†Members: Dr. J. Langley (Chair), Dr. B. Warshawsky (Vice-Chair), Dr. S. Ismail (Executive Secretary), Dr. N. Crowcroft, Ms. A. Hanrahan, Dr. B. Henry, Dr. D Kumar, Dr. S. McNeil, Dr. C. Quach-Thanh, Dr. B. Seifert, Dr. D. Skowronski, Dr. C. Cooper, Dr. B. Tan
Liaison Representatives: Dr. B. Bell (U. S. Center for Disease Control and Prevention), Ms. K. Pielak (Canadian Nursing Coalition for Immunization), Dr. S. Rechner (College of Family Physicians of Canada), Dr. M. Salvadori (Canadian Paediatric Society), S. Pelletier (Community Hospital Infection Control Association), Dr. N. Sicard (Canadian Public Health Association), Dr. V. Senikas (Society of Obstetricians and Gynaecologists of Canada), Dr. P. Plourde (Committee to Advise on Tropical Medicine and Travel), Dr. P. Van Buynder (Council of Chief Medical Officers of Health)
Ex-Officio Representatives: Ms. M. FarhangMehr (Centre for Immunization and Respiratory Infectious Diseases), Dr. S. Desai (Centre for Immunization and Respiratory Infectious Diseases), Dr. B. Law (Centre for Immunization and Respiratory Infectious Diseases), Lt.-Col. (Dr.) James Anderson (Department of National Defence), Dr. Ezzat Farzad (First Nations and Inuit Health Branch-Office of Community Medicine), Dr. J. Xiong (Biologics and Genetic Therapies Directorate), Dr. D. Elliott (Centre for Immunization and Respiratory Infectious Diseases), Dr. P. Varughese (Centre for Immunization and Respiratory Infectious Diseases)
Additional Working Group Members: A. Campbell
Preamble
The National Advisory Committee on Immunization (NACI) provides the Public Health Agency of Canada with ongoing and timely medical, scientific and public health advice relating to immunization. The Public Health Agency of Canada acknowledges that the advice and recommendations set out in this statement are based upon the best current available scientific knowledge and is disseminating this document for information purposes. People administering the vaccine should also be aware of the contents of the relevant product monograph(s). Recommendations for use and other information set out herein may differ from that set out in the product monograph(s) of the Canadian manufacturer(s) of the vaccine(s). Manufacturer(s) have sought approval of
the vaccine(s) and provided evidence as to its safety and efficacy only when it is used in accordance with the product monographs. NACI members and liaison members conduct themselves within the context of the Public Health Agency of Canada’s Policy on Conflict of Interest, including yearly declaration of potential conflict of interest.
Introduction
In 2006 the Advisory Committee on Immunization Practices (ACIP) approved a routine two-dose varicella vaccination schedule for children. The committee determined that the first dose should be administered at 12–15 months of age and the second dose between four and six years of age. The purpose of this document is to review information on the epidemiology of varicella, compare the effectiveness of one dose of varicella vaccine with two doses and consider a potential change to the current National Advisory Committee on Immunization (NACI) recommendation for a one-dose childhood varicella vaccination program.
Methods
A variety of strategies were used to obtain literature for this review. A search of the Medline database was conducted for articles published in English prior to May 1, 2008. The search terms “varicella vaccination” and “varicella immunization” were used. Additionally, previous NACI recommendation statements and the U.S. comprehensive review published in the Morbidity and Mortality Weekly Review in June 2007 by ACIP were used and then checked for relevant references. Relevant studies will be assigned a level of evidence and assessed for quality in the forthcoming NACI statement on Varicella Vaccination Two-Dose Recommendations (to be posted at: http://www.phac-aspc.gc.ca/naci-ccni/recs-eng.php).
National Goals for Varicella Disease
The National Consensus Conference for Vaccine-Preventable Diseases in Canada convened in June 2005. The purpose of the conference was to achieve consensus on national immunization coverage and disease reduction goals and targets for six vaccine-preventable diseases (VPDs) and to identify the process for their adoption and implementation at the national level. The goal established for varicella disease was to reduce illness and death due to complications from varicella through immunization. Recommendations for achievement of this goal included: 1) immunization coverage with varicella vaccine in 85% of children by their second birthday, and in 85% of susceptible individuals by their seventh and 17th birthdays, by 2010; 2) 80% reductions in varicella-related hospitalization rates and varicella-related deaths by 2010; and 3) sustained reduction in varicella incidence of 70% and 90% by 2010 and 2015 respectively (1).
Results
1. Disease Agent
Varicella zoster virus (VZV) is a DNA virus of the herpes virus family. VZV causes a primary illness of varicella (chickenpox) and establishes latency in the sensory nerve ganglia, which may reactivate later as herpes zoster (shingles). VZV can be spread through air or by direct contact with the virus shed from skin lesions, and humans are the only reservoir known to transmit the disease. The incubation period ranges from 10 to 21 days with a typical range of 14 to 16 days. Infectiousness begins one to two days before rash onset and lasts until the last lesion has crusted. The attack rate among susceptible contacts in household settings is estimated at 65% to 87% (2). Healthy children typically have 200 to 500 vesicular skin lesions. Acute varicella is typically mild and self-limiting; however, varicella-related complications have been observed. Complications include secondary bacterial infections (e.g. Group A Streptococci), dehydration, pneumonia and central nervous system involvement. Complications are more common in adolescents, adults and the immunocompromised (3).
2. Epidemiology of Varicella and Zoster in the Pre-Vaccine Era
Prior to the introduction of extensive VZV vaccination programs, varicella (chickenpox) was deemed to be primarily a benign disease in healthy children under 13 years of age. Approximately 50% and 90% of children were expected to have had an infection by the age of five years and 12 years respectively (4). In Canada there were about 350,000 cases of chickenpox each year in the pre-vaccine era (estimated incidence was 11.7 per 1,000 population). A study published in 1999 estimated the total direct costs (i.e. hospitalizations, physician consults, and medical and surgical procedures) and productivity costs (e.g. time missed from work and caregiver activities) related to childhood varicella illness to be approximately $122 million annually (5). While risk of hospitalization was low (~1 in 200–400), children accounted for 90% of the annual 1,500 to 2,000 varicella-related hospitalizations each year. Providing care for children who were ill and lost productivity accounted for 81% of the annual cost of the disease (6). Furthermore, nearly 20% of those hospitalized experienced some type of neurological complication and 8% had life-threatening infections such as necrotizing fasciitis or septicemia (6). Prior to the introduction of its universal immunization program in 1995, the United States estimated an annual incidence rate of 15 cases per 1,000 (7), and 11,000 hospitalizations and 100 deaths related to varicella annually (8,9).
Chickenpox was a nationally notifiable disease between 1924 and 1958, and was reintroduced on the list of notifiable diseases in 1986. Unfortunately, less than 10% of all varicella infections are reported to the Notifiable Diseases Reporting System (NDRS) in a given year because only laboratory-confirmed cases or clinical cases linked to laboratory-confirmed cases are captured by the case definition. Furthermore, not all provinces and territories participate in routine reporting at the national level (10).
Available supplementary surveillance information from the Immunization Monitoring Program, Active (IMPACT), a pediatric hospital-based network of 11 hospitals accounting for approximately 90% of available tertiary-care pediatric beds in Canada, revealed that varicella zoster virus was responsible for a total of 3,681 pediatric hospitalizations between 1991–1996 and 1999–2005 (Table 1) (10,11). Between 1999 and 2005, 55% of pediatric cases were male, with children 1 to 4 years old accounting for the largest percentage of hospitalizations (45%), while children 5 to 9 years old accounted for 30% of hospitalizations. Pediatric deaths among infants less than one year old were relatively uncommon (seven deaths per 100,000 population), and of the 53 deaths attributed to varicella between 1987 and 1996, 70% occurred in those >15 years of age. Also, according to the 2006 Canadian National Report on Immunization, seven deaths due to varicella and one death attributed to herpes zoster were reported to IMPACT between 1999 and 2005 (10).
Table 1. Number and Yearly Average of VZV Cases Reported in IMPACT, 1991–2005.
| Time period | Number of varicella cases | Average number of varicella cases per year |
|---|---|---|
| 1991–1996 | 1,326 | 221 |
| 1999–2005 | 2,358 (includes both varicella + zoster cases) |
337 |
3. History of Varicella Vaccination, Indications and Dosage Recommendations in Canada
Varivax® III (live attenuated, [Oka/Merck]) produced by Merck Frosst Canada Ltd. and Varilrix® (live attenuated, [Oka-strain]) produced by GlaxoSmithKline Inc. are the two varicella vaccines authorized for use in Canada. Varivax® received its first authorization for use in Canada in 1999 and Varilrix® in 2002. Each vaccine consists of lyophilized, live attenuated varicella virus designated the Oka strain, which was developed in Japan in the mid-1970s. Each 0.5 mL dose of Varivax® III contains a minimum of 1,350 Plaque Forming Units (PFU) (Product monograph Varivax® III MerckFrosst, revised September 21, 2007) (12). Each dose of Varilrix® contains a minimum of 1995 PFU (Product monograph Varilrix®, GSK amended January 3, 2007) (13).
Table 2. Historical Review of NACI Varicella Vaccination Recommendations, 1999–2004.
| Category | 1999 NACI recommendation | 2002 NACI recommendation | 2004 NACI recommendation |
|---|---|---|---|
| Routine childhood schedule | 1 dose at 12–18 months of age | No change | No change |
| Susceptible persons ≥13 years of age | 2 doses >28 days apart | No change | No change |
| Contraindications |
•
Hypersensitivity History of hypersensitivity to any vaccine component including gelatin and neomycin (history of contact dermatitis to neomycin is not a contraindication) • Immunocompromised persons Not authorized for use in persons who are immunosuppressed • Pregnancy |
Addition of details related to immunocompromised persons Any persons with blood dyscrasis, leukemia (except acute lymphoblastic leukemia), lymphomas of any type or other malignant neoplasms affecting the bone marrow or lymphatic system, or persons with other defects in cell-mediated immunity or treatment associated with T-cell abnormalities (e.g. intensive chemotherapy, high-dose steroids, cyclosporine, azathioprine, methotrexate, tacrolimus) |
No change |
| High-risk target groups | None stated | • Children and adolescents on chronic salicylic acid therapy • Persons with cystic fibrosis • Susceptible household contacts of immunocompromised persons • Susceptible health care workers • Susceptible women of child-bearing age (vaccine should not be given during pregnancy) • Solid organ transplant recipients (vaccine should be given a minimum of 4–6 weeks prior to transplantation) • HIV-infected person |
No change |
| Immunocompromised persons: special considerations | Not authorized for use | HIV-infected persons with normal immune status and solid organ transplant recipients. Some HIV-infected children should be considered for immunization if asymptomatic or mildly symptomatic in CDC class N1 or A1 with age-specific CD4+ T-lymphocyte percentages of ≥ 25%. These children should receive 2 doses 3 months apart | No change |
| Post-exposure use | No conclusive data supporting post-exposure efficacy of varicella vaccine; however, it is not harmful. Where post-exposure prophylaxis is being considered for outbreak control, consultation with the local public health department is recommended | 3–5 days post-exposure | No change |
Prince Edward Island was the first province to introduce a universal childhood immunization program in April 2000. Since then, all other provinces and territories have implemented universal varicella vaccination programs (Table 3).
Table 3. Comparison of Canadian Provincial and Territorial Varicella Vaccination Programs.
| Year implemented | Target population | Catch-up programs | |
|---|---|---|---|
| NACI recommendation | N/A | 12–18 months (1 dose) | - |
| Province/territory | |||
| British Columbia | Jan. 2005 | 12 mos. | • 4–6-year-olds • Gr. 6 • susceptible 18–47 months (ended 2007) |
| Alberta | Mar. 2001 | 12 mos. | • 4–6-year-olds (ended 2007) • Gr. 5 (ended 2007) |
| Saskatchewan | Jan. 2005 | 12 mos. | • Gr. 6 (ongoing until 2015) |
| Manitoba | Oct. 2004 | 12 mos. | • 4–6 yrs • Gr. 4 |
| Ontario | Sept. 2004 | 15 mos. | • unimmunized, susceptible • 5-year-olds |
| Quebec | Jan. 2006 | 12 mos. | • 4–6 yrs • Gr. 4 • non-immune health care workers |
| New Brunswick | Sept. 2004 | 12 mos. | • 4-year-olds (ended 2007) |
| Nova Scotia | Sept. 2002 | 12 mos. | • 1–6-year-olds • Gr. 4 (ended 2007) |
| Prince Edward Island | April 2000 | 12 mos. | |
| Newfoundland and Labrador | Jan. 2005 | 12 mos. | • 4–6-year-olds |
| Northwest Territories | Sept. 2001 | 12 mos. | • children <5 yrs |
| Yukon | Sept. 2007 | 12 mos. | |
| Nunavut | Sept. 2002 | 15 mos. |
4. Varicella Immunization Coverage
The United States introduced universal one-dose varicella immunization in 1995. Between 1997 and 2005, state-specific coverage rates increased from 27% to 88% among children 19–35 months of age. In spite of relatively high coverage rates, several varicella outbreaks have been recorded in vaccinated populations in settings ranging from child care centres and schools to households (14-16) (also see Table 7).
In Canada, universal varicella immunization programs were not implemented in all provinces and territories until 2007. The National Immunization Coverage Survey (NICS) conducted in Canada in 2004 reported a coverage rate of 32% among children 24–36 months of age. (The margin of error for the 2004 NICS is estimated to be from 4.2% to 4.4%) (10).
Unpublished data from the 2006 NICS reveal a coverage rate of 57.8% by the second birthday (95% CI: 52–62).
Unpublished data from the 2006 Adult NICS reveal a coverage rate in adults 18–64 years of age with a chronic medical condition (who report not having had “prior immunity” to varicella, based on recall of having had the disease or having been tested for immunity) of 14%a (95% CI: 4.4–23.6), and a coverage rate in health care workers in close contact with patients and no prior history of disease of 22.5% (95% CI: 20.1–24.9). Data from the 2009 NICS are not yet available.
Data from Saskatchewan, where a provincial immunization registry captures all vaccinations provided by public health (which is close to 95% of all vaccination services in the province) showed 71% of children received one dose of vaccine by two years of age in 2006 (unpublished data).
5. Post-Exposure Vaccination as a Strategy for Controlling Varicella Disease
Ideally, vaccinating persons susceptible to VZV prior to any exposure is the best method of preventing or decreasing the severity of disease should exposure occur. The NACI 2004 Update on Varicella statement (4) defines exposure for children as: living in the same household for > five minutes (some experts say >60 minutes) of face-to-face contact with another contagious child. In health care workers, having more than 15 minutes of face-to-face contact or spending >60 minutes in a room with an infected patient is considered significant exposure.
The theoretical basis for post-exposure prophylaxis relates to the ability of the varicella Oka-derived vaccines to induce cell-mediated and antibody responses within five to seven days (17). Pre-licensure studies showed that vaccination with single-antigen vaccine was 90% effective in preventing varicella in healthy persons ≥ 12 months of age if administered within three days of exposure to rash and roughly 70% effective if administered within five days, and 100% effective in modifying severe disease (7). A post-licensure study with 67 people reported vaccine effectiveness of 95% for prevention of any disease and 100% for preventing moderate or severe disease in children <13 years of age after exposure to VZV (18). Another study that looked at 10 siblings receiving post-exposure vaccination within three days demonstrated that the vaccine prevented disease in five out of 10 of these children and prevented moderate or severe disease among nine out of 10 children (19). The five children who developed mild illness were described as having between five and 83 skin lesions.
In some instances post-exposure varicella vaccination may not be possible for persons with contraindications (i.e. pregnant women, immunocompromised persons, newborn infants of mothers developing varicella during the five days before to 48 hours after delivery) if significant exposure has occurred. When this is the case, passive immunization with Varicella-Zoster Immune Globulin (VariZIGTM) is the recommended course of treatment. VariZIGTM administration within 96 hours may prolong the incubation period of VZV from 21 to 28 days. Protection after one dose lasts for approximately three weeks (20).
The 2007 ACIP Varicella Prevention statement (7) states that VariZIGTM is not indicated for persons who have received two doses of varicella vaccine and subsequently become immunocompromised as a result of disease or treatment later in life. They instead advise treatment with acylclovir.
6. Varicella Epidemiology in the Post-Vaccine Era
Follow-up data since the introduction of universal vaccination programs in Canada are not currently available. However, it is likely that similar trends will be observed here as those seen in the United States where universal programs have been in effect for more than 10 years. The U.S. data are described below.
6.1. Changes in Varicella Epidemiology in the United States after Introduction of Universal Immunization Program
A large-scale Varicella Active Surveillance Project (VASP) was set up in Antelope Valley, Calif., West Philadelphia, Pa., and Travis County, Tex., to evaluate changes in varicella epidemiology after the introduction of universal vaccination programs in these communities. The surveillance project receives information from more than 600 reporting sites across the three communities, which have a combined population of approximately 576,000. Several papers reported results on changing epidemiology, hospitalization and other varicella associated events over an 11-year surveillance period from 1995–2005 (21–23).
Population-based disease surveillance in three U.S. communities after the introduction of universal varicella vaccination programs between 1995 and 2005 reported a 90% decline in incidence case reports (21) while achieving immunization coverage levels of 74% to 84% in children aged 19 to 35 months (22). Children aged 1 to 4 years experienced the greatest decline in disease incidence.
Incidence and Outbreak Epidemiology
Incidence rates decreased significantly in the two ongoing VASP sites (surveillance in Travis County, Tex. ended earlier) between 1995 and 2005 (p<0.001). The decrease was from 10.3 cases per 1,000 population (2,934 cases) to 1.1 cases per 1,000 population (362 cases) in Antelope Valley (AV), and from 4.1 cases per 1,000 population (1,197) to 0.4 cases per 1,000 population (108 cases) in West Philadelphia (WP). This represents a 90% decrease in the incidence of cases. Age-specific rates decreased significantly for all age groups from their 1995 baseline levels (Table 4). However, an earlier published study that assessed data up to 2004 in Antelope Valley showed that while the overall varicella incidence rate had declined from baseline levels there was no substantial reduction in disease since 2001, despite vaccine coverage rates of approximately 90% (23).
Table 4. Reported Number of Varicella Cases and Incidence Rates (per 1,000 population) by Age Group in Sentinel Surveillance Sites in Antelope Valley, Calif., and West Philadelphia, Pa., 1995 and 2005.
| No. of cases | Rate per 1,000 | 2005 vs. 1995 | |||
| Antelope Valley | 1995 | 2005 | 1995 | 2005 | % Change |
| Age group (yrs.) | |||||
| <1 | 134 | 16 | 19.7 | 3.2 | -83.9 |
| 1–4 | 1,127 | 50 | 48.8 | 2.5 | -94.9 |
| 5–9 | 1,228 | 122 | 54.9 | 4.6 | -91.6 |
| 10–14 | 235 | 128 | 10.8 | 3.9 | -63.9 |
| 15–19 | 65 | 15 | 3.1 | 0.5 | -85.5 |
| ≥ 20 | 145 | 31 | 0.8 | 0.1 | -82.0 |
| Total | 2,934 | 362 | 10.3 | 1.1 | -89.8 |
| No. of cases | Rate per 1,000 | 2005 vs. 1995 | |||
| West Philadelphia | 1995 | 2005 | 1995 | 2005 | % Change |
| Age group (yrs.) | |||||
| <1 | 38 | 7 | 8.8 | 1.9 | -78.7 |
| 1–4 | 358 | 30 | 20.8 | 2.0 | -90.3 |
| 5–9 | 534 | 29 | 27.3 | 1.6 | -94.2 |
| 10–14 | 162 | 13 | 8.9 | 0.7 | -92.8 |
| 15–19 | 39 | 6 | 1.9 | 0.2 | -87.8 |
| ≥ 20 | 60 | 23 | 0.3 | 0.1 | -57.3 |
| Total | 1,197 | 108 | 4.1 | 0.4 | -90.4 |
Source: Guris D et al. Changing varicella epidemiology in active surveillance sites, United States, 1995–2005; Journal of Infectious Disease. 2008: 197 (Suppl 2) S71–5
Overall, since the introduction of universal varicella vaccination in the U.S., the number of outbreaks (characterized as ≥5 epidemiologically linked cases within one incubation period) decreased on average from 59 to 12 per year (p<.001) and the proportion of outbreaks with 25 or more cases also decreased significantly from 28% to 4% (24). Additionally, research from these surveillance sites also reported a decrease in the duration of outbreaks from 45 to 30 days (p<.001), an increase in the proportion of mild cases (less than 50 lesions) from 35% to 46% (p<.001) and a decrease from 9.3% to 3.6% (p<.001) in the number of cases with complications (24).
Age at Disease Onset
The median age at disease onset shifted upwards for both cases with and without a history of vaccination. The median age increased from five years to eight years in vaccinated cases and from five years to 13 years in unvaccinated case patients in Antelope Valley. In West Philadelphia the median age at disease onset increased from three to six years in vaccinated cases and from six to 19 years in unvaccinated cases (21).
Vaccination Status of Case Patients
In the active surveillance sites between 1995 and 2005 there was an increase in the proportion of varicella cases with a previous history of vaccination. This proportion decreased as age increased. By 2005, the proportion of vaccinated cases ≥1 year of age ranged from 57% to 64%. A history of vaccination among case patients was observed in approximately 87% to 97% of those aged 5-9 years, 38% to 45% for those 10 to 14 years, 17% to 31% for those 15 to 19 years and 7% to 9% for those patients ≥20 years of age (21). The majority of these cases would have received only one vaccination prior to disease onset. Earlier data from this population revealed that the attack rate was 15% if contacts were vaccinated and 71.5% if the contact was unvaccinated (risk ratio 0.21; 95% CI: 0.15–0.30).
Overall, vaccinated cases were half as contagious as unvaccinated cases. However, vaccinated cases with 50 lesions or more were as contagious as unvaccinated cases whereas those with fewer than 50 lesions were only one third as contagious (Table 5) (16). Specimen analysis using Polymerase Chain Reaction (PCR) from 33 vaccinated children found that 76% of those with adequate lesion sample were positive for wild-type VZV (25). Oka vaccine virus was not identified in any specimens.
Table 5. Secondary Attack Rate and Lesion Severity of Secondary Cases, Aged 1 to 14 Years, by Varicella Vaccination Status and Disease History Exposed to Previously Vaccinated Primary Cases, Antelope Valley, Calif., 1997–2001.
| Secondary contacts of primary cases previously vaccinated for varicella | |||
|---|---|---|---|
| No history of vaccination or varicella (n=70) |
History of vaccination (n=94) |
History of varicella (n=38) |
|
| Secondary attack rate, | 26 | 21 | 1 |
| no. (%) | (37) | (22.3) | (2.6) |
| Secondary cases with ≥ | 16 | 4 | 0 |
| 50 lesions, no. (%) | (61.5) | (10.2) | |
Source: Seward J, Zhang J, Maupin T et al. Contagiousness of varicella in vaccinated cases: a household contact study. JAMA. 2006; 292:704–8
Hospitalizations and Mortality
A varicella-related hospitalization (VRH), defined as an admission to an inpatient ward or an emergency room for >eight hours is a recognized complication of varicella (26). A comprehensive prospective longitudinal study found that overall between 1995 and 2005, VRH rates per 100,000 population decreased significantly from 2.54 (95% CI: 2.1–3.0) during the early vaccination period (1995–1998) to 0.6 (95% CI: 0.4–1.0) during the late vaccination period (2002–2005), p<0.01. Among those <20 years of age, rates decreased by 77% from 6.42 (95% CI: 5.3–7.8) to 1.51 (95% CI: 0.6–1.2) per 100,000 population and by 60% among adults ≥ 20 years of age from 0.85 (95% CI: 0.6–1.2) to 0.34 (95% CI: 0.2–0.6) per 100,000 population. There was also a change in the age distribution in VRH case patients. In the early vaccination period, children <10 years of age accounted for 69% of all VRHs, those 10 to 19 years of age accounted for 7.8% of VRHs, and those ≥ 20 years of age accounted for 23.3% of VRHs. In the years following 1998, children <10 years accounted for only 48.8% of VRHs, while those 10 to 19 years made up 19.5%, and adults ≥ 20 years accounted for 31.7% of VRHs. The median age for VRH case patients also increased from 4 years to 10 years; however, this increase was not statistically significant. Additionally, the number of deaths where varicella was noted as an underlying cause decreased from 115 to 16 between 1995 and 2003 (7). A similar decreasing trend in varicella-related hospitalization was observed in a large-scale Market-Scan database, which included information from approximately 40 self-insured employers from across the U.S. with about 4 million people. They reported overall hospitalization rates decreased from 2.3 to 0.3 per 100,000 population between 1994 and 2002 with the greatest declines among infants younger than one year of age (27).
A restrospective chart review of 144 patients conducted in a children’s hospital in Chicago from 1993 to 2001 reported a significant decrease (p<0.01) in the number of varicella-related Invasive Group A Streptococcal (IGAS) infections, one of the most common complications associated with varicella hospitalization in their patient population (28). While varicella infection was the most common predisposing factor to IGAS, as the vaccine coverage rate increased, the percent of IGAS cases associated with varicella decreased from 27% to 2% (28). It should be noted that four studies (8,22,29,30) with shorter lengths of follow-up (two to four years) did not observe significant declines in pediatric IGAS after the introduction of varicella vaccination programs; however, they did not exclusively examine changes in IGAS.
Varicella in Adults
It has been hypothesized that with the implementation of child varicella vaccination programs, a shift in the age distribution of varicella cases would result in an increase in incidence and morbidity in adults. However, from 1995 to 2005 varicella incidence rates declined significant from 0.50 per 100,000 population to 0.13 per 100,000 population (p<0.0001). Disease was more severe in unvaccinated adults compared with unvaccinated children. Adults had a 1.8 and 1.9 times higher risk of >500 skin lesions, a 2.0 times greater risk of developing complications and a 6.2 times higher chance of hospitalization compared with unvaccinated children. Furthermore, non-specific general symptoms, including nausea, vomiting, headache, fatigue, dizziness and appetite loss, were more prevalent in adults (one in 17 adult cases vs. one in 116 child cases). Dehydration and pneumonia also occurred more frequently in adults than children (RR 5.4 and 10.6, p<0.001) (31).
7. Vaccine Modified (or Breakthrough) Disease
The typical incubation period for wild-type varicella is 14 to 16 days; hence, rashes that occur within 14 days after vaccination are usually caused by exposure before vaccination. Between 14 and 42 days after vaccination, a vesicular rash may be due to wild virus or vaccine-strain virus; the distinction would need to be made through molecular typing or PCR testing at the National Microbiological Laboratory in Winnipeg, Man. Vaccine modified (breakthrough) disease is defined as a case of infection with wild-type VZV occurring >42 days after vaccination. Vaccinated children typically tend to have milder cases of disease with fewer lesions, shorter duration of illness and lower incidence of fever. Mild disease is defined as <50 lesions, moderate disease as 50 to 500 lesions and severe disease >500 lesions or the occurrence of serious complications such as varicella-associated pneumonia, encephalitis, hospitalization or death. The VASP in the U.S., where universal vaccination programs have been in place for more than 10 years, observed that the percent of cases with vaccine modified disease increased from 3.5% in 1997 to 24% in 2000 to 72% in 2005 in spite of increasing vaccination coverage rates (32).
8. Laboratory Testing for Immunogenicity
There are a variety of assays used for the detection of varicella antibody. In pre-licensure trials, fluorescent antibody to membrane antigen (FAMA) assay, which is a research laboratory tool, is highly correlated with neutralizing antibody titers to VZV. The later commercially developed whole-cell ELISA and glycoprotein (gp) ELISA tests developed by Merck Frosst Canada Ltd. were developed to be more sensitive in detecting vaccine-induced immune response. (4) However, during outbreaks PCR rather than serology is often used for rapid detection of infection (33-35).
9. Immunogenicity and Vaccine Effectiveness
The robustness of primary varicella antibody response six weeks after vaccination is inversely correlated with vaccine modified (breakthrough) disease rates (34). In the original clinical trials, detectable levels of antibody response >0.6 gpELISA units were used as an endpoint measurement and were observed in 97% of recipients one to 12 years of age who received one dose of varicella vaccine, and within four to six weeks post-vaccination. Later long-term follow-up data found children with varicella antibody titers <5 gpELISA units were 3.5 times more likely to have breakthrough disease than those with titers ≥5 gpELISA units. This threshold level of antibody response six weeks after vaccination was detected in 99.6% of children receiving two doses of vaccine but only in 85.7% of children receiving one dose of vaccine (36). Additionally a FAMA titer >1:4 at the time of VZV exposure and as long as one year after exposure in healthy individuals is highly correlated with protection against chickenpox after either vaccination or natural infection (37). A post-authorization study found only 76% of healthy children had FAMA seroconversion titer ratios >1:4 at 16 weeks after one dose of vaccination (7). In susceptible adults, conversion rates were 82% after one dose of vaccine and 94% after two doses (38).
Population-Based Studies
Assessment of the vaccine effectiveness (VE) in preventing disease in real-world conditions is extremely important. It is typically estimated for preventing varicella disease of any severity, and for preventing moderate to severe disease (typically >50 lesions).
Population surveillance from Antelope Valley reported VE of 89.5%, with time since vaccination greater five years identified as a risk factor for moderate to severe disease (risk ratio, 2.6; 95% CI: 1.2–5.8) (23).
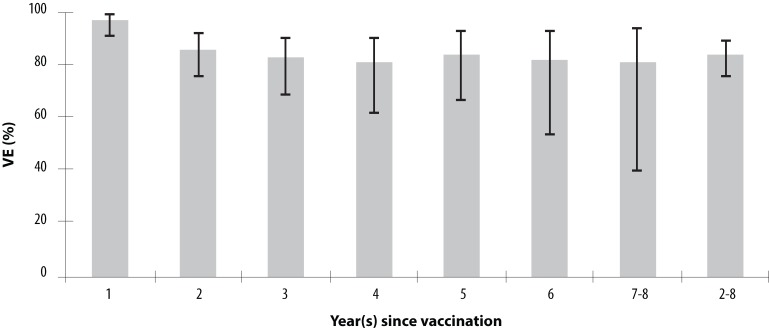
A six-year case-control study from 1997 to 2003, which assessed one-dose vaccine effectiveness against laboratory-confirmed varicella in pediatric practice centres in Connecticut, reported a vaccine effectiveness of 97% (95% CI: 91–99) in the five year after vaccination and an overall VE of 87% (95% CI: 81–91). Vaccine effectiveness was significantly higher in the first year post-vaccination than at two to eight years post-vaccination: 97% (95% CI: 91–99) vs. 84% (95% CI: 76–89) (Figure 1). Vaccine effectiveness measured one year post-vaccination was significantly lower for those receiving vaccine at <15 months of age than for those ≥15 months of age: 73% (95% CI: 43–95) vs. 99% (95% CI: 93–100), p<0.01. However, VE was similar for those vaccinated at <15 months and for those vaccinated at ≥ 15 months of age when time since vaccination was greater than one year: 81% (95% CI: 62–90) vs. 85% (95% CI: 77–90), p=0.47 (39).
Figure 1.
Effectiveness of Varicella Vaccine (by Percent) as a Function of Length of Time since Vaccination
Source: Vazquez M, LaRussa PS, Gershon AA et al. Effectiveness over time of varicella vaccine. JAMA. 2004; 291 (7): 851–5
In Israel, a retrospective cohort study followed 169,801 children for 31 months after the introduction of a voluntary one-dose vaccination program. Close to 27,000 children between the ages of 1 and 10 years without a prior history of varicella disease were vaccinated between 2000 and 2002. The investigators found that the disease incidence decreased from 86.6 to 44.6 per 1,000 population, representing nearly a 50% decline in disease incidence after authorization of varicella vaccine in that country; and VE was estimated at 92% (40).
A retrospective cohort study conducted in Canada of 431 children who received one dose of vaccine between the ages of ≥12 months to 12 years and were followed up to three years post-vaccination reported an average breakthrough rate of 3.1% per year based on definite or probable case definition, b and 2.4% breakthrough rate per year when based on a suspect case definition C (41).
These studies likely yielded higher than expected vaccine effectiveness rates, as vaccine coverage in these populations was relatively low at the time of analysis and vaccines would have the potential for immunological boosting from exposure to wild-type varicella (Table 6).
Table 6. Summary of Population-Based Varicella Studies, 1998–2004.
| Author & year published | Population | Study period | Case Ascertainment | Cases (n) | % Cases vaccinated | VE (%) | Factors for breakthrough disease |
|---|---|---|---|---|---|---|---|
| Chaves, 2007 (23) | 350,000 persons in Antelope Valley, Calif. | 1995–2004 | Phone interview collecting demographic, clinical & epidemiological data. Vaccination status obtained via self-report with 80% of reports verified against health record | 11,356 |
9.5% N=1,080 1% in 1996 18% in 2000 60% in 2004 |
89.5 | ≥ 5 years since vaccination for children 8–12 years (severe-moderate disease) RR= 2.6 (95% CI: 1.2–5.8) |
| Vazquez, 2004 (39) | 20 group practices in Connecticut | 1997–2003 | Clinical diagnosis confirmed with Polymerase Chain Reaction test result | n=339 (cases) n=669 (controls) |
n/a | 87% (81,91) | Not reported in study |
| Passwell, 2004 (40) | 161,557 children age 1–10 years in Maccabi HMO, Israel | 1998–2002 | Chart review with varicella diagnosis code | n=373 (immunized) n=35,573 (non-immunized) | 1.0% | 92.0% | Not reported in study |
| Scheifele, 2002 (41) | Three urban Canadian centres (Halifax, Ottawa, Vancouver) | 1999 | Definite case: vesicles present and physician confirmation probable case: vesicles present but no physician diagnosis | 431 vaccinated children | n/a | 3.1% per year breakthrough rate | Not reported in study |
Outbreak Studies
There have been multiple varicella outbreaks in populations with high coverage rates for one dose of varicella vaccination (Table 7). A meta analysis of 14 varicella outbreak studies reported an overall vaccine effectiveness (VE) of 72.5% (95% CI: 69.5–76.0) in 3,157 children vaccinated with one dose of attenuated Oka strain vaccine (42). Some of the studies identified risk factors for vaccine modified disease. Four studies found that time since vaccination of ≥3 years increased the risk of disease by 2.6 to 6.7 times (15,35,43,44). Table 7. Summary of Findings from Varicella Outbreak Studies, 1999–2006
Table 7. Summary of Findings from Varicella Outbreak Studies, 1999–2006.
| Author & year published | Population | Setting | Population vaccination coverage rate (%) | Attack rate in vaccinated (%) | Attack rate in un-vaccinated (%) | Vaccination history of index case | VE% overall, moderate/severe | Other Findings |
|---|---|---|---|---|---|---|---|---|
| CDC, 2006 (45) | N=142 (K–Gr. 7) 33 cases (age 5–13 yrs., median 8 yrs.) |
Elementary school in Nebraska, Aug.–Dec., 2004 | 81 (69–100) |
13 | 67 | Unvaccinated kindergarten student | 81 (66,89) 93 (82,97) |
Vaccinated student more likely to have mild disease (67% vs. 11%), fewer days of rash (5 vs. 7.3) and miss fewer days of school (3 vs. 5.2) |
| CDC, 2004 (35) | N=455 (K– Gr. 3) 66 cases |
Elementary school in Michigan, Sept.–Dec., 2003 | 97.1 | 11.8 | 76.9 | Vaccinated Grade 3 student | 84.7 (77.4, 89.7) 97.6 (95.0, 99.9) |
Vaccinated students more likely to have mild disease (84.6% vs 20.0%), less likely to have fever (44.2% vs 88.9%) and missed fewer days of school (1.3 vs. 3.5). Vaccination ≥ 4 years before outbreak, more likely to acquire varicella (RR= 4.65; 95% CI: 1.48–14.61) |
| Buchholz, 1999 (46) | N=39 12 cases (31–66 mos., mean 50 mos.) |
Child care centre in Los Angeles County, March 1998 | 87.2 | 24 | 80 | Not reported | 71 (38, 86) 93 (33,99) |
|
| N=20 12 cases (41–64 mos., mean 53 mos.) |
Child care centre in Los Angeles County, March 1998 | 30.0 | 0 | 86 | Not reported | 100 (67,100) 100 (0, 100) |
||
| Haddad, 2005 (44) | N=289 26 cases (age 5–12 yrs.) |
Elementary school in Utah, Oct.– Dec., 2002 | 77.2 | 4 | 27 | Unvaccinated Grade 1 student | 87 (71, 94) 90 (76, 96) |
Vaccinated children more likely to have mild disease (15% vs. 69%), shorter duration of illness (1.5 vs. 3 days). Children with eczema 3.8 (CI 1.8–7.1) times more likely to develop breakthrough disease. Vaccination ≥5 years before outbreak more likely to acquire varicella (RR=3.0; 95% CI:1.4–6.4) Vaccination at ≤ 18 months more likely to develop break-though disease (RR=2.6; 95% CI:1.2–5.6) |
| N=422 48 cases (age 5–12 yrs.) |
Elementary school in Utah, Oct. 2002–Feb. 2003 | 82.5 | 5 | 40.5 | 87 (78, 92) 99 (94, 99) |
|||
| Marin, 2005 (33) | N=197 48 cases (K–Gr. 3) |
Elementary school in Maine, Dec. 2002–Jan. 2003 | 74 (60–90) |
8.2 | 70.6 | Not reported | 89 (79, 94) 96 (88, 99) |
Vaccinated cases less likely to have moderate or severe disease (25% vs. 78%), had shorter duration of illness (5 vs. 7 days), missed fewer days of school (3 vs. 5 days). Eczema associated with non-significant increased risk of breakthrough disease (RR: 4.3; 95%CI: 0.8–23.5) Note: Attack rate was 5% among students with history of disease (VE 93%; 95% CI:83–97) |
| Miron, 2005 (47) | N=242 116 cases (age 3–6 yrs.) |
100 day care centres in Northern Israel (approx. 30–40 children in each centre), Jan.– June 2003 | 37 | 52 | 41.5 | Not reported | 20 (0,40) 93.4 (75,98) |
Vaccination ≥ 2 years before outbreak is a risk factor for breakthrough disease |
| Galil, 2002 (43) | N=88 25 cases (age 6 mos. to 8 yrs.; median 4.1 yrs.) |
Day care centre in New Hampshire, Dec. 2000–Jan. 2001 | 73.1 (49 of 67 eligible) | 34.7 | 44.4 | Vaccinated 4.5-year-old | 44.0 (6.9, 66.3) 86.0 (38.7, 96.8) | Vaccination ≥ 3 yrs. before outbreak increased risk for vaccine failure RR=2.6; 95% CI: 1.3–5.3) Note: Index case was healthy vaccinated child who infected more than 50% of susceptibles, suggesting breakthrough disease can be highly infectious |
| Izurieta, 1997 (14) | N=148 81 cases |
Child care centre in Georgia, Jan.–May 1996 | 44.6 | 14 | 88 | Unvaccinated 4-year-old | 86 (73, 92) 100 (96,100) |
Vaccinated children with asthma or other respiratory illness at increased risk for disease compared to vaccinated children without the same underlying condition |
| Tugwell, 2004 (15) | N=159 21 cases (age 5–11 yrs.; median 7 yrs.) |
Elementary school in Oregon Oct. 2001–Jan. 2002 | 96.8 | 12 | 42.9 | 3 unvaccinated & 1 vaccinated | 72 (3, 87) |
Vaccination >5 yrs. prior to outbreak associated with increased risk of breakthrough disease RR=6.7; 95%CI: 2.2–22.9 Early age at vaccination ≤15 months NOT associated with increased risk of breakthrough disease |
| Arnedo-Pena, 2006 (48) | N=269 148 cases (age 2–12 yrs., mean 5.7 yrs.) |
Day care & school in Spain, Dec. 2004–April 2005 | 35.7 | 22.9 | 72.8 | Unvaccinated 4-year-old | 70 (51, 82) 97 (78, 100) |
RR of disease (in vaccinated population)= 0.31(95% CI: 0.21–0.46) compared to unvaccinated population Vaccinated cases had shorter duration of disease (4.5 vs. 7.8 days), fewer cases with >500 vesicles (0% vs. 15.2%), were younger (4.0 vs. 6.0 years), and less likely to have fever ≥ 38.5C (14.3% vs. 39.0%) |
| Dworkin, 2002 (49) | N=209 35 cases (age 4–8 yrs.) |
Two schools in Illinois; Jan.–May 2001 | 69.9 | 5.5 | 42.9 | Not reported | 88 (77, 93) | Vaccination ≤ 15 months associated with increased risk of breakthrough disease; RR=3.7; 95% CI: 1.1–13.1 |
| Galil, 2002 (50) | N=100 31 cases (age 3.2 mos.–11 yrs.; median age 3.7 yrs.) |
Day care centre in Pennsylvania; Nov. 1999–April 2000 | 80 | 17.5 | 85 | Vaccinated 4-year-old | 79 (66, 88) 95 (84, 98) |
Children vaccinated at <14 months of age more likely to develop breakthrough disease (RR=3.0; 95% CI: 0.9–9.9) |
| Lee, 2004 (51) | N=154 49 cases (age 5–11 yrs.; median age 7 yrs.) |
Elementary school in Minnesota; July 2002–Jan 2003 | 76.7 | 24.6 | 55.5 | Vaccinated 6-year-old | 56 (30, 72) | Increased risk of breakthrough disease if vaccine received ≥12 months and <16 months (RR=2.1; 95% CI: 1.4–4.1) - Chronic ear infection not associated with breakthrough (RR=1.9; 95% CI: 1.0–3.5) History of respiratory syncytial virus, pneumonia or similar lung condition associated with increased risk of disease (RR=3.0; 95% CI: 1.5–5.8) |
| Lopez et al, 2006 (52) | N=395 44 cases (age 5–12 yrs.) |
Elementary school in Arkansas; Sept.–Nov. 2003 | 96 | 11.3 | 37.5 | Vaccinated Grade 2 student | 82 (76,87) |
Vaccine-Modified Disease in Canada
At the time of writing, there was no published information on breakthrough cases in Canada. Data from IMPACT regarding previously vaccinated children hospitalized with varicella are being analyzed.
10. Herpes Zoster
Herpes zoster (HZ or shingles) results from reactivation of latent varicella zoster virus in the sensory nerve ganglia and is characterized by painful vesicular dermatomal rash and may result in debilitating chronic pain known as post-herpetic neuralgia. Risk factors for HZ have been found to be greatest for immunocompromised persons and those older than 50 years of age (45-47). Other risk factors for herpes zoster include sex (higher for females), race (higher for Caucasians) and psychological stress (higher for those with recent stressful life events) (48). Factors that reduce risk of HZ are exposure to varicella or childhood vaccination with varicella vaccine as opposed to wild-type infection (48). The lifetime risk of zoster from wild-type VZV is estimated to be around 10% to 30% and incidence increases markedly with age, affecting up to 50% of people who live to 85 years (49,50). A systematic review of population-based studies in the pre-vaccination era reported overall annual HZ incidence rates ranging from 1.2 to 7.2 per 1,000 population. The rate in the oldest individuals of the populations tested (from >60 years to >75 years) was higher at 3.6 to 14.2 per 1,000 (51).
Local surveillance of HZ incidence in the U.S. reported differential findings. A study in Massachusetts reported a significant increase in HZ incidence rates from 2.99/1,000 population to 5.25/1,000 population from 1999 to 2003, p=0.0009 (47). Data from Washington State reported that the incidence of HZ remained stable between 1992 and 2003 (3.71 to 4.05 cases per 1,000 person-years) while varicella vaccination coverage rates increased to 45%–65% for children between the ages of two and six years, and varicella incidence decreased from 2.63 to 0.92 cases per 1,000 person-years. However, HZ incidence was lower in vaccinated children than in unvaccinated children up to 9 years of age (46).
Baseline surveillance data from Alberta and British Columbia demonstrated that HZ incidence was increasing prior to the implementation of universal varicella vaccination programs. In B.C. the HZ incidence rate increased from 3.2 to 3.7 per 1,000 population between 1994 and 2003, and in Alberta from 2.8 to 4.4 per 1,000 population between 1986 and 2002 (52,53). As varicella vaccination coverage rates increase, the median age of acquiring wild-type varicella may increase, hence decreasing the risk for infection in early childhood and subsequently reducing a risk factor for childhood HZ. Older age of varicella disease onset is associated with decreased risk of herpes zoster (54).
It has been hypothesized that widespread varicella vaccination may alter the risk of HZ in the population and increase the annual incidence of HZ cases as opportunity for humoral immune boosting will decrease as circulating virus dwindles (55). Model estimates predict that shingles rates will increase for the first 20 years after the elimination of varicella transmission, peaking at an incidence 51% (95% CI: 28–60) higher than in the pre-vaccine era. The incidence of zoster will gradually begin to decrease as the vaccinated cohorts begin to reach the older ages at which most zoster cases occur but would remain above the pre-vaccination level for approximately 46 years (95% CI: 35–47) after the introduction of vaccination. The mathematical model used for these predictions did not take into account the possibility of immunization of adults to boost immunity against zoster. In 2006 the United States Food and Drug Administration (FDA) licensed ZostavaxTM for the prevention of herpes zoster in persons 60 years of age and older. In August 2008, a live, injectable, attenuated herpes zoster vaccine (Zostavax™, Merck Frosst Canada Ltd.) was approved for use in Canada for the prevention of herpes zoster (shingles) infection in adults aged 60 years and older. NACI recommendations on the use of this vaccine are forthcoming. The use of this vaccine may reduce the incidence of herpes zoster in this country.
11. Two-Dose Post-Authorization Studies
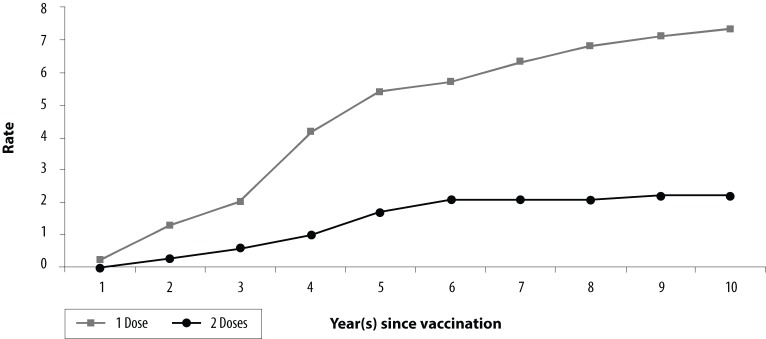
To date there is one clinical trial that compares the efficacy of a one-dose vaccination regimen with a two-dose regimen. With 10 years of follow-up data, it was found that children receiving two doses had significantly higher vaccine efficacy rates than children who received one dose [98.3% (95% CI: 97.3-99.0) vs 94.4% (95% CI: 92.9-95.7), p<0.001], and a 3.3-fold lower risk of breakthrough disease than those who received one dose (Figure 2) (36).
Figure 2.
Cumulative Varicella Disease Breakthrough Rates (per 100 Person-Years at Risk) for One and Two Doses of Single Antigen Varicella Vaccine in Children 12 months to 12 years by Number of Years after Vaccination, United States, 1993–2003
Source: Kuter B, Matthews H, Shinefield H et al. Ten-year follow-up of healthy children who received one or two injections of varicella vaccine. Pediatr Infect Dis J. 2004; 23:132–7
Two cases of zoster were reported during the 10 years of follow-up. Both cases occurred in recipients of a single dose of vaccine. The first child was vaccinated at 22 months and developed zoster 30 months post-vaccination and the second child was 13 months at vaccination and developed zoster 46 months after having been vaccinated. The two-dose group had slightly higher geometric mean titers (GMTs) in the first two years after vaccination and a larger percentage of subjects with antibody titers ≥5 gpELISA units/ml than the one-dose group in the first three years. The varicella antibody persistence rate was close to 100% throughout the nine-year follow-up for both groups and the cumulative rate of antibody persistence with both regimens remained high nine years post-vaccination [99.0% (one-dose group), 99.8% (two-dose group)]. A summary of the clinical trial is outlined in Table 8.
Table 8. Summary of Findings From 2-Dose Varicella Vaccination Clinical Trials.
| Author, (Year) | Study design | Outcomes and results | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Kuter et al. (2004) (36) |
Design: Randomized control trial Population: N=2,196 children age ≥12 months to 12 years n= 1,103 (1 dose of vaccine) (mean age 3.6 years range 1 year–12.9 years) n= 1,093 (2 doses of vaccine) (mean age 3.9 years range 1 year–12.9 years) (Oka/Merck Varivax®) Participants enrolled Dec. 1991–Jan. 1993 Setting: 18 multi-centre sites Inclusion criteria Healthy children 12 months to 12 years with no history of varicella and/or zoster Exclusion criteria Significant exposure to varicella or zoster (>1 hour of contact indoors) in the 4 weeks before entry into the study; received another vaccination within a month before or planning to receive another vaccine during the month after injection; or received any blood products during preceding 3 months. Also excluded if history of anaphylactoid reaction to neomycin, known immune deficiency or neoplastic disease or depressed immunity from disease or drugs Intervention: Administration of either 1 dose or 2 doses of vaccine (2nd dose three months after 1st) containing 2,900-9,000 PFUs of attenuated Oka/Merck Varivax® Outcomes analyzed: • Persistence of varicella antibodies (as measured by gpELISA, titers ≥ 5 considered positive) • Lab-confirmed varicella cases occurring >42 days post-vaccination (acute and convalescent titers were ≥200 gpELISA units/ml) • Suspect case if there was a 4-fold rise between acute and convalescent samples and at least one titer was between 50 and 199 gpELISA units/ml • Vaccine efficacy based on person-years at risk to calculate annual and cumulative breakthrough disease; only subjects actively followed as of March 2002 by questionnaire and/or annual blood sample contribute estimate Follow-up: 10 years of follow-up with measurements taken each year |
Variable | 1-dose regimen | 2-dose regimen | ||||||
| No. of varicella cases | 60 | 17 | ||||||||
| Median days duration of rash (range) | 4 (1–18) | 5 (1–14) | ||||||||
| Median of the daily maximum # of lesions (range) | 26 (2–272) | 20 (2–100) | ||||||||
| % with >50 lesions | 22.8 | 18.8 | ||||||||
| % with 1 or more vesicle | 77.2 | 68.8 | ||||||||
| Median of the daily maximum no. of vesicles (range) | 13 (1–272) | 5 (1–80) | ||||||||
| % with temperature ≥ 38.9○C, oral equivalent | 2.4 | 0 | ||||||||
|
Vaccine efficacy (1 dose vs. 2 doses) 94.4% (95% CI: 92.9-95.7), 98.3% (95% CI: 97.3-99.0) p<.001 Risk of developing varicella >42 days post-vaccination during 10-year period was 3.3 fold lower in children receiving 2 doses as opposed to one (p< .001) VE for prevention of varicella after household exposure (1 vs. 2 doses) 90.2% (95% CI: 83.7-96.7) vs. 96.4% (95% CI:92.4-100) p=0.112 Persistence of varicella antibody (gpELISA) 1 to 9 years post-vaccination | ||||||||||
| 1-dose regimen | 2-dose regimen (0 and 3 mos.) | |||||||||
| Time post-vaccination | N | Persistence rate (%) % | ≥5 gpELISA units/ml | GMT | N | Persistence rate (%) | % ≥5 gpELISA units/ml | GMT | ||
| 6 wk. | 881 | NA | 85.7 | 12.5 | 768 | NA | 99.6 | 142.6 | ||
| 1 yr. | 657 | 100 | 86.9 | 20.8 | 588 | 99.8 | 97.4 | 32.0 | ||
| 2 yr. | 384 | 100 | 90.9 | 23.6 | 318 | 100 | 95.0 | 24.6 | ||
| 3 yr. | 458 | 99.8 | 93.2 | 44.7 | 398 | 100 | 98.2 | 50.9 | ||
| 4 yr | 452 | 99.6 | 92.0 | 45.3 | 395 | 100 | 92.9 | 36.8 | ||
| 5 yr. | 400 | 100 | 95.5 | 50.3 | 376 | 100 | 98.1 | 44.3 | ||
| 6 yr. | 399 | 99.7 | 93.7 | 49.5 | 392 | 100 | 96.7 | 49.7 | ||
| 7 yr. | 424 | 100 | 94.3 | 54.2 | 392 | 100 | 96.2 | 54.2 | ||
| 8 yr. | 381 | 100 | 94.5 | 56.5 | 347 | 99.1 | 96.0 | 52.8 | ||
| 9 yr. | 277 | 99.6 | 95.3 | 57.8 | 237 | 99.6 | 97.0 | 61.0 | ||
Data on immune responses from the study by Kuter et al. were combined with a different study by Reisinger et al., who administered a second dose of varicella vaccine to previously vaccinated, healthy children aged four to six years (Table 9). Reisinger et al. reported similar immunological responsiveness six weeks post-second dose of a combination MMRV vaccine (56).
Table 9. Immune Response Among Children Aged 12 Months to 12 Years, Measured 6 Weeks after 1 or 2 Doses of Varicella Vaccination, United States, 1988–2002.
| 6 weeks after receiving ONE dose | 6 weeks after receiving TWO doses with 3 months between doses | 6 weeks after receiving 2nd dose at age 4–6 years | |
|---|---|---|---|
| VZV IgG gpELISA ≥5 SA units | 85.7%* | 99.6%* | 99.4%** |
| GMT | 12.5* | 142.6* | 212.4** |
* Source: Kuter B, Matthews H, Shinefield H et al. Ten-year follow-up of healthy children who received one or two injections of varicella vaccine. Pediatr Infect Dis J. 2004;23:132–7
** Reisinger K, Hoffman Brown M, Xu J et al. A combination measles, mumps, rubella and varicella vaccine (ProqUAD) given to 4-to 6-year-old, healthy children vaccinated previously with M-M-RII and Varivax. Pediatrics. 2006; 117: 265–72
12. Adverse Events
CDC Vaccine Adverse Event Reporting System
Pre-licensure clinical trials of varicella vaccine showed little or no increase in rates of fever, varicella-like rash or local reactions at the injection site in children or adults receiving varicella vaccine compared with placebo (57).
The Vaccine Adverse Event Reporting System (VAERS) is a passive surveillance system managed by the United States Centers for Disease Control and Prevention, and the U.S. Food and Drug Administration. It receives reports from health care providers, vaccine manufacturers and the public concerning adverse events temporally related to immunization. VAERS reports that between 1995 and 2005 nearly 48 million doses of varicella vaccine were distributed in the U.S. and 25,306 adverse events associated with this vaccine were reported (58). Overall the adverse events rate per 100,000 doses distributed was 52.7 (0.06%). The rate steadily declined during this time period from 245 per 100,000 in 1995 to 20.8 per 100,000 in 2005. Of all the reports, 1,276 (5.0%) were classify as serious (2.6 per 100,000 doses). The rate of serious adverse events (defined as adverse events resulting in hospitalization, death, life-threatening illness, permanent disability or certain other medically important conditions) also declined between 1995 and 2005 from 5.8 to 1.4 per 100,000 doses. There were more AE reports from those receiving varicella vaccine alone (14,780 incidents) relative to those receiving varicella vaccine in combination with another vaccine (10,526 incidents).
However, a larger percentage of AE was classified as serious in the group receiving varicella vaccine in conjunction with another vaccine relative to those receiving varicella alone (7.8% vs. 3.2%) (58).
The five most commonly reported AEs were: rash (8,262 cases or 32.6%), fever (5,451 cases or 21.5%), injection-site reaction (3,291 or 13.0%), urticaria (1,047 cases or 4.1%) and herpes zoster (981 cases or 3.9%). Sixty deaths following vaccination with varicella vaccine were reported; 23 (38.3%) followed administration of varicella vaccine alone. The median interval between vaccination and death was nine days (range, <1 day to 6.8 years). The most common fatal events reported were septicemia and multi-organ failure (11 cases or 18.3%). All deaths were in individuals with severe congenital anomalies or who had disorders that affect the immune system and 10 deaths were described as “crib-deaths.”
Laboratory testing from 338 patients with suspected adverse events after varicella vaccination found that 26% of specimens were positive for wild-type varicella, while 18% tested positive for vaccine-strain VZV. The remaining specimens either tested negative or were inadequate for laboratory testing. In 118 specimens from patients with HZ rashes, wild-type VZV was detected in 20% and vaccine-strain VZV was observed in 41%.
13. MMR and Varicella Vaccine Administration
NACI recommends the measles, mumps, rubella (MMR) vaccine at 12 months of age. This coincides with the recommendation for when the varicella vaccine should be administered. Currently, eight provinces and two territories provide a schedule whereby MMR and varicella are administered concomitantly with the expectation that decreasing the number of visits required for immunization will increase compliance. A study evaluated whether co-administration of vaccinations given six weeks apart in two separate groups of subjects would yield differences in immune responses, persistence of antibody, duration of protection against varicella or safety profiles. They found that seroconversion rates and the percent of those with glycoprotein ELISA titers ≥ 5.0 units were the same for the two groups (99.5% and 92.5% for the group with co-administered vaccines vs. 100% and 94.8% for the group given the vaccines six weeks apart, respectively (p>0.05)). However, while the seroconversion rates were similar, a statistically significant difference was observed in the GMTs between the two groups. GMTs were slightly but significantly lower in the group with concomitant MMR and varicella vaccine administration: 13.2 in the co-administered groups vs. 17.9 in the group given the vaccines six weeks apart (p<0.05) (Table 10) (59).
Table 10. Seroconversion Rate and Geometric Mean Titer for Initially Sero-Negative Varicella Subjects (gpELISA) 6 Weeks After Vaccination.
| MMR II + varicella vaccine co-administered |
MMR II + varicella vaccine given 6 weeks apart |
Fold difference in GMTs between the two groups (95% CI) | |
|---|---|---|---|
| Seroconversion Rate (%) | 99.5 (199/200) | 100 (174/174) | – |
| Geometric Mean Titer | 13.2 | 17.9 | 0.74 (0.63, 0.87) |
Source: Shinefield HR et al. Vaccination with measles, mumps and rubella vaccine and varicella vaccine: safety, tolerability, immunogenicity, persistence of antibody and duration of protection against varicella in health children. Pediatr Infect Dis J. 2002; 21: 555–61
Varicella antibody persistence rates were >98% to 100% during six years of follow-up for the two groups, and vaccine efficacy during five years of follow-up were similar between the two groups: 90.5% (95% CI: 86.2–95.0) and 88.9% (95% CI: 83.7–93.7) respectively (Table 11) (59).
Table 11. Varicella Antibody Persistence Rates and GMTs for Subjects Who Seroconverted 6 Weeks after Vaccination.
| MMR II + varicella vaccine co-administered | MMR II + varicella vaccine given 6 weeks apart | |||
|---|---|---|---|---|
| Persistence rate % | GMT (95% CI) | Persistence rate % | GMT (95% CI) | |
| 1 year | 100 | 35.3 | 98.2 | 25.7 |
| (143/143) | (28.4-44.0) | (109/111) | (20.0-33.0) | |
| 3 years | 98.8 | 40.0 | 99.1 | 36.8 |
| (82/83) | (28.7-55.9) | (112/113) | (27.4-49.3) | |
| 4 years | 99.0 | 34.4 | 100 | 29.1 |
| (95/96) | (25.6-46.4) | (76/76) | (20.1-42.3) | |
| 5 years | 100 | 49.5 | 100 | 40.2 |
| (91/91) | (35.5-69.0) | (77/77) | (27.9-57.9) | |
| 6 years | 100 | 42.9 | 98.6 | 31.3 |
| (91/91) | (31.9-57.7) | (70/71) | (20.9-46.8) | |
Source: Shinefield HR et al. Vaccination with measles, mumps and rubella vaccine and varicella vaccine: safety, tolerability, immunogenicity, persistence of antibody and duration of protection against varicella in healthy children. Pediatr Infect Dis J. 2002; 21: 555–61
14. Cost Effectiveness
In addition to intervention effectiveness studies, economic analysis is a critical factor for consideration when making population-based recommendations for vaccine use. Benefit-cost ratio is an indicator that attempts to summarize the overall value for money of a project or intervention. A value greater than one would indicate that the benefits of an intervention outweigh its cost. A 2008 U.S. study (60) conducted a comparative economic assessment of three scenarios: no varicella vaccination program; 2) a one-dose; or 3) a two-dose program. Compared with no vaccination program, both one- and two-dose programs were found to save costs, with a benefit-cost ratio (BCR) of 4.37 and 2.73 respectively. However, compared with the one-dose program, a second dose was not cost-saving either in terms of direct costs (BCR 0.13) or societal costs (BCR 0.56).
Discussion
One dose of varicella vaccine has been relatively effective at decreasing the burden of varicella-associated illness. However, breakthrough disease continues to occur as one dose has been shown to elicit a minimum threshold level of adequate immunologic protection in only about 85% of vaccine recipients. Ninety-nine percent of vaccine recipients have sufficient immune response after a second dose of vaccine is administered. A second dose is required to address primary vaccine failure and waning immunity. A similar occurrence was the reason for administering two doses of measles vaccine (61). The strain on public health resources in the mumps outbreak that occurred in populations who were either unvaccinated or vaccinated with only one dose of mumps vaccine in Nova Scotia and New Brunswick in 2007 provides some insight into what could occur in the future with varicella disease if the one-dose regimen continues.
While follow-up data show the overall incidence of varicella disease decreases with a one-dose universal child program, the evidence shows that incidence of breakthrough disease is significantly greater in those receiving one dose of varicella as compared to those receiving two doses after 10 years of follow-up. Additionally, the incidence of moderate to severe breakthrough disease cases increased as time since vaccination increased. The data also indicate that previously vaccinated persons serve as good vectors of disease transmission for those susceptible to disease (no varicella vaccination or no previous history of varicella disease) after having received only one dose of vaccine. The index case in five out of 10 of the outbreak studies had a previous history of vaccination. The same occurrence of this phenomenon with those receiving two doses of vaccine, while possible, has not been reported in any studies to date. This is important since the age distribution of varicella cases has shifted upwards since the introduction of universal vaccination and the frequency of moderate to severe disease has been shown to increase with increasing age regardless of vaccination status of the infected case. Furthermore, data from the U.S. surveillance sites showed that with time there was a greater number of cases with previous history of vaccination. This can contribute to the perception that the vaccine is ineffective in preventing varicella disease. Should this occur, parents and adults alike may be less likely to choose vaccination as an option, which would erode all the previous gains of decreased disease incidence.
Timing of the second dose will need to be considered. Currently, provinces and territories administer the first dose of varicella vaccine at either 12 or 15 months of age. A second dose would be optimally provided at either 18 months or at 4 to 6 years of age as these are the times at which the child is due for the next visit for a vaccination. Administering the dose to the 4-6 year-old age group would provide children with a boosting of immunity approximately three years after the first vaccination. This is the point in which breakthrough rates begin to increase substantially. Additionally, immune response to a second dose provided 4-6 years after the first dose has been comparable to the response observed when the second dose is administered three months after the first. Unfortunately, individuals who did not mount a sufficient response to the first vaccination would have an elevated risk of disease during the time between receiving their first and second dose; however, virus circulation would likely be sufficiently reduced, thereby reducing the risk of disease in any case. Furthermore, while this is a safe vaccine with relatively few reports of adverse events it is likely there will be some increase in the number of AEs due to the administration of a second dose.
Concern has been raised that varicella vaccination could potentially result in increased risk of herpes zoster, which is generally a more severe disease that typically impacts older populations because people previously infected with varicella will have decreased opportunities for boosting immunity by exposure to wild-type varicella zoster virus. Any protective advantage that exposure to wild-type varicella may produce would be short-lived as vaccinated cohorts become older. Additionally, a vaccine for herpes zoster was recently licensed in the U.S. (and more recently in Canada) and is recommended for use in those over the age of 60 years in the U.S. If used in Canada, it would be expected that HZ incidence and severity would decrease.
While Canada has not recommended the elimination of varicella as a national goal as is the case for rubella, the purpose of a vaccination program should be to achieve the lowest levels of morbidity and mortality possible. This will not be achieved with the current one-dose child schedule. Furthermore, breakthrough disease would likely increase in frequency and this could have a negative impact on public perception of the effectiveness and utility on varicella vaccine (and possibly other vaccines).
Footnotes
The numerator and the denominator refer to adults with chronic medical conditions who report not having “prior immunity” to varicella. “Prior immunity” is assessed based on recall of having had the disease (varicella or chickenpox) or having been tested for immunity. However, since this value is based heavily on recall of having had the disease, it is possible that the denominator is increased due to participants not recalling have had varicella/chickenpox.
Definite cases: skin lesions included vesicles and diagnosis was confirmed by a physician, or the child had been in recent contact with a physician-confirmed case of varicella. Probable case: skin lesions reportedly included vesicles.
Suspect case: skin lesions were described as nonvesicular but the child had recent contact with a case of varicella or appeared to transmit a varicella-like illness to a contact.
References
- 1.The process to establish and implement national goals and recommendations for vaccine preventable diseases in Canada under the National Immunization Strategy. Can Commun Dis Rep 2007. Dec;33(13):1–7. [PubMed] [Google Scholar]
- 2.Canadian Immunization Guide. Seventh ed. Public Health Agency of Canada. 2006. [Google Scholar]
- 3.Centers for Disease Control and Prevention. Varicella. Epidemiology and Prevention of Vaccine-Preventable Diseases. Washington, D.C.: Public Health Foundation. 2007; 175–96. [Google Scholar]
- 4.National Advisory Committee on Immunization. Update on varicella. Can Commun Dis Rep 2004. Feb;30:1–26. [PubMed] [Google Scholar]
- 5.Law B, Fitzsimon C, Ford-Jones L, McCormick J, Rivière M. Cost of chickenpox in Canada: part II. Cost of complicated cases and total economic impact. The Immunization Monitoring Program-Active (IMPACT). Pediatrics 1999. Jul;104(1 Pt 1):7–14. 10.1542/peds.104.1.7 [DOI] [PubMed] [Google Scholar]
- 6.Law BJ. Chickenpox vaccination, not chickenpox, should be routine for Canadian children. CMAJ 2001. May;164(10):1454–5. [PMC free article] [PubMed] [Google Scholar]
- 7.Marin M, Güris D, Chaves SS, Schmid S, Seward JF; Advisory Committee on Immunization Practices, Centers for Disease Control and Prevention (CDC). Prevention of varicella: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep 2007. Jun;56 RR-4:1–40. [PubMed] [Google Scholar]
- 8.Galil K, Brown C, Lin F, Seward J. Hospitalizations for varicella in the United States, 1988 to 1999. Pediatr Infect Dis J 2002. Oct;21(10):931–5. 10.1097/00006454-200210000-00009 [DOI] [PubMed] [Google Scholar]
- 9.Meyer PA, Seward JF, Jumaan AO, Wharton M. Varicella mortality: trends before vaccine licensure in the United States, 1970-1994. J Infect Dis 2000. Aug;182(2):383–90. 10.1086/315714 [DOI] [PubMed] [Google Scholar]
- 10.Public Health Agency of Canada. Canadian National Report on Immunization, 2006. Can Commun Dis Rep 2006. Nov;32 Suppl 3:1–44. [Google Scholar]
- 11.Law B, Scheifele D, MacDonald N, Halperin S, Déry P, Jadavji T et al. The Immunization Monitoring Program-active (IMPACT) prospective surveillance of varicella zoster infections among hospitalized Canadian Children: 1991-1996. Can Commun Dis Rep 2000. Aug;26(15):125–31. [PubMed] [Google Scholar]
- 12.Merck Frosst Product Monograph Varivax III. 2008
- 13.GlaxoSmithKline Inc. Varilrix Product Monograph. 2007
- 14.Izurieta HS, Strebel PM, Blake PA. Postlicensure effectiveness of varicella vaccine during an outbreak in a child care center. JAMA 1997. Nov;278(18):1495–9. 10.1001/jama.1997.03550180045035 [DOI] [PubMed] [Google Scholar]
- 15.Tugwell BD, Lee LE, Gillette H, Lorber EM, Hedberg K, Cieslak PR. Chickenpox outbreak in a highly vaccinated school population. Pediatrics 2004. Mar;113(3 Pt 1):455–9. 10.1542/peds.113.3.455 [DOI] [PubMed] [Google Scholar]
- 16.Seward JF, Zhang JX, Maupin TJ, Mascola L, Jumaan AO. Contagiousness of varicella in vaccinated cases: a household contact study. JAMA 2004. Aug;292(6):704–8. 10.1001/jama.292.6.704 [DOI] [PubMed] [Google Scholar]
- 17.Ferson MJ. Varicella vaccine in post-exposure prophylaxis. Commun Dis Intell 2001. Jan;25(1):13–5. [PubMed] [Google Scholar]
- 18.Watson B, Seward J, Yang A, Witte P, Lutz J, Chan C et al. Postexposure effectiveness of varicella vaccine. Pediatrics 2000. Jan;105(1 Pt 1):84–8. 10.1542/peds.105.1.84 [DOI] [PubMed] [Google Scholar]
- 19.Salzman MB, Garcia C. Postexposure varicella vaccination in siblings of children with active varicella. Pediatr Infect Dis J 1998. Mar;17(3):256–7. 10.1097/00006454-199803000-00020 [DOI] [PubMed] [Google Scholar]
- 20.Canadian Paediatric Society. Prevention of varicella in children and adolescents. Paediatr Child Health 2005;10(7):409–12. [Google Scholar]
- 21.Guris D, Jumaan AO, Mascola L, Watson BM, Zhang JX, Chaves SS et al. Changing varicella epidemiology in active surveillance sites--United States, 1995-2005. J Infect Dis 2008. Mar;197 Suppl 2:S71–5. 10.1086/522156 [DOI] [PubMed] [Google Scholar]
- 22.Seward JF, Watson BM, Peterson CL, Mascola L, Pelosi JW, Zhang JX et al. Varicella disease after introduction of varicella vaccine in the United States, 1995-2000. JAMA 2002. Feb;287(5):606–11. 10.1001/jama.287.5.606 [DOI] [PubMed] [Google Scholar]
- 23.Chaves SS, Gargiullo P, Zhang JX, Civen R, Guris D, Mascola L et al. Loss of vaccine-induced immunity to varicella over time. N Engl J Med 2007. Mar;356(11):1121–9. 10.1056/NEJMoa064040 [DOI] [PubMed] [Google Scholar]
- 24.Civen R, Lopez AS, Zhang J, Garcia-Herrera J, Schmid DS, Chaves SS et al. Varicella outbreak epidemiology in an active surveillance site, 1995-2005. J Infect Dis 2008. Mar;197 Suppl 2:S114–9. 10.1086/522144 [DOI] [PubMed] [Google Scholar]
- 25.Weinmann S, Chun C, Mullooly JP, Riedlinger K, Houston H, Loparev VN et al. Laboratory diagnosis and characteristics of breakthrough varicella in children. J Infect Dis 2008. Mar;197 Suppl 2:S132–8. 10.1086/522148 [DOI] [PubMed] [Google Scholar]
- 26.Reynolds MA, Watson BM, Plott-Adams KK, Jumaan AO, Galil K, Maupin TJ et al. Epidemiology of varicella hospitalizations in the United States, 1995-2005. J Infect Dis 2008. Mar;197 Suppl 2:S120–6. 10.1086/522146 [DOI] [PubMed] [Google Scholar]
- 27.Zhou F, Harpaz R, Jumaan AO, Winston CA, Shefer A. Impact of varicella vaccination on health care utilization. JAMA 2005. Aug;294(7):797–802. 10.1001/jama.294.7.797 [DOI] [PubMed] [Google Scholar]
- 28.Patel RA, Binns HJ, Shulman ST. Reduction in pediatric hospitalizations for varicella-related invasive group A streptococcal infections in the varicella vaccine era. J Pediatr 2004. Jan;144(1):68–74. 10.1016/j.jpeds.2003.10.025 [DOI] [PubMed] [Google Scholar]
- 29.Ratner AJ. Varicella-related hospitalizations: an update. Pediatr Infect Dis J 2004. Apr;23(4):377. 10.1097/00006454-200404000-00032 [DOI] [PubMed] [Google Scholar]
- 30.Rhein L, Fleisher GR, Harper MB. Lack of reduction in hospitalizations and emergency department visits for varicella in the first 2 years post-vaccine licensure. Pediatr Emerg Care 2001. Apr;17(2):101–3. 10.1097/00006565-200104000-00005 [DOI] [PubMed] [Google Scholar]
- 31.Marin M, Watson TL, Chaves SS, Civen R, Watson BM, Zhang JX et al. Varicella among adults: data from an active surveillance project, 1995-2005. J Infect Dis 2008. Mar;197 Suppl 2:S94–100. 10.1086/522155 [DOI] [PubMed] [Google Scholar]
- 32.Chaves SS, Zhang J, Civen R, Watson BM, Carbajal T, Perella D et al. Varicella disease among vaccinated persons: clinical and epidemiological characteristics, 1997-2005. J Infect Dis 2008. Mar;197 Suppl 2:S127–31. 10.1086/522150 [DOI] [PubMed] [Google Scholar]
- 33.Marin M, Nguyen HQ, Keen J, Jumaan AO, Mellen PM, Hayes EB et al. Importance of catch-up vaccination: experience from a varicella outbreak, Maine, 2002-2003. Pediatrics 2005. Apr;115(4):900–5. 10.1542/peds.2004-1162 [DOI] [PubMed] [Google Scholar]
- 34.Li S, Chan IS, Matthews H, Heyse JF, Chan CY, Kuter BJ et al. Inverse relationship between six week postvaccination varicella antibody response to vaccine and likelihood of long term breakthrough infection. Pediatr Infect Dis J 2002. Apr;21(4):337–42. 10.1097/00006454-200204000-00014 [DOI] [PubMed] [Google Scholar]
- 35.Centers for Disease Control and Prevention (CDC). Outbreak of varicella among vaccinated children--Michigan, 2003. MMWR Morb Mortal Wkly Rep 2004. May;53(18):389–92. [PubMed] [Google Scholar]
- 36.Kuter B, Matthews H, Shinefield H, Black S, Dennehy P, Watson B et al. ; Study Group for Varivax. Ten year follow-up of healthy children who received one or two injections of varicella vaccine. Pediatr Infect Dis J 2004. Feb;23(2):132–7. 10.1097/01.inf.0000109287.97518.67 [DOI] [PubMed] [Google Scholar]
- 37.Watson B. Humoral and cell-mediated immune responses in children and adults after 1 and 2 doses of varicella vaccine. J Infect Dis 2008. Mar;197 Suppl 2:S143–6. 10.1086/522130 [DOI] [PubMed] [Google Scholar]
- 38.Gershon AA, Steinberg SP, LaRussa P, Ferrara A, Hammerschlag M, Gelb L. Immunization of healthy adults with live attenuated varicella vaccine. J Infect Dis 1988. Jul;158(1):132–7. 10.1093/infdis/158.1.132 [DOI] [PubMed] [Google Scholar]
- 39.Vázquez M, LaRussa PS, Gershon AA, Niccolai LM, Muehlenbein CE, Steinberg SP et al. Effectiveness over time of varicella vaccine. JAMA 2004. Feb;291(7):851–5. 10.1001/jama.291.7.851 [DOI] [PubMed] [Google Scholar]
- 40.Passwell JH, Hemo B, Levi Y, Ramon R, Friedman N, Lerner-Geva L. Use of a computerized database to study the effectiveness of an attenuated varicella vaccine. Pediatr Infect Dis J 2004. Mar;23(3):221–6. 10.1097/01.inf.0000114906.78716.64 [DOI] [PubMed] [Google Scholar]
- 41.Scheifele DW, Halperin SA, Diaz-Mitoma F. Three-year follow-up of protection rates in children given varicella vaccine. Can J Infect Dis 2002. Nov;13(6):382–6. 10.1155/2002/907087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bayer O, Heininger U, Heiligensetzer C, von Kries R. Metaanalysis of vaccine effectiveness in varicella outbreaks. Vaccine 2007. Sep;25(37-38):6655–60. 10.1016/j.vaccine.2007.07.010 [DOI] [PubMed] [Google Scholar]
- 43.Galil K, Lee B, Strine T, Carraher C, Baughman AL, Eaton M et al. Outbreak of varicella at a day-care center despite vaccination. N Engl J Med 2002. Dec;347(24):1909–15. 10.1056/NEJMoa021662 [DOI] [PubMed] [Google Scholar]
- 44.Haddad MB, Hill MB, Pavia AT, Green CE, Jumaan AO, De AK et al. Vaccine effectiveness during a varicella outbreak among schoolchildren: Utah, 2002-2003. Pediatrics 2005. Jun;115(6):1488–93. 10.1542/peds.2004-1826 [DOI] [PubMed] [Google Scholar]
- 45.Donahue JG, Choo PW, Manson JE, Platt R. The incidence of herpes zoster. Arch Intern Med 1995. Aug;155(15):1605–9. 10.1001/archinte.1995.00430150071008 [DOI] [PubMed] [Google Scholar]
- 46.Jumaan AO, Yu O, Jackson LA, Bohlke K, Galil K, Seward JF. Incidence of herpes zoster, before and after varicella-vaccination-associated decreases in the incidence of varicella, 1992-2002. J Infect Dis 2005. Jun;191(12):2002–7. 10.1086/430325 [DOI] [PubMed] [Google Scholar]
- 47.Yih WK, Brooks DR, Lett SM, Jumaan AO, Zhang Z, Clements KM et al. The incidence of varicella and herpes zoster in Massachusetts as measured by the Behavioral Risk Factor Surveillance System (BRFSS) during a period of increasing varicella vaccine coverage, 1998-2003. BMC Public Health 2005. Jun;5(68):68. 10.1186/1471-2458-5-68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Reynolds MA, Chaves SS, Harpaz R, Lopez AS, Seward JF. The impact of the varicella vaccination program on herpes zoster epidemiology in the United States: a review. J Infect Dis 2008. Mar;197 Suppl 2:S224–7. 10.1086/522162 [DOI] [PubMed] [Google Scholar]
- 49.Brisson M, Edmunds WJ, Law B, Gay NJ, Walld R, Brownell M et al. Epidemiology of varicella zoster virus infection in Canada and the United Kingdom. Epidemiol Infect 2001. Oct;127(2):305–14. 10.1017/S0950268801005921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hope-Simpson RE. The nature of herpes zoster: a long-term study and a new hypothesis. Proc R Soc Med 1965. Jan;58:9–20. [PMC free article] [PubMed] [Google Scholar]
- 51.Thomas SL, Hall AJ. What does epidemiology tell us about risk factors for herpes zoster? Lancet Infect Dis 2004. Jan;4(1):26–33. 10.1016/S1473-3099(03)00857-0 [DOI] [PubMed] [Google Scholar]
- 52.Galanis E, Skowronski D, Naus M et al. The burden of varicella and zoster in British Columbia 1994–2003: Baseline assessment prior to universal vaccination. 2007 (unpublished). [PubMed]
- 53.Russell ML, Schopflocher DP, Svenson L, Virani SN. Secular trends in the epidemiology of shingles in Alberta. Epidemiol Infect 2007. Aug;135(6):908–13. 10.1017/S0950268807007893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Chaves SS, Santibanez TA, Gargiullo P, Guris D. Chickenpox exposure and herpes zoster disease incidence in older adults in the U.S. Public Health Rep 2007. Mar-Apr;122(2):155–9. 10.1177/003335490712200204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Brisson M, Gay NJ, Edmunds WJ et al. Exposure to varicella boosts immunity to herpes zoster: implication for mass vaccination against chickenpox. Vaccine 2002;3207:1–8. [DOI] [PubMed] [Google Scholar]
- 56.Reisinger KS, Brown ML, Xu J, Sullivan BJ, Marshall GS, Nauert B et al. ; Protocol 014 Study Group for ProQuad. A combination measles, mumps, rubella, and varicella vaccine (ProQuad) given to 4- to 6-year-old healthy children vaccinated previously with M-M-RII and Varivax. Pediatrics 2006. Feb;117(2):265–72. 10.1542/peds.2005-0092 [DOI] [PubMed] [Google Scholar]
- 57.Macartney KK, Burgess MA. Varicella vaccination in Australia and New Zealand. J Infect Dis 2008. Mar;197 Suppl 2:S191–5. 10.1086/522157 [DOI] [PubMed] [Google Scholar]
- 58.Chaves SS, Haber P, Walton K, Wise RP, Izurieta HS, Schmid DS et al. Safety of varicella vaccine after licensure in the United States: experience from reports to the vaccine adverse event reporting system, 1995-2005. J Infect Dis 2008. Mar;197 Suppl 2:S170–7. 10.1086/522161 [DOI] [PubMed] [Google Scholar]
- 59.Shinefield HR, Black SB, Staehle BO, Matthews H, Adelman T, Ensor K et al. ; Kaiser Permanente Medical Team for Varivax. Vaccination with measles, mumps and rubella vaccine and varicella vaccine: safety, tolerability, immunogenicity, persistence of antibody and duration of protection against varicella in healthy children. Pediatr Infect Dis J 2002. Jun;21(6):555–61. 10.1097/00006454-200206000-00014 [DOI] [PubMed] [Google Scholar]
- 60.Zhou F, Ortega-Sanchez IR, Guris D, Shefer A, Lieu T, Seward JF. An economic analysis of the universal varicella vaccination program in the United States. J Infect Dis 2008. Mar;197 Suppl 2:S156–64. 10.1086/522135 [DOI] [PubMed] [Google Scholar]
- 61.Arvin A, Gershon A. Control of varicella: why is a two-dose schedule necessary? Pediatr Infect Dis J 2006. Jun;25(6):475–6. 10.1097/01.inf.0000219484.55858.a2 [DOI] [PubMed] [Google Scholar]
- 62.Centers for Disease Control and Prevention (CDC). Varicella outbreak among vaccinated children--Nebraska, 2004. MMWR Morb Mortal Wkly Rep 2006. Jul;55(27):749–52. [PubMed] [Google Scholar]
- 63.Buchholz U, Moolenaar R, Peterson C, Mascola L. Varicella outbreaks after vaccine licensure: should they make you chicken? Pediatrics 1999. Sep;104(3 Pt 1):561–3. 10.1542/peds.104.3.561 [DOI] [PubMed] [Google Scholar]
- 64.Miron D, Lavi I, Kitov R, Hendler A. Vaccine effectiveness and severity of varicella among previously vaccinated children during outbreaks in day-care centers with low vaccination coverage. Pediatr Infect Dis J 2005. Mar;24(3):233–6. 10.1097/01.inf.0000154323.20387.82 [DOI] [PubMed] [Google Scholar]
- 65.Arnedo-Pena A, Puig-Barberà J, Aznar-Orenga MA, Ballester-Albiol M, Pardo-Serrano F, Bellido-Blasco JB et al. Varicella vaccine effectiveness during an outbreak in a partially vaccinated population in Spain. Pediatr Infect Dis J 2006. Sep;25(9):774–8. 10.1097/01.inf.0000232631.06763.8b [DOI] [PubMed] [Google Scholar]
- 66.Dworkin MS, Jennings CE, Roth-Thomas J, Lang JE, Stukenberg C, Lumpkin JR. An Outbreak of Varicella among children attending preschool and elementary school in Illinois. Clin Infect Dis 2002. Jul;35(1):102–4. 10.1086/340868 [DOI] [PubMed] [Google Scholar]
- 67.Galil K, Fair E, Mountcastle N, Britz P, Seward J. Younger age at vaccination may increase risk of varicella vaccine failure. J Infect Dis 2002. Jul;186(1):102–5. 10.1086/341089 [DOI] [PubMed] [Google Scholar]
- 68.Lee BR, Feaver SL, Miller CA, Hedberg CW, Ehresmann KR. An elementary school outbreak of varicella attributed to vaccine failure: policy implications. J Infect Dis 2004. Aug;190(3):477–83. 10.1086/422041 [DOI] [PubMed] [Google Scholar]
- 69.Lopez AS, Guris D, Zimmerman L, Gladden L, Moore T, Haselow DT et al. One dose of varicella vaccine does not prevent school outbreaks: is it time for a second dose? Pediatrics 2006. Jun;117(6):e1070–7. 10.1542/peds.2005-2085 [DOI] [PubMed] [Google Scholar]