Key Points
Question
Is varenicline tartrate, when added to brief cessation counseling, efficacious and safe for smoking cessation in adolescents?
Findings
In this 12-week randomized clinical trial with 157 treatment-seeking adolescent cigarette smokers, abstinence rates at the end of treatment (primary outcome) did not differ between groups, but examination of secondary findings revealed that varenicline participants achieved abstinence earlier and had higher rates of overall abstinence during treatment and at posttreatment follow-up compared with placebo participants. Treatment-emergent adverse events did not differ between groups.
Meaning
Compared with placebo, varenicline was well tolerated but did not support improved end-of-treatment abstinence for adolescent smokers.
This randomized clinical trial evaluates the efficacy and safety of varenicline for smoking cessation among a population of adolescents and young adults.
Abstract
Importance
Cigarette smoking is the leading cause of preventable morbidity and mortality in the United States and worldwide, and most tobacco users begin smoking in adolescence. Although advances have yielded efficacious pharmacotherapies to complement smoking cessation counseling in adults, far less progress has been made in addressing tobacco use in adolescence.
Objective
To evaluate the efficacy and safety of varenicline tartrate for smoking cessation in adolescents and young adults.
Design, Setting, and Participants
This 2-group randomized, placebo-controlled, double-blind intention-to-treat clinical trial enrolled a volunteer sample of treatment-seeking adolescent and young adult cigarette smokers (n = 157) aged 14 to 21 years at an outpatient clinical site in Charleston, South Carolina, from August 15, 2012, to October 20, 2017. Follow-up was completed on January 25, 2018. Data were analyzed from March 19, 2018, to August 11, 2018, with further revisions completed April 10, 2019.
Interventions
Participants were randomized in a 1:1 ratio to a 12-week course of varenicline (n = 77) or placebo (n = 80). All participants received weekly smoking cessation counseling.
Main Outcomes and Measures
The preselected primary efficacy outcome was urine cotinine level–confirmed 7-day abstinence at the end of treatment. Secondary efficacy outcomes included weekly abstinence throughout active treatment, abstinence at posttreatment follow-up visits, and time to first 7-day abstinence. The primary safety outcome was the frequency of treatment-emergent adverse events.
Results
A total of 157 participants were enrolled (94 male [59.9%]; mean [SD] age, 19.1 [1.5] years). The varenicline and placebo groups did not differ in the primary outcome of cotinine-confirmed self-reported 7-day abstinence at the end of treatment (varenicline group, 4 of 45 [8.9%]; placebo group, 4 of 45 [8.9%]; risk ratio [RR], 0.97; 95% CI, 0.29-2.99; P = .96). However, among secondary outcomes, the varenicline group achieved self-reported earlier abstinence of at least 7 days (hazard ratio, 1.91; 95% CI, 1.12-3.27) and demonstrated higher rates of self-reported weekly abstinence during the full course of treatment (RR, 1.81; 95% CI, 1.09-2.99; P = .02) and at posttreatment follow-up (RR, 1.82; 95% CI, 1.01-3.28; P = .02). Study medication was generally well tolerated, and treatment-emergent adverse events did not differ between groups (any adverse events, 55 [71.4%] in the varenicline group vs 60 [75.0%] in the placebo group; RR, 0.95; 95% CI, 0.79-1.15; P = .61).
Conclusions and Relevance
When added to weekly cessation counseling for adolescent cigarette smokers, varenicline, compared with placebo, was well tolerated but did not improve end-of-treatment abstinence. However, varenicline may hasten abstinence and yield improvements in posttreatment abstinence outcomes.
Trial Registration
ClinicalTrials.gov identifier: NCT01509547
Introduction
Tobacco use is the leading cause of preventable death in the United States and the world.1 Although rates of smoking initiation in adolescence have declined in the last decade, most adult cigarette smokers began smoking before 21 years of age, and almost all adolescents who smoke regularly will continue smoking well into adulthood, leading to a life expectancy at least 10 years shorter than that of nonsmokers.1,2,3,4 Almost two-thirds of adolescent smokers are interested in quitting, but only 4% to 6% of unassisted quit attempts are successful.3,4,5,6
Few controlled studies have evaluated adolescent smoking cessation treatments, and almost all have focused on psychosocial interventions with generally discouraging results. A meta-analysis of 48 studies7 showed a mean quit rate of 9.1% compared with 6.2% among control groups. In the interest of enhancing these very modest quit rates, a handful of randomized trials have evaluated pharmacotherapies, including nicotine replacement therapy and bupropion hydrochloride, to complement psychosocial treatment.8,9,10,11,12,13,14,15,16,17 Amid mixed findings, some evidence suggests modest efficacy during treatment but low rates of abstinence at posttreatment follow-up.
The introduction of varenicline tartrate, an α4β2 nicotinic acetylcholine receptor partial agonist, represented an advance in adult smoking cessation pharmacotherapy, demonstrating superiority to placebo (end-of-treatment odds ratio [OR] range, 3.6-5.9), bupropion (OR range, 1.7-2.2), and nicotine patch (OR, 1.7).18,19,20,21,22,23 Postmarketing concerns regarding varenicline’s neuropsychiatric safety were addressed in a large trial of adults with and without stably treated psychiatric disorders, revealing no difference in neuropsychiatric adverse events compared with bupropion, nicotine patch, or placebo.23
In contrast with the wealth of randomized clinical trials evaluating varenicline in adult smokers, little work has focused on varenicline in adolescent smokers. An initial 2-week adolescent laboratory study revealed a similar pharmacokinetic profile as in adults, with no serious adverse events or discontinuations due to adverse events,24 and a subsequent proof-of-concept pilot adolescent smoking cessation trial25 further supported the feasibility of this line of research. Encouraged by these findings, we sought to evaluate the efficacy and safety of varenicline for adolescent smoking cessation via a randomized clinical trial, hypothesizing that, when added to weekly cessation counseling, varenicline therapy would be well-tolerated and would yield higher rates of abstinence than placebo.
Methods
Trial Design
Treatment-seeking adolescent smokers were randomized in 1:1 parallel group allocation to receive a double-blind 12-week course of varenicline or placebo added to weekly cessation counseling. Self-reported smoking data were collected via daily diaries and timeline follow-back methods.26 For biomarkers of smoking, breath carbon monoxide (CO) level was measured at all visits, and urine cotinine level was measured at the end-of-treatment visit. The study was conducted within an approved US Food and Drug Administration Investigational New Drug application. The institutional review board of the University of South Carolina, Charleston, approved the study protocol (Supplement 1), which was overseen by an independent National Institute on Drug Abuse–appointed data and safety monitoring board. If the participant was younger than 18 years, a parent or guardian provided written informed consent and the adolescent provided assent. Individuals 18 years or older provided informed consent. We followed the Consolidated Standards of Reporting Trials (CONSORT) reporting guideline.
Participants
The trial was implemented in a university outpatient clinic in Charleston, South Carolina. The trial enrolled adolescent and young adult cigarette smokers aged 14 to 21 years who smoked daily for at least 6 months, had at least 1 failed prior quit attempt, and were seeking to quit. Female participants agreed to use reliable birth control methods. Individuals with a history of DSM-IV-TR mood or psychotic disorders, suicidality, homicidality, or significant hostility or aggression; with current DSM-IV-TR substance dependence other than nicotine, unstable medical disorder, pregnancy, breastfeeding, or use of medications with smoking cessation efficacy; or with known hypersensitivity to varenicline use were excluded. Recruitment was conducted from August 1, 2012, through October 31, 2017, primarily through media advertisements. Participant enrollment, occurring within this active recruitment period, spanned August 15, 2012, through October 20, 2017. Interested individuals were prescreened by telephone, and those meeting general entry criteria were scheduled for consent and screening procedures in the clinic.
General Procedures
At the baseline visit, comprehensive psychiatric and substance use diagnostic assessments, medical history, physical examination, and laboratory testing (urine pregnancy and drug tests) were performed to evaluate suitability for inclusion based on the aforementioned criteria.27,28 We used the timeline follow-back method to assess past 30-day self-reported tobacco and other substance use.26
Participants were seen weekly during the 12-week treatment, and follow-up posttreatment assessments were conducted at weeks 18 and 26. During weekly treatment visits, brief smoking cessation counseling, medication management, and adverse event assessment were provided by a study physician or physician assistant; participants completed self-assessments; and breath CO level was assessed. Follow-up was completed on January 25, 2018.
Interventions
Medication
Participants were randomized to a double-blind 12-week course of orally administered varenicline tartrate or placebo. Randomization was on a 1:1 ratio, with stratification by the prognostic covariates of age (14-17 vs 18-21 years) and baseline smoking level (<12 vs ≥12 cigarettes per day [CPD], based on median baseline smoking in a previous randomized adolescent smoking cessation trial).16 Pfizer, Inc supplied varenicline tartrate 0.5-mg and 1.0-mg tablets and matched placebo tablets to the university investigational pharmacy. All tablets were packaged in blister packs with individual labels for time and date of each dose and were dispensed in weekly supplies at study visits; medication diaries and pill counts were used to assess adherence. Consistent with recommendations from an adolescent varenicline pharmacokinetic study,14 participants weighing more than 55 kg received varenicline tartrate or placebo in a dosage of 0.5 mg once daily for 3 days, titrated to 0.5 mg twice daily for 4 days, and titrated to 1.0 mg twice daily thereafter. Participants weighing 55 kg or less received varenicline tartrate or placebo in a dosage of 0.5 mg once daily for 7 days and titrated to 0.5 mg twice daily thereafter.
Embedded Psychosocial Treatment
During the assessment visit, participants were given adolescent-targeted smoking cessation brochures and briefly counseled on cessation strategies. Participants were instructed to set a quit date 1 week after therapy initiation. At weekly visits, participants were provided with brief individual skills-based cessation counseling that paralleled psychosocial interventions in similarly structured adolescent and adult cessation pharmacotherapy studies.14,18,19,20 If participants did not succeed with the initial quit date, they were advised to select another quit date.
Outcomes
Efficacy
The primary efficacy outcome was urine cotinine level–confirmed (≤50 ng/mL) 7-day self-reported abstinence at the week 12 end-of-treatment visit. Secondary efficacy measures included weekly self-reported and breath CO–confirmed (≤8 ppm) abstinence (since the prior weekly visit) during study treatment (weeks 1-12) and 7-day abstinence at follow-up (weeks 18 and 26). In addition, time from therapy initiation to the first period of at least 7 days of sustained abstinence was measured. Timeline follow-back methods allowed for reliable collection of smoking data even during periods when study visits were missed.26
Safety and Tolerability
The primary safety outcome was frequency of treatment-emergent adverse events. General and neuropsychiatric adverse events were systematically assessed by a study physician or physician assistant at all visits.
Statistical Analysis
Sample Size Determination
Data were analyzed from March 19, 2018, to August 11, 2018, with further revisions completed April 10, 2019. All statistical analysis was conducted using SAS, version 9.4 (SAS Institute Inc), and 2-sided P < .05 indicated significance. The study was powered to detect an end-of-treatment 21% abstinence rate in the varenicline group compared with 6% in the placebo group in an intention-to-treat sample, based on estimates from previous trials.7,14,16,25 With a type I error rate of 5% and power (1 − β) of 80%, the study required randomization of 83 participants per treatment group (166 total randomized) using a 2-sided Pearson χ2 test statistic. No interim efficacy or futility analyses were specified or conducted.
Primary Analysis of Smoking Outcomes
Standard descriptive statistics were used to summarize characteristics of the study sample. The primary hypothesis was that participants in the varenicline group (varenicline participants) would have a higher probability than those in the placebo participants of end-of-treatment cotinine-confirmed 7-day abstinence. A logistic regression model with a sandwich variance estimate was used to assess this primary efficacy outcome.29 To assess the potential effect of missing outcome data on parameter estimates, sensitivity analyses were completed (1) with all available data, (2) with missing outcome data imputed as smoking (worst-case imputation), and (3) using methods of multiple imputation.30 Imputation of missing outcome data as positive for smoking is often used in cessation trials because it does not necessitate the missing-at-random assumption and allows for a correlation between missing status and smoking status.31 However, when the data are missing at random, this method may add significant bias to the parameter estimates. Further, multiple imputation was implemented using fully conditional specification with a logistic regression approach. Owing to the high rate of missing data, 100 imputation data files were created to assure reasonable relative efficiency.32 Parameter estimates were recovered from logistic regression models noted above. Imputation of cotinine-confirmed smoking status at the end of treatment was based on model variables (randomized treatment assignment, baseline-reported CPD, and participant age). No independent variables from the models were missing in the data file. Imputed values for smoking status were included in a logistic regression model and combined to generate parameter estimates and 95% CIs.33 Risk ratios (RRs) and asymptotic 95% CIs were computed for efficacy estimates.
Secondary Analysis of Smoking Outcomes
Repeated-measures logistic regression models using the methods of generalized estimating equations34 were constructed to assess self-reported and CO-confirmed abstinence at weekly treatment visits (weeks 1-12) and 7-day abstinence at posttreatment follow-up visits (weeks 18 and 26). Working correlation structures were independently compared, and the final model structure was chosen using the quasi-likelihood under the independence model criterion35 and direct comparison of the robust and model-based variance-covariance matrices; specifically autoregressive, compound symmetric, and banded structures were compared. In self-reported and CO-confirmed use data, a first-order autoregressive structure provided a better model fit than a compound symmetric or a banded structure. Risk ratios and asymptotic 95% CIs were computed for all efficacy estimates. Sensitivity analysis was completed as described above. For models with repeated outcome measures on participants, fully conditional specification methods were implemented using a logistic structure, and additional variables to account for visit number and clustering of data on participants were added to the imputation process at each visit.36 Time to first 7-day abstinence was assessed using log-rank test statistics and Cox proportional hazards regression models. All models were adjusted for baseline-reported CPD, participant age, and study visit when appropriate.
Analysis of Adverse Events
Treatment-emergent adverse event rates were compiled using all reported events after randomization, regardless of attributed medication relatedness. Negative binomial regression models were used to compare event rates (counts per participant) as well as to compare rates of participants experiencing events (any vs none). All adverse event rate comparisons used 2-sided statistical tests.
Results
Participant Characteristics
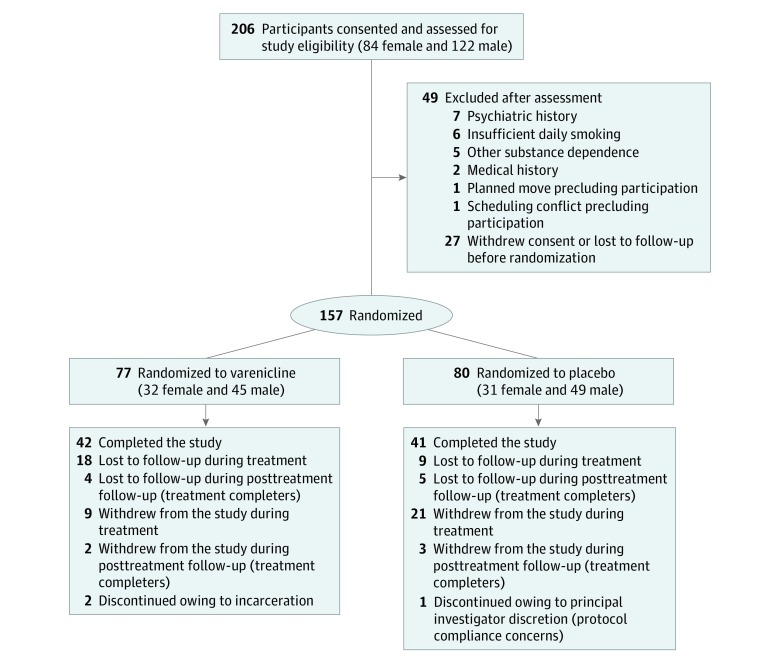
Participant demographics and baseline characteristics are presented in Table 1, and progression through trial procedures is summarized in Figure 1. Of 206 screened individuals, 157 were randomized (94 male [59.9%] and 63 female [40.1%]; mean [SD] age, 19.1 [1.5] years), 77 to varenicline and 80 to placebo. This randomized total fell slightly short of the goal of 166 participants. Although enrollment progressed at the goal rate for most of the rolling recruitment period, amid reduced rates of adolescent daily smoking it progressively declined toward the end, despite an extension of the enrollment period and redoubled recruitment efforts. This situation ultimately led the investigative team to conclude enrollment at 157 randomized participants. Of these, 146 (93.0%) attended at least 1 postrandomization visit (68 varenicline and 78 placebo participants). The end-of-treatment (week 12) visit was attended by 90 participants (57.3%) (45 varenicline and 45 placebo group participants). Participants who attended the week 12 visit, compared with those who did not, were not significantly different on any baseline measures (Table 1). Week 26 follow-up data were collected on 83 participants (52.9%). Enrolled participants were predominantly white (120 [76.4%]). At study entry, participants smoked a mean (SD) of 11.5 (6.8) CPD and had been smoking regularly for more than 4 years.
Table 1. Baseline Characteristics.
| Characteristic | Overall (N = 157) | Treatment Assignmenta | End-of-Treatment Outcomea | ||
|---|---|---|---|---|---|
| Varenicline (n = 77) | Placebo (n = 80) | Included (n = 90) | Not Included (n = 67) | ||
| Demographic | |||||
| Age, mean (SD), yb | 19.1 (1.5) | 19.1 (1.5) | 19.1 (1.4) | 19.1 (1.5) | 19.1 (1.4) |
| Male, No. (%) | 94 (59.9) | 49 (63.6) | 45 (56.3) | 52 (57.8) | 42 (62.7) |
| White, No. (%) | 120 (76.4) | 54 (70.1) | 66 (82.5) | 70 (77.8) | 50 (74.6) |
| Tobacco use | |||||
| No. of cigarettes smoked per day, mean (SD) | 11.5 (6.8) | 10.9 (5.2) | 12.0 (8.1) | 10.9 (7.3) | 12.3 (6.0) |
| Breath CO level, mean (SD), ppm | 15.2 (10.4) | 14.7 (9.9) | 15.6 (10.9) | 15.3 (9.6) | 14.9 (11.5) |
| Urine cotinine level, mean (SD), ng/mL | 1011 (652) | 1015 (632) | 1008 (677) | 989 (617) | 1041 (700) |
| Age started smoking regularly, mean (SD), y | 16.3 (1.9) | 16.2 (1.7) | 16.4 (2.1) | 16.3 (2.1) | 16.3 (1.5) |
| Duration of regular smoking, mean (SD), y | 4.4 (2.3) | 4.6 (2.3) | 4.2 (2.3) | 4.4 (2.5) | 4.3 (2.0) |
| No. of prior quit attempts, median (IQR) | 2.0 (2.0) | 2.0 (2.0) | 2.0 (1.0) | 2.0 (2.0) | 2.0 (2.0) |
| Other substance use | |||||
| Positive urine cannabinoid test, No. (%) | 92 (58.6) | 48 (62.3) | 44 (55.0) | 51 (56.7) | 41 (61.2) |
| Any illicit substances in past 30 d, No. (%) | 106 (67.5) | 54 (70.1) | 52 (65.0) | 61 (67.8) | 45 (67.2) |
| Any alcohol use in the past 30 d, No. (%) | 124 (79.0) | 65 (84.4) | 59 (73.8) | 69 (76.7) | 55 (82.1) |
| Total No. of alcohol drinks in past 30 d, mean (SD)c | 40.2 (43.7) | 35.7 (33.7) | 45.0 (52.5) | 41.7 (36.8) | 38.2 (51.5) |
Abbreviations: CO, carbon monoxide; IQR, interquartile range.
No statistically significant treatment group differences were found (all P ≥ .05).
Age range, 14 to 21 years.
For 124 participants who reported any drinking in the 30 days before study entry.
Figure 1. CONSORT Diagram.
Efficacy Outcomes
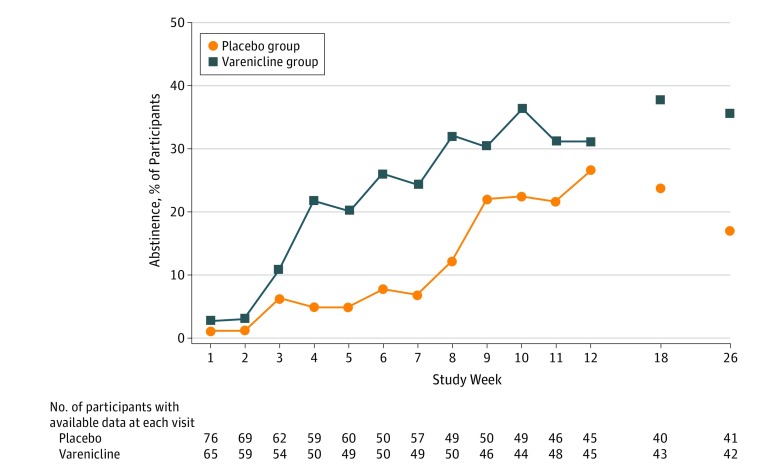
Cotinine-confirmed 7-day abstinence at the end of treatment was not statistically significantly different between randomized groups (varenicline group, 4 of 45 [8.9%]; placebo group, 4 of 45 [8.9%]; adjusted RR, 0.97; 95% CI, 0.29-2.99; P = .96). For secondary efficacy outcomes, of a possible 1884 weekly treatment visits for the 157 randomized participants, 1281 (68.0%) had associated self-reported smoking and CO measures available; 609 of 924 (65.9%) were available in the varenicline group and 672 of 960 (70.0%) in the placebo group. Abstinence rates are presented in Table 2 (with available data and with imputed missing data) and illustrated over time in Figure 2 (with available data). In the analyses using available data, compared with the placebo participants, the varenicline participants had nearly twice the probability of any self-reported abstinence during treatment (measured at weekly visits; RR, 1.81; 95% CI, 1.09-2.99; P = .02). Similarly, varenicline participants were more likely than placebo participants to report 7-day abstinence at either of the 2 posttreatment follow-up visits (RR, 1.82; 95% CI, 1.01-3.28; P = .02). Incorporating breath CO confirmation of weekly self-reported abstinence yielded similar patterns of between-group differences in efficacy outcomes during study treatment but attenuated estimates at the posttreatment follow-up visit (Table 2); however, the prevalence of cannabis use among the study sample potentially confounds interpretability of CO findings (93 participants [59.2%] had positive results of urine cannabinoid tests at baseline). When imputation methods were implemented, resulting parameter estimates were reasonably consistent with those found using available data.
Table 2. Abstinence Outcomes for Available Data and Imputed Dataa.
| End Point | No. With 7-d Abstinencea | Available Data, Estimate | Imputed Data, Estimate | |
|---|---|---|---|---|
| Impute to Smoking | Multiple Imputation | |||
| Primary Efficacy Outcome | ||||
| Cotinine-confirmed abstinence (≤50 ng/mL) | ||||
| End of treatment (week 12 only), RR (95% CI) | 8 | 0.97 (0.29-3.18) | 0.90 (0.27-3.06) | 0.95 (0.24-3.71) |
| Varenicline group, % (95% CI) | 4 | 8.9 (2.5-21.2) | 5.2 (1.4-12.8) | 8.1 (0.3-16.0) |
| Placebo group, % (95% CI) | 4 | 8.9 (2.5-21.2) | 5.0 (1.4-12.3) | 7.8 (0.3-15.5) |
| Secondary Efficacy Outcomes | ||||
| Self-reported abstinence | ||||
| During treatment (12 weekly treatment visits), RR (95% CI) | 201 | 1.81 (1.09-2.99) | 1.85 (1.06-3.23) | 2.05 (1.56-2.69) |
| Varenicline group, % (95% CI) | 131 | 21.5 (18.2-24.8) | 14.2 (11.9-16.4) | 21.7 (18.5-25.0) |
| Placebo group, % (95% CI) | 70 | 10.4 (8.1-12.7) | 7.3 (5.6-8.9) | 10.8 (8.8-13.1) |
| End of treatment (week 12 only), RR (95% CI) | 26 | 1.17 (0.63-2.20) | 1.23 (0.64-2.39) | 1.32 (0.69-2.55) |
| Varenicline group, % (95% CI) | 14 | 31.1 (17.6-44.6) | 18.2 (9.6-26.8) | 31.1 (18.5-43.6) |
| Placebo group, % (95% CI) | 12 | 26.7 (13.5-39.6) | 15.0 (7.2-22.8) | 24.0 (12.9-35.1) |
| Posttreatment follow-up (weeks 18 and 26), RR (95% CI) | 49 | 1.82 (1.01-3.28) | 1.97 (1.03-3.77) | 1.86 (1.07-3.22) |
| Varenicline group, % (95% CI) | 32 | 37.7 (27.3-48.0) | 20.8 (14.4-27.2) | 37.8 (27.7-47.8) |
| Placebo group, % (95% CI) | 17 | 21.0 (12.1-29.6) | 10.6 (5.8-15.4) | 20.9 (11.9-30.0) |
| CO-confirmed abstinence (≤8 ppm) | ||||
| During treatment (12 weekly treatment visits), RR (95% CI) | 152 | 2.09 (1.19-3.67) | 2.15 (1.15-4.00) | 2.29 (1.67-3.14) |
| Varenicline group, % (95% CI) | 103 | 16.9 (13.9-19.9) | 11.2 (9.1-13.2) | 17.0 (14.3-19.8) |
| Placebo group, % (95% CI) | 49 | 7.3 (5.2-9.4) | 5.1 (3.7-6.5) | 7.6 (5.6-9.5) |
| End of treatment (week 12 only), RR (95% CI) | 23 | 1.27 (0.64-2.50) | 1.36 (0.66-2.78) | 1.46 (0.71-2.99) |
| Varenicline group, % (95% CI) | 13 | 28.9 (15.6-42.1) | 16.9 (8.5-25.2) | 28.5 (16.4-40.6) |
| Placebo group, % (95% CI) | 10 | 22.2 (10.1-34.4) | 12.5 (5.3-19.7) | 20.0 (9.6-30.3) |
| Posttreatment follow-up (weeks 18 and 26), RR (95% CI) | 30 | 1.23 (0.57-2.66) | 1.36 (0.61-3.05) | 1.30 (0.65-2.61) |
| Varenicline group, % (95% CI) | 17 | 20.0 (11.5-28.5) | 11.0 (6.1-16.0) | 20.5 (12.1-29.0) |
| Placebo group, % (95% CI) | 13 | 16.1 (8.1-24.0) | 8.1 (3.9-12.4) | 16.3 (7.8-24.8) |
Abbreviations: CO, carbon monoxide; RR, relative risk.
Abstinence counts are collapsed across all included visits in each analysis. Presented results are adjusted for baseline number of cigarettes per day, participant age, and study visit (where appropriate).
Figure 2. Between-Visit Abstinence During Treatment and 7-Day Abstinence at Posttreatment Follow-up Visits.
Of the 157 randomized participants, 55 (35.0%) reported at least 7 consecutive abstinent days at any time during treatment (varenicline group, 31 [40.3%]; placebo group, 24 [30.0%]; log-rank P = .02). In Cox proportional hazard regression models (adjusted for baseline CPD and age), varenicline participants were nearly twice as likely as placebo participants to report at least 7 days of abstinence during treatment (hazard ratio, 1.91; 95% CI, 1.12-3.27). In those who reported at least 7 consecutive days of abstinence during treatment, the median time to the first 7 or more days of abstinence was 39 days (interquartile range, 26-59 days) in varenicline participants and 59 days (interquartile range, 45-72 days) in placebo participants.
Adverse Events
Of the 157 randomized participants, 115 (73.2%) experienced at least 1 adverse event during the study (varenicline group, 55 [71.4%]; placebo group, 60 [75.0%]; RR, 0.95; 95% CI, 0.79-1.15; P = .61). Of 391 total adverse events, 214 were reported in the varenicline group and 177 in the placebo group; most were unrelated to study treatment. Varenicline participants reported a mean (SD) of 2.8 (2.8) adverse events; placebo participants, 2.2 (2.2) adverse events (RR, 1.26; 95% CI, 0.90-1.75; P = .18). The most commonly reported categories were gastrointestinal tract and neuropsychiatric, with gastrointestinal tract events occurring in 53 participants (33.8%) (varenicline group, 29 [37.7%]; placebo group, 24 [30.0%]; P = .31) and neuropsychiatric events occurring in 50 participants (31.8%) (varenicline group, 26 [33.8%]; placebo group, 24 [30.0%]; P = .61). No medication-related serious adverse events occurred in either treatment group.
Treatment Adherence
Treatment adherence, calculated from daily medication diaries and weekly pill counts as doses taken compared with doses dispensed, was 98% in the varenicline group and 96% in the placebo group (P = .24). Similarly, 49 varenicline participants (63.6%) and 51 placebo participants (63.8%) took 100% of dispensed doses.
Discussion
Findings indicate that varenicline, when added to brief smoking cessation counseling, is adequately tolerated but does not promote end-of-treatment abstinence compared with placebo among adolescent and young adult cigarette smokers. Secondary findings suggest varenicline may hasten abstinence during treatment, allowing for continued reinforcement of abstinence and relapse prevention in the context of a structured cessation counseling program, which may translate into longer-term posttreatment sustained abstinence.37
In light of postmarketing concerns regarding neuropsychiatric adverse events with varenicline, it is encouraging that treatment-emergent adverse events, in general and specific to neuropsychiatric events, did not differ between the varenicline and placebo groups. This finding, however, occurred in the context of a trial with strict exclusion criteria (ie, history of mood or psychotic disorders, suicidality, homicidality, or significant hostility/aggression history) that reduced the likelihood of neuropsychiatric events. How the safety or efficacy findings may translate to adolescents with co-occurring psychiatric disorders, who are disproportionately represented among adolescent smokers, is not clear.38
Limitations
The use of biomarkers to verify smoking abstinence is typically regarded as a design strength, but in the present study, it may have introduced limitations. During the study’s enrollment period, the US national prevalence of adolescent tobacco use declined while rates of cannabis use increased.39 This finding was reflected in the high rates of cannabis use among enrolled participants (59.2% of participants had a positive finding of a baseline urine cannabinoid test, and 106 [67.5%] reported cannabis or other illicit substance use in the 30 days before study entry). Cannabis smoking increases breath CO level, potentially yielding a false-positive breathalyzer result40; in addition, blunts, a common mode of cannabis use, often contain residual tobacco and may yield a positive urine cotinine result.41 The US national prevalence of adolescent e-cigarette use also increased during the enrollment period39; although participants were advised not to use e-cigarettes during study participation, use may have occurred, potentially affecting urine cotinine values. Given the likelihood that bias in tobacco use self-report would be similar across treatment groups, we have secondarily presented self-report abstinence and smoking outcomes, but note that between-group comparisons incorporating biomarkers generally parallel self-report comparisons.42
Conclusions
Even amid declining rates of cigarette smoking among adolescents in the United States, the clear public health implications of adolescent-onset smoking indicate that efficacious treatments are needed for this age group, especially given that cessation during adolescence would prevent most of the morbidity and mortality incurred with years of tobacco smoking into adulthood. The present findings add to a mixed literature on adolescent smoking cessation interventions, which have generally yielded discouragingly low rates of abstinence in psychosocial and pharmacological treatment trials. Although present findings indicate that abstinence did not differ between the varenicline and placebo groups at the end of treatment, the secondary findings of earlier and sustained posttreatment follow-up response to varenicline suggest that it may provide an advantage in yielding longer-term abstinence among adolescent smokers. Future work should examine potential methods, such as the addition of behavioral incentives,16 to maximize the magnitude of smoking cessation success with varenicline and other treatments to improve health outcomes for adolescent smokers, a highly vulnerable group in need of improved treatment options.
In this study, among treatment-seeking adolescent and young adult daily cigarette smokers, a 12-week course of varenicline, compared with placebo, when added to weekly cessation counseling, was well-tolerated but did not improve end-of-treatment abstinence. However, secondary findings suggest that varenicline may promote abstinence early in treatment and increase abstinence at posttreatment follow-up.
Trial Protocol
Data Sharing Statement
References
- 1.United States Department of Health and Human Services The Health Consequences of Smoking—50 Years of Progress: A Report of the Surgeon General. Atlanta, GA: United States Dept of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health; 2014. [Google Scholar]
- 2.Backinger CL, Fagan P, Matthews E, Grana R. Adolescent and young adult tobacco prevention and cessation: current status and future directions. Tob Control. 2003;12(12)(suppl 4):IV46-IV53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chassin L, Presson CC, Sherman SJ, Edwards DA. The natural history of cigarette smoking: predicting young-adult smoking outcomes from adolescent smoking patterns. Health Psychol. 1990;9(6):701-716. doi: 10.1037/0278-6133.9.6.701 [DOI] [PubMed] [Google Scholar]
- 4.Cantrell J, Bennett M, Mowery P, et al. Patterns in first and daily cigarette initiation among youth and young adults from 2002 to 2015. PLoS One. 2018;13(8):e0200827. doi: 10.1371/journal.pone.0200827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Stanton WR, McClelland M, Elwood C, Ferry D, Silva PA. Prevalence, reliability and bias of adolescents’ reports of smoking and quitting. Addiction. 1996;91(11):1705-1714. doi: 10.1111/j.1360-0443.1996.tb02273.x [DOI] [PubMed] [Google Scholar]
- 6.Zhu SH, Sun J, Billings SC, Choi WS, Malarcher A. Predictors of smoking cessation in US adolescents. Am J Prev Med. 1999;16(3):202-207. doi: 10.1016/S0749-3797(98)00157-3 [DOI] [PubMed] [Google Scholar]
- 7.Sussman S, Sun P, Dent CW. A meta-analysis of teen cigarette smoking cessation. Health Psychol. 2006;25(5):549-557. doi: 10.1037/0278-6133.25.5.549 [DOI] [PubMed] [Google Scholar]
- 8.Jacobsen LK, Krystal JH, Mencl WE, Westerveld M, Frost SJ, Pugh KR. Effects of smoking and smoking abstinence on cognition in adolescent tobacco smokers. Biol Psychiatry. 2005;57(1):56-66. doi: 10.1016/j.biopsych.2004.10.022 [DOI] [PubMed] [Google Scholar]
- 9.Killen JD, Ammerman S, Rojas N, Varady J, Haydel F, Robinson TN. Do adolescent smokers experience withdrawal effects when deprived of nicotine? Exp Clin Psychopharmacol. 2001;9(2):176-182. doi: 10.1037/1064-1297.9.2.176 [DOI] [PubMed] [Google Scholar]
- 10.Prokhorov AV, Hudmon KS, de Moor CA, Kelder SH, Conroy JL, Ordway N. Nicotine dependence, withdrawal symptoms, and adolescents’ readiness to quit smoking. Nicotine Tob Res. 2001;3(2):151-155. doi: 10.1080/14622200110043068 [DOI] [PubMed] [Google Scholar]
- 11.Hanson K, Allen S, Jensen S, Hatsukami D. Treatment of adolescent smokers with the nicotine patch. Nicotine Tob Res. 2003;5(4):515-526. doi: 10.1093/ntr/5.4.515 [DOI] [PubMed] [Google Scholar]
- 12.Killen JD, Robinson TN, Ammerman S, et al. Randomized clinical trial of the efficacy of bupropion combined with nicotine patch in the treatment of adolescent smokers. J Consult Clin Psychol. 2004;72(4):729-735. doi: 10.1037/0022-006X.72.4.729 [DOI] [PubMed] [Google Scholar]
- 13.Moolchan ET, Robinson ML, Ernst M, et al. Safety and efficacy of the nicotine patch and gum for the treatment of adolescent tobacco addiction. Pediatrics. 2005;115(4):e407-e414. doi: 10.1542/peds.2004-1894 [DOI] [PubMed] [Google Scholar]
- 14.Muramoto ML, Leischow SJ, Sherrill D, Matthews E, Strayer LJ. Randomized, double-blind, placebo-controlled trial of 2 dosages of sustained-release bupropion for adolescent smoking cessation. Arch Pediatr Adolesc Med. 2007;161(11):1068-1074. doi: 10.1001/archpedi.161.11.1068 [DOI] [PubMed] [Google Scholar]
- 15.Rubinstein ML, Benowitz NL, Auerback GM, Moscicki AB. A randomized trial of nicotine nasal spray in adolescent smokers. Pediatrics. 2008;122(3):e595-e600. doi: 10.1542/peds.2008-0501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gray KM, Carpenter MJ, Baker NL, et al. Bupropion SR and contingency management for adolescent smoking cessation. J Subst Abuse Treat. 2011;40(1):77-86. doi: 10.1016/j.jsat.2010.08.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Scherphof CS, van den Eijnden RJ, Engels RC, Vollebergh WA. Short-term efficacy of nicotine replacement therapy for smoking cessation in adolescents: a randomized controlled trial. J Subst Abuse Treat. 2014;46(2):120-127. doi: 10.1016/j.jsat.2013.08.008 [DOI] [PubMed] [Google Scholar]
- 18.Gonzales D, Rennard SI, Nides M, et al. ; Varenicline Phase 3 Study Group . Varenicline, an α4β2 nicotinic acetylcholine receptor partial agonist, vs sustained-release bupropion and placebo for smoking cessation: a randomized controlled trial. JAMA. 2006;296(1):47-55. doi: 10.1001/jama.296.1.47 [DOI] [PubMed] [Google Scholar]
- 19.Jorenby DE, Hays JT, Rigotti NA, et al. ; Varenicline Phase 3 Study Group . Efficacy of varenicline, an α4β2 nicotinic acetylcholine receptor partial agonist, vs placebo or sustained-release bupropion for smoking cessation: a randomized controlled trial. JAMA. 2006;296(1):56-63. doi: 10.1001/jama.296.1.56 [DOI] [PubMed] [Google Scholar]
- 20.Nides M, Oncken C, Gonzales D, et al. Smoking cessation with varenicline, a selective α4β2 nicotinic receptor partial agonist: results from a 7-week, randomized, placebo- and bupropion-controlled trial with 1-year follow-up. Arch Intern Med. 2006;166(15):1561-1568. doi: 10.1001/archinte.166.15.1561 [DOI] [PubMed] [Google Scholar]
- 21.Aubin HJ, Bobak A, Britton JR, et al. Varenicline versus transdermal nicotine patch for smoking cessation: results from a randomised open-label trial. Thorax. 2008;63(8):717-724. doi: 10.1136/thx.2007.090647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Eisenberg MJ, Filion KB, Yavin D, et al. Pharmacotherapies for smoking cessation: a meta-analysis of randomized controlled trials. CMAJ. 2008;179(2):135-144. doi: 10.1503/cmaj.070256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Anthenelli RM, Benowitz NL, West R, et al. Neuropsychiatric safety and efficacy of varenicline, bupropion, and nicotine patch in smokers with and without psychiatric disorders (EAGLES): a double-blind, randomised, placebo-controlled clinical trial. Lancet. 2016;387(10037):2507-2520. doi: 10.1016/S0140-6736(16)30272-0 [DOI] [PubMed] [Google Scholar]
- 24.Faessel H, Ravva P, Williams K. Pharmacokinetics, safety, and tolerability of varenicline in healthy adolescent smokers: a multicenter, randomized, double-blind, placebo-controlled, parallel-group study. Clin Ther. 2009;31(1):177-189. doi: 10.1016/j.clinthera.2009.01.003 [DOI] [PubMed] [Google Scholar]
- 25.Gray KM, Carpenter MJ, Lewis AL, Klintworth EM, Upadhyaya HP. Varenicline versus bupropion XL for smoking cessation in older adolescents: a randomized, double-blind pilot trial. Nicotine Tob Res. 2012;14(2):234-239. doi: 10.1093/ntr/ntr130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sobell LC, Sobell MB, Leo GI, Cancilla A. Reliability of a timeline method: assessing normal drinkers’ reports of recent drinking and a comparative evaluation across several populations. Br J Addict. 1988;83(4):393-402. doi: 10.1111/j.1360-0443.1988.tb00485.x [DOI] [PubMed] [Google Scholar]
- 27.Sheehan DV, Lecrubier Y, Sheehan KH, et al. The Mini-International Neuropsychiatric Interview (MINI): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J Clin Psychiatry. 1998;59(suppl 20):22-33. [PubMed] [Google Scholar]
- 28.First M, Spitzer R, Gibbon M, Williams J. Structured Clinical Interview for Axis I Disorders, Patient Edition. New York, NY: Biometrics Research, New York State Psychiatric Institute; 2004. [Google Scholar]
- 29.Zou G. A modified Poisson regression approach to prospective studies with binary data. Am J Epidemiol. 2004;159(7):702-706. doi: 10.1093/aje/kwh090 [DOI] [PubMed] [Google Scholar]
- 30.Rubin DB. Multiple Imputation for Nonresponse in Surveys. New York, NY: John Wiley & Sons; 1987. https://onlinelibrary.wiley.com/doi/pdf/10.1002/9780470316696.fmatter. doi: 10.1002/9780470316696 [DOI] [Google Scholar]
- 31.Barnes SA, Larsen MD, Schroeder D, Hanson A, Decker PA. Missing data assumptions and methods in a smoking cessation study. Addiction. 2010;105(3):431-437. doi: 10.1111/j.1360-0443.2009.02809.x [DOI] [PubMed] [Google Scholar]
- 32.von Hippel PT. How many imputations do you need? a two-stage calculation using a quadratic rule [published online January 18, 2018]. Sociol Methods Res. doi: 10.1177/0049124117747303 [DOI] [Google Scholar]
- 33.Groenwold RHH, Moons KGM, Vandenbroucke JP. Randomized trials with missing outcome data: how to analyze and what to report. CMAJ. 2014;186(15):1153-1157. doi: 10.1503/cmaj.131353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zeger SL, Liang KY. Longitudinal data analysis for discrete and continuous outcomes. Biometrics. 1986;42(1):121-130. doi: 10.2307/2531248 [DOI] [PubMed] [Google Scholar]
- 35.Pan W. Akaike’s information criterion in generalized estimating equations. Biometrics. 2001;57(1):120-125. doi: 10.1111/j.0006-341X.2001.00120.x [DOI] [PubMed] [Google Scholar]
- 36.Huque MH, Carlin JB, Simpson JA, Lee KJ. A comparison of multiple imputation methods for missing data in longitudinal studies. BMC Med Res Methodol. 2018;18(1):168. doi: 10.1186/s12874-018-0615-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hajek P, Tønnesen P, Arteaga C, Russ C, Tonstad S. Varenicline in prevention of relapse to smoking: effect of quit pattern on response to extended treatment. Addiction. 2009;104(9):1597-1602. doi: 10.1111/j.1360-0443.2009.02646.x [DOI] [PubMed] [Google Scholar]
- 38.Griesler PC, Hu MC, Schaffran C, Kandel DB. Comorbid psychiatric disorders and nicotine dependence in adolescence. Addiction. 2011;106(5):1010-1020. doi: 10.1111/j.1360-0443.2011.03403.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Miech RA, Johnston LD, O’Malley PM, Bachman JG, Schulenber JE, Patrick ME. Monitoring the Future: National Survey Results on Drug Use, 1975–2017: Volume I, Secondary School Students. Ann Arbor: Institute for Social Research, University of Michigan; 2018. doi: 10.3998/2027.42/146530 [DOI] [Google Scholar]
- 40.Moolchan ET, Zimmerman D, Sehnert SS, Zimmerman D, Huestis MA, Epstein DH. Recent marijuana blunt smoking impacts carbon monoxide as a measure of adolescent tobacco abstinence. Subst Use Misuse. 2005;40(2):231-240. doi: 10.1081/JA-200048461 [DOI] [PubMed] [Google Scholar]
- 41.Peters EN, Schauer GL, Rosenberry ZR, Pickworth WB. Does marijuana “blunt” smoking contribute to nicotine exposure? preliminary product testing of nicotine content in wrappers of cigars commonly used for blunt smoking. Drug Alcohol Depend. 2016;168:119-122. doi: 10.1016/j.drugalcdep.2016.09.007 [DOI] [PubMed] [Google Scholar]
- 42.Blank MD, Breland AB, Enlow PT, Duncan C, Metzger A, Cobb CO. Measurement of smoking behavior: comparison of self-reports, returned cigarette butts, and toxicant levels. Exp Clin Psychopharmacol. 2016;24(5):348-355. doi: 10.1037/pha0000083 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial Protocol
Data Sharing Statement