Abstract
Alzheimer’s disease (AD) differentially and specifically affects brain regions and neuronal cell types in a predictable pattern. Damage to the brain appears to spread and worsens with time, taking over more regions and activating multiple stressors that can converge to promote vulnerability of certain cell types. At the same time, other cell types and brain regions remain intact in the face of this onslaught of neuropathology. Although neuropathologic descriptions of AD have been extensively expanded and mapped over the last several decades, our understanding of the mechanisms underlying how certain regions and cell populations are specifically vulnerable or resistant has lagged behind. In this review, we detail what is known about the selectivity of local initiation of AD pathology in the hippocampus, its proposed spread via synaptic connections, and the diversity of clinical phenotypes and brain atrophy patterns that may arise from different fibrillar strains of pathologic proteins or genetic predispositions. We summarize accumulated and emerging knowledge of the cellular and molecular basis for neuroanatomic selectivity, consider potential disease-relevant differences between vulnerable and resistant neuronal cell types and isolate molecular markers to identify them.
Introduction
Alzheimer’s disease (AD) is the most common neurodegenerative disease and cause of cognitive decline and dementia [7], and is on the rise in our aging society [39, 59]. The pathologic hallmarks of AD are misfolding, aggregation and accumulation of two proteins that are normally ubiquitously expressed in soluble form in the brain, namely amyloid-β (Aβ) in extracellular amyloid plaques and hyperphosphorylated tau as intraneuronal neurofibrillary tangles (NFTs) (Fig. 1). These pathologic changes are accompanied by early synaptic loss [30], a highly perturbed innate immune response from microglia [71, 93, 124, 171], reactive astrocytes [26, 107], reduced cerebral blood flow and neurovascular dysfunction [95], disruption of the blood brain barrier [129], and neuronal loss [188] and brain atrophy [136]; these pathologic changes present clinically as progressive cognitive decline. Pathologic Aβ and NFT assemblies progressively involve brain regions and neuronal cell types following a characteristic pattern [15], forming niches of neurodegeneration as a result of cytotoxic events. Fundamentally important to our understanding of this pattern of injury and response to injury is why it occurs in specific regions and cell types, but not in apparently similar neighboring cell types and regions. This phenomenon may be a result of selective cellular and regional vulnerability to pathologic proteins and their apparent spread through the brain. Broadly speaking, pathologic Aβ appears to accumulate first across neocortical regions, then the limbic system, diencephalon, basal forebrain, and finally cerebellum. In contrast, pathologic tau appears to follow a different sequence that involves first the entorhinal cortex and hippocampus, which are affected early by neurofibrillary degeneration, synaptic and neuronal loss and regional atrophy, followed by the locus coeruleus, basal forebrain, and association regions of the neocortex, which succumb to early to mid-stage neurofibrillary degeneration. Involvement of the primary sensory cortex follows. Interestingly, the cerebellum is resistant to neurofibrillary degeneration, unlike late stage Aβ plaque formation. It is clear that not all brain regions are equal in the face of AD. Similarly, other neurodegenerative diseases with different neuropathologies and clinical phenotypes have their own specific regional and cellular vulnerabilities [56]. The factors that dictate this selectivity have been elusive and are likely variable combinations of factors that alter the balance of stressors, injury, and response to injury within the aging brain, tipping it to fall into a cascade of cytotoxic events in some cell types and regions, but not others. These factors may include the location and arrangement of neuronal cell types, synaptic circuitry to other regions, specific species of pathologic Aβ and tau fibrillar protein strains, genetic contributions, and cellular and molecular characteristics. Furthermore, while there is an expected pattern of neuropathologic accumulation and appearance of clinical symptoms in typical AD, variations in this pattern exist which may be derived from differential selective vulnerability in different individuals. Such phenotypic heterogeneity may also arise from propagation of pathologic proteins through different pathways of circuitry or even through the differential selective targeting of different strains of misfolded proteins. In this review we describe current theories on the underlying cause of selective vulnerability in AD at the phenotypic, regional and cellular levels.
Fig. 1.
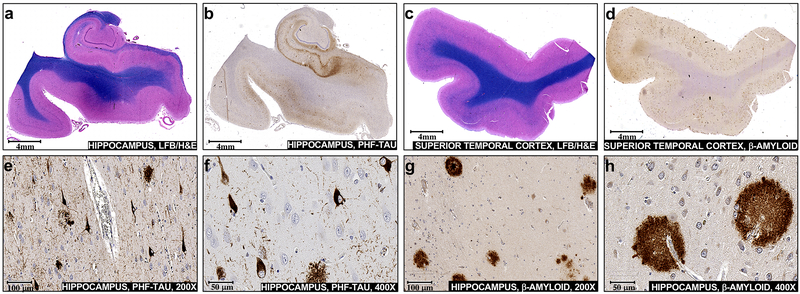
Pathologic NFT inclusions and amyloid-β plaques accumulate in vulnerable brain regions during Alzheimer’s disease. Sections of mid hippocampus were a stained with hematoxylin and eosin plus luxol fast blue (H&E/LFB) or b used for immunohistochemical staining with PHF-tau antibody. Sections of the superior temporal gyrus were c stained with H&E/LFB or d used for immunohistochemical staining with amyloid-β antibody. Neurofibrillary degeneration and scattered neuritic plaques are visualized at × 200 (e) and at × 400 (f) in the hippocampal section incubated with PHF-tau antibody. Diffuse and dense core plaques are visualized at × 200 (g) and at × 400 (h) in the superior temporal gyrus section incubated with amyloid-β Antibody
The neuropathology of Alzheimer’s disease
While the causes of sporadic AD are still not completely understood, the major players that distinguish AD from other neurodegenerative diseases are Aβ plaques, NFTs and the interplay between them. Accompanying the accumulation of pathogenic protein is neuroinflammation and gliosis, mediated in part by the reactivity of astrocytes and microglia. Aβ is formed from the endoproteolytic cleavage of amyloid precursor protein (APP) to two major isoforms: Aβ40 and Aβ42. The amyloid cascade hypothesis of disease onset proposes that alterations in proteolytic processing of APP cause dyshomeostasis of Aβ, increasing the levels of Aβ42, and initiates AD by setting off a chain of events that leads to the accumulation of NFTs and downstream neuronal cell death [163]. Despite over three decades of investigation into the physiochemistry and biological activities of Aβ, its normal physiological role is still uncertain [73, 128]. However, recent findings may be changing our fundamental understanding of this ubiquitously expressed and highly conserved peptide. The soluble form of APP has been shown to bind directly to GABABR1a and modulate synaptic transmission [146]. On the other hand, Aβ has been identified as an antimicrobial peptide [63, 167] and Aβ plaque formation in sporadic AD may be a protective response to limit the spread of neurotropic herpes virus [48] and Porphyromonas gingivalis [70] infections in brain. Evaluation of Aβ deposition is commonly accomplished by Thal phases that use immunohistochemistry to detect a variety of Aβ deposits, including plaques, in neocortical regions (Thal phase 1), the allocortex (Thal phase 2), diencephalon and striatum (Thal phase 3), brainstem nuclei (Thal phase 4) and finally in the cerebellum (Thal phase 5) [175].
While genetic evidence from patients who develop autosomal dominant, familial AD (FAD) points to Aβ as the trigger for AD, it is neurofibrillary degeneration and NFT burden rather than Aβ plaque burden that correlate relatively well with selective damage in early AD and brain atrophy in later stages of disease [89, 90, 113]. Synaptic loss in the limbic system and neocortex is the best correlate of cognitive impairment and amnestic symptoms in AD patients [41, 174]. Synaptic loss is followed by neuronal loss, together the main contributors to cortical atrophy. Although neuronal loss exceeds the number of NFTs within the same region, NFT accumulation matches the pattern of regional and laminar neuronal loss [62], which is not well correlated with Aβ plaque burden. Tau proteins are required for normal functioning of neurons. Under normal physiological conditions, tau proteins (derived from alternative splice variants of the MAPT gene [61]) stabilize microtubules within axons, facilitating axonal transport of organelles, trophic factors, neurotransmitters and other cellular constituents, and help to regulate synaptic function [5, 120]. Hyperphosphorylated tau disengages from microtubules and aggregates into paired-helical filaments (PHF), ultimately resulting in neurofibrillary degeneration in the form of NFTs, and thus losing its microtubule stabilizing functions and contributing to axonal transport deficits [6]. A persistent enigma is that, despite its axonal origin, PHF-tau accumulates primarily in the cell body and in dendrites. Using immunohistochemical staining for PHF-tau, so-called pretangle material can be observed in multiple regions, especially in locus coeruleus neurons [17]. However, formal Braak & Braak staging of neurofibrillary degeneration in AD (henceforth referred to as Braak staging, but distinct from Braak staging of Lewy bodies) begins in the transentorhinal and entorhinal cortex and the CA1 region of the hippocampus in stages I–II [14], correlating to a prolonged preclinical phase which can last up to 20 years. In stages III–IV the limbic regions are further affected and mild cognitive impairment (MCI) is often observed, whereas in stages V–VI there is also extensive neocortical involvement and usually brain atrophy accompanied by further cognitive decline. Differential vulnerability of these regions is represented by the timing and severity of their involvement in AD progression, while their selective vulnerability is highlighted by the fact that other regions of the brain like the cerebellum are resistant to the accumulation of NFTs. Strikingly, neighboring regions like the CA2-3 within the highly vulnerable hippocampus are often relatively spared compared to the CA1 region which is affected early and severely [15].
Primary age-related tauopathy (PART) and argyrophilic grain disease (AGD) show a similar pattern of pathologic tau as AD in hippocampal and peri-hippocampal subfields [38, 149]. Other tauopathies, such as a subset of frontotemporal lobar degenerations and chronic traumatic encephalopathy, show a different brain regional pattern of pathologic tau accumulation [82]. In PART, NFT accumulation is associated with atrophy and cognitive decline in the relative absence of Aβ plaques [89]. In AD, accumulation of Aβ is insufficient, but necessary for diagnosis. In fact, the synergism between Aβ deposition and neurofibrillary degeneration, rather than their additive effects, may be a better predictor of cognitive decline and progression to AD that either pathology alone [133]. The complex interplay between plaques and tangles has been studied in murine models of AD. Reduction of endogenous tau expression mitigates cognitive deficits that emerge in transgenic mice expressing human APP with mutations that increase Aβ production, while not affecting Aβ aggregation or neuritic dystrophy [148]. In another study, Aβ promoted neuronal hyperactivity, while tau suppressed and silenced neuronal activity and dominated over Aβ-dependent hyperactivity [20]. However, the presence of Aβ reduced the beneficial effects of tau reduction on neuronal impairment, again pointing to synergistically detrimental Aβ-tau interactions. A revision by the National Institute on Aging-Alzheimer’s Association (NIA-AA) that takes into account Aβ Thal stage, Braak stage, and the co-occurrence of pathologic tau within Aβ-containing neuritic plaques, is now a widely used practical guide for assessing AD neuropathologic severity [119].
In the search for effective therapeutic avenues for AD, the important question arises: which features or circumstances dictate that some neuronal cells and brain regions succumb to catastrophic fates when stressed by accumulating lesions while other cells and regions seem to have a higher threshold of withstanding stress and toxicity, and manage to retain their normal function? Addressing the cell intrinsic and extrinsic properties of this highly specific regional and cellular vulnerability to AD and subsequent synaptic loss, particularly in the context of the aging brain, will help to explain the molecular mechanisms of AD development and progression, and reveal potential compensatory mechanisms to bolster resilience.
Clinical phenotypic diversity and neuroanatomical correlates
Late onset AD clinical variants
Late onset AD (LOAD) is the most common form of AD, occurring after the age of 65 years. Although AD has stereotypical pathologic and clinical presentations, there is substantial variability among individuals in both facets of the disease. As with other neurodegenerative diseases, there are diverse clinical phenotypes that may derive from selective vulnerability of different brain regions and/or propagation through different pathways or different strains of misfolded proteins (Table 1). Classically, AD manifests as a progressive, multidomain amnestic syndrome. This begins with impairment of anterograde episodic memory and progresses to involve other cognitive domains, such as language, praxis, visuospatial functioning, and executive functioning, eventually resulting in global cognitive decline. However, individual patients have variant syndromes—different constellations of cognitive symptoms such as language, visuospatial, or behavior predominant dysfunction while memory remains relatively preserved for some time—which present major challenges for both diagnosis and monitoring of disease progression [184]. AD is a heterogeneous disease in terms of pathologic changes as well; variations in the pattern of NFT accumulation occur which do not fit readily into the Braak staging scheme. Recently, research consensus guidelines have been proposed for intra vitam biological-based categorization termed ATN, which uses flexible combinations of in vivo biomarkers for Aβ deposition (A), pathologic tau (T), and neurodegeneration (N), which include CSF concentrations of Aβ and pathologic tau, positron emission tomography (PET) tracers for Aβ, tau and neuronal hypometabolism, and structural MRI [84]. Several subtypes of AD have been proposed, based on clinical and biological features. In a retrospective study Murray et al. classified three AD subtypes based on postmortem NFT density: typical (75% of cases), hippocampal-sparing (11% of cases) and limbic-predominant AD (14% of cases) [127]. These neuropathologic subtypes also had different clinical phenotypes, with different ages of onset and rates of progression. Patients with hippocampal-sparing AD progressed more quickly than typical AD, while those with limbic-predominant AD progressed more slowly [127]. More recent studies have denoted four categories of brain atrophy in vivo by MRI: both hippocampal and cortical atrophy, hippocampal only, cortical only, and minimal atrophy groups [23, 51, 134]. Longitudinal progression over 2 years was measured and revealed that the limbic-predominant and no atrophy groups had slower progression than typical AD and hippocampal-sparing AD [51], and is concordant with other studies [80, 131, 164, 176]. Although there are significant variations in pathologic changes, atrophy, and clinical presentation in AD, in both typical and atypical AD there is good correlation among these factors: brain atrophy correlates well withNFT topography, and amnestic and non-amnestic symptoms correspond to regions of neuronal inj ury and correlate to neuronal hypometabolism (measured by PET) [80, 127, 190] (Table 1). Despite not presenting with significant atrophy and also having non-AD CSF biomarker levels, the minimal atrophy group fulfilled the diagnostic criteria for AD, but had a slow rate of disease progression. Thus, the clinical diagnosis of AD based on cognitive impairment was at odds with the common CSF AD biomarker levels and minimal brain atrophy. Interestingly, this group had significantly fewer years of education. Persson et al. hypothesized that these findings are in line with the cognitive reserve hypothesis (CRH) [134] which proposes that education is beneficial for the brain by forming more efficient and pathology-resilient networks [169], without which patients with fewer lesions may succumb more readily to neuronal injury. However, educational attainment may be a surrogate for or act in synergy with other beneficial forces such as brain size or synaptic density, lifelong cognitive or social engagement, premorbid intelligence, or benefits of higher socioeconomic status [60, 169].
Table 1.
Atypical variants of late and early onset Alzheimer’s disease
| AD subtype | Clinical presentation | Pathology | Atrophy | Progression rate | CSF Aβ levels | CSF tau levels |
|---|---|---|---|---|---|---|
| Typical LOAD (50–75% of LOAD) | Amnestic and non-amnestic | NFT counts were balanced in the hippocampus and associative cortex (lateral parietal, temporal and frontal regions) | Both hippocampal and cortical (“both impaired”) | Typical AD | Typical AD | Typical AD |
| Limbic-predominant LOAD (15–35% of LOAD) | Amnestic | NFT counts predominantly in the hippocampus | Medial-temporal lobe (“hippocampal”) | Slower than typical AD | Similar to typical AD | Similar to typical AD |
| Hippocampal-sparing LOAD (10–25% of LOAD) | Non-amnestic | NFT counts predominantly in the associative cortex | Lateral regions of the parietal, temporal and frontal lobes with relative sparing of the medial temporal lobes (“cortical”) | Faster than typical AD | Similar to typical AD | Similar to typical AD |
| Minimal atrophy LOAD (10–17% of LOAD) | Unclear | Not assessed | Minimal | Slowest | Higher than or similar to typical AD | Lower than typical AD |
| Typical EOAD (75% of EOAD) | Amnestic and non-amnestic | Higher burden of NFTs and Aβ plaques compared to LOAD | Both hippocampal and cortical (“both impaired”) | Typical AD | Similar to typical AD | Similar to typical AD |
| Atypical EOAD (25% of EOAD) | Non-amnestic | Higher burden of NFTs and Aβ plaques compared to LOAD | Posterior cortical (“cortical”) | Faster than typical AD | Similar to typical AD | Similar to typical AD |
In contrast to specific NFT accumulation and brain atrophy patterns among AD variants, Aβ accumulation appears to be similarly distributed across groups (with the exception of the minimal atrophy group) [101, 144, 151]. It should be kept in mind that these studies were performed on patients with dementia; Aβ accumulation develops decades prior to the first signs of cognitive decline and is thought to reach a plateau before achieving the clinical threshold for a diagnosis of cognitive impairment [85]. Therefore, it is still possible that Aβ accumulation initially develops differentially in AD variants before reaching a plateau at a relatively early stage.
Early onset AD clinical variants
Early Onset AD (EOAD) is differentiated from the much more common LOAD by a generally accepted (although arbitrary) cutoff where symptoms develop in individuals who are younger than 65 years of age. Epidemiological data on EOAD is sparse and the reported prevalence of EOAD among total AD patients varies between 1 and 10%; however, it is the most common cause of early onset dementia [179]. A meta-analysis based on population-enriched studies reported a rate of 5% EOAD patients among total AD patients [200]. EOAD is more likely to present with atypical clinical phenotypes including apraxia and impaired visuospatial function as compared to LOAD patients [147]. In one study, roughly 25% of EAOD patients presented with a non-amnestic phenotype in whom visual or apraxic and language phenotypes predominated [97]. In another, almost 60–70% of EOAD patients had atypical patterns of brain atrophy [147]. Different studies report different rates of EOAD progression as compared to LOAD, some suggesting that EAOD progresses faster than LOAD with earlier multidomain cognitive impairment; however, this accelerated rate may be related to the presence or absence of the APOE ε4 allele (discussed in detail below) [52, 147]. EAOD is associated with a higher burden of AD neuropathologic changes than LOAD, with a more pronounced distribution outside the medial temporal lobe [115]. MRI studies are conflicting in reporting whether regional atrophy patterns vary between LOAD and EOAD. Some have reported a higher rate of neocortical atrophy in EAOD than in LOAD, and most report greater hippocampal atrophy in LOAD than in EOAD. In contrast, others report that hippocampal atrophy and the general regional pattern of atrophy is similar between LOAD and EOAD [47]. The CSF profiles of EOAD patients are similar to that of LOAD patients, and do not differ between typical and atypical EOAD in two studies [13, 53]. However, in general, CSF concentrations of Aβ and tau levels do not vary significantly between different types of AD, although this may in part reflect a lack of sensitivity in the methods used to quantify CSF proteins. In a small percentage of EOAD cases with monogenetic, familial forms of AD (FAD), the clinical phenotypes, while still predominantly presenting as amnestic, can have specific mutation-associated heterogeneity [155]. These individuals are monitored throughout life, and will surely provide key insight into the mechanism of selective regional and cellular vulnerability in the development of AD.
Initiation and spread of protein misfolding and neurofibrillary degeneration
Initiation of local injury
In order to understand the selective formation and distribution of NFTs, it is important to look at the beginning stages of AD which occur in the transentorhinal and entorhinal cortex and hippocampus (Fig. 2). In fact, the apparent progressive spread of NFTs within specific sub-regions of this highly vulnerable region is representative of the involvement of the rest of the brain throughout the course of the disease. The initial turning point of tau to pathologic forms is its hyperphosphorylation, before the misfolding events and PHF-tau formation into tangles. Cells in this pre-tangle phase represent the earliest detectable stage of development of AD-related damage, still have their normal neuronal processes intact, and thus may have the potential for early therapeutic intervention and reversal of injury [16, 75]. In the most common case of typical AD, the transentorhinal cortex, the entorhinal cortex, and hippocampus CA1 are the first to be affected. Specifically, the first neurons in the brain to develop NFTs are the transentorhinal Pre-α projection neurons. Throughout the progression of disease, accumulation of NFTs is largely laminar in nature, initially appearing in the stellate cells and large pyramidal neurons of layer II in the transentorhinal cortex, while leaving the deeper parts of layer III to IV and layer V mostly unaffected [81]. A few isolated NFTs are also found in the entorhinal cortex proper and the CA1 region of the hippocampus, and these increase as neurofibrillary degeneration advances from Braak stage I–II to stage III–IV, including the subiculum as well [15]. Thus, the initiation of pathologic tau by hyperphosphorylation and NFT formation is localized to these regions rather than occurring arbitrarily throughout the brain. Whether or not these entorhinal-hippocampal layer II neurons are more susceptible to the initial insult as a result of cell-intrinsic properties like genetic, excitability, proteomic, molecular, and metabolic factors is discussed in detail below.
Fig. 2.
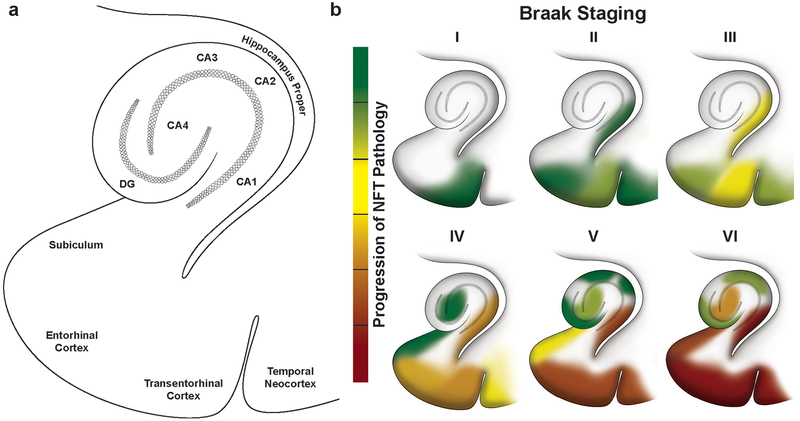
Progressive accumulation of NFTs within the transentorhinal and entorhinal cortex and hippocampal regions along Braak stages of neurofibrillary degeneration in Alzheimer’s disease, a Sub-regions within the transentorhinal and entorhinal cortex and hippocampus that are differentially vulnerable to developing NFTs and succumbing to disease, b The progression of NFT appearance and accumulation during Braak staging of AD from stage I to VI. Green color denotes low abundance of NFTs and red denotes high abundance of NFTs within a sub-region. Note that the CA4 sub-region is also referred to as the “hilus”
Spread of neurofibrillary degeneration within the hippocampus and beyond
The advancement of pathologic tau from the transentorhinal to entorhinal cortex to the CA1 region of the hippocampus does seem to be related to the spatial proximity of these regions. Additionally, the apparent spread of neurofibrillary degeneration somewhat follows the synaptic circuitry within the region since the entorhinal cortex provides input to the hippocampus directly. Specifically, the entorhinal cortex layer II neurons input via the perforant path to the dentate gyrus (DG) and the hippocampal CA3 pyramidal neurons [2]. CA3 pyramidal neurons input to the pyramidal neurons in CA1, thus forming the trisynaptic loop (EC to DG to CA3 to CA1) (Fig. 3). However, as mentioned, hippocampal regions CA2–3 and even the hilus/CA4 are relatively resistant to initial stages of neurofibrillary degeneration. The CA1 region also receives afferent input from a variety of other sources, but the most prominent arises from the ipsilateral CA3 pyramidal cells, which give rise to the Schaffer collaterals [2]. There is also direct projection from the entorhinal cortex layer III to the CA1 stratum lacunosum moleculare dendritic field [192]. Thus, to some extent the apparent spread of pathologic tau does follow the circuitry of the entorhinal-hippocampal system in that the signal initiates in the EC and inputs to the CA1, with the exception that the DG and CA3 regions mediate the signal, but do not initially display neurofibrillary change in these early stages of AD. At later stages of AD, the granule cells of the DG themselves and the hilus/CA4 region also exhibit NFTs. Support for trans-synaptic spread of tau pathology has also been demonstrated in a mouse model of tauopathy [110].
Fig. 3.
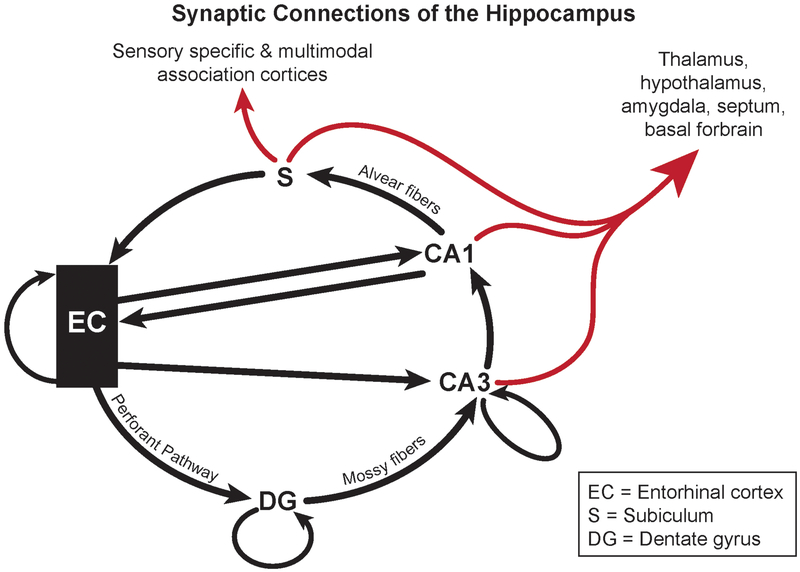
The synaptic connections of the hippocampus. The entorhinal cortex (EC) layer II neurons input to hippocampal CA3 pyramidal neurons via the dentate gyrus (DG) mossy fiber synapses of the granule cells in a laminar fashion. CA3 pyramidal neurons input to the pyramidal neurons in CA1, thus forming the trisynaptic loop (EC to DG to CA3 to CA1). CA1 neurons project to the subiculum via the Alvear fibers. The EC also directly inputs to the CA1 and CA3. The EC, DG and CA3 also project back within themselves. Black arrows indicate entorhinal-hippocampal pathways, red arrows indicate pathways leading to other parts of the brain. Adapted from Amaral and Witter’s scheme [2]
The presence of neurofibrillary degeneration correlates with synaptic loss right from the early stages of AD; however, the distinction between MCI and early AD and therefore the progression from clinically normal to diseased is not clear. MCI is a syndromic diagnostic category with much clinical diversity within it and is enriched in individuals with prodromal AD. Clinical subtypes include amnestic and non-amnestic phenotypes, with the former being at a higher risk of progressing to AD dementia. MCI subtypes as well as not cognitively impaired (NCI) cases have variable Braak stages ranging from a complete absence of NFTs (stage 0) to stage V–VI [126]. Given this heterogeneity, it is not surprising that relatively few studies have found a link between AD-like hippocampal neuropathology and MCI. Using transmission electron microscopy on autopsy tissue, Scheff et al. showed that patients with mild AD had increased synaptic loss in CA1 compared to MCI and not cognitively impaired (NCI) cases [160]. This was accompanied by loss of total CA1 volume, likely due to declining neuronal numbers in this area known to occur in mild to severe AD, but not normal aging or MCI [139]. Another study by Scheff et al. using the same methods showed that aged humans with MCI or mild AD had synaptic loss in the outer molecular layer (OML) of the DG which was greater in the mild AD group and accompanied by a loss of OML DG volume [161]. Synaptic loss in the OML of the DG did not correlate with Braak staging, while there was a significant negative correlation between synapse number and local NFT density, confirming the known association between NFT accumulation and synaptic loss. However, since Braak staging takes into account the whole brain rather than local NFT density, it may not necessarily correlate with local synaptic loss.
Apparent spread of NFTs in stage III–IV within the limbic system occurs from the entorhinal-hippocampal region to the thalamus, hypothalamus and amygdala, which are projected to from the CA1 and subiculum regions of the hippocampus. NFTs also appear in the basal forebrain magnocellular nuclei [15], which also receive input from the hippocampus. In stage V, the neocortex becomes severely affected, predominantly the large pyramidal neurons of layers III and V, while the smaller pyramidal neurons in layers II, VI, and the upper part of layer III are less affected by NFT formation [121]. The hippocampus projects trans-synaptically to the affected areas in the temporal and parietal lobes, further supporting the concept of spread through the connectome—synaptically connected regions—rather than through a concentration gradient along proximity.
Evidence for prion-like spread of pathologic tau
NFT accumulation begins in the axons of neurons where normal tau is present. Pathologic tau then becomes mislocalized from axons to cell bodies and dendrites by a thus far unknown mechanism, where it mediates synaptic dysfunction independently of neuronal degeneration [77]. Trans-synaptic spread of tau aggregates in a prion-like manner has been investigated extensively in experimental systems, where initial aggregated tau seeds the spread of pathologic tau through synaptically connected regions [65, 168, 180]. Clavaguera et al. have shown that injection of brain homogenates from human patients with different tauopathies into a mouse model that expressed full-length human tau resulted in near replication of regional patterns of neuropathologic changes seen in the human source [33]. Similarly, Boluda et al. demonstrated rapid and region-specific spread of pathological tau following injections of AD brain homogenates in transgenic mice [11]. Brain accumulation of pathologic tau can be induced in mice by intracerebral or intraperitoneal injections [11, 33, 34], and pathologic tau propagation is enhanced through increased neuronal activity [193, 195]. One consequence of these and similar experimental demonstrations of pathologic protein transmission in animal models has been the proposal that AD may be transmitted in a prion-like manner [19]. Analogous to the spread of prion protein, intracellular tau may be transferred from neuron-to-neuron via physical connections like tunneling nanotubes [172]. An alternative proposition is that tau could be released into the extracellular matrix within secreted extracellular vesicles like exosomes or microparticles [137, 156, 165]. Indeed, exosomes containing hyperphosphorylated tau are released upon depolarization of neurons in vitro [183]. While the ‘prion hypothesis’ of AD is disputed and tau pathology does not appear to be transmitted between individuals, there are several reports which indicate that amyloid pathology may be transmissible. Observational studies have shown that Aβ seeds can be transmitted to humans through cadaver-sourced dural grafts and cadaver sourced pituitary growth hormone resulting in Aβ plaques and cerebral Aβ angiopathy [72, 98]. Subsequently, Aβ seeds were demonstrated to be transmissible to mice using human cadaver-sourced pituitary growth hormone extracts [140]. Co-transmission of Aβ seeds with iatrogenic Creutzfeldt-Jakob disease has been reported [25, 46, 78, 87] and transmission through neurosurgery has been suggested [88]. If validated, these findings will radically alter the management of AD and the handling of AD biological samples, as well as present as-yet unrecognized therapeutic options.
Aβ and tau fibrillar strains
The intrinsic biochemical and structural heterogeneity of different Aβ and PHF-tau fibrils likely influence the diversity of cellular and regional responses observed in AD. Indeed, variable rates of progression and onset of the AD subtypes (shown in Table 1) are at least partially dictated by the variety of structures that Aβ, tau, and Aβ-tau aggregates can adopt [1, 138, 142].
Tau fibrillar strain spread and regional vulnerabilities
Irrespective of the precise mechanism of transmission from one cell to another, tau fibrillar strains, which have been shown to exist in multiple biochemical forms, have been associated with distinct stereotypical spread of NFT pathology in vitro and in vivo. Cell line studies, for example, have demonstrated tau aggregate uptake, conversion from monomeric to fibrillar form and transfer between cultured cells [76, 132] and different tau strains can propagate in cell line models at unique rates [55, 65] and with distinct but consistent aggregate morphologies [42, 91, 157]. Moreover, distinct tau strains from recombinant, mouse, or human sources, induce distinct neuropathologic changes in vivo, including differential regional vulnerabilities. Kaufman et al., for example, inoculated seven disparate tau fibrillar strains into six locations in the mouse brain (sensory cortex, striatum, visual cortex, hippocampus, thalamus, and inferior colliculus) [91]. While all strains produced hippocampal pathology 5 weeks after injection, the occurrence and extent of pathology in other regions varied reproducibly by strain, with one strain inducing pathology specific to only the mossy fiber tracts of the hippocampus and limited pathology in the striatum and thalamus. Collectively such observations suggest that the specific tau strain itself likely influences the type and extent of the neuropathologic changes and possibly even clinical variations observed in AD. Interestingly, in line with its central role in AD, the hippocampus was highly vulnerable to all tau strains.
Aβ fibrillar strain spread and regional vulnerabilities
Similar to tau strains, the array of structurally diffeent assemblies that Aβ oligomers and fibrils can adopt has long been proposed to result in distinct neurotoxicities and regional vulnerabilities [49, 181]. For example, using electron microscopy and solid-state nuclear magnetic resonance measurements, Petkova et al. demonstrated that the disparate molecular structures of Aβ40 fibrils, achieved simply by varying growth conditions, are differentially toxic to cultures of primary embryonic rat hippocampal neurons [135]. Qiang et al. subsequently demonstrated that distinct clinical subtypes of AD with markedly different disease durations contain distinct fibrillar Aβ structures [143]. Specifically, the cerebral cortices of individuals with a typical prolonged duration form of AD predominantly contain a single Aβ40 fibrillar structure, whereas Aβ40 fibrils prepared by seeded growth from diseased cerebral cortex extracts of patients with a rapidly progressing subtype of AD showed marked structural heterogeneity. Similarly, Cohen et al. examined structural and conformational characteristics of Aβ in brains of AD patients with varying disease duration [35]. A greater frequency and heterogeneity of Aβ42 with distinct structural characteristics was associated with more rapid clinical decline, which further reinforces the likely pathogenetic consequences of these distinct Aβ fibrillar structures. Thus, these variations in higher order molecular structure seen in vivo, which are both dependent on fibril growth conditions and are differentially neurotoxic in culture, appear to correlate with, and possibly drive, variations in disease phenotype.
Genetic predisposition to differential vulnerability
Genetic risk factors
AD is a complex disease that has up to 20-year-long latency in which many components come together to result in the injury that causes cognitive decline. Genetic associations with developing AD are important not only in predicting disease in individuals, but in helping to elucidate mechanistic pathways that result in vulnerability to disease. Evidence for a genetic basis to the etiology of AD was initially observed in the 1930 s [114] in families with multiple generations affected by early onset AD, which is now called autosomal dominant AD or FAD. As opposed to sporadic AD which is usually late onset, FAD is always early onset; however, not all EOAD is autosomal dominant. The identification of families with FAD led to the discovery of three genes involved in autosomal dominant mutations: APP (originally identified in individuals who also had cerebral amyloidosis or hemorrhagic stroke), and PSEN1 and PSEN2 which encode two enzymes involved in the endoproteolytic cleavage of APP to Aβ peptides and other products as a part of the γ-secretase complex [28, 67, 162]. These mutations converge to a general mechanism of increased Aβ42 or shift in the balance between the Aβ40/Aβ42 ratio to a lower number, which promotes its aggregation. Since the APP gene is located on chromosome 21, its expression is increased in individuals with Down syndrome. Up to 75% of individuals with Down syndrome present with early onset dementia with AD neuropathologic changes [69]. After excluding Down syndrome, the heritability of EOAD was calculated to be 92–100% [179]. However, although many dominantly inherited pathogenic mutations of APP, PSEN1 and PSEN2 have been described in FAD patients, they can only explain up to 10% of EOAD and 0.5% of total AD cases [24], implying that other recessive or incompletely penetrant genetic variants come into play in this near-completely heritable form of AD [191].
LOAD, the more common form of AD, has an estimated heritability of between 60% and 80% and is infrequently associated with a mutation in the APP, PSEN1 or PSEN2 genes [191]. LOAD has a strong polygenic component with a set of > 20 risk genes involved. The main genetic risk factor for sporadic LOAD is the APOEε4 allele, explaining approximately 25% of the heritability of AD. The APOE gene has three common alleles: ε2, ε3 and ε4, corresponding to 8.4%, 77.9% and 13.7% in worldwide frequency, respectively, which encode three different apolipoproteins, ApoE2, ApoE3, and ApoE4 [50]. The frequency of the ε4 allele increases dramatically to approximately 40% in AD patients. APOEε4 increases risk for AD in a dose dependent manner compared to the more common ε3 isoform while the APOEε2 isoform decreases the risk of LOAD [37]. ApoE is produced predominantly by astrocytes and microglia and is involved in multiple crucial homeostatic pathways in the brain. APOEε4, however, confers multiple gain of toxic functions and/or a loss of neuroprotective functions, exerting its pathogenic effects through multiple pathways. ApoE is important in maintaining lipid homeostasis in the brain by binding to cholesterol and transporting it to neurons via its receptors, which is essential for axonal growth, synaptic formation and remodeling in memory formation and learning, particularly in the hippocampus [173]. LOAD patients who are APOEε4 carriers exhibit greater medial temporal lobe atrophy particularly in the hippocampal area [108]. Additionally, EAOD patients with atypical clinical phenotypes and low hippocampal atrophy patterns seldom carry the APOEε4 allele [52]. Across AD patients the presence of APOEε4 decreases the age of onset by roughly 10 years in homozygous carriers [9]. Thus, in both EOAD and LOAD, the presence of APOEε4 alleles promotes typical amnestic and hippocampal atrophy phenotypes [43, 52] and an earlier age of onset [9]. Several studies have shown that APOEε4 transgenic mice display lower levels of total ApoE in a genotype-dependent manner (ε4 < ε3 < ε2). ApoE is also important in the clearance of Aβ by binding to and facilitating uptake of soluble Aβ by glia, where ApoE4 is less efficient at performing this function and may even inhibit Aβ clearance. ApoE can be produced by neurons themselves in a protective response to stress or injury, however, in contrast to ApoE3, ApoE4 does not protect synapses from age-related, excitotoxic or Aβ-induced degeneration in transgenic mice [79]. Interestingly, the source of ApoE4 matters: astrocyte-derived ApoE4 is relatively neuroprotective against excitotoxic injury while neuronal ApoE4 expression promotes excitotoxic cell death [18, 22]. In aging and AD, excitatory neurons are more vulnerable to degeneration and NFT pathology than inhibitory neurons [57, 158] and the excitotoxic effects of neuronal ApoE4 in response to stress and injury in these contexts may contribute to this selectivity.
Other risk factors for LOAD include low-penetrant common risk factors and rare alleles with intermediate to high penetrance. Genome-wide association studies (GWAS) have identified at least 42 common genes associated with LOAD (Reviewed here [178]), of which >20 have been confirmed in GWAS meta-analyses and are considered established risk genes for LOAD, including APOE, CLU, PICALM, CR1, B1N1, MS4A, CD2AP, CD33, EPHA1, SORL1, HLA-locus, and ABCA7 [99]. Where much of this was discovered through genotyping known common variants, whole genome and exome sequencing allowed for the identification of rare risk factors like a variant in TREM2 encoding for a loss of function mutation that increases risk for AD [64]. With the identification of increasing numbers of pathogenic risk loci for AD, network analyses could be performed analyzing the co-expression of other genes with known high-risk hub genes. Cross-referencing these hits to gene ontology pulled out disease relevant biological pathways and in this way, common biological pathways emerged pointing to the immune response, lipid metabolism, endosome-lysosome, ubiquitin-proteasome and cell adhesion molecule pathways as being involved in the manifestation of specific cell type and regional vulnerability in high risk individuals, pathways which ultimately collapse in the development of AD [56, 178].
Somatic mutations and epigenetics
Intriguingly, somatic mutations may also contribute to the extreme vulnerability of neurons in aging (compared to many other cells in the body) and their predisposition for degeneration in AD. Somatic mutations occur during both developmental mitosis and accumulate during aging resulting in genomic mosaicism where neurons from a single individual show different alterations in genomic DNA, distinct from the germline, and very likely contribute to the highly diverse phenotypes of neuronal cells within the brain [150, 177]. These DNA alterations can occur in adult neurons, for example through neurogenesis which continues throughout aging [10], or through neuronal stress. Particularly relevant for AD, adult neurogenesis occurs in two defined regions of the brain, one of which is the dentate gyrus of the hippocampus [117], thus making it particularly vulnerable for accumulating somatic mutations. Endogenous and exogenous stressors in post-mitotic, aging neurons can cause genomic alterations like DNA damage, telomere shortening and chromosomal abnormalities [177]. It has been reported that APP shows mosaic copy number variations which are increased in the human AD brain [21]; however, independent validation of this study is still pending. Recently, APP was shown to undergo gene recombination in adult neurons through a process involving APP transcription that is influenced by neural activity, DNA strand breaks and reverse transcription [100]. Single neurons express a greater number of genes than other cell types, which may contribute to transcriptional noise and proneness to transcriptional errors and mutant proteins. This transcriptome complexity is even higher in pyramidal neurons of the hippocampus than, for example, cortical pyramidal neurons or serotonergic neurons [45], further supporting a cell-intrinsic component to their early and dramatic vulnerability to AD.
Distinct from genomic mosaicism in aging neurons is the accumulation of acquired epigenetic alterations over neuronal lifespan which several studies suggest may be involved in AD etiology [141]. The most well-studied epigenetic marks in neurons are DNA methylation at cytosines (5-mC) in CpG sequences [153], which generally leads to suppression of gene expression, and histone modifications like acetylation of the ninth lysine of histone 3 (H3K9ac), which marks transcriptionally active open chromatin [96]. Mounting evidence indicates that an intricate balance of interactions between different brain cell types—neurons, astrocytes, microglia, oligodendrocytes and vascular cells—is essential for healthy brain aging and becomes disrupted in AD. Microglia and astrocytes are also long-lived cells which have the potential to accumulate various changes in response to cell-intrinsic and -extrinsic stressors. Microglial epigenetic modifications have been shown to increase with exposure to lipopolysaccharide (LPS), resultingin an innate immune memory-like phenotype, and impact on AD progression in transgenic mouse models [187]. It will be interesting to discover how epigenetic modifications increase with age in human microglia, whether certain memory-like epigenetic phenotypes are enriched in AD-prone regions like the hippocampus, and how this impacts on the interactions between cell types in the brain during AD progression. Even less is currently known about astrocyte epigenetic changes, but will likely be important, particularly in the context of their role in synaptic maintenance [130].
Cellular and molecular basis for neuroanatomical selectivity
The stressor-threshold hypothesis
As described, the hippocampal regions are affected at different stages of AD progression, reflecting in part their synaptic connectedness (Fig. 3). A multivariate stressor-threshold theory has been proposed to underlie the mechanisms that lead to selective and differential vulnerability to AD: chronic perturbations act on several critical cellular homeostatic pathways in vulnerable cells, multiple stressors can converge to promote vulnerability and reinforce a cycle of dysfunction, aging contributes to disease progression by lowering the threshold at which cells are able to deal with accumulating stressors, and systemic involvement from the periphery like increased inflammation may initiate and/or aggravate disease (reviewed here [158]). Cells can experience stress in different forms including high biosynthetic or secretory demands, oxidative stress and mitochondrial damage, high calcium fluxes and excitotoxicity, protein concentrations at near-saturation levels leading to protein misfolding and aggregation, inflammatory reactions, energy deprivation, and aging [158, 170]. Blood–brain barrier (BBB) breakdown occurs very early on in the CA1 hippocampal region (but not, for example, in the CA3 region), and CA1 projection neurons are particularly vulnerable to decreased glucose and oxygen delivery [118]. A large surface area and complex morphology as seen in large pyramidal neurons with extensive dendritic arborization likely contributes significantly to increased cellular stress on multiple levels [170]. Homeostatic pathways are able to compensate for cellular stress under normal conditions. However, these pathways are highly inter-connected, largely centering at the stress sensors associated to the endoplasmic reticulum membrane, which likely becomes overwhelmed with mounting stress from multiple sources in AD [103].
Excitotoxicity and calcium homeostasis
Generally speaking, glutamatergic excitatory neurons are highly vulnerable in AD as they are susceptible to disrupted calcium homeostasis and excitotoxicity. Particularly, the expression of specific NMD A glutamate receptors has been linked to cellular vulnerability in AD [198]. Where normal, transient activation of synaptic NMDA receptors mediates synaptic plasticity and neuroprotection and is required for neuronal survival, prolonged activation of NMDA receptors leads to increased intracellular Ca2+ levels as NMDA receptors are the primary receptors permeable to Ca2+. This overactivation, as well as secondary activation of voltage-gated Ca2+ channels, can activate phospholipases, proteases and endonucleases as a part of apoptotic cellular pathways and may result in cell toxicity and apoptosis [121]. Furthermore, Aβ can activate extra-synaptic NMD ARs, which causes synaptic loss [182].
Neurons that express high levels of calcium buffering proteins, such as parvalbumin, calbindin and calretinin are usually less vulnerable in AD. Parvalbumin-expressing neurons of the neocortex layers II-IV and V-VI are relatively resistant to AD [121]. However, while calcium buffering proteins alone do seem to offer some fortification against collapse in AD, they do not guarantee safety. In contrast to neocortical regions, axons of parvalbumin expressing neurons are affected in the hippocampus and EC in AD. Significant loss of these neurons and severe reduction in their dendritic arborization are observed in CA1. However, the morphologic changes that are observed in EC parvalbumin expressing neurons are probably secondary to the severe degeneration of the projection neurons on which they synapse within the layers of the EC itself (Fig. 3) [166]. Parvalbumin expressing neurons clearly can be damaged in AD, however not because of NFT accumulation within themselves, but rather as a result of substantial loss of their target projection neurons.
Inhibitory neurons are relatively resistant in AD and express higher levels of calcium-binding proteins. Intemeurons are usually inhibitory neurons that respond to the GABA neurotransmitter and relay signals between other neurons. More than 20 classes of interneurons have been described in CA1 and CA3, defined by their dendritic and axonal arborizations and the post-synaptic domain of the pyramidal cell that they target, as well as by their transcriptomic patterns as determined by single-cell RNA sequencing [196]. They are also characterized according to their calcium-binding protein expression patterns and their neuropeptide expression patterns including cholecystokinin, somatostatin, vasoactive intestinal polypeptide and neuropeptide Y. However, only three types of interneurons have been characterized so far in the CA2 region: basket cells, bistratified cells and Stratum Pyramidale–Stratum Radiatum intemeurons [12]. In rat and mouse, the CA2 interneuron population has been shown to be different from its neighboring CA1 and CA3 regions, with more parvalbumin- and calbindin-expressing interneurons and a higher density of reelin-expressing interneurons [12, 44]. This suggests a different inhibitory circuitry in CA2 which may represent a decrease in CA2 pyramidal cell firing and an indirectly protective role for this region against excitotoxicity related to AD. Additionally, CA2 is relatively resistant to synaptic plasticity compared to other CA regions and many of the pathways involved in this resistance overlap with a resistance to damage under disease conditions [44]. On the other hand, the disruption of the circuitry in CA2 is linked with the pathology of specific psychiatric disorders or non-AD types of neurodegeneration [31].
Protein saturation and misfolding
Proteostasis is the maintenance of normal cellular control of the synthesis, folding, trafficking and degradation of proteins, and is particularly important for long-lived cells in tissues like the brain which have a very limited capacity for self-renewal. Proteins whose cellular concentration is high relative to their solubilities are supersaturated and prone to aggregation even in healthy brains. Such proteins are considered part of a metastable subproteome [32, 54]. When stressors accumulate with age, tight maintenance of solubility can become compromised, promoting misfolding and aggregation. Both Aβ plaque and NFT components are expressed in healthy brain regions that are vulnerable to AD. However, their presence alone does not explain why early EC and hippocampal regions develop pathology first. Freer et al. analyzed microarray data from healthy brains for genes whose proteins have been found to co-aggregate with plaques and NFTs in AD and showed that the pattern of expression of groups of factors which promote the aggregation of metastable proteins in the healthy brain recapitulates the spread of pathology during AD. Highly vulnerable regions like the limbic system show high expression of aggregation-promoting factors and low expression of aggregation-protecting factors [54]. Important factors included molecular chaperones, proteins that assist in the proper folding of other proteins, and post-translational modifiers. BAG3, an autophagy facilitator and tau homeostasis regulator [54], was a hub gene in the protection against tau aggregation. BAG3 and associated aggregation protectors have been linked to a tau homeostasis signature which is more highly expressed in inhibitory neurons than excitatory neurons, mirroring their differential vulnerability [57]. Perhaps unsurprisingly, neurons expressed higher levels of tau and Aβ compared to microglia, astrocytes and endothelial cells. Furthermore, microglia, astrocytes and endothelial cells all expressed higher levels of aggregation protectors and lower levels of aggregation promoters than neurons, highlighting the specific neuronal propensity for dysfunctional proteostasis [54].
Differences in regional physiological protein expression
Neurofilament protein is a major component of the neuronal cytoskeleton and consist of high, medium and low molecular mass forms. The medium and low forms can have phosphorylated or non-phosphorylated states that are distributed axonally or in the soma, respectively. Neurofilament was initially thought to be an essential component of NFTs [3]; however, as Morrison et al. have suggested, it is rather that neurons which contain high levels of neurofilament protein are NFT-prone [121]. Large pyramidal neurons that are vulnerable in AD, primarily present in layers III and V of the hippocampus and cortex, express high amounts of neurofilament and are prone to developing NFTs in AD and to undergoing cell death, seen as a loss of cell numbers during AD progression. Similarly, in layers II and V of the EC and the pyramidal neurons of the subiculum, neurofilament-rich neurons undergo NFT accumulation and loss in AD. Interestingly, and potentially linking the calcium homeostasis theory to neurofilament, calcium-binding protein-containing intemeurons rarely exhibit neurofilament immunoreactivity [121].
Alternative splicing of tau at exon 10 is important for tau pathology as its inclusion gives rise to four-microtubule binding repeat tau rather than three-microtubule binding repeat tau (4R and 3R tau) [109]. While in the normal healthy brain, 3R and 4R tau are expressed equally, an imbalance between them has been shown to occur in various tau pathologies, including AD. In fact, there is a gradient of the 3R:4R tau ratio across the entorhinal-hippocampal region which decreases from EC-CA1-CA4 and mimics, to some degree, the spatial organization of regional vulnerability in AD. The EC is almost completely devoid of 4R+/3R− NFT inclusions while the CA4 region predominantly has 4R+/3R− and 4R+/3R+ NFTs [66, 83]. The authors suggest that there is a dynamic process taking place where there is a shift in 4R+/3R+ neurons to 4R−/3R+ neurons that corresponds to intracellular progression of NFTs from dendrites to soma, where 4R tau, initially present in the dendrites, retracts first to the soma, leaving 3R in the dendrites. This could also explain why “old” NFTs of the EC contain much more 4R−/3R+ NFTs while the relatively “younger” NFTs of CA2 and CA4 have more 4R+/3R− NFTs. The authors speculate that this regional shift in NFT tau isoform composition may provide a basis for hippocampal regional gradation of neurofibrillary degeneration. A recent study made use of integrated mouse and human genomic data to identify specific functional gene modules associated with selective neuronal vulnerability. This study demonstrated that PTB1, a splice regulator of tau at exon 10, was the most highly connected protein to tau in the EC network, further underlining the potential importance of tau isoform balance for neuronal vulnerability to neurofibrillary degeneration in AD [154].
Regional resistance to disease
In contrast to region-specific vulnerabilities is the apparent resistance of certain brain regions to AD pathology. While it has long been noted that AD neuropathologic changes follow a distinct sequence across the brain in which the regions are hierarchically involved, certain regions, in particular the visual, motor and sensory cortices, repeatedly display comparatively infrequent accumulation of NFTs. Defining common molecular profiles which correlate with resistance to AD neuropathologic changes within less NFT-affected regions (e.g. sensory cortex and motor cortex) as compared to more NFT-affected (e.g. hippocampus, entorhinal cortex and cingulate gyrus) offers the potential to identify mechanisms of intrinsic resistance and to indicate potential therapeutic avenues [29, 105, 106, 111, 112, 145]. It has even been suggested that certain regions can exhibit adaptive, protective responses specific to patients with AD. For instance Xu et al. have documented proteomic changes in the cerebella of AD patients that showed an unexpectedly distinct pattern not observed in the cerebella of age-matched non-AD controls [194]. These changes included alterations in neuronal survival pathways alongside protection against oxidative and inflammatory damage, and suggest the possibility that cells within the cerebellum mount a protective resilience response to the stressors of AD.
Vulnerable and resistant neuronal cell types
Regional organization, or cytoarchitecture, is important in determining vulnerability. The hippocampus performs complex functions and is a cellularly diverse region of the brain comprising unique sub-regional organization and complexity. Considering this diversity, cell intrinsic predisposition to lower stressor thresholds likely plays an important role in determining which cells types, brain regions, and sub-regions of the hippocampus succumb first to AD. Highly vulnerable neurons include large pyramidal neurons of the EC layer II, subiculum and CA1, and cholinergic neurons of the basal forebrain which are affected in mid-stages of AD (Summarized in Table 2) [40, 81, 116, 121, 122, 170, 189]. Large pyramidal neurons in CA2 and CA3, granule cells of the DG and mossy fiber cells of the hilus/CA4 are less vulnerable and affected late in AD progression [81, 122]. Neocortical large pyramidal neurons in layers III and V as well as noradrenergic neurons in the locus coeruleus are also affected at late stages, while pyramidal neurons of the primary visual cortex only succumb to neurofibrillary degeneration in the final stages of AD [74, 92, 102, 122]. Relatively resistant cell types in the neocortex include small pyramidal neurons in layers II, VI and the upper part of layer III, smooth and spiny stellate neurons (often called granule cells) of layer IV and inhibitory neurons [122, 152]. However, there is mounting evidence that inhibitory neurons, while they do not accumulate extensive NFTs, do still undergo significant pathologic changes which likely contribute to circuit dysfunction in AD (Reviewed here [104]). Finally, Purkinje cells of the cerebellum appear to be completely resistant to neurofibrillary degeneration in AD, although, again, there is still a loss of cell numbers, perhaps related to the presence of Aβ plaques which do accumulate in the cerebellum [15, 86, 185]. It would be highly interesting to investigate the common characteristics between resistant cell types and their major differences as compared to highly vulnerable cells, by new highly multiplexed single-cell technologies as has been done by simple immunohistochemistry in the past [121]. For example, cerebellar Purkinje cells, DG granule cells, CA2 large pyramidal neurons and hilus/CA4 mossy fiber neurons all express the PCP4 protein and are all largely resistant to neurofibrillary degeneration in AD (Table 2). PCP4 (Purkinje cell protein 4, also called Pep-19) functions as a modulator of calcium binding by calmodulin, and plays a role in synaptic plasticity, which may be beneficial in preserving cells and circuits within a potentially harmful niche [186].
Table 2.
Neuronal subtypes and their relative vulnerabilities to neurofibrillary degeneration during Alzheimer’s disease
| Brain region | Neuronal subtype | AD stage | Vulnerability to NFTs | Immunoreactivity |
|---|---|---|---|---|
| Throughout | Excitatory neurons | Throughout | Vulnerable | Glutamate receptors, especially NMDAR1; VGLUT1 |
| Entorhinal cortex layer II | Large pyramidal neurons | Early | Vulnerable | Reelin, SMI32, Rasgrp2, Sh3bgrl2 |
| Hippocampus: subiculum | Large pyramidal neurons | Early | Vulnerable | Reelin, SMI32, Cartpt |
| Hippocampus: CA1 | Large pyramidal neurons | Early | Vulnerable | Reelin, SMI32, Somatostatin receptor 4, NOV (CCN3) |
| Basal forebrain | Cholinergic neurons | Mid | Vulnerable | ChAT |
| Hippocampus: Dentate hilus | Mossy cells | Mid-late | Inconsistent | CGRP, PCP4 |
| Hippocampus: CA3 | Large pyramidal neurons | Late | Inconsistent | Reelin, SMI32, Gprin3, PKC-d |
| Hippocampus: CA2 | Large pyramidal neurons | Late | Inconsistent | Reelin, SMI32, Cacng5, PCP4, IGFBP5, NT3 |
| Hippocampus: Dentate gyrus layers III and V to VI | Granule neurons | Late | Relatively resistant | Prox1, calbindin, PCP4, IGFBP5, NT3 |
| Locus coeruleus | Noradrenergic neurons | Late | Vulnerable | NET |
| Neocortex: layer III and V | Large pyramidal neurons | Late | Vulnerable | Reelin, SMI32 |
| Primary visual cortex: layers V and VI | Pyramidal neurons | Late | Relatively resistant | Calca |
| Throughout | Inhibitory neurons | N/A | Relatively resistant | Parvalbumin, somatostatin, calbindin, calretinin; GAD; GABA receptors |
| Neocortex: layer II, upper III, VI | Small pyramidal neurons | N/A | Moderately resistant | Unknown |
| Neocortex: layer IV | Smooth stellate neurons | N/A | Relatively resistant | Unknown |
| Neocortex: layer IV | Spiny stellate neurons | N/A | Relatively resistant | Unknown |
| Cerebellum | Purkinje cells | N/A | Resistant | PCP4 |
The disease stages in which specific neuronal subtypes are affected by NFT accumulation and most distinguishing molecular markers that have been used to describe them in humans and mouse models are summarized. The level of vulnerability is colored from red (high) to green (low) and blue (resistant).
Molecular markers of vulnerability and resistance
Neuron and synapse loss in AD are the main contributors to cortical atrophy, and while the regional and laminar pattern of neuronal loss matches that of NFTs, neuronal loss within the same region exceeds the number of NFTs. Some predictive markers that distinguish vulnerable neurons from resistant neurons have been found by histological techniques, microarray analysis, bulk transcriptome sequencing of brain regions and more recently by single-cell or single-nuclear RNA sequencing (Table 2). However, these markers tend not to be for very specific cellular characteristics, and thus overlap between cell types and regions. Borders between hippocampal regions have been described by RNA sequencing with specific expression patterns that can be detected by in situ hybridization [199] and demarcate vulnerable and relatively resistant regions and specific neuronal cell types. Much work still needs to be done to map out these cells, regions and their response to toxic stimuli in AD. For example, molecular markers that distinguish between the cortical layers have been proposed [197], but markers that distinguish the specific neuronal cell types of the different cortical layers, morphologically different cell types within the EC, or between the EC and transentorhinal cortex, have not yet been found. NFT-bearing neurons in EC layer II have been shown to express increased levels of apolipoprotein-J and tissue inhibitor of metalloproteinase-3 relative to neurons that do not have NFTs, as determined by laser capture microdissection of EC regions and microarray analysis of bulk cells [106]. The question of whether these molecular changes map onto the different morphological cell types that reside in EC layer II can now be addressed with the rise of novel highly multiplexed single-cell analysis methods like CyTOF [8, 123] and single-cell RNAseq, in combination with multiplexed imaging technologies like MIBI [4, 94]. High-dimensional molecular signatures of vulnerability in single cells and assignment of genetic risk factors long known to us from GWAS studies to specific cell types by such techniques, combined with machine learning and neural networks, will very likely provide greater clarity in what defines regional and cellular vulnerability to AD.
Conclusions
The amyloid cascade hypothesis and the wealth of neuropathology that has been assembled to describe AD has led to important insights into this disease, as well as other proteinopathies; however, cellular and regional vulnerability are increasingly recognized as important factors in determining which cell types and regions succumb to the stressors within neurotoxic niches in neurodegenerative disease. These specific and characterizing susceptibilities and their complementary resiliencies, along with the pathologic lesions, determine the clinical phenotype and, likely, offer the most promising and as-yet largely under-explored therapeutic and preventative options. With the emergence of truly single-cell multiplexed analyses of complex tissues [36, 58, 68, 125], we possess for the first time the possibility to disentangle the characteristics of the convoluted populations of cells that make up the different brain regions, and what determines the differential susceptibility and resistance of cells therein.
Acknowledgements
This work was supported by grants from the Swiss National Science Foundation: P2ZHP3_181563 (D. M.); from the NIH: P50 AG005136, P50 AG047366, UF1 AG053983, P50 NS062684, UF1 AG057707, and RF1 AG053959 (T. M.), DP2 EB024246 (S. B.), R01 AG056287 and R01 AG057915 (S. B. and T. M); and by the Buster and Nancy Alvord Endowment (T. M.). We thank Dr. Brenna Cholerton for discussions, Norman L. Cyr for help with figure design, and Roomana Patel and Paochen Zhang for administrative support.
References
- 1.Aleksis R, Oleskovs F, Jaudzems K, Pahnke J, Biverstal H (2017) Structural studies of amyloid-β peptides: unlocking the mechanism of aggregation and the associated toxicity. Biochimie 140:176–192. [DOI] [PubMed] [Google Scholar]
- 2.Amaral DG, Witter MP (1989) The three-dimensional organization of the hippocampal formation: a review of anatomical data. Neuroscience 31:571–591. [DOI] [PubMed] [Google Scholar]
- 3.Anderton BH, Brion JP, Flament-Durand J, Haugh MC, Kahn J, Miller CC et al. (1987) Neurofibrillary tangles and the neuronal cytoskeleton. J Neural Transm Suppl 24:191–196. [PubMed] [Google Scholar]
- 4.Angelo M, Bendall SC, Finck R, Hale MB, Hitzman C, Borowsky AD et al. (2014) Multiplexed ion beam imaging of human breast tumors. Nat Med 20:436–442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Avila J, Lucas JJ, Perez M, Hernandez F (2004) Role of tau protein in both physiological and pathological conditions. Physiol Rev 84:361–384. [DOI] [PubMed] [Google Scholar]
- 6.Ballatore C, Lee VMY, Trojanowski JQ (2007) Tau-mediated neurodegeneration in Alzheimer’s disease and related disorders. Nat Rev Neurosci 8:663–672. [DOI] [PubMed] [Google Scholar]
- 7.Barker WW, Luis CA, Kashuba A, Luis M, Harwood DG, Loewenstein D et al. (2016) Relative frequencies of Alzheimer disease, Lewy body, vascular and frontotemporal dementia, and hippocampal sclerosis in the State of Florida Brain Bank. Alzheimer Dis Assoc Disord 16:203–212. [DOI] [PubMed] [Google Scholar]
- 8.Bendall SC, Simonds EF, Qiu P, Amir ED, Krutzik PO, Finck R et al. (2011) Single-cell mass cytometry of differential immune and drug responses across a human hematopoietic continuum. Science 332:687–696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Blacker D, Haines JL, Rodes L, Terwedow H, Go RC, Harrell LE et al. (1997) ApoE-4 and age at onset of Alzheimer’s disease: the NIMH genetics initiative. Neurology 48:139–147. [DOI] [PubMed] [Google Scholar]
- 10.Boldrini M, Fulmore CA, Tartt AN, Simeon LR, Pavlova I, Poposka V et al. (2018) Human hippocampal neurogenesis persists throughout aging. Cell Stem Cell 22:589–599.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Boluda S, Iba M, Zhang B, Raible KM, Lee VMY, Trojanowski JQ (2015) Differential induction and spread of tau pathology in young PS19 tau transgenic mice following intracerebral injections of pathological tau from Alzheimer’s disease or corticobasal degeneration brains. Acta Neuropathol 129:221–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Botcher NA, Falck JE, Thomson AM, Mercer A (2014) Distribution of interneurons in the CA2 region of the rat hippocampus. Front Neuroanat 8:1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bouwman FH, Schoonenboom NSM, Verwey NA, van Elk EJ, Kok A, Blankenstein MA et al. (2009) CSF biomarker levels in early and late onset Alzheimer’s disease. Neurobiol Aging 30:1895–1901. [DOI] [PubMed] [Google Scholar]
- 14.Braak H, Alafuzoff I, Arzberger T, Kretzschmar H, Tredici K (2006) Staging of Alzheimer disease-associated neurofibrillary pathology using paraffin sections and immunocytochemistry. Acta Neuropathol 112:389–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Braak H, Braak E (1991) Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol 82:239–259. [DOI] [PubMed] [Google Scholar]
- 16.Braak H, Braak E (1998) Evolution of neuronal changes in the course of Alzheimer’s disease In: Jellinger K, Fazekas F, Windisch M (eds) Ageing and dementia. Springer, Vienna, pp 127–140. [DOI] [PubMed] [Google Scholar]
- 17.Braak H, Thal DR, Ghebremedhin E, Del Tredici K (2011) Stages of the pathologic process in Alzheimer disease: age categories from 1 to 100 years. J Neuropathol Exp Neurol 70:960–969. [DOI] [PubMed] [Google Scholar]
- 18.Brecht WJ (2004) Neuron-specific apolipoprotein E4 proteolysis is associated with increased tau phosphorylation in brains of transgenic mice. J Neurosci 24:2527–2534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Brundin P, Melki R, Kopito R (2010) Prion-like transmission of protein aggregates in neurodegenerative diseases. Nat Rev Mol Cell Biol 11:301–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Busche MA, Wegmann S, Dujardin S, Commins C, Schiantarelli J, Klickstein N et al. (2019) Tau impairs neural circuits, dominating amyloid-β effects, in Alzheimer models in vivo. Nat Neurosci 22:57–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bushman DM, Kaeser GE, Siddoway B, Westra JW, Rivera RR, Rehen SK et al. (2015) Genomic mosaicism with increased amyloid precursor protein (APP) gene copy number in single neurons from sporadic Alzheimer’s disease brains. Elife 2015:1–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Buttini M, Masliah E, Yu GQ, Palop JJ, Chang S, Bernardo A et al. (2010) Cellular source of apolipoprotein E4 determines neuronal susceptibility to excitotoxic injury in transgenic mice. Am J Pathol 177:563–569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Byun MS, Kim SE, Park J, Yi D, Choe YM, Sohn BK et al. (2015) Heterogeneity of regional brain atrophy patterns associated with distinct progression rates in Alzheimer’s disease. PLoS One 10:e0142756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cacace R, Sleegers K, Van Broeckhoven C (2016) Molecular genetics of early-onset Alzheimer’s disease revisited. Alzheimer’s Dement 12:733–748. [DOI] [PubMed] [Google Scholar]
- 25.Cali I, Cohen ML, Haïk S, Parchi P, Giaccone G, Collins SJ et al. (2018) Iatrogenic Creutzfeldt-Jakob disease with Amyloid-β pathology: an international study. Acta Neuropathol Commun 6:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Carter SF, Herholz K, Rosa-Neto P, Pellerin L, Nordberg A, Zimmer ER (2019) Astrocyte biomarkers in Alzheimer’s disease. Trends Mol Med 25:77–95. [DOI] [PubMed] [Google Scholar]
- 27.Cembrowski MS, Wang L, Sugino K, Shields BC, Spruston N (2016) Hipposeq: a comprehensive RNA-seq database of gene expression in hippocampal principal neurons. Elife 5:1–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chartier-Harlin M-C, Crawford F, Houlden H, Warren A, Hughes D, Fidani L et al. (1991) Early-onset Alzheimer’s disease caused by mutations at codon 717 of the β-amyloid precursor protein gene. Nature 353:844–846. [DOI] [PubMed] [Google Scholar]
- 29.Chen F, Guan Q, Nie Z-Y, Jin L-J (2013) Gene expression profile and functional analysis of Alzheimer’s disease. Am J Alzheimers Dis Other Demen 28:693–701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chen M-K, Mecca AP, Naganawa M, Finnema SJ, Toyonaga T, Lin S et al. (2018) Assessing synaptic density in Alzheimer disease with synaptic vesicle glycoprotein 2A positron emission tomographic imaging. JAMA Neurol 75:1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chevaleyre V, Piskorowski RA (2016) Hippocampal area CA2: an overlooked but promising therapeutic target. Trends Mol Med 22:645–655. [DOI] [PubMed] [Google Scholar]
- 32.Ciryam P, Tartaglia GG, Morimoto RI, Dobson CM, Vendruscolo M (2013) Widespread aggregation and neurodegenerative diseases are associated with supersaturated proteins. Cell Rep 5:781–790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Clavaguera F, Akatsu H, Fraser G, Crowther RA, Frank S, Hench J et al. (2013) Brain homogenates from human tauopathies induce tau inclusions in mouse brain. Proc Natl Acad Sci USA 110:9535–9540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Clavaguera F, Hench J, Lavenir I, Schweighauser G, Frank S, Goedert M et al. (2014) Peripheral administration of tau aggregates triggers intracerebral tauopathy in transgenic mice. Acta Neuropathol 127:299–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cohen ML, Kim C, Hal diman T, ElHag M, Mehndiratta P, Pichet T et al. (2015) Rapidly progressive Alzheimer’s disease features distinct structures of amyloid-β. Brain 138:1009–1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Corces MR, Trevino AE, Hamilton EG, Greenside PG, Sinnott-Armstrong NA, Vesuna S et al. (2017) An improved ATAC-seq protocol reduces background and enables interrogation of frozen tissues. Nat Methods 14:959–962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Corder EH, Saunders AM, Strittmatter WJ, Schmechel DE, Gaskell PC, Small GW et al. (1993) Gene dose of apolipoprotein E type 4 allele and the risk of Alzheimer’s disease in late onset families. Science 261:921–923. [DOI] [PubMed] [Google Scholar]
- 38.Crary JF, Trojanowski JQ, Schneider JA, Abisambra JF, Abner EL, Alafuzoff I et al. (2014) Primary age-related tauopathy (PART): a common pathology associated with human aging. Acta Neuropathol 128:755–766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cummings JL (2004) Alzheimer’s Disease. N Engl J Med 351:56–67. [DOI] [PubMed] [Google Scholar]
- 40.Davies P (1976) Selective loss of central cholinergic neurons in Alzheimer’s Disease. Lancet 308:1403. [DOI] [PubMed] [Google Scholar]
- 41.DeKosky ST, Scheff SW (1990) Synapse loss in frontal cortex biopsies in Alzheimer’s disease: correlation with cognitive severity. Ann Neurol 27:457–464. [DOI] [PubMed] [Google Scholar]
- 42.Diamond MI, Kaufman SK, Sanders DW, Thomas TL, Ruchinskas AJ, Vaquer-Alicea J et al. (2016) Tau prion strains dictate patterns of cell pathology, progression rate, and regional vulnerability in vivo. Neuron 92(4):796–812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dickerson BC, Wolk DA (2011) Dysexecutive versus amnesic phenotypes of very mild Alzheimer’s disease are associated with distinct clinical, genetic and cortical thinning characteristics. J Neurol Neurosurg Psychiatry 82:45–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dudek SM, Alexander GM, Farris S (2016) Rediscovering area CA2: unique properties and functions. Nat Rev Neurosci 17:89–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dueck H, Khaladkar M, Kim TK, Spaethling JM, Francis C, Suresh S et al. (2015) Deep sequencing reveals cell-type-specific patterns of single-cell transcriptome variation. Genome Biol 16:1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Duyckaerts C, Sazdovitch V, Ando K, Seilhean D, Privat N, Yilmaz Z et al. (2018) Neuropathology of iatrogenic Creutzfeldt-Jakob disease and immunoassay of French cadaver-sourced growth hormone batches suggest possible transmission of tauopathy and long incubation periods for the transmission of Abeta pathology. Acta Neuropathol 135:201–212. [DOI] [PubMed] [Google Scholar]
- 47.Eckerström C, Klasson N, Olsson E, Seines P, Rolstad S, Wallin A (2018) Similar pattern of atrophy in early- and late-onset Alzheimer’s disease. Alzheimer’s Dement Diagnosis. Assess Dis Monit 10:253–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Eimer WA, Vijaya Kumar DK, Naval pur Shanmugam NK, Rodriguez AS, Mitchell T, Washicosky KJ et al. (2018) Alzheimer’s disease-associated β-amyloid is rapidly seeded by herpesviridae to protect against brain infection. Neuron 99:56–63.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Eisenberg D, Jucker M (2012) The amyloid state of proteins in human diseases. Cell 148:1188–1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Farrer LA, Cupples LA, Haines JL, Hyman B, Kukull WA, Mayeux R et al. (1997) Effects of age, sex, and ethnicity on the association between apolipoprotein E genotype and Alzheimer disease. A meta-analysis. APOE and Alzheimer Disease Meta Analysis Consortium. JAMA 278:1349–1356. [PubMed] [Google Scholar]
- 51.Ferreira D, Verhagen C, Hernández-Cabrera JA, Cavallin L, Guo C-J, Ekman U et al. (2017) Distinct subtypes of Alzheimer’s disease based on patterns of brain atrophy: longitudinal trajectories and clinical applications. Sci Rep 7:46263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.van der Flier WM, Pijnenburg YAL, Fox NC, Scheltens P (2011) Early-onset versus late-onset Alzheimer’s disease: the case of the missing APOE ε4 allele. Lancet Neurol 10:280–288. [DOI] [PubMed] [Google Scholar]
- 53.van der Flier WM, Schoonenboom SNM, Pijnenburg YAL, Fox NC, Scheltens P (2006) The effect of APOE genotype on clinical phenotype in Alzheimer disease. Neurology 67:526–527. [DOI] [PubMed] [Google Scholar]
- 54.Freer R, Sormanni P, Vecchi G, Ciryam P, Dobson CM, Vendruscolo M (2016) A protein homeostasis signature in healthy brains recapitulates tissue vulnerability to Alzheimer’s disease. Sci Adv 2:el600947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Frost B, Jacks RL, Diamond MI (2009) Propagation of Tau misfolding from the outside to the inside of a cell. J Biol Chem. 284:12845–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Fu H, Hardy J, Duff KE (2018) Selective vulnerability in neurodegenerative diseases. Nat Neurosci 21:1350–1358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Fu H, Possenti A, Freer R, Nakano Y, Villegas NCH, Tang M et al. (2019) A tau homeostasis signature is linked with the cellular and regional vulnerability of excitatory neurons to tau pathology. Nat Neurosci 22:47–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Gajera CR, Fernandez R, Postupna N, Montine KS, Fox EJ, Tebaykin D et al. (2019) Mass synaptometry: high-dimensional multi parametric assay for single synapses. J Neurosci Methods 312:73–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Gaugler J, James B, Johnson T, Scholz K, Weuve J (2018) 2018 Alzheimer’s disease facts and figures. Alzheimer’s Dement 14:367–429. [Google Scholar]
- 60.Giovacchini G, Giovannini E, Borsò E, Lazzeri P, Riondato M, Leoncini R et al. (2019) The brain cognitive reserve hypothesis: a review with emphasis on the contribution of nuclear medicine neuroimaging techniques. J Cell Physiol 234:14865–14872. [DOI] [PubMed] [Google Scholar]
- 61.Goedert M, Spillantini MG, Jakes R, Rutherford D, Crowther RA (1989) Multiple isoforms of human microtubule-associated protein tau: sequences and localization in neurofibrillary tangles of Alzheimer’s disease. Neuron 3:519–526. [DOI] [PubMed] [Google Scholar]
- 62.Gómez-Isla T, Hollister R, West H, Mui S, Growdon JH, Petersen RC et al. (1997) Neuronal loss correlates with but exceeds neurofibrillary tangles in Alzheimer’s disease. Ann Neurol 41:17–24. [DOI] [PubMed] [Google Scholar]
- 63.Gosztyla ML, Brothers HM, Robinson SR (2018) Alzheimer’s amyloid-β is an antimicrobial peptide: a review of the evidence. J Alzheimer’s Dis 62:1495–1506. [DOI] [PubMed] [Google Scholar]
- 64.Guerreiro R, Wojtas A, Bras J, Carrasquillo M, Rogaeva E, Majounie E et al. (2013) TREM2 variants in Alzheimer’s disease. N Engl J Med 368:117–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Guo JL, Lee VMY (2011) Seeding of normal tau by pathological tau conformers drives pathogenesis of Alzheimer-like tangles. J Biol Chem 286:15317–15331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hara M, Hirokawa K, Kamei S, Uchihara T (2013) Isoform transition from four-repeat to three-repeat tau underlies dendrosomatic and regional progression of neurofibrillary pathology. Acta Neuropathol 125:565–579. [DOI] [PubMed] [Google Scholar]
- 67.Hardy J (1997) Amyloid, the presenilins and Alzheimer’s disease. Trends Neurosci 20:154–159. [DOI] [PubMed] [Google Scholar]
- 68.Hartmann FJ, Bernard-Valnet R, Quériault C, Mrdjen D, Weber LM, Galli E et al. (2016) High-dimensional single-cell analysis reveals the immune signature of narcolepsy. J Exp Med. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Head E, Powell D, Gold BT, Schmitt FA (2012) Alzheimer’s disease in down syndrome. Eur J Neurodegener Dis 1:353–364. [PMC free article] [PubMed] [Google Scholar]
- 70.Hellvard A, Ryder MI, Hasturk H, Walker GD, Benedyk M, Lee A et al. (2019) Porphyromonas gingivalis in Alzheimer’s disease brains: evidence for disease causation and treatment with small-molecule inhibitors. Sci Adv 5:eaau3333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Heneka MT, Kummer MP, Stutz A, Delekate A, Schwartz S, Vieira-Saecker A et al. (2013) NLRP3 is activated in Alzheimer’s disease and contributes to pathology in APP/PS1 mice. Nature 493:674–678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hervé D, Porché M, Cabrejo L, Guidoux C, Tournier-Lasserve E, Nicolas G et al. (2018) Fatal Aβ cerebral amyloid angiopathy 4 decades after a dural graft at the age of 2 years. Acta Neuropathol. 135:801–803. [DOI] [PubMed] [Google Scholar]
- 73.Hiltunen M, van Groen T, Jolkkonen J (2009) Functional roles of amyloid-beta protein precursor and amyloid-beta peptides: evidence from experimental studies. J Alzheimers Dis 18:401–412. [DOI] [PubMed] [Google Scholar]
- 74.Hof P, Morrison J (1990) Quantitative analysis of a vulnerable subset of pyramidal neurons in Alzheimer’s disease. J Comp Neurol 301:55–64. [DOI] [PubMed] [Google Scholar]
- 75.Hof PR, Bussière T, Gold G, Kövari E, Giannakopoulos P, Bouras C et al. (2003) Stereologic evidence for persistence of viable neurons in layer II of the entorhinal cortex and the CA1 field in Alzheimer disease. J Neuropathol Exp Neurol 62:55–67. [DOI] [PubMed] [Google Scholar]
- 76.Holmes BB, Furman JL, Mahan TE, Yamasaki TR, Mirbaha H, Eades WC et al. (2014) Proteopathic tau seeding predicts tauopathy in vivo. Proc Natl Acad Sci 111 :E4376–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Hoover BR, Reed MN, Su J, Penrod RD, Kotilinek LA, Grant MK et al. (2010) Tau mislocalization to dendritic spines mediates synaptic dysfunction independently of neurodegeneration. Neuron 68:1067–1081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Hu M, Robertson NP (2018) Transmissible amyloid protein: evidence from iatrogenic CJD. J Neurol 265:1726–1729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Huang Y (2010) Aβ-independent roles of apolipoprotein E4 in the pathogenesis of Alzheimer’s disease. Trends Mol Med 16:287–294. [DOI] [PubMed] [Google Scholar]
- 80.Hwang J, Kim CM, Jeon S, Lee JM, Hong YJ, Roh JH, Lee JH et al. (2016) Prediction of Alzheimer’s disease pathophysiology based on cortical thickness patterns. Alzheimer’s Dement Diagnosis. Assess Dis Monit 2:58–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Hyman BT, Van Hoesen GW, Damasio AR, Barnes CL (1984) Alzheimer’s disease: cell-specific pathology isolates the hippocampal formation. Science 225:1168–1170. [DOI] [PubMed] [Google Scholar]
- 82.Hyman BT, Phelps CH, Beach TG, Bigio EH, Cairns NJ, Carrillo MC et al. (2012) National Institute on Aging–Alzheimer’s Association guidelines for the neuropathologic assessment of Alzheimer’s disease. Alzheimer’s Dement 8:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Iseki E, Yamamoto R, Murayama N, Minegishi M, Togo T, Katsuse O et al. (2006) Immunohistochemical investigation of neurofibrillary tangles and their tau isoforms in brains of limbic neurofibrillary tangle dementia. Neurosci Lett 405:29–33. [DOI] [PubMed] [Google Scholar]
- 84.Jack CR, Bennett DA, Blennow K, Carrillo MC, Dunn B, Haeberlein SB et al. (2018) NIA-AA Research Framework: toward a biological definition of Alzheimer’s disease. Alzheimer’s Dement 14:535–562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Jack CR, Lowe VJ, Weigand SD, Wiste HJ, Senjem ML, Knopman DS et al. (2009) Serial PIB and MRI in normal, mild cognitive impairment and Alzheimer’s disease: implications for sequence of pathological events in Alzheimer’s disease. Brain 132:1355–1365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Jacobs HIL, Hopkins DA, Mayrhofer HC, Bruner E, Van Leeuwen FW, Raaijmakers W et al. (2018) The cerebellum in Alzheimer’s disease: evaluating its role in cognitive decline. Brain 141:37–47. [DOI] [PubMed] [Google Scholar]
- 87.Jaunmuktane Z, Mead S, Ellis M, Wadsworth JDF, Nicoll AJ, Kenny J et al. (2015) Evidence for human transmission of amyloid-β pathology and cerebral amyloid angiopathy. Nature 525:247–250. [DOI] [PubMed] [Google Scholar]
- 88.Jaunmuktane Z, Quaegebeur A, Taipa R, Viana-Baptista M, Barbosa R, Koriath C et al. (2018) Evidence of amyloid-β cerebral amyloid angiopathy transmission through neurosurgery. Acta Neuropathol 135:671–679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Josephs KA, Murray ME, Tosakulwong N, Whitwell JL, Knopman DS, Machulda MM et al. (2017) Tau aggregation influences cognition and hippocampal atrophy in the absence of beta-amyloid: a clinico-imaging-pathological study of primary age-related tauopathy (PART). Acta Neuropathol 133:705–715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Josephs KA, Whitwell JL, Ahmed Z, Shiung MM, Weigand SD, Knopman DS et al. (2008) β-amyloid burden is not associated with rates of brain atrophy. Ann Neurol 63:204–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Kaufman SK, Sanders DW, Thomas TL, Ruchinskas AJ, Vaquer-Alicea J, Sharma AM et al. (2016) Tau prion strains dictate patterns of cell pathology, progression rate, and regional vulnerability in vivo. Neuron 92:796–812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Kelly SC, He B, Perez SE, Ginsberg SD, Mufson EJ, Counts SE (2017) Locus coeruleus cellular and molecular pathology during the progression of Alzheimer’s disease. Acta Neuropathol Commun 5:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Keren-Shaul H, Spinrad A, Weiner A, Matcovitch-Natan O, Dvir-Szternfeld R, Ulland TK et al. (2017) A unique microglia type associated with restricting development of Alzheimer’s disease. Cell 169:1276–1290.el7. [DOI] [PubMed] [Google Scholar]
- 94.Keren L, Bosse M, Marquez D, Angoshtari R, Jain S, Varma S et al. (2018) A structured tumor-immune microenvironment in triple negative breast cancer revealed by multiplexed ion beam imaging. Cell 174:1373–1387.el9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Kisler K, Nelson AR, Montagne A, Zlokovic BV (2017) Cerebral blood flow regulation and neurovascular dysfunction in Alzheimer disease. Nat Rev Neurosci 18:419–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Klein H-U, McCabe C, Gjoneska E, Sullivan SE, Kaskow BJ, Tang A et al. (2019) Epigenome-wide study uncovers large-scale changes in histone acetylation driven by tau pathology in aging and Alzheimer’s human brains. Nat Neurosci 22:37–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Koedam ELGE, Lauffer V, Van Der Vlies AE, Van Der Flier WM, Scheltens P, Pijnenburg YAL (2010) Early-versus late-onset Alzheimer’s disease: more than age alone. J Alzheimer’s Dis 19:1401–1408. [DOI] [PubMed] [Google Scholar]
- 98.Kovacs GG, Lutz MI, Ricken G, Ströbel T, Höftberger R, Preusser M et al. (2016) Dura mater is a potential source of Aβ seeds. Acta Neuropathol 131:911–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Lambert JC, Ibrahim-Verbaas CA, Harold D, Naj AC, Sims R, Bellenguez C et al. (2013) Meta-analysis of 74,046 individuals identifies 11 new susceptibility loci for Alzheimer’s disease. Nat Genet 45:1452–1458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Lee M-H, Siddoway B, Kaeser GE, Segota I, Rivera R, Romanow WJ et al. (2018) Somatic APP gene recombination in Alzheimer’s disease and normal neurons. Nature 563:639–645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Lehmann M, Ghosh PM, Madison C, Laforce R, Corbetta-Rastelli C, Weiner MW et al. (2013) Diverging patterns of amyloid deposition and hypometabolism in clinical variants of probable Alzheimer’s disease. Brain 136:844–858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Leuba G, Saini K (1995) Pathology of subcortical visual centres in relation to cortical degeneration in Alzheimer’s disease. Neuropathol Appl Neurobiol 21:410–422. [DOI] [PubMed] [Google Scholar]
- 103.Li J-Q, Yu J-T, Jiang T, Tan L (2015) Endoplasmic reticulum dysfunction in Alzheimer’s disease. Mol Neurobiol 51:383–395. [DOI] [PubMed] [Google Scholar]
- 104.Li Y, Sun H, Chen Z, Xu H, Bu G, Zheng H (2016) Implications of GABAergic neurotransmission in Alzheimer’s disease. Front Aging Neurosci 8:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Liang D, Han G, Feng X, Sun J, Duan Y, Lei H (2012) Concerted perturbation observed in a hub network in Alzheimer’s disease. PLoS One 7:e40498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Liang WS, Dunckley T, Beach TG, Grover A, Mastroeni D, Ramsey K et al. (2008) Altered neuronal gene expression in brain regions differentially affected by Alzheimer’s disease: a reference data set. Physiol Genom 33:240–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Liddelow SA, Guttenplan KA, Clarke LE, Bennett FC, Bohlen CJ, Schirmer L et al. (2017) Neurotoxic reactive astrocytes are induced by activated microglia. Nature 541:481–487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Liu C-C, Kanekiyo T, Xu H, Bu G (2013) Apolipoprotein E and Alzheimer disease: risk, mechanisms and therapy. Nat Rev Neurol 9:106–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Liu F, Gong CX (2008) Tau exon 10 alternative splicing and tauopathies. Mol Neurodegener 3:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Liu L, Drouet V, Wu JW, Witter MP, Small SA, Clelland C et al. (2012) Trans-synaptic spread of tau pathology in vivo. PLoS ONE 7:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Liu ZP, Wang Y, Zhang XS, Chen L (2010) Identifying dysfunctional crosstalk of pathways in various regions of Alzheimer’s disease brains. BMC Syst Biol 4 Suppl 2:S11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Loring JF, Wen X, Lee JM, Seilhamer J, Somogyi R (2001) A gene expression profile of Alzheimer’s disease. DNA Cell Biol 20:683. [DOI] [PubMed] [Google Scholar]
- 113.Lowe VJ, Wiste HJ, Senjem ML, Weigand SD, Themeau TM, Boeve BF et al. (2018) Widespread brain tau and its association with ageing, Braak stage and Alzheimer’s dementia. Brain 141:271–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Lowenberg K, Waggoner RW (1934) Familial organic psychosis (Alzheimer’s type). Arch Neurol Psychiatry 31:737. [Google Scholar]
- 115.Marshall GA, Fairbanks LA, Tekin S, Vinters HV, Cummings JL (2007) Early-onset Alzheimer’s disease is associated with greater pathologic burden. J Geriatr Psychiatry Neurol 20:29–33. [DOI] [PubMed] [Google Scholar]
- 116.Mesulam M, Shaw P, Mash D, Weintraub S (2004) Cholinergic nucleus basalis tauopathy emerges early in the aging-MCI-AD continuum. Ann Neurol 55:815–828. [DOI] [PubMed] [Google Scholar]
- 117.Ming G, Song H (2011) Adult neurogenesis in the mammalian brain: significant answers and significant questions. Neuron 70:687–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Montagne A, Nation DA, Pa J, Sweeney MD, Toga AW, Zlokovic BV (2016) Brain imaging of neurovascular dysfunction in Alzheimer’s disease. Acta Neuropathol 131:687–707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Montine TJ, Phelps CH, Beach TG, Bigio EH, Cairns NJ, Dickson DW et al. (2012) National Institute on Aging–Alzheimer’s Association guidelines for the neuropathologic assessment of Alzheimer’s disease: a practical approach. Acta Neuropathol 123:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Morris M, Maeda S, Vossel K, Mucke L (2011) The many faces of tau. Neuron 70:410–426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Morrison BM, Hof PR, Morrison JH (1998) Determinants of neuronal vulnerability in neurodegenerative diseases. Ann Neurol 44:S32–S44. [DOI] [PubMed] [Google Scholar]
- 122.Morrison JH, Hof PR (2002) Chapter 37 selective vulnerability of corticocortical and hippocampal circuits in aging and Alzheimer’s disease. In: Progress in brain research, pp 467–486. [DOI] [PubMed] [Google Scholar]
- 123.Mrdjen D, Hartmann FJ, Becher B (2017) High dimensional cytometry of central nervous system leukocytes during neuroinflammation. Humana, New York: [DOI] [PubMed] [Google Scholar]
- 124.Mrdjen D, Pavlovic A, Hartmann FJ, Schreiner B, Utz SG, Leung BP et al. (2018) High-dimensional single-cell mapping of central nervous system immune cells reveals distinct myeloid subsets in health, aging, and disease. Immunity. [DOI] [PubMed] [Google Scholar]
- 125.Mrdjen D, Pavlovic A, Hartmann FJ, Schreiner B, Utz SG, Leung BP et al. (2018) High-dimensional single-cell mapping of central nervous system immune cells reveals distinct myeloid subsets in health, aging, and disease. Immunity 48:380–395.e6. [DOI] [PubMed] [Google Scholar]
- 126.Mufson EJ, Binder L, Counts SE, DeKosky ST, de Toledo-Morrell L, Ginsberg SD et al. (2012) Mild cognitive impairment: pathology and mechanisms. Acta Neuropathol 123:13–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Murray ME, Graff-Radford NR, Ross OA, Petersen RC, Duara R, Dickson DW (2011) Neuropathologically defined subtypes of Alzheimer’s disease with distinct clinical characteristics: a retrospective study. Lancet Neurol 10:785–796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Nalivaeva NN, Turner AJ (2013) The amyloid precursor protein: a biochemical enigma in brain development, function and disease. FEBS Lett 587:2046–2054. [DOI] [PubMed] [Google Scholar]
- 129.Nation D, Sweeney M, Montagne A, Sagare A, D’Orazio L, Pachicano M et al. (2018) Blood-brain barrier breakdown is an early biomarker of human cognitive dysfunction. Nat Med 25:270–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Neal M, Richardson JR (2018) Epigenetic regulation of astrocyte function in neuroinflammation and neurodegeneration. Biochim Biophys Acta Mol Basis Dis 1864:432–443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Noh Y, Jeon S, Lee JM, Seo SW, Kim GH, Cho H et al. (2014) Anatomical heterogeneity of Alzheimer disease: based on cortical thickness on MRIs. Neurology 83:1936–1944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Nonaka T, Watanabe ST, Iwatsubo T, Hasegawa M (2010) Seeded aggregation and toxicity of α-synuclein and tau: Cellular models of neurodegenerative diseases. J Biol Chem 285:34885–34898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Pascoal TA, Mathotaarachchi S, Shin M, Benedet AL, Mohades S, Wang S et al. (2017) Synergistic interaction between amyloid and tau predicts the progression to dementia. Alzheimer’s Dement 13:644–653. [DOI] [PubMed] [Google Scholar]
- 134.Persson K, Eldholm RS, Barca ML, Cavallin L, Ferreira D, Knapskog A-B et al. (2017) MRI-assessed atrophy subtypes in Alzheimer’s disease and the cognitive reserve hypothesis. PLoS One 12:e0186595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Petkova AT, Leapman RD, Guo Z, Yau WM, Mattson MP, Tycko R (2005) Self-propagating, molecular-level polymorphism in Alzheimer’s β-amyloid fibrils. Science 307:262–5. [DOI] [PubMed] [Google Scholar]
- 136.Pini L, Pievani M, Bocchetta M, Altomare D, Bosco P, Cavedo E et al. (2016) Brain atrophy in Alzheimer’s disease and aging. Ageing Res Rev 30:25–48. [DOI] [PubMed] [Google Scholar]
- 137.Pluta R, Ułamek-Kozioł M, Januszewski S, Czuczwar SJ (2018) Exosomes as possible spread factor and potential biomarkers in Alzheimer’s disease: current concepts. Biomark Med 12:1025–1033. [Google Scholar]
- 138.Polanco JC, Li C, Bodea L-G, Martinez-Marmol R, Meunier FA, Götz J (2018) Amyloid-β and tau complexity—towards improved biomarkers and targeted therapies. Nat Rev Neurol 14:22–39. [DOI] [PubMed] [Google Scholar]
- 139.Price JL, Ko Al, Wade MJ, Tsou SK, McKeel DW, Morris (2001) Neuron number in the entorhinal cortex and CA1 in preclinical alzheimer disease. Arch Neurol 58:1395. [DOI] [PubMed] [Google Scholar]
- 140.Purro SA, Farrow MA, Linehan J, Nazari T, Thomas DX, Chen Z et al. (2018) Transmission of amyloid-β protein pathology from cadaveric pituitary growth hormone. Nature 564:415–419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Qazi TJ, Quan Z, Mir A, Qing H (2018) Epigenetics in Alzheimer’s disease: perspective of DNA methylation. Mol Neurobiol 55:1026–1044. [DOI] [PubMed] [Google Scholar]
- 142.Qiang W, Yau W-M, Lu J-X, Collinge J, Tycko R (2017) Structural variation in amyloid-β fibrils from Alzheimer’s disease clinical subtypes. Nature 541:217–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Qiang W, Yau WM, Lu JX, Collinge J, Tycko R (2017) Structural variation in amyloid-β fibrils from Alzheimer’s disease clinical subtypes. Nature 541:217–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Rabinovici GD, Furst AJ, Alkalay A, Racine CA, O’Neil JP, Janabi M et al. (2010) Increased metabolic vulnerability in early-onset Alzheimer’s disease is not related to amyloid burden. Brain 133:512–528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Ray M, Zhang W (2010) Analysis of Alzheimer’s disease severity across brain regions by topological analysis of gene co-expression networks. BMC SystBiol 4:136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Rice HC, de Malmazet D, Schreurs A, Frere S, Van Molle I, Volkov AN et al. (2019) Secreted amyloid-β precursor protein functions as a GABA B R1a ligand to modulate synaptic transmission. Science 363:eaao4827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Risacher SL, Anderson WH, Charil A, Castelluccio PF, Shcherbinin S, Saykin AJ et al. (2017) Alzheimer disease brain atrophy subtypes are associated with cognition and rate of decline. Neurology 89:2176–2186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Roberson ED, Scearce-Levie K, Pal op JJ, Yan F, Cheng IH, Wu T et al. (2007) Reducing endogenous tau ameliorates amyloid beta-induced deficits in an Alzheimer’s disease mouse model. Science 316:750–754. [DOI] [PubMed] [Google Scholar]
- 149.Rodriguez RD, Grinberg LT (2015) Argyrophilic grain disease: an underestimated tauopathy. Dement Neuropsychol 9:2–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Rohrback S, Siddoway B, Liu CS, Chun J (2018) Genomic mosaicism in the developing and adult brain. Dev Neurobiol 78:1026–1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Rosenbloom MH, Alkalay A, Agarwal N, Baker SL, O’Neil JP, Janabi M et al. (2011) Distinct clinical and metabolic deficits in PCA and AD are not related to amyloid distribution. Neurology 76:1789–1796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Rossor MN, Garrett NJ, Johnson AL, Mountjoy CQ, Roth M, Iversen LL (1982) A post-mortem study of the cholinergic and GABA systems in senile dementia. Brain 105:313–330. [DOI] [PubMed] [Google Scholar]
- 153.Roubroeks JAY, Smith RG, van den Hove DLA, Lunnon K (2017) Epigenetics and DNA methylomic profiling in Alzheimer’s disease and other neurodegenerative diseases. J Neurochem 143:158–170. [DOI] [PubMed] [Google Scholar]
- 154.Roussarie J, Yao V, Plautz Z, Kasturia S, Albomoz C, Eric F et al. (2018) Selective neuronal vulnerability in Alzheimer’s disease: a network-based analysis. bioRxiv. 10.1101/499897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Ryan NS, Rossor MN (2010) Correlating familial Alzheimer’s disease gene mutations with clinical phenotype. Biomark Med 4:99–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Saman S, Kim W, Raya M, Visnick Y, Miro S, Saman S et al. (2012) Exosome-associated tau is secreted in tauopathy models and is selectively phosphorylated in cerebrospinal fluid in early Alzheimer disease. J Biol Chem 287:3842–3849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Sanders DW, Kaufman SK, DeVos SL, Sharma AM, Mirbaha H, Li A et al. (2014) Distinct tau prion strains propagate in cells and mice and define different tauopathies. Neuron 82:1271–1288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Saxena S, Caroni P (2011) Selective neuronal vulnerability in neurodegenerative diseases: from stressor thresholds to degeneration. Neuron 71:35–48. [DOI] [PubMed] [Google Scholar]
- 159.Scharfman HE (2016) The enigmatic mossy cell of the dentate gyrus. Nat Rev Neurosci 17:562–575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Scheff SW, Price DA, Schmitt FA, Dekosky ST, Mufson EJ (2007) Synaptic alterations in CA1 in mild Alzheimer disease and mild cognitive impairment. Neurology 68:1501–1508. [DOI] [PubMed] [Google Scholar]
- 161.Scheff SW, Price DA, Schmitt FA, Mufson EJ (2006) Hippocampal synaptic loss in early Alzheimer’s disease and mild cognitive impairment. Neurobiol Aging 27:1372–1384. [DOI] [PubMed] [Google Scholar]
- 162.Selkoe DJ (1999) Translating cell biology into therapeutic advances in Alzheimer’s disease. Nature 399:A23–A31. [DOI] [PubMed] [Google Scholar]
- 163.Selkoe DJ, Hardy J (2016) The amyloid hypothesis of Alzheimer’s disease at 25 years. EMBO Mol Med 8:595–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Shiino A, Watanabe T, Maeda K, Kotani E, Akiguchi I, Matsuda M (2006) Four subgroups of Alzheimer’s disease based on patterns of atrophy using VBM and a unique pattern for early onset disease. Neuroimage 33:17–26. [DOI] [PubMed] [Google Scholar]
- 165.Simón D, García-García E, Gómez-Ramos A, Falcón-Pérez JM, Daz-Hernández M, Hernández F et al. (2012) Tau overexpression results in its secretion via membrane vesicles. Neurodegener Dis 10:73–75. [DOI] [PubMed] [Google Scholar]
- 166.Solodkin A, Veldhuizen SD, VanHoesen GW (1996) Contingent vulnerability of entorhinal parvalbumin-containing neurons in Alzheimer’s disease. J Neurosci 16:3311–3321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Soscia SJ, Kirby JE, Washicosky KJ, Tucker SM, Ingelsson M, Hyman B et al. (2010) The Alzheimer’s disease-associated amyloid β-protein is an antimicrobial peptide. PLoS One 5:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Stancu I-C, Vasconcelos B, Ris L, Wang P, Villers A, Peeraer E et al. (2015) Templated misfolding of Tau by prion-like seeding along neuronal connections impairs neuronal network function and associated behavioral outcomes in Tau transgenic mice. Acta Neuropathol 129:875–894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Stern Y (2012) Cognitive reserve in ageing and Alzheimer’s disease. Lancet Neurol 11:1006–1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Stranahan AM, Mattson MP (2010) Selective vulnerability of neurons in layer II of the entorhinal cortex during aging and Alzheimer’s disease. Neural Plast 2010:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Streit WJ, Sammons NW, Kuhns AJ, Sparks DL (2004) Dystrophic microglia in the aging human brain. Glia 45:208–212. [DOI] [PubMed] [Google Scholar]
- 172.Tardivel M, Begard S, Bousset L, Dujardin S, Coens A, Melki R et al. (2016) Tunneling nanotube (TNT)-mediated neuron-to neuron transfer of pathological Tau protein assemblies. Acta Neuropathol Commun 4:117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Tensaouti Y, Stephanz EP, Yu T-S, Kemie SG (2018) ApoE regulates the development of adult newborn hippocampal neurons. Eneuro 5:ENEURO.0155-18.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Terry RD, Masliah E, Salmon DP, Butters N, DeTeresa R, Hill R et al. (1991) Physical basis of cognitive alterations in alzheimer’s disease: synapse loss is the major correlate of cognitive impairment. Ann Neurol 30:572–580. [DOI] [PubMed] [Google Scholar]
- 175.Thal DR, Rub U, Orantes M, Braak H (2002) Phases of Aβ-deposition in the human brain and its relevance for the development of AD. Neurology 58:1791–1800. [DOI] [PubMed] [Google Scholar]
- 176.Varol E, Sotiras A, Davatzikos C (2017) HYDRA: revealing heterogeneity of imaging and genetic patterns through a multiple max-margin discriminative analysis framework. Neuroimage 145:346–364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Verheijen BM, Vermulst M, van Leeuwen FW (2018) Somatic mutations in neurons during aging and neurodegeneration. Acta Neuropathol 135:811–826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Verheijen J, Sleegers K (2018) Understanding Alzheimer disease at the interface between genetics and transcriptomics. Trends Genet 34:434–447. [DOI] [PubMed] [Google Scholar]
- 179.Vieira RT, Caixeta L, Machado S, Silva AC, Nardi AE, Arias-Carrión O et al. (2013) Epidemiology of early-onset dementia: a review of the literature. Clin Pract Epidemiol Ment Health 9:88–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Walker LC, Jucker M (2015) Neurodegenerative diseases: expanding the prion concept. Annu Rev Neurosci 38:87–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Walker LC, Schelle J, Jucker M (2016) The prion-like properties of amyloid-β assemblies: implications for Alzheimer’s disease. Cold Spring Harb Perspect Med 6:a024398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Wang R, Reddy PH (2017) Role of glutamate and NMDA receptors in Alzheimer’s disease. J Alzheimer’s Dis 57:1041–1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Wang Y, Balaji V, Kaniyappan S, Krüger L, Irsen S, Tepper K et al. (2017) The release and trans-synaptic transmission of Tau via exosomes. Mol Neurodegener 12:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Warren JD, Fletcher PD, Golden HL (2012) The paradox of syndromic diversity in Alzheimer disease. Nat Rev Neurol 8:451–464. [DOI] [PubMed] [Google Scholar]
- 185.Wegiel J, Wisniewski HM, Dziewiatkowski J, Badmajew E, Tarnawski M, Reisberg B et al. (1999) Cerebellar atrophy in Alzheimer’s disease—clinicopathological correlations. Brain Res 818:41–50. [DOI] [PubMed] [Google Scholar]
- 186.Wei P, Blundon JA, Rong Y, Zakharenko SS, Morgan JI (2011) Impaired locomotor learning and altered cerebellar synaptic plasticity in pep-19/pcp4-null mice. Mol Cell Biol 31:2838–2844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Wendeln A-C, Degenhardt K, Kaurani L, Gertig M, Ulas T, Jain G et al. (2018) Innate immune memory in the brain shapes neurological disease hallmarks. Nature 556:332–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.West MJ, Coleman PD, Flood DG, Troncoso JC (1994) Differences in the pattern of hippocampal neuronal loss in normal ageing and Alzheimer’s disease. Lancet (London, England) 344:769–772 [DOI] [PubMed] [Google Scholar]
- 189.Whitehouse P, Price D, Struble R, Clark A, Coyle J, Delon M (1982) Alzheimer’s disease and senile dementia: loss of neurons in the basal forebrain. Science 215:1237–1239. [DOI] [PubMed] [Google Scholar]
- 190.Whitwell JL, Dickson DW, Murray ME, Weigand SD, Tosakulwong N, Senjem ML et al. (2012) Neuroimaging correlates of pathologically defined subtypes of Alzheimer’s disease: a case–control study. Lancet Neurol 11:868–877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Wingo TS, Lah JJ, Levey AI, Cutler DJ (2012) Autosomal recessive causes likely in early-onset Alzheimer disease. Arch Neurol 69:59–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Witter MP, Wouterlood FG, Naber PA, Van Haeften T (2000) Anatomical organization of the parahippocampal-hippocampal network. Ann N Y Acad Sci 911:1–24. [DOI] [PubMed] [Google Scholar]
- 193.Wu JW, Hussaini SA, Bastille IM, Rodriguez GA, Mrejeru A, Rilett K et al. (2016) Neuronal activity enhances tau propagation and tau pathology in vivo. Nat Neurosci 19:1085–1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Xu J, Patassini S, Rustogi N, Riba-Garcia I, Hale BD, Phillips AM et al. (2019) Regional protein expression in human Alzheimer’s brain correlates with disease severity. Commun Biol 2:43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Yamada K, Holth JK, Liao F, Stewart FR, Mahan TE, Jiang H et al. (2014) Neuronal activity regulates extracellular tau in vivo. J Exp Med 211:3 87–393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Zeisel A, Munoz-Manchado AB, Codeluppi S, Lonnerberg P, La Manno G, Jureus A et al. (2015) Cell types in the mouse cortex and hippocampus revealed by single-cell RNA-seq. Science (80-) 347:1138–1142. [DOI] [PubMed] [Google Scholar]
- 197.Zeng H, Shen EH, Hohmann JG, Oh SW, Bernard A, Royall JJ et al. (2012) Large-scale cellular-resolution gene profiling in human neocortex reveals species-specific molecular signatures. Cell 149:483–496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Zhang Y, Li P, Feng J, Wu M (2016) Dysfunction of NMD A receptors in Alzheimer’s disease. Neurol Sci 37:1039–1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Zhao X, Lein ES, He A, Smith SC, Aston C, Gage FH (2001) Transcriptional profiling reveals strict boundaries between hippocampal subregions. J Comp Neurol 441:187–196. [DOI] [PubMed] [Google Scholar]
- 200.Zhu X, Tan L, Wang H, Jiang T, Cao L, Wang C et al. (2015) Rate of early onset Alzheimer’s disease: a systematic review and meta-analysis. Ann Transl Med 3:38. [DOI] [PMC free article] [PubMed] [Google Scholar]