Abstract
Dry eye disease (DED) is among the most common condition encountered during ophthalmic practice, reducing patient’s quality of life and work productivity. Most of DED cases have an evaporative component originated from a meibomian gland dysfunction (MGD). Conventional treatments such as tear substitute, warm compresses, topical anti-inflammatory agents and/or antibiotics often are not able to provide a complete and long-term relief of symptoms and signs. Intense pulsed light (IPL) has been widely used in the field of dermatology to treat various skin conditions, and it has been recently introduced in the ophthalmic practice for the management of DED due to MGD. To date, several clinical studies showed positive results of IPL as adjuvant therapy for DED in terms of both safety and efficacy. The treatment is usually well accepted among patients for its non-invasive nature; very rare are the major adverse reactions. Moreover, results can be maintained over time with periodic sessions of IPL. This review summarizes the clinical outcomes of IPL therapy in MGD patients pointing out its potential role in the therapeutic algorithm of the disease. Further clinical investigations are desirable to identify factors able to predict the positive outcomes of the procedure and therefore to select in advance those patients who best benefit from IPL therapy.
Keywords: intense pulsed light, meibomian gland disease, dry eye disease, evaporative dry eye
Introduction
Dry eye disease (DED) is a common ocular condition that causes ocular discomfort symptoms and reduces the vision, thus impairing patient’s quality of life and restricting daily activities and work productivity.1,2
According to the TFOS DEWS II, the new definition of DED recognizes the multifactorial nature of dry eye as a disease where loss of homeostasis of the tear film is the central pathophysiological concept replacing the old etiological classification.1
The vast majority of DED cases comprehends meibomian gland dysfunction (MGD), a condition characterized by a chronic and diffuse abnormality of the meibomian glands with obstruction of their terminal duct and qualitative/quantitative changes of glandular secretion.3,4
Until recently, treatment options for this condition have been mainly represented by tear substitutes, warm compresses, meibomian gland expression and probing, omega-3 fatty acid supplementation, topical anti-inflammatory agents and/or oral antibiotics, as well as dedicated devices administered in-office (e.g. LipiFlow).5–14 However, despite the variety of these strategies, DED patients may not experience complete or long-term relief of their symptoms suggesting the continuous need for more effective treatments.15–17
Intense pulsed light (IPL) is a relatively novel treatment for DED due to MGD. The technique has been used in dermatology for over a decade for the treatment of rosacea, acne and skin lesions like benign cavernous hemangioma and telangiectasia.18,19 The technique uses a polychromatic light with a wavelength spectrum of 500–1200 nm which is directed to the skin and absorbed by chromophores such as melanin, hemoglobin and water with the development of heat, thus inducing blood vessels ablation.18 Toyos and co-workers reported for the first time the concurrent improvement in DED symptoms in patients undergoing IPL to treat skin rosacea.20 After this anecdotal case series, there has been a growing interest among ophthalmologists in evaluating IPL as a potential therapy for DED due to MGD.21–27 Subsequently, a new generation of device, designed specifically for periocular application with calibrated sequenced light pulses delivered under the shape of regulated train pulses (intense regulated pulsed light, IRPL), has recently become commercially available.28–31
The purpose of this review is to summarize the available evidence on IPL as a novel therapeutic tool for DED. In addition, the perspectives of implementation of the instrument/procedure as well as the identification and selection of cases who best benefit from IPL procedure will be discussed.
Meibomian Gland Dysfunction
Meibomian glands are modified sebaceous glands located along the upper and lower eyelid margins. They contribute to the secretion of the lipid component of the tear film, which prevents premature tear evaporation keeping the ocular surface lubricated.4 Moreover, normal meibum contains antimicrobial properties that keep the lid margin clear from overgrowth.32
MGD is defined as a chronic and diffuse abnormality of the meibomian glands that may results in tear film instability leading to eye irritation with clinically apparent inflammation and ocular surface disease.33
In addition, chronic inflammation determines abnormal blood vessel growth that surrounds the meibomian glands and secretes inflammatory mediators that aggravate glands activity.33
According to the report of the “Definition and Classification Subcommittee of the International Workshop”, MGD can be classified as a low- or high-delivery state.33,34
The former, which is the most common stage of MGD, is associated with deficiencies in meibomian glands secretion and it may be further characterized as obstructive, with cicatricial and non-cicatricial subcategories, or hyposecretory with gland atrophy. The latter, also known as hypersecretory MGD, is characterized by the release of large amounts of meibum at the lid margin in response to pressure on the eyelid and has been associated with seborrheic dermatitis in the totality of cases.
The pathogenesis of MGD is arranged in a vicious circle: meibomian glands inflammation with dropout or blockage for ductal epithelium hyperkeratinization leads to stasis of the meibum inside the glands; the reduced gland outflow promotes the proliferation of bacteria, increasing the viscosity of the meibum, thus resulting in further blockage on the gland orifices.34
Traditional DED treatments may not provide complete relief of symptoms and signs.15–17 Moreover, multiple therapies could cause compliance and safety issues making treatments useless and harmful.
Particularly, corticosteroid therapy needs close monitoring due to its potential complications.35,36
Therefore, the development of novel therapeutic strategies is desirable in order to improve the prognosis of the disease.
IPL Transition From Dermatology To Ophthalmology
IPL consists of a non-coherent and polychromatic light source with a wavelength spectrum of 500–1200 nm, which can be easily modulated through a proper filter.18 It induces a selective photothermolysis of the irradiated tissue as light energy is preferentially absorbed by a chromophore and converted into heat, leading to coagulation and ablation of blood vessels.37 The use of polychromatic infrared light was first described in 1976 by Muhlbauer for the treatment of vascular malformations.38
The first IPL device was produced in 1990 and it was commercially available as a medical tool in 1994.39
The main field of application has been, until now, dermatology, particularly in the treatment of pigmentary, acneiform, adnexal and other inflammatory disorders, vascular lesions, scars and pre-malignant/malignant lesions such as actinic keratosis.40 The potential role of IPL in the ophthalmology field dates back to 2002, when Toyos and colleagues registered ocular symptoms improvements in patients treated with IPL for facial skin rosacea.20 Further objective examination then confirmed its positive effects on MGD and DED.20
Based on this evidence, IPL therapy started to be included in the therapeutic armamentarium of DED patients and IPL devices specifically configured for the periocular application have been developed, starting a brand-new technology. Over the following years, ophthalmologists refined the technique and the devices, aiming at improving results while minimizing complications.
Mechanisms Of Action
Despite its use in the treatment of ocular surface diseases, the mechanism of action of IPL in the setting of DED is still unclear and there is no universal consensus about it. The main speculative theories include abnormal blood vessels thrombosis, meibum heating and liquefaction, photomodulation, Demodex eradication, secretion modulation of pro and anti-inflammatory molecules and matrix metalloproteinases suppression.41
Destruction Of Superficial Blood Vessels
In patients suffering from facial skin rosacea, abnormal blood vessels release inflammatory mediators which could easily propagate to the eyelids through facial artery and orbital vasculature. It has been proposed that these inflammatory mediators could trigger the inflammation of meibomian glands leading to their dysfunction.20 The beneficial effect of IPL on vascular disorders has been extensively studied and reported.19 Light energy absorbed by chromophores such as melanin, hemoglobin and water is transformed into heat causing the localized thrombosis and destruction of superficial blood vessels, thus removing a major source of inflammation from the eyelids and meibomian glands.42
Bäumler et al, thanks to a mathematical model, demonstrated that in medium and large blood vessels (>150 μm) a single IPL pulse of 30 ms duration raises the temperature at the center of the vessel to 80–90°C, above the temperature required to cause coagulation and thrombosis.43
Fluidification Of Meibum
All patients with obstructive MGD exhibit increased meibum viscosity due to changes in meibum composition, resulting in the increase of the meibum melting temperature.44,45 Eyelid temperature significantly influences the physical properties of meibomian gland secretions.44,45 Since higher temperatures correspond to less viscous secretions, warming the eyelids could play a therapeutic effect, facilitating meibomian gland expression by reaching the phase-transition temperature.46 However, meibum modification consists of a biochemical phase transition (from gel to liquid crystalline) and not a state change (from solid to liquid).47 IPL is thought to favour the elevation in skin temperature making the meibum less viscous, thus resulting in the unclogging of the glands and promoting the normal distribution of meibum over the ocular surface.44,45,48
Down-Regulation Of Epithelial Turnover
An enhanced epithelial turnover characterizes skin rosacea and, in a mechanism like dandruff production, a large amount of dead skin cells detaches from the epidermal surface, accumulates and creates debris. Accumulation of debris on the lid margin, together with poor lid hygiene, could potentially encourage the obstruction of the gland’s orifices, leading to their dysfunction.41,49 Thus, IPL could contrast this trend by decreasing the epithelial turnover.
Photomodulation
Photomodulation consists in using light sources at different wavelengths to stimulate specific biological patterns in order to achieve a therapeutic effect. IPL produces a photochemical cascade, inducing changes in the redox properties of components along the mitochondrial respiratory chain, leading to faster electron transfer and, hence, to an increase in adenosine triphosphate production.50,51 The rise of adenosine triphosphate results in higher levels of intracellular free calcium concentration that acts as a signal to promote different physiological reactions for the cell development and growth such as increased fibroblast proliferation, enhanced collagen synthesis and local blood flow.52 The ability of IPL to activate fibroblasts and enhance collagen synthesis is the basis for the efficacy of skin rejuvenation treatments.53–55 At the eyelid skin level, this effect could contrast the natural tendency of the skin to lose rigidity and elasticity with aging, a process that could lead to poor apposition of the lid margins and incomplete blinks, resulting in reduced meibum secretion and increased tear evaporation.
Antimicrobic Effect
Another potential mechanism of action of IPL is the reduction of bacteria and parasitic growth on the eyelids and eyelashes. Demodex folliculorum, an ectoparasite living in hair follicles and sebaceous glands, retains a commensal relationship with Bacillus olerinus and together they play a role in the etiology of blepharitis and MGD.56–58 First of all, the microbial colonization of the eyelids contributes to the chronic and self-perpetuating inflammation.59–61 Secondly, Demodex and Ba. olerinus release toxic substances, including lipases, which alter meibum composition and increase its viscosity.41 Moreover, oleic acid, a product of lipase activity on the gland secretion, could play a role in the keratinization of the lid margin, thus contributing to the obstruction of the orifices and in the amplification of the inflammatory process.34
IPL could efficiently induce the coagulation and necrosis of Demodex thanks to the presence of chromophore in its pigmented exoskeleton, as confirmed by histological analysis.61,62
Anti-Inflammatory Effect
Inflammation plays a key role both in the pathogenesis and in the progression of DED as confirmed by the elevated levels of cytokines, matrix metalloproteinases and chemokines found in the tears of patients with DED compared to healthy controls.63,64 IPL could interfere this vicious circle by the upregulation of anti-inflammatory cytokines and/or the downregulation of the proinflammatory ones. Studies in the dermatological field demonstrated that IPL increased levels in the skin cells of interleukin 10 and transforming growth factor-beta and lowered levels of interleukin 6, tumor necrosis factor alpha, matrix metalloproteinases and proteolytic enzymes.65–68 The direct demonstration of IPL effect upon ocular surface inflammation has been provided by Liu et al that showed a decrease of inflammatory markers in tears after IPL treatment.69
Anti-Oxidative Effect
Above the several factors contributing to the pathogenesis of DED, it is important to cite the role of reactive oxidative species such as superoxide anions and hydroxyl radicals released by neutrophils and inflammatory cells.70,71 The effect of IPL on oxidative stress follows a biphasic dose response: at low doses, it produces an increase in reactive oxidative species together with an antimicrobial effect, whereas at higher doses it shows the reduction of reactive oxidative species levels, thus diminishing oxidative stress and inflammation.72
In contrast to the most proposed mechanisms of action, Mejia and co-authors suggested that the effects obtained from IPL do not depend on temperature rise or physical proximity but it is caused by photomodulation with a photochemical effect able to trigger biologic processes.73 Indeed, since the device is not directly applied on the eyelid and uses almost low-intensity energy (from 8.5 to 20 J/cm2) with a very subtle, short-timed and confined temperature increment they believe that the temperature cannot be the main actor of IPL effect. In this model, mitochondria play a key role with cytochrome C oxidase complex being proposed as the primary photoreceptor, yielding an increase in adenosine triphosphate production and the induction of transcription factors. Together, these effects lead to cellular stimulation and cytokines modulation.73
IPL Procedure
IPL devices allow the regulation of wavelengths, pulse duration, pulse intervals and fluence, thus facilitating the treatment of a wide spectrum of conditions in different patients using a tailored approach.74 Treatment intensity depends on patient’s skin type, which is documented by the Fitzpatrick score (patients with higher scores, i.e. more pigmented, necessitate lower energy settings to avoid risk of melanin damage and resultant hypopigmentation).75 The majority of IPL devices use low-intensity energies (8.5−20 J/cm2) and have to be set on the proprietary “dry eye mode”.
Protective eye shields must be placed over the eyes and the treatment area must be free from make-up, shaved and covered with an ultrasound gel.
IPL flashes are placed for each eye starting from the inner canthus and ending in the temporal region below the lower eyelid, with slight overlapping applications. The upper eyelids are not treated directly since there is a risk of light penetration through the eyelid and absorption within the intraocular structures such as the pigmented iris tissue.41 On the other hand, Rong et al applied IPL pulses both on the upper and lower lids and protected ocular structures by placing a Jaeger lid plate with 18 mm and 22 mm curved wide blades in the conjunctival sac. The blade moved with IPL pulses during treatment to ensure that cornea, iris and sclera were not directly exposed to IPL fluence.76,77
Usually, one full passage or, more rarely, two passages are done for each eye in order to ensure the full coverage of the treatment area. At the end of the procedure, warm compresses are usually applied along the eyelids for a few minutes and meibomian glands are manually expressed to assist meibum secretion. To date, there is no universal agreement about the therapy that patients should receive after IPL treatment. Most of the authors advised patients to continue standard ocular medications such as tear substitutes, lid hygiene with warm compresses and omega-3 supplements. Conversely, other authors treated patients with topical steroids or non-steroidal anti-inflammatory drugs for a time period ranging from 2 to 10 days after each IPL session.20,72,78,79 Most of IPL treatment protocols include 3 to 4 sessions, but the number of IPL sessions may vary depending on disease severity, patient characteristics and subjective satisfaction. The collection of further clinical data is desirable in order to identify the more efficacious number of sessions for one treatment protocol and the ideal time interval between each session.
Clinical Results
Safety
As a non-invasive and efficacious procedure, the use of IPL is increasing among ophthalmologists. For this reason, it is important to be aware of the potential adverse effects of this technique that can occur to the patient eye.
The pigmented iris absorbs light in the same wavelengths range of IPL, thus being one of the most susceptible and vulnerable targets of IPL.
Neglect for appropriate patient eye protection during IPL sessions can easily cause permanent ocular damage. Several studies documented the ocular complications related to this procedure, which ranged from anterior uveitis to permanent iris atrophy and pupillary defects with long-lasting photophobia and pain.80–82 Furthermore, posterior synechiae originating from intraocular inflammation can lead to pupillary block and secondary angle-closure glaucoma.83,84 In order to prevent these unpleasant side effects, the use of eye shields is highly recommended during each IPL session for the entire duration of the procedure.
Apart from the ocular complications secondary to the incorrect use of IPL, the procedure has been associated with minor and transient side effects such as pain, erythema, hypo- or hyperpigmentation, blistering and superficial crusts.21–23,26,30,85
In their study, Li et al aimed to determine the optimal parameters settings for patients with darker skin types (III/IV Fitzpatrick score). As treatment by laser or light sources carries greater risk for people with a higher score, this would ensure both safety and efficacy at the same time.25 They compared two types of parameters setting with the same pulse setting (2 pulses, 3.0 ms of pulse duration and 30 ms pulse delay) but with different light filters and energy densities (560 nm, 16 mJ/cm2 vs 590 nm, 14 mJ/cm2). The study showed no significant differences between the two groups in terms of clinical efficacy including tear break-up time (TBUT) and ocular surface disease index; conversely, the group treated with higher energy experienced higher discomfort symptoms compared to the lower energy-treated group. Thus, these results indicate that the setting with higher wavelength and lower energy is the best choice for patients with skin type III/IV as it provides the same efficacy with decreased side effects. Further, they attempted to characterize age-dependent therapeutic effects after the division of patients into 2 age groups ( 40 vs
40 vs 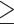 40 years). The younger group showed greater sensitivity to IPL treatment, with a better and faster improvement. The proposed explanation for this difference is the greater complexity of the ocular surface system in older patients and the better metabolism and immune functions in younger subjects.
40 years). The younger group showed greater sensitivity to IPL treatment, with a better and faster improvement. The proposed explanation for this difference is the greater complexity of the ocular surface system in older patients and the better metabolism and immune functions in younger subjects.
Efficacy
Four retrospective studies have been conducted in order to investigate the therapeutic effect of IPL in patients suffering from DED, and the major findings are summarized in Table 1.20,73,78,86
Table 1.
Retrospective Studies On IPL For DED Therapy
| Study (Year) | Design | Patients (n) | IPL Device | Frequency (Weeks) | IPL Sessions (n) | IPL Energy (J/cm2) | Adverse Events | Results |
|---|---|---|---|---|---|---|---|---|
| Gupta et al (2016)78 | Retrospective | 100 | Quadra Q4 | 3–6 | 4 | / | No | Decreased scoring of lid margin edema, lid margin telangiectasia, lid margin vascularity, meibum viscosity, OSDI score. Increased oil flow score and TBUT |
| Mejia et al (2019)73 | Retrospective cross sectional | 25 | E>Eye | 0-2-6 | 3 | 9,8–13 | / | Improved symptoms, TBUT, Schirmer and VB scores |
| Toyos et al (2015)20 | Retrospective noncomparative case series | 91 | Quadra Q4 | 4 | 7 | 8–20 | Blistering, cheek swelling, conjunctival cyst, floaters, hair loss, light sensitivity, redness | Improved TBUT and dry eye symptoms. |
| Vegunta et al (2016)86 | Retrospective | 35 | Quadra Q4 | 4–6 | 1–4 | / | No | Decreased SPEED2 Increased meibomian gland expression (MGE) |
Abbreviations: TBUT, tear break-up time; MGE, meibomian gland expressibility; VB van Bijsterveld.
Toyos and co-authors conducted a three-year, retrospective, non-comparative, interventional case series study to determine the clinical benefits of IPL therapy on DED caused by MGD in patients who received IPL for the treatment of facial skin rosacea. The study included 78 patients presenting with severe DED measured by a combination of TBUT, meibum quality, lid margin changes and ocular discomfort symptoms. Treatment consisted of an IPL session followed by meibomian gland expression at a single outpatient’s clinic. The procedure was repeated approximately every 30 days until there was adequate improvement in ocular symptoms. The evaluation of treatment efficacy was conducted through the analysis of changes in TBUT values, self-reported patient satisfaction and adverse events. Improvement in TBUT was found in 68 of 78 patients (87% of the total) with 7 treatment sessions while 93% of patients reported treatment satisfaction. No severe adverse events occurred; 14% of patients experienced blistering, cheek swelling, conjunctival cyst, floaters, hair loss at brow and forehead, light sensitivity and redness of face.20
Gupta and co-workers conducted a multicenter cohort study to evaluate the clinical outcomes of IPL in 100 patients diagnosed with MGD. Clinical parameters analyzed in this study included slit-lamp findings (eyelid and facial vascularity, edema, meibomian gland oil flow and quality score) TBUT and ocular surface disease index. Patients received on average 4 IPL sessions, each separated by 3–6 weeks, obtaining good results: there was significant decrease in scoring of lid margin edema, lid margin vascularity, meibum viscosity and ocular surface disease index while there was a significant increase in oil flow score and TBUT.78 Vegunta and co-authors adopted the same study design, analyzing medical records of 35 patients who received 1 to 4 IPL treatments every 4–6 weeks together with meibomian gland expression. The totality of patients showed a significant reduction in Standard Patient Evaluation of Eye Dryness 2 score and meibomian gland function improved significantly in 77% of patients at least in 1 eye.86
Most of the studies assessing IPL efficacy were performed in patients with an evaporative form of DED. Conversely, conflicting results are available in the literature about the effect of IPL on aqueous-deficient DED. Guilloto and co-workers conducted a prospective non-controlled study on 36 patients with different aetiologies of DED who underwent 4 IPL sessions, one every 15 days. Pure aqueous-deficient DED patients did not show any improvement after IPL treatment.31 On the other hand, Mejia and collaborators noted a statistically significant improvement in signs and symptoms not only in patients with evaporative DED, but also in those with aqueous-deficient one.73 However, the vast majority of DED cases are evaporative or mixed (evaporative plus aqueous-deficient), and IPL represents an efficient treatment strategy for both these types of disease.
Several prospective clinical trials investigating the use of IPL for the treatment of DED have been conducted. Most of them are non-randomized (Table 2) while four are randomized ones (Table 3). Craig and collaborators evaluated the effects of IPL on MGD in a prospective, double-masked, placebo-controlled and paired-eye study.28 Twenty-eight patients received IPL treatment (E > Eye E-Swin, France) in 1 eye while the other eye acted as control. The IPL sessions were performed at 1, 15 and 45 days following baseline evaluation. A significant increase in lipid layer grade and TBUT was reported in the treated eye while subjective symptom scores improved in both eyes.
Table 2.
Prospective Clinical Trials For IPL Therapy In DED
| Study (Year) | Design | Patients (n) | IPL Device | Frequency (Weeks) | IPL Sessions (n) | IPL Energy (J/cm2) | Adverse Events | Results |
|---|---|---|---|---|---|---|---|---|
| Ahmed et al (2019)87 | Prospective controlled | 12 | Philips Lumea | / | 1 | 2,5–6,5 | No | Improved tear protein and lipid content and composition |
| Albietz et al (2017)30 | Prospective noncomparative | 26 | E>Eye | 0-2-6 | 3 | 9,8–13 | No | Improved DE symptoms, MG secretion quality, expressibility and ocular surface inflammation |
| Arita et al (2018)22 | Prospective noncomparative | 31 | Lumenis | 3 | 4–8 | 11–14 | / | Improved SPEED, TBUT, CFS and MB parameters |
| Arita et al (2019)21 | Prospective controlled randomized | 45 | Lumenis | 3 | 8 | 11–14 | No | Improved LLT, TBUT, lid margin abnormalities and MGS |
| Choi et al (2019)23 | Prospective noncomparative | 30 | Lumenis | 3 | 3 | 12–14 | No | Improved MG function, tear stability and decreased inflammation (IL-4, IL-6, IL-10, IL-17A, TNF-α) |
| Dell et al (2017)24 | Prospective interventional noncomparative | 40 | Lumenis | 3 | 4 | / | / | Increased TBUT Improved SPEED, MGS, CFS and TFO |
| Guilloto et al (2017)31 | Prospective noncomparative | 36 | E>Eye | 2 | 4 | 8–20 | Redness and light sensitivity | Improved TBUT, Schirmer test and lacrimal meniscus |
| Jiang et al (2016)29 | Prospective | 40 | E>Eye | 0-2-6-10 | 4 | 9,8–13 | No | Improved TBUT, conjunctival injection, symptoms and MG parameters. |
| Karaca et al (2018)85 | Prospective noncomparative | 26 | E>Eye | 0-2-6 | 3 | / | No | Improved TBUT, Schirmer test, OSDI score and SPEED |
| Li et al (2019)25 | Prospective comparative | 40 | Lumenis | 0-2-6 | 3 | 14–16 | Erythema, edema, blister, purpura, hyperpigmentation | Improved TBUT and OSDI score. |
| Seo et al (2018)26 | Prospective noncomparative non-randomized | 17 | Lumenis | 3 | 4 | 11 | No | Long-term improvement of lower lid margin vascularity, meibum expressibility, quality and symptoms. Improved TBUT, CFS. |
| Vigo et al (2019)79 | Prospective noncomparative | 19 | E>Eye | 0-2-6 | 3 | 9–13 | / | Increased TBUT and LLT |
| Yin et al (2017)27 | Prospective comparative | 35 | Lumenis | 4 | 3 | 16–17 | No | Increased TBUT, meibum quality, MG expressibility and dropout. Improved MG microstructure and decreased inflammation |
Abbreviations: DE, dry eye; MG, meibomian glands; TBUT, tear break-up time; SPEED, standard patient evaluation of eye dryness; MGS, meibomian gland score; CFS, corneal fluorescein staining; TFO, tear film osmolarity; LLG, improved lipid layer grade; LLT, lipid layer thickness; OSDI, ocular surface disease index; MGYSS, meibomian gland yielding secretion score.
Table 3.
Randomized Clinical Trials For IPL Therapy In DED
| Study (Year) | Design | Patients (n) | IPL Device | Frequency (Weeks) | IPL Sessions (n) | IPL Energy (J/cm2) | Adverse Events | Results |
|---|---|---|---|---|---|---|---|---|
| Craig et al (2015)28 | Prospective controlled | 28 | E>Eye | 0-2-6 6 |
3 | 9–13 | / | Improved LLG, TBUT and VAS symptom score |
| Liu et al (2017)69 | Prospective controlled randomized double-masked | 44 | Lumenis | 4 | 3 | 14–16 | / | Decreased levels of inflammation markers (IL-17A, IL-6, PGE2) |
| Rong et al (2018)76 | Prospective controlled randomized double-masked | 44 | Lumenis | 4 | 3 | 14–16 | Mild pain and burning, partial eyelash loss | Improved MGYSS, TBUT, SPEED, CFS. |
| Rong et al (2018)77 | Prospective controlled randomized | 28 | Lumenis | 4 | 3 | 14–16 | No | Improved MG secretion function and TBUT by 6 months after IPL |
Abbreviations: LLG, lipid layer grade; TBUT, tear break-up time; MGYSS, meibomian gland yielding secretion score; CFS, corneal fluorescein staining; SPEED, standard patient evaluation of eye dryness; MG, meibomian gland.
Karaca and co-authors adopted the same protocol for treating 26 DED patients. Symptoms (ocular surface disease index and Standard Patient Evaluation of Eye Dryness scores), TBUT and Schirmer test significantly improved whereas corneal fluorescein staining, lid margin abnormality score, secretion quality and expressibility showed no significant changes.85
Albietz and collaborators used the same IPL device combined with meibomian gland expression at baseline, 2 weeks and 6 weeks in 26 patients.30 They found that all the parameters analyzed (meibomian gland expressibility, meibum quality, lid margin redness, ocular surface disease index, TBUT, corneal fluorescein staining, bulbar and limbal redness, eyelid margin bacteria colony count) showed significant improvement. Treatment effects were cumulative and sustained for at least 6 weeks after the final IPL procedure.
Dell and co-workers analyzed the clinical outcomes of 40 patients who underwent 4 IPL sessions, each 3 weeks apart, obtaining similar results.24 Interestingly, Yin and collaborators analyzed the effect of IPL on two indexes describing the meibomian gland microstructure: the acinar longest diameter and the acinar unit density. Both these parameters showed a significant improvement after treatment.27 Authors speculated that the changes in gland microstructure were induced by the photomodulation effect of IPL on acinar cell activity.27 Arita and co-workers evaluated 45 patients who were randomly assigned to receive either the IPL plus meibomian gland expression or meibomian gland expression alone (control group).21 The treatment consisted of 8 sessions with a time interval of 3 weeks. A significant improvement in lipid layer grade and thickness, TBUT, lid margin abnormalities and meibum grade was observed in the IPL-meibomian gland expression group but not in the control group. Significant improvements in ocular discomfort symptoms as well as in corneal fluorescein staining score were also obtained. Therefore, the study showed the superiority of IPL plus meibomian gland expression over meibomian gland expression alone in terms of efficacy and clinical results, as demonstrated by the improved tear film homeostasis and ocular symptoms obtained in patients treated with the combined approach.21
In order to overcome the drawbacks of subjective measures, Vigo and co-workers used an automated ocular surface work-up to evaluate the effect of IPL on DED.79 In this study, TBUT values significantly increased as well as tear film characteristics and quality; on the other hand, no significant changes were found for tear osmolarity, in agreement with other studies.28,30 Furthermore, no significant change was found for meibomian gland loss, in contrast to a previous study, which reported a decrease of gland loss after IPL, suggesting its possible effect on glands turnover.27,79
The anti-inflammatory effect of IPL is confirmed by the significant decrease of inflammatory markers in tears of DED subjects as shown by previous studies.23,69
In the study from Liu and co-authors, tear samples were collected and analyzed at baseline, week 4 and/or week 12 for IL-17A, IL-6 and prostaglandin E2 (PGE2). All the values of these inflammatory markers decreased during the follow-up visits compared to baseline values. Furthermore, IL-17A and IL-6 showed a significant correlation with ocular surface parameters, thus confirming the role of these cytokines in the pathogenesis of DED.69 Choi and co-workers analyzed the effect of IPL on inflammation and reported significant lower levels of IL-4, IL-6, IL-10, IL-17A and TNF-alpha after IPL. Furthermore, TNF-alpha was correlated with the improvement in meibum expressibility.23
Nevertheless, IPL was shown to improve tear protein and lipid content and composition as demonstrated by Ahmed and co-authors.87 In fact, the study showed significant improvements in tear protein concentrations and molecular weight. The most pronounced effect was seen for lysozyme, lactoferrin and albumin. Regarding the lipids, the study showed an improvement in the concentrations of total lipids, triglycerides, cholesterol, and phospholipids. Polar lipids such as phospholipids critically impact the health of the ocular surface, contributing to the stability of the tear film by providing a constant interface between non-polar lipids, such as triglycerides and cholesterol, and the hydrophilic aqueous layer.
Long-Term Results And Repeated Treatments
It is still unclear if the therapeutic effect of IPL maintains its efficacy over time. Some studies tried to clarify this issue, adopting a longer follow-up after IPL treatments. Seo and co-authors enrolled 17 patients suffering from skin rosacea with moderate and severe MGD who underwent 4 IPL sessions at 3-week intervals and were followed for a total of 12 months.26 All ocular surface parameters showed significant positive changes from baseline to follow-up visits. However, TBUT, staining score and NIBUT did not maintain their improvements at 6 and 12 months.26 Therefore, IPL may be a beneficial treatment with long-lasting effects, but repeated sessions should be performed, according to the severity of the disease. Rong and collaborators recommended the IPL treatment to be repeated every 6 months: they enrolled 44 MGD patients in which 1 eye acted as the study eye and the other as control.77 The complete therapy included 3 treatment sessions performed at 4-week intervals. Both eyes received meibomian gland expression and artificial tears. The patients were examined at 1, 3, 6 and 9 months after the treatment. A total of 28 subjects completed the treatment protocol and were included in the final analysis. IPL combined with meibomian gland expression provided sustained efficacy for at least 6 months by improving meibomian gland secretion, TBUT, ocular surface parameters and symptoms.77
Although IPL provides an early-onset clinical improvement, regular repeated treatments are usually required in order to maintain beneficial effects over the time. Further clinical investigations are clearly necessary to define the proper timing for repeated cycles of therapy.
Discussion
MGD has been identified as the most common cause of DED.34,88,89 The currently available treatments are mainly palliative solutions that often fail to achieve long-term improvement in clinical signs and ocular discomfort symptoms. IPL therapy was recently introduced in the field of Ophthalmology, leading to significantly better outcomes in MGD patients, as documented by the improvement of ocular surface parameters and the decreased levels of inflammatory cytokines detected in patients after treatment.20–31,69 Although different speculative pathophysiological theories have been proposed to explain the positive effects of IPL upon dry eye signs and symptoms, the mechanics of action are still not fully elucidated. Among these, the coagulation of superficial blood vessels carrying inflammatory mediators, the heating and liquefying of the meibum, the decrease of bacterial and parasitic load over eyelids and eyelashes are thought to be the principal ones. More recently, the enhancement in collagen synthesis and connective tissue remodeling, the reduction in skin epithelial cell turnover, and the modulation of cellular inflammatory markers have also been proposed.41
To date, most of the available studies confirmed the beneficial effect of IPL, reporting improvements in terms of lid margin features (e.g. thickening and vascularity, telangiectasia, number of plugged glands) and meibomian gland secretion quality and expressibility. Furthermore, in order to overcome the potential bias of a subjective analysis, a comprehensive ocular surface workup with automated quantitative measurements has been performed by our Group, and the efficacy of the procedure was further confirmed.79 The minority of patients who did not experience significant clinical improvement after the procedure suffered from others concomitant conditions that alter the physiological ocular surface homeostasis, including incomplete blinking, lagophthalmos, laser in situ keratomileusis, contact lens wearing, use of benzodiazepines, tricyclic antidepressants and diuretics. Furthermore, it is likely that mild and moderate forms of DED better respond to IPL compared to long-standing and end-stage disease with meibomian gland atrophy.5
IPL has demonstrated to be not only effective, but also a safe therapeutic option, as shown by a large amount of studies that reported no major adverse events.
Currently, IPL therapy for the treatment of MGD and DED is spreading among ophthalmologists and is a flourishing field of interest as it is also indicated by different ongoing studies (Table 4).
Table 4.
Ongoing Clinical Trials For IPL Therapy In DED
| Study | Design | Condition | Patients (n) | Control Arm | IPL Device | Number Of IPL Sessions | Frequency | Outcomes | Concomitant Therapy |
|---|---|---|---|---|---|---|---|---|---|
| Dell et al (2018) | Interventional Randomized Single-masked | MGD | 50 | Sham IPL + MGX | Lumenis | 4 | 2 | TBUT; OSDI; EDS; Eyelid appearance; meiboscore; incidence of adverse events; pain during procedure; biomicroscopy |
MGX |
| Pyacomn et al (2018) | Interventional Randomized Double masked | MGD | 114 | Sham IPL | E>Eye | 3 | 1-2-6 | TBUT; OSDI; LLT; meiboscore; CFS; MGE; meibum quality; TFO (6 months follow-up) ST; IL-1Ra; IL-6 | Warm compression, lid scrub, non preservative ocular lubricants |
| Wood et al (2017) | Interventional Randomized Double-masked | Evaporative DED | 14 | Sham IPL | / | 4 | / | TBUT (4 months follow up); OSDI (7 months follow up) | / |
| Shen et al (2017) | Interventional randomized open label | Ocular rosacea | 20 | MGX | / | 4 | 4 | OSDI (4 months follow-up); pathologic microbial load (4 months follow-up); TGF-B1 (4 months follow-up) | MGX, topical anesthetic for DED |
| Zadock et al (2017) | Interventional randomized single-masked | MGD | 24 | Sham IPL + MGX | Lumenis | 4 | 2 | TBUT (10 weeks follow-up); MG secretion score; OSDI; MGYLS; TFO; meiboscore; eyelid appearance (10 weeks follow-up) | MGX |
| Dell et al (2015) | Interventional noncomparative | MGD | 44 | / | Lumenis | 4 | / | TBUT (3 weeks after each IPL session); CFS; MGS; SPEED; TFO; LLT; incidence of adverse events; immediate/short term skin response; subjective level of pain/discomfort | MGX |
Abbreviations: TBUT, tear break-up time; OSDI, ocular surface disease index; EDS, eye dryness score; LLT, lipid layer thickness; CFS, corneal fluorescein staining; MGE, meibomian gland expressibility; TFO, tear film osmolarity; MGLYS, meibomian glands yielding liquid secretion; SPEED, standard patient evaluation of eye dryness.
Despite its clinical efficacy, currently there are some limitations: the technology cannot be used in patients with a Fitzpatrick score higher than IV, as deeper pigmented subjects are at a higher risk of skin damage. Moreover, the upper eyelid cannot be directly treated because it is possible for the broad-spectrum light to penetrate and reach underlying ocular structures with potential damages. However, some authors demonstrated that also upper meibomian glands showed an improvement in their function after IPL treatment limited in the lower lids area.78 Other current limitations are related to lack of evidence about the number of sessions that should be performed for each treatment cycle and to the proper interval time that should be waited between each IPL session.
Finally, as several options are available in the armamentarium of MGD therapies, studies aiming at identifying predictive factors for a better response to IPL procedure are desirable.
Conclusion
IPL therapy is a safe and effective adjuvant therapeutic strategy for DED due to MGD. The treatment is usually well accepted among patients for its non-invasive nature; rare are main adverse reactions. Furthermore, the procedure is quick and has shown good ability to improve signs and symptoms in the early phases after treatment. However, repeated treatments are often required to maintain the beneficial effects over time. In addition, in order to further optimize outcomes, the procedure should be combined with other DED therapies (e.g. meibomian gland expression, tear substitute use, eyelid hygiene). Future perspectives should be addressed to the identification of parameters able to predict those patients who will most likely benefit from IPL procedure and to the identification of the ideal number and timing of sessions for repeated treatments.
Acknowledgment
Giuseppe Giannaccare and Leonardo Taroni share the first authorship.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Craig JP, Nichols KK, Akpek EK, et al. TFOS DEWS II definition and classification report. Ocul Surf. 2017;15:276–283. doi: 10.1016/j.jtos.2017.05.008 [DOI] [PubMed] [Google Scholar]
- 2.Uchino M, Uchino Y, Dogru M, et al. Dry eye disease and work productivity loss in visual display users: the Osaka study. Am J Ophthalmol. 2014;157:294–300. doi: 10.1016/j.ajo.2013.10.014 [DOI] [PubMed] [Google Scholar]
- 3.Qiao J, Yan X. Emerging treatment options for meibomian gland dysfunction. Clin Ophthalmol. 2013;7:1797–1803. doi: 10.2147/OPTH.S33182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nichols KK, Foulks GN, Bron AJ, et al. The international workshop on meibomian gland dysfunction: executive summary. Invest Ophthalmol Vis Sci. 2011;52:1922–1929. doi: 10.1167/iovs.10-6997a [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Giannaccare G, Pellegrini M, Sebastiani S, et al. Efficacy of omega-3 fatty acid supplementation for treatment of dry eye disease: a meta-analysis of randomized clinical trials. Cornea. 2019;38:565–573. doi: 10.1097/ICO.0000000000001884 [DOI] [PubMed] [Google Scholar]
- 6.Lindsley K, Matsumara S, Hatef E, Akpek EK. Interventions for chronic blepharitis. Cochrane Database Syst Rev. 2012. doi: 10.1002/14651858.CD005556.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Thode AR, Latkany RA. Current and emerging therapeutic strategies for the treatment of meibomian gland dysfunction (MGD). Drugs. 2015;75(11):1177–1185. doi: 10.1007/s40265-015-0432-8 [DOI] [PubMed] [Google Scholar]
- 8.Villani E, Garoli E, Canton V, Pichi F, Nucci P, Ratiglia R. Evaluation of a novel eyelid-warming device in meibomian gland dysfunction unresponsive to traditional warm compress treatment: an in vivo confocal study. Int Ophthalmol. 2015;35:319–323. doi: 10.1007/s10792-014-9947-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Doan S, Chiambaretta F, Baudouin C; ESPOIR study group. Evaluation of an eyelid warming device (Blephasteam) for the management of ocular surface diseases in France: the ESPOIR study. J Fr Ophtalmol. 2014;37:763–772. doi: 10.1016/j.jfo.2014.06.004 [DOI] [PubMed] [Google Scholar]
- 10.Purslow C. Evaluation of the ocular tolerance of a novel eyelid-warming device used for meibomian gland dysfunction. Contact Lens Anterior Eye. 2013;36:226–231. doi: 10.1016/j.clae.2013.02.009 [DOI] [PubMed] [Google Scholar]
- 11.Greiner JV. A single LipiFlow® Thermal Pulsation System treatment improves meibomian gland function and reduces dry eye symptoms for 9 months. Curr Eye Res. 2012;37:272–278. doi: 10.3109/02713683.2011.631721 [DOI] [PubMed] [Google Scholar]
- 12.Friedland BR, Fleming CP, Blackie CA, Korb DR. A novel thermodynamic treatment for meibomian gland dysfunction. Curr Eye Res. 2011;36:79–87. doi: 10.3109/02713683.2010.509529 [DOI] [PubMed] [Google Scholar]
- 13.Korb DR, Blackie CA. Restoration of meibomian gland functionality with novel thermodynamic treatment device-a case report. Cornea. 2010;29:930–933. doi: 10.1097/ICO.0b013e3181ca36d6 [DOI] [PubMed] [Google Scholar]
- 14.Goto E, Monden Y, Takano Y, et al. Treatment of non-inflamed obstructive meibomian gland dysfunction by an infrared warm compression device. Br J Ophthalmol. 2002;86:1403–1407. doi: 10.1136/bjo.86.12.1403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lienert JP, Tarko L, Uchino M, Christen WG, Schaumberg DA. Long-term natural history of dry eye disease from the patient’s perspective. Ophthalmology. 2016;123:425–433. doi: 10.1016/j.ophtha.2015.10.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zeev MSB, Miller DD, Latkany R. Diagnosis of dry eye disease and emerging technologies. Clin Ophthalmol. 2014;8:581–590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Alves M, Fonseca EC, Alves MF, et al. Dry eye disease treatment: a systematic review of published trials and a critical appraisal of therapeutic strategies. Ocul Surf. 2013;11:181–192. doi: 10.1016/j.jtos.2013.02.002 [DOI] [PubMed] [Google Scholar]
- 18.Raulin C, Greve B, Grema H. IPL technology: a review. Lasers Surg Med. 2003;32:78–87. doi: 10.1002/lsm.10145 [DOI] [PubMed] [Google Scholar]
- 19.Papageorgiou P, Clayton W, Norwood S, Chopra S, Rustin M. Treatment of rosacea with intense pulsed light: significant improvement and long-lasting results. Br J Dermatol. 2008;159:628–632. doi: 10.1111/j.1365-2133.2008.08702.x [DOI] [PubMed] [Google Scholar]
- 20.Toyos R, McGill W, Briscoe D. Intense pulsed light treatment for dry eye disease due to meibomian gland dysfunction; a 3-year Retrospective Study. Photomed Laser Surg. 2015;33:41–46. doi: 10.1089/pho.2014.3819 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Arita R, Fukuoka S, Morishige N. Therapeutic efficacy of intense pulsed light in patients with refractory meibomian gland dysfunction. Ocul Surf. 2019;17:104–110. doi: 10.1016/j.jtos.2018.11.004 [DOI] [PubMed] [Google Scholar]
- 22.Arita R, Mizoguchi T, Fukuoka S, Morishige N. Multicenter study of intense pulsed light therapy for patients with refractory meibomian gland dysfunction. Cornea. 2018;37:1566–1571. doi: 10.1097/ICO.0000000000001687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Choi M, Han SJ, Ji YW, et al. Meibum Expressibility improvement as a therapeutic target of intense pulsed light treatment in meibomian gland dysfunction and its association with tear infammatory cytokines. Sci Rep. 2019;9:1–8. doi: 10.1038/s41598-018-37186-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dell SJ, Gaster RN, Barbarino SC, Cunningham DN. Prospective evaluation of intense pulsed light and meibomian gland expression efficacy on relieving signs and symptoms of dry eye disease due to meibomian gland dysfunction. Clin Ophthalmol. 2017;11:817–882. doi: 10.2147/OPTH.S130706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li D, Lin S, Cheng B. Intense pulsed light treatment for meibomian gland dysfunction in skin types III/IV. Photobiomodul Photomed Laser Surg. 2019;37:70–76. doi: 10.1089/photob.2018.4509 [DOI] [PubMed] [Google Scholar]
- 26.Seo KY, Kang SM, Ha DY, Chin HS, Jung JW. Long-term effects of intense pulsed light treatment on the ocular surface in patients with rosacea-associated meibomian gland dysfunction. Cont Lens Anterior Eye. 2018;41:430–435. doi: 10.1016/j.clae.2018.06.002 [DOI] [PubMed] [Google Scholar]
- 27.Yin Y, Liu N, Gong L, Song N. Changes in the meibomian gland after exposure to intense pulsed light in meibomian gland dysfunction (MGD) Patients. Curr Eye Res. 2017;00:1–6. [DOI] [PubMed] [Google Scholar]
- 28.Craig JP, Chen YH, Turnbull PRK. Prospective trial of intense pulsed light for the treatment of meibomian gland dysfunction. Invest Ophthalmol Vis Sci. 2015;56:1965–1970. doi: 10.1167/iovs.14-15764 [DOI] [PubMed] [Google Scholar]
- 29.Jiang X, Lv H, Song H, et al. Evaluation of the safety and effectiveness of intense pulsed light in the treatment of meibomian gland dysfunction. J Ophthalmol. 2016;1910694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Albietz JM, Schmid KL. Intense pulsed light treatment and meibomian gland expression for moderate to advanced meibomian gland dysfunction. Clin Exp Optom. 2018;101:23–33. doi: 10.1111/cxo.12541 [DOI] [PubMed] [Google Scholar]
- 31.Guilloto Caballero S, García Madrona JL, Colmenero Reina E. Effect of pulsed laser light in patients with dry eye syndrome. Arch Soc Esp Oftalmol. 2017;92:509–515. doi: 10.1016/j.oftal.2016.12.018 [DOI] [PubMed] [Google Scholar]
- 32.Mudgil P. Antimicrobial role of human meibomian lipids at the ocular surface. Invest Ophthalmol Vis Sci. 2014;55:7272–7277. doi: 10.1167/iovs.14-15512 [DOI] [PubMed] [Google Scholar]
- 33.Nelson JD, Shimazaki J, Benitez-Del-Castillo JM, et al. The international workshop on meibomian gland dysfunction: report of the definition and classification subcommittee. Invest Ophthalmol Vis Sci. 2011;52:1930–1937. doi: 10.1167/iovs.10-6997b [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Baudouin C, Messmer EM, Aragona P, et al. Revisiting the vicious circle of dry eye disease: a focus on the pathophysiology of meibomian gland dysfunction. Br J Ophthalmol. 2016;100:300–306. doi: 10.1136/bjophthalmol-2015-307415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wordinger RJ1. Clark AF. Effects of glucocorticoids on the trabecular meshwork: towards a better understanding of glaucoma. Prog Retin Eye Res. 1999;18:629–667. [DOI] [PubMed] [Google Scholar]
- 36.James ER. The etiology of steroid cataract. J Ocul Pharmacol Ther. 2007;23:403–420. doi: 10.1089/jop.2006.0067 [DOI] [PubMed] [Google Scholar]
- 37.Vora GK, Gupta PK. Intense pulsed light therapy for the treatment of evaporative dry eye disease. Curr Opin Ophthalmol. 2015;26:314–318. doi: 10.1097/ICU.0000000000000166 [DOI] [PubMed] [Google Scholar]
- 38.Muhlbauer W, Nath G, Kreitmair A. Treatment of capillary hemangiomas and nevi flammei with light. Langenbecks Arch Chir. 1976;(suppl):91–94. [PubMed] [Google Scholar]
- 39.Li D, Lin S, Cheng B. Intense pulsed light: from the past to the future. Photomed Laser Surg. 2016;34:435–447. doi: 10.1089/pho.2016.4139 [DOI] [PubMed] [Google Scholar]
- 40.Wat H, Wu DC, Rao J, Goldman MP. Application of intense pulsed light in the treatment of dermatologic disease: a systematic review. Dermatol Surg. 2014;40:359–377. doi: 10.1111/dsu.12424 [DOI] [PubMed] [Google Scholar]
- 41.Dell SJ. Intense pulsed light for evaporative dry eye disease. Clin Ophtalmol. 2017;11:1167–1173. doi: 10.2147/OPTH.S139894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Piccolo D, Di Marcantonio D, Crisman G, et al. Unconventional use of intense pulsed light. Biomed Res Int. 2014;2014:618206. doi: 10.1155/2014/618206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bäumler W, Vural E, Landthaler M, Muzzi F, Shafirstein G. The effects of intense pulsed light (IPL) on blood vessels investigated by mathematical modeling. Lasers Surg Med. 2007;39:132–139. doi: 10.1002/lsm.20408 [DOI] [PubMed] [Google Scholar]
- 44.Nagymihályi A, Dikstein S, Tiffany J. The influence of eyelid temperature on the delivery of meibomian oil. Exp Eye Res. 2004;78:367–370. doi: 10.1016/s0014-4835(03)00197-0 [DOI] [PubMed] [Google Scholar]
- 45.Borchman D, Foulks G, Yappert M, et al. Human meibum lipid conformation and thermodynamic changes with meibomian-gland dysfunction. Invest Ophthalmol Vis Sci. 2011;52:3805–3817. doi: 10.1167/iovs.10-6514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Finis D, Hayajneh J, König C, Schrader S, Geerling G. Evaluation of an automated thermodynamic treatment (LipiFlow) system for meibomian gland dysfunction: a prospective, randomized, observer-masked trial. Ocul Surf. 2014;12:146–154. doi: 10.1016/j.jtos.2013.12.001 [DOI] [PubMed] [Google Scholar]
- 47.Arita R, Morishige N, Shirakawa R, Sato Y, Amano S. Effects of eyelid warming devices on tear film parameters in normal subjects and patients with meibomian gland dysfunction. Ocul Surf. 2015;13:321–330. doi: 10.1016/j.jtos.2015.04.005 [DOI] [PubMed] [Google Scholar]
- 48.Borchman D. The optimum temperature for the heat therapy for meibomian gland dysfunction. Ocul Surf. 2019;17:360–364. doi: 10.1016/j.jtos.2019.02.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Geerling G, Tauber J, Baudouin C, et al. The International Workshop on Meibomian Gland Dysfunction: report of the Subcommittee on management and treatment of meibomian gland dysfunction. Invest Ophthalmol Vis Sci. 2011;52:2050–2064. doi: 10.1167/iovs.10-6997g [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Karu T. Primary and secondary mechanisms of action of visible to near-IR radiation on cells. J Photochem Photobiol B. 1999;49:1–17. doi: 10.1016/S1011-1344(98)00219-X [DOI] [PubMed] [Google Scholar]
- 51.Farivar S, Malekshahabi T, Shiari R. Biological effects of low-level laser therapy. J Lasers Med Sci. 2014;5:58–62. [PMC free article] [PubMed] [Google Scholar]
- 52.Takezaki S, Omi T. Sato, S Kawana S. Ultrastructural observations of human skin following irradiation with visible red light-emitting diodes (LEDs): a preliminary in vivo report. Laser Ther. 2005;14:153–160. doi: 10.5978/islsm.14.153 [DOI] [Google Scholar]
- 53.Barolet D, Roberge C, Auger F, Boucher A, Germain L. Regulation of skin collagen metabolism in vitro using a pulsed 660 nm LED light source: clinical correlation with a single-blinded study. J Invest Dermatol. 2009;129:2751–2759. doi: 10.1038/jid.2009.186 [DOI] [PubMed] [Google Scholar]
- 54.Cuerda-Galindo E, Díaz-Gil G, Palomar-Gallego M, Linares-García-Valdecasas R. Increased fibroblast proliferation and activity after applying intense pulsed light 800–1200 nm. Ann Anat. 2015;198:66–72. doi: 10.1016/j.aanat.2014.11.005 [DOI] [PubMed] [Google Scholar]
- 55.Goldberg D. Current trends in intense pulsed light. J Clin Aesthet Dermatol. 2012;5:45–53. [PMC free article] [PubMed] [Google Scholar]
- 56.Liu J, Sheha H, Tseng S. Pathogenic role of Demodex mites in blepharitis. Curr Opin Allergy Clin Immunol. 2010;10:505–510. doi: 10.1097/ACI.0b013e32833df9f4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Szkaradkiewicz A, Chudzicka-Strugała I, Karpiński T, et al. Bacillus oleronius and Demodex mite infestation in patients with chronic blepharitis. Clin Microbiol Infect. 2012;18:1020–1025. doi: 10.1111/j.1469-0691.2011.03704.x [DOI] [PubMed] [Google Scholar]
- 58.Li J, O’Reilly N, Sheha H, et al. Correlation between ocular Demodex infestation and serum immunoreactivity to Bacillus proteins in patients with facial rosacea. Ophthalmology. 2010;117:870–877. doi: 10.1016/j.ophtha.2009.09.057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Margalit A, Kowalczyk M, Żaba R, Kavanagh K. The role of altered cutaneous immune responses in the induction and persistence of rosacea. J Dermatol Sci. 2016;82:3–8. doi: 10.1016/j.jdermsci.2015.12.006 [DOI] [PubMed] [Google Scholar]
- 60.Lacey N, Delaney S, Kavanagh K, Powell F. Mite-related bacterial antigens stimulate inflammatory cells in rosacea. Br J Dermatol. 2007;157(3):474–481. doi: 10.1111/j.1365-2133.2007.08028.x [DOI] [PubMed] [Google Scholar]
- 61.Prieto V, Sadick N, Lloreta J, Nicholson J, Shea C. Effects of intense pulsed light on sun-damaged human skin, routine, and ultrastructural analysis. Lasers Surg Med. 2002;30:82–85. doi: 10.1002/lsm.10042 [DOI] [PubMed] [Google Scholar]
- 62.Kirn T. Intense pulsed light eradicates Demodex mites. Skin Allergy News. 2002;33:37. [Google Scholar]
- 63.Enríquez-de-Salamanca A, Castellanos E, Stern M, et al. Tear cytokine and chemokine analysis and clinical correlations in evaporative-type dry eye disease. Mol Vis. 2010;16:862–873. [PMC free article] [PubMed] [Google Scholar]
- 64.Stevenson W, Chauhan S, Dana R. Dry eye disease: an immune-mediated ocular surface disorder. Arch Ophthalmol. 2012;130:90–100. doi: 10.1001/archophthalmol.2011.364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Byun J, Choi H, Myung K, Choi YW. Expression of IL-10, TGF-β1 and TNF-α in cultured keratinocytes (HaCaT Cells) after IPL treatment or ALA-IPL photodynamic treatment. Ann Dermatol. 2009;21:12–17. doi: 10.5021/ad.2009.21.1.12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Huang J, Luo X, Lu J, et al. IPL irradiation rejuvenates skin collagen via the bidirectional regulation of MMP-1 and TGF-β1 mediated by MAPKs in fibroblasts. Lasers Med Sci. 2011;26:381–387. doi: 10.1007/s10103-010-0870-1 [DOI] [PubMed] [Google Scholar]
- 67.Taylor M, Porter R, Gonzalez M. Intense pulsed light may improve inflammatory acne through TNF-α down-regulation. J Cosmet Laser Ther. 2014;16:96–103. doi: 10.3109/14764172.2013.864198 [DOI] [PubMed] [Google Scholar]
- 68.Wong WR, Shyu WL, Tsai JW, Hsu KH, Lee HY, Pang JH. Intense pulsed light modulates the expressions of MMP-2, MMP-14 and TIMP-2 in skin dermal fibroblasts cultured within contracted collagen lattices. J Dermatol Sci. 2008;51:70–73. doi: 10.1016/j.jdermsci.2008.02.011 [DOI] [PubMed] [Google Scholar]
- 69.Liu R, Rong B, Tu P, et al. Analysis of cytokine levels in tears and clinical correlations after intense pulsed light treating meibomian gland dysfunction. Am J Ophthalmol. 2017;183:81–90. doi: 10.1016/j.ajo.2017.08.021 [DOI] [PubMed] [Google Scholar]
- 70.Jones D. Reactive oxygen species and rosacea. Cutis. 2009;74:17–20, 32–34. [PubMed] [Google Scholar]
- 71.Huang YY, Chen AH, Carroll J, Hamblin MR. Biphasic dose response in low level light therapy. Dose Response. 2009;7:358–383. doi: 10.2203/dose-response.09-027.Hamblin [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Augustin A, Spitznas M, Kaviani N, et al. Oxidative reactions in the tear fluid of patients suffering from dry eyes. Graefes Arch Clin Exp Ophthalmol. 1995;233:694–698. doi: 10.1007/bf00164671 [DOI] [PubMed] [Google Scholar]
- 73.Mejía LF, Gil JC, Jaramillo M. Intense pulsed light therapy: a promising complementary treatment for dry eye disease. Arch Soc Esp Oftalmol. 2019;94:331–336. doi: 10.1016/j.oftal.2019.03.009 [DOI] [PubMed] [Google Scholar]
- 74.Babilas P, Schreml S, Szeimies R, Landthaler M. Intense pulsed light (IPL): a review. Lasers Surg Med. 2010;42:93–104. doi: 10.1002/lsm.20877 [DOI] [PubMed] [Google Scholar]
- 75.Fitzpatrick TB. The validity and practicality of sun-reactive skin types I through VI. Arch Dermatol. 1988;124:869–871. doi: 10.1001/archderm.124.6.869 [DOI] [PubMed] [Google Scholar]
- 76.Rong B, Tang Y, Tu P, et al. Intense pulsed light applied directly on eyelids combined with meibomian gland expression to treat meibomian gland dysfunction. Photomed Laser Surg. 2018;36:326–332. doi: 10.1089/pho.2017.4402 [DOI] [PubMed] [Google Scholar]
- 77.Rong B, Tang Y, Liu R, et al. Long-term effects of intense pulsed light combined with meibomian gland expression in the treatment of meibomian gland dysfunction. Photomed Laser Surg. 2018;36:562–567. doi: 10.1089/pho.2018.4499 [DOI] [PubMed] [Google Scholar]
- 78.Gupta PK, Vora GK, Matossian C, Kim M, Stinnett S. Outcomes of intense pulsed light therapy for treatment of evaporative dry eye disease. Can J Ophthalmol. 2016;51:249–253. doi: 10.1016/j.jcjo.2016.01.005 [DOI] [PubMed] [Google Scholar]
- 79.Vigo L, Giannaccare G, Sebastiani S, Pellegrini M, Carones F. Intense pulsed light for the treatment of dry eye owing to meibomian gland dysfunction. J Vis Exp. 2019;146:1–7. [DOI] [PubMed] [Google Scholar]
- 80.Crabb M, Chan WO, Taranath D, Huilgol SC. Intense pulsed light therapy (IPL) induced iritis following treatment for a medial canthal capillary malformation. Australas J Dermatol. 2014;55:289–291. doi: 10.1111/ajd.12137 [DOI] [PubMed] [Google Scholar]
- 81.Lee WW, Murdock J, Albini TA, O’Brien TP, Levine ML. Ocular damage secondary to intense pulse light therapy to the face. Ophthal Plast Reconstr Surg. 2011;27:263–265. doi: 10.1097/IOP.0b013e31820c6e23 [DOI] [PubMed] [Google Scholar]
- 82.Pang AL, Wells K. Bilateral anterior uveitis after intense pulsed light therapy for pigmented eyelid lesions. Dermatol Surg. 2008;34:1276–1279. doi: 10.1111/j.1524-4725.2008.34274.x [DOI] [PubMed] [Google Scholar]
- 83.Goldman MP, Weiss RA, Weiss MA. Intense pulsed light as a nonablative approach to photoaging. Dermatol Surg. 2005;31:1179–1187. doi: 10.1111/j.1524-4725.2005.31924 [DOI] [PubMed] [Google Scholar]
- 84.Javey G, Schwartz SG, Albini TA. Ocular complication of intense pulsed light therapy: iris photoablation. Dermatol Surg. 2010;36:1466–1468. doi: 10.1111/j.1524-4725.2010.01661.x [DOI] [PubMed] [Google Scholar]
- 85.Karaca EE, Kemer OE, Özek D. Intense regulated pulse light for the meibomian gland dysfunction. Eur J Ophthalmol. 2018;1–4. [DOI] [PubMed] [Google Scholar]
- 86.Vegunta S, Patel D, Shen JF. Combination therapy of intense pulsed light therapy and meibomian gland expression (IPL/MGX) can improve dry eye symptoms and meibomian gland function in patients with refractory dry eye: a retrospective analysis. Cornea. 2016;35:318–322. doi: 10.1097/ICO.0000000000000735 [DOI] [PubMed] [Google Scholar]
- 87.Ahmed SA, Taher IME, Ghoneim DF, Sawfat AEM. Effect of intense pulsed light therapy on tear proteins and lipids in meibomian gland dysfunction. J Ophthalmic Vis Res. 2019;14:3–10. doi: 10.4103/jovr.jovr_12_18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Lemp MA, Crews LA, Bron AJ, Foulks GN, Sullivan BD. Distribution of aqueous-deficient and evaporative dry eye in a clinic-based patient cohort: a retrospective study. Cornea. 2012;31:472–478. doi: 10.1097/ICO.0b013e318225415a [DOI] [PubMed] [Google Scholar]
- 89.Schaumberg DA, Nichols JJ, Papas EB, Tong L, Uchino M, Nichols KK. The international workshop on meibomian gland dysfunction: report of the subcommittee on the epidemiology of, and associated risk factors for, MGD. Invest Ophthalmol Vis Sci. 2011;52:1994–2005. doi: 10.1167/iovs.10-6997e [DOI] [PMC free article] [PubMed] [Google Scholar]


