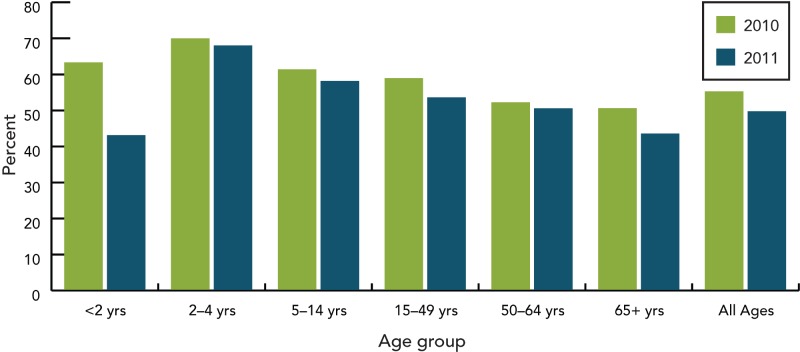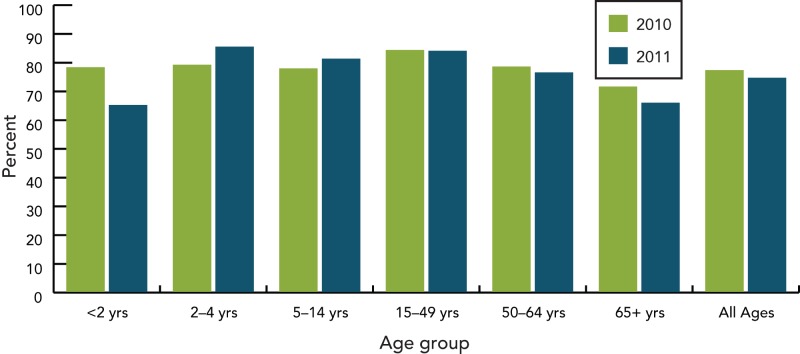†NACI Members: Dr. B. Warshawsky (Chair), Dr. I. Gemmill (Vice-Chair), Dr. N. Crowcroft, Dr. B. Henry, Dr. D. Kumar, Dr. Quach-Thanh, Dr. M. Salvadori, Dr. B. Seifert, Dr. N. Sicard, Dr. W. Vaudry, and Dr. R. Warrington.
Liaison Representatives: Dr. J. Blake (Society of Obstetricians and Gynaecologists of Canada), Dr. A. Corriveau (Council of Chief Medical Officers of Health), Dr. S. Deeks (Canada Public Health Association), Dr. A. Mawle (U.S. Centers for Disease Control and Prevention), Dr. D. Moore (Canadian Paediatric Society), Dr. A. Pham-Huy (Canadian Association for Immunization Research and Evaluation).
Ex-Officio Representatives: Dr. M. Carew (First Nations and Inuit Health Branch, Health Canada), Lt.-Col. (Dr.) P. Eagan (Canadian Forces Health Services Group, National Defence), Dr. A. Klein (Biologics and Genetic Therapies Directorate, Health Canada), Dr. B. Law (Centre for Immunization and Respiratory Infectious Diseases, Public Health Agency of Canada), Dr. B. Raymond (Centre for Immunization and Respiratory Infectious Diseases, PHAC/Canadian Immunization Committee), Dr. E. Taylor (Marketed Health Products Directorate, Health Canda) and Ms. M. St-Laurent (Centre for Immunization and Respiratory Infectious Diseases, Public Health Agency of Canada).
Preamble
The National Advisory Committee on Immunization (NACI) provides the Public Health Agency of Canada (PHAC) with ongoing and timely medical, scientific and public health advice relating to immunization. PHAC acknowledges that the advice and recommendations set out in this statement are based upon the best current available scientific knowledge and is disseminating this document for information purposes. People administering the vaccine should also be aware of the contents of the relevant product monograph(s). Recommendations for use and other information set out herein may differ from that set out in the product monograph(s) of the Canadian manufacturer(s) of the vaccine(s). Manufacturer(s) have sought approval of the vaccine(s) and provided evidence as to its safety and efficacy only when it is used in accordance with the product monographs. NACI members and liaison members conduct themselves within the context of PHAC’s Policy on Conflict of Interest, including yearly declaration of potential conflict of interest.
Summary Table of Information Contained in this NACI Statement
The following table highlights key information for immunization providers. Please refer to the remainder of the statement for details.
| 1. What |
|---|
| What is Pneumococcal Disease? |
| Streptococcus pneumoniae is a Gram-positive bacterium that is known to cause invasive disease such as sepsis, meningitis and pneumonia. Symptoms depend on the site of infection. It is a major cause of morbidity and mortality in children, the elderly population, and individuals with immunosuppression and other chronic conditions. Additional information can be found on the Public Health Agency of Canada website (www.phac-aspc.gc.ca/im/vpd-mev/pneumococcal-eng.php) |
| What are pneumococcal vaccines? |
| Two forms of pneumococcal vaccine are available; conjugated vaccines (PNEU-C-13 and PNEU-C-10) and polysaccharide vaccines (PNEU-P-23). |
| 2. Who |
| Who should be immunized? |
| This statement relates to adults (18 years and older). More specifically: 1. Adults with hematopoietic stem cell transplants (HSCT) 2. Adults with HIV 3. Adults with immunosuppressive conditions Recommendations for children are available on the NACI website (www.phac-aspc.gc.ca/publicat/ccdr-rmtc/10vol36/acs-12/index-eng.php) |
| 3. How |
|
Dose and Schedule For PNEU-C-13 vaccine, the dose is 0.5mL administered IM. |
| More specifically: 1. Adults with HSCT: 3 doses of PNEU-C-13 starting 3–9 months after transplant. These doses should be administered at least 4 weeks apart, followed by a booster dose of PNEU-P-23 12 to 18 months post transplant (6 to 12 months after the last dose of PNEU-C-13). 2. Adults with HIV: 1 dose of PNEU-C-13 followed 8 weeks later by one dose of PNEU-P-23. There is currently no evidence that a PNEU-C-13 booster dose adds any benefit. The PNEU-C-13 dose should be administered at least one year after any previous dose of PNEU-P-23. 3. Adults with immunosuppressive conditions: 1 dose of PNEU-C-13 followed 8 weeks later by one dose of PNEU-P-23. There is currently no evidence that a PNEU-C-13 booster dose adds any benefit. The PNEU-C-13 dose should be administered at least one year after any previous dose of PNEU-P-23. |
|
Precautions/Contraindications PNEU-C-13 is contraindicated in any individual with a history of anaphylaxis to any component of the vaccine including diphtheria toxoid. |
|
Co-administration PNEU-C-13 can be co-administered (using a different site) with other vaccines. |
| 4. Why |
| Current evidence supports the use of PNEU-C-13 in immunocompromised adults, as they are at a higher risk of invasive pneumococcal disease (IPD) and given the higher efficacy/effectiveness and/or immunogenicity of conjugated pneumococcal vaccine in certain immunocompromised groups. Pneumococcal infection can cause severe infections. The most effective way to prevent these infections is through immunization. |
I. Introduction
This statement will supplement previous conjugate pneumococcal statements (1–3) and provide information regarding a newly authorized indication for the use of the 13-valent conjugate vaccine against pneumococcal disease, Prevnar® 13 (PNEU-C-13): As of January 2012, PNEU-C-13 has been authorized for use in adults aged 50 years and older for the prevention of invasive pneumococcal disease (IPD), including sepsis, meningitis, bacteremia, and pneumonia (with or without empyema) with bacteremia. As new data becomes available on the use of PNEU-C-13 in adults, NACI will review these data and provide new guidance as required. The 23-valent polysaccharide vaccine (PNEU-P-23) will not be discussed at length in this statement. Recommendations for its use remain unchanged. www.phac-aspc.gc.ca/publicat/cig-gci/index-eng.php
This statement will:
• Update the epidemiology of pneumococcal disease in Canada relevant to the introduction of PNEU-C-13;
• Provide a review of the literature on the use of conjugate pneumococcal vaccines in adult populations (PNEU-C-7 and PNEU-C-13 when available); and
• Provide recommendations for the use of the 13-valent conjugate vaccine in adults (PNEU-C-13), and on the use of PNEU-P-23 related to the use of PNEU-C-13. Although PNEU-C-13 was authorized for use in adults aged 50 years and over, we will be considering adults (all age groups) with high-risk conditions for IPD and the healthy 65 years and older age group because of the burden of illness that led to the recommendation of PNEU-P-23 use in this age group.
II. Methods
NACI reviewed (4) such considerations as the burden of disease and the target population, safety, immunogenicity, efficacy, effectiveness of the vaccine(s), vaccine schedules, and other aspects of the overall immunization strategy. Following critical appraisal of individual studies, summary tables with ratings of the quality of the evidence were prepared using NACI’s methodological hierarchy (Tables 6 and 7), and proposed recommendations for vaccine use were developed. The Working Group chair presented the evidence and proposed recommendations to NACI. Following thorough review of the evidence and consultation at NACI meetings, the committee voted on specific recommendations. The description of relevant considerations, rationale for specific decisions, and knowledge gaps are described in the text of this statement. PHAC maintains documentation of these processes throughout knowledge synthesis and recommendation development.
III. Epidemiology of pneumococcal disease in Canada
III.1. Disease Description
Invasive pneumococcal disease (IPD) is a serious illness caused by the bacterium Streptococcus pneumoniae. There are currently 92 serotypes recognized worldwide, 15 of which cause the majority of disease. S. pneumoniae can be spread from an infected person to another person by droplets from the nose or mouth, by sneezing or coughing. Infections caused by S. pneumoniae are a major cause of morbidity and mortality worldwide. It is estimated that approximately one million children die of pneumococcal disease each year; the majority of which are young children in developing countries. In developed countries, a large burden of disease also exists among elderly persons.
Children and adults often are asymptomatically colonized with S. pneumoniae in their upper respiratory and nasopharynx. IPD is a severe form of infection that occurs when the bacterium invades normally sterile sites, such as the bloodstream and central nervous system. The symptoms or clinical manifestations depend on the site of infection. Invasive disease may lead to several syndromes including bacteremia, meningitis, and/or pneumonia (with or without empyema) with bacteremia.
Certain conditions predispose to complications of pneumococcal infections, including sickle-cell disease, other haemoglobinopathies, chronic renal failure, chronic liver disease, immunosuppression, anatomic or functional asplenia, cerebrospinal fluid leaks, diabetes mellitus and HIV infection. Older individuals, especially those over 65 years of age, are also at increased risk for pneumococcal disease (5).
Epidemiological data were collected from two sources of Canadian pneumococcal surveillance data: the Canadian Notifiable Disease Surveillance System (CNDSS) and the National Microbiology Laboratory (NML).
III.2. Disease Distribution
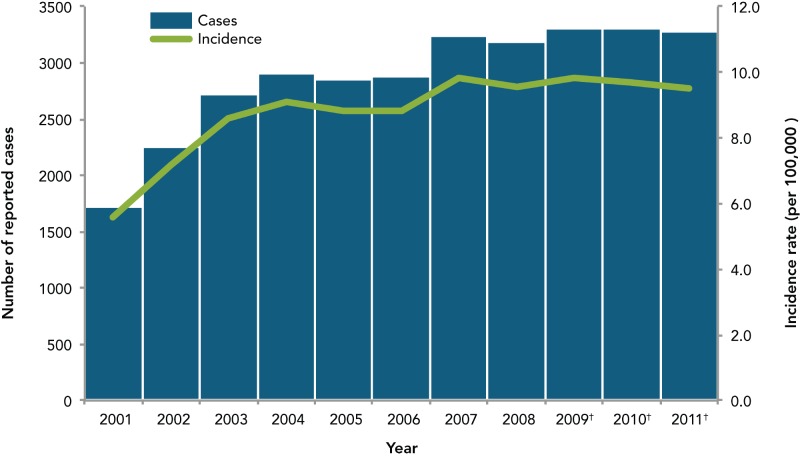
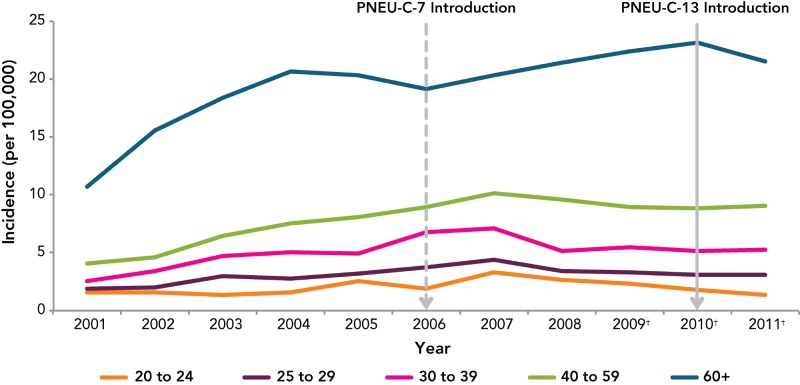
Since IPD became nationally notifiable in Canada in 2000, the number of reported cases and incidence rates have increased but have been stable over the past 5 years at an incidence rate of 10 cases per 100,000 population (Figure 1). The increase in incidence rates from 2001 to 2004 was likely—at least partially—secondary to a reporting bias, where an increase in IPD case reporting occurred as the passive surveillance system was better understood and known.
Figure 1.
Reported number of cases and incidence rate of invasive pneumococcal disease, Canada, 2001–2011
* Data obtained from the Canadian Notifiable Disease Surveillance System
† Based on preliminary data
III.3. Geographical Distribution
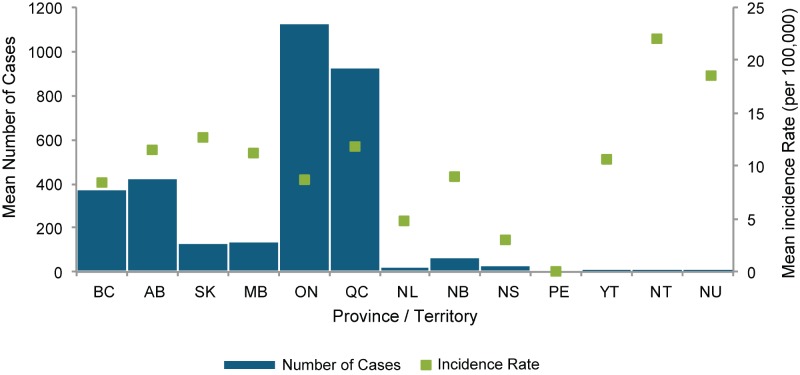
On average, Ontario and Quebec have the highest number of reported cases, which is expected given the large provincial population. However, the incidence rate per 100,000 population in the territories is high with high variability from year to year, most likely due to the small population size (Figure 2, Tables 1 and 2). Of note, the high incidence rate in British Columbia (BC), Alberta (AB), Saskatchewan (SK) and Manitoba (MB) was thought to be due to large outbreaks of serotype 5 in these provinces from 2006 to 2008. There was no outbreak explaining the higher incidence rates in Quebec.
Figure 2.
Mean number of cases and mean incidence rate of invasive pneumococcal disease (per 100,000 population), by province and territory, Canada, 2007–2011
Table 1. Number of reported cases of invasive pneumococcal disease, by province/territory and year, Canada, 2007–2011.
| Year | Province / Territory | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| BC | AB | SK | MB | ON | QC | NL | NB | NS | PE | YT | NT | NU | Canada | |
| Mean 2001–2006 | 322 | 401 | 76 | 93 | 760 | 833 | 24 | 21 | 14 | 0 | 4 | 5 | 5 | 2557 |
| 2007 | 552 | 601 | 148 | 101 | 941 | 786 | 13 | 47 | 26 | 0 | 2 | 9 | 9 | 3235 |
| 2008 | 402 | 454 | 133 | 145 | 1065 | 877 | 35 | 46 | 14 | 0 | 1 | 7 | 7 | 3186 |
| 2009† | 324 | 371 | 112 | 138 | 1224 | 989 | 36 | 75 | 20 | 0 | 7 | 11 | 4 | 3311 |
| 2010† | 258 | 340 | 126 | 176 | 1185 | 1066 | 23 | 82 | 35 | 0 | 3 | 10 | 5 | 3309 |
| 2011† | 327 | 358 | 134 | 128 | 1235 | 914 | 17 | 89 | 51 | 0 | 5 | 11 | 5 | 3274 |
| Mean 2007–2011 | 373 | 425 | 131 | 138 | 1130 | 926 | 25 | 68 | 29 | 0 | 4 | 10 | 6 | 3263 |
* Data obtained from the Canadian Notifiable Disease Surveillance System
† Based on preliminary data.
Table 2. Incidence rate of invasive pneumococcal disease (per 100,000 population), by year and Province/Territory, Canada, 2007 to 2011.
| Year | Province / Territory | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| BC | AB | SK | MB | ON | QC | NL | NB | NS | PE | YT | NT | NU | Canada | |
| Mean 2001–2006 | 8.05 | 12.04 | 9.95 | 9.52 | 7.33 | 11.43 | 4.71 | 5.63 | 2.22 | 0.07 | 11.81 | 15.84 | 16.84 | 8.81 |
| 2007 | 12.81 | 17.11 | 14.80 | 8.46 | 7.36 | 10.22 | 2.57 | 6.30 | 2.78 | 0.00 | 6.14 | 20.67 | 28.78 | 9.82 |
| 2008 | 9.17 | 12.64 | 13.12 | 12.03 | 8.23 | 11.32 | 6.91 | 6.16 | 1.49 | 0.00 | 3.02 | 16.03 | 22.13 | 9.56 |
| 2009† | 7.26 | 10.10 | 10.88 | 11.32 | 9.36 | 12.64 | 7.07 | 10.00 | 2.13 | 0.00 | 20.78 | 25.21 | 12.41 | 9.82 |
| 2010† | 5.70 | 9.14 | 12.07 | 14.26 | 8.96 | 13.48 | 4.50 | 10.89 | 3.70 | 0.00 | 8.68 | 22.82 | 15.23 | 9.70 |
| 2011† | 7.15 | 9.47 | 12.67 | 10.24 | 9.24 | 11.45 | 3.33 | 11.78 | 5.39 | 0.00 | 14.42 | 25.19 | 15.01 | 9.49 |
| Mean 2007–2011 | 8.37 | 11.62 | 12.69 | 11.27 | 8.64 | 11.83 | 4.88 | 9.04 | 3.10 | 0.00 | 10.68 | 21.98 | 18.60 | 9.68 |
* Data obtained from the Canadian Notifiable Disease Surveillance System.
† Based on preliminary data
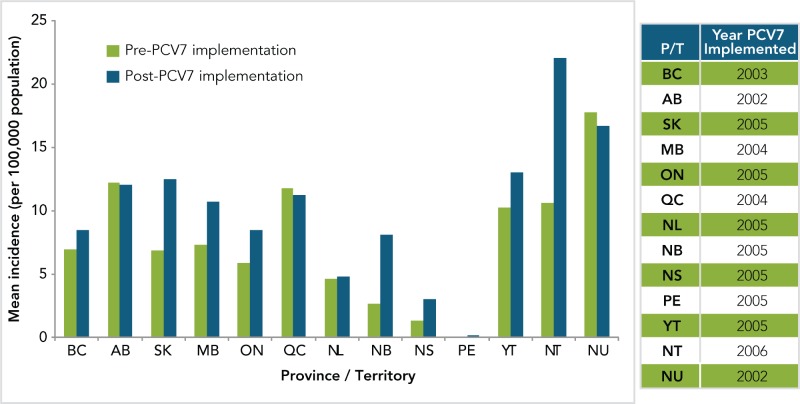
The PNEU-C-7 vaccine was introduced in all provincial and territorial pediatric vaccination programs by 2006 and PNEU-C-13 by 2011. Figure 3 shows the mean incidence of invasive pneumococcal disease by province and territory (P/T), both pre and post implementation of the PNEU-C-7 vaccine.
Figure 3.
Mean annual incidence of invasive pneumococcal disease by province and territory – all age groups (per 100,000 population), pre and post PNEU-C-7 implementation, Canada, 2001–2011
*Data obtained from the Canadian Notifiable Disease Surveillance System (Preliminary data from 2009–2011)
**The year in which the program was initiated is included in pre-implementation. Data were available from 2001 to 2011 for all Provinces /Territories.
III.4. Age Distribution
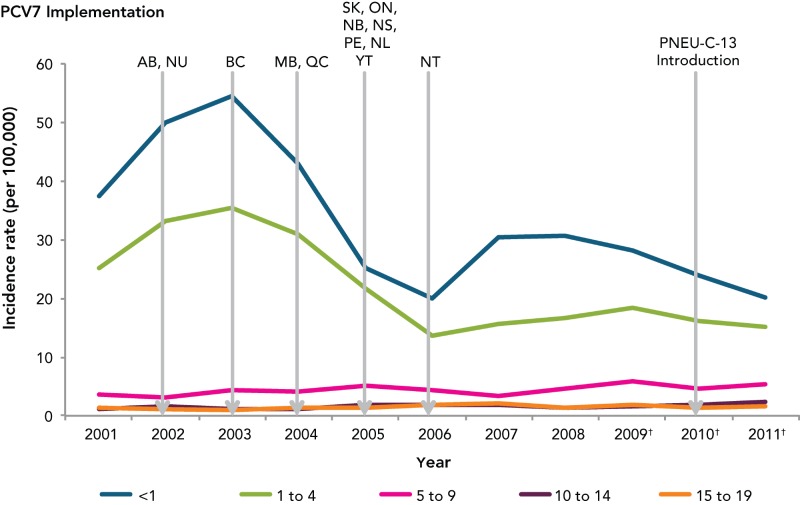
Data on IPD by age group shows that up until 2005, children < age of 5 were most affected (Figure 4). The PNEU-C-7 vaccine was introduced in all provincial and territorial vaccination programs by 2006, and national data suggests that the introduction of PNEU-C-7 led to a decreased incidence rate among <5 year olds. Although, an increase in IPD incidence rate was observed from 2006 to 2008, the incidence rate was still below rates observed before vaccine introduction. A steady increase in the incidence rate among adults aged 60 years and older has occurred since 2006.
Figure 4.
Incidence rate of invasive pneumococcal disease in < 20 years old, by age group and year, Canada, 2001–2011
* Data obtained from the Canadian Notifiable Disease Surveillance System
† Based on preliminary data
Note: PNEU-C-10 was introduced in some Canadian provinces and territories in 2009.
Incidence rates of IPD among adults seem to increase with age, with those aged 60 years and over most affected (Figure 5). Incidence rates among older individuals has increased over time in almost all age groups, most markedly among those aged 40 to 59 and 60+.
Figure 5.
Incidence rate of IPD cases aged 20 years and older, by age group and year, Canada, 2001–2011
*Data obtained from the Canadian Notifiable Disease Surveillance System.
† Based on preliminary data
Table 3. Number of reported cases of invasive pneumococcal disease in Canada, by age group and year, Canada, 2001–2011.
| Year | Age Groups | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| <1 | 1 to 4 | 5 to 9 | 10 to 14 | 15 to 19 | 20 to 24 | 25 to 29 | 30 to 39 | 40 to 59 | 60+ | |
| 2001 | 124 | 359 | 72 | 24 | 30 | 33 | 39 | 124 | 358 | 558 |
| 2002 | 164 | 465 | 62 | 33 | 25 | 34 | 42 | 166 | 421 | 828 |
| 2003 | 180 | 490 | 85 | 25 | 21 | 30 | 63 | 223 | 602 | 1002 |
| 2004 | 145 | 425 | 80 | 25 | 28 | 36 | 60 | 230 | 713 | 1161 |
| 2005 | 85 | 296 | 97 | 38 | 28 | 58 | 69 | 223 | 784 | 1171 |
| 2006 | 70 | 188 | 81 | 38 | 40 | 44 | 82 | 303 | 887 | 1133 |
| 2007 | 110 | 221 | 62 | 40 | 48 | 75 | 99 | 319 | 1008 | 1252 |
| 2008 | 114 | 237 | 81 | 29 | 30 | 61 | 79 | 234 | 957 | 1364 |
| 2009† | 107 | 271 | 104 | 31 | 41 | 54 | 78 | 252 | 897 | 1475 |
| 2010† | 92 | 242 | 83 | 36 | 29 | 42 | 73 | 235 | 900 | 1573 |
| 2011† | 78 | 233 | 96 | 46 | 36 | 32 | 74 | 246 | 921 | 1511 |
*Data obtained from the Canadian Notifiable Disease Surveillance System
† Based on preliminary data
Table 4. Incidence rate (per 100,000) of invasive pneumococcal disease by age group and year, Canada, 2001–2011.
| Year | Age Group | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| <1 | 1 to 4 | 5 to 9 | 10 to 14 | 15 to 19 | 20 to 24 | 25 to 29 | 30 to 39 | 40 to 59 | 60+ | |
| 2001 | 37.37 | 25.17 | 3.57 | 1.15 | 1.42 | 1.57 | 1.88 | 2.54 | 4.08 | 10.71 |
| 2002 | 50.00 | 33.24 | 3.12 | 1.56 | 1.18 | 1.59 | 2.02 | 3.45 | 4.66 | 15.53 |
| 2003 | 54.51 | 35.55 | 4.36 | 1.17 | 0.99 | 1.38 | 3.00 | 4.74 | 6.49 | 18.33 |
| 2004 | 42.90 | 31.04 | 4.20 | 1.17 | 1.31 | 1.63 | 2.83 | 5.00 | 7.50 | 20.70 |
| 2005 | 25.06 | 21.60 | 5.20 | 1.79 | 1.29 | 2.60 | 3.21 | 4.92 | 8.08 | 20.36 |
| 2006 | 19.99 | 13.58 | 4.44 | 1.81 | 1.81 | 1.95 | 3.75 | 6.74 | 9.00 | 19.11 |
| 2007 | 30.54 | 15.75 | 3.44 | 1.95 | 2.14 | 3.30 | 4.44 | 7.09 | 10.16 | 20.37 |
| 2008 | 30.56 | 16.56 | 4.52 | 1.44 | 1.33 | 2.67 | 3.45 | 5.17 | 9.58 | 21.43 |
| 2009† | 28.16 | 18.51 | 5.78 | 1.57 | 1.82 | 2.33 | 3.32 | 5.53 | 8.92 | 22.43 |
| 2010† | 24.03 | 16.10 | 4.60 | 1.86 | 1.30 | 1.78 | 3.05 | 5.11 | 8.89 | 23.15 |
| 2011† | 20.26 | 15.17 | 5.26 | 2.42 | 1.64 | 1.33 | 3.06 | 5.30 | 9.06 | 21.55 |
*Data obtained from the Canadian Notifiable Disease Surveillance System.
† Based on preliminary data
Data on underlying medical conditions unavailable
III.5. Serotype Distribution of IPD in Canada
PNEU-C-7 contained the following serotypes: 4, 6B, 9V, 14, 18C, 19F, and 23F. PNEU-C-13 contains these additional serotypes: 1, 3, 5, 6A, 7F, and 19A; whereas PNEU-P-23 contains these additional serotypes: 2, 8, 9N, 10A, 11A, 12F, 15B, 17F, 20, 22F, and 33F, but does not include serotype 6A.
National laboratory surveillance of IPD serotypes in Canada is currently accomplished through a voluntary, passive system where isolates are submitted to the National Microbiology Laboratory (NML) for diagnostic reference services. This passive surveillance system is limited by reporting differences between jurisdictions; variability in sample sizes amongst the smaller jurisdictions that result in small counts representing large relative proportions; and the availability of bacterial isolates submitted for testing.
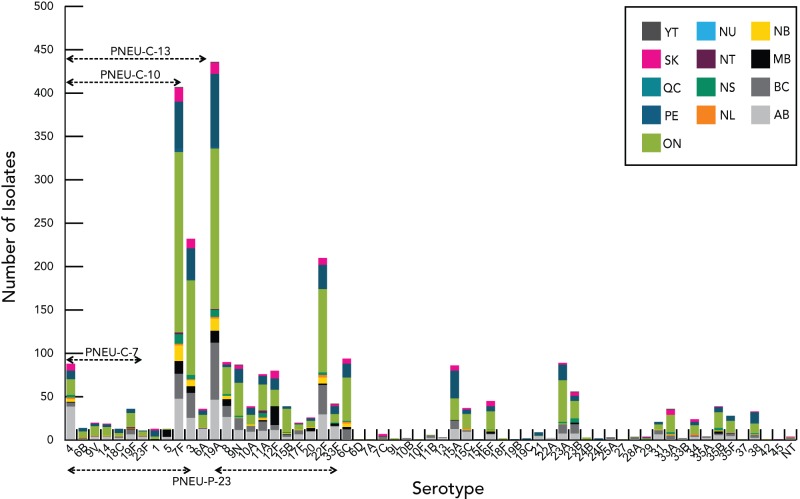
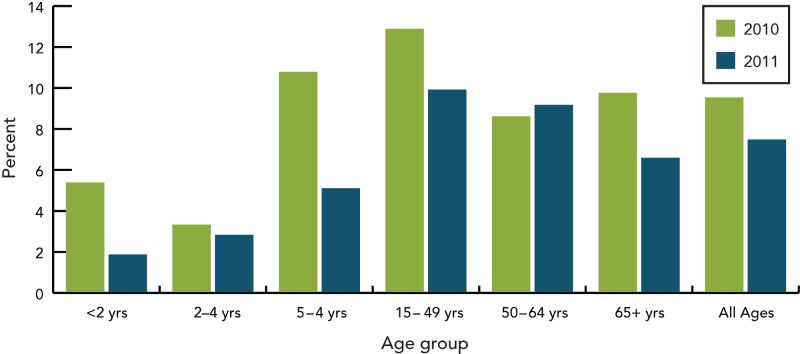
Based on isolates submitted by the provinces and territories to the NML, as well as supplemental serotyping information provided to NML by the Laboratoire de santé publique du Québec (LSPQ) and the Toronto Invasive Bacterial Disease Network (TIBDN), the most predominant serotypes in Canada in 2011 were 19A, 7F, 3 (all three contained in PNEU-C-13 and PNEU-P-23 but not in PNEU-C-7), 22F (contained in PNEU-P-23) and 6C (not a vaccine serotype), together accounting for 51% (n=1252) of IPD isolates tested in Canada (Figure 6). Serotype 19A, continues to be the most predominant serotype in 2011 among isolates from the <2 (23%, n=38), 2-4 (41%, n=59), 50-64 (16%, n=108) and ≥65 (14%, n=126) year olds, whereas 7F continues to be predominant in the 5-14 (28%, n=39) and 15-49 (21%, n=134) year old age groups. Serotype 3 is relatively prevalent in the 2-4; 50-64 and ≥65 year olds, representing 11% (n=16), 8% (n=57) and 11% (n=99) of the age group isolates respectively. Serotype 22F tends to be relatively prevalent in the older age groups 15-49 (7%, n=46), 50-64 (6%, n=43) and ≥65 (10%, n=90) year olds; whereas serotype 6C is prevalent in the ≥65 year olds representing 7% (n=60) of the isolates tested. The proportion of PNEU-C-13 serotypes has decreased from 2010 to 2011 in the <2 year-old age group accounting for 63% (n=118) in 2010 to 43% (n=70) in 2011. PNEU-P-23 serotypes have remained relatively constant in all age groups over the two years (Figures 7 to 9) (6-8).
Figure 6.
Regional distribution of S. pneumoniae serotypes in Canada, 2011
Figure 7.
Proportion of PNEU-C-7 serotypes by age group, 2010–2011
Figure 8.
Proportion of PNEU-C-13 serotypes by age group, 2010–2011
Figure 9.
Proportion of PNEU-P-23 serotypes by age group, 2010–2011
IV. Vaccine
IV.1. Preparation(s) authorized for use in Canada
Two forms of pneumococcal vaccine are available in Canada; conjugated vaccines (PNEU-C-13 and PNEU-C-10) and polysaccharide vaccines (PNEU-P-23).
Prevnar13 ®, a conjugated pneumococcal vaccine, is a sterile solution of polysaccharide capsular antigen of 13 serotypes of Streptococcus pneumoniae (1, 3, 4, 5, 6A, 6B, 7F, 9V, 14, 18C, 19A, 19F and 23F). The polysaccharides are conjugated individually to a diphtheria CRM197 protein carrier prior to compounding to a multivalent vaccine. The CRM197 protein carrier is adsorbed on aluminum phosphate as an adjuvant. Each 0.5mL dose of vaccine contains 4.4 mcg of the 6B polysaccharide, and 2.2 mcg each of the remaining polysaccharides.
PNEU-C-13 is the same as PNEU-C-7 with the exception of the additional six serotypes, a modification of the conjugation for serotype 19F to improve stability, the addition of 0.02% polysorbate (Tween) 80 (an excipient), and the addition of a succinate buffer. The syringe stopper is latex free.
PNEU-C-13 is marketed in a single dose, prefilled syringe containing 0.5mL of vaccine.
IV.2. Efficacy – direct and indirect (Table 1)
Please refer to Tables 1 for a detailed description of studies
There are currently no efficacy data available for PNEU-C-13 for any adult indication. It is anticipated that efficacy data on PNEU-C-13 for the prevention of community-acquired pneumonia in adults aged 65 years and over will be available in the next few years. A thorough literature review did not reveal efficacy studies using PNEU-C-7 or -10 in healthy adults. One vaccine efficacy study done in HIV-seropositive adults was found and is summarized below.
PNEU-C-7 has a good vaccine efficacy in HIV-positive participants. French et al. randomized 439 HIV-positive patients from Malawi, who had recovered from a documented IPD, to receive either 2 doses of PNEU-C-7 or placebo. The endpoint was a new episode of IPD from one of the vaccine serotype and 6A, which was chosen because of potential cross-protection. The adjusted vaccine efficacy of 2 doses of PNEU-C-7 compared to placebo was 69% (95%CI 16 – 89) (9).
IV.3. Effectiveness (Table 1)
There is currently no data on the effectiveness of PNEU-C-13 use in adults. The only vaccine effectiveness (VE) studies that exist were done in HIV-seropositive adults and in the elderly with PNEU-P-23 and are summarized below.
HIV-seropositive adults
Even with appropriate healthcare and treatment, IPD continues to be a significant disease in HIV-seropositive adults. Siemieniuk et al. recently published a Canadian study describing 68 cases of IPD among 1946 HIV-positive adults in Southern Alberta between 2000 and 2010. The crude incidence rate, in this study, was 342/100,000 person-years. Overall, 429 (22%) patients had never received a PNEU-P-23. Among those vaccinated, 34% received at least 2 doses of PNEU-P-23. PNEU-P-23 vaccination was associated with a decrease in IPD incidence for vaccine serotypes (720 vs. 189/100,000 person-years before vaccination and after vaccination respectively; p<0.0001) and for all serotypes (768 vs. 244/100,000 person-years; p<0.001). Viral load at time of vaccination, adjusted for CD4 count, was an independent predictor of IPD. Compared to patients who had received PNEU-P-23 at least once every 5 years, patients who had received less often than every 5 years had an adjusted OR of 2.3 in their risk of IPD (95%CI 1.0-5.4), illustrating the limited duration of PNEU-P-23 effectiveness in this population, as shown in other publications (10). Although 78% of the population was vaccinated, 74% of IPD cases were caused by PNEU-P-23 serotypes, broken down as follows: 66% of serotypes isolated from people previously immunized were caused by PNEU-P-23 serotypes compared to 86% of serotypes isolated from people who had not been previously immunized.
Previous studies have shown the sub-optimal effectiveness of PNEU-P-23 in HIV patients in various contexts. Breiman et al. reported an adjusted vaccine effectiveness of PNEU-P-23 of 49% (95%CI 12-70), using a case-control design. In their study, race played an important role in vaccine effectiveness: the adjusted VE was 74% (95%CI 8 – 93%) in Whites and 8% (95% -112 – 60%) in Blacks (11). Hung et al., in a prospective observational study of 507 HIV-positive patients (305 vaccinated and 203 not vaccinated with PNEU-P-23), reported an adjusted OR (aOR) for pneumococcal bacteremia of 0.22 (95%CI 0.02-2.56), giving a VE of 78% (95%CI -156 – 98) and an aOR for all pneumococcal disease of 0.09 (95%CI 0.01 – 0.74) for a VE of 91% (95%CI 26 – 99) (12). French et al. had randomized 1392 HIV-infected adults in Uganda to receive either PNEU-P-23 (n=697) or placebo and reported that the hazard ratio (HR) for a first IPD episode was 1.48 (95%CI 0.7 – 3.3); the HR for an IPD due to vaccine-specific serotypes was 2.14 (95%CI – 5.31), for all-cause pneumonia HR=2.02 (95%CI – 3.45), and for all pneumococcal events HR = 1.46 (95%CI 0.73 – 2.91). The authors therefore concluded that PNEU-P-23 was ineffective in preventing pneumococcal disease in HIV-positive individuals (13).
Elderly population
Vila-Corcoles et al. studied PNEU-P-23 VE in adults aged 65 years and older in a prospective cohort study (n = 4986 unvaccinated and n = 6255 vaccinated before study entry). The adjusted VE for the prevention of hospitalization for pneumonia was 19% (95%CI -30 – 49%). The VE for all-cause pneumonia was 15% (95%CI -31 – 44%), the VE for death from pneumonia was 72% (95%CI 20 – 91%), and VE for all-cause mortality was 33% (95%CI 17 – 46%) (14). In a second study where 11,241 adults aged 65 years and over were followed after receipt of PNEU-P-23, Vila-Corcoles et al. showed that the adjusted VE for IPD due to vaccine-related serotypes was 39% (95%CI -176 – 87%). The VE for the prevention of pneumococcal pneumonia was 45% (95%CI 12 – 66%), the VE for the prevention of hospitalization for pneumonia was 26% (95%CI 8 – 41%), and the VE for the prevention of overall pneumonia was 21% (95%CI 2-36%). Once stratified for influenza vaccination status, the authors reported a PNEU-P-23 VE in decreasing the risk of hospitalization for pneumonia in influenza non-vaccinated individuals of 35% (95%CI 1 – 57%) (15).
Mooney et al. also looked at PNEU-P-23 VE in adults aged 65 years and over in Scotland, using a retrospective cohort design, for the prevention of IPD. The adjusted VE (excluding high risk patients) was 61.7% (95%CI 45.1 – 73.2%), giving a number needed to vaccinate of 5,206 (95%CI 4388 – 7122). The adjusted VE for adults aged 65 – 74 years, excluding high-risk patients, was 54.4% (95%CI 20.1 – 74%) and it was 68.8% (95%CI 52.0 – 79.8%) in adults aged 75 years and older (16).
Melegaro et al. published a meta-analysis of PNEU-P-23 VE. Pooled estimates of VE against pneumococcal pneumonia using random effects model, excluding high-risk groups, gave an estimate of 16% (95%CI -50 – 50%); when high-risk groups were included, VE was -20% (95%CI -92 – 25%). The pooled VE estimate against IPD in the general elderly population was 65% (95%CI -42 – 92%); VE in high-risk groups was 20% (95%CI -187 – 78%) (17). This meta-analysis showed that PNEU-P-23 had a very limited VE in the elderly population.
IV.4. Immunogenicity (Table 1)
The following at-risk groups were reviewed separately: transplant recipients (solid organ and hematopoietic stem cells), HIV seropositive subjects, and the elderly population (aged 65 years and over).
Transplant Recipients
A) Solid Organ Transplant (SOT) Recipients:
Kumar et al. published a randomized, placebo-controlled trial (RCT) of 130 participants who had undergone a liver transplant at least 3 months before study enrolment and, if previously vaccinated with PNEU-P-23, had received this vaccine at least 5 years prior to study enrolment. Sixty-five participants were randomized to receive a placebo followed by PNEU-P-23 eight weeks later or PNEU-C-7 followed by PNEU-P-23 eight weeks later. There was no difference in the proportion of participants who had previously been vaccinated with PNEU-P-23 (8% in placebo vs. 14% in PNEU-C-7, p=0.17), who were receiving prednisone (p=0.09), or had previously received anti-thymocyte globulin therapy (p=0.06). There was no difference in the proportion of liver transplant recipients who responded to at least 1 serotype at 16 weeks (85.7% vs. 91.2% for placebo and PNEU-C-7, respectively) or in the mean number of serotypes with a response (3.7±2.3 vs. 4.4±2.2 for placebo and PNEU-C-7, respectively). There was no difference in the serotype-specific geometric mean titers (GMT – antibody level) at 16 weeks after the first vaccine dose or in the opsonophagocytic activity (OPA – considered the best functional measure, as it represents the host’s primary defense mechanism against pneumococcal infections18) when comparing placebo and PNEU-C-7, each followed by PNEU-P-23 (19).
Kumar et al. also published a double-blinded RCT of 60 participants who had undergone a renal transplant 3 months to 3 years before the study. Splenectomized patients or those who had received PNEU-P-23 in the last 5 years were excluded. The majority of participants were still receiving cyclosporine, corticosteroids, or Mycophenolate mofetil (MMF) at the time of vaccination. Thirty participants in each group were randomized to receive either PNEU-P-23 or PNEU-C-7. Baseline characteristics in both groups were similar. There was no significant difference in the serotype-specific GMT mean fold-increase at eight weeks compared to baseline for the two study groups for 5 of 7 serotypes (4, 6B, 14, 18C, 19F). GMT increase was higher after PNEU-C-7 compared to PNEU-P-23 for serotype 23F and had a trend towards better response for serotype 9 (p=0.09). The proportion of participants who responded (ELISA titers at least 2-fold increase compared to baseline with absolute titer of at least 1 µg/mL) to at least 1 serotype was not statistically significantly different in both groups (73.3% vs. 53.3% for PNEU-C-7 and PNEU-P-23, respectively; p=0.11). The median number of serotypes with a response was 2.5 (PNEU-C-7) and 1.0 (PNEU-P-23) p=0.069. There was no difference between groups for OPA titers-fold increase: an OPA response to any serotype was present in 80% of participants after PNEU-C-7 and in 83.3% of participants after PNEU-P-23 (20). A follow-up 3 years later (PNEU-C-7 [n=23] or PNEU-P-23 [n=24]) showed that there was no difference in the proportion of participants who maintained a serotype-specific response at 3 years for all 7 serotypes. The overall GMT at 3 years declined significantly for 6 of 7 serotypes compared to the eight-week titers, except for serotype 19F (not different). However, the 3-year titers were significantly greater than pre-vaccination titers for all serotypes except 14 in both groups. The rate of decline in GMT was similar in both groups (21).
Tobudic et al. (22) completed a randomized single blind controlled study to investigate the use of PNEU-C-7 or PNEU-P-23 as priming doses to a PNEU-P-23 boost one year later in adult renal transplant recipients. Transplantation had occurred at least 6 months prior to study enrollment. Exclusion criteria included splenectomy, pneumococcal vaccine within the preceding 5 years, treatment for allograft rejection, IVIG within the previous 6 weeks and an acute febrile illness within 2 weeks of study enrollment. A total of 80 participants were randomized to receive either PNEU-C-7 or PNEU-P-23 followed by a booster dose of PNEU-P-23 1 year later. Immunogenicity results were compared after the second dose of pneumococcal vaccine in both groups, but were also compared to immunogenicity results obtained after the initial dose of PNEU-P-23 received upon enrolment. Outcomes measures were a 2-fold increase in the antibody concentration from baseline and an absolute post vaccination value of > 1µg/mL. After the initial dose, in the PNEU-C-7 group, 77.1% of subjects had a response to at least one serotype as compared to 93.1% in the PNEU-P-23 group (p=0.046). One year later, after a second vaccination with PNEU-P-23, the response to at least one serotype was 87.5% in the PNEU-C-7/PNEU-P-23 group and 87.1% in the PNEU-P-23/PNEU-P-23 group. The median number of serotypes with a response was not statistically different between the two groups. Based on these findings, the authors concluded that a single dose of PNEU-P-23 should continue to be the standard of care in adult renal transplant recipients.
B) Hematopoietic Stem Cell Transplant (HSCT) Recipients:
Cordonnier et al. published a multicenter RCT where 158 allogeneic HSCT recipients were randomized to receive either PNEU-C-7 at 3, 4, and 5 months post-HSCT followed by PNEU-P-23 (early group; n=75) at 12 months post-HSCT or PNEU-C-7 at 9, 10, and 11 months post-HSCT followed by PNEU-P-23 at 18 months post-HSCT (late group; n=83). Baseline characteristics were similar in both groups but differed significantly in the proportion of participants with antibody titers ≥ 0.15 µg/mL to all 7 serotypes prior to PNEU-C-7 vaccination (44% in early group vs. 9% in late group; p<0.001). One month after the 3rd dose of PNEU-C-7, 79 and 82% of participants had an antibody titer ≥ 0.15 µg/mL to all 7 serotypes and 56 vs. 54% had a response to all 7 serotypes (GMT≥0.5µg/mL) in the early and late group, respectively. At 24 months post-HSCT, 59% vs. 83% (p=0.013) of participants had an antibody titer ≥ 0.15 µg/mL to all 7 serotypes and 34% vs. 55% had a response to all 7 serotypes (GMT≥0.5µg/mL) in the early and late group, respectively. One month after the 3rd dose of PNEU-C-7, GMTs were not different for any of the serotypes in both groups. However, at 24 months post-HSCT, GMTs were significantly lower for all serotypes except 23F in the early vaccination group (23).
Molrine et al. studied immunization of donors in a RCT of allogeneic HSCT recipients. Donors were immunized or not with PNEU-C-7 10 days prior to harvest and transplantation. All recipients received PNEU-C-7 at 3, 6, and 12 months post-HSCT. Donors for 30 HSCT recipients were immunized; donors for 35 were unimmunized, and 31 recipients were not evaluable (21 died in the peri-transplantation period, 5 were excluded prior to transplantation, 2 were excluded because of protocol violation, and 3 relapsed). There was no difference in the proportion of recipients who had a GMT ≥ 0.5 µg/mL at 3, 12, and 13 months post-HSCT when comparing both groups. Differences between GMTs were significantly higher at 6 and 12 months post transplant for serotypes 6B, 9V, 18C, 19F, and 23F when the donor was immunized. Although difficult to interpret, the authors compared GMT concentrations after PNEU-C-7 in their study with results from a previous study of allogeneic HSCT participants who had received PNEU-P-23 at 12 months post transplant. GMT concentrations were significantly higher for the 7 common serotypes after PNEU-C-7 than after PNEU-P-23 (24).
Kumar et al. randomized 64 donor-recipient allogeneic HSCT pairs (32 pairs in each group) so that each pair received either PNEU-P-23 or PNEU-C-7: donors were vaccinated at least 2 weeks prior to harvesting and HSCT recipients received 1 dose at 6 months post-HSCT. Both groups (donors and recipients) were similar in terms of baseline characteristics. Donors’ GMTs – 2 weeks after vaccination – were not different between both groups. At 6 months post-HSCT, GMTs were similar for all 7 serotypes, except for 6B (higher after PNEU-C-7). At 12 months post-HSCT, GMTs were higher after PNEU-C-7 for serotypes 14 and 18C. A response to at least 1 serotype (≥ 0.35 µg/mL) occurred in 38.1% and 0% of HSCT recipients after PNEU-C-7 and PNEU-P-23 at 6 months post-HSCT, respectively (p=0.003). At 12 months post-HSCT, the proportion of respondents was 90.8% vs. 55.6% in the PNEU-C-7 vs. PNEU-P-23 groups, respectively (p=0.02) (25).
HIV-seropositive participants
Feikin et al. randomized 90 HIV-positive patients (CD4 of at least 200 cells/µl) to receive 2 doses of a combination of placebo, PNEU-C-7, and PNEU-P-23. There were 67 evaluable participants. Participants receiving PNEU-C-7 tended to have an improved response compared to PNEU-P-23 for 3 of 5 serotypes tested (23F, 4, 9V). However, the 2nd dose of vaccine did not add any additional benefit: after the 2nd dose geometric mean concentrations (GMC) were equivalent for participants who had received PNEU-C-7/ PNEU-C-7 or PNEU-C-7/PNEU-P-23. OPA titers were significantly better after PNEU-C-7 compared to PNEU-P-23 for the 5 serotypes tested. Again, the 2nd dose did not further increase OPA titers (26).
Penaranda et al. randomized 220 HIV-positive patients (CD4: 200–500 cells/µl, viral load <5 log copies/mL) to receive either PNEU-C-7/PNEU-P-23 or PNEU-P-23 (n=110 in each group). The baseline characteristics were similar in both groups with a high proportion of patients on highly active antiretroviral therapy (HAART) (98 and 91%). There was no statistical difference in the proportion of responders at 8 weeks, based on IgG 2-fold increase with a minimum level of 1 µg/mL, and no difference in IgG avidity (27).
Crum-Cianflone et al. randomized 131 HIV-positive participants to be revaccinated with PNEU-C-7 and 73 to PNEU-P-23, 3–8 years after their previous dose of PNEU-P-23. Twenty-five HIV-negative participants who were previously unvaccinated received PNEU-C-7. The median CD4 count was 533 and 513 cells/mm3 in the PNEU-C-7 and the PNEU-P-23 group, respectively. Participants in the PNEU-C-7 group were more likely to be on HAART (84.7% vs. 56%). The proportion of subjects with a 2-fold increase in their IgG titers at day 60 for 2 of 4 serotypes (4, 9V, 14, 19F) was higher in the group revaccinated with PNEU-C-7 (57%), compared to PNEU-P-23 (36%); p=0.004. The proportion of respondent in HIV-negative participants was 88%. In HIV-infected participants, the adjusted OR (adjusted for CD4 count, age, ethnicity, prior pneumonia, HAART at baseline, and HIV viral load) for response to 2 of 4 serotypes, when comparing PNEU-C-7 and PNEU-P-23 was 2.6 (95%CI 1.4-5.0) (28).
Lesprit et al. randomized 212 HIV-positive patients with CD4 counts between 200–500 cells/µl to receive either PNEU-C-7/ PNEU-P-23 (n=106) or PNEU-P-23 alone. Baseline IgG titers and characteristics were similar in both groups. Four weeks after the last vaccine dose, IgG titers were similar in both groups except for an improved response after PNEU-C-7/ PNEU-P-23 for serotypes 18C and 23F. At week 24, IgG titers were higher in the PNEU-C-7/PNEU-P-23 group for 4 of 7 serotypes. There was a higher proportion of subjects with a response (2-fold increase in IgG levels and a minimum level of 1 mg/L) to 5 of 7 serotypes in the prime-boost arm: with a 59% response vs. 40% in the PNEU-P-23 alone; p=0.005. The same was found at 24 weeks: 30% vs. 10% responded to 5 of 7 serotypes in the prime-boost and PNEU-P-23 group, respectively (p=0.003) (29).
Elderly population
Two multicenter studies looked at the immunogenicity of the concomitant administration of the trivalent influenza vaccine (TIV) with PNEU-C-13 in healthy participants, previously not immunized with any pneumococcal vaccine, aged 50–59 years (U.S. study) (30) and 65 years and over (European study) (31). These studies compared the administration of PNEU-C-13/ TIV followed 1 month later by placebo vs. TIV/placebo followed 1 month later by PNEU-C-13. No comparison was made with PNEU-P-23. Schwarz et al. reported 13 serotypes GMCs between 1.08 (serotype 3) and 11.93 (19A) µg/mL in the concomitant group and between 1.15 (3) and 17.10 (19A) µg/mL when PNEU-C-13 was administered alone, with GMC ratios (concomitant/PNEU-C-13 alone) < 1.0 – all reaching the pre-determined non-inferiority criterion except for serotype 19F. Response to TIV was similar in both groups.31 Similarly, Frenck et al. reported GMCs between 1.15 (serotype 3) and 16.80 (19A) µg/mL in concomitant group and between 1.46 (3) and 18.84 (19A) µg/mL when PNEU-C-13 was administered alone. OPA GMTs were between 61 (serotype 3) and 2421 (6B) in the concomitant group and between 78 (3) and 3215 (6B) when PNEU-C-13 was administered alone. In this study, although GMC ratios were all <1, all serotypes reached the non-inferiority criterion. However, for OPA GMT ratios, all were also <1; and 5 of 13 serotypes (1, 5, 7F, 9V, and 19F) did not met the non-inferiority criterion (lower bound of 95% CI <0.5) (30).
Jackson et al randomized healthy adults aged 70 to 79 years, who had received PNEU-P-23 at or after their 65th birthday (at least 5 years before the study) to receive either a dose of PNEU-P-23 or PNEU-C-7 at doses of 0.5, 1, or 2 mL. Post vaccination titers were significantly higher with the 2-mL dose compared to 0.5-mL dose for 3 of 7 serotypes (6B, 9V, 23F) and significantly higher with the 1-mL and 2-mL dose, compared to 0.5 mL for serotypes 18C and 19. One year after vaccination, GMC for all serotypes were similar to pre-vaccine titers, regardless of vaccine and dose administered. There was no significant difference in GMC between PNEU-C-7 (0.5 mL) and PNEU-P-23; however, once adjusted for baseline GMC, 2 serotypes met criteria for superior immunogenicity in the PNEU-C-7 group. OPA GMTs were significantly higher after PNEU-C-7 (0.5 mL) compared to PNEU-P-23 for serotypes 9V and 23F. However, there was no significant difference in OPA GMTs 1 year after the vaccine (32).
De Roux et al. randomized 219 adults aged 70 years and over to receive either PNEU-C-7 (n=110) or PNEU-P-23 (n=109) as their first dose, followed one year later with either PNEU-C-7 or PNEU-P-23 for participants who had received PNEU-C-7 as their first vaccine dose, or PNEU-C-7 if the first dose was PNEU-P-23. Serotype specific IgG titers were significantly higher one month after a single dose of PNEU-C-7 compared with PNEU-P-23. Titers were significantly lower in the group who received PNEU-P-23 followed 1 year later by PNEU-C-7 when measured one month after PNEU-C-7 compared to the group that received a single dose of PNEU-C-7. There was no added benefit in a second dose of PNEU-C-7 one year after a primary PNEU-C-7 dose. OPA GMTs followed the same trends as IgG titers (33).
Miernyk et al. randomized Alaskan Natives aged 55–70 years, who were not previously vaccinated against pneumococcus, to receive either PNEU-P-23 (n=28), PNEU-C-7 followed 2 months later by PNEU-P-23 (n=29), or PNEU-C-7 followed 6 months later by PNEU-P-23 (n=29). Baseline characteristics of all groups were similar. There was a significant increase in GMCs to all vaccine serotypes compared to baseline among the groups (PNEU-P-23 [1 group] and PNEU-C-7 [2 groups]) and the increase was similar for the 3 groups except for serotype 1 (higher after PNEU-P-23). Two months after the final dose, there was no difference in GMCs for all serotypes among the 3 groups. OPA titers 1 month after the 1st dose and 2 months after the final dose were similar for the 3 groups (34).
Goldblatt et al. randomized patients aged 50 to 80 years who were not vaccinated against pneumococcus in the previous 5 years to receive either PNEU-C-7 and PNEU-C-7 6 months later (n = 133); PNEU-C-7 and PNEU-P-23 6 months later (n = 171), PNEU-P-23 (n = 159), or PNEU-C-7 (n = 136). One month after PNEU-C-7 or PNEU-P-23, serotypes 4, 9, and 23F had higher GMCs after PNEU-C-7; however, one year after vaccination, there was no significant difference between the 2 groups for 6 of 7 serotypes (23F had higher GMC after PNEU-P-7). One month after the 2nd dose, there was no difference in GMCs for 6 of 7 serotypes (19F had higher GMC after PNEU-C-7/PNEU-P-23 compared with PNEU-C-7/PNEU-C-7). One year after PNEU-C-7/PNEU-C-7 vs. PNEU-P-23, the proportion of subjects with serotype-specific antibody concentrations > 1µg/mL was not different (35).
Schenkein et al. randomized 45 adults aged < 45 years and 58 adults aged 65 years and over to receive PNEU-P-23. There was no difference in GMC found between the younger and the older age groups. However, there was a significantly higher antibody potency (opsonization titers) for all serotypes in the younger age group (36).
Musher et al. randomized adults who were either previously vaccinated (no minimal interval between previous dose of PNEU-P-23 and study enrolment) or not vaccinated with PNEU-P-23, aged 50–65 years and 65 years and over, to receive PNEU-P-23 or placebo. They reported that, for all serotypes, except 8 and 14 in the 65 years and over and 14 in the 50–65 years, there was no significant difference in IgG titers between groups who were vaccinated for the first time and the group that was being revaccinated, when measured at 30 and 60 days (37).
IV.5. Vaccine Administration and Schedule
The dose of PNEU-C-13 for adults is 0.5 mL administered IM once, followed 8 weeks later by PNEU-P-23 for patients at high-risk of IPD. HSCT recipients should receive a priming series composed of 3 doses of PNEU-C-13 given at least 4 weeks apart. The exact timing of the series initiation should be assessed on a case-by-case basis and discussed with hemato-oncologists. The literature shows that this priming series can be started between 3 and 9 months after HSCT. This primary series should be boosted with one dose of PNEU-P-23 given 6 to 12 months later.
IV.6. Serological Testing
There is no indication for routine pre- or post-immunization serology.
IV.7. Storage Requirements
The vaccine should be refrigerated, with the temperature maintained between 2ºC to 8ºC. Vaccine, which has been frozen, should be discarded. In cases of temporary breaks in the cold chain, PNEU-C-13 has been shown to be stable at temperatures up to 40ºC for up to 4 days (38). However, practitioners should adhere to usual provincial procedures for deciding on vaccine viability following breaks in the cold chain.
IV.8. Simultaneous Administration with Other Vaccines
PNEU-C-13 has only been administered concomitantly with TIV in the adult population (30,31) and seemed to provide a similar response to TIV, whether PNEU-C-13 was given concomitantly or alone. PNEU-C-13 serotype specific titers were not different when given alone or concomitantly in most instances. One study showed a decrease in OPA GMTs when PNEU-C-13 was given with TIV but the clinical implications for these findings are unclear.
PNEU-C-13 has been studied when given concomitantly with a number of vaccine antigens used in a childhood schedule in Canada with no adverse effect on immunogenicity or safety profile. These antigens include: diphtheria, tetanus, acellular pertussis, Haemophilus influenzae type b, inactivated poliomyelitis, hepatitis B, meningococcal serogroup C, measles, mumps, rubella and varicella (39). As with general guidance given for all vaccines, PNEU-C-13 should be administered at a different site than concomitantly administered vaccines, using a different needle and syringe.
IV.9. Adverse Events
PNEU-C-13 was administered to 2,276 adults aged 50 years and over in two distinct studies. A total of 943 received PNEU-C-13 alone; 453 aged 50–59 years and 470 aged 65 years and older. In these participants, 607/926 (66%) reported a local reaction, the most frequent being pain at the injection site in 587/923 (64%) of participants. Systemic adverse events (any) were reported in 562/912 (62%) of participants, with fatigue (305/795; 38%), headache (283/787; 36%), and new onset myalgia (312/798; 39%) being the most frequent. Fever (38 degrees C and higher) was reported in 21/663 (3%) of participants (30,31).
Safety was assessed in 6 clinical trials (including the 2 trials previously described)38: PNEU-C-13 was administered to 5,667 adults; 2,616 adults were aged 50 to 64 years and 3,051 adults were 65 years and older. Of the PNEU-C-13 recipients, 1,916 adults were previously vaccinated with PNEU-P-23 at least 3 years prior, and 3,751 adults had never been vaccinated with PNEU-P-23. The results are summarized below:
Local reactions (38):
In adults aged 50–59 years, previously unvaccinated with PNEU-P-23, pain at injection site was the most frequently reported adverse event (88.8%) but in the majority of cases (85.9%), pain was mild in intensity. Other reported local adverse events were: limitation of arm movement (40.7% - majority was mild in intensity), swelling (21.7%), and redness (15.8%).
In adults aged 60–64 years, previously unvaccinated with PNEU-P-23, pain was reported in 80.1% and 69.2% of participants (2 different studies), which was higher than after PNEU-P-23 (73.4% and 58.3%). Pain was mainly mild in intensity. Limitation of arm movement was reported in 28.5% and 23.5% (lower than after PNEU-P-23), redness in 20.2% and 12.2%, and swelling in 19.3% and 10%.
In adults aged 68 years and over, previously vaccinated with PNEU-P-23, pain was reported in 51%, limitation of arm movement in 16.2%, redness in 14.3%, and swelling in 12.8%. In adults aged
70 years and over, previously vaccinated with PNEU-P-23, pain was reported in 51.7%, limitation of arm movement in 10.5%, redness in 10.8%, and swelling in 10,4% - significantly lower than after PNEU-P-23 for limitation of arm movement (27.6%), redness (22.2%), and swelling (23.1%).
Systemic events (38):
In adults aged 50–59 years, previously unvaccinated with PNEU-P-23, headache was the most commonly reported event (65.9%), followed by fatigue (63.3%), myalgia (61.8%), arthralgia (31.5%), anorexia (25.3%), chills (19.6%), and rash (14.2%).
In the two studies done in adults aged 60–64 years who were previously unvaccinated with PNEU-P-23, fatigue was reported in 63.2% and 50.5%, headache in 54% and 49.7%, myalgia in 56.2% and 46.9%, arthralgia in 24.4% and 15.5%, chills in 23.5% and 19.9%, and rash in 16.5% and 8.6% in the 2 studies respectively. In one study, the proportion of adverse events reported after PNEU-C-13 does not differ from those reported after PNEU-P-23. In the second study, arthralgia (23.8%) and anorexia (23%) were reported more commonly after PNEU-P-23 than after PNEU-C-13.
In adults aged 68 years and over, previously vaccinated with PNEU-P-23, fatigue was reported in 34.4%, headache in 26.1%, myalgia in 25.3%, diarrhea in 14.5%, arthralgia in 12.8%, anorexia in 11.2%, rash in 8.4%, and chills in 7.5%. In adults aged 70 years and over, previously vaccinated with PNEU-P-23, myalgia was reported in 36.8%, fatigue in 34%, headache in 23.7%, arthralgia in 12.6%, anorexia in 10.4%, chills in 7.9%, and rash in 7.3%. The following were solicited adverse events that occurred significantly more commonly following PNEU-P-23 in the same study: fatigue (43.3%), myalgia (44.7%), and rash (16.4%).
Any fever was reported in 1.1 to 4.2% of participants after PNEU-C-13, compared to 1.1 to 2.3% of participants after PNEU-P-23. Most fever episodes were between 38–38.50C.
IV.10. Contraindications and Precautions
PNEU-C-13 is contraindicated in any individual with a history of anaphylaxis to any component of the vaccine, including diphtheria toxoid.
V. Summary
The evidence reviewed show improved immune response of pneumococcal conjugate vaccines compared to PNEU-P-23 for adults who have undergone a HSCT; the evidence for SOT is not as compelling. PNEU-P-23 VE in HIV-positive subjects is low – at best – but most often not protective, whereas PNEU-C-7 has shown better protection. There is no efficacy or effectiveness study on the use of pneumococcal conjugate vaccines in the elderly and results from immunogenicity studies that compared the use of PNEU-C-7 and PNEU-P-23 showed similar antibody titers one year after vaccination for the seven common serotypes. There is currently no published immunogenicity study comparing the use of PNEU-C-13 and PNEU-P-23 in the elderly population and no established correlates of protection to allow for the interpretation of immunogenicity results in this population.
VI. Recommendations
Please note that provinces and territories must consider economic factors and other local programmatic/operational factors when considering inclusion of the following recom-mendations in publicly funded immunization programs.
As new data become available, these recommendations will be reviewed and updated.
Recommendation #1:
NACI concludes that there is good evidence to recommend the use of PNEU-C-13 for HSCT recipients given the improved immunogenicity of pneumococcal conjugate vaccines compared to PNEU-P-23 in HSCT recipients. HSCT recipients should receive a primary series of 3 doses of PNEU-C-13 starting 3–9 months after transplant, after discussion with transplant specialists. The primary series (3 doses) should be administered at least 4 weeks apart, followed by a booster dose of PNEU-P-23 12 to 18 months post transplant (6 to 12 months after the last dose of PNEU-C-13). (NACI recommendation A)
Recommendation #2:
NACI concludes that there is good evidence to recommend the use of PNEU-C-13 for HIV-positive patients given the improved efficacy and effectiveness of pneumococcal conjugate vaccine (PNEU-C-7) in HIV-positive subjects. HIV-positive subjects should receive one dose of PNEU-C-13 followed 8 weeks later by one dose of PNEU-P-23. There is currently no evidence that a PNEU-C-13 booster dose adds any benefit. (NACI recommendation A)
Most published studies excluded participants who had received a dose of PNEU-P-23 in the 5 years preceding study enrolment. Therefore data on the minimal interval between a previous PNEU-P-23 dose and PNEU-C-13 is unknown. However, given the potential for decrease in antibody titers following PNEU-P-23, severity of IPD in immunocompromised individuals, and benefits from PNEU-C-13 in this patient population, NACI recommends to administer PNEU-C-13 at least one year after any previous dose of PNEU-P-23. (Experts’ opinions)
Re-immunization with one lifetime booster dose of PNEU-P-23 is recommended for those at highest risk of IPD, i.e. HIV-positive individuals. HIV-positive patients who previously received PNEU-P-23 and who require an additional dose of PNEU-P-23 should therefore receive it no sooner than 8 weeks after PNEU-C-13 and no sooner than 5 years after the most recent dose of PNEU-P-23. (Experts’ opinion)
Recommendation #3:
NACI concludes that there is currently fair evidence to recommend the use of PNEU-C-13 for subjects with other immunocompromising conditions including:
• Asplenia (anatomical or functional)
• Sickle cell disease or other hemoglobinopathies
• Congenital immunodeficiencies involving any part of the immune system, including B-lymphocyte (humoral) immunity, T-lymphocyte (cell) mediated immunity, complement system (properdin, or factor D deficiencies), or phagocytic functions
• Immunosuppressive therapy including use of long term corticosteroids, chemotherapy, radiation therapy, post-organ-transplant therapy, biologic and non-biologic immunosuppressive therapies for rheumatologic and other inflammatory diseases.
• Malignant neoplasms including leukemia and lymphoma
• Solid organ or islet cell transplant (candidate or recipient).
These immunocompromised patients should receive one dose of PNEU-C-13 followed 8 weeks later by one dose of PNEU-P-23. There is currently no evidence that a PNEU-C-13 booster dose adds any benefit. (NACI recommendation B)
Published studies excluded participants who had received a dose of PNEU-P-23 in the 5 years preceding study enrolment. Therefore data on the minimal interval between a previous PNEU-P-23 dose and PNEU-C-13 is unknown. However, given the potential for decrease in antibody titers following PNEU-P-23, severity of IPD in immunocompromised individuals, and benefits from PNEU-C-13 in this patient population, NACI recommends to administer PNEU-C-13 at least one year after any previous dose of PNEU-P-23. (Experts’ opinions)
Re-immunization with one lifetime booster dose of PNEU-P-23 is recommended for those at highest risk of IPD, i.e. those with functional or anatomic asplenia or sickle cell disease; and immunosuppression related to disease or therapy, including solid organ transplant recipients. Individuals from these high-risk categories who have previously received PNEU-P-23 and who require an additional dose of PNEU-P-23 should therefore receive it no sooner than 8 weeks after PNEU-C-13 and no sooner than 5 years after the most recent dose of PNEU-P-23. (Experts’ opinion)
There are currently no available studies for all of the above-mentioned conditions. However, based on data from solid organ transplants recipients, the recommendation has been generalized to other immunosuppressed individuals.
Recommendation #4:
NACI concludes that there is currently insufficient evidence to recommend the use of PNEU-C-13 in patients with chronic conditions without immunosuppression (listed below). However, other factors may influence decision-making. Recommendations for vaccination with PNEU-P-23 have not changed. (NACI recommendation I)
Patients with chronic conditions without immunosuppression include:
• Chronic CSF leak
• Cochlear implants
• Chronic neurologic condition that may impair clearance of oral secretions
• Chronic cardiac or pulmonary disease
• Diabetes mellitus
• Chronic kidney disease, including nephrotic syndrome
• Chronic liver disease (including hepatitis B and C, and hepatic cirrhosis due to any cause)
Recommendation #5:
NACI concludes that there is currently insufficient evidence to recommend the use of PNEU-C-13 in healthy adults aged 65 years and over. However, other factors may influence decision-making. Recommendations for vaccination with PNEU-P-23 have not changed. (NACI recommendation I)
NACI considers that the following critical information is currently missing to allow for a recommendation of PNEU-C-13 use in healthy adults aged 65 and over:
Efficacy or effectiveness data – in comparison to placebo and ideally PNEU-P-23 – on the ability of PNEU-C-13 to prevent IPD in this population
Efficacy or effectiveness data – in comparison to placebo and ideally PNEU-P-23 – on the ability of PNEU-C-13 to prevent community-acquired pneumonia in this population
Correlates of protection that would allow for the interpretation of immunogenicity data that are currently available for PNEU-C-13
The indirect protection provided by the infant and childhood PNEU-C-13 program on IPD and serotypes causing IPD in the adult population.
VII. Surveillance and Research Priorities
The epidemiology of invasive pneumococcal disease is changing in Canada and elsewhere, both due to and independent of the use of pneumococcal vaccines. Ongoing changes are expected as PNEU-C-13 vaccines are used routinely in childhood vaccination programs. Nationwide surveillance systems to detect these changes over time are essential. Optimal decisions about the use of pneumococcal vaccines requires ongoing surveillance for serotype-specific rates of invasive pneumococcal disease, and other disease syndromes, serotype-specific estimates of the efficacy of different vaccines, and continuing assessment of the effectiveness and cost-effectiveness of different vaccination schedules over time.
Surveillance and research which addresses the following outstanding questions is particularly encouraged:
• What is the efficacy and effectiveness of PNEU-C-13 in adults in the prevention of IPD or community-acquired pneumonia, compared to PNEU-P-23?
• What is the impact of conjugate pneumococcal vaccines use on IPD in adults aged 65 years and over– in particular in terms of serotypes involved?
• What is the serotype-specific efficacy and effectiveness of PNEU-C-13?
• What antibody concentrations, or other immunologic markers, correlate best with protection against invasive pneumococcal disease, pneumococcal pneumonia, acute otitis media and nasopharyngeal carriage?
• Will serotype replacement offset the benefits of pneumococcal conjugate vaccine use in children?
• What are the determinants of indirect protection of adults from pediatric vaccines?
• What factors other than immunization influence changes in the incidence of disease due to different serotypes over time?
• Does previous vaccination with PNEU-P-23 impact the effectiveness of PNEU-C-13 in adults?
• Is there a need for a booster dose of PNEU-C-13 or PNEU-P-23 in adults?
Tables
Table 5. Summary of Evidence for NACI Recommendation(s):
| Evidence for Efficacy | ||||||
|---|---|---|---|---|---|---|
| STUDY DETAILS | SUMMARY | |||||
| Study | Vaccine | Study Design | Participants | Summary of Key Findings Using Text or Data | Level of Evidence | Quality |
| French, 2010 (9) | PNEU-C-7 vs. Placebo Patients recruited from Queen Elizabeth central hospital in Malawi |
Double-blind, Randomized controlled trial Length of follow-up: Feb 2003–October 2007 (median: 1.2 year) HIV+ adolescent and adults who had recuperated from IPD episode |
496 patients of whom 439 were HIV+ were randomly assigned to receive two doses of vaccine or placebo four weeks apart Vaccine group N= 248, Age Complete follow-up available on 239 HIV+ patients |
Endpoint: New episode of IPD from one of the vaccine serotypes + 6A (in light of potential cross-protection) Primary endpoint (1st episode of IPD of vaccine serotypes or 6A) – ITT: HR (adjusted) = 0.31 (0.11-0.84) VE (adjusted) = 0.69 (0.16-0.89) Per-protocol: HR (adjusted) = 0.26 (0.08-0.78) |
Level I | Good |
| Evidence for Effectiveness | ||||||
|---|---|---|---|---|---|---|
| STUDY DETAILS | SUMMARY | |||||
| Study | Vaccine | Study Design | Participants | Summary of Key Findings Using Text or Data | Level of Evidence | Quality |
| French, 2010 (9) | PNEU-C-7 vs. Placebo Patients recruited from Queen Elizabeth central hospital in Malawi |
Double-blind, randomized controlled trial Length of follow-up: Feb 2003 –October 2007 (median: 1.2 year) HIV+ adolescent and adults who had recuperated from IPD episode |
496 patients of whom 439 were HIV+ were randomly assigned to receive two doses of vaccine or placebo four weeks apart Vaccine group N= 248, Age Complete follow-up available on 239 HIV+ patients |
Endpoint: New episode of IPD from one of the vaccine serotypes + 6A (in light of potential cross-protection) Primary endpoint (1st episode of IPD of vaccine serotypes or 6A) – ITT: HR (adjusted) = 0.31 (0.11-0.84) VE (adjusted) = 0.69 (0.16-0.89) Per-protocol: HR (adjusted) = 0.26 (0.08-0.78) |
Level I | Good |
| Breiman, 2000 (11) |
PNEU-P-23 Dose: n/a Route: n/a Sched |
Retrospective matched case-control study Study period: Feb 1992–April 1995 Determination of vaccination status: physician ns contacted and immunization record obtained |
N= 503 Cases: 176 HIV-infected adults hospitalized with IPD Controls = 327 HIV-infected adults admitted to hospital, matched on age, hospital of admission, CD4 count, clinical stage of HIV |
Vaccine effectiveness (VE): 49 (95% CI 12, 70) adjusted for all variables, no interaction term. VE with interaction term race*vaccination status: Whites: 74% (95%CI 8, 93) VE in Blacks: 8% (95%CI -112, 60) |
Level II-2 | Good |
| Hung, 2004 (12) | PNEU-P-23 Route: n/a Dose: n/a Schedule: n/a |
Prospective observational study Length of follow-up: 641 days in vaccinees 500 days in non-vaccinees Period: 1 June 2001–31 October 2002 |
N1= 305 HIV-infected vaccinated Mean age: 37 M/F: 280/25 Baseline CD4+ < 200: 54.6% AIDS associated oral infection necessitating antimicrobial therapy: 23.6% N2= 203 HIV-infected non vaccinated Mean age: 33 M/F: 183/20 Baseline CD4+ <200 – 50.6% AIDS associated oral infection necessitating antimicrobial therapy: 23.6% Setting: National Taiwan University Hospital |
Incidence of pneumococcal disease: Vaccinated: 2.1/1000 patient-years (95%CI 1.7-2.5) Unvaccinated: 21.8/1000 patient-years (95%CI 20.1-23.7) Incidence of BSI: Vaccinated: 2.1/1000 patient-years (95%CI 1.7-2.5) Unvaccinated: 7.3/1000 patient years (95%CI 7.0-7.6) A-OR for pneumococcal disease: 0.085 (95% CI, 0.01 -0.735) VE = 91.5% (26.5 -99.9) A-OR for bacteremia = 0.22 (95%CI 0.018-2.561) VE = 88% (-156%; 98.2%) A-OR for all-cause community acquired pneumonia: 1.876 (95% CI, 0.785 -4.485) New AIDS-defining opportunistic illness 0.567 (95% CI 0.217-1.484) Adjusted Hazard ratios for death: 0.733 (95% CI 0.236-2.274) |
Level II-2 | Good |
| French, 2000 (13) | PNEU-P-23 vs. placebo | Randomized, placebo controlled trial | N= 1392 HIV-infected adults in WHO clinical stage 1,2 or 3 randomized 1:1 |
First IPD episode: HR =1.48; 95% CI 0.7-3.3 IPD due to Vaccine-specific serotypes: HR=2.14; 95% CI 0.86-5.31 All cause pneumonia: HR = 2.02; 95%CI 1.19-3.45 All pneumococcal events: HR = 1.46; 95%CI 0.73-2.91 Death: HR = 1.08; 95%CI 0.87-1.33 |
Level I | Good |
| Vila-Corcoles, 2005 (14) | PNEU-P-23 | Prospective cohort study Length of follow-up: 12 months Cohort of patients followed in specific clinics. Outcomes identified through electronic databases and chart reviews |
N = 11,241 Vaccinated before entry = 4986 Not vaccinated before entry = 6255 Vaccinated after entry = 720 Baseline characteristics of community-dwelling subjects, ≥ 65 years (vaccinated vs. unvaccinated prior to entry) differed significantly in age, comorbidities and immunecompromised status were significantly greater in the vaccinated group than in the unvaccinated group. Percentage of smokers was significantly greater in the unvaccinated group. |
Adjusted Hazard Ratios for primary endpoints: Hospitalization for pneumonia (adjusted sex, age, obesity, chronic lung disease, immunecompromised status and receipt or non-receipt of influenza vaccine in the previous year): 0.81 (95% CI 0.51-1.30); p = 0.387 Overall pneumonia (adjusted for sex, age, chronic cardiopathy, chronic lung disease, immunocompromised status, obesity and receipt of influenza vaccine in the previous year): 0.85 (95% CI 0.56-1.31); p = 0.467 Death from pneumonia (adjusted for age, immunocompromised status, receipt of influenza vaccine in the previous year): 0.28 (95% CI 0.09-0.80); p = 0.018 Death from all causes (adjusted for age, immunocompromised status, sex, chronic lung disease, hypertension): 0.67 |
Level II-2 | Fair |
| Vila-Corcoles, 2006 (15) | PNEU-P-23: offered free of charge to 65 years and over but no specific campaign Vaccine: time-dependent variable |
Prospective cohort study Length of follow-up: 40 month Included all communitydwelling aged 65 years and over assigned to 1 of 8 primary health care centers Primary outcomes: IPD, CAP, pneumococcal pneumonia, death occurring within 30 days from pneumonia Diagnostic evaluations: Blood and sputum cultures, serological testing of paired sera, and urinary antigen test (as determined by treating physician) Pneumonia Acute infiltrate on CXR Cases identified using ICD-9 codes (discharge summaries) and laboratory data |
Total cohort: 11,241 N (vaccinated prior to study) = 4986 N (unvaccinated before study) = 6255 All subjects were ≥ 65 year old community dwellers Baseline characteristics between the two groups were very dissimilar. These differences were found in age group 65–74 years (46.2% vs. 62.4%. p <0.001), history of hospitalization for pneumonia in previous 2 years (1.7% vs 0.8%; p <0.01), rate of influenza vaccination in previous year |
Adjusted HR (95% CI) - 1. Incidence and risk of IPD due to vaccine-related serotypes 0.61 (0.13-2.76); p =0.517 (adjusted for immune-status, smoking, receipt of influenza vaccine in the previous year) 2. Incidence of all IPD casea: 0.60 (0.22-1.65); p = 0.324 (Adjusted for age, smoking, immune-competence, receipt of influenza vaccine in the previous year) 3. Incidence of pneumococcal pneumonia with bacteremia: 0.45 (0.15-1.40); p = 0.452 (Adjusted for age, smoking, immune-competence, receipt of influenza vaccine in the previous year) 4. Incidence of pneumococcal pneumonia without bacteremia: Adjusted HR: 0.61 (0.35-1.06); p = 0.81 (Adjusted for sex, age, history of hospitalization for pneumonia in the previous 2 years, DM, CLD) 5. Overall incidence of pneumococcal pneumonia: 0.55 (0.34-0.88); p = 0.013 (Adjusted for age, sex, CLD and immune-compromised status) 6. Risk of hospitalization due to CAP 0.74 (0.59-0.92); p = 0.007 (Adjusted for age, sex, # outpatient visits in previous 2 years, history of hospitalization for pneumonia in previous 2 years, CLD, chronic cardiopathy, immune-competence) 7. Risk for outpatient CAP: 0.90 (0.59-1.37); p = 0.619 (Adjusted for age number of outpatient visits in the previous 2 years, CLD, immune-competence, receipt of influenza vaccine in the previous year) 8. Overall risk of CAP: 0.79 (0.64-0.98); p = 0.032 adjusted for age, sex, # outpatient visits in previous 2 years, history of hospitalization for pneumonia in the previous 2 years, CLD, chronic cardiopathy, immune-competence, receipt or no-receipt of influenza vaccine. 9. Death due to pneumococcal infection: 0.50 (0.13-2.02) p =0.332 adjusted for age, sex, CLD, IC, receipt of influenza vaccine in the previous year 10. Death due to pneumonia: 0.41 (0.23-0.72); p =0.002 adjusted for variables as #9 and obesity 11. Death due to any cause: 0.97 (0.86-1.09); p =0.595 adjusted for sex, age, CLD, IC, chronic cardiopathy, presence of DM, smoking, hypertension, obesity and influenza vaccine status. Stratifying for influenza vaccination status: PNEU-P-23 VE in decreasing risk of hospitalization for pneumonia in influenza non-vaccinated: HR=0.65 (95% 0.43-0.99) Analysis restricted to influenza season: PNEU-P-23 vaccination reduced risk of Pneumococcal pneumonia (HR 0.39; 95%CI 0.21-0.74) Hospitalizations for pneumonia (HR 0.69; 95%CI 0.49-0.97) Death due to pneumonia (HR 0.44; 95%CI 0.20-0.98) |
Level II-2 | Good |
| Mooney, 2008 (16) | PNEU-P-23 | Retrospective cohort design Primary outcome measure: Incidence rates of IPD during winter 2003/2004 in the vaccine targeted population aged 65 and over. Data extracted from national HPS database for IPD cases PNEU-P-23 uptake estimated through sentinel network (7% representative sample of Scottish population) For each IPD case: chart review (primary care) and postal questionnaire |
N (IPD cases): 396 N (IPD and >/ 65 years): 170 N (IPD and ≥65 yrs + known vaccination status): 145 N (IPD cases +65 years vaccinated): 63 No significant difference in incidence of IPD among vaccine recipients aged 65+ years with or without respiratory disease, cardiovascular disease or more than one risk factor. % male (all IPD): 51.4 % female (all IPD): 36.7 Estimated vaccine uptake: Males 68.1% Females 65.5% |
Age 65+ years: Adjusted % VE (excluding very high risk): 61.7 (95% CI 45.1-73.2) NNV: 5,206 (95% CI 4388-7122) Age 65–74 years: VE (excluding very high risk): 54.4% (95% CI 20.1-74.0) VE (male): 41.0% (95% CI -31.7-73.6) VE (female): 64.6% (95% CI 21.1-84.1) Age 75+ years: VE (excluding very high risk): 68.8% (95% CI 52.0-79.8) VE (male): 60.5% (95% CI 23.3-79.6) VE (female): 73.9% (95% CI 53.3-85.4) Adjusted RR of mortality All age 65+ years: 0.89 (95% CI 0.49-1.63) NNV to prevent 1 death: 14,810 |
Level II-2 | Good |
| Melagaro, 2004 (17) | PNEU-P-23 Vs. Placebo | Meta-analysis Medline and PubMed literature search for studies on PNEU-P-23 efficacy without any year or language restriction. Outcomes evaluated: Pneumococcal pneumonia and IPD |
Studies included: RCTs = 6 Quasi-Randomized = 3 High-risk groups = 4 Inclusion: Randomized and quasirandomized controlled trials with a well-defined randomization or quasirandomization process; targeted immunecompetent or immunecompromised elderly >50 years, assessing pneumo-coccal pneumonia and/or IPD Two independent reviewers; Us |
VE against pneumococcal pneumonia n studies analyzed with this end-point: 7 Pooled VE estimates using random effects model including non-high-risk groups trials: 16% (95% CI: -50-53%) Pooled VE estimates using random effects model including high-risk groups (HRG): -20% (-92-25%) Vaccine efficacy against IPD Studies included: 6 General population): 2 HRG – randomized): 4 HRG – quasi-randomized): 2 Pooled VE estimate against IPD in the general elderly population: 65% (-42-92%) Pooled VE estimate E against IPD in HRG excluding quasirandomized: 20% (-187-78%) Pooled VE estimate against IPD in HRG including quasi-randomized: 44% (-45-79%) |
Level I | Good |
| Evidence for Immunogenicity | ||||||
|---|---|---|---|---|---|---|
| STUDY DETAILS | SUMMARY | |||||
| Study | Vaccine | Study Design | Participants | Summary of Key Findings Using Text or Data | Level of Evidence | Quality |
| Kumar, 2008 (19) | Placebo-PNEU-P-23 PNEU-C-7/PNEU-P-23 Route: IM Schedule: 0 weeks, 8 weeks Dose: 0.5 mL single dose |
Randomizeddouble-blind, Placebo-controlled trial Length of follow-up: 16 weeks Power: 80% to detect a 25% difference Response: ≥ 2-fold increase and level at least 0.35 mg/L |
N= 130 N1= 65 N2 = 65 Baseline characteristics –patients post-liver transplant ≥ 3 months earlier; PNEU-P-23 ≥ 5 years prior. Previous PNEU-P-23 (%): 8%, 14% P = 0.17 Prednisone (%): 28, 44 (P = 0.09) Previous anti-thymoglobulin therapy p = 0.064 In multivariate and univariate analysis, corticosteroid use was significantly associated with lower antibody response (OR = 5.0; 95% CI 1.29 – 19.6; p = 0.02) |
Immunogenicity Response to at least 1 serotype of ≥ 2 fold increase in titer from baseline at 16 weeks: 85.7% vs. 91.2%; p = NS Mean number of serotypes to respond at week 16: 3.7 ± 2.3 vs., 4.4 ± 2.2 (p= NS) Response to each serotype at week 16 (%): 48.2-76.8 vs. 36.8 – 64.9 (p = NS) Serotype-specific GMT at week 16 (μg/mL): Serotype 4: 0.67 vs. 1.24; p = 0.25 Serotype 6B: 1.8 vs. 2.63; p = 0.56 Serotype 9V: 2.37 vs. 1.96; p = 0.28 Serotype 14: 12.42 vs. 15.49; p = 0.84 Serotype 18C: 4.34 vs. 3.79 p = 0.46 Serotype 19F: 4.95 vs. 5.10; p = 0.78 Serotype 23 F: 1.54 vs. 2.57; p = 0.22 Opsonophagocytic activity ≥ 4 fold from baseline(%): 16.7 – 45.8 vs. 11.5 – 42.3 (p = NS for 6/7 serotypes) |
Level I | Good |
| Kumar, 2003 (20) | PNEU-C-7 PNEU-P-23 Dose = 0.5 mL Route: IM Study period: Sept–Dec 2001 |
Randomized double-blind Controlled trial Length of follow-up: 8 weeks Renal transplant: 3 months to 3 years post transplant Exclusions: splenectomy or PNEU-P-23 in past 5 years |
N = 60 – Renal transplant N= 30 (PNEU-P-23) N= 30 (PNEU-C-7) Baseline: 2 groups similar in demographic and clinical characteristics including renal function, time since transplantation and immunesuppression. Response: ELISA: ≥ 2-fold increase with absolute titer of at least 1μg/mL OPA: ≥4-fold increase with absolute value >1:8 |
Immunogenicity (IgG): Serotype-specific GMT mean fold-increase at 8 weeks compared to baseline (PNEU-C-7 vs. PNEU-P-23): Serotype 4, 6B, 14, 18C, 19F; p = NS Serotype 23F: 16.8 (range, 0.8 -160) vs. 2.2 (range, 0.4 -23.5); p = 0.046 Serotype 9: 17 vs. 2.6, p = 0.09 Proportion that responded (PNEU-C-7 vs. PNEU-P-23): Serotypes 4, 9V, 18C, 19F: PNEU-C-7>PNEU-P-23; p = NS Serotype 23F (%): 40 vs. 16.7; p = 0.046 Serotype 6B: 33.7 vs. 13.3; p = 0.067 ≥1 serotype (%): 53.3 vs. 73.3 Median number of serotypes responding: 1.0 vs. 2.5; p= 0.069 Serotype-specific OPA titers –fold increase: No significant difference between two groups for all 7 serotypes. Serotype-specific OPA response rate: 83.3 vs. 80; p = NS Median number of serotype-specific OPA response: 2.0 vs. 3.0; p = NS. |
Level I | Good |
| Kumar, 2007 (21) | PNEU-C-7 PNEU-P-23 Original dose: 0.5 mL |
Three-year follow up prospective cohort study of a RCT Renal transplant patients At 3 years, majority of patients were receiving calcineurininhibitors (95.7%) and MMF (78.7%) |
60 randomized Renal transplant 47 analyzed = PNEU-C-7 = 23 PNEU-P-23 = 24 14: lost to follow-up due to refusal of consent (7), death (1), unreachable (5) Baseline characteristics between the two groups were similar with respect to mean age, gender, time from transplant, imunonosuppression, graft loss, serum creatinine, acute rejection during study period. |
Immunogenicity: Overall (PNEU-P-23 and PNEU-C-7) serotype-specific response rate (≥ 0.35 μg/mL and 2-fold increase from baseline) to each of 7 serotypes at 8 weeks: 12.8% (Serotype 6B)-44.7% (serotype 9V) At 3 years: 42.1% (serotype 4) -85.0% (serotype 14) of initial responders at 8 weeks. No difference in the percentage of patients maintaining a serotypespecific response at 3 years between PNEU-C-7 and PNEU-P-23 groups for all 7 serotypes. P = NS Overall GMT at 8 weeks postvaccination was significantly greater for all 7 serotypes compared to baseline; p < 0.001 Overall GMT at 3 years declined significantly for 4, 6B, 9V, 14, 18C, 23F as compared to eight-week titers; p < 0.001 except serotype 19F; p = NS Significantly greater than prevaccination titers (p < 0.05) for all serotypes except serotype 14 No significant difference in decline of titer between PNEU-C-7 or PNEU-P-23 group at 3 years compared to 8 week titers; p = NS Single factor predictive of response durability by logistic regression analysis - the number of serotypes patient responded to at 8 weeks (OR 2.63, 95% CI 1.49 -4.76, p = 0.0007) At 13 months: similar for both groups except serotype 6B - 14.8 μg/mL vs. 4.22 μg/mL p = 0.046 |
Level I | Good |
| Tobudic et al. (22) | PNEU-C-7 PNEU-P-23 |
Single center randomized, single blind, controlled trial | N=80 (renal transplant recipients) 9 withdrew 9 lost to follow-up 62 analyzed |
Immunogenicity Response Rate (GMT 8 weeks post dose of PNEU-C-7 vs. PNEU-P-23) all had significant rise as compared to baseline except serotypes 6B and 19F in PNEU-P-23. Response Rate – one year post initial vaccination: (GMT) declined in for all serotypes in both vaccines Response Rate – 8 weeks post booster with PNEU-P-23 (GMT) Serotypes (4,6B,14,18C,23F) had better responses than in recipients of one dose PNEU-P-23 Vaccine response ≥ 2-fold rise in antibody concentration and an absolute value of >1ug/mL. • A response to >1 serotype after one vaccination: 77.1% PNEU-C-7 93.1% PNEU-P-23 (p=0.046) • A response to >1 serotype after boost with PNEU-P-23: 87.5% PNEU-C-7/PNEU-P-23 87.1% PNEU-P-23/PNEU-P-23 (not significant) • Median number of serotypes with response: 3.5 in PNEU-C-7/PNEU-P-23 5 in PNEU-P-23 |
Level 1 | Good |
| Cordonnier 2009 (23) | PNEU-C-7 PNEU-P-23 Schedule: 3 doses of PNEU-C-7 given 1 month apart at: 3 (early) or 9 (late) months post-transplant PNEU-P-23 at 12 (early) or 18 (late) months post-transplant |
Multicenter, randomized, non-inferiority study. Non inferiority margin = -20% Length of follow-up: 24 months Response = titer at least 0.15 mg/L 1 month after 3rd PNEU-C-7 |
N = 158 (Allogeneic HSCT) Early group = 75 Late group = 83 Analyzed after 3rd dose of PNEU-C-7: 57 (early) 57 (late). Baseline: Similar Both groups were balanced with respect to underlying disease, status of HSCT, source of stem cells, Donor age, sex and type, prior vaccination of donor, acute and chronic GVHD, and time from HSCT to first dose of PNEU-C-7. The 2 groups differed significantly in the % of patients with antibody titer ≥ 0.15 μg/mL prior to PNEU-C-7 vaccination. |
Immunogenicity Early vs. Late Response rate (antibody titers ≥ 0.15 μg/mL to all 7 PNEU-C-7 serotypes at 1 month after 3rd dose of vaccine): 79% vs. 82%, (90% CI, -15.6 to 8.6) Response rate (GMT ≥ 0.5 μg/mL to all 7 antibodies at 1 month after 3rd dose): 56% vs. 54% (p = 0.85, 90% CI -13.5 – 17) Response rate (GMT ≥ 0.15μg/mL to all 7 PNEU-C-7 serotypes at 24 months post-transplantation): 59% vs. 83%; p = 0.013 Response rate (GMT ≥ 0.5 μg/mL to all 7 serotypes at 24 months post-transplantation): 34% vs. 55%; p = 0.054 GMT for all 7 antibodies at 1 month after 3rd dose of PNEU-C-7 were not significantly different between the two groups except for 19F p < 0.001 GMT at 24 months after transplantation following the 3rd dose of PNEU-C-7 and 1 dose of PNEU-P-23 were significantly lower in the early vaccination group than in late vaccination group for 7 serotypes, except 23F p = 0.12 |
Level I | Good |
| Molrine, 2003 (24) | PNEU-C-7 Single dose IM Schedule Donors and recipients randomized to PNEU-C-7 vs. nothing 10 days pre-HSCT All HSCT recipients: PNEU-C-7 at 3, 6, 12 months post-HSCT Serotypes tested: 4, 6B, 9V, 14, 18C, 19F, 23F Non T-cell depleted allogeneic HSCT Donors who had received PNEU-P-23 in past 6 years were excluded |
Randomized-controlled trial Length of follow up: 13 months *comparison of GMT made with patients in another study with similar baseline clinical characteristics, who received PNEU-P-23 at 12 months Randomization stratified by age Subjects ≥ 2 years |
96 pairs randomized – Allo HSCT Evaluable immunized donors = 30 Evaluable unimmunized donors = 35 Non-evaluable = 31 Baseline: similar in both groups for recipient age, donor age, race, sex, diagnosis, total body irradiation, presence of GVHD, and IVIG after HSCT. *Donors immunized with a single dose of PNEU-C-7 had a significantly higher GMT to all 7 vaccine serotypes compared to unimmunized donors. |
Immunogenicity Response rate (%) – immunized vs. unimmunized donors (GMT of ≥0.5 μg/mL to all 7 serotypes) at 3 months: 57 vs. 54%; p=NS 6 months: 67 vs.36%; p=0.05 12 months: 60 vs. 35%; p= NS 13 months: 75 vs.64%; p= NS Differences in GMT between 2 groups at: 6 months (after 1st dose): significant for 5 of 7 serotypes 6B (p =0.036) 9V (p = 0.019) 18C (p = 0.039) 19F (p = 0.043) 23F (p = 0.03) 12 months (after 2nd dose): significant for 4 of 7 serotypes 6B (p = 0.013) 14 (p = 0.019) 19F (p = 0.023) 23F (p = 0.0004) *GMT concentrations in group who received PNEUMO-C-7 at 3, 6, 12 months (n= 45) was significantly higher for all 7 serotypes compared with GMT of PNEU-P-23 group of another study (n= 22) who received initial dose of PNEU-P-23 at 12 months. Factors independently influencing GMT following HSCT using linear regression analysis: Donor immunization – higher GMT Administration of IVIG prior to PNEU-C-7 after HSCT – higher GMT at 3 months Level I Fair |
Level I | Fair |
| Kumar, 2007 (25) | PNEU-P-23 PNEU-C-7 Dose: 0.5 mL Donors – recipients pairs Donors: 1 dose of either vaccine ≥ 2 weeks before harvesting Recipient: 1 dose of either vaccine 6 months after HSCT. Route: IM Serotypes tested: 4, 6B, 14, 18C, 19F, 23F. |
Randomized, double-blind, controlled trial. Pairs randomized Length of follow-up: 12 months post HSCT. Response: 2-fold increase in GMT and absolute titer ≥ 0.35 μ/mL |
N = 64 donorrecipient pairs (Allo HSCT) PNEU-C-7 pairs = 32 PNEU-P-23 pairs = 32 Baseline: Similar for donors and recipients |
Immunogenicity GMT in donor: no difference between PNEU-P-23 and PNEU-C-7 GMT at 6 months post-HSCT: No difference for 6 of 7 serotypes, except 6B (p< 0.05) – higher after PNEU-C-7 GMT at 12 months post-HSCT: No difference for 5 of 7 serotypes, except 14 (p=0.034) and 18C (p=0.013) – higher after PNEU-C-7 Response to at least 1 serotype (≥ 0.35 μg/mL at 6 months post-HSCT): PNEU-C-7 vs. PNEU-P-23 = 38.1 vs. 0%; p = 0.003 Mean # of serotypes with response at 6 months post HSCT: PNEU-C-7 vs. PNEU-P-23 = 0.7 vs. 0 Response to at least 1 serotype (≥ 0.35 μg/mL at 12 months) PNEU-C-7 vs. PNEU-P-23 = 90.9 vs. 55.6%; p = 0.02 Response rate was generally poor in both groups. *Factors shown to impact vaccine response at 12 months by multivariate analysis: vaccine type OR 8.85 (95% CI, 1.62 – 47.6) |
Level I | Good |
| Feikin, 2002 (26) | Combination of PNEU-C-7, PNEU-P-23, or placebo PNEU-C-7/PNEU-C-7 PNEU-C-7/PNEU-P-23 Placebo/PNEU-P-23 Placebo/Placebo Route: Intramuscular deltoid injection Schedule: 0, 8 weeks. |
Randomized controlled trial. Length of follow up: 24 weeks Serotypes tested: 4, 6B, 9V, 14, 23F GMC (IgG) GMT (OPA) |
N = 90 N = 67 (completed study) HIV-infected adults with ≥ 200 CD4 cells/μl received 2-dose combinations of vaccines |
Immunogenicity Serotype specific IgG (ELISA) Serotype 23F: PNEU-C-7/PNEU-C-7>Placebo/PNEU-P-23; p=0.024 Serotype 4: PNEU-C-7/ PNEU-P-23> Placebo/PNEU-P-23; p=0.011 Serotype 6B: PNEU-C-7/ PNEU-P-23> Placebo/PNEU-P-23; p=0.01 Serotype 9V: PNEU-C-7/ PNEU-P-23> Placebo/PNEU-P-23; p=0.024 *2nd dose not adding any additional benefit After 2nd dose: GMC equivalent for PNEU-C-7/PNEU-C-7 and PNEU-C-7/PNEU-P-23 OPA titers Serotype 9V: PNEU-C-7/ PNEU-P-23> Placebo/PNEU-P-23; p= 0.034 Serotype 23F: PNEU-C-7/ PNEU-P-23> Placebo/PNEU-P-23; p=0.028 Serotype 4: PNEU-C-7/PNEU-C-7> Placebo/PNEU-P-23; p <0.005 Serotype 6B: PNEU-C-7/PNEU-C-7> Placebo/PNEU-P-23; p=0.012 Serotype 9V: PNEU-C-7/PNEU-C-7> Placebo/PNEU-P-23; p=0.038 Proportion of subjects with 4-fold rises in titers after 2nd dose higher in groups who received PNEU-C-7 compared to group who received placebo/PNEU-P-23 *2nd dose not adding any increase in OPA titers |
Level I | Good |
| Penaranda 2010 (27) | PNEU-C-7 PNEU-P-23 Schedule Group 1: Single dose PNEU-C-7 at 0 weeks followed by single dose PNEU-P-23 at 4 weeks. Group 2:PNEU-P-23 at 0 week. |
Randomized, open-label multicenter study Previously unimmunized adults with HIV and CD4 count between 200–500 cells/μl and HIV viral load <5 log copies/mL Length of follow-up: 8 weeks. |
N= 220 (110 each group) Randomized to: PNEU-C-7/PNEU-P-23 4 weeks later OR PNEU-P-23 alone Patient characteristics: Similar at baseline except for proportion of patients taking HAART: 98% in group 1 vs. 91% in group 2 |
Primary end points 1) Specific IgG ≥ 2 fold 2) Specific IgG increase 2-fold AND reaching 1μg/mL Percentage of responders at 8 weeks using criteria 1 and 2: No difference between 2 groups except for serotype 23F: 26% (combined) vs. 14% responders (alone) OR = 2.2; 95% CI 1.07-4.56 No difference in avidity |
Level I | Good |
| Crum-Cianflone, 2010 (28) | Revaccination using PNEU-C-7 or PNEU-P-23 Single 3 to 8 years after previous PNEU-P-23 |
Randomized controlled trial. HIV infected randomized 2:1 (PNEU-C-7: PNEU-P-23) HIV uninfected: 1 dose PNEU-C-7 Patients: 18–60 years Length of follow-up: 180 days. Duration of study: Feb 2006–September 2008 |
N = 131 (PNEU-C-7) N = 73 (PNEU-P-23) HIV-infected adults vaccinated with PNEU-P-23 3–8 years earlier N= 25 in HIV-uninfected arm not vaccinated a priori Baseline: similar (median CD4 count > 500 cells/mm3) except for HAART (%) |
Primary end point: Proportion of subjects with positive antibody response to at least 2 of 4 (4, 9V, 14, 19F) serotypes at day 60. Positive response: ≥ 2 fold response in IgG level Proportion with response to 2 of 4 serotypes at 60 days: PNEU-C-7 = 57% vs. PNEU-P-23 = 36%; p=0.004 (HIV uninfected = 88%) Mean changes in IgG concentration from baseline to Day 60: PNEU-C-7 > PNEU-P-23 for serotypes 4, 9V, 19F (p < 0.05), not for serotype 14. Day 180: PNEU-C-7 = PNEU-P-23 HIV-uninfected group: greater response in frequency and magnitude compared to HIV-infected group. Adjusted OR for response to 2 of 4 serotypes HIV infected: PNEU-C-7 vs. PNEU-P-23: OR = 2.6 (1.4-5.0) PNEU-C-7: HIV infected vs. uninfected: OR = 0.2 (0.1-0.7) |
Level I | Good |
| Lesprit, 2007 (29) | PNEU-C-7/PNEU-P-23 at 4 weeks later OR PNEU-P-23 at 4 weeks Dose – n/a Route – n/a |
Randomized controlled trial – stratified on CD4 at baseline and prior ARV Length of follow-up: 24 weeks Inclusion: HIV1 with CD4: 200-500 VL: <50,000 copies/mL; ARV naive or stable regimen x at least 3 months; no pneumococcal infection in previous 5 years Patients screened between December 2002 and December 2003 |
N = 212 (HIV-infected patients) Randomized to: PNEU-C-7/PNEU-P-23 (106) PNEU-P-23 alone (106) Baseline: No difference in baseline characteristics between 2 groups |
Immunogenicity Outcomes a) 2-fold increase in serotype-specific IgG levels b) 2-fold increase AND level of at least 1 mg/L Baseline: IgG titers not different in both groups At week 8 (4 weeks after PNEU-P-23 in both groups): IgG titers same for both groups except for 18C and 23F (higher with combined schedule) At week 24: IgG titers same for both groups except for 18C, 23F, 14, and 19F. Proportion of subjects who met criteria b above: No difference between groups at 8 weeks, except for response to 5 of 7 serotypes (59% of combined vs. 40% of PNEU-P-23, p= 0.005) and same for levels at week 24, except for response to 5 of 7 serotypes (30% of combined vs. 10% of PNEU-P-23, p=0.003). |
Level I | Good |
| Frenck, 2012 (30) | PNEU-C-13/TIV – placebo (1 month later) vs. Placebo/TIV – PNEUC-13 1 month later 0.5 mL IM |
Randomized controlled trial Population: Healthy 50–59 years of age. No previous pneumococcal vaccination |
Multicentric – U.S. N = 1158 enrolled N = 1116 randomized N1 = 554 (PNEU-C-13/TIV) N2 = 562 (TIV then PNEU-C-13) |
PNEU-C-13 IgG serotype specific response (ELISA) 1 month after pneumococcal vaccination OPA: serotype specific response 1. GMCs between 1.15 (serotype 3) and 16.80 (19A) μg/mL in concomitant group and between 1.46 (3) and 18.84 (19A) μg/mL in PNEU-C-13 group 2. OPA GMTs between 61 (serotype 3) and 2421 (6B) in concomitant group and between 78 (3) and 3215 (6B) in PNEU-C-13 group 3. Response to TIV: similar in both groups 4. Response to PNEU-C-13: a) GMCs tended to be lower in the concomitant administration group with ratios from 0.69 to 0.89 – none reached the inferiority criterion; b) OPA GMTs also tended to be lower in the concomitant group with ratios ranging from 0.5 to 0.8 – serotypes 1, 5, 7F, 9V, and 19F met the inferiority criterion (lower bound of 95% CI <0.5) |
Level I | Good |
| Schwarz, 2011 (31) | PNEU-C-13/TIV – placebo (1 month later) vs. Placebo/ TIV – PNEU-C-13 1 month later 0.5 mL IM | Randomized controlled trial All PNEU-C-13 serotypes tested Healthy 65 years and over. No previous pneumococcal vaccination Follow-up until 1 month after last vaccine administered |
Multicentric – Europe N = 1190 participants enrolled; 1160 randomized – 580 in each group 1096 evaluable |
IgG specific GMCs in each arm – ratio of GMCs 1. GMCs between 1.08 (serotype 3) and 11.98 (19A) μg/mL in concomitant group and between 1.15 (3) and 17.10 (19A) μg/mL in PNEU-C-13 group 2. Response to TIV: similar in both groups 3. Response to PNEU-C-13: GMCs tended to be lower in the concomitant administration group with ratios from 0.65 to 0.97 – only serotype 19F was below the non-inferiority value (lower bound CI <0.5). |
Level I | Good |
| Jackson, 2007 (32) | PNEU-C-7 PNEU-P-23 Dose (PNEU-C-7): 0.1 mL or 0.5 mL or 1 mL or 2 mL or *PNEU-C-7 licensed at 0.5 mL in children Dose (PNEU-P-23): 0.5 mL IM 0.1 Ml challenge with PNEU-P-23 dose at 1 year |
Randomized controlled trial. Length of follow-up: 1 year and 4 weeks. Serotypes tested: 4, 6B, 9V, 14, 18C, 19F, 23F. Population: Aged 70–79 years who had received PNEU-P-23 on or after 65th birthday, at least 5 years prior |
N = 220 Randomized 4:1 by group of 55 to PNEU-C-7 at 0.1, 0.5, 1, and 2 mL vs. PNEU-P-23 N = 219 (analyzed after 1st vaccination) N = 208 (before challenge PNEU-P-23 vaccine) N = 203 (analyzed post-challenge vaccination) |
Immunogenicity Serotype-specific GMC post enrolment vaccination (0.5 mL vs. 1 mL and 2 mL; 95% CI) Post-vaccination titers were greater with 1- and 2-mL doses for all serotypes except serotype 14: 0.5 mL-8.8 (5.8-13.2) vs. 1 mL - 7.3(4.7-11.3) vs. 2 mL - 8.9(5.5-14.8) Post vaccination titers were significantly higher at 2-mL dose than at 0.5-mL dose for: Serotype 6B; p < 0.05 At 0.5 mL - 2.6 (1.6-4.2) 2 mL - 4.7 (2.9-7.6) Serotype 9V; p< 0.05 0.5 mL- 3.2 (2.0- 4.9) 2 mL - 6.4 (4.2-9.2) Serotype 23F: p < 0.05 0.5 mL - 3.4 (1.9-5.9) 2 mL - 7.2 (4.3-12.1) Post vaccination titers were significantly higher at 1 mL and 2 mL than 0.5 mL for: Serotype 18C; p < 0.05 0.5 mL - 5.0 (3.3-7.7) 1 mL - 8.8 (6.5-12.0) 2 mL - 8.7 (5.8-13.1) Serotype 19F; p < 0.05 0.5 mL - 3.4 (2.4-4.9 1 mL - 6.0 (4.0-8.9) 2 mL - 5.6 (4.1-7.8) After 1 year (pre-challenge): GMC by ELISA were similar to pre-vaccine, regardless of vaccine given and dose Adjusted for baseline GMC post-PCV7 vs. PPV23 No significant difference between 0.5 mL PNEU-C-7 group and PNEU-P-23 group for all serotypes but when adjusted for baseline GMC: 2 serotypes met criteria for superior immunogenicity for PNEU-C-7 vs. PNEU-P-23 GMC for 1 mL & 2 mL: PNEU-C-7 > PNEU-P-23 for all serotypes with significant difference in GMC titers for: (Data shown for PNEU-C-7 1 mL, 2 mL and PNEU-P-23): Serotype 4: 2.8 (1.6-4.9) vs. 2.8(1.8-4.3) vs. 1.1 (0.7-1.8) Serotype 9V: 5.3 (3.4-8.0) vs. 6.2 (4.1-9.2) vs. 2.7 (1.8-3.9) Serotype 18C: 8.8 (6.5- 12.0) vs. 8.7 (5.8-13.1) vs. 4.5 (3.0- 6.5) Serotype 23F: 5.9 (3.3-10.8) vs. 7.2(4.3-12.1) vs. 2.3 (1.5- 3.5) When adjusted for baseline GMC: 1 and 2 mL of PNEU-C-7 = 6 serotypes met superiority criteria compared with PNEU-P-23 PNEU-C-7 vs. PNEU-P-23 OPA GMC titers; (95% CI) Significantly higher at 0.5 mL for 2 of 7 serotypes: Serotype 9V: 332 (147- 752) vs. 59 (24-146) Serotype 23F: 4572 (2674- 7818) vs. 502 (207 -1217) Significantly higher at 1 mL and 2 mL for serotypes 4, 6B, 9V, 18C & 23F There was no significant difference in pre and post-challenge vaccination titers for all serotypes for all four groups |
Level I | Good |
| De Roux, 2008 (33) | PNEU-C-7 followed by either PNEU-C-7 or PNEU-P-23 one year later OR PNEU-P-23 followed by PNEU-C-7 one year later |
Randomized open-labeled phase 2 study Length of follow-up: 13 months Population: 70 years and older with NO previous pneumococcal vaccination |
N= 219 ambulatory elderly adults ≥ 70 years PNEU-C-7 1st dose = 110 PNEU-P-23 1st dose = 109 PNEU-C-7/ PNEU-C-7 = 43 PNEU-C-7/ PNEU-P-23 = 38 PNEU-P-23/ PNEU-C-7 = 78 Baseline: pre-immunization antibody levels were similar between initial groups. Mean age at entry was 75 years. |
Immunogenicity Serotype specific IgG antibody level (μg/mL) (measured 1 month after last dose): Single dose PNEU-C-7 vs. single dose PNEU-P-23: Significantly higher (p < 0.01) for serotype 4, 6B, 9V, 14, 18C & 23 F. Difference not significant for serotype 19F PNEU-P-23/ PNEU-C-7 vs. single dose PNEU-C-7: Significantly lower for all serotypes; p ≤ 0.05 PNEU-C-7/ PNEU-C-7 vs. single dose PCV7: Significantly lower for serotype 9V; p = 0.02 Difference not significant for serotypes 4, 6B, 9V, 14, 18C & 23F. PNEU-C-7/PNEU-P-23 vs. single dose PNEU-C-7: Significantly higher for serotypes 19F and 23 F. No significant difference for serotypes 4, 6B, 9V, 14, 18C. GMTs of OPA after 1st and 2nd doses: PNEU-C-7 vs. PNEU-P-23: Significantly higher for all serotypes tested; p < 0.01 - except 6B and 19F PNEU-P-23/ PNEU-C-7 vs. single dose PNEU-C-7: Significantly lower for all serotypes tested (p < 0.01) except serotype 19F PNEU-C-7/ PNEU-C-7 vs. single dose PNEU-C-7: No difference for all serotype except 23 F; p < 0.01. PNEU-C-7/PNEU-P-23 vs. Single dose PNEU-P-23: Significantly higher for serotypes 4, 9V, 14, 18C, 19F, 23 F; p < 0.01 Difference not significant for serotype 6B. |
Level I | Good |
| Miernyk, 2009 (34) | PNEU-P-23 PNEU-C-7 Dose: 0.5 mL IM Group 1: PNEU-P-23 Group 2: PNEU-C-7/PNEU-P-23 2 months later Group 3: PNEU-C-7/PNEU-P-23 6 months later Serotypes tested: 1, 4, 6B, 14, 19F. |
Randomized controlled trial Population: Alaskan Natives aged 55–70 years Not previously vaccinated against pneumo |
PNEU-P-23 = 28 PNEU-C-7/PNEU-P-23 2 months later = 29 PNEU-C-7/PNEU-P-23 6 months later =29 Baseline: study groups did not differ significantly in age or gender. |
Immunogenicity Serotype-specific IgG (GMC μg/mL) (95% CI) No difference in baseline IgG GMCs for serotypes 1, 4, 6B, 14 & 19F between groups 1,2 &3; p > 0.3 *2 months after 1st dose (group 1 = PNEU-P-23 vs. group 2&3 = PNEU-C-7) All study groups showed significant increases in GMCs to all vaccine specific serotypes compared to baseline and were similar in all groups except for: Serotype 1: 1.95 (1.21-3.15) after PNEU-P-23 vs. 0.43 (0.31-0.59) after PNEU-C-7; p < 0.001 No significant difference between groups 1,2 & 3 for Serotypes 4, 6b, 14, 19F; p> 0.2 *2 months after final dose: No difference between groups 1, 2&3 for all serotypes tested; p> 0.3 Median OPA (Range) Baseline OPA s against all serotypes tested were similar among all 3 study groups; p > 0.05 *2 months after 1st dose (group 1 vs. groups 2&3) All study groups showed significant increase in GMCs against vaccine specific serotypes except Serotype 1 which showed no increase in all 3 groups Serotype 4 which showed nonsignificant increase in group 3; p = 0.122 No difference between groups 1, 2, &3 for all serotypes tested; p > 0.15 *2 months after final dose: No difference between groups 1, 2, &3 for all serotypes tested; p > 0.2 |
Level I | Good |
| Goldblatt, 2009 (35) | PNEU-C-7 PNEU-P-23 Group 1: PNEU-C-7/ PNEU-C-7 6 months later Group 2: PNEU-C-7/ PNEU-P-23 6 months later Group 3: PNEU-P-23 Group 4: PNEU-C-7 |
Randomized open-labeled controlled trial Length of follow-up: 12 months IgG titers cut-off: 0.35ug/mL |
Group 1=133 Group 2=171 Group 3=159 Group 4=136 Study patients were 50–80 yrs, did not receive either pneumo vaccine in 3 ≤ 5 yrs before recruitment. There were similar numbers of individuals in the 50–59, 60–69, 70–79 age in each group. No baseline characteristics provided |
Immunogenicity GMC (serotype-specific IgG) at 4–6 weeks after vaccination (PNEU-C-7 vs PNEU-P-23): Serotypes 4,9, 23F: PNEU-C-7 > PNEU-P-23 Serotype 19F: PNEU-P-23 > PNEU-C-7 GMC at 1 year (PNEU-C-7 vs. PNEU-P-23) No significant difference for serotypes 4, 6B, 9V, 14, 18C, 19F. Serotype 23F: 4.11 (PNEU-C-7; 95%CI, 3.19-5.29) vs. 2.3 (PNEU-P-23; 95% CI1.81-2.92) GMC 4–6 weeks after 2nd dose: Serotype 19 F: 9.9 (PNEU-C-7/PNEU-P-23; 95% CI 8.27 -11.85) > 6.74 (PNEU-C-7/PNEU-C-7; 95% CI 5.76 -7.19) No difference between PNEU-C-7/PNEU-P-23 and PNEU-C-/PNEU-C-7 for remaining 6 serotypes. Proportion of subjects with serotype-specific IgG concentration > 1.0 μg/mL at 12 months after PNEU-C-7/PNEU-C-7 vs. PNEU-P-23 alone for serotypes: 4B: 0.74 (0.65-0.81) vs. 0.67 (0.59-0.75) 6B: 0.86 (0.79-0.92) vs. 0.80 (0.73-0.86) 9V: 0.94 (0.89-0.98) vs. 0.84 (0.77-0.89) 14: 0.98 (0.94-1.0) vs. 0.95 (0.91-0.98) 18C: 0.95 (0.9-0.98) vs. 0.91 (0.86-0.95) 19F: 0.97 (0.92-0.99) vs. 0.96 (0.91-0.99) 23F: 0.86 (0.79-0.92) s. 0.74 (0.66-0.81) Overall PNEU-C-7/PNEU-C-7 = PNEU-P-23 (p = NS) |
Level I | Good |
| Schenkein, 2008 (36) | PNEU-P-23 0.5 mL x1 IM Serotypes tested: 4, 6B, 9V, 14, 18C, 19F, 23F tested 28 days post vaccine |
Randomized controlled trial Comparative study of immune response in those aged 65 years and above vs. aged 45 years and less |
<45 years =45 ≥ 65 years =58 Older adults were community dwelling individuals taken from control group subset of a previously reported randomized, double-blind, placebo controlled trial. Younger adults were healthy volunteers recruited from lab research personnel from large university medical center. |
Immunogenicity Serotype-specific IgG GMC: No difference in GMC between younger and older age group for serotypes 4, 6B, 9V, 14, 18C & 23F. Serotype 19F: 9.14 vs. 5.24; p = 0.001 Serotype specific OPA titers (95% CI) Serotype 4: 1735 (1074-2804) vs. 453 (250-821); p < 0.01 Serotype 6B: 1707(1046-2787) vs. 377 (188-756); p < 0.01 Serotype 9V: 3957(1921-8151) vs. 411(203-832); p < 0.001 Serotype 14: 3715 (1888-7309) vs. 1250 (717-2179); p <0.01 Serotype 18C: 1344 (721-2505) vs. 933 (500-1740); p =0.09 Serotype 19F: 1254 (707-2223) vs. 266 (133-532) p< 0.01 Serotype 23F: 766 (377-1554) vs. 147 (76-285); p < 0.001 Antibody potency (OT/IgG): Significantly higher for all serotypes in the younger age group than in the older age group; p<0.05 for all serotypes. |
Level I | Good |
| Musher, 2010 (37) | PNEU-P-23 Placebo Followed 30 days later by other product in alternate arm Route: IM Follow-up: 5 years – yearly blood samples thereafter |
Randomized controlled trial Exclusion: prior IPD, splenectomy, immune suppression Stratified by age group and prior history of pneumococcal vaccination (at least 3 years before for those aged 50–64 and 3 to 5 years before for those aged 65 years and older) |
50–65 yrs, primary vaccination = 222 50–65 yrs, revaccination= 157 ≥ 65, primary vaccination = 222 ≥65 revaccination = 407 Total n = 1008; 721 participated in persistence up to 5 years (average 3.9 years) Baseline characteristics similar between four groups except revaccination subjects within each age group were more likely to report risk factors IPD. Baseline IgG levels similar between younger and older adults who had not previously received PNEU-P=23 and between younger and older adults who had been previously vaccinated. Baseline IgG levels were 2–3 fold higher in those previously vaccinated and not vaccinated. |
Immunogenicity Serotype-specific IgG at Day 30: Level significantly higher compared to baseline for all 4 groups Primary vs. revaccination IgG levels at Day 30 ≥65 years: Same in 2 groups for all serotypes except Serotype 8: 8.5 vs. 6.6 (p< 0.5) Serotype 14: 28.7 vs. 20.8 (p< 0.5) 50–65 years: Same in 2 groups for all serotypes except Serotype 14: 36.1 vs. 24.6 (p<0.5) For all other serotypes, no significant difference between primary and revaccination groups in both age groups, although marginally higher in the primary vaccination group. Serotype specific IgG at day 60: Higher compared to day 30 in older revaccination and older primary vaccination and younger revaccination group for all serotypes; p = NS Higher in younger revaccination group for serotypes 4, 6B, 14, 23F; p = NS Serotype-specific IgG at 5 years: Serotype 3 IgG levels returned to baseline in all 4 study groups by year 2. Re-vaccinated patients: For remaining 7 serotypes (except 3), IgG levels were similar compared to baseline (p = NS) and at least 2-fold higher than vaccine naive recipients (baseline of primary vaccines) Primary vaccinees: In 50–65 group, levels were greater than baseline for serotypes 4,6B, 8, 9V, 12 F, 14 and 23 F; p =N In ≥ 65 years serotypes 4, 6B, 9V, 12F, 14 and 23F; p = NS |
Level I | Good |
| Evidence for Safety | ||||||
|---|---|---|---|---|---|---|
| STUDY DETAILS | SUMMARY | |||||
| Study | Vaccine | Study Design | Participants | Summary of Key Findings Using Text or Data | Level of Evidence | Quality |
| Frenck, 2012 (30) | PNEU-C-13/TIV – placebo (1 month later) vs. Placebo/ TIV – PNEU-C-13 1 month later 0.5 mL IM |
Randomized controlled trial Population: Healthy 50–59 years of age. No previous pneumococcal vaccination |
Multicentric – U.S. N = 1158 enrolled N = 1116 randomized N1 = 554 (PNEU-C-13/TIV) N2 = 562 (TIV then PNEU-C-13) |
Safety: a) Local reactions: proportion significantly greater in concomitant vs. TIV alone but no difference vs. PNEU-C-13 alone b) Systemic reactions: increased proportion of participants with any systemic event, headache, chills, rash, decreased appetite, vomiting, joint and muscle pain vs. TIV alone but when compared to PNEU-C-13 alone: only higher proportion of any systemic AE and headache |
Level I | Good |
| Schwarz, 2011 (31) | PNEU-C-13/TIV – placebo (1 month later) vs. Placebo/ TIV – PNEU-C-13 1 month later 0.5 mL IM |
Randomized controlled trial All PNEU-C-13 serotypes tested Healthy 65 years and over. No previous pneumococcal vaccination Follow-up until 1 month after last vaccine administered |
Multicentric – Europe N = 1190 participants enrolled; 1160 randomized – 580 in each group 1096 evaluable |
Safety: a) Local reactions: similar in both groups b) Systemic reactions: increased proportion of participants with chills, rash, muscle pain, any systemic event in the concomitant administration group compared to TIV alone c) Increased proportion of participants with fatigue, headache, chills, decreased appetite, joint pain, any systemic even in the concomitant administration compared to PNEU-C013 alone |
Level I | Good |
Table 6. Levels of Evidence Based on Research Design.
| I | Evidence from randomized controlled trial(s). |
| II-1 | Evidence from controlled trial(s) without randomization. |
| II-2 | Evidence from cohort or case–control analytic studies, preferably from more than one centre or research group using clinical outcome measures of vaccine efficacy. |
| II-3 | Evidence obtained from multiple time series with or without the intervention. Dramatic results in uncontrolled experiments (such as the results of the introduction of penicillin treatment in the 1940s) could also be regarded as this type of evidence. |
| III | Opinions of respected authorities, based on clinical experience, descriptive studies and case reports, or reports of expert committees |
Table 7. Quality (internal validity) Rating of Evidence.
| Good | A study (including meta-analyses or systematic reviews) that meets all design-specific criteria* well. |
| Fair | A study (including meta-analyses or systematic reviews) that does not meet (or it is not clear that it meets) at least one design-specific criterion* but has no known “fatal flaw”. |
| Poor | A study (including meta-analyses or systematic reviews) that has at least one design-specific* “fatal flaw”, or an accumulation of lesser flaws to the extent that the results of the study are not deemed able to inform recommendations. |
* General design specific criteria are outlined in Harris et al., 20011
Table 8. NACI Recommendation for Immunization – Grades.
| A | NACI concludes that there is good evidence to recommend immunization. |
| B | NACI concludes that there is fair evidence to recommend immunization. |
| C | NACI concludes that the existing evidence is conflicting and does not allow making a recommendation for or against immunization, however other factors may influence decision-making. |
| D | NACI concludes that there is fair evidence to recommend against immunization. |
| E | NACI concludes that there is good evidence to recommend against immunization. |
| I | NACI concludes that there is insufficient evidence (in either quantity and/or quality) to make a recommendation, however other factors may influence decision-making. |
List of Abbreviations
| Abbreviation | Term |
| ARV | Anti-retroviral |
| CAP | Community Acquired Pneumonia |
| CNDSS | Canadian Notifiable Disease Surveillance System |
| GMC | Geometric mean concentration |
| GMT | Geometric mean titer |
| HAART | Highly active antiretroviral therapy |
| HSCT | Hematopoietic stem cell transplant |
| IM | Intramuscular |
| IPD | Invasive pneumococcal disease |
| LSPQ | Laboratoire de santé publique du Québec |
| MMF | Mycophenolate mofetil |
| NML | National Microbiology Laboratory |
| OPA | Opsophanocytic activity |
| PNEU-C-13 | Pneumococcal conjugate vaccine – 13-valent |
| PNEU-P-23 | Pneumococcal polysaccharide vaccine – 23-valent |
| RCT | Randomized controlled trial |
| SOT | Solid organ transplant |
| TIBDN | Toronto Invasive Bacterial Disease Network |
| TIV | Trivalent influenza vaccine |
| VE | Vaccine effectiveness |
Acknowledgments
NACI gratefully acknowledges the contribution of Lt.-Col. Dr. J. Anderson, Dr. J. Brophy, Dr. P. De Wals, Dr. E. Farzad, Ms. M. Helferty, Dr. J. Johnstone, Dr. J. Kellner, Dr. J. Laroche, Dr. S. McNeil, Dr. H. Morrison, Dr. A. Opavsky, Dr. S. Rechner, Ms. L. Sherrard, Dr. G. Tyrrell, Dr. P. Van Buynder and Dr. V. Senikas.
Footnotes
Harris RP, Helfand M, Woolf SH, et al. Current methods of the US Preventive Services Task Force: a review of the process. Am J Prev Med 2001;20:21–35.
References
- 1.National Advisory Committee on Immunization. An Advisory Committee Statement (ACS). National Advisory Committee on Immunization (NACI). Statement on recommended use of pneumococcal conjugate vaccine. Can Commun Dis Rep 2002. Jan;28 ACS-2:1–32. [PubMed] [Google Scholar]
- 2.National Advisory Committee on Immunization (NACI); An Advisory Committee Statement (ACS). Update on the recommendations for the routine use of pneumococcal conjugate vaccine for infants. Can Commun Dis Rep 2006. May;32 ACS-4:1–6.16718922 [Google Scholar]
- 3.National Advisory Committee on Immunization (NACI). Update on the Use of Pneumococcal Vaccines in Childhood. Can Commun Dis Rep 2010;36(ACS-12):1–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.National Advisory Committee on Immunization (NACI); An Advisory Committee Statement (ACS). Evidence-based recommendations for immunization--methods of the National Advisory Committee on Immunization. Can Commun Dis Rep 2009. Jan;35 ACS-1:1–10. [PubMed] [Google Scholar]
- 5.World Health Organization. Pneumococcal Disease. 2012; www.who.int/ith/diseases/pneumococcal/en/index.html
- 6.Demczuk WH, Martin I, Griffith A, Lefebvre B, McGeer A, Shane A et al. ; Toronto Invasive Bacterial Diseases Network; Canadian Public Health Laboratory Network. Serotype distribution of invasive Streptococcus pneumoniae in Canada during the introduction of the 13-valent pneumococcal conjugate vaccine, 2010. Can J Microbiol 2012. Aug;58(8):1008–17. 10.1139/w2012-073 [DOI] [PubMed] [Google Scholar]
- 7.National Microbiology Laboratory. National Laboratory Surveillance of Streptococcus pneumoniae and Streptococcus pyogenes in Canada: Annual Summary 2010 2010.
- 8.National Microbiology Laboratory. National Laboratory Surveillance of Streptococcus pneumoniae and Streptococcus pyogenes in Canada: Annual Summary 2011 2011.
- 9.French N, Gordon SB, Mwalukomo T, White SA, Mwafulirwa G, Longwe H et al. A trial of a 7-valent pneumococcal conjugate vaccine in HIV-infected adults. N Engl J Med 2010. Mar;362(9):812–22. 10.1056/NEJMoa0903029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Siemieniuk RA, Gregson DB, Gill MJ. The persisting burden of invasive pneumococcal disease in HIV patients: an observational cohort study. BMC Infect Dis 2011. Nov;11:314. 10.1186/1471-2334-11-314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Breiman RF, Keller DW, Phelan MA, Sniadack DH, Stephens DS, Rimland D et al. Evaluation of effectiveness of the 23-valent pneumococcal capsular polysaccharide vaccine for HIV-infected patients. Arch Intern Med 2000. Sep;160(17):2633–8. 10.1001/archinte.160.17.2633 [DOI] [PubMed] [Google Scholar]
- 12.Hung CC, Chen MY, Hsieh SM, Hsiao CF, Sheng WH, Chang SC. Clinical experience of the 23-valent capsular polysaccharide pneumococcal vaccination in HIV-1-infected patients receiving highly active antiretroviral therapy: a prospective observational study. Vaccine 2004. May;22(15-16):2006–12. 10.1016/j.vaccine.2003.10.030 [DOI] [PubMed] [Google Scholar]
- 13.French N, Nakiyingi J, Carpenter LM, Lugada E, Watera C, Moi K et al. 23-valent pneumococcal polysaccharide vaccine in HIV-1-infected Ugandan adults: double-blind, randomised and placebo controlled trial. Lancet 2000. Jun;355(9221):2106–11. 10.1016/S0140-6736(00)02377-1 [DOI] [PubMed] [Google Scholar]
- 14.Vila-Córcoles A, Ochoa-Gondar O, Llor C, Hospital I, Rodríguez T, Gómez A. Protective effect of pneumococcal vaccine against death by pneumonia in elderly subjects. Eur Respir J 2005. Dec;26(6):1086–91. 10.1183/09031936.05.00030205 [DOI] [PubMed] [Google Scholar]
- 15.Vila-Córcoles A, Ochoa-Gondar O, Hospital I, Ansa X, Vilanova A, Rodríguez T et al. ; EVAN Study Group. Protective effects of the 23-valent pneumococcal polysaccharide vaccine in the elderly population: the EVAN-65 study. Clin Infect Dis 2006. Oct;43(7):860–8. 10.1086/507340 [DOI] [PubMed] [Google Scholar]
- 16.Mooney JD, Weir A, McMenamin J, Ritchie LD, Macfarlane TV, Simpson CR et al. The impact and effectiveness of pneumococcal vaccination in Scotland for those aged 65 and over during winter 2003/2004. BMC Infect Dis 2008. Apr;8:53. 10.1186/1471-2334-8-53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Melegaro A, Edmunds WJ. The 23-valent pneumococcal polysaccharide vaccine. Part I. Efficacy of PPV in the elderly: a comparison of meta-analyses. Eur J Epidemiol 2004;19(4):353–63. 10.1023/B:EJEP.0000024701.94769.98 [DOI] [PubMed] [Google Scholar]
- 18.Nahm MH, Romero-Steiner S. Functional Assays for Pneumococcal Antibody. In: Siber G, Klugman KP, Makela PH, eds. Pneumococcal Vaccines - The Impact of Conjugate Vacine. Washington, DC: ASM Press; 2008:449. [Google Scholar]
- 19.Kumar D, Chen MH, Wong G, Cobos I, Welsh B, Siegal D et al. A randomized, double-blind, placebo-controlled trial to evaluate the prime-boost strategy for pneumococcal vaccination in adult liver transplant recipients. Clin Infect Dis 2008. Oct;47(7):885–92. 10.1086/591537 [DOI] [PubMed] [Google Scholar]
- 20.Kumar D, Rotstein C, Miyata G, Arlen D, Humar A. Randomized, double-blind, controlled trial of pneumococcal vaccination in renal transplant recipients. J Infect Dis 2003. May;187(10):1639–45. 10.1086/374784 [DOI] [PubMed] [Google Scholar]
- 21.Kumar D, Welsh B, Siegal D, Chen MH, Humar A. Immunogenicity of pneumococcal vaccine in renal transplant recipients--three year follow-up of a randomized trial. Am J Transplant 2007. Mar;7(3):633–8. 10.1111/j.1600-6143.2007.01668.x [DOI] [PubMed] [Google Scholar]
- 22.Tobudic S, Plunger V, Sunder-Plassmann G, Riegersperger M, Burgmann H. Randomized, single blind, controlled trial to evaluate the prime-boost strategy for pneumococcal vaccination in renal transplant recipients. PLoS One 2012;7(9):e46133. 10.1371/journal.pone.0046133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cordonnier C, Labopin M, Chesnel V, Ribaud P, De La Camara R, Martino R et al. ; Infectious Diseases Working Party of the European Group for Blood and Marrow Transplantation. Randomized study of early versus late immunization with pneumococcal conjugate vaccine after allogeneic stem cell transplantation. Clin Infect Dis 2009. May;48(10):1392–401. 10.1086/598324 [DOI] [PubMed] [Google Scholar]
- 24.Molrine DC, Antin JH, Guinan EC, Soiffer RJ, MacDonald K, Malley R et al. Donor immunization with pneumococcal conjugate vaccine and early protective antibody responses following allogeneic hematopoietic cell transplantation. Blood 2003. Feb;101(3):831–6. 10.1182/blood-2002-03-0832 [DOI] [PubMed] [Google Scholar]
- 25.Kumar D, Chen MH, Welsh B, Siegal D, Cobos I, Messner HA et al. A randomized, double-blind trial of pneumococcal vaccination in adult allogeneic stem cell transplant donors and recipients. Clin Infect Dis 2007. Dec;45(12):1576–82. 10.1086/523583 [DOI] [PubMed] [Google Scholar]
- 26.Feikin DR, Elie CM, Goetz MB, Lennox JL, Carlone GM, Romero-Steiner S et al. Randomized trial of the quantitative and functional antibody responses to a 7-valent pneumococcal conjugate vaccine and/or 23-valent polysaccharide vaccine among HIV-infected adults. Vaccine 2001. Nov;20(3-4):545–53. 10.1016/S0264-410X(01)00347-4 [DOI] [PubMed] [Google Scholar]
- 27.Peñaranda M, Payeras A, Cambra A, Mila J, Riera M; Majorcan Pneumococcal Study Group. Conjugate and polysaccharide pneumococcal vaccines do not improve initial response of the polysaccharide vaccine in HIV-infected adults. AIDS 2010. May;24(8):1226–8. 10.1097/QAD.0b013e3283389de5 [DOI] [PubMed] [Google Scholar]
- 28.Crum-Cianflone NF, Huppler Hullsiek K, Roediger M, Ganesan A, Patel S, Landrum ML et al. ; Infectious Disease Clinical Research Program HIV Working Group. A randomized clinical trial comparing revaccination with pneumococcal conjugate vaccine to polysaccharide vaccine among HIV-infected adults. J Infect Dis 2010. Oct;202(7):1114–25. 10.1086/656147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lesprit P, Pédrono G, Molina JM, Goujard C, Girard PM, Sarrazin N et al. ; ANRS 114-Pneumovac Study Group. Immunological efficacy of a prime-boost pneumococcal vaccination in HIV-infected adults. AIDS 2007. Nov;21(18):2425–34. 10.1097/QAD.0b013e3282887e91 [DOI] [PubMed] [Google Scholar]
- 30.Frenck RW Jr, Gurtman A, Rubino J, Smith W, van Cleeff M, Jayawardene D et al. Randomized, controlled trial of a 13-valent pneumococcal conjugate vaccine administered concomitantly with an influenza vaccine in healthy adults. Clin Vaccine Immunol 2012. Aug;19(8):1296–303. 10.1128/CVI.00176-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schwarz TF, Flamaing J, Rümke HC, Penzes J, Juergens C, Wenz A et al. A randomized, double-blind trial to evaluate immunogenicity and safety of 13-valent pneumococcal conjugate vaccine given concomitantly with trivalent influenza vaccine in adults aged ≥65 years. Vaccine 2011. Jul;29(32):5195–202. 10.1016/j.vaccine.2011.05.031 [DOI] [PubMed] [Google Scholar]
- 32.Jackson LA, Neuzil KM, Nahm MH, Whitney CG, Yu O, Nelson JC et al. Immunogenicity of varying dosages of 7-valent pneumococcal polysaccharide-protein conjugate vaccine in seniors previously vaccinated with 23-valent pneumococcal polysaccharide vaccine. Vaccine 2007. May;25(20):4029–37. 10.1016/j.vaccine.2007.02.062 [DOI] [PubMed] [Google Scholar]
- 33.de Roux A, Schmöle-Thoma B, Siber GR, Hackell JG, Kuhnke A, Ahlers N et al. Comparison of pneumococcal conjugate polysaccharide and free polysaccharide vaccines in elderly adults: conjugate vaccine elicits improved antibacterial immune responses and immunological memory. Clin Infect Dis 2008. Apr;46(7):1015–23. 10.1086/529142 [DOI] [PubMed] [Google Scholar]
- 34.Miernyk KM, Butler JC, Bulkow LR, Singleton RJ, Hennessy TW, Dentinger CM et al. Immunogenicity and reactogenicity of pneumococcal polysaccharide and conjugate vaccines in alaska native adults 55-70 years of age. Clin Infect Dis 2009. Jul;49(2):241–8. 10.1086/599824 [DOI] [PubMed] [Google Scholar]
- 35.Goldblatt D, Southern J, Andrews N, Ashton L, Burbidge P, Woodgate S et al. The immunogenicity of 7-valent pneumococcal conjugate vaccine versus 23-valent polysaccharide vaccine in adults aged 50-80 years. Clin Infect Dis 2009. Nov;49(9):1318–25. 10.1086/606046 [DOI] [PubMed] [Google Scholar]
- 36.Schenkein JG, Park S, Nahm MH. Pneumococcal vaccination in older adults induces antibodies with low opsonic capacity and reduced antibody potency. Vaccine 2008. Oct;26(43):5521–6. 10.1016/j.vaccine.2008.07.071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Musher DM, Manof SB, Liss C, McFetridge RD, Marchese RD, Bushnell B et al. Safety and antibody response, including antibody persistence for 5 years, after primary vaccination or revaccination with pneumococcal polysaccharide vaccine in middle-aged and older adults. J Infect Dis 2010. Feb;201(4):516–24. 10.1086/649839 [DOI] [PubMed] [Google Scholar]
- 38.Pfizer Canada. Product Monograph - Prevnar 13. 2012; www.pfizer.ca/en/our_products/products/monograph/232. Accessed 2012-08-28.
- 39.Vanderkooi OG, Scheifele DW, Girgenti D, Halperin SA, Patterson SD, Gruber WC et al. ; Canadian PCV13 Study Group. Safety and immunogenicity of a 13-valent pneumococcal conjugate vaccine in healthy infants and toddlers given with routine pediatric vaccinations in Canada. Pediatr Infect Dis J 2012. Jan;31(1):72–7. 10.1097/INF.0b013e318233049d [DOI] [PubMed] [Google Scholar]