Abstract
This cohort study uses Swedish Medical Birth Register data to investigate risk of birth defects in infants born to women who had Roux-en-Y bariatric surgery between 2007 and 2014.
Maternal body mass index (BMI) and glucose control are associated with offspring birth defects.1,2 Bariatric surgery results in weight loss and glucose normalization but is also associated with nutritional deficiencies and substance abuse,3 which could cause birth defects as hypothesized based on case series.4
It could be unethical to randomize pregnant women to bariatric surgery, and it is impossible to perform a randomized trial requiring pregnancy after alternative interventions. We conducted a nationwide matched cohort study to investigate major birth defect risk in infants born to women after gastric bypass surgery vs infants born to comparable women without bariatric surgery.
Methods
We identified live-born singleton infants in the Swedish Medical Birth Register born in 2007 to 2014 to women receiving Roux-en-Y gastric bypass surgery during the same period (ascertained from the Scandinavian Obesity Surgery Register) and to women without bariatric surgery.
Using coarsened exact matching, controls were matched by major birth defects in previous pregnancies, presurgery BMI and diabetes (early-pregnancy BMI and preconception diabetes used for controls), delivery year, and a propensity score (estimated using logistic regression) including maternal age, smoking, alcohol/substance use, parity, psychiatric drugs, and number of prescription drugs.
Major birth defects, excluding genetic syndromes, defined according to the EUROCAT classification (which does not include minor defects), were identified via the National Patient Register (including inpatient and hospital-based outpatient care) and Causes of Death Register through 2015, permitting 1 year of follow-up.
Using generalized linear models with robust sandwich estimators in SAS version 9.4 (SAS Institute Inc), we estimated risk ratios in infants born after gastric bypass surgery vs control infants assuming a binomial distribution. A 2-sided P < .05 indicates statistical significance. Sensitivity analyses were conducted excluding infants with chromosomal abnormalities, restricting analysis to the first postsurgery birth, and excluding strata with high coarsened exact matching weights (>20).
The study was approved by the regional ethics committee in Stockholm, Sweden. Informed consent was not required.
Results
Matched controls were found for 97.4% (2921/2998) of postsurgery-born infants. The groups were well balanced on maternal characteristics (Table). In the surgery group, mean presurgery BMI (calculated as weight in kilograms divided by height in meters squared) was 43.5 and mean body weight was 122 kg; median surgery-to-conception interval was 1.6 years; mean weight loss was 40 kg, resulting in a body weight of 82 kg; and diabetes drug use decreased from 9.7% before surgery to 1.5% during the 6 months before conception.
Table. Maternal Characteristics for Singleton Infants Born to Women With Gastric Bypass Surgery and Matched Controls.
| Characteristicsa | Women With Gastric Bypass Surgery (n = 2921) | Matched Controls (n = 30 573)b | Standardized Difference |
|---|---|---|---|
| Maternal age, mean (SD), y | 31 (5) | 31 (6) | 0.028 |
| Maternal body mass index, No. (%)c | |||
| 30-30.9 | 6 (0.2) | 63 (0.2) | 0.000 |
| 31-31.9 | 6 (0.2) | 63 (0.2) | 0.000 |
| 32-32.9 | 7 (0.2) | 73 (0.2) | 0.000 |
| 33-33.9 | 17 (0.6) | 178 (0.6) | 0.000 |
| 34-34.9 | 39 (1.3) | 408 (1.3) | 0.000 |
| 35-39.9 | 706 (24) | 7389 (24) | 0.000 |
| 40-44.9 | 1178 (40) | 12 330 (40) | 0.000 |
| 45-49.9 | 626 (21) | 6552 (21) | 0.000 |
| ≥50 | 336 (12) | 3517 (12) | 0.000 |
| Pregnancy-related covariates, No. (%) | |||
| Primiparous | 1192 (41) | 11 574 (38) | 0.043 |
| History of major birth defectsd | 67 (3.9) | 701 (3.6) | 0.007 |
| Smokinge | 427 (15) | 4292 (14) | 0.012 |
| Year of delivery | |||
| 2007-2009 | 73 (2.5) | 764 (2.5) | 0.000 |
| 2010-2012 | 1148 (39) | 12 016 (39) | 0.000 |
| 2013-2014 | 1700 (58) | 17 793 (58) | 0.000 |
| Comorbidity, No. (%)f | |||
| Diabetes | 283 (9.7) | 2962 (9.7) | 0.000 |
| History of alcohol or substance use | 149 (5.1) | 1410 (4.6) | 0.016 |
| Drug use, No. (%)f | |||
| Any psychiatric drug | 873 (30) | 9551 (31) | 0.021 |
| No. of unique prescription drugs | |||
| 0-1 | 300 (10) | 3050 (10) | 0.007 |
| 2-3 | 495 (17) | 5283 (17) | 0.006 |
| 4-5 | 504 (17) | 5223 (17) | 0.003 |
| ≥6 | 1622 (56) | 17 017 (56) | 0.002 |
All variables originate from the Medical Birth Register, except for presurgery body mass index (from the Scandinavian Obesity Surgery Register), history of major birth defects (from the National Patient Register and Causes of Death Register), diabetes and substance use (from the National Patient Register and Prescribed Drug Register), and drug use (from the Prescribed Drug Register).
Exact matching by presurgery body mass index and diabetes (early-pregnancy body mass index and preconception diabetes used for controls), maternal history of major birth defects in previous pregnancies, delivery year, and a propensity score based on maternal age, alcohol/substance use, smoking, parity, psychiatric drug use, and number of prescription drugs.
Presurgery data for the surgery group; early-pregnancy (body mass index) or preconception (comorbidity and drug use) data for controls. Body mass index calculated as weight in kilograms divided by height in meters squared.
Only possible for parous women. Percentages were calculated based on births to parous women.
The 0.9% missing data on smoking status (surgery: n = 127; controls: n = 284) was imputed using the mode.
During the 24 months before surgery for the surgery group and during the 24 months before conception for controls.
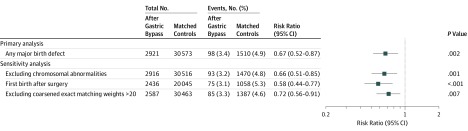
Major birth defects were recorded in 3.4% (98/2921) of infants born to mothers with gastric bypass surgery vs 4.9% (1510/30 573) of controls (risk ratio, 0.67 [95% CI, 0.52-0.87]; risk difference, −1.6% [95% CI, −2.7% to −0.6%]) (Figure). Major heart defects accounted for 60% (n = 58) of birth defects among postsurgery-born infants. There were no cases of neural tube defects in the surgery group and 20 cases (0.07%) among controls. The lower risk remained in sensitivity analyses (Figure).
Figure. Major Birth Defects in Infants Born to Women With Gastric Bypass Surgery and Matched Controls.
Matched controls: exact matching by maternal presurgery body mass index and diabetes (early-pregnancy body mass index and preconception diabetes used for controls), history of major birth defects in previous pregnancies, delivery year, and a propensity score based on maternal age, alcohol and substance use, smoking, parity, psychiatric drug use, and number of prescription drugs. For first birth after gastric bypass surgery, restriction was performed to avoid clustering effects (13% of mothers had >1 birth after surgery). Weights from coarsened exact matching were used to account for different sizes of matching strata.
Discussion
In this nationwide matched cohort study, infants born to women with Roux-en-Y gastric bypass surgery had lower risk of major birth defects than infants born to matched control women.
Obesity is associated with poor glucose control, which is teratogenic.1,2 In this study, after bariatric surgery, women lost weight and diabetes drug use decreased. If the observed association is true, a mechanism could be that surgery-induced improvements in glucose metabolism, and potentially other beneficial physiologic changes, led to a reduction of major birth defect risk to a level similar to that of the general population (3.5%).1
Concerns were raised by case series that gastric bypass–induced folate deficiency could increase risk of neural tube defects.4 In this study, no neural tube defects were found in postsurgery-born infants.
A systematic review of bariatric surgery and birth defects5 could not draw conclusions from available studies because of inadequate statistical power,6 a mix of procedures with different physiologic effects, unclear outcome ascertainment, and limited confounder control. The current study had sufficient statistical power, analyzed gastric bypass surgery only, used a well-established birth defect classification applied to prospectively recorded outcome data including defects detected after delivery hospital discharge, and matched for a range of potential confounders.
Limitations include that pregnancy termination data were not available, stillbirths were not included, and individual birth defects could not be analyzed because of small numbers.
Section Editor: Jody W. Zylke, MD, Deputy Editor.
References
- 1.Persson M, Cnattingius S, Villamor E, et al. Risk of major congenital malformations in relation to maternal overweight and obesity severity: cohort study of 1.2 million singletons. BMJ. 2017;357:j2563. doi: 10.1136/bmj.j2563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ludvigsson JF, Neovius M, Söderling J, et al. Periconception glycaemic control in women with type 1 diabetes and risk of major birth defects: population based cohort study in Sweden. BMJ. 2018;362:k2638. doi: 10.1136/bmj.k2638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Arterburn DE, Courcoulas AP. Bariatric surgery for obesity and metabolic conditions in adults. BMJ. 2014;349:g3961. doi: 10.1136/bmj.g3961 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Haddow JE, Hill LE, Kloza EM, Thanhauser D. Neural tube defects after gastric bypass. Lancet. 1986;1(8493):1330. doi: 10.1016/S0140-6736(86)91252-3 [DOI] [PubMed] [Google Scholar]
- 5.Benjamin RH, Littlejohn S, Mitchell LE. Bariatric surgery and birth defects: a systematic literature review. Paediatr Perinat Epidemiol. 2018;32(6):533-544. doi: 10.1111/ppe.12517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Johansson K, Cnattingius S, Näslund I, et al. Outcomes of pregnancy after bariatric surgery. N Engl J Med. 2015;372(9):814-824. doi: 10.1056/NEJMoa1405789 [DOI] [PubMed] [Google Scholar]