Abstract
Sleep and brain glutamatergic signaling are homeostatically regulated. Recovery sleep following prolonged wakefulness restores efficient functioning of the brain, possibly by keeping glutamatergic signaling in a homeostatic range. Evidence in humans and mice suggested that metabotropic glutamate receptors of subtype-5 (mGluR5) contribute to the brain’s coping mechanisms with sleep deprivation. Here, proton magnetic resonance spectroscopy in 31 healthy men was used to quantify the levels of glutamate (Glu), glutamate-to-glutamine ratio (GLX), and γ-amino-butyric-acid (GABA) in basal ganglia (BG) and dorsolateral prefrontal cortex on 3 consecutive days, after ~8 (baseline), ~32 (sleep deprivation), and ~8 hours (recovery sleep) of wakefulness. Simultaneously, mGluR5 availability was quantified with the novel radioligand for positron emission tomography, [18F]PSS232, and the blood levels of the mGluR5-regulated proteins, fragile X mental retardation protein (FMRP) and brain-derived neurotrophic factor (BDNF) were determined. The data revealed that GLX (p = 0.03) in BG (for Glu: p < 0.06) and the serum concentration of FMRP (p < 0.04) were increased after sleep loss. Other brain metabolites (GABA, N-acetyl-aspartate, choline, glutathione) and serum BDNF levels were not altered by sleep deprivation (pall > 0.6). By contrast, the night without sleep enhanced whole-brain, BG, and parietal cortex mGluR5 availability, which was normalized by recovery sleep (pall < 0.05). The findings provide convergent multimodal evidence that glutamatergic signaling is affected by sleep deprivation and recovery sleep. They support a role for mGluR5 and FMRP in sleep–wake regulation and warrant further studies to investigate their causality and relevance for regulating human sleep in health and disease.
Clinical Trial Registration: www.clinicaltrials.gov (study identifier: NCT03813082)
Keywords: PET-MRS imaging, sleep homeostasis, FMRP, BDNF, plasticity
Statement of Significance.
The molecular substrates of increased sleep need and intensity after prolonged wakefulness—referred to as sleep homeostasis—are currently unknown. The glutamatergic system has recently moved to center stage in the search for the molecules underlying sleep homeostasis, yet the evidence is virtually limited to preclinical studies. By combining multi-modal brain imaging (simultaneous proton magnetic resonance spectroscopy and positron emission tomography) and blood sampling, we demonstrate convergent changes in different markers of glutamatergic signaling across prolonged wakefulness and recovery sleep in humans. The findings suggest that glutamatergic signaling in distinct regions of the human brain play an important role in sleep homeostasis and highlight the possible importance of sleep in regulating glutamatergic system activity in health and disease.
Introduction
Sleep has been conserved throughout evolution and is generally assumed to fulfill vital biological functions. This notion is corroborated by the general principle referred to as sleep homeostasis, which assumes that the lack of sleep is predictably compensated by increased sleep need and intensity as reflected by electroencephalographic (EEG) slow-wave activity (SWA; activity in the ~ 0.75–4.5 Hz range) in non-rapid-eye-movement (NREM) sleep [1]. Prevailing current hypotheses posit that sleep homeostasis serves the normalization of synaptic long-term potentiation (LTP) occurring during wakefulness, by synaptic long-term depression (LTD) occurring during NREM sleep [2–4].
Glutamate (Glu) plays an essential role in the fine-tuned molecular processes underpinning LTP and LTD [5–7]. Overstimulation of metabotropic and ionotropic Glu receptors by excess extracellular Glu is a major culprit of neuronal excitotoxicity and contributes to neuropsychiatric and neurodevelopmental disorders that can be exacerbated by inadequate sleep [8–10]. Suggesting an important contribution of glutamatergic signaling to sleep homeostasis and a role for sleep in keeping extracellular Glu in a homeostatic range, Glu levels in the frontal cortex of freely moving rats rose during prolonged wakefulness and rapid-eye-movement (REM) sleep and decreased during NREM sleep [11]. No comparable data are currently available in humans.
Nevertheless, two key players of glutamatergic signaling were recently identified that may orchestrate synaptic plasticity across the sleep–wake cycle: Homer1a and metabotropic Glu receptors of subtype-5 (mGluR5). Homer1a uncouples mGluR5 from their downstream signaling partners, which leads to synaptic LTD [12–14]. Biochemical, proteomic, and imaging studies in mice demonstrated that Homer1a and signaling from group-I mGluRs (mGluR1/5) drive the homeostatic downscaling of excitatory synapses during sleep [15]. In humans, mGluR5 show high expression in brain regions regulating sleep, and their functional availability was increased after prolonged wakefulness [16]. Furthermore, increased mGluR5 availability correlated with behavioral and neurophysiological markers of elevated sleep need, including self-rated sleepiness, unintended sleep during prolonged wakefulness, as well as SWA and slow (<1 Hz) oscillatory activity in the NREM sleep EEG [16, 17].
Apart from interacting with Homer1a, activation of mGluR5 regulates the expression of fragile X mental retardation protein (FMRP) and brain-derived neurotrophic factor (BDNF), which both play important roles in neuronal plasticity [5, 18–23]. Work in Drosophila suggested that the dFmr1 gene is a molecular regulator of sleep need [24], and that the expression of FMRP controls sleep time and the sleep loss-induced sleep rebound [25]. Similarly, the expression of BDNF protein in mice has been associated with the rebound in SWA following sleep deprivation [26]. Whereas the effects of prolonged waking on the concentration of FMRP in humans are unknown, for BDNF either an increase or a decrease have been reported [27, 28].
Based upon the evidence outlined above, in this study in healthy human volunteers dynamic changes in brain metabolites, including Glu, glutamate-to-glutamine ratio (GLX), and γ-amino-butyric-acid (GABA), were quantified in dorsolateral prefrontal cortex (dlPFC) and basal ganglia (BG) simultaneously with cerebral mGluR5 availability, as well as FMRP and BDNF levels in blood serum after prolonged wakefulness and following recovery sleep. It was hypothesized that sleep loss increases these potential markers of elevated sleep need and expected that recovery sleep normalizes the waking-induced changes. With the exception of BDNF and GABA, all markers quantified revealed sleep loss-induced changes and in part reverted to baseline (BL) following recovery sleep, suggesting that glutamatergic signaling involving mGluR5 contributes to the regulation of sleep–wake dependent synaptic plasticity in humans.
Materials and Methods
To investigate the interplay of mGluR5 with its potential molecular signaling partners in sleep–wake regulation, a controlled in-lab study was designed, in which 3-Tesla PET/MR-Spectroscopy scanning and blood sampling were conducted three times, at the same circadian time in BL, after a night without sleep, and again following recovery sleep. Concentrations of Glu, GLX, and GABA in BG and dlPFC were measured with dedicated PRESS/MEGAPRESS magnetic resonance spectroscopy (MRS) sequences. The mGluR5 availability was quantified with the novel PET radioligand [18F]PSS232 which is a noncompetitive selective antagonist of mGluR5 [29, 30]. Circulating levels of BDNF and FMRP in human blood were quantified with ELISA.
Study participants
The study protocol and all experimental procedures were approved by the ethics committee of the Canton of Zürich for research on human participants. All participants provided written informed consent prior to the experiments and received financial compensation for their participation, in accordance with the principles in the Declaration of Helsinki.
Thirty-one healthy men completed a within-subject, 1-week sleep deprivation protocol after being screened for medical history and psychological state. All participants were nonsmokers, in good health, had no history of neurologic or psychiatric disease and were instructed not to take any medications or consumed any illicit drugs 2 months prior to the study. Participants were excluded if they traveled across multiple time zones or performed shift work 3 months prior to study participation. Participants who prior to the study were not aware of the presence of any sleep–wake disturbances, yet the polysomnographic screening night in the sleep laboratory revealed a sleep efficiency < 75%, sleep apnea or periodic leg movements during sleep (PLMS) with an index of 5 or more per hour of sleep, were excluded from participation and study enrollment. Table 1 summarizes lifestyle and demographic characteristics of the healthy study sample derived from validated.
Table 1.
Demographic data of study participants
| Demographic variable | |
|---|---|
| Age (years) | 41.44 ± 20.86 |
| Body mass index (kg/m2) | 23.85 ± 2.37 |
| Caffeine consumption (mg/day) | 176.32 ± 144.64 |
| Alcohol consumption (drinks/week) | 2.98 ± 2.67 |
| Daytime sleepiness | 7.14 ± 3.27 |
| Habitual sleep duration (hours) | 7.44 ± 0.55 |
| Sleep quality | 3.05 ± 1.46 |
| Diurnal preference | 56.00 ± 10.31 |
| Trait anxiety | 29.68 ± 7.55 |
| Eysenck personality traits | |
| Psychoticism | 1.95 ± 1.68 |
| Extraversion | 7.32 ± 3.40 |
| Neuroticism | 2.18 ± 2.68 |
| Lie scale | 3.68 ± 2.51 |
| Depression score | 3.45 ± 4.64 |
| Cognitive assessment | 29.14 ± 1.04 |
Values represent means ± SD (n = 31). Caffeine consumption was calculated based on the following amounts per serving: coffee: 100 mg; Ceylon or green tea: 30 mg; cola drink: 40 mg (2 dL); energy drink: 80 mg (2 dL); chocolate: 50 mg (100 g). Diurnal preference: Horne-Östberg Morningsness-Eveningness Questionnaire [31]; daytime sleepiness: Epworth Sleepiness Scale [32]; depression score: Beck Depression Inventory [33]; personality traits: Eysenck Personality Questionnaire [34]; cognitive assessment: Montreal Cognitive Assessment [35]; trait anxiety: State-Trait Anxiety Inventory [36]; sleep quality: Pittsburgh Sleep Quality Index [37].
German translations and versions of questionnaires used to assess lifestyle and personality traits.
Pre-experimental procedure and experimental protocol
Two weeks prior to the study, participants were required to refrain from all sources of caffeine and wear a wrist activity monitor on the non-dominant arm. During the 5 days prior to the study, they were asked to abstain from alcohol intake and to maintain a regular 8-hour nighttime sleep schedule, corresponding approximately to the participants’ habitual sleep times. Daily log-books and wrist actigraphy verified compliance with the pre-study instructions. Additionally, caffeine and ethanol concentrations in saliva and breath were tested upon entering the laboratory, to confirm participants’ abstinence.
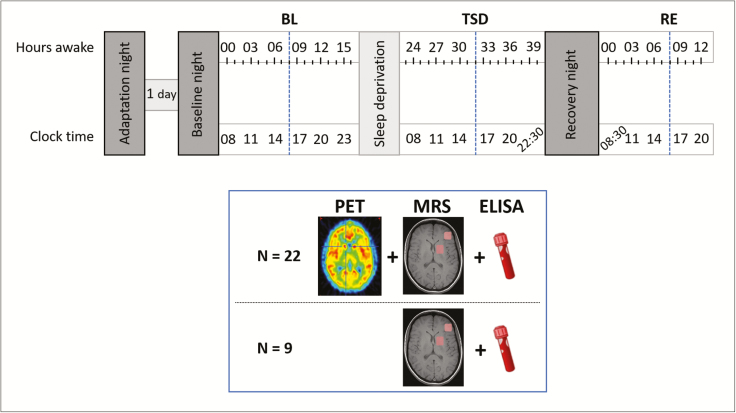
Under constant supervision, all participants completed a sleep deprivation protocol (Figure 1), consisting of an 8 hours adaptation and BL night (time in bed: 11:00 pm–07:00 am), followed by 40 hours of continuous wakefulness, and terminated by a 10-hour recovery night. In BL, total sleep deprivation (TSD) and recovery (RE) conditions, 22 participants underwent a combined positron emission tomography (PET) with [18F]PSS232 to quantify mGluR5 availability in the brain and proton magnetic resonance spectroscopy (1H-MRS) examination (Division of Nuclear Medicine, University Hospital Zürich). To minimize confounding circadian effects, all measurements were conducted at the same circadian timepoint, starting at 04:23 pm ± 23 minutes. Due to time and logistic constraints, only two participants could be PET scanned per experimental week. To optimize data collection, one additional participant was included in each study block (n = 9 in total) as a back-up candidate, participating in the entire experimental protocol, MR imaging, and blood sampling, but without [18F]PSS232 injection and PET scanning.
Figure 1.
Experimental protocol. After an adaptation and baseline night, participants underwent 40 hours of prolonged wakefulness followed by a recovery night. At baseline (BL), after sleep deprivation (TSD), and again after recovery sleep (RE), levels of mGluR5 were measured using positron emission tomography (PET) with [18F]PSS232 at the same circadian timepoint (blue dotted lines). Furthermore, distinct brain metabolites were measured with magnetic resonance spectroscopy (MRS). Blue box summarizes type of data collection and number of subjects at the imaging sessions in BL, TSD and RE conditions (blue dotted lines). Blood samples for the quantification of FMRP and BDNF levels were drawn at these timepoints. In addition, a cognitive test session was performed, consisting of vigilance (Psychomotor Vigilance Task [PVT]) [38], sleepiness (Karolinska Sleepiness Scale [KSS]) [39], tiredness symptoms (Tiredness Symptoms Scale [TSS]) [40], and affective state (Visual Analogue Scales [VAS]) [41] testing.
MRS data acquisition and analysis
The 1H-MRS data were acquired simultaneously with the PET data using a GE 3T combined PET/MR scanner (SIGNA PET/MR; GE Healthcare). Single-voxel edited 1H-MR spectra were acquired from two voxels of interest (VOI) in the left dorsolateral prefrontal cortex (dlPFC; 30 × 25 × 40 mm3) and in the BG (35 × 30 × 25 mm3) using the MEscher-GArwood Point RESolved Spectroscopy (MEGAPRESS) method to quantify GABA as well as Glx and Glu [42]. In addition, a third VOI in the BG (25 × 25 × 25 mm3) was measured with the Point RESolved Spectroscopy (PRESS) method to quantify just Glx and Glu [42]. To ensure a consistent MRS voxel position between participants, the voxel was carefully positioned based on anatomical landmarks on the T1 image. The T1 weighted MR images were also used to correct for partial volume effects related to the cerebrospinal fluid (CSF) content in the MRS voxel, as well as for gray/white matter (GM/WM) correction.
MEGAPRESS:
A total of 320 spectra were averaged to obtain the final spectrum. Individual spectra were acquired with a repetition time (TR) of 1800 ms, an echo time (TE) of 68 ms, and an eight-step phase cycle, resulting in a total acquisition time of ~10 minutes. For each metabolite spectrum, 16 water reference lines were also acquired as part of the standard PROBE acquisition.
PRESS:
The PRESS spectra were acquired with an TE of 35 ms and a TR of 3 ms. One hundred sixty spectral averages were acquired to obtain the final spectrum resulting in an acquisition time of 9 minutes.
Data analysis
The MR spectra were analyzed with LCModel v. 6.3-1 [43], which is a fully automated spectral fitting method. For the MEGAPRESS data, edited spectra were analyzed with a simulated basis set providing metabolite concentrations for Gln, Glu, GLX, GABA, N-acetyl-aspartate, and glutathione. The control parameter sptype = “megapress-2” was used to avoid mis-assignment of the BL to GABA. For the PRESS spectra, a standard experimental basis set was used, from which data for creatine, glutamate to glutamine, myo-inositol, N-acetyl-aspartate, and total choline were extracted (Supplementary Figure S1). For all spectra, peaks that were poorly fitted, resulting in Cramer-Rao minimum variance bounds of more than 20% as reported by LCModel or demonstrating apparent artifacts were excluded from further analyses. Specifically, six dlPFC spectra from five participants could not be included in the statistical analyses. Moreover, some BG spectra measured with PRESS were unfortunately automatically overwritten by the scanner software, resulting in missing data points. The numbers of included participants are indicated throughout the results and in all graphs and/or legends to the Figures.
PET image acquisition
A T1-weighted, whole-brain, three-dimensional magnetic resonance (MR) image (resolution: 1 × 1 × 1 mm) was obtained for each participant in parallel to the PET imaging (SIGNA PET/MR 3T whole-body PET/MR unit equipped with an 8-channel head coil; GE Healthcare), to exclude morphological abnormalities and as anatomical standard for the quantification of the PET images. After an automated standard single bolus injection of [18F]PSS232, dynamic PET brain imaging was performed for 60 minutes. Images were acquired in 3D Mode with Time of flight fully iterative reconstruction (VPFX) using standard MRAC based attenuation correction with a resolution of 1.17 × 1.17 × 2.78 mm3 and Matrix size of 256 × 256 × 89 voxels binned into 43 timeframes (11 × 1 minutes, 22 × 2 minutes, 10 × 1 minutes). Participants were instructed to not fall asleep during image acquisition. To verify wakefulness, participants were required to gently press the button of a response box, generating as little movements as possible. As soon as participants stopped pressing the response box, they were alerted via an intercom. Direct contact was avoided to minimize movement artifacts. Due to technical issues with tracer synthesis, some participants could not be scanned in sleep deprivation and recovery conditions, resulting in missing data points.
Neither injected tracer activity (BL: 164.7 ± 5.2 MBq; TSD: 159.1 ± 3.7 MBq; RE: 154.7 ± 3.1 MBq; p > 0.32, factor “condition”), total activity at the end of synthesis (BL: 2.16 ± 0.10 GBq; TSD: 2.15 ± 0.14 GBq; RE: 1.86 ± 0.12 GBq; p > 0.23), nor injected patient dose of [18F]PSS232 (BL: 2.00 ± 0.22 mg; TSD: 1.55 ± 0.17 mg; RE: 1.75 ± 0.16 mg; p > 0.18) differed between the conditions.
Image processing and quantification
All processing and quantification analyses were conducted with a dedicated brain PET/MR analysis tool (PNEURO, version 3.7) provided by PMOD Technologies LLC. PET image processing consisted of within-subject rigid-body motion correction followed by time-series alignment to the MR-T1 image for between scan comparisons. For PET quantification, the T1 image was automatically segmented, separating the MR image into GM, WM, and CSF probability maps. After matching the T1 MR image to the functional PET images, the specific neocortical and subcortical (core brain segments) brain regions were determined using the Hammers-N30R83 brain atlas. Partial volume correction (PVC) was performed automatically in the PNEURO toolbox. A time-activity curve (TAC) was calculated for each VOI. Because a single bolus injection was used, the binding potential (BPND) was quantified with standard SRTM2 (Simplified Reference Tissue Model with fixed k2 [44]) modeling. For modeling, TACs of receptor-rich regions (GM VOIs) were compared to the TAC of a receptor-less region (cerebellum) assumed mainly to entail nonspecific binding [30].
Assessment of proteins in human serum
Fresh blood was collected immediately before the PET/MRS scans in two 10-mL clot activator tubes (BD Vacutainer CAT). The samples were allowed to clot for about 30 minutes at room temperature (RT) before centrifugation (2.000 relative centrifugal force [RCF] for 10 minutes). 1.9 mL serum was extracted and purified by a second centrifugation step (12.000 RCF for 5 minutes). The purified serum was aliquoted into multiple 255 μL samples and stored in Eppendorf tubes (SafeSeal micro tube 1.5 mL, PP, Sarstedt, Nümbrecht) The probes were then snap-frozen in liquid nitrogen and stored at −80°C for future analysis.
Fragile X mental retardation protein
FMRP was studied by a quantitative sandwich enzyme-linked immunosorbent assay (ELISA) purchased prefabricated and ready to use (Human Fragile X mental retardation 1 ELISA kit, MyBioSource, San Diego, CA). The detection range of this assay is 15.6–1000 pg/mL. A 96-well microplate was pre-coated with an FMRP-specific antibody. Each sample was quantified at least twice for independent confirmation according to the manufacturer’s instructions and guidelines (coefficients of inter-assay variation: BN: CV = 14.23 ± 1.52%, TSD: CV = 11.39 ± 1.68%, RE: CV = 13.52 ± 1.37%). The data were normally distributed (D = 0.08, Pr > D > 0.15, Kolmogorov–Smirnov) and sleep deprivation and recovery sleep did not affect the number of monocytes per mL of blood sample used for FMRP analyses (p > 0.69). For technical reasons, some samples could not be reliably quantified and were excluded from the analyses.
Brain-derived neurotrophic factor
Quantification of serum BDNF levels was conducted at the Department of Clinical Psychology and Psychotherapy at the University of Zurich using a 96-Well MULTI-ARRAY BDNF Assay purchased from Meso-Scale Discovery (MSD, Rockville, MD), according to the manufacturer’s instructions. No estimate of coefficients of inter-assay variation could be obtained.
Statistical analyses
All statistical analyses were performed with SAS 9.4 software (SAS Institute, Cary, NC). If not stated otherwise, numbers represent mean ± standard error of the mean (SEM) and the error bars shown in the figures represent the SEM of between-subjects variability. Mixed-effect repeated-measures analyses of variance (ANOVAs) of blood protein, PET, and 1H-MRS data included the within-subjects factor “condition” (BL, TSD, RE; Supplementary Table S2). The p-values of post hoc analyses to localize significant differences between the experimental conditions were corrected as follows: Base upon a priori hypotheses, the statistical analyses of global mGluR5 availability and FMRP and BDNF levels consisted of three-condition (BL, TSD, RE) Tukey-Kramer correction. The secondary analyses, including 81 comparisons (15 pre-selected PET VOIs across three conditions and six MRS metabolites in two brain regions across three conditions) were corrected by the Benjamini-Hochberg procedure to reduce the false discovery rate. If not mentioned otherwise, only findings with a corrected p-value below the threshold of α < 0.05 were considered significant (Supplementary Table S3). Following significant main effects of “condition” and post hoc testing, Mann-Whitney U tests of the relative data were performed to illustrate the individual changes in % due to the experimental interventions.
Results
Thirty-one healthy men completed this strictly controlled study (Table 1 for demographics; the numbers of study participants contributing to each analysis are specified below). Following 8-hour adaptation and BL sleep opportunities in the sleep laboratory, all volunteers stayed awake under constant supervision for 40 hours, followed by a 10-hour recovery sleep opportunity. All measurements in BL, TSD, and RE started at the same circadian time, at 4:23 pm ± 23 minutes (Figure 1). The prolongation of wakefulness increased self-reported sleepiness and symptoms of tiredness and lowered the levels of mood and energy. Recovery sleep reversed these changes in self-reported state (Supplementary Table S1).
Sleep deprivation increases Glu and GLX levels in the BG
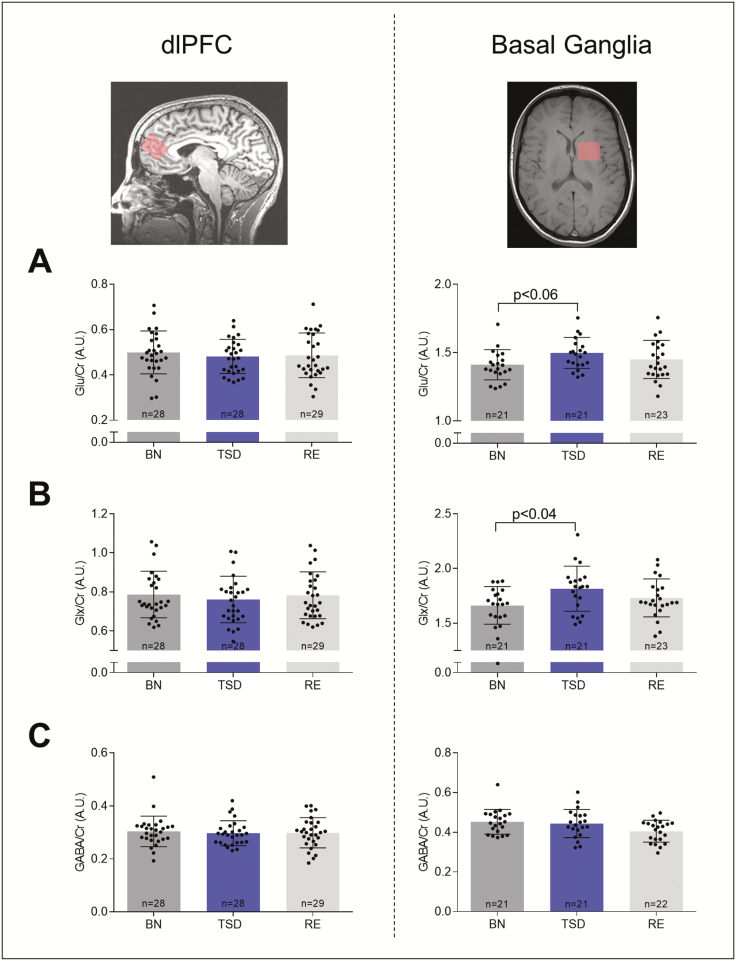
Methodological advances in 1H-MRS have recently permitted the noninvasive detection of naturally occurring changes in tightly regulated metabolite concentrations in circumscribed areas of the human brain. Whereas one recent study suggested that GLX levels in the left parietal lobe decrease overnight [45], previous data from this lab revealed no significant changes after sleep deprivation in GLX/Glu and GABA in the medial prefrontal cortex [17]. Thus, the exact roles in humans of the main excitatory and inhibitory neurotransmitters in circadian and homeostatic sleep–wake regulation remain unclear. Here, the effects of prolonged wakefulness and recovery sleep on the extracellular concentrations of Glu, GLX, and GABA in two pre-defined voxels located in cortex (dlPFC) and BG were quantified at the same circadian time in a separate study in 31 newly recruited study participants. Both these regions show pronounced waking-induced changes in mGluR5 availability [17], and are thought to contribute importantly to sleep homeostasis [46–48]. Consistent with our previous study [17], sleep deprivation caused no reliable changes in these metabolites in the cortex (Figure 2, left-hand panel). By contrast, Glu levels in the BG were increased after prolonged waking in 17 of 21 study participants when compared to BL (Figure 2, right-hand panel). The mean increase equaled 6.31 ± 2.06%, which tended to be significant (BL: 1.41 ± 0.02 [arbitrary units]; TSD: 1.50 ± 0.03; TSD vs. BL: p < 0.06, n = 21). Similarly, sleep loss increased the GLX concentration in the BG in 16 of 21 participants, and the mean increase equaled 9.02 ± 2.53 % (BL: 1.66 ± 0.04; TSD: 1.81 ± 0.05; TSD vs. BL: p < 0.04, n = 21). When relative changes were analyzed, a sleep deprivation-induced increase in both Glu and GLX levels in the BG was confirmed (p < 0.01, Mann-Whitney U tests). Although both, Glu (TSD: 1.50 ± 0.03; RE: 1.45 ± 0.03; RE vs. TSD: 2.8% reduction) and GLX (TSD: 1.81 ± 0.05; RE: 1.73 ± 0.04; RE vs. TSD: 4.2% reduction) were slightly reduced after recovery sleep when compared to sleep deprivation, these changes did not reach statistical significance.
Figure 2.
Effects of sleep deprivation and recovery sleep on endogenous brain metabolites in dorsolateral prefrontal cortex (dlPFC, left) and basal ganglia (BG, right). Magnetic resonance spectroscopy yielded levels of glutamate (Glu; A), glutamate/glutamine ratio (GLX; B) and γ-amino-butyric-acid (GABA; C) relative to creatine in baseline (BL, dark gray), sleep deprivation (TSD, blue) and recovery (RE, light gray) conditions. Bars represent means of arbitrary units (A.U.) ± standard error of the mean (SEM). Numbers on the bars indicate the number of contributing individuals. Black dots represent individual participants. Missing data points were caused by technical problems during 1H-MRS quantification. Data for Glu and GLX were acquired with PRESS and data for GABA with MEGAPRESS sequences. p-values: Benjamini-Hochberg corrected paired, t-tests.
The levels of GABA remained stable in the BG following sleep deprivation and recovery sleep (BL: 0.45 ± 0.01; TSD: 0.45 ± 0.007; RE: 0.41 ± 0.01; TSD vs. BL: p > 0.8; RE vs. TSD: p > 0.9, n = 21; Figure 2C). Similarly, no significant changes in other metabolites (N-acetyl-aspartate, glutathione, choline) were detected (Supplementary Table S3).
Whole-brain mGluR5 availability is elevated after sleep deprivation and normalized after recovery sleep
To quantify sleep–wake associated changes in the availability of mGluR5 that may occur simultaneously with the above described local changes in Glu and GLX, the newly developed, highly selective, noncompetitive mGluR5 antagonist for PET brain imaging, [18F]PSS232, was employed [29, 30].
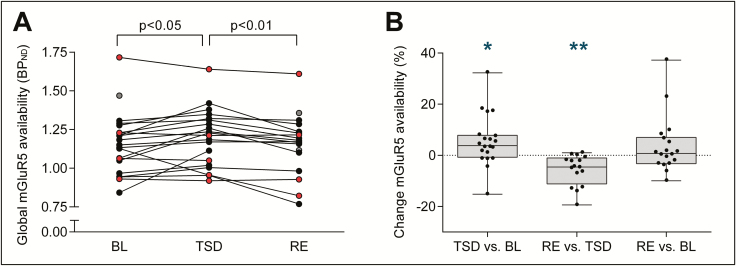
When compared to BL, sleep deprivation induced a consistent increase in whole-brain [18F]PSS232 binding potential reflecting elevated cerebral mGluR5 availability (BL: 1.16 ± 0.04; TSD:1.20 ± 0.04; TSD vs. BL: p < 0.05, n = 20; Figure 3). The [18F]PSS232 binding increased from BL to TSD in 15 of 20 participants in whom PET scans in both conditions were available. On average, the sleep deprivation-induced increase in whole-brain mGluR5 availability equaled 5.53 ± 2.22%.
Figure 3.
Effects of sleep deprivation and recovery sleep on whole-brain metabotropic glutamate receptor subtype 5 (mGluR5) availability. (A) Global NonDisplaceable binding potential (BPND) after [18F]PSS232 uptake in human brain. Individual data points in baseline (BL, n = 22) and following total sleep deprivation (TSD, n = 20) and recovery sleep (RE, n = 18) are plotted. Connecting lines represent within-subjects changes from BL to TSD and from TSD to RE. The color code identifies individuals exhibiting an increase from BL to TSD (filled black circles) and individuals exhibiting a decrease from BL to TSD (filled red circles); filled gray circles: TSD condition missing. p-values: Tukey-Kramer corrected paired, t-tests following significant mixed-model ANOVA with the within-subject factor “condition” (F2,36 = 4.52, p < 0.02). (B) Box plots of relative changes in global mGluR5 availability from BL to TSD, TSD to RE, and BL to RE. Black dots represent individual participants. Asterisks indicate significant change scores: *p < 0.03, **p < 0.01 (Mann-Whitney U tests).
To examine whether recovery sleep reverses the wakefulness-induced changes, PET scans were also performed after the recovery night. In 13 of 16 study participants in whom TSD and RE data were available, whole-brain [18F]PSS232 binding was reduced in RE when compared to TSD (TSD: 1.21 ± 0.05; RE: 1.14 ± 0.04; RE vs. TSD: p < 0.01, n = 16). The reduction in mGluR5 availability from TSD to RE equaled 5.77 ± 1.50 %. No difference in [18F]PSS232 binding potential between BL and RE was detected, suggesting that recovery sleep normalized the waking-induced enhancement in mGluR5 availability.
Wake-sleep dependent changes in mGluR5 availability in BG, amygdala and parietal cortex
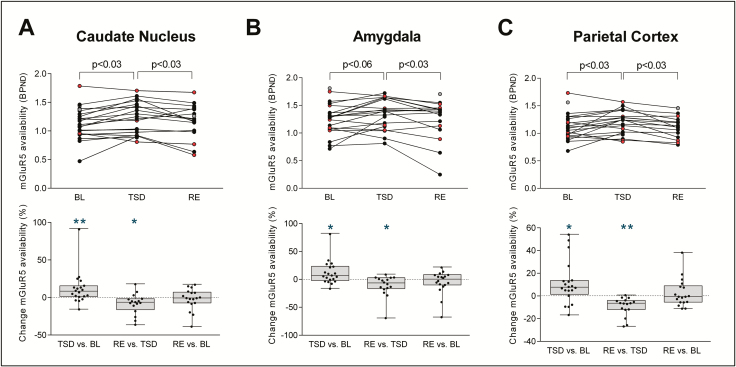
Fourteen VOIs previously associated with sleep–wake regulation [16, 17, 49] were selected for secondary PET image analyses. These VOIs included: caudate nucleus, putamen, ventral striatum, amygdala, dlPFC, orbitofrontal cortex, medial superior frontal cortex, anterior cingulate cortex, parietal cortex, inferior parietal cortex, precuneus, medial temporal lobe, parahippocampal gyrus, hippocampus, and insula. Increased [18F]PSS232 binding after prolonged waking was observed in caudate nucleus (BL: 1.15 ± 0.06; TSD: 1.25 ± 0.06; increase: 8.71 ± 4.82%; TSD vs. BL: p < 0.03) and parietal cortex (BL: 1.12 ± 0.05; TSD: 1.19 ± 0.05; increase: 6.58 ± 4.46%; TSD vs. BL: p < 0.03), and tended to be increased in the amygdala (BL: 1.27 ± 0.07; TSD: 1.38 ± 0.07; increase: 8.66 ± 4.72%; TSD vs. BL: p < 0.06; n = 20; Supplementary Table S3). When relative changes were analyzed, an increase in mGluR5 availability by sleep deprivation was present in all these three brain regions (Figure 4, lower panel). Similar to the whole-brain data, recovery sleep normalized mGluR5 availability in caudate nucleus (TSD: 1.25 ± 0.06; RE: 1.14 ± 0.06; reduction: 8.59 ± 3.46%; RE vs. TSD: p < 0.03), amygdala (TSD: 1.38 ± 0.07; RE: 1.23 ± 0.07; reduction: 11.31 ± 4.71%; RE vs. TSD: p < 0.03) and parietal cortex (TSD: 1.19 ± 0.05; RE: 1.13 ± 0.05; reduction: 5.51 ± 1.95%; RE vs. TSD: p < 0.03; n = 16) to the level of BL (RE vs. BL: pall > 0.5, n = 16; Figure 4).
Figure 4.
Regional differences in the effect of sleep deprivation and recovery sleep on metabotropic glutamate receptor subtype 5 (mGluR5) availability. Upper panel: NonDisplaceable binding potential (BPND) after [18F]PSS232 uptake in Caudate nucleus (A), amygdala (B) and parietal cortex (C). Individual data points in baseline (BL, n = 22) and following total sleep deprivation (TSD, n = 20) and recovery sleep (RE, n = 18) are plotted. Connecting lines represent within-subjects changes from BL to TSD and from TSD to RE. The color code identifies individuals exhibiting an increase from BL to TSD (filled black circles) and individuals exhibiting a decrease from BL to TSD (filled red circles); filled gray circles: TSD condition missing. p-values: Benjamini-Hochberg corrected paired, t-tests following significant mixed-model ANOVA with the within-subject factor “condition” (Caudate nucleus: F2,36 = 6.25, p < 0.01; amygdala: F2,36 = 5.54, p < 0.01; parietal cortex: F2,36 = 6.85, p < 0.01). Lower panel: Box plots of relative changes in mGluR5 availability in Caudate nucleus (A), amygdala (B), and parietal cortex (C) from BL to TSD, TSD to RE, and BL to RE. Black dots represent individual participants. Asterisks indicate significant change scores: *p < 0.03, **p < 0.01 (Mann-Whitney U tests).
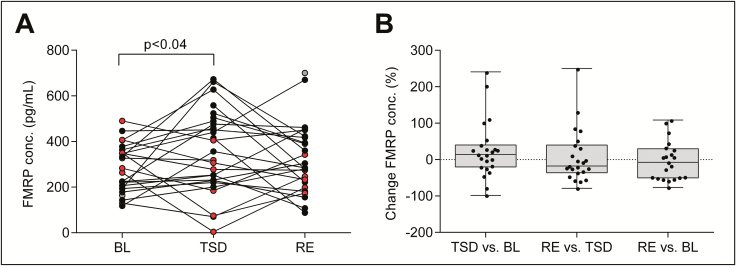
Sleep deprivation increases FMRP concentration in blood serum
To tackle the question whether the wake-sleep-related changes in Glu/GLX concentrations and mGluR5 availability in the brain are mimicked by changes in mGluR5-regulated proteins in peripheral blood, circulating FMRP and BDNF in serum were quantified with ELISA in BL, TSD, and RE conditions. Intriguingly, prolonged waking increased the blood FMRP concentration in 13 of 23 participants (BL: 268.52 ± 33.76 pg/mL; TSD: 370.86 ± 31.93 pg/mL; mean increase: 25.86 ± 16.39%, TSD vs. BL: p < 0.04, n = 23; Figure 5). Although the FMRP concentration in RE tended to revert to BL and the mean FMRP levels in these two conditions did not differ, a difference was neither observed between RE and TSD conditions (RE: 333.89 ± 33.51 pg/mL; RE vs. BL: p > 0.25, n = 21; RE vs. TSD: p > 0.6, n = 23).
Figure 5.
Effects of sleep deprivation and recovery sleep on serum fragile X mental retardation protein (FMRP) levels. (A) Circulating FMRP concentration in human blood serum. Individual data points in baseline (BL, n = 24) and following total sleep deprivation (TSD, n = 27) and recovery sleep (RE, n = 26) are plotted. Connecting lines represent within-subjects changes from BL to TSD and from TSD to RE. The color code identifies individuals exhibiting an increase from BL to TSD (filled black circles) and individuals exhibiting a decrease from BL to TSD (filled red circles); filled gray circles: TSD condition missing. p-values: Tukey-Kramer corrected paired, t-tests following significant mixed-model ANOVA with the within-subject factor “condition” (F2,44 = 3.37, p < 0.05). (B) Box plots of relative changes in blood FMRP levels from BL to TSD, TSD to RE, and BL to RE. Black dots represent individual participants.
In contrast to FMRP, the levels of BDNF were not affected by prolonged waking (Supplementary Figure S2).
Discussion
Glu is the primary excitatory neurotransmitter of the human brain. Although basic research in vitro and in animal models highlights a prominent role for glutamatergic mechanisms in regulating sleep–wake homeostasis [11, 15, 17, 50–52], knowledge about glutamatergic signaling as a function of waking and sleep in humans is scarce. This study suggests an important relationship between glutamatergic signaling and sleep in humans and supports a role of the BG in sleep homeostatic mechanisms. More specifically, the data revealed that one night without sleep elicited reliable increases in cerebral Glu/GLX levels and mGluR5 availability, particularly in the BG, as well as in the concentration of the mGluR5-regulated protein, FMRP, in the blood stream. Given that most of these wakefulness-induced molecular changes tended to normalize after recovery sleep, the findings suggest that sleep may be beneficial to keep glutamatergic signaling in a homeostatic range. In other words, sleep in humans may counteract neuronal dysfunction and degeneration, which can be caused by excessive Glu [8–10], on multiple levels of the metabotropic glutamatergic signaling cascade. Nevertheless, because the concentrations of Glu/GLX and FMRP were not fully restored by recovery sleep, a single recovery night is probably insufficient for the glutamatergic system to fully recover after a night of total sleep deprivation.
Sleep deprivation and recovery sleep induce dynamic changes in BG Glu levels
The levels of Glu in the rat cortical extra-synaptic space rise during waking and decrease during NREM sleep [11], yet it is currently unknown whether similar changes also occur in the human brain. To examine a glutamatergic contribution to the relief of depressive symptoms after wake therapy, brain levels of Glu, GLX, and GABA were previously measured with 1H-MRS in depressed patients undergoing acute and repeated therapeutic sleep deprivation [53–55]. No significant alterations in GLX or its elements were found in different cortical regions (dlPFC, anterior cingulate cortex, and parieto-occipital cortex), yet preliminary data indicated that sleep loss increased GLX in subcortical brain regions [53]. Because the BL levels of GLX and Glu in cerebral cortex differ between depressed patients and healthy controls [56, 57], it is unclear whether these older studies are directly comparable with the present investigation. Nevertheless, previous [17] and the current work in healthy controls is consistent with the data in depressed patients [54, 55]. It indicates that prolonged wakefulness does not reliably alter the MRS signal compatible with GLX and its constituents in anterior cingulate cortex and dlPFC. It cannot be excluded, however, that the lack of a significant change in GLX in the dlPFC voxel could be related to the voxel composition, which, compared to the BG voxel was composed of a higher fraction of GM.
The data collected in the BG strongly suggest that sleep loss indeed affects glutamatergic signaling on different levels. More specifically, prolonged wakefulness increased Glu, GLX, and mGluR5 availability in sub-regions of the BG, and some of these changes were re-normalized after recovery sleep. The findings corroborate and expand previously published observations from this group, showing that mGluR5 availability was increased after sleep deprivation [16]. The investigation of different brain regions indicated that the BG are a brain structure that reliably shows sleep–wake related changes in the glutamatergic balance in humans. The dorsal (caudate nucleus and putamen) and ventral (nucleus accumbens and olfactory tubercle) parts of the striatum and the amygdala showed increased mGluR5 availability after sleep loss [16]. Together, the data strengthen the emerging hypothesis that the BG are a key player in sleep–wake regulation [58–60]. Whereas the observed increase by 5–10% in Glu levels and mGluR5 availability after extended wakefulness may be considered as small or moderate, the simultaneous changes could mutually amplify each other and cause a substantial increase in glutamatergic signaling after sleep deprivation. Importantly, the present new data demonstrate that recovery sleep is associated with reduced mGluR5 availability, supporting a restorative role for sleep and providing complementary evidence for the mGluR5 signaling cascade to contribute to sleep–wake regulation.
Sleep deprivation impacts on the expression of FMRP
Currently, the most specific molecular marker of sleep need is the immediate early gene Homer1a, which uncouples mGluR5 from its downstream signaling partners, leading to synaptic LTD [12–15, 50, 61, 62]. This form of synaptic plasticity may ultimately support sleep-dependent recovery processes [15, 63, 64]. The mGluR5 has been specifically associated with two proteins that may be important for sleep–wake regulation: FMRP and BDNF. Consistent with experiments in Drosophila [24], the present data reveal elevated FMRP levels after prolonged wakefulness when compared to BL. A prolonged effect of sleep deprivation, or insufficient recovery, might explain the incomplete normalization after recovery sleep. In contrast to the findings in vivo, the FMRP concentration in cultured neural cells of sleep-deprived rats appeared to decrease with sleep deprivation [65]. Further research is needed to clarify the potential role for FMRP in sleep–wake regulation. Moreover, the concentration of FMRP in human blood serum is low (in the pg range), rendering its quantification difficult, and depends on various possible factors, including genetic influences [66]. Cautious interpretation and independent replication of this result are, thus, crucial. Similarly, the evidence for a suggested role of BDNF in regulating sleep homeostasis and LTP-like plasticity after sleep deprivation [28, 67] has been equivocal. Here, neither sleep deprivation nor recovery sleep revealed consistent effects on BDNF levels in the human serum as quantified with ELISA. The establishment of a reliable method to assess blood serum BDNF still remains a clinical challenge. The discrepancies among the available studies may reflect the methodological difficulties in the reliable quantification of BDNF serum concentration.
Concluding remarks
Although the findings cannot be generalized to female and patient populations because only healthy men were investigated, this study provides convergent evidence that sleep deprivation and recovery sleep affect glutamatergic signaling in distinct regions of the human brain that play an important role in sleep–wake regulation. Nevertheless, the questions remain whether the observed molecular changes regulate the need for sleep or whether they reflect secondary changes associated with the expression of wakefulness and sleep, or both. The present findings warrant further studies to elucidate the mechanisms that link the homeostatic regulation of sleep and glutamatergic system activity in health and disease.
Supplementary Material
Acknowledgments
We thank I. Clark, D.M. Baur, S.M. Pereira Soares, A. Dieffenbacher, and S. Brühlmeier for their help with data collection and analyses. Furthermore, we thank S. Geistlich for the help and production of the radioactive ligand and Prof. Dr. M. Kohler, as well as Prof. Dr. S. Brown for providing access to their laboratories for blood processing and analyses. Finally, we thank two anonymous reviewers for their careful evaluation of our submission; their insightful comments and suggestions helped to improve the paper.
Funding
This work was supported by the Swiss National Science Foundation grant #320030_163439 and the Clinical Research Priority Program Sleep & Health of the University of Zurich (to H-P.L.).
Author contributions
S.W.: Data curation, Formal analysis, Supervision, Investigation, Visualization, Writing original draft, Writing-review and editing; S.C.H.: Data curation, Formal analysis, Supervision, Funding acquisition, Investigation, Visualization, Writing original draft, Writing-review and editing; V.T.: Resources, Methodology, Supervision, Writing-review and editing; R.L.O.T.: Software, Methodology, Data curation, Writing-review, and editing; J.M.: Data curation, Formal analysis, Investigation; S.M.A. and A.B.: Resources, Methodology, Project administration; H-P.L.: Conceptualization, Resources, Data curation, Supervision, Funding Acquisition, Writing-original draft, Project administration, Writing-review, and editing.
Conflict of interest statement. None declared.
References
- 1. Achermann P, et al. Sleep homeostasis and models of sleep regulation. In: Kryger M, Roth T, Dement W, eds. Principles and Practices of Sleep Medicine. 6th ed. Philadelphia, PA: Elsevier; 2017: 377–387. [Google Scholar]
- 2. Tadavarty R, et al. Long-term depression of excitatory synaptic transmission in rat hippocampal CA1 neurons following sleep-deprivation. Exp Neurol. 2009;216(1):239–242. [DOI] [PubMed] [Google Scholar]
- 3. Pigeat R, et al. Sleep slow wave-related homo and heterosynaptic LTD of intrathalamic GABAAergic synapses: involvement of T-type Ca2+ channels and metabotropic glutamate receptors. J Neurosci. 2015;35(1):64–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Tononi G, et al. Sleep and the price of plasticity: from synaptic and cellular homeostasis to memory consolidation and integration. Neuron. 2014;81(1):12–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Huber KM, et al. Altered synaptic plasticity in a mouse model of fragile X mental retardation. Proc Natl Acad Sci U S A. 2002;99(11):7746–7750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Marshall L, et al. Boosting slow oscillations during sleep potentiates memory. Nature. 2006;444(7119):610–613. [DOI] [PubMed] [Google Scholar]
- 7. Kauer JA, et al. Synaptic plasticity and addiction. Nat Rev Neurosci. 2007;8(11):844–858. [DOI] [PubMed] [Google Scholar]
- 8. Sanacora G, et al. Targeting the glutamatergic system to develop novel, improved therapeutics for mood disorders. Nat Rev Drug Discov. 2008;7(5):426–437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ahmed I, et al. Glutamate NMDA receptor dysregulation in Parkinson’s disease with dyskinesias. Brain. 2011;134(Pt 4):979–986. [DOI] [PubMed] [Google Scholar]
- 10. Averill LA, et al. Glutamate dysregulation and glutamatergic therapeutics for PTSD: Evidence from human studies. Neurosci Lett. 2017;649:147–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Dash MB, et al. Long-term homeostasis of extracellular glutamate in the rat cerebral cortex across sleep and waking states. J Neurosci. 2009;29(3):620–629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kammermeier PJ, et al. Homer 1a uncouples metabotropic glutamate receptor 5 from postsynaptic effectors. Proc Natl Acad Sci U S A. 2007;104(14):6055–6060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Berridge MJ. The inositol trisphosphate/calcium signaling pathway in health and disease. Physiol Rev. 2016;96(4):1261–1296. [DOI] [PubMed] [Google Scholar]
- 14. Ronesi JA, et al. Homer interactions are necessary for metabotropic glutamate receptor-induced long-term depression and translational activation. J Neurosci. 2008;28(2):543–547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Diering GH, et al. Homer1a drives homeostatic scaling-down of excitatory synapses during sleep. Science. 2017;355(6324):511–515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hefti K, et al. Increased metabotropic glutamate receptor subtype 5 availability in human brain after one night without sleep. Biol Psychiatry. 2013;73(2):161–168. [DOI] [PubMed] [Google Scholar]
- 17. Holst SC, et al.. Cerebral mGluR5 availability contributes to elevated sleep need and behavioral adjustment after sleep deprivation. Elife. 2017;6:e28751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Comery TA, et al. Abnormal dendritic spines in fragile X knockout mice: maturation and pruning deficits. Proc Natl Acad Sci U S A. 1997;94(10):5401–5404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Li J, et al. Reduced cortical synaptic plasticity and GluR1 expression associated with fragile X mental retardation protein deficiency. Mol Cell Neurosci. 2002;19(2):138–151. [DOI] [PubMed] [Google Scholar]
- 20. Restivo L, et al. Enriched environment promotes behavioral and morphological recovery in a mouse model for the fragile X syndrome. Proc Natl Acad Sci U S A. 2005;102(32):11557–11562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Bramham CR, et al. BDNF function in adult synaptic plasticity: the synaptic consolidation hypothesis. Prog Neurobiol. 2005;76(2):99–125. [DOI] [PubMed] [Google Scholar]
- 22. Desai NS, et al. Early postnatal plasticity in neocortex of Fmr1 knockout mice. J Neurophysiol. 2006;96(4):1734–1745. [DOI] [PubMed] [Google Scholar]
- 23. Lu B, et al. BDNF and synaptic plasticity, cognitive function, and dysfunction. Handb Exp Pharmacol. 2014;220:223–250. [DOI] [PubMed] [Google Scholar]
- 24. Bushey D, et al. The Drosophila fragile X mental retardation gene regulates sleep need. J Neurosci. 2009;29(7):1948–1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Bushey D, et al. Sleep and synaptic homeostasis: structural evidence in Drosophila. Science. 2011;332(6037):1576–1581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Huber R,, et al. Exploratory behavior, cortical BDNF expression, and sleep homeostasis. Sleep. 2007;30(2):129–139. [DOI] [PubMed] [Google Scholar]
- 27. Schmitt K, et al. BDNF in sleep, insomnia, and sleep deprivation. Ann Med. 2016;48(1-2):42–51. [DOI] [PubMed] [Google Scholar]
- 28. Kuhn M, et al. Sleep recalibrates homeostatic and associative synaptic plasticity in the human cortex. Nat Commun. 2016;7:12455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Sephton SM, et al. Preclinical evaluation and test-retest studies of [(18)F]PSS232, a novel radioligand for targeting metabotropic glutamate receptor 5 (mGlu5). Eur J Nucl Med Mol Imaging. 2015;42(1):128–137. [DOI] [PubMed] [Google Scholar]
- 30. Warnock G, et al. A first-in-man PET study of [18F]PSS232, a fluorinated ABP688 derivative for imaging metabotropic glutamate receptor subtype 5. Eur J Nucl Med Mol Imaging. 2018;45(6):1041–1051. [DOI] [PubMed] [Google Scholar]
- 31. Horne JA, et al. A self-assessment questionnaire to determine morningness-eveningness in human circadian rhythms. Int J Chronobiol. 1976;4(2):97–110. [PubMed] [Google Scholar]
- 32. Bloch KE, et al. German version of the Epworth Sleepiness Scale. Respiration. 1999;66(5):440–447. [DOI] [PubMed] [Google Scholar]
- 33. Beck AT, et al. An inventory for measuring depression. Arch Gen Psychiatry. 1961;4:561–571. [DOI] [PubMed] [Google Scholar]
- 34. Francis LJ, et al. The Short-Form Revised Eysenck Personality Questionnaire (EPQ-S): A German Edition. 2006. glyndwr.collections.crest.ac.uk. [Google Scholar]
- 35. Nasreddine ZS, et al. The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc. 2005;53(4):695–699. [DOI] [PubMed] [Google Scholar]
- 36. Spielberger CD, et al.. Manual for the State-Trait Anxiety Inventory. Palo Alto, CA: Consulting Psychologists Press; 1970. [Google Scholar]
- 37. Buysse DJ, et al. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res. 1989;28(2):193–213. [DOI] [PubMed] [Google Scholar]
- 38. Dinges DF, et al. Microcomputer analyses of performance on a portable, simple visual reaction task during sustained operations. Behav Res Methods Instrum Comput. 1985;17:652–655. [Google Scholar]
- 39. Akerstedt T, et al. Subjective and objective sleepiness in the active individual. Int J Neurosci. 1990;52(1–2):29–37. [DOI] [PubMed] [Google Scholar]
- 40. Schulz H, et al.. Measuring tiredness by symptoms. Sleep Res. 1991;20A:515. [Google Scholar]
- 41.Bodenmann S, et al. Pharmacogenetics of modafinil after sleep loss: catechol-O-methyltransferase genotype modulates waking functions but not recovery sleep. Clin Pharmacol Ther. 2009;85(3):296–304. [DOI] [PubMed] [Google Scholar]
- 42. Mescher M, et al. Simultaneous in vivo spectral editing and water suppression. NMR Biomed. 1998;11(6):266–272. [DOI] [PubMed] [Google Scholar]
- 43. Provencher SW. Estimation of metabolite concentrations from localized in vivo proton NMR spectra. Magn Reson Med. 1993;30(6):672–679. [DOI] [PubMed] [Google Scholar]
- 44. Wu Y, et al. Noise reduction in the simplified reference tissue model for neuroreceptor functional imaging. J Cereb Blood Flow Metab. 2002;22(12):1440–1452. [DOI] [PubMed] [Google Scholar]
- 45. Volk C, et al. Diurnal changes in glutamate + glutamine levels of healthy young adults assessed byproton magnetic resonance spectroscopy. Hum Brain Mapp. 2018;39(10):3984–3992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Dahan L, et al. Prominent burst firing of dopaminergic neurons in the ventral tegmental area during paradoxical sleep. Neuropsychopharmacology. 2007;32(6):1232–1241. [DOI] [PubMed] [Google Scholar]
- 47. Léna I, et al. Variations in extracellular levels of dopamine, noradrenaline, glutamate, and aspartate across the sleep-wake cycle in the medial prefrontal cortex and nucleus accumbens of freely moving rats. J. Neurosci. 2005;30:4382–4389. [DOI] [PubMed] [Google Scholar]
- 48. Guillaumin MCC, et al. Cortical region-specific sleep homeostasis in mice: effects of time of day and waking experience. Sleep. 2018;41(7). doi: 10.1093/sleep/zsy079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Dang-Vu TT, et al. Functional neuroimaging insights into the physiology of human sleep. Sleep 2010;33:1589–1603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Maret S, et al. Homer1a is a core brain molecular correlate of sleep loss. Proc Natl Acad Sci U S A. 2007;104(50):20090–20095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Ahnaou A, et al. Relevance of the metabotropic glutamate receptor (mGluR5) in the regulation of NREM-REM sleep cycle and homeostasis: evidence from mGluR5 (-/-) mice. Behav Brain Res. 2015;282:218–226. [DOI] [PubMed] [Google Scholar]
- 52. Halassa MM, et al. Integrated brain circuits: astrocytic networks modulate neuronal activity and behavior. Annu Rev Physiol. 2010;72:335–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Murck H, et al. Increase in amino acids in the pons after sleep deprivation: a pilot study using proton magnetic resonance spectroscopy. Neuropsychobiology. 2002;45(3):120–123. [DOI] [PubMed] [Google Scholar]
- 54. Murck H, et al. The glutamatergic system and its relation to the clinical effect of therapeutic-sleep deprivation in depression - an MR spectroscopy study. J Psychiatr Res. 2009;43(3):175–180. [DOI] [PubMed] [Google Scholar]
- 55. Benedetti F, et al. Spectroscopic correlates of antidepressant response to sleep deprivation and light therapy: a 3.0 Tesla study of bipolar depression. Psychiatry Res. 2009;173(3):238–242. [DOI] [PubMed] [Google Scholar]
- 56. Järnum H, et al. Longitudinal MRI study of cortical thickness, perfusion, and metabolite levels in major depressive disorder. Acta Psychiatr Scand. 2011;124(6):435–446. [DOI] [PubMed] [Google Scholar]
- 57. Njau S, et al. Neurochemical correlates of rapid treatment response to electroconvulsive therapy in patients with major depression. J Psychiatry Neurosci. 2017;42(1):6–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Lazarus M, et al. Role of the basal ganglia in the control of sleep and wakefulness. Curr Opin Neurobiol. 2013;23(5):780–785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Holst SC, et al. Sleep homeostasis, metabolism, and adenosine. Current Sleep Medicine Reports. 2015;1:1–11.26618103 [Google Scholar]
- 60. Holst SC, et al. Sleep-wake neurochemistry. Sleep Med Clin. 2018;13(2):137–146. [DOI] [PubMed] [Google Scholar]
- 61. Mackiewicz M, et al. Analysis of the QTL for sleep homeostasis in mice: Homer1a is a likely candidate. Physiol Genomics. 2008;33(1):91–99. [DOI] [PubMed] [Google Scholar]
- 62. Ménard C, et al. Group 1 metabotropic glutamate receptor function and its regulation of learning and memory in the aging brain. Front Pharmacol. 2012;3:182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Krueger JM, et al. Sleep: a synchrony of cell activity-driven small network states. Eur J Neurosci. 2013;38(2):2199–2209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. de Vivo L, et al. Ultrastructural evidence for synaptic scaling across the wake/sleep cycle. Science. 2017;355(6324):507–510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Kwon KJ, et al. The potential role of melatonin on sleep deprivation-induced cognitive impairments: implication of FMRP on cognitive function. Neuroscience. 2015;301:403–414. [DOI] [PubMed] [Google Scholar]
- 66. LaFauci G, et al. Detection and quantification of the fragile X Mental Retardation Protein 1 (FMRP). Genes 2016;7:e121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Faraguna U, et al. A causal role for brain-derived neurotrophic factor in the homeostatic regulation of sleep. J Neurosci. 2008;28(15):4088–4095. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.