Abstract
Study Objectives
Both periodic limb movements during sleep (PLMS) and arousals are associated with sympathetic nervous system activation and may be arrhythmogenic. We hypothesize a temporal relationship exists between individual PLMS, particularly with arousal, and nonsustained ventricular tachycardia (NSVT) events.
Methods
A bidirectional time-stratified case-crossover design was used to assess temporal associations between PLMS and NSVT during sleep in 49 Osteoporotic Fractures in Men Sleep Study participants with NSVT in a community-based cohort (n = 2,911). Sleep time was divided into approximate 30-min segments. For each NSVT (n = 141), we selected a preceding 30-s hazard period and three randomly chosen 30-s control periods from sleep within the same segment and evaluated for PLMS, respiratory events, minimum saturation, and arousals. Odds ratios and 95% confidence intervals—OR (95% CI)—were determined by conditional logistic regression; covariates included EEG arousals, minimum saturation, and respiratory events in the same hazard/control period.
Results
Participants with NSVT were 79.5 ± 6.2 years with a PLMS index of 32.1 (IQR: 10.1, 61.4) and apnea–hypopnea index of 17.1 (IQR: 9.4, 26.1). PLMS without arousal were not significantly associated with NSVT (OR = 0.80, 95% CI: 0.41–1.59). PLMS with arousal were associated with NSVT in unadjusted analyses (OR = 2.50, 95% CI: 1.11–5.65) and after adjustment (OR = 2.31, 95% CI: 1.02–5.25). Arousals associated with PLMS were associated with NSVT in unadjusted (OR = 2.84, 95% CI: 1.23–6.56) and adjusted analyses (OR = 2.61, 95% CI: 1.13–6.05).
Conclusions
PLMS with (but not without) arousals are temporally associated with a greater than twofold higher odds of subsequent NSVT episodes. PLMS-related arousals may be physiologically important ventricular arrhythmia triggers.
Clinical Trial Registration
ClinicalTrials.gov, NCT00070681.
Keywords: periodic limb movements, arrhythmia, nonsustained tachycardia
Statement of Significance.
Nonsustained ventricular tachycardia is an arrhythmia that is associated with worse cardiovascular outcomes. Identifying new causes or contributors to arrhythmia can help with better arrhythmia prevention strategies. Periodic limb movements and arousals, brief periods of brain awakening, increase sympathetic nervous system activation. Sympathetic nervous system activation increase the chance for arrhythmias. This study evaluated whether periodic limb movements are linked with nonsustained ventricular tachycardia and found that in older men periodic limb movements during sleep with arousal double the odds of having a subsequent nonsustained ventricular tachycardia.
Introduction
Accumulating data support an association of sleep disruption and adverse cardiovascular outcomes. Although the majority of these data have addressed relationships between sleep disordered breathing (SDB) and cardiovascular disease, emerging clinic-based and epidemiologic data suggest an independent association of periodic limb movements during sleep (PLMS) and increased cardiovascular morbidity including cardiac arrhythmia [1–4]. Purported mechanisms linking PLMS and cardiac arrhythmia include increased sympathetic outflow, impaired baroreflex function, altered heart rate variability, and altered chemoreflex sensitivity [5, 6]. Individual limb movements comprising PLMS events are associated with immediate, discrete increases in blood pressure of 10–20 mmHg and rises in heart rate of ~10 bpm [7, 8]. Notably, limb movements with accompanying arousals appear to elicit a more pronounced cardiovascular physiologic perturbation in blood pressure and heart rate than those without accompanying arousals and may be more likely to be associated with cardiac arrhythmia [9].
Expanding upon prior work [2], we analyzed a large cohort of older men with polysomnography-identified PLMS and cardiac arrhythmia to investigate the association of PLMS and discrete nonsustained ventricular tachycardia (NSVT) episodes. We hypothesize that PLMS, particularly when associated with arousals, are temporally associated with NSVT events even after accounting for SDB influences.
Methods
Participants and overall cohort study design
Analyses for this study were conducted using data from the MrOS Sleep Study, an ancillary study the Osteoporotic Fractures in Men (MrOS) study (http://mrosdata.sfcc-cpmc.net). The MrOS study design, methods, and demographics have been previously published [10–12]. The institutional review board for each site and the study coordinating center granted ethics approval and written informed consent was obtained from all participants.
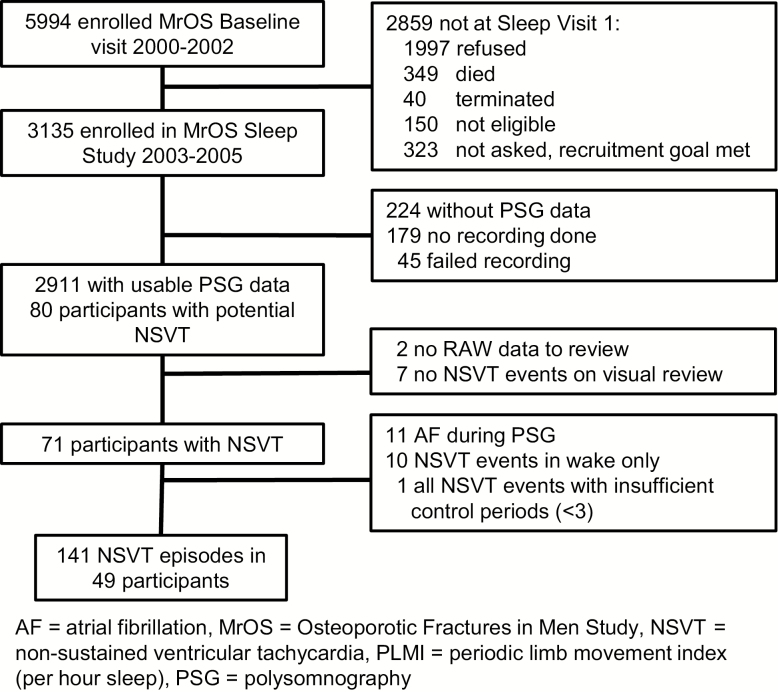
The MrOS study enrolled 5,994 community-living men aged 65 and older, able to ambulate without assistance from another person, and without bilateral hip replacement history from six centers (Birmingham, Alabama; Minneapolis, Minnesota; Monongahela Valley near Pittsburgh, Pennsylvania; Palo Alto, California; Portland, Oregon; San Diego, California) [10, 11]. The MrOS Sleep Study recruited 3,135 participants, a subset of the MrOS study, from December 2003 to March 2005 for a comprehensive sleep assessment. MrOS Sleep Study participants were screened for nightly sleep equipment use including continuous positive airway pressure or bi-level positive airway pressure, mouthpiece for snoring or sleep apnea, or nocturnal oxygen therapy; those who could not forgo device use during polysomnography (PSG) recording were excluded. Of the 2,859 men who did not participate in the MrOS Sleep Study, 349 died before the sleep visit, 40 had already terminated the study, 323 were not asked because recruitment goals had already been met, 150 were ineligible, and 1,997 refused. Of the 3,135 MrOS Sleep Study participants recruited, 179 did not participate in polysomnography (PSG) secondary to refusal or contemporaneous SDB treatment, and 45 men had a failed sleep study (1.5%), resulting in 2,911 participants.
Only those participants with NSVT episodes occurring during a sleep epoch were retained in the final analytic cohort. Eighty participants were identified with NSVT possibly occurring during sleep—defined as at least three consecutive heartbeats originating below the atrioventricular node with a rate of at least 100 beats per minute. Visual review of the raw data was conducted to verify NSVT occurrence and timing; two subjects did not have available raw data files for review and seven other participants did not have NSVT after visual review and were excluded. In addition, because of overlap and synergies of sympathetic nervous system activation and risk factors common to atrial fibrillation and NSVT, 11 participants with atrial fibrillation during the sleep study were excluded. The final analytic sample consisted of 2,911 individuals of whom 49 participants had 141 episodes of NSVT (Figure 1).
Figure 1.
Study recruitment, retention, and attrition.
Case crossover study design
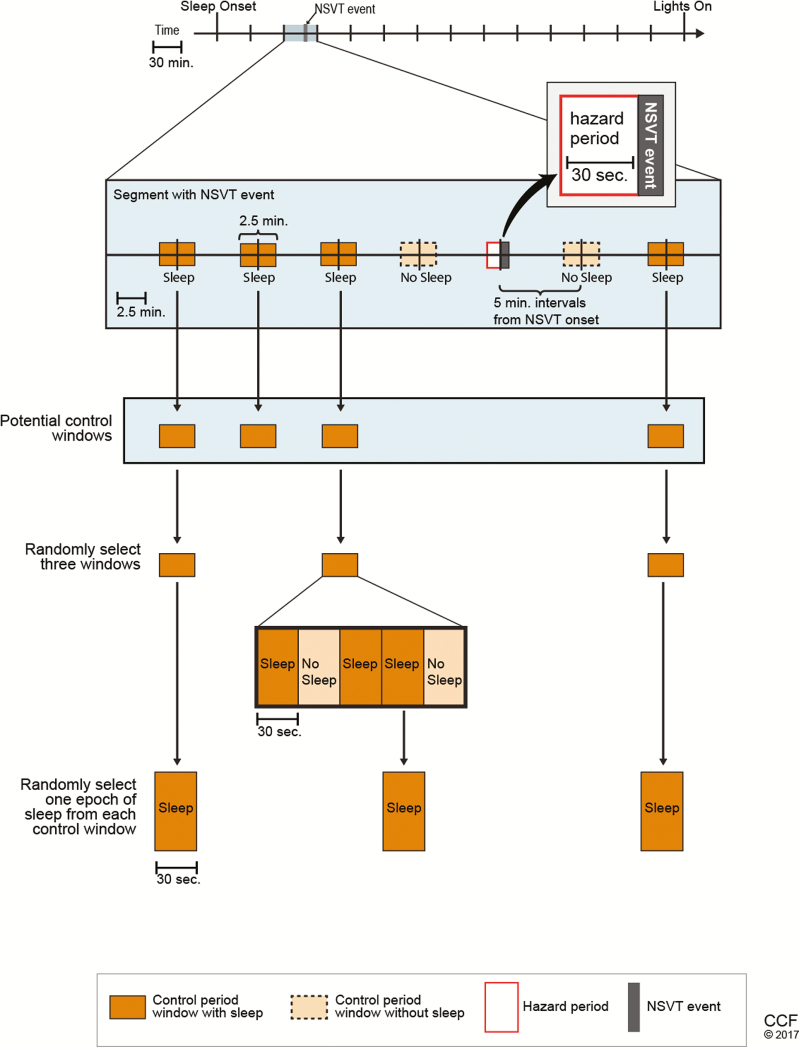
We utilized the bidirectional time-stratified case-crossover study design in which each individual serves as his own control with a hazard period directly preceding the event of interest (NSVT) and control periods sampled at regular intervals from both before and after the NSVT event (Figure 2). This approach is well-suited to determine relationships between outcomes with a discrete onset (NSVT) and exposures with intermittent timing and immediate and transient effects on risk (PLMS). Because both hazard and control periods are taken from the same person, each participant serves as his own control for variables which remain constant throughout the night (e.g. age, sex, comorbidities, medications). Since PLMS vary in distribution across the sleep period (i.e. declining across the sleep period [13]), the bidirectional time-stratified case-crossover design was used to address confounding by time-varying trends of PLMS (PLMS distribution occurrence skewed toward earlier compared with later time points).
Figure 2.
Bidirectional case-crossover design used in this analysis. Each segment with an NSVT event was analyzed for hazard period (30 s preceding the onset of NSVT) and control periods selected from 2.5-min control windows spaced at 5-min intervals from the NSVT event. Three 2.5-min control windows with at least 1 30-s epoch of sleep is selected. If there is more than 1 epoch of sleep within the control window, 1 epoch of sleep is randomly selected to serve as the control period.
The study design is presented in Figure 2. First, the sleep period, defined as sleep onset to last epoch of sleep, was divided into approximately equal 30-min. Segments were of equal duration in each participant but differed between participants (range: 30.0–33.6 min) because of modest differences in sleep duration as assessed using standardized approaches [14]. Thirty-second hazard and control time windows were chosen based on duration of physiologic changes seen on sleep studies in prior work [9]. Within each such time segment with NSVT, a 30-s hazard period directly before the NSVT event and three control periods were selected by a random number generator. The control periods were selected in a bidirectional manner from sleep epochs in the 2.5-min window centered at 5-min intervals from the epoch with NSVT event onset. The 5-min intervals are equally spaced within the 30-min segment centered on the epoch with NSVT onset and can be either before or after the NSVT. If there were more than three potential control periods identified in the time segment, three control periods were randomly selected. NSVT events with fewer than three control periods were excluded from the main analysis (seven events excluded) but were included in sensitivity analyses. Each 30-s hazard and control period was examined for PLMS, respiratory events, and arousals. An EEG arousal, respiratory event, and/or PLMS were considered to be within the hazard or control window if any portion was within that 30-s epoch.
Polysomnography data collection and scoring
The recording montage consisted of C3/A2 and C4/A1 electroencephalograms (EEG), bilateral electrooculograms, a bipolar submental electromyogram, thoracic and abdominal respiratory inductance plethysmography, airflow, finger pulse oximetry, lead II electrocardiogram (250 Hz), body position, and bilateral anterior tibialis piezoelectric movement sensors. Additional information on polysomnography recording acquisition is in the Supplementary Material.
PLMS were scored in accordance with the 1993 American Academy of Sleep Medicine criteria in which individual leg movements are defined by a clear amplitude increase from baseline lasting between 0.5 and 5 s. Leg movements were considered to be periodic if at least four movements occurred in succession 5–90 s apart. This approach resulted in a PLMS index that is highly correlated (r = 0.83) with the PLMS index derived using the 2013 [14] scoring criteria using leg EMG sensors [15]. Leg movements after respiratory events were not included unless part of a movement cluster with two or more leg movements occurring independent of respiratory events [16]. Arousals were scored according to American Sleep Disorders Association criteria, defined as an abrupt shift in electroencephalogram frequency lasting at least 3 s and starting after at least 10 continuous seconds of sleep [17]. In rapid eye movement sleep, electroencephalogram frequency shift required a simultaneous increase in chin electromyography amplitude. Periodic limb movements during sleep associated with arousals (PLMA) were defined as a PLMS which occurs during or within 0.5 s of an arousal [14, 18]. EEG arousals were subclassified as either associated with PLMS (ArousalsPLMS)—an arousal occurring during or within 0.5 s of a PLMS—or not (Arousalsno PLMS) [18]. Apnea was defined as 10 or more seconds of complete or near complete airflow cessation [19]. Hypopneas required a minimum 30% reduction in breathing amplitude compared with baseline accompanied by arterial desaturation of at least 3% lasting for 10 or more seconds [19]. Respiratory-associated arousals were defined as arousals occurring within 3 s of the end of a respiratory event. SDB severity was determined by the apnea–hypopnea index (AHI) which was calculated as the total number of apneas and hypopneas associated with a ≥3% desaturation per hour of sleep [20].
Outcome measures
Any participant with three or more consecutive ventricular ectopic beats was marked for secondary review. NSVT was defined as at least three consecutive beats arising from below the atrioventricular node with a rate >100 beats/min and lasting less than 30 s. Only those NSVT episodes which occurred during a sleep epoch and were at least 5 min apart—which limits potentiation from rapidly sequential NSVT events—were retained (n = 96 events in 41 participants) and comprised the final analytic sample.
Other measures
All participants completed questionnaires at the sleep visit, which included demographics, medical history, smoking status, and alcohol use questions. Medications used in the last month were recorded (details in the Supplementary Material). Physical activity was assessed using the Physical Activity Scale for the Elderly [21]. Coronary artery disease (CAD) was defined by a history of myocardial infarction, angioplasty, and/or coronary artery bypass graft surgery. Cerebrovascular disease was defined as a history of stroke, transient ischemic attack, or cerebrovascular.
Body mass index (BMI, kg/m2) was calculated from body weight and height. Cholesterol was measured during the MrOS baseline visit 3 years prior.
Statistical analysis
Participant characteristics were summarized as mean ± standard deviation (SD), median (interquartile range, IQR), or n (%) and compared using Fisher’s exact tests for categorical variables, t-tests for normally distributed continuous variables, and Wilcoxon rank sum for continuous variables with skewed distributions.
Conditional logistic regression was used to determine the relationship between the PLMS exposures (PLMS and PLMA) and subsequent NSVT likelihood; results are reported as an odds ratio (95% confidence interval). Because respiratory events and arousals can trigger sympathetic nervous system activation, models were further adjusted for minimum oxygen saturation and respiratory events and arousals during the same hazard/control period as covariates. Multivariable models examining (1) PLMA or (2) arousals associated with PLMS (ArousalsPLMS) included the following covariates from the same hazard/control period: respiratory events, minimum oxygen saturation, and Arousalsno PLMS. For the primary analysis, each sleep-related event was coded as a binary variable (present or absent) if any part of it was within the 30-s hazard/control period.
Methods for secondary and sensitivity analyses are presented in the Supplementary Material. Secondary analyses include stratified analyses based on periodic limb movement index (≥5 vs. <5 and ≥15 vs. <15) and subset analyses based on coronary artery disease diagnosis. Interactions between PLMS and respiratory events or arousals were examined. Interaction between those participants with one event versus several were assessed. Sensitivity analyses include evaluating only one NSVT event per participant, including all events with at least one control period, and evaluating events (PLMS, PLMA, respiratory events, and arousals) as graded exposures. All significance levels reported are two-sided and all analyses were conducted using R version 3.4.3 [22].
Results
Participant characteristics
The 49 participants with NSVT in the study cohort (n = 2,911) were predominantly Caucasian (98%), had an average age of 79.5 ± 6.2 years and body mass index of 26.5 ± 4.2 kg/m2 with a PLMI of 32.1 (IQR: 10.1, 61.4), PLMA index of 2.2 (IQR: 1.0, 8.4), and apnea–hypopnea index of 17.1 (IQR: 9.4, 26.1); further demographic, relevant medical history and medication use, and polysomnography characteristics are given in Table 1. The participants in the final analytic sample—those with NSVT—compared with those not included were significantly older (mean 79.5 vs. 76.3 years) with higher prevalence of CAD (47.9% vs. 25.9%) and heart failure (14.3% vs. 5.8%), and a lower weekly alcohol consumption (65.3% vs. 46.3% <1 drink). Although the analytic cohort had increased PLMS and PLMA indices (32.1 [IQR: 10.1, 61.4] vs. 23.8 [IQR: 3.2, 56.1] and 2.2 [IQR: 1.0, 8.4] vs. 1.8 [IQR: 0.3, 5.5], respectively), no significant differences were found in any sleep variables.
Table 1.
Baseline cohort characteristics
| Analytic sample with NSVT (n = 49) | Sample without NSVT (n = 2,862) | P | |
|---|---|---|---|
| Age | 79.5 ± 6.2 | 76.3 ± 5.5 | <0.001 |
| Race | 0.52 | ||
| Caucasian | 48 (98.0) | 2593 (90.6) | |
| African American | 0 (0.0) | 99 (3.5) | |
| Asian | 0 (0.0) | 84 (2.9) | |
| Other | 1 (2.0) | 86 (3.0) | |
| Body mass index, kg/m2 | 26.5 ± 4.2 | 27.2 ± 3.8 | 0.18 |
| History of | |||
| Coronary artery disease* | 23 (47.9) | 740 (25.9) | 0.001 |
| Heart Failure | 7 (14.3) | 167 (5.8) | 0.03 |
| Cerebrovascular disease† | 5 (10.2) | 315 (11.0) | >0.99 |
| Hypertension | 23 (46.9) | 1430 (50.0) | 0.77 |
| Diabetes mellitus | 7 (14.3) | 380 (13.3) | 0.83 |
| Renal disease | 0 (0.0) | 30 (1.0) | >0.99 |
| Current medication use | |||
| Anti-arrhythmic‡ | 27 (55.1) | 1513 (52.9) | 0.87 |
| Anti-hypertensive | 34 (69.4) | 1799 (62.9) | 0.43 |
| Dopaminergics | 1 (2.0) | 75 (2.6) | >0.99 |
| Dopamine antagonists§ | 2 (4.1) | 31 (1.1) | 0.11 |
| SSRI/SNRI | 1 (2.0) | 126 (4.4) | 0.72 |
| Current smoker | 1 (2.0) | 56 (2.0) | 0.63 |
| Alcohol use, drinks/week | 0.08 | ||
| <1 | 32 (65.3) | 1326 (46.3) | |
| 1–13 | 16 (32.7) | 1366 (47.7) | |
| 14+ | 1 (2.0) | 155 (5.4) | |
| PASE physical activity score | 125.7 [68.2, 162.4] | 141.1 [95.9, 187.3] | 0.08 |
| Rapid eye movement % | 18.8 ± 8.2 | 19.2 ± 6.6 | 0.66 |
| Apnea–hypopnea index | 17.1 [9.4, 26.1] | 16.7 [8.8, 28.6] | 0.83 |
| PLMI | 32.1 [10.1, 61.4] | 23.8 [3.2, 56.1] | 0.08 |
| PLMAI | 2.2 [1.0, 8.4] | 1.8 [0.3, 5.5] | 0.051 |
| Arousal index | 20.9 [16.3, 28.3] | 21.2 [15.3, 29.5] | 0.90 |
Values presented as n(%), mean ± standard deviation, or median [interquartile range]. PLMAI, periodic limb movement with arousal index; PLMI, periodic limb movement during sleep index; SNRI, selective norepinephrine reuptake inhibitor; SSRI, selective serotonin reuptake inhibitors.
*Includes myocardial infarction, coronary bypass surgery, and angioplasty.
†Includes stroke, transient ischemic event, or past history of cerebrovascular disease.
‡Includes calcium channel blockers, nonophthalmic beta-blockers, cardiac glycosides, and anti-arrhythmic medications—cardiac sodium channel blockers and potassium channel blockers.
§Includes antipsychotics, domperidone, prochlorperazine, perphenazine, chlorpromazine, metoclopramide, and tricyclic antidepressants.
NSVT and periodic limb movements during sleep
Reflective of the overall sleep state distribution, 20% of NSVT events occurred during rapid eye movement sleep which comprised 18.8 ± 8.2% of the sleep period. Most NSVT events occurred during N1 or N2 sleep (71.6%), and 8.5% during N3 sleep. Among participants with NSVT events, the majority had one NSVT event (n = 30), nine participants had two NSVT events, four participants had three to five NSVT events, and six had more than five NSVT events.
PLMS with or without associated arousal occurred in 34 of the 141 hazard periods (24.1%) and 90 of the 423 control periods (21.3%). Of 124 PLMS events, 36 (29.0%) were PLMA. PLMA occurred in 14 of 141 (9.9%) hazard periods and 22 of 423 (5.2%) control periods.
Case-crossover analyses
Primary analysis results are displayed in Table 2. There was no statistically significant association between PLMS—reflecting combined PLMS both with and without arousals—and NSVT events in unadjusted or adjusted analyses (OR = 1.41, 95% CI: 0.73–2.75 and OR = 1.43, 95% CI: 0.73–2.81, respectively). Moreover, when only PLMS not associated with arousals were examined, there was no association identified with NSVT. In contrast, PLMA were associated with a greater than twofold higher subsequent NSVT odds in unadjusted models (OR = 2.50, 95% CI: 1.11–5.65), which persisted after adjusting for respiratory events, minimum oxygen saturation and Arousalsno PLMS (OR = 2.31, 95% CI: 1.02–5.25).
Table 2.
Odds ratios (95% confidence intervals) of nonsustained ventricular tachycardia after exposure
| Unadjusted odds ratio (95% CI) | Multivariable adjusted odds ratio (95% CI) | |
|---|---|---|
| PLMS type | ||
| No PLMS | Reference | Reference |
| PLMS with or without associated arousal | 1.41 (0.73–2.75) | 1.43 (0.73–2.81)* |
| PLMS without arousal | 0.80 (0.41–1.55) | 0.80 (0.41–1.59)* |
| PLMA | 2.50 (1.11–5.65) | 2.31 (1.02–5.25)† |
| Respiratory events | 1.17 (0.75–1.84) | 1.19 (0.76–1.87)‡ |
| Minimum oxygen saturation | 0.99 (0.98–1.00) | 0.99 (0.98–1.00)§ |
| Arousal | ||
| No arousal | Reference | Reference |
| All arousals | 1.00 (0.61–1.63) | 0.96 (0.59–1.57)|| |
| Arousalsno PLMS | 0.61 (0.34–1.11) | 0.65 (0.36–1.19)¶ |
| ArousalsPLMS | 2.84 (1.23–6.56) | 2.61 (1.13–6.05)† |
Arousals PLMS, arousals associated with PLMS; Arousalsno PLMS, arousals not associated with PLMS; CI, confidence interval; PLMA, periodic limb movement associated with arousal; PLMS, periodic limb movement during sleep.
*Adjusted for respiratory events, minimum saturation, and arousals.
†Adjusted for respiratory events, minimum saturation, and Arousalsno PLMS.
‡Adjusted for minimum saturation, arousals, and any PLMS.
§Adjusted for respiratory events, arousals, and any PLMS.
||Adjusted for respiratory events, minimum saturation, and any PLMS.
¶Adjusted for respiratory events, minimum saturation, and PLMA.
Arousals (including all arousals regardless of association to PLMS or not) were not significantly associated with NSVT events. When arousals were stratified into those associated with PLMS or not, both adjusted and unadjusted models of Arousalsno PLMS were not significantly associated with NSVT (OR = 0.61, 95% CI: 0.34–1.11 and OR = 0.65, 95% CI: 0.36–1.19, respectively). Only ArousalsPLMS were significantly associated with NSVT in unadjusted models (OR = 2.84, 95% CI: 1.23–6.56), and this significance persisted after adjustment for Arousalsno PLMS, respiratory events, and minimum oxygen saturation (OR = 2.61, 95% CI: 1.13–6.05). Respiratory events (apneas and hypopneas) and minimum oxygen saturation were not associated with NSVT events in unadjusted or adjusted models.
Secondary and sensitivity analysis are presented in the Supplementary Material. Stratified analyses identified associations between PLMA and ArousalsPLMS relative to subsequent NSVT. These associations are preserved in those with PLMS index ≥5 (Supplementary eTable 1) and PLMS index ≥ 15 (Supplementary eTable 2), although with loss of significance for PLMA likely secondary to decreased power. Results were stronger in those without prevalent heart disease (Supplementary eTable 3). Results were robust to sensitivity analyses (Supplementary Results).
Discussion
This is the first study of which we are aware to evaluate the temporal relationship between PLMS with and without arousal on arrhythmogenesis. Our findings provide novel insights pertaining to PLMA as a potential trigger of ventricular arrhythmogenesis. We identify that in this cohort of older men PLMA are associated with an approximate twofold higher odds of subsequent NSVT compared with similar sleep periods (matched in sleep time) without PLMA. Correspondingly, only arousals associated with PLMS were temporally associated with NSVT. In contrast, no such association was observed for PLMS without associated arousals or arousals not associated with PLMS. The strength of associations persisted after adjustment for potential confounding by other sleep-related disturbances such as respiratory events, minimal period saturation, and arousals without associated PLMS. This association between PLMA and arousals associated with PLMS relative to NSVT was strongest in those without prevalent cardiovascular disease. Results were robust to multiple sensitivity analyses. These findings are of potential clinical importance given that NSVT is associated with a twofold increased risk of sudden cardiac death in men without heart disease NSVT as well as predicts sudden cardiac death after myocardial infarction [23, 24]. Our sensitivity analyses support associations between PLMA and NSVT in individuals without known cardiac disease, a group that may not be routinely recognized as at increased risk for arrhythmias.
There is a biological rationale for the observed association of PLMS, especially with arousal, and cardiac arrhythmia. Limb movements, particularly when associated with arousal, are associated with autonomic nervous system dysregulation in sleep which persists into the wake state, and this upregulation of autonomic activity has been implicated as a strong etiologic basis in arrhythmia generation [9, 25–29]. PLMS distort the normal autonomic nervous system balance characterized by sympathetic nervous system dominance over parasympathetic output and decreased heart rate variability during PLMS events [28–30]. Autonomic tone changes associated with PLMS are also observed before ventricular tachycardia onset [25, 26, 31–33]. Taken together, these data suggest that PLMS may exacerbate cardiac structural changes and provoke arrhythmias.
Another mechanism that may link PLMS and NSVT in those without self-reported cardiac disease may be via subclinical cardiac disease. It is possible that recurrent surges in blood pressure with PLMA results in cardiac remodeling, increasing propensity for NSVT [34, 35]. Interestingly, we observed a stronger association in individuals without known cardiac disease. This could reflect competitive risk factors, or potentially protective effects of common cardiac medications (e.g. beta-blockers) on arrhythmogenesis, particularly in those without known cardiac disease.
An inherent strength of this study is the case-crossover design which is ideally suited to examine temporal associations for outcomes with a discrete onset such as NSVT. Additionally, since all participants served as their own controls, all characteristics that are constant during the one-night observation period—demographics, medical history, medication use, etc.—have inherent perfect subject matching. In this epidemiologic cohort of community-dwelling older men, data integrity was maximized with standardized protocols and procedures.
There are several study limitations. Given the cohort characteristics, findings may not be generalizable beyond men aged 65 years and older, a population at high risk for cardiac arrhythmias. There is also possibility of misclassification due to analysis of polysomnography data from a single-night. Although night-to-night variability in PLMS frequency may limit assessment of absolute level of risk attributable to PLMS, it is unlikely that the relative risk estimates would substantially vary [36]. Periods with ectopy (including other NSVT events in the same sleep period as the index NSVT event) were not excluded from eligibility for control periods and may have biased the results toward the null. Leg movements were assessed using piezoelectric sensors, which do not reflect the standard anterior tibialis electromyography sensors and did not allow quantification of amplitude changes in movement. The limb scoring used does not correspond with the current standard, which impacts the generalizability of the study to studies using different scoring approaches; therefore, results should be interpreted accordingly. There is also the possibility of residual confounding by events outside the hazard period which may influence arrhythmogenesis. The small number of NSVT episodes in this study may have had insufficient power to detect a meaningful temporal association between predictors (e.g. respiratory obstructive events, hypoxemia) and NSVT. Competing risks in those with cardiac disease may have precluded identification of a significant association in this group. Additional studies that confirm and expand on this work are needed.
Future investigation should be focused on addressing the role of PLMS on arrhythmia risk in other populations, including women and younger populations. Clarification of the relationship between PLMA and cardiac structural changes via echocardiography may further characterize the pathophysiology of PLMA-induced arrhythmogenesis. Finally, further work is needed to clarify whether targeted PLMA-directed interventions mitigate cardiac arrhythmogenesis.
In conclusion, PLMA and arousals associated with PLMS are temporally associated with a twofold increase in odds of subsequent nocturnal NSVT in older men with persistence after numerous sensitivity analyses. Results were stronger in those without prevalent heart disease.
Funding
The Osteoporotic Fractures in Men (MrOS) Study is supported by National Institutes of Health funding. The following institutes provide support: the National Institute on Aging (NIA), the National Institute of Arthritis and Musculoskeletal and Skin Diseases (NIAMS), the National Center for Advancing Translational Sciences (NCATS), and NIH Roadmap for Medical Research under the following grant numbers: U01 AG027810, U01 AG042124, U01 AG042139, U01 AG042140, U01 AG042143, U01 AG042145, U01 AG042168, U01 AR066160, and UL1 TR000128. The National Heart, Lung, and Blood Institute (NHLBI) provides funding for the MrOS Sleep ancillary study “Outcomes of Sleep Disorders in Older Men” under the following grant numbers: R01 HL071194, R01 HL070848, R01 HL070847, R01 HL070842, R01 HL070841, R01 HL070837, R01 HL070838, R01 HL070839, R21 HL108226, and R01 HL109493. S.R. was supported by R35HL135818. The analysis and manuscript composition was supported by NIH T32 HL/NS 07913 Sleep Medicine Neurobiology and Epidemiology and the ASPIRE fellowship. The sponsors did not participate in the design or conduct of the study; collection, analysis, and interpretation of the data; or preparation, review, or approval of the article.
Supplementary Material
Acknowledgments
Investigators in the Outcomes of Sleep Disorders in Older Men study (MrOS Sleep): Coordinating Center (California Pacific Medical Center Research Institute and University of California, San Francisco): K.L. Stone (Principal Investigator), D.C. Bauer (co-Investigator), S.R. Cummings (co-Investigator), N. Goldschlager (co-Investigator), P. Varosy (co-Investigator), K. Yaffe (co-Investigator), P.M. Cawthon (co-Investigator), R. Fullman (Project Director), R. Benard, T. Blackwell, L. Concepcion, J. Diehl, S. Ewing, C. Fox, M. Jaime-Chavez, E. Kwan, S. Litwack, W. Liu, L.Y. Lui, J. Schneider, R. Scott, D. Tanaka, J. Ziarno; Administrative Center (Oregon Health & Sciences University): E. Orwoll (Principal Investigator), K. Phipps (co-Investigator), L. Marshall (co-Investigator), J. Babich Blank (Project Director), L. Lambert, B. Chan, D. Neevel; University of Alabama, Birmingham: C.E. Lewis (Principal Investigator), J. Shikany (co-Investigator), P. Johnson (Project Director), C. Oden, S. House, N. Webb, K. Hardy, S. Felder, J. Wilkoff, J. King, T. Johnsey, M. Young, J. Smith, C. Sassaman, C. Collier, C. Atkins; University of Minnesota: K. Ensrud (Principal Investigator), H. Fink (co-Investigator), D. King (Program Manager), N. Michaels (Asst. Program Manager), N. Nelson (Clinic Coordinator), C. Bird, D. Blanks, F. Imker-Witte, K. Moen, M. Paudel, M. Slindee; Stanford University: M. Stefanick (Principal Investigator), A. Hoffman (co-Investigator), K. Kent, B. Malig, S. Wong; University of Pittsburgh: J. Cauley (Principal Investigator), J. Zmuda (co-Investigator), M. Danielson (Study Administrator), L. Harper (Project Director), L. Buck (Clinic Coordinator), M. Nasim, D. Cusick, M. Gorecki, N. Watson, C. Bashada, C. Newman; University of California, San Diego: E. Barrett-Connor (Principal Investigator), S. Ancoli-Israel (co-Investigator), T. Dam (co-Investigator), ML Carrion-Petersen (Project Director), P. Miller, N. Kamantigue; Central Sleep Reading Center: S. Redline (Principal Investigator), S. Surovec (Project Administrator), N. Scott (Chief Polysomnologist), M. Rueschman and N. Johnson (Programmer Analyst), J. Arnold (Polysomnologist), R. Nawabit (Polysomnologist), J. Romaniuk (Polysomnologist), S. Seicean (Polysomnologist).
Conflict of interest statement. A.M.M., R.D.M., J.B., L.W., K.M., K.L.S., E.B.C., B.B.K., J.W.W., S.R., and M.A.M. have no relevant disclosures. R.M. reports her institution has received positive airway pressure machines and equipment from Philips Respironics and Resmed for use in research. R.M. has received honorarium from the American Academy of Sleep Medicine and royalties from Up to Date.
Contributor Information
for the Osteoporotic Fractures in Men (MrOS) Study Group:
K L Stone, D C Bauer, S R Cummings, N Goldschlager, P Varosy, K Yaffe, P M Cawthon, R Fullman, R Benard, T Blackwell, L Concepcion, J Diehl, S Ewing, C Fox, M Jaime-Chavez, E Kwan, S Litwack, W Liu, L Y Lui, J Schneider, R Scott, D Tanaka, J Ziarno, E Orwoll, K Phipps, L Marshall, J Babich Blank, L Lambert, B Chan, D Neevel, C E Lewis, J Shikany, P Johnson, C Oden, S House, N Webb, K Hardy, S Felder, J Wilkoff, J King, T Johnsey, M Young, J Smith, C Sassaman, C Collier, C Atkins, K Ensrud, H Fink, D King, N Michaels, N Nelson, C Bird, D Blanks, F Imker-Witte, K Moen, M Paudel, M Slindee, M Stefanick, A Hoffman, K Kent, B Malig, S Wong, J Cauley, J Zmuda, M Danielson, L Harper, L Buck, M Nasim, D Cusick, M Gorecki, N Watson, C Bashada, C Newman, E Barrett-Connor, S Ancoli-Israel, T Dam, M L Carrion-Petersen, P Miller, N Kamantigue, S Redline, S Surovec, N Scott, M Rueschman, N Johnson, J Arnold, R Nawabit, J Romaniuk, and S Seicean
References
- 1. Koo BB, et al. ; Osteoporotic Fractures in Men (MrOS) Study Group. Association of incident cardiovascular disease with periodic limb movements during sleep in older men: outcomes of sleep disorders in older men (MrOS) study. Circulation. 2011;124(11):1223–1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Koo BB, et al. ; Osteoporotic Fractures in Men (MrOS) Study Group. Periodic limb movements during sleep and cardiac arrhythmia in older men (MrOS sleep). J Clin Sleep Med. 2014;10(1):7–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. May AM, et al. ; Osteoporotic Fractures in Men (MrOS) Study Group. Longitudinal relationships of periodic limb movements during sleep and incident atrial fibrillation. Sleep Med. 2016;25:78–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Nannapaneni S, et al. Periodic limb movements during sleep and their effect on the cardiovascular system: is there a final answer? Sleep Med. 2014;15(4):379–384. [DOI] [PubMed] [Google Scholar]
- 5. Cuellar NG. The effects of periodic limb movements in sleep (PLMS) on cardiovascular disease. Heart Lung. 2013;42(5):353–360. [DOI] [PubMed] [Google Scholar]
- 6. Gottlieb DJ, et al. Restless legs syndrome and cardiovascular disease: a research roadmap. Sleep Med. 2017;31:10–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Pennestri MH, et al. Nocturnal blood pressure changes in patients with restless legs syndrome. Neurology. 2007;68(15):1213–1218. [DOI] [PubMed] [Google Scholar]
- 8. Siddiqui F, et al. Rise of blood pressure with periodic limb movements in sleep and wakefulness. Clin Neurophysiol. 2007;118(9):1923–1930. [DOI] [PubMed] [Google Scholar]
- 9. Winkelman JW. The evoked heart rate response to periodic leg movements of sleep. Sleep. 1999;22(5):575–580. [DOI] [PubMed] [Google Scholar]
- 10. Orwoll E, et al. Design and baseline characteristics of the osteoporotic fractures in men (MrOS) study—a large observational study of the determinants of fracture in older men. Contemp Clin Trials. 2005;26(5):569–585. [DOI] [PubMed] [Google Scholar]
- 11. Blank JB, et al. Overview of recruitment for the osteoporotic fractures in men study (MrOS). Contemp Clin Trials. 2005;26(5):557–568. [DOI] [PubMed] [Google Scholar]
- 12. Mehra R, et al. Nocturnal arrhythmias across a spectrum of obstructive and central sleep-disordered breathing in older men: outcomes of sleep disorders in older men (MrOS sleep) study. Arch Intern Med. 2009;169(12):1147–1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Pollmächer T, et al. Periodic leg movements (PLM): their relationship to sleep stages. Sleep. 1993;16(6):572–577. [PubMed] [Google Scholar]
- 14. Berry RB, et al. The AASM Manual for the Scoring of Sleep and Associated Events: Rules, Terminology and Technical Specifications, Version 2.0.2. Darien, IL: American Academy of Sleep Medicine; 2013. [Google Scholar]
- 15. Claman DM, et al. ; Study of Osteoporotic Fractures Research Group. Periodic leg movements are associated with reduced sleep quality in older men: the MrOS Sleep Study. J Clin Sleep Med. 2013;9(11):1109–1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Fulda S, et al. Characteristics and determinants of respiratory event associated leg movements. Sleep. 2018; 41( 2):zsx206. [DOI] [PubMed] [Google Scholar]
- 17. American Sleep Disorders Association. EEG arousals: scoring rules and examples: a preliminary report from the Sleep Disorders Atlas Task Force of the American Sleep Disorders Association. Sleep. 1992;15(2):173–184. [PubMed] [Google Scholar]
- 18. Ferri R, et al. An evidence-based analysis of the association between periodic leg movements during sleep and arousals in restless legs syndrome. Sleep. 2015;38(6):919–924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Berry RB, et al. ; American Academy of Sleep Medicine. Rules for scoring respiratory events in sleep: update of the 2007 AASM manual for the scoring of sleep and associated events. Deliberations of the sleep apnea definitions task force of the American Academy of Sleep Medicine. J Clin Sleep Med. 2012;8(5):597–619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Quan SF, et al. The Sleep Heart Health Study: design, rationale, and methods. Sleep. 1997;20(12):1077–1085. [PubMed] [Google Scholar]
- 21. Washburn RA, et al. The Physical Activity Scale for the Elderly (PASE): development and evaluation. J Clin Epidemiol. 1993;46(2):153–162. [DOI] [PubMed] [Google Scholar]
- 22. R Core Team. R: A Language and Environment for Statistical Computing. Vienna, Austria: R Foundation for Statistical Computing; 2013. ISBN 3-900051-07-0; 2014. http://www.R-project.org/. [Google Scholar]
- 23. Bikkina M, et al. Prognostic implications of asymptomatic ventricular arrhythmias: the Framingham Heart Study. Ann Intern Med. 1992;117(12):990–996. [DOI] [PubMed] [Google Scholar]
- 24. de Sousa MR, et al. Non-sustained ventricular tachycardia as a predictor of sudden cardiac death in patients with left ventricular dysfunction: a meta-analysis. Eur J Heart Fail. 2008;10(10):1007–1014. [DOI] [PubMed] [Google Scholar]
- 25. Osaka M, et al. Changes in autonomic activity preceding onset of nonsustained ventricular tachycardia. Ann Noninvasive Electrocardiol. 1996;1(1):3–11. [DOI] [PubMed] [Google Scholar]
- 26. Shusterman V, et al. Autonomic nervous system activity and the spontaneous initiation of ventricular tachycardia. ESVEM Investigators. Electrophysiologic study versus electrocardiographic monitoring trial. J Am Coll Cardiol. 1998;32(7):1891–1899. [DOI] [PubMed] [Google Scholar]
- 27. Ferri R, et al. Heart rate and spectral EEG changes accompanying periodic and non-periodic leg movements during sleep. Clin Neurophysiol. 2007;118(2):438–448. [DOI] [PubMed] [Google Scholar]
- 28. Lavoie S, et al. Influence of sleep stage and wakefulness on spectral EEG activity and heart rate variations around periodic leg movements. Clin Neurophysiol. 2004;115(10):2236–2246. [DOI] [PubMed] [Google Scholar]
- 29. Sforza E, et al. Time-dependent variation in cerebral and autonomic activity during periodic leg movements in sleep: implications for arousal mechanisms. Clin Neurophysiol. 2002;113(6):883–891. [DOI] [PubMed] [Google Scholar]
- 30. Guggisberg AG, et al. The significance of the sympathetic nervous system in the pathophysiology of periodic leg movements in sleep. Sleep. 2007;30(6):755–766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Antoine S, et al. Pathophysiologic mechanisms in heart failure: role of the sympathetic nervous system. Am J Med Sci. 2017;353(1):27–30. [DOI] [PubMed] [Google Scholar]
- 32. Cohn JN, et al. Cardiac remodeling—concepts and clinical implications: a consensus paper from an international forum on cardiac remodeling. Behalf of an International Forum on Cardiac Remodeling. J Am Coll Cardiol. 2000;35(3):569–582. [DOI] [PubMed] [Google Scholar]
- 33. Iaccarino G, et al. Role of the sympathetic nervous system in cardiac remodeling in hypertension. Clin Exp Hypertens. 2001;23(1–2):35–43. [DOI] [PubMed] [Google Scholar]
- 34. Giannaki CD, et al. Periodic limb movements in sleep contribute to further cardiac structure abnormalities in hemodialysis patients with restless legs syndrome. J Clin Sleep Med. 2013;9(2):147–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Mirza M, et al. Frequent periodic leg movement during sleep is an unrecognized risk factor for progression of atrial fibrillation. PLoS ONE. 2013;8(10):e78359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Ferri R, et al. Night-to-night variability of periodic leg movements during sleep in restless legs syndrome and periodic limb movement disorder: comparison between the periodicity index and the PLMS index. Sleep Med. 2013;14(3):293–296. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.