Abstract
The “hunger” hormone ghrelin regulates food-intake and preference for high-calorie (HC) food through modulation of the mesocortico-limbic dopaminergic pathway. Laparoscopic sleeve gastrectomy (LSG) is an effective bariatric surgery to treat morbid obesity. We tested the hypothesis that LSG-induced reductions in appetite and total ghrelin levels in blood are associated with reduced prefrontal brain reactivity to food cues. A functional magnetic resonance imaging (fMRI) cue-reactivity task with HC and low-calorie (LC) food pictures was used to investigate brain reactivity in 22 obese participants tested before and one month after bariatric surgery (BS). Nineteen obese controls (Ctr) without surgery were also tested at baseline and one-month later. LSG significantly decreased (1) fasting plasma concentrations of total ghrelin, leptin and insulin, (2) craving for HC food, and (3) brain activation in the right dorsolateral prefrontal cortex (DLPFC) in response to HC vs. LC food cues (PFWE < 0.05). LSG-induced reduction in DLPFC activation to food cues were positively correlated with reduction in ghrelin levels and reduction in craving ratings for food. Psychophysiological interaction (PPI) connectivity analyses showed that the right DLPFC had stronger connectivity with the ventral anterior cingulate cortex (vACC) after LSG; and changes in BMI were negatively correlated with changes in connectivity between the right DLPFC and vACC in the LSG group only. These findings suggest that LSG-induced weight-loss may be related to reductions in ghrelin, possibly leading to decreased food craving and hypothetically reducing DLPFC response to the HC food cues.
Keywords: obesity, bariatric surgery, ghrelin, dorsolateral prefrontal cortex, craving, fMRI
Introduction
Laparoscopic sleeve gastrectomy (LSG) is a surgical procedure that leads to the removal of the gastric fundus. It is currently one of the most effective treatments for morbid obesity (Diamantis et al., 2014).
Ghrelin acts as a hunger regulating peptide and is mainly produced by endocrine cells in the gastric fundus. Ghrelin is the only known peripheral hormone that has orexigenic properties, as it stimulates appetite and increases short-term food-intake (Papailiou et al., 2010). Several studies have reported significant reduction in both appetite and fasting blood ghrelin concentrations at one-month post-LSG (Tsoli et al., 2013; Langer et al., 2005). The reduction in ghrelin concentrations remained stable at 6-and 12-months postoperatively (Tsoli et al., 2013; Langer et al., 2005; Ramon et al., 2012; Karamanakos et al., 2008). However, fasting ghrelin changes after LSG are different from the short-term diet-induced weight-loss and after laparoscopic adjustable gastric banding (LAGB) or gastric bypass surgery in obese subjects. That is, after an energy-restricted diet, fasting plasma ghrelin has been shown to increase (Lean and Malkova, 2016). In another study, fasting ghrelin levels remained unchanged at 6-months, but increased at 12-months after LAGB (Hanusch-Enserer et al., 2003). However, ghrelin levels significantly decreased after gastric bypass surgery in some (Cummings et al., 2002), but not all studies (Faraj et al., 2003; Holdstock et al., 2003).
Ghrelin’s actions are not limited to control of basic food intake; it also increases incentive salience to food reward (Skibicka et al., 2011) via activation of the mesocorticolimbic dopaminergic system (Skibicka et al., 2013; Abizaid et al., 2006). Ghrelin signaling in the ventral tegmental area appears to play a role in food-choice behavior (i.e., increased intake of palatable food) (Egecioglu et al., 2010). Rats who are knock-out for the ghrelin receptor gain less weight, eat less, and have higher brown adipose tissue (Zallar et al., 2018). A food-cue reactivity study in humans revealed that fasting ghrelin concentrations were associated with the hedonic effects of food pictures and with enhanced subjective craving when confronted with reward cues (Kroemer et al., 2013). In summary, ghrelin plays an important but complex and not fully understood role in food intake, food reward and in weight loss after LSG.
Neuroimaging studies in obese patients have shown functional abnormalities in the frontal-mesolimbic circuitry (Bohon, 2014; Tracy et al., 2015; Volkow et al., 2008), including the prefrontal cortex (PFC), particularly the dorsolateral prefrontal cortex (DLPFC) (Hare et al., 2009) and mesolimbic regions (Killgore et al., 2013). Brain regions within the frontal-mesolimbic circuitry encompasses the PFC, which is crucial for executive function (Lowe et al., 2009). In particular, the capacity of the DLPFC to modulate eating-behavior in response to tempting food is strongly linked to several aspects of impulse-control, such as inhibitory-control (Batterink et al., 2010), executive-attention (Hofmann et al., 2009) and emotion-regulation (Gruber and McDonald, 2012). There is strong evidence for impairments in these brain regions and/or circuits in obesity, which have been associated with elevated food craving (Pepino et al., 2009) and over-eating (Volkow et al., 2011). Neuroimaging studies have also revealed alterations occurring in homeostatic/hedonic neurocircuits after bariatric surgery, including an association between lessened postoperative craving for HC food and diminished activity within mesolimbic areas/DLPFC (Ochner et al., 2012; Bruce et al., 2012).
It remains unclear whether LSG-induced modulation of the prefrontal-mesolimbic neurocircuitry is associated with significant changes in peripheral appetitive hormones. Here, we employed functional magnetic resonance imaging (fMRI) with a food-cue-reactivity paradigm in conjunction with a psychophysiological interaction (PPI) analysis to investigate the alterations of cue-induced brain activation/connectivity in obese patients at one-month after bariatric surgery (BS). We hypothesized that changes in fasting plasma ghrelin levels post-LSG would be associated with decreased craving for HC food-cues and attenuation of HC food-cue induced activation in brain regions implicated in inhibitory-control. Clearly, the neurobiological mechanisms contributing to weight loss following LSG are quite complex and likely to depend on the balance among several hormones, as well as short-term and long-term adaptations of several endocrine pathways, including hormones that, unlike ghrelin, execute anorexigenic effects like leptin and insulin. As such, in addition to ghrelin, these hormones were also measured in the present study.
Materials and Methods
Subjects
The experimental protocol was approved by the Institutional-Review-Board of Xijing Hospital of the Fourth Military Medical University in Xi’an, China and registered in the Chinese Clinical Trial Registry Center as: ChiCTR-OOB-15006346 (http://www.chictr.org.cn). The experiments were conducted in accordance with the Declaration of Helsinki. All participants were informed of the nature of the research and provided written informed consent. Participants with psychiatric/neurological diseases, previous intestinal surgery/inflammatory intestinal disease/organ dysfunction or taking any current medication that could affect the CNS were excluded. Individuals who had a waist-circumference (WC) > the interior diameter of the MRI scanner were excluded (Zhang et al., 2016). Thirty-five morbidly obese patients were recruited for laparoscopic sleeve gastrectomy at Xijing Gastrointestinal Hospital. Given the exclusion criteria, six candidates were disqualified (two had WC > the interior diameter of the scanner, three due to metal implants, and one subject’s imaging data were lost due to technical problems). Seven obese subjects reported having significant weight loss after surgery via their local clinics. However, these subjects could not return for follow-up MRI assessment due to long distance travel. As a result, 22 patients remained in the BS group. The patients completed the pre-surgical MRI scan (Pre-BS) then underwent surgery. In LSG; the greater curvature, including the complete fundus, was resected from the antrum to the angle of His. The stomach was reduced to a narrow gastric tube at the lesser curvature over a 36-French bougie. As the integrity of the vagus nerve remains preserved in LSG; no pyloroplasty was performed. At the end of resection, the stapled line was covered by a running suture (Langer et al., 2005). The same MRI scans were performed one-month after surgery (Post-BS). Nineteen obese patients who did not receive LSG surgery were recruited as controls (Ctr). The BS and Ctr groups were matched for BMI, age, and sex (Table 1). The Ctr group completed two identical MRI scans mirroring BS, one (CtrT) at baseline, and a retest (CtrRT) one-month later.
Table 1.
Demographic and clinical information of obese subjects in both BS and Ctr groups.
| Pre-BS (22) (Mean ± SE) |
Post-BS (22) (Mean ± SE) |
CtrT (19) (Mean ± SE) |
CtrRT (19) (Mean ± SE) |
Pre-BS vs. CtrT |
|||
|---|---|---|---|---|---|---|---|
| T | P | d | |||||
| Age (yrs) | 26.64 ± 1.83 | 26.64 ± 1.83 | 28.63 ± 2.06 | 28.63 ± 2.06 | 0.977 | 0.334 | 0.306 |
| Gender | 9M/13F | 9M/13F | 12M/7F | 12M/7F | 2.020* | 0.155 | - |
| Duration of Obesity (yrs) | 12.75 ± 2.19 | 12.75 ± 2.19 | 12.05 ± 1.16 | 12.05 ± 1.16 | 0.004 | 0.997 | 0.001 |
| Weight (Kg) | 109.92 ± 3.77 | 98.43 ± 3.71 | 104.71 ± 4.02 | 103.68 ± 3.91 | 0.932 | 0.357 | 0.291 |
| BMI (Kg/m2) | 38.11 ± 1.32 | 34.03 ± 1.31 | 35.27 ± 1.01 | 35.14 ± 1.04 | 1.646 | 0.108 | 0.515 |
| WC (cm) | 117.09 ± 3.08 | 108.59 ± 2.93 | 114.81 ± 2.57 | 112.89 ± 2.23 | 0.558 | 0.580 | 0.174 |
| YFAS | 4.95 ± 0.59 | 2.81 ± 0.41 | 3.37 ± 0.56 | 3.42 ± 0.53 | 1.906 | 0.064 | 0.596 |
| Food HC | 65.00 ± 5.26 | 31.48 ± 4.66 | 66.67 ± 6.43 | 62.22 ± 4.83 | 0.148 | 0.877 | 0.046 |
| Craving LC | 46.17 ± 4.56 | 42.74 ± 5.99 | 49.17 ± 6.62 | 48.33 ± 4.99 | 0.571 | 0.568 | 0.179 |
| HAMD | 12.50 ± 2.35 | 10.45 ± 1.65 | 8.05 ± 1.84 | 8.16 ± 2.02 | 1.213 | 0.232 | 0.379 |
| HAMA | 10.59 ± 1.77 | 7.73 ± 1.36 | 7.74 ± 1.76 | 6.21 ± 1.38 | 1.133 | 0.264 | 0.354 |
: chi-square test.
Abbreviation: BS, bariatric surgery; Ctr, obese control group; BMI, body mass index; WC, waist circumference; YFAS, Yale Food Addiction Scale; HC, High calorie; LC, Low calorie; HAMD, Hamilton Depression Rating Scale; HAMA, Hamilton Anxiety Rating Scale; Pre-BS, obese patients who received MRI scan before surgery; Post-BS, obese patients who received MRI scan at one month after surgery; CtrT, control subjects who received MRI scan at baseline; CtrRT, control subjects who received MRI scan one month after the first scan; SE, standard error.
Experimental design
All participants underwent 12-hours overnight fasting and were instructed to rate their hunger level at a scale from 1–5. Fasting blood samples were taken and MRI scans were performed between 9 AM and 10 AM.
Fasting blood samples were obtained before and one-month after surgery and stored at −80 °C until assayed. Plasma concentrations of total ghrelin, leptin and insulin were measured using a Bio-Plex 200™ suspension array system (BIO-RAD, Inc, Hercules, California, USA), according to the manufacturers’ instructions (please see Supplementary Information-SI for detailed information) (Goldstone et al., 2014; Nagasaki and Ohta, 2015). We only assessed the association of peripheral hormones with brain activity during the fasting state.
Questionnaires
A designated clinician rated the severity of the subjects’ anxiety using the Hamilton Anxiety Rating Scale (HAMA) (Hamilton, 1959), and depression using the Hamilton Depression Rating Scale (HAMD) (Hamilton, 1960). Subjects were required to complete the Yale Food Addiction Scale (YFAS) evaluation (Clark and Saules, 2013) (Table 1). All clinical measurements were conducted before (baseline) and one-month after surgery, and the same surgeon performed all surgical procedures.
fMRI food-cue-reactivity task
Food-cue stimuli consisted of 88 unique HC and 88 LC food pictures selected from the International Affective Picture System (IAPS) (Bradley and Lang, 2007) and others (Killgore et al., 2013; Stoeckel et al., 2008; Dimitropoulos et al., 2012). Negative images with uncomfortable content such as those that would induce disgust, anger, anxiety were avoided. We modified the images to a consistent resolution/saturation/brightness/size via Adobe Photoshop. To increase the task’s validity, all participants were familiarized with the items. Food images were randomly selected for each subject and the same images were used but they were randomly presented for each subject at baseline and one month later. The software E-Prime (Psychology Software Tools, INC) was used to present visual images. The stimulation consisted of three HC and three LC food-cue blocks, and they were presented in a pseudorandom order. Each block lasted 30 seconds, during which 10 pictures were presented for 3 seconds each without inter-trial-interval. Finally, there were 30 seconds between blocks.
Craving ratings
After the food-cue-reactivity task, participants were instructed to rate their level of craving for HC/LC food using a visual-analog-scale (range 0–100) (Ochner et al., 2011). Statistical analyses of behavioral performance data were carried out using SPSS 22 (Armonk, NY, USA). A two-way ANOVA was conducted to model the effects of group (BS, Ctr), condition (HC, LC) and time (Baseline, 1 Month) on behavioral/clinical data.
MRI acquisition
The experiment was carried out using a 3T GE (Signa Excite HD, Milwaukee, WI, USA) scanner. First, a high-resolution structural image for each subject was acquired using three-dimensional magnetization-prepared rapid acquisition gradient-echo-sequences with a voxel size of 1 mm3 and with an axial fast spoiled gradient-echo-sequence (TR=7.8ms, TE=3.0ms, matrix size=256×256, field-of-view=256×256mm2, slice thickness=1mm and 166 slices). Then, a gradient-echo T2*-weighted echo-planar-imaging sequence was used for acquiring functional images with the following parameters: TR=2000ms, TE=30ms, matrix size=64×64, field-of-view=256×256mm2, flip angle=90 degrees, in-plane resolution of 4mm2, slice thickness=4mm and 32 axial slices. The scan for functional imaging lasted 360 seconds. Subjects were instructed to open their eyes and watch the instruction and food pictures during the entire scanning procedure. A radiologist examined the imaging data to rule out abnormalities in brain structure.
Image processing
All imaging data were analyzed using Statistical Parametric Mapping 12 (SPM12, http://www.fil.ion.uclac.uk/spm). The functional images first underwent conventional slice-timing, head movement correction. The echo-planar images were co-registered to everyone’s T1 anatomical image, and spatially normalized to the template of the Montreal-Neurological-Institute and resampled to a voxel size of 3 mm3. An isotropic Gaussian kernel (full-width-at-half-maximum=6mm3) was used to spatially smooth the images.
For each subject, a general linear model (GLM) including HC/LC food-cue condition regressors was constructed. Each regressor was created by convolving the canonical hemodynamic response function with a box-car function corresponding to the onset/duration of each condition. Additionally, six realignment parameters were also included in the GLM as covariates. Finally, individual contrast images for HC vs. LC food-cues were computed and submitted to second-level whole brain repeated-measures ANOVAs employing the factor group (BS, Ctr) and time (Baseline, 1 Month). In addition, the beta images responded to HC and LC food stimulation were also generated at the first-level and were used in the repeated-measures ANOVAs to examine the factor condition (HC and LC) and time (Baseline, 1 Month) in each group. Results were corrected for multiple comparisons using family wise error (FWE) corrections at the cluster level correction approach (PFWE<0.05) with a minimum cluster size of k=100 voxels and a cluster defining threshold of P<0.001 (uncorrected at the voxel level).
PPI analysis
The clusters with significant group × time interaction effects were selected as the seed regions, then whole-brain PPI analyses were performed to investigate alterations in task-related functional connectivity in response to HC vs. LC food-cues following surgery in the BS group (cluster level correction PFWE<0.05, k>100; please see SI for detailed information).
Association between behaviors and brain response/functional-connectivity
We conducted a partial-correlation analysis with age/gender as covariates to assess the association between ROI brain responses and food-craving, and between brain responses and clinical data, including BMI/YFAS which showed interaction (group × time) effects, as well as correlations between changes in ROI brain responses and changes in behavioral measurements. Similarly, correlation analyses were also performed between PPI values and behavioral measurements. Bonferroni-correction was applied for multiple-comparisons; and level of significance was set at P<0.008 (0.05/6).
Results
Demographic characteristics
At baseline, there were no significant differences in age, gender, duration of obesity, weight, BMI, WC and scores on the YFAS, HAMD and HAMA questionnaires between BS and Ctr groups (Table 1). There were significant group × time interaction effects for weight (F(1, 39)=43.2, P<0.001), BMI (F(1, 39)=43.1, P<0.001),WC (F(1, 39)=14.7, P<0.001), and YFAS (F(1, 39)=10.0, P=0.003) due to significant weight loss (t=−7.3, P < 0.001, d=1.6), reductions of BMI (t=−8.8, P<0.001, d=1.9) and WC (t=−6.6, P<0.001, d=1.4), and decreased YFAS score (t=−3.4, P=0.003, d=0.7) in the BS group, but not in the Ctr group (Supplementary Table 1).
Peripheral hormone measurements
At baseline, there were no significant differences in ghrelin (t=0.7, P=0.494, d=0.1), leptin (t=0.3, P=0.773, d=0.1) and insulin (t=0.3, P=0.758, d=0.1) levels between BS and Ctr groups. In the BS group, out of the three hormones investigated here, only fasting plasma ghrelin levels were negatively correlated with BMI (r=−0.54, P=0.014, Fig 1A) and positively correlated with craving for HC food (r=0.60, P=0.004, Fig 1B) before LSG. After the surgery, plasma ghrelin, insulin, and leptin levels were significantly lower than before the surgery (P<0.001, Fig 1C–E). The leptin/BMI ratio was also significantly lower after surgery (P<0.001, Fig 1F).
Figure 1.
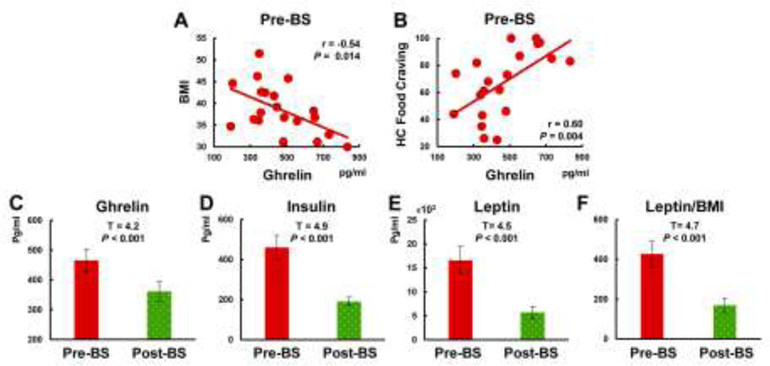
Changes in plasma peripheral hormones before and after LSG surgery and correlations between peripheral hormones and behavioral measurements. A-B. In the BS group, only fasting plasma total ghrelin (neither insulin nor leptin) levels were negatively correlated with BMI and positively correlated with craving for HC food. C-F. Total ghrelin, insulin, leptin levels and leptin/BMI ratio were lower than before the surgery.
Abbreviation: LSG; laparoscopic sleeve gastrectomy; BMI, body mass index; Pre-BS, obese patients who received MRI scan before surgery; Post-BS, obese patients who received MRI scan at one month after surgery.
Food craving
There were no significant interaction effects (time × group, F(1,39) = 0.8, P = 0.360) for hunger levels (Pre-BS:3.27±0.09; Post-BS:3.18±0.12; CtrT:3.21±0.14; CtrRT: 3.31±0.15). There was no significant difference in HC/LC food craving between BS and Ctr group at baseline condition (Fig 2, Supplementary Table 2). The ANOVA showed significant condition × time × group interaction effects for food craving (F(1,39)=7.5, P=0.009) (Fig 2). Further post-hoc tests showed that Pre-BS and Ctr groups (both CtrT and CtrRT) had higher craving for HC/LC food-cues (Pre-BS: t=3.6, P=0.002, d=0.8; CtrT: t=2.6, P=0.017, d=0.6; CtrRT: t=2.3, P=0.035, d=0.5). There was a significant reduction of craving for HC food in the BS group after surgery (t=−6.0, P<0.001, d=1.2), and the reduction in HC food-cue cravings post-surgery were larger than for LC food-cues (t=3.1, P=0.005, d=0.7) (Fig 2). Food craving for LC food did not significantly change in either group. After surgery, there was a trend for a positive correlation between the changes in ghrelin (ΔPost-Pre) and the changes in craving for the HC vs. LC food-cues contrast, such that the larger the ghrelin decreases, the greater the decline in food-craving post-surgery (r=0.46, P=0.041, Supplementary Fig 1).
Figure 2.
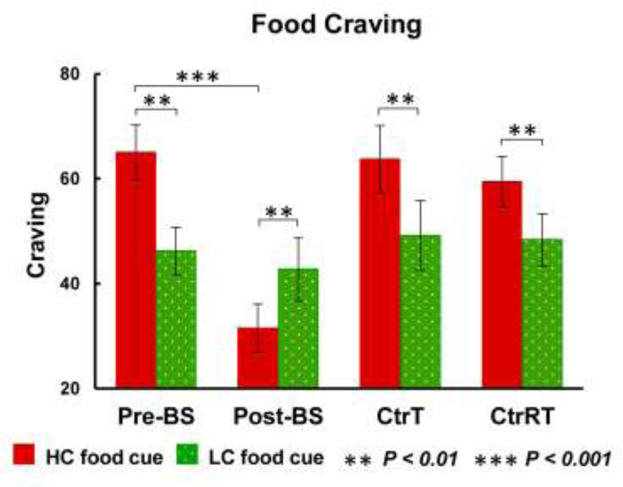
ANOVAs for food craving and post-hoc tests. There was condition × time × group interaction effects for food craving, time × group interaction effects for HC food craving, and food × time interaction effects in the BS groups. Both Pre-BS and Ctr groups (CtrT, CtrRT) had higher craving for HC than LC food cues. There was a significant reduced craving for HC food in the BS group after surgery, and HC food cue cravings were lower than LC food cues after surgery; there were no changes in craving for LC food in either group.
Abbreviation: Pre-BS, obese patients who received MRI scan before surgery; Post-BS, obese patients who received MRI scan at one month after surgery; CtrT, control subjects who received MRI scan at baseline; CtrRT, control subjects who received MRI scan one month after the first scan.
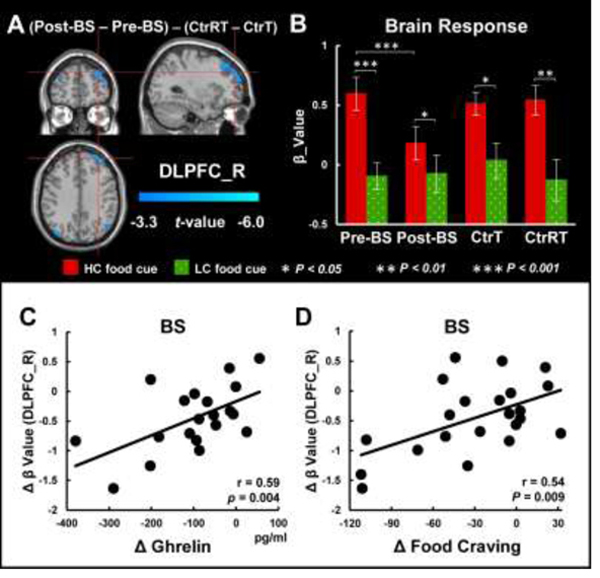
Brain response to food cues
At baseline condition, both Pre-BS and CtrT had activation in the right DLPFC and occipital gyrus in response to HC vs. LC food-cues, and there was no difference between the two groups. There were significant interaction effects (group × time) on brain responses to HC vs. LC food-cues in the right DLPFC (PFWE<0.05, Fig 3A, Supplementary Table 3) due to significant activation reduction in the BS group after surgery (t=3.5, P=0.002, d=0.7) (Fig 3B). All groups showed greater responses for HC than for LC food-cues (Pre-BS: t=6.6, P<0.001, d=1.4; Post-BS: t=2.3, P=0.032, d=0.5; CtrT: t=2.6, P=0.018, d=0.6; CtrRT: t=3.1, P=0.006, d=0.7), and LSG decreased DLPFC activation induced by HC food cues in the BS group (t=3.7, P<0.001, d=0.8). The Ctr group did not show significant changes in brain activation between measures at baseline and one-month later (Fig 3B). Changes in DLPFC activation in response to HC vs. LC food-cues were positively correlated with changes in ghrelin levels (r=0.59, P=0.004, Fig 3C) and changes in craving for HC versus LC food-cues (r=0.54, P=0.009, Fig 3D) following surgery in the BS group, such that the greater the decreases in ghrelin post-surgery the greater the attenuation of the DLPFC responses and the greater the attenuation of the DLPFC, the larger the decreases in food-craving. There were no significant associations of food craving with plasma insulin and leptin levels.
Figure 3.
Whole brain ANOVAs for brain responses to food cues and post-hoc tests. A. There was a significant interaction effect (group × time) for brain responses to HC vs. LC food cues in the DLPFC. B. BS group showed significant reduction in brain responses to HC vs. LC food cues after surgery. There were significant group × time interaction effects for brain responses to HC food cues, and significant condition × time interaction effects for brain activations in the DLPFC in the BS group. C. Changes in DLPFC activation in response to HC vs. LC food cues were positively correlated with changes in ghrelin. D. Positive correlation between changes in craving for HC versus LC food cues and changes in DLPFC activation in response to HC vs. LC food cues following LSG surgery in the BS group.
Abbreviation: BS, bariatric surgery; Pre-BS, obese patients who received MRI scan before surgery; Post-BS, obese patients who received MRI scan at one month after surgery; CtrT, control subjects who received MRI scan at baseline; CtrRT, control subjects who received MRI scan one month after the first scan; DLPFC, dorsolateral prefrontal cortex; HC, high-calorie; LC, low calorie.
PPI connectivity analysis
The functional-connectivity between the right DLPFC seed and the ventral anterior cingulate cortex (vACC) showed condition × group interaction effects, such that calorie-related increases in connectivity (i.e., HC vs. LC food-cues) were stronger for Post-BS than for Pre-BS (PFWE<0.05, K>100, Fig 4A). The results suggested surgery-increased functional connectivity between the right DLPFC and vACC during exposure to HC vs. LC. Moreover, the correlation analysis revealed that changes in connectivity of the vACC post-surgery were negatively correlated with the changes in BMI, such that increases in connectivity between right DLPFC and vACC post-surgery were associated with greater reduction in BMI (r=−0.48, P=0.017, Fig 4B). Higher BMI prior to surgery also correlated with lower PPI connectivity between the right DLPFC and vACC (r=−0.58, P=0.004, Fig 4C).
Figure 4.
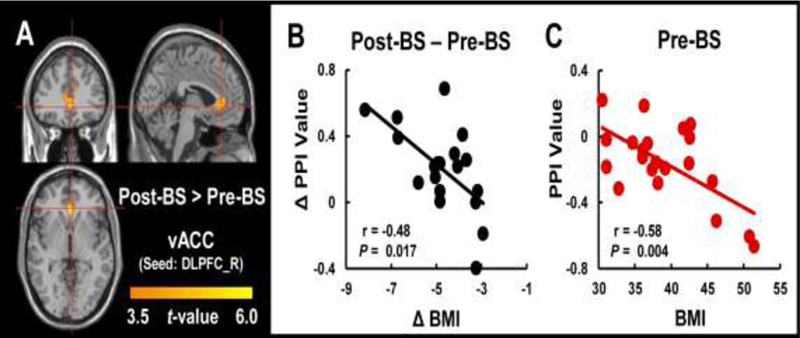
Altered PPI connectivity between the DLPFC and vACC in the BS group following surgery and correlation analysis between PPI connectivity and behavior measures. A. There was elevated PPI connectivity between the DLPFC and vACC when exposed to HC vs. LC food cues in Post-BS compared to Pre-BS group (PFWE<0.05). B. Prior to surgery, higher BMI was correlated with lower PPI connectivity between the right DLPFC and vACC. C. After the surgery, greater decreases in BMI were correlated with greater increases in PPI connectivity.
Abbreviation: Pre-BS, obese patients who received MRI scan before surgery; Post-BS, obese patients who received MRI scan at one month after surgery; DLPFC, dorsolateral prefrontal cortex; vACC, ventromedial anterior cingulate cortex; BMI, body mass index.
Discussion
We found LSG significantly decreased plasma ghrelin concentrations, craving for HC food, and decreased brain activation in DLPFC regions involved with executive-control in response to HC versus LC food-cues in the BS group. The decreased DLPFC activation in response to HC vs. LC food-cues post-surgery was positively correlated with decreased ghrelin levels and reduced craving for HC versus LC food-cues. Additionally, LSG increased the connectivity between the right DLPFC and vACC since there was a greater increase in connectivity with a larger reduction in BMI post-surgery.
DLPFC plays important roles in the central regulation of eating-behavior (Hare et al., 2009; Tataranni et al., 1999) and in effectively down-regulating the motivation to consume desirable food (Hare et al., 2009; Lavagnino et al., 2016). The activation of the DLPFC may inhibit activation within the striatum and motor cortex during food-cue stimulation (Killgore et al., 2003). HC food stimuli were shown to activate the DLPFC (Hare et al., 2009). Greater PFC activation in response to HC foods may be due to inhibitory processes in response to increased reward value of HC foods (Killgore et al., 2003). Similar results were also found in studies showing PFC activation during inhibition of food-intake (DelParigi et al., 2007). Conscious attempts to inhibit eating-behavior are processed primarily in the DLPFC (DelParigi et al., 2007), and are reciprocally activated with reward-related regions in response to HC food (Burger and Stice, 2011). Our results showed significantly decreased brain activation in the right DLPFC in response to HC versus LC food-cues after surgery, which is consistent with findings by Ochner et al. (Ochner et al., 2011) who reported decreases in HC food-cue induced DLPFC activation one-month after gastric bypass surgery. Our findings suggest beneficial effects of LSG surgery in the normalization of DLPFC hyperactivity in responses to HC food-cues.
The findings of positive correlations between changes in DLPFC activation in response to HC vs. LC food-cues and changes in ghrelin levels and changes in craving for HC versus LC food-cues suggest that changes in the stomach-derived hormone ghrelin after LSG surgery may underlie the changes in brain reactivity and eating-behaviors. Notably, similar correlations were not found for insulin or leptin, which are also key appetitive hormones produced by the pancreas and adipose tissue, respectively. This difference suggests a potential predominant role of ghrelin, over other endocrine pathways, at least in the short-term (one month) effects following LSG. While speculative, this interpretation would be consistent with the fact that LSG results in an acute manipulation of the gut-brain axis, while more systemic changes and adaptation involving other endocrine pathways may take place at a later time. The significant associations between ghrelin, craving and DLPFC activation mirrored concurrent postsurgical reduction in the desire to eat (craving under HC food-cues) (Ochner et al., 2011). The reduction in brain activation in DLPFC may be related to decreased brain activation in the mesolimbic-reward pathway, as found in previous studies (Ochner et al., 2012; Ochner et al., 2011). The association between changes in ghrelin levels and changes in DLPFC activation suggests that ghrelin and gut hormone-mediated shift in hunger/satiety would simply lead to reduced reward value of food, and hence reduced need for DLPFC response when exposed to HC food cues. While the present study does not show a causality role of ghrelin, it is possible to speculate that ghrelin may represent a peripheral biomarker of the here described LSG-induced changes in food craving and DLPFC activation; or could be implicated in the mechanisms of how LSG leads to changes in brain areas involved in food reward and food craving, or both.
To further assess the connectivity of the right DLPFC and its associated brain regions, we used PPI analysis. Under HC food-cues stimuli, the seed in the right DLPFC had stronger connectivity (increased PPI value) with the vACC after the surgery, and changes in BMI were negatively correlated with changes in connectivity between the right DLPFC and vACC. The ACC is implicated in the executive-control of internal/external stimuli-related, context-dependent behaviors involving evaluation of salience of emotional information and modulation of the emotional response (Cohen et al., 2005; Bush et al., 2000). The ACC may contribute to an imbalance between cognitive and emotional processing and consequentially an increased risk of overeating (Cohen et al., 2005). The area in the vACC that showed that the increased connectivity corresponds to Brodmann Area (BA) 25, is implicated in emotional regulation, presumably through its connection with hypothalamic, limbic, thalamic and brain stem nuclei (Hamani et al., 2011).
Increased connectivity of the right DLPFC and vACC may reflect an enhanced ability of the DLPFC to resolve emotional conflict processed by vACC (Wallis et al., 2017). The negative correlation between changes in BMI and changes in connectivity of the right DLPFC and vACC may indicate that greater modulation of the vACC by the DLPFC, which might then prevent impulsive/compulsive overeating and thus lead to reductions in BMI.
Limitations
There are several limitations in this study that need to be taken into account, as they limit the generalizability of our results and require follow-up investigation. Specifically, 1. Due to strict exclusion criteria and the difficulty in retaining patient’s post-surgery for follow-up scanning, we did not have a larger cohort for the BS group pre-and post-surgery, including the controls, and we did not have longer post-surgery assessments and scans. 2. We assessed obese participants at two-time points only. Multiple time-point assessments are warranted for future investigations on the progression of food-induced decreases in craving and DLPFC activation after surgery. 3. There was no standardized diet for participants followed outside of the study. 4. We only assessed the association of peripheral hormones with brain activity during the fasting state. Further studies are needed to assess these gut hormones and their association with brain reactivity at fasting and postprandial conditions. 5. We measured total ghrelin even if acyl-ghrelin is the form of the peptide that stimulates appetite (Filigheddu et al., 2007). While this approach is a limitation, we elected to measure total ghrelin as it is easier to measure, and it is likely that total and acyl-ghrelin highly correlate with each other. Nonetheless, future studies should measure both total ghrelin and acyl-ghrelin concentration and calculate the acyl-to-total ghrelin ratio.
Conclusion
This study investigated the association of ghrelin and brain changes under food-cue exposures after LSG surgery. We found that the reductions in fasting ghrelin levels was correlated with reduced craving for HC food-cues and reduced activation in the right DLPFC under HC versus LC food-cue stimuli along with strengthening of connectivity of the right DLPFC with vACC, which are regions implicated in executive-control and self-regulation. These associations seem specific for ghrelin, as we did not find similar results for leptin and insulin, at least within the short-term post-surgery assessment conducted in this study. These findings suggest that LSG-induced weight-loss one-month after surgery may be in part due to changes in fasting ghrelin. A shift in hunger/satiety as a result of decreased ghrelin levels would simply lead to reduced craving for food, hence a reduced need for DLPFC response when exposed to HC food cues. Future studies are needed to test the causal role of the ghrelin system in the changes in food craving and in brain activity after bariatric surgery and to investigate the potential role of the ghrelin system as a treatment target for sustained weight loss after the surgery.
Supplementary Material
Highlights.
-
○
LSG decreased fasting plasma ghrelin levels, food craving, and brain activation in right DLPFC.
-
○
Reduction in ghrelin levels and food craving correlated with reduction in DLPFC activation.
-
○
Changes in BMI negatively correlated with changes in connectivity between DLPFC and vACC.
Acknowledgements
This work was supported by the National Natural Science Foundation of China [grants numbers 61431013, 81470816, 81601563, 81730016, and 81501543]; National Natural Science Foundation of Shaanxi Province [grants number 2018JM3007]; National Clinical Research Center for Digestive Diseases, Xi’an, China [grants number 2015BAI13B07]; and support in part from the Intramural Research Program of the United States National Institute on Alcohol Abuse and Alcoholism [grants number Z01AA3009 (DT, CEW, NDV, GJW)] and from the Intramural Research Programs of the United States National Institute on Alcohol Abuse and Alcoholism and National Institute on Drug Abuse[grants number ZIA-AA000218 (Lorenzo L)].
Footnotes
Conflict of interest
The authors declare no conflict of interest.
References
- Diamantis T, Apostolou KG, Alexandrou A, Griniatsos J, Felekouras E & Tsigris C 2014. Review of long-term weight loss results after laparoscopic sleeve gastrectomy. Surg Obes Relat Dis, 10(1), 177–183. doi: 10.1016/j.soard.2013.11.007 [DOI] [PubMed] [Google Scholar]
- Papailiou J, Albanopoulos K, Toutouzas KG, Tsigris C, Nikiteas N & Zografos G 2010. Morbid obesity and sleeve gastrectomy: how does it work? Obes Surg, 20(10), 1448–1455. doi: 10.1007/s11695-010-0148-5 [DOI] [PubMed] [Google Scholar]
- Tsoli M, Chronaiou A, Kehagias I, Kalfarentzos F & Alexandrides TK 2013. Hormone changes and diabetes resolution after biliopancreatic diversion and laparoscopic sleeve gastrectomy: a comparative prospective study. Surg Obes Relat Dis, 9(5), 667–677. doi: 10.1016/j.soard.2012.12.006 [DOI] [PubMed] [Google Scholar]
- Langer FB, Reza HM, Bohdjalian A, Felberbauer FX, Zacherl J, Wenzl E, Schindler K, Luger A, Ludvik B & Prager G 2005. Sleeve gastrectomy and gastric banding: effects on plasma ghrelin levels. Obes Surg, 15(7), 1024–1029. doi: 10.1381/0960892054621125 [DOI] [PubMed] [Google Scholar]
- Ramon JM, Salvans S, Crous X, Puig S, Goday A, Benaiges D, Trillo L, Pera M & Grande L 2012. Effect of Roux-en-Y gastric bypass vs sleeve gastrectomy on glucose and gut hormones: a prospective randomised trial. J Gastrointest Surg, 16(6), 1116–1122. doi: 10.1007/s11605-012-1855-0 [DOI] [PubMed] [Google Scholar]
- Karamanakos SN, Vagenas K, Kalfarentzos F & Alexandrides TK 2008. Weight loss, appetite suppression, and changes in fasting and postprandial ghrelin and peptide-YY levels after Roux-en-Y gastric bypass and sleeve gastrectomy: a prospective, double blind study. Ann Surg, 247(3), 401–407. doi: 10.1097/SLA.0b013e318156f012 [DOI] [PubMed] [Google Scholar]
- Lean ME & Malkova D 2016. Altered gut and adipose tissue hormones in overweight and obese individuals: cause or consequence? Int J Obes (Lond), 40(4), 622–632. doi: 10.1038/ijo.2015.220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanusch-Enserer U, Brabant G & Roden M 2003. Ghrelin concentrations in morbidly obese patients after adjustable gastric banding. N Engl J Med, 348(21), 2159–2160. doi: 10.1056/NEJM200305223482125 [DOI] [PubMed] [Google Scholar]
- Cummings DE, Weigle DS, Frayo RS, Breen PA, Ma MK, Dellinger EP & Purnell JQ 2002. Plasma ghrelin levels after diet-induced weight loss or gastric bypass surgery. N Engl J Med, 346(21), 1623–1630. doi: 10.1056/NEJMoa012908 [DOI] [PubMed] [Google Scholar]
- Faraj M, Havel PJ, Phelis S, Blank D, Sniderman AD & Cianflone K 2003. Plasma acylation-stimulating protein, adiponectin, leptin, and ghrelin before and after weight loss induced by gastric bypass surgery in morbidly obese subjects. J Clin Endocrinol Metab, 88(4), 1594–1602. doi: 10.1210/jc.2002-021309 [DOI] [PubMed] [Google Scholar]
- Holdstock C, Engstrom BE, Ohrvall M, Lind L, Sundbom M & Karlsson FA 2003. Ghrelin and adipose tissue regulatory peptides: effect of gastric bypass surgery in obese humans. J Clin Endocrinol Metab, 88(7), 3177–3183. doi: 10.1210/jc.2002-021734 [DOI] [PubMed] [Google Scholar]
- Skibicka KP, Hansson C, Alvarez-Crespo M, Friberg PA & Dickson SL 2011. Ghrelin directly targets the ventral tegmental area to increase food motivation. Neuroscience, 180, 129–137. doi: 10.1016/j.neuroscience.2011.02.016 [DOI] [PubMed] [Google Scholar]
- Skibicka KP, Shirazi RH, Rabasa-Papio C, Alvarez-Crespo M, Neuber C, Vogel H & Dickson SL 2013. Divergent circuitry underlying food reward and intake effects of ghrelin: dopaminergic VTA-accumbens projection mediates ghrelin’s effect on food reward but not food intake. Neuropharmacology, 73, 274–283. doi: 10.1016/j.neuropharm.2013.06.004 [DOI] [PubMed] [Google Scholar]
- Abizaid A, Liu Z, Andrews ZB, Shanabrough M, Borok E, Elsworth JD, Roth RH, Sleeman MW, Picciotto MR, Tschop MH, Gao X & Horvath TL 2006. Ghrelin modulates the activity and synaptic input organization of midbrain dopamine neurons while promoting appetite. Journal of Clinical Investigation, 116(12), 3229–3239. doi: 10.1172/JCI29867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egecioglu E, Jerlhag E, Salome N, Skibicka KP, Haage D, Bohlooly-Y M, Andersson D, Bjursell M, Perrissoud D, Engel JA & Dickson SL 2010. Ghrelin increases intake of rewarding food in rodents. Addict Biol, 15(3), 304–311. doi: 10.1111/j.1369-1600.2010.00216.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zallar LJ, Tunstall BJ, Richie CT, Zhang YJ, You ZB, Gardner EL, Heilig M, Pickel J, Koob GF, Vendruscolo LF, Harvey BK & Leggio L 2018. Development and initial characterization of a novel ghrelin receptor CRISPR/Cas9 knockout wistar rat model. International Journal of Obesity. doi: 10.1038/s41366-018-0013-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroemer NB, Krebs L, Kobiella A, Grimm O, Pilhatsch M, Bidlingmaier M, Zimmermann US & Smolka MN 2013. Fasting levels of ghrelin covary with the brain response to food pictures. Addiction Biology, 18(5), 855–862. doi: 10.1111/j.1369-1600.2012.00489.x [DOI] [PubMed] [Google Scholar]
- Bohon C 2014. Greater emotional eating scores associated with reduced frontolimbic activation to palatable taste in adolescents. Obesity (Silver Spring), 22(8), 1814–1820. doi: 10.1002/oby.20759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tracy AL, Wee CJ, Hazeltine GE & Carter RA 2015. Characterization of attenuated food motivation in high-fat diet-induced obesity: Critical roles for time on diet and reinforcer familiarity. Physiol Behav, 141, 69–77. doi: 10.1016/j.physbeh.2015.01.008 [DOI] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Telang F, Fowler JS, Thanos PK, Logan J, Alexoff D, Ding YS, Wong C, Ma Y & Pradhan K 2008. Low dopamine striatal D2 receptors are associated with prefrontal metabolism in obese subjects: possible contributing factors. Neuroimage, 42(4), 1537–1543. doi: 10.1016/j.neuroimage.2008.06.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hare TA, Camerer CF & Rangel A 2009. Self-control in decision-making involves modulation of the vmPFC valuation system. Science, 324(5927), 646–648. doi: 10.1126/science.1168450 [DOI] [PubMed] [Google Scholar]
- Killgore WD, Weber M, Schwab ZJ, Kipman M, DelDonno SR, Webb CA & Rauch SL 2013. Cortico-limbic responsiveness to high-calorie food images predicts weight status among women. Int J Obes (Lond), 37(11), 1435–1442. doi: 10.1038/ijo.2013.26 [DOI] [PubMed] [Google Scholar]
- Lowe MR, van Steenburgh J, Ochner C & Coletta M 2009. Neural correlates of individual differences related to appetite. Physiol Behav, 97(5), 561–571. doi: 10.1016/j.physbeh.2009.04.001 [DOI] [PubMed] [Google Scholar]
- Batterink L, Yokum S & Stice E 2010. Body mass correlates inversely with inhibitory control in response to food among adolescent girls: an fMRI study. Neuroimage, 52(4), 1696–1703. doi: 10.1016/j.neuroimage.2010.05.059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofmann W, Friese M & Roefs A 2009. Three ways to resist temptation: The independent contributions of executive attention, inhibitory control, and affect regulation to the impulse control of eating behavior. Journal of Experimental Social Psychology(45), 431–435. doi: 10.1111/j.1745-6924.2009.01116.x [DOI] [Google Scholar]
- Gruber AJ & McDonald RJ 2012. Context, emotion, and the strategic pursuit of goals: interactions among multiple brain systems controlling motivated behavior. Front Behav Neurosci, 6, 50. doi: 10.3389/fnbeh.2012.00050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pepino MY, Finkbeiner S & Mennella JA 2009. Similarities in food cravings and mood states between obese women and women who smoke tobacco. Obesity (Silver Spring), 17(6), 1158–1163. doi: 10.1038/oby.2009.46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ & Baler RD 2011. Reward, dopamine and the control of food intake: implications for obesity. Trends Cogn Sci, 15(1), 37–46. doi: 10.1016/j.tics.2010.11.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ochner CN, Stice E, Hutchins E, Afifi L, Geliebter A, Hirsch J & Teixeira J 2012. Relation between changes in neural responsivity and reductions in desire to eat high-calorie foods following gastric bypass surgery. Neuroscience, 209, 128–135. doi: 10.1016/j.neuroscience.2012.02.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruce JM, Hancock L, Bruce A, Lepping RJ, Martin L, Lundgren JD, Malley S, Holsen LM & Savage CR 2012. Changes in brain activation to food pictures after adjustable gastric banding. Surg Obes Relat Dis, 8(5), 602–608. doi: 10.1016/j.soard.2011.07.006 [DOI] [PubMed] [Google Scholar]
- Zhang Y, Ji G, Xu M, Cai W, Zhu Q, Qian L, Zhang YE, Yuan K, Liu J, Li Q, Cui G, Wang H, Zhao Q, Wu K, Fan D, Gold MS, Tian J, Tomasi D, Liu Y, Nie Y & Wang GJ. 2016. Recovery of brain structural abnormalities in morbidly obese patients after bariatric surgery. Int J Obes (Lond), 40(10), 1558–1565. doi: 10.1038/ijo.2016.98 [DOI] [PubMed] [Google Scholar]
- Langer FB, Hoda M, Bohdjalian A, Felberbauer FX, Zacherl J, Wenzl E, Schindler K, Luger A, Ludvik B & Prager G 2005. Sleeve gastrectomy and gastric banding: Effects on plasma ghrelin levels. OBESITY SURGERY, 15(7), 1024–1029. doi: 10.1381/0960892054621125 [DOI] [PubMed] [Google Scholar]
- Goldstone AP, Prechtl CG, Scholtz S, Miras AD, Chhina N, Durighel G, Deliran SS, Beckmann C, Ghatei MA, Ashby DR, Waldman AD, Gaylinn BD, Thorner MO, Frost GS, Bloom SR & Bell JD 2014. Ghrelin mimics fasting to enhance human hedonic, orbitofrontal cortex, and hippocampal responses to food. Am J Clin Nutr, 99(6), 1319–1330. doi: 10.3945/ajcn.113.075291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagasaki H & Ohta T 2015. Extra-uterine growth and adipocytokines in appropriate-for-gestational-age preterm infants. Pediatr Int. doi: 10.1111/ped.12896 [DOI] [PubMed] [Google Scholar]
- Hamilton M 1959. The assessment of anxiety states by rating. Br J Med Psychol, 32(1), 50–55 [DOI] [PubMed] [Google Scholar]
- Hamilton M 1960. A rating scale for depression. J Neurol Neurosurg Psychiatry, 23, 56–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark SM & Saules KK 2013. Validation of the Yale Food Addiction Scale among a weight-loss surgery population. Eat Behav, 14(2), 216–219. doi: 10.1016/j.eatbeh.2013.01.002 [DOI] [PubMed] [Google Scholar]
- Bradley M & Lang P (2007). The International Affective Picture System (IAPS) in the study of emotion and attention In Coan J & Allen J (Eds.), Handbook of Emotion Elicitation and Assessment (pp. 29–46). New York: Oxford University Press. [Google Scholar]
- Stoeckel LE, Weller RE, Cook ER, Twieg DB, Knowlton RC & Cox JE 2008. Widespread reward-system activation in obese women in response to pictures of high-calorie foods. Neuroimage, 41(2), 636–647. doi: 10.1016/j.neuroimage.2008.02.031 [DOI] [PubMed] [Google Scholar]
- Dimitropoulos A, Tkach J, Ho A & Kennedy J 2012. Greater corticolimbic activation to high-calorie food cues after eating in obese vs. normal-weight adults. Appetite, 58(1), 303–312. doi: 10.1016/j.appet.2011.10.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ochner CN, Kwok Y, Conceicao E, Pantazatos SP, Puma LM, Carnell S, Teixeira J, Hirsch J & Geliebter A 2011. Selective reduction in neural responses to high calorie foods following gastric bypass surgery. Ann Surg, 253(3), 502–507. doi: 10.1097/SLA.0b013e318203a289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tataranni PA, Gautier JF, Chen K, Uecker A, Bandy D, Salbe AD, Pratley RE, Lawson M, Reiman EM & Ravussin E 1999. Neuroanatomical correlates of hunger and satiation in humans using positron emission tomography. Proc Natl Acad Sci U S A, 96(8), 4569–4574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavagnino L, Arnone D, Cao B, Soares JC & Selvaraj S 2016. Inhibitory control in obesity and binge eating disorder: A systematic review and meta-analysis of neurocognitive and neuroimaging studies. Neurosci Biobehav Rev, 68, 714–726. doi: 10.1016/j.neubiorev.2016.06.041 [DOI] [PubMed] [Google Scholar]
- Killgore WD, Young AD, Femia LA, Bogorodzki P, Rogowska J & Yurgelun-Todd DA 2003. Cortical and limbic activation during viewing of high-versus low-calorie foods. Neuroimage, 19(4), 1381–1394 [DOI] [PubMed] [Google Scholar]
- DelParigi A, Chen K, Salbe AD, Hill JO, Wing RR, Reiman EM & Tataranni PA 2007. Successful dieters have increased neural activity in cortical areas involved in the control of behavior. Int J Obes (Lond), 31(3), 440–448. doi: 10.1038/sj.ijo.0803431 [DOI] [PubMed] [Google Scholar]
- Burger KS & Stice E 2011. Relation of dietary restraint scores to activation of reward-related brain regions in response to food intake, anticipated intake, and food pictures. Neuroimage, 55(1), 233–239. doi: 10.1016/j.neuroimage.2010.12.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen MX, Heller AS & Ranganath C 2005. Functional connectivity with anterior cingulate and orbitofrontal cortices during decision-making. Brain Res Cogn Brain Res, 23(1), 61–70. doi: 10.1016/j.cogbrainres.2005.01.010 [DOI] [PubMed] [Google Scholar]
- Bush G, Luu P & Posner MI 2000. Cognitive and emotional influences in anterior cingulate cortex. Trends Cogn Sci, 4(6), 215–222 [DOI] [PubMed] [Google Scholar]
- Hamani C, Mayberg H, Stone S, Laxton A, Haber S & Lozano AM 2011. The subcallosal cingulate gyrus in the context of major depression. Biol Psychiatry, 69(4), 301–308. doi: 10.1016/j.biopsych.2010.09.034 [DOI] [PubMed] [Google Scholar]
- Wallis CU, Cardinal RN, Alexander L, Roberts AC & Clarke HF 2017. Opposing roles of primate areas 25 and 32 and their putative rodent homologs in the regulation of negative emotion. Proc Natl Acad Sci U S A, 114(20), E4075–E4084. doi: 10.1073/pnas.1620115114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filigheddu N, Gnocchi VF, Coscia M, Cappelli M, Porporato PE, Taulli R, Traini S, Baldanzi G, Chianale F, Cutrupi S, Arnoletti E, Ghe C, Fubini A, Surico N, Sinigaglia F, Ponzetto C, Muccioli G, Crepaldi T & Graziani A 2007. Ghrelin and des-acyl ghrelin promote differentiation and fusion of C2C12 skeletal muscle cells. Mol Biol Cell, 18(3), 986–994. doi: 10.1091/mbc.E06-05-0402 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.