Abstract
Study Objective
To evaluate long-term efficacy and safety of phrenic nerve stimulation (PNS) in patients with moderate-to-severe central sleep apnea (CSA) through 3 years of therapy.
Methods
Patients in the remedē System Pivotal Trial were observed every 3 months after implant until US Food and Drug Administration approval. At the time of approval and study closure, all patients completed 24 months of follow-up; 33 patients had not reached the 36-month visit. Sleep metrics (polysomnography) and echocardiographic parameters are reported at baseline, 12, 18, and 24 months, in addition to available 36-month sleep results from polygraphy. Safety was assessed through 36 months; however, analysis focused through 24 months and available 36-month results are provided.
Results
Patients were assessed at 24 (n = 109) and 36 (n = 60) months. Baseline characteristics included mean age 64 years, 91% male, and mean apnea–hypopnea index 47 events per hour. Sleep metrics (apnea–hypopnea index (AHI), central apnea index, arousal index, oxygen desaturation index, rapid eye movement sleep) remained improved through 24 and 36 months with continuous use of PNS therapy. At least 60% of patients in the treatment group achieved at least 50% reduction in AHI through 24 months. Serious adverse events (SAEs) related to the remedē System implant procedure, device, or therapy through 24 months were reported by 10% of patients, no unanticipated adverse device effects or deaths, and all events resolved. No additional related SAEs were reported between 24 and 36 months.
Conclusion
These data suggest beneficial effects of long-term PNS in patients with CSA appear to sustain through 36 months with no new safety concerns.
Trial Registration
Keywords: central sleep apnea, phrenic nerve stimulation, transvenous stimulation
Statement of Significance.
Central sleep apnea (CSA) is a highly common comorbidity in heart failure (HF) patients, but also affects sleep and quality of life in non-HF patients. CSA also results in alterations in autonomic, chemical, rhythmic, and inflammatory processes affecting overall individual health and is associated with increased morbidity and mortality. Thus, therapeutic options to treat CSA are needed. Phrenic nerve stimulation (PNS) is a recent US Food and Drug Administration-approved technology shown to be safe and effective in adult patients for the treatment of moderate-to-severe CSA through 12 months. The importance of the current analysis is that safety and effectiveness of PNS therapy is extended out through 36 months with no unanticipated adverse device effects related to the device or delivered therapy.
Introduction
Epidemiological and interventional studies have shown that adverse health outcomes are associated with sleep-disordered breathing (SDB) [1]. SDB is typically classified into two predominant types: obstructive sleep apnea (OSA) and central sleep apnea (CSA). Regardless of concomitant pathology, patients may have mixed OSA and CSA, but one or the other type of SDB is generally predominant [2]. CSA is frequent in patients with underlying cardiovascular diseases and especially with heart failure (HF) [3, 4]. Neurophysiologically, CSA is a manifestation of central breathing instability with transient inhibition of ventilatory motor [5]. Depending on the underlying cause of the CSA, treatment options vary. These include positive pressure devices, pharmacological therapy, and phrenic nerve stimulation (PNS) [5]. Patients with CSA have limited treatment options because mask-based therapies are associated with poor patient adherence, mask and pressure intolerance and, more recently, a contraindication for some devices (adaptive servo-ventilation) in patients with decreased cardiac systolic function.
Independent of what therapeutic option is used, the main goal of the treatment is to eliminate abnormal respiratory events and stabilize sleep infrastructure with the hope to eliminate CSA-associated symptoms and improve quality of life [5]. Given various causes and pathophysiological conditions associated with CSA, long-term randomized controlled trials are needed to ascertain the long-term effectiveness of individual treatment modalities [5]. So far such studies are scarce.
The recent randomized Treatment of Sleep-Disordered Breathing with Predominant Central Sleep Apnea by Adaptive Servo-Ventilation in Patients with Heart Failure (SERVE-HF) study demonstrated an increase in mortality in the adaptive servo-ventilation treatment group compared to control in patients with reduced ejection fraction and predominant CSA [6]; thus, novel therapeutic approaches are critically needed [7, 8].
Transvenous PNS is a promising therapy shown to be safe and to effectively treat events and symptoms of CSA [9]. Improvements in sleep indices, sleep quality, and quality of life observed in the remedē System Pivotal Trial were sustained through 12 months of active therapy in patients with and without HF [10]. The remedē System (Respicardia, Inc., Minnetonka, MN) is a fully implantable neurostimulation system that delivers PNS continuously throughout the night to treat CSA, mitigating therapy compliance issues that often occur with mask-based therapies [11]. Despite the sustained benefit and safety of PNS through 12 months, longer-term data are essential because the disorders underlying CSA are typically chronic and progressive. Therefore, the objective of this paper was to show both therapeutic effectiveness measures as well as safety profile indicators through 24 and 36 months of active remedē System therapy.
Methods
Design, methods, oversight, primary results as well as 12-month and HF results of the remedē System Pivotal Trial (NCT01816776) have previously been reported [9, 10]. Briefly, the remedē System Pivotal Trial was a prospective, multicenter, randomized, open-label controlled trial in patients with predominant CSA of different etiologies to assess transvenous unilateral PNS versus no stimulation [9]. Patients in the control group had the device implanted at time of randomization but not activated until 6-month effectiveness endpoints were assessed, ending the randomized portion of the trial; this cohort is referred to as former control once therapy was activated. Patients were observed every 3 months post implant until US Food and Drug Administration (FDA) approval of the remedē System (October 2017). As a result, the patients analyzed here have different durations of active therapy. All patients remaining in the trial at study closure had been followed for a minimum of 24 months after implant, but 33 patients had not yet reached the 36-month visit when the study was closed. In agreement with FDA, ongoing patients were asked to enroll into the remedē System Post Approval Study (NCT03425188).
Effectiveness of PNS was assessed by full overnight, attended polysomnography (PSG) completed at baseline and at 6-month intervals through 24 months, adding a final home sleep test of cardiorespiratory polygraphy (PG) using a NOX-T3 apparatus (ResMed Corp., San Diego, CA) at 36 months. Typical sleep measurements were analyzed including the apnea–hypopnea index (AHI) and its components (components central apnea index [CAI], obstructive apnea index [OAI], mixed apnea index [MAI], hypopnea index [HI]), oxygen desaturation ≥4% index (ODI4), arousal index (ArI), and sleep stages in PSG. A PSG/PG core laboratory (Registered Sleepers, Leicester, NC) performed all scoring and was blinded to randomization assignment. Standard criteria were used to score sleep studies as detailed previously Costanzo Lancet [9]. However, hypopneas were not scored as central versus obstructive as part of this trial. Echocardiograms were performed at the time of sleep studies through 24 months and were interpreted by a core laboratory blinded to randomization assignment (United Heart and Vascular Center, St. Paul, MN). The former control patients, due to their 6-month delay of active therapy, had their last PSG and echocardiogram at 18 months of active therapy.
The protocol was approved by local ethics or institutional review boards; all patients provided written informed consent. The study was conducted in accordance with the Declaration of Helsinki, Good Clinical Practice, and ISO 14155:2011 and registered at ClinicalTrials.gov (Identifier: NCT01816776).
Statistical analysis
Observed effectiveness results are presented separately for each group (treatment and former control) based on months since therapy activation using the predefined per protocol population. Owing to the exploratory nature of this analysis, statistical test results for change from baseline are not included and imputation was not performed for missing data.
Multivariate analysis to find subgroups that may respond better or worse included identification of covariates that may have a univariate association with achieving at least 50% reduction in AHI from baseline to 24 months. Covariates with univariate p value less than 0.2 from logistic regression were included in a backward selection multivariate logistic regression model to determine a final model (p value of 0.1 used to retain variables in the model). The analysis was repeated using pooled data from the treatment and former control patients (after having therapy turned on) following 18 months of active therapy in order to have a larger sample size.
Safety, reported as freedom from related serious adverse events (SAEs) through 24 months, was summarized as a binomial proportion. Overall survival in the pooled group of treatment and former control patients who had therapy initiated was assessed using the Kaplan–Meier method, censoring patients who did not die at the date of study exit. Deaths were also summarized by modality (pump failure, sudden cardiac death, other cardiovascular and noncardiovascular), as adjudicated by an independent Clinical Events Committee (CEC).
The Kaplan–Meier method was used to estimate median time to battery depletion. SAS, version 9.4 (Cary, NC) was used for all analyses.
Results
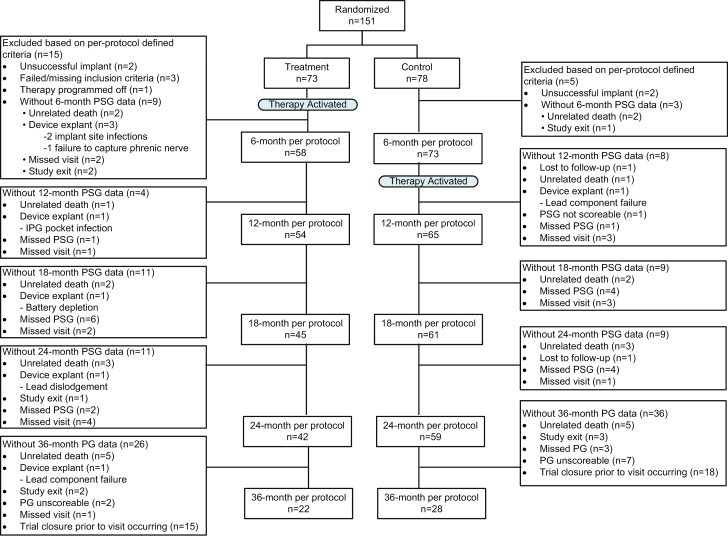
At the time of the Pivotal Trial closure, the original 151 patients had been followed for 32 ± 13 months (median = 35, maximum = 52 months) and 94 patients were ongoing at the time of trial closure. All subjects remaining in the trial at the time of closure had completed a minimum of 24 months of follow-up; however, 33 patients had not yet reached the 36-month visit. Reasons for patient exclusion or withdrawal from analysis (e.g. device, therapy, or implant related) at each visit are displayed in the CONSORT diagram Figure 1. Patient demographics for treatment and control patients at baseline as well as for pooled patients at both 24 months (n = 109) and 36 months (n = 60) are reported in Table 1. Patients with HF were on optimal guideline recommended therapy before enrollment and throughout the duration of follow-up [9]. Patient scheduled site follow-up visits occurred every 3 months per the trial design and additionally as needed to titrate therapy.
Figure 1.
CONSORT diagram. Composition of the per protocol population with sleep study data through the 36-month post-therapy initiation visit.
Table 1.
Baseline characteristics (per protocol population)
| Baseline characteristics | Treatment (n = 58) | Control (n = 73) | Pooled 24 months* (n = 109) | Pooled 36 months† (n = 60) |
|---|---|---|---|---|
| Age (years) | 63 ± 12 | 64 ± 14 | 64 ± 13 | 64 ± 13 |
| Men | 51 (88%) | 68 (93%) | 99 (91%) | 52 (87%) |
| White | 57 (98%) | 69 (95%) | 106 (97%) | 58 (97%) |
| Body mass index (kg/m2) | 30 ± 5 | 31 ± 7 | 31 ± 6 | 32 ± 6 |
| Neck circumference (cm) | 42 ± 5 | 43 ± 5 | 43 ± 5 | 42 ± 5 |
| Heart rate (beats per minute) | 76 ± 13 | 73 ± 14 | 75 ± 14 | 76 ± 15 |
| Systolic blood pressure (mm Hg) | 124 ± 18 | 124 ± 18 | 126 ± 18 | 126 ± 18 |
| Diastolic blood pressure (mm Hg) | 74 ± 11 | 75 ± 12 | 76 ± 11 | 76 ± 10 |
| Respiration rate (breaths per minute) | 18 ± 3 | 17 ± 3 | 17 ± 3 | 17 ± 3 |
| AHI (events/hour) | 50 ± 19 | 44 ± 17 | 47 ± 19 | 48 ± 19 |
| CAI (events/hour) | 32 ± 19 | 26 ± 16 | 29 ± 17 | 29 ± 17 |
| OAI (events/hour) | 2 ± 2 | 2 ± 3 | 2 ± 3 | 3 ± 3 |
| MAI (events/hour) | 3 ± 4 | 2 ± 3 | 3 ± 4 | 3 ± 4 |
| HI (events/hour) | 13 ± 11 | 13 ± 12 | 13 ± 12 | 15 ± 13 |
| Percent of sleep with oxygen saturation <90% (%) | 17 ± 18 | 11 ± 12 | 14 ± 16 | 16 ± 18 |
| Oxygen desaturation ≥4% index (events/hour) | 44 ± 22 | 37 ± 18 | 41 ± 20 | 42 ± 20 |
| Epworth Sleepiness Scale (points) | 11 ± 5 | 9 ± 6 | 10 ± 6 | 10 ± 5 |
| Atrial fibrillation | 22 (38%) | 29 (40%) | 39 (36%) | 19 (32%) |
| LVEF ≤ 45% | 32/57 (56%) | 42/70 (60%) | 59/106 (56%) | 34/60 (57%) |
| HF | 35 (60%) | 45 (62%) | 62 (57%) | 32 (53%) |
| New York Heart Association class | ||||
| I | 5 (9%) | 12 (16%) | 15 (14%) | 11 (18%) |
| II | 14 (24%) | 20 (27%) | 27 (25%) | 16 (27%) |
| III | 16 (28%) | 13 (18%) | 20 (18%) | 5 (8%) |
| IV | 0% | 0% | 0% | 0% |
| Coronary artery disease | 33 (57%) | 42 (58%) | 61 (56%) | 33 (55%) |
| Hypertension | 42 (72%) | 55 (75%) | 79 (72%) | 41 (68%) |
| Diabetes mellitus | 20 (34%) | 17 (23%) | 31 (28%) | 18 (30%) |
| Prior stroke | 4 (7%) | 5 (7%) | 7 (6%) | 4 (7%) |
| Renal impairment | 11 (19%) | 20 (27%) | 23 (21%) | 9 (15%) |
| Concomitant cardiac devices | 24 (41%) | 30 (41%) | 40 (37%) | 20 (33%) |
| Implantable cardioverter defibrillator | 14 (24%) | 13 (18%) | 20 (18%) | 12 (20%) |
| Cardiac resynchronization therapy defibrillator | 8 (14%) | 9 (12%) | 11 (10%) | 4 (6%) |
| Noncardiac resynchronization therapy pacemaker | 2 (3%) | 8 (11%) | 9 (8%) | 4 (6%) |
| Medications | ||||
| Angiotensin-converting enzyme inhibitor | 28 (48%) | 35 (48%) | 52 (48%) | 29 (48%) |
| Angiotensin receptor blocker | 9 (16%) | 13 (18%) | 17 (16%) | 8 (13%) |
| Aldosterone-blocking agent | 25 (43%) | 17 (23%) | 30 (28%) | 12 (20%) |
| Beta-blocker | 36 (62%) | 47 (64%) | 66 (61%) | 36 (60%) |
| Loop diuretic | 26 (45%) | 26 (36%) | 40 (37%) | 18 (30%) |
| Thiazide diuretic | 15 (26%) | 16 (22%) | 23 (21%) | 8 (13%) |
| Thiazide-like diuretic | 5 (9%) | 2 (3%) | 6% (6) | 4 (7%) |
| Antiarrhythmic | 4 (7%) | 7 (10%) | 8 (7%) | 2 (3%) |
| Digoxin | 10 (17%) | 12 (16%) | 17 (16%) | 8 (13%) |
Continuous variables reported as mean ± SD and categorical display n (percent).
*24-month visit represents 24 months of therapy for treatment and 18 for former control.
†The 36-month visit represents 36 months of therapy for treatment and 30 months for former control; 33 subjects remaining in the trial at time of FDA approval and study closure had not reached the 36-month visit.
Sleep metrics
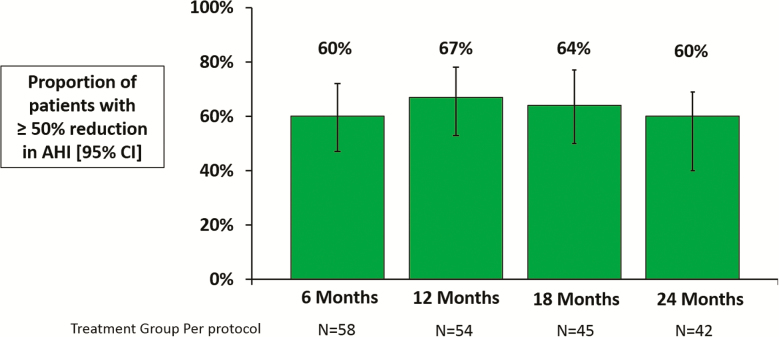
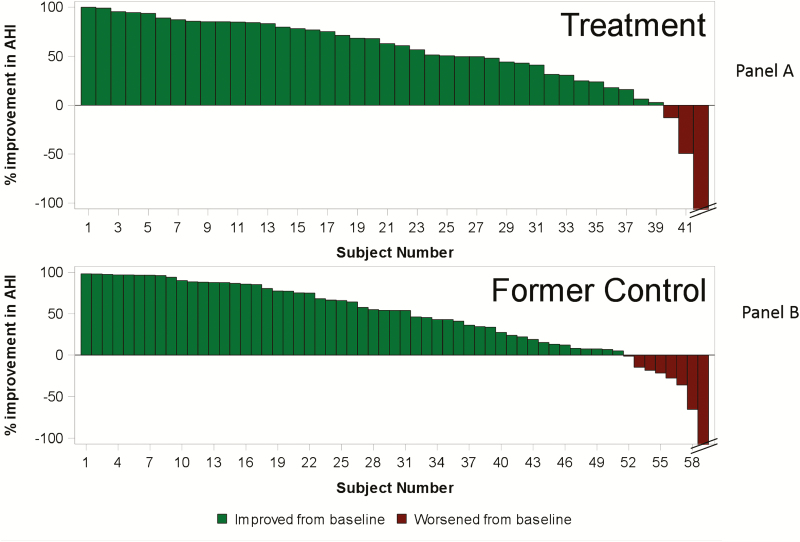
The primary effectiveness endpoint in the Pivotal Trial was a 50% or greater reduction in AHI from baseline. In the per protocol population, 60% of treatment group patients achieved this target at 6 months (11% in untreated control) and effectiveness remained high through 24 months of therapy, with 67%, 64%, and 60% at 12 (n = 54), 18 (n = 45), and 24 (n = 42) months (Figure 2). Furthermore, 93% of treatment group patients (39/42) had a reduction in AHI at the 24-month visit (Figure 3, panel A). Notably, these results were maintained with minimal additional titration visits in which programming changes were made (mean <1 programming change, median = 0) for each 6-month interval after the 6-month visit.
Figure 2.
Proportion of treatment group patients that achieved a ≥50% reduction in AHI at 6, 12, 18, and 24-months post-therapy initiation.
Figure 3.
Percentage change in AHI after 24 months of therapy for each treatment group patient (panel A) and 18 months of active therapy for each former control group patient (panel B). Panel A: The improvements represent the percentage change in AHI from baseline to 24 months of active therapy for each patient in the treatment group. Patients with any decrease in AHI from baseline are shown in green bars and patients with any increase in AHI from baseline are shown in red bars. Panel B: The improvements represent the percentage change in AHI from baseline (prior to therapy activation) to 18 months of active therapy for each patient in the former control group. Patients with any decrease in AHI from baseline are shown in green bars and patients with any increase in AHI from baseline are shown in red bars.
Multivariate analysis modeling of 24-month treatment group results retained three variables in the model to determine what characteristics may lead to therapy response (50% reduction in AHI): body mass index (BMI), patient height, and HI. The analysis suggests patients may be more likely to respond to PNS with lower BMI and higher HI. Repeating the multivariate analysis using the pooled treatment and former control patients following 18 months of active therapy also retained BMI as the most significant predictor. Other variables retained were sex (female more likely to respond) and oxygen desaturation ≥4% index (ODI4; higher ODI4 more likely to respond), however, only 10 females had data of which 9 experienced 50% AHI reduction compared to 53% (51/96) of males.
Analyses to determine subgroups that may be more or less likely to respond to therapy did not yield definitive results. However, the data demonstrate that most subgroups seem to improve to varying degree following PNS. The forest plot displaying response rates at 24 months of therapy for various subgroups (i.e. subjects <65 and ≥65) indicates a majority of subgroups appear to have reasonable chance to have at least half of the patients achieve a 50% or greater reduction in AHI at 24 months (Supplementary Figure S1).
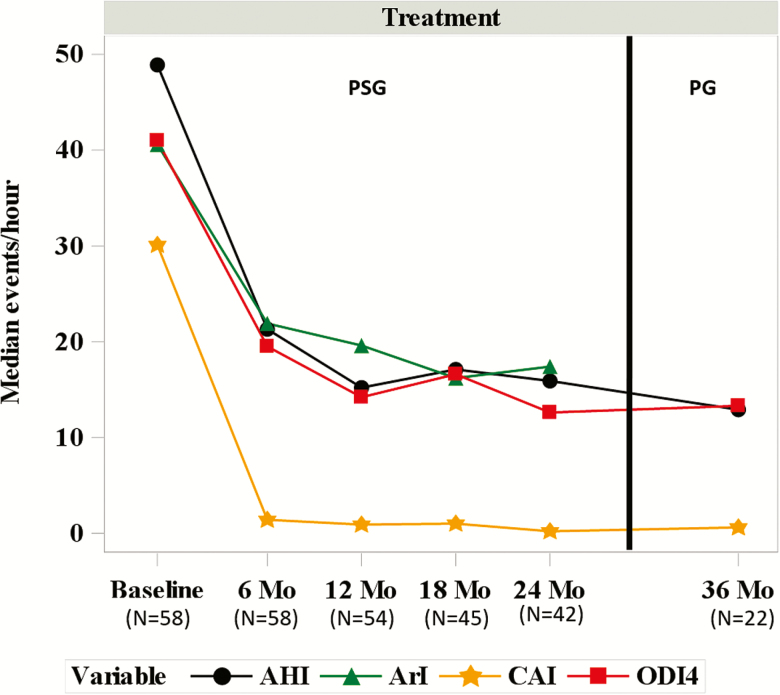
Sleep metrics show consistent improvement throughout the follow-up period. Median events per hour AHI [interquartile range] at 12, 18, and 24 months were 15 [9, 31], 17 [10, 25], and 16 [7, 37], respectively (Figure 4 and Table 2). Median central apnea index (CAI) was less than or equal to 1.0 event per hour and median OAI was 2–3 events per hour at 12 months and beyond. ODI4 improved similarly to the AHI improvement [median 41 [30, 56] events per hour at baseline and 14 [7, 26], 17 [8, 23], and 13 [5, 34] at 12, 18, and 24 months, respectively]. One of the unique findings of the Pivotal Trial was that PNS additionally improved the ArI at 6 months and long-term data through 24 months suggest persistent improvements in ArI (median 41 [34, 64] events per hour at baseline and 20 [14, 32], 16 [12, 30], and 17 [9, 29] at 12, 18, and 24 months, respectively). Moreover, median percentage of rapid eye movement (REM) sleep improved from 10% at baseline to 14%, 15%, and 19% at each follow-up visit. In addition, the percentage of sleep with oxygen saturation less than 90% was approximately half compared to baseline levels (median 9%) at each subsequent visit (medians ranged from 3% to 5%).
Figure 4.
Treatment group sleep indices by visit. Median AHI, ArI, CAI, and ODI4 are displayed by visit for subjects in the treatment group. A PSG was performed at baseline, 6, 12, 18, and 24 months. A PG was performed at 36 months (33 subjects had not reached this visit at time of study closure). AHI = apnea-hyponea index; ArI = arousal index; CAI = central apnea index; Mo = month; ODI4 = oxygen desaturation ≥4% index.
Table 2.
Treatment group PSG results by visit (per protocol population)
| Baseline (n = 58) | 6 months (n = 58) | 12 months (n = 54) | 18 months (n = 45) | 24 months (n = 42) | |
|---|---|---|---|---|---|
| Proportion with 50% reduction in AHI from baseline [% (95% CI)] | N/A | 60% (47% to 72%) | 67% (53% to 78%) | 64% (50% to 77%) | 60% (44% to 73%) |
| AHI (events/hour) | 49 [35, 60] | 21 [11, 35] | 15 [9, 31] | 17 [10, 25] | 16 [7, 37] |
| CAI (events/hour) | 30 [16, 43] | 1 [0, 7] | 1 [0, 3] | 1 [0, 3] | 0 [0, 3] |
| OAI (events/hour) | 2 [1, 3] | 4 [2, 9] | 2 [1, 7] | 2 [1, 6] | 3 [0, 8] |
| MAI (events/hour) | 1 [0, 4] | 0 [0, 0] | 0 [0, 0] | 0 [0, 1] | 0 [0, 0] |
| HI (events/hour) | 13 [3, 20] | 9 [3, 19] | 8 [5, 16] | 10 [5, 16] | 8 [3, 19] |
| ODI4 (events/hour) | 41 [30, 56] | 19 [8, 37] | 14 [7, 26] | 17 [8, 23] | 13 [5, 34] |
| ArI (events/hour) | 41 [34, 64] | 22 [16, 33] | 20 [14, 32] | 16 [12, 30] | 17 [9, 29] |
| Sleep with oxygen saturation <90% (%) | 9 [5, 27] | 5 [1, 20] | 3 [1, 14] | 4 [2, 12] | 5 [1, 21] |
| Sleep with oxygen saturation <90% (min) | 33 [16, 87] | 14 [2, 44] | 8 [2, 26] | 11 [5, 33] | 15 [3, 53] |
| Percent of sleep in REM (%) | 10 [6, 15] | 14 [5, 18] | 14 [6, 21] | 15 [9, 22] | 19 [8, 23] |
Continuous variables reported as median [interquartile range]. CI = confidence interval; N/A = not applicable; ODI4 = oxygen desaturation ≥4% index.
In addition, 22 treatment group patients that reached the 36-month visit prior to the primary trial closure underwent PG examinations, with results showing enduring and similar effectiveness measurements. Although PG results are not fully comparable with PSG results, at the 36-month visit the median number of events per hour were AHI = 13, CAI = 1, OAI = 4, MAI = 0, and HI = 6 (Table 3).
Table 3.
PG results at 36-month visit (per protocol population)
| Treatment 36-month active therapy (n = 22) |
Former control 30-month active therapy (n = 28) |
|
|---|---|---|
| AHI (events/hour) | 13 [8, 37] | 14 [8, 18] |
| CAI (events/hour) | 1 [0, 3] | 2 [0, 4] |
| OAI (events/hour) | 4 [1, 11] | 5 [2, 7] |
| MAI (events/hour) | 0 [0, 0] | 0 [0, 0] |
| HI (events/hour) | 6 [3, 10] | 5 [3, 10] |
Reported as median [interquartile range]. Not all subjects had reached the 36-month visit at the time of study closure. AHI = apnea-hypopnea index; ArI = arousal index; CAI = central apnea index; HI = hypopnea index; MAI = mixed apnea index; N/A = not applicable; OAI = obstructive apnea index; ODI4 = oxygen desaturation ≥4% index; REM = rapid eye movement.
Former control group patients had PSG results available through 18 months of active therapy and a PG at 30 months of active therapy. PSG analysis demonstrated results comparable to the treatment group, with 55%, 52%, and 53% of patients experiencing a 50% or greater improvement in AHI at 6 (n = 65), 12 (n = 61), and 18 (n = 59) months of active therapy, respectively (Table 4). Eighty-six percent (51/59) of patients had a reduction in AHI at 18 months of active therapy (Figure 3, panel B). Moreover, additional sleep metrics and parameters revealed comparable results for this group through 18 months of active therapy (Table 4). The available results from patients completing a PG prior to trial closure at 30 months of active therapy (n = 28) demonstrated similar improvement to that observed in the treatment group (median AHI = 14 events per hour, CAI = 2, OAI = 5, MAI = 0, and HI = 5) (Table 3).
Table 4.
Former control group PSG results by months of active therapy (per protocol population)
| Baseline (n = 73) | 6-month active therapy (n = 65) | 12-months active therapy (n = 61) | 18-month active therapy (n = 59) | |
|---|---|---|---|---|
| Proportion with 50% reduction in AHI from baseline [% (95% CI)] | N/A | 55% (43% to 67%) | 52% (40% to 64%) | 53% (40% to 65%) |
| AHI (events/hour) | 42 [32, 60] | 18 [8, 32] | 22 [10, 34] | 17 [6, 30] |
| CAI (events/hour) | 22 [10, 35] | 2 [0, 5] | 1 [0, 5] | 1 [0, 3] |
| OAI (events/hour) | 2 [1, 4] | 2 [1, 8] | 3 [1, 10] | 2 [1, 7] |
| MAI (events/hour) | 1 [0, 5] | 0 [0, 1] | 0 [0, 1] | 0 [0, 1] |
| HI (events/hour) | 11 [4, 18] | 7 [3, 15] | 9 [4, 19] | 8 [3, 18] |
| ODI4 (events/hour) | 39 [26, 57] | 17 [7, 30] | 18 [9, 32] | 15 [5, 27] |
| ArI (events/hour) | 37 [25, 56] | 19 [13, 30] | 19 [14, 34] | 17 [12, 35] |
| Sleep with oxygen saturation <90% (%) | 8 [2, 19] | 4 [1, 16] | 4 [1, 11] | 4 [0, 15] |
| Sleep with oxygen saturation <90% (min) | 28 [8, 63] | 13 [2, 36] | 11 [3, 27] | 13 [2, 33] |
| Percent of sleep in REM | 11 [6, 16] | 16 [10, 20] | 16 [7, 22] | 16 [9, 21] |
Continuous variables reported as median [interquartile range]. CI = confidence interval; N/A = not applicable; ODI4 = oxygen desaturation ≥4% index.
Echocardiographic metrics
Left ventricular ejection fraction (LVEF) showed small but significant improvements for the treatment group throughout follow-up in the subset of patients with HF, baseline ejection fraction less than or equal to 45% and no permanent atrial fibrillation. Although median LVEF at baseline was 27% (n = 19), paired changes from baseline to each visit reveal slight improvements in absolute percentages of 4%, 8%, and 6% at 12 (n = 17), 18 (n = 14), and 24 (n = 12) months after therapy activation, respectively. Former control group experienced smaller levels of improvement, with a median LVEF at baseline of 34% (n = 26) and paired changes to each visit of 2% at both 12 (n = 22) and 18 (n = 21) months of active therapy.
Safety
This study provides safety data of the remedē System therapy in a long-term setting. SAEs adjudicated as related to the implant procedure, device, or delivered therapy occurred in 10% of patients (15/151) through the 24-month visit, which is similar to the 9% rate reported through 12 months. The types of events experienced are expected for an implantable neurostimulation device, including concomitant device interactions (three patients), lead component failure (two), lead dislodgment (two), implant site infection (two), impending pocket erosion (two), inadequate lead position (one), lead displacement (one), feeling a sensation in an area remote from the diaphragm (one), implant site hematoma (one), noncardiac chest pain (one), and elevated transaminase (one) (Table 5). All procedure-related events resolved with routine care and all device- or therapy-related events resolved with remedē System revisions or programming. Further safety data beyond the 24 months follow-up will be collected as part of the ongoing remedē System Post Approval Study (NCT03425188), which is following patients from the Pivotal Trial throughout 5 years post implant. However, no additional related SAEs were reported between 24 and 36 months in the Pivotal Trial.
Table 5.
Implant procedure-, device-, or delivered-therapy-related serious adverse events through 24 months of follow-up
| Event | Pooled groups (N = 151) |
|---|---|
| Any event | 15 (10%) |
| Concomitant device interaction | 3 (2%) |
| Lead component failure | 2 (1%) |
| Lead dislodgment | 2 (1%) |
| Implant site infection | 2 (1%) |
| Impending pocket erosion | 2 (1%) |
| Inadequate lead position | 1 (1%) |
| Lead displacement | 1 (1%) |
| Feeling sensation in an area remote from the diaphragm | 1 (1%) |
| Implant site hematoma | 1 (1%) |
| Noncardiac chest pain | 1 (1%) |
| Elevated transaminase | 1 (1%) |
Reported as number with event (percent). All events resolved with routine care, system revisions, or programming changes.
The median months to battery depletion estimated from Kaplan–Meier analysis was 45.1 months for a left stimulation lead and 34.6 for a right stimulation lead, with a combined estimate of 39.4 months. If a device was replaced prior to full battery depletion, the date of the replacement was used in the calculation so the actual duration may be underestimated. There were three implant site infections following the initial implant procedure, however, no implant site infections have been reported following any remedē System implantable pulse generator replacement or lead revision procedure.
Survival
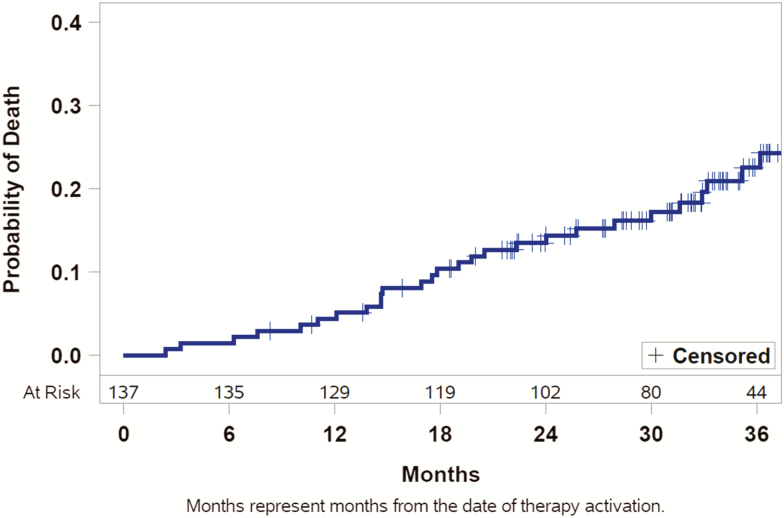
Overall survival through 3 years is illustrated in a Kaplan–Meier curve (Figure 5) for all patients who had therapy activated (both treatment and former control groups pooled). Through 24 months, the independent CEC adjudicated deaths to the following modalities: 8 (5% of 151 patients) noncardiovascular, 6 (4%) pump failure including 2 control patients prior to therapy activation, 4 (3%) experienced sudden cardiac death (2 out of hospital cardiac arrests, one due to ventricular arrhythmia [per device interrogation], and one other cardiovascular death), and 1 (1%) intracranial bleed subsequent to a fall. Beyond 24 months, five additional pump failure deaths, three noncardiovascular deaths, one sudden cardiac death (asystole in the setting of end stage HF [per device interrogation]), and one other cardiovascular death had been reported prior to study closure. The survival rates remain constant over time, suggesting no additional risk based on duration of therapy. Critically, no deaths have been associated with the device, therapy, or implant procedure.
Figure 5.
Kaplan–Meier curve of mortality. Kaplan–Meier curve showing estimated mortality through 36 months of active therapy using the pooled treatment and former control groups. Patients who did not die were censored at last contact if they did not reach 36 months of active therapy.
Discussion
This study provides the first data on long-term safety and effectiveness of the novel PNS for the treatment of moderate-to-severe CSA through 36 months of patient follow-up. This study shows therapy effectiveness remains consistent and reliable for 36 months of active therapy. Ninety-three percent of patients in the Pivotal Trial treatment group and 86% of the former control group had a sustained reduction in AHI at the 24-month PSG. CAI was less than or equal to 1 event per hour at 12 months and beyond and more importantly the PNS system improved the ArI in this long-term study [median 41 [34, 64] events per hour at baseline and 20 [14, 32], 16 [12, 30], and 17 [9, 29] at 12, 18, and 24 months]. Moreover, PNS therapy improved REM sleep and the percentage and minutes of sleep with oxygen saturation less than 90%, which is an independent predictor of all-cause mortality in chronic HF as recently demonstrated [12]. LVEF showed small but measurable improvements with this therapy.
Above all, this study demonstrates evidence of enduring safety using PNS as a long-term CSA therapy, with SAEs adjudicated to the implant procedure, device, or delivered therapy in 10% of patients through 24 months. This is only one percentage point higher than at 12 months, indicating most complications occur in the first year. All of the events resolved with routine care, remedē System revisions or programming changes. Importantly, although the patient populations are quite different, the rate of subjects experiencing sudden cardiac death is lower than the control group in the SERVE-HF trial. However, this finding should be confirmed in larger trials with particular attention paid to adjudicating any cardiac death. Overall survival demonstrates constant survival rates during the trial follow-up, suggesting no additional risk with increasing duration of PNS therapy, as depicted in the Kaplan–Meier survival curve in Figure 5. This is very important as clinicians consider appropriate therapy options for their patients with moderate-to-severe CSA.
As the first description of the remedē System by Augostini et al. [13], interest was high in this novel approach of treating CSA, but interventionalists and sleep specialists wanted to see evidence of longer-term effectiveness and safety. The randomized controlled Pivotal Trial achieved the primary effectiveness endpoint with an AHI reduction from baseline of 50% or greater at 6 months of follow-up that was significantly higher in the treatment group (51%) than in the control group (11%) in the intent-to-treat population [9]. Also, 91% of patients were free from SAEs related to the implant procedure, device, or delivered therapy through 12 months [9]. Subsequent analysis of 12 months follow-up demonstrated sustained at least 50% reduction in AHI in 67% of the treatment group and 55% of the former control group experienced at least 50% reduction in AHI following 6 months of active therapy [10]. In addition, patient global assessment was markedly or moderately improved at 6 and 12 months in 60% of treatment patients [10]. The data in this manuscript show long-term performance of PNS remains consistent with previously reported data.
REM sleep is essential for any human. Patients with CSA and cardiovascular disease, especially those with HF, are often deprived of REM sleep and experience poor sleep quality [14]. Although the debate on this topic continues, REM sleep deprivation is associated with emergence of anxiety, irritability, hallucinations, impairment in concentration, and an increase in appetite, which are aspects that can be found in patients with HF. Reduced sleep quality is not only associated with impaired quality of life but also with increased mortality in patients with HF [15]. Less restorative sleep, changes in sympathovagal balance and impaired resetting of important reflexes may contribute to worse cardiovascular outcomes in HF patients with CSA [14], but CSA treatment has been shown to improve the proportion of sleep in REM [15]. This analysis from the remedē System Pivotal Trial shows similar improvements for REM sleep through the use of PNS in a long-term setting, whereas a recent publication demonstrated PNS to be associated with benefits on HF quality of life [16]. This is of particular importance, as more than 50% of patients with CSA have either overt or yet undiagnosed underlying HF [4].
The forest plot (Supplementary Figure S1) showing the proportion of subjects achieving at least 50% reduction in AHI for subgroups of interest suggests that all subgroups displayed have a reasonable probability of subjects reaching 50% improvement in AHI. The few subgroups that have an estimated proportion below 50% are associated with wide confidence intervals that have the upper limit extend beyond 50%, meaning the true proportion may exceed 50%. Our study results show that PNS using the remedē System can be performed with high procedural success and low rates of SAEs, close to what is reported in CRT trials [17]. Moreover, the battery longevity of the remedē System is close to 4 years for a stimulation lead implanted on the left side where the majority of the study implants occurred and within the expected range of 17–55 months based on high and low-energy use conditions; the replacement procedure is comparable to other cardiac devices [11].
The 24-month multivariate analysis showed a lower BMI and higher HI to be predictors of better response to PNS. Higher HI may indicate patients that are easier to treat with the assumption that a majority of the hypopneas are central. However, as hypopneas were not scored as central versus obstructive in this trial, we cannot determine whether that is true. In general, known risk factors for OSA include obesity, large neck circumference, male gender, increasing age, alcohol use, smoking, menopausal status, and black race so it may not be surprising that a higher BMI could limit PNS [18, 19]. We repeated the analysis using pooled (treatment and former control) 18-month results and found BMI, sex, and ODI4 to be predictors of response. The interpretation for the sex finding is limited by small sample size (9/10 females had 50% AHI reduction). The ODI4 may simply suggest that patients with more severe disease more easily improve AHI by 50%. The common finding of BMI in both analyses may suggest that patients with high BMI are less likely to attain 50% reduction in AHI following PNS, although a particular BMI cutoff where that happens was not identified. However, future investigations are needed to confirm and clarify these findings.
LVEF slightly improved. Similar ranges of LVEF improvements have been reported in earlier trials when CSA was effectively controlled [20–22], but whether this finding is of any clinical relevance will be a cornerstone of future clinical trials [8].
This is the first trial to provide long-term data through 36 months on the treatment of CSA with PNS and its results are consistent with previously reported data. Importantly, the enduring improvement in sleep indices and patient safety may indicate that this novel technology can be an option for long-term treatment of CSA. Therapy effectiveness was shown to be reproducible in the former control group with comparable sustained therapy affect supporting enduring and reliable treatment. This study adds confirmation to available data investigating PNS for CSA treatment and supports PNS to be an effective and safe treatment improving sleep and quality of life in CSA patients.
One of our limitations in the study design is that the control group was followed only for 6 months prior to activating therapy so control data are somewhat limited. However, at the time of the study design, it was felt that depriving patients with symptomatic CSA of any treatment for longer than 6 months was unethical. Importantly, in assessing long-term safety and effectiveness, the ability to pool the former control patients with the therapy group provided more patients in which to assess the safety profile as well as effectiveness of the therapy. Another limitation is that not all patients completed 36 months at the time the Pivotal Trial was closed following FDA approval. However, the data collected through 24 and 36 months indicate continued effectiveness and safety based on the available PG results and reported adverse events (AEs). Moreover, additional AEs after 24 months may be reported in the ongoing remedē System Post Approval Study (NCT03425188) that is following patients from the Pivotal Trial through 5 years post implant.
In conclusion, this 36-month long-term analysis establishes that unilateral transvenous PNS with the remedē System in patients with CSA is associated with a high response to therapy as indicated by sustained improvement in key sleep indices through 36-months follow-up as well as echocardiography parameters through 24 months. Also, the remedē System demonstrates a strong safety profile through 36 months. The beneficial effects of long-term PNS in patients with CSA appear to be sustained through 36 months with no new safety concerns.
Supplementary Material
Acknowledgments
The authors wish to acknowledge the technical expertise of United Heart and Vascular Clinic’s Echocardiography Core Laboratory, supervised by Dr. Alan Bank (St. Paul, MN) as well as Registered Sleepers Sleep, Inc. Core Laboratory, supervised by Tim Winchester (Leicester, NC).
Funding
Conflict of interest statement. This trial was funded by Respicardia. The authors have following conflicts of interest to declare: Fox: no conflict of interest to declare regarding this manuscript. Oldenburg: no conflict of interest to declare regarding this manuscript. Javaheri: personal fees from Respicardia. Ponikowski: research grants from Respicardia and Coridea; personal fees from Respicardia, Coridea. Augostini: Consultant/Speaker Bureau, Respicardia; Advisory Board, Respicardia and Philips. Goldberg: research grants and consulting fees from Respircardia. Stellbrink: research grants from Respicardia, St. Jude Medical, Biotronik, Medtronic, and Sorin/LivaNova; advisory board for Sorin/LivaNova. McKane and Meyer: Employees of Respicardia. Abraham: research grants to institution from Respicardia; personal fees from Respicardia (consulting, Advisory Board). Costanzo: personal fees from Respicardia (consulting and study principal investigator for remedē System Pivotal Trial).
References
- 1. Linz D, et al. The importance of sleep-disordered breathing in cardiovascular disease. Clin Res Cardiol. 2015;104(9):705–718. [DOI] [PubMed] [Google Scholar]
- 2. Anker SD, et al. Sleep-disordered breathing and cardiovascular disease. Indian Heart J. 2016;68(Suppl 1):S69–S76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Fox H, et al. Prevalence of sleep-disordered breathing and patient characteristics in a coronary artery disease cohort undergoing cardiovascular rehabilitation. J Cardiopulm Rehabil Prev. 2016;36(6):421–429. [DOI] [PubMed] [Google Scholar]
- 4. Arzt M, et al. Phenotyping of sleep-disordered breathing in patients with chronic heart failure with reduced ejection fraction-the SchlaHF registry. J Am Heart Assoc. 2017;6(12):1– 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Javaheri S, et al. Sleep apnea: types, mechanisms, and clinical cardiovascular consequences. J Am Coll Cardiol. 2017;69(7):841–858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Cowie MR, et al. Adaptive servo-ventilation for central sleep apnea in systolic heart failure. N Engl J Med. 2015;373(12):1095–1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Oldenburg O, et al. Addendum zum Positionspapier „Schlafmedizin in der Kardiologie. Update 2014”. Somnologie. 2017;21(1):51–52. [Google Scholar]
- 8. Oldenburg O, et al. Adaptive servoventilation to treat sleep-disordered breathing in cardiac patients. Somnologie. 2017;21(1):82–83. [Google Scholar]
- 9. Costanzo MR, et al. ; remedé System Pivotal Trial Study Group. Transvenous neurostimulation for central sleep apnoea: a randomised controlled trial. Lancet. 2016;388(10048):974–982. [DOI] [PubMed] [Google Scholar]
- 10. Costanzo MR, et al. ; remedē System Pivotal Trial Study Group. Sustained 12 month benefit of phrenic nerve stimulation for central sleep apnea. Am J Cardiol. 2018;121(11):1400–1408. [DOI] [PubMed] [Google Scholar]
- 11. Fox H, et al. Long-term experience with first-generation implantable neurostimulation device in central sleep apnea treatment. Pacing Clin Electrophysiol. 2017;40(5):498–503. [DOI] [PubMed] [Google Scholar]
- 12. Oldenburg O, et al. Nocturnal hypoxaemia is associated with increased mortality in stable heart failure patients. Eur Heart J. 2016;37(21):1695–1703. [DOI] [PubMed] [Google Scholar]
- 13. Augostini R. A novel approach to the treatment of central sleep apnea in patients with heart failure. Herzschrittmacherther Elektrophysiol. 2012;23(1):9–13. [DOI] [PubMed] [Google Scholar]
- 14. Türoff A, et al. Sleep duration and quality in heart failure patients. Sleep Breath. 2017;21(4):919–927. [DOI] [PubMed] [Google Scholar]
- 15. Heider K, et al. Adaptive servo-ventilation and sleep quality in treatment emergent central sleep apnea and central sleep apnea in patients with heart disease and preserved ejection fraction. Clin Res Cardiol. 2018;107(5):421–429. [DOI] [PubMed] [Google Scholar]
- 16. Costanzo MR, et al. ; remedē® System Pivotal Trial Study Group. Phrenic nerve stimulation to treat patients with central sleep apnoea and heart failure. Eur J Heart Fail. 2018;20(12):1746–1754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Al-Majed NS, et al. Meta-analysis: cardiac resynchronization therapy for patients with less symptomatic heart failure. Ann Intern Med. 2011;154(6):401–412. [DOI] [PubMed] [Google Scholar]
- 18. Malhotra A, et al. Obstructive sleep apnoea. Lancet. 2002;360(9328):237–245. [DOI] [PubMed] [Google Scholar]
- 19. Davies RJ, et al. Neck circumference and other clinical features in the diagnosis of the obstructive sleep apnoea syndrome. Thorax. 1992;47(2):101–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Aurora RN, et al. The treatment of central sleep apnea syndromes in adults: practice parameters with an evidence-based literature review and meta-analyses. Sleep. 2012;35(1):17–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Fietze I, et al. Bi-level positive pressure ventilation and adaptive servo ventilation in patients with heart failure and Cheyne-Stokes respiration. Sleep Med. 2008;9(6):652–659. [DOI] [PubMed] [Google Scholar]
- 22. Pepperell JC, et al. A randomized controlled trial of adaptive ventilation for Cheyne-Stokes breathing in heart failure. Am J Respir Crit Care Med. 2003;168(9):1109–1114. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.