Abstract
Background:
In US cystic fibrosis (CF) patients, methicillin-resistant Staphylococcus aureus (MRSA) rates have tripled in the past 2 decades. Known clinical risk factors include exposure to a healthcare setting, Pseudomonas aeruginosa and CF-related diabetes. Area-level socio-environmental exposures have not been evaluated. We explored the association of area-level deprivation with MRSA prevalence in a pediatric CF Center in the Southeastern United States.
Methods:
Patients’ residential addresses were geocoded and linked to a composite Area Deprivation Index and Rural-Urban Commuting Area scores. The association of MRSA with Area Deprivation Index and Rural-Urban Commuting Area scores was evaluated using logistic regression with robust standard errors adjusted for sociodemographic covariates (age, sex, race, mother’s and father’s education and household income), clinical risk factors (P. aeruginosa, CF-related diabetes, hospitalizations and number of clinic visits) and clustering.
Results:
The study included all pediatric patients (N = 231; mean age 12) at a single CF Center. MRSA was present in 44% of subjects. Higher area-level deprivation was correlated with rural residence, lack of parental college education and lower household income (P < 0.001 for each). In a multiple regression model fully adjusted for patient-level sociodemographic covariates, clinical risk factors and clustering, neighborhood deprivation was associated with more than 2-fold increase in the odds of having MRSA [OR 2.26 (1.14–4.45), P < 0.05].
Conclusions:
Neighborhood deprivation is a risk factor for MRSA in pediatric CF, doubling the odds of infection. Community-level socioeconomic risk factors should be considered when developing prevention strategies and treatment plans for MRSA infection in pediatric patients with CF.
Keywords: methicillin-resistant Staphylococcus aureus, cystic fibrosis, neighborhood characteristics, area deprivation, rurality
Cystic fibrosis (CF) is the most common autosomal recessive genetic disorder among Caucasians, affecting 1 in 2500 live births and ~30,000 individuals in the United States.1 Despite marked improvements in outcomes and survival, the primary causes of death in CF patients remain respiratory.1 As such, prevention and treatment of bacterial lung infections remain major priorities in CF care.2,3
In addition to known pathogens, such as Pseudomonas aeruginosa and Burkholderia cepacia complex, methicillin-resistant Staphylococcus aureus (MRSA) has emerged as a particularly concerning organism. Between 2002 and 2017, MRSA infection rates in US CF patients have nearly tripled, increasing from 9.2% to 25.9%.1 In one CF center with high MRSA prevalence, MRSA rates have increased from 1% to 49% between 1997 and 2009.4
Persistent infection with MRSA is a known contributor to CF morbidity and mortality. CF patients with persistent MRSA infection have lower lung function,5–7 accelerated lung function decline8,9 and impaired lung function recovery after exacerbation10 and require increased maintenance and antibiotic therapies.6,11,12 MRSA-infected CF patients have a 27% higher risk of death and a 6.2-year shorter life than non-infected counterparts.13
Previously reported clinical risk factors for MRSA infection include co-infection with P. aeruginosa,14,15 cystic fibrosis-related diabetes (CFRD),15 exposure to a healthcare setting4,14–16 and receiving care at a CF Center with high prevalence of MRSA.15 There is also evidence for environmental risks, such as higher ambient temperature,17 air pollution18 and tobacco smoke exposure.19 A recent retrospective study by Jennings et al15 reported that private health insurance is associated with lower risk of persistent MRSA infection. Additionally, population-based studies have shown that community deprivation is associated with higher odds of MRSA in the general population.20 The role of such socio-environmental factors for MRSA acquisition in CF has not been explored. The current study evaluated the association between MRSA prevalence and area-level socioeconomic deprivation. We hypothesized that neighborhood deprivation is associated with increased prevalence of MRSA infection in pediatric CF patients.
MATERIALS AND METHODS
Study Design and Participants
This was a cross-sectional observational study of pediatric patients treated at the University of Alabama at Birmingham CF Center from August 1, 2017 to July 31, 2018. Clinical and sociodemographic data of the cohort were obtained from the CF Center’s Patient Registry, through an ongoing protocol approved by the Institutional Review Board (IRB-000509005) of the University of Alabama at Birmingham. Area-level data were obtained by geocoding the residential addresses of patients to US Census block groups—small geographic units that serve as proxies for neighborhoods—and linking them to existing area-level measures as described further.
Measures
The primary outcome measure was MRSA prevalence. MRSA-positive status was defined as at least 1 positive respiratory culture during the 12-month observation period, while MRSA-negative status was defined as no positive cultures during the same period.
Area deprivation was assessed with the 2013 Area Deprivation Index (ADI) for the State of Alabama. The ADI is an existing factor-based composite measure of socioeconomic deprivation, available in a national (1–100 scale) and state-specific (1–10 scale) versions, with higher values indicating higher deprivation.21,22 The ADI is constructed from 17 variables in the domains of income, education, employment and housing quality collected by the American Community Survey and aggregated to US Census block groups.21,22 In our study, each CF patient was assigned a neighborhood ADI value according to the Census block group in which he or she resided. Because the relationship between area deprivation and health outcomes is nonlinear, we grouped neighborhoods into 2 disadvantage levels based on the ADI distribution as done previously23: areas with ADI values in the bottom 50% for the sample were classified as less disadvantaged, while those in the top 50% were classified as more disadvantaged (ADI scores 1–4 vs ≥5).
Rurality was assessed with the 2010 Rural-Urban Commuting Area codes, a classification that combines US Census Bureau definitions with commuting information and ranks Census tracts on a scale of 1–10, with higher values indicating higher rurality.24 We dichotomized the measure as metro/non-metro (Rural-Urban Commuting Area codes scores 1–3 vs ≥4).
Individual socioeconomic status (SES) measures included the educational attainment of the mother and the father (college degree vs less) and the annual household income dichotomized at the median for both the sample and the State of Alabama ($50,000).
Covariates included age, sex, race and known risk factors for MRSA: P aeruginosa, CFRD status, smoke exposure, hospitalizations and number of clinic visits in the past 12 months.
Statistical Analysis
The distribution of individual- and area-level characteristics was obtained by MRSA status and for the overall sample. Bivariate relationships between MRSA status and every covariate were estimated using simple logistic regression. Multiple logistic regression was used to estimate models of individual- and area-level characteristics. As 86% of Census block groups included only 1 CF patient, multilevel modeling was not feasible, but we accounted for clustering of individuals within Census block groups by using robust standard errors.25 Interactions between area deprivation and rurality were explored, as well as interactions between individual- and area-level SES, and none were found to be significant. Statistical tests were 2-sided and were performed using a 5% significance level (α = 0.05). Analyses were performed using Stata software, version 15 (StataCorp LLC, College Station, TX).
RESULTS
The study population included 231 patients residing in 196 Census block groups. Characteristics of the sample (N = 231), by MRSA status and overall, are presented in Table 1. Mean patient age was 12 years (range 0–22, SD = 6), 53% were males and 91% were non-Hispanic White. In terms of individual SES, 68% of fathers and 56% of mothers did not have college education, and 51% had annual household income less than $50,000 (the median for the State as well as the sample). MRSA was present in 44% of patients.
TABLE 1.
Descriptive Statistics of the Study Sample: Overall and by MRSA Status (N = 231)
| Overall | MRSA status |
P | ||
|---|---|---|---|---|
| Positive, n = 101 (44%) |
Negative, n = 130 (56%) |
|||
| Individual level | ||||
| Sociodemographic | ||||
| Age, year | 11.9 (SD 6.0) | 13.1 (5.2) | 10.9 (6.4) | 0.006 |
| Male, % | 53.2 | 53.5 | 53.1 | 0.953 |
| White, % | 90.5 | 89.1 | 91.5 | 0.534 |
| Lack of college education in father, % | 68.0 | 74.3 | 63.1 | 0.072 |
| Lack of college education in Mother , % | 56.3 | 60.4 | 53.1 | 0.266 |
| Household income <$50,000, % | 51.1 | 50.2 | 53.9 | 0.336 |
| Smoke exposure, % | 26.0 | 29.7 | 23.1 | 0.256 |
| Clinical | ||||
| Pseudomonas aeruginosa, % | 25.5 | 22.8 | 27.7 | 0.396 |
| ≥4 clinic visits/year, % | 76.2 | 83.2 | 70.8 | 0.030 |
| CFRD, % | 14.0 | 14.9 | 13.3 | 0.439 |
| Area level | ||||
| High area deprivation (ADI), % | 43.3 | 51.5 | 36.9 | 0.036 |
| Metro area, % | 67.5 | 68.3 | 66.9 | 0.830 |
The geographic location of participants by MRSA status is depicted on Fig. 1. Each CF subject is represented by a single dot, with red indicating MRSA-positive status, and blue indicating MRSA-negative status. MRSA-positive status was associated with older patient age (P = 0.006), more clinic visits (P = 0.030) and area-level deprivation (P = 0.036). In bivariate analysis, higher neighborhood deprivation (ADI ≥ 5) was associated with a non-metro residence [OR 2.57 (95% CI 1.72–3.84), P < 0.001], lack of college education [OR 7.50 (3.42–16.45) for fathers; OR 2.99 (1.63–5.46) for mothers; P < 0.001 for both] and household income <$50,000 [OR 2.95 (1.59–5.48), P = 0.001].
FIGURE 1.
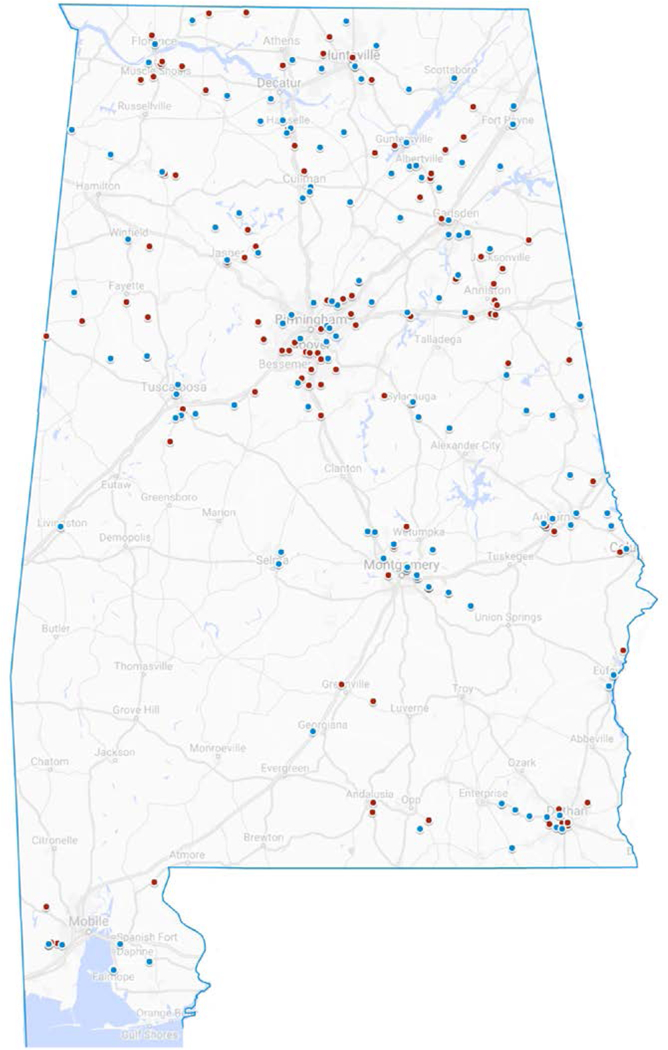
Geographic location of CF patients by MRSA status (N = 231). Red dot, MRSA positive; blue dot, MRSA negative.
Table 2 presents results of multiple logistic regression of MRSA prevalence. To understand the relationship between individual-level SES and area-level deprivation and their contributions to MRSA prevalence, we estimated 3 separate multiple regression models. Model 1 includes only individual-level SES (education of the mother and father and household income); Model 2 includes only area-level SES (area deprivation and rurality) and Model 3 (the full model) includes both individual and area-level SES to define the independent contribution of each. All 3 models control for the same covariates: age, sex, race, P. aeruginosa, CFRD, smoke exposure, number of hospitalizations and number of clinic visits in the past 12 months. Model 1 shows that low paternal education (no college) is associated with a 2-fold higher odds of MRSA risk [OR 2.18 (1.06–4.49), P < 0.05]. Model 2 shows that high area-level deprivation (ADI ≥ 5) is associated with twice the odds of having MRSA [OR 2.51 (1.34–4.71), P < 0.01]. When individual- and area-level measures are included in the same fully adjusted model (Model 3), area deprivation remains significant [OR 2.26 (1.14–4.45), P < 0.05] while low paternal education does not, indicating that area deprivation captures the association of individual SES.
TABLE 2.
Multiple Logistic Regression Models of MRSA Status (N=231)
| Model 1 Individual SES |
Model 2 Area SES |
Model 3 Individual + area SES |
||||
|---|---|---|---|---|---|---|
| OR | 95% CI | OR | 95% CI | OR | 95% CI | |
| Individual | ||||||
| Father no college | 2.18* | 1.06–4.49 | – | – | 2.00 | 0.94–4.26 |
| Mother no college | 1.15 | 0.59–2.23 | – | – | 1.13 | 0.56–2.26 |
| Income <$50,000 | 1.65 | 0.79–3.45 | – | – | 1.71 | 0.78–3.74 |
| Age, years | 1.09† | 1.03–1.15 | 1.11‡ | 1.05–1.18 | 1.10† | 1.04–1.17 |
| ≥4 clinic visits/year | 2.42* | 1.13–5.18 | 3.15† | 1.41–7.07 | 2.69* | 1.17–6.21 |
| Pseudomonas aeruginosa | 0.50 | 0.24–1.03 | 0.41* | 0.19–0.90 | 0.42* | 0.19–0.95 |
| Area | ||||||
| High deprivation | – | – | 2.51† | 1.34–4.71 | 2.26* | 1.14–4.45 |
| Metro area | – | – | 1.65 | 0.85–3.23 | 1.86 | 0.92–3.74 |
All models adjusted for sex, race, tobacco smoke exposure, hospitalizations and CFRD status. Bold font indicates statistical significance (P < 0.05, P < 0.01, or P < 0.001), two-tailed tests.
P < 0.05, two-tailed tests.
P < 0.01, two-tailed tests.
P < 0.001, two-tailed tests.
Patient age and ≥4 clinic visits annually were significant risk factors in all models. Each year of age was associated with a 10% increase in the odds of having MRSA, while ≥4 annual clinic visits more than doubled the odds of having MRSA.
DISCUSSION
We conducted a cross-sectional analysis of data from a single pediatric CF Center to quantify the contributions of area-level socioeconomic factors for MRSA prevalence in this population. Our findings show that high neighborhood deprivation is a risk factor for MRSA. CF patients residing in deprived neighborhoods have twice the odds of being MRSA-positive than those in more affluent areas, after adjusting for individual SES and previously identified risk factors such as age, P aeruginosa, CFRD, smoke exposure and encounters with the health system.
This is the first report documenting a relationship between neighborhood socioeconomic measures and MRSA risk in pediatric CF. Earlier studies have found an increased likelihood of chronic P. aeruginosa infection in UK patients with CF residing in the most deprived geographic areas compared with those in the most affluent areas.26 Our findings expand the adverse effects of area deprivation to include MRSA infection. Multiple mechanisms may be contributing to this association. Collective and concentrated poverty may affect exposure to environmental risk factors such as tobacco smoke, indoor and outdoor air quality and pathogens. For example, exposure to fine particulate matter based on air pollution monitors within 30 miles of place of residence has been associated with an increased risk of P. aeruginosa acquisition in a cohort of 3575 children aged 6 years or younger.27 It is possible that such mechanisms facilitate MRSA acquisition as well. Further study of potential mediators of the link between area deprivation and MRSA risk is warranted.
Prior studies have associated the increased presence of MRSA with living in warmer climates.17,28 Indeed, CF Centers in the Southern United States have the highest proportion of patients with MRSA (41.9%)28 However, the South is also characterized by lower education, employment, income and healthcare coverage than the rest of the United States.29 For example, Alabama is among the bottom 5 US states on all these indicators. The US states in the top quartile for average annual temperature also have 9 times the odds of being in the top quartile for average area deprivation (OR 9.2, P = 0.003) calculated using the national ADI scores (scale range 1–100). These data, along with our study findings, highlight the potential for unmeasured socioeconomic confounders in the association between warmer climate and MRSA infection in CF. We show that ADI can be used to account for community-level conditions under a single metric that predicts MRSA acquisition in pediatric patients with CF.
Analyzing the association between mean annual ambient temperature and MRSA, Collaco et al17 controlled for health insurance as a marker of SES but not for other individual-level socio-economic indicators or area-level disadvantage. Higher MRSA prevalence in the South may be associated with area characteristics beyond ambient temperature. Resource-deprived areas have increased levels of air pollution,30,31 community smoke exposure32,33 and low-quality housing that exposes residents to allergens,34,35 semi-volatile organic contaminants36 and inadequate ventilation.37,38 Additionally, neighborhood socioeconomic vulnerability is a known risk factor for heat illness in Georgia39 and heat-related deaths in Arizona,40 and area deprivation has been integrated into the Heat Exposure Integrated Deprivation Index to predict vulnerability during extreme hot weather.41
In our study, higher number of clinic visits predicted MRSA-positive status. This finding corroborates prior reports of exposure to healthcare settings as a risk factor for MRSA acquisition.4,14,16 For patients receiving care in CF centers with high MRSA rates,15 the combination of socio-environmental and healthcare exposures may result in a risk proliferation that exacerbates inequities. Importantly, area deprivation was not associated with number of clinic visits or hospitalizations, confirming previous reports that disparities in pediatric CF outcomes are not driven by differential healthcare access.42–44
Notably, we do not know if the MRSA strains in the current sample are healthcare associated or community associated (CA),45 but previous research indicates a marked increase in CA-MRSA strains among pediatric CF patients in recent years.14 CF patients with CA-MRSA tend to be younger and less frequently co-infected with P. aeruginosa.46 In our study, P. aeruginosa had a negative association with MRSA [adjusted OR 0.42 (0.19–0.95) P < 0.05). Along with the reported significance of area characteristics, this may be indicative of an increased prevalence of CA-MRSA in the sample. Future studies considering the effect of community-level factors should include information about the strains and types of MRSA infection.
Finally, despite an increased scientific interest in the role of socio-environmental factors for CF outcomes, patient registries and medical records rarely include sufficient patient-level socioeconomic data. This study shows that area-level socioeconomic measures can serve as a proxy of patient-level data. It also highlights the need for expanding health records with spatial data relevant for clinical decision-making. Small-area measures can be a clinically useful tool for identifying high-risk CF patients who may benefit from protocolized MRSA prevention and eradication approaches.47 We show that ADI can be used to account for community-level conditions under a single metric that predicts MRSA acquisition in pediatric patients with CF.
This study has several limitations. The cross-sectional design prevents us from making causal inferences about the observed relationship between area deprivation and MRSA prevalence. Results may not be generalizable to areas outside of the Southern United States region characterized by high ambient temperature, humidity and socioeconomic deprivation. Finally, the reported associations may not be applicable to adult CF populations.
The increasing prevalence of MRSA in individuals with CF, particularly in the United States, demands careful assessment of risk factors and mechanisms of MRSA infection in this population. Our study provides initial evidence of the role of the socioeconomic environment for MRSA infection in pediatric CF patients. Future research should investigate the association of area deprivation with MRSA infection in a geographically diverse CF sample from multiple US regions. MRSA risk prediction models that incorporate area-level exposures then need to be developed.
CONCLUSION
Neighborhood deprivation is a risk factor for MRSA in pediatric CF, increasing the odds of infection more than 2-fold. Beyond established clinical variables such as frequency of clinical encounters, living in a resource-deprived community doubles the rate of MRSA acquisition. Area-level measures may be used as a proxy of patient-level socioeconomic data that are not available in clinical settings. Community-level socioeconomic risk factors should be considered when developing prevention strategies and treatment plans for MRSA infection in pediatric patients with CF.
Acknowledgments
This study was supported by grants from the NIH (P30DK72482) and the CF Foundation (CC032).
G.R.O. conceived and designed the study, performed the analysis and interpreted the data, drafted the paper, approved the final version. W.T.H. interpreted the data, revised the draft critically for important intellectual content, approved the final version. S.M.R. interpreted the data, revised the draft critically for important intellectual content, approved the final version. G.M.S. facilitated the data collection, interpreted the data, revised the draft critically for important intellectual content, approved the final version. S.D. collected the data, contributed data or analysis tools, drafted the paper, approved the final version. A.Z. collected the data, contributed data or analysis tools, revised the draft critically, approved the final version. W.C.H. interpreted the data, revised the draft critically for important intellectual content, approved the final version. H.H.G. facilitated the data collection, interpreted the data, revised the draft critically for important intellectual content, approved the final version.
Footnotes
The authors have no conflicts of interest to disclose.
REFERENCES
- 1.Cystic Fibrosis Foundation Patient Registry, 2017 Annual Data Report. Bethesda, Maryland: Cystic Fibrosis Foundation; 2018. [Google Scholar]
- 2.Flume PA, Mogayzel PJ Jr, Robinson KA, et al. ; Clinical Practice Guidelines for Pulmonary Therapies Committee. Cystic fibrosis pulmonary guidelines: treatment of pulmonary exacerbations. Am J Respir Crit Care Med 2009;180:802–808. [DOI] [PubMed] [Google Scholar]
- 3.Flume PA, O’Sullivan BP, Robinson KA, et al. ; Cystic Fibrosis Foundation, Pulmonary Therapies Committee. Cystic fibrosis pulmonary guidelines: chronic medications for maintenance of lung health. Am J Respir Crit Care Med 2007;176:957–969. [DOI] [PubMed] [Google Scholar]
- 4.Harik NS, Com G, Tang X, et al. Clinical characteristics and epidemiology of methicillin-resistant Staphylococcus aureus (MRSA) in children with cystic fibrosis from a center with a high MRSA prevalence. Am J Infect Control. 2016;44:409–415. [DOI] [PubMed] [Google Scholar]
- 5.Ren CL, Morgan WJ, Konstan MW, et al. ; Investigators and Coordinators of the Epidemiologic Study of Cystic Fibrosis. Presence of methicillin resistant Staphylococcus aureus in respiratory cultures from cystic fibrosis patients is associated with lower lung function. Pediatr Pulmonol 2007;42:513–518. [DOI] [PubMed] [Google Scholar]
- 6.Sawicki GS, Rasouliyan L, Pasta DJ, et al. The impact of incident methicillin resistant Staphylococcus aureus detection on pulmonary function in cystic fibrosis. Pediatr Pulmonol 2008;43:1117–1123. [DOI] [PubMed] [Google Scholar]
- 7.Miall LS, McGinley NT, Brownlee KG, et al. Methicillin resistant Staphylococcus aureus (MRSA) infection in cystic fibrosis. Arch Dis Child. 2001;84:160–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dasenbrook EC, Merlo CA, Diener-West M, et al. Persistent methicillin-resistant Staphylococcus aureus and rate of FEV1 decline in cystic fibrosis. Am J Respir Crit Care Med 2008;178:814–821. [DOI] [PubMed] [Google Scholar]
- 9.Vanderhelst E, De Meirleir L, Verbanck S, et al. Prevalence and impact on FEV(1) decline of chronic methicillin-resistant Staphylococcus aureus (MRSA) colonization in patients with cystic fibrosis. A single-center, case control study of 165 patients. J Cyst Fibros. 2012;11:2–7. [DOI] [PubMed] [Google Scholar]
- 10.Sanders DB, Bittner RC, Rosenfeld M, et al. Failure to recover to base-line pulmonary function after cystic fibrosis pulmonary exacerbation. Am J Respir Crit Care Med 2010;182:627–632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stone A, Saiman L. Update on the epidemiology and management of Staphylococcus aureus, including methicillin-resistant Staphylococcus aureus, in patients with cystic fibrosis. Curr Opin Pulm Med 2007;13: 515–521. [DOI] [PubMed] [Google Scholar]
- 12.Hubert D, Reglier-Poupet H, Sermet-Gaudelus I, et al. Association between Staphylococcus aureus alone or combined with Pseudomonas aeruginosa and the clinical condition of patients with cystic fibrosis. J Cyst Fibros. 2013;12:497–503. [DOI] [PubMed] [Google Scholar]
- 13.Dasenbrook EC, Checkley W, Merlo CA, et al. Association between respiratory tract methicillin-resistant Staphylococcus aureus and survival in cystic fibrosis. JAMA. 2010;303:2386–2392. [DOI] [PubMed] [Google Scholar]
- 14.Muhlebach MS, Heltshe SL, Popowitch EB, et al. ; STAR-CF Study Team. Multicenter observational study on factors and outcomes associated with various methicillin-resistant Staphylococcus aureus types in children with cystic fibrosis. Ann Am Thorac Soc 2015;12:864–871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jennings MT, Dasenbrook EC, Lechtzin N, et al. Risk factors for persistent methicillin-resistant Staphylococcus aureus infection in cystic fibrosis. J Cyst Fibros. 2017;16:681–686. [DOI] [PubMed] [Google Scholar]
- 16.Nadesalingam K, Conway SP, Denton M. Risk factors for acquisition of methicillin-resistant Staphylococcus aureus (MRSA) by patients with cystic fibrosis. J Cyst Fibros. 2005;4:49–52. [DOI] [PubMed] [Google Scholar]
- 17.Collaco JM, Raraigh KS, Appel LJ, et al. Respiratory pathogens mediate the association between lung function and temperature in cystic fibrosis. J Cyst Fibros. 2016;15:794–801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Psoter KJ, De Roos AJ, Wakefield J, et al. Air pollution exposure is associated with MRSA acquisition in young U.S. children with cystic fibrosis. BMC Pulm Med 2017;17:106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kopp BT, Sarzynski L, Khalfoun S, et al. Detrimental effects of second-hand smoke exposure on infants with cystic fibrosis. Pediatr Pulmonol 2015;50:25–34. [DOI] [PubMed] [Google Scholar]
- 20.Casey JA, Cosgrove SE, Stewart WF, et al. A population-based study of the epidemiology and clinical features of methicillin-resistant Staphylococcus aureus infection in Pennsylvania, 2001–2010. Epidemiol Infect 2013;141:1166–1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.University of Wisconsin School of Medicine and Public Health. Area Deprivation Index. 2018. Available at: https://www.neighborhoodatlas.medicine.wisc.edu/. Accessed June 15, 2019.
- 22.Kind AJH, Buckingham WR. Making neighborhood-disadvantage metrics accessible - the neighborhood atlas. N Engl J Med 2018;378:2456–2458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hu J, Kind AJH, Nerenz D. Area deprivation index predicts readmission risk at an urban teaching hospital. Am J Med Qual 2018;33:493–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.United States Department of Agriculture, Economic Research Service. Rural-Urban Commuting Area Codes. 2016. https://www.ers.usda.gov/data-products/rural-urban-commuting-area-codes/. Accessed June 15, 2019.
- 25.Rogers WH. Regression standard errors in clustered samples. Stata Technical Bulletin. 1994;13:19–23. [Google Scholar]
- 26.Taylor-Robinson DC, Smyth RL, Diggle PJ, et al. The effect of social deprivation on clinical outcomes and the use of treatments in the UK cystic fibrosis population: a longitudinal study. Lancet Respir Med 2013;1:121–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Psoter KJ, De Roos AJ, Mayer JD, et al. Fine particulate matter exposure and initial Pseudomonas aeruginosa acquisition in cystic fibrosis. Ann Am Thorac Soc 2015;12:385–391. [DOI] [PubMed] [Google Scholar]
- 28.Kopp BT, Nicholson L, Paul G, et al. Geographic variations in cystic fibrosis: an analysis of the U.S. CF Foundation Registry. Pediatr Pulmonol 2015;50:754–762. [DOI] [PubMed] [Google Scholar]
- 29.Oates GR, Jackson BE, Partridge EE, et al. Sociodemographic patterns of chronic disease: how the mid-south region compares to the rest of the country. Am J Prev Med 2017;52(1S1):S31–S39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Brunt H, Barnes J, Jones SJ, et al. Air pollution, deprivation and health: understanding relationships to add value to local air quality management policy and practice in Wales, UK. J Public Health (Oxf). 2017;39:485–97. [DOI] [PubMed] [Google Scholar]
- 31.Morelli X, Rieux C, Cyrys J, et al. Air pollution, health and social deprivation: a fine-scale risk assessment. Environ Res 2016;147:59–70. [DOI] [PubMed] [Google Scholar]
- 32.Wilson KM, Klein JD, Blumkin AK, et al. Tobacco-smoke exposure in children who live in multiunit housing. Pediatrics. 2011;127:85–92. [DOI] [PubMed] [Google Scholar]
- 33.Benowitz NL, Jain S, Dempsey DA, et al. Urine cotinine screening detects nearly ubiquitous tobacco smoke exposure in urban adolescents. Nicotine Tob Res 2017;19:1048–1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rosenfeld L, Rudd R, Chew GL, et al. Are neighborhood-level characteristics associated with indoor allergens in the household? J Asthma. 2010;47:66–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Adamkiewicz G, Zota AR, Fabian MP, et al. Moving environmental justice indoors: understanding structural influences on residential exposure patterns in low-income communities. Am J Public Health. 2011;101(suppl 1):S238–S245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bi C, Maestre JP, Li H, et al. Phthalates and organophosphates in settled dust and HVAC filter dust of U.S. low-income homes: association with season, building characteristics, and childhood asthma. Environ Int 2018;121 (pt 1):916–930. [DOI] [PubMed] [Google Scholar]
- 37.Adamkiewicz G, Spengler JD, Harley AE, et al. Environmental conditions in low-income urban housing: clustering and associations with self-reported health. Am J Public Health. 2014;104:1650–1656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zota A, Adamkiewicz G, Levy JI, et al. Ventilation in public housing: implications for indoor nitrogen dioxide concentrations. Indoor Air. 2005;15: 393–01. [DOI] [PubMed] [Google Scholar]
- 39.Pillai SK, Noe RS, Murphy MW, et al. Heat illness: predictors of hospital admissions among emergency department visits-Georgia, 2002–2008. J Community Health. 2014;39:90–98. [DOI] [PubMed] [Google Scholar]
- 40.Harlan SL, Declet-Barreto JH, Stefanov WL, et al. Neighborhood effects on heat deaths: social and environmental predictors of vulnerability in Maricopa County, Arizona. Environ Health Perspect. 2013;121:197–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Krstic N, Yuchi W, Ho HC, et al. The Heat Exposure Integrated Deprivation Index (HEIDI): a data-driven approach to quantifying neighborhood risk during extreme hot weather. Environ Int 2017;109:42–52. [DOI] [PubMed] [Google Scholar]
- 42.Schechter MS, Shelton BJ, Margolis PA, et al. The association of socioeconomic status with outcomes in cystic fibrosis patients in the United States. Am J Respir Crit Care Med 2001;163:1331–1337. [DOI] [PubMed] [Google Scholar]
- 43.Schechter MS, McColley SA, Silva S, et al. ; Investigators and Coordinators of the Epidemiologic Study of Cystic Fibrosis; North American Scientific Advisory Group for ESCF. Association of socioeconomic status with the use of chronic therapies and healthcare utilization in children with cystic fibrosis. J Pediatr 2009;155:634–9.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Taylor-Robinson D, Schechter MS. Health inequalities and cystic fibrosis. BMJ. 2011;343:d4818. [DOI] [PubMed] [Google Scholar]
- 45.Tenover FC, McAllister S, Fosheim G, et al. Characterization of Staphylococcus aureus isolates from nasal cultures collected from individuals in the United States in 2001 to 2004. J Clin Microbiol 2008;46:2837–2841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Muhlebach MS, Miller M, LaVange LM, et al. Treatment intensity and characteristics of MRSA infection in CF. J Cyst Fibros. 2011;10:201–206. [DOI] [PubMed] [Google Scholar]
- 47.Muhlebach MS, Beckett V Popowitch E, et al. ; STAR-too study team. Microbiological efficacy of early MRSA treatment in cystic fibrosis in a randomised controlled trial. Thorax. 2017;72:318–326. [DOI] [PMC free article] [PubMed] [Google Scholar]


