This cluster randomized clinical trial investigates the effect of clinical geriatric assessments and collaborative medication reviews by geriatrician and family physician on health-related quality of life and other patient-relevant outcomes in home-dwelling older patients receiving polypharmacy.
Key Points
Question
Can clinical geriatric assessments and collaborative medication reviews carried out by a geriatrician in cooperation with the patient’s family physician have positive effects on health-related quality of life in older patients receiving polypharmacy?
Findings
In this cluster randomized clinical trial that included 70 participating family physicians with 174 patients, health-related quality of life after 16 weeks was statistically significantly better in patients who received the intervention compared with those who received usual care.
Meaning
Clinical geriatric assessments and collaborative medication reviews have the potential to improve health-related quality of life among older patients exposed to polypharmacy.
Abstract
Importance
Polypharmacy and inappropriate drug regimens are major health concerns among older adults. Various interventions focused on medication optimization strategies have been carried out, but the effect on patient-relevant outcomes remains uncertain.
Objective
To investigate the effect of clinical geriatric assessments and collaborative medication reviews by geriatrician and family physician (FP) on health-related quality of life and other patient-relevant outcomes in home-dwelling older patients receiving polypharmacy.
Design, Setting, and Participants
Cluster randomized, single-blind, clinical trial. Norwegian FPs were recruited from March 17, 2015, to March 16, 2017, to participate in the trial with their eligible patients. Participants were home-dwelling patients 70 years or older, using at least 7 medications regularly, and having their medications administered by the home nursing service. Patients in the control group received usual care. Randomization occurred at the FP level. A modified intent-to-treat analysis was used.
Intervention
The intervention consisted of 3 main parts: (1) clinical geriatric assessment of the patients combined with a thorough review of their medications; (2) a meeting between the geriatrician and the FP; and (3) clinical follow-up.
Main Outcomes and Measures
The primary outcome was health-related quality of life as assessed by the 15D instrument (score range, 0-1; higher scores indicate better quality of life, with a minimum clinically important change of ±0.015) at week 16. Secondary outcomes included changes in medication appropriateness, physical and cognitive functioning, use of health services, and mortality.
Results
Among 174 patients (mean [SD] age, 83.3 [7.3] years; 67.8% women; 87 randomized to the intervention group and 87 randomized to the control [usual care] group) in 70 FP clusters (36 intervention and 34 control), 158 (90.8%) completed the trial. The mean (SD) 15D instrument score at baseline was 0.708 (0.121) in the intervention group and 0.714 (0.113) in the control group. At week 16, the mean (SD) 15D instrument score was 0.698 (0.164) in the intervention group and 0.655 (0.184) in the control group, with an estimated between-group difference of 0.045 (95% CI, 0.004-0.086; P = .03). Several secondary outcomes were also in favor of the intervention. There were more drug withdrawals, reduced dosages, and new drug regimens started in the intervention group.
Conclusions and Relevance
This study’s findings indicate that, among older patients exposed to polypharmacy, clinical geriatric assessments and collaborative medication reviews carried out by a geriatrician in cooperation with the patient’s FP can result in positive effects on health-related quality of life.
Trial Registration
ClinicalTrials.gov identifier: NCT02379455
Introduction
Older patients are prescribed an increasing number of medications.1,2 Polypharmacy is associated with negative health outcomes,3 although many drugs may have good clinical indications individually. Evidence-based methods to manage complex treatment regimens in a way that ensures positive effects on clinical and patient-relevant outcomes are lacking to date. Therefore, there is a need for strategies that can guide clinicians on how to provide the benefits of drug treatment for these patients but at the same time avoid negative consequences.
Previous studies aimed at improving drug treatment for older patients have mainly studied effects on surrogate clinical outcomes, such as potentially inappropriate medications.4,5 Numerous tools to assess medication appropriateness have been developed, but effects on such criteria-based outcomes do not necessarily mean that the patient has benefited from the intervention.6 Although some studies have included clinical outcomes, the results have been inconclusive.7,8 We hypothesized that most improvements in drug treatment (eg, better pain control, enhanced symptom control in heart failure, or less iatrogenic dehydration or sedation) have the potential to improve health-related quality of life (HRQoL). In our opinion, HRQoL is thus an appropriate outcome measure when the aim is to improve drug treatment in an individualized manner across a broad spectrum of drug classes. Two core outcome sets for polypharmacy interventions have been developed, both highlighting HRQoL as the most important patient-related outcome to assess.9,10 So far, it is unclear whether interventions to improve pharmacotherapy result in clinical improvements, and there is no evidence regarding an effect on HRQoL.11
Geriatricians are trained in assessments of multimorbidity and polypharmacy. A closer cooperation between geriatricians and family physicians (FPs), who have a key role in the follow-up of patients over time, might thus be beneficial. We investigated whether clinical geriatric assessments and collaborative medication reviews carried out by a geriatrician in cooperation with the patient’s FP could have positive effects on HRQoL and other patient-relevant outcomes in home-dwelling older patients receiving polypharmacy.
Methods
Trial Oversight
This was a cluster randomized, single-blind, clinical trial with follow-up at 16 weeks and 24 weeks. The trial protocol has previously been published,12 and the version submitted to the institutional review boards is available in Supplement 1. Inclusion of patients was based on written informed consent. Patients unable to give a valid consent because of dementia were included based on written informed consent from a close relative in combination with assent from the patient. The trial was approved by the Regional Committee for Medical and Health Research Ethics and by the Data Protection Officer at Oslo University Hospital (Oslo, Norway) and was carried out in accord with the Declaration of Helsinki.13 This study followed the Consolidated Standards of Reporting Trials (CONSORT) reporting guidelines.
Participants
Family physicians from the counties of Akershus and Oslo, Norway, were invited to participate in the trial with their eligible patients. Patients were eligible for enrollment if they were home-dwelling individuals, were 70 years or older, used at least 7 systemic medications taken regularly, and had their medications administered by the home nursing service. Patients were excluded if they were expected to die or become permanently institutionalized within 6 months, if the FP discouraged participation, or if valid information was unavailable. Details are available in eAppendix 1 and eTable 1 in Supplement 2.
Trial Procedures
Our intervention consisted of 3 main parts. First was geriatric assessment consisting of a medical history, systematic screening for current problems, clinical examination of the patient, and relevant supplementary tests as well as a detailed review of each medication in use, with emphasis on indication, dosage, possible adverse effects, and interactions. Assessments were done by a physician trained in geriatric medicine, supervised by a senior consultant. On average, 1 hour was spent on each clinical consultation. Second was a meeting between the geriatrician and the FP, with discussion of each medication, establishing a collaborative plan for adjustments and follow-up. Approximately 15 minutes were spent discussing each patient. Third was clinical follow-up by the geriatrician or FP, as agreed on. Follow-up was in general done by the FP. Details on the various components of the intervention are provided in eAppendix 1, eFigure 1, and eFigure 2 in Supplement 2. The control group received usual care.
Cluster randomization at the FP level was performed to avoid between-group contamination. To avoid large variations in cluster sizes, each FP participated with a maximum of 5 patients, and stratification was performed based on the number of contributing patients (1-2 vs 3-5). Randomization was computer generated and carried out in blocks of unknown and variable size. A statistician not otherwise involved in trial procedures prepared the allocation sequence. The research assistant, who provided all assessments, was blinded with respect to allocation. The patients received 3 home visits from the research assistant: at baseline, 16 weeks, and 24 weeks. Detailed descriptions of the trial procedures are given in eAppendix 1 and eTable 2 in Supplement 2.
Outcomes
The primary outcome was HRQoL, measured by the 15D instrument at 16 weeks.14,15 The 15D instrument is a generic, 15-dimensional measure assessing mobility, vision, hearing, breathing, sleeping, eating, speech, elimination, usual activities, mental function, discomfort/symptoms, depression, distress, vitality, and sexual activity. Each dimension is rated by the respondent on an ordinal scale with 5 levels. Single index scores are calculated by population-based utility weights and range from 0 to 1, with higher scores indicating better HRQoL.16 A change of ±0.015 or more is considered clinically important, and a change of more than 0.035 in the positive direction represents “much better HRQoL.”17
Secondary outcomes were appropriateness of drug regimens as assessed by the Medication Appropriateness Index and the Assessment of Underutilization18,19; physical functioning as assessed by the Short Physical Performance Battery, gait speed, and grip strength20,21; cognitive functioning as assessed by the Digit Span Forward and Digit Span Backward, Trail Making Test A and Trail Making Test B, and Five Digits Test22,23,24; physical and cognitive disability as assessed by the Functional Independence Measure25; and caregiver burden as assessed by the Relative Stress Scale.26 We also assessed orthostatic blood pressure, falls, weight, hospital admissions, the number of days the patient spent in his/her own home during follow-up, use of the home nursing service, admission to permanent institutional care, and mortality. Details on secondary outcomes are listed in eTable 3 in Supplement 2.
Statistical Analysis
Detailed power calculations are included in eAppendix 2 and eTable 4 in Supplement 2. We planned to randomly assign 200 patients (100 per trial group), which was expected to give greater than 80% power to detect a difference of 0.035 in the 15D instrument score after 16 weeks, at a 2-sided significance level of 5%.
In the primary analysis, all participants were kept in the treatment group to which their FP had been randomly assigned. However, a strict intent-to-treat analysis was not possible because outcome data were missing for some patients. According to protocol, an analysis of covariance model was used, with the 15D instrument score at 16 weeks as the dependent variable, randomization group as the fixed factor, and cluster size and baseline 15D instrument score as covariates. A clustered sandwich estimator of the SE with FP as the cluster was applied. Missing data were imputed by multiple imputation, as explained in eAppendix 2 in Supplement 2. Distributional assumptions were checked by visual inspection of residual plots. Secondary analysis of the primary outcome included adjustment for other covariates expected a priori to be prognostic of the outcome. These included age, sex, comorbidity (Cumulative Illness Rating Scale27), dementia severity (Clinical Dementia Rating Scale Sum of Boxes28,29), and use of the home nursing service (hours per week), all measured at baseline. If the introduction of a covariate to the model changed the effect estimate for the randomization variable by at least 10%, the covariate was incorporated in a final model that included all variables with an effect of this size. We also carried out a linear mixed model analysis, adjusting for cluster size, applying an unstructured covariance matrix, and using a clustered sandwich estimator to estimate SE. The same analytic approach was used for 15D instrument scores at 24 weeks. We performed multiple additional sensitivity analyses, described in eAppendix 2 in Supplement 2. Analyses of the primary outcome were carried out by a statistician blinded to group allocation.
Responder analyses classified all patients with an improvement of at least 0.015 on the 15D instrument as responders. These analyses were performed by logistic regression after adjusting for cluster size and covariates as described above, using the clustered sandwich estimator to estimate SE.
Secondary outcomes with repeated measurements were analyzed by using a linear mixed model as described above. When distributional assumptions were violated, percentile CIs were estimated by 100 bootstrap replications, with FP as the unit of resampling. Outcomes measured only once were analyzed by multiple linear regression or logistic regression as appropriate. The analyses were adjusted for age, sex, dementia severity, and use of the home nursing service at baseline, and the clustered sandwich estimator was used to estimate SE. Use of the Cumulative Illness Rating Scale did not affect any of the estimates, and this scale was not included as a covariate for adjustment.
Statistical analyses were performed with software programs. These included SPSS (version 25.0.0.1; IBM) and Stata (version 15; StataCorp).
Results
Participants
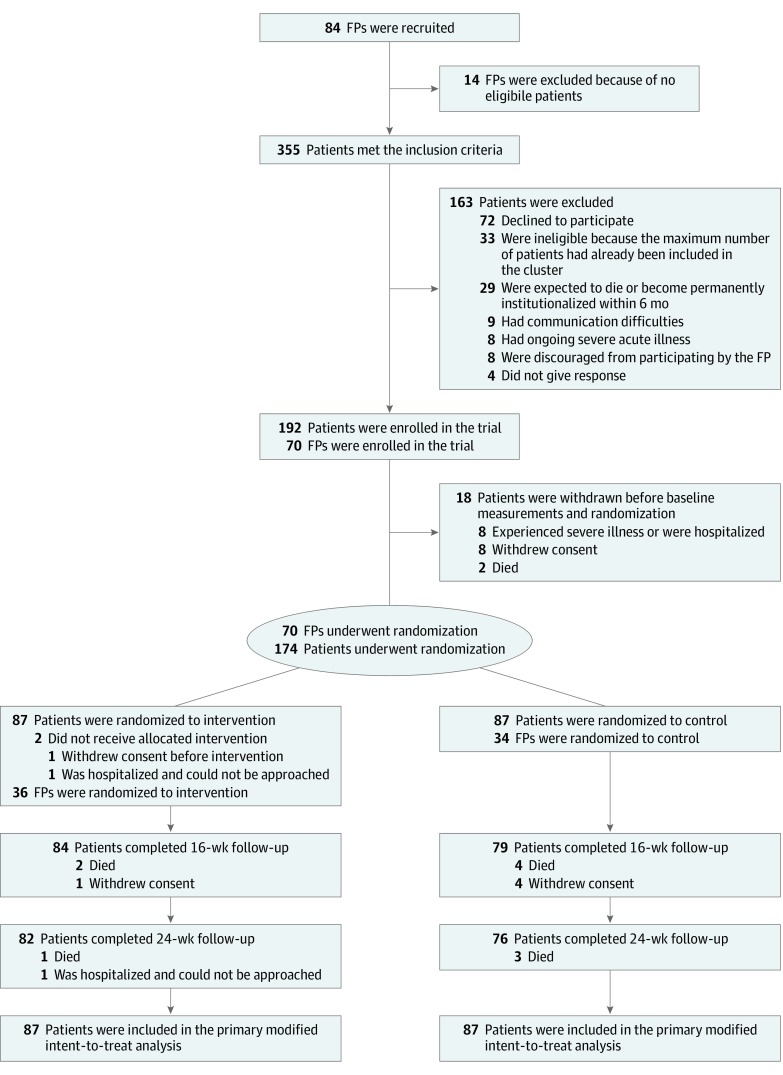
From March 17, 2015, to March 16, 2017, we recruited 84 Norwegian FPs to participate in the trial with their eligible patients. The screening procedure (eAppendix 1 in Supplement 2) identified 355 patients who met the inclusion criteria. Of these, 163 were excluded and 18 were withdrawn before baseline measurements and randomization. Fourteen FPs did not have eligible patients. The modified intent-to-treat analysis is thus based on 70 FPs and 174 patients who underwent randomization (Figure 1). Demographic and baseline data are listed in Table 1. Among 174 patients (mean [SD] age, 83.3 [7.3] years; 67.8% [118 of 174] women; 87 intervention and 87 control) in 70 FP clusters (36 intervention and 34 control), 158 (90.8%) completed the trial. The mean (SD) 15D instrument score at baseline was 0.708 (0.121) in the intervention group and 0.714 (0.113) in the control group.
Figure 1. Consolidated Standards of Reporting Trials (CONSORT) Diagram of Participants in the Study.
Through the screening procedure, described in eAppendix 1 in Supplement 2, a total of 355 patients from 84 family physicians (FPs) were identified as meeting the inclusion criteria.
Table 1. Baseline Characteristics of Participants.
| Characteristic | Intervention (n = 87) | Control (n = 87) |
|---|---|---|
| Age, mean (SD), y | 82.2 (7.6) | 84.4 (6.9) |
| Females, No. (%) | 52 (59.8) | 66 (75.9) |
| Cumulative Illness Rating Scale summary score, mean (SD) | 16.8 (4.4) | 16.6 (4.1) |
| Clinical Dementia Rating Scale Sum of Boxes score, mean (SD) | 2.9 (3.7) | 1.8 (2.8) |
| Regularly used drugs, mean (SD), No. | 10.1 (2.7) | 9.5 (2.6) |
| Medication Appropriateness Index, mean (SD) | 16.3 (9.2) | 14.6 (7.2) |
| Assessment of Underutilization score, mean (SD) | 0.49 (0.70) | 0.55 (0.74) |
| 15D instrument score, mean (SD) | 0.708 (0.121) | 0.714 (0.113) |
| Short Physical Performance Battery score, mean (SD) | 4.8 (3.3) | 4.3 (2.8) |
| Gait speed | ||
| No. of patients | 81 | 81 |
| Mean (SD), m/s | 0.62 (0.21) | 0.61 (0.20) |
| Grip strength, mean (SD), kg | 19.4 (7.7) | 17.7 (8.4) |
| Digit Span Forward | ||
| No. of patients | 87 | 86 |
| Maximum span, mean (SD) | 4.69 (0.92) | 4.57 (0.95) |
| Digit Span Backward | ||
| No. of patients | 87 | 85 |
| Maximum span, mean (SD) | 2.94 (0.96) | 2.96 (0.97) |
| Trail Making Test Aa | ||
| No. of patients | 72 | 70 |
| Mean (SD), s | 163 (138) | 130 (104) |
| Trail Making Test Ba | ||
| No. of patients | 70 | 69 |
| Mean (SD), s | 359 (161) | 398 (151) |
| Five Digits Test 1a | ||
| No. of patients | 77 | 74 |
| Mean (SD), s | 47 (27) | 48 (43) |
| Five Digits Test 2a | ||
| No. of patients | 77 | 73 |
| Mean (SD), s | 56 (62) | 51 (49) |
| Five Digits Test 3a | ||
| No. of patients | 76 | 72 |
| Mean (SD), s | 108 (86) | 83 (64) |
| Five Digits Test 4a | ||
| No. of patients | 74 | 70 |
| Mean (SD), s | 229 (124) | 202 (127) |
| Use of the home nursing service, mean (SD), min/wk | 155 (173) | 181 (268) |
| Functional Independence Measure score, mean (SD) | 111 (11) | 111 (11) |
| Relative Stress Scale score | ||
| No. of patients | 81 | 77 |
| Mean (SD) | 14.4 (11.9) | 11.8 (10.1) |
| Change in SBP after standing 1 min | ||
| No. of patients | 82 | 77 |
| Mean (SD), mm Hg | −9.7 (19.3) | −9.9 (22.8) |
Abbreviation: SBP, systolic blood pressure.
Values for patients unable to complete the test because of cognitive difficulties were imputed as described in eTable 3 in Supplement 2.
Primary Outcome
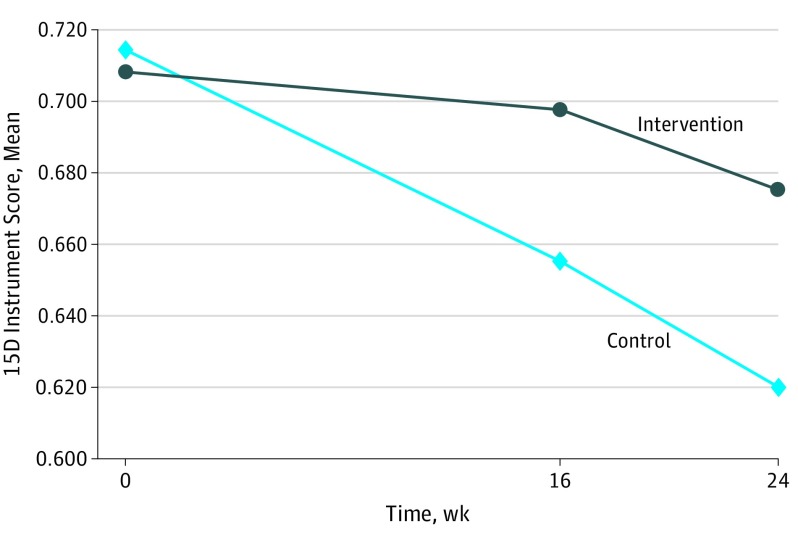
At week 16, the mean (SD) 15D instrument score was 0.698 (0.164) in the intervention group and 0.655 (0.184) in the control group, with an estimated between-group difference of 0.045 (95% CI, 0.004-0.086; P = .03). Dementia severity, measured by the Clinical Dementia Rating Scale Sum of Boxes, was the only prespecified covariate that influenced the effect estimate for the randomization variable by at least 10%. After adjustment for the Clinical Dementia Rating Scale Sum of Boxes score, the between-group difference was 0.055 (95% CI, 0.014-0.096; P = .01). Analyzed by linear mixed model, the between-group difference was 0.048 (95% CI, 0.006-0.090; P = .03). All sensitivity analyses gave similar results (eAppendix 3 and eTable 5 in Supplement 2). The proportion of responders was higher in the intervention group (41 of 86 patients [47.7%]) compared with the control group (18 of 83 patients [21.7%]) (adjusted odds ratio, 3.32; 95% CI, 1.47-7.46; P = .004).
The mean 15D instrument score decreased in both groups but at a slower pace in the intervention group (Figure 2). At week 24, the mean (SD) 15D instrument score was 0.675 (0.186) in the intervention group and 0.620 (0.216) in the control group, with an estimated between-group difference of 0.052 (95% CI, −0.002 to 0.105; P = .06). After adjustment for the Clinical Dementia Rating Scale Sum of Boxes score, the between-group difference was 0.064 (95% CI, 0.011-0.116; P = .02). Analyzed by linear mixed model, the between-group difference was 0.061 (95% CI, 0.004-0.118; P = .04). The proportion of responders at week 24 was higher in the intervention group (37 of 85 patients [43.5%]) compared with the control group (19 of 83 patients [22.9%]) (adjusted odds ratio, 2.74; 95% CI, 1.13-6.65; P = .03).
Figure 2. Primary Outcome of Health-Related Quality of Life as Measured by the 15D Instrument.
Shown are mean (SD) 15D instrument scores at baseline, week 16, and week 24. The score range is 0 to 1, with higher scores indicating better quality of life.
Secondary Outcomes
Secondary outcomes are listed in Table 2 and eTable 6 in Supplement 2. Medication appropriateness as assessed by the Medication Appropriateness Index and the Assessment of Underutilization improved in the intervention group compared with the control group at 16 weeks and 24 weeks. There was also a suggestion toward positive effects of the intervention on several of the physical and cognitive tests (Table 2). Of those completing the study, 31 of 82 patients in the intervention group (37.8%) and 17 of 76 patients in the control group (22.4%) had been hospitalized during follow-up (adjusted odds ratio, 2.03; 95% CI, 0.98-4.24; P = .06). There were no statistically significant differences between groups regarding orthostatic blood pressure, falls, weight, relative stress, disability (as assessed with the Functional Independence Measure), the number of days the patient spent in his or her own home during follow-up, use of the home nursing service, admission to permanent institutional care, or mortality.
Table 2. Change in Secondary Outcomes From Baseline to Week 16 and Week 24a.
| Outcome | Change From Baseline to Week 16 | Change From Baseline to Week 24 | ||||
|---|---|---|---|---|---|---|
| Intervention (n = 84) | Control (n = 79) | Estimated Effect of Intervention (95% CI) | Intervention (n = 82) | Control (n = 76) | Estimated Effect of Intervention (95% CI) | |
| Medication Appropriateness Index, mean (SD) | −6.6 (7.1) | −0.1 (4.3) | −6.5 (−8.6 to −4.3) | −7.2 (7.2) | −0.4 (4.9) | −6.9 (−9.1 to −4.7) |
| Short Physical Performance Battery score | ||||||
| No. of patients | 83 | 76 | NA | 79 | 73 | NA |
| Mean (SD) | −0.15 (1.52) | 0.03 (1.28) | −0.17 (−0.58 to 0.23) | −0.29 (1.60) | −0.18 (1.29) | −0.09 (−0.51 to 0.33) |
| Gait speed | ||||||
| No. of patients | 74 | 68 | NA | 69 | 66 | NA |
| Mean (SD), m/s | 0.02 (0.12) | 0.00 (0.08) | 0.01 (−0.02 to 0.05) | 0.02 (0.12) | −0.02 (0.09) | 0.04 (0.00 to 0.07) |
| Grip strength | ||||||
| No. of patients | 84 | 78 | NA | 80 | 75 | NA |
| Mean (SD), kg | −0.4 (2.5) | −1.3 (2.2) | 1.0 (0.2 to 1.7) | −1.4 (3.1) | −2.0 (3.9) | 0.6 (−0.4 to 1.7) |
| Digit Span Forward | ||||||
| No. of patients | 83 | 76 | NA | 78 | 74 | NA |
| Maximum span, mean (SD) | −0.07 (0.91) | −0.33 (0.62) | 0.23 (−0.01 to 0.48) | −0.08 (0.98) | −0.41 (0.72) | 0.30 (0.03 to 0.58) |
| Digit Span Backward | ||||||
| No. of patients | 83 | 76 | NA | 78 | 74 | NA |
| Maximum span, mean (SD) | 0.12 (0.77) | 0.00 (0.69) | 0.12 (−0.08 to 0.33) | 0.03 (0.93) | −0.26 (0.85) | 0.27 (−0.01 to 0.56) |
| Trail Making Test A | ||||||
| No. of patients | 60 | 58 | NA | 60 | 56 | NA |
| Mean (SD), s | −5.4 (55.5) | 11.0 (28.1) | −15.0 (−31.7 to −2.9)b | 9.3 (90.7) | 35.0 (81.5) | −23.9 (−58.5 to 7.4)b |
| Trail Making Test B | ||||||
| No. of patients | 59 | 57 | NA | 59 | 57 | NA |
| Mean (SD), s | 23.6 (131.6) | 25.0 (133.1) | −1.8 (−44.0 to 31.4)b | 16.2 (159.3) | 35.7 (133.6) | −19.5 (−61.1 to 24.1)b |
| Five Digits Test 1 | ||||||
| No. of patients | 67 | 61 | NA | 62 | 59 | NA |
| Mean (SD), s | 3.7 (28.3) | 12.2 (45.1) | −6.5 (−17.8 to 4.8)b | 8.0 (40.0) | 18.6 (53.6) | −6.7 (−21.4 to 8.0)b |
| Five Digits Test 2 | ||||||
| No. of patients | 67 | 61 | NA | 61 | 59 | NA |
| Mean (SD), s | 6.2 (43.7) | 9.7 (40.7) | −10.7 (−34.2 to 6.2)b | 5.2 (69.1) | 18.7 (61.3) | −26.6 (−69.5 to 6.2)b |
| Five Digits Test 3 | ||||||
| No. of patients | 66 | 60 | NA | 60 | 58 | NA |
| Mean (SD), s | 8.3 (66.2) | 5.4 (15.3) | −0.3 (−12.6 to 14.5)b | 4.5 (51.7) | 14.2 (37.8) | −8.9 (−26.4 to 10.1)b |
| Five Digits Test 4 | ||||||
| No. of patients | 63 | 57 | NA | 59 | 55 | NA |
| Mean (SD), s | 5.2 (66.0) | 19.0 (56.0) | −13.1 (−35.3 to 10.2)b | 17.4 (67.5) | 41.3 (83.0) | −24.4 (−52.6 to 3.2)b |
| Functional Independence Measure score, mean (SD) | −2.4 (8.5) | −1.6 (3.6) | −0.7 (−3.0 to 1.6) | −5.5 (14.2) | −3.1 (4.9) | −2.2 (−5.6 to 1.1) |
| Relative Stress Scale score | ||||||
| No. of patients | 74 | 71 | NA | 75 | 67 | NA |
| Mean (SD) | −0.2 (6.3) | −0.6 (4.8) | 0.4 (−1.4 to 2.2) | −1.0 (6.5) | −0.5 (4.6) | −0.3 (−2.2 to 1.7) |
| Change in SBP after standing 1 min | ||||||
| No. of patients | 77 | 69 | NA | 73 | 67 | NA |
| Mean (SD), mm Hg | −0.3 (22.9) | 1.4 (21.2) | −0.3 (−10.5 to 9.9) | −1.0 (21.9) | 0.4 (20.0) | 2.3 (−7.8 to 12.4) |
Abbreviations: NA, not applicable; SBP systolic blood pressure.
The results were derived by linear mixed model after adjustment for baseline values, cluster size, age, sex, severity of dementia, and use of the home nursing service at baseline.
Bootstrap (100 replications) with percentile CIs. Values for patients unable to complete the test because of cognitive difficulties were imputed as described in eTable 3 in Supplement 2.
Changes in drug regimens from baseline to week 16 are listed in Table 3, and eTable 7 in Supplement 2 summarizes drug regimens at baseline. There were more drug withdrawals, reduced dosages, and new drug regimens started in the intervention group in the period from baseline to week 16, but there were no statistically significant differences between groups in the period from week 16 to week 24. At week 16, only 1 of 84 patients (1.2%) in the intervention group had not experienced any drug changes at all compared with 28 of 79 patients (35.4%) in the control group.
Table 3. Changes in Drug Regimens From Baseline to Week 16.
| Drug Change | No. of Occurrences | |||||||
|---|---|---|---|---|---|---|---|---|
| Drug Withdrawals | Reduced Dosages | New Drug Regimens Started | Increased Dosages | |||||
| Intervention (n = 84) | Control (n = 79) | Intervention (n = 84) | Control (n = 79) | Intervention (n = 84) | Control (n = 79) | Intervention (n = 84) | Control (n = 79) | |
| Total No. of drug changes | 224 | 56 | 84 | 18 | 109 | 50 | 38 | 29 |
| Alimentary tract and metabolism (ATC group A) | 53 | 13 | 17 | 4 | 47 | 15 | 6 | 6 |
| Blood and blood-forming organs (ATC group B) | 31 | 7 | 4 | 1 | 12 | 5 | 4 | 0 |
| Cardiovascular system (ATC group C) | 68 | 14 | 35 | 5 | 19 | 3 | 13 | 5 |
| Genitourinary system and reproductive hormones (ATC group G) | 11 | 3 | 2 | 0 | 2 | 2 | 0 | 0 |
| Systemic hormonal preparations, excluding reproductive hormones and insulin (ATC group H) | 2 | 0 | 2 | 1 | 0 | 0 | 2 | 0 |
| Anti-infective agents for systemic use (ATC group J) | 4 | 0 | 0 | 0 | 0 | 1 | 0 | 0 |
| Antineoplastic and immunomodulating agents (ATC group L) | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 0 |
| Musculoskeletal system (ATC group M) | 5 | 2 | 1 | 0 | 1 | 0 | 0 | 1 |
| Nervous system (ATC group N) | 37 | 15 | 21 | 6 | 24 | 21 | 11 | 15 |
| Respiratory system (ATC group R)a | 13 | 2 | 2 | 1 | 3 | 2 | 1 | 2 |
| Various (ATC group V) | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 |
Abbreviation: ATC, Anatomical Therapeutic Classification.30
Includes codeine used as an analgesic (in combination with acetaminophen).
Discussion
This cluster randomized clinical trial shows that clinical geriatric assessments and collaborative medication reviews carried out by a geriatrician in cooperation with the patient’s FP may have a positive effect on HRQoL among home-dwelling older patients receiving polypharmacy. Secondary outcomes suggested positive effects also on several physical and cognitive tests. We believe that the intervention could be implemented within the framework of a geriatric outpatient clinic. Whether comparable results could be achieved by other health care professionals using a similar method can be the topic of future studies.
The 15D instrument assesses different dimensions of HRQoL that in our experience are perceived as important for older patients. Although 15D instrument scores declined in both groups, we found a statistically significant between-group difference in favor of the intervention group. The responder analyses indicate that a higher proportion of patients in the intervention group experienced clinically significant improvements in 15D instrument scores compared with the control group. Therefore, we regard our results to be clinically relevant.
Medication appropriateness improved in the intervention group compared with the control group. Our results also suggested a positive effect on most secondary outcomes assessing physical and cognitive functioning. There were no statistically significant effects regarding orthostatic blood pressure, falls, weight, relative stress, activities of daily living functioning, or use of formal care resources. For these outcomes, other aspects of the patient’s health and social situation might be of greater importance.
There were more hospital admissions in the intervention group than in the control group. Although the difference was not statistically significant, it cannot be excluded that this was due in part to negative effects from medication changes after the intervention. However, some patients were hospitalized because examinations carried out during the intervention procedure identified severe illness. For these patients, being admitted to the hospital was a positive event. Data on hospital admissions were incomplete for patients who withdrew consent or died, and the analysis only included patients still participating after 24 weeks. Because more patients died in the control group, it is possible that they would have contributed with hospital admissions related to their terminal illness if all patients had been considered for this outcome.
The intervention group experienced more drug withdrawals, reduced dosages, and prescriptions of new drugs compared with the control group. The number of medication changes that indicate reduced treatment intensity (eg, drug withdrawals and reduced dosages) outnumbered new prescriptions. Deprescribing is a process focused on gradual withdrawal of inappropriate or unnecessary medications, a process that becomes increasingly important the more frail a patient gets.31,32,33 At the same time, even in the context of polypharmacy, an optimized pharmacotherapy sometimes involves initiation of new drug regimens.
A possible reason for our positive results is that the collaborative medication reviews were led by a physician experienced in evaluating geriatric pharmacotherapy. Older people exposed to polypharmacy are heterogeneous, and our aim was to assess their diverse clinical problems and thereby personalize the pharmacotherapy. We presume that clinical examinations and relevant supplementary tests are necessary for medication reviews to be effective in this population. The clinician must carefully balance potential benefits and harms of all medications while taking the patient’s wishes into consideration. Our intervention was time-consuming, but the results indicate that such thorough evaluations are beneficial for patients with pronounced and complex polypharmacy. Interventions that only use standardized prescription tools or guidelines and do not include individual clinical assessments are less likely to provide health benefits.34
Another potentially important factor was the involvement of FPs, who are physicians with a key role in patient follow-up over time. This close cooperation between hospital specialists and the primary health care system is innovative and combines the strengths of both specialties. Many participating FPs knew their patients well and contributed with valuable input in the discussions on medication changes. However, most FPs had limited experience and confidence regarding performing structured evaluations of complex pharmacotherapy. Time constraints were also highlighted as a reason for why the FPs rarely performed equally comprehensive assessments. The patients included in our trial were clinically stable, and the FPs seldom had any specific concerns about their drug regimens. Although the geriatrician could suggest changes to the drug regimen, the FP retained the medical responsibility for the patient and was in charge of all medication changes. Therefore, the discussion between the 2 physicians was important to reach a common understanding, achieve implementation of suggested medication changes, and ensure further follow-up.
Strengths and Limitations
A strength of our trial was the combination of a rigorous design with an examination of real-life scenarios involving older patients with multiple comorbidities. Our focus on patient-related outcomes provides valuable knowledge regarding clinical effects of collaborative medication reviews.
This study also has some limitations. Our use of a complex, pragmatic, and not completely standardized intervention might be viewed as a limitation with regard to replication. We have provided a detailed description of the intervention in eAppendix 1 in Supplement 2. However, recommended medication changes are inevitably dependent on the competence of the physician performing the assessments. Because all interventions were carried out by a single physician, we do not know if other geriatricians would have achieved similar results. The inability to blind patients to group allocation was a possible source of bias. Although we repeatedly instructed patients not to reveal their allocation group to the research assistant, such revelations may have occurred. The recommendations resulting from the geriatric assessment were focused on medication use and not on other aspects of the patient’s situation. In a few cases, however, the FP was advised to refer patients to a specialist for further investigation. In such situations, as well as when patients were admitted to the hospital because of severe illness revealed by the geriatric assessment, the intervention could have led to improved HRQoL beyond our recommendations on medication use.
Conclusions
In older, home-dwelling patients exposed to polypharmacy, clinical geriatric assessments and comprehensive drug reviews carried out by a geriatrician in cooperation with the patient’s FP may constitute a beneficial model of care. This can result in positive effects on HRQoL as measured by the 15D instrument.
Trial Protocol
eAppendix 1. Methods
eAppendix 2. Statistical Analysis
eAppendix 3. Results
eFigure 1. Patient Assessments Carried Out by the Geriatrician
eFigure 2. Key Elements of the Medication Review Carried Out by the Geriatrician
eTable 1. Patient Inclusion and Exclusion Criteria
eTable 2. Study Assessment Procedures and Timetable
eTable 3. Details on Secondary Outcomes
eTable 4. Estimation of Power in Different Scenarios, Provided a Total of 200 Participants
eTable 5. Estimated Effect of Intervention by Various Analyses of the Primary Outcome at Week 16
eTable 6. Secondary Outcomes
eTable 7. Drugs in Use at Baseline
eReferences.
Data Sharing Statement
References
- 1.Hovstadius B, Hovstadius K, Astrand B, Petersson G. Increasing polypharmacy: an individual-based study of the Swedish population 2005-2008. BMC Clin Pharmacol. 2010;10:16. doi: 10.1186/1472-6904-10-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kantor ED, Rehm CD, Haas JS, Chan AT, Giovannucci EL. Trends in prescription drug use among adults in the United States from 1999-2012. JAMA. 2015;314(17):1818-1831. doi: 10.1001/jama.2015.13766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fried TR, O’Leary J, Towle V, Goldstein MK, Trentalange M, Martin DK. Health outcomes associated with polypharmacy in community-dwelling older adults: a systematic review. J Am Geriatr Soc. 2014;62(12):2261-2272. doi: 10.1111/jgs.13153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gallagher PF, O’Connor MN, O’Mahony D. Prevention of potentially inappropriate prescribing for elderly patients: a randomized controlled trial using STOPP/START criteria. Clin Pharmacol Ther. 2011;89(6):845-854. doi: 10.1038/clpt.2011.44 [DOI] [PubMed] [Google Scholar]
- 5.Clyne B, Smith SM, Hughes CM, et al. ; OPTI-SCRIPT Study Team . Effectiveness of a multifaceted intervention for potentially inappropriate prescribing in older patients in primary care: a cluster-randomized controlled trial (OPTI-SCRIPT study). Ann Fam Med. 2015;13(6):545-553. doi: 10.1370/afm.1838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Masnoon N, Shakib S, Kalisch-Ellett L, Caughey GE. Tools for assessment of the appropriateness of prescribing and association with patient-related outcomes: a systematic review. Drugs Aging. 2018;35(1):43-60. doi: 10.1007/s40266-018-0516-8 [DOI] [PubMed] [Google Scholar]
- 7.Lehnbom EC, Stewart MJ, Manias E, Westbrook JI. Impact of medication reconciliation and review on clinical outcomes. Ann Pharmacother. 2014;48(10):1298-1312. doi: 10.1177/1060028014543485 [DOI] [PubMed] [Google Scholar]
- 8.Huiskes VJ, Burger DM, van den Ende CH, van den Bemt BJ. Effectiveness of medication review: a systematic review and meta-analysis of randomized controlled trials. BMC Fam Pract. 2017;18(1):5. doi: 10.1186/s12875-016-0577-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Beuscart JB, Knol W, Cullinan S, et al. International core outcome set for clinical trials of medication review in multi-morbid older patients with polypharmacy. BMC Med. 2018;16(1):21. doi: 10.1186/s12916-018-1007-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rankin A, Cadogan CA, Ryan C, Clyne B, Smith SM, Hughes CM. Core outcome set for trials aimed at improving the appropriateness of polypharmacy in older people in primary care. J Am Geriatr Soc. 2018;66(6):1206-1212. doi: 10.1111/jgs.15245 [DOI] [PubMed] [Google Scholar]
- 11.Rankin A, Cadogan CA, Patterson SM, et al. Interventions to improve the appropriate use of polypharmacy for older people. Cochrane Database Syst Rev. 2018;9:CD008165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Romskaug R, Molden E, Straand J, et al. Cooperation between geriatricians and general practitioners for improved pharmacotherapy in home-dwelling elderly people receiving polypharmacy: the COOP study: study protocol for a cluster randomised controlled trial. Trials. 2017;18(1):158. doi: 10.1186/s13063-017-1900-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.World Medical Association World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA. 2013;310(20):2191-2194. doi: 10.1001/jama.2013.281053 [DOI] [PubMed] [Google Scholar]
- 14.Sintonen H. The 15D instrument of health-related quality of life: properties and applications. Ann Med. 2001;33(5):328-336. doi: 10.3109/07853890109002086 [DOI] [PubMed] [Google Scholar]
- 15.Sintonen H. The 15-D Measure of Health Related Quality of Life: Reliability, Validity and Sensitivity of Its Health State Descriptive System. Parkville, Melbourne, Australia: Centre for Health Program Evaluation; October 1994. Working Paper 41. http://business.monash.edu/__data/assets/pdf_file/0009/391374/wp41-1.pdf. Accessed January 24, 2019. [Google Scholar]
- 16.15D instrument. http://www.15d-instrument.net/15d/. Accessed May 6, 2019.
- 17.Alanne S, Roine RP, Räsänen P, Vainiola T, Sintonen H. Estimating the minimum important change in the 15D scores. Qual Life Res. 2015;24(3):599-606. doi: 10.1007/s11136-014-0787-4 [DOI] [PubMed] [Google Scholar]
- 18.Hanlon JT, Schmader KE, Samsa GP, et al. A method for assessing drug therapy appropriateness. J Clin Epidemiol. 1992;45(10):1045-1051. doi: 10.1016/0895-4356(92)90144-C [DOI] [PubMed] [Google Scholar]
- 19.Wright RM, Sloane R, Pieper CF, et al. Underuse of indicated medications among physically frail older US veterans at the time of hospital discharge: results of a cross-sectional analysis of data from the Geriatric Evaluation and Management Drug Study. Am J Geriatr Pharmacother. 2009;7(5):271-280. doi: 10.1016/j.amjopharm.2009.11.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Guralnik JM, Ferrucci L, Pieper CF, et al. Lower extremity function and subsequent disability: consistency across studies, predictive models, and value of gait speed alone compared with the Short Physical Performance Battery. J Gerontol A Biol Sci Med Sci. 2000;55(4):M221-M231. doi: 10.1093/gerona/55.4.M221 [DOI] [PubMed] [Google Scholar]
- 21.Studenski S. Bradypedia: is gait speed ready for clinical use? J Nutr Health Aging. 2009;13(10):878-880. doi: 10.1007/s12603-009-0245-0 [DOI] [PubMed] [Google Scholar]
- 22.Randolph C, Tierney MC, Mohr E, Chase TN. The Repeatable Battery for the Assessment of Neuropsychological Status (RBANS): preliminary clinical validity. J Clin Exp Neuropsychol. 1998;20(3):310-319. doi: 10.1076/jcen.20.3.310.823 [DOI] [PubMed] [Google Scholar]
- 23.Reitan RM. Validity of the Trail Making Test as an indicator of organic brain damage. Percept Mot Skills. 1958;8:271-276. doi: 10.2466/pms.1958.8.3.271 [DOI] [Google Scholar]
- 24.Sedó M. FDT: Test de los Cinco Digitos. Madrid, Spain: TEA Ediciones; 2007. [Google Scholar]
- 25.Pollak N, Rheault W, Stoecker JL. Reliability and validity of the FIM for persons aged 80 years and above from a multilevel continuing care retirement community. Arch Phys Med Rehabil. 1996;77(10):1056-1061. doi: 10.1016/S0003-9993(96)90068-4 [DOI] [PubMed] [Google Scholar]
- 26.Greene JG, Smith R, Gardiner M, Timbury GC. Measuring behavioural disturbance of elderly demented patients in the community and its effects on relatives: a factor analytic study. Age Ageing. 1982;11(2):121-126. doi: 10.1093/ageing/11.2.121 [DOI] [PubMed] [Google Scholar]
- 27.Salvi F, Miller MD, Grilli A, et al. A manual of guidelines to score the modified Cumulative Illness Rating Scale and its validation in acute hospitalized elderly patients. J Am Geriatr Soc. 2008;56(10):1926-1931. doi: 10.1111/j.1532-5415.2008.01935.x [DOI] [PubMed] [Google Scholar]
- 28.Hughes CP, Berg L, Danziger WL, Coben LA, Martin RL. A new clinical scale for the staging of dementia. Br J Psychiatry. 1982;140:566-572. doi: 10.1192/bjp.140.6.566 [DOI] [PubMed] [Google Scholar]
- 29.O’Bryant SE, Waring SC, Cullum CM, et al. ; Texas Alzheimer’s Research Consortium . Staging dementia using Clinical Dementia Rating Scale Sum of Boxes scores: a Texas Alzheimer’s Research Consortium study. Arch Neurol. 2008;65(8):1091-1095. doi: 10.1001/archneur.65.8.1091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.WHO Collaborating Centre for Drug Statistics Methodology ATC classification system. www.whocc.no/atc/structure_and_principles/. Published 2018. Accessed September 28, 2019.
- 31.Reeve E, Gnjidic D, Long J, Hilmer S. A systematic review of the emerging definition of “deprescribing” with network analysis: implications for future research and clinical practice. Br J Clin Pharmacol. 2015;80(6):1254-1268. doi: 10.1111/bcp.12732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Scott IA, Hilmer SN, Reeve E, et al. Reducing inappropriate polypharmacy: the process of deprescribing. JAMA Intern Med. 2015;175(5):827-834. doi: 10.1001/jamainternmed.2015.0324 [DOI] [PubMed] [Google Scholar]
- 33.Fried TR, Mecca MC. Medication appropriateness in vulnerable older adults: healthy skepticism of appropriate polypharmacy. J Am Geriatr Soc. 2019;67(6):1123-1127. doi: 10.1111/jgs.15798 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang-Hansen MS, Wyller TB, Hvidsten LT, Kersten H. Can screening tools for potentially inappropriate prescriptions in older adults prevent serious adverse drug events? Eur J Clin Pharmacol. 2019;75(5):627-637. doi: 10.1007/s00228-019-02624-1 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial Protocol
eAppendix 1. Methods
eAppendix 2. Statistical Analysis
eAppendix 3. Results
eFigure 1. Patient Assessments Carried Out by the Geriatrician
eFigure 2. Key Elements of the Medication Review Carried Out by the Geriatrician
eTable 1. Patient Inclusion and Exclusion Criteria
eTable 2. Study Assessment Procedures and Timetable
eTable 3. Details on Secondary Outcomes
eTable 4. Estimation of Power in Different Scenarios, Provided a Total of 200 Participants
eTable 5. Estimated Effect of Intervention by Various Analyses of the Primary Outcome at Week 16
eTable 6. Secondary Outcomes
eTable 7. Drugs in Use at Baseline
eReferences.
Data Sharing Statement