Abstract
Extracellular vesicles (EVs) have recently attracted substantial attention due to the potential diagnostic and therapeutic relevance. Although a variety of techniques have been used to isolate and analyze EVs, it is still far away from satisfaction. Size-exclusion chromatography (SEC), which separates subjects by size, has been widely applied in protein purification and analysis. The purpose of this chapter is to show the applications of size-exclusion high-performance liquid chromatography (HPLC) as methods for EV characterization of impurities or contaminants of small size, and thus for quality assay for the purity of the samples of EVs.
Keywords: Size-exclusion chromatography, High-performance liquid chromatography (HPLC), Extracellular vesicles, Exosomes
1. Introduction
Extracellular vesicles (EVs), which include exosomes, are membrane enclosed vesicles that are secreted by all cells, circulated in blood and are readily accessible in most body fluids [1–7]. Carrying abundant biomolecules like proteins, RNAs, DNAs, metabolites and lipids, these vesicles are important messengers and mediators in intercellular communications, and play pivotal role in tumor progression and metastasis [8]. In addition to the efforts to understand the biology of EVs in different disease, extensive attention has been recently also paid to the studies of these nanoparticles as biomarker and the engineering to be used as therapeutic vehicles for drug and gene delivery. All these research activities using different approaches including proteomics [9–11], transcriptomics [10, 11], lipidomics [10, 12] and vesicle engineering [13, 14] demand samples of high purity.
However, current isolation and characterization methods are far to be satisfied to assure high purity EVs for clinic use. Various methods have been used for vesicle isolation, including ultracentrifugation [15], size exclusion (filtration or chromatography) [15–19], immunoaffinity isolation [15, 20], precipitation (ExoQuick or “salting-out”) [21, 22] or the combinations of above techniques. Each of these methods has advantages and disadvantages. There is a lack of clear consensus for an optimal method of isolation as well as characterization of the pure samples of these EVs [23]. A few techniques have been commonly used to characterize isolated vesicles, such as transmission electron microscopy (TEM) or Cryo-EM [24], nanoparticle tracking analysis (NTA, NanoSight) [25] and western blot for protein marker confirmation [15]. Recently, Webber et al. proposed to use the ratio of EVs counts to protein concentration as an indirect means to check the purity of EVs preparation [26]. All these approaches are based on the presence of vesicular particles. On the other hand for impurity detection, while the presence of protein biomarkers that are not expressed by vesicles [26] was proposed for quality assay, the selection of these markers is challenging and may not be accurate due to the limited understanding of how proteins are packaged into EVs.
To assure high quality EVs, not only the desired population of EVs must be confirmed to be present, but contaminants and impurities must also be demonstrated to be absent. Unfortunately, there is no robust and reproducible method available to rule out the presence of contaminants. We hereby describe a simple SE-HPLC analysis to detect water-soluble impurities of small size in the samples of exosomes or extracellular vesicles. SE-HPLC is widely used and accessible in most institutions and could be used along with other current techniques to characterize EVs [27]. It provides a convenient and robust means to quantify the EV products for further biology and function studies.
2. Materials
Chemicals and reagents are purchased from commercial sources and used without further processing unless indicated otherwise.
A Beckman Coulter Optima LE-80K ultracentrifuge with an SW28 rotor for ultracentrifugation.
An Agilent 1260 Infinity high-performance liquid chromatography (HPLC) system equipped with an autosampler and a variable wavelength UV detector.
A size-exclusion column (Superose 12 10/300 GL from GE Healthcare) (9–13 μm) for HPLC.
DPBS buffer (pH 7.4) (Hyclone Laboratories, Inc.) for HPLC mobile phase and the ultracentrifugation.
The Gel Filtration HMW Calibration Kits (GE Healthcare) which contains five proteins from 43 kD to 669 kD and Blue Dextran 2000.
0.45 μm and 0.22 μm polyvinylidene fluoride (PVDF) syringe filters (Sigma-aldrich).
Dulbecco’s Modified Eagle’s Medium (DMEM, Thermo Fisher Scientific).
Fetal bovine serum, FBS (research grade and sterile triple 100 nm filtered, Thermo Fisher Scientific).
The EV-depleted fetal bovine serum (FBS) used for cell culture are processed in-house from the commercial available FBS as shown below.
3. Methods
3.1. Preparation of Vesicle-Depleted Fetal Bovine Serum (FBS)
The commercial FBS is subject to ultracentrifugation at 120,000 × g overnight (15 h).
The upper liquid of 80% volume is reserved for cell culture while the remaining bottom liquid was used for EV preparation from FBS itself (see Note 1).
The EV-depleted FBS is frozen at −20 °C for storage.
3.2. Preprocessed Cell Culture Supernatant for Isolation of EVs
Cells such as MDA-MB-231, MSTO-211H, and endothelial cells are maintained at 37 °C at 5% CO2 in Dulbecco’s Modified Eagle’s Medium supplemented with 4% EV-depleted FBS (see Note 2).
When cell confluence reached 90%, the supernatant is collected.
The supernatant is preprocessed by subsequent centrifugations at 400 × g for 5 min and 3000 × g for 30 min to remove cell debris.
The preprocessed supernatant is used immediately or kept at −80 °C for future use.
Before ultracentrifugation, the preprocessed supernatant is sequentially filtered through 0.45 μm and 0.22 μm PVDF filters (see Note 3).
3.3. EV Isolation from FBS or Preprocessed Cell Culture Supernatant
The preprocessed supernatant or FBS is ultracentrifuged using SW28 flying rotor at 120,000 × g for 90 min to pellet EVs (see Note 4).
The EV pellet is resuspended, washed with DPBS and ultracentrifuged at 120,000 × g for 90 min, and the washing step is repeated multiple times, during which SE-HPLC can be used to monitor the removal of contaminants.
The final EV pellet is resuspended in DPBS and aliquots are used immediately for various characterizations or stored at −80 °C.
3.4. Size-Exclusion HPLC Analysis
A superpose 12 10/300 GL column with exclusion limit of 2,000,000 Da is selected for impurity analysis (see Note 5, Table 1).
The column is calibrated with high molecular weight (HMW) kit, which contains five proteins from 43 kD to 669 kD and Blue Dextran 2000 (Fig. 1).
DPBS buffer (pH 7.4) is used as mobile phase (see Note 6).
The flow rate is set for 1 mL/min (see Note 7).
50–100 μL of an aliquot is injected via the autosampler (see Note 8).
UV absorbance is detected at a wavelength of 254 nm (see Notes 9 and 10).
Table 1.
Comparison of HPLC size-exclusion columns
| Manufacturer | Product | Pore diameter (nm) | Particle (mm) | Exclusion limit Protein M | Optimal range | pH range |
|---|---|---|---|---|---|---|
| Bio-Rad (silica-based diol) | Bio-Sil SEC 125 | 12.5 | 5 | 100 kD | 5–100 kD | 2.0–8.0 |
| Bio-Sil SEC 250 | 25 | 5 | 300 kD | 10–300 kD | ||
| Bio-Sil SEC 400 | 40 | 5 | 1000 kD | 20–1000 kD | ||
| MICRA (silica-based diol) | SynChropak GPC Peptide | 5 | 5 | 35 kD | 1–35 kD | 2.0–8.0 |
| SynChropak GPC100 | 10 | 5 | 500 kD | 5–160 kD | ||
| SynChropak GPC300 | 30 | 5 | 2000 kD | 10–500 kD | ||
| SynChropak GPC500 | 50 | 7 | 5000 kD | 40–1000 kD | ||
| SynChropak GPC1000 | 100 | 7 | 10,000 kD | 40–10,000 kD | ||
| SynChropak GPC4000 | 400 | 10 | >10,000kD | |||
| GE Healthcare (semirigid agarose) | Superose 6 | 13 | 40,000 kD | 5–5000 kD | 1.0–14 | |
| Superose 12 | 10 | 2000 kD | 1–300 kD | 3–12 | ||
| Superdex 75 | 11–15 | 100 kD | 3–70 kD | |||
| Superdex 200 | 11–15 | 1300 kD | 10–600 kD | |||
| Agilent Technologies (silica-based diol, stabilized) | ZORBAX GF-250 | 15 | 5 | 400 kD | 4–400 kD | 2.5–8.5 |
| ZORBAX GF-450 | 30 | 6 | 1000 kD | 10–1000 kD | ||
Data from manufacturer’s catalogs
Fig. 1.
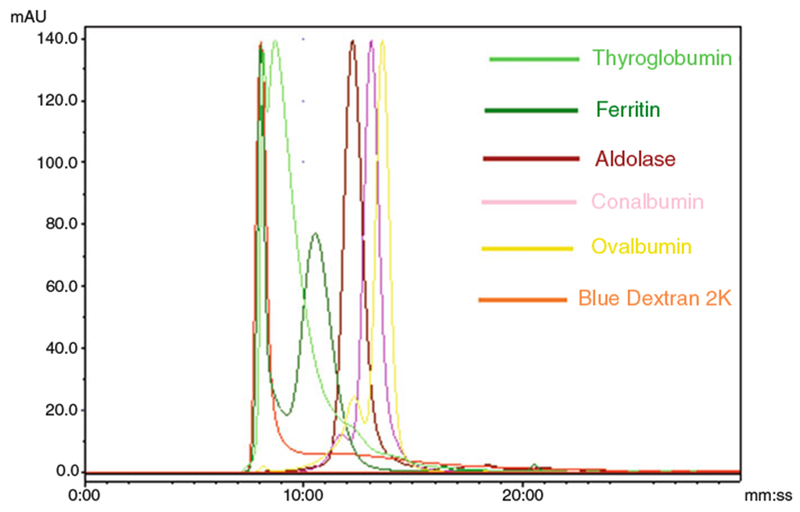
The column calibration with HMW kit provided by the column manufacturer. Elution profiles of Calibration Kit standards: Thyroglobumin (669 kD, 8 min 43 s and 8 min 4 s), Ferritin (440 kD, 10 min 34 s and 8 min 4 s), Aldolase (158 kD, 12 min 17 s), Conalbumin (78 kD, 13 min 6 s), Ovalbumin (44 kD, 13 min 35 s), and Blue Dextran 2000 (2000 kD, 8 min 4 s) (Courtesy of Journal of Circulation Biomarkers)
Fig. 2.
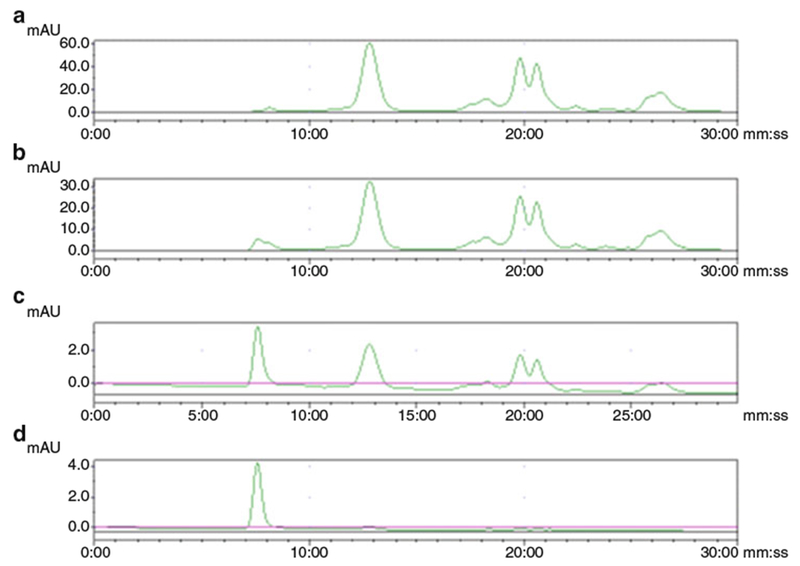
Representative chromatograms of extracellular vesicles from MSTO cell culture supernatant. Aliquots after once (a), twice (b), three times (c), and four times (d) ultra-centrifugations were analyzed. The peak with a retention time of 7.5 min stands for the exosome particles while the rest peaks are impurities of small size. (Courtesy of Journal of Circulation Biomarker)
Fig. 3.
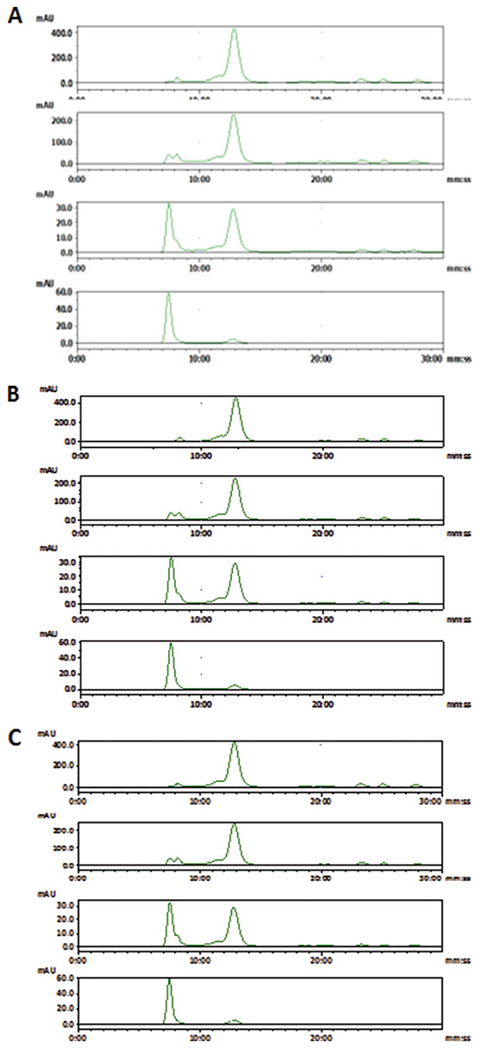
SE-HPLC chromatogram of extracellular vesicles purified from FBS: (a), (b) and (c) represent HPLC results from three batches of EV isolations demonstrating high reproducibility. Four chromatograms from top to bottom in (a) or (b) or (c) are representing aliquots after once, twice, three times, and four times ultracentrifugations respectively. The peak with a retention time of 7.5 min stands for the exosome particles while the rest peaks are impurities of small size. (Courtesy of Journal of Circulation Biomarkers)
4 Notes
FBS is used as nutrients during cell culture. To avoid irrelevant vesicle contamination for further biological analysis and studies, it is critical to deplete those vesicles from FBS before adding it into cell media.
A variety of cell lines could be cultured to “produce” EVs. In the authors’ lab, MDA-MB-231 cancer cells, MSTO-211H cancer cells, and endothelial cells have been used for study.
Filtration further reduced cell debris or large vesicle particles although particles larger than 220 nm could still squeezed through the filter. Filtration through 0.45 μm first makes it easier to filter further through 0.22 μm. Otherwise, the filter will get clogged.
The ultracentrifugation speed and time can vary depending on the equipment and rotor type [15, 23].
The selection of size-exclusion column is critically important. Packing materials, resolving limit, pressure sustainability, and so forth should be taken into consideration. The high exclusion limit of 2 × 106 Daltons was used in this study with the aim to give a wide detection range and better resolution of small impurities for the extracellular vesicles with size less than around 200 nm. Other range of exclusion limit may give different results depending on the vesicles and the sources to prepare these vesicles. From our experience, size-exclusion columns with higher exclusion limit may be able to resolve large particles but will give less desirable resolution of small size molecules. (Figs. 2 and 3).
Size-exclusion columns for HPLC are usually packed with materials based on silica which has been covered with a carbohydrate bonded phase. The particle size for SE-HPLC column packing is usually much smaller than that for low pressure SEC, which provides better resolution at higher pressure. Parameters of some commercially available columns for SE-HPLC are listed in the Table 1, from which an appropriate column can be selected for each application.
Here we used DPBS buffer as mobile phase since the EV samples was isolated through multiple DPBS wash steps and kept in DPBS. Generally, it is critical to choose suitable mobile phase in size-exclusion chromatography to ensure the mobile phase can solubilize the sample and maintain low viscosity. Also the mobile phase could influence the interactions between the stationary phase and the sample materials. If there is mobile phase recommended by column providers, it is wise to follow because it should be optimized for the specific packing materials.
Generally, low flow rates are desired to get optimal resolution. For commercially available columns, flow rate range and maximal are recommended. Take this column as an example, 0.5–1 mL/min is recommended with a maximum of 1.5 mL/min.
The loading capacity for each analysis is related to multiple factors such as column size and the sample characters. As a rule of thumb, the loading volume should be no more than 2% of the column volume otherwise band spreading may occur. According to this empirical rule, the maximal loading volume for this column is 500 μL.
To make small molecule impurity detectable, the most commonly used 254 nm was selected where RNAs, DNAs and proteins are all detectable. Other wavelength could also be used in case by case.
The intention of this study is to test if SE-HPLC can be used as a means for quality analysis of soluble proteins and other impurities of small size. For a detailed size measurement by HPLC, more evaluations are needed. It would be best to use the simple and straightforward SE-HPLC to qualify the vesicle preparation followed by other characterizations such as TEM, NTA, western blot and flow cytometry.
References
- 1.Thery C, Zitvogel L, Amigorena S (2002) Exosomes: composition, biogenesis and function. Nat Rev Immunol 2:569–579 [DOI] [PubMed] [Google Scholar]
- 2.Thind A, Wilson C (2016) Exosomal miRNAs as cancer biomarkers and therapeutic targets. J Extracell Vesicles 19(5):31292. doi: 10.3402/jev.v5.31292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Conde-Vancells J, Rodriguez-Suarez E, Embade N et al. (2008) Characterization and comprehensive proteome profiling of exosomes secreted by hepatocytes. J Proteome Res 7(12):5157–5166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Van der Pol E, Boing AN, Harrison P et al. (2012) Classification, functions, and clinical relevance of extracellular vesicles. Pharmacol Rev 64(3):676–705 [DOI] [PubMed] [Google Scholar]
- 5.Wang F, Nazarali AJ, Ji S (2016) Circular RNAs as potential biomarkers for cancer diagnosis and therapy. Am J Cancer Res 6 (6):1167–1176 [PMC free article] [PubMed] [Google Scholar]
- 6.Pisitkun T, Shen RF, Knepper MA (2004) Identification and proteomic profiling of exosomes in human urine. Proc Natl Acad Sci 101 (36):13368–133373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Srivastava A, Babu A, Filant J et al. (2016) Exploitation of Exosomes as Nanocarriers for Gene-, chemo-, and immune-therapy of cancer. J Biomed Nanotechnol 12(6):1159–1173 [DOI] [PubMed] [Google Scholar]
- 8.Kahlert C, Kalluri R (2013) Exosomes in tumor microenvironment influence cancer progression and metastasis. J Mol Med 91(4):431–437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Simpson RJ, Jensen SS, Lim JW (2008) Proteomic profiling of exosomes: current perspectives. Proteomics 8(19):4083–4099 [DOI] [PubMed] [Google Scholar]
- 10.Choi DS, Kim DK, Kim YK et al. (2013) Proteomics, transcriptomics and lipidomics of exosomes and ectosomes. Proteomics 13(10–11):1554–1571 [DOI] [PubMed] [Google Scholar]
- 11.Xiao D, Ohlendorf J, Chen Y et al. (2012) Identifying mRNA, microRNA and protein profiling of melanoma exosomes. PLoS One 7(10):e46874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Subra C, Laulagnier K, Perret B et al. (2007) Exosome lipidomics unravels lipid sorting at the level of multivesicular bodies. Biochimie 89(2):205–212 [DOI] [PubMed] [Google Scholar]
- 13.Johnsen KB, Gudbergsson JM, Skov MN et al. (2014) A comprehensive overview of exosomes as drug delivery vehicles-endogenous nanocarriers for targeted cancer therapy. Biochim Biophys Acta 1846(1):75–87 [DOI] [PubMed] [Google Scholar]
- 14.Van Dommelen SM, Vader P, Lakhal S et al. (2012) Microvesicles and exosomes: opportunities for cell-derived membrane vesicles in drug delivery. J Control Release 161(2):635–644 [DOI] [PubMed] [Google Scholar]
- 15.Thery C, Amigorena S, Raposo G, et al. (2006) Isolation and characterization of exosomes from cell culture supernatants and biological fluids. Curr Protoc Cell Biol Chapter 3: Unit 3.22 [DOI] [PubMed] [Google Scholar]
- 16.Muller L et al. (2014) Isolation of biologically-active exosomes from human plasma. J Immunol Methods 411:55–65. doi: 10.1016/j.jim.2014.06.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tan SS, Yin Y, Lee T et al. (2013) Therapeutic MSC exosomes are derived from lipid raft microdomains in the plasma membrane. J Extracell Vesicles 2:22614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Arroyo JD, Chevilleta JR, Kroha EM et al. (2011) Argonaute2 complexes carry a population of circulating microRNAs independent of vesicles in human plasma. Proc Natl Acad Sci 108(12):5003–5008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Boing AN, van der Pol E, Grootemaat AE et al. (2014) Single-step isolation of extracellular vesicles by size-exclusion chromatography. J Extracell Vesicles 3:23430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Clayton A, Court J, Navabi H et al. (2001) Analysis of antigen presenting cell derived exosomes, based on immune-magnetic isolation and flow cytometry. J Immunol Methods 247:163–174 [DOI] [PubMed] [Google Scholar]
- 21. http://www.systembio.com/downloads/ExoQuick_ProdSheet.pdf.
- 22.Brownlee Z, Lynn KD, Thorpe PE et al. (2014) A novel “salting-out” procedure for the isolation of tumor-derived exosomes. J Immunol Methods 407:120–126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Witwer KW, Buzas EI, Bemis LT et al. (2013) Standardization of sample collection, isolation and analysis methods in extracellular vesicle research. J Extracell Vesicles 2:20360. doi: 10.3402/jev.v2i0.20360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yuan Y, Koning RI, Kuil ME et al. (2013) Cryo-electron microscopy of extracellular vesicles in fresh plasma. J Extracell Vesicles 2:21494. doi: 10.3402/jev.v2i0.21494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gardiner C, Ferreira YJ, Dragovic RA et al. (2013) Extracellular vesicle sizing and enumeration by nanoparticle tracking analysis. J Extracell Vesicles 2:19671. doi: 10.3402/jev.v2i0.19671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Webber J, Clayton A (2013) How pure are your vesicles? J Extracell Vesicles 2:19861. doi: 10.3402/jev.v2i0.19861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Huang T, Banizs AB, Shi W et al. (2015) Size-exclusion HPLC detection of small-size impurities as a complementary means for quality analysis of extracellular vesicles. J Circ Biomark doi: 10.5772/61148 [DOI] [PMC free article] [PubMed] [Google Scholar]


