Abstract
This study aims to explore the effect of environmental factors (temperature, light, storage time) on germination response and dormancy patterns in eight Mediterranean native wildplants, belonging to the Euphorbia L. genus. In detail, we considered E. amygdaloides subsp. arbuscula, E. bivonae subsp. bivonae, E. ceratocarpa, E. characias, E. dendroides, E. melapetala, E. myrsinites, and E. rigida. We collected seeds from natural plant populations and performed germination assays in climatic chambers at seven constant temperatures (from 5 to 35°C, with 5°C increments), and four fluctuating temperature regimes (8/15, 8/20, 8/25, and 8/30°C, with a 12/12 hr thermoperiod). Germination assays were set up both in dark (D) and in light/dark conditions (L/D, 12/12 hr photoperiod), after short and long seed storage (SS around 30 days and LS around 150 days). For all these species, except E. amygdaloides subsp. arbuscula, results show that the final germinated proportions were improved by a long storage period (>150 days), which supports the existence of nondeep physiological dormancy. Optimal temperature levels ranged from 14.3 to 21.3°C and base temperatures ranged from 5.6 to 12.1°C, while ceiling temperatures from 25.6 to 34.7°C. For none of these species, germinations were favored by an alternating daily temperature regime, while in several instances, germinations were quicker and more complete in darkness, than in an alternating light/dark regime. In some instances, extreme temperature levels (5 and 30°C) induced dormancy and germinations did not resume when seeds were exposed at optimal temperature levels. Results are discussed in terms of the dynamics of emergences and how this might be affected by climate changes.
Keywords: cardinal temperatures, germination strategy, perennial Euphorbia species, seed dormancy, storage time, thermal time, thermo‐inhibition
Most of the Euphorbia species under investigation showed physiological dormancy, which was released following an after‐ripening period (nondeep Physiological Dormancy; PD). On average, germinations were quickest and most complete at temperature levels ranging from 14 to 21°C, while there was no or little effect of light and alternating temperature regimes. The germination of most Euphorbia species was obstacled at temperatures as low as 5°C and as high as 35°C, due to either thermo‐inhibition or thermo‐dormancy. Under present climatic conditions, germination occurs mainly in the autumn season. Due to thermal requirements, the germination of these species may be vulnerable to climate change, which may narrow the favorable germination season and affect the dynamics of plant populations.

1. INTRODUCTION
Seed germination behavior is a fundamental trait to determine the ability of plants to successfully establish in a given habitat or geographical region. Indeed, seeds need to be able to germinate in the right timing and location, so that the chances of seedling survival and establishment are maximized. A key role is played by several environmental factors, including temperature (T), light, and water availability. In particular, the effect of temperature is related to threshold levels for seed germination, such as base temperature (T b), optimum temperature (T o), and maximum or ceiling temperature (T c), which form the basis for the concept of thermal time.
Germination is inhibited for T < T b and T > T c (thermo‐inhibition). Thermo‐inhibition at high temperatures is very important for many perennial species or winter annual species adapted to warm climates, which have an optimum germination temperature between 10 and 20°C (Thanos, Georghiou, & Skarou, 1989; Thompson, 1973; Washitani & Masuda, 1990). Thermo‐inhibition is also very common in species of Mediterranean ecosystems, as well as in Euphorbia species inhabiting warm seasonal ecosystems (Lavorel, Debussche, Lebreton, & Lepart, 1993; Washitani & Masuda, 1990). In some cases, high (and low) temperatures can also induce secondary dormancy, according to a phenomenon known as thermo‐dormancy (Bewley & Black, 1985). This was observed especially in annual plants of deserts as well as in some Mediterranean species. In both cases, thermo‐dormancy could play a strategic role (Maher, Gerasopoulos, & Maloupa, 2000; Thanos et al., 1989) in preventing seed germination during occasional rain in hot and dry summers (Gutterman, 2002). Thermo‐dormancy has also been found in seeds of Lactuca sativa and Euphorbia nicaeensis (Narbona, Arista, & Ortiz, 2007a; Thanos et al., 1989; Vidaver & Hsiao, 1975).
Apart from the prevailing temperature level, thermal history also plays an important role, in the sense that several plant species may prefer a fluctuating temperature regime and may not germinate with constant daily temperatures (Cristaudo, Gresta, Catara, & Mingo, 2014; Masin, Onofri, Gasparini, & Zanin, 2017).
Light is also important, and, in this respect, previous research has shown that some plants give better germination capabilities when exposed to an alternating light/dark regime (Catara et al., 2016).
In addition to the climatic variables, germination is also controlled by endogenous factors, such as primary dormancy. Although this is a potentially expensive trait (Willis et al., 2014), it has been recognized as an adaptive mechanism, by which the plant avoids germination in unfavorable conditions for seedling establishment (e.g., excess heat and drought) and postpones them until the season is more favorable (Baskin & Baskin, 2014).
Among the endogenous factors, several studies have shown that seed germination is genetically determined and the phylogenetic signal may be a significant constraint to interspecific variation within a genus (Zhang, Du, & Chen, 2004). This was found, for example, for some Romulea species in Mediterranean habitats (Carta, Hanson, & Müller, 2016), even though a contrasting behavior was observed with other genera, such as Stellaria and Nothofagus, which showed considerable interspecific variability in germination behavior (Arana et al., 2016; Vandelook, Van de Moer, & Van Assche, 2008).
Morphological traits, such as seed size, seed mass, seed shape, and seed dispersal, can also play a role in seed germination behavior as shown, for example, in Verbascum sp. pl., Euphorbia humifusa and Solanum nigrum (Catara et al., 2016; Wang et al., 2016).
Studying the relationship between environmental factors and plant physiology is fundamental to understand and predict ecological dynamics, depending on the climate characteristics of a certain location, such as latitude, altitude, soil moisture, temperature and rainfall patterns, light, and photoperiod (Baskin & Baskin, 2014; Cristaudo, Gresta, Restuccia, Catara, & Onofri, 2016; Gresta, Cristaudo, Onofri, Restuccia, & Avola, 2010; Zhang et al., 2017). This is particularly important for Mediterranean regions, which are characterized by a high variability of environmental conditions, where wet and mild periods are alternated with dry and hot periods, which make seedling survival very difficult. In this respect, climate change and global warming should be carefully considered, as they can pose additional problems for seed germination and plant recruitment (Baskin & Baskin, 2014; Mondoni, Rossi, Orsenigo, & Probert, 2012; Walck, Hidayati, Dixon, Thompson, & Poschlod, 2011), which can modify the geographical distribution of plant species (Poschlod et al., 2013).
Climate change has been shown to produce a more erratic rainfall pattern, with higher temperatures and more frequent conditions of water shortage. A few studies have addressed the effects of water stress on germination in Mediterranean species. Conifers have shown high tolerance to water stress (Boydak, Dirik, Tilki, & Çalikoğlu, 2003; Thanos & Skordilis, 1987), whereas shrub species have shown variable responses, from high (e.g., Antyllis cytisoides; Ibanez & Passera, 1997) to low or moderate tolerance (e.g., Genista scorpius, Cistus monspeliensis, C. salviifolius, Calicotome villosa; Bochet, García‐Fayos, Alborch, & Tormo, 2007; Chamorro, Luna, & Moreno, 2017; Pérez‐Fernández, Calvo‐Magro, & Ferrer‐Castán, 2006). Negative effects of water stress can persist for several years, through maternal effects which lead to less viable seeds with lowest germination capacity. Unfortunately, the effects of climate change on seed germination seem to be highly variable, depending on plant species, populations, and sites, which makes it difficult to make predictions for a given species at a specific site. This motivates research on the germination responses to climate factors, such as temperature, light, and water availability.
In this study, we present a novel assessment of seed germination strategies, focusing on some taxa of the Euphorbia L. genus (Euphorbiaceae Juss.). This is the largest genera of the Euphorbiaceae family, also known as spurge family, and it is one of the five most species‐rich genera of the angiosperm group, with around 2,000 species (Frodin, 2004; Govaerts, Frodin, & Radcliffe‐Smith, 2000), that occur in all temperate and tropical regions. Some of them have a considerable economic importance in medicine (Rahman & Akter, 2013) or for revegetation, landscaping, and xero‐gardening in semiarid environments (Benvenuti, 2014; Franco, Martínez‐Sánchez, Fernández, & Bañón, 2005).
We focused on eight perennial taxa, on which no pertinent information about the germination traits is available from literature: Euphorbia amygdaloides L. subsp. arbuscula Meusel, E. bivonae Steud. subsp. bivonae, E. ceratocarpa Ten., E. characias L., E. dendroides L., E. melapetala Gasp. ex Guss., E. myrsinites L., and E. rigida M. Bieb. (Figure 1). The investigated species are phylogenetically related and belong to the monophyletic subgenus Esula Pers., one of the four major clades within the genus Euphorbia (Geltman, 2015; Riina et al., 2013). Some of these species are widespread across the Mediterranean region, in a wide range of altitudes, while three of them are narrowly distributed (endemic).
Figure 1.

Photographs of the eight Euphorbia study species
The seeds of all species in the group possess a caruncle, but differences in the size and shape of the caruncle are pronounced. Seed can have different sizes and, above all, different shapes (ovoidal, quadrangular or strongly compressed laterally), ornamentation (smooth or vermiculate‐rugulose) and color (brown or gray) (Figure 2).
Figure 2.
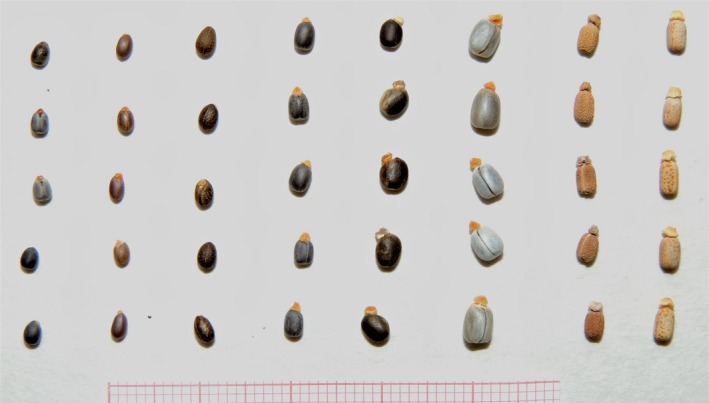
Photograph about seeds of Euphorbia study species
Some of these species, such as E. characias, E. dendroides, E. myrsinites, and E. rigida, are characterized by interesting biotechnical and/or ornamental value, both for architectural shapes and fascinating foliage (La Mantia et al., 2012; Pahlevani, Geltman, & Riina, 2011; Riina et al., 2013; Sari & Karaşah, 2015).
Considering those eight species, the objectives of this study were as follows: (a) evaluate the relationships between temperature, light, storage time, and germination behavior; (b) estimate threshold temperatures for seed germination and thermal time; and (c) verify the existence of thermo‐inhibition and/or thermo‐dormancy.
2. MATERIALS AND METHODS
2.1. Fruit collection and seed storage
Seeds were collected in four provinces of Eastern and Western Sicily (Catania, Messina, Palermo, Trapani) where the plants are abundant under wild conditions. The main characteristics of the species under investigation are reported in Table 1.
Table 1.
Chorological, ecological, environmental, and morphological characteristics of the Euphorbia species under investigation
| Species | Chorological type | Life form | Habitat | Altitudinal range (m a.s.l) | Environmental characteristics | Seed mass (mg per seed) (1) | ||
|---|---|---|---|---|---|---|---|---|
| Climate | Light | Soil moisture | ||||||
| E. amygdaloides subsp. arbuscula | Endemic to Sicily, Sardinia and Calabria | Chamaephytes perennial herb, woody at the base | Oak and beech‐woods | 600–1,500 | Temperate (cool and moist) | Partial shade | Moist | 4.38 (0.17) |
| E. bivonae | Stenomediterranean | Nanophanerophytes perennial woody | Rocky ridges of the coastal lands, calcareous rocks | 0–300 | Mediterranean (hot and dry) | Full sun | Dry | 5.04 (0.05) |
| E. ceratocarpa | Endemic to Sicily and southern Italy | Chamaephytes perennial herb, woody at the base | Disturbed soils, road edges | 0–850 | Intermediate between Mediterranean and temperate | Full sun | Dry to moist | 5.78 (0.13) |
| E. characias | Stenomediterranean | Nanophanerophytes perennial woody | Quercus ilex woods, scrublands, garrigues | 0–1,000 | Mediterranean | Full sun to partial shade | Dry | 9.63 (0.37) |
| E. dendroides | Stenomediterranean | Nanophanerophytes perennial woody | Rocky limestone and volcanic slopes | 0–800 | Mediterranean | Full sun | Dry | 9.61 (0.35) |
| E. melapetala | Endemic to Sicily and Malta | Nanophanerophytes perennial woody | Scrublands, dry slopes | 150–1,400 | From Mediterranean to temperate | Partial shade | Dry to moist | 17.49 (0.57) |
| E. myrsinites | S‐European‐Asia Minor | Chamaephytes perennial herb, woody at the base | Mountain pastures, rocky dry slopes, calcareous slopes | 600–1,900 | From Mediterranean to temperate | Full sun | Dry | 8.96 (0.12) |
| E. rigida | S‐European | Chamaephytes perennial herb, woody at the base | Dry rocky, exposed, limestone and volcanic slopes; dry river beds | 400–1,500 | From Mediterranean to temperate | Full sun | Dry | 10.70 (0.21) |
Nomenclature of plant species follows the Planetary Biodiversity Inventory (PBI) Euphorbia database (http://app.tolkin.org/projects/72/taxa; Riina & Berry, 2017).
Seeds of all eight Euphorbia species were collected from native populations (Table 2, Figure 3), during May, June, and July 2012, 2013, and 2014, from mature capsules (schizocarp fruits, called cocci) ready for dispersal. Only one harvest was made for each species; seeds were immediately stored and submitted to germination tests. Large and vigorous populations were selected, with highest densities and proportions of reproductive output. A GPS (iFINDER HUNT™, Lowrance Electronics Inc.) was used to record positioning and altitude for each collection site (Table 2).
Table 2.
Characteristics of the sampling sites, for each of the eight Euphorbia species
| Species | Date of collection | Province | District | Collection sites | Altitude (m a.s.l.) | Geographical coordinates WGS84 |
|---|---|---|---|---|---|---|
| 1. E. amygdaloides subsp. arbuscula | 16/06/2013 | Palermo | Petralia Sottana | P.no di Farina (Madonie mountains) | 1,381 |
37 51 42,73 N 14 04 43,78 E |
| 2. E. bivonae | 14/05/2013 | Trapani | Castellammare del Golfo | Mt. Inici | 230 |
38 01 48,00 N 12 52 22,75 E |
| 3. E. ceratocarpa | 25/07/2013 | Palermo | Monreale | Contrada Strasatto | 830 |
38 00 26,4 N 13 14 49,2 E |
| 4. E. characias | 26/05/2012 | Messina | Francavilla di Sicilia | C.da Serro Piddu | 570 |
37 54 40,22 N 15 04 21,41 E |
| 5. E. dendroides | 14/05/2013 | Palermo | Palermo | Mt. Pellegrino | 39 |
38 11 40,70 N 13 20 17,08 E |
| 6. E. melapetala | 14/05/2014 | Palermo | Palermo | Mt. Pellegrino | 140 |
38 11 09,20 N 13 20 43,35 E |
| 7. E. myrsinites | 16/06/2013 | Palermo | Petralia Sottana | P.no Battaglia (Madonie mountains) | 1,636 |
37 52 27,60 N 14 01 41,98 E |
| 8. E. rigida | 27/05/2012 | Catania | Castiglione di Sicilia | Regional road “Mareneve” (Etna volcano) | 956 |
37 51 15,62 N 15 00 31,75 E |
Figure 3.
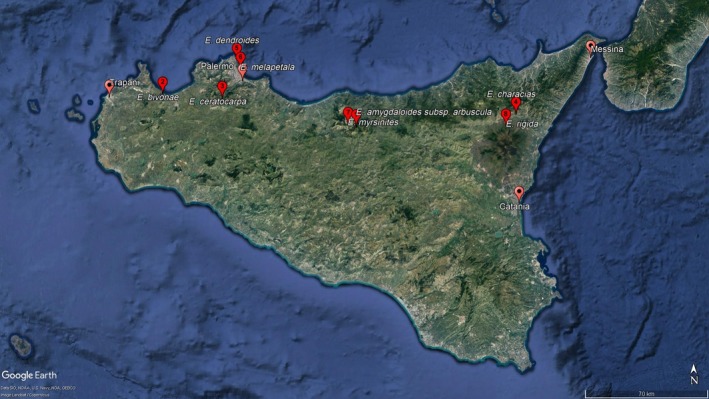
Map of sampling sites for the eight study species across northern Sicily. Numbers coincide with site numbers in Table 2
Fruits were collected from randomly chosen individuals (>50 individuals per species) in a relatively big area (200–500 m2) to obtain an adequate representation of genetic diversity. Plants were randomly selected from the middle of each population, in order to avoid any edge effects and any disturbances to the natural spread of the population (sustainable harvesting).
In the laboratory, the fruits were air‐dried at room temperature (25°C ± 2°C), for about 3 weeks, in plastic box perforated for ventilation with bottom surfaces covered with blotting paper, to absorb humidity; mesh lids were used, to ensure air circulation and prevent seed losses, due to the “explosion” of fruits. After their release, seeds were cleaned from the remaining fruit tissues using a stack of sieves. For each taxon, the weight of five replicates of 20 randomly chosen seeds was determined (Table 1). The average weight of one dry seed, expressed in mg, of each species was determined using a precision balance with an accuracy of 0.0001 g (Mettler AE 50). A preliminary cut test on a subsample of seeds showed that the viability was nearly 100%, for all species. The collected seeds were stored in paper bags in laboratory conditions (22 ± 2°C, 50% RH), until the beginning of the germination experiments, starting approximately 1 month from the collection, for all species, regardless of the sampling year.
2.2. Experimental design
Germination experiments were carried out in automatic temperature‐, humidity‐, and light‐controlled growth chambers (Sanyo ‐ model MLR‐351H). Light was delivered via cool white fluorescent tubes (Osram FL 40 SS W/37), with a photon flux density of 50 μmol m−2s−1.
For all species, we considered seven constant temperature regimes (from 5 to 35°C, with 5°C increments) and four fluctuating temperature regimes (8/15, 8/20, 8/25, and 8/30°C) with a 12/12 hr thermoperiod. A further constant temperature treatment at 8°C was added as control for fluctuating temperatures. Temperature regimes were chosen considering the typical Mediterranean conditions, where 10°C corresponds to the average daytime temperature in late autumn or winter, while 20°C corresponds to the average daytime temperature early in autumn and late in spring. The highest temperatures (30 and 35°C) are common in Mediterranean summer conditions, and they were included to test for possible thermo‐inhibition or thermo‐dormancy phenomena in imbibed seeds. Maximum and minimum daily temperatures for fluctuating regimes were selected to represent the typical extent of daily fluctuations.
All regimes were set up in continuous darkness (D) and in light/dark conditions (L/D, 12/12 hr photoperiod, with the lowest temperature during the dark period).
Germination assays were repeated twice, considering the same populations at two different storage times: short storage (SS: around 30 days) and long storage (LS: around 150 days). Germination assays at fluctuating temperatures were performed only after long storage.
For each germination assay, four replicates of 25 randomly selected seeds were used for each treatment and studied species. Seeds were placed in 9 cm diameter Petri dishes, on top of three layers of filter papers (Whatmann No. 1), previously moistened with 5 ml of distilled water and incubated in growth chambers at the different temperature regimes.
For darkness treatments, the Petri dishes were wrapped in two layers of aluminum foil and kept in the same growth chamber. All Petri dishes were sealed using Parafilm® to avoid moisture losses; water was added to the dishes as needed to keep an adequate moisture level.
Seed germination was monitored daily, and germinated seeds were counted and removed from Petri dishes. Seeds were considered as germinated when the radicle protrusion was about 2 mm. In continuous darkness treatments, counts were made under green‐filtered light (Philips PF710E), which had not shown any effect on seed germination, as assessed by preliminary assays. In L/D, germination counts were performed during the light period. Experiments were continued for 30 days. At the end of incubation period, the viability of the remaining seeds was estimated by cut test; seeds with white, hard embryos were considered to be alive. Empty and dead seeds were excluded from the calculation of final germination percentages.
After the end of assays, ungerminated seeds exposed at 5 and 30°C for six Euphorbia species (E. amygdaloides subsp. arbuscula, E. bivonae subsp. bivonae, E. ceratocarpa, E. characias, E. dendroides, and E. rigida) were moved to a growth chamber at 20°C (close to optimal for all species), to assess whether they could recover germination or whether they had become dormant.
2.3. Data analyses
For each Petri dish, the observed counts were used to parameterize a log‐logistic germination model, by using a time‐to‐event modeling platform (Onofri, Benincasa, Mesgaran, & Ritz, 2018; Ritz, Pipper, & Streibig, 2013). The fitted model was used to derive the final percentage of germinated seeds (FPG) and the time to 50% germination (T 50), which were submitted to ANOVA. FPGs were arcsine‐square‐root transformed and T 50 were log‐transformed prior to analyses, in order to meet the basic assumptions for linear models; back‐transformed means were used for tables and graphs.
Considering the assays at constant temperature, the number of days to achieve 10% and 30% germination was also derived from the fitted germination models and their reciprocal values, together with the reciprocal of T 50 values, were taken as the germination rates respectively for the 10th percentile (GR10), 30th percentile (GR30), and 50th percentile (GR50) (Bierhuizen & Wagenvoort, 1974).
The observed GRs for each species, light regime, and storage time were used to parameterize the following thermal‐time model, derived from Mesgaran, Onofri, Mashhadi, and Cousens (2017):
where g is the percentile (10, 30, or 50th), T is the temperature, ΘT(g) is the thermal time to germination for the g‐th percentile, T b is the base temperature, and k relates to the decrease of germination velocity when temperature exceeds the optimal level.
3. RESULTS
3.1. Germination capability
For all species, the ANOVA showed that germination capability was significantly influenced by temperature regimes, light conditions, and storage times. Germination occurred across a relatively large range of temperatures, although this appeared to be species‐dependent. Some species germinated from 15 to 25°C (E. ceratocarpa, E. characias, E. melapetala), and other species germinated from 10 to 25°C (E. bivonae, E. dendroides), while one species germinated between 10 and 20°C (E. rigida) and another one germinated only at 20°C (E. myrsinites; Figures 4 and 5).
Figure 4.
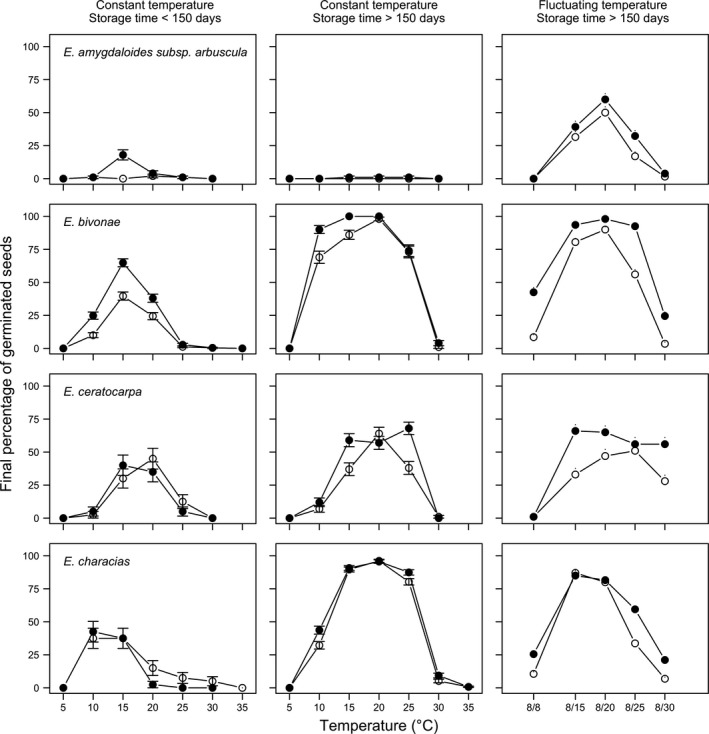
Final germination percentages of Euphorbia amygdaloides subsp. arbuscula, E. bivonae, E. ceratocarpa, and E. characias at two storage periods (<150 and >150 days), two light conditions (photoperiod of 12/12 hr, with the highest temperature during daytime; darkness), at constant and fluctuating temperature. (LEGEND: open symbols represent the alternating light/darkness regime while closed symbols represent the continuous darkness regime). Vertical bars represent standard errors
Figure 5.
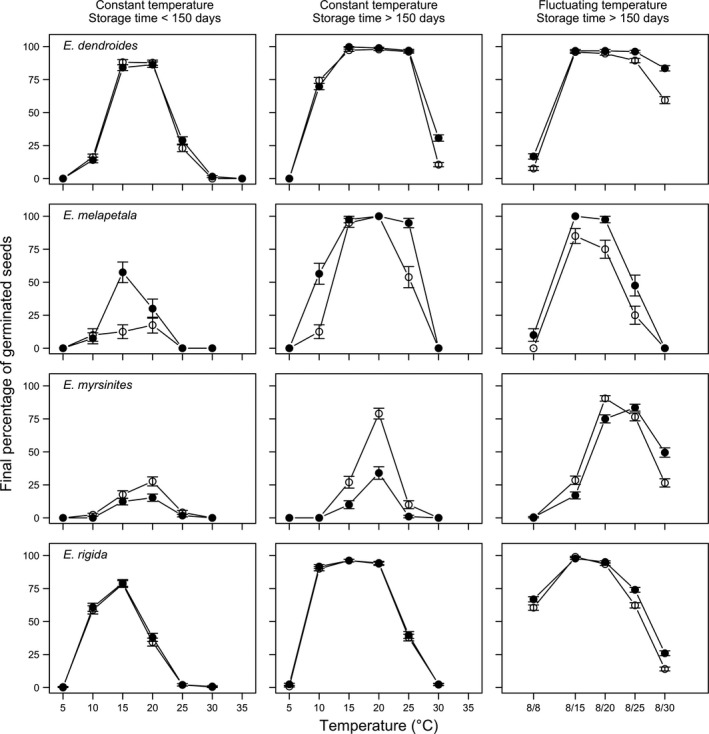
Final germination percentages of Euphorbia dendroides, E. melapetala, E. myrsinites, and E. rigida at two storage periods (<150 and >150 days), two light conditions (photoperiod of 12/12 hr, with the highest temperature during daytime; darkness), at constant and fluctuating temperature (LEGEND: open symbols represent the alternating light/darkness regime while closed symbols represent the continuous darkness regime). Vertical bars represent standard errors
In general, all species reached the highest germination capability from 15 to 20°C. Outside this range, the capability was progressively reduced, as temperature increased/decreased. It is important to note that E. amygdaloides subsp. arbuscula showed little or no germination at constant temperatures (<18%; Figure 4).
Differences between light regimes were rather small, although most species (except E. characias and E. myrsinites) gave slightly higher FGPs in continuous darkness, especially at suboptimal or supra‐optimal temperature levels (Figures 4 and 5). Differences were most visible in E. bivonae (especially at short storage time and with fluctuating temperature), E. ceratocarpa, and E. melapetala.
As for storage time, FGPs were often higher with long storage. Notable effects were noted with E. bivonae, which gave higher FGPs with long storage time, together with a wider temperature range for germination (10–25°C). Improved germination with long storage was also noted with E. ceratocarpa, E. characias, E. melapetala, and E. myrsinites (Figures 4 and 5). For this latter species, a high proportion of seeds were dormant at all constant temperatures with short storage.
Considering fluctuating temperatures, the effects were rather small, although higher FGPs were noted with E. amygdaloides subsp. arbuscula and E. myrsinites (Figures 3 and 4).
3.2. Thermo‐dormancy and/or thermo‐inhibition
For all species, no germination was observed at 5°C for 30 days. However, when seeds were reincubated at 20°C (benefit temperature) under the same light conditions (L/D and D), E. characias, E. dendroides, and E. rigida exhibited a high germination capability, with FPG values ranging from 60% to 100%, both in L/D and in D. E. amygdaloides subsp. arbuscula, E. bivonae, and E. ceratocarpa showed the ability to germinate, although the FGPs were rather low (Figure 6).
Figure 6.
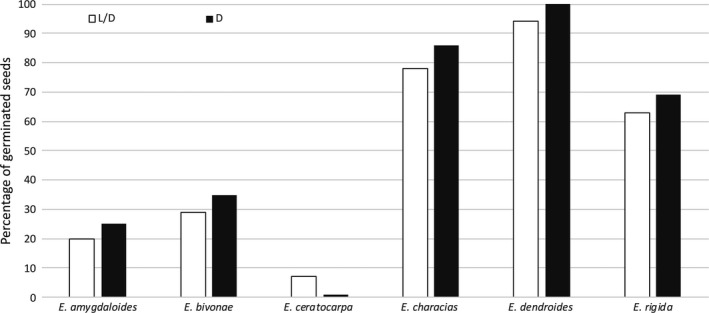
Germination of nongerminated seeds of six Euphorbia species after 30 days of exposure at the benefit temperature (20°C). Seeds were first exposed at 5°C for 30 days, where no germinations were observed (see Figures 3 and 4). Immediately afterward, seeds were reincubated at 20°C for 30 days
Considering 30°C, for all species the germination capacity was rather low or null. When ungerminated seeds were reincubated at 20°C under the same light conditions (L/D or D), E. bivonae, E. characias, and E. dendroides showed high germination capacities (>80°C) while E. amygdaloides subsp. arbuscula, E. ceratocarpa and E. rigida, although viable, showed little or no germination (Figure 7).
Figure 7.
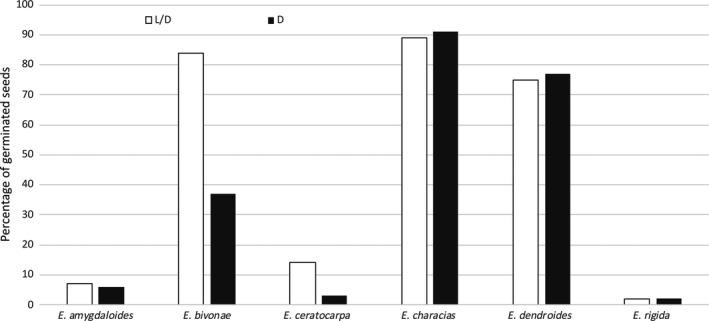
Germination of nongerminated seeds of six Euphorbia species after 30 days of exposure at benefit temperature (20°C). Seeds were first exposed at 30°C for 30 days, where no germinations were observed (see Figures 3 and 4). Immediately afterward, seeds were reincubated at 20°C for 30 days
3.3. Germination velocity
Considering the germinated fraction, the time (days) taken for 50% of seeds to germinate (T 50) generally decreased as temperature increased from 5 to 20°C (Figures 8 and 9). All species showed quickest germinations between 15 and 20°C, requiring <10 days to reach 50% germination. On average, the ranking among species at constant 20°C, short storage, and in dark conditions was: E. myrsinites < E. rigida < E. dendroides < E. characias < E. melapetala < E. ceratocarpa < E. bivonae with, respectively, 3.0 (SE = 0.37), 5.1 (0.44), 5.8 (0.42), 6.5 (1.63) 7.9 (1.15), 9.3 (1.17), and 11.6 (0.87) days. For all species, T 50 was remained constant or increased slightly in alternating light conditions, although such an effect was significant only with E. characias (from 6.5 to 9.5 days) and, particularly with E. melapetala (from 7.9 to 14.9 days). A long storage time caused an increase of T 50 in dark conditions for E. myrsinitis (from 3.0 to 7.5 days) and a decrease for E. melapetala (from 7.9 to 4.5 days), E. ceratocarpa (from 9.3 to 5.8 days), and E. bivonae (from 11.6 to 5.0 days).
Figure 8.
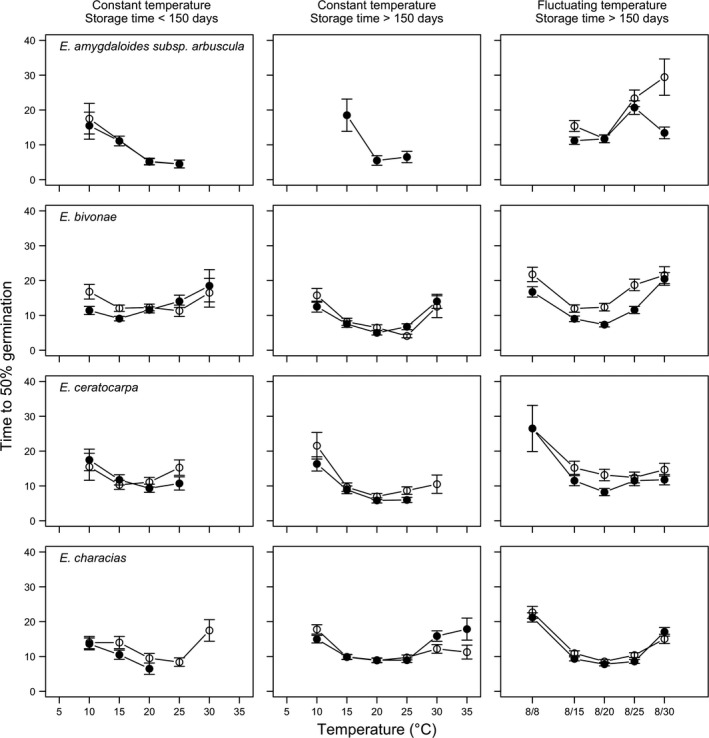
Time to 50% germination (relative to FGP) for Euphorbia amygdaloides subsp. arbuscula, E. bivonae, E. ceratocarpa, and E. characias at two storage periods (<150 and >150 days), two light conditions (photoperiod of 12/12 hr, with the highest temperature during daytime; darkness), at constant and fluctuating temperature. (LEGEND: open symbols represent the alternating light/darkness regime while closed symbols represent the continuous darkness regime). Vertical bars represent standard errors
Figure 9.
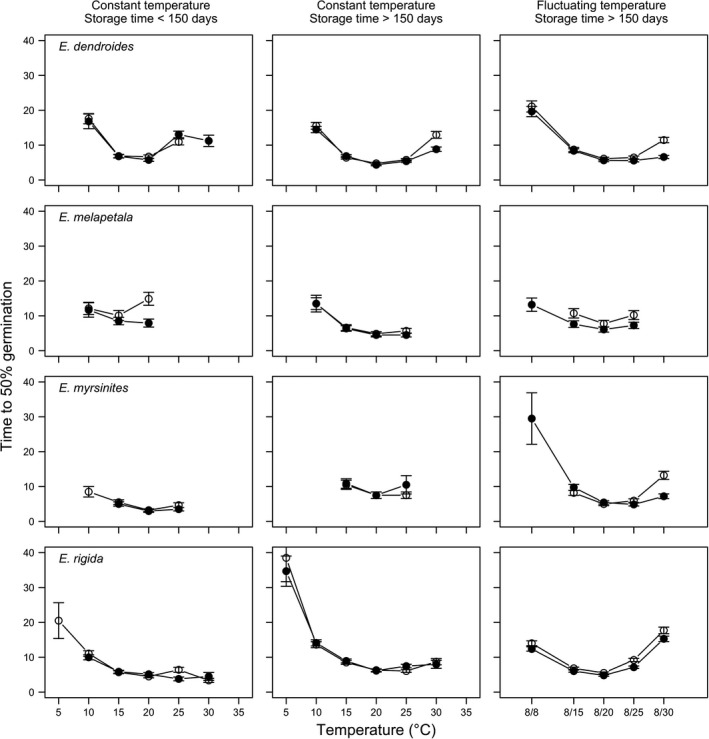
Time to 50% germination (relative to FGP) for Euphorbia dendroides, E. melapetala, E. myrsinites, and E. rigida at two storage periods (<150 and >150 days), two light conditions (photoperiod of 12/12 hr, with the highest temperature during daytime; darkness), at constant and fluctuating temperature (LEGEND: open symbols represent the alternating light/darkness regime while closed symbols represent the continuous darkness regime). Vertical bars represent standard errors
3.4. Thermal‐time parameters
Threshold temperatures (T b, T o, and T c) are reported in Table 3. In general, in almost all the Euphorbia species with long storage, base, optimal, and ceiling temperatures were lower than with short storage, both in continuous darkness and alternating light regime. T b values showed some variability among tested species, ranging from −0.5°C (E. characias) to 13°C (E. myrsinites). The T b for germination of E. bivonae, E. dendroides, and E. ceratocarpa indicated that these three species were able to germinate already at temperatures ranging from 7.6 to 9.5°C. E. myrsinites required warmer temperatures (13°C), and E. rigida, which is usually found in wide altitudinal range, started germinating at cooler temperatures (6.2°C). T o values fall in a relatively narrow interval, ranging approximately from 14.3 to 21.3°C, according to the species, light conditions, and storage time. T c changed from a minimum of 21.1°C (E. characias in the L/D regime) to a maximum of 34.3 (E. characias in the L/D regime), in short storage, and from a minimum of 25.6°C (E. myrsinites) to a maximum of 34.7°C (E. dendroides), in long storage.
Table 3.
Cardinal temperatures for germination for seven Euphorbia species exposed to a constant temperature regime (T b: base temperature; T c: ceiling temperature; T o: optimal temperature), together with thermal times to germination for the 10th (Θ10), 30th (Θ30), and 50th (Θ50) percentile for the germinated fraction (SE: standard errors)
| Species | Light–storage | T b | SE | T c | SE | T o | SE | Θ10 | SE | Θ30 | SE | Θ50 | SE |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| E. bivonae | D–SS | 7.8 | 0.22 | 26.1 | 0.17 | 17.0 | 0.12 | 34.3 | 1.53 | 36.3 | 1.67 | 41.7 | 2.11 |
| D–LS | 7.6 | 0.52 | 32.4 | 0.51 | 20.0 | 0.28 | 29.1 | 2.29 | 30.9 | 2.51 | 33.8 | 2.88 | |
| L/D–SS | 8.4 | 0.16 | 26.4 | 0.15 | 17.4 | 0.09 | 42.1 | 1.55 | 48.6 | 1.95 | 54.1 | 2.36 | |
| L/D–LS | 9.0 | 0.96 | 31.7 | 1.08 | 20.3 | 0.62 | 23.7 | 4.56 | 25.9 | 5.25 | 28.5 | 6.17 | |
| E. ceratocarpa | D–SS | 8.8 | 0.18 | 27.6 | 0.24 | 18.2 | 0.12 | 43.3 | 2.03 | 43.7 | 2.05 | 44.9 | 2.14 |
| D–LS | 8.5 | 0.65 | 30.7 | 0.57 | 19.6 | 0.38 | 25.3 | 3.03 | 28.4 | 3.67 | 31.6 | 4.44 | |
| L/D–SS | 9.2 | 0.13 | 27.5 | 0.19 | 18.4 | 0.10 | 41.4 | 1.50 | 42.5 | 1.56 | 44.5 | 1.68 | |
| L/D–LS | 9.5 | 0.37 | 31.2 | 0.42 | 20.4 | 0.25 | 27.2 | 2.10 | 33.9 | 3.07 | 38.0 | 3.76 | |
| E. characias | D–SS | 7.6 | 0.17 | 21.1 | 0.11 | 14.3 | 0.08 | 23.0 | 0.96 | 24.0 | 1.02 | 31.7 | 1.53 |
| D–LS | 5.6 | 1.09 | 33.7 | 0.98 | 19.6 | 0.49 | 50.1 | 6.03 | 55.3 | 6.89 | 57.6 | 7.37 | |
| L/D–SS | −0.5 | 3.98 | 34.3 | 1.79 | 16.9 | 1.47 | 91.2 | 19.49 | 91.9 | 19.66 | 99.9 | 22.0 | |
| L/D–LS | 6.8 | 0.41 | 32.7 | 0.37 | 19.7 | 0.21 | 47.1 | 2.56 | 49.3 | 2.75 | 53.7 | 3.13 | |
| E. dendroides | D–SS | 9.1 | 0.47 | 30.6 | 0.45 | 19.9 | 0.29 | 22.6 | 2.07 | 28.0 | 2.97 | 33.1 | 4.08 |
| D–LS | 7.9 | 0.51 | 34.7 | 0.77 | 21.3 | 0.35 | 26.8 | 2.10 | 29.6 | 2.50 | 32.3 | 2.88 | |
| L/D–SS | 8.7 | 0.15 | 28.7 | 0.25 | 18.7 | 0.12 | 23.2 | 0.80 | 26.9 | 1.01 | 31.3 | 1.29 | |
| L/D–LS | 7.9 | 0.34 | 33.4 | 0.42 | 20.6 | 0.20 | 27.0 | 1.44 | 29.5 | 1.65 | 31.8 | 1.91 | |
| E. melapetala | D–SS | 8.9 | 0.18 | 23.6 | 0.39 | 16.3 | 0.18 | 18.8 | 1.23 | 21.3 | 1.53 | 28.3 | 2.24 |
| D–LS | 11.1 | 1.15 | 30.2 | 0.51 | 20.6 | 0.56 | 18.9 | 3.10 | 19.1 | 3.19 | 19.5 | 3.31 | |
| L/D–SS | 4.2 | 0.86 | 25.2 | 0.27 | 14.7 | 0.41 | 61.5 | 5.19 | 61.5 | 5.19 | 65.1 | 5.71 | |
| L/D–LS | 10.6 | 0.75 | 30.1 | 0.30 | 20.4 | 0.36 | 19.8 | 1.86 | 21.0 | 2.03 | 22.6 | 2.30 | |
| E. myrsinites | D–SS | 12.4 | 0.10 | 27.5 | 0.10 | 20.0 | 0.04 | 19.1 | 0.44 | 19.1 | 0.44 | 21.4 | 0.51 |
| D–LS | 12.1 | 0.01 | 25.6 | 0.00 | 18.9 | 0.00 | 24.6 | 0.04 | 24.6 | 0.04 | 24.7 | 0.04 | |
| L/D–SS | 13.0 | 0.36 | 26.8 | 0.33 | 19.9 | 0.18 | 17.6 | 1.82 | 18.1 | 1.89 | 20.8 | 2.29 | |
| L/D–LS | 11.6 | 0.40 | 30.2 | 0.20 | 20.9 | 0.20 | 30.8 | 1.98 | 31.2 | 2.02 | 31.2 | 2.02 | |
| E. rigida | D–SS | 6.2 | 0.57 | 27.1 | 0.40 | 16.6 | 0.04 | 25.5 | 2.05 | 28.4 | 2.36 | 29.8 | 2.56 |
| D–LS | 7.0 | 0.51 | 31.1 | 0.37 | 19.1 | 0.00 | 32.2 | 2.28 | 34.8 | 2.60 | 38.7 | 3.08 | |
| L/D–SS | 6.7 | 0.81 | 27.2 | 0.64 | 16.9 | 0.18 | 24.1 | 3.10 | 25.7 | 3.40 | 27.7 | 3.80 | |
| L/D–LS | 7.6 | 0.69 | 32.6 | 0.71 | 20.1 | 0.20 | 30.2 | 3.19 | 32.3 | 3.55 | 35.4 | 4.07 |
No germinations were observed at constant temperature for Euphorbia amygdaloides subsp. arbuscula.
Abbreviations: D, complete darkness; L/D, light/darkness (12/12 hr photoperiod); LS, long storage; SS, short storage.
Seed germination in response to temperature was well described by the thermal‐time model at suboptimal temperatures. Thermal times (°Cd) calculated for different germination percentiles (Θ10, Θ30, and Θ50) are shown in Table 3. Values for Θ50 ranged between 20 and 100°Cd. The extremes were the E. myrsinites, which had a high T b (ca. 13.0°C) and low thermal time (ca. 21 degree days), and E. characias which had a low estimated T b (ca. 0.5°C) and a high thermal time (100 degree days).
4. DISCUSSION
Our results show that, in absence of other limiting factors (e.g., water), germination capability of the eight Euphorbia species was significantly influenced by temperature regimes, light conditions, and storage times, although the effects were species‐dependent.
In more detail, for all these species, except E. amygdaloides subsp. arbuscula, results show that the final germinated proportions were improved by a long storage period (>150 days). This finding supports the idea that most of these species are characterized by primary dormancy, which is released following an after ripening period (nondeep Physiological Dormancy; PD). Narbona, Arista, and Ortiz (2007b) have documented that also the seeds of E. boetica are affected by nondeep physiological dormancy. This might be regarded as an adaptation mechanism, by which germination is prevented immediately after seed dispersal, in May–June, when the season is becoming unfavorable for seedling establishment, and postponed to the beginning of the rainy season (García‐Fayos, García‐Ventoso, & Cerda, 2000; Quilichini & Debussche, 2000).
We tried to classify the abovementioned dormancy, according to Soltani, Baskin, and Baskin (2017). In this respect, it is possible to note that storage time did not only influence germination capability, and it also increased ceiling temperatures for E. bivonae, E. ceratocarpa, E. characias, E. dendroides, E. melapetala, and E. rigida. Accordingly, these species should be classified in the Type 1 nondeep PD category. On the contrary, storage time did not affect ceiling temperature in E. myrsinites, at least for germination in complete darkness. This behavior might support the idea that this species should be included in the Type 5 nondeep PD category (Soltani et al., 2017).
Once primary dormancy has been released, in the absence of other limiting factors (e.g., water), seed germination of the eight Euphorbia species seemed to be mainly driven by temperature. The highest germination capabilities and speeds were reached at optimal temperatures levels, ranging from 14.3 to 21.3°C. Base temperatures ranged from 5.6 to 12.1°C, while ceiling temperatures ranged from 25.6 to 34.7°C. In Mediterranean regions, under present climatic conditions, these results support a germination peak from early autumn to early winter, while mid‐winter germination is prevented by temperatures below the base level requirements. Early spring germination is also possible, although germinations may progressively become more difficult as the season progresses, because of water shortage (Fenner & Thompson, 2005). In summer, high temperatures (above the T c level) may prevent germinations, until the unfavorable conditions are overcome. These results confirm that cardinal temperatures for germination represent a mechanism of adaptation, by which a given species can match its germination timing to favorable conditions for seedling recruitment (Huang, Liu, Bradford, Huxman, & Venable, 2016; Qiu, Bai, BiFu, & Wilmshurst, 2010; Thompson, 1970). Furthermore, the evident reduction in the germinative performance at both high (≥30°C) and low (5°C) constant temperatures may also dictate the altitudinal and latitudinal limits for the geographical distribution of these species.
During the unfavorable periods (winter and summer), the germination of these eight Euphorbia species may be obstacled either by thermo‐inhibition or by thermo‐dormancy. Our results show that seeds of E. bivonae, E. characias, and E. dendroides were thermo‐inhibited at high temperatures (Horowitz & Taylorson, 1983), while seeds of E. amygdaloides subsp. arbuscula, E. ceratocarpa, and E. rigida showed the existence of some mechanisms of thermo‐dormancy. Likewise, low temperatures caused thermo‐inhibition in three of the species under investigation (E. characias, E. dendroides, and E. rigida), while they caused thermo‐dormancy in the other species. Thermo‐inhibition has been documented in many perennial species or winter annual species in warm climates (Thanos et al., 1989; Thompson, 1973; Washitani & Masuda, 1990), including some Euphorbia species (Narbona et al., 2007a). Thermo‐dormancy has been also documented in annual desert plants (Gutterman, 1990; Gutterman, 2002; Thanos et al., 1989).
We also tested the effect of fluctuating temperatures, and we observed that only E. amygdaloides subsp. arbuscula and E. myrsinitis were favoured, in respect to a constant daily temperature regime. For all the other species, fluctuating temperatures induced an improvement of germination only for the highest levels of maximum daily temperatures (>25°C). This is probably due to the fact that, with the 8/25 and 8/30°C regimes, the average daily temperature was, respectively, 16.5 and 19°C, which is close to the optimal temperature level for all species.
The effect of light conditions was smaller than that of temperature. However, the results showed that, at fluctuating temperatures, seeds of E. amygdaloides subsp. arbuscula, E. bivonae, E. ceratocarpa, and E. melapetala were favoured by dark conditions. Likewise, E. characias and E. dendroides were slightly favoured, especially at the warmest temperatures (8/25 and 8/30°C). A good germination capacity in dark conditions was also observed in other Euphorbia species, such as E. heterophylla, E. esula, and E. nicaeensis (Best, Bowes, Thomas, & Maw, 1980; Brecke, 1995; Narbona, Ortiz, & Arista, 2006). It is possible that this phenomenon can be related to their large seeds or processes of seed dispersal. Indeed, many Euphorbia species are diplochorous and they are characterized by a primary explosive dispersal system and a secondary ant dispersal system, aided by the presence of the caruncle. Ants may bury the seeds inside their nest or may abandon them outside, on waste piles. In these conditions, the capacity to germinate in darkness can be advantageous. Some of the examined taxa have been confirmed to be myrmecochores (i.e., E. characias, E. melapetala), while some others may potentially be so.
Considering the abovementioned thermal requirements, the germination of these species may be vulnerable to climate change, which may narrow the favorable germination season and affect the dynamics of plant populations. However, the high habitat heterogeneity of the Mediterranean regions could ensure the future persistence of these plant species at local scale, allowing their migration to more favorable nearby ecological niches. More specifically, the expected reduction of precipitation in summer and winter seasons and substantial temperature increase should be less harmful for plant species of the thermo‐Mediterranean zone (i.e., E. dendroides, E. bivonae), through a slight altitudinal rise of communities and species migration.
In conclusion, most of the eight species of Euphorbia under investigation showed physiological dormancy, which was released following an after ripening period (nondeep Physiological Dormancy; PD). Germination was mainly affected by temperature, with optimal levels ranging from 14 to 21°C. The light regime showed lower effects with respect to temperature. These common traits seem to be rather typical for plant species growing in Mediterranean regions. In agreement with the results of Skordilis and Thanos (1995) relating to seeds of Pinus brutia and P. halepensis, our results support the idea that all the Euphorbia species under investigation exhibit a “Mediterranean germination syndrome,” which may be regarded as an adaptation strategy to typical elements of Mediterranean climates, that is, autumn seasons with mild temperatures associated to the restart of the rainy period. As the consequence, for all these species, germination should mainly occur during the autumn season.
Apart from the above similarities, the eight species also showed several differences, for example, in terms of threshold temperatures. Furthermore, suboptimal or supraoptimal temperature levels gave thermo‐inhibition in some species or thermo‐dormancy in other species. All these differences between species in terms of germination requirements may support their specific adaptation to local ecological factors. Furthermore, they demonstrate that Euphorbia species have neither similar germination traits, nor niche preferences, nor germination strategies, which suggests that seed germination is not likely to be a phylogenetically conserved trait.
Climate change, impacting on ambient temperatures, can strongly influence the dynamics and emergence patterns of Mediterranean Euphorbia species, restricting their favorable germination season.
These aspects are to be taken in account when defining the best measures for species conservation, either in situ or ex situ, particularly when plants must be propagated for vegetation restoration as well as for low‐maintenance Mediterranean gardens (i.e., E. characias, E. dendroides, E. myrsinites and E. rigida).
CONFLICT OF INTEREST
The authors declare that they have no conflict of interest.
AUTHOR CONTRIBUTIONS
AC, SC, AM, and AR conceived the ideas and designed methodology; AC, SC, and AR collected the data; AO and AM analyzed the data; AC and AO led the writing of the manuscript. All authors contributed critically to the drafts and gave final approval for publication.
ACKNOWLEDGMENTS
This work was supported by the SiMaSeed Project “Protecting biodiversity in Siciy‐Malta Natura 2000 sites through Seed Banks and population reinforcement.” Programme INTERREG V‐A Italia‐Malta 2014‐2020. Priority Axis III, Specific Objective 3.1, Project code C1‐3.1‐16.
Cristaudo A, Catara S, Mingo A, Restuccia A, Onofri A. Temperature and storage time strongly affect the germination success of perennial Euphorbia species in Mediterranean regions. Ecol Evol. 2019;9:10984–10999. 10.1002/ece3.5535
DATA AVAILABILITY STATEMENT
The main dataset is available at https://doi.org/10.5061/dryad.m1b1k35.
REFERENCES
- Arana, M. V. , Gonzalez‐Polo, M. , Martinez‐Meier, A. , Gallo, L. A. , Benech‐Arnold, R. L. , Sánchez, R. A. , & Batlla, D. (2016). Seed dormancy responses to temperature relate to Nothofagus species distribution and determine temporal patterns of germination across altitudes in Patagonia. New Phytologist, 209, 507–520. 10.1111/nph.13606 [DOI] [PubMed] [Google Scholar]
- Baskin, C. C. , & Baskin, J. M. (2014). Seeds: Ecology, biogeography, and evolution of dormancy and germination (2nd ed.). London, UK: Elsevier Academic Press. [Google Scholar]
- Benvenuti, S. (2014). Wildflower green roofs for urban landscaping, ecological sustainability and biodiversity. Landscape and Urban Planning, 124, 151–161. 10.1016/j.landurbplan.2014.01.004 [DOI] [Google Scholar]
- Best, K. F. , Bowes, G. G. , Thomas, A. G. , & Maw, M. G. (1980). The biology of Canadian weeds. 39. Euphorbia esula L. Canadian Journal of Plant Science, 60, 651–663. 10.4141/cjps80-092 [DOI] [Google Scholar]
- Bewley, J. D. , & Black, M. (1985). Seeds: Physiology of development and germination. New York, NY: Plenum Press. [Google Scholar]
- Bierhuizen, J. F. , & Wagenvoort, W. A. (1974). Some aspects of seed germination in vegetables. 1. The determination and application of heat sums and minimum temperature for germination. Scientia Horticulturae, 2, 213–219. 10.1016/0304-4238(74)90029-6 [DOI] [Google Scholar]
- Bochet, E. , García‐Fayos, P. , Alborch, B. , & Tormo, J. (2007). Soil water availability effects on seed germination account for species segregation in semiarid roadslopes. Plant and Soil, 295, 179–191. 10.1007/s11104-007-9274-9 [DOI] [Google Scholar]
- Boydak, M. , Dirik, H. , Tilki, F. , & Çalikoğlu, M. (2003). Effects of water stress on germination in six provenances of Pinus brutia seeds from different bioclimatic zones in Turkey. Turkish Journal of Agriculture and Forestry, 27, 91–97. [Google Scholar]
- Brecke, B. J. (1995). Wild poinsettia (Euphorbia heterophylla) germination and emergence. Weed Science, 43(1), 103–106. [Google Scholar]
- Carta, C. , Hanson, S. , & Müller, J. V. (2016). Plant regeneration from seeds responds to phylogenetic relatedness and local adaptation in Mediterranean Romula (Iridaceae) species. Ecology and Evolution, 6, 4166–4178. 10.1002/ece3.2150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catara, S. , Cristaudo, A. , Gualtieri, A. , Galesi, R. , Impelluso, C. , & Onofri, A. (2016). Threshold temperatures for seed germination in nine species of Verbascum (Scrophulariaceae). Seed Science Research, 26(1), 30–46. 10.1017/S0960258515000343 [DOI] [Google Scholar]
- Chamorro, D. , Luna, B. , & Moreno, J. M. (2017). Germination responses to current and future temperatures of four seeder shrubs across a latitudinal gradient in western Iberia. American Journal of Botany, 104(1), 83–91. 10.3732/ajb.1600278 [DOI] [PubMed] [Google Scholar]
- Cristaudo, A. , Gresta, F. , Catara, S. , & Mingo, A. (2014). Assessment of daily heat pulse regimes on the germination of six Amaranthus species. Weed Research, 54, 366–376. 10.1111/wre.12085 [DOI] [Google Scholar]
- Cristaudo, A. , Gresta, F. , Restuccia, A. , Catara, S. , & Onofri, A. (2016). Germinative response of redroot pigweed (Amaranthus retroflexus L.) to environmental conditions: Is there a seasonal pattern? Plant Biosystems, 150(3), 583–591. 10.1080/11263504.2014.987845 [DOI] [Google Scholar]
- Fenner, M. , & Thompson, K. (2005). The ecology of seeds. Cambridge, UK: Cambridge University Press. [Google Scholar]
- Franco, J. A. , Martínez‐Sánchez, J. J. , Fernández, J. A. , & Bañón, S. (2005). Selection and nursery production of ornamental plants for landscaping and xerogardening in semi‐arid environments. The Journal of Horticultural Science and Biotechnology, 81(1), 3–17. 10.1080/14620316.2006.11512022 [DOI] [Google Scholar]
- Frodin, D. G. (2004). History and concepts of big plant genera. Taxon, 53, 753–776. 10.2307/4135449 [DOI] [Google Scholar]
- García‐Fayos, P. , García‐Ventoso, B. , & Cerda, A. (2000). Limitations to plant establishment on eroded slopes in southeastern Spain. Journal of Vegetation Science, 11, 77–86. 10.2307/3236778 [DOI] [Google Scholar]
- Geltman, D. V. (2015). Phytogeographical analysis of Euphorbia subgenus Esula (Euphorbiaceae). Polish Botanical Journal, 60(2), 147–161. 10.1515/pbj-2015-0024 [DOI] [Google Scholar]
- Govaerts, R. , Frodin, D. , & Radcliffe‐Smith, A. (2000). World checklist and bibliography of Euphorbiaceae (and Pandaceae) (Vol. 4, p. 1621). Kew, UK: Royal Botanic Gardens. [Google Scholar]
- Gresta, F. , Cristaudo, A. , Onofri, A. , Restuccia, A. , & Avola, G. (2010). Germination response of four pasture species to temperature, light, and post‐harvest period. Plant Biosystems, 144(4), 849–856. 10.1080/11263504.2010.523549 [DOI] [Google Scholar]
- Gutterman, Y. (1990). Do germination mechanisms differ in plants originating in deserts receiving winter or summer rain? Israel Journal of Botany, 39, 355–372. [Google Scholar]
- Gutterman, Y. (2002). Survival strategies of annuals desert plants. Berlin, Germany: Springer. [Google Scholar]
- Horowitz, M. , & Taylorson, R. B. (1983). Effect of high temperatures on imbibition, germination, and thermal death of velvetleaf (Abutilon theophrasti) seeds. Canadian Journal of Botany, 61, 2269–2276. [Google Scholar]
- Huang, Z. , Liu, S. , Bradford, K. J. , Huxman, T. E. , & Venable, D. L. (2016). The contribution of germination functional traits to population dynamics of a desert plant community. Ecology, 97, 250–261. 10.1890/15-0744.1 [DOI] [PubMed] [Google Scholar]
- Ibanez, A. N. , & Passera, C. B. (1997). Factors affecting the germination of albaida (Anthyllis cytisoides L.), a forage legume of the Mediterranean coast. Journal of Arid Environments, 35, 225–231. [Google Scholar]
- La Mantia, T. , Messana, G. , Billeci, V. , Dimarca, A. , Del Signore, M. B. , Leanza, M. , … Pasta, S. (2012). Combining bioengineering and plant conservation on a Mediterranean islet. iForest ‐ Biogeosciences and Forestry, 5, 296–305. 10.3832/ifor0638-005 [DOI] [Google Scholar]
- Lavorel, S. , Debussche, M. , Lebreton, J. D. , & Lepart, J. (1993). Seasonal patterns in the seed bank of Mediterranean old‐fields. Oikos, 67, 114–128. 10.2307/3545102 [DOI] [Google Scholar]
- Maher, J. , Gerasopoulos, D. , & Maloupa, E. (2000). Temperature and light effects on germination of Lavandula stoechas seeds. Acta Horticulturae, 541, 261–264. 10.17660/ActaHortic.2000.541.38 [DOI] [Google Scholar]
- Masin, R. , Onofri, A. , Gasparini, V. , & Zanin, G. (2017). Can alternating temperatures be used to estimate base temperature for seed germination? Weed Research, 57, 390–398. 10.1111/wre.12270 [DOI] [Google Scholar]
- Mesgaran, M. B. , Onofri, A. , Mashhadi, H. R. , & Cousens, R. D. (2017). Water availability shifts the optimal temperatures for seed germination: A modelling approach. Ecological Modelling, 351, 87–95. 10.1016/j.ecolmodel.2017.02.020 [DOI] [Google Scholar]
- Mondoni, A. , Rossi, G. , Orsenigo, S. , & Probert, R. J. (2012). Climate warming could shift the timing of seed germination in alpine plants. Annals of Botany, 110, 155–164. 10.1093/aob/mcs097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narbona, E. , Arista, M. , & Ortiz, P. L. (2007a). High temperature and burial inhibit seed germination of two perennial Mediterranean Euphorbia species. Botanica Helvetica, 117, 169–180. 10.1007/s00035-007-0816-9 [DOI] [Google Scholar]
- Narbona, E. , Arista, M. , & Ortiz, P. L. (2007b). Seed germination ecology of the perennial Euphorbia boetica, an endemic spurge of the southern Iberian Peninsula. Annales Botanici Fennici, 44, 276–282. [Google Scholar]
- Narbona, E. , Ortiz, P. L. , & Arista, M. (2006). Germination variability and the effect of various pre‐treatment on germination in the perennial spurge Euphorbia nicaeensis All. Flora, 201, 633–641. 10.1016/j.flora.2006.02.004 [DOI] [Google Scholar]
- Onofri, A. , Benincasa, P. , Mesgaran, M. B. , & Ritz, C. (2018). Hydrothermal‐time‐to‐event models for seed germination. European Journal of Agronomy, 101, 129–139. 10.1016/j.eja.2018.08.011 [DOI] [Google Scholar]
- Pahlevani, A. , Geltman, D. V. , & Riina, R. (2011). Taxonomic revision of Euphorbia subsect. Myrsiniteae in Iran. Annales Botanici Fennici, 48, 483–493. [Google Scholar]
- Pérez‐Fernández, M. A. , Calvo‐Magro, E. , & Ferrer‐Castán, D. (2006). Simulation of germination of pioneer species along an experimental drought gradient. Journal of Environmental Biology, 27, 679–685. [PubMed] [Google Scholar]
- Poschlod, P. , Abedi, M. , Bartelheimer, M. , Drobnik, J. , Rosbakh, S. , & Saatkamp, A. (2013). Seed ecology and assembly rules in plant communities In van der Maarel E. & Franklin J. (Eds.), Vegetation ecology (pp. 164–202). Chichester, UK: John Wiley & Sons. [Google Scholar]
- Qiu, J. , Bai, Y. , BiFu, Y. , & Wilmshurst, J. F. (2010). Spatial variation in temperature thresholds during seed germination of remnant Festuca hallii populations across the Canadian prairie. Environmental and Experimental Botany, 67, 479–486. 10.1016/j.envexpbot.2009.09.002 [DOI] [Google Scholar]
- Quilichini, A. , & Debussche, M. (2000). Seed dispersal and germination patterns in a rare Mediterranean island endemic (Anchusa crispa Viv., Boraginaceae). Acta Oecologica, 21, 303–313. 10.1016/S1146-609X(00)01089-4 [DOI] [Google Scholar]
- Rahman, A. H. M. M. , & Akter, M. (2013). Taxonomy and medicinal uses of Euphorbiaceae (Spurge) family of Rajshahi, Bangladesh. Research in Plant Sciences, 1(3), 74–80. 10.12691/plant-1-3-5 [DOI] [Google Scholar]
- Riina, R. , & Berry, P. E. (coordinators) (2017). Euphorbia planetary biodiversity inventory database. Tolkin; Retrieved from http://app.tolkin.org/projects/72/taxa [Google Scholar]
- Riina, R. , Peirson, J. A. , Geltman, D. V. , Molero, J. , Frajman, B. , Pahlevani, A. , … Berry, P. E. (2013). A worldwide molecular phylogeny and classification of the leafy spurges, Euphorbia subgenus Esula (Euphorbiaceae). Taxon, 62, 316–342. 10.12705/622.1 [DOI] [Google Scholar]
- Ritz, C. , Pipper, C. B. , & Streibig, J. C. (2013). Analysis of germination data from agricultural experiments. European Journal of Agronomy, 45, 1–6. 10.1016/j.eja.2012.10.003 [DOI] [Google Scholar]
- Sari, D. , & Karaşah, B. (2015). Green roofs and xeriscape planting that contribute to sustainable urban green space Conference: ICSAUD 2015: 17th International Conference on Sustainable Architecture and Urban Design. Japan, Kyoto, 17(11), 962–966. [Google Scholar]
- Skordilis, A. , & Thanos, C. A. (1995). Seed stratification and germination strategy in the Mediterranean pines Pinus brutia and P. halepensis . Seed Science Research, 5, 151–160. 10.1017/S0960258500002774 [DOI] [Google Scholar]
- Soltani, E. , Baskin, C. C. , & Baskin, J. M. (2017). A graphical method for identifying the six types of non‐deep physiological dormancy in seeds. Plant Biology, 19(5), 673–682. 10.1111/plb.12590 [DOI] [PubMed] [Google Scholar]
- Thanos, C. A. , Georghiou, K. , & Skarou, F. (1989). Glacium flavum seed germination ‐ An ecophysiological approach. Annals of Botany, 63, 121–130. 10.1111/plb.12590 [DOI] [Google Scholar]
- Thanos, C. A. , & Skordillis, A. (1987). The effects of light, temperature and osmotic stress on the germination of Pinus halepensis and P. brutia seeds. Seed Science and Technology, 15, 163–174. [Google Scholar]
- Thompson, P. A. (1970). Germination of species of Caryophyllaceae in relation to their geographical distribution in Europe. Annals of Botany, 34, 427–449. 10.1093/oxfordjournals.aob.a084381 [DOI] [Google Scholar]
- Thompson, P. A. (1973). Geographical adaptation of seed In Heydecker W. (Ed.), Seed ecology (pp. 31–58). London, UK: Butterworths. [Google Scholar]
- Vandelook, F. , Van de Moer, D. , & Van Assche, J. A. (2008). Environmental signals for seed germination reflect habitat adaptations in four temperate Caryophyllaceae. Functional Ecology, 22, 470–478. 10.1111/j.1365-2435.2008.01385.x [DOI] [Google Scholar]
- Vidaver, W. , & Hsiao, A. I. (1975). Secondary dormancy in light‐sensitive lettuce seeds incubated anaerobically or at elevated temperature. Canadian Journal of Botany, 53, 2557–2560. 10.1139/b75-281 [DOI] [Google Scholar]
- Walck, J. L. , Hidayati, S. N. , Dixon, K. W. , Thompson, K. E. N. , & Poschlod, P. (2011). Climate change and plant regeneration from seed. Global Change Biology, 17, 2145–2161. 10.1111/j.1365-2486.2010.02368.x [DOI] [Google Scholar]
- Wang, Z. , Wang, L. , Liu, Z. , Li, Y. , Liu, Q. , & Liu, B. (2016). Phylogeny, seed trait, ecological correlates of seed germination at the community level in a degraded sandy grassland. Frontiers in Plant Science, 7, 1532 10.3389/fpls.2016.01532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Washitani, I. , & Masuda, M. (1990). A comparative study of the germination characteristics of seeds of a moist tall grassland community. Functional Ecology, 4, 543–557. 10.2307/2389322 [DOI] [Google Scholar]
- Willis, C. G. , Baskin, C. C. , Baskin, J. M. , Auld, J. R. , Venable, D. L. , Cavender‐Bares, J. , … Rubio de Casas, R. (2014). The evolution of seed dormancy: Environmental cues, evolutionary hubs, and diversification of the seed plants. New Phytologist, 203, 300–309. 10.1111/nph.12782 [DOI] [PubMed] [Google Scholar]
- Zhang, R. , Baskin, J. M. , Baskin, C. C. , Mo, Q. , Chen, L. , Hu, X. , & Wang, Y. (2017). Effect of population, collection year, after‐ripening and incubation condition on seed germination of Stipa bungeana . Scientific Reports, 7(13893), 1–11. 10.1038/s41598-017-14267-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, S. T. , Du, G. Z. , & Chen, J. K. (2004). Seed size in relation to phylogeny, growth form and longevity in a subalpine meadow on the east of the Tibetan Plateau. Folia Geobotanica, 39, 129–142. 10.1007/BF02805242 [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The main dataset is available at https://doi.org/10.5061/dryad.m1b1k35.


