Abstract
Study Objectives
To asses the long-term safety and efficacy of pitolisant, an histamine H3-receptor antagonist, on narcolepsy.
Methods
This open-label, single-arm, pragmatic study, recruited adult patients with narcolepsy and Epworth Sleepiness Scale (ESS) score ≥12. After a titration period, patients were treated for up to 1 year with oral pitolisant once-a-day at up to 40 mg. Concomitant stimulants and anti-cataplectic agents were allowed. The primary endpoint was safety; secondary endpoints included ESS, cataplexy, and other diary parameters.
Results
Patients (n = 102, 75 with cataplexy) received pitolisant, for the first time in 73 of them. Sixty-eight patients (51 with cataplexy) completed the 12-month treatment. Common treatment-emergent adverse events were headache (11.8% of patients), insomnia (8.8%), weight gain (7.8%), anxiety (6.9%), depressive symptoms (4.9%), and nausea (4.9%). Seven patients had a serious adverse effect, unrelated to pitolisant except for a possibly related miscarriage. One-third of patients stopped pitolisant, mostly (19.6%) for insufficient benefit. ESS score decreased by 4.6 ± 0.6. Two-thirds of patients completing the treatment were responders (ESS ≤ 10 or ESS decrease ≥ 3), and one third had normalized ESS (≤10). Complete and partial cataplexy, hallucinations, sleep paralysis, and sleep attacks were reduced by 76%, 65%, 54%, 63%, and 27%, respectively. Pitolisant as monotherapy (43% of patients) was better tolerated and more efficacious on ESS than on add-on, but efficacy was maintained in this last case.
Conclusions
Long-term safety and efficacy of pitolisant on daytime sleepiness, cataplexy, hallucinations, and sleep paralysis is confirmed.
Keywords: pitolisant, narcolepsy, cataplexy, excessive daytime sleepiness
Statement of Significance.
This open-label, naturalistic, prospective longitudinal uncontrolled, multi-center international trial confirmed the long-term safety, tolerability, and efficacy of 12-month therapy with pitolisant. However, one-third of the patients left the trial before 1 year, mainly for insufficient perceived efficacy. This new wake-promoting and anti-cataplectic agent works as a histamine H3R inverse agonist/antagonist in narcolepsy patients with or without cataplexy who experience persistent daytime sleepiness. Once-daily treatment with pitolisant at 40 mg is well tolerated and improves excessive daytime sleepiness, generalized and partial cataplexy, hallucinations, and sleep paralysis in patients with narcolepsy.
Introduction
Narcolepsy is a chronic, debilitating neurological disorder characterized by excessive daytime sleepiness (EDS) and abnormal rapid eye movement (REM) sleep manifestations including cataplexy, hallucinations, and sleep paralysis [1, 2]. The treatment of narcolepsy symptoms mainly include modafinil for EDS, antidepressants (selective serotonin and dual serotonin and noradrenaline reuptake inhibitors) for cataplexy, and sodium oxybate for both symptoms [1, 3, 4]. Alternatively, methylphenidate and, more rarely, amphetamines are used. However, the quality of studies supporting these guidelines varies widely, long-term treatment studies are rare and not controlled, several treatments are used on an empirical basis (namely antidepressants, see [5]) and few studies compared the efficacy of different treatments [3, 4, 6–8].
A new class of drugs based on boosting histamine transmission recently emerged, with pitolisant, a H3-receptor inverse agonist/antagonist, as the first-developed drug in this field [9, 10]. One single-blind proof-of-concept [11] and two recent pivotal double-blind phase III trials demonstrated that pitolisant, was well tolerated and reduced EDS and cataplexy compared with placebo in patients with narcolepsy [12, 13]. Pitolisant decreased EDS on both subjective (ESS) and objective (maintenance of wakefulness test, MWT) assessments, improved attention (sustained attention to response task, SART), and reduced the frequency of cataplexies and of hypnagogic hallucinations. These results suggested that pitolisant offers a new treatment option for patients with narcolepsy [14, 15] and its use as a first-line treatment was suggested [16]. However, previous trials had a relatively short-term duration (i.e. 3 months) and could not exclude that tolerance may develop and that new treatment-emergent adverse events (TEAEs) arise on continuation.
The main aim of this open-label study was to address the long-term (12 months) safety and efficacy of pitolisant in the treatment of EDS and other symptoms in adult patients with narcolepsy, with and without cataplexy, with persistent sleepiness despite established treatments. This study aimed also to assess the frequency of generalized and partial cataplexy, sleep attacks, hypnagogic hallucinations and sleep paralysis and included patients with comorbidities or patients that could not be enrolled in a placebo-controlled study, that is, aimed to be closer to “real life” prescriptions. CNS stimulants or anti-cataplectic drugs were allowed as co-medications to assess the safety and efficacy of pitolisant combined with other therapies in treating patients with narcolepsy.
Methods
Patients
Seven centers in France and one in Hungary enrolled adult narcoleptic patients according to ICSD-2 criteria with persistent daytime sleepiness (ESS ≥ 12). When typical cataplexy was not present, an overnight polysomnogram followed by a positive Multiple Sleep Latency Test within the past 5 years had to show a mean sleep latency ≤8 minutes with two or more sleep-onset rapid eye movement periods (SOREMPs)
Patients could not take part if they had any other cause of daytime sleepiness, including an untreated sleep apnea syndrome (according to the investigator judgment) or history of substance abuse, severe psychiatric or neurological disorder, serious cardiovascular disorder, severe hepatic or renal impairment. Women with child-bearing potential had to use a reliable birth control method.
Enrolled patients could be naïve to pitolisant (“de novo” subgroup) or formerly treated with pitolisant (“exposed” subgroup) during previous single- or double-blind studies (P05-03, HARMONY I, and HARMONY Ibis) or have been switched from the French Compassionate Use Program (CUP) to this study. Co-medications with ongoing CNS stimulants and anti-cataplectic agents were allowed. Tricyclic antidepressants and H1-receptor antagonists, that may block the effect of pitolisant by abrogating the effect of endogenous histamine released as a result of H3R blockade, were prohibited.
The study was approved by local ethics committees in each country. Patients provided written informed consent.
Study management
An independent Data Safety Monitoring Board regularly reviewed the trial safety. The study was monitored by Bioprojet. Statistical analysis was performed by Ascopharm (France) and reviewed by an independent expert (Prof P Lehert, Belgium).
The study was registered in ClinicalTrials.gov, identifier NCT01399606.
Procedures
Eligible patients went through a 1-month individual titration period at the initiation of the treatment with pitolisant, except for patients coming from the CUP who were already treated by pitolisant and could continue at their established dose at inclusion. The titration scheme was: 5 mg pitolisant hydrochloride once daily (OD) for the first 7 days, and 10 mg for the next 7 days. Then, during the third week, the dose could be increased up to 20 mg OD if safety and tolerability were good and, during the fourth week, doses could be adjusted according to individual benefit/tolerance ratio between 5 to 20 mg OD. After 1 month, the dose could be increased to 40 mg OD if the investigator judged that the efficacy of 20 mg was not sufficient. Thereafter, the dose remained stable for a 2-month period. During the follow-up visits scheduled in all patients at 3, 6, 9, and 12 months, an individual dose adjustment could be performed again (5, 10, 20, or 40 mg OD).
Six patients dropped out before being titrated to 40 mg (4 at 1 month and 2 at 3 months).
Outcome measures
The primary endpoint was the incidence of TEAEs at 12 months. Other safety measurements included vital signs, physical examination, laboratory tests, electrocardiograms, and Beck Depression Inventory (BDI-13).
Efficacy measurements included ESS score, Clinical Global Impressions of Change (CGI-C), European Quality of life questionnaire (EQ-5D), and compliance. Patients were also instructed to report all partial and generalized cataplexy attacks, hypnagogic hallucinations, sleep paralysis, and sleep attacks in their sleep diaries completed during the 7 days prior to each visit. Cataplexy attacks were defined as sudden and transient episodes (ranging from several seconds to a few minutes) of loss of muscle tone triggered by emotion. Cataplexy was partial when hypotonic phenomena mainly occurred in the face and neck, upper or lower limb drop. It was generalized when hypotonia affected the whole body, leading to impaired postural control or falls.
Statistical analysis
Safety and efficacy analysis were descriptive, considering all included patients having received at least one dose of pitolisant during their participation in this trial (safety population and intent to treat population). Treatment-emergent AEs were classified using the MedDRA dictionary version 15.1. Changes from baseline and abnormal values were calculated for other safety parameters. Safety was also evaluated in subgroups of patients with specific concomitant treatments for narcolepsy such as CNS stimulants and/or anti-cataplectic drugs.
Efficacy results were reported based on pitolisant treatment history (de novo versus exposed patients) and concomitant treatments during the 12-month period. Changes from baseline were calculated. Responders on EDS at 12 months (patients with an ESS difference between baseline and final evaluation ≥3) were compared to nonresponders in terms of baseline characteristics to identify a potential profile of responders on ESS if any. The same analysis was performed in patients having reached a normal daytime sleepiness (final ESS ≤ 10).
Missing values at baseline (D0) for vital signs, BDI, ESS, CGI-Severity, and EQ-5D were replaced by value at screening (D-7) if available. For ESS and in case of premature withdrawal, missing data at V7 (12-month visit) were replaced using the Last Observation Carried Forward (LOCF) method. A sensitivity analysis without any replacement was also performed.
To compare results with other studies, the effect sizes of change in time were calculated as the standardizing mean changes (and 95% CI) for ESS and cataplexy at final values.
We used SAS stat package version 9.3 (Copyright 2002–2010 by SAS Institute Inc., Cary, NC).
The significance of difference of proportions was assessed by chi-square or Fisher exact tests. For continuous endpoints, the normality was first checked with the Shapiro-Wilk test on untransformed and log-transformed values (in case of expected log-normality). T-tests were used in case of normality and Mann-Whitney tests otherwise. No type 1 error correction was used.
Role of the funding source
Bioprojet funded and monitored the trial.
Results
Participants
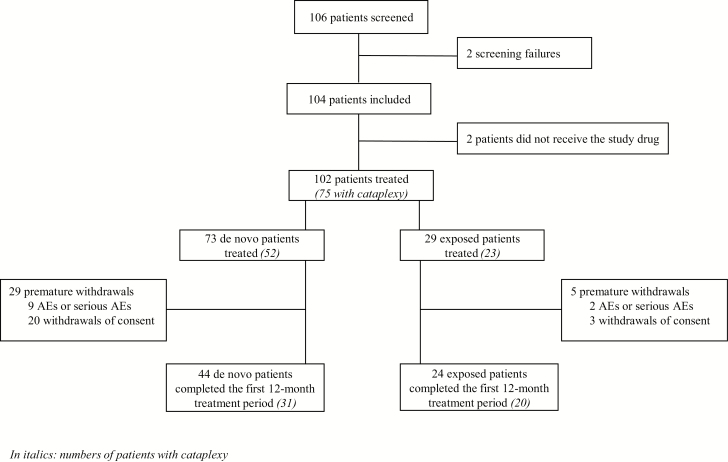
Between May 2011 and October 2013, 104 patients (79 in France, 25 in Hungary) were included, of whom 102 received pitolisant (Figure 1). The group included 73 de novo patients (52 with cataplexy) and 13 exposed patients (11 with cataplexy) with a period of at least 3 months without pitolisant between a previous participation in a pitolisant trial (except one with only a 1-week washout); all 86 patients had an up-titration at the start of the present study. The other 16 exposed patients (12 with cataplexy) were directly switched from the French CUP and were included at their previous established dose without titration. Hence the length of exposure to pitolisant was longer for the subgroup of previously exposed patients (mean 548 days ± 308 days) as some of them were treated since more than 1 year in the CUP before being enrolled in this study, whereas “de novo” patients were exposed for a maximum of 1 year (mean 260 ± 143 days). Two thirds (N = 68) of treated patients completed the 12-month treatment period: 60.3% of the de novo patients (N = 44, 31 with cataplexy) and 82.8% of the previously exposed patients (N = 24, 20 with cataplexy, Figure 1).
Figure 1.
Study flow chart.
At inclusion, as expected, the subgroup of exposed patients (N = 29), including those already treated in the CUP, had a lower mean ESS score than de novo patients (Table 1) namely in relation with the lower value in the CUP patients (14.6 ± 0.8). They also had a better health status evaluated with EQ-5D VAS and less depressive symptoms as assessed by a lower BDI-13 score. Eighteen patients of the whole population (17.6%) had history of depression or depressive syndrome, with 9 (8.8%) suffering from an ongoing depression at baseline. As it was a naturalistic trial, other medications were allowed and, at inclusion, a large number of patients (35.3%) were taking narcolepsy co-medications such as CNS stimulants and/or anti-cataplectic drugs, in addition to pitolisant in both subgroups (Table 1). During the 12 months of treatment, 52.9% of patients had such co-medications, the most frequent being methylphenidate (22.5%) and modafinil (17.6%) (Table 1). The co-medications taken at inclusion remained unchanged during the study in 37% of patients, increased (or new treatment added) in 50%, decreased in 7.4% or were discontinued in 5.5% (Table 1).
Table 1.
Demographic and clinical characteristics of patients at baseline (intent-to-treat population)
| Measure | De novo patientsa (N = 73) | Exposed patientsb (N = 29) | Between-group p-value |
|---|---|---|---|
| Age (years), Mean (SE) | 38.5 (1.70) | 36.8 (2.95) | 0.481 |
| Men, n (%) | 31 (42.5) | 14 (48.3) | 0.594 |
| Weight (kg) at inclusion, Mean (SE) | 74.8 (2.06) | 81.8 (4.01) | 0.223 |
| Duration since narcolepsy diagnosis (years) (SE) | 12.3 (1.56) | 13.8 (1.76) | 0.595 |
| QTc interval on EKG at screening, Mean (SE) | 405 (2.96) | 410.5 (5.35) | 0.291 |
| ESS score at inclusion, Mean (SE) | 17.6 (0.35) | 15.6 (0.54) | 0.004 |
| CUP patients sub group | 14.50 (0.8) | ||
| History of cataplexy, n (%) | 52 (71.2) | 23 (79.3) | 0.404 |
| History of treatments for narcolepsy – Stimulants prior inclusion, n (%) | |||
| Modafinil | 54 (74.0) | 26 (89.7) | |
| Methylphenidate | 41 (56.2) | 25 (86.2) | |
| Mazindol | 12 (16.4) | ||
| Dextroamphetamine | 2 (2.7) | 3 (10.3) | |
| History of treatments for cataplexy prior inclusion, n (%) | |||
| Sodium oxybate | 16 (21.9) | 11 (37.9) | |
| SSRI | 3 (4.1) | 2 (6.9) | |
| Venlafaxine | 5 (6.8) | 4 (13.8) | |
| Daily number of generalized cataplexy at inclusion (sleep diary), n | 58c | 13c | |
| Mean value (SE) | 0.25 (0.18) | 0.09 (0.09) | 0.682 |
| Daily number of partial cataplexy at inclusion (sleep diary), n | 58 | 13 | |
| Mean value (SE) | 0.64 (0.22) | 0.66 (0.40) | 0.970 |
| Multiple sleep latency test, n | 67 | 24 | |
| Mean sleep latency, minute (SE) | 5.3 (0.32) | 4.8 (0.53) | 0.454 |
| History of associated symptoms, n (%) | |||
| Hallucinations | 43 (58.9) | 17 (58.6) | 0.979 |
| Automatic behaviors | 33 (45.2) | 13 (44.8) | 0.972 |
| Dyssomnia | 37 (50.7) | 17 (58.6) | 0.469 |
| Sleep paralysis | 37 (50.7) | 13 (44.8) | 0.593 |
| Clinical general impression severity of narcolepsy at inclusion, n | 72 | 25 | |
| 3 = mildly ill, n (%) | 1 (1.4) | 3 (12.0) | 0.169 |
| 4 = moderately ill, n (%) | 10 (13.9) | 5 (20.0) | |
| 5 = markedly ill, n (%) | 23 (31.9) | 6 (24.0) | |
| 6 = severely ill, n (%) | 35 (48.6) | 11 (44.0) | |
| 7 = among the most extremely ill, n (%) | 3 (4.2) | - | |
| Quality of life (EQ-5D VAS score) at inclusion, Mean (SE) | 62.2 (1.92) | 74.8 (2.12) | <0.001 |
| Beck Depression Inventory – 13 Item Score at inclusiond, n | 71 | 25 | 0.013 |
| Mean (SE) | 4.8 (0.53) | 2.8 (0.58) | |
| Ongoing treatment for sleepiness or cataplexy at inclusion, n (%) | 26 (35.6) | 10 (34.5) | |
| Methylphenidate | 10 (13.7) | 3 (10.3) | |
| Modafinil | 8 (11.0) | 2 (6.9) | |
| Venlafaxine | 8 (11.0) | 1 (3.4) | |
| Sodium oxybate | 3 (4.1) | 4 (13.8) | |
| Mazindol | 1 (1.4) | 2 (6.9) | |
| Citalopram | 1 (1.4) | - | |
| Fluoxetine | 1 (1.4) | - | |
| Concomitant treatment for sleepiness or cataplexy during the trial, n (%) | 40 (54.8) | 14 (48.3) | |
| Methylphenidate | 18 (24.7) | 5 (17.2) | |
| Modafinil | 15 (20.5) | 3 (10.3) | |
| Venlafaxine | 12 (16.4) | 2 (6.9) | |
| Sodium oxybate | 6 (8.2) | 5 (17.2) | |
| Mazindol | 3 (4.1) | 1 (3.4) | |
| Escitalopram | 3 (4.1) | 2 (6.9) | |
| Paroxetine, Citalopram, Fluoxetine | 3 (4.1) | 2 (6.9) | |
| Clomipramine | 1 (1.4) | - |
Data are median (range), mean ± SE, or n (%). MSLT = Multiple Sleep Latency Test; EDS = Excessive Daytime Sleepiness; BDI = Beck Depression Inventory; ESS = Epworth Sleepiness Scale. Significant p-values are bolded.
aDe novo: patients naïve to pitolisant prior to this study.
bExposed: 13 patients having received pitolisant in previous trial or 16 patients already treated in CUP.
cNumber of patients for which the information was available (including patients without history of cataplexy).
dOne de novo patient was included despite a BDI score of 24 and item G > 0 (3 at screening; 1 at inclusion); this was a major deviation. Nevertheless, as the primary endpoint was safety, this patient was taken into account in the analysis.
The protocol allowed individual dose adjustment with an up-titration for patients who were not receiving pitolisant at baseline through the CUP. At 3 months of treatment, 67.5% (56/83) of patients were taking 40 mg pitolisant OD. At the end of the 12 months, 76.5% (52/68) of the completers were treated with the 40 mg daily dose and among them 65.4% were on monotherapy. Patients took in average 90% of the prescribed dose throughout their participation, reflecting a good compliance on the treatment.
Overall, 34 (33.3%) patients prematurely discontinued the trial, mainly during the first 3 months (31/34), including 29 de novo patients (39.7% of this subgroup) and five (17.2%) exposed patients. The most frequently reported reason was a perceived insufficient efficacy in 20 patients (18 de novo patients, two exposed). However, a treatment response was noted in five of them by their ESS score (decrease of at least three points between the inclusion and withdrawal) in spite of perceived inefficacy. In addition, 11 patients discontinued for AEs and three for other reasons.
Safety
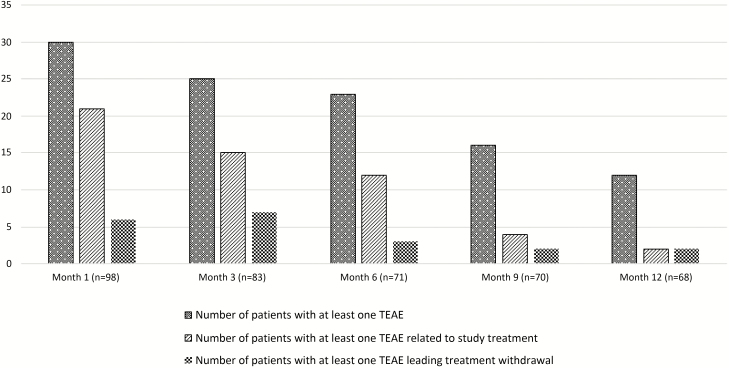
Pitolisant was well tolerated overall when used either as monotherapy or in association with other anti-narcoleptic drugs. Approximately half the patients (N = 58; 56.9%) reported TEAEs (N = 168). The TEAE frequency tended to decrease along treatment: 54.8% (92/168) of them were observed during the first 3 months and 12.5% (21/168) during the last 3 months (Figure 2). Overall, 43.5% of TEAEs were considered related to the study drug, and included mostly psychiatric (38.4% of the related TEAEs) and nervous system disorders (20.5%). The most common ones were headache (11.8% of patients), insomnia (8.8%), weight gain (7.8%), anxiety (6.9%), depressive symptoms (4.9%), and nausea (4.9%) (Table 2). Most TEAEs were mild to moderate; only 22 (13.1%) were severe, of which only half were considered related to the study drug: migraine (n = 2), insomnia (1), irregular sleep (1), nausea (1), depression (1), rash (1), vertigo (1), libido decrease (1), premature ejaculation (1), spontaneous abortion (1). All patients with a severe TEAE recovered except one (non-serious libido decrease), either spontaneously (18% of them), or after temporary (41%) or permanent (41%) discontinuation of pitolisant. Seven patients (6.9%) experienced a serious TEAE, always non-life-threatening (Table 2). They all recovered, either spontaneously (3/7), or after temporary (3/7) or permanent (1/7) discontinuation of pitolisant. All serious TEAEs were unrelated to pitolisant, except one miscarriage that was possibly related. The proportion of patients with treatment-related TEAEs was twice greater in the subgroup who took additional anti-narcoleptic agents in comparison to patients treated with pitolisant alone (53.7% versus 29.2%, p = 0.012) (Table 2). TEAE frequency or nature was not substantially increased or different in other specific subpopulations such as the elderly (≥65 years) patients, patients with depressive symptoms at inclusion, patients with cardiovascular or gastrointestinal disorders, renal impairment, hepatic impairment, patients with allergies, patients receiving a concomitant treatment with a possible cytochrome P450 interaction (e.g. paroxetine), or patients treated with selective serotonin reuptake inhibitors only. Five cases of depression were reported during the 12-month treatment period. Two of them were considered related to the study drug whereas one case occurred after 1 day of treatment at the lowest dosage (5 mg OD) and was judged unlikely related and the two others were aggravation of preexisting depression judged as non-related to the study drug by the investigators. The BDI was also used for all patients throughout the study to evaluate any potential worsening of mood. The mean BDI score remained stable between the baseline (4.1 ± 0.42) and the end of 12-month treatment period (3.8 ± 0.49). Moreover, the proportion of patients with moderate or severe depressive symptoms (defined as a BDI score of 8 or greater) was relatively stable during the trial (16.6% at baseline versus 19.1% at 12 months). The Data Safety Monitoring Board did not issue any concern about depression during data reviews that took place on a regular basis to assess any safety risk during the study. No safety issue was identified regarding vital signs, physical examination, blood chemistry, and hematological parameters (data not shown). ECG were performed at entry in the study (baseline) and at 6 and 12 months, and patients who completed the 12 months of treatment did not show any significant change, including in the QTc (409 ± 25 ms at baseline and 416 ± 25 ms after 12 months—data available for 67/68 patients).
Figure 2.
Changes over 12 months in the number of TEAEs.
Table 2.
Number of patients with at least one TEAE when on pitolisant as monotherapy or combined with narcolepsy co-medication at least once during the trial (safety population)
| Parameter | Patients on pitolisant monotherapya N = 48 |
Patients with additional anti-narcolepsy treatmentb N = 54 |
Between-group test |
|---|---|---|---|
| All TEAEs (treatment related or not) | 20 (41.7) | 38 (70.4) | 0.003 |
| Headache | 4 (8.3) | 8 (14.8) | |
| Insomnia | 4 (8.3) | 5 (9.3) | |
| Weight increase | 3 (6.3) | 5 (9.3) | |
| Anxiety | 3 (6.3) | 4 (7.4) | |
| Depression | 1 (2.1) | 4 (7.4) | |
| Irritability | 0 (0.0) | 4 (7.4) | |
| Nausea | 2 (4.2) | 3 (5.6) | |
| Vertigo | 1 (2.1) | 3 (5.6) | |
| Vomiting | 2 (4.2) | 2 (3.7) | |
| Treatment related TEAEs | 14 (29.2) | 29 (53.7) | 0.012 |
| Psychiatric disorders | 7 (14.6) | 13 (24.1) | |
| Insomnia | 3 (6.3) | 5 (17.2) | |
| Irritability | 0 (0.0) | 4 (13.8) | |
| Anxiety | 2 (4.2) | 3 (10.8) | |
| Nervous system disorders | 3 (6.3) | 11 (20.4) | |
| Headache | 3 (6.3) | 6 (20.7) | |
| Investigations (weight increase or decrease) | 3 (6.3) | 6 (11.1) | |
| Weight increasedc | 3 (6.3) | 4 (13.8) | |
| Gastrointestinal disorders | 5 (10.4) | 1 (1.9) | |
| Severe TEAEs (treatment related or not) | 6 (12.5) | 9 (16.7) | 0.553 |
| Serious TEAEs (treatment related or not) | 2 (4.2) | 5 (9.3) | 0.442 |
| TEAEs leading to treatment withdrawal (treatment related or not) | 9 (18.8) | 10 (18.5) | 0.976 |
| TEAEs leading to treatment withdrawal (treatment-related) | 7 (14.6) | 4 (7.4) |
Data are number of patients (%) or p-value. Significant p-values are bolded.
aPatients who did not take any concomitant psychostimulant or anti-cataplectic agent.
bPatients who received concomitant psychostimulant (modafinil, methylphenidate, or mazindol) and/or anti-cataplectic (sodium oxybate, SSRIs, or clomipramine) agent.
cBetween 2% and 12% of body weight.
Efficacy
Sleepiness.
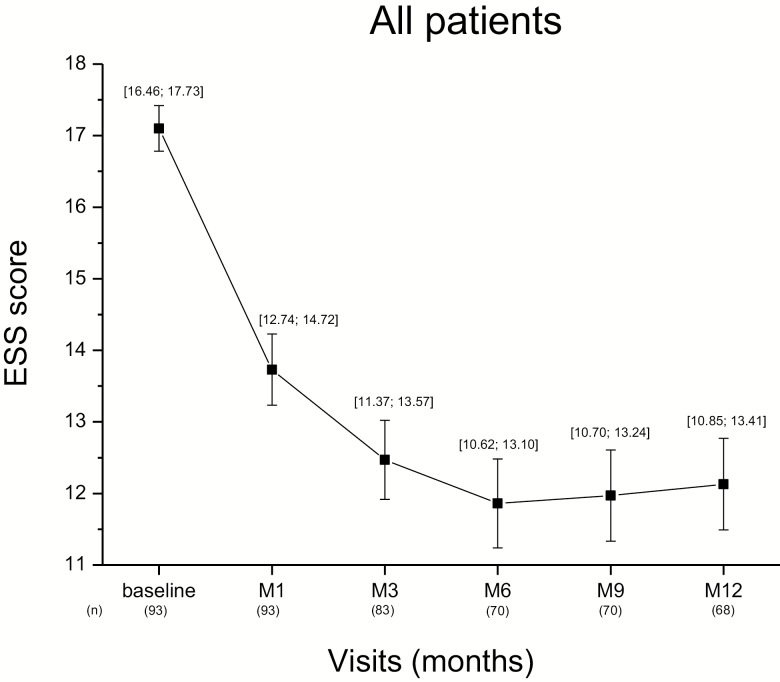
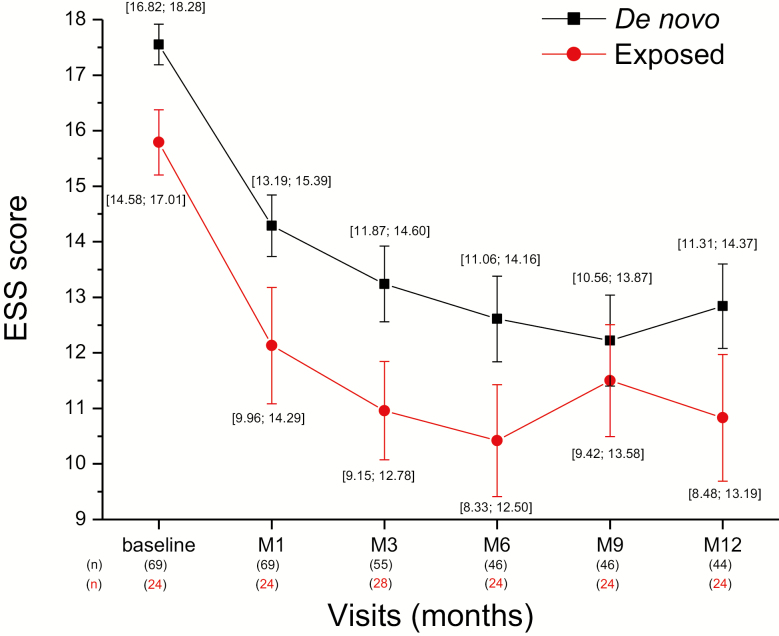
The ESS score, assessed at each visit, decreased along the 12-month period (Figure 3). Compared to baseline, the mean score (±SE) decreased from the first month of treatment (−3.37 ± 0.42; n = 93) and continued to decline after 3 (−4.39 ± 0.51) and 6 months (−4.90 ± 0.54). This change occurred at a similar rate in the de novo or previously exposed patients (Figure 4). In the whole patient population who completed the 12-month treatment (n = 68), the mean decrease from baseline in ESS score was −4.6 ± 0.59 at the end of the period (Table 3). With LOCF method applied to the missing data of the whole population (N = 102, i.e. taking into account the patients having left the trial before 12 months), the reduction was −4.0 ± 0.49. The decrease was significant whether patients had previously been exposed to pitolisant or not (p < 0.001 for both) and of similar magnitude in both subgroups (−4.2 and −4.9, respectively; Figure 5). The improvement of EDS was observed whether the patient was treated with pitolisant only or in combination with other treatments for narcolepsy such as sodium oxybate, stimulants or antidepressants used as anti-cataplectic agents. The decline in ESS score was clinically relevant (≥3 units) in all subgroups except one and the highest improvement was obtained in de novo patients on pitolisant monotherapy (−6.5 ± 1.3) whereas the smallest improvement was observed in de novo patients receiving sodium oxybate as co-medication (Table 3). However, the number in the last subgroup was too small to make a firm conclusion.
Figure 3.
Epworth sleepiness score over 12 months in the total intention-to-treat population, without replacement of missing values. Data are the mean (±SE) and (95% CI) of number of patients (n).
Figure 4.
ESS score over 12 months in the subgroups of de novo and previously exposed to pitolisant patients intention-to-treat, without replacement of missing values. Data are the means (±SE) and (95% CI) of values.
Table 3.
Efficacy results: changes in ESS, CGI, and EQ-5D VAS scores between baseline and 12 months in de novo and exposed patients on monotherapy or receiving various narcolepsy co-medications (intent-to-treat population)
| Endpoint | De novo patients (N = 44) | Exposed patients (N = 24) | Total (N = 68) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n | Baselinea | Finala | Changea | p-value | n | Baselinea | Finala | Changea | p-value | n | Baselinea | Finala | Changea | |
| ESS (without replacement of missing values) | ||||||||||||||
| Whole population | ||||||||||||||
| ESS score, mean (SE) | 44 | 17.7 ± 0.5 | 12.8 ± 0.7 | −4.9 ± 0.7 | <0.001 | 24 | 15.0 ± 0.6 | 10.8 ± 1.1 | −4.2 ± 1.1 | 0.001 | 68 | 16.8 ± 0.4 | 12.1 ± 0.6 | −4.6 ± 0.6 |
| 95% CI | [16.78; 18.68] | [11.31; 14.37] | [−6.29; −3.49] | [13.71; 16.29] | [8.48; 13.19] | [−6.50; −1.84] | [15.95; 17.58] | [10.85; 13.41] | [−5.82; −3.44] | |||||
| Respondersb, n (%) | 44 | 31 (70.5) | 24 | 13 (54.2) | 68 | 44 (64.7) | ||||||||
| Normalizedb, n (%) | 44 | 12 (27.3) | 24 | 13 (54.2) | 68 | 25 (36.8) | ||||||||
| Concomitant sodium oxybate subgroupc | ||||||||||||||
| ESS score, mean (SE) | 4 | 18.0 ± 2.0 | 16.0 ± 3.1 | −2.0 ± 1.7 | 0.320 | 5 | 15.2 ± 1.3 | 10.0 ± 2.5 | −5.2 ± 2.7 | 0.063 | 9 | 16.4 ± 1.2 | 12.7 ± 2.1 | −3.8 ± 1.7 |
| 95% CI | [11.50; 24.50] | [6.19; 25.81] | [−7.36; 3.36] | [11.43; 18.97] | [2.92; 17.08] | [−12.82; 2.42] | [13.67; 19.22] | [7.77; 17.56] | [−7.69; 0.14] | |||||
| Respondersb, n (%) | 4 | 2 (50.0) | 5 | 3 (60.0) | 9 | 5 (55.6) | ||||||||
| Concomitant psychostimulants only subgroupc | ||||||||||||||
| ESS score | 14 | 17.9 ± 0.9 | 14.2 ± 1.1 | −3.7 ± 0.9 | 0.001 | 4 | 15.5 ± 2.2 | 11.8 ± 4.2 | −3.8 ± 3.1 | 0.312 | 18 | 17.4 ± 0.8 | 13.7± 1.2 | −3.7 ± 0.9 |
| 95%CI | [16.01; 19.84] | [11.71; 16.72] | [−5.64; −1.79] | [8.32; 22.68] | [−1.84; 25.34] | [−13.59; 6.09] | [15.59; 19.19] | [11.02; 16.31] | [−5.67; −1.78] | |||||
| Respondersb, n (%) | 14 | 8 (57.1) | 4 | 2 (50.0) | 18 | 10 (55.6) | ||||||||
| Concomitant anti-cataplectics only subgroupc | ||||||||||||||
| ESS score, mean (SE) | 7 | 18.0 ± 0.9 | 14.4 ± 1.7 | −3.6 ± 1.4 | 0.039 | 4 | 15.8 ± 1.6 | 9.8 ± 3.3 | −6.0 ± 3.4 | 0.175 | 11 | 17.2 ± 0.9 | 12.7 ± 1.6 | −4.5 ± 1.4 |
| 95% CI | [15.61; 20.39] | [10.40; 18.45] | [−6.90; −0.24] | [10.66; 20.84] | [−0.67; 20.17] | [−16.79; 4.79] | [15.24; 19.12] | [9.06; 16.40] | [−7.68; −1.23] | |||||
| Respondersb, n (%) | 7 | 5 (71.4) | 4 | 3 (75.0) | 11 | 8 (72.7) | ||||||||
| Concomitant psychostimulants and anti-cataplecticsc | ||||||||||||||
| ESS score, mean (SE) | 6 | 20.5 ± 0.7 | 15.8 ± 1.2 | −4.7 ± 1.0 | 0.006 | 4 | 12.8 ± 1.4 | 9.3 ± 0.6 | −3.5 ± 1.3 | 0.077 | 10 | 17.4 ± 1.4 | 13.2 ± 1.3 | −4.2 ± 0.8 |
| 95% CI | [18.54; 22.46] | [12.76; 18.90] | [−7.29; −2.04] | [8.18; 17.32] | [7.25; 11.25] | [−7.71; 0.71] | [14.14; 20.66] | [10.26; 16.14] | [−5.98; −2.42] | |||||
| Respondersb, n (%) | 6 | 5 (83.3) | 4 | 3 (75.0) | 10 | 8 (80.0) | ||||||||
| Pitolisant monotherapy | ||||||||||||||
| ESS score, mean (SE) | 17 | 16.5 ± 0.7 | 10.0 ± 1.3 | −6.5 ± 1.4 | <0.001 | 12 | 15.3 ± 0.7 | 11.4 ± 1.6 | −3.9 ± 1.7 | 0.048 | 29 | 16.0 ± 0.5 | 10.6 ± 1.0 | −5.4 ± 1.1 |
| 95% CI | [14.92; 18.02] | [7.26; 12.74] | [−9.57; −3.37] | [13.68; 16.99] | [7.88; 14.95] | [−7.79; −0.04] | [14.91; 17.09] | [8.54; 12.63] | [−7.73; −3.10] | |||||
| Respondersb, n (%) | 17 | 13 (76.5) | 12 | 5 (41.7) | 29 | 18 (62.1) | ||||||||
| ESS (with replacement of missing valuesd) | ||||||||||||||
| ESS score, n (%) | 69 | 17.6 ± 0.3 | 13.6 ± 0.5 | −3.9 ± 0.5 | <0.001 | 29 | 15.1 ± 0.6 | 10.9 ± 0.9 | −4.3 ± 1.0 | <0.001 | 98 | 16.8 ± 0.3 | 12.8 ± 0.5 | −4.0 ± 0.46 |
| 95% CI | [16.82;18.28] | [12.48; 14.76] | [−4.94; −2.91] | [13.98; 16.30] | [8.92; 12.81] | [−6.29; −2.27] | [16.19; 17.48] | [11.81; 13.81] | [−4.94; −3.12] | |||||
| Improvement at CGI of changee (score = 1, 2, or 3) | 44 | 41 (93.2) | 23 | 22 (95.6) | 67 | 63 (94.0) | ||||||||
| EQ-5D VAS, mean (SE) 95% CI |
44 | 62.1 ± 2.4 [57.2; 67.0] |
71.2 ± 2.6 [65.9; 76.5] |
9.1 ± 2.3 [4.5; 13.7] |
<0.001 | 24 | 71.8 ± 3.0 [65.6; 78.0] |
74.5 ± 2.9 [68.4; 80.5] |
2.7 ± 3.1 [−3.7; 9.0] |
0.395 | 68 | 65.5 ± 1.9 [61.6; 69.4] |
72.4 ± 2.0 [68.4; 76.3] |
6.8 ± 1.8 [3.1; 10.6] |
ESS = Epworth Sleepiness Scale; CGI of change = Clinical Global Impression of change; EQ-5D = European Quality of life questionnaire (100 corresponds to the best health and 0 to the worst). Significant p-values are bolded.
aBaseline, final, change: mean ± SD or n (%); Change: Change from Baseline.
bResponders were defined as patients with ESSB – ESSF ≥ 3; Normalized patients were patients with ESSF ≤ 10.
cSubgroup of patients with concomitant sodium oxybate = patients who received sodium oxybate at least once in addition to pitolisant; Subgroup of patients with concomitant psychostimulants = patients who received modafinil, methylphenidate, or mazindol in addition to pitolisant, but no other concomitant anti-cataplectic agent, during the study; Subgroup of patients with concomitant anti-cataplectic agents = patients who received sodium oxybate, SSRIs, or clomipramine in addition to pitolisant, but no other concomitant psychostimulant, during the study; Subgroup of patients with concomitant psychostimulants and anti-cataplectics = patients who received both concomitant psychostimulants and anti-cataplectic agents in addition to pitolisant during the study; Subgroup of patients with concomitant SSRIs = patients who received SSRIs for narcolepsy, in addition to pitolisant, but no other treatment for narcolepsy; Subgroup of patients with cataplexy and concomitant SSRIs = patients who had history of cataplexy at baseline and took concomitant SSRIs at least once during the study.
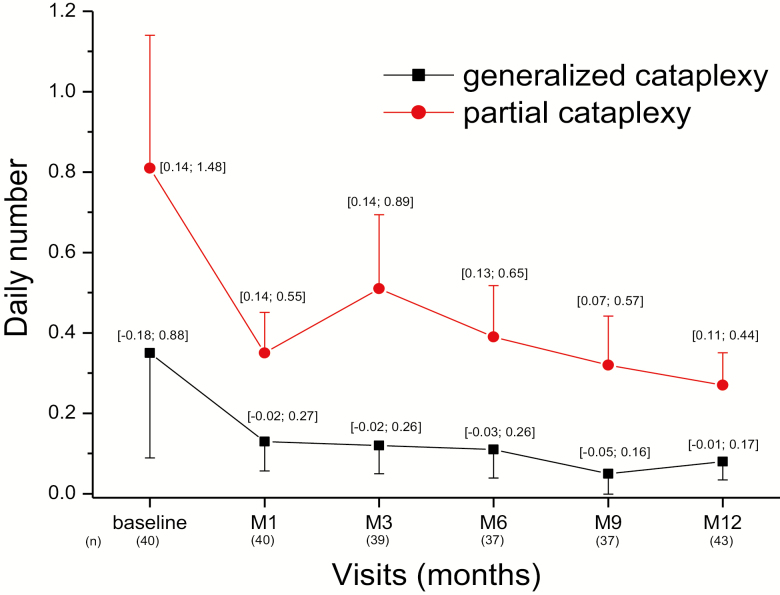
Figure 5.
Frequency of episodes of generalized and partial cataplexy over time for the whole ITT population. Data are the means (±SE) and (95% CI) in 43 patients with cataplexy who completed the diaries until the 12-month visit.
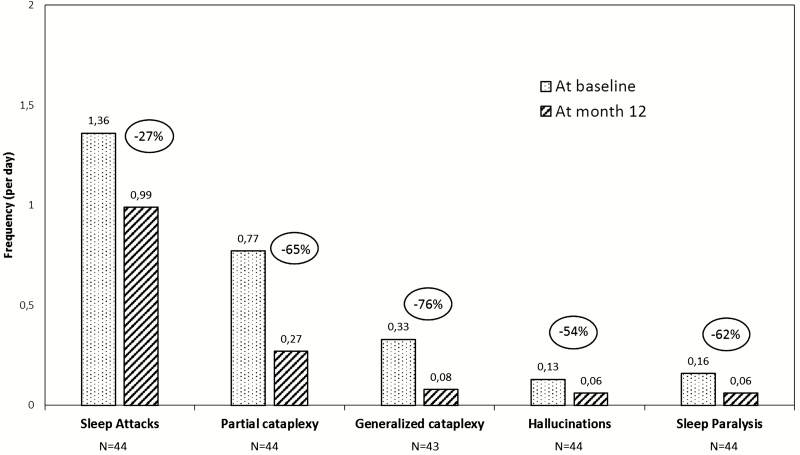
At the end of the 12-month treatment period, two-thirds of patients were responders for ESS with minimum decrease of 3 units; the highest responder rate was observed in the de novo subgroup (70.5%). More than one-third of patients (25/68) had normalized sleepiness (ESS < 11) at 12 months (27.3% for de novo patients and 54.2% for exposed patients); their mean ESS score decreased from 15.3 ± 0.6 at baseline to 6.6 ± 0.6 at 12 months. Responders were less likely to have a history of automatic behavior (32.6% versus 60.0%, chi-square test p = 0.027) and of irregular sleep (41.9% versus 72.0%, p = 0.016) at baseline than nonresponders had. Patients with normalized ESS had less often a history of hallucinations (44.0% versus 69.8%, p = 0.036) and of automatic behavior (24.0% versus 53.5%, p = 0.018) at baseline than non-normalized patients. Other baseline characteristics (age, gender, disease course since narcolepsy diagnosis, history and frequency of complete or partial cataplexy, mean sleep latency at the multiple sleep latency test, preexisting co-medications for narcolepsy) were similar between responders and non-responders as well as between patients with and without normalized ESS (data not shown). In the 44 patients (among 68) who completed a diary at 12 months, the mean daily number of sleep attacks decreased by 27% (from 1.36 ± 0.21 to 0.99 ± 0.14; change −0.37; 95% CI [−0.80; 0.06]).
Cataplexy.
The daily frequency of cataplexy was collected in 44 of the 68 patients (including only one CUP patient) who completed the 12-month visit. All cataplexy measures improved. The mean daily number of generalized cataplexy episodes decreased by 76% between baseline and 12 months (from 0.33 ± 0.25 to 0.08 ± 0.05 per day; change −0.25; 95% CI [−0.67; 0.17]), and by 65% (from 0.77 ± 0.37 to 0.27 ± 0.08 per day; change −0.49; 95% CI [−1.09; 0.10]) for partial cataplexy (Figure 5). The mean daily number of all (generalized and partial) cataplexy episodes decreased by 68% between baseline and 12 months (1.09 ± 0.53 to 0.35 ± 0.10 per day; p = 0.055). When selecting patients with partial cataplexy at baseline (N = 39), the mean geometric rate ratio between 12 months and baseline was 0.29 (95% CI [0.16, 0.52], p < 0.001, paired t-test on log-counts difference), thus the rate of partial cataplexy reduced of 71% from baseline. When restricted to patients with generalized cataplexy at baseline (n = 14), the mean geometric rate ratio between 12 months and baseline was 0.12 (95% CI [0.04, 0.29], p < 0.001), thus the rate of generalized cataplexy reduced of 88% from baseline. These results were consistent among narcolepsy co-medications subgroups, the greatest decrease being observed in patients receiving pitolisant alone (Table 4). If one considers the subgroup of de novo patients on pitolisant monotherapy (N = 15), generalized and partial cataplexy attacks were reduced by 80% (0.71 to 0.14 per day) and 82% (0.93 to 0.17 per day), respectively. The effect size of the studied drug on cataplexy change measured by the standardized mean of the difference was 0.78 (95% CI [0.13–1.43]).
Table 4.
Cataplexy change between baseline and M12 in the pitolisant monotherapy and co-medications for narcolepsy subgroups (ITT population – LOCF)
| Subgroups (Concomitant treatments) | Pitolisant+stimulants (n = 11) (modafinil, methylphenidate, mazindol) | Pitolisant+Anti-cataplectics (n = 7) (sodium oxybate, SSRIs, clomipramine) | Pitolisant+stimulants and anti-cataplectics (n = 4) (modafinil, methylphenidate, mazindol, SSRIs) | Pitolisant monotherapy (n = 21) (No additional narcolepsy treatment) | ||||
|---|---|---|---|---|---|---|---|---|
| Generalized CTP | Partial CTP | Generalized CTP | Partial CTP | Generalized CTP | Partial CTP | Generalized CTP | Partial CTP | |
| Baseline | ||||||||
| Mean nb cataplexies per day (SE) | 0.12 (0.07) | 0.25 (0.16) | 0.08 (0.05) | 0.83 (0.48) | 0.09 (0.18) | 0.97 (0.86) | 0.56 (0.48) | 0.98 (1.0) |
| Final (12-month) | ||||||||
| Mean nb of cataplexies per day (SE) | 0.00 (0.00) | 0.26 (0.15) | 0.00 (0.00) | 0.43 (0.33) | 0.04 (0.02) | 0.23 (0.06) | 0.16 (0.15) | 0.24 (0.17) |
| Change from baseline | ||||||||
| Mean nb of cataplexies per day (SE) 95% CI |
−0.12 (0.07) [−0.29; 0.06] |
0.02 (0.01) [−0.22; 0.25] |
−0.08 (0.14) [−0.21; 0.05] |
−0.40 (1.69) [−1.96; 1.16] |
−0.05 (0.07) [−0.39; 0.28] |
−0.74 (0.26) [−1.87; 0.40] |
−0.41 (0.72) [−1.30; 0.48] |
−0.74 (0.27) [−1.92; 0.43] |
| Variation | −100% | +4% | −100% | −48% | −66% | −77% | −71.5% | −75.5% |
Other symptoms.
The mean frequency of hallucinations decreased by 54% between baseline and 12 months (from 0.13 ± 0.06 to 0.06 ± 0.03 per day; change −0.06; 95% CI [−0.14; 0.01]). The mean frequency of sleep paralysis was reduced by 63% (from 0.16 ± 0.06 to 0.06 ± 0.04, change −0.10; 95% CI [−0.21; 0.00] p = 0.023 Figure 6). The health status (self-evaluated using the EQ-5D VAS) improved in de novo (from 62.1 ± 2.4 at baseline to 71.2 ± 2.6 at 12 months, p < 0.001) patients and, to a lesser extent, in previously exposed patients (from 71.8 ± 3.0 at baseline to 74.5 ± 2.9 at 12 months; Table 3). The Clinical Global Impression of Change improved for almost all patients who completed the 12-month treatment period (93.2% and 95.6% of de novo and exposed patients, respectively; Table 3). The total duration of nocturnal sleep remained unchanged.
Figure 6.
Changes in narcolepsy symptoms after a 12-month treatment with pitolisant. Results for complete and partial cataplexy episodes (in the subgroup of patients having cataplexy, i.e. at least one episode at baseline or during the treatment period [n = 43] and having completed the 12-month treatment). Results for other symptoms are described in 44 patients who correctly filled out their sleep diaries until the 12-month visit.
Discussion
This open-label multicentric study was conducted in a real-world setting and naturalistic conditions, with prospective longitudinal follow-up for 12 months to assess the long-term safety and effectiveness of pitolisant given in a pragmatic way either in de novo patients (the largest selected group here), or in patients previously exposed to pitolisant. The main objective was to confirm the long-term safety profile of pitolisant administered in real-life conditions in adult narcoleptic patients with and without cataplexy, some of whom were being treated with other narcolepsy medications (such as stimulants, antidepressants used as anti-cataplectics, and sodium oxybate) but were still experiencing persistent sleepiness.
In this realistic study, the good safety and tolerability profile of pitolisant was confirmed over the 12-month treatment period in a majority of patients. The safety results of this trial are consistent with those reported in previous studies when pitolisant was administered for a shorter duration [17, 18]. No new adverse event was identified. The frequency of adverse event was highest during the first 3 months in patients with monotherapy or with combined therapies, indicating that practitioners and patients should be more attentive to them during these first months. In total, 10.8% of patients dropped out from the trial related to adverse events (11/102 patients), mostly during this period. After these 3 months, emergent adverse effects were rare. TEAEs were minor and the most common (>5%) were headache, insomnia, weight gain, and anxiety. Notably, only 43.5% of them were considered related to the study drug. Adverse events were mainly disorders from the psychiatric (insomnia, irritability, anxiety, depression) and nervous system (headache) systems which is consistent with the high selectivity of pitolisant for the H3 receptor and the expression of the latter essentially restricted to the CNS [9, 17]. Treatment-emergent depressive symptoms during this 1-year treatment were observed in five patients (4.9%) but only two were considered as possibly related to pitolisant, all stopped when stopping the drug, did not lead patients to suicidal ideas and the mean Beck Depression Index was not modified. Notably, no specific comorbid condition influenced the TEAEs frequency or nature. In particular, patients suffering from depression or depressive symptoms prior to pitolisant did not seem to be exposed to a higher risk of psychiatric side effects when treated with pitolisant (with a caveat due to the relatively low number of patients exposed both to depression and pitolisant). One miscarriage, possibly related to pitolisant, was observed. As most miscarriages occur spontaneously in the general female population, it is impossible to definitively link miscarriage risk and pitolisant, but this isolated observation reinforces the caveat of stopping the drug when pregnancy is planned. Adverse events were almost twice as frequent when pitolisant was combined with stimulants and anti-cataplectic drugs than when it was taken alone. These side effects could be caused by pitolisant itself, by the concomitant medications (modafinil, sodium oxybate, methylphenidate), which are also known to induce headaches, insomnia, and anxiety or to additive effects of these combinations. The low number of each adverse event together with the large variety of associated drugs does not allow any conclusion regarding the responsibility of any association. All in all, this result suggests to be more cautious when using pitolisant as an add-on drug in patients with narcolepsy. However, very few adverse effects (e.g. headaches) were important enough to stop pitolisant. They disappeared after discontinuing the drug, indicating that, adverse events do not persist when the drug is stopped.
This study confirms the efficacy of pitolisant for improving daytime sleepiness over a 12-month period. The improvement in EDS, as assessed by a reduction in the ESS, started during the first month of treatment, increased up to 6 months, and was maintained until the end of the 12-month period. This rather long time to achieve the maximum benefit on sleepiness (up to 6 months) is similar to that observed in trials of sodium oxybate, with a median time of 106 (85–164) days to reach a maximum effect [18]. The ESS improvement was in the same range as that found in these trials of sodium oxybate as well as in a long-term trial with modafinil [19].
A large proportion of patients who completed the 1-year treatment with pitolisant were responders on EDS, that is, they had a decrease of at least 3 points on ESS from baseline, whether they were receiving concomitant narcolepsy treatments or not. The highest decrease in mean ESS score was observed when pitolisant was given as monotherapy with a mean of 5.4 units. However, the change was also clinically relevant (mean decrease in ESS score ≥ 3) when stimulants and anti-cataplectic agents were concomitantly administered. Moreover, more than one-third of patients who took pitolisant for 12 months were normalized on sleepiness (i.e. ESS ≤ 10).
This study also confirms the significant improvement in the cataplexy frequency observed in previous short-term double-blind studies [8, 12, 13]. The daily frequency of both partial and generalized cataplexies decreased by 65% to 76% after 12 months of treatment, whether patients were treated with co-medications or not, and whether they had already been exposed to pitolisant or not. The decrease in partial cataplexies was the highest (76%) with pitolisant monotherapy, whereas generalized cataplexies were totally suppressed with associations of pitolisant with antidepressants or psychostimulants (Table 4). In de novo patients on pitolisant monotherapy, the decrease was even higher (80%). Similar results were found for hallucinations and sleep paralysis. These results demonstrate that pitolisant, used as monotherapy or in combination with other narcolepsy medications, is effective for improving not only EDS but also cataplexy, hallucinations and sleep paralysis in patients with narcolepsy, with a sustained effect over 1 year.
Our study has limitations. It is an open-label naturalistic trial without a placebo control for the formal assessment of efficacy and without laboratory testing of the efficacy on EDS, for example, using polysomnography and MWT. Another study limitation is the potential for selection bias, both in the patients who entered the study from the CUP who had been on pitolisant previously, as well as from those who dropped out, nearly one-third, during the 1-year treatment period. This level of drop out is common in real-life open-label studies in narcolepsy [3, 6, 19], especially among de novo subjects, our larger subgroup. Patients already exposed to pitolisant are more likely to be compliant, being a priori good responders with good tolerance, a bias that we tried to circumvent by separately analyzing naïve and previously exposed groups. Among the included patients, some were concomitantly treated with stimulants and anti-cataplectic drugs at inclusion or during the trial, which may also bring a risk of methodological bias, as it may select multi-resistant patients and may increase (as observed here) the risk of observing additional side effects. But this is the real-life for a clinician treating narcolepsy and the interaction should be disclosed, as some patients may need antidepressants for other reasons than cataplexy (e.g. depressive symptoms, anxiety, pain). Eventually, the results from several subgroup analysis, especially those in de novo patients, may allow us to generalize our findings to the patients with narcolepsy associated or not with cataplexy, and treated or not with combined therapies. Finally, since the trial started before the publication of ICSD-3, narcolepsy was diagnosed according to ICSD-2, and low CSF hypocretin-1 levels was not an inclusion criterion for the diagnosis of narcolepsy type 1.
In spite of these limitations, this first long-term pitolisant study in narcolepsy in realistic conditions confirms that this drug, given OD, is well tolerated, improves most major narcolepsy symptoms when given alone or in combination with other anti-narcoleptic agents, for a long period. It remains to be definitively determined whether it constitutes a useful first-line therapy for patients with narcolepsy.
Funding
The trial was funded by Bioprojet
Acknowledgments
We are grateful to the members of HARMONY-III study group: Isabelle Arnulf, Sleep Disorder Unit, Hôpital la Pitié-Salpêtrière, Paris, France (patients included = 32); Hélène Bastuji, Sleep Disorder Unit, Neurologic hospital, Bron, France (patients included = 5); Yves Dauvilliers, Sleep Unit, Department of Neurology, Gui-de-Chauliac Hospital, Montpellier, France (patients included = 19); Marie Françoise Vieccherini, Sleep Disorder unit, Hôtel Dieu, Paris, France (patients included = 4); Jean Louis Pepin, Unité Epreuves Fonctionnelles Respiratoires, Michallon Hospital, La Tronche, France (patients included = 7); Maria Antonia Quera Salva, Sleep Disorder unit, R. Poincaré Hospital, Garches, France (patients included = 3); Anne Thibault Stoll, Neurology clinic, Strasbourg, France (patients included = 7); Zoltan Szakacs, State Health Center, Budapest, Hungary (patients included = 25).
Contributor Information
HARMONY III study group:
Isabelle Arnulf, Hélène Bastuji, Yves Dauvilliers, Marie Françoise Vieccherini, Jean Louis Pepin, Maria Antonia Quera Salva, Anne Thibault Stoll, and Zoltan Szakacs
Conflict of interest statement. Y.D. received funds for seminars, board engagements and travel to conferences by UCB Pharma, Jazz, Theranexus, Idorsia, Takeda, Flamel, and Bioprojet. I.A. was investigator in the study Harmony III of Bioprojet. She had paid speaking engagements for UCB Pharma and received consultancy fees by Novartis and by Roche Pharma. S.L-S. was investigator in the study Harmony III of Bioprojet. She had paid speaking engagements for UCB Pharma. Z.S. received funds for travel to conferences from Bioprojet. I.L., C.S-G., J-M.L., and J-C.S. are employees of Bioprojet.
References
- 1. Scammell TE. Narcolepsy. N Engl J Med. 2015;373(27):2654–2662. [DOI] [PubMed] [Google Scholar]
- 2. Kornum BR, et al.. Narcolepsy. Nat Rev Dis Primers. 2017;3:16100. [DOI] [PubMed] [Google Scholar]
- 3. Morgenthaler TI, et al.; Standards of Practice Committee of the American Academy of Sleep Medicine Practice parameters for the treatment of narcolepsy and other hypersomnias of central origin. Sleep. 2007;30(12):1705–1711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kallweit U, et al.. Pharmacological management of narcolepsy with and without cataplexy. Expert Opin Pharmacother. 2017;18(8):809–817. [DOI] [PubMed] [Google Scholar]
- 5. Vignatelli L, et al. Antidepressant drugs for narcolepsy. Cochrane Database Syst Rev. 2008;( 1):CD003724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Drakatos P, et al.. Safety and efficacy of long-term use of sodium oxybate for narcolepsy with cataplexy in routine clinical practice. Sleep Med. 2017;35:80–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Thorpy MJ, et al.. Clinical and practical considerations in the pharmacologic management of narcolepsy. Sleep Med. 2015;16(1):9–18. [DOI] [PubMed] [Google Scholar]
- 8. Lehert P, et al.. Multiple treatment comparison in narcolepsy: a network meta-analysis. Sleep. 2018;41(12). doi: 10.1093/sleep/zsy185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ligneau X, et al.. BF2.649 [1-{3-[3-(4-Chlorophenyl)propoxy]propyl}piperidine, hydrochloride], a nonimidazole inverse agonist/antagonist at the human histamine H3 receptor: preclinical pharmacology. J Pharmacol Exp Ther. 2007;320(1):365–375. [DOI] [PubMed] [Google Scholar]
- 10. Schwartz JC. The histamine H3 receptor: from discovery to clinical trials with pitolisant. Br J Pharmacol. 2011;163(4):713–721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Lin JS, et al.. An inverse agonist of the histamine H(3) receptor improves wakefulness in narcolepsy: studies in orexin-/- mice and patients. Neurobiol Dis. 2008;30(1):74–83. [DOI] [PubMed] [Google Scholar]
- 12. Dauvilliers Y, et al.; HARMONY I study group Pitolisant versus placebo or modafinil in patients with narcolepsy: a double-blind, randomised trial. Lancet Neurol. 2013;12(11):1068–1075. [DOI] [PubMed] [Google Scholar]
- 13. Szakacs Z, et al.; HARMONY-CTP study group Safety and efficacy of pitolisant on cataplexy in patients with narcolepsy: a randomised, double-blind, placebo-controlled trial. Lancet Neurol. 2017;16(3):200–207. [DOI] [PubMed] [Google Scholar]
- 14. Kollb-Sielecka M, et al.. The European Medicines Agency review of pitolisant for treatment of narcolepsy: summary of the scientific assessment by the Committee for Medicinal Products for Human Use. Sleep Med. 2017;33:125–129. [DOI] [PubMed] [Google Scholar]
- 15. Calik MW. Update on the treatment of narcolepsy: clinical efficacy of pitolisant. Nat Sci Sleep. 2017;9:127–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Romigi A, et al.. Profile of pitolisant in the management of narcolepsy: design, development, and place in therapy. Drug Des Devel Ther. 2018;12:2665–2675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Arrang JM, et al.. Highly potent and selective ligands for histamine H3-receptors. Nature. 1987;327(6118):117–123. [DOI] [PubMed] [Google Scholar]
- 18. Bogan RK, et al.. Time to response with sodium oxybate for the treatment of excessive daytime sleepiness and cataplexy in patients with narcolepsy. J Clin Sleep Med. 2015;11(4):427–432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Mitler MM, et al.. Long-term efficacy and safety of modafinil (PROVIGIL(®)) for the treatment of excessive daytime sleepiness associated with narcolepsy. Sleep Med. 2000;1(3):231–243. [DOI] [PubMed] [Google Scholar]