Abstract
The transcription factor signal transducer and activator of transcription 3 (STAT3) plays a central role in cell survival and function. STAT3 has been demonstrated to participate in the maintenance of bone homeostasis in osteoblasts, but its role in osteoclasts in vivo remains poorly defined. Here, we generated a conditional knockout mouse model in which Stat3 was deleted in osteoclasts using a cathepsin K-Cre (Ctsk-Cre) driver. We observed that osteoclast-specific Stat3 deficiency caused increased bone mass in mice, which we attributed to impaired bone catabolism by osteoclasts. Stat3-deficient bone marrow macrophages (BMMs) showed decreased expression of nuclear factor of activated T cells, cytoplasm 1 (NFATc1), and reduced osteoclast differentiation determined by decreases in osteoclast number, tartrate-resistant acid phosphatase activity, and expression of osteoclast marker genes. Enforced expression of NFATc1 in Stat3-deficient BMMs rescued the impaired osteoclast differentiation. Mechanistically, we revealed that STAT3 could drive the transcription of NFATc1 by binding to its promoter. Furthermore, preventing STAT3 activation by using an inhibitor of upstream phosphorylases, AG490, also impaired osteoclast differentiation and formation in a similar way as gene deletion of Stat3. In summary, our data provide the first evidence that STAT3 is significant in osteoclast differentiation and bone homeostasis in vivo, and it may be identified as a potential pharmacological target for the treatment of bone metabolic diseases through regulation of osteoclast activity.
Keywords: bone, osteoporosis, STAT3, osteoclast, NFAT transcription factor, transgenic mice
Introduction
Excess osteoclastic activity abolishes the balance in bone remodeling and causes skeletal metabolic diseases (1). Osteoporosis is a bone metabolic disease with the highest prevalence, which seriously affects the quality of life of patients and causes tremendous economic costs in both developing and developed countries (2). Even in the United States, more than 1.5 million fractures are caused by osteoporosis each year (3). However, according to a clinical guideline published in The New England Journal of Medicine in 2016, Food and Drug Administration (FDA)5-approved drugs for osteoporosis treatment are still limited (3). In addition, patients using bisphosphonates, the first-line antiresorptive therapy, are at risk of adverse effects, including osteonecrosis of the jaw (4). It is therefore important to understand the molecular mechanisms underlying the formation and activity of osteoclasts to improve the treatment of bone diseases at an earlier and more controllable stage.
Osteoclasts are specialized cells derived from the monocyte/macrophage hematopoietic lineage. There are two critical extracellular factors that are essential for the differentiation of osteoclast precursors, macrophage colony-stimulating factor (M-CSF) and receptor activator of NF-κB ligand (RANKL) (1). M-CSF and RANKL promote the expression of transcriptional factors such as nuclear factor of activated T cells, cytoplasm 1 (NFATc1), and c-Fos, to induce osteoclast differentiation. NFATc1 belongs to the NFAT family, which plays a critical role in osteoclast formation and function (5). NFATc1 is functionally linked with another transcription factor c-Fos, which may bind to the promoter region of NFATc1 to induce NFATc1 expression (6, 7). NFATc1 rescues osteoclastogenesis in osteoclast precursors lacking c-Fos (8). In recent years, increasing numbers of researchers have reported the involvement of other signaling molecules in regulating osteoclast precursor differentiation and function, particularly in the RANKL-induced expression and activity of c-Fos–NFATc1 (1, 9, 10), including IL-6–JAK–STAT signaling.
The signal transducer and activator of transcription (STAT) family, which transmits signals from the cell membrane to the nucleus, plays a central role in cell survival and functional activities (11). It is well-known that STATs regulate cell immunity and cancer progression (12). Inflammatory signaling such as IL-6–JAK–STAT has recently been defined as a trigger involved in bone resorption due to its critical role in inducing expression of RANKL (13, 14). In addition, evidence has shown that IL-6 directly promotes osteoclastogenesis (15), although this remains controversial (16). There are also reports of the involvement of the classic inflammatory cytokines such as STATs in maintaining the balance of bone metabolism. Among the seven STATs, STAT3 is reported to be the most relevant to bone homeostasis (17). Specific inactivation of STAT3 in osteoblasts in vivo decreases bone formation (18, 19). As for osteoclasts, several studies have reported that protein inhibitor of activated STAT3 (PIAS3) diminishes osteoclastogenesis (20, 21), and knockdown of STAT3 in vitro using shRNA attenuates RANKL-induced osteoclastogenesis (22). Nevertheless, direct evidence demonstrating the participation of STAT3 in osteoclast formation and bone metabolism in vivo is still required, and the underlying mechanism still needs to be further explored.
In this study, we provided the first evidence that STAT3 participated in osteoclast differentiation and bone metabolism in vivo with a conditional knockout mouse model. Additionally, we showed that STAT3 regulated the transcription of NFATc1 signaling in osteoclasts. This study not only highlighted the significance of STAT3 in osteoclast differentiation and bone catabolism, but also provided a potential molecular target for treatment of osteoclast-induced bone metabolic diseases.
Results
Deletion of Stat3 in osteoclasts led to an increase of bone mass
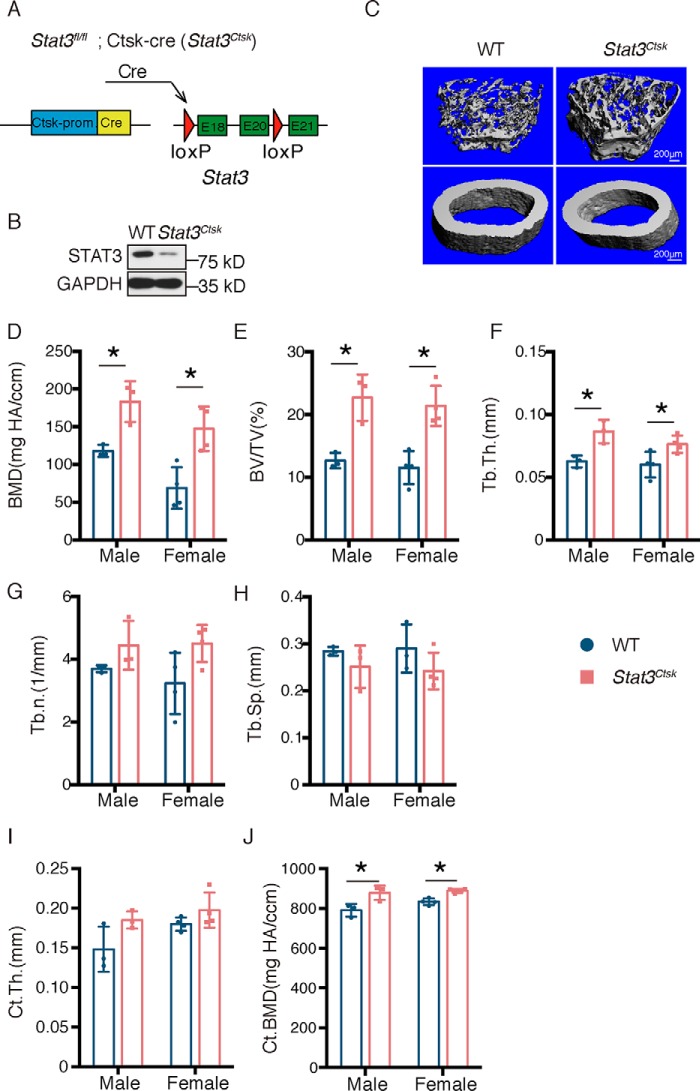
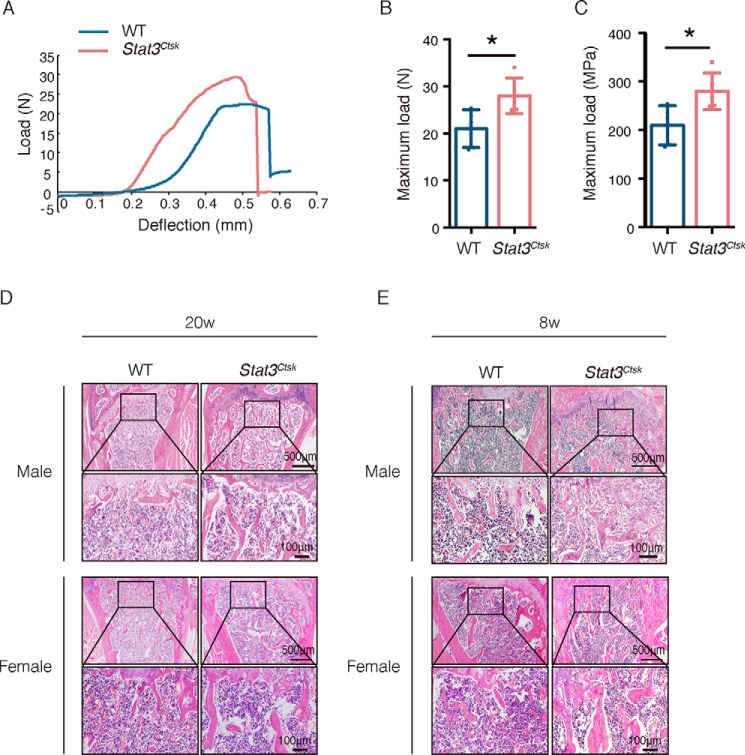
To determine the specific role of STAT3 in osteoclasts, Stat3fl/fl mice (hereafter called WT) were crossed with Ctsk-Cre mice, in which the osteoclast-specific expression of Cre was driven by the promoter of cathepsin K (Ctsk). Stat3 was conditionally knocked out in osteoclasts by generating Stat3fl/fl;Ctsk-Cre mice (hereafter called Stat3Ctsk, Fig. 1A). Western blotting confirmed the effective depletion of STAT3 protein in bone marrow macrophages (BMMs) in Stat3Ctsk mice compared with the WT mice (Fig. 1B). Micro-CT was used to analyze the changes of bone mass and microarchitecture in Stat3-deificient mice (Fig. 1C). Analysis of the quantitative microarchitectural parameters of femora from 20-week-old male and female Stat3Ctsk mice revealed marked increases of trabecular bone mass compared with their Stat3fl/fl littermates, such as bone mineral density (BMD, Fig. 1D), bone volume fraction (BV/TV, Fig. 1E), and trabecular thickness (Tb.Th., Fig. 1F), trabecular number (Tb.N., Fig. 1G), and trabecular separation (Tb.Sp., Fig. 1H). Although there was no difference in cortical bone thickness (Ct.Th., Fig. 1I), bone mineral density of cortical bone was increased in the Stat3Ctsk mice compared with the control group (Ct.BMD, Fig. 1J). We next analyzed the structural integrity of femora from 20-week-old Stat3Ctsk and Stat3fl/fl littermate mice via three-point bending tests, which showed increased bone stiffness of Stat3Ctsk femora compared with controls (Fig. 2, A–C). H&E staining confirmed increased trabecular bone mass and Tb.Th. of the femur from male and female 20-week-old Stat3Ctsk mice compared with WT mice (Fig. 2D). Furthermore, 8-week-old male and female mice were also included in this study to determine the effect of STAT3 on the early stage of bone formation during development. H&E staining showed the same trend of increasing bone mass in 8-week-old Stat3Ctsk mice compared with WT mice (Fig. 2E). These results indicated that deletion of Stat3 in osteoclasts resulted in an increase of bone mass.
Figure 1.
Deletion of Stat3 in osteoclasts led to an increase of bone mass. A, illustration of Stat3 deletion in Ctsk-expressing osteoclasts. B, Western blotting of STAT3 in BMMs from WT and Stat3Ctsk mice cultured with M-CSF and RANKL for 7 days. GAPDH, glyceraldehyde-3-phosphate dehydrogenase. C, three-dimensional micro-CT reconstruction images of femora from 20-week-old WT and Stat3Ctsk mice. The top panel shows trabecular bone, and the bottom panel represents cortical bone. A total of 1-mm-wide trabecular bone close to the distal growth plate and a 1-mm-wide section of cortical bone from the middle of the femur were three-dimensionally reconstructed. Representative examples are shown. D–J, quantitative microarchitectural parameters of micro-CT: BMD, BV/TV, Tb.Th., Tb.N., Tb.Sp., Ct.Th., and Ct.BMD. Three pairs of male Stat3Ctsk and their Stat3fl/fl littermates and four pairs of female Stat3Ctsk and their Stat3fl/fl littermates were included in the measurement. Error bars represent mean ± S.D.; *, p < 0.05.
Figure 2.
Deletion of Stat3 in osteoclasts led to an increase of bone stiffness. A, representative load-deflection diagram from a three-point bending test performed on femora of 20-week-old Stat3Ctsk mice and their Stat3fl/fl littermates. B and C, maximum load measured during the test. Error bars are represented as mean ± S.D.; *, p < 0.05. Five pairs of Stat3Ctsk mice and their Stat3fl/fl littermates were tested. D and E, H&E staining of the femora of male and female 20-week-old (D) and 8-week-old (E) WT and Stat3Ctsk mice. The bottom panel shows high-power images of the primary spongiosa. Five pairs of male and female Stat3Ctsk mice and their Stat3fl/fl littermates were included, and representative examples are shown.
Ablation of Stat3 in osteoclasts resulted in decreased bone resorption without influencing bone formation
Increased bone mass can be the result of increased bone formation, decreased bone resorption, or a combination of both effects. To determine whether the increased bone mass in Stat3Ctsk mice was the result of the effects on bone resorption or bone formation, or both, histological analyses of femora were performed. First, bone formation activity was compared between femora from 8-week-old male and female WT and Stat3Ctsk mice by calcein and alizarin red double-labeling. The results indicated that neither trabecular nor cortical bone formation activities were influenced in Stat3Ctsk mice (Fig. 3A), as represented by mineral apposition rate (MAR, Fig. 3, B and C). These results excluded the possibility that deletion of Stat3 in osteoclasts induced an increase of bone mass through indirect promotion of osteogenesis. We then focused on the changes in bone resorption in Stat3Ctsk mice. As shown by tartrate-resistant acid phosphatase (TRAP) staining, there were far fewer TRAP-positive multinuclear osteoclasts detected surrounding the trabecular bone of male and female Stat3Ctsk mice compared with WT mice (Fig. 3, D–F). We confirmed the decrease in the number of osteoclasts in samples from 8- and 20-week-old Stat3Ctsk mice, which indicated that the decrease in bone resorption might be the main contributor to the increased bone mass in Stat3Ctsk mice. These data implied that the increased bone mass caused by ablation of Stat3 in osteoclasts resulted from decreased bone resorption rather than increased bone formation.
Figure 3.
Ablation of Stat3 in osteoclasts resulted in decreased bone resorption without influencing bone formation. A, osteogenic activity in the femora of 8-week-old WT and Stat3Ctsk mice represented by calcein and alizarin red double-staining. Seven-week-old mice received an intraperitoneal injection of 20 mg/kg calcein on day 0 and 40 mg/kg alizarin red on day 4 and then were sacrificed on day 7. The top panel shows a representative image of cortical bone, and the bottom panel shows a representative image of trabecular bone. B and C, MAR evaluated by histomorphometric analysis. Five pairs of male and female Stat3Ctsk mice and their Stat3fl/fl littermates were included. Error bars represent mean ± S.D.; *, p < 0.05. D, TRAP staining of femora from 8- and 20-week-old WT and Stat3Ctsk mice. Black triangles indicate TRAP+-multinucleated osteoclasts. E and F, number of TRAP+ multinucleated osteoclasts was counted. Five pairs of male and female Stat3Ctsk mice and their Stat3fl/fl littermates were included. Error bars represent mean ± S.D.; *, p < 0.05.
Stat3-driven osteoclast differentiation and formation in a cell-autonomous manner
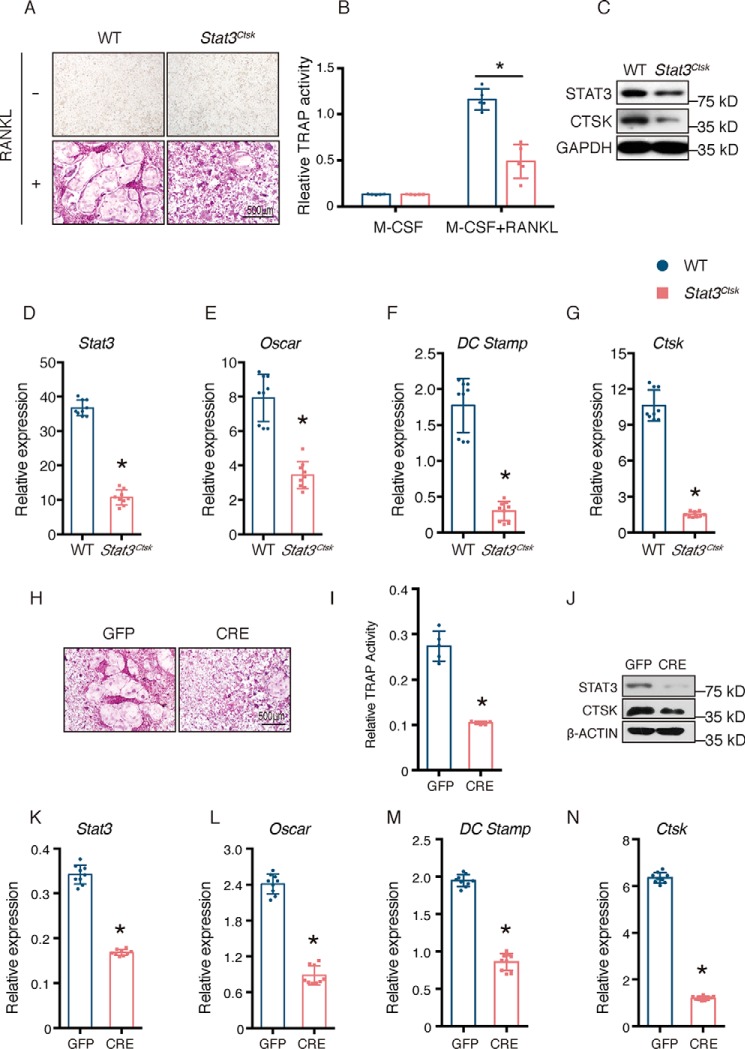
Having determined that the bone mass increase in Stat3Ctsk mice was mainly the result of a direct decrease in bone resorption, we wondered whether Stat3 was the driver of osteoclast differentiation in a cell-autonomous manner. To test this, we isolated BMMs from 4-week-old WT and Stat3Ctsk mice and cultured them with 20 ng/ml M-CSF and 20 ng/ml RANKL for 7 days. The cells seeded into 96-well plates were examined by TRAP staining. As shown in Fig. 4, A and B, there were fewer TRAP+ giant multinuclear osteoclasts formed, accompanied by relatively lower TRAP activity of culture supernatant in BMMs from Stat3Ctsk mice compared with WT mice 7 days after seeding. Western blotting confirmed the decreased level of STAT3 protein in Stat3Ctsk BMMs (Fig. 4C). Furthermore, the expression of osteoclast-specific marker genes during osteoclast differentiation, such as osteoclast-associated receptor (Oscar), dendritic cell-specific transmembrane protein (Dc Stamp), and Ctsk were determined by quantitative PCR (qPCR), and the protein level of CTSK was determined by Western blotting. The down-regulation of these marker genes in BMMs from Stat3Ctsk mice indicated that osteoclast differentiation was impaired due to deletion of Stat3 in a cell-autonomous manner (Fig. 4, D–G). To exclude the possibility that impaired osteoclast differentiation was due to differences in the cell populations, we next infected BMMs from 4-week-old Stat3fl/fl mice with adenovirus expressing GFP (Ad-GFP) and CRE recombinase (Ad-CRE) to knock out Stat3 in vitro. The number of TRAP+ osteoclasts and the level of TRAP activity in the culture supernatant of infected BMMs were decreased in Ad-CRE–expressed cells compared with the Ad-GFP–infected group (Fig. 4, H and I). Western blotting indicated a decrease in STAT3 at the protein level in BMMs infected by Ad-CRE (Fig. 4J). Expression of osteoclast-specific genes was also impeded in the Ad-CRE group when evaluated by qPCR and Western blotting (Fig. 4, K–N). All these data supported the notion that the increase of bone mass in Stat3Ctsk mice resulted from the reduced catabolism secondary to impeded osteoclast differentiation and formation as well as a cell-autonomous action through which STAT3 drove osteoclast differentiation.
Figure 4.
Stat3 deficiency impaired osteoclast differentiation. A, BMMs from 4-week-old WT and Stat3Ctsk mice were cultured with M-CSF and RANKL until mature multinucleated osteoclasts formed (5–7 days). TRAP staining was performed to evaluate osteoclasts. B, relative TRAP activity in the culture supernatants of WT and Stat3Ctsk BMMs on day 7 after addition of M-CSF. Five wells in each group were tested in this experiment, and more than three independent experiments were performed. Error bars represent mean ± S.D.; *, p < 0.05. C, levels of STAT3 and CTSK proteins in WT and Stat3Ctsk BMMs. Cells were cultured with M-CSF and RANKL until mature multinuclear osteoclasts formed (5–7 days), and then expression levels were evaluated by Western blotting. D–G, expression of Stat3 and the osteoclast differentiation markers Oscar, Dc Stamp, and Ctsk by WT and Stat3Ctsk BMMs cultured with M-CSF and RANKL until formation of mature multinuclear osteoclasts (5–7 days). Three independent cell isolations were collected, and tests were performed three times. Experiments were repeated more than three times independently. Error bars represent mean ± S.D.; *, p < 0.05. H, BMMs from 4-week-old Stat3fl/fl mice were infected with adenovirus expressing GFP and CRE recombinase and then cultured with M-CSF and RANKL until mature multinuclear osteoclasts formed (5–7 days). TRAP staining was performed to evaluate osteoclast numbers. I, relative TRAP activity in the culture supernatant of BMMs grown in 96-well plates and evaluated on day 7 after addition of M-CSF. Five wells in each group were tested. Error bars represented mean ± S.D.; *, p < 0.05. J, levels of STAT3 and CTSK protein in BMMs infected with Ad-GFP or Ad-CRE, then cultured with M-CSF and RANKL until formation of mature multinuclear osteoclasts was observed (5–7 days), and tested by Western blotting. K–N, Stat3 and osteoclast-specific gene expression of BMMs infected with Ad-GFP or Ad-CRE and then cultured with M-CSF and RANKL until mature multinuclear osteoclasts formed (5–7 days). Three independent cell isolations were collected and were tested three times. Error bars represent mean ± S.D.; *, p < 0.05.
STAT3 promoted osteoclast differentiation through regulating NFATc1 expression
NFATc1 plays a critical role in osteoclast formation and function (5), and several studies have reported that PIAS3 impairs osteoclastogenesis accompanied by down-regulation of NFATc1 (20, 21). To determine whether NFATc1 was the direct downstream target of Stat3 in osteoclasts, we examined the expression of NFATc1 in BMMs isolated from WT and Stat3Ctsk mice by qPCR and Western blotting. The expression of NFATc1 was down-regulated at both the mRNA and protein level in STAT3-deficient BMMs (Fig. 5, A and B). The same results were obtained by deleting Stat3 in vitro by using adenovirus (Fig. 5, C and D). In addition, immunofluorescence staining displayed a decreased expression of NFATc1 in Stat3Ctsk BMMs compared with WT BMMs (Fig. 5, E and F). As we observed that the mRNA level of NFATc1 was affected in Stat3Ctsk BMMs, we hypothesized that STAT3 might regulate the transcription of NFATc1. By using sequence analysis, we identified two canonical STAT3-binding motifs (TTXXXGGAA) in the promoter of NFATc1. We then constructed a luciferase reporter driven by the NFATc1 promoter in the pGL3 vector to analyze the effect of STAT3 on the transcriptional activity of NFATc1. Indeed, we found that the activity of the luciferase reporter driven by the NFATc1 promoter was enhanced by STAT3 expression, which suggested that STAT3 promoted the transcriptional activity of NFATc1 (Fig. 5G). Furthermore, we directly determined whether STAT3 could bind to the NFATc1 promoter by using ChIP assay. As shown in Fig. 5H, STAT3 was enriched in the promoter of NFATc1 when the antibody against STAT3 was used for immunoprecipitation compared with immunoprecipitation with control IgG, indicating that STAT3 is the transcription factor for NFATc1 gene expression.
Figure 5.
STAT3 promoted osteoclast differentiation through regulation of NFATc1. A, NFATc1 expression in WT and Stat3Ctsk BMMs cultured with M-CSF and RANKL until mature multinuclear osteoclasts formed (5–7 days). Three independent cell isolations were collected and were tested three times. Experiments were repeated more than three times independently. Error bars represent mean ± S.D.; *, p < 0.05. B, level of NFATc1 protein in WT and Stat3Ctsk BMMs cultured with M-CSF and RANKL until mature multinuclear osteoclasts formed (5–7 days) and analyzed by Western blotting. Proteins were tested in the same samples used in Fig. 4C. C, NFATc1 expression of BMMs infected with Ad-GFP or Ad-CRE and then cultured with M-CSF and RANKL until mature multinuclear osteoclasts formed (5–7 days). Three independent cell isolations were collected and were tested three times. Error bars represent mean ± S.D.; *, p < 0.05. D, level of NFATc1 protein in BMMs infected with Ad-GFP or Ad-CRE followed by culture with M-CSF and RANKL until mature multinuclear osteoclasts formed (5–7 days) and then analyzed by Western blotting. Proteins were tested in the same samples used in Fig. 4J. E, immunofluorescence staining of NFATc1 (green) and CTSK (red) in WT and Stat3Ctsk BMMs cultured with M-CSF and RANKL on day 5. Three pairs of Stat3Ctsk mice and their Stat3fl/fl littermates were included, and representative examples are shown. F, ratio of NFATc1+ cells in WT and Stat3Ctsk BMMs cultured with M-CSF and RANKL on day 5. Three pairs of Stat3Ctsk mice and their Stat3fl/fl littermates were included. Error bars represent mean ± S.D.; *, p < 0.05. G, effect of STAT3 on activation of the NFATc1 promoter and evaluated using a luciferase expression reporter system performed in HEK293T cells. Four independent cell isolations were collected and tested. Error bars represent mean ± S.D.; *, p < 0.05. H, chromatin immunoprecipitation (ChIP)-qPCR analysis of STAT3 in NFATc1 promoter in RAW264.7 cells. Immunoprecipitation was performed with anti-STAT3, and IgG was used as a negative control. Precipitated DNA was detected by qPCR with specific primers. I, 293T cells were transfected with NFATc1-Luc, together with or without STAT3 or c-Fos. Three independent cell isolations were collected and tested. Error bars represent mean ± S.D.; *, p < 0.05. J, co-immunoprecipitation analysis of STAT3 with c-Fos was performed. A FLAG–c-Fos expression plasmid was co-transfected with Myc-STAT3 into 293T cells. Whole cell lysates (WCL) were used for immunoprecipitation (IP) and then immunoblotting (IB) with the indicated antibodies. Immunoprecipitation products were detected by Western blotting with the indicated antibodies.
It has been reported that c-Fos is the essential transcription factor for NFATc1 expression (6, 7). Thus, we were wondering about the relationship between STAT3 and c-Fos in regulating NFATc1 expression. Sequence analysis revealed that the canonical STAT3-binding motifs were close to c-Fos–binding motifs in the promotor of NFATc1. Therefore, we speculated that STAT3 might functionally cooperate with c-Fos to regulate NFATc1 transcription. To test this hypothesis, we co-transfected STAT3 and c-Fos in 293T cells and examined their effect on NFATc1 reporter activity. We found that c-Fos increased NFATc1 promoter activity, and STAT3 could further promote this effect (Fig. 5I). Moreover, we found that STAT3 and c-Fos formed a physical complex. The co-immunoprecipitation (co-IP) assay identified a protein interaction between STAT3 and c-Fos (Fig. 5J). These data suggested a potential cooperative action between STAT3 and c-Fos in regulating the transcription of NFATc1.
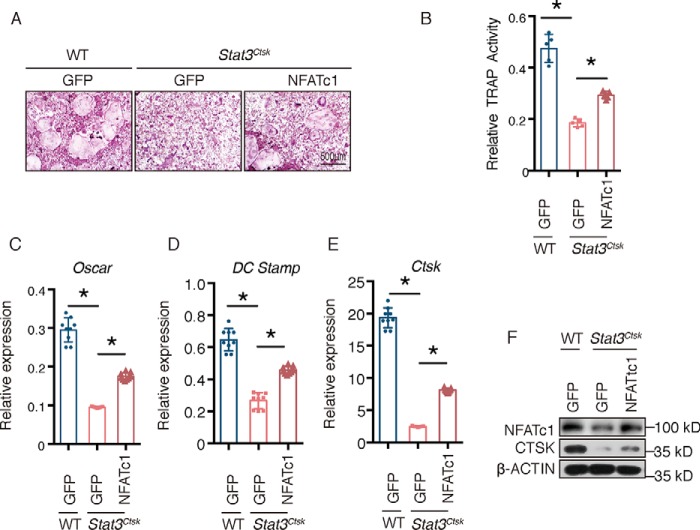
To further verify whether decreased NFATc1 transcription contributed to the impaired osteoclast differentiation caused by STAT3 deficiency, we ectopically expressed NFATc1 in Stat3-deficient BMMs using lentivirus (Lenti-NFATc1 and Lenti-GFP). As shown in Fig. 6, A and B, the impaired osteoclast differentiation and formation were restored in the Lenti-NFATc1 group, as determined by an increase of TRAP+ osteoclasts and TRAP activity in the culture supernatant (Fig. 6, A and B). These results were consistent with increased expression of osteoclast marker genes in Lenti-NFATc1–infected Stat3Ctsk BMMs compared with the Stat3Ctsk BMMs infected with Lenti-GFP (Fig. 6, C–F). Western blotting confirmed the ectopic expression of NFATc1 in Stat3Ctsk BMMs (Fig. 6F). These data demonstrated that STAT3–NFATc1 signaling was critical for osteoclast differentiation and formation.
Figure 6.
NFATc1 rescued the impaired osteoclast differentiation of Stat3Ctsk BMMs. A, BMMs from 4-week-old WT and Stat3Ctsk mice were infected with lentiviruses expressing GFP or NFATc1 and then cultured with M-CSF and RANKL until mature multinuclear osteoclasts formed (5–7 days). TRAP staining was performed to evaluate osteoclast numbers. B, relative TRAP activity of culture supernatants from WT and Stat3Ctsk BMMs infected with lentiviruses expressing GFP or NFATc1 and cultured with M-CSF and RANKL until mature multinuclear osteoclasts formed (5–7 days). Five wells in each group were tested. Error bars represent mean ± S.D.; *, p < 0.05. C–E, Stat3 and osteoclast-specific gene expression of WT and Stat3Ctsk BMMs infected with Lenti-GFP or Lenti-NFATc1 and cultured with M-CSF and RANKL until mature multinuclear osteoclasts formed (5–7 days). Three independent cell isolations were collected and were tested three times. Error bars represent mean ± S.D.; *, p < 0.05. F, Western blotting assay of WT and Stat3Ctsk BMMs infected with Lenti-GFP or Lenti-NFATc1 and cultured with M-CSF and RANKL until mature multinuclear osteoclasts formed (5–7 days).
STAT3 might act as a pharmacological target in osteoclasts
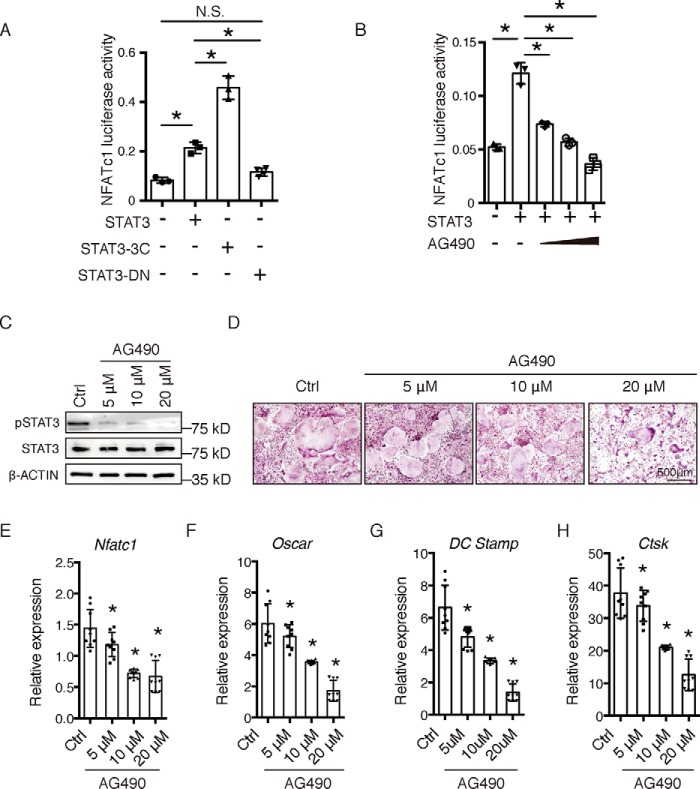
STAT3 has been considered as a molecular target in a series of diseases, including cancer and immune disorders (12); therefore, we wondered whether STAT3 could be a suitable pharmacological target to regulate the activity of osteoclasts. Interestingly, we found that different mutant forms of STAT3 may affect its activity on the NFATc1 promoter. As shown in Fig. 7A, constitutively-active STAT3 (STAT3-3C, a gift from Dr. Feng (23)) promoted NFATc1 promoter activity more dramatically than STAT3, but a dominant-negative mutation of STAT3 (STAT3-DN, a gift from Dr. Feng (23)) had no effect on the activity of the NFATc1 promoter. Furthermore, we found that AG490, the classical JAK2–STAT3 inhibitor that impairs the phosphorylation and transcriptional activity of STAT3, could impair the activity of STAT3 on NFATc1 transcription (Fig. 7B). Subsequently, we determined the effects of AG490 on RANKL-induced osteoclast differentiation of BMMs in vitro. BMMs obtained from 4-week-old WT mice were treated with M-CSF and RANKL in the absence or presence of different concentrations of AG490 according to a previously-described method (24). The phosphorylation level of STAT3 decreased in the groups treated with 5, 10, and 20 μm AG490 compared with the control group (Fig. 7C). The osteoclast number (Fig. 6D) and the expression of osteoclast markers decreased in all three AG490-treated groups compared with the control group (Fig. 7, E–H). These results indicated that STAT3 may be a potential pharmacological target for the treatment of bone metabolic diseases through the regulation of osteoclast activity and bone resorption.
Figure 7.
STAT3 might act as a pharmacological target in osteoclasts. A, 293T cells were transfected with STAT3 WT (STAT3), or variants (STAT-3C or STAT3-DN), together with NFATc1-Luc. Three independent cell isolations were collected and tested. Error bars represent mean ± S.D.; *, p < 0.05; N.S., not significant. B, 293T cells were transfected with STAT3, together with NFATc1-Luc, and then treated with increased amounts of STAT3 inhibitor AG490. Three independent cell isolations were collected and tested. Error bars represent mean ± S.D.; *, p < 0.05. C, Western blotting assay of BMMs from 4-week-old WT mice cultured with M-CSF and RANKL in the absence or presence of different concentrations of AG490 for 1 h. D, TRAP staining of BMMs cultured with M-CSF and RANKL in the absence or presence of different concentrations of AG490 until mature multinuclear osteoclasts formed (5–7 days). E–H, NFATc1 and osteoclast-specific gene expression of BMMs cultured with M-CSF and RANKL in the absence or presence of different concentrations of AG490 until mature multinuclear osteoclasts formed (5–7 days). Three independent cell isolations were collected and were tested three times. Experiments were repeated more than three times independently. Error bars represent mean ± S.D.; *, p < 0.05.
Discussion
Bone remodeling is a balance between anabolism and catabolism. Osteoclasts, one of the most important cell types in these processes, interact with osteoblasts through complex cross-talk (25). Even a small dysfunction in osteoclasts may disturb the balance of bone remodeling and cause skeletal diseases, such as osteoporosis, periodontal disease, and rheumatoid arthritis (1, 9, 10). Considering the enormous population of patients suffering from osteoporosis and the huge economic loss to society (2), the development of effective and safe therapies is both necessary and urgent. However, the FDA-approved drugs for osteoporosis treatment are limited, and their side effects cannot be ignored (3). Clarifying the precise mechanisms involved in osteoclast differentiation and function is critical for developing specific treatment targets of bone metabolic diseases. In this study, we revealed the important role of STAT3 in osteoclast differentiation and the underlying mechanism.
Specific inactivation of STAT3 in osteoblasts in vivo decreases bone formation (18, 19). Nevertheless, whether STAT3 participates in osteoclast formation in vivo has not been unveiled. In this study, in order to specifically deplete Stat3 in osteoclasts, Stat3fl/fl mice were crossed with Ctsk-Cre mice. Stat3 depletion in osteoclasts caused a marked increase of trabecular bone mass in both 20-week-old mice and in younger adult mice 8 weeks old. Although there was no difference in Ct.Th., Ct.BMD was increased in the Stat3Ctsk mice compared with the control group. Histological analysis in femora from 8- and 20-week-old mice indicated similar results. These effects were not related to sex, because both male and female mice showed the same changes. These results were similar to the osteopetrotic phenotype induced by overexpression of PIAS3 in osteoclasts, which was initially identified as a molecule that inhibited DNA binding of STAT3 and its transcriptional activity (21). These data indicated that STAT3 in osteoclasts played an important role in maintaining bone volume and the balance of bone metabolism.
Bone volume is maintained by balanced activity between osteoblasts and osteoclasts. In this mouse model, we found that deletion of Stat3 in osteoclasts with Ctsk-Cre did not influence the activity of osteoblasts, as indicated by comparable bone formation rates and osteoblast marker gene expression in Stat3Ctsk and WT mice. These results excluded the possibility that deletion of Stat3 with Ctsk-Cre induced increased bone volume by promoting bone formation of osteoblasts directly or indirectly, although it has been reported that Stat3 plays an important role in osteoblasts and that Ctsk-Cre may mark a specific osteoblast precursor (19, 26, 27). In contrast, we found that deletion of Stat3 in osteoclasts impeded bone catabolism, indicated by decreased osteoclast number. These data imply that ablation of Stat3 in osteoclasts resulted in decreased bone resorption rather than influencing bone formation, which is in accordance with the decreased bone resorption observed in osteoclast-specific overexpression of PIAS (21).
Differentiation of osteoclast precursors is a key regulator of osteoclast formation and bone resorption (1). Here, we analyzed the influences of deletion of Stat3 on RANKL-induced osteoclast differentiation using our previously-described method (24). BMMs from Stat3Ctsk mice showed impaired osteoclast differentiation in TRAP-positive osteoclasts, reduced TRAP activity, and osteoclast-specific gene expression. To exclude the possibility that the impaired osteoclast differentiation was due to the difference in cell populations, we knocked down Stat3 in Stat3fl/fl BMMs in vitro by Ad-CRE. The results suggested that knockdown of Stat3 in BMMs in vitro also inhibited osteoclast differentiation, as indicated by decreased osteoclast number, TRAP activity, and osteoclast-specific gene expression. These data were supported by a previous study showing that knockdown of Stat3 in vitro using shRNA attenuates RANKL-induced osteoclastogenesis (22). These data combined with the decreased number of osteoclasts in vivo indicated that STAT3 could drive osteoclast differentiation and formation in a cell-autonomous manner.
The transcription factor NFATc1 is known to be a master transcriptional regulator of osteoclast differentiation and formation (5, 28), which activates many of the signaling pathways involved in osteoclastogenesis (29). Several studies have reported that PIAS3 impairs osteoclastogenesis accompanied by down-regulation of NFATc1 (20, 21), which indicates that NFATc1 might participate in STAT3-driven osteoclast differentiation. In this study, we found that the drop in NFATc1 expression was also observed in Stat3-deficient BMMs at both mRNA and protein levels. Thus, we hypothesized that STAT3 might affect the transcriptional activity of NFATc1. We constructed a luciferase reporter driven by the NFATc1 promoter to analyze whether STAT3 could directly bind to the promotor of NFATc1 to activate its transcriptional activity. Our results indicated that STAT3 promoted activity of the NFATc1 promoter. ChIP assay confirmed that STAT3 could directly bind to the NFATc1 promoter. Another critical transcription factor, c-Fos, is essential for NFATc1 transcription by directly binding to and activating its promotor (6, 7). There are binding motifs of c-Fos in the promotor of NFATc1 (30), and we also identified two canonical STAT3-binding motifs (TTXXXGGAA). Hence, we wondered whether STAT3 could participate in c-Fos–NFATc1 transcriptional regulation, which has not been reported. Our results indicated that STAT3 could interact with c-Fos directly at the protein level. Furthermore, STAT3 promoted the c-Fos–driven NFATc1 promotor activity. These data indicated that STAT3 cooperated with c-Fos to drive the transcription of NFATc1 via its promoter. Furthermore, overexpression of NFATc1 in Stat3-deficient BMMs apparently rescued osteoclast differentiation, as indicated by increased osteoclast number, TRAP activity, and expression of osteoclastic marker genes. In short, STAT3 could drive osteoclast differentiation by regulating the transcription of the critical transcriptional factor NFATc1 through its promoter.
As we have shown, STAT3-3C induced NFATc1 promoter activity more dramatically than STAT3, although STAT3-DN could not promote NFATc1 promoter activity. AG490, the inhibitor of JAK2–STAT3 signaling inhibited the activity of STAT3 on the NFATc1 promoter. These results indicated that STAT3 may act as a potential molecular therapeutic target for osteoclast disorders, as it has already been used in the treatment of cancer and immune diseases (12). In this study, we found that AG490 inhibited osteoclast differentiation of BMMs in vitro as suggested by decreased osteoclast number, reduced TRAP activity, and osteoclast-specific gene expression, which indicated that STAT3 was a potential target for regulation of osteoclast activity. This was consistent with the reports of Li et al. (31). Moreover, STAT3 may play an important role in osteoblasts, as suggested by deletion of Stat3 in osteoblast-induced osteoporosis in mice. Hence, further specific pharmacological research into the influence of STAT3 signaling on bone metabolism should be carried out to refine the treatment strategy of STAT3 in bone metabolic diseases.
In summary, to our knowledge, we demonstrated for the first time that specific inhibition of STAT3 in osteoclasts increased bone volume and improved mechanical properties while decreasing bone catabolism in vivo. Mechanistically, we found that STAT3 could drive the transcription of NFATc1 and osteoclast differentiation. These findings not only revealed the mechanisms via which STAT3 controlled osteoclast differentiation and bone resorption, but also provided an alternative pharmacological molecular target for the treatment of bone metabolic diseases.
Experimental procedures
Mice
Stat3fl/fl mice (stock no. D000527) were purchased from GemPharmatech Co., Ltd. (Nanjing, Jiangsu, China). Stat3fl/fl mice were crossed with cathepsin K-Cre mice (Ctsk-cre; provided by S. Kato, University of Tokyo, Tokyo, Japan (32)) to generate Stat3fl/fl;Ctsk-Cre mice (hereafter called Stat3Ctsk). Mice were bred and maintained under specific pathogen-free conditions in the institutional animal facility of the Shanghai Institute of Biochemistry and Cell Biology, Chinese Academy of Sciences. All animal experiments were performed according to a protocol approved by the Animal Care and Use Committee of Shanghai Institute of Biochemistry and Cell Biology, Chinese Academy of Sciences.
Micro-CT analysis
Femora from 20-week-old male and female mice were dissected and stored in ethanol and then scanned with a micro-CT scanner (vivaCT 80; Scanco Medical AG, Bassersdorf, Switzerland). A total of 1 mm width of trabecular bone close to the distal growth plate of the femora were three-dimensionally reconstructed and analyzed for microarchitectural parameters of BMD, BV/TV, Tb.Th., Tb.N., and Tb.Sp. In addition, a 1-mm-wide section of cortical bone from the middle of the femur was analyzed for Ct.BMD.
Three-point bending test
Femora from 8- and 20-week-old WT and Stat3Ctsk mice were collected and stored in 75% ethanol. Three-point bending tests were performed using an Instron 3345 universal testing machine (Instron, Canton, MA) at the femur midshaft with a displacement rate of 0.03 mm/s until the bone fractured (33). Maximum load was determined using load-deflection diagrams.
Histological analysis
Femora from 8- and 20-week-old WT and Stat3Ctsk mice were fixed in 4% paraformaldehyde for 48 h, followed by decalcification in 15% EDTA for 1 month. Specimens were then embedded in paraffin and cut into 5-μm-thick sections. H&E staining and TRAP staining (TRAP kit 387A, Sigma) were performed as described previously (24, 34). Images were captured with a microscope (BX51, Olympus, Tokyo, Japan).
Calcein–alizarin red double labeling
Seven-week-old mice received intraperitoneal injection of 20 mg/kg calcein (1 mg/ml in 2% NaHCO3 solution) on day 0, and 40 mg/kg alizarin red S (AL, 2 mg/ml in H2O) on day 4. Mice were sacrificed on day 7, and isolated tibiae were dehydrated and embedded in polymethylmethacrylate. Samples were cut into 5-μm sections with a hard tissue cutter (RM2265, Leica, Wetzlar, Germany), and fluorescence-labeled images were captured using a microscope (BX51, Olympus). The bone formation activity represented by MAR was measured according to a previously-described protocol (35).
Cell culture
BMMs were washed out of the femora and tibiae of 4-week-old WT and Stat3Ctsk mice as described previously (24, 34). After centrifugation (800 × g, 3 min), cells were resuspended as a single-cell suspension and cultured in 10-cm dishes with α-MEM (Corning) containing 10% fetal bovine serum and 1% penicillin/streptomycin (hereafter called α-MEM) in an incubator (37 °C, 5% CO2) for 24 h. Nonadherent cells were collected, centrifuged (800 × g, 3 min), and resuspended in α-MEM supplemented with 10 ng/ml M-CSF (AF-315-02, Sigma). Cells were then seeded into 96-well plates at a density of 4 × 106 cells/ml and into 24-well plates at a density of 2 × 106 cells/ml. Three days after seeding, BMMs were cultured in osteoclast differentiation medium consisting of α-MEM supplemented with 20 ng/ml M-CSF and 20 ng/ml RANKL (462-TEC-010, R&D, Minneapolis, MN). The culture medium was replaced after 2 days and every day thereafter until mature multinuclear osteoclasts formed (5–7 days). Cells in 96-well plates were stained for TRAP activity (TRAP kit, 387A, Sigma), and the culture supernatants were prepared for TRAP quantification. Cells in 24-well plates were prepared for RNA and protein assay.
TRAP activity quantification
The culture supernatants in 96-well plates were collected and incubated with a 0.33 m tartrate solution containing phosphatase substrate (387A, Sigma) at 37 °C. The reaction was stopped after 2 h with 3 n NaOH, and TRAP activity was quantified by colorimetric analysis at a maximum wavelength of 405 nm (24).
RT-PCR analysis
Total RNA was extracted from osteoclasts using TRIzol (T9424, Sigma) and was reverse-transcribed into cDNA using the PrimeScript RT master kit (RR036A, TakaRa Bio Inc., Shiga, Japan). Real-time reverse transcription PCR (RT-PCR) was performed using the Bio-Rad CFX96 system. The primer sets used were as follows: hypoxanthine-guanine phosphoribosyltransferase, 5′-GTAATGATCAGTCAACGGGGGAC-3′ and 5′-CCAGCAAGCTTGCAACCTTAACCA-3′; Oscar, 5′-CCTAGCCTCATACCCCCAG-3′ and 5′-CGTTGATCCCAGGAGTCACAA-3′; Dc Stamp, 5′-GGGGACTTATGTGTTTCCACG-3′ and 5′-ACAAAGCAACAGACTCCCAAAT-3′; Ctsk, 5′-GAAGAAGACTCACCAGAAGCAG-3′ and 5′-TCCAGGTTATGGGCAGAGATT-3′; NFATc1, 5′-GACCCGGAGTTCGACTTCG-3′ and 5′-TGACACTAGGGGACACATAACTG-3′; Stat3, 5′-CATCCTGAAGCTGACCCAGG-3′ and 5′-TATTGCTGCAGGTCGTTGGT-3′.
Western blotting
Total proteins were extracted from osteoclasts with 1× SDS-PAGE lysis buffer (Takara Bio Inc.) containing protease inhibitor (B14001, Bimake, Houston, TX) and phosphatase inhibitors (B15001-A and 9B, Bimake). Lysates were centrifuged at 12,000 × g for 10 min, and the supernatants containing proteins were collected. Lysates containing 30 μg of protein were separated by 10% SDS-PAGE, transferred to a polyvinylidene difluoride membrane, and blocked with 5% skimmed milk in TBS (TBST) at room temperature for 1 h. Then the membrane was incubated with primary antibodies in 5% BSA–TBST at 4 °C with gentle shaking overnight. The antibodies used were STAT3 (sc-482, Santa Cruz Biotechnology, Santa Cruz, CA), pSTAT3 (no. 9138, Cell Signaling Technology, Danvers, MA), NFATc1 (ab25916, Abcam, Cambridge, UK), Ctsk (sc48353; Santa Cruz Biotechnology), β-actin (no. 4970; Cell Signaling Technology), and glyceraldehyde-3-phosphate dehydrogenase (G8795; Sigma).
Adenoviruses
BMMs were reseeded at 2 × 106/cm2. Twenty four hours later, BMMs were infected with adenovirus expressing either CRE recombinase or GFP at a multiplicity of infection of 20. Then, BMMs were cultured in α-MEM with 20 ng/ml M-CSF and 20 ng/ml RANKL.
Plasmid and lentivirus
cDNA of NFATc1 and c-Fos were cloned into a phage-based plasmid. The Myc-tagged Stat3, FLAG-tagged Stat3-3c, and FLAG-tagged Stat3-dn plasmids were gifts from Dr. Feng Xinhua's lab (23). HEK293T cells were transfected with NFATc1 or egfp vector and package vectors to generate lentivirus expressing NFATc1 (Lenti-NFATc1) and lentivirus expressing GFP (Lenti-GFP).
Immunofluorescence
BMMs from 4-week-old WT and Stat3Ctsk mice cultured in 24-well plates as described previously were fixed with 4% paraformaldehyde for 30 min at room temperature. After washing in PBS, cells were permeabilized with 0.1% Triton X-100 and blocked with 5% BSA for 60 min. Samples were incubated with NFATc1 and CTSK antibodies overnight at 4 °C. The next day, the samples were washed and incubated with goat anti-mouse cy3 (AS008, ABclonal, Wuhan, Hubei, China) and goat anti-rabbit Alexa Fluor 488 (AS053, ABclonal) as secondary antibody for 1 h at room temperature and then counterstained with 4,6-diamidino-2-phenylindole (D8417, Sigma). Images were captured with a microscope (IX83, Olympus).
Luciferase reporter assay
The NFATc1 promoter-driven pGL3-based luciferase reporter was synthesized. HEK293T cells were plated at a density of 2 × 105 cells/well in a 24-well plate for 1 day. Plasmids containing NFATc1-luc, Stat3, c-Fos, Stat3-3c, Stat3-dn, and Renilla were transfected into HEK293T cells with Lipofectamine 2000 (Life Technologies, Inc.) according to a previously-reported protocol (36). At 36–48 h after transfection, the luciferase activity was assayed in cell extract supernatants using the Dual-Luciferase Reporter Assay System according to the manufacturer's protocol (E1910; Promega, Madison, WI).
Co-immunoprecipitation and Western blotting
Co-immunoprecipitation was performed following a previously described method (37). HEK293T cells transfected with Myc–Stat3 and FLAG–c-Fos were lysed, and whole-cell lysates were used for immunoprecipitation by FLAG antibody (Sigma) at 4 °C overnight. Western blotting assay was performed with FLAG (F3165, Sigma) and Myc (AE010, ABclonal) antibody.
ChIP and qPCR
ChIP analysis in RAW264.7 cells was performed using an enzymatic chromatin immunoprecipitation kit (EZ ChIPTM no. 17-371, Merck-Millipore, Darmstadt, Germany) following the manufacturer's instructions. Briefly, RAW264.7 cells were cross-linked with 1% formaldehyde for 10 min at room temperature followed by quenching with glycine. Chromatin digestion was performed to obtain DNA fragments from 150 to 900 bp by micrococcal nuclease. Immunoprecipitation was performed with STAT3 (no. 1264, Cell Signaling Technology), and IgG was used as a negative control. Precipitated DNA was detected by qPCR with specific primers. Primers for the STAT3-binding site in the NFATc1 promotor were 5′-GCTAGAAAATGACCCCACC-3′ and 5′-ATTCCCAACATGGCTTCTCT-3′.
Statistical analysis
Statistical analysis was performed using SPSS 16.0 (IBM, Armonk, NY) and GraphPad Prism 6.0 (GraphPad, San Diego). Data are expressed as mean ± S.D. Student's t tests were performed to evaluate the differences between the experimental and control groups. p < 0.05 was considered statistically significant.
Author contributions
Y. Y., W. Z., Q. D., and L. J. conceptualization; Y. Y., W. Z., Q. D., and L. J. resources; Y. Y. data curation; Y. Y. and Q. D. formal analysis; Y. Y., W. Z., Q. D., and L. J. validation; Y. Y., M. R. C., S. Z., X. G., H. X., Y. H., A. J., and X. H. investigation; Y. Y., W. Z., and Q. D. visualization; Y. Y., M. R. C., S. Z., X. G., H. X., Y. H., A. J., X. H., W. Z., Q. D., and L. J. methodology; Y. Y. writing-original draft; Y. Y., W. Z., Q. D., and L. J. writing-review and editing; W. Z., Q. D., and L. J. supervision; W. Z., Q. D., and L. J. project administration; Q. D. and L. J. funding acquisition.
Acknowledgments
We thank Prof. Xinhua Feng and S. Kato for reagents and mice and the members of the Zou laboratory for useful discussions. We thank Prof. Qian Bian, Shanghai Institute of Precision Medicine, Ninth People's Hospital, Shanghai Jiao Tong University School of Medicine, for critical reading and helpful discussion. We also thank the Cell Biology Core Facility and the Animal Core Facility of Shanghai Institute of Biochemistry and Cell Biology and the Laboratory for Digitized Stomatology and Research Center for Craniofacial Anomalies of Shanghai Ninth People's Hospital for assistance.
This work was supported in part by National Natural Science Foundation of China (NSFC) Grants 81570950, 81870740, and 81800949; Shanghai Summit & Plateau Disciplines; the SHIPM-mu fund from Shanghai Institute of Precision Medicine, Shanghai Ninth People's Hospital, Shanghai Jiao Tong University School of Medicine Grant JC201809; and the Incentive Project of High-level Innovation Team for Shanghai Jiao Tong University School of Medicine. This work was also supported by Scholar of the Outstanding Youth Medical Talents, Shanghai “Rising Stars of Medical Talent” Youth Development Program and the “Chen Xing” project from Shanghai Jiaotong University. The authors declare that they have no conflicts of interest with the contents of this article.
- FDA
- Food and Drug Administration
- M-CSF
- macrophage colony-stimulating factor
- Ctsk
- cathepsin K
- BMM
- bone marrow macrophage
- RANKL
- receptor activator of NF-κB ligand
- BMD
- bone mineral density
- BV/TV
- bone volume fraction
- Tb.Th
- trabecular thickness
- Tb.N.
- trabecular number
- Tb.Sp.
- trabecular separation
- Ct.Th.
- cortical bone thickness
- Ct.BMD.
- bone mineral density of cortical bone
- H&E
- hematoxylin and eosin
- MAR
- mineral apposition rate
- TRAP
- tartrate-resistant acid phosphatase
- qPCR
- quantitative PCR
- α-MEM
- α-minimum Eagle's medium
- STAT
- signal transducer and activator of transcription.
References
- 1. Boyle W. J., Simonet W. S., and Lacey D. L. (2003) Osteoclast differentiation and activation. Nature 423, 337–342 10.1038/nature01658 [DOI] [PubMed] [Google Scholar]
- 2. Cummings S. R., and Melton L. J. (2002) Epidemiology and outcomes of osteoporotic fractures. Lancet 359, 1761–1767 10.1016/S0140-6736(02)08657-9 [DOI] [PubMed] [Google Scholar]
- 3. Black D. M., and Rosen C. J. (2016) Clinical practice. Postmenopausal osteoporosis. N. Engl. J. Med. 374, 254–262 10.1056/NEJMcp1513724 [DOI] [PubMed] [Google Scholar]
- 4. Wotton C. J., Green J., Brown A., Armstrong M. E. G., Floud S., Beral V., Reeves G. K., and Million Women Study collaborators. (2019) Use of oral bisphosphonates and risk of hospital admission with osteonecrosis of the jaw: large prospective cohort study in UK women. Bone 124, 69–74 10.1016/j.bone.2019.04.003 [DOI] [PubMed] [Google Scholar]
- 5. Takayanagi H., Kim S., Koga T., Nishina H., Isshiki M., Yoshida H., Saiura A., Isobe M., Yokochi T., Inoue J., Wagner E. F., Mak T. W., Kodama T., and Taniguchi T. (2002) Induction and activation of the transcription factor NFATc1 (NFAT2) integrate RANKL signaling in terminal differentiation of osteoclasts. Dev. Cell 3, 889–901 10.1016/S1534-5807(02)00369-6 [DOI] [PubMed] [Google Scholar]
- 6. Ikeda F., Nishimura R., Matsubara T., Tanaka S., Inoue J., Reddy S. V., Hata K., Yamashita K., Hiraga T., Watanabe T., Kukita T., Yoshioka K., Rao A., and Yoneda T. (2004) Critical roles of c-Jun signaling in regulation of NFAT family and RANKL-regulated osteoclast differentiation. J. Clin. Invest. 114, 475–484 10.1172/JCI200419657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Takayanagi H. (2007) The role of NFAT in osteoclast formation. Ann. N.Y. Acad. Sci. 1116, 227–237 10.1196/annals.1402.071 [DOI] [PubMed] [Google Scholar]
- 8. Matsuo K., Galson D. L., Zhao C., Peng L., Laplace C., Wang K. Z., Bachler M. A., Amano H., Aburatani H., Ishikawa H., and Wagner E. F. (2004) Nuclear factor of activated T-cells (NFAT) rescues osteoclastogenesis in precursors lacking c-Fos. J. Biol. Chem. 279, 26475–26480 10.1074/jbc.M313973200 [DOI] [PubMed] [Google Scholar]
- 9. Yang W., Han W., Qin A., Wang Z., Xu J., and Qian Y. (2018) The emerging role of Hippo signaling pathway in regulating osteoclast formation. J. Cell. Physiol. 233, 4606–4617 10.1002/jcp.26372 [DOI] [PubMed] [Google Scholar]
- 10. Ikeda K., and Takeshita S. (2016) The role of osteoclast differentiation and function in skeletal homeostasis. J. Biochem. 159, 1–8 10.1093/jb/mvv112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Hirano T., Ishihara K., and Hibi M. (2000) Roles of STAT3 in mediating the cell growth, differentiation and survival signals relayed through the IL-6 family of cytokine receptors. Oncogene 19, 2548–2556 10.1038/sj.onc.1203551 [DOI] [PubMed] [Google Scholar]
- 12. Yu H., Lee H., Herrmann A., Buettner R., and Jove R. (2014) Revisiting STAT3 signalling in cancer: new and unexpected biological functions. Nat. Rev. Cancer 14, 736–746 10.1038/nrc3818 [DOI] [PubMed] [Google Scholar]
- 13. Mori T., Miyamoto T., Yoshida H., Asakawa M., Kawasumi M., Kobayashi T., Morioka H., Chiba K., Toyama Y., and Yoshimura A. (2011) IL-1β and TNFα-initiated IL-6-STAT3 pathway is critical in mediating inflammatory cytokines and RANKL expression in inflammatory arthritis. Int. Immunol. 23, 701–712 10.1093/intimm/dxr077 [DOI] [PubMed] [Google Scholar]
- 14. Wu Q., Zhou X., Huang D., Ji Y., and Kang F. (2017) IL-6 enhances osteocyte-mediated osteoclastogenesis by promoting JAK2 and RANKL activity in vitro. Cell. Physiol. Biochem. 41, 1360–1369 10.1159/000465455 [DOI] [PubMed] [Google Scholar]
- 15. Harmer D., Falank C., and Reagan M. R. (2018) Interleukin-6 interweaves the bone marrow microenvironment, bone loss, and multiple myeloma. Front. Endocrinol. 9, 788 10.3389/fendo.2018.00788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Johnson R. W., McGregor N. E., Brennan H. J., Crimeen-Irwin B., Poulton I. J., Martin T. J., and Sims N. A. (2015) Glycoprotein130 (Gp130)/interleukin-6 (IL-6) signalling in osteoclasts promotes bone formation in periosteal and trabecular bone. Bone 81, 343–351 10.1016/j.bone.2015.08.005 [DOI] [PubMed] [Google Scholar]
- 17. Li J. (2013) JAK-STAT and bone metabolism. JAKSTAT 2, e23930 10.4161/jkst.23930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Itoh S., Udagawa N., Takahashi N., Yoshitake F., Narita H., Ebisu S., and Ishihara K. (2006) A critical role for interleukin-6 family-mediated Stat3 activation in osteoblast differentiation and bone formation. Bone 39, 505–512 10.1016/j.bone.2006.02.074 [DOI] [PubMed] [Google Scholar]
- 19. Zhou H., Newnum A. B., Martin J. R., Li P., Nelson M. T., Moh A., Fu X. Y., Yokota H., and Li J. (2011) Osteoblast/osteocyte-specific inactivation of Stat3 decreases load-driven bone formation and accumulates reactive oxygen species. Bone 49, 404–411 10.1016/j.bone.2011.04.020 [DOI] [PubMed] [Google Scholar]
- 20. Kim K., Lee J., Kim J. H., Jin H. M., Zhou B., Lee S. Y., and Kim N. (2007) Protein inhibitor of activated STAT 3 modulates osteoclastogenesis by down-regulation of NFATc1 and osteoclast-associated receptor. J. Immunol. 178, 5588–5594 10.4049/jimmunol.178.9.5588 [DOI] [PubMed] [Google Scholar]
- 21. Hikata T., Takaishi H., Takito J., Hakozaki A., Furukawa M., Uchikawa S., Kimura T., Okada Y., Matsumoto M., Yoshimura A., Nishimura R., Reddy S. V., Asahara H., and Toyama Y. (2009) PIAS3 negatively regulates RANKL-mediated osteoclastogenesis directly in osteoclast precursors and indirectly via osteoblasts. Blood 113, 2202–2212 10.1182/blood-2008-06-162594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Joung Y. H., Darvin P., Kang D. Y., Sp N., Byun H. J., Lee C. H., Lee H. K., and Yang Y. M. (2016) Methylsulfonylmethane inhibits RANKL-induced osteoclastogenesis in BMMs by suppressing NF-κB and STAT3 activities. PLoS ONE 11, e0159891 10.1371/journal.pone.0159891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Wang G., Yu Y., Sun C., Liu T., Liang T., Zhan L., Lin X., and Feng X. H. (2016) STAT3 selectively interacts with Smad3 to antagonize TGF-β signalling. Oncogene 35, 4388–4398 10.1038/onc.2015.446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Dai Q., Xie F., Han Y., Ma X., Zhou S., Jiang L., Zou W., and Wang J. (2017) Inactivation of regulatory-associated protein of mTOR (Raptor)/Mammalian target of rapamycin complex 1 (mTORC1) signaling in osteoclasts increases bone mass by inhibiting osteoclast differentiation in mice. J. Biol. Chem. 292, 196–204 10.1074/jbc.M116.764761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Chen X., Wang Z., Duan N., Zhu G., Schwarz E. M., and Xie C. (2018) Osteoblast–osteoclast interactions. Connect. Tissue Res. 59, 99–107 10.1080/03008207.2017.1290085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Han Y., Feng H., Sun J., Liang X., Wang Z., Xing W., Dai Q., Yang Y., Han A., Wei Z., Bi Q., Ji H., Kang T., and Zou W. (2019) Lkb1 deletion in periosteal mesenchymal progenitors induces osteogenic tumors through mTORC1 activation. J. Clin. Invest. 129, 1895–1909 10.1172/JCI124590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Debnath S., Yallowitz A. R., McCormick J., Lalani S., Zhang T., Xu R., Li N., Liu Y., Yang Y. S., Eiseman M., Shim J. H., Hameed M., Healey J. H., Bostrom M. P., Landau D. A., and Greenblatt M. B. (2018) Discovery of a periosteal stem cell mediating intramembranous bone formation. Nature 562, 133–139 10.1038/s41586-018-0554-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Aliprantis A. O., Ueki Y., Sulyanto R., Park A., Sigrist K. S., Sharma S. M., Ostrowski M. C., Olsen B. R., and Glimcher L. H. (2008) NFATc1 in mice represses osteoprotegerin during osteoclastogenesis and dissociates systemic osteopenia from inflammation in cherubism. J. Clin. Invest. 118, 3775–3789 10.1172/JCI35711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Sitara D., and Aliprantis A. O. (2010) Transcriptional regulation of bone and joint remodeling by NFAT. Immunol. Rev. 233, 286–300 10.1111/j.0105-2896.2009.00849.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Chuvpilo S., Jankevics E., Tyrsin D., Akimzhanov A., Moroz D., Jha M. K., Schulze-Luehrmann J., Santner-Nanan B., Feoktistova E., König T., Avots A., Schmitt E., Berberich-Siebelt F., Schimpl A., and Serfling E. (2002) Autoregulation of NFATc1/A expression facilitates effector T cells to escape from rapid apoptosis. Immunity 16, 881–895 10.1016/S1074-7613(02)00329-1 [DOI] [PubMed] [Google Scholar]
- 31. Li C. H., Zhao J. X., Sun L., Yao Z. Q., Deng X. L., Liu R., and Liu X. Y. (2013) AG490 inhibits NFATc1 expression and STAT3 activation during RANKL induced osteoclastogenesis. Biochem. Biophys. Res. Commun. 435, 533–539 10.1016/j.bbrc.2013.04.084 [DOI] [PubMed] [Google Scholar]
- 32. Nakamura T., Imai Y., Matsumoto T., Sato S., Takeuchi K., Igarashi K., Harada Y., Azuma Y., Krust A., Yamamoto Y., Nishina H., Takeda S., Takayanagi H., Metzger D., Kanno J., et al. (2007) Estrogen prevents bone loss via estrogen receptor α and induction of Fas ligand in osteoclasts. Cell 130, 811–823 10.1016/j.cell.2007.07.025 [DOI] [PubMed] [Google Scholar]
- 33. Sun J., Ermann J., Niu N., Yan G., Yang Y., Shi Y., and Zou W. (2018) Histone demethylase LSD1 regulates bone mass by controlling WNT7B and BMP2 signaling in osteoblasts. Bone Res. 6, 14–14 10.1038/s41413-018-0015-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Zou W., Greenblatt M. B., Brady N., Lotinun S., Zhai B., de Rivera H., Singh A., Sun J., Gygi S. P., Baron R., Glimcher L. H., and Jones D. C. (2013) The microtubule-associated protein DCAMKL1 regulates osteoblast function via repression of Runx2. J. Exp. Med. 210, 1793–1806 10.1084/jem.20111790 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Dai Q., Zhou S., Zhang P., Ma X., Ha N., Yang X., Yu Z., Fang B., and Jiang L. (2017) Force-induced increased osteogenesis enables accelerated orthodontic tooth movement in ovariectomized rats. Sci. Rep. 7, 3906 10.1038/s41598-017-04422-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Liu Z., Yao X., Yan G., Xu Y., Yan J., Zou W., and Wang G. (2016) Mediator MED23 cooperates with RUNX2 to drive osteoblast differentiation and bone development. Nat. Commun. 7, 11149–11149 10.1038/ncomms11149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Dai Q., Xu Z., Ma X., Niu N., Zhou S., Xie F., Jiang L., Wang J., and Zou W. (2017) mTOR/Raptor signaling is critical for skeletogenesis in mice through the regulation of Runx2 expression. Cell Death Differ. 24, 1886–1899 10.1038/cdd.2017.110 [DOI] [PMC free article] [PubMed] [Google Scholar]