Abstract
Regorafenib is a sorafenib-derived chemotherapy drug belonging to the multikinase inhibitor family. This agent effectively targets a wide range of tyrosine kinases involved in cancer biology, such as those implicated in oncogenesis, angiogenesis, and tumor microenvironment control. The beneficial effects of regorafenib in clinical trials of patients who suffer from advanced hepatocellular carcinoma (HCC), colorectal cancer (CRC) or gastrointestinal stromal tumors (GISTs) refractory to standard treatments led to regorafenib monotherapy approval as a second-line treatment for advanced HCC and as a third-line treatment for advanced CRC and GISTs. Multiple in vitro and in vivo studies have been performed over the last decade to reveal the molecular mechanisms of the favorable actions exerted by regorafenib in patients. Given the hypothetical loss of sensitivity to regorafenib in tumor cells, preclinical research is also searching for novel therapeutic approaches consisting of co-administration of this drug plus other agents as a strategy to improve regorafenib effectiveness. This review summarizes the anti-tumor effects of regorafenib in single or combined treatment in preclinical models of HCC, CRC and GISTs and discusses both the global and molecular effects that account for its anti-cancer properties in the clinical setting.
Subject terms: Liver cancer, Targeted therapies, Colorectal cancer, Cancer models
Regorafenib: Combination therapy in liver and gastrointestinal cancer
The cancer drug regorafenib exhibits a broad range of anti-tumor activities that could be enhanced by combination with other treatments. A team led by José L. Mauriz from the University of León, Spain, review the ways in which regorafenib, blocking several enzymes involved in cancer biology, has been shown to shrink tumors in different models of liver, colon and gastrointestinal cancer. Its mechanisms of action include blockade of new blood vessel formation, induction of cell death and modulation of the immune microenvironment. Research studies show that co-administration of regorafenib with other drugs directed at various molecular targets or immune pathways produces synergistic effects against cancer cells. The preclinical data highlights the potential of combination drug regimens to improve outcomes among patients eligible for regorafenib treatment.
Introduction
Regorafenib is an orally available multitargeted tyrosine kinase inhibitor (TKI)1–3 that emerged from the process of optimizing sorafenib efficacy by modulating its molecular structure4. The difference between these TKIs is the addition of a unique fluorine atom to the central phenyl ring of sorafenib4, which confers a broader inhibitory profile and greater pharmacological activity to regorafenib5. Regarding sorafenib and regorafenib common targeting profile, both drugs share the ability to inhibit tyrosine kinases involved in signaling pathways driving tumorigenesis and cancer progression. These kinases are isoforms of rapidly accelerated fibrosarcoma (RAF) RAF-1, B-RAF, vascular endothelial growth factor receptor (VEGFR) 1–3, and platelet-derived growth factor receptor (PDGFR) β6–8. However, regorafenib also blocks additional tyrosine kinases indispensable for oncogenesis, angiogenesis and tumor microenvironment maintenance in addition to those previously mentioned. In line with this effect, regorafenib inhibits B-RAFV600E (a mutant isoform of B-RAF), the oncogenic kinases KIT and RET, angiopoietin 1 receptor (TIE2)2,6,9, PDGFRα and fibroblast growth factor receptors (FGFRs) 1 and 21,4. It has been proposed that sorafenib may even target B-RAFV600E, KIT and RET kinases6,7; nonetheless, inhibition would require much higher sorafenib doses than regorafenib doses6. These results were also reported when comparing the capability of both TKIs to suppress the tyrosine kinases included in the common inhibitory profile6; thus, sorafenib is an inhibitor with weaker kinase affinity. The blockade of a high number of tyrosine kinases that cooperate in tumor initiation and progression, in addition to the stronger inhibitory effectiveness of this drug than sorafenib, means that regorafenib is a more potent TKI with promising applications in cancer when standard chemotherapies fail. In fact, as a consequence of its high efficacy in clinical trials, regorafenib was approved as second-line therapy for advanced hepatocellular carcinoma (HCC)4,5,10 and as a third-line treatment for advanced colorectal cancer (CRC)4,9,11 and gastrointestinal stromal tumors (GISTs)4,12. Apart from updating the current therapeutic landscape for advanced-stage HCC, CRC, and GISTs, this review focuses on the anti-tumor actions of regorafenib monotherapy in HCC, CRC, and GISTs preclinical models, as well as on the anti-cancer properties resulting from combining this drug with other compounds as new treatment approaches against these cancer types, detailing not only the global effects but also the molecular mechanisms.
Regorafenib and HCC
Therapeutic approaches for advanced HCC
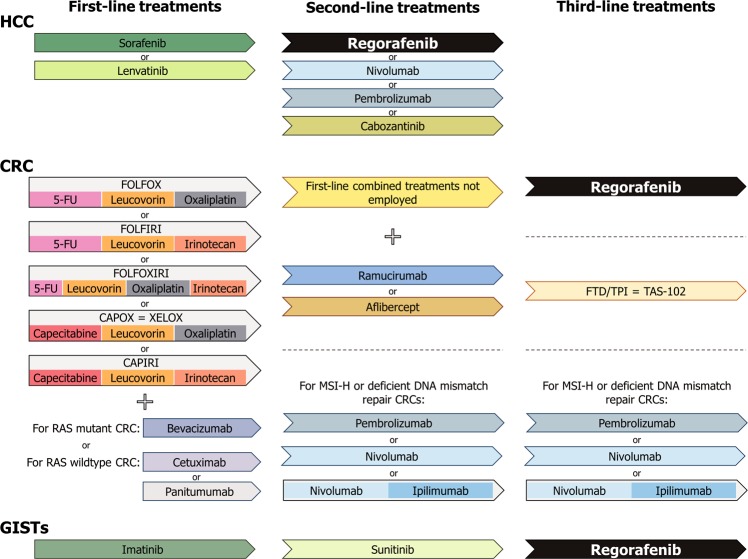
Liver cancer ranks as the sixth most diagnosed cancer and fourth leading cause of cancer-related death worldwide13. A total of 75–85% of primary liver tumors are classified as HCC13, a major health problem whose incidence and mortality rates are continuously increasing8,10,14. The main etiological factors for HCC are hepatitis B or C virus infection, aflatoxin B1 exposure, alcohol intake, and metabolic syndrome related to obesity and type 2 diabetes5,13. HCC is usually diagnosed at advanced stages, when no healing treatments are available8,15; the survival rate at 5 years is only 3%8, and sorafenib remained the only approved front-line treatment until 20185 (Fig. 1). Systemic treatment with sorafenib has become the standard therapy for advanced HCC16,17 since the Food and Drug Administration (FDA) approved it in 20078, and this treatment has prolonged overall survival (OS) by almost 3 months17. Despite this breakthrough for the management of advanced HCC5, sorafenib has important shortcomings, such as its low response rate16. This is related to the development of somatic mutations by HCC cells after prolonged treatment and the resulting drug resistance acquisition15,18.
Fig. 1. Sequential treatment schedule comprising the currently approved chemotherapeutic options for HCC, CRC, and GISTs in advanced stages.
5-FU: 5-fluorouracil; CRC: colorectal cancer; FTD: trifluridine; GISTs: gastrointestinal stromal tumors; HCC: hepatocellular carcinoma; MSI-H: microsatellite instability-high; TPI: tipiracil
There have been multiple attempts to develop molecular targeted drugs better than sorafenib capable of acting as alternatives in the first-line setting or even in the second-line setting after sorafenib failure. However, every phase 3 trial assessing new single-agents from 2007 to 2016 obtained negative results and did not achieve statistical superiority over sorafenib10,16. Regarding first-line treatment options apart from sorafenib, lenvatinib was recently approved for patients with advanced HCC, being the unique drug that has demonstrated mild positive outcomes8,16 (Fig. 1). Eight second-line placebo-controlled phase 3 clinical trials were conducted in patients who progressed after sorafenib administration, but all of them were ineffective16. It was not until the publication of regorafenib results in 2017 and its consecutive FDA approval that the first encouraging outcomes were found in this field10,16 (Fig. 1). Regorafenib was evaluated in the randomized, double-blind, placebo-controlled phase 3 RESORCE trial, where this drug demonstrated efficacy and safety in patients with HCC who experienced disease progression during sorafenib treatment. This trial was the first to indicate a significant OS benefit compared with placebo in HCC patients refractory to sorafenib, with an OS of 10.6 months in the regorafenib group versus 7.8 months in the placebo group (HR = 0.63, CI 0.50–0.79, p < 0.0001)19.
The FDA newly authorized cabozantinib20 and granted accelerated but conditional approval of two human IgG4 monoclonal antibodies against programmed cell death-1 (PD-1), nivolumab and pembrolizumab, as second-line treatments for advanced HCC5,8,17 (Fig. 1). Additional clinical trials testing different molecular targeted agents and immune checkpoint inhibitors are currently ongoing, and better outcomes are expected to expand the therapy spectrum for HCC5,8,14,16.
Regorafenib for advanced HCC: evidences in preclinical models
The results from RESORCE suggested high pharmacological activity of regorafenib when sorafenib is ineffective5. Considering this finding, experimental research has focused on determining the anti-tumor effects of regorafenib on preclinical models of HCC. A preliminary study with Hep3B, HepG2, and PLC/PRF/5 human HCC cell lines revealed that regorafenib reduces cell proliferation in a concentration- and time-dependent manner21. It was found that Hep3B and PLC/PRF/5 cells respond similarly to the drug, having an IC50 near 5 µM for regorafenib, while HepG2 cells exhibited greater sensitivity, with an approximate IC50 value of 1 µM21,22. Moreover, 10-fold lower doses of regorafenib than previously mentioned were needed to decrease alpha-fetoprotein (AFP) levels in AFP-positive HepG2 and PLC/PRF/5 cells, which supports the inhibitory activity of regorafenib on HCC cells proliferation22. Likewise, Tsai et al.23 and Liu et al.24 independently demonstrated the dose- and time-dependent cytotoxic effects of this drug in the SK-HEP-1 HCC cell line, whereas Tai et al.25 showed similar results in HA59T cells. Regorafenib administration at 20 mg/kg/day resulted in anti-HCC effects in PLC/PRF/525, SK-HEP-1/luc2, and Hep3B 2.1-7-bearing subcutaneous xenograft mice, suppressing tumor growth25,26 as well as reducing tumor weight and size25. Furthermore, regorafenib administered once daily at 10 mg/kg has been shown to be enough to significantly extend survival time in an H129 hepatoma in vivo model and delay tumor growth in 8/10 patient-derived (PD) HCC xenograft (HCC-PDX) mouse models27.
It has been reported that regorafenib induces cell death by apoptosis in a concentration-dependent manner in Hep3B21,25, PLC/PRF/5, HepG2, SK-HEP-1, and HA59T HCC cells25. This agent reduced Bcl-2, Bcl-2-like protein 1 (Bcl-xL), survivin21, induced myeloid leukemia cell differentiation protein (Mcl-1), and cyclin D125 protein levels; enhanced caspases 3, 8, and 9 cleavage; and stimulated pro-apoptotic Bcl-2 associated X (Bax) expression21 and poly(ADP-ribose) polymerase (PARP) activation25. Consistent with these results, the apoptotic mediator phospho-c-Jun N-terminal kinase (JNK) and its target phospho-c-Jun were upregulated in Hep3B cells after regorafenib treatment21. Additional data from HCC in vivo studies indicated that apart from downregulation of full-length PARP, regorafenib can increase the expression of the pro-apoptotic Bcl-2-associated agonist of cell death (BAD)27 and decrease cyclin D1 levels26. On the other hand, this drug enhanced microtubule-associated protein 1 light chain 3 II (LC3-II) and Beclin 1 expression in Hep3B cells, suggesting the induction of autophagy, a process that is related to drug-mediated tumor cell growth inhibition21. These results are supported by a study performed with HepG2 and Hep3B cell lines where regorafenib activated pro-death autophagy due to protein kinase B (AKT)/mammalian target of rapamycin (mTOR) signaling abrogation28.
The transducer and activator of transcription 3 (STAT3)-related signaling pathway, which includes some of the previously mentioned anti-apoptotic proteins, has been strongly associated with cancer progression and advanced-stage HCC. Regorafenib has been shown to downregulate this pathway by directly relieving the autoinhibited N-Src homology region 2 (SH2) domain of SH2 domain-containing phosphatase 1 (SHP-1), a negative regulator of STAT3, in both in vitro and in vivo HCC models25. Constitutive activation of nuclear factor-ĸB (NF-ĸB) in cancer cells, which depends on upstream kinases such as extracellular signal-regulated kinase (ERK), has been linked to downstream anti-apoptotic proteins upregulation and apoptosis evasion. An in vitro investigation using SK-HEP-1 cells reported that regorafenib suppresses the expression of NF-ĸB, anti-apoptotic X-linked inhibitor of apoptosis (XIAP), Mcl-1 and cellular FLICE-like inhibitory protein (c-FLIP) while increasing cytochrome c levels and the sub-G1 cell population23. Regarding ERK protein levels, regorafenib inhibited ERK phosphorylation in SK-HEP-123, Hep3B21,22, and PLC/PRF/5 cells21. Indeed, regorafenib was found to reduce the expression of phospho-mitogen-activated protein kinase (MAPK) 7 (MEK), an upstream molecule of ERK22. All this evidence seems to indicate that regorafenib induces both extrinsic and intrinsic apoptotic pathways through suppressing ERK/NF-ĸB activation23. Such findings agree with those observed in an in vivo study employing SK-HEP-1/luc2 and Hep3B 2.1-7-bearing mice26. In contrast, although most investigations have demonstrated that regorafenib abrogates RAS/RAF/MEK/ERK signaling, there is in vivo evidence showing that the drug can promote the phosphorylation of several components of the MAPK pathway. This unexpected induction, which is accompanied by an uncommon increase in the proliferation marker pS10 histone H3, has to be thoroughly analyzed27.
Sustained angiogenesis, invasion and metastasis constitute key cancer hallmarks whose effector proteins are also modulated by NF-ĸB. Regorafenib has been shown to block this transcription factor signal cascade and drive anti-angiogenic and anti-metastatic effects in SK-HEP-1 cell line24. The results obtained included decreased levels and secretion of the angiogenesis-associated proteins VEGF, tumor necrosis factor-α (TNF-α), interleukin-1β (IL-1β), and IL-6; reduced expression and secretion of the metastasis-associated markers matrix metalloproteinase-2 (MMP-2) and MMP-9; and inhibition of cell invasion24. Furthermore, Chen et al.29 disclosed that regorafenib impedes epithelial-to-mesenchymal transition (EMT) through suppressing the ERK and STAT3 pathways and consequent inhibition of hepatocyte growth factor (HGF)-mediated Snail upregulation in HCC cells. These anti-angiogenic and anti-metastatic properties were supported by data from in vitro assays using Hep3B, HepG2, and PLC/PRF/5 cells, as well as by results from an in vivo experiment using SK-HEP-1/luc2 and Hep3B 2.1-7-bearing mouse models26. In addition, regorafenib has been shown to control the balance between MMPs and endogenous tissue inhibitors of metalloproteinases (TIMPs), a critical ratio that determines invasion and metastatic potential, in both SMMC-7721 and Hep3B cell lines30.
Regarding the possibility that regorafenib could have some effect on immune-related proteins, this drug has been shown to attenuate the expression of PD-1 ligand 1 (PD-L1) checkpoint in BALB/c nude mice inoculated with Hep3B cells. Downregulation of immunosuppressive molecules such as PD-L1 could lead to reactivation of the immune response against HCC and prevent the characteristic immune evasion of tumor cells31. Moreover, Carr et al.21 reported that incubation with regorafenib for several weeks causes Hep3B cell quiescence. This finding could supply a useful model to investigate dormancy, a clinical issue that most likely hides behind cancer recurrence after definitive primary treatment in patients21.
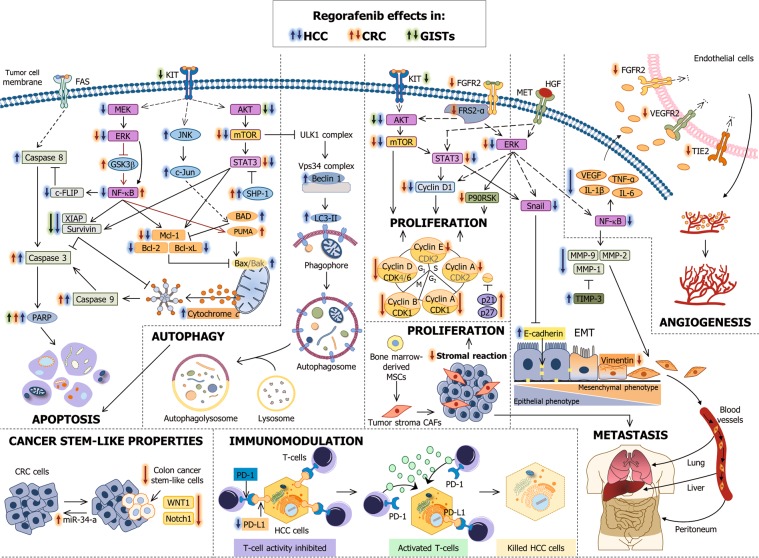
In light of these results, the inhibition of tumor growth, angiogenesis and metastasis, in addition to cell apoptosis promotion and immune response recovery, explains the great anti-tumoral activity exerted by regorafenib in HCC (Fig. 2). Its considerable ability to target cancer-related processes and signaling pathways highlights the importance of regorafenib administration in cases of sorafenib-insensitive advanced HCC patients.
Fig. 2. Effect of regorafenib on the main cancer-related signaling pathways and processes involved in HCC, CRC, and GISTs tumor cells survival.
AKT: protein kinase B; BAD: Bcl-2 associated agonist of cell death; Bak: Bcl-2 antagonist/killer; Bax: Bcl-2 associated X; Bcl-xL: Bcl-2-like protein 1; c-FLIP: cellular FLICE-like inhibitory protein; CAF: carcinoma-associated fibroblast; CDK1: cyclin-dependent kinase 1; CDK6: cyclin-dependent kinase 6; CRC: colorectal cancer; EMT: epithelial-to-mesenchymal transition; ERK: extracellular signal-regulated kinase; FAS: tumor necrosis factor receptor superfamily member 6; FGFR2: fibroblast growth factor receptor 2; FRS2-α: FGFR substrate 2-α; GISTs: gastrointestinal stromal tumors; GSK3β: glycogen synthase kinase 3β; HCC: hepatocellular carcinoma; HGF: hepatocyte growth factor receptor; IL-1β: interleukin-1β; IL-6β: interleukin-6β; JNK: c-Jun N-terminal kinase; LC3-II: microtubule-associated protein 1 light chain 3 II; Mcl-1: induced myeloid leukemia cell differentiation protein; MEK: MAPK 7; MET: HGF receptor; MMP-1: matrix metalloproteinase-1; MMP-2: matrix metalloproteinase-2; MMP-9: matrix metalloproteinase-9; MSC: mesenchymal stem cell; mTOR: mammalian target of rapamycin; NF-ĸB: nuclear factor-ĸB; Notch1: Notch receptor 1; P90RSK: 90-kDa ribosomal protein S6 kinase 1; PARP: poly(ADP-ribose) polymerase; PD-1: programmed cell death-1; PD-L1: PD-1 ligand 1; PUMA: p53-upregulated modulator of apoptosis; SHP-1: SH2 domain-containing phosphatase 1; STAT3: transducer and activator of transcription 3; TIE2: angiopoietin 1 receptor; TIMP-3: MMP inhibitor-3; TNF-α: tumor necrosis factor-α; ULK-1: unc-51 like autophagy activating kinase 1; VEGF: vascular endothelial growth factor; VEGFR: VEGF receptor; Vps34: PI3K catalytic subunit type 3; WNT1: Wnt family member 1; XIAP: X-linked inhibitor of apoptosis
Emerging combined treatment strategies with regorafenib against advanced HCC
Although clinical and preclinical data have demonstrated great regorafenib efficacy against HCC, several regorafenib-combined therapies targeting parallel pathways have emerged with the aim of improving its anti-tumor actions and resolving its main drawbacks. Given that overactivation of the phosphatidylinositol 3 kinase (PI3K)/AKT and MAPK pathways is a well-recognized trait in cancer, heightened suppression of these signaling routes represents an attractive approach to strengthen regorafenib properties. Administration of AKT inhibitors (such as perifosine and MK2206) or PI3K inhibitors (such as PX-866) have individually been shown to cooperate with regorafenib to inhibit HepG2, Hep3B, and Huh7 liver cancer cells proliferation32.
Annexin A3 (ANXA3) is known to play a crucial role in promoting tumor aggressiveness, impeding apoptosis and inducing pro-survival autophagy in sorafenib-resistant HCC cells. Tong et al.33 have shown in sorafenib non-responsive HCC in vivo models that co-administration of regorafenib and an anti-ANXA-3 monoclonal antibody can enhance apoptotic induction because of the abolishment of autophagy. Similarly, navitoclax, a specific inhibitor of the Bcl-2 and Bcl-xL anti-apoptotic proteins, increased the sensitivity of Hep3B and HepG2 cells to regorafenib, as shown by increased general apoptotic features34.
Mitogens such as insulin-like growth factor 1 (IGF1) are involved in HCC growth. Simultaneous treatment with vitamin K1 (VK1), a non-toxic natural compound with anti-tumor properties, and IGF1 receptor (IGF1R) antagonists (such as GSK1838705A or OSI-906) enhanced the pro-apoptotic and anti-proliferative actions of regorafenib in vitro. This combination blocked the IGF1R downstream cascades MAPK and PI3K/AKT and significantly impaired cell migration through decreased actin polymerization35. Gankyrin, a protein that plays a crucial role in malignant cancer development, is responsible for desensitizing human HCC cells to chemotherapy by upregulating c-Myc and hence reprogramming glucose metabolism. Accordingly, the use of a glycolytic inhibitor (2-DG), a glutaminase 1 inhibitor (BPTES) or a c-Myc inhibitor (10058-F4), in addition to regorafenib, effectively inhibited the growth of HCC-PDX tumors with high gankyrin expression36.
Natural compounds, such as the above mentioned VK1, are promising resources for combined therapeutic strategies. Given its anti-tumor effects displayed in liver cancer, oleanolic acid coupled to regorafenib has been tested for advanced HCC. Both agents acted synergistically to inhibit the proliferation, EMT, migration and invasion of the PLC/PRF/5 cell line, which agrees with the suppression of tumor growth and lung metastasis in PLC-bearing mice37. In the same way, chlorogenic acid, a polyphenol present in the human diet, has shown positive effects in vitro in conjunction with regorafenib. Its co-administration caused beneficial effects on cell growth inhibition and apoptosis induction, as well as on cell cycle progression inhibition, MAPK and PI3K/AKT/mTOR signaling repression, and migrating cancer cell percentage decrease38.
On the other hand, the anti-cancer compound CDDP coupled to regorafenib synergistically inhibited HepG2 and Hep3B cells growth28. The combination of regorafenib plus metformin, the main drug used to treat type 2 diabetes, caused greater inhibition of MHCC97H HCC cells proliferation and stimulated cell death, also preventing tumor relapse and metastasis in an in vivo model. This two-drug treatment reduced the expression of hypoxia-inducible factor 2α (HIF-2α), a key factor in tumor cell adaption to oxygen depletion, which led to negative modulation of EMT by upregulation of the 30-kDa HIV Tat-interacting protein (TIP30)39.
All these preclinical studies support the synergistic activity of regorafenib in combination with different compounds (Table 1), resulting in enhanced anti-tumoral actions of this drug compared with the effects of single administration. These findings confirm the outstanding role of drug co-administration for improving regorafenib-based second-line treatment and thus the outcomes of late-stage HCC patients.
Table 1.
Results of regorafenib-based combined treatments in preclinical HCC, CRC and GISTs models
| Cancer type | Model | Regorafenib-combined treatment schedule | Global effects | Molecular effects | Reference |
|---|---|---|---|---|---|
| HCC | HepG2, Hep3B, and Huh7 cell lines | Perifosine, MK2206 or PX-866 | Enhanced cell death | – | 32 |
| Sorafenib-resistant HepG2 xenografts | Anti-ANXA3 monoclonal antibody |
Tumor growth suppression Apoptosis induction Reduced autophagosome formation Inhibition of pro-survival autophagy |
↓ ANXA3 and LC3-II | 33 | |
| Genetically engineered immune-competent sorafenib non-responsive HCC mouse model | |||||
| HepG2 and Hep3B cell lines | Navitoclax (ABT-263) | Promotion of mitochondrial caspase-cell death |
↓ Mcl-1 ↑ Bim, caspase 3 activity, cytochrome c release, PARP cleavage |
34 | |
| PLC/PRF/5, HLF and HepG2 cell lines | VK1 plus GSK1838705A or OSI-906 |
Enhanced anti-proliferative and pro-apoptotic effects of regorafenib Cell migration impairment via actin depolymerization |
↓ AFP secretion (PLC/PRF/5 and HepG2) ↑ caspase 3/7 activation Loss of cytoplasm F-actin fibers but redistribution around de nucleus (HLF) ↓ p-ERK, p-AKT, p-p38, p-JNK, p-TSC2, p-S6 (PLC/PRF/5) |
35 | |
| HCC-PDX with high gankyrin levels | 2-DG, BPTES or 10058-F4 | Tumor growth repression | – | 36 | |
| PLC/PRF/5 cells | OA | Inhibition of cell growth, migration and invasion | – | 37 | |
| PLC-bearing mice | Reduction of tumor volume, lung metastasis, EMT, migration and invasion |
↑ E-cadherin ↓ Vimentin, MMP-2, MMP-9 |
|||
| PLC/PRF/5 and HepG2 cell lines | CGA |
Decreased cell proliferation and cell cycle progression from S to G2/M phase Apoptosis promotion Cell migration inhibition |
↓ Ki-67 ↑ Annexin V, Bax, caspase 3/7 activation ↓ Bcl-2, Bcl-xL ↓ p-JNK, p-p38, p-S6, p-TSC2, p-ERK, p-AKT |
38 | |
| HepG2 and Hep3B cells | CDDP | Synergistical inhibition of cell growth | – | 28 | |
| MHCC97H cells | Metformin |
Reduction of cell proliferation EMT suppression Apoptosis induction |
↓ HIF-2α, N-cadherin ↑ TIP30, E-cadherin |
39 | |
| Orthotopic MHCC97H mouse model |
Inhibition of postoperative recurrence and lung metastasis Apoptosis induction |
↓ Ki-67, N-cadherin ↑ TUNEL positive cells ↑ TIP30, E-cadherin |
|||
| CRC | COLO205, HT29, LoVo, HCT15 cells | Pimasertib | Synergistic effects on growth inhibition | – | 61 |
| HCT15 cells | Apoptosis induction |
↓ p-MAPK, p-AKT, p-4E-BP1, p-p70S6K, cyclin D1 ↑ p27 ↑ cleaved caspase 3, PARP |
|||
| SW620, SW480, HT29, and HCT116 cell lines | PX-866 | Enhanced cell death | – | 32 | |
| HCT116 cells | MK2206 | ||||
| HCT116 mouse model | MK2206 | Suppression of tumor growth | – | ||
| HCT116, SW480, HT29, and HCT116 p53−/− cells | 5-FU | Reduced cell viability |
↓ Mcl-1, Bcl-xL ↑ PUMA (HCT116 p53−/−) |
48 | |
| 5-FU resistant HCT116 (HCT116R) and DLD-1 (DLD-1R) cells | 5-FU |
Overcoming of 5-FU resistance Decrease of cell viability and tumor spheres formation |
– | 58 | |
| DLD-1R mouse model | Inhibition of tumor growth and tumor spheres formation |
↓ ABCG2, β-catenin, WNT1 ↑ Bax |
|||
| Oxaliplatin-refractory CRC-PDX | Irinotecan | Tumor growth delay | – | 50 | |
| HCT116 cells | 5-FU, oxaliplatin or cetuximab | Increased percentage of apoptotic cells | ↑ PUMA | 56 | |
| Mice with HCT116 xenograft tumors | 5-FU | Tumor volume decrease and apoptosis activation | ↑ TUNEL positive cells, active caspase 3 | ||
| HT29, SW620, LoVo, HCT15, SW48, SW480, HCT116, GEO and cetuximab-resistant GEO (GEO-CR), and SW48 (SW48-CR) cells | Cetuximab | Enhanced growth inhibition and apoptotic cells percentage (HT29, SW480, SW620, HCT116, LoVo, HCT15, SW48-CR, GEO-CR) | ↓ p-AKT, p-S6, p-MAPK (SW480, SW620, SW48-CR, GEO-CR, HCT116, LoVo, HCT15) | 51 | |
| Subcutaneous HCT15, HCT116, GEO-CR, and SW48-CR xenograft mouse models | Greater tumor volume reduction | – | |||
| Orthotopic HCT116 xenograft mouse model |
Inhibition of tumor growth in the cecum and metastasis formation Suppression of neovascularization |
– | |||
| SW620, HCT116, and HT29 cell lines | FTD | Inhibition of FTD incorporation into DNA |
↓ p-ERK ↓ TS |
62 | |
| FTD → regorafenib |
Higher survival inhibition Lower FTD incorporation into DNA Apoptosis induction (SW620) |
↑ cleaved PARP (SW620) ↓ p-ERK, TS |
|||
| SW620 and COLO205 xenograft mouse models | FTD/TPI → regorafenib | Higher inhibition of tumor growth | – | ||
| HCT116, HCT116 p53−/−, RKO and HT29 cells | CRT0066101 |
Cell growth inhibition Clonogenic growth inhibition (HCT116 and RKO) Apoptosis induction (RKO) |
↑ cleaved PARP (RKO) ↓ p-HSP27 (RKO) ↓ p-PKD2, p-AKT, p-ERK (RKO) ↓ p-PKD2 (HCT116) ↓ NF-κB activity (HCT116 and RKO) |
63 | |
| Mitoxantrone-resistant BCRP-overexpressing S1-M1-80 cells | Mitoxantrone or SN-38 |
Reversion of BCRP-mediated MDR Improvement of cells sensitivity to mitoxantrone or SN-38 Raised [3H]-mitoxantrone cellular retention via BCRP efflux impairment |
Interaction with the BCRP transmembrane domain | 64 | |
| Mitoxantrone-resistant BCRP-overexpressing S1-M1-80 xenografts | Topotecan | Reduced tumor volume and weight | – | ||
| Doxorubicin-resistant ABCB1-overexpressing SW620 cells (SW620/Ad300) | Paclitaxel |
Overcoming of ABCB1-mediated MDR Increased [3H]-paclitaxel cellular accumulation via ABCB1 efflux impairment |
↓ ABCB1 ATPase activity Interaction with the ABCB1 transmembrane domain |
65 | |
| SW620/Ad300 xenograft mouse model |
Synergistic effect on tumor growth inhibition Higher intratumoral paclitaxel concentration Increased plasma regorafenib concentration |
– | |||
| SW620/Ad300 cells | Paclitaxel, doxorubicin or vincristine |
Overcoming of ABCB1-mediated MDR Reduced resistance fold |
|||
| HCT116 and SW620 cell lines | Lapatinib |
Decreased survival rate Cell cycle arrest in G0/G1 phase (↑ G0/G1 phase cells and ↓ G2/M phase cells) (HCT116) Apoptosis induction |
↓ cyclins A, B, D1, E, CDK1, CDK6 ↓ p-AKT, p-ERK, Bcl-2, Mcl-1, XIAP, survivin ↑ cleaved PARP, Bax |
52 | |
| Subcutaneous HCT116 xenograft mouse model |
Tumor growth inhibition Diminished tumor volume and weight Inhibition of cell growth and angiogenesis |
↓ Ki-67, p-AKT, CD34 ↑ cleaved caspase 3, Bax |
|||
|
HCT116 and HT29 human cells CT26 and MCC38 mouse cells |
Sildenafil and neratinib |
Elevated cell death Increased toxic autophagosome formation (HCT116 and CT26) Activation of death receptor signaling (HCT116 and CT26) Lysosomal disfunction and release of cathepsin B (HCT116 and CT26) Mitochondrial disfunction and release of AIF (HCT116 and CT26) Modulation of tumor cells immunogenicity via autophagy-dependent regulation of HDAC proteins (CT26 and MCC38) |
↑ p-eIF2α, p-ATM, p-AMPK, p-ULK-1, p-S317, p-ATG13 (HCT116 and CT26) ↓ p-mTOR, p-AKT, p-p70S6K, p-ERK (HCT116 and CT26) ↑ ATG5, Beclin 1 (HCT116 and CT26) ↓ Mcl-1, Bcl-xL (HCT116 and CT26) ↓ p-GSK3, β-catenin (HCT116 and CT26) ↑ Frizzled (HCT116 and CT26) ↑ CD95 plasma membrane levels (CT26) ↓ HDAC proteins (CT26) ↓ PD-L1, IDO-1 (CT26 and MCC38) ↓ PD-L2 (CT26) ↑ MHCA (CT26 and MCC38) |
66 | |
| Mouse CT26 tumors | Enhanced reduction of tumor growth | – | |||
| D5D-knocking down HCA-7 colony 29 and HT29 cells | DGLA | Improvement of regorafenib inhibitory effect on cell viability and colony formation | – | 67 | |
| SW620, SW480, HCT15, HCT116, LoVo, SW48, GEO, SW48-CR, and GEO-CR | Silybin | Further cell growth suppression | – | 68 | |
| HCT15, SW480, SW48 and SW48-CR |
Reduced colony formation Higher ROS generation Apoptosis induction |
↑ cleaved PARP ↑ caspase 3, pro-caspase 9 (SW48, SW48-CR and HCT15) ↓ p-AKT, p70S6K, p-4E-BP1 |
|||
| EpCAM-positive HCT8 xenograft mouse model | CAR-modified NK-92 cells with specificity against EpCAM |
Lower tumor volume and weight Increased persistence of NK cells in the tumor |
– | 69 | |
| GISTs | Imatinib-resistant GIST430-654 cells | TL32711 or LCL161 | Increased pro-apoptotic activity |
↓ p-KIT, p-AKT, cIAP1, XIAP, survivin ↑ cleaved PARP |
78 |
4E-BP1 eukaryotic initiation factor 4E binding protein 1, 5-FU 5-fluorouracil, ABCB1 multidrug resistance protein 1, ABCG2 (BCRP) ATP-binding cassette sub-family G member 2, AFP alpha-fetoprotein, AIF apoptosis inducing factor, AKT protein kinase B, AMPK AMP‐dependent protein kinase, ANXA3 Annexin A3, ATG5 autophagy related protein 5, ATG13 autophagy related protein 13, Bax Bcl-2 associated X, Bcl-xL Bcl-2-like protein 1, Bim Bcl-2-like protein 11, CAR chimeric antigen receptor, CD34 hematopoietic progenitor cell antigen CD34, CD95 FAS cell surface death receptor, CDK1 cyclin-dependent kinase 1, CDK6 cyclin-dependent kinase 6, CGA chlorogenic acid, cIAP1 cellular inhibitor of apoptosis protein 1, CRC colorectal cancer, D5D delta-5-desaturase, DGLA dihomo-γ-linolenic acid, eIF2α eukaryotic translation initiation factor 2α, EMT epithelial-to-mesenchymal transition, EpCAM epithelial cell adhesion molecule, ERK extracellular signal-regulated kinase, FTD trifluridine, GISTs gastrointestinal stromal tumors, GSK3 glycogen synthase kinase 3, HCC hepatocellular carcinoma, HDAC histone deacetylase, HIF-2α hypoxia-inducible factor 2α, HSP27 heat shock protein beta-1, IDO-1 indoleamine‐pyrrole 2,3‐dioxygenase, JNK c-Jun N-terminal kinase, Ki-67 proliferation marker protein Ki-67, LC3-II microtubule-associated protein 1 light chain 3 II, MAPK mitogen-activated protein kinase, Mcl-1 induced myeloid leukemia cell differentiation protein, MDR multidrug resistance, MHCA major histocompatibility complex A, MMP-2 matrix metalloproteinase-2, MMP-9 matrix metalloproteinase-9, mTOR mammalian target of rapamycin, NF-ĸB nuclear factor-ĸB, NK natural killer, OA oleanolic acid, p phospho, p38 p38 MAPK, p70S6K ribosomal protein S6 kinase, PARP poly(ADP-ribose) polymerase, PD-L1 programmed cell death-1 ligand 1, PD-L2 programmed cell death-1 ligand 2, PDX patient-derived xenograft, PKD2 protein kinase D2, PUMA p53-upregulated modulator of apoptosis, ROS reactive oxygen species, S6 S6 ribosomal protein, TIP30 30 kDa HIV Tat-interacting protein, TPI tipiracil, TS thymidylate synthase, TSC2 tuberin, TUNEL terminal deoxynucleotidyl transferase dUTP nick end labeling, ULK-1 unc-51 like autophagy activating kinase 1, VK1 vitamin K1, WNT1 Wnt family member 1, XIAP X-linked inhibitor of apoptosis
Regorafenib and CRC
Therapeutic approaches for advanced CRC
Globally, CRC is the second cancer with higher mortality rate and the third in terms of incidence, which means that ~1 of every 10 cancer cases and deaths is due to CRC13. One of the main risk factors is age, with a positive correlation between the increasing aging population and CRC incidence40. Inadequate lifestyle habits, such as excessive alcohol consumption, smoking, high intake of red processed meat13,40,41, obesity, diabetes41, and inflammatory bowel disease also contribute to the development of this cancer40,41. In addition, CRC has a hereditary component in which Lynch and familial adenomatous polyposis syndromes are included40. Metastasis exists in 25% of patients at the time of diagnosis, and it is estimated that 50% of tumors metastasize in the short term4,9,42. Although advanced-stage CRC patient survival has more than doubled in the last 20 years43, the 5-year survival of these individuals is ~10%41. Many therapies have been recently developed; however, 5-fluorouracil (5-FU) is still the mainstay of advanced CRC treatment42.
Single 5-FU intravenous administration has been the only available therapy for advanced CRC over several decades, but basic first-line treatment options currently comprise combined regimens involving 5-FU plus cytotoxic agents such as oxaliplatin and irinotecan. The combination of these compounds has resulted in the chemotherapy regimens FOLFOX (5-FU + leucovorin + oxaliplatin), FOLFIRI (5-FU + leucovorin + irinotecan), and FOLFOXIRI (5-FU + leucovorin + oxaliplatin + irinotecan)40,42. There is also the possibility of replacing 5-FU by oral-administered capecitabine in the FOLFOX and FOLFIRI therapies, thus generating CAPOX (or XELOX) and CAPIRI regimens, respectively11. All of these therapeutic strategies have yielded great tumor growth management40 and increased the median progression-free survival (PFS) to 8 months9. The PFS increased to 9–12 months with posterior incorporation into the pre-existing first-line regimens of molecularly targeted drugs against VEGFR and epidermal growth factor receptor (EGFR)9. Using one or other targeted biologic inhibitor depends on the molecular profile of the tumor4. While the anti-VEGFR monoclonal antibody bevacizumab is used for patients with RAS mutant advanced CRC, cetuximab and panitumumab, two anti-EGFR monoclonal antibodies, are approved for individuals with RAS wild-type disease11,44 (Fig. 1).
Upon tumor progression, chemotherapy regimens not employed as first-line treatment should be given in the second-line setting11. Ramucirumab, an anti-VEGFR2 monoclonal antibody, or aflibercept, a recombinant fusion protein targeting placental growth factor, can also be introduced into the combined protocols to enhance treatment efficacy. Despite the improvement in survival rates, patients with advanced CRC inevitably develop chemoresistance11,42. If patients in this situation maintain good organ function and performance status, they are candidates for regorafenib or trifluridine/tipiracil (FTD/TPI or TAS-102) administration4,42,44. Moreover, the FDA recently granted accelerated approval to pembrolizumab, and nivolumab alone or in combination with ipilimumab (an anti-cytotoxic T-lymphocyte antigen-4 inhibitor), in the second- and later-setting for patients with microsatellite instability-high (MSI-H) or deficient DNA mismatch repair metastatic CRC that has progressed following typical chemotherapy44 (Fig. 1).
Regorafenib as monotherapy is the standard treatment for refractory advanced CRC11,40. The international, multicenter, randomized, placebo-controlled phase 3 CORRECT trial showed for the first time the benefit of regorafenib in patients with metastatic CRC that progresses after all available therapies. Regorafenib treatment significantly increased the median OS to 6.4 months from 5.0 months in the placebo group (HR = 0.77, CI 0.64–0.94, p = 0.0052)45, implying that this drug can reduce death risk by 23%4. Given these positive outcomes, the FDA approved regorafenib in 2012 to become the first drug applicable in patients after conventional therapy failure4,9,44. Regorafenib efficacy and safety were also evaluated in a randomized, double-blind, placebo-controlled, phase 3 trial (CONCUR) performed in Asian patients with refractory metastatic CRC. This study reported an OS of 8.8 months in the treated group versus 6.3 months in the placebo group (HR = 0.55, CI 0.40–0.77, p = 0.00016)4,9,46. The difference between the OS values in both clinical trials can be explained by the fact that patients enrolled in the CORRECT trial received more pre-treatment and were further deteriorated than those in the CONCUR study4,42.
Although there is a large list of approved agents for treating advanced CRC, searching for more potent and effective treatments will continue to prolong patient survival beyond 30 months4,11,42–44.
Regorafenib for advanced CRC: evidences in preclinical models
A study performed with the human CRC cell lines HCT15, DLD-1, HT29, and HCT116 showed a drastic decrease in cell viability when regorafenib was administered at doses higher than 5 µM47, an effect that was also observed in SW48048, KM12SM, Caco-2, LoVo, WiDr, and RKO CRC cells49. Cell proliferation inhibition was also corroborated in a panel of in vitro models50–52, as well as in an HCT116 nanoimprinting 3D culture53 and in cell lines established from CRC primary tumors54. Moreover, daily regorafenib administration at 30 mg/kg suppressed tumor growth in a highly aggressive murine CT26 metastatic colon cancer model55. Although similar results were observed in different HCT116-bearing mouse models53,56, it has been noted that a lower regorafenib dose, such as 10 mg/kg/day, can delay tumor growth47, even in oxaliplatin-refractory CRC-PDX50.
Regarding apoptosis status after regorafenib treatment, several researches have shown that this drug induces apoptosis both in vitro48,57,58 and in vivo55,59. In this way, regorafenib has been shown to downregulate phospho-STAT3 and its pro-survival targets cyclin D1 and Mcl-1 and upregulate cleavage of PARP and caspase 9 in different CRC cells. Regorafenib-mediated STAT3 inhibition was due to an increase in SHP-1 tyrosine phosphatase activity by direct impairment of the association between N-SH2 and the catalytic domain of SHP-1, as we have already described in the HCC section. These findings were reported in not only CRC cells but also in HCT116 xenograft models47. Another investigation using 5-FU-resistant in vitro models supports the regorafenib-mediated downregulation of the oncogenic STAT3 pathway, finding that it takes place in parallel with the inhibition of its upstream molecule phospho-mTOR58.
It is known that p53-upregulated modulator of apoptosis (PUMA) evokes CRC cell apoptosis. Chen et al.56 reported that regorafenib treatment enhances PUMA expression in HCT116, Lim2405, LoVo, Lim1215, SW48, and RKI CRC cells in a dose- and time-dependent manner and in xenograft tumors; moreover, this effect correlated with apoptosis induction. Further analyses revealed that activation of PUMA-mediated apoptosis occurs via ERK inhibition56, the subsequent activation of glycogen synthase kinase 3β (GSK3β) and finally the binding of the p65 NF-ĸB subunit to the proximal PUMA promoter56. According to these findings, regorafenib administration also causes ERK downregulation in different in vitro50 and in vivo models49.
FGFR signaling participates in cancer cell proliferation, angiogenesis, and migration. Cha et al.57 observed that regorafenib treatment of NCI-H716 CRC cells leads to the inhibition of FGFR2 phosphorylation and its downstream molecules, abrogating pro-survival FGFR2 signaling. With respect to tumor vascularization and angiogenesis inhibition, regorafenib significantly decreased the microvessel area and VEGFR2- and TIE2-positive vessels in a highly aggressive murine metastatic CRC model55. Similarly, this drug suppressed tumor vascularity and tumor perfusion in HT29 carcinoma xenografts59, in a KM12SM-bearing mouse model49 and in CRC-PDX models50.
Regorafenib at clinically effective concentrations exhibits a potent inhibitory effect on the migration ability of CRC cells49, thus decreasing pro-EMT vimentin levels58 and reducing small tumors spread in the abdominal cavity in vivo53. There is evidence that SHP-1 impedes transforming growth factor β1 (TGF-β1)-induced EMT through phospho-STAT3 downregulation. Because regorafenib activates SHP-1, which results in low phospho-STAT3 levels, it is not surprising that the drug shows anti-EMT activity via targeting this pathway, inhibiting invasion in vitro and lung metastasis in vivo60. In agreement, regorafenib has also been shown to avoid liver metastasis50,55 and other extra-hepatic nodule formation in vivo50, in addition to decreasing the infiltration of tumor-associated macrophages, which are crucial for angiogenesis and metastatic spreading55. Moreover, when bone marrow-derived mesenchymal stem cells (MSCs) migrate to the tumor stroma, they differentiate into carcinoma-associated fibroblasts (CAFs), stimulating cell proliferation and invasion. A study using KM12SM + MSCs-bearing mice revealed that regorafenib completely blocks lymph node metastasis, inhibits tumor growth and stromal reaction, and prompts apoptosis49.
On the other hand, experiments employing CRC cell lines resistant to 5-FU reported a meaningful miR-34a-associated reduction in colon cancer stem-like phenotypes after regorafenib addition. This effect was related to decreased tumor sphere formation and side populations and reduced expression of the stemness markers Wnt family member 1 (WNT1) and Notch receptor 1 (Notch1)58.
Numerous preclinical researches have demonstrated the broad activity of regorafenib in advanced CRC. Similar to the results reported in advanced HCC, regorafenib mainly abrogates tumor cell proliferation and promotes apoptosis, in addition to reducing tumor vascularity and invasion ability (Fig. 2). This evidence suggests that regorafenib is a useful therapeutic option for refractory metastatic CRC.
Emerging combined treatment strategies with regorafenib against advanced CRC
Some studies have analyzed the potential effect of regorafenib co-treatment with other compounds. This could provide new therapeutic insights that could reinforce the anti-tumoral potency of regorafenib to prolong CRC patient survival. The combination of regorafenib with the selective MEK1/2 inhibitor pimasertib synergistically reduced the proliferation of pimasertib-resistant CRC cells and induced apoptosis, which were associated with the inhibition of the pro-survival intracellular signals MAPK and AKT61. Similarly, the AKT inhibitor MK2206 and the PI3K inhibitor PX-866 have separately been demonstrated to augment regorafenib lethality in colon cancer cells32.
The co-administration of regorafenib with other drugs approved for CRC has been evaluated. Regorafenib coupled with 5-FU caused a greater decrease in HCT116, HT29, and SW480 cells growth48. In 5-FU-resistant in vitro and in vivo models, this combination also inhibited the appearance of cancer-starting cell phenotypes through Wnt/β-catenin signaling impairment, which thereby reversed 5-FU resistance58. Moreover, regorafenib combined with irinotecan showed greater tumor growth inhibition of oxaliplatin-refractory CRC-PDX50. Regorafenib was also able to increase cell sensitivity to oxaliplatin and cetuximab in vitro and to 5-FU in vivo through the induction of PUMA-mediated apoptosis56. Similarly, a study using cetuximab-resistant CRC cell lines reported that regorafenib overcomes primary and acquired resistance to anti-EGFR therapy by targeting the MAPK and AKT pathways51. The chemosensitizer ability of regorafenib against anti-EGFR inhibitors was also supported by an assay using GEO and SW48 cetuximab-resistant xenograft mouse models51. The combination of both drugs has also been demonstrated to be effective in HCT116 orthotopic xenografts, as shown by growth regression and neovascularization and metastasis abolishment51. In addition, the combination of regorafenib plus FTD revealed that cell survival is reduced to a higher extent when FTD is administered prior to regorafenib; this can be explained by a greater decrease in phospho-ERK1/2 and thymidylate synthase (TS) expression, which induces cell death62. These findings are supported by increased cytotoxic effects in SW620 and COLO205-bearing mice when FTD/TPI is added followed by regorafenib62.
Protein kinase D (PKD) constitutes a key mediator of multiple biological processes implicated in cancer. It has been found that co-treatment with a PKD-specific inhibitor (CRT0066101) exerts synergistic effects against CRC cells through the inhibition of proliferation and clonogenic growth and the activation of apoptosis. These results were associated with the suppression of PKD2-mediated RAS/RAF/ERK, PI3K/AKT and NF-ĸB signaling cascades63. Breast cancer resistance protein (BCRP or ABCG2) participates in the acquisition of resistance to multiple drugs (MDR). Zhang and colleagues64 observed that regorafenib improves BCRP-overexpressing S1-M1-80 CRC cells sensitivity to mitoxantrone and SN-38, both substrates of BCRP, due to its interaction with the BCRP transmembrane domain and its ability to impair BCRP efflux and increase intracellular drug retention. Regorafenib and topotecan also increased cytotoxicity against BCRP-overexpressing CRC xenografts64. Likewise, regorafenib overcame MDR in in vitro and in vivo CRC models that overexpress multidrug resistance protein 1 (ABCB1)65. Wang et al.65 confirmed that regorafenib stimulates cell sensitivity to paclitaxel, an ABCB1 substrate, increasing the intracellular paclitaxel level via inhibition of ABCB1-associated chemotherapy efflux.
Lapatinib, a TKI approved for breast cancers that overexpress HER2 (or ERBB2), has been shown to improve anti-tumor regorafenib properties against metastatic CRC. Combined treatment meaningfully arrested the cell cycle in the G0/G1 phase and decreased the expression of several cyclins and cyclin-dependent kinases (CDKs) in vitro. This treatment also modulated the apoptotic balance leading to cell death and suppressed tumor growth and angiogenesis in an HCT116 xenograft tumor model52. Another research focused on triple treatment with regorafenib plus sildenafil (a phosphodiesterase 5 inhibitor) and neratinib (an ERBB1/2/4 inhibitor) found a more than additive inhibitory effect on CRC cells growth in vitro and in vivo, enhancing harmful autophagosome formation, death receptor signaling activation and mitochondrial and lysosomal dysfunction. Furthermore, these agents increased the levels of major histocompatibility complex A (MHCA) and reduced the expression of biomarkers related to tumor immune response, such as PD-L166.
With respect to the co-administration of regorafenib plus natural substances, a study employing dihomo-γ-linolenic acid (DGLA) has shown this molecule to complement the anti-cancer properties of regorafenib in CRC cells with knockdown of delta-5-desaturase, the enzyme that converts DGLA into arachidonic acid67. Regorafenib was also tested in conjunction with silybin, a natural plant extract that has biological activity, in several CRC cell lines, such as HCT15, SW480 and cetuximab-resistant and cetuximab-sensitive SW48 CRC cells; this combination was found to increase the inhibition of cell growth and colony formation and promote apoptosis. These results were associated with the inactivation of PI3K/AKT/mTOR signaling and the increased production of reactive oxygen species (ROS)68.
Finally, regarding immunomodulatory activity, which could allow the reactivation of the immune response against cancer cells, Zhang et al.69 verified that co-administration of regorafenib with chimeric antigen receptor (CAR)-modified NK-92 cells (CAR-NK-92) with specificity against epithelial cell adhesion molecule (EpCAM) displays beneficial anti-cancer properties in EpCAM-positive HCT8 tumor xenografts.
Simultaneous administration of regorafenib with other drugs or natural compounds has been shown to enhance the anti-cancer properties of regorafenib alone by targeting some of the major tumor-related pathways or increasing the intracellular drug concentration (Table 1). Despite the significant results of regorafenib monotherapy, a combination strategy represents a breakthrough in therapeutic approaches for advanced stages of CRC.
Regorafenib and GISTs
Therapeutic approaches for advanced GISTs
GISTs are unusual neoplasms, accounting for 0.1–3% of all gastrointestinal malignant tumors12,70, and they represent the most common mesenchymal cancer in the digestive tract1,12,70–74. GISTs annual incidence is approximately 14–20 per million in Northern Europe72 and 10–15 per million in the United States1,12, with nearly 5000 new cases diagnosed each year3,74. The most frequent locations where these tumors originate are the stomach (60%) and small intestine (30%)2,3,12. Although GISTs can appear at any time, advanced age is considered the principal risk factor, as most GISTs arise after 60 years of age70,72. Nearly 15–50% of patients display advanced and metastatic disease12,70, for which there was formerly no effective treatment due to poor response to classical cytotoxic drugs1,74.
Imatinib constitutes the first-line treatment for metastatic and unresectable GISTs70–73,75, being able to raise advanced GISTs patients OS to ~60 months versus 18 months achieved with traditional therapies3,70,72,73. For those individuals who do not tolerate imatinib or present primary or secondary imatinib resistance, sunitinib is available in the second-line setting1,2,12,73,75. Unfortunately, almost all metastatic GISTs ultimately also develop sunitinib resistance. For these reasons, regorafenib received FDA approval in 2013 as a third-line treatment, becoming the only functional therapy for advanced GISTs when both imatinib and sunitinib fail1,3,12,71,73,75 (Fig. 1). Regorafenib approval was based on positive outcomes derived from an international, multicenter, prospective, randomized, placebo-controlled phase 3 trial (GRID) enrolling patients with GISTs refractory to both imatinib and sunitinib. This trial showed an increased PFS (4.8 months in the regorafenib arm versus 0.9 in the placebo arm, HR = 0.27, CI 0.19–0.39, p < 0.0001) and improved disease control rate (52.6% for regorafenib versus 9.1% for placebo) in the regorafenib group76. Currently, several studies testing new treatment options for advanced GISTs, either immunotherapy or novel molecular targeted agents, are underway1,71.
Regorafenib in single or combined treatment for advanced GISTs: evidences in preclinical models
Few studies evaluating regorafenib effects on in vitro and in vivo GISTs models have been performed (Fig. 2). Van Looy et al.77 reported that regorafenib suppresses tumor growth in a GIST-PDX model (UZLX-GIST9) through necrosis induction, microvessel density decrease and KIT, AKT and the eukaryotic initiation factor 4E binding protein 1 (4E-BP1) inactivation. Experiments conducted with imatinib-resistant GIST430-654 and GIST48 cells support the pro-apoptotic activity of regorafenib, as evidenced by KIT and AKT inhibition, survivin and XIAP downregulation and cleaved PARP upregulation. In addition, the combination of regorafenib plus TL32711 or LCL161, both mimetics of second mitochondria-derived activator of caspases (SMAC), displayed agonistic anti-cancer properties against GIST430-654 cells78.
Few investigations have been performed with regorafenib alone or co-treatment for advanced GISTs. Co-administration of regorafenib with SMAC mimetics has exhibited beneficial cytotoxic effects against this cancer (Table 1). Nonetheless, further studies are required to determine the mechanisms underlying the actions of regorafenib in GISTs as a third-line therapy and, overall, to evaluate possible regorafenib-based combinations with the objective of enhancing the last treatment option for this tumor type.
Conclusions and future perspectives
Regorafenib represents the gold standard for advanced HCC, CRC, and GISTs when first-line or even second-line therapies fail. This article summarizes the broad subset of anti-tumor actions exhibited by regorafenib in preclinical models of three cancer types, highlighting its anti-proliferative, pro-apoptotic, anti-angiogenic, anti-metastatic and immunomodulatory properties, as well as its ability to regulate autophagy and stemness markers (Fig. 2). Regorafenib effectiveness is clear and well-established given the preclinical outcomes discussed here and the clinical evidence that resulted in regorafenib approval. Nevertheless, because regorafenib is employed in the final stages when tumors become refractory to standard chemotherapy, it would be advantageous to search for new treatment strategies that enhance OS beyond regorafenib monotherapy. Among these strategies, co-administration of this drug with other agents capable of potentiating its efficacy by targeting the same signaling pathways or even complementary cancer-related routes has arisen as a promising therapeutic approach. In this review, we describe favorable results obtained from the investigations that have been carried out to date that evaluate combined regorafenib-based treatments (Table 1).
In regard to improving regorafenib via combination therapies, it is important to note that some of the principal causes underlying loss of chemotherapy sensitivity and resistance acquisition are augmented ABC transporter activity65, the hypoxic microenvironment18, immune response evasion31, and miRNA expression modulation79.
ABC transporter proteins are responsible for the acceleration of drug efflux from tumor cells. Thus, the exploration of ABC transporters mediating regorafenib efflux will be critical for the design of new combined therapies able to increase its intracellular concentration, leading to the considerable enhancement of its anti-cancer actions. Likewise, due to the important role played by the hypoxia response in chemotherapy resistance, especially in HCC patients where hypoxia appears as a direct consequence of the anti-angiogenic activity of sorafenib, it would be interesting to co-administer regorafenib with compounds targeting the oxygen-deficient microenvironment. For example, co-treatment with regorafenib plus hypoxia-activated pro-drugs (HAPs), agents that facilitate oxygen delivery in tumor hypoxic areas (e.g., YQ23), or compounds targeting the main mediators of the cell response to hypoxia, the hypoxia-inducible factors (HIFs)18.
Immunotherapy for cancer-specific antigens has emerged as one of the main interests in the cancer therapy landscape. In particular, immune checkpoint inhibitors such as nivolumab and pembrolizumab, both human antibodies targeting PD-1, have become useful for treating advanced HCC and CRC after first-line treatment failure. Accordingly, future studies focused on the combination of regorafenib with nivolumab, pembrolizumab or novel drugs that impede immune response escape by cancer cells represent a great approach to optimizing the effects of regorafenib through immunomodulation enhancement31.
Another promising research direction aimed at improving anti-tumor regorafenib properties could be the administration of this drug in conjunction with antagonists or mimetics of well-known cancer-related miRNAs. The first case proposed refers to inhibitors targeting miRNAs with oncogenic actions, whereas the mimetics option is based on the functional re-establishment of miRNAs whose tumor-suppressor role has been lost79.
On the other hand, because cancer cells are prone to acquire drug resistance and because regorafenib seems to be effective after previously unsuccessfully administered chemotherapy, several in vitro and in vivo studies have analyzed the effects of regorafenib on tumors for which it is not yet indicated. Encouraging outcomes have been observed in gastric cancer57,80,81, adenoid cystic carcinoma82, bladder carcinoma83, breast cancer84, thyroid, prostate and endometrial neoplasms85, lung squamous cell carcinoma86, meningioma87, multiple myeloma88, and neuroblastoma89.
Although further preclinical studies will assist in providing novel insights into the subjacent molecular mechanisms of regorafenib activity as well as the suitability of new combination strategies, the transfer of the basic and translational findings detailed in this article to the clinical area is also an important need. This will undoubtedly enhance the therapeutic potency of regorafenib and extend its employment to other cancers where providing new treatment options is of utmost importance.
Acknowledgements
CIBERehd is funded by the Instituto de Salud Carlos III, Spain. F.F. and P.F.P. are supported by the Ministry of Education of Spain (Becas FPU16/05277 and FPU17/01995, respectively), and C.M.B. is supported by the Asociación Española Contra el Cáncer (AECC)-Junta Provincial de León.
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
These authors contributed equally: Javier González-Gallego, José L. Mauriz
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Mei L, Du W, Idowu M, von Mehren M, Boikos SA. Advances and challenges on management of gastrointestinal stromal tumors. Front. Oncol. 2018;8:135. doi: 10.3389/fonc.2018.00135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ferraro D, Zalcberg J. Regorafenib in gastrointestinal stromal tumors: clinical evidence and place in therapy. Ther. Adv. Med. Oncol. 2014;6:222–228. doi: 10.1177/1758834014544892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schroeder B, Li Z, Cranmer LD, Jones RL, Pollack SM. Targeting gastrointestinal stromal tumors: the role of regorafenib. Onco. Targets Ther. 2016;9:3009–3016. doi: 10.2147/OTT.S104081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.de la Fouchardière C. Regorafenib in the treatment of metastatic colorectal cancer. Futur. Oncol. 2018;14:2239–2246. doi: 10.2217/fon-2017-0512. [DOI] [PubMed] [Google Scholar]
- 5.Llovet JM, Montal R, Sia D, Finn RS. Molecular therapies and precision medicine for hepatocellular carcinoma. Nat. Rev. Clin. Oncol. 2018;15:599–616. doi: 10.1038/s41571-018-0073-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Eso Y, Marusawa H. Novel approaches for molecular targeted therapy against hepatocellular carcinoma. Hepatol. Res. 2018;48:597–607. doi: 10.1111/hepr.13181. [DOI] [PubMed] [Google Scholar]
- 7.Kudo M. Targeted and immune therapies for hepatocellular carcinoma: predictions for 2019 and beyond. World J. Gastroenterol. 2019;25:789–807. doi: 10.3748/wjg.v25.i7.789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mody K, Abou-Alfa GK. Systemic therapy for advanced hepatocellular carcinoma in an evolving landscape. Curr. Treat. Options Oncol. 2019;20:3. doi: 10.1007/s11864-019-0601-1. [DOI] [PubMed] [Google Scholar]
- 9.Røed Skårderud M, Polk A, Kjeldgaard Vistisen K, Larsen FO, Nielsen DL. Efficacy and safety of regorafenib in the treatment of metastatic colorectal cancer: a systematic review. Cancer Treat. Rev. 2018;62:61–73. doi: 10.1016/j.ctrv.2017.10.011. [DOI] [PubMed] [Google Scholar]
- 10.Forner A, Reig M, Bruix J. Hepatocellular carcinoma. Lancet. 2018;391:1301–1314. doi: 10.1016/S0140-6736(18)30010-2. [DOI] [PubMed] [Google Scholar]
- 11.Martini G, et al. Present and future of metastatic colorectal cancer treatment: a review of new candidate targets. World J. Gastroenterol. 2017;23:4675–4688. doi: 10.3748/wjg.v23.i26.4675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Keung EZ, Raut CP. Management of gastrointestinal stromal tumors. Surg. Clin. North Am. 2017;97:437–452. doi: 10.1016/j.suc.2016.12.001. [DOI] [PubMed] [Google Scholar]
- 13.Bray F, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. Ca. Cancer J. Clin. 2018;68:394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 14.Llovet JM, et al. Hepatocellular carcinoma. Nat. Rev. Dis. Prim. 2016;2:16018. doi: 10.1038/nrdp.2016.18. [DOI] [PubMed] [Google Scholar]
- 15.Prieto-Domínguez N, et al. Melatonin-induced increase in sensitivity of human hepatocellular carcinoma cells to sorafenib is associated with reactive oxygen species production and mitophagy. J. Pineal Res. 2016;61:396–407. doi: 10.1111/jpi.12358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kudo M. Systemic therapy for hepatocellular carcinoma: latest advances. Cancers (Basel). 2018;10:412. doi: 10.3390/cancers10110412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhu Jing, Yin Tailang, Xu Yong, Lu Xiao‐Jie. Therapeutics for advanced hepatocellular carcinoma: Recent advances, current dilemma, and future directions. Journal of Cellular Physiology. 2019;234(8):12122–12132. doi: 10.1002/jcp.28048. [DOI] [PubMed] [Google Scholar]
- 18.Méndez-Blanco C, Fondevila F, García-Palomo A, González-Gallego J, Mauriz JL. Sorafenib resistance in hepatocarcinoma: role of hypoxia-inducible factors. Exp. Mol. Med. 2018;50:134. doi: 10.1038/s12276-018-0159-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bruix J, et al. Regorafenib for patients with hepatocellular carcinoma who progressed on sorafenib treatment (RESORCE): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2017;389:56–66. doi: 10.1016/S0140-6736(16)32453-9. [DOI] [PubMed] [Google Scholar]
- 20.U. S. Food and Drug Administration. FDA approves cabozantinib for hepatocellular carcinoma. https://www.fda.gov/Drugs/InformationOnDrugs/ApprovedDrugs/ucm629512.htm (2019).
- 21.Carr BI, et al. Fluoro-sorafenib (regorafenib) effects on hepatoma cells: growth inhibition, quiescence, and recovery. J. Cell. Physiol. 2013;228:292–297. doi: 10.1002/jcp.24148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Carr BI, et al. Effects of low concentrations of regorafenib and sorafenib on human HCC cell AFP, migration, invasion and growth in vitro. J. Cell. Physiol. 2013;228:1344–1350. doi: 10.1002/jcp.24291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tsai JJ, Pan PJ, Hsu FT. Regorafenib induces extrinsic and intrinsic apoptosis through inhibition of ERK/NF-κB activation in hepatocellular carcinoma cells. Oncol. Rep. 2017;37:1036–1044. doi: 10.3892/or.2016.5328. [DOI] [PubMed] [Google Scholar]
- 24.Liu YC, Wu RH, Wang WS. Regorafenib diminishes the expression and secretion of angiogenesis and metastasis associated proteins and inhibits cell invasion via NF-κB inactivation in SK-Hep1. Cells Oncol. Lett. 2017;14:461–467. doi: 10.3892/ol.2017.6142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tai WT, et al. STAT3 mediates regorafenib-induced apoptosis in hepatocellular carcinoma. Clin. Cancer Res. 2014;20:5768–5776. doi: 10.1158/1078-0432.CCR-14-0725. [DOI] [PubMed] [Google Scholar]
- 26.Weng MC, et al. Regorafenib inhibits tumor progression through suppression of ERK/NF-κB activation in hepatocellular carcinoma bearing mice. Biosci. Rep. 2018;38:BSR20171264. doi: 10.1042/BSR20171264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kissel M, et al. Antitumor effects of regorafenib and sorafenib in preclinical models of hepatocellular carcinoma. Oncotarget. 2017;8:107096–107108. doi: 10.18632/oncotarget.22334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Han R, Li S. Regorafenib delays the proliferation of hepatocellular carcinoma by inducing autophagy. Pharmazie. 2018;73:218–222. doi: 10.1691/ph.2018.7988. [DOI] [PubMed] [Google Scholar]
- 29.Chen W, et al. Regorafenib reverses HGF-induced sorafenib resistance by inhibiting epithelial-mesenchymal transition in hepatocellular carcinoma. FEBS Open Bio. 2019;9:335–347. doi: 10.1002/2211-5463.12578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.He X, et al. Molecularly targeted anti-cancer drugs inhibit the invasion and metastasis of hepatocellular carcinoma by regulating the expression of MMP and TIMP gene families. Biochem. Biophys. Res. Commun. 2018;504:878–884. doi: 10.1016/j.bbrc.2018.08.203. [DOI] [PubMed] [Google Scholar]
- 31.Qiu M, et al. Effects of liver-targeted drugs on expression of immune-related proteins in hepatocellular carcinoma cells. Clin. Chim. Acta. 2018;485:103–105. doi: 10.1016/j.cca.2018.06.032. [DOI] [PubMed] [Google Scholar]
- 32.Sajithlal GB, et al. Sorafenib/regorafenib and phosphatidyl inositol 3 kinase/thymoma viral proto-oncogene inhibition interact to kill tumor cells. Mol. Pharmacol. 2013;84:562–571. doi: 10.1124/mol.113.088005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tong M, et al. Efficacy of annexin A3 blockade in sensitizing hepatocellular carcinoma to sorafenib and regorafenib. J. Hepatol. 2018;69:826–839. doi: 10.1016/j.jhep.2018.05.034. [DOI] [PubMed] [Google Scholar]
- 34.Tutusaus A, et al. Antiapoptotic BCL-2 proteins determine sorafenib/regorafenib resistance and BH3-mimetic efficacy in hepatocellular carcinoma. Oncotarget. 2018;9:16701–16717. doi: 10.18632/oncotarget.24673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Refolo MG, et al. IGF-1R tyrosine kinase inhibitors and Vitamin K1 enhance the antitumor effects of regorafenib in HCC cell lines. Oncotarget. 2017;8:103465–103476. doi: 10.18632/oncotarget.21403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Liu R, et al. Gankyrin drives metabolic reprogramming to promote tumorigenesis, metastasis and drug resistance through activating β-catenin/c-Myc signaling in human hepatocellular carcinoma. Cancer Lett. 2019;443:34–46. doi: 10.1016/j.canlet.2018.11.030. [DOI] [PubMed] [Google Scholar]
- 37.Wang H, et al. Oleanolic acid inhibits epithelial–mesenchymal transition of hepatocellular carcinoma by promoting iNOS dimerization. Mol. Cancer Ther. 2019;18:62–74. doi: 10.1158/1535-7163.MCT-18-0448. [DOI] [PubMed] [Google Scholar]
- 38.Refolo MG, et al. Chlorogenic acid improves the regorafenib effects in human hepatocellular carcinoma cells. Int. J. Mol. Sci. 2018;19:1518. doi: 10.3390/ijms19051518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yang Q, Guo X, Yang L. Metformin enhances the effect of regorafenib and inhibits recurrence and metastasis of hepatic carcinoma after liver resection via regulating expression of Hypoxia Inducible Factors 2α (HIF-2α) and 30 kDa HIV Tat-Interacting Protein (TIP30) Med. Sci. Monit. 2018;24:2225–2234. doi: 10.12659/MSM.906687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kuipers EJ, et al. Colorectal cancer. Nat. Rev. Dis. Prim. 2015;1:15065. doi: 10.1038/nrdp.2015.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Brenner H, Kloor M, Pox CP. Colorectal cancer. Lancet. 2014;383:1490–1502. doi: 10.1016/S0140-6736(13)61649-9. [DOI] [PubMed] [Google Scholar]
- 42.Sánchez-Gundín J, Fernández-Carballido AM, Martínez-Valdivieso L, Barreda-Hernández D, Torres-Suárez AI. New trends in the therapeutic approach to metastatic colorectal cancer. Int. J. Med. Sci. 2018;15:659–665. doi: 10.7150/ijms.24453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Van Cutsem E, et al. ESMO consensus guidelines for the management of patients with metastatic colorectal cancer. Ann. Oncol. 2016;27:1386–1422. doi: 10.1093/annonc/mdw235. [DOI] [PubMed] [Google Scholar]
- 44.Bekaii-Saab Tanios, Kim Richard, Kim Tae Won, O’Connor Juan Manuel, Strickler John H., Malka David, Sartore-Bianchi Andrea, Bi Feng, Yamaguchi Kensei, Yoshino Takayuki, Prager Gerald W. Third- or Later-line Therapy for Metastatic Colorectal Cancer: Reviewing Best Practice. Clinical Colorectal Cancer. 2019;18(1):e117–e129. doi: 10.1016/j.clcc.2018.11.002. [DOI] [PubMed] [Google Scholar]
- 45.Grothey A, et al. Regorafenib monotherapy for previously treated metastatic colorectal cancer (CORRECT): an international, multicentre, randomised, placebo-controlled, phase 3 trial. Lancet. 2013;381:303–312. doi: 10.1016/S0140-6736(12)61900-X. [DOI] [PubMed] [Google Scholar]
- 46.Li J, et al. Regorafenib plus best supportive care versus placebo plus best supportive care in Asian patients with previously treated metastatic colorectal cancer (CONCUR): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2015;16:619–629. doi: 10.1016/S1470-2045(15)70156-7. [DOI] [PubMed] [Google Scholar]
- 47.Fan LC, et al. SHP-1 is a target of regorafenib in colorectal cancer. Oncotarget. 2014;5:6243–6251. doi: 10.18632/oncotarget.2191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Marks EI, et al. Regorafenib with a fluoropyrimidine for metastatic colorectal cancer after progression on multiple 5-FU-containing combination therapies and regorafenib monotherapy. Cancer Biol. Ther. 2015;16:1710–1719. doi: 10.1080/15384047.2015.1113355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Takigawa H, et al. Multikinase inhibitor regorafenib inhibits the growth and metastasis of colon cancer with abundant stroma. Cancer Sci. 2016;107:601–608. doi: 10.1111/cas.12907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Schmieder R, et al. Regorafenib (BAY 73-4506): antitumor and antimetastatic activities in preclinical models of colorectal cancer. Int. J. Cancer. 2014;135:1487–1496. doi: 10.1002/ijc.28669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Napolitano S, et al. Primary and acquired resistance of colorectal cancer to anti-EGFR monoclonal antibody can be overcome by combined treatment of regorafenib with cetuximab. Clin. Cancer Res. 2015;21:2975–2983. doi: 10.1158/1078-0432.CCR-15-0020. [DOI] [PubMed] [Google Scholar]
- 52.Zhang WJ, et al. Synergistic antitumor activity of regorafenib and lapatinib in preclinical models of human colorectal cancer. Cancer Lett. 2017;386:100–109. doi: 10.1016/j.canlet.2016.11.011. [DOI] [PubMed] [Google Scholar]
- 53.Yoshii Y, et al. Regorafenib as a potential adjuvant chemotherapy agent in disseminated small colon cancer: drug selection outcome of a novel screening system using nanoimprinting 3-dimensional culture with HCT116-RFP cells. Int. J. Oncol. 2016;48:1477–1484. doi: 10.3892/ijo.2016.3361. [DOI] [PubMed] [Google Scholar]
- 54.Lange F, et al. Biological and molecular effects of small molecule kinase inhibitors on low-passage human colorectal cancer cell lines. Biomed. Res. Int. 2014;2014:568693. doi: 10.1155/2014/568693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Abou-Elkacem L, et al. Regorafenib inhibits growth, angiogenesis, and metastasis in a highly aggressive, orthotopic colon cancer model. Mol. Cancer Ther. 2013;12:1322–1331. doi: 10.1158/1535-7163.MCT-12-1162. [DOI] [PubMed] [Google Scholar]
- 56.Chen D, Wei L, Yu J, Zhang L. Regorafenib inhibits colorectal tumor growth through PUMA-mediated apoptosis. Clin. Cancer Res. 2014;20:3472–3484. doi: 10.1158/1078-0432.CCR-13-2944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Cha Y, et al. FGFR2 amplification is predictive of sensitivity to regorafenib in gastric and colorectal cancers in vitro. Mol. Oncol. 2018;12:993–1003. doi: 10.1002/1878-0261.12194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Cai MH, et al. Regorafenib suppresses colon tumorigenesis and the generation of drug resistant cancer stem-like cells via modulation of miR-34a associated signaling. J. Exp. Clin. Cancer Res. 2018;37:151. doi: 10.1186/s13046-018-0836-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Cyran CC, et al. Regorafenib effects on human colon carcinoma xenografts monitored by dynamic contrast-enhanced computed tomography with immunohistochemical validation. PLoS ONE. 2013;8:e76009. doi: 10.1371/journal.pone.0076009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Fan LC, et al. Regorafenib (Stivarga) pharmacologically targets epithelial-mesenchymal transition in colorectal cancer. Oncotarget. 2016;7:64136–64147. doi: 10.18632/oncotarget.11636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Martinelli E, et al. Antitumor activity of pimasertib, a selective MEK 1/2 inhibitor, in combination with PI3K/mTOR inhibitors or with multi-targeted kinase inhibitors in pimasertib-resistant human lung and colorectal cancer cells. Int. J. Cancer. 2013;133:2089–2101. doi: 10.1002/ijc.28236. [DOI] [PubMed] [Google Scholar]
- 62.Matsuoka K, et al. Effective sequential combined chemotherapy with trifluridine/tipiracil and regorafenib in human colorectal cancer cells. Int. J. Mol. Sci. 2018;19:2915. doi: 10.3390/ijms19102915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wei N, Chu E, Wu S, Wipf P, Schmitz JC. The cytotoxic effects of regorafenib in combination with protein kinase D inhibition in human colorectal cancer cells. Oncotarget. 2014;6:4745–4756. doi: 10.18632/oncotarget.2938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zhang YK, et al. Regorafenib antagonizes BCRP-mediated multidrug resistance in colon cancer. Cancer Lett. 2019;442:104–112. doi: 10.1016/j.canlet.2018.10.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wang YJ, et al. Regorafenib overcomes chemotherapeutic multidrug resistance mediated by ABCB1 transporter in colorectal cancer: in vitro and in vivo study. Cancer Lett. 2017;396:145–154. doi: 10.1016/j.canlet.2017.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Booth L, et al. Neratinib augments the lethality of [regorafenib + sildenafil] J. Cell. Physiol. 2019;234:4874–4887. doi: 10.1002/jcp.27276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Xu Y, et al. Knockdown of delta-5-desaturase promotes the anti-cancer activity of dihomo-γ-linolenic acid and enhances the efficacy of chemotherapy in colon cancer cells expressing COX-2. Free Radic. Biol. Med. 2016;96:67–77. doi: 10.1016/j.freeradbiomed.2016.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Belli V, et al. Regorafenib in combination with silybin as a novel potential strategy for the treatment of metastatic colorectal cancer. Oncotarget. 2017;8:68305–68316. doi: 10.18632/oncotarget.20054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zhang Q, et al. Combination therapy with EpCAM-CAR-NK-92 cells and regorafenib against human colorectal cancer models. J. Immunol. Res. 2018;2018:4263520. doi: 10.1155/2018/4263520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.El-Menyar A, Mekkodathil A, Al-Thani H. Diagnosis and management of gastrointestinal stromal tumors: an up-to-date literature review. Clin. Res. 2017;13:889–900. doi: 10.4103/0973-1482.177499. [DOI] [PubMed] [Google Scholar]
- 71.Lim KT, Tan KY. Current research and treatment for gastrointestinal stromal tumors. World J. Gastroenterol. 2017;23:4856–4866. doi: 10.3748/wjg.v23.i27.4856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Miettinen M, Lasota J. Gastrointestinal stromal tumors. Gastroenterol. Clin. North Am. 2013;42:399–415. doi: 10.1016/j.gtc.2013.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Poveda A, et al. GEIS guidelines for gastrointestinal sarcomas (GIST) Cancer Treat. Rev. 2017;55:107–119. doi: 10.1016/j.ctrv.2016.11.011. [DOI] [PubMed] [Google Scholar]
- 74.Oppelt PJ, Hirbe AC, Van Tine BA. Gastrointestinal stromal tumors (GISTs): point mutations matter in management, a review. J. Gastrointest. Oncol. 2017;8:466–473. doi: 10.21037/jgo.2016.09.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Overton LC, Heinrich MC. Regorafenib for treatment of advanced gastrointestinal stromal tumors. Expert Opin. Pharmacother. 2014;15:549–558. doi: 10.1517/14656566.2014.877888. [DOI] [PubMed] [Google Scholar]
- 76.Demetri GD, et al. Efficacy and safety of regorafenib for advanced gastrointestinal stromal tumours after failure of imatinib and sunitinib: an international, multicentre, prospective, randomised, placebo-controlled phase 3 trial (GRID) Lancet. 2013;381:9863. doi: 10.1016/S0140-6736(12)61857-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Van Looy T, et al. Characterization and assessment of the sensitivity and resistance of a newly established human gastrointestinal stromal tumour xenograft model to treatment with tyrosine kinase inhibitors. Clin. Sarcoma Res. 2014;4:10. doi: 10.1186/2045-3329-4-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Falkenhorst J, et al. Inhibitor of Apoptosis Proteins (IAPs) are commonly dysregulated in GIST and can be pharmacologically targeted to enhance the pro-apoptotic activity of imatinib. Oncotarget. 2016;7:41390–41403. doi: 10.18632/oncotarget.9159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Galun D, Srdic-Rajic T, Bogdanovic A, Loncar Z, Zuvela M. Targeted therapy and personalized medicine in hepatocellular carcinoma: drug resistance, mechanisms, and treatment strategies. J. Hepatocell. Carcinoma. 2017;4:93–103. doi: 10.2147/JHC.S106529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Huynh H, Ong R, Zopf D. Antitumor activity of the multikinase inhibitor regorafenib in patient-derived xenograft models of gastric cancer. J. Exp. Clin. Cancer Res. 2015;34:132. doi: 10.1186/s13046-015-0243-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Lin XL, et al. Regorafenib inhibited gastric cancer cells growth and invasion via CXCR4 activated Wnt pathway. PLoS ONE. 2017;12:e0177335. doi: 10.1371/journal.pone.0177335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Chen C, et al. A multiplex preclinical model for adenoid cystic carcinoma of the salivary gland identifies regorafenib as a potential therapeutic drug. Sci. Rep. 2017;7:11410. doi: 10.1038/s41598-017-11764-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Hsu FT, et al. Regorafenib induces apoptosis and inhibits metastatic potential of human bladder carcinoma cells. Anticancer Res. 2017;37:4919–4926. doi: 10.21873/anticanres.11901. [DOI] [PubMed] [Google Scholar]
- 84.Stalker L, Pemberton J, Moorehead RA. Inhibition of proliferation and migration of luminal and claudin-low breast cancer cells by PDGFR inhibitors. Cancer Cell Int. 2014;14:89. doi: 10.1186/s12935-014-0089-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Mirantes C, et al. Effects of the multikinase inhibitors sorafenib and regorafenib in PTEN deficient neoplasias. Eur. J. Cancer. 2016;63:74–87. doi: 10.1016/j.ejca.2016.04.019. [DOI] [PubMed] [Google Scholar]
- 86.Hu X, et al. The anti-tumor effect of regorafenib in lung squamous cell carcinoma in vitro. Biochem. Biophys. Res. Commun. 2018;503:1123–1129. doi: 10.1016/j.bbrc.2018.06.129. [DOI] [PubMed] [Google Scholar]
- 87.Tuchen M, et al. Receptor tyrosine kinase inhibition by regorafenib/sorafenib inhibits growth and invasion of meningioma cells. Eur. J. Cancer. 2017;73:9–21. doi: 10.1016/j.ejca.2016.12.004. [DOI] [PubMed] [Google Scholar]
- 88.Breitkreutz I, et al. The orally available multikinase inhibitor regorafenib (BAY 73-4506) in multiple myeloma. Ann. Hematol. 2018;97:839–849. doi: 10.1007/s00277-018-3237-5. [DOI] [PubMed] [Google Scholar]
- 89.Chen Z, et al. Small molecule inhibitor regorafenib inhibits RET signaling in neuroblastoma cells and effectively suppresses tumor growth in vivo. Oncotarget. 2017;8:104090–104103. doi: 10.18632/oncotarget.22011. [DOI] [PMC free article] [PubMed] [Google Scholar]