Abstract
Background
Jinmaitong (JMT) has been used to prevent and treat diabetic peripheral neuropathy (DPN) for decades. The present study aimed to elucidate the effects of JMT on thioredoxin-interacting protein (TXNIP) and Nod-like receptor protein 3 (NLRP3) inflammasome activation in the streptozotocin (STZ)-induced rat model.
Methods
The diabetic rat model was induced by a single intraperitoneal injection of 55 mg/kg STZ. The rats were divided into 3 groups (n = 8–10 per group): diabetic control, JMT (0.876 g/kg/d), and alpha-lipoic acid (ALA; 100 mg/kg/d). Body weight and blood glucose levels were monitored every 4 weeks for 12 weeks. Mechanical allodynia and myelin sheath injury of sciatic nerves (SNs) were assessed using the mechanical withdrawal threshold (MWT) test and Luxol fast blue staining. Serum T-superoxide dismutase (T-SOD), malondialdehyde (MDA), and catalase (CAT) levels were measured using commercially available kits. TXNIP/NLRP3 inflammasome proteins, including TXNIP, NLRP3, pro-caspase-1, and cleaved -caspase-1, and the downstream protein interleukin (IL)-1β, were measured using immunohistochemistry and Western blot. Gasdermin D (GSDMDC1) protein expression was analyzed using Western blot, and serum IL-1β and IL-18 levels were detected using ELISA.
Results
JMT did not significantly affect body weight or level of fasting blood glucose but improved mechanical allodynia and myelin sheath injury of SNs at 12 weeks following treatment. Moreover, JMT increased serum levels of the anti-oxidative enzymes CAT and T-SOD, and decreased MDA levels. Both JMT and ALA decreased expression of TXNIP, NLRP3, and cleaved-caspase-1 protein. JMT and ALA also decreased IL-1β, IL-18, and GSDMDC1 protein expression.
Conclusion
The current study demonstrated that TXNIP/NLRP3 inflammasome activation is involved in the molecular mechanisms underlying JMT’s protective effects in the STZ-induced diabetic rat model, which provides novel evidence to support the future clinical use of JMT.
Keywords: Jinmaitong, diabetic peripheral neuropathy, TXNIP/NLRP3 inflammasome, diabetic rat model, alpha lipoic acid
Introduction
Diabetic peripheral neuropathy (DPN), the most common complication in diabetes patients, is characterized by chronic pain and loss of sensation in the feet and other areas of the body.1 Epidemiological studies suggest that up to 60% of long-standing diabetic patients suffer from DPN.2 Although DPN has been broadly investigated in the past few decades, its pathogenesis remains to be defined and there is still no validated curative clinical therapy.1 Cellular oxidative stress and inflammation are known to be important risk factors of DPN.3 However, there are no therapies for diabetic neuropathy that effectively target oxidative stress and inflammation. Thus, there is an urgent need to explore new clinical therapies for DPN.
Thioredoxin-interacting protein (TXNIP) is a multifunctional protein that is also referred to as Vitamin D3 up-regulating protein 1 or thioredoxin binding protein-2. TXNIP is an important regulator of cell proliferation and apoptosis, as well as glucose and lipid metabolism. It contains an SH-3-domain and a WW-domain for binding Nod-like receptor protein 3 (NLRP3).4 It is reported that the NLRP3 inflammasome includes apoptosis-associated speck-like adaptor protein (ASC), NLRP3, and pro-caspase-1. The activated NLRP3 inflammasome will cleave pro-caspase-1 into caspase-1, which regulates the maturation of pro-inflammatory cytokines interleukin (IL)-1β and IL-18, and the induction of pyroptosis.5,6 In addition, Zhou et al,7 reported that TXNIP/NLRP3 inflammasome activation may be linked to IL-1β in the pathogenesis of diabetes, as well as regulating neuropathic pain mediated by microRNA (miR)-23a in spinal glial cells.8 Xu et al,9 suggested that the increased TXNIP/NLRP3 complex can induce IL-1β up-regulation and enhance inflammation. Thus, TXNIP may be a potential therapeutic target to treat neuropathy in prediabetic patients.
Jinmaitong (JMT) is a traditional Chinese compound that has been used for decades and has been reported to have remarkable clinical efficacy for preventing and treating DPN. The chemical profile of JMT was characterized by UPLC/Q-TOF-MS analysis and a total of 72 compounds were putatively identified. We recently published a representative chromatogram summarizing this characterization.10 According to our previous studies, JMT improved the clinical symptoms of numbness, cold, and pain experience in DPN patients, decreased blood glucose levels and lipid metabolism, and accelerated nerve conduction velocity, evidenced by clinical double-blind randomized controlled studies.11,12 Pharmacologically, JMT can reduce DNA oxidative damage to the sciatic nerves (SNs) in streptozotocin (STZ)-induced diabetic rats.12 Additionally, JMT regulated peripheral neuronal cell apoptosis-related gene expression, such as Bcl-2 and caspase 3.12 Thus, we speculate that JMT may ameliorate DPN through activating the TXNIP/NLRP3 inflammasome. The present study aimed to elucidate the molecular mechanisms by which JMT confers protection involving TXNIP/NLRP3 inflammasome activation in the STZ-induced diabetic rat model. The findings presented here provide new therapeutic evidence for using JMT to clinically treat DPN.
Materials And Methods
Preparation Of JMT
JMT is composed of twelve drugs, including Cuscuta chinensis Lam., Ligustrum lucidum Ait., Eclipta prostrata L., Prunella vulgaris L., Litchi chinensis Sonn., Buthus martensii Karsch., Cinnamomum cassia Presl., Corydalis yanhusuo w. T., Prunus persica L., Cassia obtuse folia L., Asarum heterotropiodes F. Schmidt, and Whitmania pigra Whitman. The crude drugs were purchased from Tong Ren Tang Lit. Corp (Beijing, China) and authenticated by Professor Xiaochun Liang according to the rigid specifications set in the Chinese Pharmacopoeia (2010 Edition). Detailed information on the drug materials and the scan of the vouchers are summarized in Supplementary Table 1. The voucher specimens were deposited at the Department of Traditional Chinese Medicine, Peking Union Medical College Hospital (PUMCH, Beijing, China). All drugs were ground into powder at a ratio of 10:10: 10:10: 30:3: 10:10: 10:30: 3:3 (w/w). The dosage of JMT was calculated based on a well-mixed JMT powder. Alpha-lipoic acid (ALA) was used as a positive control and was purchased from Shandong Qidu Pharmaceutical Co., Ltd (Zibo City, Shandong, China, lot number H20100152, 0.3 g/tablet).
Diabetic Rat Model: Induction And Treatment
This study was approved by the Institutional Animal Care and Use Committee of PUMCH and followed the Guidelines of the Care and Use of Laboratory Animals issued by the Chinese Council on Animal Research. Specifically, male Sprague–Dawley rats weighing 180–200 g were purchased from Vital River Laboratory Animal Technology Co., Ltd (Beijing, China; Certificate No. SCXK (Beijing) 2011-0004). All rats were fed a chow diet ad libitum and were housed in a temperature-controlled (22°C) and light-controlled environment (12 hrs light/dark cycle). The diabetic rat model was induced by a single intraperitoneal injection of 55 mg/kg STZ (Sigma-Aldrich, St Louis, MO, USA) in 0.1 mol/L citrate buffer (pH 4.5) after fasting overnight. Normal control animals received only citrate buffer. At 72 hrs after STZ injection, blood glucose levels were measured from a tail snip after overnight fasting using a blood glucose meter (MediSense® OptiumTM; Abbott Laboratories, Chicago, IL, USA). Only rats with a fasting blood glucose level ≥16.7 mmol/L were considered diabetic. Diabetic rats were further divided into 3 groups (n = 8–10 per group): diabetic control treated with vehicle, JMT (10 times the dose recommended for a human adult, 0.876 g/kg/d), and ALA positive control (100 mg/kg/d). The normal control rats (n = 8), matched in age and body weight, were administered vehicle alone. JMT powder and ALA tablets were freshly dissolved in distilled water, and homogeneously mixed, then gavaged immediately (10 mL/kg/d). The doses chosen were based on both our studies and previous reports.10,13 The treatment was started on day 4 after STZ injection and then administered by gavage every day for the following 12 weeks. Body weight and fasting blood glucose levels were measured before and after STZ administration, as well as during drug treatment at intervals of 4 weeks. Upon sacrifice, all animals were deprived of food, but not water, overnight. The SNs on one side of the rats were snap frozen in liquid nitrogen and stored at −80°C for Western blot analysis. The other side of the SNs was fixed in 10% phosphate-buffered formalin, processed, embedded in paraffin, and cut into 4 μm sections for Luxol fast blue staining and immunohistochemistry analysis.
Mechanical Withdrawal Threshold (MWT) Assessment
A transparent organic glass box (22 cm × 22 cm × 22 cm) with a metal mesh (1 cm2) at the bottom was used to assess MWT. The rats were placed in the box, which was situated 50 cm above the experimental bench. After acclimating for 15 mins, the rear toes of the rats were vertically stimulated using the Electronic Von Frey Filaments (Cat. No. 2391, IITC Life Science Inc., Woodland Hills, CA, USA) with a single-stimulus duration of ≤1 s and a stimulus interval of 10 s. The intensity of stimulus (g) was measured during the test when the rats lifted or licked their feet. MWT was calculated as the average of the five times the intensity that caused the rats to lift or lick their feet as previously described.14
Biochemical Assay
Blood samples were collected from the carotid arteries of rats after overnight fasting. Serum was obtained by centrifuging for 20 mins at 1000 × g. Serum T-superoxide dismutase (T-SOD), malondialdehyde (MDA), and catalase (CAT) levels were determined using commercially available kits (Nanjing Jiancheng Bioengineering Institute, Nanjing, China) following the manufacturer’s instructions. Additionally, serum IL-1β and IL-18 levels were measured using ELISA kits (eBioscience, San Diego, CA, USA and Abcam, Cambridge, MA, USA).
Luxol Fast Blue Staining
After deparaffinization and rehydration, SN tissue sections were incubated in Luxol Fast Blue Solution (Biossci, Wuhan, China) for 24 hrs at room temperature and then rinsed with 70% ethanol and distilled water. Sections were then differentiated in Lithium Carbonate Solution (Biossci, Wuhan, China) for 15–20 s, followed by incubation in 70% ethanol until the background became colorless. Hematoxylin and eosin were used as the counterstain. The sections were mounted after dehydration and clearing in xylene and examined under a light microscope (Carl Zeiss, Thornwood) with a digital camera (Olympus, Tokyo, Japan).
Immunohistochemistry
Following deparaffinization and rehydration, the SN sections were microwaved in citric acid (pH 6.0). After washing with phosphate-buffered saline with Tween-20 (PBST, pH 7.4), the sections were incubated in 3% H2O2 solution and blocked with 10% normal goat serum (Boster, Wuhan, China) for 20 mins at room temperature. Then, the sections were incubated with TXNIP (1:100, Abcam, Cambridge, MA, USA), NLRP3 (1:200, Abcam, Cambridge, MA, USA), and IL-1β (1:150, R&D, Minneapolis, MN, USA) primary antibodies at 4°C overnight and subsequently incubated at room temperature for 30 mins with an HRP-conjugated secondary antibody (Dako, Glostrup, Denmark), followed by staining with a diaminobenzidine (DAB) solution (Dako, Glostrup, Denmark). Sections were counterstained with hematoxylin, dehydrated, and mounted. Stained images were captured using a light microscope (Carl Zeiss, Thornwood) with a digital camera (Olympus, Tokyo, Japan). Positive DAB stained areas were quantified using the NIH Image J software (National Institute of Heath, Bethesda, MD, USA). Detailed information on antibodies used in the present study is presented in Supplementary Table 2.
Western Blot
Total protein was homogenized with RIPA lysis buffer on ice and the protein concentration was determined using the BCA protein assay kit (Beyotime, Shanghai, China). Protein samples were separated using 10% SDS-PAGE (Beyotime, Shanghai, China) and transferred to a nitrocellulose membrane (Pall Gelman, Ann Arbor, MI, USA). The membranes were blocked with 5% fat-free milk and incubated with primary antibodies against TXNIP (1:1,000), NLRP3 (1:1,000), caspase-1 (1:1,000) (Abcam, Cambridge, MA, USA), IL-1β (1:1,000), GSDMDC1 (1:500), and β-actin (1:3,000) (Santa Cruz Biotechnology, Santa Cruz, Dallas, TX, USA) overnight at 4°C, washed with Tris-buffered saline with Tween 20 (TBST) three times, and incubated with HRP-conjugated secondary antibodies (1:3,000, Zhongshan Golden Bridge Biotechnology, Beijing, China) for 1 hr at room temperature. An ECL kit (Roche, Penzberg, Germany) was used to visualize the protein bands. The relative levels of each protein to β-actin were determined using densitometry analysis with the Gel Image System ver.4.00 (Tanon, Shanghai, China). Detailed information on antibodies used in the present study is shown in Supplementary Table 2.
Statistical Analyses
Data are presented as means ± S.E.M, calculated using GraphPad Prism 7.0 software. Differences were analyzed using one-way ANOVA with GraphPad Prism 7.0 software or the unpaired two-tailed Student’s t-test. P < 0.05 was considered statistically significant.
Results
JMT Did Not Significantly Affect Body Weight And Fasting Blood Glucose Levels In Diabetic Rats At 12 Weeks
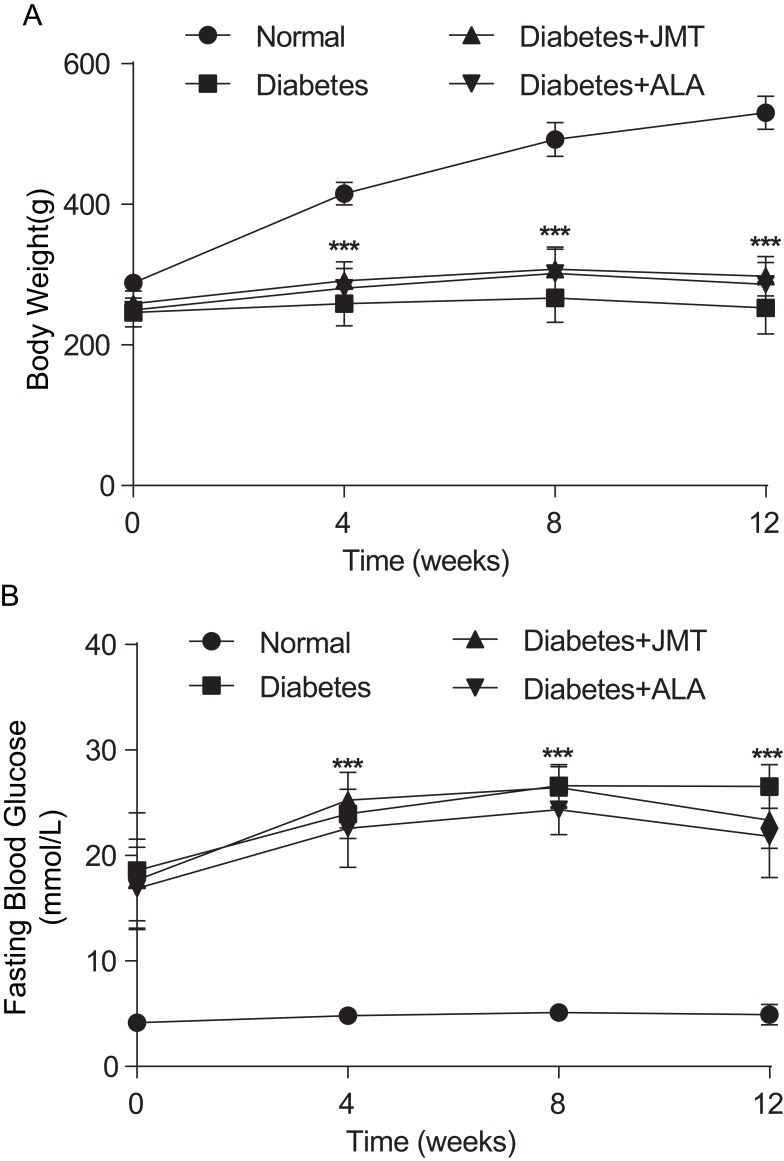
As shown in Figure 1, body weight was significantly decreased in diabetic rats at all tested time points (Figure 1A). In addition, fasting blood glucose levels were significantly increased in the diabetic rats (Figure 1B). There was no significant difference in either body weight or fasting blood glucose levels between the diabetic group and the JMT or ALA-treated diabetic groups at all tested time points (Figure 1A and B).
Figure 1.
Effects of JMT on body weight and fasting blood glucose in diabetic rats at 12 weeks.
Notes: Body weight (A) and fasting blood glucose levels (B) were measured every 4 weeks. Data are expressed as the means ± SEM (n = 8). ***P < 0.001 vs normal group.
Abbreviations: JMT, Jinmaitong; SEM, standard error of mean.
JMT Improved Mechanical Allodynia And Myelin Sheath Injury Of SNs In Diabetic Rats
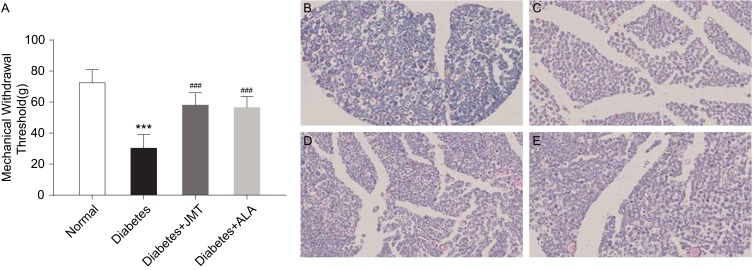
The STZ-induced rat model has been reported as a stable DPN model with thermal hyperalgesia and mechanical allodynia.15 We measured the MWT in the present study to evaluate the effects of JMT on mechanical allodynia in diabetic rats. As shown in Figure 2, diabetic rats showed a marked decrease in MWT levels compared to the normal control group. JMT and ALA partly reversed the diabetes-induced decrease in MWT levels (Figure 2A). In addition, we stained myelin with Luxol fast blue dye to observe SNs demyelination and axon atrophy. Luxol dye binds and stains the myelin sheath blue, leaving a clear background with an unstained axon and other structures. As shown in Figure 2B, the myelin sheaths of normal SNs were stained blue and the structure was regular and uniform, with an obvious outline. In contrast, the myelinated nerve fibers were arranged irregularly in the diabetic group (Figure 2C). In addition, the myelin sheaths were unevenly stained a light blue color. We observed that the vacuolization of nerve fibers with decreased myelination and the degree of degeneration were ameliorated in the treatment groups (Figure 2D and E).
Figure 2.
JMT improved mechanical allodynia and myelin sheath injury of SNs in diabetic rats.
Notes: The MWT in different groups of rats was assessed at 12 weeks after STZ injection (A). Data are expressed as the means ± SEM (n = 8). ***P < 0.001 vs normal group, ###P < 0.001 vs diabetes group. Representative images of Luxol fast blue staining in SN sections (magnification, ×400) (B–E). Normal control (B), Diabetes (C), Diabetes + JMT (D), Diabetes + ALA (E).
Abbreviations: JMT, Jinmaitong; SN, sciatic nerve; MWT, mechanical withdrawal threshold; STZ, streptozotocin; SEM, standard error of mean; ALA, alpha-lipoic acid.
JMT Attenuated Serum Oxidative Stress In Diabetic Rats
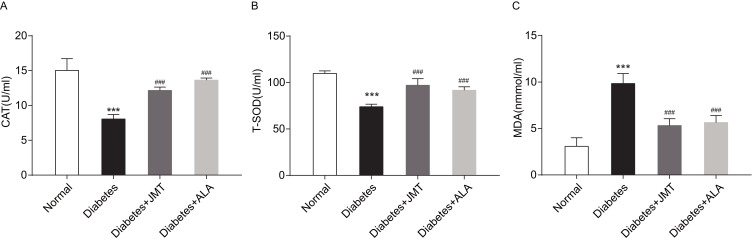
To observe the anti-oxidative effects of JMT, we measured the serum levels of antioxidant enzymes including CAT and T-SOD, and the oxidase enzyme MDA. As shown in Figure 3A and B, CAT and T-SOD expression decreased in the diabetic group and treatment with JMT and ALA restored CAT and T-SOD expression. MDA expression was significantly increased in the diabetic rats, while both JMT and ALA treatment reversed the STZ-induced increase in MDA in the diabetic rats (Figure 3C).
Figure 3.
JMT attenuated serum oxidative stress in diabetic rats.
Notes: Serum CAT (A), T-SOD (B), and MDA (C) levels were assayed using biochemical kits (n = 8). ***P < 0.001 vs normal group, ###P < 0.001 vs diabetes group.
Abbreviations: JMT, Jinmaitong; CAT, catalase; T-SOD, total superoxide dismutase; MDA, malonyldialdehyde.
JMT Suppressed TXNIP Expression In SNs Of Diabetic Rats
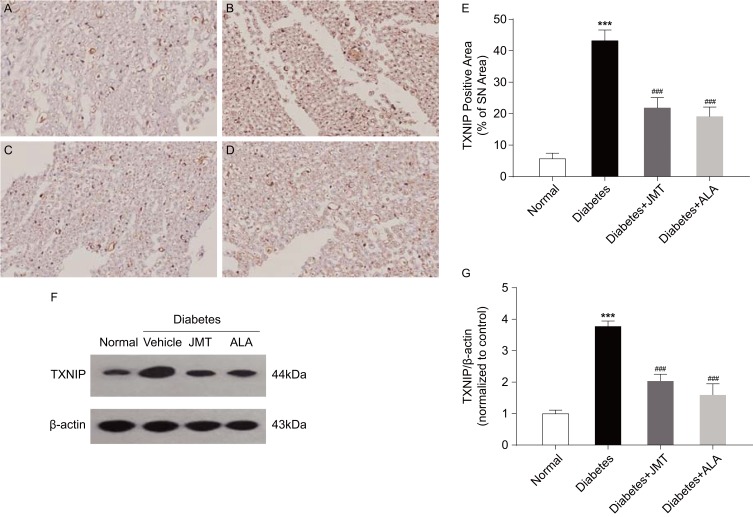
TXNIP protein distribution and expression were assessed using immunohistochemical and Western blot assays. As shown in Figure 4A–D, TXNIP expression was mainly localized in the axons and Schwann cells of the SNs. Quantitative analysis showed that TXNIP protein expression increased in diabetic rats, and both JMT and ALA significantly restored the protein levels (Figure 4E). These results are consistent with the Western blot analysis, which demonstrated that TXNIP expression was increased in the diabetic group and decreased in the JMT and ALA-treated diabetic groups (Figure 4F and G), suggesting that TXNIP is involved in JMT’s protective effects in diabetic rats.
Figure 4.
JMT suppressed TXNIP expression in SNs of diabetic rats.
Notes: Representative staining of TXNIP (magnification, ×400) (A–D). Brown color corresponds to protein of interest, and blue color corresponds to nuclear staining. Normal control (A), Diabetes (B), Diabetes + JMT (C), Diabetes + ALA (D). Quantitative analysis of positive-stained areas relative to total tissue areas (E). Representative Western blot images of TXNIP (F). Densitometric analysis of TXNIP/β-actin protein expression normalized to the normal control group (G). Data are the mean ± SEM from three independent experiments. ***P < 0.001 vs normal group, ###P < 0.001 vs diabetes group.
Abbreviations: JMT, Jinmaitong; TXNIP, thioredoxin-interacting protein; SN, sciatic nerve; ALA, alpha-lipoic acid; SEM, standard error of mean.
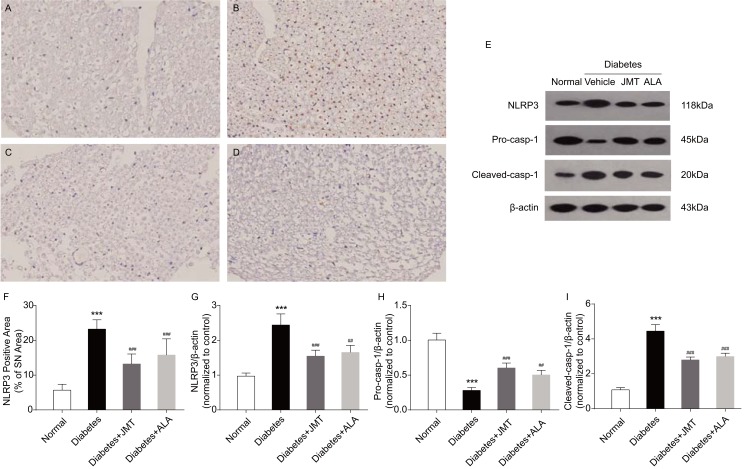
JMT Regulated Expression Of NLRP3 Inflammasome Proteins In SNs Of Diabetic Rats
The NLRP3 inflammasome includes NLRP3, the cysteine protease pro-caspase-1 and the adaptor protein ASC. Inflammasome formation can induce autocatalysis and activation of caspase-1.16 Results of the present study suggest that NLRP3 protein expression, predominantly located in the axons of SNs in diabetic rats (Figure 5A–D), was increased in the diabetic group compared to the normal control group (Figure 5E–G). JMT and ALA treatment significantly attenuated the effects of diabetes on NLRP3 protein expression (Figure 5E–G). In addition, protein expression of cleaved-caspase-1 was higher and the caspase-1 precursor was lower in the diabetic rats compared to the control rats (Figure 5E, H and I), indicating that caspase-1 was activated in our model. JMT and ALA treatment markedly reduced caspase-1 cleavage (Figure 5E, H and I). In summary, JMT may inhibit NLRP3 inflammasome activation in SN tissue under chronic hyperglycemia conditions.
Figure 5.
JMT regulated NLRP3 inflammasome expression in SNs of diabetic rats.
Notes: Representative staining of NLRP3 (magnification, ×400) (A–D). Brown color corresponds to protein of interest, and blue color corresponds to nuclear staining. Normal control (A), Diabetes (B), Diabetes + JMT (C), Diabetes + ALA (D). Representative Western blot images of NLRP3, pro-caspase-1, and cleaved-caspase-1 (E). Quantitative analysis of positive-stained areas relative to total tissue areas (F). Densitometric analysis of NLRP3, pro-caspase-1, and cleaved-caspase-1 protein expression normalized to β-actin (G–I). Data are the mean ± SEM from three independent experiments. ***P < 0.001 vs normal group, ##P < 0.01 vs diabetes group, ###P < 0.001 vs diabetes group.
Abbreviations: JMT, Jinmaitong; NLRP3, NACHT, LRR and PYD domains-containing protein 3; SN, sciatic nerve; ALA, alpha-lipoic acid; SEM, standard error of mean.
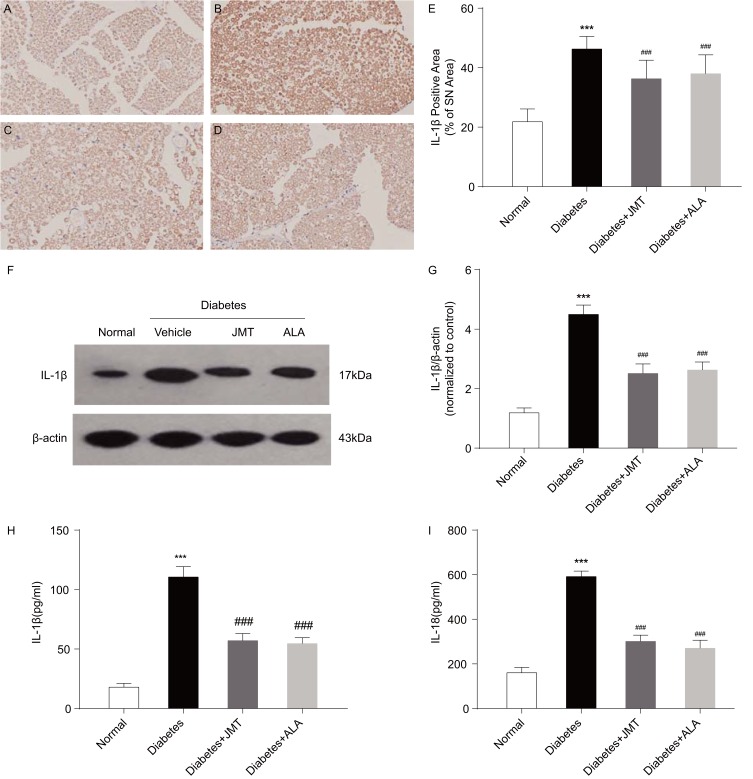
JMT Decreased IL-1β And IL-18 Expression In Both The Serum And SNs Of Diabetic Rats
Activated caspase-1 has been reported to induce maturation of IL-1β and IL-18 and cause pyroptosis.6 Thus, we also measured IL-1β and IL-18 protein expression in our model. Immunostaining revealed IL-1β positive staining primarily in the myelin and Schwann cells of SNs of diabetic rats (Figure 6A–D). IL-1β protein expression was increased in the diabetic group compared to the normal control group, and JMT and ALA treatment normalized IL-1β expression (Figure 6E–G). Serum levels of IL-1β and IL-18, used as biological markers for caspase-1-induced inflammation, were also measured using ELISA. The ELISA results were generally consistent with the Western blot analysis, indicating that serum IL-1β and IL-18 were increased in the diabetic group, and JMT and ALA treatment normalized their expression (Figure 6H and I).
Figure 6.
JMT decreased IL-1β and IL-18 expression in serum and SNs of diabetic rats.
Notes: Representative staining of IL-1β (magnification, ×400) (A–D). Brown color corresponds to protein of interest, and blue color corresponds to nuclear staining. Normal control (A), Diabetes (B), Diabetes + JMT (C), Diabetes + ALA (D). Quantitative analysis of positive-stained areas relative to total tissue areas (E). Representative Western blot images of IL-1β (F). Densitometric analysis of IL-1β/β-actin protein expression normalized to the normal control group (G). Serum IL-1β and IL-18 levels analyzed using ELISA (H, I). Data are the mean ± SEM from three independent experiments. ***P < 0.001 vs normal group, ###P < 0.001 vs diabetes group.
Abbreviations: JMT, Jinmaitong; IL, interleukin; SN, sciatic nerve; ALA, alpha-lipoic acid; ELISA, enzyme-linked immunosorbent assay; SEM, standard error of mean.
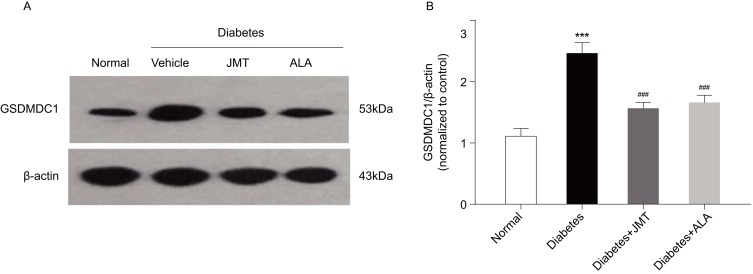
JMT Reduced GSDMD Expression In SNs Of Diabetic Rats
Gasdermin D (GSDMD) has been reported to be required for caspase-1 activation. Thus, we also measured GSDMDC1 protein expression using Western blot analysis. GSDMDC1 protein expression was increased in the diabetic group compared to the normal control group, and JMT and ALA treatment down-regulated GSDMDC1 expression (Figure 7A and B), further suggesting that GSDMD is involved JMT’s protective effects in diabetic rats.
Figure 7.
JMT reduced GSDMD expression in SNs of diabetic rats.
Notes: Representative Western blot images of GSDMDC1 (A). Densitometric analysis of GSDMDC1/β-actin protein expression normalized to the normal control group (B). Data are the mean ± SEM from three independent experiments. ***P < 0.001 vs normal group, ###P < 0.001 vs diabetes group.
Abbreviations: JMT, Jinmaitong; GSDMD, gasdermin D; SEM, standard error of mean.
Discussion
JMT is a traditional Chinese compound prescription that has been used in the Department of Tradition Chinese Medicine, PUMCH. JMT is formulated according to the basic theory of Traditional Chinese Medicine17 and is reported to nourish the qi and yin of the kidney, activate blood circulation, and warm and dilate tendons and blood vessels. In the STZ-induced DPN rat model, JMT has also been reported to alleviate peripheral nerve injury by increasing levels of insulin growth factor-I (IGF-I), its receptor IGF-IR,10 and ciliary neurotrophic factor (CNTF),17 and by decreasing DNA oxidative damage and apoptosis of the SNs.12 In vitro, JMT was reported to induce autophagy,18 prevent apoptosis,19 and increase proliferation20 of Schwann cells. The present study is the first to report that JMT can ameliorate DPN through suppressing TXNIP/NLRP3 inflammasome activation in the STZ-induced diabetic rat model.
STZ can eliminate or destroy islet β-cells. As such the STZ-induced diabetic rat model is representative of Type I diabetes.21 This model can also be used as a stable DPN model with thermal hyperalgesia and mechanical allodynia, and is a well-known animal model for exploring the mechanisms of neuropathy and evaluating the effects of target therapeutics.14,22 The STZ-induced diabetic rats in the present study had decreased body weights and increased fasting blood glucose levels. Additionally, STZ caused mechanical allodynia, as evidenced by the MWT assay and myelin sheath injury of SNs, and as demonstrated by Luxol fast blue staining. These results confirm that we successfully established a model of DPN. ALA has been reported to improve neuropathic symptoms in diabetic patients23 and prevent the progression of neuropathic impairments by mediating anti-oxidative effects.24 In the present study, JMT and ALA had no obvious effects on either body weight and fasting blood glucose levels. However, both JMT and ALA improved mechanical allodynia and myelin sheath injury of SNs in the diabetic rats. These findings confirm our previous study, which showed that the MWT increased and the neurological morphology of the SNs was improved by JMT in STZ-induced diabetic rats.10
JMT has also been reported to reduce SNs DNA oxidative damage and apoptosis in STZ-induced diabetic rats.12 In the present study, JMT and ALA increased the serum levels of antioxidant enzymes, including CAT and T-SOD, and the oxidase enzyme MDA, as evidenced by ELISA assays. These data suggest that JMT exerts anti-oxidative effects to protect against SNs injury in STZ-induced diabetes. Moreover, recent evidence suggests that NLRP3 plays an important role in the pathogenesis of diabetes and its complications. The NLRP3 inflammasome includes the apoptosis-associated speck-like adaptor protein (ASC), NLRP3, and pro-caspase-1. Upon activation, NLRP3 becomes ligated to ASC and then binds to pro-caspase-1. This binding activates cleavage and transformation of pro-caspase-1 to caspase-1, which in turn regulates the maturation of pro-inflammatory cytokines IL-1β and IL-18 and induces pyroptosis.5,6 TXNIP has been reported to be upstream of NLPP3, and the complex of these two proteins is necessary for inflammasome activation.7 Results of the present study showed that both JMT and ALA can suppress TXNIP and NLRP3 inflammasome protein expression, as demonstrated by immunostaining and Western blot analysis of SNs in the diabetic rats. In addition, cleaved-caspase-1 protein expression was higher and the caspase-1 precursor level was lower in the diabetic rats compared to the control rats, indicating activation of caspase-1. Thus, we also measured the serum levels of IL-1β and IL-18. Results suggest that JMT and ALA decrease IL-1β and IL-18 expression in serum and SNs of diabetic rats. In summary, JMT may suppress TXNIP/NLRP3 inflammasome activation, as well as IL-1β and IL-18 in the STZ-induced diabetic rat model.
GSDMD is a newly identified protein that is a member of the NLRP3 inflammasome.25 Mastrocola et al,26 reported that cleavage of GSDMDC1 in the inflammasome is required for caspase-1 activation. In the present study, GSDMDC1 protein expression was detected using Western blot analysis. Results suggest that JMT and ALA decrease GSDMDC1 protein expression in the diabetic rat model. This further indicates that JMT may suppress caspase-1 activation and thus suppress TXNIP/NLRP3 inflammasome activation in the STZ-induced diabetic rat model.
Conclusion
In conclusion, the present study is the first to report that JMT ameliorates DPN through suppressing TXNIP/NLRP3 inflammasome activation in the STZ-induced diabetic rat model. This study provides new evidence for the clinical use of JMT as a traditional Chinese prescription for preventing and treating DPN. More research is needed to explore the key effective constituents of JMT in future studies.
Acknowledgments
This study was supported in part by grants from the National Natural Sciences Foundation of China (#81603481), the PUMCH Science Fund for Junior Faculty (#pumch-2013-140), and CAMS Innovation Fund for Medical Sciences (#2017-I2M-1-009). Present Address for Chao Wang: Department of Oncology of Integrative Chinese and Western Medicine, China-Japan Friendship Hospital, Beijing, China
Disclosure
The authors declare that there are no conflicts of interest in this work.
References
- 1.Pop-Busui R, Boulton AJ, Feldman EL, et al. Diabetic neuropathy: a position statement by the American Diabetes Association. Diabetes Care. 2017;40(1):136–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Iqbal Z, Azmi S, Yadav R, et al. Diabetic peripheral neuropathy: epidemiology, diagnosis and pharmacotherapy. Clin Ther. 2018;40(6):828–849. [DOI] [PubMed] [Google Scholar]
- 3.Vincent AM, Callaghan BC, Smith AL, et al. Diabetic neuropathy: cellular mechanisms as therapeutic targets. Nat Rev Neurol. 2011;7(10):573–583. [DOI] [PubMed] [Google Scholar]
- 4.Zhang PX, Pang XL, Tu YY. Thioredoxin-interacting proteins as a common regulating target for multiple drugs in clinical therapy/application. Cancer Transl Med. 2015;1(1):26–30. [Google Scholar]
- 5.Xiao YD, Huang YY, Wang HX, et al. Thioredoxin-interacting protein mediates NLRP3 inflammasome activation involved in the susceptibility to ischemic acute kidney injury in diabetes. Oxid Med Cell Longev. 2016;2386068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.He Y, Hara H, Nunez G. Mechanism and regulation of NLRP3 inflammasome activation. Trends Biochem Sci. 2016;41(12):1012–1021. doi: 10.1016/j.tibs.2016.09.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhou R, Tardivel A, Thorens B, Choi I, Tschopp J. Thioredoxin-interacting protein links oxidative stress to inflammasome activation. Nat Immunol. 2010;11(2):136–140. doi: 10.1038/ni.1831 [DOI] [PubMed] [Google Scholar]
- 8.Pan Z, Shan Q, Gu P, et al. MiRNA-23a/CXCR4 regulates neuropathic pain via directly targeting TXNIP/NLRP3 inflammasome axis. J Neuroinflammation. 2018;15(1):29. doi: 10.1186/s12974-018-1220-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Xu L, Lin X, Guan M, et al. Verapamil attenuated prediabetic neuropathy in high-fat diet-fed mice through inhibiting TXNIP-mediated apoptosis and inflammation. Oxid Med Cell Longev. 2019;1896041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Song W, Jiang W, Wang C, et al. Jinmaitong, a traditional chinese compound prescription, ameliorates the streptozocin-induced diabetic peripheral neuropathy rats by increasing sciatic nerve IGF-1 and IGF-1R Expression. Front Pharmacol. 2019;29(10):255. doi: 10.3389/fphar.2019.00255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liang X, Cui L, Guo S. Clinical study on Jinmaitong composita on diabetic peripheral neuropathy. Zhongguo Zhong Xi Yi Jie He Za Zhi. 1999;19(9):517–519. [PubMed] [Google Scholar]
- 12.Yin DH, Liang XC, Zhao L, et al. Jinmaitong decreases sciatic nerve DNA oxidative damage and apoptosis in a streptozotocin-induced diabetic rat model. Exp Ther Med. 2015;10:778–786. doi: 10.3892/etm.2015.2543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jiang YJ, Gong DX, Liu HB, et al. Ability of alpha-lipoic acid to reverse the diabetic cystopathy in a rat model. Acta Pharmacol Sin. 2008;29(6):713–719. doi: 10.1111/j.1745-7254.2008.00790.x [DOI] [PubMed] [Google Scholar]
- 14.Vrinten DH, Hamers FF. “CatWalk” automated quantitative gait analysis as a novel method to assess mechanical allodynia in the rat: a comparison with von Frey testing. Pain. 2003;102:203–209. doi: 10.1016/s0304-3959(02)00382-2 [DOI] [PubMed] [Google Scholar]
- 15.Liao C, Yang M, Zhong W, et al. Stable rat model of mechanical allodynia in diabetic peripheral neuropathy: the role of nerve compression. J Reconstr Microsurg. 2018;34(4):264–269. doi: 10.1055/s-0037-1621723 [DOI] [PubMed] [Google Scholar]
- 16.Sutterwala FS, Haasken S, Cassel SL. Mechanism of NLRP3 inflammasome activation. Ann N Y Acad Sci. 2014;1319:82–95. doi: 10.1111/nyas.2014.1319.issue-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shi Y, Liang XC, Wu QL, et al. Effects of Jinmaitong capsule on ciliary neurotrophic factor in sciatic nerves of diabetes mellitus rats. Chin J Integr Med. 2013;19(2):104–111. doi: 10.1007/s11655-013-1352-7 [DOI] [PubMed] [Google Scholar]
- 18.Qu L, Zhang H, Gu B, et al. Jinmaitong alleviates the diabetic peripheral neuropathy by inducing autophagy. Chin J Integr Med. 2016;22(3):185–192. doi: 10.1007/s11655-015-2164-8 [DOI] [PubMed] [Google Scholar]
- 19.Wang PY, Liang XC, Zhang H, et al. Effect of serum containing Jinmaitong capsule on rats’ Schwann cell apoptosis induced by high glucose concentration. Chin J Integr Med. 2013;19(7):517–523. doi: 10.1007/s11655-013-1506-7 [DOI] [PubMed] [Google Scholar]
- 20.Qu L, Liang XC, Zhang H, et al. Effect of Jinmaitong serum on the proliferation of rat Schwann cells cultured in high glucose medium. Chin J Integr Med. 2008;14(4):293–297. doi: 10.1007/s11655-008-0293-z [DOI] [PubMed] [Google Scholar]
- 21.Biessels GJ, Bril V, Calcutt NA, et al. Phenotyping animal models of diabetic neuropathy: a consensus statement of the diabetic neuropathy study group of the EASD (Neurodiab). J Peripher Nerv Syst. 2014;19(2):77–87. doi: 10.1111/jns5.12072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Guo N, Li C, Liu Q, et al. Maltol, a food flavor enhancer, attenuates diabetic peripheral neuropathy in streptozotocin-induced diabetic rats. Food Funct. 2018;9(12):6287–6297. doi: 10.1039/c8fo01964a [DOI] [PubMed] [Google Scholar]
- 23.Ziegler D, Ametov A, Barinov A, et al. Oral treatment with alpha-lipoic acid improves symptomatic diabetic polyneuropathy: the SYDNEY 2 trial. Diabetes Care. 2006;29(11):2365–2370. doi: 10.2337/dc06-1216 [DOI] [PubMed] [Google Scholar]
- 24.Ziegler D, Low PA, Litchy WJ, et al. Efficacy and safety of antioxidant treatment with alpha-lipoic acid over 4 years in diabetic polyneuropathy: the NATHAN 1 trial. Diabetes Care. 2011;34(9):2054–2060. doi: 10.2337/dc11-0503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liu X, Zhang Z, Ruan J, et al. Inflammasome-activated gasdermin D causes pryrotosis by forming membrane pores. Nature. 2016;535(7610):153–158. doi: 10.1038/nature18629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mastrocola R, Penna C, Tullio F, et al. Pharmacological inhibition of NLRP3 inflammasome attenuates myocardial ischemia/reperfusion injury by activation of RISK and mitochondrial pathways. Oxid Med Cell Longev. 2016. doi:10.1155/2016/ 5271251. [DOI] [PMC free article] [PubMed] [Google Scholar]