Abstract
The study demonstrates a sustainable process for production of bio-crude oil via hydrothermal liquefaction of microbial biomass generated through co-cultivation of microalgae and bacteria coupled with wastewater remediation. Biomass concentration and wastewater treatment efficiency of a tertiary consortium (two microalgae and two bacteria) was evaluated on four different wastewater samples. Total biomass concentration, total nitrogen and COD removal efficiency was found to be 3.17 g L−1, 99.95% and 95.16% respectively when consortium was grown using paper industry wastewater in a photobioreactor under batch mode. Biomass concentration was enhanced to 4.1 g L−1 through intermittent feeding of nitrogen source and phosphate. GC-MS and FTIR analysis of bio-crude oil indicates abundance of the hydrocarbon fraction and in turn, better oil quality. Maximum distillate fraction of 30.62% lies within the boiling point range of 200–300 °C depicting suitability of the bio-crude oil for conversion into diesel oil, jet fuel and fuel for stoves.
Subject terms: Biotechnology, Bioremediation
Introduction
Unfettered exploitation of the fossil fuels integrated with unimpeded population growth and brisk industrialization are the root causes of the escalating world energy crisis and intensifying global warming by and large. Global disquietude for energy security and environmental sustainability has propelled the scientific community to contemplate conceivable energy resources as prospective substitutes. Presently, several countries across the globe are capitalizing on biomass-, wastes-, solar-, wind-, hydro- and geothermal energy sources, with net-zero carbon emissions, as futuristic alternatives to fossil based fuels. Microalgal biofuel has been gaining impetus owing to their several advantages such as high photosynthetic efficiency coupled with sequestration of carbon dioxide, high growth rate, momentous capability to accumulate lipids/carbohydrates and significant tolerance towards manifold cultivation conditions. An auxiliary characteristic is the wide paradigm of value-added bio-products which can be obtained from these organisms, besides the fuel counterpart. Though the concept of biofuel production using microalgal feedstock is gaining ground, yet certain challenges pose a major hindrance towards commercialization of this scientific know-how. The high production cost of microalgal biomass using fresh water coupled with energy and cost intensive downstream processing constitute the dominant threat towards the economic feasibility of this technology.
A realistic solution to the techno-economic infeasibility of microalgal biofuel technology can be the concept of co-cultivation of microalgae and bacteria in wastewater as an integrated system. Several studies have been reported in the literatures on production of biofuels using mixed-culture algal biomass grown in wastewater1,2. However, in recent years microalgae-bacteria consortia have been gaining impetus in generation of biomass feedstock coupled with wastewater treatment. This may be attributed to various advantages which microalgae-bacteria consortia might offer over mixed-culture algal system: (i) ability to function under fluctuating environmental conditions and nutrient loading owing to their diverse metabolic activities and adaptation ability to different growth conditions; (ii) microalgae and bacteria can form micro-ecosystem where they can positively influence the growth of each other in different ways3; and (iii) certain bacteria can facilitate the harvesting ability of algal cells4. There are several studies which have reported the enhanced growth of microalgae when co-cultured with bacteria5,6. Wastewater is a convenient source of nitrogen, phosphorous and micronutrients, essential for the growth of microalgae, besides being a reasonable replacement of fresh water. Microalgae have been reported to possess noteworthy potential to utilize inorganic nutrients, copious in wastewater, to produce biofuels, fertilizers or other bio products supplementing the wastewater treatment7,8. Microalgae cultivation in wastewater therefore offers a two-fold advantage: (i) minimizes the cost of biomass production and (ii) aids in wastewater remediation. An accretion in nutrient removal efficiency from wastewater was reported when microalgae were grown in consortium with bacteria9,10. This improved biomass concentration and nutrient removal efficiency may be attributed to the concerted interplay between microalgae and bacteria within their micro-ecosystem through various means of interaction e.g., exchange of gases or growth promoting factors, reduction of photosynthetic oxygen tension, bacterial decomposition of dissolved organic nitrogen etc.6,8,11–13.
High cost of downstream processing is another prime drawback towards sustainable biofuel production using microalgal biomass feedstock. Transesterification, the traditional method for producing biodiesel from lipid rich microalgal biomass is energy intensive as it requires biomass drying; expensive; time consuming; and employs hazardous organic solvents. Moreover, post transesterification the residual major fraction of the biomass, comprising of protein and carbohydrate, remain unutilized and hence, the overall process efficiency is compromised. In order to address the existing challenges as imposed by the traditional transesterification method, researchers are now focusing on hydrothermal liquefaction (HTL) of biomass into biofuel. The key advantage of HTL includes direct conversion of wet algal biomass (~80% water content, w/w) into bio-crude oil and hence, bypassing the need for drying. Unlike transesterification, where only lipid fraction of the microalgal biomass is converted into biofuel, HTL facilitates conversion of the entire fraction of biomass (lipids, proteins and carbohydrates) into bio-crude oil14. Further, microbial consortium including bacteria, wastewater sludge and fast growing algae with low lipid content can successfully be converted into biofuel via HTL15.
The present study demonstrates a sustainable process for production of biofuel using microbial biomass as potential feedstock. Sustainability was aimed to be achieved through a combinatorial approach of: (i) cultivation of a microbial consortium comprising of two microalgae and two bacteria; (ii) utilization of wastewater as a cheaper source of nutrients and replacement of fresh water; (iii) improving biomass productivity through intermittent feeding of limiting nutrients and mutualistic growth of the microbes; (iv) direct conversion of wet biomass into bio-crude oil and (v) biomass generation coupled with wastewater treatment. In an earlier study, a tertiary consortium comprising of two microalgae and two bacteria was shown to exhibit superior performance in terms of biomass concentration and nutrient removal efficiency from the artificial wastewater and raw dairy wastewater16. In the first step, with the aim of establishing the feasibility of application at industrial scale, performance of this tertiary consortium in terms of biomass concentration and wastewater treatment efficiency was evaluated on four different types of wastewater: paper industry wastewater (PWW), textile industry wastewater (TWW), leather industry wastewater (LWW) and municipal wastewater (MWW). Based on the microbial biomass concentration and nutrient removal efficiency obtained in different wastewaters, PWW was selected for subsequent experimentation. In the next step, with the objective of maximization of microbial biomass concentration, a process engineering strategy was implemented in an automated photobioreactor involving intermittent feeding of the limiting nutrients under fed-batch mode of cultivation of the consortium on PWW. The wet microbial biomass thus obtained from fed-batch cultivation was further subjected to HTL for direct conversion into bio-crude oil. Extensive characterization of the bio-crude oil was carried out to assess its potential as an alternative to conventional fossil fuels.
Results and Discussion
Characterization of tertiary consortium on different wastewater samples
With the aim of assessing its potential to be exploited at commercial scale, the tertiary consortium has been characterized in terms of its growth and wastewater treatment efficiency on different wastewater samples. Four different types of wastewater were considered in the present study: PWW, TWW, LWW and MWW. These wastewaters differed in terms of concentration of the nutrients and pH. Major nutrients such as total nitrogen, phosphate and COD content were found to be abundant in case of PWW and MWW (Table 1). However, these nutrients were present in lesser amount in case of TWW and LWW. All the wastewater samples contained significant amount of heavy metals, chromium and nickel, with the exception of MWW (Table 1).
Table 1.
Analysis of wastewater samples.
| Wastewater Type |
Parameters | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| pH | Total Nitrogen (mg L−1) |
NO3−-N (mg L−1) |
NO2−-N (mg L−1) |
NH4+-N (mg L−1) |
Phosphate (mg L−1) |
COD (mg L−1) |
Chromium (mg L−1) |
Nickel (mg L−1) |
|
| Paper Industry | 6.29 ± 0.09 | 490 ± 0.02 | 187 ± 0.02 | 0 | 303 ± 0.02 | 77.70 ± 0.073 | 5250.0 ± 0.27 | 0.98 ± 0.02 | 0.53 ± 0.04 |
| Textile Industry | 7.74 ± 0.01 | 230 ± 0.07 | 110 ± 0.04 | 0.29 ± 0.14 | 118.71 ± 0.03 | 53.16 ± 0.012 | 735.00 ± 0.31 | 0.27 ± 0.02 | 0.43 ± 0.07 |
| Leather Industry | 7.53 ± 0.04 | 250 ± 0.04 | 162 ± 0.03 | 0.1 ± 0.03 | 89.9 ± 0.06 | 48.30 ± 0.061 | 400.50 ± 0.14 | 0.69 ± 0.06 | 1.01 ± 0.03 |
|
Municipal Wastewater |
7.42 ± 0.04 | 540 ± 0.02 | 112 ± 0.01 | 0 | 428 ± 0.03 | 69.20 ± 0.049 | 1650.00 ± 0.25 | *ND | *ND |
*ND: Not Detected.
Figure 1 elucidates the comparative performance of the tertiary consortium in different wastewater samples with respect to growth and removal efficiency (%) of total nitrogen, phosphate and COD. Maximum total microbial biomass concentration of 2.97 g L−1 was achieved when tertiary consortium was grown in PWW, followed by 2.14 g L−1 in MWW. Growth performance of the consortium was inferior in both LWW and TWW (Fig. 1A). Further, an improved microalgal growth was observed in case of PWW and MWW with a final chlorophyll-a concentration of 31.79 µg mL−1 (10.7 µg mg biomass−1) and 21.87 µg mL−1 (10.22 µg mg biomass−1) respectively (see Supplementary Fig. S1). In spite of the wide variation in the initial concentrations of total nitrogen, phosphate and COD, these nutrients were exhausted within similar cultivation period for all wastewater samples (see Supplementary Fig. S1), depicting higher utilization rates of these nutrients by the microbes when grown on PWW and MWW and in turn higher growth rate of the microbes. A biomass concentration of 826 mg L−1 was reported when C. vulgaris was co-cultivated with indigenous bacteria in municipal wastewater17. Das et al.18 has reported a chlorophyll-a concentration of 12.5 µg mL−1 when a marine species of Chlorella was grown in consortium with Phormidium sp. in tannery wastewater. Better growth performance of the consortium in PWW and MWW may be attributed to higher amount of inorganic nutrients (total nitrogen and phosphate) primarily supporting growth of the microalgae19,20 and higher amount of COD primarily favoring growth of the bacteria21 in comparison to TWW and LWW. Microalgae assimilates inorganic nitrogen sources into organic macromolecules and genetic materials and hence, critically essential for their growth22. Phosphorous also play crucial role in microalgal energy metabolism via synthesis of nucleic acids, lipids, proteins and the intermediates of carbohydrate23. In a microalgae-bacteria consortium, bacterial strains remove COD through aerobic biological treatment involving oxidative degradation of the organic compounds for both energy and carbon usages24.
Figure 1.
(A) Total biomass concentration (DCW, g L−1), (B) total nitrogen removal efficiency (%), (C) phosphate removal efficiency (%) and (D) COD removal efficiency (%) of the tertiary consortium grown on paper industry wastewater (PWW), textile industry wastewater (TWW), leather industry wastewater (LWW) and municipal wastewater (MWW). The asterisk sign represents the significant difference between the biomass concentration or nitrate removal efficiency or phosphate removal efficiency or COD removal efficiency obtained for different wastewater samples analyzed using one-way analysis of variance based on Tukey’s method. Biomass concentration or nitrate removal efficiency or phosphate removal efficiency or COD removal efficiency that do not share a common symbol are significantly different.
This improved growth performance of the consortium in PWW and MWW correlated well with the removal efficiency of the key nutrients viz., total nitrogen, phosphate and COD. The highest nitrogen removal efficiency of 89.3% was observed in case of PWW followed by MWW (78.48%) and TWW (70.99%) (Fig. 1B). The nitrogen removal efficiency was the lowest at a value of 55.66% when grown on LWW. Phosphate was completely exhausted for all wastewater samples as seen in Fig. 1C. While, COD removal efficiency was maximum at a considerably higher value of 94.23% in case of PWW, the same was merely in the range of 52–62% for rest of the wastewater samples (Fig. 1D). Total nitrogen and phosphate removal efficiency of 95.9% and 94.4% respectively was achieved from cultivation of C. vulgaris with indigenous bacteria in municipal wastewater17. An algae-bacteria combined system of C. vulgaris and Bacillus licheniformis when grown in municipal wastewater resulted in removal of ammoniacal nitrogen and phosphate with an efficiency of 78% and 92% respectively25. Bioremediation of tannery wastewater by a consortium of marine Chlorella sp. and Phormidium sp. resulted in 91.16% nitrogen and 88.88% phosphate removal18. COD removal efficiency of ~91% was achieved when microalgae-bacteria consortia was cultivated in wastewater from potato processing plant21, dairy industry26 and municipality27. While, a commendable removal efficiency of both the heavy metals (61.95% for Ni and 45.71% for Cr) was observed in PWW, the same was lower in LWW (Table 2). However, an effective Cr removal (56.38%) was observed in case of TWW. Das et al.18 has reported 94.45% removal of Cr from tannery wastewater employing a consortium of Chlorella sp. and Phormidium sp. Based on the maximum total biomass concentration and nutrient removal efficiency, PWW was selected for subsequent characterization and process development in the bioreactor. A detailed material balance for total nitrogen, phosphate and COD has been shown in Supplementary Table S1.
Table 2.
Heavy metal removal efficiency (%) of tertiary consortium grown in different types of wastewater.
| Wastewater Type | Nickel (Ni) | Chromium (Cr) |
|---|---|---|
| Shake flask experiments | ||
| Paper Industry | 61.95 ± 0.07 | 45.71 ± 0.09 |
| Textile Industry | 30.00 ± 0.04 | 56.38 ± 0.02 |
| Leather Industry | 35.50 ± 0.05 | 22.07 ± 0.04 |
| Municipal Wastewater | *ND | *ND |
| Bioreactor | ||
| Paper Industry | 74.54 ± 0.04 | 80.00 ± 0.07 |
*ND: Not Detected.
All the experiments were performed in duplicate and the data were expressed as mean ± standard error.
Batch and fed-batch mode of cultivation of tertiary consortium in the bioreactor utilizing paper industry wastewater
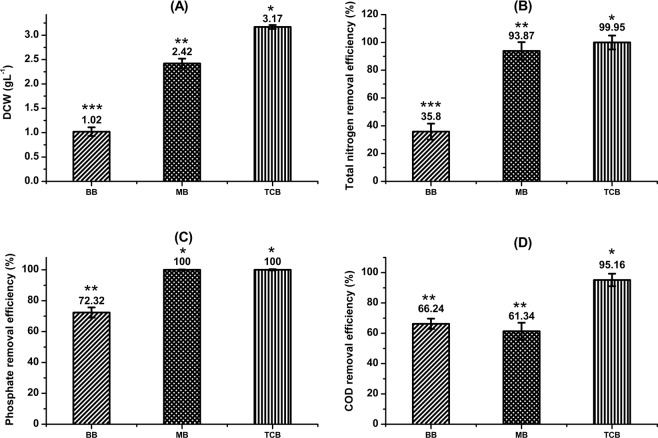
In order to evaluate the performance of the tertiary consortium in PWW at a larger scale of operation, characterization was carried out in 7.5 L automated bioreactor with a working volume of 4 L. A condescending performance in terms of growth and nutrient removal efficiency was exhibited by the tertiary consortium (TCB) in comparison with bacteria (BB) and microalgae (MB) when cultivated individually in batch mode of operation (Fig. 2). For instance, an increment in total biomass concentration of 211% and 31% was achieved for the TCB (3.17 g L−1) compared to BB and MB respectively (Fig. 2A). For TCB, the final chlorophyll-a concentration was evaluated to be 32.50 µg mL−1 which was 1.2 folds higher than the chlorophyll-a concentration obtained (27.30 µg mL−1) in the MB. However, the normalized chlorophyll-a concentration was evaluated to be 10.25 µg mg biomass−1 and 11.28 µg mg biomass−1 for TCB and MB respectively. Cho et al.28 reported a biomass concentration of 3.31 g L−1 when C. vulgaris was co-cultivated with four different growth enhancing bacteria. However, removal efficiency for total nitrogen was found to be analogous for both TCB (99.95%) and MB (93.87%), albeit a reduction by 2.7 folds in case of BB (35.8%) (Fig. 2B). Similar trend was observed in case of phosphate removal. While, phosphate was completely exhausted for both TCB and MB, 72.32% removal was observed in case of BB (Fig. 2C). Interestingly, an uppermost COD removal by 95.16% was recorded for TCB, significantly higher than both MB and BB (Fig. 2D). The total nitrogen, phosphate and COD removal efficiency of TCB, achieved in the present study, was found to be better than majority of literatures reported till date. For instance, phosphate and nitrogen removal efficiency of 47% and 62% respectively was reported for co-cultivation of immobilized C. sorokiniana and Azospirillum brasilense on unsterile municipal wastewater29. Phosphate, total nitrogen and COD removal efficiency of 72.8%, 71% and 46% respectively was achieved for combined growth of C. sorokiniana and Pseudomonas H4 in wastewater30. However, a complete removal of both nitrogen and phosphate along with 97% removal of COD was observed on co-cultivation of C. vulgaris FACHB-30 and P. putida in municipal wastewater31. Removal efficiency of Ni and Cr was observed to be significantly enhanced to 74.54% and 80% respectively as compared to growth in shake flasks utilizing PWW (Table 2).
Figure 2.
(A) Total biomass concentration (DCW, g L−1), (B) total nitrogen removal efficiency (%), (C) phosphate removal efficiency (%) and (D) COD removal efficiency (%) of the tertiary consortium grown on paper industry wastewater in photobioreactor under batch mode of cultivation. BB represents bacterial batch involving two bacteria; MB represents microalgal batch involving two microalgae and TCB represents tertiary consortium batch involving two microalgae and two bacteria. The asterisk sign represents the significant difference between the biomass concentration or nitrate removal efficiency or phosphate removal efficiency or COD removal efficiency obtained for BB, MB and TCB analyzed using one-way analysis of variance based on Tukey’s method. Biomass concentration or nitrate removal efficiency or phosphate removal efficiency or COD removal efficiency that do not share a common symbol are significantly different.
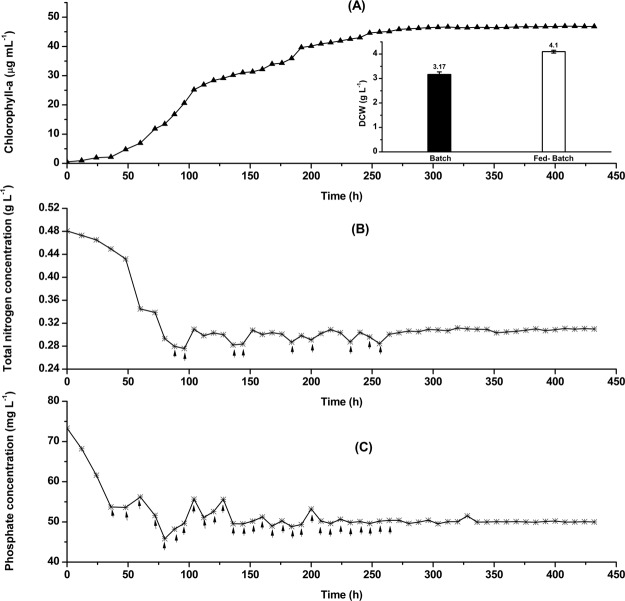
With the aim of improving the biomass concentration, cultivation of tertiary consortium was conducted through intermittent feeding of nitrogen source and phosphate in order to maintain their respective optimal concentrations throughout the entire course of fermentation. As observed from the figure (Fig. 3B,C), concentration of total nitrogen and phosphate in the culture broth was maintained at approximately 0.29 g L−1 and 49.5 mg L−1 respectively. Total biomass concentration was evaluated to be 4.1 g L−1, an increment by 29.3% when compared to the batch mode of cultivation (Fig. 3A). A total biomass concentration in the range of 2.23–3.15 g L−1 was obtained when cyanobacterium Arthrospira platensis and microalga C. vulgaris were cultivated in fed-batch mode in wastewater derived from the anaerobic digestion of poultry litter depending on different concentrations of ammoniacal nitrogen32. The maximum concentration of chlorophyll-a was observed to be 46.92 µg mL−1 (11.44 µg mg biomass−1) when the tertiary consortium was cultivated in PWW in fed-batch mode of operation. This chlorophyll-a concentration was found to be 1.44 times higher than that obtained in batch mode.
Figure 3.
Dynamic profiles for (A) growth of microalgae (Chlorophyll-a, µg mL−1); (B) total nitrogen (g L−1) and (C) phosphate (mg L−1). The tertiary consortium was grown on paper industry wastewater in a photobioreactor under fed-batch mode with intermittent feeding of nitrogen source and phosphate. The graph in the inset of panel A compares total biomass concentration obtained in batch and fed-batch cultivation.
Symbiotic growth of microalgae and bacteria
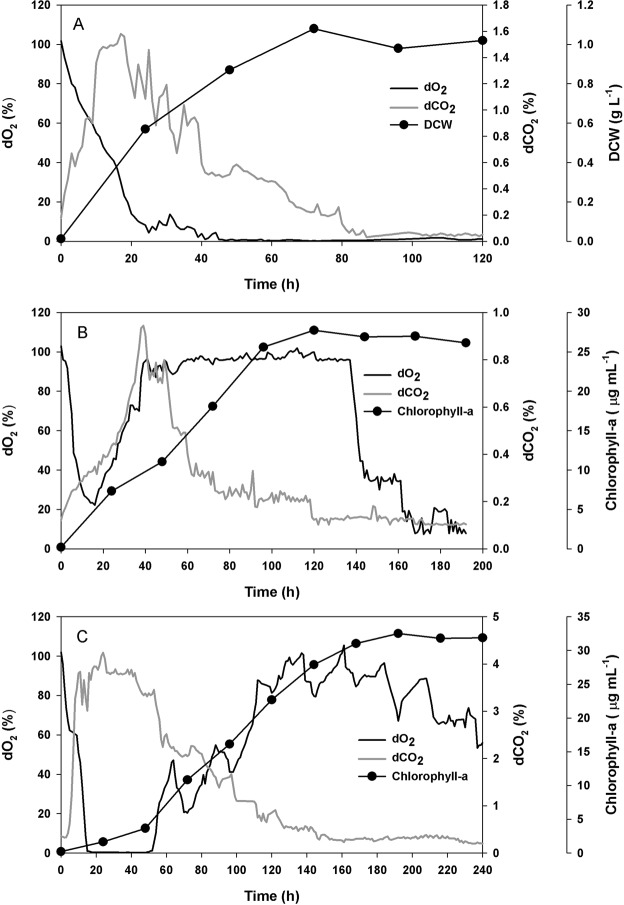
In the present study, an enhancement in total biomass concentration and in turn, nutrient removal efficiency was observed when a consortium of two microalgae and two bacteria were grown together (TCB) as compared to growth of two bacteria (BB) or two microalgae (MB) alone. The possible reasons for this improved biomass concentration and nutrient removal efficiency may be attributed to various mutualistic interactions between microalgae and bacteria e.g., symbiotic exchange of CO2 and O213 and supply of micronutrients and growth promoting factors from bacteria28,33,34. An inhibition in the growth of the bacteria in BB was expected post 40 h of cultivation as the dO2 level dipped significantly below saturation value and in turn, resulted in limitation to the oxygen supply (Fig. 4A). However, in this batch the peak dCO2 concentration was recorded to be ~1.5% during exponential phase of the bacterial growth (Fig. 4A). Contrary to the BB, in the MB an initial drop in the dO2 level was marked till 20 h followed by a sharp increase and its maintenance close to the saturation value till ~140 h (Fig. 4B). While the initial drop in the dO2 level may be due to the consumption of oxygen by the native bacteria present in the wastewater, increase in the dO2 level can be attributed to the initiation of exponential growth of the microalgae after 20 h which continued till 120 h (Fig. 4B). A concomitant drop in dO2 level was observed with the attainment of stationary phase of microalgal growth. Nevertheless, in this batch, rate of CO2 supply appeared to be the rate controlling step as evident from lower dCO2 level (maximum of <1%) as compared to the BB. Interestingly, in case of TCB while dO2 level above 40% was maintained for entire cultivation period except the initial hours where a sudden decrease in the dO2 level was due to combined growth of the native bacteria present in the wastewater and the bacteria present in the consortium (Fig. 4C). The maintenance of dO2 level above 40% post 50 h of cultivation corroborated well with the growth of microalgae contributing to continuous release of oxygen in the culture broth (Fig. 4C). A higher dissolved carbon dioxide tension in the range of 1–4% was maintained in TCB for a prolonged cultivation period of 120 h, which might be attributed to the sustained growth of the bacteria in the consortium (Fig. 4C). While the bacteria consumed oxygen produced by microalgae during photosynthesis as an electron acceptor to degrade organic matter (COD) present in the wastewater, the microalgae fixed CO2 released as by-product of bacterial oxidative degradation. This mutualistic interaction between microalgae and bacteria in terms of exchange of CO2 and O2 promoted the individual growth of these organisms present in the tertiary consortium, which resulted in improved total biomass concentration and better nutrient removal efficiency. In a previous study, glucose removal efficiency was reported to increase from 50% to 100% in batch mode of operation and 73% to 100% in continuous mode of operation of the bioreactor when immobilized microalgae C. vulgaris was co-cultivated with P. putida13. This improvement in glucose utilization was attributed to a symbiotic CO2/O2 gas exchange between these two microbes. Further, an exponential growth of the microalgae was observed during the symbiosis when compared to unaerated condition13. However, in the present study existence of any other possible interaction may not be ruled out for growth upliftment.
Figure 4.
Dynamic profiles for: (A) dO2 (%), dCO2 (%) & growth of bacteria (DCW, g L−1) in case of bacterial batch (BB); (B) dO2 (%), dCO2 (%) & growth of microalgae (Chlorophyll-a, µg mL−1) in case of microalgal batch (MB); and (C) dO2 (%), dCO2 (%) & growth of microalgae (Chlorophyll-a, µg mL−1) in case of tertiary consortium batch (TCB). The experiment was carried out in a photobioreactor under batch mode of cultivation.
Characterization of bio-crude oil produced via hydrothermal liquefaction of microbial biomass
The yield of bio-crude oil obtained from HTL of microbial biomass generated by growing tertiary consortium in PWW was 15% (w/w). The bio-crude oil yield obtained for TCB was found to be significantly higher than BB (4.4%, w/w) and MB (8.2%, w/w). This better bio-crude oil yield in case of TCB might be due to difference in composition of biomass feedstock as compared to BB and MB. However, the bio-crude oil yield obtained from TCB of the present study was found to be 41.69% and 39.02% lower than that reported for mixed culture of microalgae1 or microalgae-bacteria consortia35, respectively. This inferior yield might be due to the un-optimized process parameters and quality of the biomass feedstock. The lower yield of biocrude-oil from TCB can be improved by optimizing the HTL parameters e.g., biomass loading, residence time and reaction temperature36. Further, in-situ catalytic treatment is expected to increase the bio-crude oil yield37. Elemental composition of the bio-crude oil in terms of C, H, N, S and O (%) was found to be 63.16, 8.11, 5.37, 0.21 and 23.15 respectively. This elemental composition of the bio-crude oil obtained in the present study was found to be comparable with the biocrude obtained via HTL of other microalgae38. However, heavy metals and inorganic phosphorous was not found to be present in the bio-crude oil.
The FT-IR spectra of bio-crude oil exhibited characteristic absorption peaks reflecting presence of various functional groups and in turn, corresponding compounds (Fig. 5A). The band at 3265 cm−1 refers to the O-H stretching indicating presence of water or alcohol in the bio-crude oil. The bands at 2965, 2930, 2852, 1450 and 1377 cm−1 depict C-H vibrations, indicating the presence of alkanes. The C=O stretching vibration at 1652 cm−1 attributed to the presence of ketones, aldehydes and carboxylic acids. C-O stretching at 1265, 1170, 1060 and 970 cm−1 represents presence of primary, secondary and tertiary alcohols in the bio-crude oil. Existence of phenols, esters, ethers and aromatic compounds are represented by the presence of O-H bends at 872, 735 and 700 cm−1. These results corroborate with the findings reported by Shuping et al.39.
Figure 5.
(A) FTIR spectra and (B) TGA curve of bio-crude oil obtained via hydrothermal liquefaction of microbial biomass.
GC-MS analysis (Table 3) revealed that more than 82% of the bio-crude oil was composed of hydrocarbons, alcohols, amines, amides, aldehydes and ketones. These findings complement the results obtained from FTIR analysis. The list of all major compounds with a score of 75 on a scale of 100 have been enlisted in Table 3. Percentage of hydrocarbon content (1, 2, 4, 6, 7, 8, 11, 12, 13, 14, 17, 18, 19, 20, 25, 26 and 28) in the bio-crude oil was found to be high, depicting good oil quality. The presence of nitrogenous compounds (5, 9 and 10) in bio-crude oil indicates thermal conversion of the protein fraction present in the biomass. The oxygen content in the bio-crude oil may be attributed to the presence of oxygen containing functional groups such as ketones, aldehydes, alcohols and esters produced by the decomposition of proteins and polysaccharide fractions of the microbial biomass. This oxygen and nitrogen content could interfere with the quality of the bio-crude oil and hence, additional up gradation in terms of deoxygenation and denitrogenation is essential prior to its application as fuel oil. The boiling point distribution of the bio-crude oil has been estimated using TGA in nitrogen atmosphere (Fig. 5B). As per the analysis, the maximum distillate fraction of 30.62% lies within the boiling point range of 200–300 °C (Table 4) depicting suitability of the bio-crude oil for conversion into diesel oil, jet fuel and fuel for stoves. It is interesting to note that N, P and chromium was completely absent in HTL aqueous phase with presence of nickel in trace amount (0.04 mg L−1). Therefore, this post HTL aqueous phase can be recycled for subsequent cultivation of microalgae or can be used as utility in other industrial applications.
Table 3.
Major chemical compounds present in the bio-crude oil obtained from hydrothermal liquefaction of tertiary consortium biomass grown in paper industry wastewater.
| Sl. No. | Retention time (min) | Name of the compound | Area (%) |
|---|---|---|---|
| 1 | 3.11 | Undecane | 1.16 |
| 2 | 3.21 | Benzene, 1-ethyl-3-(1-methylethyl) - | 1.20 |
| 3 | 3.25 | Bicyclo [2.2.1] heptan-2-ol, 4-chloro-1, 7, 7-trimethyl-, exo- | 1.48 |
| 4 | 7.51 | Pyrene, 1, 3-dimethyl- | 1.95 |
| 5 | 8.01 | benzene acetonitrile, 4, 4′-[1, 2-ethenediyl] bis- | 1.51 |
| 6 | 8.73 | Tetracosane | 1.44 |
| 7 | 9.49 | 1, 4-Dimethyl-6-phenyl-naphthalene | 1.90 |
| 8 | 9.80 | Phenanthrene, 2, 3, 5-trimethyl- | 3.78 |
| 9 | 10.73 | 2-Phenazinecarbonitrile, 7-amino- | 5.37 |
| 10 | 27.00 | Dibenzepin | 0.50 |
| 11 | 27.10 | Fluoranthene | 1.39 |
| 12 | 27.39 | n-Heptadecene | 0.99 |
| 13 | 27.59 | Octadecane, 2, 6, 10, 14-tetramethyl- | 0.49 |
| 14 | 27.70 | Phenanthrene, 4, 5-dimethyl- | 0.61 |
| 15 | 27.84 | 10-Hydroxynortriptyline | 10.72 |
| 16 | 28.18 | 3, 7-Dimethyldibenzothiophene | 1.92 |
| 17 | 28.44 | 5-Methoxy (5 H) dibenzo [a, d] cycloheptene | 0.57 |
| 18 | 28.65 | Bicyclo [2.2.1] heptane, 3-methylene-2-(3-phenylprop-1-en2yl)- | 10.55 |
| 19 | 28.69 | Naphthalene, 1-(phenylmethyl) - | 2.60 |
| 20 | 28.76 | Phenanthrene, 3, 6-dimethyl- | 5.80 |
| 21 | 29.18 | 3-(3-Indolyl)−5-oxo-3-pyrazoline-4-carbonitrile | 0.56 |
| 22 | 29.26 | Normethadone | 3.05 |
| 23 | 29.37 | Benzo[b]naphtha [2, 3-d] thiophene, 7, 8, 9, 10-tetrahydro- | 2.16 |
| 24 | 30.03 | 3-Naphthalen-2-yl-3-piperidin-1-yl-propan-1-ol | 2.09 |
| 25 | 30.18 | Phenanthrene, 3, 6-dimethyl | 0.61 |
| 26 | 30.45 | Phenanthrene, 2, 5-dimethyl- | 3.82 |
| 27 | 30.67 | Isobenzofuran, 1, 3-dihydro-1, 1-dimethyl-3-phenyl- | 0.83 |
| 28 | 30.92 | Pyrene, 4, 5, 9, 10-tetrahydro | 3.20 |
| 29 | 31.08 | 4-(1-Methyl-1-siletanyl) phenol | 9.90 |
| 30 | 31.61 | Benzo[b]selenophene-3-carboxaldehyde, 2-methyl- | 0.50 |
| Total area (%) | 82.65 | ||
Table 4.
Distillate range of different fractions of bio-crude oil obtained via thermogravimetric analysis.
| Distillate Range (°C) | Typical Applications | Fraction of bio-crude oil (%) |
|---|---|---|
| 25–100 | Bottle gas and chemicals | 2.33 |
| 100–200 | Gasoline | 23.20 |
| 200–300 | Jet fuel, fuel for stoves and diesel oil | 30.62 |
| 300–400 | Lubricating oil for engines, fuel for ships and machines | 12.51 |
| 400–550 | Lubricants and candles | 14.11 |
| 550–700 | Fuel for ships and factories | 2.60 |
Conclusions
A sustainable process was demonstrated towards production of bio-crude oil from microbial biomass feedstock via combinatorial approach of: (i) improved biomass concentration through intermittent feeding of limiting nutrients and mutualistic growth of microalgae-bacteria; (ii) utilization of wastewater as cheap source of nutrients and water; (iii) direct conversion of wet biomass into bio-crude oil via hydrothermal liquefaction and (iv) simultaneous remediation of wastewater. The process may be a potential technology platform towards sustainable production of bio-crude oil from microbial biomass utilizing wastewater.
Methods
Sampling and characterization of different wastewaters
Four different types of wastewater samples were collected from effluent treatment plants of the Guwahati Municipal Corporation (GMC) and different industries across Guwahati (26.1445 °N, 91.7362 °E), Assam, India. These consisted of samples from (i) paper industry, (ii) textile industry, (iii) leather industry and (iv) GMC. The wastewater samples were allowed to settle down in order to eliminate the interference of total suspended particles. Subsequent to physical separation of the suspended particles, the pH, total nitrogen, phosphorus, chemical oxygen demand (COD) and concentrations of two heavy metals, namely Chromium (Cr) and Nickel (Ni) present in the wastewater samples, were measured.
Microalgae and bacteria consortium
In a previous study, Makut et al.16 designed a tertiary consortium comprising of two microalgae and two bacteria towards generation of biomass feedstock utilizing wastewater as cheaper source of nutrients. These organisms were isolated from oil refinery wastewater sample collected from Indian Oil Corporation Limited, Noonmati (26.19 °N, 91.80 °E), Guwahati, Assam, India. The microalgae were identified as Chlorella sorokiniana strain DBWC2 & Chlorella sp. strain DBWC7 and the bacteria were identified as Klebsiella pneumoniae strain ORWB1 & Acinetobacter calcoaceticus strain ORWB316. In the present study, this tertiary consortium was used for development of a sustainable process for production of bio-crude oil using microbial biomass as potential feedstock.
Characterization of growth and wastewater treatment efficiency of the tertiary consortium on different types of wastewater
With the aim of assessing the performance of the tertiary consortium in terms of biomass concentration and wastewater treatment efficiency, the pH values of all the four wastewater samples were adjusted to the optimal value of 8.616. For TWW and LWW, the concentration of total nitrogen was adjusted to the optimal value of 0.29 g L−1 16. The phosphorus concentration of only LWW was adjusted to the optimal value of 49.5 mg L−1 as reported by Makut et al.16. The composition of PWW and MWW was kept unaltered. Thereafter, the wastewater samples were inoculated with 18% (v/v) inoculum in which microalgae and bacteria were extant in the ratio of 1:116. The inoculated wastewater samples were incubated for 7 days in an orbital shaker (Multitron-Pro, Infors HT, Switzerland) at 150 rpm, 30 °C under 100 μE m−2 s−1 light intensity with a photoperiod of 16:8 h light and dark cycle. All the experiments were carried out in duplicate in 500 mL shake flasks. At the end of the incubation period, the wastewater samples were characterized and screened on the basis of total biomass concentration along with removal efficiency of nutrients and heavy metals. The selected wastewater was then considered for subsequent process development. Dynamic profiles for growth of microalgae, utilization of total nitrogen, phosphate and COD were obtained by analyzing the samples at every 24 h interval. The total biomass (microalgae plus bacteria) concentration achieved at the end of each batch was determined in terms of dry cell weight (DCW). Heavy metal removal efficiency was calculated by analyzing the fermentation broth at the end of the cultivation. The removal efficiency of total nitrogen, phosphate, COD and heavy metals was calculated using Eq. (1):
| 1 |
where, Si and Sf are the concentrations of a specific nutrient or heavy metal before and after cultivation respectively.
Batch and fed-batch mode of cultivation of tertiary consortium in automated bioreactor utilizing paper industry wastewater
Based on the characterization of tertiary consortium on different wastewater samples (detailed in the previous section), PWW was selected as the best culturing medium supporting maximum growth and nutrient removal efficiency. With the aim of evaluating the performance of tertiary consortium on PWW at a larger scale of operation, further experiment was performed in a 7.5 L automated photobioreactor (Biojenik Engineering, India) with a working volume of 4 L in batch mode of cultivation wherein the wastewater was inoculated (inoculum size of 18%, v/v) with (i) two bacteria only (designated as bacterial batch, BB); (ii) two microalgae only (designated as microalgal batch, MB) and (iii) two microalgae & two bacteria (designated as tertiary consortium batch, TCB) present in the tertiary consortium. The photobioreactor was operated at 30 °C with an agitation of 150 rpm, aeration and light intensity being 1 vvm and 250 μE m−2 s−1 respectively for a light:dark cycle of 16:8 h. The BB was conducted for 5 days while the MB and the TCB were run for 10 days. For all the three batches, the initial pH value, total nitrogen and phosphate concentrations were adjusted to their corresponding optimal values as detailed by Makut et al.16. Sampling was carried out at regular intervals of 24 h for the analysis of total nitrogen, phosphate and COD. Dynamic profiles for growth of microalgae (in case of MB and TCB) and bacteria (only for BB) were obtained by measuring chlorophyll-a content and DCW respectively. Final microbial biomass (microalgae plus bacteria) concentration in terms of DCW was measured at the end of each batch. Dynamic profiles of dissolved oxygen (dO2) and dissolved carbon dioxide (dCO2) concentrations were obtained for all the three individual batches. Removal efficiency of the nutrients and heavy metals was estimated as described before.
In the next step, a fed-batch strategy was implemented to maximize the microbial biomass concentration. The fed-batch was operated for a period of 18 days under same cultivation conditions as detailed for the batch mode. An intermittent feeding of the key nutrients, nitrogen source and phosphate, was performed in order to maintain their concentrations at their respective optimal values of 0.29 g L−1 and 49.5 mg L−1 throughout the entire course of fermentation. The concentrations of these key nutrients in the culture broth were monitored every 8 h. The growth of microalgae (in terms of chlorophyll-a content) and COD were estimated every 24 h. At the end of the experiment, the biomass was collected, centrifuged at 8000 rpm and processed for HTL. The total biomass (microalgae plus bacteria) concentration achieved was determined in terms of DCW.
Analysis of growth, nutrient utilization, COD and heavy metal ion removal
Chlorophyll-a was estimated following the protocol described by Makut et al.16. The total nitrogen content was determined as the summation of the individual concentrations of nitrate, nitrite and ammoniacal nitrogen. Estimation of nitrate in the supernatant was carried out using the salicylic acid method with sodium nitrate as the standard40. The mole fraction and the corresponding nitrogen concentration in nitrite and ammonia was measured and calculated according to the Standard Methods for Examination of Water and Wastewater41. Phosphate concentration was estimated using the ascorbic acid method with potassium hydrogen phosphate (dibasic) as standard42. The COD was analyzed with HACH COD reagents and quantified in DR900 colorimeter (Hach, USA). For determining the heavy metal concentration, the samples were centrifuged at 13,000 rpm for 5 min and the clear supernatant was analyzed using atomic absorption spectroscopy (Varian AA240, Australia). Standard procedures41,43 were followed for the analysis. All chemicals and reagents were procured from Hi Media, India and were of analytical grade.
Hydrothermal liquefaction of microbial biomass and sample preparation
HTL experiments were carried out for all three types of biomass feedstock obtained from TCB, MB and BB. The HTL experiment was performed using a stainless steel stirred tank batch reactor (Amar Equipments Pvt. Ltd., India) with a capacity of 750 mL. The biomass loading was kept at 15% (w/v) of the total working volume of 200 mL. The reaction temperature was maintained at 310 °C for 55 min at corresponding pressure with a heating rate of 10 °C min−1 35. The reaction residence time was calculated from the time point the reactor reached the desired temperature. Supply of heat to the reactor was stopped on attaining the desired holding time. Dichloromethane (DCM) (99%, Hi-media) was added into the reactor once the reaction was complete and subsequently cooled, in order to separate the reaction mixture into the water soluble phase and DCM soluble phase. Then this mixture was filtered using Whatman ash less grade-41 filter paper (20 µm) to separate the biochar. The extraction and separation of the organic phase and the aqueous phase was successively carried out using DCM in the separating funnel. The solvent from the organic phase was removed by evaporation under vacuum in a rota evaporator unit (R-300 digital, Buchi, Switzerland) at 60 °C to obtain the bio-crude oil. The bio-crude yield was calculated using the following Eq. (2):
| 2 |
Analysis of bio-crude oil
Gas chromatography-Mass spectrometry
The composition of the bio-crude oil was analyzed by Gas chromatography-Mass spectrometry (GC-MS) (Agilent Technologies, USA) using HP5-MS capillary column (30 m, 0.25 mm id, 0.25 mm film thickness). The inlet temperature and split ratio were maintained at 300 °C and 20:1 respectively. 2 µL of the sample was injected into the GC–MS system consisting of an Agilent 7890B gas chromatograph and Agilent 5977B mass selective detector. The temperature of the column was initially held at 50 °C for 5 min and then ramped to 300 °C at a rate of 10 °C min−1. On attaining 300 °C, the temperature was maintained isothermally for 4 min, thereby amounting to a total run time of 37 min. Helium was used as the carrier gas with a constant flow rate 1.6 mL min−1. Data acquisition of the chromatogram peaks was carried out using the Mass Hunter WorkStation and the probable compounds were identified using NIST Mass Spectral Database (NIST 14).
Thermogravimetric analysis
Thermogravimetric analysis (TGA) of the bio-crude oil was performed using a thermogravimetric analyser (STA7200, Hitachi, Japan) in an inert atmosphere of nitrogen. The flow rate of nitrogen was maintained at 50 mL min−1. For TGA analysis of the bio-crude oil, 10 µL of the sample was taken in the alumina crucible and heated from room temperature to 700 °C at a heating rate of 20 °C min−1. The sample weight loss with respect to rise in temperature was recorded to estimate the boiling point distribution.
Fourier transform infrared spectroscopy
Fourier transform infrared spectroscopy (FTIR) analysis of the bio-crude oil was conducted using IR-Affinity-1 (Shimadzu, Japan) in order to analyze the functional groups present in the sample. The infrared spectrum range was between 400 cm−1 and 4000 cm−1 and scanning was executed at the rate of 20 with a step size of 4 cm−1.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Supplementary information
Acknowledgements
This research work was partially supported by the Ministry of Human Resource and Development and is gratefully acknowledged.
Author contributions
Conceptualization and design of experiments was done by Debasish Das. Gargi Goswami and Bidhu Bhusan Makut did execution of the experiments. The manuscript was prepared by Gargi Goswami, Bidhu Bhusan Makut and Debasish Das.
Data availability
The data that support the findings of this study are available on request from the corresponding author.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Gargi Goswamia and Bidhu Bhusan Makut.
Supplementary information
is available for this paper at 10.1038/s41598-019-51315-5.
References
- 1.Chen WT, et al. Hydrothermal liquefaction of mixed-culture algal biomass from wastewater treatment system into bio-crude oil. Bioresour. Technol. 2014;152:130–139. doi: 10.1016/j.biortech.2013.10.111. [DOI] [PubMed] [Google Scholar]
- 2.Roberts GW, Fortier M-OP, Sturm BSM, Stagg-Williams SM. Promising pathway for algal biofuels through wastewater cultivation and hydrothermal conversion. Energ. Fuel. 2013;27:857–867. doi: 10.1021/ef3020603. [DOI] [Google Scholar]
- 3.Subashchandrabose SR, Ramakrishnan B, Megharaj M, Venkateswarlu K, Naidu R. Consortia of cyanobacteria / microalgae and bacteria: biotechnological potential. Biotechnol. Adv. 2011;29:896–907. doi: 10.1016/j.biotechadv.2011.07.009. [DOI] [PubMed] [Google Scholar]
- 4.Powell RJ, Hill RT. Mechanism of algal aggregation by Bacillus sp. strain RP1137. Appl. Environ. Microbiol. 2014;80:4042–4050. doi: 10.1128/AEM.00887-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Guo Z, Tong YW. The interactions between Chlorella vulgaris and algal symbiotic bacteria under photoautotrophic and photoheterotrophic conditions. J. Appl. Phycol. 2014;26:1483–1492. doi: 10.1007/s10811-013-0186-1. [DOI] [Google Scholar]
- 6.Du J, et al. Growth stimulation of Microcystis aeruginosa by a bacterium from hyper-eutrophic water (Taihu Lake, China) Aquat. Ecol. 2013;47:303–313. doi: 10.1007/s10452-013-9445-0. [DOI] [Google Scholar]
- 7.Mujtaba G, Lee K. Advanced treatment of wastewater using symbiotic co-culture of microalgae and bacteria. Appl. Chem. Eng. 2016;27:1–9. doi: 10.14478/ace.2016.1002. [DOI] [Google Scholar]
- 8.Samorì G, Samorì C, Guerrini F, Pistocchi R. Growth and nitrogen removal capacity of Desmodesmus communis and of a natural microalgae consortium in a batch culture system in view of urban wastewater treatment: Part I. Water Res. 2013;47:791–801. doi: 10.1016/j.watres.2012.11.006. [DOI] [PubMed] [Google Scholar]
- 9.Medina M, Neis U. Symbiotic algal bacterial wastewater treatment: Effect of food to microorganism ratio and hydraulic retention time on the process performance. Water Sci. Technol. 2007;55:165–171. doi: 10.2166/wst.2007.351. [DOI] [PubMed] [Google Scholar]
- 10.Munoz R, Jacinto M, Guieysse B, Mattiasson B. Combined carbon and nitrogen removal from acetonitrile using algal-bacterial bioreactors. Appl. Microbiol. Biotechnol. 2005;67:699–707. doi: 10.1007/s00253-004-1811-3. [DOI] [PubMed] [Google Scholar]
- 11.De‐Bashan LE, Antoun H, Bashan Y. Involvement of indole‐3‐acetic acid produced by the growth‐promoting bacterium Azospirillum sp. in promoting growth of Chlorella vulgaris. J. Phycol. 2008;44:938–947. doi: 10.1111/j.1529-8817.2008.00533.x. [DOI] [PubMed] [Google Scholar]
- 12.Fuentes J, et al. Impact of microalgae-bacteria interactions on the production of algal biomass and associated compounds. Mar. Drugs. 2016;14:100. doi: 10.3390/md14050100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Praveen P, Loh KC. Photosynthetic aeration in biological wastewater treatment using immobilized microalgae-bacteria symbiosis. Appl. Microbiol. Biotechnol. 2015;99:10345–10354. doi: 10.1007/s00253-015-6896-3. [DOI] [PubMed] [Google Scholar]
- 14.Vlaskin MS, Chernova NI, Kiseleva SV, Zhuk AZ. Hydrothermal liquefaction of microalgae to produce biofuels: State of the art and future prospects. Therm. Eng. 2017;64:627–636. doi: 10.1134/S0040601517090105. [DOI] [Google Scholar]
- 15.Zhou Y, Schideman L, Yu G, Zhang Y. A synergistic combination of algal wastewater treatment and hydrothermal biofuel production maximized by nutrient and carbon recycling. Energy Environ. Sci. 2013;6:3765–3779. doi: 10.1039/c3ee24241b. [DOI] [Google Scholar]
- 16.Makut BB, Das D, Goswami G. Production of microbial biomass feedstock via co-cultivation of microalgae-bacteria consortium coupled with effective wastewater treatment: A sustainable approach. Algal Res. 2019;37:228–239. doi: 10.1016/j.algal.2018.11.020. [DOI] [Google Scholar]
- 17.Ryu BG, et al. Simultaneous treatment of municipal wastewater and biodiesel production by cultivation of Chlorella vulgaris with indigenous wastewater bacteria. Biotechnol. Bioprocess Eng. 2014;19:201–210. doi: 10.1007/s12257-013-0250-3. [DOI] [Google Scholar]
- 18.Das C, Ramaiah N, Pereira E, Naseera K. Efficient bioremediation of tannery wastewater by monostrains and consortium of marine Chlorella sp. and Phormidium sp. Int. J. Phytoremediation. 2018;20:284–292. doi: 10.1080/15226514.2017.1374338. [DOI] [PubMed] [Google Scholar]
- 19.Choi HJ, Lee SM. Effects of microalgae on the removal of nutrients from wastewater: various concentrations of Chlorella vulgaris. Environ. Eng. Res. 2012;17:S3–S8. doi: 10.4491/eer.2012.17.S1.S3. [DOI] [Google Scholar]
- 20.Pathak VV, Singh DP, Kothari R, Chopra AK. Phycoremediation of textile wastewater by unicellular microalga Chlorella pyrenoidosa. Cell Mol. Biol. 2014;60:35–40. [PubMed] [Google Scholar]
- 21.Rasouli Z, Valverde-Pérez B, D’Este M, De Francisci D, Angelidaki I. Nutrient recovery from industrial wastewater as single cell protein by a co-culture of green microalgae and methanotrophs. Biochem. Eng. J. 2018;134:129–135. doi: 10.1016/j.bej.2018.03.010. [DOI] [Google Scholar]
- 22.Barsanti, L. & Gualtieri, P. Algae: Anatomy, Biochemistry, and Biotechnology, second ed., CRC Press, USA (2006).
- 23.Cai T, Park SY, Li Y. Nutrient recovery from wastewater streams by microalgae: status and prospects. Renew. Sust. Energ. Rev. 2013;19:360–369. doi: 10.1016/j.rser.2012.11.030. [DOI] [Google Scholar]
- 24.Mujtaba G, Rizwan M, Lee K. Simultaneous removal of inorganic nutrients and organic carbon by symbiotic co-culture of Chlorella vulgaris and Pseudomonas putida. Biotechnol. Bioprocess Eng. 2015;20:1114–1122. doi: 10.1007/s12257-015-0421-5. [DOI] [Google Scholar]
- 25.Liang Z, et al. Efficiency assessment and pH effect in removing nitrogen and phosphorus by algae-bacteria combined system of Chlorella vulgaris and Bacillus licheniformis. Chemosphere. 2013;92:1383–1389. doi: 10.1016/j.chemosphere.2013.05.014. [DOI] [PubMed] [Google Scholar]
- 26.Tricolici O, Bumbac C, Postolache C. Microalgae–bacteria system for biological wastewater treatment. J. Environ. Prot. Ecol. 2014;15:268–276. [Google Scholar]
- 27.Su Y, Mennerich A, Urban B. Synergistic cooperation between wastewater-born algae and activated sludge for wastewater treatment: Influence of algae and sludge inoculation ratios. Bioresour. Technol. 2012;105:67–73. doi: 10.1016/j.biortech.2011.11.113. [DOI] [PubMed] [Google Scholar]
- 28.Cho DH, et al. Organic carbon, influent microbial diversity and temperature strongly influence algal diversity and biomass in raceway ponds treating raw municipal wastewater. Bioresour. Technol. 2015;191:481–487. doi: 10.1016/j.biortech.2015.02.013. [DOI] [PubMed] [Google Scholar]
- 29.Covarrubias SA, de-Bashan LE, Moreno M, Bashan Y. Alginate beads provide a beneficial physical barrier against native microorganisms in wastewater treated with immobilized bacteria and microalgae. Appl. Microbiol. Biotechnol. 2012;93:2669–2680. doi: 10.1007/s00253-011-3585-8. [DOI] [PubMed] [Google Scholar]
- 30.Chen T, Zhao Q, Wang L, Xu Y, Wei W. Comparative metabolomics analysis of the green microalga Chlorella sorokiniana cultivated in the single culture and a consortium with bacteria for wastewater remediation. Biotechnol. Appl. Biochem. 2017;183:1062–1075. doi: 10.1007/s12010-017-2484-6. [DOI] [PubMed] [Google Scholar]
- 31.Shen Y, Gao J, Li L. Municipal wastewater treatment via co-immobilized microalgal-bacterial symbiosis: Microorganism growth and nutrients removal. Bioresour. Technol. 2017;243:905–913. doi: 10.1016/j.biortech.2017.07.041. [DOI] [PubMed] [Google Scholar]
- 32.Markou G. Fed-batch cultivation of Arthrospira and Chlorella in ammonia-rich wastewater: Optimization of nutrient removal and biomass production. Bioresour. Technol. 2015;193:35–41. doi: 10.1016/j.biortech.2015.06.071. [DOI] [PubMed] [Google Scholar]
- 33.Droop MR. Vitamins, phytoplankton and bacteria: symbiosis or scavenging? J. Plankton Res. 2007;29:107–113. doi: 10.1093/plankt/fbm009. [DOI] [Google Scholar]
- 34.Hernandez JP, de-Bashan LE, Rodriguez DJ, Rodriguez Y, Bashan Y. Growth promotion of the freshwater microalga Chlorella vulgaris by the nitrogen-fixing, plant growth-promoting bacterium Bacillus pumilus from arid zone soils. Eur. J. Soil. Biol. 2009;45:88–93. doi: 10.1016/j.ejsobi.2008.08.004. [DOI] [Google Scholar]
- 35.Boëns B, Pilon G, Bourdeau N, Adjallé K, Barnabé S. Hydrothermal liquefaction of a wastewater native Chlorella sp. bacteria consortium: Biocrude production and characterization. Biofuels. 2016;7:611–619. doi: 10.1080/17597269.2016.1168027. [DOI] [Google Scholar]
- 36.Chiaramonti D, Prussi M, Buffi M, Rizzo AM, Pari L. Review and experimental study on pyrolysis and hydrothermal liquefaction of microalgae for biofuel production. Appl. Energy. 2017;185:963–972. doi: 10.1016/j.apenergy.2015.12.001. [DOI] [Google Scholar]
- 37.Nam H, Kim C, Capareda SC, Adhikari S. Catalytic upgrading of fractionated microalgae bio-oil (Nannochloropsis oculata) using a noble metal (Pd/C) catalyst. Algal Res. 2017;24:188–198. doi: 10.1016/j.algal.2017.03.021. [DOI] [Google Scholar]
- 38.Biller P, Sharma BK, Kunwar B, Ross AB. Hydroprocessing of bio-crude from continuous hydrothermal liquefaction of microalgae. Fuel. 2015;159:197–205. doi: 10.1016/j.fuel.2015.06.077. [DOI] [Google Scholar]
- 39.Shuping Z, et al. Production and characterization of bio-oil from hydrothermal liquefaction of microalgae Dunaliella tertiolecta cake. Energy. 2010;35:5406–5411. doi: 10.1016/j.energy.2010.07.013. [DOI] [Google Scholar]
- 40.Cataldo DA, Maroon M, Schrader LE, Youngs VL. Rapid colorimetric determination of nitrate in plant tissue by nitration of salicylic acid. Commun. Soil Sci. Plant Anal. 1975;6:71–80. doi: 10.1080/00103627509366547. [DOI] [Google Scholar]
- 41.APHA, Standard Methods for the Examination of Water and Wastewater, 23rd edition, American Public Health Association, American Water Works Association and Water Environmental Federation, Washington DC (2017).
- 42.Parsons, T. R. A Manual of Chemical and Biological Methods for Seawater Analysis, pp. 3–33 (1984).
- 43.Trivedi RK, Khatavkar SB, Goel PK. Characterization, treatment and disposal of wastewater in a textile industry. J. Ind. Pollut. Control. 1986;2:1–12. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author.