Supplement). Lastly, there may be many different reasons for the income changes (for example, loss of a spouse), which may meaningfully influence CVD risk apart from the magnitude of the change.
Conclusions
In conclusion, in this large cohort of middle-aged, community-dwelling participants, we found that an income drop was associated with a significantly higher risk of subsequent cardiovascular disease, and an income rise was associated with a significantly lower risk of cardiovascular disease, even after adjustment for sociodemographic variables, health behaviors, and biomarkers. This study reinforces the need to increase awareness among health professionals of the influence of income changes on health to optimize patient treatment.
eTable 1. Socio-demographic variables, health behaviors, and biomarkers, of individuals prior to income change (ARIC visit 1) and after income change (ARIC visit 3)
eTable 2. Change in biomarkers from before income change (visit 1) to after income change (visit 3), by income change category
eTable 3. Association of income change categories of income drop, income unchanged, and income rise (using 25% and 50% income changes) with risk of incident cardiovascular disease in participants free of cardiovascular disease and post income change
eTable 4. Association of income change categories with incident cardiovascular disease, stratified by race and gender
eTable 5. Association of income change categories of income drop (n=900), income unchanged (n=6284), and income rise (n=1805) with incident cardiovascular disease
eTable 6. Association of income change categories of income drop (n=900), income unchanged (n=6284), and income rise (n=1805) with incident cardiovascular disease
References
- 1.Link BG, Phelan J. Social conditions as fundamental causes of disease. J Health Soc Behav. 1995;(Spec No):80-94. doi: 10.2307/2626958 [DOI] [PubMed] [Google Scholar]
- 2.Berkman LF, Kawachi I, Glymour MM. Social Epidemiology Second Edition. New York, NY: Oxford University Press; 2014. [Google Scholar]
- 3.Fretz A, Schneider AL, McEvoy JW, et al. The association of socioeconomic status with subclinical myocardial damage, incident cardiovascular events, and mortality in the ARIC Study. Am J Epidemiol. 2016;183(5):452-461. doi: 10.1093/aje/kwv253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vart P, Matsushita K, Rawlings AM, et al. SES, heart failure, and N-terminal pro-b-type natriuretic peptide: the Atherosclerosis Risk in Communities study. Am J Prev Med. 2018;54(2):229-236. doi: 10.1016/j.amepre.2017.10.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Marshall IJ, Wang Y, Crichton S, McKevitt C, Rudd AG, Wolfe CD. The effects of socioeconomic status on stroke risk and outcomes. Lancet Neurol. 2015;14(12):1206-1218. doi: 10.1016/S1474-4422(15)00200-8 [DOI] [PubMed] [Google Scholar]
- 6.Mani A, Mullainathan S, Shafir E, Zhao J. Poverty impedes cognitive function. Science. 2013;341(6149):976-980. doi: 10.1126/science.1238041 [DOI] [PubMed] [Google Scholar]
- 7.Tawakol A, Osborne MT, Wang Y, et al. Stress-associated neurobiological pathway linking socioeconomic disparities to cardiovascular disease. J Am Coll Cardiol. 2019;73(25):3243-3255. doi: 10.1016/j.jacc.2019.04.042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Freeman A, Tyrovolas S, Koyanagi A, et al. The role of socio-economic status in depression: results from the COURAGE (aging survey in Europe). BMC Public Health. 2016;16(1):1098. doi: 10.1186/s12889-016-3638-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lett HS, Blumenthal JA, Babyak MA, et al. Depression as a risk factor for coronary artery disease: evidence, mechanisms, and treatment. Psychosom Med. 2004;66(3):305-315. [DOI] [PubMed] [Google Scholar]
- 10.Siahpush M, Farazi PA, Maloney SI, Dinkel D, Nguyen MN, Singh GK. Socioeconomic status and cigarette expenditure among US households: results from 2010 to 2015 Consumer Expenditure Survey. BMJ Open. 2018;8(6):e020571. doi: 10.1136/bmjopen-2017-020571 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cerdá M, Johnson-Lawrence VD, Galea S. Lifetime income patterns and alcohol consumption: investigating the association between long- and short-term income trajectories and drinking. Soc Sci Med. 2011;73(8):1178-1185. doi: 10.1016/j.socscimed.2011.07.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kendzor DE, Businelle MS, Costello TJ, et al. Financial strain and smoking cessation among racially/ethnically diverse smokers. Am J Public Health. 2010;100(4):702-706. doi: 10.2105/AJPH.2009.172676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kirkpatrick SI, Dodd KW, Reedy J, Krebs-Smith SM. Income and race/ethnicity are associated with adherence to food-based dietary guidance among US adults and children. J Acad Nutr Diet. 2012;112(5):624-635.e6. doi: 10.1016/j.jand.2011.11.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Meltzer DO, Jena AB. The economics of intense exercise. J Health Econ. 2010;29(3):347-352. doi: 10.1016/j.jhealeco.2010.03.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Elfassy T, Swift SL, Glymour MM, et al. Associations of income volatility with incident cardiovascular disease and all-cause mortality in a US cohort. Circulation. 2019;139(7):850-859. doi: 10.1161/CIRCULATIONAHA.118.035521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.The ARIC investigators The Atherosclerosis Risk in Communities (ARIC) study: design and objectives. Am J Epidemiol. 1989;129(4):687-702. doi: 10.1093/oxfordjournals.aje.a115184 [DOI] [PubMed] [Google Scholar]
- 17.Eriksson H, Caidahl K, Larsson B, et al. Cardiac and pulmonary causes of dyspnoea: validation of a scoring test for clinical-epidemiological use: the Study of Men Born in 1913. Eur Heart J. 1987;8(9):1007-1014. doi: 10.1093/oxfordjournals.eurheartj.a062365 [DOI] [PubMed] [Google Scholar]
- 18.Loehr LR, Rosamond WD, Chang PP, Folsom AR, Chambless LE. Heart failure incidence and survival (from the Atherosclerosis Risk in Communities study). Am J Cardiol. 2008;101(7):1016-1022. doi: 10.1016/j.amjcard.2007.11.061 [DOI] [PubMed] [Google Scholar]
- 19.Young-Hoon KN. A longitudinal study on the impact of income change and poverty on smoking cessation. Can J Public Health. 2012;103(3):189-194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Armour P, Burkhauser RV, Larrimore J. Using the Pareto distribution to improve estimates of topcoded earnings. Econ Inq. 2016;54:1263-1273. doi: 10.1111/ecin.12299 [DOI] [Google Scholar]
- 21.Siedel J, Hägele EO, Ziegenhorn J, Wahlefeld AW. Reagent for the enzymatic determination of serum total cholesterol with improved lipolytic efficiency. Clin Chem. 1983;29(6):1075-1080. [PubMed] [Google Scholar]
- 22.Folsom AR, Yamagishi K, Hozawa A, Chambless LE; Atherosclerosis Risk in Communities Study Investigators . Absolute and attributable risks of heart failure incidence in relation to optimal risk factors. Circ Heart Fail. 2009;2(1):11-17. doi: 10.1161/CIRCHEARTFAILURE.108.794933 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lustgarten JA, Wenk RE. Simple, rapid, kinetic method for serum creatinine measurement. Clin Chem. 1972;18(11):1419-1422. [PubMed] [Google Scholar]
- 24.Richardson MT, Ainsworth BE, Wu HC, Jacobs DR Jr, Leon AS. Ability of the Atherosclerosis Risk in Communities (ARIC)/Baecke Questionnaire to assess leisure-time physical activity. Int J Epidemiol. 1995;24(4):685-693. doi: 10.1093/ije/24.4.685 [DOI] [PubMed] [Google Scholar]
- 25.Folsom AR, Arnett DK, Hutchinson RG, Liao F, Clegg LX, Cooper LS. Physical activity and incidence of coronary heart disease in middle-aged women and men. Med Sci Sports Exerc. 1997;29(7):901-909. doi: 10.1097/00005768-199707000-00009 [DOI] [PubMed] [Google Scholar]
- 26.Rosamond WD, Folsom AR, Chambless LE, et al. Stroke incidence and survival among middle-aged adults: 9-year follow-up of the Atherosclerosis Risk in Communities (ARIC) cohort. Stroke. 1999;30(4):736-743. doi: 10.1161/01.STR.30.4.736 [DOI] [PubMed] [Google Scholar]
- 27.White AD, Folsom AR, Chambless LE, et al. Community surveillance of coronary heart disease in the Atherosclerosis Risk in Communities (ARIC) study: methods and initial two years’ experience. J Clin Epidemiol. 1996;49(2):223-233. doi: 10.1016/0895-4356(95)00041-0 [DOI] [PubMed] [Google Scholar]
- 28.Prineas RJ, Crow RS, Zhang Z-M. The Minnesota Code Manual of Electrocardiographic Findings. London, England: Springer Science & Business Media; 2009. [Google Scholar]
- 29.Koton S, Schneider AL, Rosamond WD, et al. Stroke incidence and mortality trends in US communities, 1987 to 2011. JAMA. 2014;312(3):259-268. doi: 10.1001/jama.2014.7692 [DOI] [PubMed] [Google Scholar]
- 30.Mellis AM, Athamneh LN, Stein JS, Sze YY, Epstein LH, Bickel WK. Less is more: negative income shock increases immediate preference in cross commodity discounting and food demand. Appetite. 2018;129:155-161. doi: 10.1016/j.appet.2018.06.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Drewnowski A, Specter SE. Poverty and obesity: the role of energy density and energy costs. Am J Clin Nutr. 2004;79(1):6-16. doi: 10.1093/ajcn/79.1.6 [DOI] [PubMed] [Google Scholar]
- 32.Holahan J. The 2007-09 recession and health insurance coverage. Health Aff (Millwood). 2011;30(1):145-152. doi: 10.1377/hlthaff.2010.1003 [DOI] [PubMed] [Google Scholar]
- 33.Reitzel LR, Langdon KJ, Nguyen NT, Zvolensky MJ. Financial strain and smoking cessation among men and women within a self-guided quit attempt. Addict Behav. 2015;47:66-69. doi: 10.1016/j.addbeh.2015.03.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shaw BA, Agahi N, Krause N. Are changes in financial strain associated with changes in alcohol use and smoking among older adults? J Stud Alcohol Drugs. 2011;72(6):917-925. doi: 10.15288/jsad.2011.72.917 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pool LR, Burgard SA, Needham BL, Elliott MR, Langa KM, Mendes de Leon CF. Association of a negative wealth shock with all-cause mortality in middle-aged and older adults in the United States. JAMA. 2018;319(13):1341-1350. doi: 10.1001/jama.2018.2055 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable 1. Socio-demographic variables, health behaviors, and biomarkers, of individuals prior to income change (ARIC visit 1) and after income change (ARIC visit 3)
eTable 2. Change in biomarkers from before income change (visit 1) to after income change (visit 3), by income change category
eTable 3. Association of income change categories of income drop, income unchanged, and income rise (using 25% and 50% income changes) with risk of incident cardiovascular disease in participants free of cardiovascular disease and post income change
eTable 4. Association of income change categories with incident cardiovascular disease, stratified by race and gender
eTable 5. Association of income change categories of income drop (n=900), income unchanged (n=6284), and income rise (n=1805) with incident cardiovascular disease
eTable 6. Association of income change categories of income drop (n=900), income unchanged (n=6284), and income rise (n=1805) with incident cardiovascular disease