Abstract
Background:
Irritable bowel syndrome (IBS) is a stress-sensitive disorder of brain-gut interactions associated with a higher prevalence of early adverse life events (EALs). However, it is incompletely understood how trauma severity or disclosure influence the risk of developing IBS or symptom severity.
Aims:
To determine whether: 1) IBS patients report a greater number of EALs compared with healthy controls (HCs), 2) trauma severity and first age of EAL increase the odds of IBS, 3) confiding in others reduces the odds of IBS, 4) the number, trauma severity, and first age of EAL are associated with symptom severity, 5) sex differences exist.
Methods:
In total, 197 IBS patients (72% women, mean age=30.28 years) and 165 HCs (59% women, mean age=30.77 years) completed the Childhood Traumatic Events Scale (CTES), measuring severity of EALs and degree of confiding in others. Regression analyses were used to predict IBS status from EALs and association between gastrointestinal (GI) symptoms and EALs.
Results:
A greater number of EALs [odds ratio (OR)=1.36, 95% confidence interval (CI), 1.14–1.62; p<0.001) and higher perceived trauma severity (OR=1.13, 95% CI, 1.08–1.19; p<0.001) were associated with increased odds of IBS. Confiding in others decreased the odds of having IBS (OR=0.83, 95% CI, 0.72–0.96; p=0.012). The first age of EAL was not predictive of IBS. No sex differences were found.
Conclusions:
Assessing the traumatic severity of EALs and amount of confiding in others is important as they can affect the risk of having IBS. Our findings emphasize early intervention to improve health outcomes in individuals with EALs.
Keywords: early adverse life events, irritable bowel syndrome, social support, stress, trauma
Introduction
Irritable bowel syndrome (IBS) is a stress-sensitive gastrointestinal (GI) disorder of brain-gut interactions characterized by abdominal pain with changes in stool form and/or frequency [1, 2]. Previous studies have found that patients with IBS have a higher prevalence of early adverse life events (EALs) compared to healthy individuals and those with organic disorders, such as inflammatory bowel disease [3–7]. EALs are defined as reported traumatic experiences during childhood, which can include physical violence, sexual abuse, illness, or injury [8]. We have recently demonstrated that 75% of 148 IBS patients recruited from the community report EALs that occurred before the age of 18 compared to 58% of 154 healthy controls (HCs) [7]. We have also shown that as the number of EALs increases, the odds of having IBS increases [3, 7]. Specifically, a history of EALs was associated with a two-fold higher odds of developing IBS and correlated with severity of overall IBS symptoms, abdominal pain [7], poorer daily functioning, and greater health care utilization [4, 9, 10]. In addition, the association of EALs and IBS was predominantly seen in women and less so in men, although this may be due to the smaller number of men studied [3]. It is hypothesized that a history of EALs can increase the risk for developing IBS as well as other chronic health problems, via epigenetic mechanisms resulting in a dysregulation of the hypothalamic-pituitary-adrenal (HPA) axis and autonomic nervous system regulation of GI function (e.g., motility, intestinal permeability, sensation) [11, 12].
Although a history of EALs is associated with an increase in odds of having IBS and other chronic illnesses, and is thus a risk factor, there is clearly significant variation in the extent to which affected individuals develop these negative health outcomes [13]. Few research studies have directly evaluated the context in which EALs occur and how individual characteristics moderate the impact of EAL on health outcomes. For instance, there is a dearth in research addressing the role of perceived trauma severity of EALs in predicting health outcomes, though studies suggest populations with chronic illness report greater trauma severity. Patients with overactive bladder rated their experience of childhood sexual events as more traumatic compared to controls, but there was no difference between patients with overactive bladder and healthy controls in the perceived severity of trauma of childhood violence, deaths, parental upheaval, or illness/injury [14]. Scores for severity of EALs have also been found to be significantly correlated with scores for frequency and severity of post-traumatic stress disorder (PTSD) in patients with schizophrenia and substance abuse [15]. Moreover, it is not known if there is a critical age of exposure that increases the risk of developing IBS. A study of women in a gastroenterology clinic found no differences in the health status of women who first experienced sexual or physical abuse in childhood versus adulthood [16]. Fifty percent of IBS patients reported having first had symptoms of IBS before the age of 35, but there was a lack of data on the temporal association of EAL and onset of IBS symptoms [17]. Animal studies have helped to elucidate the mechanisms underlying the potential critical time period during which EALs are more likely to affect the development of IBS, such as altered emotionality, increased stress responsivity, and heightened visceral pain perception [18, 19].
Furthermore, it is important to determine if confiding in others reduces the risk of having IBS or serves as a protective factor. It has been hypothesized that confiding in others may be indicative of support resources or active coping abilities that may buffer against long-term adverse physical and mental health outcomes [20, 21]. Nondisclosure of trauma is associated with heightened inhibition, which can serve as a cumulative, low-level stressor leading to adverse health outcomes [8, 22]. Women with interstitial cystitis/painful bladder syndrome endorsed less confiding in others regarding EALs than healthy controls (HCs) [23]. Drossman et al [24] found that 44% of IBS patients report a history of physical or sexual abuse, but almost a third have never discussed their abuse with anyone.
Data from the Adverse Childhood Experiences (ACE) module of the Behavioral Risk Factor Surveillance System (BRFSS) indicate that women experience higher rates of most EALs, particularly sexual abuse and living with family members who are mentally ill or abusing substances [25]. Sex differences have also been a factor in the impact of EALs on negative health outcomes. A 2012 Canadian Community Health Survey of 23,395 participants revealed slightly stronger effects for women in the association between child abuse and increased odds of back problems, chronic pulmonary disease, cancer, and chronic fatigue syndrome [26]. An analysis of 111,964 participants from the BRFSS suggests that almost all types of ACEs, including physical, verbal, and emotional abuse increased odds of cancer for women, whereas only emotional abuse was associated with increased odds of cancer for men [27]. While IBS is more common in women than men, less is known about sex differences in EALs and IBS. We previously found that the higher number of EAL events reported by IBS patients compared with HCs was significantly different within women but less so in men, though this could also be due to smaller numbers of men in the study [3]. Sex differences have been found among IBS patients in biobehavioral stress responses and hypersensitivity to visceral stimuli [28–31]. Men with IBS have described their relationships as less supportive and experience more interpersonal difficulties than women [31]. Thus, men may underreport traumatic events and be less likely to confide in others compared to women [32].
The impact of the perceived severity of the EALs, the age of occurrence, sex, and presence or absence of concurrent social support are potential key factors that have not been well studied in IBS. Based on the lack of direct evidence for how contextual variables may moderate the impact of EALs on IBS, the current study aims to better understand the risk and protective factors related to EALs that affect the odds of having IBS or the severity of symptoms. Specifically, this study aims to test the following hypotheses: 1) IBS patients will report a greater number of EALs compared to HCs, 2) in individuals with a history of EALs, greater trauma severity and earlier first age of EAL will increase the odds of having IBS, 3) in individuals with a history of EALs, confiding in others about the EAL will reduce the odds of having IBS, 4) in IBS patients with EALs, the number of EALs, trauma severity, and first age of EAL will be associated with more severe GI and non-GI symptoms, 5) women will report a significantly greater prevalence of EALs, trauma severity, and confiding in others compared to men in both IBS and HC populations.
Methods
Study Participants
Participants with IBS and HCs at least 18 years of age were recruited through flyers and community advertisements for studies conducted at the G. Oppenheimer Family Center for Neurobiology of Stress and Resilience between August 2007 and August 2016. Patients with IBS completed a Bowel Symptom Questionnaire (BSQ) and fulfilled the ROME III diagnostic criteria [33]. IBS diagnosis and HCs were confirmed by a clinician (e.g., gastroenterologist or nurse practitioner) with expertise in IBS after a medical history and physical examination. HCs did not have a history of IBS or other chronic pain conditions, a history of active psychiatric illness, or use of psychotropic agents. Exclusion criteria for all participants included other chronic medical illnesses (e.g., renal or cardiac disease, autoimmune disorders, diabetes), other GI disorders (e.g., inflammatory bowel disease, ulcerative colitis, diverticulitis), and active psychiatric illness as assessed by a structured clinical interview based on DSM-IV criteria. Body mass index (BMI) was determined during the screening visit by measurement of height and weight using a calibrated scale. The study was approved by the University of California, Los Angeles Institutional Review Board. Participants signed written informed consent and were compensated for their participation in the study.
Questionnaires
The Childhood Traumatic Events Scale (CTES) is a 6-item measure of traumatic events (e.g., sexual, violence, injury, family events) prior to the age of 17 that asks age of the traumatic event, trauma severity (1=not at all traumatic, 4=somewhat traumatic, to 7=extremely traumatic), and how much one confided in others at the time (1=not at all to 7=a great deal) [8]. The CTES has been used with various populations, including patients with urologic chronic pelvic pain syndromes, overactive bladder, hypochondriasis, and PTSD [14, 15, 34, 35]. The number of EALs was defined as the total sum of reported EALs. Trauma severity was defined as the sum of the trauma severity ratings of the EALs.
The BSQ includes ROME III symptom-based diagnostic questions about IBS and measures current severity of overall IBS symptoms (0=no symptoms to 20=the most intense symptoms imaginable), usual severity of IBS symptoms (1=no symptoms to 5=very severe: markedly affects my lifestyle), and abdominal pain (0=no pain to 20=the most intense pain imaginable) using numerical scales [33]. Participants with IBS were also asked the age of onset of IBS symptoms [36].
Other Psychometric Measures
The Hospital Anxiety and Depression Scale (HADS) measures current anxiety and depression [37]. Appraisals of stress over the past month were measured using the10-item Perceived Stress Scale (PSS) [38]. The Personal Health Questionnaire (PHQ-12) was modified from PHQ-15 to assess severity of common non-GI symptoms (e.g. headache, back pain, breathing problems, etc.) [39]. The Visceral Sensitivity Index (VSI) measures GI symptom anxiety and has been validated in IBS [40].
Statistical Analysis
Descriptive statistics of demographic and clinical measures were summarized for categorical (n [%]) and continuous (mean±SD) variables. Wilcoxon rank sums and t-tests were utilized to test for differences between IBS and HC participants among continuous/ordinal variables; Fisher’s exact or chi-squared tests were used for categorical variables. For aim 1, logistic regressions were used to predict the odds of having IBS from the number of EALs while adjusting for sex and BMI as potential confounders. For aim 2 and aim 3, logistic regression was used to predict the odds of having IBS from trauma severity, first age of EAL, and confiding in others about the EAL among individuals with a history of EAL. In addition, linear regression was used to determine the association between first age of EAL and trauma severity. For aim 4, linear regression was used to evaluate whether the number of EALs, trauma severity, and first age of EAL are associated with GI and non-GI symptoms among IBS patients with EALs. Lastly, for aim 5, linear regression was utilized to determine sex differences in the prevalence of EALs, trauma severity, and confiding in others within IBS subjects and HCs. BMI was adjusted for due to a significant difference between HCs and IBS, and IBS was predominant in women. A Bonferroni-adjusted significance level of 0.05/3=0.017 was used to account for the multiple comparisons for the three factors related to EAL (i.e., trauma severity, confiding in others, and first age of EAL). All analyses were performed using R version 3.2.0 1 (http://cran.r-project.org/) and all tests were two-tailed.
The CTES has not been used to evaluate EALs in GI disease. Hence, sample size was estimated a priori using effect size estimates provided by Lai et al [14] where childhood trauma severity was compared between HCs and subjects with overactive bladders. Using G*Power 3.1.9.2 and a two-tailed t-test based on an α error rate of 5%, a total of 156 subjects (78 HCs and 78 IBS) were needed to detect a small to moderate effect (Cohen’s d=0.45) with 80% power.
Results
Study participants included 197 IBS patients (72% women, mean age=30.28 years old) and 165 HCs (59% women, mean age=30.77 years old). Compared to HCs, the IBS group was comprised of significantly more women, had higher scores for HAD anxiety and depression symptoms, and greater non-GI symptom severity (Table 1).
Table 1:
Clinical characteristics
| HCs (N=165) | IBS (N=197) | p-value | |
|---|---|---|---|
| Age (SD) | 30.77 (11.057) | 30.284 (9.866) | 0.959 |
| BMI (SD) | 26.482 (5.018) | 24.746 (4.811) | p<0.001 |
| Female: n (%) | 97 (59%) | 141 (72%) | 0.015 |
| Hispanic: n (%) | 37 (23%) | 43 (23%) | 0.999 |
| Race: n (%) | 0.104 | ||
| Asian | 39 (23.64%) | 31 (15.74%) | |
| African American | 21 (12.73%) | 15 (7.61%) | |
| Caucasian | 71 (43.03%) | 103 (52.28%) | |
| Other/Mixed | 23 (13.94%) | 35 (17.77%) | |
| Decline to respond | 11 (6.66%) | 13 (6.60%) | |
| Education: n (%) | 0.547 | ||
| Some high school or less | 1 (0.61%) | 1 (0.51%) | |
| High school graduate | 12 (7.27%) | 9 (4.57%) | |
| Some college | 70 (42.42%) | 75 (38.07%) | |
| College graduate | 40 (24.24%) | 51 (25.89%) | |
| Any post graduate work | 32 (19.39%) | 49 (24.87%) | |
| Decline to respond | 10 (6.06%) | 12 (6.09%) | |
| CTES | |||
| First Age of EAL (years) | 9.377 (4.714) | 8.873 (4.502) | 0.3805 |
| Total Number of EAL (0‒6) | 1.115 (1.118) | 1.614 (1.556) | 0.009 |
| Total Trauma Burden (0‒42) | 6.655 (5.098) | 11.338 (8.34) | p<0.001 |
| Average Trauma Severity (0‒7) | 3.805 (1.695) | 4.509 (1.601) | 0.001 |
| Average Amount of Confiding (0‒7) | 3.170 (2.02) | 2.579 (1.665) | 0.035 |
| HAD Anxiety (0‒21) | 3.835 (3.036) | 7.86 (4.547) | p<0.001 |
| HAD Depression (0‒21) | 1.518 (1.923) | 3.637 (3.271) | p<0.001 |
| PSS Score (0‒40) | 11.723 (6.342) | 17.056 (7.295) | p<0.001 |
| VSI Score (0‒90) | 3.903 (6.274) | 38.353 (17.607) | p<0.001 |
| PHQ-12 Score (0‒24) | 2.248 (2.468) | 11.089 (4.751) | p<0.001 |
| GI Symptoms | |||
| Overall Severity (0‒20) | 9.09 (4.38) | ||
| Abdominal Pain (0‒20) | 8.77 (4.27) | ||
| Usual Severity (1‒5) | 3.28 (0.66) |
Abbreviations: SD, standard deviation shown for continuous variables; HCs, healthy controls; IBS, irritable bowel syndrome; BMI, body mass index; ACE, Adverse Childhood Experiences; CTES, Childhood Traumatic Events Scale (CTES); EAL, Early adverse life events; GI, Gastrointestinal; HAD, Hospital Anxiety and Depression Scale; PHQ-12, Personal Health Questionnaire-12; PSS, Perceived Stress Scale; VSI, Visceral Sensitivity Index.
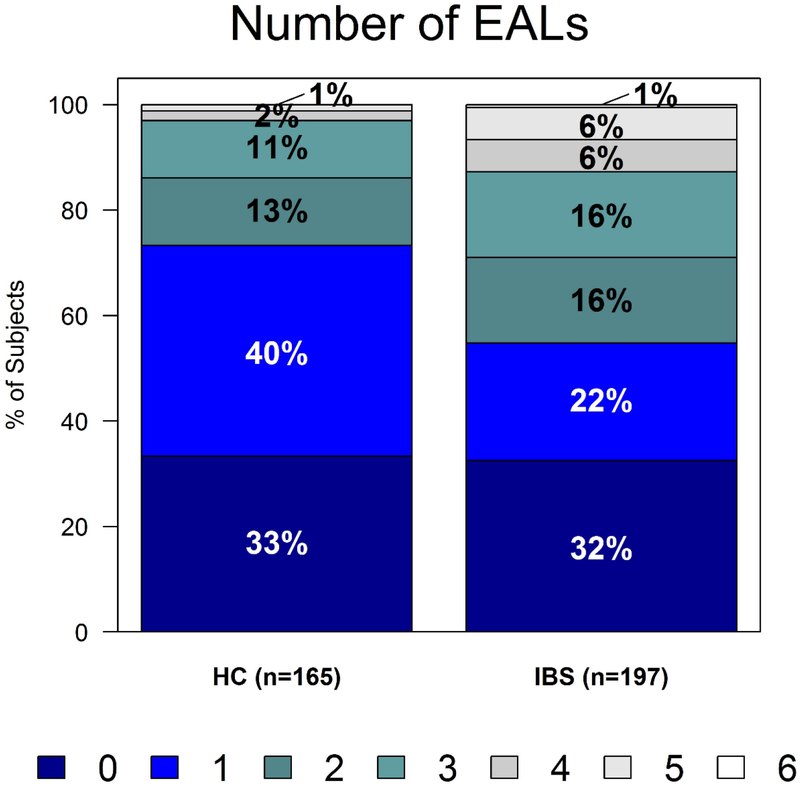
IBS patients reported a greater number of EALs than HCs, even after adjusting for sex and BMI. While about two-thirds of both IBS patients and HCs reported at least one EAL, 45% of IBS patients had two or more traumatic events compared to 27% of HCs with two or more traumatic events (Figure 1, OR=1.36, 95% CI, 1.14–1.62; p<0.001). Experiencing sexual trauma (p=0.002), being the victim of violence (p=0.006), and experiencing any other major upheaval (p<0.001) were associated with increased odds of IBS (Table 2). Notably, the first age of EAL was not predictive of having IBS (p=0.33) and did not correlate with the age of onset of IBS symptoms (p=0.37).
Figure 1.
IBS patients report a greater number of EALs than healthy controls (p<0.001). EAL indicates early adverse life event; HC, healthy controls; IBS, irritable bowel syndrome.
Table 2:
Types of EALs and odds of having IBS
| Types of EALs: | HCs (N=165) N (%) | IBS (N=197) N (%) | Odds Ratio (Confidence Interval) | p-value |
|---|---|---|---|---|
| Death of close family or friend | 49 (29.7%) | 64 (32.49%) | 1.15 (0.72‒1.84) | 0.563 |
| Major upheaval between parents | 62 (37.58%) | 76 (38.58%) | 1.10 (0.69‒1.72) | 0.695 |
| Traumatic sexual experience | 10 (6.06%) | 32 (16.24%) | 3.36 (1.54‒7.34) | 0.002 |
| Victim of violence | 15 (9.09%) | 37 (18.78%) | 2.59 (1.31‒5.12) | 0.006 |
| Extreme illness or injury | 14 (8.48%) | 26 (13.2%) | 1.75 (0.84‒3.62) | 0.134 |
| Any other major upheaval | 34 (20.61%) | 83 (42.13%) | 2.74 (1.67‒4.49) | <0.001 |
Abbreviations: HCs, healthy controls; IBS, irritable bowel syndrome; EAL, early adverse life events.
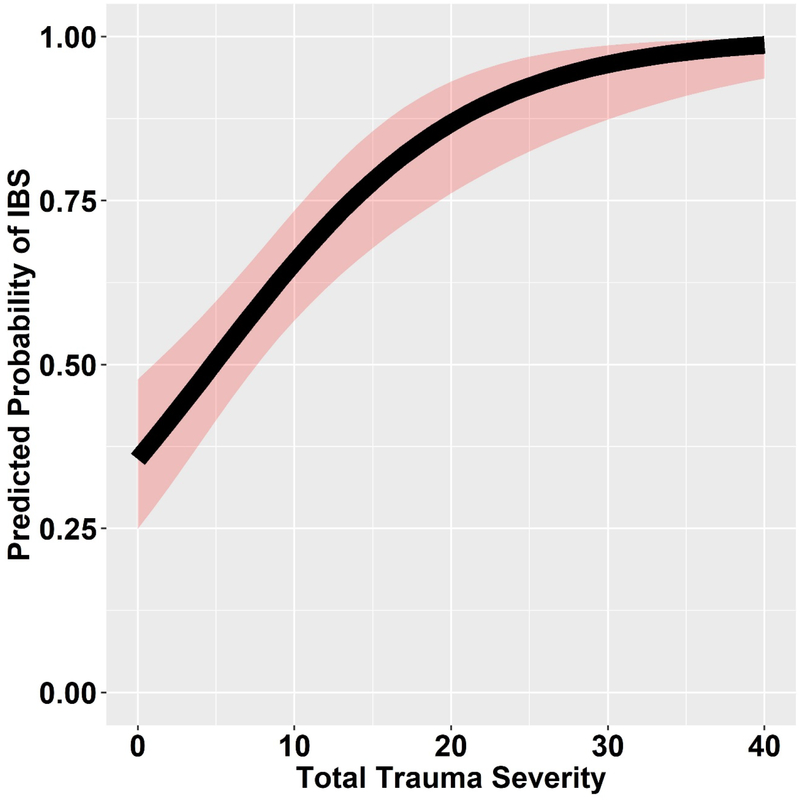
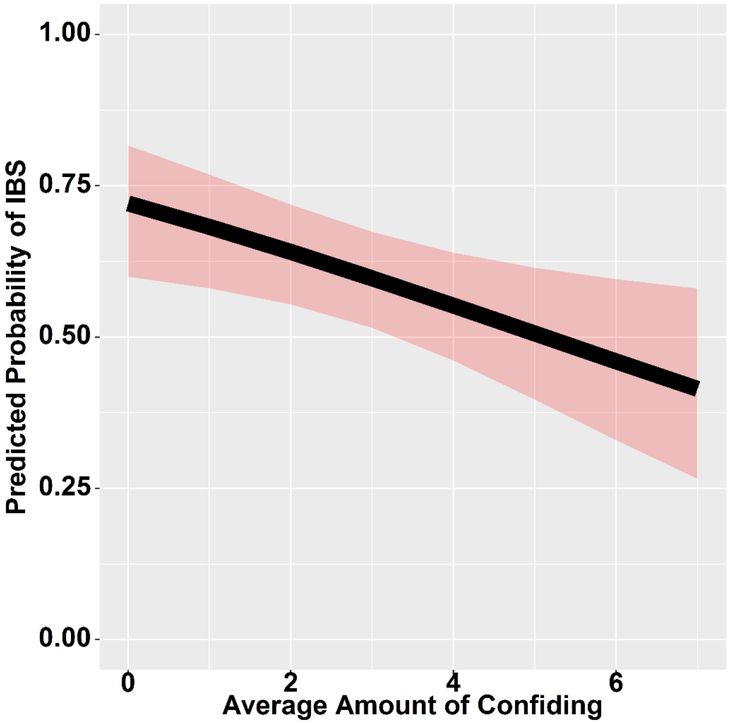
In the 243 subjects who reported a history of EALs, a greater total trauma severity increased the odds of having IBS (Figure 2, OR=1.13, 95% CI, 1.08–1.19; p<0.001). In contrast, confiding in others about the traumatic event at the time decreased the odds of having IBS (Figure 3, OR=0.83, 95% CI, 0.72–0.96; p=0.012). In other words, confiding in others about an EAL reduced the odds of having IBS by 17%.
Figure 2.
Total trauma severity increases the odds of having IBS, with the shaded areas representing the 95% CI for the predicted probability of IBS (OR=1.13, 95% CI, 1.08–1.19; p<0.001). CI indicates confidence interval; IBS, irritable bowel syndrome.
Figure 3.
Confiding in others decreases the odds of having IBS, with the shaded areas representing the 95% CI for the predicted probability of IBS (OR=0.83, 95% CI, 0.72–0.96; p=0.012). CI indicates confidence interval; IBS, irritable bowel syndrome.
There were trends for an association of increasing trauma severity with increasing current overall IBS symptoms (p=0.05) and abdominal pain (p=0.07), but there were no other associations between EAL measures and IBS symptom severity. Trauma severity and confiding in others were not significantly associated with non-GI symptom severity, including current anxiety and depression symptoms, perceived stress, somatic symptom severity, and GI symptom anxiety.
No sex differences were found in the prevalence or type of EALs, trauma severity, and confiding in others within IBS (p-value range: 0.74–0.95) or HCs (p-value range: 0.17–0.78). Even when comparing individual items on the CTES, there were no significant sex differences within IBS (p-value range: 0.15–0.99) or HCs (p-value range: 0.10–0.99).
Discussion
This study found that a greater number of EALs was associated with an increased odds of having IBS, consistent with our previous studies using other EAL questionnaires, including the ACE [7] and ETI-SR [3]. The OR in the present study was consistent with the OR range of previous estimates of EALs and chronic disease outcomes [41]. This study also confirms our previous findings and others’ studies that there is a graded relationship between the number of EALs and IBS status [3, 6, 7]. That is, a higher number of EALs is associated with increased odds of having IBS. A graded relationship between number of EALs and presence of chronic disease has been similarly shown with cardiac and lung disease, obesity, diabetes, and stroke, among others [13, 42]. However, not all individuals who experience an EAL will develop IBS. Our results go beyond prior studies by demonstrating the relevant risk and protective factors that moderate the association of EALs and IBS. Specifically, the perceived severity of the traumatic events and the type of trauma increased the odds of having IBS, while confiding in others about the EAL was a protective factor. In our study, the age of first EAL was neither a risk nor protective factor.
Consistent with our hypothesis, a greater perceived severity of a traumatic event increased the odds of having IBS. This finding may be due to having greater fear associated with the EAL. We have previously found that IBS patients reported higher prevalence of peritraumatic fear (“Did you experience emotions of fear, horror or helplessness?”) associated with EALs compared to HCs [43]. Furthermore, peritraumatic fear was found to be an independent predictor of IBS. IBS patients have been shown to have dysregulated abdominal pain-related fear learning and memory processes mediated by the amygdala, cingulate cortex, prefrontal areas, and hippocampus [44]. In addition, IBS patients have an enhanced startle response to abdominal threat [45]. Peritraumatic fear may increase the salience of an EAL and be associated with a greater emotional and physiological stress response [43]. A history of EALs in IBS has been shown to be associated with an enhanced stress response (i.e., HPA axis dysregulation) [11, 46], functional and structural alterations in core brain regions involved in emotional regulation and salience [47, 48], and decreased resilience, which is the ability to recover and adapt positively to stress [49]. Stress via altered autonomic nervous system activity has also been shown to affect GI motility, sensation, and intestinal permeability in IBS [50, 51].
Contrary to our hypothesis, the age of first EAL was not associated with IBS status or age of onset of IBS symptoms. Previous studies have only assessed if EALs occurring in childhood and/or adulthood increase the risk of having IBS but not the actual age of occurrence. Leserman et al found no difference in pain severity, non-GI medical symptoms, psychological distress, and functional disability between first abuse occurring in childhood versus adulthood among women with functional or organic GI disorders [16]. However, Talley et al has shown that there may be an additive effect of abuse such that those who experienced abuse during both childhood and adulthood were three times more likely to have IBS whereas those with abuse in either childhood or adulthood were only twice as likely to have IBS [4]. Furthermore, patients reporting multiple forms of abuse were found to have greater IBS symptom severity and worse health-related quality of life [13].
Although prior studies have focused on risk factors of IBS, few have studied protective factors. This study demonstrates that if individuals confided in others about an EAL at the time, their odds of having IBS significantly decreased. However, studies have demonstrated that most IBS patients do not disclose a history of EALs to others, including their physicians [5]. A study by Drossman et al found that only about 17% of patients informed their doctors of their abuse history [24], though this study was conducted over 25 years ago. Our findings suggest that early intervention addressing the occurrence of EALs may help to reduce the odds of having IBS possibly by mitigating maladaptive stress responses, although further studies are needed. Health care providers can aid in symptom management in IBS patients who have experienced EALs through the use of cognitive behavioral therapy and parental education (for pediatric patients) [52].
The finding that confiding in others reduced the odds of IBS underscores the importance of cathartic expression and social support in disease processes. Confiding in others regarding traumatic experiences may be indicative of greater social support resources, which can buffer the negative effects of stress and reduce symptom severity, and more effective coping strategies that can extend from childhood to adulthood [5, 53, 54]. The benefits of confiding in others can also be limited by interpersonal barriers, such that it can be detrimental when there is an unsupportive response to the disclosure. For example, a younger age of disclosure correlated with a greater risk for negative reactions [55]. Studies of disclosure have mainly been conducted among victims of sexual abuse, which found that approximately two-thirds of victims of sexual abuse confided in someone about their childhood sexual abuse, but about half delayed disclosure until five or more years after the event [56]. Although research is limited, there is some evidence to suggest that time of disclosure (i.e., during childhood or adulthood) is not related to severity of abuse, but results are inconsistent in regards to whether earlier or later disclosure is linked to better psychological adjustment later in life [20, 55]. Future studies should determine the factors associated with confiding in others about an EAL (e.g., amount, depth, characteristics and response of the confidant, etc.) that are most beneficial and whether confiding in others when the trauma occurred versus later as an adult has a similar or greater effect on health outcomes.
The main limitation of the current as well as previous studies about EAL effects is the exclusive reliance on subjective retrospective reporting of age of IBS symptom onset and trauma occurrences. Due to the limitations of the study design, the results are based on associations and cannot be directly used to infer causality. In addition, retrospective self-report may not capture the role of events in prenatal or infancy, such as noxious stimulation at birth or neonatal maternal separation, that could influence visceral hypersensitivity or psychological hypervigilance to GI symptoms [57, 58]. Furthermore, the CTES does not include items directly addressing emotional abuse, which has been shown to be one of the strongest predictors of IBS [3]. The study participants were recruited predominantly from the community, did not have active psychiatric disease, and the vast majority were not taking psychotropic agents; thus, our findings may not be applicable to other, more severe patient populations. Lastly, no sex differences were found in the EAL variables in this study, although they have been found in prior studies of EALs [26, 59, 60]. The lack of sex differences in our study may have been due to the relatively smaller sample size of each sex.
In summary, the number and traumatic severity of EALs, but not the first age of when an EAL occurred, increase the risk of having IBS. In contrast, confiding in others about the traumatic event at the time decreased the risk of having IBS. These findings can help identify individuals at greater risk of developing IBS and are consistent with previous studies that show a beneficial effect of seeking support from others, emphasizing the importance of early intervention to improve health outcomes in individuals with EALs.
Abbreviations:
- ACE
Adverse Childhood Experiences
- BMI
Body mass index
- BSQ
Bowel Symptom Questionnaire
- CTES
Childhood Traumatic Events Scale
- CI
Confidence interval
- EALs
Early adverse life events
- ETISR-SF
Early Trauma Inventory Self Report-Short Form
- GI
Gastrointestinal
- HCs
Healthy controls
- HADS
Hospital Anxiety and Depression Scale
- HPA axis
Hypothalamic-pituitary-adrenal axis
- IBS
Irritable bowel syndrome
- OR
Odds ratio
- PHQ-12
Personal Health Questionnaire
- PSS
Perceived Stress Scale
- SD
Standard deviation
- VSI
Visceral Sensitivity Index
Footnotes
Disclosures: None
References
- 1.Mayer EA, Naliboff BD, Chang L, et al. V. Stress and irritable bowel syndrome. Am J Physiol Gastrointest Liver Physiol. 2001;280:G519–524. [DOI] [PubMed] [Google Scholar]
- 2.Blanchard EB, Lackner JM, Jaccard J, et al. The role of stress in symptom exacerbation among IBS patients. J Psychosom Res. 2008;64:119–128. [DOI] [PubMed] [Google Scholar]
- 3.Bradford K, Shih W, Videlock EJ, et al. Association between early adverse life events and irritable bowel syndrome. Clin Gastroenterol Hepatol. 2012;10:385–390.e381–383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Talley NJ, Fett SL, Zinsmeister AR, et al. Gastrointestinal tract symptoms and self-reported abuse: a population-based study. Gastroenterology. 1994;107:1040–1049. [DOI] [PubMed] [Google Scholar]
- 5.Drossman DA, Talley NJ, Leserman J, et al. Sexual and physical abuse and gastrointestinal illness. Review and recommendations. Ann Intern Med. 1995;123:782–794. [DOI] [PubMed] [Google Scholar]
- 6.Halland M, Almazar A, Lee R, et al. A case-control study of childhood trauma in the development of irritable bowel syndrome. Neurogastroenterol Motil. 2014;26:990–998. [DOI] [PubMed] [Google Scholar]
- 7.Park SH, Videlock EJ, Shih W, et al. Adverse childhood experiences are associated with irritable bowel syndrome and gastrointestinal symptom severity. Neurogastroenterol Motil. 2016;28:1252–1260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pennebaker JW and Susman JR. Disclosure of traumas and psychosomatic processes. Soc Sci Med. 1988;26:327–332. [DOI] [PubMed] [Google Scholar]
- 9.Drossman DA, Li Z, Leserman J, et al. Health status by gastrointestinal diagnosis and abuse history. Gastroenterology. 1996;110:999–1007. [DOI] [PubMed] [Google Scholar]
- 10.Goldberg RT, Pachas WN and Keith D. Relationship between traumatic events in childhood and chronic pain. Disabil Rehabil. 1999;21:23–30. [DOI] [PubMed] [Google Scholar]
- 11.Videlock EJ, Adeyemo M, Licudine A, et al. Childhood trauma is associated with hypothalamic-pituitary-adrenal axis responsiveness in irritable bowel syndrome. Gastroenterology. 2009;137:1954–1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Heim C and Nemeroff CB. The role of childhood trauma in the neurobiology of mood and anxiety disorders: preclinical and clinical studies. Biol Psychiatry. 2001;49:1023–1039. [DOI] [PubMed] [Google Scholar]
- 13.Kanuri N, Cassell B, Bruce SE, et al. The impact of abuse and mood on bowel symptoms and health-related quality of life in irritable bowel syndrome (IBS). Neurogastroenterol Motil. 2016;28:1508–1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lai HH, Morgan CD, Vetter J, et al. Impact of childhood and recent traumatic events on the clinical presentation of overactive bladder. Neurourol Urodyn. 2016;35:1017–1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Scheller-Gilkey G, Moynes K, Cooper I, et al. Early life stress and PTSD symptoms in patients with comorbid schizophrenia and substance abuse. Schizophr Res. 2004;69:167–174. [DOI] [PubMed] [Google Scholar]
- 16.Leserman J, Drossman DA, Li Z, et al. Sexual and physical abuse history in gastroenterology practice: how types of abuse impact health status. Psychosom Med. 1996;58:4–15. [DOI] [PubMed] [Google Scholar]
- 17.Canavan C The epidemiology of irritable bowel syndrome. 2014;6:71–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.O’Mahony SM, Hyland NP, Dinan TG, et al. Maternal separation as a model of brain-gut axis dysfunction. Psychopharmacology (Berl). 2011;214:71–88. [DOI] [PubMed] [Google Scholar]
- 19.Greenwood-Van Meerveld B, Prusator DK and Johnson AC. Animal models of gastrointestinal and liver diseases. Animal models of visceral pain: pathophysiology, translational relevance, and challenges. Am J Physiol Gastrointest Liver Physiol. 2015;308:G885–903. [DOI] [PubMed] [Google Scholar]
- 20.O’Leary P, Coohey C and Easton SD. The effect of severe child sexual abuse and disclosure on mental health during adulthood. J Child Sex Abus. 2010;19:275–289. [DOI] [PubMed] [Google Scholar]
- 21.Glover DA, Loeb TB, Carmona JV, et al. Childhood sexual abuse severity and disclosure predict posttraumatic stress symptoms and biomarkers in ethnic minority women. J Trauma Dissociation. 2010;11:152–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pennebaker JW, Hughes CF and O’Heeron RC. The psychophysiology of confession: linking inhibitory and psychosomatic processes. J Pers Soc Psychol. 1987;52:781–793. [DOI] [PubMed] [Google Scholar]
- 23.Nickel JC, Tripp DA, Pontari M, et al. Childhood sexual trauma in women with interstitial cystitis/bladder pain syndrome: a case control study. Can Urol Assoc J. 2011;5:410–415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Drossman DA, Leserman J, Nachman G, et al. Sexual and physical abuse in women with functional or organic gastrointestinal disorders. Ann Intern Med. 1990;113:828–833. [DOI] [PubMed] [Google Scholar]
- 25.Centers for Disease Control and Prevention. Adverse childhood experiences reported by adults --- five states, 2009. MMWR Morb Mortal Wkly Rep. 2010;59:1609–1613. [PubMed] [Google Scholar]
- 26.Afifi TO, MacMillan HL, Boyle M, et al. Child abuse and physical health in adulthood. Health Rep. 2016;27:10–18. [PubMed] [Google Scholar]
- 27.Alcala HE, Tomiyama AJ and von Ehrenstein OS. Gender Differences in the Association between Adverse Childhood Experiences and Cancer. Womens Health Issues. 2017;27:625–631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Saito YA, Locke GR, Talley NJ, et al. A comparison of the Rome and Manning criteria for case identification in epidemiological investigations of irritable bowel syndrome. Am J Gastroenterol. 2000;95:2816–2824. [DOI] [PubMed] [Google Scholar]
- 29.Chang L and Heitkemper MM. Gender differences in irritable bowel syndrome. Gastroenterology. 2002;123:1686–1701. [DOI] [PubMed] [Google Scholar]
- 30.Chang L, Mayer EA, Labus JS, et al. Effect of sex on perception of rectosigmoid stimuli in irritable bowel syndrome. Am J Physiol Regul Integr Comp Physiol. 2006;291:R277–284. [DOI] [PubMed] [Google Scholar]
- 31.Thakur ER, Gurtman MB, Keefer L, et al. Gender differences in irritable bowel syndrome: the interpersonal connection. Neurogastroenterol Motil. 2015;27:1478–1486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Alaggia R Disclosing the Trauma of Child Sexual Abuse:A Gender Analysis. Journal of Loss and Trauma. 2005;10:453–470. [Google Scholar]
- 33.Longstreth GF, Thompson WG, Chey WD, et al. Functional bowel disorders. Gastroenterology. 2006;130:1480–1491. [DOI] [PubMed] [Google Scholar]
- 34.Naliboff BD, Stephens AJ, Afari N, et al. Widespread Psychosocial Difficulties in Men and Women with Urologic Chronic Pelvic Pain Syndromes (UCPPS): Case-control findings from the MAPP Research Network. Urology. 2015;85:1319–1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Noyes R Jr., Stuart S, Langbehn DR, et al. Childhood antecedents of hypochondriasis. Psychosomatics. 2002;43:282–289. [DOI] [PubMed] [Google Scholar]
- 36.Munakata J, Naliboff B, Harraf F, et al. Repetitive sigmoid stimulation induces rectal hyperalgesia in patients with irritable bowel syndrome. Gastroenterology. 1997;112:55–63. [DOI] [PubMed] [Google Scholar]
- 37.Zigmond AS and Snaith RP. The hospital anxiety and depression scale. Acta Psychiatr Scand. 1983;67:361–370. [DOI] [PubMed] [Google Scholar]
- 38.Cohen S, Kamarck T and Mermelstein R. A global measure of perceived stress. J Health Soc Behav. 1983;24:385–396. [PubMed] [Google Scholar]
- 39.Spiller RC, Humes DJ, Campbell E, et al. The Patient Health Questionnaire 12 Somatic Symptom scale as a predictor of symptom severity and consulting behaviour in patients with irritable bowel syndrome and symptomatic diverticular disease. Aliment Pharmacol Ther. 2010;32:811–820. [DOI] [PubMed] [Google Scholar]
- 40.Labus JS, Bolus R, Chang L, et al. The Visceral Sensitivity Index: development and validation of a gastrointestinal symptom-specific anxiety scale. Aliment Pharmacol Ther. 2004;20:89–97. [DOI] [PubMed] [Google Scholar]
- 41.Felitti VJ, Anda RF, Nordenberg D, et al. Relationship of childhood abuse and household dysfunction to many of the leading causes of death in adults. The Adverse Childhood Experiences (ACE) Study. Am J Prev Med. 1998;14:245–258. [DOI] [PubMed] [Google Scholar]
- 42.Gilbert LK, Breiding MJ, Merrick MT, et al. Childhood adversity and adult chronic disease: an update from ten states and the District of Columbia, 2010. Am J Prev Med. 2015;48:345–349. [DOI] [PubMed] [Google Scholar]
- 43.Videlock EJ, Liu C, Shih W, et al. The Association of Early Adverse Life Events and Irritable Bowel Syndrome (IBS) Is Amplified by the Presence of Peritraumatic Fear. Gastroenterology. 2015;148:S656–S657. [Google Scholar]
- 44.Icenhour A, Langhorst J, Benson S, et al. Neural circuitry of abdominal pain-related fear learning and reinstatement in irritable bowel syndrome. Neurogastroenterology & Motility. 2015;27:114–127. [DOI] [PubMed] [Google Scholar]
- 45.Naliboff BD, Waters AM, Labus JS, et al. Increased Acoustic Startle Responses in IBS Patients During Abdominal and Non-Abdominal Threat. The Journal of urology. 2009;181:2127–2133.19286199 [Google Scholar]
- 46.Videlock EJ, Shih W, Adeyemo M, et al. The effect of sex and irritable bowel syndrome on HPA axis response and peripheral glucocorticoid receptor expression. Psychoneuroendocrinology. 2016;69:67–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gupta A, Labus J, Kilpatrick LA, et al. Interactions of early adversity with stress-related gene polymorphisms impact regional brain structure in females. Brain Struct Funct. 2016;221:1667–1679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gupta A, Mayer EA, Acosta JR, et al. Early adverse life events are associated with altered brain network architecture in a sex- dependent manner. Neurobiol Stress. 2017;7:16–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Park SH, Naliboff BD, Shih W, et al. Resilience is decreased in irritable bowel syndrome and associated with symptoms and cortisol response. Neurogastroenterol Motil. 2017;30:e13155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Vanuytsel T, van Wanrooy S, Vanheel H, et al. Psychological stress and corticotropin-releasing hormone increase intestinal permeability in humans by a mast cell-dependent mechanism. Gut. 2014;63:1293–1299. [DOI] [PubMed] [Google Scholar]
- 51.Chang L The role of stress on physiologic responses and clinical symptoms in irritable bowel syndrome. Gastroenterology. 2011;140:761–765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chitkara DK, van Tilburg MA, Blois-Martin N, et al. Early life risk factors that contribute to irritable bowel syndrome in adults: a systematic review. Am J Gastroenterol. 2008;103:765–774; quiz 775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lackner JM, Brasel AM, Quigley BM, et al. The ties that bind: perceived social support, stress, and IBS in severely affected patients. Neurogastroenterol Motil. 2010;22:893–900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tener D and Murphy SB. Adult Disclosure of Child Sexual Abuse: A Literature Review. Trauma Violence Abuse. 2015;16:391–400. [DOI] [PubMed] [Google Scholar]
- 55.Roesler TA. Reactions to disclosure of childhood sexual abuse. The effect on adult symptoms. J Nerv Ment Dis. 1994;182:618–624. [DOI] [PubMed] [Google Scholar]
- 56.Ullman SE. Social reactions to child sexual abuse disclosures: a critical review. J Child Sex Abus. 2003;12:89–121. [DOI] [PubMed] [Google Scholar]
- 57.Coutinho SV, Plotsky PM, Sablad M, et al. Neonatal maternal separation alters stress-induced responses to viscerosomatic nociceptive stimuli in rat. Am J Physiol Gastrointest Liver Physiol. 2002;282:G307–316. [DOI] [PubMed] [Google Scholar]
- 58.Anand KJ, Runeson B and Jacobson B. Gastric suction at birth associated with long-term risk for functional intestinal disorders in later life. J Pediatr. 2004;144:449–454. [DOI] [PubMed] [Google Scholar]
- 59.Cunningham TJ, Ford ES, Croft JB, et al. Sex-specific relationships between adverse childhood experiences and chronic obstructive pulmonary disease in five states. Int J Chron Obstruct Pulmon Dis. 2014;9:1033–1042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Haatainen KM, Tanskanen A, Kylmä J, et al. Gender differences in the association of adult hopelessness with adverse childhood experiences. Social Psychiatry and Psychiatric Epidemiology. 2003;38:12–17. [DOI] [PubMed] [Google Scholar]