Key Points
Question
Is transcranial direct current stimulation (tDCS) a safe and effective add-on therapy for negative symptoms in schizophrenia?
Findings
In this randomized clinical trial of 100 patients with schizophrenia with predominant negative symptoms, active tDCS was superior to sham in ameliorating negative symptoms, with superior response rates (20% improvement) for negative symptoms. These effects were sustained at follow-up, and tDCS was not associated with significant adverse effects.
Meaning
Transcranial direct current stimulation is an affordable, safe, and effective add-on treatment for negative symptoms in schizophrenia.
This randomized clinical trial studies the efficacy and safety of transcranial direct current stimulation compared with sham as an add-on treatment for patients with schizophrenia with predominant negative symptoms.
Abstract
Importance
Negative symptoms represent a substantial burden in schizophrenia. Although preliminary studies have suggested that transcranial direct current stimulation (tDCS) is effective for some clusters of symptoms, the clinical benefits for negative symptoms are unclear.
Objective
To determine the efficacy and safety of tDCS vs sham as an add-on treatment for patients with schizophrenia and predominant negative symptoms.
Design, Setting, and Participants
The double-blind Schizophrenia Treatment With Electric Transcranial Stimulation (STARTS) randomized clinical trial was conducted from September 2014 to March 2018 in 2 outpatient clinics in the state of São Paulo, Brazil. Patients with schizophrenia with stable negative and positive symptoms and a minimum score of 20 points in the negative symptoms subscale of the Positive and Negative Syndrome Scale (PANSS) were included.
Interventions
Ten sessions of tDCS performed twice a day for 5 days or a sham procedure. The anode and the cathode were positioned over the left prefrontal cortex and the left temporoparietal junction, respectively.
Main Outcomes and Measures
Change in the PANSS negative symptoms subscale score at week 6 was the primary outcome. Patients were followed-up for an additional 6 weeks.
Results
Of the 100 included patients, 20 (20.0%) were female, and the mean (SD) age was 35.3 (9.3) years. A total of 95 patients (95.0%) finished the trial. In the intention-to-treat analysis, patients receiving active tDCS showed a significantly greater improvement in PANSS score compared with those receiving the sham procedure (difference, 2.65; 95% CI, 1.51-3.79; number needed to treat, 3.18; 95% CI, 2.12-6.99; P < .001). Response rates for negative symptoms (20% improvement or greater) were also higher in the active group (20 of 50 [40%]) vs the sham group (2 of 50 [4%]) (P < .001). These effects persisted at follow-up. Transcranial direct current stimulation was well tolerated, and adverse effects did not differ between groups, except for burning sensation over the scalp in the active group (43.8%) vs the sham group (14.3%) (P = .003).
Conclusions and Relevance
Transcranial direct current stimulation was effective and safe in ameliorating negative symptoms in patients with schizophrenia.
Trial Registration
ClinicalTrials.gov identifier: NCT02535676
Introduction
Schizophrenia is a severe mental illness presenting a substantial, increasing burden.1 Its negative symptoms include flattened affect, loss of interest, and emotional withdrawal and are associated with poor functional outcomes.2 Most antipsychotic drugs are not effective for such symptoms and present important adverse effects3 and low tolerability.4 Nonpharmacological interventions are also limited.5
The pathophysiology of negative symptoms has been associated with decreased activity of the prefrontal cortex (PFC).6,7 Thus, several studies used high-frequency (excitatory) repetitive transcranial magnetic stimulation (rTMS) protocols over the left PFC, showing moderate but significant results for improving negative symptoms.8 However, rTMS use is limited because of high costs and a small risk of seizures.9
Transcranial direct current stimulation (tDCS) is a noninvasive neuromodulatory technique that presents low costs, portability, ease of use, and no serious adverse effects.10,11,12 The technique injects weak, direct currents via scalp electrodes. A current fraction penetrates the brain, increasing or decreasing the neuronal excitability of regions near the anode or the cathode, respectively.11 Mimicking rTMS studies, tDCS trials have used anodal stimulation over the left PFC aiming to ameliorate negative symptoms.8 In a seminal study, Brunelin et al13 used a frontotemporoparietal montage in 30 patients with schizophrenia and demonstrated large effect sizes for improvement of negative symptoms and auditory hallucinations (AHs). However, the findings by Brunelin et al13 were not consistently replicated by later studies using different tDCS parameters, including cathode positioning (left temporal vs right supraorbital), unilateral vs bilateral prefrontal anodal stimulation, and number of sessions.14,15,16,17 Nonetheless, most studies presented low sample sizes and were not adequately powered. In fact, a 2018 meta-analysis8 found only 5 tDCS trials (n = 134 patients) investigating negative symptoms, and not necessarily as the primary outcome, emphasizing the need for larger studies.
Therefore, we evaluated the efficacy of tDCS on the treatment of negative symptoms of schizophrenia, as measured by the negative subscale of the Positive and Negative Syndrome Scale (PANSS),18 at 6 weeks after trial onset (primary end point). Secondary outcome measures were changes in other scales, response rates, treatment tolerability, and adverse effects at 12 weeks (secondary end point).
Methods
The Schizophrenia Treatment With Electric Transcranial Stimulation (STARTS) trial was a double-blind, placebo-controlled randomized clinical trial that enrolled patients with schizophrenia with negative symptoms. Randomization was performed using random block sizes from a computer-generated list. We used opaque, sealed envelopes for allocation concealment. The study protocol was described elsewhere19 and performed with no significant changes (Supplement 1).
The STARTS trial was conducted at 2 study centers (Institute of Psychiatry, General Hospital of the University of São Paulo Medical School, São Paulo, Brazil and Instituto Bairral de Psiquiatria, Itapira, São Paulo, Brazil) from September 2014 to March 2018. The study was registered on ClinicalTrials.gov (NCT02535676), reported per the Consolidated Standards of Reporting Trials (CONSORT) reporting guideline for nonpharmacological treatments,20 and approved by the Comitê de Ética em Pesquisa do Hospital das Clínicas da Faculdade de Medicina da Universidade de São Paulo (main site) and the ethics committee of Instituto Bairral (secondary site). Participants signed informed consent forms per the Declaration of Helsinki guidelines.21
Participants
Participants were recruited through media advertisements and physician referrals. We included patients with schizophrenia diagnosed by trained psychiatrists using the Portuguese version of the Structured Clinical Interview for Diagnostic and Statistical Manual of Mental Disorders (Fourth Edition).22 Only patients aged 18 to 55 years with prominent negative symptoms (based on psychiatric assessment and 20 points or greater on the PANSS negative symptoms subscale, similar to the approach used by Mogg et al23) and stable positive and negative symptoms for 4 weeks or more (based on medical records, clinical judgment, and psychiatric interview, including no history of hospital admissions, acute exacerbations, or treatment regimen changes during this period) were included. Exclusion criteria were unstable medical conditions, pretreatment with rTMS or tDCS, previous (past 6 months) or current treatment with electroconvulsive therapy, psychiatric comorbidities (such as mood and personality disorders), substance use disorders (except for tobacco use disorders), and specific contraindications to tDCS, such as metal implants in the head.
Regarding pharmacotherapy, participants were in a proper antipsychotic treatment regimen with stable doses for 4 weeks or more before trial onset. Doses remained stable throughout the study. Antidepressant drugs were washed out for 4 weeks or more before trial onset, and benzodiazepines were allowed up to a maximum dosage of 10 mg per day of diazepam equivalents to minimize the interactions of pharmacological treatments with tDCS.24,25
Interventions
We used the same protocol as Brunelin et al.13 Therefore, we chose the same tDCS montage (anodal over the left PFC and cathode over the left temporoparietal junction) and treatment schedule (twice daily sessions, with a minimum interval between sessions of 3 hours, over 5 consecutive days from Monday to Friday). In fact, tDCS is a nonfocal noninvasive brain stimulation approach, and both left frontotemporoparietal and bifrontal montages may be used for targeting the left PFC.26,27
We opted for not increasing the number of sessions, as Brunelin et al13 showed sustained and increased effects for up to 3 months after treatment. This is in line with prior observations from tDCS depression trials24,28,29,30 that optimal clinical effects of tDCS may take several weeks to develop after the acute treatment phase.
We used DC-Stimulator tDCS devices (Neuroconn) to perform the tDCS sessions. The devices presented a study mode function, which can be customized, in which a 5-digit code is imputed that determines, without staff awareness, whether active or sham tDCS was applied.
Participants laid in reclinable, comfortable chairs to receive the treatment, which lasted 20 minutes (ramp-up and ramp-down periods of 40 seconds). The following stimulation parameters were used: 2 mA; 5 × 7 cm2 electrodes, with the anode centered over the area corresponding to the left dorsolateral PFC and the cathode centered over the area corresponding to the left temporoparietal junction; and use of the electroencephalography 10–20 system (F3 and T3P3 areas, respectively), with both electrodes’ large axes (7 cm) perpendicular to the skull’s circumference. Computational simulation of the current distribution can be found elsewhere.19
For sham tDCS, the same procedures were used, including the ramp-up and ramp-down periods of 40 seconds, with a stimulation duration of 30 seconds at 2 mA between the ramp phases. Blinding efficacy was assessed at the end point by asking participants to guess their allocation group.
Assessments
Assessments were performed by trained psychiatrists and psychologists blinded for patients’ condition. Participants were assessed at baseline and then 5 days, 2 weeks, 4 weeks, 6 weeks (primary end point), and 12 weeks (secondary end point) after treatment onset. The measurements from the assessment immediately after the end of the acute tDCS phase were not selected as the primary outcome, as we considered that negative symptoms represent a more underlying and complex pathophysiology that, in contrast with AHs in the study by Brunelin et al,13 would not acutely improve after 1 week of treatment. Adverse effects were recorded at 5 days, 6 weeks, and 12 weeks after treatment onset.
The primary outcome was the change in score on the negative symptoms subscale of PANSS over time. Secondary outcomes included clinical response defined as 20% or greater improvement in the negative symptoms subscale score on PANSS, according to a previous large rTMS trial31 (the 20% cutoff has also been commonly used in pharmacological studies32) as well as changes in PANSS score (both positive symptoms subscale and total scores), Calgary Depression Scale for Schizophrenia (CDSS) score,33 Auditory Hallucinations Rating Scale score,34 Global Assessment of Functioning (GAF) score,35 frequency of adverse effects,10,36 and Scale for the Assessment of Negative Symptoms (SANS) score.37 Sociodemographic and clinical variables were collected at baseline and analyzed as predictors of response (eAppendix 1 in Supplement 2).
Statistical Analysis
The sample size was estimated for a power of 80% and a 2-tailed α level of 5% for the negative symptoms subscale of PANSS. Our study was powered to detect a between-group difference of at least 3 points. We estimated an attrition rate of 15%. Therefore, a targeted sample of 100 patients (50 per group) was obtained.
Data were analyzed in the intention-to-treat sample. Analyses were performed using the lme4 package of R version 3.5.2 (The R Foundation).38 Results were significant at a P value less than .05. Effect sizes were calculated as Cohen d and odds ratios for continuous and binary outcomes, respectively. We provided the number needed to treat, which assesses the effectiveness of a clinical intervention, for all outcomes.39 For continuous outcomes, they were obtained by transformation of Cohen d using the cumulative distribution function of the standard normal distribution.40 For all continuous outcomes, we calculated 3-level linear mixed-effects regression models (LMM), assuming a linear relationship over time with 5 (up to week 6) or 6 (up to week 12) repeated measurements per patient, respectively (eAppendix 2 in Supplement 2).
Binary outcomes of treatment response were modeled using 2-level mixed logistic regression models (patients clustered in centers) at week 6 and week 12. Adverse effects were compared between groups by Fisher exact test or χ2 test. We investigated predictors of tDCS response by testing the interaction of each predictor with the group. In these analyses, change of the negative symptoms from baseline to week 6 was the dependent variable.
Finally, 3 post hoc analyses were conducted to make our results comparable with literature and to better investigate whether findings were clinically meaningful. Post hoc analyses included similar LMM analyses corrected for multiple comparisons (false discovery rate method) for each individual symptom of the PANSS negative symptoms subscale, an analysis using PANSS Factor Score for Negative Symptoms (FSNS), which is more specific for negative symptoms,41 and an analysis of response rates using a 25% cutoff.42
Results
Participants
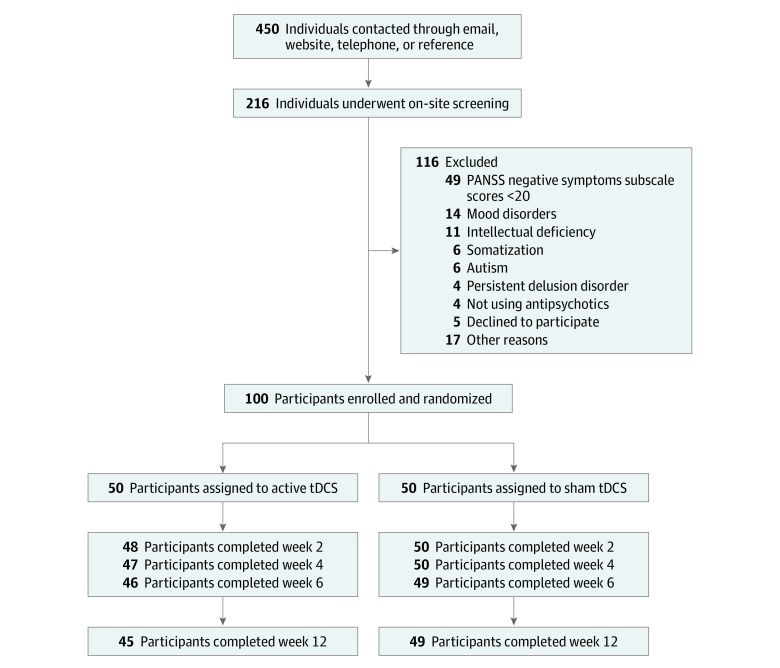
Of 450 volunteers, 100 patients initiated the study; of these, 20 (20.0%) were female, and the mean (SD) age was 35.3 (9.3) years. A total of 95 and 94 participants completed the study at the primary and secondary end points, respectively. Dropouts were balanced between groups (Figure 1) (Table 1). The relatively low number of hospitalizations in both groups, despite the long illness durations, might be attributed to strict policies against psychiatric hospitalization in Brazil44 and chronic shortage of psychiatric beds in the public health system.45
Figure 1. Study Flowchart.
Analysis was performed in the intention-to-treat sample. PANSS indicates Positive and Negative Syndrome Scale; tDCS, transcranial direct current stimulation.
Table 1. Clinical and Demographic Characteristics of the Samplea.
| Characteristic | tDCS Group, Mean (SD) | |
|---|---|---|
| Active (n = 50) | Sham (n = 50) | |
| Age, y | 34.6 (8.4) | 35.9 (10.1) |
| Women, No. (%) | 9 (18) | 11 (22) |
| Years of study | 11.6 (3.1) | 10.5 (3.0) |
| Unemployed, No. (%) | 39 (78) | 38 (76) |
| Not married, No. (%) | 40 (80) | 41 (82) |
| Self-declared white race, No. (%) | 15 (30) | 12 (24) |
| Smoker, No. (%) | 15 (30) | 14 (28) |
| Duration of disease, y | 14.2 (8.1) | 14.1 (8.7) |
| No. of hospitalizations | 0.93 (1.52) | 1.84 (2.07) |
| Previous clozapine use, No. (%) | 19 (38) | 19 (38) |
| Treatment-resistant schizophrenia, No. (%)b | 38 (76) | 35 (70) |
| Ultra–treatment-resistant schizophrenia, No. (%)c | 24 (48) | 20 (40) |
| Haloperidol dose equivalents, mg/dd | 9.53 (4.41) | 10.38 (7.93) |
| ECT, No. (%) | 2 (4) | 5 (10) |
| PANSS score | ||
| Positive symptoms | 14.26 (4.27) | 14.24 (4.09) |
| Negative symptoms | 25.00 (3.93) | 25.10 (3.44) |
| General symptoms | 34.36 (10.21) | 34.58 (8.66) |
| Total symptoms | 73.62 (15.76) | 73.92 (13.36) |
| PANSS FSNS score | 24.22 (5.13) | 24.22 (3.56) |
| CDSS score | 2.32 (3.77) | 2.26 (3.15) |
| AHRS score | 9.44 (11.91) | 7.66 (12.74) |
| GAF score | 46.47 (12.40) | 46.40 (11.04) |
| SANS score | 60.12 (13.80) | 62.32 (11.11) |
| Patients, No. (%) | ||
| With no auditory hallucinations per AHRS score | 29 (58) | 35 (70) |
| With no major depressive episode per CDSS score | 43 (86) | 45 (90) |
Abbreviations: AHRS, Auditory Hallucinations Rating Scale; CDSS, Calgary Depression Scale for Schizophrenia; ECT, electroconvulsive therapy; FSNS, Factor Score for Negative Symptoms; GAF, Global Assessment of Functioning; PANSS, Positive and Negative Syndrome Scale; SANS, Scale for the Assessment of Negative Symptoms; tDCS, transcranial direct current stimulation.
There were no statistically significant differences between groups, except number of hospitalizations, which was higher in the sham group.
Treatment-resistant schizophrenia was defined as lack of satisfactory clinical response to treatment with at least 2 antipsychotic drugs from different groups used with therapeutic doses and for 6 or more weeks.
Ultra–treatment-resistant schizophrenia was defined as those with treatment-resistant disease who did not respond to at least 6 months of clozapine in dosages of 300 mg/d or more.32,46
Haloperidol dose equivalents was defined per Andreasen et al.43
Primary Outcome
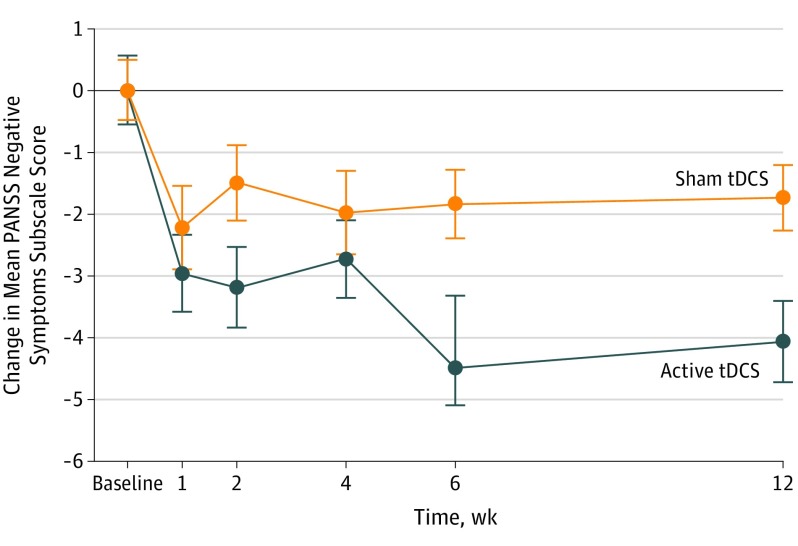
Linear mixed-effects regression analysis revealed a significant time × group interaction (F1394.11 = 12.47; P < .001). Active tDCS was superior to sham tDCS (PANSS score point difference, 2.65; 95% CI, 1.51-3.79; number needed to treat, 3.18; 95% CI, 2.12-6.99; P < .001) (Figure 2) (eTables 1 and 2 in Supplement 2).
Figure 2. Change in Negative Symptoms Subscale Score on the Positive and Negative Syndrome Scale (PANSS).
The mean reduction in the PANSS negative symptoms subscale scores (intention-to-treat analysis) in the active transcranial direct current stimulation (tDCS) and sham tDCS treatment groups from baseline to week 12. Scores on the PANSS negative symptoms subscale range from 7 to 26, with higher scores indicating more severe negative symptoms. Treatment with active tDCS was superior to sham tDCS at week 6 (PANSS score point difference, 2.65; 95% CI, 1.51-3.79; P < .001) and at week 12 (PANSS score point difference, 2.35; 95% CI, 1.02-3.67; P < .001). Error bars represent 1 SE.
Secondary Outcomes
Effects were maintained over the course of 12 weeks, sustaining superiority of the active tDCS treatment. For the active and sham groups, 20 participants (40%) and 2 participants (4%), respectively, presented a response (ie, 20% improvement) at week 6 (P < .001), and 19 participants in the active group (38%) and 2 participants in the sham group (4%) presented a response at week 12 (P < .001). We observed no significant time × group interactions for the other secondary scales (PANSS positive symptoms subscale score, total PANSS score, CDSS score, Auditory Hallucinations Rating Scale score, GAF score, and SANS score) (Figure 2) (Table 2) (eTables 1 and 3 in Supplement 2).
Table 2. Primary and Secondary Outcomes.
| Outcome | tDCS Group, Mean (SD) | Sham tDCS Group vs Active tDCS Group | ||||
|---|---|---|---|---|---|---|
| Sham (n = 50) | Active (n = 50) | Difference in Score (95% CI)a | P Value | Cohen d (95% CI) | NNT (95% CI)b | |
| Primary Outcome | ||||||
| Change in PANSS negative symptoms subscale score at week 6 | −1.84 (1.82) | −4.49 (3.50) | 2.65 (1.51 to 3.79) | <.001 | 0.57 (0.26 to 0.89) | 3.18 (2.12 to 6.99) |
| Secondary Outcomes | ||||||
| Change in score at week 6 (primary end point) | ||||||
| PANSS positive symptoms subscalec | −1.70 (3.99) | −1.46 (3.55) | −0.24 (−1.77 to 1.28) | .49 | −0.13 (−0.48 to 0.23) | −14.00 (−3.73 to 7.72) |
| PANSS general symptoms subscale | −2.76 (5.11) | −1.98 (7.96) | −0.78 (–3.53 to 1.98) | .68 | −0.13 (−0.36 to 0.11) | −14.10 (−4.91 to 15.69) |
| PANSS total | −6.61 (7.68) | −8.02 (11.47) | 1.41 (−2.60 to 5.42) | .49 | 0.14 (−0.13 to 0.42) | 12.37 (−13.32 to 4.28) |
| PANSS FSNS | −2.20 (2.42) | −4.49 (4.24) | 2.28 (0.85 to 3.72) | .02 | 0.38 (0.08 to 0.68) | 4.72 (2.69 to 23.41) |
| CDSSc | −0.71 (2.31) | −1.15 (2.75) | 0.44 (−0.60 to 1.48) | .40 | 0.10 (−0.14 to 0.34) | 17.66 (−12.96 to 5.30) |
| AHRS | −0.94 (7.71) | 0.24 (6.61) | −1.18 (−4.12 to 1.75) | .43 | −0.10 (−0.33 to 0.14) | −18.52 (−5.34 to 12.39) |
| GAF | 1.73 (9.78) | 2.80 (10.70) | 1.07 (−3.40 to 5.54) | .70 | 0.07 (−0.23 to 0.38) | 24.28 (−7.67 to 4.74) |
| SANS | −8.08 (11.08) | −8.15 (14.20) | 0.07 (−5.15 to 5.29) | .97 | 0.03 (−1.55 to 1.60) | 59.70 (−1.38 to 1.35) |
| Response, No. (%) | ||||||
| ≥20% Improvement | 2 (4) | 20 (40) | 17.78 (6.87 to 28.68)d | <.001 | NA | 2.78 (1.98 to 4.68) |
| ≥25% Improvement | 0 | 12 (24) | NA | <.001e | NA | NA |
| Change in score at week 12 (follow-up) | ||||||
| PANSS negative symptoms subscale | −1.74 (1.86) | −4.09 (4.17) | 2.35 (1.02 to 3.67) | <.001 | 0.51 (0.27 to 0.75) | 3.56 (2.47 to 6.71) |
| PANSS positive symptoms subscale | −1.42 (2.92) | −1.27 (2.86) | −0.15 (−1.34 to 1.04) | .63 | −0.06 (−0.29 to 0.17) | −31.47 (−6.24 to 10.25) |
| PANSS general symptoms subscale | −2.21 (5.65) | −2.51 (7.69) | 0.30 (−2.50 to 3.10) | .56 | 0.05 (0.13 to 0.23) | −34.80 (−7.63 to 13.50) |
| PANSS total | −5.40 (7.42) | −8.04 (11.63) | 2.65 (−1.41 to 6.71) | .42 | 0.21 (−0.16 to 0.58) | 8.58 (−10.95 to 3.16) |
| PANSS FSNS | −2.19 (2.87) | −4.25 (5.01) | 2.06 (0.34 to 3.78) | .001 | 0.38 (0.15 to 0.61) | 4.71 (2.98 to 12.01) |
| CDSSc | −0.73 (2.45) | −0.96 (3.30) | 0.22 (−0.98 to 1.42) | .43 | 0.05 (−0.22 to 0.32) | 35.29 (−7.95 to 5.51) |
| AHRS | −0.02 (7.82) | −0.02 (5.94) | 0 (−2.83 to 2.83) | .54 | −0.05 (−0.23 to 0.12) | −35.46 (−7.74 to 14.79) |
| GAF | 2.15 (8.20) | 3.10 (9.83) | 0.95 (−3.06 to 4.97) | .77 | 0.06 (−0.21 to 0.34) | 27.34 (−8.52 to 5.28) |
| SANS | −10.08 (11.57) | −9.16 (17.74) | −0.93 (−7.14 to 5.29) | .73 | −0.15 (−0.97 to 0.67) | −11.84 (−1.97 to 2.75) |
| Response, No. (%) | ||||||
| ≥20% Improvement | 2 (4) | 19 (38) | 16.29 (5.37 to 27.20)d | <.001 | NA | 2.88 (2.02 to 4.98) |
| ≥25% Improvement | 0 | 15 (30) | NA | <.001e | NA | NA |
Abbreviations: AHRS, Auditory Hallucinations Rating Scale; CDSS, Calgary Depression Scale for Schizophrenia; FSNS, Factor Score for Negative Symptoms; GAF, Global Assessment of Functioning; NA, not applicable; NNT, number needed to treat; PANSS, Positive and Negative Syndrome Scale; SANS, Scale for the Assessment of Negative Symptoms; tDCS, transcranial direct current stimulation.
Change in score was calculated by obtaining the slope (average change between measurements) and multiplying it by the number of observations from baseline.
Negative NNT represents better outcome in sham tDCS group than in active tDCS group.
Model included random slopes because improved model fit was indicated by χ2 likelihood ratio test.
Odds ratio (95% CI).
Determined with Fisher exact test for count data because there were 0 events in one group.
An additional analysis was performed in the subsample with a CDSS score greater than 6, which had 82% sensitivity and 85% specificity for major depression.47 A difference of 3.23 points (95% CI, −1.17 to 7.64; P = .08) favored active tDCS in the LMM model.
Adverse Effects and Safety
The rate of adverse effects between groups was similar, except for burning sensation (Table 3). No serious adverse effects (such as acute psychosis, hospitalization, or suicide attempts) were reported.
Table 3. Frequency of Adverse Effects.
| Adverse Effect | tDCS Group, No. (%) | P Value | |
|---|---|---|---|
| Active (n = 50) | Sham (n = 50) | ||
| Day 5 | |||
| Headache | 7 (14) | 3 (6) | .20a |
| Neck pain | 7 (14) | 2 (4) | .09a |
| Scalp pain | 4 (8) | 2 (4) | .43a |
| Burning sensation | 21 (42) | 7 (14) | .003b |
| Tinnitus | 5 (10) | 1 (2) | .11a |
| Skin redness | 14 (28) | 8 (16) | .18b |
| Sleepiness | 13 (26) | 17 (34) | .60b |
| Trouble concentrating | 13 (26) | 10 (20) | .59b |
| Week 6 | |||
| Headache | 7 (14) | 3 (6) | .18a |
| Neck pain | 4 (8) | 4 (8) | >.99a |
| Scalp pain | 6 (12) | 5 (10) | .87b |
| Burning sensation | 10 (20) | 10 (20) | >.99b |
| Tinnitus | 3 (6) | 3 (6) | >.99a |
| Skin redness | 12 (24) | 9 (18) | .46b |
| Sleepiness | 9 (18) | 11 (22) | .98b |
| Trouble concentrating | 5 (10) | 10 (20) | .35b |
| Week 12 | |||
| Headache | 3 (6) | 4 (8) | >.99a |
| Neck pain | 3 (6) | 3 (6) | >.99a |
| Scalp pain | 6 (12) | 5 (10) | .79b |
| Burning sensation | 8 (16) | 6 (12) | .55b |
| Tinnitus | 0 | 3 (6) | .25a |
| Skin redness | 10 (20) | 9 (18) | .72b |
| Sleepiness | 5 (10) | 13 (26) | .13b |
| Trouble concentrating | 5 (10) | 10 (20) | .41b |
Abbreviation: tDCS, transcranial direct current stimulation.
Fisher exact test.
χ2 Test.
Predictors of Response
Patients with treatment-resistant schizophrenia showed a smaller reduction of negative symptoms after active vs sham tDCS. Similar effects were observed for those with ultra–treatment-resistant schizophrenia, those who used clozapine, and those who took higher haloperidol dose equivalents (eTables 4 and 5 in Supplement 2).
Post Hoc Analyses
Analyses of individual items in the PANSS negative symptoms subscale showed significant improvements in all items except for passive/apathetic withdrawal and stereotyped thinking (eTable 6 and eFigure 1 in Supplement 2). Analyses for the PANSS FSNS showed superiority of active tDCS at both time points. Using the response cutoff of 25%, there were 12 and 0 responders in the active and sham tDCS groups, respectively (Table 2) (eFigure 2 in Supplement 2).
Integrity of Blinding
Participants were unable to guess their actual group beyond chance. In the active and sham groups, of 79 surveyed participants, 32 participants and 12 participants, respectively, correctly identified their group (χ2 = 0.45; P = .50).
Discussion
Main Findings
In line with our primary hypothesis, 10 tDCS sessions within 5 days (ie, twice a day) were effective in ameliorating negative symptoms in schizophrenia 6 weeks after treatment onset. This effect presented a medium effect size, was reflected in higher response rates for negative symptoms, and persisted during the follow-up phase.
The treatment was tolerable and safe, with no reports of serious adverse effects. The safety profile is an appealing characteristic for tDCS, as antipsychotic drugs have adverse effects limiting treatment adherence.4
Individual item analyses showed that improvement occurred in all PANSS negative symptoms subscale scores except for passive/apathetic withdrawal and stereotyped thinking. Interestingly, a meta-analysis of the PANSS factor structure48 revealed that stereotyped thinking might not pertain to the negative domain but rather to a cognitive domain. In addition, the improvement in the PANSS FSNS score was superior in the active tDCS group compared with the sham group. These findings further reinforce the efficacy of tDCS for negative symptoms of schizophrenia.
Higher haloperidol dose equivalents and use of clozapine were associated with decreased tDCS effects. In fact, medications can change tDCS plasticity,25 eg, sulpiride (D2 blocker) can eliminate anodal excitability-enhancing effects49 and citalopram, which modulates the serotonergic system as clozapine, has complex effects in tDCS excitability.50
Treatment resistance was associated with lower tDCS effects. This was also observed for depression24,51 and indicates lower tDCS efficacy in these samples.
Outcomes in Other Secondary Scales
No improvement in AHs was observed. In contrast, Brunelin et al13 and Kantrowitz et al52 reported AH improvement using the same parameters as we used. However, their participants presented moderate to severe AH symptomatology per eligibility criteria. In turn, we did not adopt such inclusion criterion. Importantly, only 36 of 100 patients in our sample (36.0%) presented any AH symptom (ie, Auditory Hallucinations Rating Scale score greater than 0). For instance, the mean baseline Auditory Hallucinations Rating Scale score in the study by Brunelin et al13 was 27.75, whereas ours was 8.55. Therefore, the lack of effects in this scale might reflect the absence of prominent AH symptomatology in our sample.
The absence of significant findings for CDSS can be explained by the fact that depression is not a negative symptom. In fact, CDSS and negative symptoms of PANSS are not correlated.53 Moreover, only 12 patients presented a clinically meaningful depressive episode per the CDSS score. The effects of tDCS in improving depressive symptoms in schizophrenia should be investigated.
The GAF score remained basically unchanged throughout the trial, whereas an improvement of global functioning vis-à-vis negative symptoms’ reduction could have been expected. A ceiling effect could explain the absence of GAF score changes, as our sample presented a relatively high GAF score at baseline. For instance, data from a European Schizophrenia Cohort Study linked a PANSS score of 70 to a GAF score of 38,54 lower than our baseline GAF scores. Nonetheless, such discrepancy can be partly attributed to differences in eligibility criteria and strategies for sample recruitment. In addition, it may take longer for an improvement of negative symptoms to lead to better functional outcomes (for instance, more social interest may require some time to foster new relationships and develop new social skills).
Finally, improvement in SANS score was observed to a similar extent in both active and sham groups. In fact, SANS and PANSS negative symptoms subscale scores are only moderately correlated55 and differ regarding domain coverage.56 Moreover, although SANS is meant to focus on negative symptoms, it also includes attention, which does not pertain to the cognitive domain, and assesses anhedonia and asociality together.56 For these reasons, its content validity for evaluating negative symptoms has been challenged recently.57 Interestingly, neither Brunelin et al13 nor Kantrowitz et al52 used SANS as an outcome scale; therefore, we cannot compare our findings with others to assess whether this scale is sensible to the effects of frontotemporoparietal tDCS in negative symptoms of schizophrenia.
Clinical and Research Implications
There is an unmet clinical need for the treatment of negative symptoms in schizophrenia. A 2017 trial58 showed that cariprazine was more effective than risperidone in ameliorating negative symptoms. To compare the results, we performed a post hoc analysis to estimate the PANSS FSNS score, which was the primary outcome in that study, obtaining a significant absolute decrease of approximately 4.5 points at our primary and secondary end points. This is discreetly lower than the decrease of cariprazine (approximately 6 to 7 points) and risperidone (approximately 5 to 6 points) in the same time frame; however, direct comparisons between randomized clinical trials are limited.
Therefore, our results point toward a clinically meaningful effect for tDCS, fostering further studies examining this intervention vs antipsychotic pharmacotherapy regarding efficacy and risks, using longer periods of observation, and assessing its cost-effectiveness. In fact, given its acceptability, tolerability, and short treatment protocol, tDCS could be evaluated as an add-on intervention for patients with schizophrenia with negative symptoms in outpatient settings. Remotely supervised home treatment of tDCS could be used for prolonged administration.59,60
Moreover, strategies for enhancing tDCS effects should be pursued. These include administration of a cognitive task concomitantly to tDCS,61 use of high-definition tDCS to increase current focality in potential regions of interest,11 and identifying preferential responders to the intervention.62
Strengths and Limitations
Regarding study strengths, we used the same montage as Brunelin et al13 and a 2019 replication trial.52 This allows evaluation of tDCS reproducibility across trials and future individual patient data meta-analysis. Moreover, the attrition rate was low, and blinding was effective. Finally, patients were observed for a long period compared with other tDCS trials in schizophrenia.52,63
This study had limitations. There was no stratified randomization for clozapine or antipsychotic drug use, although groups were overall balanced. No adjunct magnetic resonance imaging study was performed; thus, data on magnetic resonance imaging–based prediction or electric field models are lacking. Additionally, as we aimed to reproduce the findings by Brunelin et al,13 other electrode montages and protocols should be further investigated. Although differences between the active and sham groups were significant for the primary outcome, absolute active and sham changes were relatively low. This issue has already been observed in well-powered noninvasive brain stimulation trials31,52 and could be explained by distinct placebo effects produced by medical devices, in which issues such as response conditioning and expectancy might operate differently.64
Conclusions
Frontotemporoparietal tDCS was an effective and safe add-on treatment for patients with schizophrenia with prominent negative symptoms. Our findings encourage the use and optimization of this technique in patients with psychotic disorders.
Trial Protocol.
eAppendix 1. Sociodemographic variables analyzed as predictors of response.
eAppendix 2. Detailed statistical analysis.
eTable 1. Statistical analysis of primary and secondary outcomes.
eTable 2. Change of PANSS negative symptoms subscale in percentages.
eTable 3. Summary of all scales used in the trial at each measurement.
eTable 4. Results of moderation analyses.
eTable 5. Change in PANSS negative symptoms subscale score for subgroups showing significant moderation effects.
eTable 6. Group differences in PANSS negative symptoms subscale single item scores.
eFigure 1. Changes in individual items of the PANSS negative symptoms subscale.
eFigure 2. Plot showing individual trajectories for patients’ PANNS negative symptoms subscale scores.
Data Sharing Statement.
References
- 1.Charlson FJ, Ferrari AJ, Santomauro DF, et al. Global epidemiology and burden of schizophrenia: findings from the Global Burden of Disease Study 2016. Schizophr Bull. 2018;44(6):1195-1203. doi: 10.1093/schbul/sby058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ferrari AJ, Saha S, McGrath JJ, et al. Health states for schizophrenia and bipolar disorder within the Global Burden of Disease 2010 Study. Popul Health Metr. 2012;10(1):16. doi: 10.1186/1478-7954-10-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Leucht S, Cipriani A, Spineli L, et al. Comparative efficacy and tolerability of 15 antipsychotic drugs in schizophrenia: a multiple-treatments meta-analysis. Lancet. 2013;382(9896):951-962. doi: 10.1016/S0140-6736(13)60733-3 [DOI] [PubMed] [Google Scholar]
- 4.Lieberman JA, Stroup TS, McEvoy JP, et al. ; Clinical Antipsychotic Trials of Intervention Effectiveness (CATIE) Investigators . Effectiveness of antipsychotic drugs in patients with chronic schizophrenia. N Engl J Med. 2005;353(12):1209-1223. doi: 10.1056/NEJMoa051688 [DOI] [PubMed] [Google Scholar]
- 5.Veerman SRT, Schulte PFJ, de Haan L. Treatment for negative symptoms in schizophrenia: a comprehensive review. Drugs. 2017;77(13):1423-1459. doi: 10.1007/s40265-017-0789-y [DOI] [PubMed] [Google Scholar]
- 6.Andreasen NC, O’Leary DS, Flaum M, et al. Hypofrontality in schizophrenia: distributed dysfunctional circuits in neuroleptic-naive patients. Lancet. 1997;349(9067):1730-1734. doi: 10.1016/S0140-6736(96)08258-X [DOI] [PubMed] [Google Scholar]
- 7.Cohen JD, Perlstein WM, Braver TS, et al. Temporal dynamics of brain activation during a working memory task. Nature. 1997;386(6625):604-608. doi: 10.1038/386604a0 [DOI] [PubMed] [Google Scholar]
- 8.Aleman A, Enriquez-Geppert S, Knegtering H, Dlabac-de Lange JJ. Moderate effects of noninvasive brain stimulation of the frontal cortex for improving negative symptoms in schizophrenia: meta-analysis of controlled trials. Neurosci Biobehav Rev. 2018;89:111-118. doi: 10.1016/j.neubiorev.2018.02.009 [DOI] [PubMed] [Google Scholar]
- 9.Brunoni AR, Sampaio-Junior B, Moffa AH, et al. Noninvasive brain stimulation in psychiatric disorders: a primer. Braz J Psychiatry. 2019;41(1):70-81. doi: 10.1590/1516-4446-2017-0018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brunoni AR, Amadera J, Berbel B, Volz MS, Rizzerio BG, Fregni F. A systematic review on reporting and assessment of adverse effects associated with transcranial direct current stimulation. Int J Neuropsychopharmacol. 2011;14(8):1133-1145. doi: 10.1017/S1461145710001690 [DOI] [PubMed] [Google Scholar]
- 11.Bikson M, Brunoni AR, Charvet LE, et al. Rigor and reproducibility in research with transcranial electrical stimulation: an NIMH-sponsored workshop. Brain Stimul. 2018;11(3):465-480. doi: 10.1016/j.brs.2017.12.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bikson M, Grossman P, Thomas C, et al. Safety of transcranial direct current stimulation: evidence based update 2016. Brain Stimul. 2016;9(5):641-661. doi: 10.1016/j.brs.2016.06.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brunelin J, Mondino M, Gassab L, et al. Examining transcranial direct-current stimulation (tDCS) as a treatment for hallucinations in schizophrenia. Am J Psychiatry. 2012;169(7):719-724. doi: 10.1176/appi.ajp.2012.11071091 [DOI] [PubMed] [Google Scholar]
- 14.Lee EHM, Chan PY, Law EYL, et al. Efficacy of transcranial direct current stimulation (tDCS) as a treatment for persistent hallucinations in patients with schizophrenia: a systematic review and meta-analysis. Schizophr Res. 2018;202:423-425. doi: 10.1016/j.schres.2018.06.069 [DOI] [PubMed] [Google Scholar]
- 15.Osoegawa C, Gomes JS, Grigolon RB, et al. Non-invasive brain stimulation for negative symptoms in schizophrenia: an updated systematic review and meta-analysis. Schizophr Res. 2018;197:34-44. doi: 10.1016/j.schres.2018.01.010 [DOI] [PubMed] [Google Scholar]
- 16.Kennedy NI, Lee WH, Frangou S. Efficacy of non-invasive brain stimulation on the symptom dimensions of schizophrenia: a meta-analysis of randomized controlled trials. Eur Psychiatry. 2018;49:69-77. doi: 10.1016/j.eurpsy.2017.12.025 [DOI] [PubMed] [Google Scholar]
- 17.Palm U, Keeser D, Hasan A, et al. Prefrontal transcranial direct current stimulation for treatment of schizophrenia with predominant negative symptoms: a double-blind, sham-controlled proof-of-concept study. Schizophr Bull. 2016;42(5):1253-1261. doi: 10.1093/schbul/sbw041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kay SR, Fiszbein A, Opler LA. The Positive and Negative Syndrome Scale (PANSS) for schizophrenia. Schizophr Bull. 1987;13(2):261-276. doi: 10.1093/schbul/13.2.261 [DOI] [PubMed] [Google Scholar]
- 19.Valiengo L, Gordon PC, de Carvalho JB, et al. Schizophrenia Treatment With Electric Transcranial Stimulation (STARTS): design, rationale and objectives of a randomized, double-blinded, sham-controlled trial. Trends Psychiatry Psychother. 2019;41(2):104-111. doi: 10.1590/2237-6089-2018-0047 [DOI] [PubMed] [Google Scholar]
- 20.Boutron I, Moher D, Altman DG, Schulz KF, Ravaud P; CONSORT Group . Extending the CONSORT statement to randomized trials of nonpharmacologic treatment: explanation and elaboration. Ann Intern Med. 2008;148(4):295-309. doi: 10.7326/0003-4819-148-4-200802190-00008 [DOI] [PubMed] [Google Scholar]
- 21.World Medical Association World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA. 2013;310(20):2191-2194. doi: 10.1001/jama.2013.281053 [DOI] [PubMed] [Google Scholar]
- 22.Del-Ben CM, Vilela JAA, Crippa JA de S, Hallak JEC, Labate CM, Zuardi AW. Reliability of the Structured Clinical Interview for DSM-IV–Clinical Version translated into Portuguese. Br J Psychiatry. 2001;23(3):156-159. doi: 10.1590/S1516-44462001000300008 [DOI] [Google Scholar]
- 23.Mogg A, Purvis R, Eranti S, et al. Repetitive transcranial magnetic stimulation for negative symptoms of schizophrenia: a randomized controlled pilot study. Schizophr Res. 2007;93(1-3):221-228. doi: 10.1016/j.schres.2007.03.016 [DOI] [PubMed] [Google Scholar]
- 24.Brunoni AR, Valiengo L, Baccaro A, et al. The sertraline vs. electrical current therapy for treating depression clinical study: results from a factorial, randomized, controlled trial. JAMA Psychiatry. 2013;70(4):383-391. doi: 10.1001/2013.jamapsychiatry.32 [DOI] [PubMed] [Google Scholar]
- 25.McLaren ME, Nissim NR, Woods AJ. The effects of medication use in transcranial direct current stimulation: a brief review. Brain Stimul. 2018;11(1):52-58. doi: 10.1016/j.brs.2017.10.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Brunoni AR, Shiozawa P, Truong D, et al. Understanding tDCS effects in schizophrenia: a systematic review of clinical data and an integrated computation modeling analysis. Expert Rev Med Devices. 2014;11(4):383-394. doi: 10.1586/17434440.2014.911082 [DOI] [PubMed] [Google Scholar]
- 27.Lee WH, Kennedy NI, Bikson M, Frangou S. A computational assessment of target engagement in the treatment of auditory hallucinations with transcranial direct current stimulation. Front Psychiatry. 2018;9:48. doi: 10.3389/fpsyt.2018.00048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Brunoni AR, Moffa AH, Sampaio-Junior B, et al. ; ELECT-TDCS Investigators . Trial of electrical direct-current therapy versus escitalopram for depression. N Engl J Med. 2017;376(26):2523-2533. doi: 10.1056/NEJMoa1612999 [DOI] [PubMed] [Google Scholar]
- 29.Sampaio-Junior B, Tortella G, Borrione L, et al. Efficacy and safety of transcranial direct current stimulation as an add-on treatment for bipolar depression: a randomized clinical trial. JAMA Psychiatry. 2018;75(2):158-166. doi: 10.1001/jamapsychiatry.2017.4040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Valiengo LC, Goulart AC, de Oliveira JF, Benseñor IM, Lotufo PA, Brunoni AR. Transcranial direct current stimulation for the treatment of post-stroke depression: results from a randomised, sham-controlled, double-blinded trial. J Neurol Neurosurg Psychiatry. 2017;88(2):170-175. doi: 10.1136/jnnp-2016-314075 [DOI] [PubMed] [Google Scholar]
- 31.Wobrock T, Guse B, Cordes J, et al. Left prefrontal high-frequency repetitive transcranial magnetic stimulation for the treatment of schizophrenia with predominant negative symptoms: a sham-controlled, randomized multicenter trial. Biol Psychiatry. 2015;77(11):979-988. doi: 10.1016/j.biopsych.2014.10.009 [DOI] [PubMed] [Google Scholar]
- 32.Howes OD, McCutcheon R, Agid O, et al. Treatment-resistant schizophrenia: Treatment Response and Resistance in Psychosis (TRRIP) working group consensus guidelines on diagnosis and terminology. Am J Psychiatry. 2017;174(3):216-229. doi: 10.1176/appi.ajp.2016.16050503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Addington D, Addington J, Maticka-Tyndale E. Specificity of the Calgary Depression Scale for Schizophrenics. Schizophr Res. 1994;11(3):239-244. doi: 10.1016/0920-9964(94)90017-5 [DOI] [PubMed] [Google Scholar]
- 34.Hoffman RE, Hawkins KA, Gueorguieva R, et al. Transcranial magnetic stimulation of left temporoparietal cortex and medication-resistant auditory hallucinations. Arch Gen Psychiatry. 2003;60(1):49-56. doi: 10.1001/archpsyc.60.1.49 [DOI] [PubMed] [Google Scholar]
- 35.American Psychiatric Association Diagnostic and Statistical Manual of Mental Disorders. 4th ed, text revision Washington, DC: American Psychiatric Association; 2000. [Google Scholar]
- 36.Aparício LVM, Guarienti F, Razza LB, Carvalho AF, Fregni F, Brunoni AR. A systematic review on the acceptability and tolerability of transcranial direct current stimulation treatment in neuropsychiatry trials. Brain Stimul. 2016;9(5):671-681. doi: 10.1016/j.brs.2016.05.004 [DOI] [PubMed] [Google Scholar]
- 37.Andreasen NC. The Scale for the Assessment of Negative Symptoms (SANS): conceptual and theoretical foundations. Br J Psychiatry Suppl. 1989;(7):49-58. doi: 10.1192/S0007125000291496 [DOI] [PubMed] [Google Scholar]
- 38.Bates D, Mächler M, Bolker B, Walker S. Fitting linear mixed-effects models using lme4. J Stat Softw. 2015;67(1). doi: 10.18637/jss.v067.i01 [DOI] [Google Scholar]
- 39.Kraemer HC, Kupfer DJ. Size of treatment effects and their importance to clinical research and practice. Biol Psychiatry. 2006;59(11):990-996. doi: 10.1016/j.biopsych.2005.09.014 [DOI] [PubMed] [Google Scholar]
- 40.Preti A. How to calculate the number needed to treat (NNT) from Cohen’s d or Hedges’ g. https://rpubs.com/RatherBit/78905. Accessed June 4, 2019.
- 41.Marder SR, Davis JM, Chouinard G. The effects of risperidone on the five dimensions of schizophrenia derived by factor analysis: combined results of the North American trials. J Clin Psychiatry. 1997;58(12):538-546. doi: 10.4088/JCP.v58n1205 [DOI] [PubMed] [Google Scholar]
- 42.Leucht S, Davis JM, Engel RR, Kissling W, Kane JM. Definitions of response and remission in schizophrenia: recommendations for their use and their presentation. Acta Psychiatr Scand Suppl. 2009;(438):7-14. doi: 10.1111/j.1600-0447.2008.01308.x [DOI] [PubMed] [Google Scholar]
- 43.Andreasen NC, Pressler M, Nopoulos P, Miller D, Ho B-C. Antipsychotic dose equivalents and dose-years: a standardized method for comparing exposure to different drugs. Biol Psychiatry. 2010;67(3):255-262. doi: 10.1016/j.biopsych.2009.08.040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Miliauskas CR, Faus DP, Junkes L, Rodrigues RB, Junger W. Association between psychiatric hospitalizations, coverage of psychosocial care centers (CAPS) and primary health care (PHC) in metropolitan regions of Rio de Janeiro (RJ) and São Paulo (SP), Brazil. Cien Saude Colet. 2019;24(5):1935-1944. doi: 10.1590/1413-81232018245.18862017 [DOI] [PubMed] [Google Scholar]
- 45.Candiago RH, Saraiva S da S, Gonçalves V, Belmonte-de-Abreu P. Shortage and underutilization of psychiatric beds in southern Brazil: independent data of Brazilian mental health reform. Soc Psychiatry Psychiatr Epidemiol. 2011;46(5):425-429. doi: 10.1007/s00127-010-0207-1 [DOI] [PubMed] [Google Scholar]
- 46.Correll CU, Kishimoto T, Nielsen J, Kane JM. Quantifying clinical relevance in the treatment of schizophrenia. Clin Ther. 2011;33(12):B16-B39. doi: 10.1016/j.clinthera.2011.11.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Addington D, Addington J, Maticka-Tyndale E. Assessing depression in schizophrenia: the Calgary Depression Scale. Br J Psychiatry Suppl. 1993;(22):39-44. doi: 10.1192/S0007125000292581 [DOI] [PubMed] [Google Scholar]
- 48.Shafer A, Dazzi F. Meta-analysis of the Positive and Negative Syndrome Scale (PANSS) factor structure. J Psychiatr Res. 2019;115:113-120. doi: 10.1016/j.jpsychires.2019.05.008 [DOI] [PubMed] [Google Scholar]
- 49.Nitsche MA, Lampe C, Antal A, et al. Dopaminergic modulation of long-lasting direct current-induced cortical excitability changes in the human motor cortex. Eur J Neurosci. 2006;23(6):1651-1657. doi: 10.1111/j.1460-9568.2006.04676.x [DOI] [PubMed] [Google Scholar]
- 50.Nitsche MA, Kuo MF, Karrasch R, Wächter B, Liebetanz D, Paulus W. Serotonin affects transcranial direct current-induced neuroplasticity in humans. Biol Psychiatry. 2009;66(5):503-508. doi: 10.1016/j.biopsych.2009.03.022 [DOI] [PubMed] [Google Scholar]
- 51.Brunoni AR, Moffa AH, Fregni F, et al. Transcranial direct current stimulation for acute major depressive episodes: meta-analysis of individual patient data. Br J Psychiatry. 2016;208(6):522-531. doi: 10.1192/bjp.bp.115.164715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kantrowitz JT, Sehatpour P, Avissar M, et al. Significant improvement in treatment resistant auditory verbal hallucinations after 5 days of double-blind, randomized, sham controlled, fronto-temporal, transcranial direct current stimulation (tDCS): a replication/extension study. Brain Stimul. 2019;12(4):981-991. doi: 10.1016/j.brs.2019.03.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lançon C, Auquier P, Reine G, Bernard D, Toumi M. Study of the concurrent validity of the Calgary Depression Scale for Schizophrenics (CDSS). J Affect Disord. 2000;58(2):107-115. doi: 10.1016/S0165-0327(99)00075-0 [DOI] [PubMed] [Google Scholar]
- 54.Samara MT, Engel RR, Millier A, Kandenwein J, Toumi M, Leucht S. Equipercentile linking of scales measuring functioning and symptoms: examining the GAF, SOFAS, CGI-S, and PANSS. Eur Neuropsychopharmacol. 2014;24(11):1767-1772. doi: 10.1016/j.euroneuro.2014.08.009 [DOI] [PubMed] [Google Scholar]
- 55.Rabany L, Weiser M, Werbeloff N, Levkovitz Y. Assessment of negative symptoms and depression in schizophrenia: revision of the SANS and how it relates to the PANSS and CDSS. Schizophr Res. 2011;126(1-3):226-230. doi: 10.1016/j.schres.2010.09.023 [DOI] [PubMed] [Google Scholar]
- 56.Daniel DG. Issues in selection of instruments to measure negative symptoms. Schizophr Res. 2013;150(2-3):343-345. doi: 10.1016/j.schres.2013.07.005 [DOI] [PubMed] [Google Scholar]
- 57.Garcia-Portilla MP, Garcia-Alvarez L, Saiz PA, et al. Psychometric evaluation of the negative syndrome of schizophrenia. Eur Arch Psychiatry Clin Neurosci. 2015;265(7):559-566. doi: 10.1007/s00406-015-0595-z [DOI] [PubMed] [Google Scholar]
- 58.Németh G, Laszlovszky I, Czobor P, et al. Cariprazine versus risperidone monotherapy for treatment of predominant negative symptoms in patients with schizophrenia: a randomised, double-blind, controlled trial. Lancet. 2017;389(10074):1103-1113. doi: 10.1016/S0140-6736(17)30060-0 [DOI] [PubMed] [Google Scholar]
- 59.Borrione L, Moffa AH, Martin D, Loo CK, Brunoni AR. Transcranial direct current stimulation in the acute depressive episode: a systematic review of current knowledge. J ECT. 2018;34(3):153-163. doi: 10.1097/yct.0000000000000512 [DOI] [PubMed] [Google Scholar]
- 60.Palm U, Kumpf U, Behler N, et al. Home use, remotely supervised, and remotely controlled transcranial direct current stimulation: a systematic review of the available evidence. Neuromodulation. 2018;21(4):323-333. doi: 10.1111/ner.12686 [DOI] [PubMed] [Google Scholar]
- 61.Sathappan AV, Luber BM, Lisanby SH. The dynamic duo: combining noninvasive brain stimulation with cognitive interventions. Prog Neuropsychopharmacol Biol Psychiatry. 2019;89:347-360. doi: 10.1016/j.pnpbp.2018.10.006 [DOI] [PubMed] [Google Scholar]
- 62.Koutsouleris N, Wobrock T, Guse B, et al. Predicting response to repetitive transcranial magnetic stimulation in patients with schizophrenia using structural magnetic resonance imaging: a multisite machine learning analysis. Schizophr Bull. 2018;44(5):1021-1034. doi: 10.1093/schbul/sbx114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lindenmayer JP, Kulsa MKC, Sultana T, et al. Transcranial direct-current stimulation in ultra-treatment-resistant schizophrenia. Brain Stimul. 2019;12(1):54-61. doi: 10.1016/j.brs.2018.10.002 [DOI] [PubMed] [Google Scholar]
- 64.Burke MJ, Kaptchuk TJ, Pascual-Leone A. Challenges of differential placebo effects in contemporary medicine: the example of brain stimulation. Ann Neurol. 2019;85(1):12-20. doi: 10.1002/ana.25387 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial Protocol.
eAppendix 1. Sociodemographic variables analyzed as predictors of response.
eAppendix 2. Detailed statistical analysis.
eTable 1. Statistical analysis of primary and secondary outcomes.
eTable 2. Change of PANSS negative symptoms subscale in percentages.
eTable 3. Summary of all scales used in the trial at each measurement.
eTable 4. Results of moderation analyses.
eTable 5. Change in PANSS negative symptoms subscale score for subgroups showing significant moderation effects.
eTable 6. Group differences in PANSS negative symptoms subscale single item scores.
eFigure 1. Changes in individual items of the PANSS negative symptoms subscale.
eFigure 2. Plot showing individual trajectories for patients’ PANNS negative symptoms subscale scores.
Data Sharing Statement.