Key Points
Question
Does cardiovascular risk increase and lead to the development of pathologic cardiovascular phenotypes among US football players?
Findings
In this multiyear, multicenter, longitudinal and observational cohort study of 126 collegiate US football athletes, weight gain, increased systolic blood pressure, and arterial stiffening and reduced diastolic function occurred over 3 years. Increased weight and blood pressure were associated with arterial stiffening and the development of concentric left ventricular hypertrophy.
Meaning
Throughout collegiate US football exposure, weight gain and hypertension progressed and were associated with the development of a pathologic cardiovascular phenotype characterized by concentric left ventricular hypertrophy, arterial stiffening, and reduced left ventricular diastolic function.
This cohort study assesses collegiate US football players to quantify the emergence and progression of multiple independent factors associated with cardiovascular risk across serial years of football participation.
Abstract
Importance
Former US football athletes are at increased risk of cardiovascular (CV) morbidity and mortality compared with the general population and other professional athletes. However, responsible maladaptive CV phenotypes have not been fully characterized.
Objective
To address the emergence and progression of multiple independent factors associated with CV risk across serial years of collegiate US football participation.
Design, Setting, and Participants
Collegiate US football athletes from 2 National Collegiate Athletic Association Division I programs were recruited as freshmen between June 2014 and June 2017 and analyzed at multiple points throughout 3 complete years of collegiate US football participation (until January 2019). Excluded athletes were those who did not complete any season of US football training because of injury, illness, or leaving the team. Factors associated with CV risk assessed clinically, by transthoracic echocardiography, and by vascular applanation tonometry were recorded.
Exposures
The exposure of interest was seasonal US football exposure, including training, competition, and the training environment.
Main Outcomes and Measures
Primary outcome measures were left ventricular mass index and geometry (cardiac structure), early diastolic myocardial relaxation velocity (E′; diastolic function), and pulse-wave velocity (arterial stiffness).
Results
Of 186 individuals recruited as freshmen, 126 athletes were included in analyzed data. Collegiate US football athletes (62 white individuals [49%]; 63 black individuals [50%]; 77 nonlinemen [61%]; 49 linemen [39%]; 126 male individuals [100%]) weighed a mean (SD) of 101.1 (21.0) kg, with a mean systolic blood pressure of 129.1 (11.6) mm Hg at baseline of the freshman season. Adjusting for race, height, and player position, there were significant increases in weight (mean [SE] Δ, 4.74 [0.6] kg; P < .001), systolic blood pressure (mean [SE] Δ, 11.6 [1.6] mm Hg; P < .001), and pulse-wave velocity (mean [SE] Δ, 0.24 [0.09] m/s; P = .007), and significant declines in E′ (mean [SE] Δ, −1.7 [0.3] cm/s; P < .001) across 3 years of US football participation. Weight gain was associated with both arterial stiffening (increased pulse-wave velocity, β = 0.01 [SE, 0.004]; P = .003) and the development of concentric left ventricular hypertrophy (odds ratio, 1.09 [95% CI, 1.05-1.14]; P < .001); increased systolic blood pressure was also associated with arterial stiffening (β = 0.01 [SE, 0.003]; P = .007) and the development of concentric left ventricular hypertrophy (odds ratio, 1.04 [95% CI, 1.01-1.07]; P = .02).
Conclusions and Relevance
Collegiate US football athletes who gain weight and develop increased systolic blood pressure levels are at risk for the development of a pathologic CV phenotype characterized by concentric left ventricular hypertrophy, arterial stiffening, and reduced left ventricular diastolic function. Future work aimed at optimizing CV health in this population, who are young but uniquely at risk, is warranted.
Introduction
Early-life participation in competitive endurance and team-based sports reduces later-life health care utilization and improves longevity.1,2 To what degree this paradigm applies to US football, the most popular team sport in the United States, remains incompletely understood and actively debated.3,4 Mortality studies comparing former US football athletes with the general population document increased cardiovascular (CV) mortality among US football athletes with playing-time body mass index values (calculated as weight in kilograms divided by height in meters squared) greater than 35,5 as well as those that played at a lineman (LM) field position.6 Further, a recent study comparing longevity among former professional baseball vs US football players, a study design that minimized the influence of a healthy-worker effect,7 found higher CV and all-cause mortality among the former US football athletes.8 To date, the CV pathology underlying the inherent risk associated with US football participation remains incompletely understood.
Cross-sectional studies have documented left ventricular (LV) hypertrophy9 and hypertension among active professional US football players.10 However, professional US football athletes accrue years of US football exposure prior to joining the National Football League. The transition from high school to the collegiate US football ranks has been identified as a critical period of CV maladaptation,11,12 and weight gain during collegiate US football participation is independently associated with later-life CV morbidity.12 Observational studies of freshman collegiate US football athletes have also documented the emergence of concentric LV hypertrophy with relative functional impairments,13,14 hypertension,15 and arterial stiffening.16 However, contemporary understanding of how US football participation affects the CV system is limited by the relatively short duration of prior longitudinal studies and their focus on single components of cardiac health.
We sought to address this knowledge gap by examining the emergence and progression of multiple independent factors associated with CV risk across serial years of collegiate US football participation. We hypothesized that established determinants of CV risk, including weight gain, hypertension, concentric LV hypertrophy with functional impairment, and arterial stiffening, would develop and progress over the course of a multiyear collegiate US football career. To address this hypotheses, we enrolled a multicenter cohort of US football athletes and performed serial multimodality CV phenotyping throughout the collegiate US football experience.
Methods
We recruited US football athletes from National Collegiate Athletic Association Division I programs at Georgia Institute of Technology (Atlanta, Georgia) and Furman University (Greenville, South Carolina) between June 2014 and June 2017. Any athlete with known hypertension requiring pharmacotherapy at enrollment was excluded. Only US football athletes 18 years or older available for all longitudinal data collection points were eligible for analysis. All US football athletes leaving the team for any reason, including experiencing a season-ending injury or illness, at any point during the study period were excluded from analysis. Clinical characteristics, anthropometric measurements, complete transthoracic echocardiographic findings, and vascular applanation tonometry findings were longitudinally captured for all eligible participants at multiple points over 3 competitive collegiate US football seasons. The Emory institutional review board approved all aspects of the study, and participants provided written informed consent.
Study Population
Participants were enrolled in serial fashion across the study period, with new freshman athletes recruited and followed up longitudinally each year and studied at predefined prospective points. Point 1 was defined as the preseason of the freshman US football season; point 2 was the immediate beginning of the freshman postseason, approximately 5 to 6 months later; point 3 was defined as the immediate beginning of the sophomore postseason, approximately 1 year after point 2; and point 4 was defined as the immediate beginning of the junior postseason, approximately 1 year after point 3. Two points for freshman US football athletes were chosen because of the considerable CV plasticity previously demonstrated among first-year college athletes in response to the hemodynamic stress of athletic training.13 Because collegiate US football athletes at the participating institutions engage in consistent levels of US football training throughout the calendar year after their freshman season, only annual postseason points were captured after the first year.
The field position for each US football participant was classified as either LM or nonlinemen (NLM), as previously proposed.17 Specifically, LM positions included players at the tackle, guard, center, or defensive end positions, while NLM positions included quarterbacks, running backs, wide receivers, tight ends, linebackers, cornerbacks, safeties, kickers, and punters. All US football participants were subject to testing for performance-enhancing drugs, as dictated by National Collegiate Athletic Association standards.
Anthropometric and clinical data collected included age (in years), weight (in kilograms), height (in centimeters), current prescription medication use, systolic (SBP) and diastolic blood pressure measurements (in millimeters of mercury; measured using a manual aneroid sphygmomanometer and an appropriately sized cuff after >15 minutes of rest), complete transthoracic echocardiography results, and vascular applanation tonometry results. A family history of hypertension and early coronary artery disease and participant height (in centimeters) were recorded at point 1. Race was self-reported by participants to assess for associations with our primary outcome measures. Participants were required to abstain from exercise for 24 hours or more prior to all data collection points.
Cardiac Structure and Function
Transthoracic echocardiography was performed using a commercially available system (Vivid-I [GE Healthcare]) after 20 minutes of rest. Two-dimensional, tissue-Doppler, and speckle-tracking imaging from standard parasternal and apical positions was performed by experienced sonographers from Digirad and Athletic Heart, both mobile ultrasonography organizations in the United States. Throughout the study period, the sonographers involved with this study (n = 7) were consistent; all were employed at institutions accredited by the Intersocietal Commission for the Accreditation of Echocardiography Laboratories. All studies were obtained prospectively and only images with adequate LV endocardial definitions were recorded for analysis. For the primary outcome variables and measurements of cardiac structure, we obtained adequate images for analysis on all participants available at the study points. All data were stored digitally, and poststudy offline data analysis (EchoPAC version 7 [GE Healthcare]) was performed (J.H.K.). Definitions of normality for cardiac structure and function were adopted from the most recent guidelines.18 Left ventricular mass was calculated using the area-length method.18 Relative wall thickness was calculated as (interventricular septum thickness + posterior wall thickness [in millimeters]) / LV end-diastolic diameter (in millimeters). Concentric LV hypertrophy was defined as a relative wall thickness greater than 0.42 with an LV mass index greater than 102 g/m2; concentric LV remodeling was defined as a relative wall thickness greater than 0.42 with an LV mass index of 102 g/m2 or less; and eccentric LV hypertrophy was defined as a relative wall thickness of 0.42 or less with an LV mass index greater than 102 g/m2.18 Measurements were adjusted for body surface area when appropriate.19 Left ventricular ejection fraction, end-diastolic volume, and end-systolic volume were calculated using the modified biplane technique.18 Comprehensive assessment of regional myocardial systolic and diastolic function using speckle-tracking and tissue-Doppler imaging was performed. Longitudinal myocardial tissue velocities (early relaxation E′, A′, and S′) were measured from color-coded images at the lateral and septal mitral annulus. The E′ value was then reported as the mean value between the 2 measurements. Global LV longitudinal strain was measured in the apical 4-chamber view.
Arterial Function
Indices of arterial stiffness were measured using high-fidelity applanation tonometry (SphygmoCor [Atcor Medical]), which records sequential high-quality pressure waveforms at peripheral pulse sites. Full details of tonometer technology and measurement algorithms have been previously detailed.20 The primary measure of arterial function was the carotid-femoral pulse-wave velocity (PWV), which is the gold standard index of arterial stiffness and a validated surrogate of CV disease risk.20,21,22,23,24 All applanation tonometry measurements were made with participants in the supine position. Pulse-wave velocity was measured by acquisition of pressure waveforms within the carotid and femoral arteries. The distance between the carotid and femoral measurement sites was recorded manually using the so-called foot-to-foot method.21 Pulse-wave analysis derivation greater than 80% of the operator index and PWV with less than 10% SDs were required for quality control. Studies were performed by experienced device operators (J.H.K. and C.H.), with the software programmed to acknowledge internal quality control. We obtained adequate quality control on all participants available at the study points.
Statistical Analysis
Descriptive continuous variables are presented as the mean (SD) and categorical variables as percentages. Three a priori primary outcome variables were chosen to reflect cardiac structure (body surface area − indexed LV mass), cardiac function (early diastolic LV relaxation velocity [E′]), and arterial function (PWV). Separate linear mixed models were constructed to evaluate the change of the primary outcome measures across the study period by using time as the independent variable (baseline visit as reference) and adjusting for race (black or white), height, player position (LM or NLM), weight, SBP, and the 2 other respective primary outcome variables incorporated as time-varying and adjusted measurements (least square means) at all study points. Participant-specific random intercepts were incorporated to account for within-participant correlations. Similarly, linear mixed models were used to investigate the change in key clinical measurements (weight, SBP, diastolic blood pressure, and heart rate) and other basic echocardiographic parameters across the study period, adjusting for race, height, and player position. Additional subgroup analyses investigating the change in weight, SBP, PWV, E′, and LV mass index across the study period, stratified by player position (after adjusting for race and height) and participants with vs without concentric LV hypertrophy (after adjusting for race, height, and player position) were performed. The percentage of athletes who developed concentric LV hypertrophy at each point was calculated and compared with the Cochran Q test. Finally, a generalized linear mixed model (with logit link) was constructed to evaluate the associations between race, player position, weight, height, SBP, PWV, and E′ and the development of concentric LV hypertrophy. Analyses were performed with SAS software version 9.4 (SAS Institute). A P value of .05 or less was considered significant.
Results
US Football Player Characteristics
Between 2014 and 2017, a total of 186 freshman US football athletes were serially enrolled in this study; the last data collection point was in January 2019. At the conclusion of the study period, 126 US football athletes (126 male and 0 female athletes) were eligible for analysis, which included 55 athletes who reached point 4. The final study cohort was evenly distributed by race (62 white individuals [49%]; 63 African American individuals [50%]) and contained slightly more NLM field position players (77 NLM [61%] vs 49 LM [39%]). A family history of hypertension and early coronary artery disease was reported by 50 participants (39.7%) and 11 participants (8.7%), respectively. At baseline (preseason freshman year), the athletes had a mean (SD) age of 18.1 (0.2) years old, a mean (SD) weight of 101.1 (21.0) kg, and a mean (SD) height of 186.0 (0.5) cm tall. Across the entire study period, no field position switches (NLM becoming LM or vice versa) occurred.
Changes in Weight, SBP, and CV Structure and Function
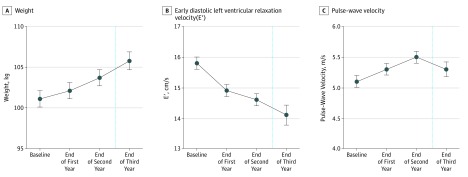
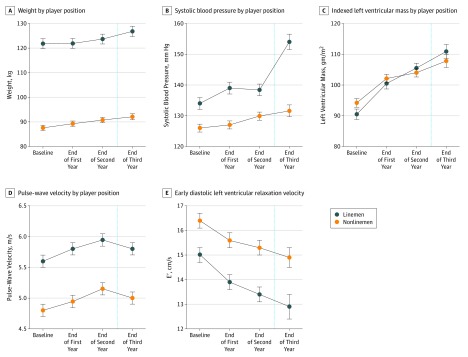
Adjusted anthropometric, structural, and functional cardiac parameters and PWV across the study period are shown in Table 1. Adjusting for height, ethnicity, and player position, collegiate US football athletes demonstrated progressive increases in SBP (cumulative mean [SE] Δ, 11.6 [1.6] mm Hg; P < .001) and weight (cumulative mean [SE] Δ, 4.74 [0.6] kg; P < .001). These findings were observed in parallel with a significant decline in CV efficiency as manifest by concomitant reductions in E′ (cumulative mean [SE] Δ, −1.7 [0.3] cm/s; P < .001) and increases in PWV (cumulative mean [SE] Δ, 0.24 [0.09] m/s; P = .007; Figure 1). Mild subclinical reductions in global LV systolic function, estimated by both global longitudinal strain and ejection fraction, also occurred across the study period. When stratified by player position and adjusted for race and height, LM demonstrated higher SBP levels (mean [SE]: baseline: 134 [1.9] vs 126 [1.3] mm Hg; point 3: 153.8 [3.5] vs 132.9 [2.4] mm Hg), lower E' (mean [SE]: baseline: 15.0 [0.3] vs 16.4 [0.3] cm/s; point 3: 12.6 [0.5] vs 14.5 [0.4] cm/s), and higher PWV (mean [SE]: baseline: 5.6 [0.1] vs 4.8 [0.1] m/s; point 3: 5.9 [0.2] vs 5.1 [0.1] m/s) at all time points (Figure 2). However, the trends of change for these adjusted measurements across the study period for each outcome measure were not significantly different among NLM and LM (Figure 2). There was no difference in the change over time for LV mass index as a function of player field position.
Table 1. Adjusted Weight, Blood Pressure, and Cardiovascular Structural and Functional Measurements Throughout Collegiate US Football Participation.
| Characteristic | Mean (SE) | P Valuea | End of Year 3, Mean (SE) | P Valueb | ||
|---|---|---|---|---|---|---|
| Baseline (N = 126) | End of Year 1 | End of Year 2 | ||||
| Anthropometric measures | ||||||
| Weight, kg | 101.1 (1.0) | 102.1 (1.0) | 103.7 (1.0) | <.001 | 105.8 (1.1) | <.001 |
| BMI | 29.3 (0.3) | 29.6 (0.3) | 30.1 (0.3) | <.001 | 30.4 (0.3) | <.001 |
| Blood pressure | ||||||
| Systolic blood pressure, mm Hg | 129.2 (1.1) | 131.7 (1.1) | 133.2 (1.1) | .001 | 140.8 (1.6) | <.001 |
| Diastolic blood pressure, mm Hg | 76.4 (0.8) | 75.2 (0.8) | 75.6 (0.9) | .52 | 75.4 (1.2) | .50 |
| Heart rate, bpm | 75.9 (1.1) | 74.4 (1.1) | 75.1 (1.1) | .54 | 76 (1.6) | .97 |
| Cardiac structure and function | ||||||
| Mean LV wall thickness, mm | 8.9 (0.1) | 9.9 (0.1) | 10.3 (0.1) | <.001 | 12.3 (0.2) | <.001 |
| LV internal diameter end-diastole/body surface area, mm/m2 | 23.2 (0.2) | 23.5 (0.2) | 23.4 (0.2) | .06 | 23.7 (0.2) | .004 |
| LV internal diameter end systole/body surface area, mm/m2 | 14.9 (0.2) | 15.2 (0.2) | 15.2 (0.2) | .08 | 15.5 (0.2) | .02 |
| LV mass/body surface area, gm/m2 | 92.8 (1.1) | 101.4 (1.1) | 104.5 (1.0 | <.001 | 108.9 (1.5) | <.001 |
| Relative wall thickness | 0.35 (0.0) | 0.38 (0.0) | 0.39 (0.0) | <.001 | 0.39 (0.0) | <.001 |
| Ejection fraction, % | 61.5 (0.4) | 60.6 (0.4) | 60.8 (0.4) | .18 | 58.6 (0.7) | <.001 |
| Global longitudinal strain, % | 19.3 (0.2) | 19.1 (0.2) | 18.6 (0.2) | .005 | 18.4 (0.3) | .005 |
| Diastolic function measurements | ||||||
| E wave velocity, cm/s | 84.5 (1.3) | 82.2 (1.3) | 78.1(1.3) | <.001 | 75.5 (1.8) | <.001 |
| A wave velocity, cm/s | 46.1 (0.9) | 42.5 (0.9) | 44.1 (0.9) | .07 | 41.4 (1.4) | .002 |
| E wave velocity–A wave velocity ratio | 1.93 (0.0) | 2.0 (0.0) | 1.9 (0.0) | .29 | 1.9 (0.1) | .98 |
| Tissue-Doppler LV mean E′, cm/s | 15.8 (0.2) | 14.9 (0.2) | 14.6 (0.2) | <.001 | 14.1 (0.3) | <.001 |
| Arterial function | ||||||
| Pulse-wave velocity (m/s) | 5.1 (0.1) | 5.3 (0.1) | 5.5 (0.1) | <.001 | 5.3 (0.1) | .007 |
Abbreviations: BMI, body mass index (calculated as weight in kilograms divided by height in meters squared); bpm, beats per minute; LV, left ventricular.
Linear mixed model comparison of end of year 2 with baseline value adjusted for height, player position, and race.
Linear mixed model comparison of end of year 3 with baseline value adjusted for height, player position, and race; 55 US football athletes were analyzed at the end of year 3.
Figure 1. Temporal Changes in Weight, Early Diastolic Myocardial Relaxation Velocity (E′), and Pulse-Wave Velocity Throughout Collegiate US Football Participation, Adjusted for Race, Height, and Player Position.
A total of 126 participants were included. At the end of the third year, 55 athletes were included in the analysis because of dropout, either from ineligibility for serial enrollment or exclusions for injury or leaving the team.
Figure 2. Progression of Key Outcome Measures Throughout Collegiate US Football Participation, Stratified by Player Position and Adjusted for Race and Height.
Forty-nine linemen and 77 nonlinemen were included. At the end of the third year, 55 athletes (32 nonlinemen and 23 linemen) were included in the analysis after participant ineligibility from serial enrollment or exclusions for injury or leaving the team.
Factors Associated With Declining E' and Increasing PWV
The results of multivariable linear mixed models to determine factors associated with declining E′ and increasing PWV are shown in Table 2. With adjustment for race, body size, player position, and SBP level, weight gain emerged as an independent factor associated with increasing PWV (β = 0.01 [SE, 0.004]; P = .003) and had a nonsignificant association with declining E′ (β = −0.02 [SE, 0.01]; P = .08). Systolic blood pressure was independently associated with increasing PWV (β = 0.01 [SE, 0.003]; P = .007) but not decreasing E′ (β = −0.01 [SE, 0.01]; P = .29). Importantly, SBP and weight levels were incorporated as adjusted, time-varying measurements in our models. No factors significantly associated with increasing LV mass index were identified.
Table 2. Factors Associated With E′, Pulse-Wave Velocity, Indexed Left Ventricular Mass, and Concentric Left Ventricular Hypertrophy.
| Characteristic | Multivariable Analysis | |
|---|---|---|
| Estimate (SE) | P Value | |
| Tissue-Doppler E′ velocitya | ||
| Race, white vs black | −0.71 (0.34) | .03 |
| Height, cm | 0.03 (0.03) | .46 |
| Player position, NLM vs LM | −0.72 (0.50) | .16 |
| Weight, kgb | −0.02 (0.01) | .08 |
| Systolic blood pressure, mm Hgb | −0.01 (0.01) | .29 |
| Pulse-wave velocity, m/sb | −0.58 (0.18) | .001 |
| LV mass index, gm/m2b | 0.01 (0.01) | .41 |
| Pulse-wave velocity | ||
| Race, white vs black | −0.09 (0.10) | .34 |
| Height, cm | 0.01 (0.01) | .43 |
| Player position, NLM vs LM | 0.21 (0.15) | .16 |
| Weight, kgb | 0.01 (0.00) | .003 |
| Systolic blood pressure, mm Hgb | 0.01 (0.00) | .007 |
| Tissue-Doppler E′ velocity, cm/sa,b | −0.04 (0.01) | .002 |
| LV mass index, gm/m2b | −0.003 (0.00) | .25 |
| LV mass index | ||
| Race, white vs black | 3.2 (1.7) | .07 |
| Height, cm | 0.1 (0.2) | .69 |
| Player position, NLM vs LM | −3.3 (2.6) | .21 |
| Weight, kgb | 0.11 (0.1) | .13 |
| Systolic blood pressure, mm Hgb | 0.07 (0.05) | .16 |
| Pulse-wave velocity, m/sb | −1.1 (0.9) | .25 |
| Tissue-Doppler E′ velocity, cm/sb | 0.21 (0.3) | .42 |
| Concentric LV hypertrophy, odds ratios (95% CIs) | ||
| Height, cm | 0.94 (0.85-1.04) | .22 |
| Race, white vs black | 2.02 (0.73-5.56) | .18 |
| Player position, NLM vs LM | 0.03 (0.01-0.17) | <.001 |
| Weight, kgb | 1.09 (1.05-1.14) | <.001 |
| Systolic blood pressure, mm Hgb | 1.04 (1.01-1.07) | .02 |
| Pulse-wave velocity, m/sb | 0.99 (0.57-1.72) | .98 |
| Tissue-Doppler E′ velocity, cm/sa,b | 0.83 (0.71-0.98) | .03 |
Abbreviations: LM, linemen; LV, left ventricle, NLM, nonlinemen.
Mean E′ velocity (cm/s).
Covariate incorporated as a time-varying measurement (adjusted least square means) at all the study points.
Weight Gain and Concentric LV Hypertrophy
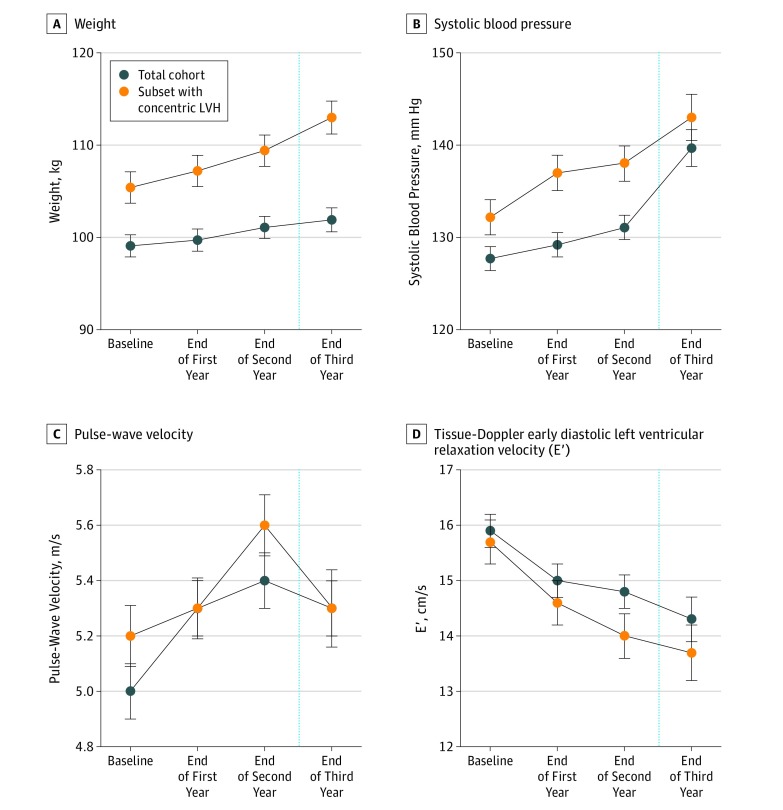
Across the span of collegiate US football participation, athletes demonstrated increased LV mass index with contributions from evolving eccentric hypertrophy, the well-established adaptive form of exercise-induced cardiac remodeling,25 and concentric hypertrophy (Table 1). Most noteworthy was the continual increase in prevalent concentric LV hypertrophy across the study period. Specifically, there was a significant increase in the percentage of athletes with concentric LV hypertrophy across the study period from baseline (4 of 126 [3.2%]) to the freshman postseason (15 of 126 [11.9%]), sophomore postseason (23 of 126 [18.3%]), and junior postseason (14 of 55 [25.4%], including 8 new cases of concentric LV hypertrophy; P = .001). Adjusting for race, height, and player position, we found that US football athletes who developed concentric LV hypertrophy demonstrated higher weights (mean [SE]: baseline, 105.4 [1.7] vs 99.1 [1.2] kg; point 3, 113.0 [1.8] vs 101.9 [1.3] kg), higher SBP level (mean [SE], baseline: 132.2 [1.9] vs 127.7 [1.3] mm Hg; point 3, 143.0 [2.5] vs 139.7 [2.0] mm Hg), higher PWV (mean [SE]: baseline, 5.2 [0.1] vs 5.0 [0.1] m/s; point 3, 5.3 [0.1] vs 5.3 [0.1] m/s), and lower E' (mean [SE]: baseline, 15.7 [0.4] vs 15.9 [0.3] cm/s; point 3, 13.7 [0.5] vs 14.3 [0.4] cm/s) compared with the athletes without concentric LV hypertrophy (Figure 3). In addition, after adjustments for race, player position, weight, SBP, and measures of CV efficiency, weight gain (odds ratio, 1.09 [95% CI, 1.05-1.14]; P < .001) and increased SBP (odds ratio, 1.04 [95% CI, 1.01-1.07]; P = .02) emerged as independent factors strongly associated with concentric LV hypertrophy (Table 2). We incorporated SBP, PWV, E′, and weight as adjusted, time-varying measurements in this model.
Figure 3. Comparison of US Football Athletes Who Developed Concentric Left Ventricular Hypertrophy and Those Without Concentric Left Ventricular Hypertrophy, Adjusted for Race, Height, and Player Position.
The total cohort included 126 individuals. At the end of the third year, 55 athletes were included in the analysis after participant ineligibility from serial enrollment or exclusions for injury or leaving the team.
Discussion
To our knowledge, this study represents the longest-duration prospective study of US football athletes and is the first to serially examine numerous complementary metrics of CV health. In summary, we observed the emergence and progression of a maladaptive CV phenotype over several years of collegiate US football participation. Specifically, collegiate US football athletes appear to be at risk of developing hypertensive SBP, concentric LV hypertrophy with relative impairments in diastolic function, and arterial stiffening. Importantly, these analyses identified weight gain throughout collegiate US football participation, a factor that has recently been associated with CV morbidity later in life among former professional US football players12 as a potential unifying mechanistic factor in the development of this constellation of early life subclinical CV pathology.
Prior epidemiologic data consistently demonstrate that former professional US football athletes who played at an LM field position or who had obese playing-time BMI levels experience increased CV mortality compared with the general population.5,6,26 In a recent mortality study designed to minimize the influence of a healthy worker bias,7 professional US football athletes were found to have higher all-cause (hazard ratio, 1.26 [95% CI, 1.10-1.44]) and CV disease mortality (hazard ratio, 2.40 [95% CI, 2.03-2.84]) compared with former professional baseball players, thereby indicating differential sport-specific health outcomes.8 To date, the underlying explanatory CV pathology remains incompletely understood. Cross-sectional and longitudinal studies of relatively short duration of active US football athletes have reported weight gain,11,14,15,16,27 hypertension,10,15 arterial stiffness,16 and subclinical decrements in LV systolic14 and diastolic function.11,13 The present study, an effort designed to capture the majority of the collegiate US football experience and examine CV health in comprehensive fashion, suggests novel clarification of the link between US football participation, weight gain, rising blood pressure, and the emergence of a subclinical maladaptive CV phenotype.
Findings from this study also provide novel insight into the contemporary understanding of the condition termed athlete heart. As suggested by Morganroth and colleagues28 in 1975 and confirmed by others,25 athletes across different sporting disciplines demonstrate variable geometric forms of LV hypertrophy. Specific to strength athletes, concentric LV remodeling is common with the conventional mechanistic explanation associated with repetitive surges in systemic afterload.25 However, the existence of a link between high-intensity isometric exercise and LV afterload has been challenged,29 and at least 1 longitudinal study30 of adults who underwent strength training, a cohort that maintained normal resting blood pressure levels, failed to detect acquired concentric remodeling. Data from the current study suggests that factors including weight gain and resting blood pressure levels are more potent determinants of which athletes who undergo strength training experience concentric LV remodeling. Importantly, the emergence of concentric LV hypertrophy, coupled with reductions in LV diastolic function and arterial stiffening, strongly suggest that this is not an adaptive response to sport but rather a form of subclinical pathology that should not be labeled athlete heart.
Defining the underlying US football mechanisms associated with weight gain and hypertension that lead to corollary CV pathology remain largely speculative. Factors that may contribute to the emergence of subclinical cardiac pathology among US football athletes include obstructive sleep apnea,27,31,32 nonsteroidal anti-inflammatory use, intake of energy and other dietary supplements, and excess sodium or calorie intake. The inclusion of the primary outcome measures used in this study should be considered as end points in the careful evaluation of each of these factors in future mechanistic analyses.
There are clinical implications taken from these data. At present, practitioners and athletic trainers charged in the care of US football athletes are faced with the challenge of identifying US football athletes at high risk, who warrant close CV monitoring. Our findings demonstrate that serial anthropometric and blood pressure measurements, coupled with the consideration of differential LV structural phenotypes, represent easily accessible metrics that can be used to identify US football athletes who may benefit from lifestyle counseling, and when indicated, guideline-based medical therapies. We also observed a dynamic interaction between the LV and arterial system that appears to emerge during US football exposure. While not directly measured, independent associations between declining E′ and increasing PWV over time suggests an element of ventricular-arterial uncoupling, a standard estimate of CV inefficiency,33 which is independently associated with adverse CV outcomes in both adult and pediatric populations.34,35,36,37,38,39 Our results set the stage for the inclusion of validated ventricular-arterial coupling measurements35 in future US football outcomes research to determine whether these early subclinical functional alterations can further reclassify risk among active US football athletes. Strengths of this study include the longitudinal assessment of CV risk and pathology over multiple years of collegiate US football participation, the inclusion of 2 large National Collegiate Athletic Association Division I athletic programs, the use of mixed models that were adequately powered to address our primary a priori outcome measures, and the even distribution of race in our data set.
Limitations
Nonetheless, we acknowledge several limitations with this study. First, although prior analyses have replicated similar longitudinal changes in CV phenotypes among freshman US football athletes,11,13,14,16 we did not have a concurrent control group followed across this same 3-year time frame. Second, we acknowledge there were incomplete cases of follow-up at point 4 (n = 42) because of the serial enrollment procedures, which limited our power at this final point. Further, we were unable to assess our primary outcomes at a senior year postseason point because of the logistical challenges of retaining and studying graduating senior-level students. Third, we acknowledge the comparison of the subcohort of US football athletes with concentric LV hypertrophy to the total cohort was limited by multiple subgroup tests. The rationale for this subanalysis was to support our primary findings. Fourth, we were unable to assess the influence of detraining on the primary outcome measures. Fifth, this study was not designed to address potential US football mechanistic factors responsible for the pathophysiology observed. Finally, this study was confined to the collegiate US football experience. Longitudinal studies of professional US football athletes and former collegiate US football athletes who are not pursuing a professional career are warranted.
Conclusions
Multiple independent and likely synergistic indices of CV risk, including increased SBP, arterial stiffening, and concentric LV hypertrophy with relative impairments in diastolic function, develop and progress over the course of a multiyear collegiate US football career. Our analyses suggest that weight gain and increased SBP during the years of collegiate US football exposure are associated with the development of this complex maladaptive CV phenotype, which may be used to identify US football athletes that warrant close clinical surveillance both during and after US football participation. Clarification of the mechanistic link between deliberate weight gain and the emergence of CV pathology, coupled with the development of directed preventive strategies designed to minimize later-life morbidity and mortality in this population, represent essential future directives.
References
- 1.Sarna S, Sahi T, Koskenvuo M, Kaprio J. Increased life expectancy of world class male athletes. Med Sci Sports Exerc. 1993;25(2):237-244. doi: 10.1249/00005768-199302000-00013 [DOI] [PubMed] [Google Scholar]
- 2.Kujala UM, Sarna S, Kaprio J, Koskenvuo M. Hospital care in later life among former world-class Finnish athletes. JAMA. 1996;276(3):216-220. doi: 10.1001/jama.1996.03540030050031 [DOI] [PubMed] [Google Scholar]
- 3.Kim JH, Zafonte R, Pascuale-Leon A, et al. American-style football and cardiovascular health. J Am Heart Assoc. 2018;7(8):e008620. doi: 10.1161/JAHA.118.008620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Araton H. For N.F.L. players, another risk: heart disease. https://www.nytimes.com/2010/11/04/sports/football/04nflhearts.html. Published November 3, 2010. Accessed June 15, 2019.
- 5.Lincoln AE, Vogel RA, Allen TW, et al. Risk and causes of death among former National Football League Players (1986-2012). Med Sci Sports Exerc. 2018;50(3):486-493. doi: 10.1249/MSS.0000000000001466 [DOI] [PubMed] [Google Scholar]
- 6.Baron S, Rinsky R Rate and causes of death of National Football League Players. https://www.cdc.gov/niosh/hhe/reports/pdfs/1988-0085-letter.pdf?id=10.26616/NIOSHHETA88085. Published January 10, 1994. Accessed September 9, 2019.
- 7.Arrighi HM, Hertz-Picciotto I. The evolving concept of the healthy worker survivor effect. Epidemiology. 1994;5(2):189-196. doi: 10.1097/00001648-199403000-00009 [DOI] [PubMed] [Google Scholar]
- 8.Nguyen VT, Zafonte RD, Chen JT, et al. Mortality among professional American-style football players and professional American baseball players. JAMA Netw Open. 2019;2(5):e194223. doi: 10.1001/jamanetworkopen.2019.4223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Abernethy WB, Choo JK, Hutter AM Jr. Echocardiographic characteristics of professional football players. J Am Coll Cardiol. 2003;41(2):280-284. doi: 10.1016/S0735-1097(02)02633-5 [DOI] [PubMed] [Google Scholar]
- 10.Tucker AM, Vogel RA, Lincoln AE, et al. Prevalence of cardiovascular disease risk factors among National Football League players. JAMA. 2009;301(20):2111-2119. doi: 10.1001/jama.2009.716 [DOI] [PubMed] [Google Scholar]
- 11.Kim JH, Hollowed C, Patel K, et al. Temporal changes in cardiovascular remodeling associated with football participation. Med Sci Sports Exerc. 2018;50(9):1892-1898. doi: 10.1249/MSS.0000000000001631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Churchill TW, Krishnan S, Weisskopf M, et al. Weight gain and health affliction among former National Football League players. Am J Med. 2018;131(12):1491-1498. doi: 10.1016/j.amjmed.2018.07.042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Baggish AL, Wang F, Weiner RB, et al. Training-specific changes in cardiac structure and function: a prospective and longitudinal assessment of competitive athletes. J Appl Physiol (1985). 2008;104(4):1121-1128. doi: 10.1152/japplphysiol.01170.2007 [DOI] [PubMed] [Google Scholar]
- 14.Lin J, Wang F, Weiner RB, et al. Blood pressure and LV remodeling among American-style football players. JACC Cardiovasc Imaging. 2016;9(12):1367-1376. doi: 10.1016/j.jcmg.2016.07.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Weiner RB, Wang F, Isaacs SK, et al. Blood pressure and left ventricular hypertrophy during American-style football participation. Circulation. 2013;128(5):524-531. doi: 10.1161/CIRCULATIONAHA.113.003522 [DOI] [PubMed] [Google Scholar]
- 16.Kim JH, Sher S, Wang F, et al. Impact of American-style football participation on vascular function. Am J Cardiol. 2015;115(2):262-267. doi: 10.1016/j.amjcard.2014.10.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Croft LB, Belanger A, Miller MA, Roberts A, Goldman ME. Comparison of National Football League linemen versus nonlinemen of left ventricular mass and left atrial size. Am J Cardiol. 2008;102(3):343-347. doi: 10.1016/j.amjcard.2008.03.065 [DOI] [PubMed] [Google Scholar]
- 18.Lang RM, Badano LP, Mor-Avi V, et al. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr. 2015;28(1):1-39.e14. doi: 10.1016/j.echo.2014.10.003 [DOI] [PubMed] [Google Scholar]
- 19.Mosteller RD. Simplified calculation of body-surface area. N Engl J Med. 1987;317(17):1098. doi: 10.1056/NEJM198710223171717 [DOI] [PubMed] [Google Scholar]
- 20.Laurent S, Cockcroft J, Van Bortel L, et al. ; European Network for Non-invasive Investigation of Large Arteries . Expert consensus document on arterial stiffness: methodological issues and clinical applications. Eur Heart J. 2006;27(21):2588-2605. doi: 10.1093/eurheartj/ehl254 [DOI] [PubMed] [Google Scholar]
- 21.Yasmin, Brown MJ. Similarities and differences between augmentation index and pulse wave velocity in the assessment of arterial stiffness. QJM. 1999;92(10):595-600. doi: 10.1093/qjmed/92.10.595 [DOI] [PubMed] [Google Scholar]
- 22.Mitchell GF, Hwang SJ, Vasan RS, et al. Arterial stiffness and cardiovascular events: the Framingham Heart Study. Circulation. 2010;121(4):505-511. doi: 10.1161/CIRCULATIONAHA.109.886655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Woodman RJ, Kingwell BA, Beilin LJ, Hamilton SE, Dart AM, Watts GF. Assessment of central and peripheral arterial stiffness: studies indicating the need to use a combination of techniques. Am J Hypertens. 2005;18(2 pt 1):249-260. doi: 10.1016/j.amjhyper.2004.08.038 [DOI] [PubMed] [Google Scholar]
- 24.Willum-Hansen T, Staessen JA, Torp-Pedersen C, et al. Prognostic value of aortic pulse wave velocity as index of arterial stiffness in the general population. Circulation. 2006;113(5):664-670. doi: 10.1161/CIRCULATIONAHA.105.579342 [DOI] [PubMed] [Google Scholar]
- 25.Baggish AL, Wood MJ. Athlete’s heart and cardiovascular care of the athlete: scientific and clinical update. Circulation. 2011;123(23):2723-2735. doi: 10.1161/CIRCULATIONAHA.110.981571 [DOI] [PubMed] [Google Scholar]
- 26.Baron SL, Hein MJ, Lehman E, Gersic CM. Body mass index, playing position, race, and the cardiovascular mortality of retired professional football players. Am J Cardiol. 2012;109(6):889-896. doi: 10.1016/j.amjcard.2011.10.050 [DOI] [PubMed] [Google Scholar]
- 27.Kim JH, Hollowed C, Irwin-Weyant M, et al. Sleep-disordered breathing and cardiovascular correlates in college football players. Am J Cardiol. 2017;120(8):1410-1415. doi: 10.1016/j.amjcard.2017.07.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Morganroth J, Maron BJ, Henry WL, Epstein SE. Comparative left ventricular dimensions in trained athletes. Ann Intern Med. 1975;82(4):521-524. doi: 10.7326/0003-4819-82-4-521 [DOI] [PubMed] [Google Scholar]
- 29.Haykowsky MJ, Samuel TJ, Nelson MD, La Gerche A. Athlete’s heart: is the Morganroth hypothesis obsolete? Heart Lung Circ. 2018;27(9):1037-1041. doi: 10.1016/j.hlc.2018.04.289 [DOI] [PubMed] [Google Scholar]
- 30.Spence AL, Naylor LH, Carter HH, et al. A prospective randomised longitudinal MRI study of left ventricular adaptation to endurance and resistance exercise training in humans. J Physiol. 2011;589(pt 22):5443-5452. doi: 10.1113/jphysiol.2011.217125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rice TB, Dunn RE, Lincoln AE, et al. ; National Football League Subcommittee on Cardiovascular Health . Sleep-disordered breathing in the National Football League. Sleep. 2010;33(6):819-824. doi: 10.1093/sleep/33.6.819 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.George CF, Kab V, Kab P, Villa JJ, Levy AM. Sleep and breathing in professional football players. Sleep Med. 2003;4(4):317-325. doi: 10.1016/S1389-9457(03)00113-8 [DOI] [PubMed] [Google Scholar]
- 33.Chantler PD, Lakatta EG, Najjar SS. Arterial-ventricular coupling: mechanistic insights into cardiovascular performance at rest and during exercise. J Appl Physiol (1985). 2008;105(4):1342-1351. doi: 10.1152/japplphysiol.90600.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ky B, French B, May Khan A, et al. Ventricular-arterial coupling, remodeling, and prognosis in chronic heart failure. J Am Coll Cardiol. 2013;62(13):1165-1172. doi: 10.1016/j.jacc.2013.03.085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Antonini-Canterin F, Poli S, Vriz O, Pavan D, Bello VD, Nicolosi GL. The ventricular-arterial coupling: from basic pathophysiology to clinical application in the echocardiography laboratory. J Cardiovasc Echogr. 2013;23(4):91-95. doi: 10.4103/2211-4122.127408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Capone CA, Lamour JM, Lorenzo J, et al. Ventricular arterial coupling: a novel echocardiographic risk factor for disease progression in pediatric dilated cardiomyopathy. Pediatr Cardiol. 2019;40(2):330-338. doi: 10.1007/s00246-018-2021-6 [DOI] [PubMed] [Google Scholar]
- 37.Antonini-Canterin F, Enache R, Popescu BA, et al. Prognostic value of ventricular-arterial coupling and B-type natriuretic peptide in patients after myocardial infarction: a five-year follow-up study. J Am Soc Echocardiogr. 2009;22(11):1239-1245. doi: 10.1016/j.echo.2009.08.009 [DOI] [PubMed] [Google Scholar]
- 38.Lam CS, Shah AM, Borlaug BA, et al. Effect of antihypertensive therapy on ventricular-arterial mechanics, coupling, and efficiency. Eur Heart J. 2013;34(9):676-683. doi: 10.1093/eurheartj/ehs299 [DOI] [PubMed] [Google Scholar]
- 39.Her AY, Kim JY, Choi EY, et al. Value of ventricular stiffness index and ventriculoarterial interaction in patients with nonischemic dilated cardiomyopathy. Circ J. 2009;73(9):1683-1690. doi: 10.1253/circj.CJ-09-0046 [DOI] [PubMed] [Google Scholar]